Abstract
Background
There is a significant need for rapid and cost-effective biomarkers of Alzheimer’s disease (AD) for advancement of clinical practice and therapeutic trials.
Objective
The aim of the current study was to cross-validate our previously published serum-based algorithm on an independent assay platform as well as validate across tissues and species. Preliminary analyses were conducted to examine the utility in distinguishing AD from non-AD neurological disease (Parkinson’s Disease).
Methods
Serum proteins from our previously published algorithm were quantified from 150 AD cases and 150 controls on the Meso Scale Discovery (MSD) platform. Serum samples were analyzed from 49 Parkinson’s disease (PD) cases and compared to a random sample of 51 AD cases and 62 controls. Support vector machines (SVM) were used to discriminate PD vs. AD vs. NC. Human and AD mouse model microvessel images were quantified with HAMAMATSU imaging software. Mouse serum biomarkers were assayed via MSD.
Results
Analysis of 21 serum proteins from 150 AD cases and 150 controls yielded an algorithm with sensitivity and specificity of 0.90 for correctly classifying AD. This multi-marker approach was then validated across species and tissue. Assay of the top proteins in human and AD mouse model brain microvessels correctly classified 90–100% of the samples. SVM analyses were highly accurate at distinguishing PD vs. AD vs. NC.
Conclusions
This serum-based biomarker panel should be tested in a community-based setting to determine its utility as a first-line screen for AD and non-AD neurological diseases for primary care providers.
Keywords: Alzheimer’s Disease, blood-based biomarkers, tissue, species, serum
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative dementia with over 5 million Americans affected by the disease. Every 68 seconds an American develops AD, which is the fifth leading cause of death for those over the age of 65 [1]. AD has an annual health care cost similar to that of cardiovascular disease (CVD) and higher than cancer [2]. While death rates from CVD and cancer have declined in recent decades, death rates have steadily increased for AD [3]. However, there are currently no rapid, cost-effective means for detecting the disease, which is needed to move clinical and research efforts forward. Current state-of-the-art diagnosis of AD is obtained through specialty clinic settings incorporating medical examinations, neuropsychological evaluations, clinical blood work, and neuroimaging [4]; this process has been validated against autopsy findings [5]. On the other hand, AD is common but under diagnosed in primary care settings [5–7].
While the search for blood-based biomarkers of AD was largely unsuccessful for decades, recent work shows great promise. In a seminal study, Ray and colleagues [8] analyzed 120 plasma- proteins and developed an algorithm that accurately distinguished AD patients from healthy controls (89% accuracy) as well as accurately identified patients with mild cognitive impairment (MCI) patients who progressed to AD (81% accuracy) [8]. This study represented the first major support for the notion that an AD biomarker profile can yield excellent accuracy. However, enthusiasm waned when the findings did not cross-validate on an independent assay platform [9]. Despite this initial setback, others have continued to identify promising signals in peripheral blood suggesting that a blood-based AD screen is on the horizon [10–21].
We have analyzed serum proteins from AD and control subjects in the Texas Alzheimer’s Research & Care Consortium (TARCC) to generate [15] and refine [13] a biomarker algorithm that yielded excellent accuracy for AD identification [13,15]. Additionally, our group was the first to cross-validate a blood-based approach across independent cohorts [16], which has now been accomplished by the Australian Imaging, Biomarkers and Lifestyle (AIBL) group [20]. However, a large portion of the recent literature including those from the TARCC [15], the Alzheimer’s Disease Neuroimaging Initiative (ADNI)[16, 22], AIBL [20], Washington University St. Louis [22], and University of Pennsylvania [22] have utilized the same luminex-based multi-plex platform rather than seek cross-validation in independent assay platforms, which is the point where the Ray et al procedure failed to validate [9]. Kiddle et al [23] recently demonstrated that select blood biomarkers of AD can be cross-validated across an independent platform. The identification of a blood-based AD screening tool that can be implemented by any clinical or research laboratory across the globe would be an important advancement and is preferable to a model requiring a single isolated reference laboratory. The primary purpose of the current study was to cross-validate our prior blood-based algorithm on an independent assay platform that can be easily implemented across multiple laboratory settings. The current study was also undertaken to directly compare serum vs. plasma based biomarker algorithms. We also sought to validate the approach across tissues (serum and brain microvessels) and species (humans and AD mouse models) given the recent suggestion that biomarkers identified in human studies should be translated back to animal models for further validation [24]. Lastly, we sought to conduct preliminary analyses on the ability of the algorithm to distinguish AD from a non-AD neurological disease (Parkinson’s disease).
Methods
Participants
AD and control patients
Serum samples from TARCC participants (150 AD cases, 150 controls) were analyzed. Details on blood collection methods are described elsewhere [15, 16]. Additionally, 200 plasma samples (100 AD cases and 100 controls), from the same TARCC subject group were analyzed. Briefly, each participant undergoes an annual standardized assessment at one of the five participating TARCC sites that includes a medical evaluation, neuropsychological testing, and a blood draw. Diagnosis of AD is based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [25]. Controls participants were those within the sample who were determined through a consensus review process to have performed within normal limits on psychometric testing. Normal limits were defined as performance for which is expected given age and education level and for which did not meet NINCDS-ADRDA criteria for cognitive impairment. Institutional Review Board approval was obtained at each site and written informed consent is obtained for all participants. Parkinson’s disease (PD) patients. Serum samples from 49 patients (28 males and 21 females) diagnosed with Parkinson’s disease (PD) came from the University of Texas Southwestern Medical Center (UTSW) Movement Disorders Clinic.
Blood Sample Collection
Serum
Samples were collected as follows: (1) non-fasting serum samples were collected in 10ml tiger-top tubes, (2) allowed to clot for 30 minutes at room temperature in a vertical position, (3) centrifuged for 10 minutes at 1300 × g within one hour of collection, (4) 1.0 ml aliquots of serum were transferred into cryovial tubes, (5) Freezerworks™ barcode labels were firmly affixed to each aliquot, and (6) samples placed into −80° C freezer within two hours of collection for storage until use in an assay. Plasma. (1) non-fasting blood was collected into 10ml lavender-top tubes and gently invert 10–12 times, (2) centrifuge tubes at 1300 × g for 10 minutes within one hour of collection, (3) transfer 1ml aliquots to cryovial tubes, (4) affix Freezerworks™ barcode labels, and (5) placed in −80° C freezer within 2 hours of collection for storage.
Serum from control and transgenic AD mice
Serum samples from nine 3XTg AD (B6;129-Psen1tm1Mpm Tg [APPSwe,tauP301L]1Lfa/Mmjax; Stock # 004807, The Jackson Laboratory, Bar Harbor, ME) and 9 control mice were analyzed using electrochemiluminescence (ECL) as was done with the human samples. Based on the findings from our human serum assays, we choose to analyze 4 serum protein markers in the mice (IL5, IL6, IL10 and TNFα). Institutional Animal Ethics approval was obtained.
Assays
All samples were assayed in duplicate via a multi-plex biomarker assay platform using ECL on the SECTOR Imager 2400A from Meso Scale Discovery (MSD; http://www.mesoscale.com). The MSD platform has been used extensively to assay biomarkers associated with a range of human diseases including AD [26–30]. ECL measures have well-established properties of being more sensitive and requiring less sample volume than conventional ELISAs [28], the gold standard for most assays. The markers assayed were from our previously generated and cross-validated AD algorithm[13,15,16] and included: fatty acid binding protein (FABP3), beta 2 microglobulin, pancreatic polypeptide (PPY), sTNFR1, CRP, VCAM1, thrombopoeitin (THPO), α2 macroglobulin (A2M), exotaxin 3, tumor necrosis factor α, tenascin C, IL-5, IL6, IL7, IL10, IL18, I309, Factor VII, TARC, SAA, and ICAM1. Mouse serum samples were assayed using ECL and the Proinflammatory Panel 1 (mouse) from MSD.
Immunofluorescent Staining of Human and Mouse Cerebromicrovasculature for IL-6 and TNFα
Based on our human data, we chose to assay IL-6 and TNFα in microvessels of humans and mouse AD models. Adult (12 month old) wild-type (control) and Tg2576 mice were purchased from Taconic (Hudson, NY). Immunofluorescent analysis of human and mouse brain tissue sections was performed as previously described [31, 32]. Briefly, human or mice brain tissue paraffin sections (5–7 µm) were deparaffinized in xylene, hydrated through a graded alcohol series, and then rinsed for 5 min in deionized water. Sections were subjected to heat-induced epitope antigen retrieval, washed with Tris-buffered saline and incubated at 4°C overnight with primary antibodies against IL-6 (ab6672, Abcam, Cambridge, MA), TNFα (ab6671, Abcam) or the control-marker - endothelial cell marker von Willebrand Factor (vWF, sc8068, Santa Cruz Biotechnology, Santa Cruz, CA) in IHC-Tek antibody diluent (IHCWORLD, Woodstock, MD). Sections were then washed, incubated with appropriate secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 594 viewed using an Olympus IX71 microscope and images quantified with HAMAMATSU imaging software (www.hamamatsu.com).
Statistical Analyses
Chi square and t-tests were used to compare case versus controls for categorical variables (APOE ε4 allele frequency, gender, race, ethnicity, presence of cardiovascular risk factors) and continuous variables (age, education, Mini Mental State Exam [MMSE] and clinical dementia rating sum of boxes scores [CDR-SB]), respectively. The biomarker data were transformed using the Box-Cox transformation. The random forest (RF) prediction model was performed using R package randomForest (V 4.5) [33], with all software default settings. The ROC (receiver operation characteristic) curves were analyzed using R package and AUC (area under the curve) was calculated using R package DiagnosisMed (V 0.2.2.2). The AD and control samples were randomly divided into training and test samples separately for serum and plasma markers. The RF model was generated in the training set and then applied to the test set. Logistic regression was used to combine demographic data (i.e. age, gender, education, and APOE4 presence [yes/no]) with the RF risk score as was done in our prior work [13, 15–17]. Clinical variables were added to create a more robust diagnostic algorithm given the prior work documenting a link between such variables and cognitive dysfunction in AD [34–37]. In order to further refine the algorithm, the biomarker risk score was limited to the smallest set of markers that retained optimal diagnostic accuracy as a follow-up analysis. In order to demonstrate that our approach cross-validates using human and mouse-model microvessel data, logistic regression was utilized to categorize AD versus normal control using proteins that overlap with those from our algorithm (IL6 and TNFα). Disease status (AD vs. normal) was the outcome variable with both markers entered simultaneously as predictor variables. For the peripheral mouse serum analyses, a logistic regression model was created with AD vs. control as the outcome variable with the four markers (IL5, IL6, IL10 and TNFα) entered as the predictor variables. For the AD vs. PD vs. NC analyses, a random sample of 51 AD cases and 62 controls utilized in the first set of analyses was selected and combined with serum data 49 PD cases. Due to small sample size per diagnostic group, the sample could not be split into a training and test sample. Therefore, the SVM profile was generated in the entire sample and applied to the total sample using five-fold cross-validation. SVM is based on the concept of decision planes that define decision boundaries and is primarily a method that performs classification tasks by constructing hyperplanes in a multidimensional space that separates cases of different class labels.
Results
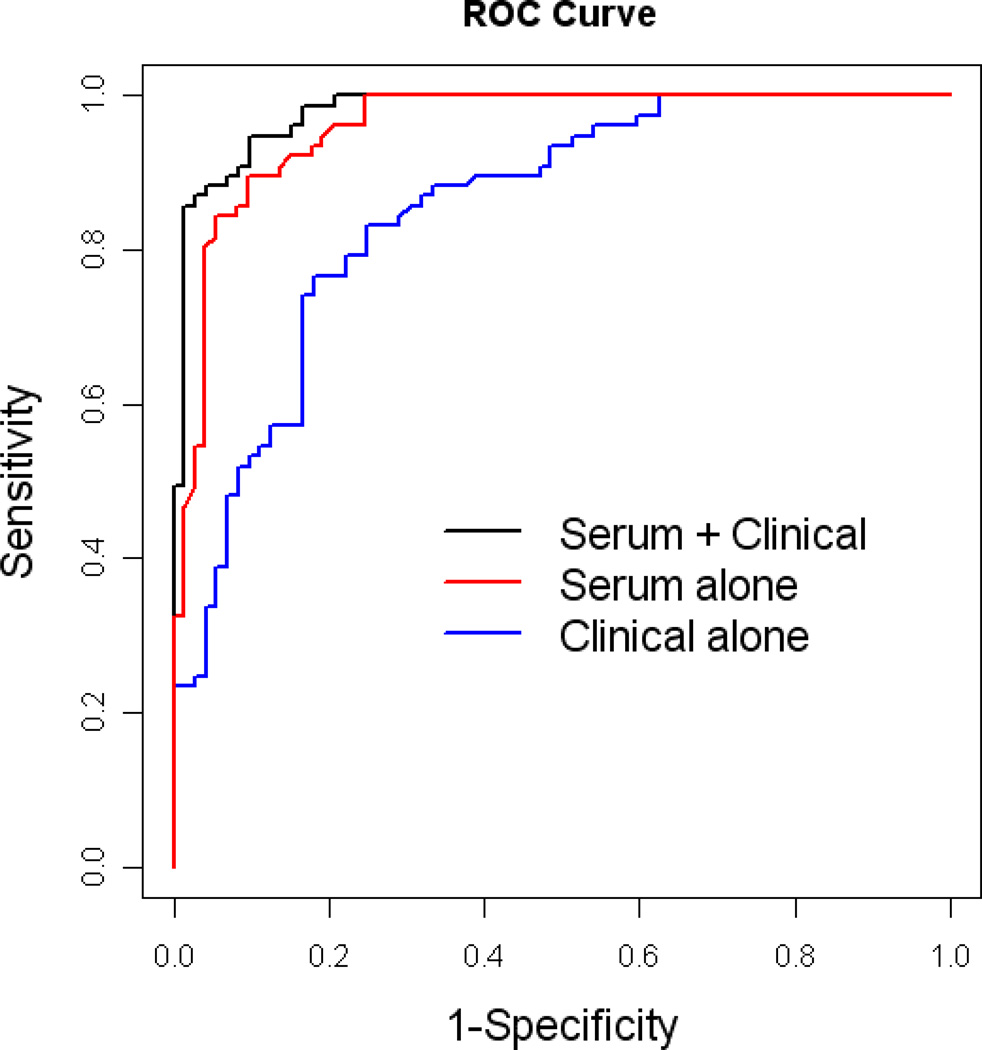
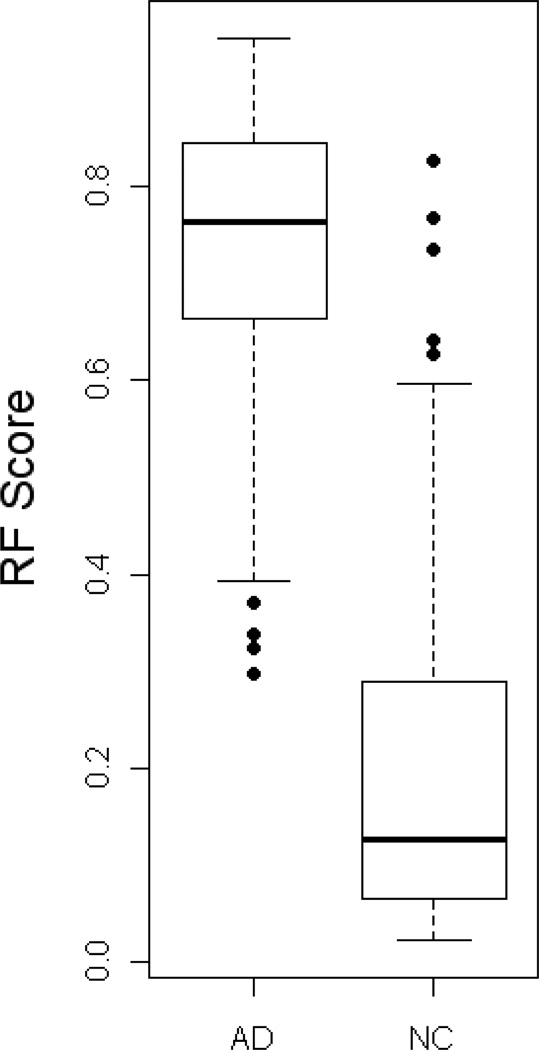
The demographic characteristics of the AD and normal control samples are presented in Table 1. There were 150 AD subjects and 150 normal controls used for serum analysis examining levels of 21 proteins. Using random forest (RF) analysis of the serum protein levels, we found a sensitivity (SN) of 0.90, specificity (SP) of 0.90 and area under the ROC curve (AUC) of 0.96 (See Figures 1–3 and Table 2). Examination of plasma samples from the same subjects resulted in an algorithm with a much lower accuracy estimate of SN, SP, and AUC of 0.65, 0.79, and 0.76, respectively. Therefore, the remaining analyses focused solely on serum. Inclusion of age, gender, education, and APOE4 genotype into the algorithm with the RF biomarker profile increased SN, SP, and AUC to 0.95, 0.90, and 0.98, respectively (See Table 2).
Table 1.
Demographic Characteristics of Cohort
| AD (N=150) |
Control (N=150) |
P-value | |
|---|---|---|---|
| Gender (male) | 35% | 31% | 0.46 |
| Age (years) | 78.0(8.2) 57–94 |
70.6(8.9) 52–90 |
<0.001 |
| Education (years) | 14.0 (3.4) 0–22 |
15.6(2.7) 10–23 |
<0.001 |
| APOE4 presence (yes/no) | 61% | 26% | <0.001 |
| Hispanic Ethnicity | 5% | 5% | 0.61 |
| Race (non-Hispanic white) | 95% | 97% | 0.49 |
| MMSE | 19.2(6.1) 1–30 |
29.4(0.9) 26–30 |
<0.001 |
| CDR-SB | 7.8(4.4) 1–18 |
0.0(0.04) 0–1 |
<0.001 |
| Hypertension (% yes) | 56% | 59% | 0.73 |
| Dyslipidemia (% yes) | 53% | 56% | 0.49 |
| Diabetes (% yes) | 12% | 13% | 0.60 |
| Obese (% yes) | 13% | 24% | 0.04 |
NOTE: CDR-SB, Clinical Dementia Rating Scale Sum of Boxes scores; MMSE, Mini-Mental State Examination. SD is given in parentheses.
Figure 1.
ROC plot for the AD serum biomarker profile using 21 serum analytes.
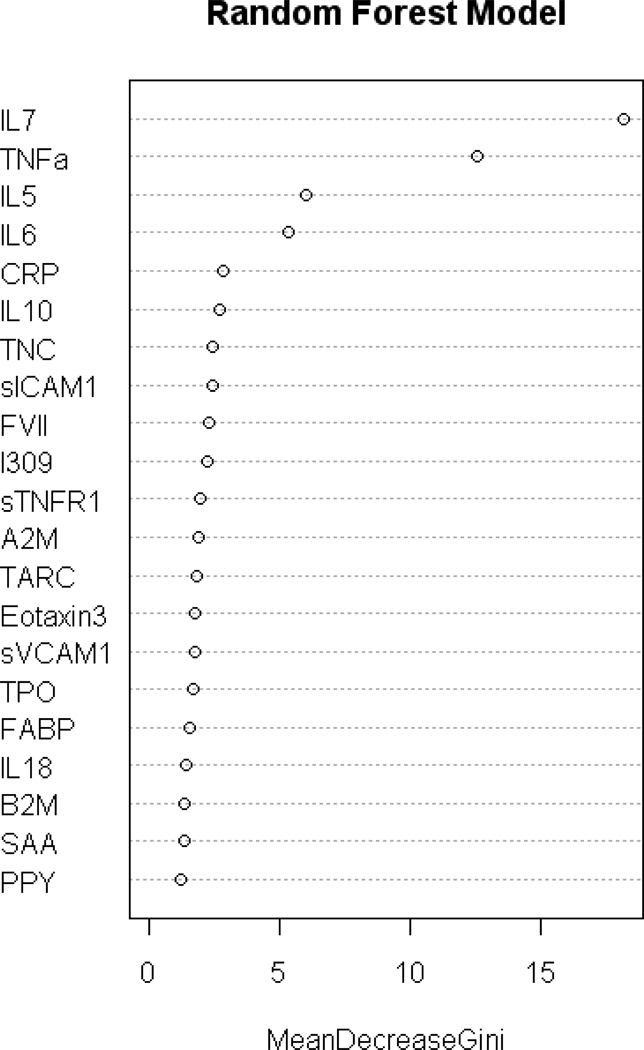
Figure 3.
Gini Plot from Random Forest Biomarker Model demonstrating variable importance and differential expression
Table 2.
Statistical results for AD biomarker sensitivity and specificity and area under the receiver operating characteristic curve (AUC) for samples run on the ECL platform (n=150 AD and n=150 controls).
| AUC | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|
| Serum Biomarker alone | 0.96 | 0.90 (0.81,0.95) | 0.90 (0.82, 0.95) |
| Clinical variables alone | 0.85 | 0.77 (0.66, 0.85) | 0.82 (0.72, 0.89) |
| Biomarkers + Clinical variables | 0.98 | 0.95 (0.87, 0.98) | 0.90 (0.81, 0.95) |
| Abbreviated Biomarker Profile (8 proteins) |
0.95 | 0.88 (0.79, 0.94) | 0.92 (0.83, 0.96) |
| Abbreviated Biomarker Profile (8 proteins) + Clinical Variables |
0.98 | 0.92 (0.84, 0.96) | 0.94 (0.87, 0.98) |
| Plasma Biomarker alone | 0.76 | 0.65 (0.46, 0.74) | 0.79 (0.69, 0.95) |
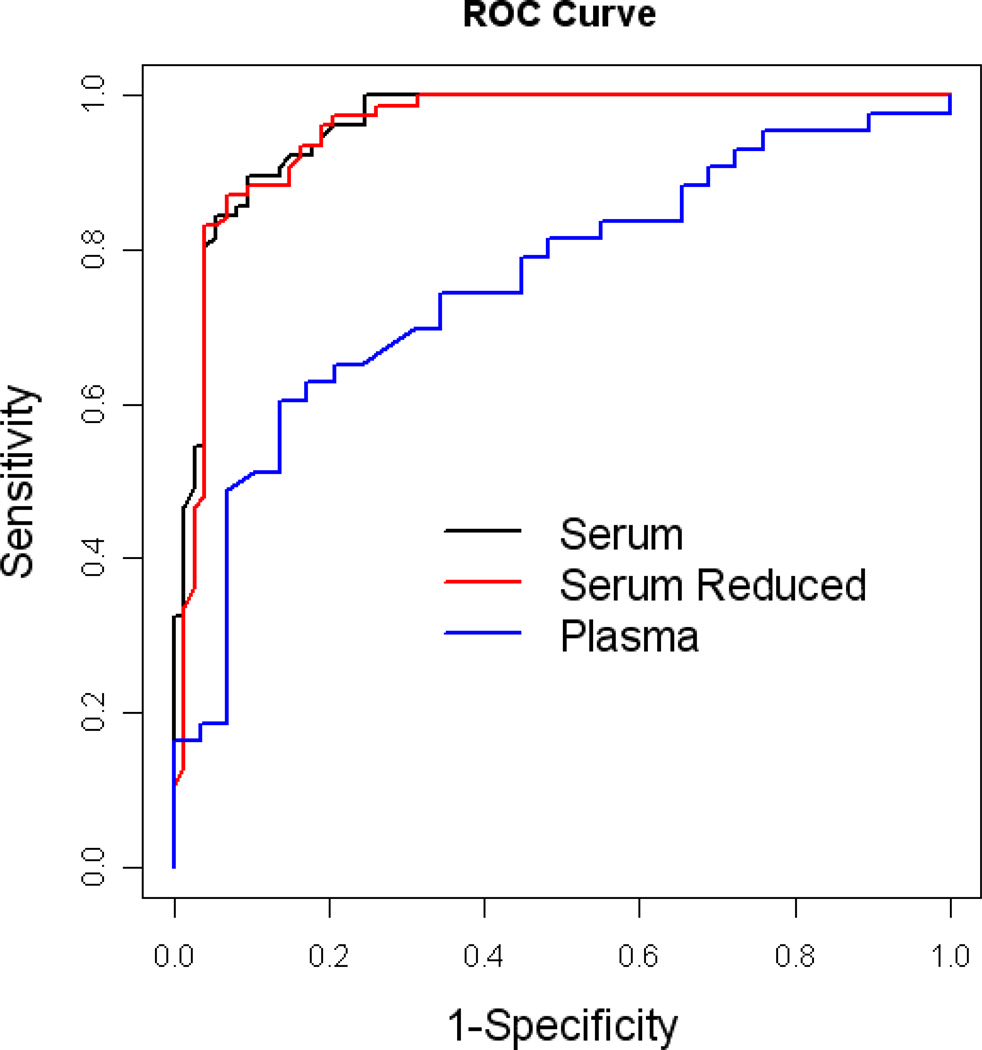
The RF analysis was re-run to determine the optimal algorithm for predicting AD using the smallest number of serum biomarkers. Using only the top 8 markers from the biomarker profile (Figure 4), it yielded a SN, SP, and AUC of 0.88, 0.92 and 0.95, respectively (see Table 2). The addition of age, gender, education and APOE4 genotype increased SN, SP, and AUC to 0.92, 0.94, and 0.98, respectively.
Figure 4.
ROC plot using only the top 8 biomarkers for the AD algorithm
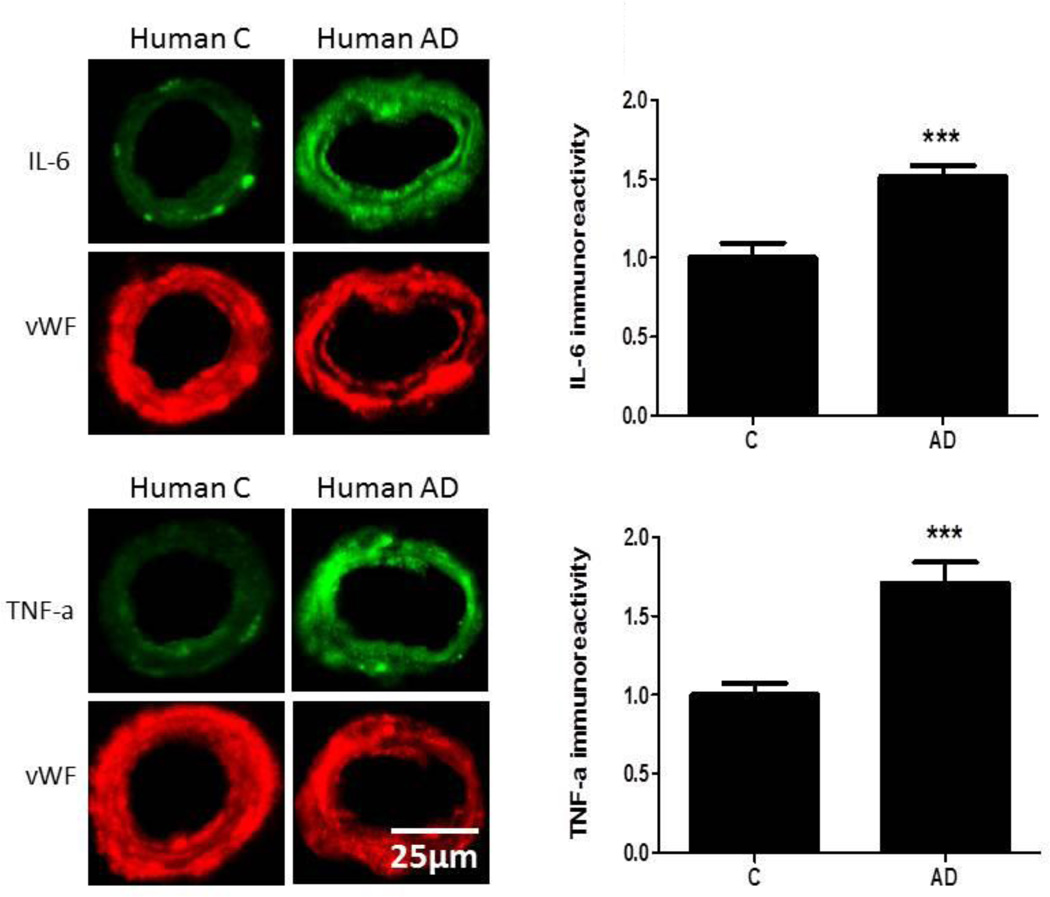
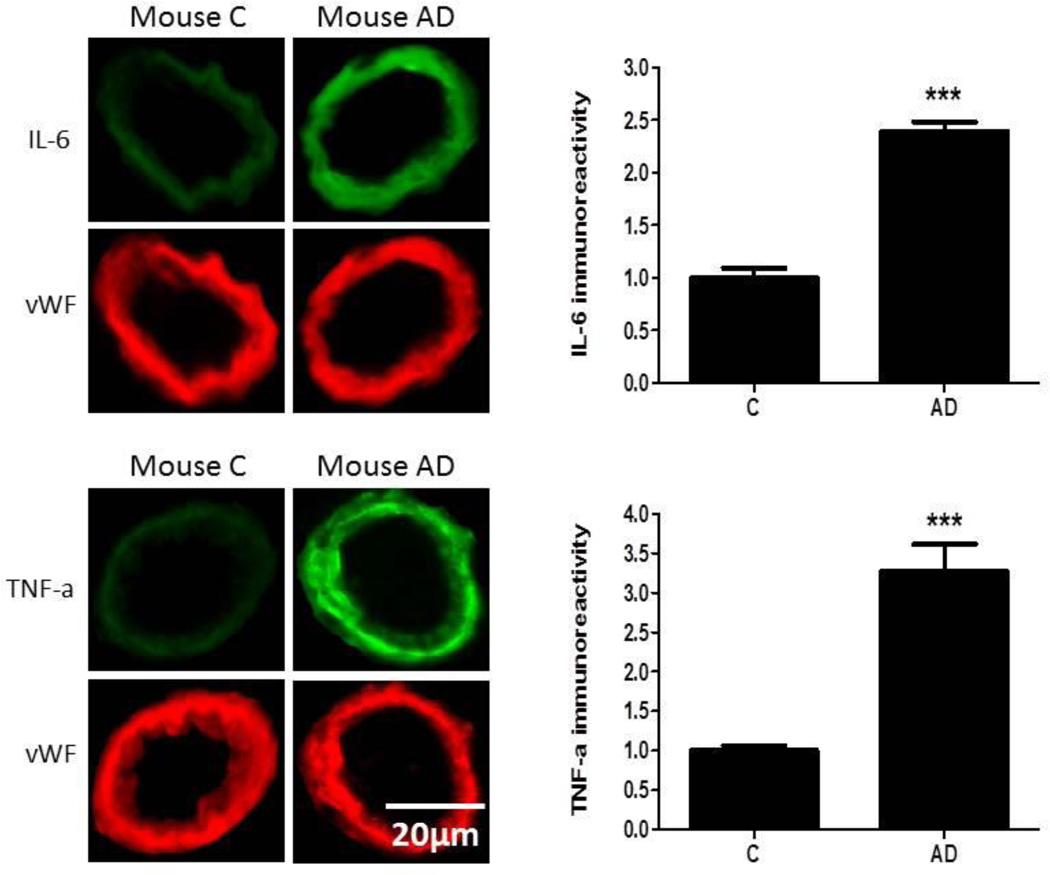
Expression of IL-6 and TNFα was significantly higher (p<0.001) in the cerebromicrovasculature of AD patients (n=9) compared to vessels from control tissue sections (n=9; Figure 5). Similarly, a comparison between control and AD transgenic mice showed that there was a significant increase (p<0.001) in cerebrovascular expression in AD mice of IL-6 and TNFα (n=6/group; Figure 6). In the logistic regression model, IL-6 and TNFα successfully classified 100% of the human and mouse model microvessels demonstrating that these biomarkers can be combined to classify brain samples from diseased versus non-diseased humans and mice. In addition, we analyzed peripheral serum from 3XTg AD mice and control mice (n=9/group) using the Meso Scales Discovery (MSD) panel, and analyzed 4 of the top 8 markers (IL7, IL5, IL6, TNFα). Using logistic regression, 100% of the mice were correctly classified using all 4 markers. For comparison purposes, using only these 4 of the top 8 markers, the overall accuracy in our human samples was 86% in logistic regression analyses (SN = 0.85, SP = 0.87) (AD n=150, NC n=150). The overall accuracy of the SVM was 100% in discriminating AD vs. PD vs. NC with all of the individuals being correctly classified within their respective categories. Interestingly, the inflammatory and vascular markers were most significantly different between the AD and PD groups (i.e. IL7, TNFα, thrombopoietin, VCAM1) were elevated in the AD as compared to PD cases (p<0.0001 for each).
Figure 5.
Brain tissue sections from human control and AD patients were fixed and immunostained with primary antibodies to IL-6 or TNFα and fluorescence-labeled secondary antibody (green). The bar graph denotes signal intensities of microvessels normalized to endothelial specific marker von Willebrand factor (vWF, red) and control values set to 1. Data are from 9 control and 9 AD cases. ***p<0.001
NOTE: C=control. Change graphs to read “Fluorescence Intensity” for the TNFa, and IL-6 plots.
Figure 6.
Brain tissue from control and AD Tg2576 mice were fixed and immunostained with primary antibodies to IL-6 or TNFα and fluorescence-labeled secondary antibody (green). The bar graph denotes signal intensities of microvessels normalized to endothelial specific marker von Willebrand factor (vWF, red) and control values set to 1. Data are from 6 mice per group. ***p<0.001
NOTE: C= control. Change graphs to read “Fluorescence Intensity” for the TNFa, and IL-6 plots.
Discussion
The cumbersome nature of the current AD diagnostic process is a hindrance to both clinical care and therapeutic trials. Biomarkers of disease presence would greatly facilitate access to care as well as streamline therapeutic trial subject identification. In fact, several ongoing studies utilize neuroimaging biomarkers (i.e. amyloid-β scans) as patient selection criteria. While tremendous strides have been accomplished in the areas of neuroimaging and CSF biomarkers of AD, their limited accessibility and cost make them unsuitable for first-line screening [38]. Blood-based biomarkers are ideally suited as the first-step in a multi-stage diagnostic process, and they would greatly enhance the utility of neuroimaging and CSF biomarkers. In fact, it has been proposed that blood-based biomarkers “will most likely be the prerequisite to future sensitive screening of large populations at risk of AD and the baseline in a diagnostic flow approach to AD [38].” From a clinical standpoint, while fewer than half of the physicians interviewed in a recent study felt screenings for AD were important, the vast majority of the general public and caregivers felt such screenings are very important [39]. Additionally, the average physician visit duration in an ambulatory setting for those age 65 and above is approximately 18 minutes [40], making limited time available for even brief cognitive assessments. A blood-based screening tool would fit within the existing medical model following the state-of-the-art practices currently in wide-spread use in the areas of cardiology, oncology, infectious disease and others. Specifically, a positive blood test finding results in follow-up neuroimaging, CSF or specialty clinic examination for confirmatory diagnosis.
Here we cross-validate a blood-based biomarker profile with an independent assay platform. In the current study, a set of serum-based biomarker proteins from our prior work (using a Luminex-based platform) was assayed on an assay platform using ECL. The resulting algorithm with 21 serum-proteins alone yielded an AUC=0.96, which is substantially better than most prior findings. As has been the case in our prior work, the inclusion of demographic factors (age, gender, education, and APOE4 status) yielded an even better AUC (0.98). Using the most informative 8 protein markers, the algorithm yielded an AUC of 0.95 alone and 0.98 when combined with demographic factors. The 8 proteins in this algorithm are IL7, TNFα, IL5, IL6, CRP, IL10, tenascin C, and ICAM1. While there has been a tremendous surge in efforts aimed at identifying blood-based tools for detecting AD, many of these approaches are very laborious [19] or require specialty reference laboratories [15]. The current findings demonstrate that an 8–21 protein algorithm can yield excellent accuracy on an ECL platform that is currently in place in both academic and industry settings globally.
A noteworthy finding is the clear superiority of serum to plasma-based biomarker profiles in this study. The accuracy of the plasma-based markers alone (AUC = 0.76) is similar to our prior work using 11-proteins that spanned both serum and plasma from TARCC and ADNI [16]. There is a tremendous inconsistency in the literature as to which blood fraction should be utilized in the search for blood-biomarkers of AD; however, our work has previously shown that the particular blood fraction is an important consideration [41]. In the current study, using the same 21 proteins from the same research participants at the same blood draw, the serum-based algorithm was clearly superior to that obtained from plasma.
The pathobiologic relevance of proteins in the 8-biomarker algorithm, such as IL-6, TNFα and tenascin C, is supported by data in the current study as well as published results which show their up-regulation in the AD brain. The immunofluorescent data presented herein that demonstrate an increase in IL-6 and TNFα levels in the cerebrovasculature of AD patients, relative to controls, are in agreement with our previously published results that document elevated levels of several inflammatory proteins, including IL-6 and TNFα, in isolated microvessels from human AD brains compared to controls [42, 43]. We have previously documented in the LaFerla triple transgenic AD mouse an increase in inflammatory proteins, including IL-6 [31], and here further highlight the importance of this protein in the Tg2576 mouse model of AD. The demonstration that tenascin C, a protein associated with matrix metalloproteinase (MMP) metabolism [44], is elevated in serum from AD patients agrees with data showing an increase in MMP-2 and MMP-9 in the cerebrovasculature of AD patients [43]. Taken together, the current study provides an important link between inflammatory proteins in serum and inflammatory changes in the AD brain, in both human AD and animal models. These results suggest that the 8-protein algorithm, validated clinically, also closely reflects pathologic processes ongoing in the AD brain. Combining these individual markers into a multi-marker profile, as is done with our human-serum based approach, classified 100% of human and mouse model brain tissue as well as mouse model AD versus control using peripheral serum. Therefore, we have now validated our algorithmic approach across species (mouse and human) and tissue (serum and brain) demonstrating the robustness of the methodology.
A key need for the AD field is the identification of patients who do not have established AD (i.e. not using case-control design) as well as the identification of pre-AD. Therefore, the next step for this line of work is to examine the performance of the serum-based tool in a non-specialty clinic population, where base rates of disease presence are substantially lower. All of the major findings on blood-based biomarkers of AD have been conducted on known-case designs with expert diagnoses. If a blood-based tool is to be utilized as the first step in a multi-stage diagnostic process, it is important that the PPV and NPV be calculated within a community-based or non-specialty-based ambulatory clinic given the substantial differences in base rates of disease presence [3]. For example, if one utilizes the SN and SP of 0.94 and 0.92, respectively to calculate PPV and NPV in a dementia clinic with a base rate of AD of 50%, the resulting PPV and NPV would be 0.92 and 0.94 respectively. However, with an estimated 11% base rate of AD among those ages 65 and above [3], the same SN and SP would yield a PPV and NPV of 0.59 and 0.99, respectively. That is, a clinician would be correct nearly 100% of the time if s/he simply said none of his/her patients age 65 and above suffered from AD; however, a 41% false negative rate would be obtained. Here we held SP at 0.98 allowing a resulting decrease in SN to 0.86. With an 11% base rate, PPV and NPV were 0.84 and 0.98, respectively using the current 8-protein screener, which is excellent for a front-line screening tool. For comparison purposes, the 11% base rate was applied to several of the recently published blood-based tools, including those from the current group. As can be seen from Table 3, no prior work yields acceptable predictive power within a community-based setting. Such a clinical trial on the effectiveness of this 8-protein tool is warranted. Additional work is needed to “lock down” the pre-analytic protocols and analytic protocols to set the stage for independent verification across cohorts and laboratories.
Table 3.
Positive and Negative Predictive Values for the current biomarker findings along with those from previously published biomarker findings when applied to the estimated base rate of AD from the general population (i.e. 11%)
| Base Rate = 11% | ||||
|---|---|---|---|---|
| SN | SP | PPV | NPV | |
| Current findings | 0.86 | 0.98 | 0.84 | 0.98 |
| 1998 Consensus Report minimal guidelines45 | 0.80 | 0.80 | 0.33 | 0.97 |
| Our Prior work15 | 0.94 | 0.84 | 0.42 | 0.99 |
| Our Prior work13 | 0.89 | 0.85 | 0.42 | 0.98 |
| Our Prior work16 | 0.75 | 0.91 | 0.50 | 0.97 |
| AIBL study20 | 0.85 | 0.85 | 0.41 | 0.98 |
| Peptoid approach19 | 0.94 | 0.94 | 0.66 | 0.99 |
| Laske and colleagues12 | 0.94 | 0.80 | 0.37 | 0.99 |
Note: BR = base rate, SN = sensitivity, SP=specificity, PPV = positive predictive value, NPV=negative predictive value.
Preliminary results suggest that the algorithmic approach can be useful in discriminating AD from non-AD neurodegenerative disease (PD). These results must be cross-validated in a larger sample preferably with multiple non-AD disease states in order for analyses to examine (1) AD-specific protein signatures, (2) non-AD protein signatures, as well as (3) general neurodegenerative disease protein signatures. Such work could have significant implications for the utility of the blood-based screening approach within non-specialty clinics as well as the search for novel therapeutic interventions for AD and non-AD neurodegenerative diseases.
Figure 2.
Box Plot of Random Forest Risk Scores for AD vs. normal controls (NC)
Acknowledgement
Research reported in this publication was supported by the National Institute on Aging under Award Numbers R01AG039389, P30AG12300, AG020569, and AG028367. This research was also made possible in part by the Texas Council on Alzheimer’s Disease and Related Disorders to the Texas Alzheimer’s Research & Care Consortium (TARCC)a. Human brain tissue samples were obtained from the New York Brain Bank, Columbia University ADRC, and the Garrison Institute on Aging Brain Bank. The National Institutes of Health had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
*Investigators from the Texas Alzheimer’s Research and Care Consortium: Baylor College of Medicine: Rachelle Doody MD, PhD, Susan Rountree MD, Valory Pavlik PhD, Wen Chan PhD, Paul Massman PhD, Eveleen Darby, Tracy Evans RN, Aisha Khaleeq; Texas Tech University Health Sciences Center: Chuang-Kuo Wu MD, PhD, Kim Johnson PsyD, Victoria Perez, Michelle Hernandez; University of North Texas Health Science Center: Thomas Fairchild PhD, Janice Knebl DO, Leigh Johnson PhD, James Hall PhD, Douglas Mains, Lisa Alvarez, Rosemary McCallum, Tori Conger; University of Texas Southwestern Medical Center: Perrie Adams PhD, Roger Rosenberg MD, Myron Weiner MD, Benjamin Williams MD, PhD, Mary Quiceno MD, Danielle Kalwat, Janet Smith; University of Texas Health Science Center – San Antonio: Donald Royall MD, Raymond Palmer PhD, Marsha Polk.
Contributor Information
Sid E O’Bryant, Email: Sid.O'Bryant@unthsc.edu.
Guanghua Xiao, Email: Guanghua.Xiao@UTSouthwestern.edu.
Fan Zhang, Email: Fan.Zhang@unthsc.edu.
Melissa Edwards, Email: MelissaEdwards@my.unt.edu.
Dwight C German, Email: Dwight.German@UTSouthwestern.edu.
Xiangling Yin, Email: Xiangling.Yin@ttuhsc.edu.
Tori Como, Email: Tori.Como@unthsc.edu.
Joan Reisch, Email: Joan.Reisch@UTSouthwestern.edu.
Ryan M Huebinger, Email: Ryan.Huebinger@UTSouthwestern.edu.
Neill Graff-Radford, Email: Graffradford.neill@mayo.edu.
Dennis Dickson, Email: Dickson.dennis@mayo.edu.
Robert Barber, Email: Robert.Barber@unthsc.edu.
James Hall, Email: James.Hall@unthsc.edu.
Padraig O’Suilleabhain, Email: Pedraig.osuilleabhan@UTsouthwestern.edu.
Paula Grammas, Email: Paula.Grammas@ttuhsc.edu.
References
- 1.Alzheimer's Association. Alzheimer's Disease Facts and Figures. Alzheimers Dement. 2012;8:1–72. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary Costs of Dementia in the United States. N Engl J Med. 2013;369:489–490. doi: 10.1056/NEJMc1305541. [DOI] [PubMed] [Google Scholar]
- 3.Association Association. 2013 Alzheimer's Disease Facts and Figures. Alzheimers Dement. 2013;9:1–72. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, Scheltens P, Tariska P, Winblad B EFNS. Recommendations for the diagnosis and management of Alzheimer's disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1–e26. doi: 10.1111/j.1468-1331.2006.01605.x. [DOI] [PubMed] [Google Scholar]
- 5.Kennard M. Diagnostic markers for Alzheimer's disease. Neurobiol Aging. 1998;19:131–132. doi: 10.1016/s0197-4580(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 6.Boustani M, Callahan CM, Unverzagt FW, Austrom MG, Perkins AJ, Fultz BA, Hui SL, Hendrie HC. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20:572–577. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter VT. Alzheimer's disease: issues and challenges in primary care. Nurs Clin North Am. 2006;41:83–93. doi: 10.1016/j.cnur.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Ray S, Britschqi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, SoYT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 9.Soares HD, Chen Y, Sabbagh M, Roher A, Schrijvers E, Breteler M. Identifying early markers of Alzheimer's disease using quantitative multiplex proteomic immunoassay panels. Ann NY Acad Sci. 2009;1180:56–76. doi: 10.1111/j.1749-6632.2009.05066.x. [DOI] [PubMed] [Google Scholar]
- 10.Booij BB, Lindahl T, Wetterberg P, Skaane NV, Saebo S, Feten G, Rye PD, Kristiansen LI, Hagen N, Jensen M, Bardsen K, Winblad B, Sharma P, Lonneborg A. A gene expression pattern in blood for the early detection of Alzheimer's disease. J Alzheimers Dis. 2011;23:109–119. doi: 10.3233/JAD-2010-101518. [DOI] [PubMed] [Google Scholar]
- 11.Buerger K, Ernst A, Ewers M, Uspenskaya O, Omerovic M, Morgenthaler NG, Knauer K, Bergmann A, Hampel H. Blood-based microcirculation markers in Alzheimer's disease-diagnostic value of midregional pro-atrial natriuretic peptide/C-terminal endothelin-1 precursor fragment ratio. Biol Psychiatry. 2009;65:979–984. doi: 10.1016/j.biopsych.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Laske C, Leyhe T, Stransky E, Hoffmann N, Fallgatter AJ, Dietzsch J. Identification of a blood-based biomarker panel for classification of Alzheimer's disease. Int J Neuropsychopharmacol. 2011;14:1147–1155. doi: 10.1017/S1461145711000459. [DOI] [PubMed] [Google Scholar]
- 13.O'Bryant SE, Xiao G, Barber R, Reisch J, Hall J, Cullum CM, Doody R, Fairchild T, Adams P, Wilhelmsen K, Diaz-Arrastia R Texas Alzheimer's Research and Care Consortium. A blood-based algorithm for the detection of Alzheimer's disease. Dement Geriatr Cogn Disord. 2011;32:55–62. doi: 10.1159/000330750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laske C, Schmohl M, Leyhe T, Stransky E, Maetzler W, Berg D, Fallgatter AJ, Joos T, Dietzsch J. Immune profiling in blood identifies sTNF-R1 performing comparably well as biomarker panels for classification of Alzheimer's disease patients. J Alzheimers Dis. 2013;34:367–375. doi: 10.3233/JAD-121558. [DOI] [PubMed] [Google Scholar]
- 15.O'Bryant SE, Xiao G, Barber R, Reisch J, Doody R, Fairchild T, Adams P, Waring S, Diza-Arrastia R Texas Alzheimer's Research Consortium. A serum protein-based algorithm for the detection of Alzheimer disease. Arch Neurol. 2010;67:1077–1081. doi: 10.1001/archneurol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Bryant SE, Xiao G, Barber R, Huebinger R, Wilhelmsen K, Edwards M, Graff-Radford N, Doody R, Diaz-Arrastia R Texas Alzheimer's Research and Care Consortium, Alzheimer's Disease Neuroimaging Initiative (2011) A blood-based screening tool for Alzheimer's disease that spans serum and plasma: Findings from TARC and ADNI. PLoS ONE. 6:e28092. doi: 10.1371/journal.pone.0028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Bryant SE, Xiao G, Edwards M, Devous M, Gupta VB, Martins R, Zhang F, Barber R Texas Alzheimer's Research and Care Constortium (TARCC) Biomarkers of Alzheimer's disease among Mexican Americans. J Alzheimers Dis. 2013;34:841–849. doi: 10.3233/JAD-122074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagele E, Han M, DeMarshall C, Belinka B, Nagele R. Diagnosis of Alzheimer's disease based on disease-specific autoantibody profiles in human sera. PLoS ONE. 2011;6:e23112. doi: 10.1371/journal.pone.0023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy MM, Wilson R, Wilson J, Connell S, Gocke A, Hynan L, German D, Kodadek T. Identification of candidate IgG biomarkers for Alzheimer's disease via combinatorial library screening. Cell. 2011;144:132–142. doi: 10.1016/j.cell.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, Mondal A, Bedo J, Bush AI, Brown B, De Ruyck K, Ellis KA, Fowler C, Gupta VB, Head R, Macaulay SL, Pertile K, Rowe CC, Rembach A, Rodrigues M, Rumble R, Szoeke C, Taddei T, Trounson B, Ames D, Masters CL, Martins RN Alzheimer's Disease Neuroimaging Initiative, Australian Imaging Biomarker and Lifestyle Research Group. Blood-based protein biomarkers for the diagnosis of Alzheimer's disease. Arch Neurol. 2012;69:1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nature Medicine. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu WT, Holtzman DM, Fagan AM, Shaw LM, Perrin R, Arnold SE, Grossman M, Xiong C, Craig-Schapiro R, Clark CM, Pickering E, Kuhn M, Chen Y, Van Deerlin VM, McCluskey L, Elman L, Karlawish J, Chen-Plotkin A, Hurtig HI, Siderowf A, Swenson F, Lee VM, Morris JC, Trojanowski JQ, Soares H Alzheimer's Disease Neuroimaging Initiative. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer Disease. Neurology. 2012;79:897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiddle SJ, Sattlecker M, Proitsi P, Simmons A, Westman E, Bazenet C, Nelson SK, Williams S, Hodges A, Johnston C, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Newhouse S, Lovestone S, Dobson RJB. Candidate blood proteome markers of Alzheimer's disease onset and progression: A systematic review and replication study. Journal of Alzheimer's Disease. 2014;38:515–531. doi: 10.3233/JAD-130380. [DOI] [PubMed] [Google Scholar]
- 24.Sabbagh JJ, Kinney JW, Cummings JL. Alzheimer's disease biomarkers: Correspondence between human studies and animal models. Neurobiol Dis. 2013;56:116–130. doi: 10.1016/j.nbd.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 25.McKhann D, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsater H, Anckarsater R, Andreasen N, Zetterberg H, Andreasson U, Blennow K. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis. 2010;2010:1–11. doi: 10.4061/2010/986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL. Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhle J, Regeniter A, Leppert D, Mehling M, Kappos L, Lindberg RL, Petzold A. A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J Neuroimmunol. 2010;220:114–119. doi: 10.1016/j.jneuroim.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Oh ES, Mielke MM, Rosenberg PB, Jain A, Fedarko NS, Lyketsos CG, Mehta PD. Comparison of conventional ELISA with electrochemiluminescence technology for detection of amyloid-β in plasma. J Alzheimers Dis. 2010;21:769–773. doi: 10.3233/JAD-2010-100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alves G, Bronnick K, Aarsland D, Blennow K, Zetterberg H, Ballard C, Kurz MW, Andreasson U, TYsnes OB, Larsen JP, Mulugeta E. CSF amyloid-β and tau proteins, and cognitive performance, in early and untreated Parkinson's Disease: The Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 31.Tripathy D, Sanchez A, Yin X, Luo J, Martinez J, Grammas P. Thrombin as a mediator of cerebrovascular inflammation in AD and hypoxia. Front Aging Neurosci. 2013;5:19. doi: 10.3389/fnagi.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grammas P, Tripathy D, Sanchez A, Yin X, Luo J. Brain microvasculature and hypoxia-related proteins in Alzheimer's disease. Int J Clin Exp Pathol. 2011;4:616–627. [PMC free article] [PubMed] [Google Scholar]
- 33.Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- 34.Dickstein DL, Walsh J, Brautigam H, Stockton SD, Jr., Gandy S, Hof PR. Role of vascular risk factors and vascular dysfunction in Alzheimer's disease. Mt Sinai J Med. 2010;77:82–102. doi: 10.1002/msj.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piazza F, Galimberti G, Conti E, Isella V, Perlangeli MV, Speranza T, Borroni B, Pogliani EM, Padovani A, Ferarese C. Increased tissue factor pathway inhibitor and homocysteine in Alzheimer's disease. Neurobiol Aging. 2010;33:226–233. doi: 10.1016/j.neurobiolaging.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Okereke OI, Selkoe DJ, Pollak MN, Stampfer MJ, Hu FB, Hankinson SE, Grodstein F. A profile of impaired insulin degradation in relation to late-life cognitive decline: A preliminary investigation. Int J Geriatr Psychiatry. 2009;24:177–182. doi: 10.1002/gps.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 38.Schneider P, Hampel H, Buerger K. Biological marker candidates of Alzheimer's disease in blood, plasma, and serum. CNS Neurosci Ther. 2009;15:358–374. doi: 10.1111/j.1755-5949.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bond J, Graham N, Padovani A, Mackell J, Knox S, Atkinson J. Screening for cognitive impairment, Alzheimer's disease and other dementias: Opinions of European caregivers, payors, physicians and the general public. J Nutr Health Aging. 2010;14:558–562. doi: 10.1007/s12603-010-0268-6. [DOI] [PubMed] [Google Scholar]
- 40.Lo A, Ryder K, Shorr RI. Relationship between patient age and duration of physician visit in ambulatory setting: Does one size fit all? J Am Geriatr Soc. 2005;53:1162–1167. doi: 10.1111/j.1532-5415.2005.53367.x. [DOI] [PubMed] [Google Scholar]
- 41.Huebinger R, Xiao G, Wilhelmsen K, Diaz-Arrastia R, Zhang F, O'Bryant SE, Barber R. Comparison of protein concentrations in serum versus plasma from Alzheimer's patients. Advances in Alzheimers Dis. 2012;1:51–58. [Google Scholar]
- 42.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 43.Thirumangalakudi L, Samany PG, Owoso A, Wiskar B, Grammas P. Angiogenic proteins are expressed by brain blood vessels in Alzheimer's disease. J Alzheimers Dis. 2006;10:111–118. doi: 10.3233/jad-2006-10114. [DOI] [PubMed] [Google Scholar]
- 44.Sakamoto N, Hoshino Y, Misaka T, Mizukami H, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Serum tenascin-C level is associated with coronary plaque rupture in patients with severe acute coronary syndrome. Heart Vessels. 2013 doi: 10.1007/s00380-013-0341-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Anonymous. Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer's Disease". The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Neurobiol Aging. 1998;19:109–116. [PubMed] [Google Scholar]