Abstract
FoxP3 determines the development of CD4+CD25+ regulatory T (Treg) cells and represses interleukin-2 (IL-2) expression in Treg cells. However, human immunodeficiency virus type 1 (HIV-1) infects and replicates efficiently in FoxP3+ Treg cells. We report that, while inhibiting IL-2 gene expression, FoxP3 enhances gene expression from HIV-1 long terminal repeat (LTR). This FoxP3 activity requires both the N- and C-terminal domains and is inactivated by human IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) mutations. FoxP3 enhances HIV-1 LTR via its specific NFκB binding sequences in an NFκB-dependent fashion in T cells but not in HEK293 cells. FoxP3 decreases level of histone acetylation at the interleukin-2 locus but not at the HIV-1 LTR. Although NFκB nuclear translocation is not altered, FoxP3 enhances NFκB-p65 binding to HIV-1 LTR. These data suggest that FoxP3 modulates gene expression in a promoter sequence-dependent fashion by modulating chromatin structure and NFκB activity. HIV-1 LTR has evolved to both highjack the T-cell activation pathway for expression and to resist FoxP3-mediated suppression of T-cell activation.
Although clearly generated in the thymus (1), CD4+CD25+ Treg2 cells can also be generated from mature T cells in the peripheral organs (2, 3). Recent genetic studies in both mouse and human have identified FoxP3, a Forkhead transcription factor, as a master determinant of Treg development and function (4–7). Mutations in the FoxP3 gene in Scurfy mice or human IPEX patients lead to lymphoproliferative and autoimmune phenotypes due to impaired Treg cells. Importantly, ectopic expression of FoxP3 in naïve CD4+CD25− T cells leads to inhibition of IL-2 expression and Treg-like suppression activity (8). FoxP3+ Treg cells have been implicated in a number of pathological processes, including elevated levels in cancers (9–11) and infectious diseases (12–15), and reduced levels in autoimmune diseases (16–20). With chronic HCV (hepatitis C virus) infection in human and chimps (21–23), and friend leukemia virus in mice (14), Treg cells are induced to subdue the anti-viral immune responses and allow persistent infection.
HIV-1 disease or AIDS progression, however, is associated with overt immune activation. Since the beginning of the AIDS epidemic, it has been documented that chronic immune activation is a reliable predictor of AIDS progression (24–26). Interestingly, SIV infection in its native hosts is non-pathogenic, characterized by high SIV replication, limited immune responses, and long-lasting Treg induction (27–29). In HIV-1-infected patients, conflicting findings have been reported regarding Treg cells. FoxP3+ Treg cells are generally depleted in parallel to the total CD4+ T-cell population during disease progression (30). In some reports, increased levels of Treg cells are documented in lymphoid tissues (31, 32), whereas others have observed decreased levels of Treg cells and FoxP3 expression in peripheral blood (13, 33–35). However, in rhesus monkeys acutely infected with SIV, FoxP3+ Treg cells are induced and are productively infected by SIV (36). Thus, it is not clear how HIV-1 infects and replicates in Treg cells in vivo.
One clear function of FoxP3 is to suppress expression of IL-2 in Treg cells. Direct FoxP3 binding with NFAT has been reported to prevent its association with AP-1 and contribute to suppression of IL-2 gene expression (37). Moreover, FoxP3 has been demonstrated to occupy the IL-2 promoter to inhibit gene expression by altering histone acetylation levels, thus changing the chromatin structure (38). In addition, FoxP3 has been reported to inhibit NFκB activity in transfected 293 cells or T cells (39, 40); however, others have failed to show such inhibitory effect in human T cells (37). Both NFAT and NFκB are also involved in regulating HIV-1 gene expression (41). Although NFκB activates HIV-1 LTR, the effect of NFAT on LTR is controversial. NFATc1 is reported to up-regulate HIV-1 gene expression by binding to the overlapping NFκB sites (41), and NFATc2 has also been shown to up-regulate HIV-1 replication in primary CD4 T cells (42). In other reports, however, NFATc2 is shown to competitively bind to the LTR NFκB sites and inhibit LTR activity (43, 44).
Given that FoxP3 inhibits T-cell activation and expression of cytokines such as IL-2 via inhibiting NFAT and NFκB, one surprising recent finding reports that Treg cells support higher levels of infection by HIV-1 or feline immunodeficiency virus (FIV) compared with FoxP3-CD4+T cells in vitro (13, 45). Two lines of evidence have also indicated that HIV-1 infection and replication in Treg cells may be important in vivo. First, recent reports have documented that, although <5% of total CD4+ T cells from peripheral blood are CD25+FoxP3+ Treg cells, up to 50% of CD4+ T cells express FoxP3 in mucosal lymphoid organs from HIV-1 (46) or SIV (36) infected human or monkeys, respectively. Therefore, the FoxP3+ Treg cells can provide a significant number of target cells for HIV-1 infection in lymphoid organs. Remarkably, 13% of the FoxP3+ T cells are shown to be productively infected by SIV in the lymphoid organs of acutely infected animals (36). Therefore, FoxP3+ Treg cells are important target cells for HIV-1 infection and replication, at least in mucosal lymphoid tissues during acute infection. Treg induction in HIV-1-infected lymphoid organs may contribute to suppressed anti-HIV immunity and establishment of persistent HIV infection. It is therefore critical to investigate how HIV-1 infects and replicates in these T cells for both virological and immunopathogenic reasons.
Here we report that FoxP3 enhances HIV-1 LTR but inhibits IL-2 promoter activity. This FoxP3 activity requires both the N-and C-terminal domains and maps to the N terminus (including the proline-rich region) and the C-terminal Forkhead domain and is inactivated by human IPEX mutations. We also demonstrate that FoxP3 enhances HIV-1 LTR via specific NFκB binding sequences in an NFκB-dependent fashion. FoxP3 also enhances a minimal promoter with the NFκB sequences derived from LTR but not from the MHC promoter in Jurkat cells but represses both the HIV-1 LTR and MHC promoter in 293T cells. Interestingly, FoxP3 reduces the acetylation level of the IL-2 promoter but increases acetylation of HIV-1 LTR. Furthermore, FoxP3 enhances NFκB occupancy at HIV-1 promoter in T cells but does not alter NFκB nuclear translocation or levels. The data suggest that FoxP3 modulates gene expression in a promoter sequence-dependent fashion by modulating chromatin structure and NFκB activity. HIV-1 LTR has evolved to both highjack the T-cell activation pathway for expression and to resist FoxP3-mediated suppression of T-cell activation.
EXPERIMENTAL PROCEDURES
Plasmids, Antibodies, and Cell Lines
FoxP3 was cloned into the HSPG retrovirus vector (47). LTR linker scanning mutants (48), LTR-luc, LTR deletion or point mutants, minimal promoter with LTR 3xκB-luc (41) were previously reported. Mis-sense mutations identified from IPEX patients (8) (I363V, F371C, A384T, and R397W) were introduced in the FoxP3 cDNA by site-directed mutagenesis. Deletion mutants in FoxP3 were generated by PCR amplification to specifically remove the N terminus (aa 1–189) as in FoxP3-ZFLZFKH, the N terminus, including the zinc finger (aa 1–241) as in FoxP3-LZFKH, or the N terminus, including all domains except Forkhead (aa 333–429) as in FoxP3-FKH. MHC-3XκB-luc and IκB-SR plasmids were provided by A. Baldwin (University of North Carolina-Chapel Hill). Jurkat cells were maintained in RPMI 1640 (Invitrogen), 293T cells were maintained in Dulbecco’s modified Eagle’s medium, and primary human T cells were maintained in Iscove’s minimal essential medium, supplemented with 10% fetal bovine serum (Sigma), 2 mM L-glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin. For T-cell activation, mouse anti-CD3 and -CD28 (BD Biosciences) and goat anti-mouse IgG (Caltag) antibodies were used. To determine T-cell purity, anti-human CD25-PE (Miltenyi) and CD4-FITC (BD Biosciences) were used. 7AAD (7-actinomycin D) (Molecular Probes) was used to determine cell viability.
Retrovirus Production and Transduction
293T cells were co-transfected with retroviral vector-, VSV-G-, and gag/pol-containing plasmids as previously described (47). For production of single cycle HIV-Luc reporter virus, 293T cells were transfected with VSV-G and HIV-Luc plasmids by Effectene (Qiagen). For retroviral transduction, Jurkat cells were spin-inoculated with HSPG control or FoxP3 retrovirus to >95% efficiency as measured by GFP expression (47).
Cell Culture and T-cell Purification and Transfection
CD4+ primary T cells were purified from Ficoll-separated PBMC (peripheral blood mononuclear cell) by MACS human CD4+ T-cell isolation kit (>95% purity, Miltenyi-Biotech, Auburn, CA). CD4+ T cells were activated by anti-CD3/CD28 monoclonal antibody cross-linked by plate-bound anti-mouse IgG as previously described (49). Briefly, purified cells are stained with anti-CD3 (0.5 μg/ml) and anti-CD28 (1 μg/ml) and incubated on plated-bound goat anti-mouse IgG. Purified primary CD4+ T cells were transfected by using the Amaxa nucleofector kit (Amaxa Biosystems, Gaithersburg, MD). Briefly, 5 × 106 unstimulated CD4+ T cells were transfected with 5 μg of plasmid DNA (>40% efficiency by GFP expression), and cultured for 48 h before infection with the VSV-G pseudotyped HIV-luciferase reporter virus and activation by CD3 and CD28 cross-linked with plate-bound IgG. Cells were activated for 48 h, and luciferase was measure by luciferase assay. Alternatively, T cells were transduced with vector or FoxP3 retrovirus (>60% efficiency by GFP) and infected with VSV-G pseudotyped HIV-luciferase virus.
IL-2 Measurement by Real-time qPCR and Enzyme-linked Immunosorbent Assay
Amaxa-transfected primary CD4+ T cells were sorted for GFP expression and activated with anti-CD3/CD28 as above. IL-2 mRNA levels were determined following activation. 5 × 103 cells were lysed in TRIzol reagent (Invitrogen) and column-purified (RNeasy, Qiagen). cDNA was generated using Cells-to-cDNA II kit (Ambion, Austin, TX), and quantitative PCR was performed on an ABI Prism 7000 (Applied Biosystems) using TaqMan IL-2 probe/primer set (Applied Biosystems). Each sample was normalized to 18 s. To measure IL-2 production in Jurkat T cells, transduced Jurkat cells were stimulated with PHA (phytohemagglutinin) and ionomycin (1 μM) for 12 h (41), and supernatants were collected. Secreted IL-2 was quantitated by the Human IL-2 enzyme-linked immunosorbent assay kit (BD Biosciences).
Transfection of Jurkat T Cells and Luciferase Assay
Jurkat cells were transfected using Geneporter transfection reagent (Genlantis, San Diego, CA), and transfection efficiency was monitored by GFP expression or co-transfection with pAX-βgal reporter plasmid. Cells were lysed, and luciferase expression was determined by Luciferase Assay System (Promega). Experiments were done in triplicates and repeated at least three times.
ChIP Assays
Jurkat T cells with a stably integrated HIV-1 LTR were transduced with control or FoxP3-expressing vectors. Sonicated chromatin from the Jurkat cells was immunoprecipitated with control IgG (IgG) or p65 (a kind gift from Al Baldwin) or anti-AcH3 by chromatin immunoprecipitation assay kit per the manufacturer’s instruction (Upstate, Millipore). Samples were purified and subject to real-time qPCR analysis. The relative p65 binding or acetylation of IL-2 or LTR promoter is expressed with ratios of ChIP-specific signal divided by signals from control 10% input chromatins. IL-2 promoter was analyzed with TaqMan reagents using the following primers: 5′-cac cta agt gtg tgg gct aat gta ac-3′and 5′-ctg atg act ctt tgg aat ttc ttt aaa cc-3′ and a FAM:TAM probe 5′-aga ggg att tca cct aca tcc att cag tca gtc-3′. This amplicon spans −226 to −133 in the human IL-2 promoter and spans the NFκB and AP-1 sites. HIV-1 LTR was analyzed with SYBR Green reagents (Abgene, UK) using the primers (5′-agc cct cag atc ctg cat ata agc a-3′ and 5′-gtt agc ca gaga gct ccc agg ctc a-3′) that yield an amplicon spanning −44 to −49.
EMSA
Standard electrophoretic mobility shift assays were performed (50). Briefly, probes containing the NFκB site (GGGATTCCCC) from the MHC gene or HIV-1 LTR were labeled with [α-32P]dCTP using Klenow. 10 μg of nuclear extract was incubated with probe. For antibody supershift or blocking, 2 μg of α-p65, α-p50, or control IgG were added to nuclear extract and labeled probe.
Western Blot
1 × 106 Jurkat T cells were lysed in 0.5% Nonidet P-40 lysis buffer and resolved by 10% SDS-PAGE. Approximately 25 μg of proteins was used, and FoxP3 was probed with an anti-FoxP3 antiserum (51), α-rabbit-IgG-horseradish peroxidase and visualized by ECL (Amersham Biosciences).
Statistical Analysis
For statistical analysis, a two-tailed Student t test is employed where p < 0.05 is considered significant.
RESULTS
FoxP3 Differentially Modulates IL-2 and HIV-1 Gene Expression by Inhibiting IL-2 but Enhancing LTR Promoter Activity
To investigate the mechanism of HIV-1 replication in FoxP3+ T cells, we expressed FoxP3 in the human CD4+ T leukemia (Jurkat) cells. When Jurkat cells were infected with VSV-G pseudotyped HIV-1 containing luciferase reporter gene, elevated levels of HIV-1 gene expression were detected in both unstimulated and stimulated FoxP3+ T cells (5- to 8-fold, Fig. 1A). Retrovirus-mediated expression of FoxP3 in Jurkat cells inhibited the expression of IL-2 upon activation (>6-fold, Fig. 1B). To determine if FoxP3 similarly regulates expression of IL-2 and HIV-1 in primary CD4+ T cells, we expressed FoxP3 in purified primary CD4+ T cells. HIV-1 gene expression was significantly enhanced in Th cells previously transduced with FoxP3-expressing retroviral vector (Fig. 1C) or by Amaxa nucleofection (data not shown). In contrast, ectopic expression of FoxP3 in CD4+ T cells efficiently suppressed expression from the endogenous IL-2 gene (Fig. 1D). Thus, FoxP3 inhibits IL-2 expression but enhances HIV-1 gene expression in human T cells.
FIGURE 1. FoxP3 inhibits IL-2, but enhances HIV-1 gene expression in T cells.
A, vector or FoxP3-transduced Jurkat T cells were infected with VSV-G-pseudotyped NL4-Luc virus and non-activated or CD3/CD28-stimulated. At 48 h post-infection, cells were lysed and assayed for luciferase expression. B, vector control and FoxP3 transduced Jurkat cell were non-activated or activated with PMA and ionomycin for 24 h, and supernatants were assayed for IL-2 by enzyme-linked immunosorbent assay. C, primary CD4+ T cells transduced with FoxP3 or control vector and infected with VSV-G/NL-4-Luciferase virus and relative HIV-1 gene expression was measured at 48 h post-infection. D, primary CD4+ T cells transfected with control vector or FoxP3 plasmid (sorted based on GFP) were activated with anti-CD3/CD28 and cultured for 2 days. Cells were harvested for relative IL-2 expression by real-time RT-PCR. Data represents three independent experiments done in triplicates (*, p < 0.05).
To determine if FoxP3 regulates expression of IL-2 and HIV-1 at the promoter level, we transfected IL-2 or HIV-1 LTR promoter constructs driving the luciferase gene into Jurkat T cells. Similar to NL4-luciferase virus infection, FoxP3 also enhanced gene expression from HIV-LTR (Fig. 2A). FoxP3 efficiently suppressed IL-2 gene expression from both the endogenous IL-2 gene (Fig. 1, B and D) and from the transfected IL-2 promoter (Fig. 2B). The HIV-1 LTR co-transfection experiment was performed in the absence of Tat. Therefore, HIV-1 Tat is not required for the FoxP3 activity.
FIGURE 2. FoxP3 inhibits IL-2 promoter but enhances HIV-1 LTR expression in T cells.

Jurkat T cells were transiently transfected with HIV-1 LTR (A) or IL-2 promoter (B) driven luciferase constructs in the presence of control vector or FoxP3 plasmid. For IL2-luciferase assay, Jurkat cell were non-activated or activated with PMA and ionomycin at 36 h post-transfection. Luciferase gene expression relative to vector control cells was measured at 48 h post-infection. Data represents >5 independent experiments done in triplicates (*, p < 0.05).
Forkhead Domain and N Terminus of FoxP3 Are Critical for FoxP3-mediated Enhancement of HIV-1 LTR
The Forkhead domain and distinct residues in FoxP3 are critical for FoxP3 function as indicated by mutations in the scurfy mouse and in human IPEX patients (8). To define the FoxP3 domains that contribute to this LTR-enhancing activity, we generated FoxP3 deletion mutants and transduced Jurkat T cells. Loss of the N terminus, including the proline-rich region, inhibited FoxP3 enhancement (Fig. 3A), whereas expression of the N terminus alone was not able to enhance LTR activity (data not shown). We also tested the known mis-sense mutations derived from IPEX patients by single site-directed mutagenesis introduction into full-length FoxP3. Interestingly, all four such mis-sense mutations tested also inactivated the FoxP3 activity in enhancing HIV-1 LTR (Fig. 3B). Similar levels of the mutant FoxP3 proteins were expressed (supplemental Fig. S1). Therefore, the FoxP3 activity in enhancing HIV-1 LTR depends on both the N-terminal domain and critical residues in the forkhead domain, which are also critical for the function of the FoxP3 protein in humans.
FIGURE 3. A critical activity of FoxP3 is required for LTR enhancement.
A, deletion mutants of FoxP3 were made as described under “Experimental Procedures.” Jurkat cells transduced with retrovirus containing no insert control (PG), wild type FoxP3 (FP3), FoxP3 C terminus (FP3C, aa 191–420), FoxP3 lacking the N terminus and zinc finger (FP3LZFKH, aa 241–420), or the Forkhead domain of FoxP3 (FP3-FKH, aa 333–420) were infected with the VSV-G NL4-Luciferase virus, and luciferase activity was measured 48 h post-infection. Proline-rich region (PRR), zinc finger (ZF), leucine zipper (LZ), and Forkhead domain (FKH) are labeled. B, FoxP3 genes with indicated IPEX mis-sense mutations are co-transfected into Jurkat cells with HIV-1 LTR-luciferase (*, p < 0.05).
Two NFκB Sites in the LTR Enhancer Core Are Both Required for FoxP3 Enhancement
We have mapped the cis-acting elements in HIV-1 LTR that respond to FoxP3 by employing the 26 linker scanning mutants (LS1–LS26) of HIV-1 LTR (48). The mutations in LS20 (−111 to −94) and LS21 (−93 to −76), which inactivate the two NFκB binding sites in the LTR enhancer core, significantly reduced response to FoxP3 (Fig. 4A). Deletion of the NFκB/NFAT enhancer core completely abolished LTR response to FoxP3 (Fig. 4B). The two NFκB sites also overlap with the sites for NFAT binding (41). Point mutations in either NFκB site (LTR mκB1 and mκB2) significantly reduced FoxP3-mediated enhancement, whereas mutations in both sites (mnκB) led to complete loss of enhancement by FoxP3 (Fig. 4B). Therefore the two NFκB sites in the LTR enhancer core are both required for FoxP3 enhancement.
FIGURE 4. FoxP3-mediated LTR enhancement maps to the NFκB sites.
A, linker scanning (LS1–LS26) mutants (48) replacing every 18 bp of HIV-1 LTR were co-transfected into Jurkat cells with vector or FoxP3 as above, and lysates were assayed for LTR-driven luciferase expression. Data are representative of three independent experiments each done in triplicates. LS20/LS21 mutants affect the two NFκB sites in the LTR. B, HIV-1 LTR containing mutations ablating specific binding by NFκB (41) were co-transfected with control vector or FoxP3 in Jurkat cells, and results are summarized from three independent experiments.
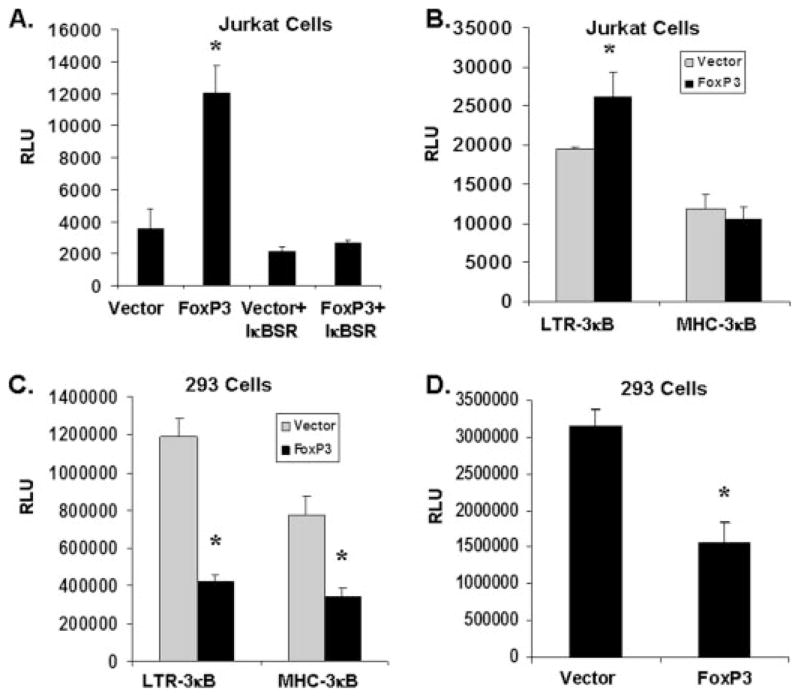
FoxP3 Enhances HIV-1 LTR Activity in NFκB Sequence- and Cell Type-specific Fashions
To further elucidate the role of NFκB, we blocked NFκB activation using the IκB super-repressor in T cells. Consistent with results that the NFκB sites in LTR were critical for FoxP3-mediated activation, FoxP3 was unable to enhance HIV-1 LTR activity when NFκB function was inhibited (Fig. 5A). We also determined if FoxP3 enhancement was specific to the LTR-derived NFκB sequences. A luciferase reporter gene driven by a minimal promoter with three NFκB sites derived from HIV-1 LTR (LTR-3κB-luc) or from the MHC gene (MHC-3κB-luc) was studied in Jurkat cells. Under the same experimental conditions, FoxP3 enhanced LTR-3κB-luc expression but not the MHC-3κB-luc expression in T cells (Fig. 5B). Interestingly, both LTR-3κB-luc and MHC-3kB-luc, as well as the LTR-luc were inhibited by FoxP3 in 293T cells (Fig. 5, C and D), corroborating results previously described for FoxP3 in 293T cells (39, 40). Thus, FoxP3 modulates HIV-1 LTR activity via its specific NFκB sites in T cells.
FIGURE 5. FoxP3 affects LTR expression via an NFκB sequence-dependent and cell type-dependent mechanism.
A, LTR enhancement by FoxP3 is ablated by IκBα-super-repressor (IκB-SR (50)). LTR-luciferase is transfected with IκB-SR and vector or FoxP3 plasmid into Jurkat cells. B and C, LTR enhancement by FoxP3 is specific for the LTR NFκB sites. Luciferase reporters driven by multiple copies of LTR or MHC II (50) NFκB sites were transfected into Jurkat cells (B) or 293T cells (C) with vector control or FoxP3. D, FoxP3 represses in 293T cells. 293T cells transfected with vector control or FoxP3 with HIV-1 LTR luciferase reporter plasmids. Experiments were done in triplicates and lysates are assayed for luciferase expression.
FoxP3 Differentially Alters the Acetylation Level of the IL-2 Promoter and HIV LTR
We tested if FoxP3 affects the chromatin structure at the IL-2 promoter and the HIV-1 LTR by ChIP assays with anti-acetylated histone H3-K9 (AcH3) antibodies. Relative levels of AcH3 were determined by qPCR of the NFκB and NFAT/AP-1 region (−226 to −133) in the human IL-2 promoter after ChIP (Fig. 6A). FoxP3 reduced the AcH3 level or the “openness” of the IL-2 promoter in Jurkat cells either unstimulated or after stimulation. When the stably integrated HIV-1 LTR in the same Jurkat A82 (52) cell was analyzed, we discovered that FoxP3 either had no effect (unstimulated) or increased (stimulated) AcH3 acetylation level at the NFκB/Sp1 enhancer core sequences of the LTR (1.8-fold, p < 0.05, Fig. 6B). Thus, FoxP3 reduced the histone acetylation level of the IL-2 promoter but had no effect or increased that of the HIV-1 LTR in T cells.
FIGURE 6. FoxP3 differentially affects the histone 3 acetylation level of the IL2 and HIV-1 LTR promoters.

Jurkat A82 T cells with a copy of stably integrated HIV-1 LTR were transduced with vector or FoxP3 (>95% GFP+). Sonicated chromatins from activated Jurkat cells were immunoprecipitated with control IgG or anti-acetyl-H3 antibody (AcH3). A, FoxP3 reduces the acetylation of histone H3 at the IL-2 promoter. ChIP analysis of vector or FoxP3 transduced Jurkat A82 T cells was performed. Samples were purified and subject to qPCR analysis with the IL-2 promoter amplicon spanning the NFκB and NFAT/AP-1 sites (−226 to −133) in the human IL-2 promoter. B, FoxP3 increases H3 acetylation at integrated HIV-1 LTR locus. The same Jurkat A82 T cells with a stably integrated HIV-1 LTR were analyzed by anti-AcH3 ChIP for HIV-1 LTR core enhancer sequences by qPCR. Error bars indicate ± S.D. of triplicate samples (*, p < 0.05).
FoxP3 Does Not Alter NFκB Nuclear Translocation but Modulates NFκB Occupancy at the HIV-1 LTR Promoter
Given that FoxP3 enhancement is both NFκB sequence- and cell type-specific, it is unlikely that a simple increase in the nuclear levels of NFκB is involved. Indeed, FoxP3 did not enhance NFκB nuclear localization in Jurkat cells by Western blot analysis (Fig. 7A). Using a consensus NFκB probe, similar levels of p65/p50 and p50/p50 NFκB DNA binding activity were detected in FoxP3+ and vector control cells (Fig. 7B). We next wanted to determine if FoxP3 was able to modulate NFκB occupancy at the HIV-1 LTR promoter in vivo by ChIP using specific anti-p65 antibodies. In a Jurkat T cell line stably integrated with the HIV-1 LTR (53), FoxP3 enhanced the association of p65 at the LTR promoter (~2-fold, Fig. 7C) but not at the IL-2 promoter (Fig. 7D). Therefore, although NFκB nuclear translocation was unchanged, FoxP3 enhanced p65 occupancy specifically at the HIV-1 LTR in T cells.
FIGURE 7. FoxP3 alters NFκB occupancy to the LTR promoter.
A, FoxP3 expression does not affect NFκB localization in the nucleus. Vector control and FoxP3 transduced Jurkat T cells were fractionated, and cytoplasmic and nuclear lysates were separated by SDS-PAGE. Membrane was probed for NFκB using anti-p65 antibody. B, FoxP3 does not alter NFκB p65 and p50 binding to the MHC II-derived consensus NFκB probe. Nuclear extract of transduced Jurkat T cells were left unstimulated or PMA/ionomycin stimulated for 30 min, and NFκB binding was assessed. Antibodies specific for p65 and p50 were used for supershift. C and D, Jurkat T cells with a stably integrated HIV-1 LTR were transduced with control or FoxP3-expressing vectors. Sonicated chromatins were immunoprecipitated with control IgG or anti-p65 antibody. Samples were purified and subjected to real-time qPCR analysis. Input chromatin (10% relative to each immunoprecipitation) was used to normalize samples. The relative p65 occupancy at the HIV-1 LTR (C) or IL-2 promoter (D) from a representative experiment is shown as relative anti-p65-specific ChIP signals divided by signals from control input chromatins (**, p < 0.01).
DISCUSSION
FoxP3 inhibits T-cell activation and IL-2 expression in T cells, but high levels of HIV-1 or feline immunodeficiency virus infection are reported in FoxP3+ Treg cells in vitro (13, 45, 54). Because HIV-1 LTR activity is closely coupled to T-cell activation and IL-2 gene expression, we studied how FoxP3 modulates IL-2 gene expression and HIV-1 replication in T cells. We report that, while inhibiting IL-2 promoter, FoxP3 enhances HIV-1 LTR. This FoxP3 activity is inactivated by human IPEX mutations. FoxP3 enhances HIV-1 LTR via its specific NFκB binding sequences in an NFκB-dependent fashion in T cells but not in HEK293 cells. FoxP3 decreases the level of histone acetylation at the IL2 locus but not at the HIV-1 LTR. Although NFκB nuclear translocation is not altered, FoxP3 enhances NFκB binding to HIV-1 LTR. These data suggest that FoxP3 modulates gene expression in a promoter sequence-dependent fashion by modulating chromatin structure and NFκB activity. HIV-1 LTR has evolved to both highjack the T-cell activation pathway for expression and to resist FoxP3-mediated suppression of T-cell activation.
HIV-1 infection of Treg cells is well supported by a number of studies in vitro and in animal models in vivo. In fact, HIV-1 (or feline immunodeficiency virus) can replicate more efficiently in human (or feline) Treg cells in vitro compared with Th cells (13, 45, 54). It is likely that HIV-1 LTR has evolved to resist FoxP3-mediated mechanisms that inhibit IL-2 expression in FoxP3+ Treg cells. Several recent reports have indicated that: 1) Treg cells can serve as a significant target population for HIV-1 infection in vivo, and 2) these FoxP3+ Treg cells support productive HIV-1 infection in vivo. Although ~5% of total CD4+ T cells from normal peripheral blood are CD25+FoxP3+ Treg cells, up to 50% of CD4+ T cells express FoxP3 in mucosal lymphoid organs from HIV-1 (46)- or SIV (36)-infected human or monkeys. In addition, it has been reported that virtually all human CD4+ T cells up-regulate FoxP3 upon activation and have transient suppressive activity (55). Thus, FoxP3+ T cells can provide a significant number of target cells for HIV-1 infection. Remarkably, 13% of the FoxP3+ T cells are shown to be productively infected by SIV in the lymphoid organs of acutely infected animals (36), whereas 30–60% of memory CD4 T cells in these organs have been reported to harbor SIV proviral genomes (56–58). In comparison, <1% total infection are detected by qPCR of HIV-1 proviral genomes in blood CD4+ memory T cells from HIV-1-infected patients (59, 60). Therefore, FoxP3+ Treg cells are important target cells for HIV-1 infection and replication, at least in mucosal lymphoid tissues during acute infection.
The distinct modulation of LTR and IL2 expression by FoxP3 may be due to specific LTR NFκB binding sequence, or to the unique FoxP3-mediated NFκB activity and chromatin remodeling. Based on a recent report (61), the HIV-1 LTR-derived NFκB site would have a 2- to 3-fold lower affinity relative to the MHC- or IL2-derived NFκB sites.3 FoxP3 may differentially affect the IL-2 and HIV-1 promoter by altering the cooperative binding of p65-p50 with other transcription factors in T cells. Although our data suggest that NFκB is critical for FoxP3 enhancement of HIV-1 LTR, the role of NFAT should be clarified, because NFκB and NFAT share overlapping binding sequences at the LTR enhancer core. IL-2 expression is critically dependent on NFAT and AP-1 activity. Wu et al. (37) describe a model in which FoxP3 binds NFAT to displace AP-1, thus inhibiting IL-2 expression. The direct inhibition of NFAT transcriptional activity by FoxP3 (8, 39) may also lead to preferential inhibition of IL-2. Interestingly, FoxP3 binding at the promoters of CD25 and CTLA-4 is also accompanied by increased NFAT binding, suggesting that FoxP3 stabilizes NFAT on those promoters (37). Although NFATc1 and NFATc2 have both been reported to enhance HIV-1 gene expression in some reports (41, 42), NFATc2 (or NFAT1) can also inhibit HIV-1 LTR by competitively binding to the NFκB core sequences (43, 44). It is thus likely that FoxP3 may inhibit NFATc2 binding at competing LTR NFκB sites and lead to increased NFκB binding and LTR expression. However, our data do not support the involvement of NFAT. First, FoxP3 enhanced HIV-1 LTR expression in unstimulated Jurkat cells (Figs. 1A and 2A) where no NFAT activity is detected. Second, mutations in the LTR NFκB binding sites blocked FoxP3-dependent enhancement, but mutations that specifically inhibit NFAT binding to the LTR (41) did not affect the enhancement (Fig. 4).3 Finally, inhibition of NFAT activation by FK506 did not affect FoxP3-mediated enhancement of LTR,3 whereas enhancement of LTR by FoxP3 depended on NFκB activation (Fig. 5A). Thus a new FoxP3 activity may be involved in modulating the NFκB activity and in enhancing HIV-1 gene expression.
Similar to stabilized NFAT and FoxP3 binding at the promoters of IL-2, CD25, and CTLA-4 (37), the increase in NFκB binding at the LTR in Jurkat cells may be due to a similar FoxP3-mediated stabilization. This is supported by the fact that IPEX mutations in the forkhead DNA binding domain ablated the activity of FoxP3 on LTR (Fig. 3). However, it is not clear if FoxP3 directly binds to specific LTR sequences. The physical interaction of FoxP3 with NFκB is also controversial. Although FoxP3 is shown to interact with NFκB when overexpressed in 293T cells (39), others (37) and our study3 have failed to detect such interaction. Similar to its interaction with NFAT (37), FoxP3 may also interact with NFκB in a DNA sequence-dependent fashion.
A recent report (40) has documented that FoxP3 represses HIV-1 LTR by inhibiting NFκB in T cells as well as in 293 cells. In contrast, we observed that FoxP3 inhibited HIV-1 LTR in 293T cells but enhanced its activity in T cells (Fig. 5). The apparent discrepancy in T cells may be due to different experimental models and procedures. We analyzed HIV-1 gene expression mostly from T cells infected with reporter HIV-1 pseudotyped with VSV-G, whereas the previous report is based only on transient transfection of promoter-reporter assays. The FoxP3 effect on HIV-1 LTR also depends on the activation condition of T cells. We observed enhancement of LTR by FoxP3 in T cells either unstimulated or stimulated with anti-CD3/CD28. However, low or no significant enhancement by FoxP3 was observed following stimulation with PMA and ionomycin in T cells.3 The activation conditions used in the previous study are not clear (40). Therefore, transfection or infection methods and relative levels of activation may contribute to the discrepancy. In addition, the C-terminal Forkhead domain of FoxP3 is required for the reported FoxP3 activity in 293T cells but not in T cells (40). Most human IPEX mutations reside in the Fork-head domain, suggesting that the reported FoxP3 activity in inhibiting NFκB and HIV-1 LTR in T cells may not be a critical FoxP3 activity. Our data conclude that both the N terminus and Forkhead domains are essential for FoxP3 to enhance HIV-1 LTR, consistent with the findings by Wu et al. (37) that both domains of FoxP3 are required for repression of IL-2 and activation of CD25 and CTLA-4.
FoxP3 has recently been demonstrated to inhibit IL-2 and INF-γ gene expression by reducing the histone acetylation and thus altering their chromatin structure. Conversely, promoters activated by FoxP3 (such as CD25, GITR, and CTLA-4) have increased levels of acetylation in FoxP3-expressing cells (38). Our findings support a model wherein expression of FoxP3 leads to increased NFκB occupancy at the HIV-1 LTR and elevated histone acetylation in T cells. The altered chromatin structure at the LTR may promote NFκB occupancy in FoxP3-expressing T cells. Because the FoxP3-mediated effect is correlated with increased AcH3 level at the LTR, CD25, and CTLA4 loci but decreased AcH3 level at the IL2 gene locus (Fig. 7A and Ref. 38), FoxP3 may modulate activity of specific HDAC or HAT in a promoter-dependent fashion in T cells. Alternatively, recruitment of HDAC or HAT by NFκB protein to HIV-1 LTR (62, 63) may be altered by FoxP3 and contribute to the differential gene modulation.
Supplementary Material
Acknowledgments
We are grateful to Drs. A. Baldwin, R. Swanstrom, R. Tisch, B. Damania, D. Margolis, and D. Unutmaz for critical reading and/or discussion of the manuscript. We thank Dr. Y. Liu for providing the FoxP3 cDNA, Dr. A. Baldwin for the anti-NFκB antibodies, IkB super-repressor, and MHC-3x NFκB-luciferase constructs, Dr. S. Kinoshita for the LTR mutants, and Drs. Y. Zhang and A. Baldwin for advice regarding ChIP assays. We thank R. Hunt and Dedeke Brouwer for technical supports and members of the Su laboratory for their input and assistance during this project.
Footnotes
The project was supported by National Institutes of Health Grants AI048407 (to L. S.) and 5T32AI07273 (to G. K.). The authors declare no competing financial interests.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
The abbreviations used are: Treg, regulatory T cell; IPEX, immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome; IL-2, interleukin-2; HIV-1, human immunodeficiency virus, type 1; SIV, simian immunodeficiency virus; LTR, long terminal region; MHC, major histocompatibility complex; aa, amino acid(s); NFAT, nuclear factor of activated T cells; GFP, green fluorescent protein; VSV-G, vesicular stomatitis virus glycoprotein; qPCR, quantitative PCR.
D. Holmes and L. Su, unpublished results.
References
- 1.Sakaguchi S. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apostolou I, von Boehmer H. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Science. 2003;299:1057–1061. [Google Scholar]
- 6.Khattri R, Cox T, Yasayko SA, Ramsdell F. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler SF. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 9.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 10.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 11.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 12.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 13.Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, Unutmaz D. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, Evans LH, Peterson KE, Yang G, Hasenkrug KJ. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 15.Beilharz MW, Sammels LM, Paun A, Shaw K, van Eeden P, Watson MW, Ashdown ML. J Immunol. 2004;172:4917–4925. doi: 10.4049/jimmunol.172.8.4917. [DOI] [PubMed] [Google Scholar]
- 16.Torgerson TR, Ochs HD. Curr Opin Allergy Clin Immunol. 2002;2:481–487. doi: 10.1097/00130832-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 18.Morse SS, Sakaguchi N, Sakaguchi S. J Immunol. 1999;162:5309–5316. [PubMed] [Google Scholar]
- 19.Hornum L, Markholst H. Curr Diab Rep. 2004;4:135–142. doi: 10.1007/s11892-004-0069-6. [DOI] [PubMed] [Google Scholar]
- 20.Boyer O, Saadoun D, Abriol J, Dodille M, Piette JC, Cacoub P, Klatzmann D. Blood. 2004;103:3428–3430. doi: 10.1182/blood-2003-07-2598. [DOI] [PubMed] [Google Scholar]
- 21.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsacker F, Thimme R. J Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto K, Kaplan DE, Ikeda F, Ding J, Schwartz J, Nunes FA, Alter HJ, Chang KM. J Virol. 2005;79:6976–6983. doi: 10.1128/JVI.79.11.6976-6983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. J Acquir Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 25.Giorgi JV. Ann N Y Acad Sci. 1993;677:126–137. doi: 10.1111/j.1749-6632.1993.tb38771.x. [DOI] [PubMed] [Google Scholar]
- 26.Giorgi JV, Nishanian PG, Schmid I, Hultin LE, Cheng HL, Detels R. J Clin Immunol. 1987;7:140–150. doi: 10.1007/BF00916008. [DOI] [PubMed] [Google Scholar]
- 27.Silvestri G, Fedanov A, Germon S, Kozyr N, Kaiser WJ, Garber DA, McClure H, Feinberg MB, Staprans SI. J Virol. 2005;79:4043–4054. doi: 10.1128/JVI.79.7.4043-4054.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestri G. J Med Primatol. 2005;34:243–252. doi: 10.1111/j.1600-0684.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 29.Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, Mc-Clure HM, Staprans SI, Feinberg MB. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 30.Montes M, Lewis DE, Sanchez C, de Castilla DL, Graviss EA, Seas C, Gotuzzo E, White AC., Jr AIDS. 2006;20:1669–1671. doi: 10.1097/01.aids.0000238415.98194.38. [DOI] [PubMed] [Google Scholar]
- 31.Tsunemi S, Iwasaki T, Imado T, Higasa S, Kakishita E, Shirasaka T, Sano H. AIDS. 2005;19:879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- 32.Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, Shearer GM, Chougnet CA. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 33.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, Planta M, McGlaughlin M, Jackson R, Ziegler SF, Fauci AS. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apoil PA, Puissant B, Roubinet F, Abbal M, Massip P, Blancher A. J Acquir Immune Defic Syndr. 2005;39:381–385. doi: 10.1097/01.qai.0000169662.30783.2d. [DOI] [PubMed] [Google Scholar]
- 35.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, Mugyenyi P, Cao H. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 36.Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, Schacker T, Picker LJ, Watkins DI, Lifson JD, Reilly C, Carlis J, Haase AT. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. J Biol Chem. 2006;281:36828–36834. doi: 10.1074/jbc.M608848200. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E, Dastrange M, Oukka M. Proc Natl Acad Sci U S A. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant C, Oh U, Fugo K, Takenouchi N, Griffith C, Yao K, Newhook TE, Ratner L, Jacobson S. PLoS Pathog. 2006;2:e33. doi: 10.1371/journal.ppat.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 42.Cron RQ, Bartz SR, Clausell A, Bort SJ, Klebanoff SJ, Lewis DB. Clin Immunol. 2000;94:179–191. doi: 10.1006/clim.1999.4831. [DOI] [PubMed] [Google Scholar]
- 43.Giffin MJ, Stroud JC, Bates DL, von Koenig KD, Hardin J, Chen L. Nat Struct Biol. 2003;10:800–806. doi: 10.1038/nsb981. [DOI] [PubMed] [Google Scholar]
- 44.Macian F, Rao A. Mol Cell Biol. 1999;19:3645–3653. doi: 10.1128/mcb.19.5.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshi A, Garg H, Tompkins MB, Tompkins WA. J Virol. 2005;79:4965–4976. doi: 10.1128/JVI.79.8.4965-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM, Andersson J, Chougnet C. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coffield VM, Jiang Q, Su L. Nat Biotechnol. 2003;21:1321–1327. doi: 10.1038/nbt889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeichner SL, Kim JY, Alwine JC. J Virol. 1991;65:2436–2444. doi: 10.1128/jvi.65.5.2436-2444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovalev GI, Franklin DS, Coffield VM, Xiong Y, Su L. J Immunol. 2001;167:3285–3292. doi: 10.4049/jimmunol.167.6.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 51.Jiang Q, Su H, Knudsen G, Helms W, Su L. BMC Immunol. 2006;7:6. doi: 10.1186/1471-2172-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan A, Defechereux P, Verdin E. EMBO J. 2001;20:1726–1738. doi: 10.1093/emboj/20.7.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jordan A, Bisgrove D, Verdin E. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joshi A, Vahlenkamp TW, Garg H, Tompkins WA, Tompkins MB. Virology. 2004;321:307–322. doi: 10.1016/j.virol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haase AT. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 57.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 58.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 59.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenchley JM, Ruff LE, Casazza JP, Koup RA, Price DA, Douek DC. J Virol. 2006;80:6801–6809. doi: 10.1128/JVI.00070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Udalova IA, Mott R, Field D, Kwiatkowski D. Proc Natl Acad Sci U S A. 2002;99:8167–8172. doi: 10.1073/pnas.102674699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L, Fischle W, Verdin E, Greene WC. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 63.Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.