Abstract
Data are presented which suggest the existence of a light-harvesting pigment-protein complex which is functionally and structurally associated with photosystem I (PSI) reaction centers. These observations are based on techniques which allow isolation of PSI using minimal concentrations of Triton X-100. Properties of density and self aggregation allowed purification of a “native” PSI complex.
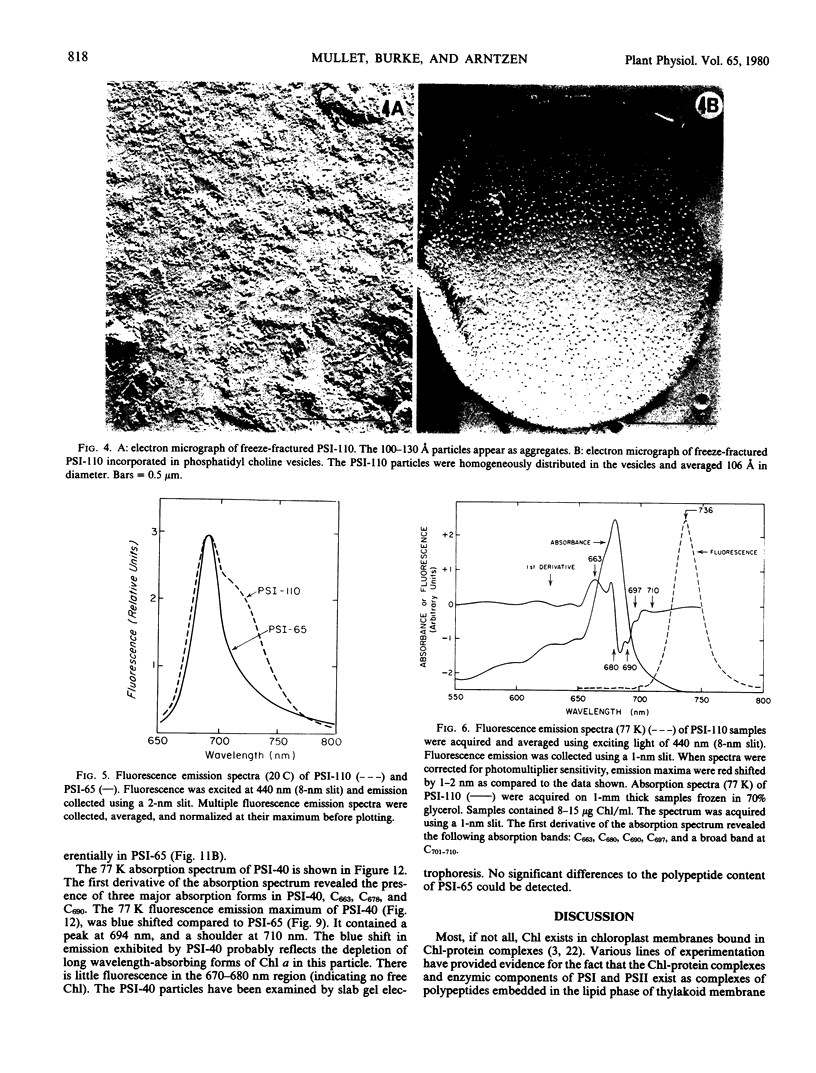
The isolated PSI particles appear as 106 Å spherical subunits when viewed by freeze fracture microscopy. When incorporated into phosphatidyl choline vesicles, the particles lose self-aggregation properties and disperse uniformly within the lipid membrane.
The isolated PSI preparation contains 100 ± 10 chlorophylls/P700 (Chl a/b ratio greater than 18); this represents a recovery of 27% of the original chloroplast membrane Chl. These particles were enriched in Chl a forms absorbing at 701 to 710 nm. Chl fluorescence at room temperature exhibited a maximum at 690 nm with a pronounced shoulder at 710 nm. At 77 K, peak fluorescence emission was at 736 nm; in the presence of dithionite an additional fluorescence maximum at 695 nm was obtained at 77 K. This dual fluorescence emission peak for the PSI particles is evidence for at least two Chl populations within the PSI membrane subunit. The fluorescence emission observed at 695 nm was identified as arising from the core of PSI which contains 40 Chl/P700 (PSI-40). This core complex, derived from native PSI particles, was enriched in Chl a absorbing at 680 and 690 nm and fluorescing with maximal emission at 694 nm at 77 K. PSI particles consisting of the PSI core complex plus 20 to 25 Chl antennae (65 Chl/P700) could also be derived from native PSI complexes. These preparations were enriched in Chl a forms absorbing at 697 nm and exhibited a 77 K fluorescence emission maximum at 722 nm.
A comparison of native PSI particles which contain 110 Chl/P700 (PSI-110) and PSI particles containing 65 Chl/P700 (PSI-65) provides evidence for the existence of a peripheral Chl-protein complex tightly associated in the native PSI complex. The native PSI subunits contain polypeptides of 22,500 to 24,500 daltons which are not found in the PSI-65 or PSI-40 subfractions. It is suggested that these polypeptides function to bind 40 to 45 Chl per structural complex, including the Chl which emits fluorescence at 736 nm.
A model for the organization of Chl forms is presented in which the native PSI membrane subunit consists of a reaction center core complex plus two regions of associated light-harvesting antennae. The presence of energy “sinks” within the antennae is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Staehelin L. A., Arntzen C. J. Spatial relationship of photosystem I, photosystem II, and the light-harvesting complex in chloroplast membranes. J Cell Biol. 1977 May;73(2):400–418. doi: 10.1083/jcb.73.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Ditto C. L. Effects of cations upon chloroplast membrane subunit. Interactions and excitation energy distribution. Biochim Biophys Acta. 1976 Nov 9;449(2):259–274. doi: 10.1016/0005-2728(76)90138-9. [DOI] [PubMed] [Google Scholar]
- BUTLER W. L. A far-red absorbing form of chlorophyll. in vivo. Arch Biochem Biophys. 1961 May;93:413–422. doi: 10.1016/0003-9861(61)90287-9. [DOI] [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W., Anderson J. M. Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Aug;56(2):586–593. doi: 10.1073/pnas.56.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W. Sensitive fluorescence method for the determination of chlorophyll a-chlorophyll b ratios. Biochim Biophys Acta. 1971 Nov 2;253(1):222–231. doi: 10.1016/0005-2728(71)90248-9. [DOI] [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Ditto C. L., Arntzen C. J. Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys. 1978 Apr 15;187(1):252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Tredwell C. J., Malkin R., Barber J. The relationship between the lifetime and yield of the 735 nm fluorescence of chloroplasts at low temperatures. Biochim Biophys Acta. 1979 Feb 8;545(2):309–315. doi: 10.1016/0005-2728(79)90208-1. [DOI] [PubMed] [Google Scholar]
- Gasanov R. A., French C. S. Chlorophyll composition and photochemical activity of photosystems detached from chloroplast grana and stroma lamellae. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2082–2085. doi: 10.1073/pnas.70.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami I. Fluorescence changes related in the primary photochemical reaction in the P-700-enriched particles isolated from spinach chloroplasts. Biochim Biophys Acta. 1976 Nov 9;449(2):245–258. doi: 10.1016/0005-2728(76)90137-7. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Butler W. L. Light-induced absorbance changes in chloroplasts mediated by photosystem I and photosystem II at low temperature. Biochim Biophys Acta. 1974 Mar 26;333(3):465–480. doi: 10.1016/0005-2728(74)90131-5. [DOI] [PubMed] [Google Scholar]
- Markwell J. P., Thornber J. P., Boggs R. T. Higher plant chloroplasts: Evidence that all the chlorophyll exists as chlorophyll-protein complexes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1233–1235. doi: 10.1073/pnas.76.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. A developmental study of photosystem I peripheral chlorophyll proteins. Plant Physiol. 1980 May;65(5):823–827. doi: 10.1104/pp.65.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson K. D., Sato V. L., Sauer K. Exciton interaction in the photosystem I reaction center from spinach chloroplasts. Absorption and circular dichroism difference spectra. Biochemistry. 1972 Nov 21;11(24):4591–4595. doi: 10.1021/bi00774a027. [DOI] [PubMed] [Google Scholar]
- Satoh K., Butler W. L. Low temperature spectral properties of subchloroplast fractions purified from spinach. Plant Physiol. 1978 Mar;61(3):373–379. doi: 10.1104/pp.61.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Alberte R. S., Thornber J. P. The P700-chlorophyll a-protein. Isolation and some characteristics of the complex in higher plants. Arch Biochem Biophys. 1974 Nov;165(1):388–397. doi: 10.1016/0003-9861(74)90177-5. [DOI] [PubMed] [Google Scholar]
- Strasser R. J., Butler W. L. Fluorescence emission spectra of photosystem I, photosystem II and the light-harvesting chlorophyll a/b complex of higher plants. Biochim Biophys Acta. 1977 Nov 17;462(2):307–313. doi: 10.1016/0005-2728(77)90129-3. [DOI] [PubMed] [Google Scholar]




