Abstract
Abnormalities of GABAergic interneurons are some of the most consistent findings from post-mortem studies of schizophrenia. However, linking these molecular deficits with in vivo observations in patients – a critical goal in order to evaluate interventions that would target GABAergic deficits – presents a challenge. Explanatory models have been developed based on animal work and the emerging experimental literature in schizophrenia patients. This literature includes: neuroimaging ligands to GABA receptors, magnetic resonance spectroscopy (MRS) of GABA concentration, transcranial magnetic stimulation of cortical inhibitory circuits and pharmacologic probes of GABA receptors to dynamically challenge the GABA system, usually in combination with neuroimaging studies. Pharmacologic challenges have elicited behavioral changes, and preliminary studies of therapeutic GABAergic interventions have been conducted. This article critically reviews the evidence for GABAergic dysfunction from each of these areas. These methods remain indirect measures of GABAergic function, and a broad array of dysfunction is linked with the putative GABAergic measures, including positive symptoms, cognition, emotion, motor processing and sensory processing, covering diverse brain areas. Measures of receptor binding have not shown replicable group differences in binding, and MRS assays of GABA concentration have yielded equivocal evidence of large-scale alteration in GABA concentration. Overall, the experimental base remains sparse, and much remains to be learned about the role of GABAergic interneurons in healthy brains. Challenges with pharmacologic and functional probes show promise, and may yet enable a better characterization of GABAergic deficits in schizophrenia.
Keywords: fMRI, MRS, TMS, PET, psychosis, benzodiazepine
1.0 Introduction
Post-mortem studies of schizophrenia have yielded definitive evidence of GABAergic abnormalities (Akbarian and Huang, 2006; Benes, 2010; Lewis et al., 2012; Nakazawa et al., 2012), but what is not clear is how these GABAergic abnormalities cause symptoms of the illness. While animal models provide helpful test beds for modeling schizophrenia and designing pharmacologic interventions, functional targets in patients linking behavior with the underlying molecular mechanisms are needed. In this article, we will critically review methodologies used for assaying in vivo function of GABA in schizophrenia, focusing on neuroimaging studies, transcranial magnetic stimulation (TMS) probes and pharmacologic challenges.
2.0 Post-mortem GABA findings in schizophrenia
GABAergic interneurons are the predominant machinery of inhibition in the brain. In schizophrenia, the most replicated findings are reductions in the 67-kDa isoform of the synthetic enzyme for GABA, glutamic acid decarboxylase (GAD67; Curley et al., 2011; Guidotti et al., 2000). This reduction has been localized to parvalbumin (PV) positive interneurons (Hashimoto et al., 2003) and has been observed in the dorsolateral prefrontal cortex (dLPFC; Akbarian et al., 1995), anterior cingulate cortex (ACC; Woo et al., 2004), motor cortex (Hashimoto et al., 2008b), visual cortex (Hashimoto et al., 2008b) and hippocampus (Benes et al., 2007; Knable et al., 2004). Levels of PV mRNA are also reduced in schizophrenia, and some findings show that the density of these interneurons does not differ from comparison groups (Beasley et al., 2002; Hashimoto et al., 2003; Woo et al., 1997), suggesting no loss of PV-positive inhibitory interneurons in schizophrenia. However, using different techniques for counting PV-positive neurons, as well as cresyl violet stains useful for counting the entire population of interneurons, other groups have found reduced cell counts of PV-positive and other interneurons in hippocampus (Benes et al., 1998; Konradi et al., 2011) and layers II/III of the anterior cingulate gyrus (Benes et al., 1991). Reductions in other GABAergic-related markers have also been noted, such as mRNA levels of neuropeptide Y, somatostatin and cholecystokinin (Hashimoto et al., 2008a). Evidence suggests that the changes are not due to extraneous factors such as age, sex of medication effects. In sum, a variety of techniques have been applied to examine GABAergic systems, which have not always yielded consistent findings, but it is clear that GABA-related dysfunction is an important feature of schizophrenia.
Focusing on the PV-positive interneurons, at least two varieties are relevant for schizophrenia: Fast-spiking PV-positive basket cells (PVBC) synapse on the pyramidal cell body and dendrites in layer III, whereas chandelier cells (PVChC) synapse on the axon initial segment (AIS) of the pyramidal cells (for review, see (Markram et al., 2004)). Perisomatic GABA receptors (GABAR) of the PVBC neurons, predominantly containing the α-1 subunit, show mRNA reductions, while the GABAR on the AIS, predominantly containing the α-2 subunit, appear to be upregulated (Beneyto et al., 2011; Volk et al., 2002), along with down regulation of PVChC GAT1 (GABA membrane transporter; Pierri et al., 1999). Importantly, PVChCs appear to depolarize and excite pyramidal cells when releasing GABA at the AIS (Szabadics et al., 2006; Woodruff et al., 2009), whereas the PVBCs inhibit pyramidal cells (Markram et al., 2004). In schizophrenia, these changes could be compensations, possibly for reduced excitatory drive to the layer III pyramidal cells, implied by reduced dendritic spine density (Lewis et al., 2012). In this conceptualization, the findings may reflect alterations in the excitatory/inhibitory balance of the cellular micro-circuits.
Interestingly, PVBCs are associated with cortical gamma oscillations (Bartos et al., 2007; Fuchs et al., 2007; Sohal et al., 2009). These cortical rhythms are associated with integrative cortical function (Buzsaki and Draguhn, 2004), including attention (Fries et al., 2001), working memory (Tallon-Baudry et al., 1998) and perception (Gray et al., 1989). Abnormal gamma oscillations have also been implicated in schizophrenia – a potential link between the PV abnormalities and cognitive dysfunction observed in the illness (Gonzalez-Burgos et al., 2011; Uhlhaas and Singer, 2010). However, since abnormal gamma oscillations can arise from a variety of pathological mechanisms – not necessarily GABAergic -- in the sections that follow, we will consider other methodologies that more specifically target GABA function in schizophrenia.
Given that many of these studies combine samples of schizophrenia and schizoaffective disorder, we will use ‘schizophrenia’ to refer to both diagnostic groups.
3.0 In vivo measurement of GABA and GABARs
3.1 Radioligand studies
Several radioligands exist to measure the availability of GABARs in the brain. Positron emission tomographic (PET) studies have used [11C]flumazenil, or a fluoridated derivative [18F]fluoroflumazenil, which bind to α-1,2,3,6 subunits (See Figure 1 for an overview of the GABAR subunit pharmacology). Single photon emission computed tomography (SPECT) studies have employed [123I]iomazenil, with similar pharmacology except for inverse agonist properties. PET studies have more recently used [11C]Ro15-4513, an inverse agonist binding to α-1,5. Although these neuroimaging ligand studies permit reliable quantification of receptor densities, the diversity of GABAR and differential distribution in the brain presents an interpretative challenge when assaying for pathological changes.
Figure 1. Pharmacology of GABA receptor subunits.
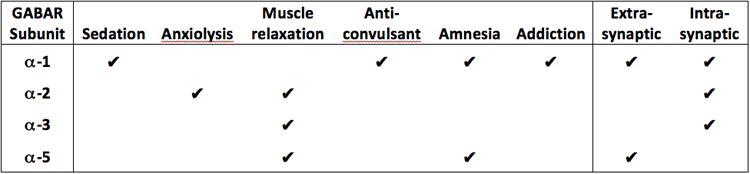
The GABAR is a heteropentameric glycoprotein, composed of combinations of at least 19 different subunits in 7 families, affecting function as well as ligand affinity, of which the α-subunit family is most relevant for BDZ pharmacology. The figure shows the functions and distributions of the subunits most relevant for schizophrenia. Information in the figured derived from (Brickley and Mody, 2012; Tan et al., 2011)
Overall, the density of GABAR in schizophrenia does not appear abnormal in radioligand studies (see Table 1). SPECT studies with [123I]iomazenil reported no group differences in comparison with control subjects (Abi-Dargham et al., 1999; Ball et al., 1998; Busatto et al., 1997; Verhoeff et al., 1999), as did a PET study with [11C]Ro15-4513 (Asai et al., 2008). One group found that medial frontal cortex binding was positively associated with total symptoms, but negatively associated with outcome measures across the brain after displacement of the ligand following a diazepam infusion (Schroder et al., 1997). While other groups also found symptom correlations with binding (Ball et al., 1998; Busatto et al., 1997), another group failed to replicate these symptom correlations using a quantitative measurement of binding not sensitive to cerebral blood flow (Abi-Dargham et al., 1999). Considering what is now known from the post-mortem findings, these essentially negative findings should not be too surprising. If the α-1 subunit GABAR's are downregulated at the pyramidal cell body and the α-2 subunit GABAR's are upregulated at the AIS of the pyramidal cells, a relatively non-selective ligand is not likely to show aggregate differences. Furthermore, 45% of the GABAergic cells in dLPFC are calretinin containing (not PV), which are not compromised in schizophrenia (Lewis et al., 2012). Using more selective agents, such as [11C]Ro15-4513, in combination with a pharmacologic challenge may provide a more promising approach. Recently, tiagabine, which increases GABA levels by blocking GAT1, has been shown to demonstrate shifts in α-1 binding due to endogenous GABA in healthy individuals (Stokes et al., 2014). Such a strategy may be effective at evaluating the α-1 subunit changes reported in schizophrenia (Beneyto et al., 2011; Volk et al., 2002).
Table 1. Radioligand studies of GABAR binding in schizophrenia and schizoaffective disorder.
| Study | # SZ M/F | SZ Age | #HC M/F | HC Age | Subject type1 | Med status | Ligand | Analysis | Results |
|---|---|---|---|---|---|---|---|---|---|
| Busatto et al, 1997; Ball et al, 1998 | 14/1 | 29 | 11/1 | 28 yr | Mixed | Mixed | IMZ | ROI | Group comparison n.s.; L med temp neg corr w/pos Sx; MFC neg corr w/neg Sx ; OC pos corr w/GAF; 10 ROIs have many pos corr w/cognitive measures and a few neg corr w/in SZ but not HC2 |
| Schroder et al, 1997 | 20 | 30 yr | -- | -- | Chronic | Med | IMZ | ROI | MFC pos corr w/BPRS total before DZP; after DZP: MFC, TPC, OC neg corr w/outcome2 |
| Verhoeff et al, 1999 | 25/0 | 41 yr | 24 | 37 yr | Chronic | Mixed | IMZ | WB | Relative binding SZ<HC in L BA6, (t) in R BA9/44; n.s. correlations for PANSS |
| Abi-Dargham et al, 1999 | 16/0 | 44 yr | 16/0 | 45 yr | Chronic | Med | IMZ | ROI/WB | Group comparison n.s.; n.s. w/BPRS total or subscales |
| Asai et al, 2008 | 6/5 | 33 yr | 12/0 | 29 yr | Mixed | Mixed | Ro15 | ROI | Group comparison n.s; PFC neg corr w/PANSS negative, general, total; HIPP neg corr w/PANSS neg2 |
| Lee et al, 2013 | 8/9 | 29 yr | 9/9 | 26 yr | Chronic | Med | FMZ | WB | SZ<HC in BA25, L BA38; HC<SZ in BA192 |
Acute = average duration of illness < 3 years; Chronic = average duration of illness ≥ 3 years
Corrections for multiple comparisons not done
Abbreviations: SZ, Schizophrenia/schizoaffective subjects; HC, healthy control subjects; M, male; F, female; Med, medicated; Unmed, unmedicated; IMZ, [123I]iomazenil; Ro15, [11C]Ro15-4513;FMZ, [18F]fluoroflumazenil; ROI, region of interest; WB, whole brain voxel-wise; n.s., no significant differences/correlations; neg corr w/, negative correlation with; pos corr w/, positive correlation with; (t), trend; pos Sx, positive symptoms; neg Sx, negative symptoms; L med temp, left medial temporal; MFC, medial frontal cortex; BPRS, Brief Psychiatric Rating Scale; DZP, diazepam; TPC, temporo-parietal cortex; OC, occipital cortex; GAF, Global Assessment of Functioning; BA, Brodman Area; PANSS, positive and negative syndrome scale; PFC, prefrontal cortex; HIPP, hippocampus;
3.2 Magnetic resonance spectroscopy studies
Magnetic resonance spectroscopy (MRS) is another imaging technique, which, in distinction to PET/SPECT studies, can assay GABA concentrations in vivo. However, measurement is limited by low signal-to-noise ratio, large voxel sizes, typically around 18 cm3, and sparse sampling, usually no more than 3 voxels sampled with a 3-tesla magnet. In contrast to radioligand studies of GABAR, a prediction of reduced GABA concentration from MRS is more straightforward, although MRS cannot separate intra-cellular from extra-cellular GABA, somewhat complicating interpretation. However, results to date are mixed, as Table 2 demonstrates. Of 17 voxels studied, 4 show reduced GABA, 3 reported increased GABA and 10 showed no difference. Reduced GABA in occipital cortex has been associated with impaired performance on visual orientation surround suppression, a GABAergic-dependent early visual process deficient in schizophrenia (Yoon et al., 2010). Linking MRS-measured GABA with disrupted visual processing in schizophrenia represents an important step, although another group failed to find a relationship between another visual processing measure (contrast sensitivity) and GABA levels (Kelemen et al., 2013).
Table 2. MRS studies of GABA in schizophrenia and schizoaffective disorder.
| Study | # SZ M/F | SZ Age | #HC M/F | HC Age | Subject Type1 | Med status | Measure | Voxel | Result |
|---|---|---|---|---|---|---|---|---|---|
| Goto et al, 2009a, b | 9/9 | 29 yr | 9/9 | 30 yr | ? | Med | 3T GABA/Cr | Frontal | n.s.; trend neg corr w/perseverative errors on WCST |
| L BG | HC>Sz | ||||||||
| POC | n.s. | ||||||||
| Tayoshi et al, 2010 | 20/18 | 34 yr | 17/12 | 34 yr | Chronic | Med | 3T GABA | ACC | n.s.; neg corr w/APD |
| L BG | n.s.; pos corr w/APD | ||||||||
| Ongur et al, 2010 | 14/7 | 39 yr | 12/7 | 36 yr | Chronic | Med | 4T GABA/Cr | ACC | SZ>HC2 |
| POC | SZ>HC2 | ||||||||
| Yoon et al, 2010 | 11/2 | 27 yr | 11/2 | 28 yr | Mixture | Mixture | 3T GABA/Cr | Occ | HC>SZ; pos corr w/visual OSSS |
| Kegeles et al, 2012 | 11/5 | 32 yr | 14/8 | 33 yr | Chronic | Unmed | 3T GABA/w | MPFC3 | SZ>HC, unmed(t) >med |
| L dLPFC | n.s. | ||||||||
| 11/5 | 32 yr | “ | “ | Chronic | Med | MPFC3 | n.s. | ||
| dLPFC | n.s. | ||||||||
| Rowland et al, 2013 | 9/2 | 30 yr | 5/5 | 33 yr | Chronic | Med | 3T GABA/w | ACC | n.s. |
| CSO | n.s. | ||||||||
| 7/3 | 51 yr | 7/3 | 49 yr | Chronic | Med | ACC | HC>SZ | ||
| CSO | n.s. | ||||||||
| Rowland et al, 2013 | 8/2 | 43 yr | Chronic | Med | 3T GABA/w | ACC | Neg corr w/theta-alpha and beta ratio in auditory paired click | ||
| Kelemen et al, 2013 | 18/10 | 25 yr | 14/6 | 24 yr | Acute | Unmed/med4 | 3T GABA/Cr | Occ | HC>SZ pre and post medication; n.s. corr w/visual contrast sensitivity |
| Chen et al, 20145 | 9/3 | 31 yr | 6/6 | 33 yr | Chronic | Mixture | 3T GABA/w | L dLPFC | Pos corr w/surface gamma amplitude at rest and during working memory (both groups combined) |
Acute = average duration of illness < 3 years; Chronic = average duration of illness ≥ 3 years
Effect of diagnosis not significant when patients on anti-convulsants excluded
Voxel overlaps with rostral ACC voxels
Patients studied medication-free and 8 weeks after various pharmacotherapy regimens begun
Partial overlap with sample of Kegeles et al, 2012
Abbreviations: SZ, Schizophrenia/schizoaffective subjects; HC, healthy control subjects; M, male; F, female; Med, medicated; Unmed, unmedicated; GABA/Cr, GABA/creatine ratio; GABA/w, GABA/water ratio; n.s., no significant differences; neg corr w/, negative correlation with; pos corr w/, positive correlation with; APD, anti-psychotic dose; L BG, left basal ganglia; WCST, Wisconsin Card Sort; POC, midline parieto-occipital cortex; ACC, anterior cingulate cortex; L dLPFC, left dorsolateral prefrontal cortex; OSSS, orientation-specific surround suppression; (t), trend; MPFC, medial prefrontal cortex; CSO, centrum semiovale white matter;
Reliable measurement of GABA is relatively new, with the first report in schizophrenia appearing in 2009 (Goto et al., 2009b). Medication status may be a confounding factor, as suggested by one study finding increased GABA only in unmedicated subjects in a medial prefrontal cortex (MPFC) voxel (Kegeles et al., 2012)], although another report found reduced GABA in occipital cortex in unmedicated patients (Kelemen et al., 2013). Sampling of voxels across the brain has also been sparse due to long acquisition times for single voxels, and it is too early to tell whether or not there is regional variation in GABA abnormalities based on MRS findings. One promising development is linking GABA levels to risk genes for schizophrenia that are involved in GABA synthesis, such as the gene for GAD1 (Marenco et al., 2010) or Erbb4, associated with risk and interneuron development (Marenco et al., 2011). Another interesting approach found a positive correlation between GABA levels and gamma activity over the dLPFC (Chen et al., 2014). In summary, while studies to date have not provided confirmation of reduced GABA levels in schizophrenia, the power of this technology has yet to be fully exploited.
4.0 GABA pharmacologic challenge and cognition
Cognitive impairment is a prominent feature of schizophrenia, and several investigators have proposed theories linking cognitive dysfunction to GABAergic abnormalities in schizophrenia (Gonzalez-Burgos et al., 2011; Lewis et al., 2012; Lisman et al., 2008). A critical constituent of some of these theories has been hypofunction of the excitatory N-methyl-d-aspartic acid glutamate receptors (NMDAR; Coyle, 2004; Javitt and Zukin, 1991; Olney and Farber, 1995). NMDAR antagonists, such as ketamine and PCP, cause schizophrenia-like symptoms when administered to healthy volunteers, including interpersonal withdraw and cognitive impairment not seen with dopaminergic agonists (Krystal et al., 1994; Lahti et al., 1995b). In animal models, NMDA antagonists inhibit GABAergic interneurons, leading to excitation of the disinhibited pyramidal cells (Homayoun and Moghaddam, 2007), accounting for observations from neuroimaging studies in humans of increased cerebral blood flow and metabolism after ketamine challenge (Holcomb et al., 2005; Lahti et al., 1995a; Vollenweider, 1998). Animal work has shown that down-regulation of the GAD67 enzyme occurs as a response of the interneuron to NMDA antagonism, which has been attributed to reduced Ca++ flux from the hypofunctioning NMDAR (Kinney et al., 2006; Zhang et al., 2008), and reduction of the NR2A subunit of the NMDAR has been associated with PV neurons in schizophrenia (Woo et al., 2004). Putting this work together, Lisman has suggested that GABAergic interneurons might falsely sense reduced pyramidal cell activity, and reduced GAD67 production may reflect the attempt to rebalance excitation/inhibition (Lisman, 2012). As mentioned above, Lewis and colleagues have also suggested that a re-setting of the excitatory/inhibitory balance occurs in conjunction with reduced layer III excitation, inferred from reduced dendritic spine density (Lewis et al., 2012). In both interpretations, the implication is that overall cortical capacity is reduced, potentially linked to a reduction in the gamma-oscillations mediated by PVBCs and PVChCs.
To address GABAergic mechanisms more specifically, Menzies and colleagues conducted a pharmacologic fMRI study, in which schizophrenia patients performed a working memory task (‘n-back’) after taking lorazepam (a benzodiazepine [BDZ], which allosterically modulates the GABAR and potentiates inhibitory function), flumazenil (an antagonist at the BDZ receptor on the GABAR with partial inverse agonist properties) and saline (Menzies et al., 2007). Working memory performance was worsened by lorazepam and improved by flumazenil in the schizophrenia patients, changes not seen in the controls. Overall, lorazepam and flumazenil both decreased activation and deactivation in the controls, whereas lorazepam did not reduce deactivation and flumazenil tended to enhance deactivation in the schizophrenia patients. Regional drug-by-group interactions were also noted in several brain areas. Their results confirm differential potency of GABAergic inhibitory systems in the schizophrenia brain and also highlight the difficulty of straightforward predictions in this paradigm. The use of the challenging n-back task may have confounded their experiment, since performance in the schizophrenia patients was at chance level for many of the experimental conditions. Together, these findings illustrate the importance of considering the balance between inhibitory and excitatory systems, as reflected by the fact that both potentiating and reducing overall GABA tone, in the healthy subjects, resulted in a similar change in overall activation and deactivation.
The Menzies study illustrates – albeit in a very small sample -- the possibility of targeting the BDZ site on the GABAR to improve cognition in schizophrenia. Benzodiazepines bind relatively non-selectively to the α-1,2,3,5 subunits, and it has been suggested that drugs with subtype selectivity, e. g. for the α-2,3 subunits (Guidotti et al., 2005; Korpi and Sinkkonen, 2006; Rudolph and Mohler, 2014) or the α-5 subunit (Lodge and Grace, 2011) may be more efficacious in treating schizophrenia. A recent trial with an α-2,3 selective agent, MK-0777 (also known as TPA-023) showed promise in a small, proof-of-concept trial, improving performance on several cognitive tasks and increasing gamma band power (Lewis et al., 2008). Unfortunately, the drug failed to improve cognition or symptoms in a larger trial (Buchanan et al., 2011), but relatively poor affinity for the α-2 subunit make MK-0777 (Rudolph and Knoflach, 2011) an imperfect test of the hypothesis that these subunit selective compounds can play a role in schizophrenia therapeutics.
5.0 TMS studies of GABA and motor activity
TMS has been employed as another tool to study GABA-related inhibition in schizophrenia. TMS delivers a pulsed magnetic field that stimulates electrical current in nervous tissue. The most common paradigms use TMS to stimulate over the motor cortex, leading to contraction of a peripheral muscle, typically of the thumb or finger (motor evoked potential, MEP). One paradigm, called short-interval intra-cortical inhibition (SICI), involves the delivery of a sub-threshold conditioning stimulus (CS), which does not elicit a motor response by itself, approximately 1-4 msec prior to a suprathreshold test stimulus (TS), which does elicit a motor response (Kujirai et al., 1993). The pairing of CS with TS reduces the amplitude of the MEP compared to the TS alone, referred to as inhibition. Of relevance for schizophrenia, pharmacologic evidence indicates the SICI is mediated by GABAA interneurons (Di Lazzaro et al., 2000; Ziemann, 2003), probably with the α-2,3 subunit (Di Lazzaro et al., 2006). A recent meta-analysis of 12 published studies found a significant reduction in SICI in schizophrenia patients (Hedge's g = 0.476, p < 0.0005), and no effect of age or medication status (Radhu et al., 2013). As Table 3 shows, reduced SICI has been reported across all phases of the illness. Some groups have found symptom correlations (less inhibition with greater symptom severity) (Daskalakis et al., 2002; Daskalakis et al., 2008; Liu et al., 2009), but this has not been found in other centers (see Table 3). Interestingly, one recent report found a correlation between greater SICI inhibition and working memory performance (Takahashi et al., 2013). Although these studies cannot rule out non-GABAergic causes of reduced SICI, this literature provides some of the most direct evidence for reduced inhibitory function predicted by the post-mortem GABA findings.
Table 3. TMS studies of short interval cortical inhibition (SICI) in schizophrenia.
| Study | # SZ M/F | SZ Age | #HC M/F | HC Age | Subject type1 | Med status | SICI 2 | Correlations3 |
|---|---|---|---|---|---|---|---|---|
| Pascual-Leone et al, 2002 | 7/1 | 41 yr | 6/1 | 41 yr | Chronic | Unmed | n.s. | |
| 6/1 | 46 yr | -- | -- | Chronic | Med | Med <Unmed, HC | ||
| Daskalakis et al, 2002 | 8/7 | 33 yr | 10/5 | 28 yr | Chronic | Unmed | Unmed<HC; Unmed (t)<Med | All SZ: pos corr w/PANSS positive, global, (t)negative |
| 10/5 | 32 yr | -- | -- | Chronic | Med | Med(t)<HC | ||
| Fitzgerald et al, 2002a | 17/5 | 29 yr | 14/7 | 32 yr | Chronic | Med | n.s. | n.s. with PANSS |
| Fitzgerald et al, 2002b | 32/8 | 28 yr | 15/7 | 32 yr | Chronic | Med | n.s. | |
| Fitzgerald et al, 2004 | 8/2 | 33 yr | 15/3 | 31 yr | Chronic | Unmed | n.s. | n.s. with PANSS |
| 10/6 | 32yr | -- | -- | Chronic | Med | Med<HC | ||
| Oxley et al, 2004 | 8/4 | 34 yr | 8/4 | 28 yr | Chronic | Mixed | SZ<HC | |
| Eichhammer et al, 2004 | 10/11 | 29 yr | 10/11 | 31 yr | Acute | Unmed | SZ(t)<HC | |
| Wobrock et al, 2008, 2009 | 21/8 | 30 yr | 23/21 | 34 yr | Acute | Med | SZ<HC | n.s. with PANSS, CGI, GAF |
| Daskalakis et al, 2008 | 4/2 | 32 yr | 7/3 | 33 yr | Chronic | Unmed | n.s. | All SZ: pos corr w/PANSS positive & total |
| 7/3 | 30 yr | -- | -- | Chronic | Med | n.s. | ||
| Liu et al, 2009 | 78 | 36 yr | 38 | 34 yr | Chronic | Mixed | SZ(t)< HC; SZ clozapine < other med SZ, HC | Pos corr w/PANSS positive |
| Hasan et al, 2011 | 6/3 | 29 yr | 13/9 | 30 yr | Acute | Mixed | All SZ<HC | |
| 9/4 | 36 yr | -- | -- | Chronic | Mixed | |||
| Hasan et al, 2012 | 14/4 | 25 yr | 14/4 | 24 yr | Acute | Med | n.s. | n.s. with PANSS |
| Takahashi et al, 2013 | 9/11 | 27 yr | 10/10 | 28 yr | Acute | Mixed | SZ<HC | n.s with PANSS, neg corr w/digit span |
| Strube et al, 20144 | 29/12 | 30 yr | 37/22 | 32 yr | Acute | Mixed | 3 groups ANOVA (t); 2 groups: SZ<HC | All SZ: n.s. with PANSS |
| 33/9 | 38 yr | -- | -- | Chronic | Mixed |
Acute = average duration of illness < 3 years; Chronic = average duration of illness ≥ 3 years
Results coded positively for greater inhibition, either smaller % of control MEP or smaller ratio in comparison with control MEP
Correlations reported as positive for less inhibition associating with more symptoms or worse performance
Includes subjects from Wobrock et al 2008 and Hasan et al 2011
Abbreviations: SZ, Schizophrenia/schizoaffective subjects; HC, healthy control subjects; M, male; F, female; Med, medicated; Unmed, unmedicated; SICI, short interval cortical inhibition; n.s., no significant differences/correlations; neg corr w/, negative correlation with; pos corr w/, positive correlation with; PANSS, positive and negative syndrome scale; (t), trend; L, left; R, Right;
Expanding the reach of TMS probes outside the motor cortex and dependency upon measuring the MEP is desirable if one wishes to study more complex cortical functions. Recent innovations have combined TMS with other modalities, such as EEG, MEG and fMRI. For instance, using interleaved TMS and EEG, Ferrarelli and colleagues demonstrated decreased evoked gamma oscillations in fronto-central cortex after TMS stimulation of pre-motor cortex in schizophrenia (Ferrarelli et al., 2008). While gamma oscillations have been linked with GABAergic abnormalities (Gonzalez-Burgos et al., 2011; Uhlhaas and Singer, 2010), it is not entirely clear that this TMS-EEG paradigm implicates GABA deficits. However, with additional study and pharmacologic manipulation, it may yet be possible to use such a paradigm to link with the underlying molecular deficits.
6.0 GABA and emotion processing
It is well established that drugs acting on GABARs, such as valproate and BDZ, affect emotion regulation and have been used to treat schizophrenia, e. g. (Wassef et al., 1999). These drugs treat anxiety associated with psychosis, but GABAergic systems are also more directly implicated in psychotic symptoms. Benzodiazepines can prevent a psychotic episode in the early stages of relapse (Carpenter et al., 1999), and blocking GABAR activity with iomazenil, an antagonist and partial inverse agonist of the BDZ receptor, can induce psychotic symptoms in clinically stable schizophrenia patients (Ahn et al., 2011). Furthermore, considering that GAD67 deficits have been identified in patients with bipolar disorder (Guidotti et al., 2000; Torrey et al., 2005; Woo et al., 2008), affect may be an appropriate behavioral target for a schizophrenia drug aimed at one of the α-subunits of GABARs.
Benzodiazepine challenges during fMRI have been used to address GABAergic mechanisms of emotion processing in schizophrenia. Using a lorazepam challenge while subjects viewed salient visual stimuli, Taylor and colleagues (Taylor et al., 2014) found that schizophrenia patients exhibited a predicted group by drug interactions in the dorsal MPFC (dMPFC), as well as right superior frontal gyrus and left and right occipital regions. Instead of lorazepam causing a decreased BOLD signal as it did in the healthy subjects, it caused either less signal drop or, in the case of the dMPFC, increased BOLD signal in schizophrenia patients. These findings are consistent with the GABAR changes from the post-mortem data, because decreased α-1 GABAR would lead to less effect of lorazepam inhibiting neuronal activity, whereas increased α-2 GABAR on the AIS would lead to increased excitation (Lewis et al., 2012). Interestingly, the magnitude of BOLD change with lorazepam correlated with several measures of negative affect, in both the schizophrenia patients and the comparison subjects, suggesting that GABAergic function is associated with negative affect in schizophrenia, on a continuum with normal behavior.
Using an alprazolam challenge while identifying emotional faces, Wolf and colleagues found that the first degree relatives of schizophrenia patients exhibited a stronger reduction in the amygdala and hippocampus than control subjects, who showed no BOLD signal change in these regions (Wolf et al., 2011), findings which may be consistent with at least one study showing decreases in high affinity GABA uptake sites in the amygdala (Simpson et al., 1989). Differences between these two BDZ challenge studies with emotional stimuli, as well as the BDZ challenge during a cognitive task described in section 4.0, may reflect the differential effects of task to uncover different aspects of abnormal GABA function. While these preliminary pharmaco-fMRI studies implicate GABAR abnormalities in schizophrenia, the fMRI signal is a composite of many physiological factors, and alternative interpretations are difficult to rule out.
7.0 Conclusions
GABAergic abnormalities in post-mortem studies of schizophrenia provide a rich database and fertile ground for theoretical models, although as this review demonstrates, functional implications of this post-mortem work have not been definitively established. The in vivo studies face a number of challenges, such as: 1) considerable variability exists at the subject level in all studies of post-mortem and in vivo GABA markers; 2) uncertainty exists as to whether or not a single or multiple etio-pathological processes characterizes patient samples gathered under the label of ‘schizophrenia;’ 3) not all findings have been replicated by all groups in all samples; 4) even if one assumes a single etio-pathological process and 100% replication, it remains difficult to predict implications of molecular level abnormalities on large-scale, aggregate measures of behavior; 5) regional variation across brain structures is poorly characterized; and 6) limited understanding of the molecular mechanisms and microcircuitry of the brain hinders our ability to interpret and predict effects on behavior. Most of the specific studies cited here have small sample sizes, usually less than 20 subjects, meaning that heterogeneity may lead to null results. Lastly, the experimental base for studies aimed at functional implications provides very sparse coverage of different functions and brain regions.
In spite of these limitations, several conclusions may be offered. The indirect measures available with current methodologies implicate a wide range of functions and brain regions associated with GABAergic abnormalities. In other words, it may be premature to limit attention to the dLPFC, hippocampus and cognitive function, when motor cortex and visual cortex may be involved, along with simple motor activity, perception, emotion processing and positive symptoms. As mentioned above, the post-mortem findings have been observed across all cortical regions examined, and it is easy to imagine that a pan-cortical defect in the microarchitecture might characterize some individuals with schizophrenia. Obviously, much work remains, but combinations of existing methodologies, such as pharmacologic challenges with neuroimaging studies, have the potential to yield new information that may refine our understanding of treatment targets and, ultimately, benefit those patients suffering from this devastating disorder.
Acknowledgments
none
References
- Abi-Dargham A, Laruelle M, Krystal J, D'Souza C, Zoghbi S, Baldwin RM, Seibyl J, Mawlawi O, de Erasquin G, Charney D, Innis RB. No evidence of altered in vivo benzodiazepine receptor binding in schizophrenia. Neuropsychopharmacology. 1999;20(6):650–661. doi: 10.1016/S0893-133X(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Ahn K, Gil R, Seibyl J, Sewell RA, D'Souza DC. Probing GABA receptor function in schizophrenia with iomazenil. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(3):677–683. doi: 10.1038/npp.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain research reviews. 2006;52(2):293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene Expression for Glutamic Acid Decarboxylase is Reduced Without Loss of Neurons in Prefrontal Cortex of Schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Asai Y, Takano A, Ito H, Okubo Y, Matsuura M, Otsuka A, Takahashi H, Ando T, Ito S, Arakawa R, Asai K, Suhara T. GABAA/Benzodiazepine receptor binding in patients with schizophrenia using [11C]Ro15-4513, a radioligand with relatively high affinity for alpha5 subunit. Schizophrenia research. 2008;99(1-3):333–340. doi: 10.1016/j.schres.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ball S, Busatto GF, David AS, Jones SH, Hemsley DR, Pilowsky LS, Costa DC, Ell PJ, Kerwin RW. Cognitive functioning and GABAA/benzodiazepine receptor binding in schizophrenia: a 123I-iomazenil SPET study. Biol Psychiatry. 1998;43(2):107–117. doi: 10.1016/s0006-3223(97)00300-4. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature reviews Neuroscience. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biological psychiatry. 2002;52(7):708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44(2):88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48(11):996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21(5):999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73(1):23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RS, Lieberman JA, Barch DM, Csernansky JG, Goff DC, Gold JM, Green MF, Jarskog LF, Javitt DC, Kimhy D, Kraus MS, McEvoy JP, Mesholam-Gately RI, Seidman LJ, Ball MP, McMahon RP, Kern RS, Robinson J, Marder SR. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biological psychiatry. 2011;69(5):442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busatto GF, Pilowsky LS, Costa DC, Ell PJ, David AS, Lucey JV, Kerwin RW. Correlation between reduced in vivo benzodiazepine receptor binding and severity of psychotic symptoms in schizophrenia. Am J Psychiatry. 1997;154(1):56–63. doi: 10.1176/ajp.154.1.56. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Buchanan RW, Kirkpatrick B, Breier AF. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299–303. doi: 10.1176/ajp.156.2.299. [DOI] [PubMed] [Google Scholar]
- Chen CM, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, Schroeder CE, Kegeles LS. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68(8):1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. The American journal of psychiatry. 2011;168(9):921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59(4):347–354. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Moller B, Fountain SI, Chen R. Increased cortical inhibition in persons with schizophrenia treated with clozapine. Journal of psychopharmacology. 2008;22(2):203–209. doi: 10.1177/0269881107084002. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2000;111(5):794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. The Journal of physiology. 2006;575(Pt 3):721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhammer P, Wiegand R, Kharraz A, Langguth B, Binder H, Hajak G. Cortical excitability in neuroleptic-naive first-episode schizophrenic patients. Schizophrenia research. 2004;67(2-3):253–259. doi: 10.1016/S0920-9964(03)00223-8. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, Tononi G. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. The American journal of psychiatry. 2008;165(8):996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry research. 2002a;114(1):11–22. doi: 10.1016/s0925-4927(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of the effects of olanzapine and risperidone on motor cortical excitability in patients with schizophrenia. Psychopharmacology. 2002b;162(1):74–81. doi: 10.1007/s00213-002-1068-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NA, Oxley T, De Castella A, Daskalakis ZJ, Kulkarni J. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophrenia research. 2004;71(1):17–26. doi: 10.1016/j.schres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53(4):591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Kakeda S, Moriya J, Hayashi K, Ikenouchi-Sugita A, Umene-Nakano W, Hori H, Ueda N, Korogi Y, Nakamura J. Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Hum Psychopharmacol. 2009a;24(8):639–645. doi: 10.1002/hup.1070. [DOI] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Hayashi K, Oonari N, Korogi Y, Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophrenia research. 2009b;112(1-3):192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of general psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180(2):191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, Falkai P, Wobrock T. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behavioural brain research. 2011;224(1):15–22. doi: 10.1016/j.bbr.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Hasan A, Wobrock T, Grefkes C, Labusga M, Levold K, Schneider-Axmann T, Falkai P, Muller H, Klosterkotter J, Bechdolf A. Deficient inhibitory cortical networks in antipsychotic-naive subjects at risk of developing first-episode psychosis and first-episode schizophrenia patients: a cross-sectional study. Biological psychiatry. 2012;72(9):744–751. doi: 10.1016/j.biopsych.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008a;13(2):147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008b;165(4):479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23(15):6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Cullen T, Tamminga CA. Effects of noncompetitive NMDA receptor blockade on anterior cingulate cerebral blood flow in volunteers with schizophrenia. Neuropsychopharmacology. 2005;30(12):2275–2282. doi: 10.1038/sj.npp.1300824. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. The American journal of psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Archives of general psychiatry. 2012;69(5):449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Kiss I, Benedek G, Keri S. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Progress in neuro-psychopharmacology & biological psychiatry. 2013;47:13–19. doi: 10.1016/j.pnpbp.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(5):1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9(6):609–620. 544. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1-3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Sinkkonen ST. GABA(A) receptor subtypes as targets for neuropsychiatric drug development. Pharmacol Ther. 2006;109(1-2):12–32. doi: 10.1016/j.pharmthera.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. The Journal of physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995a;6(6):869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995b;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee JD, Park HJ, Oh MK, Chun JW, Kim SJ, Kim E, Kim JJ. Is the GABA System Related to the Social Competence Improvement Effect of Aripiprazole? An (18)F-Fluoroflumazenil PET Study. Psychiatry Investig. 2013;10(1):75–80. doi: 10.4306/pi.2013.10.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165(12):1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in neurosciences. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Current opinion in neurobiology. 2012;22(3):537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biological psychiatry. 2009;65(6):503–509. doi: 10.1016/j.biopsych.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in pharmacological sciences. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Geramita M, van der Veen JW, Barnett AS, Kolachana B, Shen J, Weinberger DR, Law AJ. Genetic association of ErbB4 and human cortical GABA levels in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(32):11628–11632. doi: 10.1523/JNEUROSCI.1529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Savostyanova AA, van der Veen JW, Geramita M, Stern A, Barnett AS, Kolachana B, Radulescu E, Zhang F, Callicott JH, Straub RE, Shen J, Weinberger DR. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(8):1708–1717. doi: 10.1038/npp.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nature reviews Neuroscience. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Menzies L, Ooi C, Kamath S, Suckling J, McKenna P, Fletcher P, Bullmore E, Stephenson C. Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch Gen Psychiatry. 2007;64(2):156–167. doi: 10.1001/archpsyc.64.2.156. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biological psychiatry. 2010;68(7):667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T, Fitzgerald PB, Brown TL, de Castella A, Daskalakis ZJ, Kulkarni J. Repetitive transcranial magnetic stimulation reveals abnormal plastic response to premotor cortex stimulation in schizophrenia. Biological psychiatry. 2004;56(9):628–633. doi: 10.1016/j.biopsych.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Manoach DS, Birnbaum R, Goff DC. Motor cortical excitability in schizophrenia. Biological psychiatry. 2002;52(1):24–31. doi: 10.1016/s0006-3223(02)01317-3. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156(11):1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2013;124(7):1309–1320. doi: 10.1016/j.clinph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Edden RA, Kontson K, Zhu H, Barker PB, Hong LE. GABA predicts inhibition of frequency-specific oscillations in schizophrenia. The Journal of neuropsychiatry and clinical neurosciences. 2013a;25(1):83–87. doi: 10.1176/appi.neuropsych.11120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, Holcomb HH, Barker PB. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophrenia bulletin. 2013b;39(5):1096–1104. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10(9):685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder J, Bubeck B, Demisch S, Sauer H. Benzodiazepine receptor distribution and diazepam binding in schizophrenia: an exploratory study. Psychiatry research. 1997;68(2-3):125–131. doi: 10.1016/s0925-4927(96)02843-0. [DOI] [PubMed] [Google Scholar]
- Simpson MD, Slater P, Deakin JF, Royston MC, Skan WJ. Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neuroscience letters. 1989;107(1-3):211–215. doi: 10.1016/0304-3940(89)90819-7. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes PR, Myers JF, Kalk NJ, Watson BJ, Erritzoe D, Wilson SJ, Cunningham VJ, Barros DR, Hammers A, Turkheimer FE, Nutt DJ, Lingford-Hughes AR. Acute increases in synaptic GABA detectable in the living human brain: A [C]Ro15-4513 PET study. NeuroImage. 2014 doi: 10.1016/j.neuroimage.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Strube W, Wobrock T, Bunse T, Palm U, Padberg F, Malchow B, Falkai P, Hasan A. Impairments in motor-cortical inhibitory networks across recent-onset and chronic schizophrenia: a cross-sectional TMS Study. Behavioural brain research. 2014;264:17–25. doi: 10.1016/j.bbr.2014.01.041. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311(5758):233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Ukai S, Kose A, Hashimoto T, Iwatani J, Okumura M, Tsuji T, Shinosaki K. Reduction of cortical GABAergic inhibition correlates with working memory impairment in recent onset schizophrenia. Schizophrenia research. 2013;146(1-3):238–243. doi: 10.1016/j.schres.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(11):4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Rudolph U, Luscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends in neurosciences. 2011;34(4):188–197. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Demeter E, Phan KL, Tso IF, Welsh RC. Abnormal GABAergic function and negative affect in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(4):1000–1008. doi: 10.1038/npp.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Ueno S, Harada M, Ohmori T. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophrenia research. 2010;117(1):83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biological psychiatry. 2005;57(3):252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature reviews Neuroscience. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Verhoeff NP, Soares JC, D'Souza CD, Gil R, Degen K, Abi-Dargham A, Zoghbi SS, Fujita M, Rajeevan N, Seibyl JP, Krystal JH, van Dyck CH, Charney DS, Innis RB. [123I]Iomazenil SPECT benzodiazepine receptor imaging in schizophrenia. Psychiatry Res. 1999;91(3):163–173. doi: 10.1016/s0925-4927(99)00027-x. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12(10):1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX. Advances and pathophysiological models of hallucinogenic drug actions in humans: a preamble to schizophrenia research. Pharmacopsychiatry. 1998;31(Suppl 2):92–103. doi: 10.1055/s-2007-979353. [DOI] [PubMed] [Google Scholar]
- Wassef AA, Dott SG, Harris A, Brown A, O'Boyle M, Meyer WJ, 3rd, Rose RM. Critical review of GABA-ergic drugs in the treatment of schizophrenia. J Clin Psychopharmacol. 1999;19(3):222–232. doi: 10.1097/00004714-199906000-00004. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Hasan A, Malchow B, Wolff-Menzler C, Guse B, Lang N, Schneider-Axmann T, Ecker UK, Falkai P. Increased cortical inhibition deficits in first-episode schizophrenia with comorbid cannabis abuse. Psychopharmacology. 2010;208(3):353–363. doi: 10.1007/s00213-009-1736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Schneider M, Kadovic D, Schneider-Axmann T, Ecker UK, Retz W, Rosler M, Falkai P. Reduced cortical inhibition in first-episode schizophrenia. Schizophrenia research. 2008;105(1-3):252–261. doi: 10.1016/j.schres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Satterthwaite TD, Loughead J, Pinkham A, Overton E, Elliott MA, Dent GW, Smith MA, Gur RC, Gur RE. Amygdala abnormalities in first-degree relatives of individuals with schizophrenia unmasked by benzodiazepine challenge. Psychopharmacology. 2011;218(3):503–512. doi: 10.1007/s00213-011-2348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–277. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. The American journal of psychiatry. 1997;154(7):1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Archives of general psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30(10):3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. Journal of neurophysiology. 2008;100(2):959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. Pharmacology of TMS. Suppl Clin Neurophysiol. 2003;56:226–231. [PubMed] [Google Scholar]


