Abstract
The immune system has the power to modulate the expression of radiation-induced normal and tumor tissue damage. On the one hand, it can contribute to cancer cure, on the other it can influence acute and late radiation side effects, which in many ways resemble acute and chronic inflammatory disease states. The way radiation-induced inflammation feeds into adaptive antigen-specific immune responses adds another dimension to the tumor-host crosstalk during radiation therapy and to possible radiation-driven autoimmune responses. Understanding how radiation impacts inflammation and immunity is therefore critical if we are to effectively manipulate these forces for benefit in radiation oncology treatments.
Keywords: radiation, inflammation, ROS, cytokines, danger, macrophages, TLR
1 RADIATION DAMAGE & ROS: A gift that keeps on giving
Radiation-damaged tissues often show cardinal signs of inflammation. In fact, rubor, tumor, calor, dolor and functio laesa may be reasons for the Radiation Oncologist to limit treatment dose. While the physicochemical and free radical events are over within a microsecond of radiation exposure, the inflammatory response that ensues perpetuates the response by generating recurring waves of ROS, cytokines, chemokines and growth factors with associated inflammatory infiltrates.1, 2 As a result, radiation-induced damage in normal tissues evolves with time as it is impacted by the various regulatory immune mechanisms associated with wound healing.3 There are many aspects to these complex scenarios but the progressive nature of events is in common. One example is the rapid remodeling of the extracellular matrix in an irradiated site that goes on for long after “healing” seems to be over.4 Some of the ongoing events may be impacted by processes that are specific to irradiation. For example, although hypoxia is part of normal wound healing control mechanisms that drive angiogenesis, this may be dysregulated by radiation damage to the microvasculature causing the default vasculogenesis pathway to be engaged in an irradiated site.5
Importantly, the persistent crosstalk between the radiation-damaged lesion and the immune system in turn initiates inflammatory loops that feed back to the bone marrow to mobilize inflammatory cells, with further systemic consequences, as will be discussed later. One such consequence of radiation-induced inflammation is that it can lead to out-of-field effects.1, 6 That the damage response can extend beyond the locally irradiated area was shown for example by bilateral pneumonitis that can occur after unilateral irradiation.7 Indirect support for a self-perpetuating pro-oxidant and pro-inflammatory scenario stems from the fact that non-steroidal anti-inflammatory drugs and antioxidants can alleviate some of that latent damage, at least in vivo, as well as reduce inflammation-induced mutations.8-10
Multiple features determine the host tissue response. There is a genetic component that dictates how different tissues deal with external and internal threats such as pathogens11, 12 and injury, and the acute and late effects that are manifested following irradiation reflect this link. For example, different strains of mice develop pneumonitis or fibrosis following lung irradiation depending on the genetics of the host.13 The nature of the response that is stimulated however depends also on the type of the tissue.14 This is most obvious in tumor models where the tumor-host relationships are dictated by the type of the tumor and the genetic make-up of the host. As a result, palpable tumors have a chronic host cell infiltrate that may be dynamic, but is relatively stable functionally and phenotypically. This is exemplified by the fact that experimentally transplantable tumors have highly reproducible, characteristic infiltrates.15 This will not necessarily be the case for early lesions, which generate acute responses that may feed back and shape the tumor phenotype through immunoediting.16 Acute pro-inflammatory responses with highly oxidative stress may also contribute to tumor heterogeneity by generating additional genomic diversity.
In most cases, by the time radiation therapy is initiated the cancer already exists in a relatively stable state of equilibrium with inflammatory host cells and hypoxia already present. The impact of irradiation, and probably the outcome of treatment, will therefore depend upon the preexisting status quo. One contribution to the preexisting condition is the oxidative stress that tumors are under. This is often poorly defined but its consequences can be seen in the abnormalities in anti-oxidant genes, such as those in the NF-E2-related factor 2 (Nrf2) pathway in many human cancers. For example, Kelch-like ECH-associated protein 1 (Keap1) the factor that controls Nrf2 expression is frequently mutated (loss-of-function) in adenocarcinomas of the lung while Nrf2 itself is often mutated (gain-of-function) in lung squamous cell.17 Since Nrf2 controls numerous anti-oxidant genes, such targeted mutations that enhance expression may have major consequences for response to radiation therapy.18-20 Furthermore, Nrf2, by controlling the redox balance in the cell can also dictate the Th1-M1/Th2-M2 equilibrium.21 When Nrf2 is absent, for instance, murine splenocytes respond to external stimulation with a seriously magnified IFN-γ profile while the IL-4/Th2 axis seems unaffected (Figure 1). Tumors that have Nrf2 pathway activating mutations are therefore likely to be in an anti-oxidant state, less likely to generate inflammation following irradiation, less likely to generate anti-tumor immunity, and less likely to respond to radiation therapy.
Figure 1.
Nrf2 prevents excessive Th1 signaling in activated T cells. Splenocytes were isolated from Nrf2−/− mice and stimulated in vitro with aCD3/CD28 with or without LPS. IL-4 and IFN-γ releasing splenocytes were enumerated 36h later by ELISPOT and compared to splenocyte responses from C57Bl/6 WT mice.
2 RESPONSE: Balances of opposing forces
General principles of immune regulation in normal and tumor tissues have been derived from animal models. These have taught us that irradiated tissues generally interact with the innate immune system in a manner similar to those damaged by pathogens.22 One common resulting paradigm is stimulation of granulocyte-macrophage colony formation with entry of early myeloid progenitors into the circulation and into the inflamed site within hours of irradiation.23 Such cells are often referred to as myeloid-derived suppressor cells (MDSC)24 but they may be of a broader functional phenotype as they are generated under the influence of multiple cytokine pathways, with SDF-1 and CSF-1 probably the most important.25-27 MDSC differentiate into more mature granulocytes and macrophages with time, the granulocytes being short-lived while the macrophage response may increase over 1-2 weeks with the potential of being long lasting. In irradiated tumors, granulocytes gravitate towards areas of necrosis, while tumor-associated macrophages (TAM) infiltrate areas of hypoxia amplified by radiation-induced vascular damage.28 These myeloid cells may differentiate further and join pre-existing TAMs throughout the tumor mass. In normal and tumor tissues, these infiltrating macrophages should be considered as being distinct from resident tissue macrophages and pre-existing TAMs as they have obvious inflammatory functions.
Inflammatory macrophages are fascinating cells with a myriad of tools at their disposal including the ability to produce high levels of pro-inflammatory or anti-inflammatory cytokines and ROS/RNS and high pro-/anti-oxidants. They may even differentiate into antigen presenting cells in the right milieu and attract lymphocytic immune cell infiltrates so as to allow immunologically adaptive responses or, alternatively, they may drive angiogenesis and wound healing.3 They also appear to be required for stimulating growth of primary tumors and metastases.23, 28
These divergent functions reflect a basic principle of the immune system, namely a network of finely tuned but opposing cellular and soluble forces that appear functionally linked to classes of cytokines and to redox status.2 In the world of inflammation orchestrated by macrophages, the extremes of these forces are exemplified by two major phenotypes: the pro-inflammatory, iNOS expressing and ROS/IL-12 releasing M1 “killer” type that enhances antigen-specific responses and that are induced by Toll-like receptors and interferon gamma and the alternatively activated M2 “healer” type that is induced by IL-4/IL-13, IL-10, or TGF-β and that are characterized by production of factors such as HIF-1, arginase, IL-10, IL-6 and VEGF which push angiogenesis, wound healing, and fibrosis and that are generally immunosuppressive.29 M1/2 are equivalents to the Th1/2 system in T cell biology and M1 and T1 cells tend to collaborate to reinforce their pro-inflammatory functions in cellular immunity, while Th2 and Treg cells do the same with M2 cells to emphasize an immunosuppressive environment.
This immune dichotomy is generally valid and important in responses to pathogens and tumors. TAMs in most tumors seem to lean towards an M2 phenotype that promotes angiogenesis, tumor growth and metastasis.30, 31 However, in more immunogenic tumors, M1 and Th1 functions may predominate, at least while tumors are of moderate size. Such tumors proliferate rapidly and do not appear to metastasize because the small metastatic deposits they generate are effectively eliminated by the adaptive immune system.32 When such tumors are small they appear able to escape the immune system by inducing tolerance.33 It is only when they grow larger that they can break through to switch on anti-tumor immunity. At this time TAMs that can present tumor antigens34 can be detected but the immune response is rapidly down regulated by Tregs and M2 cells that emerge to control the Th1/M1 response, ultimately allowing these tumors once more to escape immune surveillance.35 As they become very large non-specific, alternatively activated, immunosuppressive macrophages effectively shut down the immune system making individuals unresponsive to most antigens.35 Importantly, primary tumors of this type are easily controlled by radiation therapy with a good prognosis.32 In contrast, poorly immunogenic tumors tend to metastasize early and are more likely to generate MDSCs and M2 populations. Such tumors typically portend a poor patient prognosis.36-38
A major issue for understanding this aspect of radiation therapy is the extent to which tumor irradiation can switch the anti-oxidant/anti-inflammatory status of the tumor microenvironment to one that is pro-oxidant/pro-inflammatory and that favors tumor immunity. We know that in vitro irradiation can enhance the maturation of antigen presenting dendritic cells that makes them more able to cross present tumor antigens, in addition to the phenotypic changes that accompany pro-inflammatory cytokine production.39 In general, macrophages seem more adaptable with respect to their phenotypes than dendritic cells. In fact, macrophages appear to have a plasticity unmatched by any other cell type and they readily respond to environmental cues. This plasticity may be one reason why the literature can be conflicting concerning their roles. For example, iNOS production can be associated with tumor growth promoting M2 TAMs40 as well as being an M1 characteristic; perhaps different levels of iNOS being involved. It should also be remembered that there are different macrophage subtypes, that resident macrophages in tissues are functionally very different from bone-marrow mobilized macrophages and that resident macrophages show distinct tissue-specific features. They also respond to in vivo/in vitro handling and simply by placing them in vitro one may stimulate high levels of cytokine production,41 making it difficult to interpret any response to irradiation or other stimuli.
In vivo, it seems that radiation, at least in the tumor models that have been studied, mostly reinforces the preexisting phenotypic proclivities.42, 43 This may also be the case in normal tissues. Some of the contradictory reports on the effects of radiation on macrophage polarization seem primarily due to differences in the genetic make-up of the model host that can dictate preferential M1/M2 polarization at baseline as well as following treatment.44 However, irradiation may alter the preexisting equilibrium under some circumstances and it seems important to understand how this might be modulated. One way is through infiltrating MDSCs that are generally immunosuppressive but may be modifiable.27 There is some evidence that radiation at lower doses can reprogram macrophages towards a iNOS/M1 phenotype that in turn mediates vascular normalization, T cell recruitment and promotes efficient tumor rejection,45 although the general applicability of this concept has yet to be tested. The role of T cells in determining TAM status has however considerable support. In models where irradiation activates tumor immunity, Th1 cells appear to play a major role in redirecting the tumor microenvironment, including TAMs to M1, and towards enhanced anti-tumor cellular responses. It has long been known that the size of the radiation dose matters in particular for late responding normal tissues, but it also influences the extent of vascular damage and tumor cell death. This could be an important determinant of subsequent immune and inflammatory disturbances, be they pro- or anti-inflammatory.46, 47 Clearly, one cannot ignore the potential implication of this in light of the recent trends in treating tumors with more sophisticated IMRT-based, higher dose per fraction radiation delivery systems and increased efforts to combine radiation with immunotherapy. It should be said that often dose schedules that are isoeffective with respect to tumor cure are not compared where tumor immunity is concerned, leaving some issues unanswered.
3 CONSEQUENCES: Radiation damage links inflammation to immune activation
It is becoming increasingly clear that one way to immunologically target a tumor is to re-connect a broken link between an innate immune recognition of the tumor with the initiation of the adaptive arm of the immune system. It appears that the Type I IFN signature in the tumor microenvironment can do just that, namely initiate antigen cross-presentation by DCs, efficient T cells priming and tumor rejection.48-51
We know that irradiated tissues can have the same IFN signature 50 presumably through the release of “danger” signals (or Damage-Associated Molecular Pattern molecules; DAMPs) such as dsDNA, RNA, chromatin and HMGB-1 from stressed and dying cells. DAMPs then get recognized through common pattern recognition receptors (PRR) of the Toll like receptor (TLR) family, analogous to the recognition of pathogenic danger signals (or PAMPs). 52-57 The immune system has evolved to constantly scan for these danger signals and to swiftly act upon their detection. This is mostly done by macrophages through cytokine and chemokine networks that guide other immune cells to eliminate that danger and to turn the pro-inflammatory, prooxidant environment into one that is more compatible with homeostasis and healing.2, 3 Indeed there is increasing evidence in the literature for radiation-driven TLR activation.58 A case in point is the observation that 4Gy irradiation of primary human (but not murine) lymphocytes can drive gene expression of most of the TLR family members.59 Some of this innate TLR induction may actually be an integral part of the DNA damage response following radiation exposure and even lead to broad stress adaptation.60
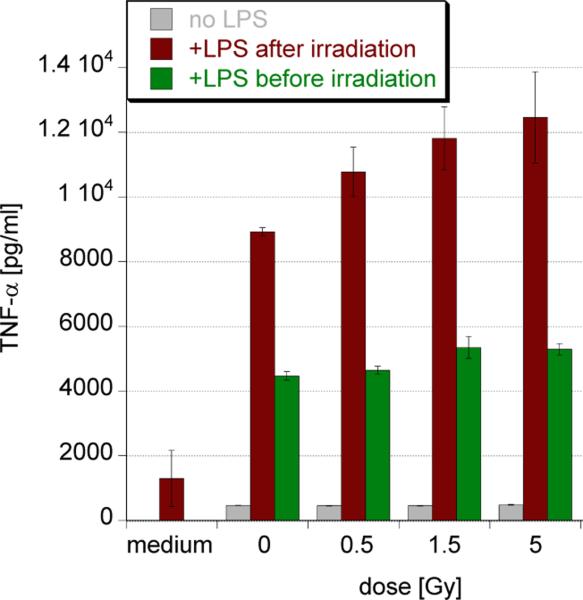
A crucial consideration of the role of danger signaling in radiation responses is how it interacts with more classical triggers. From in vitro studies we know that danger signals such as LPS potently stimulate murine macrophages to release TNF-α, a bonafide pro-inflammatory cytokine, and that this is more intense in macrophages that had previously been irradiated (Figure 2). However, this is not the case when radiation is given after LPS. Irradiation therefore primes these cells for further TLR responses, which could be clinically highly relevant to the generation of immunity. In light of the fact that LPS and many other TLR agonists are much more potent radioprotectors61, 62 than they are mitigators63 at least of acute hematopoietic radiation syndrome in vivo (Cheng, unpublished), one has to wonder whether these observations are in fact related to danger perception and signaling by macrophages and what importance has the timing between signal 1 (DAMPs/PAMPs) to signal 2 (radiation) and the order in which they are received.
Figure 2.
Radiation primes macrophages for pro-inflammatory signaling when given prior to TLR engagement. RAW264.7 murine macrophages were stimulated with 10ng/ml LPS either 30mins before or after X-irradiation. Supernatants were harvested after 4h of stimulation and assayed for TNF-α production by ELISA.
Of the diverse signaling network that lies downstream of TLR activation,64 it seems that the STING pathway sensing danger from cytosolic DNA may be a critical link between radiation-damaged, dying normal and tumor cells and type I IFN induction by DCs that drive adaptive immunity.49, 65 Indeed, the effectiveness of many cancer therapies, including radiation, seems to partially rely on intact danger signaling as patients without functional TLR4 were found to relapse quicker, even though it is not clear whether this extends to all of the TLR signaling network and to all tumors.66-68 Indirect evidence comes from activated lymphocytes being found in irradiated tissues both during acute and chronic phases and from radiation-induced autoimmunity.69-75
Conversely, one would expect that inhibiting this kind of danger signaling and hence immune recognition in the aftermath of radiation exposure would limit immune activation and progressive normal tissue inflammation and in doing so, aid recovery.76 In fact, this is precisely what we observed in lethally, whole-body irradiated mice that better survived acute radiation syndrome when treated with the inhibitor of HMGB-1 danger signaling, glycyrrhizin, (Figure 3). This dichotomy between macrophage-driven inflammatory resolution and normal tissue protection versus prolonged, pro-inflammatory tissue damage and anti-tumor immune activation was eloquently discussed by Gough et al.77 and is not unfamiliar to radiation oncologists thriving to maximize tumor cell kill at minimum normal tissue damage. In other words, the crucial link to anti-tumor immune activation may be time-sensitive and potentially lost as the normal tissue attempts to heal and resolve inflammation in the aftermath of radiation exposure (Figure 4).
Figure 3.
Inhibition of HMGB-1 aids the recovery of whole-body irradiated mice. Male C3H mice were lethally irradiated with 8Gy and treated with s.c. 330mg/kg Glycyrrhizin everyday for 5 days starting 24h post exposure. Survival was monitored over 30days. N=8 animals/group.
Figure 4.
Recurring waves of inflammation link radiation-induced tissue damage to anti-tumor immune activation with potentially excessive normal tissue damage. This is being offset by attempts to resolve the inflammatory lesion, to heal and to regulate immunity at the price of possible tumor immune escape. Black (tumor), red (inflammation), green (healing).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kim K, McBride WH. Modifying radiation damage. Curr Drug Targets. 2010;11:1352–1365. doi: 10.2174/1389450111009011352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaue D, McBride WH. Links between innate immunity and normal tissue radiobiology. Radiat Res. 2010;173:406–417. doi: 10.1667/RR1931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment -tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 5.Chen FH, Chiang CS, Wang CC, et al. Vasculatures in tumors growing from preirradiated tissues: formed by vasculogenesis and resistant to radiation and antiangiogenic therapy. Int J Radiat Oncol Biol Phys. 2011;80:1512–1521. doi: 10.1016/j.ijrobp.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 6.Rastogi S, Coates PJ, Lorimore SA, et al. Bystander-type effects mediated by long-lived inflammatory signaling in irradiated bone marrow. Radiat Res. 2012;177:244–250. doi: 10.1667/rr2805.1. [DOI] [PubMed] [Google Scholar]
- 7.Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys. 1995;31:361–369. doi: 10.1016/0360-3016(94)00477-3. [DOI] [PubMed] [Google Scholar]
- 8.Khan MA, Hill RP, Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int J Radiat Oncol Biol Phys. 1998;40:467–476. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee D, Coates PJ, Lorimore SA, et al. The in vivo expression of radiation-induced chromosomal instability has an inflammatory mechanism. Radiat Res. 2012;177:18–24. doi: 10.1667/rr2793.1. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D, Coates PJ, Lorimore SA, et al. Responses to ionizing radiation mediated by inflammatory mechanisms. J Pathol. 2014;232:289–299. doi: 10.1002/path.4299. [DOI] [PubMed] [Google Scholar]
- 11.van de Vosse E, van Dissel JT, Ottenhoff TH. Genetic deficiencies of innate immune signalling in human infectious disease. Lancet Infect Dis. 2009;9:688–698. doi: 10.1016/S1473-3099(09)70255-5. [DOI] [PubMed] [Google Scholar]
- 12.Jin P, Wang E. Polymorphism in clinical immunology - From HLA typing to immunogenetic profiling. J Transl Med. 2003;1:8. doi: 10.1186/1479-5876-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 15.McBride WH. Phenotype and functions of intratumoral macrophages. Biochim Biophys Acta. 1986;865:27–41. doi: 10.1016/0304-419x(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 17.Clinical Lung Cancer Genome P, Network Genomic M A genomics-based classification of human lung tumors. Science translational medicine. 2013;5:209ra153. doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald JT, Kim K, Norris AJ, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70:8886–8895. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A, Bodas M, Wakabayashi N, et al. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal. 2010;13:1627–1637. doi: 10.1089/ars.2010.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata T, Kokubu A, Saito S, et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864–873. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockwell CE, Zhang M, Fields PE, et al. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J Immunol. 2012;188:1630–1637. doi: 10.4049/jimmunol.1101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBride WH, Chiang CS, Olson JL, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 23.Ahn GO, Tseng D, Liao CH, et al. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Xie C, Ma H, et al. Gr-1+CD11b+ cells facilitate Lewis lung cancer recurrence by enhancing neovasculature after local irradiation. Sci Rep. 2014;4:4833. doi: 10.1038/srep04833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozin SV, Kamoun WS, Huang Y, et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie MW, Gorodetsky R, Micewicz ED, et al. Marrow-derived stromal cell delivery on fibrin microbeads can correct radiation-induced wound-healing deficits. J Invest Dermatol. 2013;133:553–561. doi: 10.1038/jid.2012.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen FH, Chiang CS, Wang CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res. 2009;15:1721–1729. doi: 10.1158/1078-0432.CCR-08-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- 32.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229–1235. [PubMed] [Google Scholar]
- 33.McBride WH, Howie SE. Induction of tolerance to a murine fibrosarcoma in two zones of dosage--the involvement of suppressor cells. Br J Cancer. 1986;53:707–711. doi: 10.1038/bjc.1986.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dougherty GJ, McBride WH. Immunoregulating activity of tumor-associated macrophages. Cancer Immunol Immunother. 1986;23:67–72. doi: 10.1007/BF00205558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howie S, McBride WH. Tumor-specific T helper activity can be abrogated by two distinct suppressor cell mechanisms. Eur J Immunol. 1982;12:671–675. doi: 10.1002/eji.1830120809. [DOI] [PubMed] [Google Scholar]
- 36.Cole S, Montero A, Garrett-Mayer E, et al. Elevated Circulating Myeloid Derived Suppressor Cells (MDSC) Are Associated with Inferior Overall Survival (OS) and Correlate with Circulating Tumor Cells (CTC) in Patients with Metastatic Breast Cancer. Cancer Res. 2009;69 Abstract nr 4135. [Google Scholar]
- 37.Takanami I, Takeuchi K, Kodaira S. Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology. 1999;57:138–142. doi: 10.1159/000012021. [DOI] [PubMed] [Google Scholar]
- 38.Busuttil RA, George J, Tothill RW, et al. A signature predicting poor prognosis in gastric and ovarian cancer represents a coordinated macrophage and stromal response. Clin Cancer Res. 2014;20:2761–2772. doi: 10.1158/1078-0432.CCR-13-3049. [DOI] [PubMed] [Google Scholar]
- 39.Liao YP, Wang CC, Butterfield LH, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immunol. 2004;173:2462–2469. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 40.Tsai CS, Chen FH, Wang CC, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys. 2007;68:499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 41.Chiang CS, Chen FH, Hong JH, et al. Functional phenotype of macrophages depends on assay procedures. Int Immunol. 2008;20:215–222. doi: 10.1093/intimm/dxm137. [DOI] [PubMed] [Google Scholar]
- 42.Chiang CS, Fu SY, Wang SC, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell JS, Brown JM. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery. Front Physiol. 2013;4:157. doi: 10.3389/fphys.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coates PJ, Rundle JK, Lorimore SA, et al. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. 2008;68:450–456. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- 45.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Rodel F, Frey B, Multhoff G, et al. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2013 doi: 10.1016/j.canlet.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Hellevik T, Martinez-Zubiaurre I. Radiotherapy and the Tumor Stroma: The Importance of Dose and Fractionation. Front Oncol. 2014;4:1. doi: 10.3389/fonc.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X, Zhang X, Fu ML, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 53.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 54.Sato Y, Goto Y, Narita N, et al. Cancer Cells Expressing Toll-like Receptors and the Tumor Microenvironment. Cancer Microenviron. 2009;2(Suppl 1):205–214. doi: 10.1007/s12307-009-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 56.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 58.El-Saghire H, Michaux A, Thierens H, et al. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med. 2013;32:1407–1414. doi: 10.3892/ijmm.2013.1514. [DOI] [PubMed] [Google Scholar]
- 59.Menendez D, Shatz M, Azzam K, et al. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 2011;7:e1001360. doi: 10.1371/journal.pgen.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ermolaeva MA, Segref A, Dakhovnik A, et al. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature. 2013;501:416–420. doi: 10.1038/nature12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C, Zhang C, Mitchel RE, et al. A critical role of toll-like receptor 4 (TLR4) and its' in vivo ligands in basal radio-resistance. Cell Death Dis. 2013;4:e649. doi: 10.1038/cddis.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shakhov AN, Singh VK, Bone F, et al. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). PLoS One. 2012;7:e33044. doi: 10.1371/journal.pone.0033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 65.Kondo T, Kobayashi J, Saitoh T, et al. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A. 2013;110:2969–2974. doi: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 67.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao C, Kozlowska A, Nechaev S, et al. TLR9 signaling in the tumor microenvironment initiates cancer recurrence after radiotherapy. Cancer Res. 2013;73:7211–7221. doi: 10.1158/0008-5472.CAN-13-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong JH, Jung SM, Tsao TC, et al. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int J Radiat Biol. 2003;79:159–167. doi: 10.1080/0955300031000076894. [DOI] [PubMed] [Google Scholar]
- 70.Chiang CS, Liu WC, Jung SM, et al. Compartmental responses after thoracic irradiation of mice: strain differences. Int J Radiat Oncol Biol Phys. 2005;62:862–871. doi: 10.1016/j.ijrobp.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 71.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. doi: 10.1080/02841860510007440. [DOI] [PubMed] [Google Scholar]
- 72.Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008;25:51–58. doi: 10.1007/s10014-008-0233-9. [DOI] [PubMed] [Google Scholar]
- 73.Moravan MJ, Olschowka JA, Williams JP, et al. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. 2011;176:459–473. doi: 10.1667/rr2587.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toma CL, Serbescu A, Alexe M, et al. The bronchoalveolar lavage pattern in radiation pneumonitis secondary to radiotherapy for breast cancer. Maedica (Buchar) 2010;5:250–257. [PMC free article] [PubMed] [Google Scholar]
- 75.Teymoortash A, Simolka N, Schrader C, et al. Lymphocyte subsets in irradiation-induced sialadenitis of the submandibular gland. Histopathology. 2005;47:493–500. doi: 10.1111/j.1365-2559.2005.02256.x. [DOI] [PubMed] [Google Scholar]
- 76.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010:2010. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gough MJ, Young K, Crittenden M. The impact of the myeloid response to radiation therapy. Clin Dev Immunol. 2013;2013:281958. doi: 10.1155/2013/281958. [DOI] [PMC free article] [PubMed] [Google Scholar]