Abstract
Background
Prostaglandin D2 (PGD2) and cysteinyl leukotrienes (cysLTs) are lipid mediators derived from mast cells, which activate TH2 cells. The combination of PGD2 and cysLTs (notably cysteinyl leukotriene E4 [LTE4]) enhances TH2 cytokine production. However, the synergistic interaction of cysLTs with PGD2 in promoting TH2 cell activation is still poorly understood. The receptors for these mediators are drug targets in the treatment of allergic diseases, and hence understanding their interaction is likely to have clinical implications.
Objective
We aimed to comprehensively define the roles of PGD2, LTE4, and their combination in activating human TH2 cells and how such activation might allow the TH2 cells to engage downstream effectors, such as neutrophils, which contribute to the pathology of allergic responses.
Methods
The effects of PGD2, LTE4, and their combination on human TH2 cell gene expression were defined by using a microarray, and changes in specific inflammatory pathways were confirmed by means of PCR array, quantitative RT-PCR, ELISA, Luminex, flow cytometry, and functional assays, including analysis of downstream neutrophil activation. Blockade of PGD2 and LTE4 was tested by using TM30089, an antagonist of chemoattractant receptor-homologous molecule expressed on TH2 cells, and montelukast, an antagonist of cysteinyl leukotriene receptor 1.
Results
PGD2 and LTE4 altered the transcription of a wide range of genes and induced diverse functional responses in TH2 cells, including cell adhesion, migration, and survival and cytokine production. The combination of these lipids synergistically or additively enhanced TH2 responses and, strikingly, induced marked production of diverse nonclassical TH2 inflammatory mediators, including IL-22, IL-8, and GM-CSF, at concentrations sufficient to affect neutrophil activation.
Conclusions
PGD2 and LTE4 activate TH2 cells through different pathways but act synergistically to promote multiple downstream effector functions, including neutrophil migration and survival. Combined inhibition of both PGD2 and LTE4 pathways might provide an effective therapeutic strategy for allergic responses, particularly those involving interaction between TH2 cells and neutrophils, such as in patients with severe asthma.
Key words: Prostaglandin D2, leukotriene E4, chemoattractant receptor-homologous molecule expressed on TH2 cells, TH2 cells, neutrophils
Abbreviations used: CAIA, Cell activation–induced aggregation; CRTH2, Chemoattractant receptor-homologous molecule expressed on TH2 cells; cysLT, Cysteinyl leukotriene; CysLT1, Cysteinyl leukotriene receptor 1; CysLT2, Cysteinyl leukotriene receptor 2; ICAM, Intercellular adhesion molecule; LTC4, Cysteinyl leukotriene C4; LTD4, Cysteinyl leukotriene D4; LTE4, Cysteinyl leukotriene E4; PGD2, Prostaglandin D2; PI3K, Phosphoinositide 3-kinase; PMA, Phorbol 12-myristate 13-acetate; qPCR, Quantitative PCR; RORγt, Retinoic acid–related orphan receptor γt
Prostaglandin D2 (PGD2) and cysteinyl leukotrienes (cysLTs) are lipid mediators released from mast cells.1 Both are detected in high concentrations at sites of allergic inflammation, playing critical roles in the pathogenesis of allergic disorders.2,3
A significant contribution of PGD2 to the development of allergic inflammation has been suggested by the observation of enhanced eosinophilic lung inflammation and cytokine release in transgenic mice overexpressing PGD2 synthase.4 Two PGD2 receptors have been identified: D prostanoid receptor 1 and chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2).5 CRTH2 is highly expressed in human eosinophils, basophils, TH2 cells, and group 2 innate lymphoid cells.6-8 Evidence suggests that the proinflammatory role of PGD2 in these cells is predominantly mediated by CRTH2. Through CRTH2, PGD2 elicits chemotaxis,6,7,9 stimulates type 2 cytokine production,9-11 and suppresses apoptosis.12
CysLTs, including cysteinyl leukotriene C4 (LTC4), cysteinyl leukotriene D4 (LTD4), and cysteinyl leukotriene E4 (LTE4), are derived from the 5-lipoxygenase pathway of arachidonic acid metabolism. Two G protein–coupled receptors for cysLTs have been characterized and designated as cysteinyl leukotriene receptor 1 (CysLT1) and cysteinyl leukotriene receptor 2 (CysLT2).13,14 CysLT1 mediates bronchoconstriction and proinflammatory effects, including activation and migration of leukocytes.15,16 CysLT1 antagonists, including montelukast, are approved for clinical use in patients with asthma and allergic rhinitis.
We reported recently that cysLTs potentiated type 2 cytokine production from human TH2 cells in response to PGD2.17 The combination of a CRTH2 antagonist and montelukast was required to completely inhibit type 2 cytokine production induced by mast cell supernatants. These data highlighted an interaction between PGD2 and cysLTs in promoting mast cell–mediated TH2 cell activation. To date, understanding of the synergistic effects of these lipids on TH2 cell function is limited to type 2 cytokine production. Hence we investigated their effects on additional mediators of allergic inflammation and their roles in triggering diverse TH2 cell responses. In particular, we addressed their ability to crosstalk with neutrophils, which are critical players in allergic inflammation, particularly in patients with severe asthma.18,19 Because our previous studies had identified LTE4 as the most potent cysLT in TH2 cytokine production,17 we focused on the effects of combining PGD2 and LTE4.
Our data demonstrate that the proinflammatory effects of both PGD2 and LTE4 in human TH2 cells reach far beyond type 2 cytokine production. Indeed, we find that these lipids synergistically upregulated expression of a range of genes associated with inflammation and confirm that this gene regulation enhances TH2 cell adhesiveness, migration, and survival and promotion of TH2 crosstalk with neutrophils in vitro. Hence we suggest that the synergistic action of PGD2 and LTE4 could contribute to neutrophilia in patients with severe asthma by inducing neutrophil chemokine and growth factor production by TH2 cells.
Methods
TH2 lymphocytes
TH2 cells were isolated from buffy coats (National Blood Service, Bristol, United Kingdom), as described in the Methods section in this article's Online Repository at www.jacionline.org.11 They are memory cells showing a CD4+CRTH2+CD45RO+GATA3+CCR6−CD45RA− retinoic acid–related orphan receptor γt (RORγt)− phenotype with relatively high purity (see Fig E1, A, in this article's Online Repository at www.jacionline.org).
For analysis of gene regulation and cytokine production, TH2 cells were treated with PGD2 or LTE4 alone or their combination in X-VIVO 15 medium (Lonza, Basel, Switzerland) in the presence or absence of antagonist compounds for 2.5 hours (microarray, PCR array, and quantitative PCR [qPCR]) or 4 hours (Luminex).
Cells were treated with the same compounds in serum-free RPMI medium for 4 hours to prepare TH2 cell–conditioned media for neutrophil assays.
Neutrophils
Human neutrophils were isolated from fresh whole blood. Briefly, the red blood cell pellet was collected after Ficoll-Paque Plus density gradient, suspended in HBSS, and mixed with 3% dextran. Neutrophil-rich supernatant was collected and treated in a 0.2% NaCl solution for red blood cell lysis and resuspended in RPMI medium.
Microarrays
Total cellular RNA was extracted with RNeasy Mini kits (Qiagen, Hilden, Germany). Microarrays were performed by Cambridge Genomic Services (Cambridge, United Kingdom) using a HumanHT-12 v4 chip. Genes significant at a P value of less than .05 were analyzed by using the Venn Diagram module within GenePattern.20 Pathway analyses were conducted with IPA (Ingenuity Systems, www.ingenuity.com). Heat maps were generated by using GENE-E software (http://www.broadinstitute.org/cancer/software/GENE-E/index.html).
PCR arrays
PCR arrays were performed with an RT2 Profiler PCR Array Human Common Cytokines kits (SABiosciences, Frederick, Md) in a LightCycler 480 Real-Time PCR System (Roche, Mannheim, Germany).
Luminex
Cytokines were measured with a Procarta Human Cytokine Immunoassay kit (Affymetrix, Santa Clara, Calif). The results were obtained with a Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, Calif).
qPCR
qPCR was performed, as described previously.17 Primers and probes (Roche) used are listed in Table E1 in this article's Online Repository at www.jacionline.org.
ELISA
Cytokines were assayed with ELISA kits (R&D Systems, Minneapolis, Minn). The results were measured in a FLUOstar OPTIMA luminescence plate reader (BMG LabTech, Ortenberg, Germany).
Flow cytometric analysis
Cells were labeled with antibody to CD16–fluorescein isothiocyanate or Annexin V–allophycocyanin and then acquired with an LSR II Flow Cytometer (BD Biosciences, San Jose, Calif).
Cell aggregation analysis
Cell aggregation was photographed with a Nikon Eclipse TS100 microscope (Nikon, Tokyo, Japan). Images were analyzed with CellProfiler 2 software (Broad Institute, Cambridge, Mass; also see the Methods section in this article's Online Repository).21
Chemotaxis assays
Chemotaxis assays were conducted, as described previously.9
Statistics
Data were analyzed by using 1-way ANOVA, followed by the Newman-Keuls test. P values of less than .05 were considered statistically significant.
Results
Effect of PGD2 and LTE4 on the gene expression profile of TH2 cells
PGD2 and LTE4 synergistically evoke type 2 cytokine production from human TH2 cells.17 To understand their broader synergistic effects on TH2 cell function, we investigated the transcriptional responses to LTE4 or PGD2 added either alone or in combination by using RNA microarrays. Three experimental replicates were prepared for each of the 4 groups (control, LTE4, PGD2, and their combination). The concentrations of LTE4 (50 nmol/L) and PGD2 (100 nmol/L) used for the treatments were close to their relative median effective concentration values for type 2 cytokine production in TH2 cells.17
The data showed broad transcriptional changes after treatment. The mRNA levels of 1344, 4750, and 5868 genes were significantly (P < .05) modulated (including upregulation and downregulation) by LTE4, PGD2, or their combination, respectively (Fig 1, A). The effect of PGD2 was much broader than that of LTE4. Although some (approximately 675) of the gene responses overlapped, most of them were regulated distinctly: 669 only by LTE4 and 4075 only by PGD2. The combination of LTE4 and PGD2 amplified significantly the range of the transcriptional response. Expression of a group of 1885 genes was altered only by combination treatment, indicating the combinatorial effect of PGD2 and LTE4 on gene expression. Among the modulated genes, about half were upregulated and half were downregulated (Fig 1, B).
Fig 1.
Gene regulation in TH2 cells by PGD2 and LTE4 detected by using a microarray. A, Venn diagram representing total numbers of genes regulated significantly. B, Venn diagrams and heat map showing numbers of genes downregulated or upregulated significantly. P < .05.
The genes regulated included those involved in the pathways critical for T-cell intrinsic functions and interactions with other cell types (through cell-surface receptors and secreted mediators). To define the significance of the gene expression changes specifically in relation to TH2-mediated allergic inflammation, we analyzed first the effect of lipid mediators on T-cell intrinsic functions and then the effect on cytokine-driven crosstalk with downstream effector cells.
Effects of PGD2 and LTE4 on the apoptosis and migration of TH2 cells
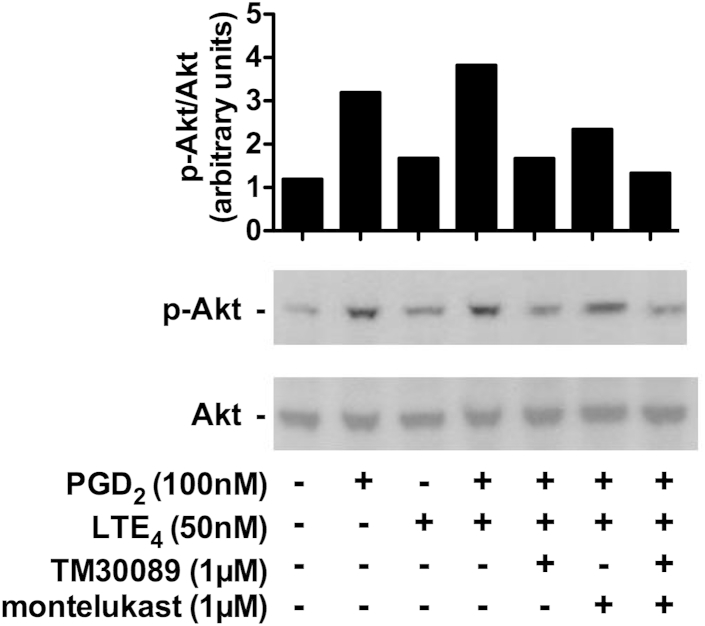
Ingenuity pathway analysis of the microarray data suggested that PGD2 and LTE4 treatment altered the expression of clusters of genes associated with distinct cell-signaling pathways in TH2 cells, including the phosphoinositide 3-kinase (PI3K) and apoptosis pathways (see Fig E2 in this article's Online Repository at www.jacionline.org). Western blotting for phospho-Akt also confirmed that activation of the PI3K pathway by PGD2 and LTE4 was inhibited by TM30089 and montelukast (see Fig E3 in this article's Online Repository at www.jacionline.org). PI3K pathway signaling is critical in mediating the antiapoptotic and chemotactic roles of PGD2/CRTH2.12,22 Therefore we further addressed the effect of PGD2, LTE4, and their combination on these functions (Fig 2).
Fig 2.
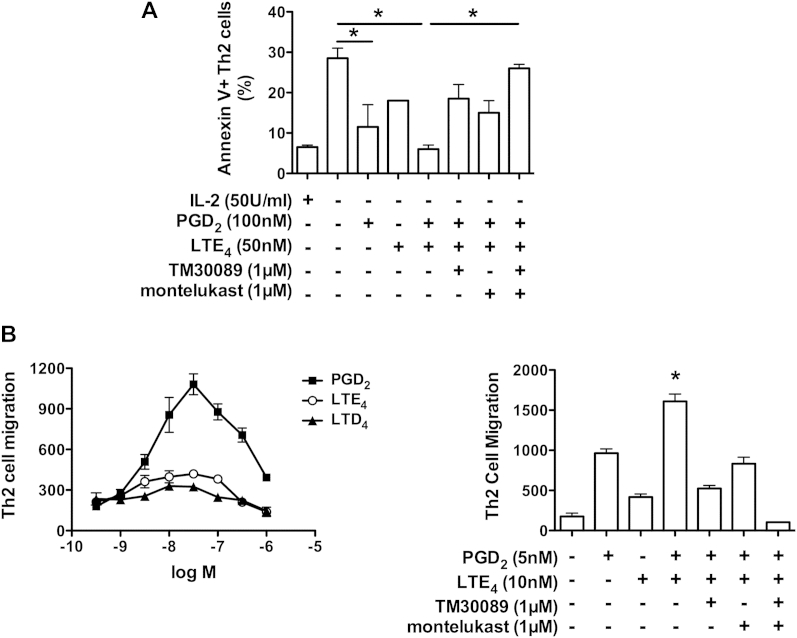
Effects of PGD2 and LTE4 on the apoptosis and migration of TH2 cells. A, Detection of Annexin V in TH2 cells treated with IL-2 deprivation in the presence of compounds, as indicated. B, Cell migration in response to PGD2, LTE4, or LTD4(left panel) or the combination of indicated compounds (right panel). *P < .05 between indicated treatments (Fig 2, A) or between PGD2 plus LTE4 and other treatments (Fig 2, B). n = 3.
In the case of apoptosis, both PGD2 and LTE4 markedly reduced upregulation of Annexin V in TH2 cells after 16 hours of IL-2 withdrawal (Fig 2, A). The combination of 2 mediators additively enhanced this effect.
In chemotaxis assays we first compared the chemotactic effect of PGD2 with that of cysLTs, including LTD4 and LTE4 (Fig 2, B, left panel). These induced migration in a dose-dependent manner, peaking around 30 nmol/L for PGD2 and LTE4 and 20 nmol/L for LTD4. The maximum response achieved by LTE4 was higher (approximately 2-fold) than that elicited by LTD4 but only approximately 27% of that elicited by PGD2. The combination of PGD2 and LTE4 at concentrations close to their median effective concentration synergistically enhanced cell migration (Fig 2, B, right panel).
In both apoptosis and chemotaxis assays the contribution of PGD2 and LTE4 was ablated by TM30089 and montelukast, respectively, and the combination of TM30089 and montelukast inhibited almost all cell responses induced by the combination of PGD2 and LTE4.
Effects of PGD2 and LTE4 on expression of adhesion molecules in TH2 cells
Inspection of the microarray data indicated induction by PGD2 and LTE4 of a particularly large number of transcripts associated with leukocyte adhesion (Table I). Prominent among these were the integrins αV (ITGAV), α2 (ITGA2), αE (ITGAE), and α11 (ITGA11), which constitute subunits of the leukocyte receptors α2βL for intercellular adhesion molecule 1 (ICAM-1) on endothelium, αEβ7 for E-cadherins on epithelium, and α2β/αVβ3 for collagen and vitronectin in tissue extracellular matrix. In addition, PGD2 and LTE4 induced expression of transcripts encoding ICAM-1 (ICAM1) and ICAM-2 (ICAM2), as well as the homophilic adhesion molecule CD31 (PECAM1) and the cadherin/catenin protein family members CTNNAL1, CTNNA1, CTNND1, PCDHA1, PCDHA4, and CDH1, which also act as homophilic adhesion receptors. The majority of these genes (n = 14) were induced by PGD2 alone, whereas only 3 (ITGAV, CTNND1, and ITGA11) were induced by LTE4. Upregulation of some genes (IGSF3, CTNNA1, NINJ1, DCHS1, PCDHA4, and ITGA2) was amplified by the combination of PGD2 and LTE4.
Table I.
List of genes encoding adhesion molecules upregulated by PGD2, LTE4, or their combination in TH2 cells detected by using a microarray∗
| Gene | Protein | Sample treatment |
||
|---|---|---|---|---|
| PGD2 | LTE4 | PGD2 + LTE4 | ||
| FBLN7 | Fibulin 7 | ++ | + | ++ |
| IGSF3 | Immunoglobulin superfamily, member 3 | + | ++ | |
| CTNNAL1 | Catenin alpha-like 1 | ++ | ++ | |
| CTNNA1 | Catenin alpha-1 | + | + | ++ |
| NINJ1 | Ninjurin-1 | + | ++ | |
| CEACAM1 | CD66a | + | + | |
| PECAM1 | Platelet endothelial cell adhesion molecule | + | + | + |
| CD226 | CD226 molecule | + | + | + |
| CD9 | CD9 molecule | + | + | + |
| DCHS1 | Dachsous 1 | + | ||
| ICAM1 | CD54 | + | + | |
| PCDHA4 | Protocadherin alpha-4 | + | ||
| LAMA5 | Laminin, alpha 5 | + | + | |
| SELE | E-selectin | + | ||
| ITGAV | Integrin alpha-V | + | + | |
| ITGAX | CD11c | + | + | |
| CD44 | CD44 molecule | + | + | |
| ITGA2 | CD49b | + | ||
| ITGB1BP1 | Integrin beta-1–binding protein 1 | + | + | |
| CTNND1 | Catenin delta-1 | + | + | |
| ICAM2 | CD102 | + | ||
| CIB2 | Calcium and integrin binding family member 2 | + | ||
| PCDHA1 | Protocadherin alpha-1 | + | ||
| CD151 | CD151 molecule (Raph blood group) | + | ||
| ITGAE | Integrin, alpha E | + | ||
| CDH1 | Cadherin-1 | + | ||
| ITGA11 | Integrin alpha-11 | + | ||
++, Fold change greater than 3.
The concentrations of PGD2 and LTE4 were 100 and 50 nmol/L, respectively.
To explore the consequences of such increased integrin expression on TH2 cell-cell adhesion, we used an in vitro cell activation–induced aggregation (CAIA) assay.23 Stimulation with either lipid alone caused marked CAIA that formed within 0.5 and 1 hours and persisted for 4 to 6 hours (Fig 3, A). The combination of both mediators further enhanced the aggregation. To verify the involvement of integrins, which are critically dependent on Ca2+ ions, we tested the effect of EDTA, an inhibitory chelating agent, and MnCl2, an activator of integrin function, on PGD2/LTE4-induced CAIA. As shown in Fig 3, B, EDTA (5 mmol/L) inhibited and MnCl2 (1 mmol/L) prolonged the CAIA. Blocking antibodies to CD54 (ICAM1) and CD31 (PECAM1) were used to further confirm the contribution of integrins to the CAIA (Fig 3, C). Both antibodies partially reduced the intensity of CAIA in a concentration-dependent manner. The inhibitory effect of anti-CD54 was slightly more potent than that of anti-CD31, and the combination showed a marginal additive effect.
Fig 3.
Involvement of integrins in CAIA of TH2 cells induced by PGD2 and LTE4. Cell aggregation after incubation with indicated treatments for 2 hours (A), for 2 or 9 hours in the presence of EDTA or MnCl2(B), or for 1 hour in the presence of the indicated antibodies (C). Scale bar = 0.5 mm. *P < .05 between PGD2 plus LTE4 and other treatments or between indicated conditions (n = 2-5).
Enhancement of proinflammatory protein production by PGD2 and LTE4 in TH2 cells
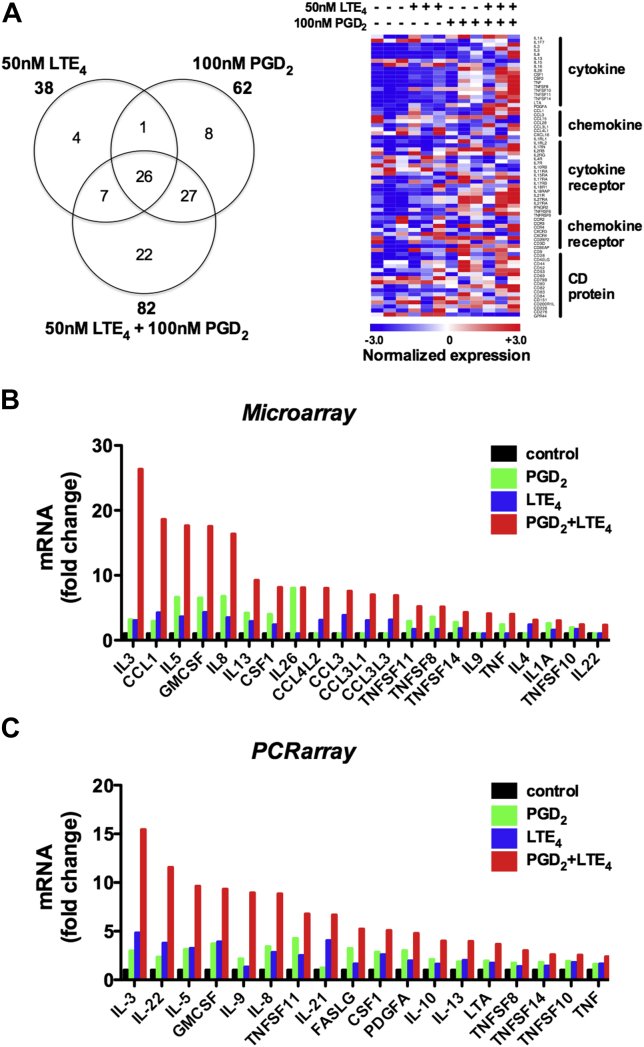
To focus on gene regulation potentially relevant to T cell–mediated diseases, we next analyzed the genes encoding cytokines, chemokines, their receptors, and cluster of differentiation (CD) molecules detected by the microarray (Fig 4). A total of 95 of these genes were significantly modulated, most of them upregulated (Fig 4, A, and see Table E2 in this article's Online Repository at www.jacionline.org), most significantly the cytokines and chemokines. Although some of these effects were induced by PGD2 alone (eg, IL26, IL1RL1, and CCR4) or LTE4 alone (eg, CCL3, CCL3L1, CCL3L3, and CCL4L2), most were driven by their combination. In addition to type 2 cytokine genes, the expression of many other genes was also synergistically enhanced by the combination treatment (Fig 4, B). A number of genes were downregulated (see Table E2), notably transcription of GPR44, the gene for CRTH2 (CD294), which was downregulated by 2.4-fold by PGD2 alone and 3.8-fold by combination treatment. Importantly, these microarray data were largely confirmed also by using a PCR array assay for human common cytokines, including 84 cytokine genes, among which approximately 30 showed significant changes (see Table E3 in this article's Online Repository at www.jacionline.org), although some effects (IL10 and IL21) were only detected by using the PCR array (Fig 4, C).
Fig 4.
PGD2 and LTE4 modulate gene transcription of cytokines, chemokines, and surface receptors in TH2 cells determined by means of microarray (A and B) or PCR array (C). Fig 4, A, Venn diagram and heat map showing the number and distribution of genes significantly regulated. Fig 4, B and C, Strongly upregulated genes. P < .05 (n = 3).
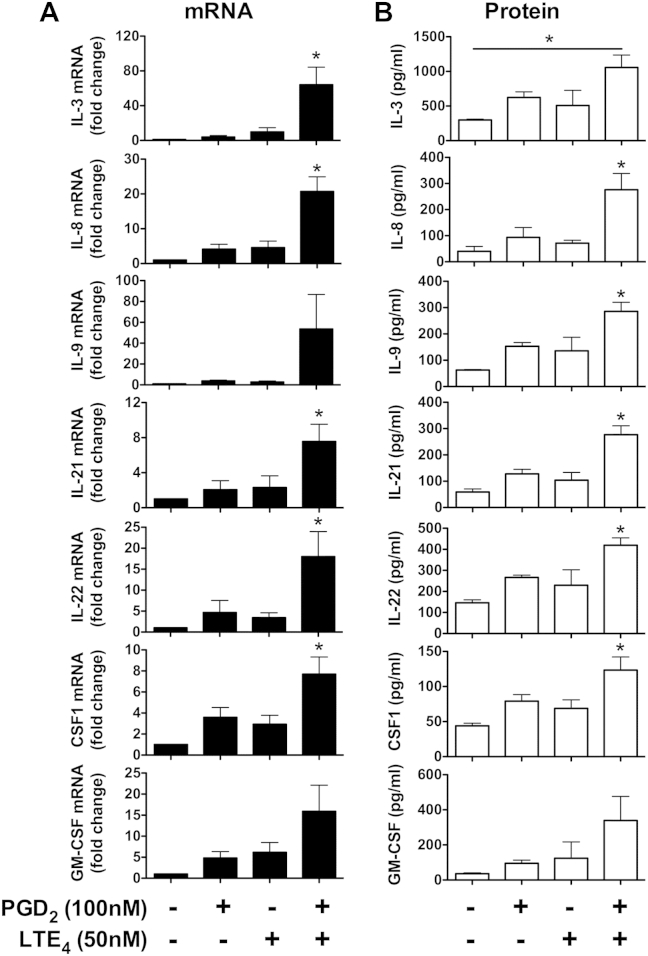
To further verify our findings, we also conducted qPCR (Fig 5, A) and Luminex (Fig 5, B) assays on selected cytokines. The qPCR data strongly supported the synergistic effects of PGD2 and LTE4. At the protein level, combination treatment either additively (IL-3, IL-22, and macrophage colony-stimulating factor) or synergistically (IL-8, IL-9, IL-21, and GM-CSF) enhanced the cytokine production. A similar cytokine profile was also observed after stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin in both cultured TH2 cells (by means of intracellular cytokine staining) and ex vivo TH2 cells (by means of qPCR/Luminex; see Fig E1, B and C). As expected, TM30089 or montelukast only partially inhibited cytokine upregulation after PGD2 plus LTE4, but a combination of TM30089 and montelukast completely blocked this effect (see Fig E4 in this article's Online Repository at www.jacionline.org).
Fig 5.
Effects of PGD2 and LTE4 on production of selected cytokines in TH2 cells. A, Levels of mRNA measured by using qPCR. The mRNA levels in control samples were treated as 1-fold. B, Protein levels were detected with the Luminex assay. *P < .05 between PGD2 plus LTE4 and other conditions or the indicated condition (n = 3).
Effect of cytokines induced by PGD2 and LTE4 from TH2 cells on neutrophil function
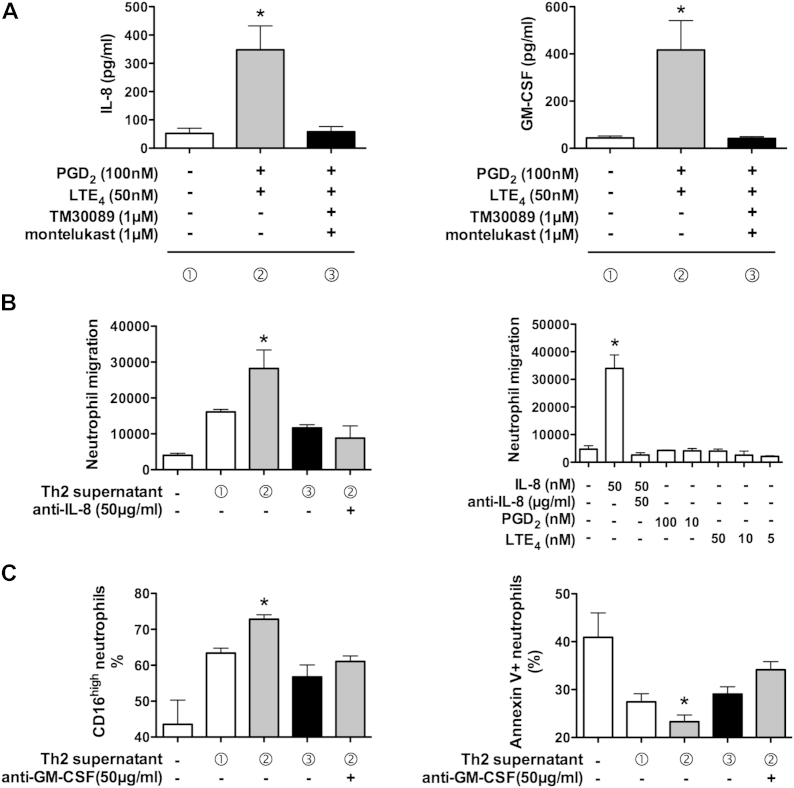
Neutrophilia is detected in the majority of patients with severe asthma,19 and the cytokines induced by activation of TH2 cells included several that could potentially interact with neutrophils. To explore this possibility, we tested the ability of IL-8 and GM-CSF produced by TH2 cells to elicit relevant changes in human neutrophil behavior (Fig 6). As indicated by the data in Fig 6, A, treatment with PGD2 and LTE4 promoted secretion of IL-8 and GM-CSF from resting levels (<90 and <70 pg/mL, respectively, in supernatant 1 to more than 560 and 650 pg/mL, respectively, in supernatant 2; Fig 6, A). This was inhibited by coincubation with TM30089 and montelukast (approximately 94 pg/mL IL-8 and approximately 50 pg/mL GM-CSF in supernatant 3).
Fig 6.
TH2-derived cytokines activate neutrophils. A, IL-8 and GM-CSF levels in stimulated TH2 cell supernatants assigned as supernatants 1 (white bars), 2 (gray bars), and 3 (black bars, n = 4). B, Effect of supernatants (left panel), IL-8, PGD2, LTE4(right panel), and anti–IL-8 antibody on neutrophil migration. C, Effect of supernatants and anti–GM-CSF antibody on expression of CD16 (left panel) and Annexin V (right panel) in neutrophils determined by using fluorescence-activated cell sorting. *P < .05 between the indicated treatment and other conditions (n = 2-7).
First, we addressed the effect of endogenous IL-8 on neutrophil chemotaxis. Recombinant IL-8 induced neutrophil migration with a typical chemotaxis dose curve (see Fig E5, A, in this article's Online Repository at www.jacionline.org). This effect could be inhibited by a neutralizing antibody against IL-8 in a dose-dependent manner (see Fig E5, B). The TH2 supernatant containing high levels of IL-8 (supernatant 2) had a strong capacity to induce neutrophil migration (Fig 6, B, left panel). Inhibition of IL-8 production by TM30089 and montelukast caused substantial (approximately 43%) reduction of the chemotactic activity of supernatant 3. The neutrophil migration to supernatant 2 was mostly blocked by IL-8 neutralizing antibody. To rule out the possibility that cell migration was induced directly by PGD2 or LTE4 (used to prepare supernatant 2), we examined the effects of these mediators (Fig 6, B, right panel). Neither was chemotactic for neutrophils.
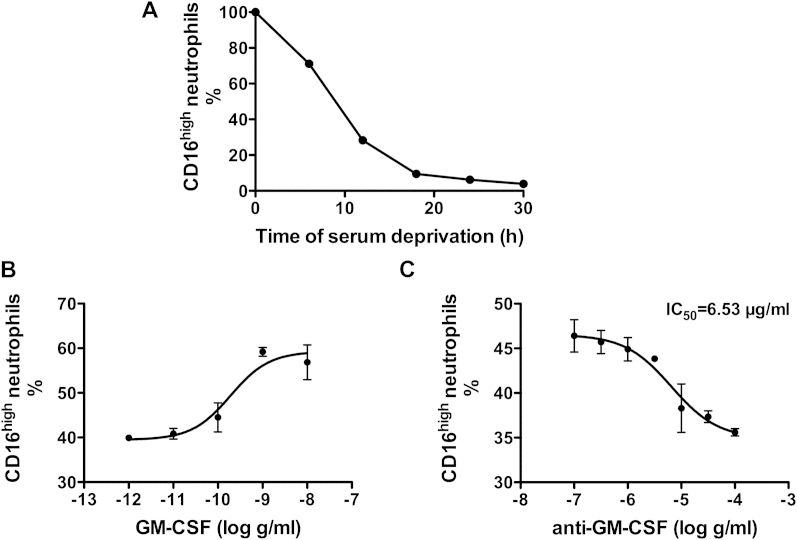
Next, we studied the influence of endogenous GM-CSF on neutrophil behavior by measuring its ability to rescue cells from serum starvation–induced apoptosis using CD16 (FcγRIII) expression as a biomarker of cell integrity (see Fig E6, A, in this article's Online Repository at www.jacionline.org).24 Confirming the validity of the assay, the numbers of CD16high neutrophils decreased after serum withdrawal, which was inhibited by recombinant GM-CSF in a dose-dependent manner (see Fig E6, B). The inhibitory effect of GM-CSF was reversed by a neutralizing antibody against human GM-CSF (see Fig E6, C). Importantly, similar protection against apoptosis was observed when TH2 cell supernatants, particularly supernatant 2, were substituted for recombinant GM-CSF (Fig 6, C, left panel). The protective capacity depended on the level of GM-CSF in the supernatant, which was reduced either by the inhibition of GM-CSF production in TH2 culture (supernatant 3) or GM-CSF neutralizing antibody. To further confirm the antiapoptotic activity of the endogenous GM-CSF induced by PGD2 and LTE4, we also examined the expression of Annexin V in neutrophils (Fig 6, C, right panel). As expected, TH2 supernatants, especially supernatant 2, reduced the numbers of Annexin V–positive cells markedly, which was partially inhibited by the GM-CSF antibody.
Discussion
TH2 cells play an important role in type II immunity, particularly in mast cell–mediated allergic responses, by releasing high levels of type 2 cytokines.25 Our previous study demonstrated that PGD2 and cysLTs are the dominant mediators from activated mast cells that induce TH2 cytokine production.17 A combination of these mediators, particularly PGD2 and LTE4, synergistically enhances this response. In this study we further explored the role of PGD2 and LTE4 and their synergistic effect on diverse TH2 cell functions and revealed that the proinflammatory effects of these mediators are broader than previously recognized. Through activation of CRTH2 and a montelukast-sensitive receptor, gene expression was widely regulated; a number of cytokines, chemokines, and adhesion molecules were upregulated; and several cell-signaling pathways associated with cell adhesion and migration and apoptosis were activated. The upregulated cytokines and other proteins were functional to amplify proinflammatory responses of both TH2 cells themselves and downstream effectors. Combinations of PGD2 and LTE4 showed synergistic effects on these responses. Our findings provide novel insight into the critical role of PGD2 and LTE4, which contribute to important aspects of mast cell/TH2 cell–mediated allergic disorders.
Both PGD2 and LTE4 are lipid mediators involved in a wide range of chronic inflammatory disorders, including allergic asthma and rhinitis.26,27 Bronchoalveolar lavage fluid PGD2 and urinary LTE4 levels are increased in asthmatic patients. The role of PGD2 has been well studied, but the role of LTE4 and the molecular mechanisms used by these mediators are still obscure.10-12 For the first time, we show microarray analysis of the effects of these mediators in human TH2 cells, which suggested that their effects are not limited to type 2 cytokines but rather include a broad range of different genes. In general, the responses to PGD2 were more pronounced than those to LTE4. Although many genes are regulated by both lipids, these lipids seem to use different signaling mechanisms because most genes detected in the microarray were regulated distinctly. However, these mechanisms interact with each other because the combination of the lipids enhances gene regulation through both the intensity and number of genes. Although it has been reported that LTE4 activates the extracellular signal-regulated kinase pathway in human mast cells,28 no phosphorylation of extracellular signal-regulated kinase was detected in PGD2-activated TH2 cells.24 Intriguingly, the microarray data in this study indicate that the PI3K pathway is involved in both responses. Therefore further studies will be required to understand how the signals from these lipids interact to synergistically amplify their proinflammatory effects.
It is well established that activation of TH2 cells is characterized by production of high levels of type 2 cytokines that in turn promote type 2 responses in patients with allergic diseases. A subset of TH2 cells (CD4+CRTH2+CCR6+RORγt+) can also produce IL-17.29 In this study we demonstrated that TH2 cells activated by PGD2/LTE4 and other stimulations, such as PMA/ionomycin, could produce many other proinflammatory cytokines and chemokines that could also play important roles in orchestrating TH2-mediated immune responses. IL-8 and CCL3 are potent chemokines for neutrophils,30 a cell type that is associated with severe asthma.18,19 IL-8 is likely also secreted from other cell types in asthmatic patients, including bronchial epithelial cells. IL-21 is involved in allergic disorders, controlling the differentiation and function of T and B cells.31 IL-22, an IL-10 family cytokine expressed by cell types, including TH17, TH22, γδ T cells, natural killer, and group 3 innate lymphoid cells, is bifunctional, with both proinflammatory and protective effects on tissues depending on the inflammatory context. IL-22–producing cells and plasma concentrations of IL-22 are increased with the severity of atopic dermatitis and asthma.32,33 GM-CSF is critical for granulocyte survival and enhances their activities.34 Increases in GM-CSF levels are detected in patients with allergic asthma, and anti–GM-CSF antibodies administered during allergen challenge of sensitized mice inhibited airway inflammation and mucus production.35 Our data also confirmed that the cytokines induced by PGD2 and LTE4 from TH2 cells are functional, suggesting that these cytokines could make an important contribution to the downstream effects of TH2 cell activation. The tested cytokines (IL-8 and GM-CSF) potently promoted neutrophil activation, although neutralizing antibodies against these cytokines could not completely inhibit the neutrophil activation in response to TH2 supernatants, indicating other products, such as CCL3, might also contribute.
It has been recognized that numbers of neutrophils, as well as eosinophils, are increased in the sputum of patients with severe persistent asthma.19,36,37 The interaction between TH2 cells and eosinophils through type 2 cytokines has been well established. Here we reveal novel mechanisms linking TH2 activation and neutrophilia. Considering that upregulation of the PGD2 pathway and CRTH2 levels is also linked with severe and poorly controlled asthma,27 PGD2/CRTH2 might contribute to severe asthma through neutrophil recruitment and activation. A contribution of PGD2/CRTH2 to neutrophilic inflammation has also been demonstrated by the observation of a role of CRTH2 in contact hypersensitivity–induced skin neutrophil inflammation in the mouse.38
TH2 cells are enriched at the site of allergic inflammation, but the mechanism involved in recruitment of the cells remains obscure. Immune cells undergo a series of sequential steps during extravasation from blood to tissue, including tethering, rolling, adhesion, crawling, and transmigration.39 Our data suggested that PGD2 and LTE4 contribute to the TH2 recruitment cascade through promoting selectin-mediated rolling and integrin-dependent adhesion. Several adhesion molecules could enhance this because antibodies to CD54 and CD31 only partially reduced CAIA. The present study demonstrated the important roles of PGD2 and LTE2 in the TH2 cell infiltration seen during allergic inflammation.
The effect of PGD2 on TH2 cells is mediated by CRTH2 because it can be completely abolished by a selective CRTH2 antagonist but not by the inhibitor of D prostanoid receptor 1.11 The receptor mediating the biological activities of LTE4 is still uncertain, although CysLT1 and CysLT2 are both expressed by human TH2 cells and the effects of cysLTs, including LTE4, can be inhibited by the CysLT1 antagonist.16,17 The activity of LTE4 is unlikely to be mediated by these receptors because of their low affinity for this leukotriene compared with LTD4 and LTC4.13,14 The CysLT1-mediated calcium flux in response to cysLTs in human TH2 cells showed a rank order of potency as follows: LTD4 > LTC4 > LTE4.16 However, the proinflammatory efficacy of LTE4, used alone or in combination with PGD2, is much higher than that of LTD4 and LTC4.17 LTE4 can also stimulate inflammatory responses through mechanisms independent of CysLT1 or CysLT2.28,40 It has been proposed that both montelukast and LTE4 can interact with P2Y-like receptors41,42; however, P2Y12 activation is undetectable in TH2 cells.16,17 Therefore it is possible that the effect of LTE4 is mediated by a montelukast-sensitive receptor that is distinct from the established cysLT receptors.
In summary, this study highlights the broad proinflammatory functions of PGD2 and cysLTs, particularly LTE4, in TH2 cells. They combine to upregulate the expression of many proinflammatory molecules, promote cell adhesion and migration, suppress cell apoptosis, and induce neutrophil activation. These observations indicate how these 2 mast cell products can promote allergic responses and point to potential improved therapies for allergic inflammation.
Key messages.
-
•
The effect of PGD2 and LTE4 on activation of TH2 cells is much broader than previously recognized, which might contribute to the etiology of IgE/mast/TH2 cell–mediated allergic inflammation.
-
•
The combination of PGD2 and LTE4 synergistically enhances proinflammatory responses in TH2 cells.
-
•
The combination of PGD2 and LTE4 promotes TH2 cell/neutrophil crosstalk.
Acknowledgments
We thank Dr Natasha Sahgal for her help in the analysis of microarray data, Anna Barrow for help with chemotaxis assays, and Professor Ian Pavord for critical reading of this article. The microarray was conducted by the Cambridge Gene Service, University of Cambridge.
Footnotes
Supported by the Wellcome Trust (WT091663MA to P.K. and 092871/Z/10/Z to J.F.), the Medical Research Council (CFRXNC00/CF00.G1 to G.O. and D.G.J.), the NIHR (Biomedical Research Centre Programme to L.X., G.O., and M.S.), the Oxford Martin School (to P.K.), the British Medical Association (James Trust 2011; to G.O., P.K., and L.X.), the Oxfordshire Health Services Research Committee Research Grant (1075 to L.X.), the Oxford Dominions Trust (to J.E.U.), the European Molecular Biology Organization long-term fellowship (ALTF1161-2012 to A.N.H.), the Marie Curie Fellowship (330621 to A.N.H.), and the National Institutes of Health (U19AI082630 to L.X. and P.K.). P.K. is a National Institute for Health Research Senior Investigator and is funded by WT091663 MA.
Disclosure of potential conflict of interest: L. Xue has received research support from the BMA James Trust (2011) and the National Institute for Health Research (NIHR) (Biomedical Research Centre, Oxford). J. Fergusson has received research support from the Wellcome Trust (092871/Z/10/Z). A. N. Hegazy has been supported by an EMBO Postdoctoral Fellowship (ALTF1161-2012). D. G. Jackson has received research support from the Medical Research Council (MRC). M. G. Hunter is a board member for and has received consultancy fees from Orca Pharmaceuticals LLC, is employed by Oxagen Ltd and Atopix Therapeutics, and has stock/stock options in Atopix Therapeutics and Orca Pharmaceuticals LLC. R. Pettipher is employed by and has stock/stock options in Atopix Therapeutics. G. Ogg has received research support from the MRC (CFRXNC00/CF00.G1), BMA (2011), NIHR (Biomedical Research Centre, Oxford), and British Skin Foundation; has received consultancy fees from Janssen Pharmaceutical, Novartis, and Lilly; is employed by the University of Oxford and Oxford University Hospitals NHS Trust; has a patent with and has received royalties from Novo Nordisk; and has received travel support from L'Oreal. P. Klenerman has received research support from the NIHR (Biomedical Research Centre, Oxford) and the Wellcome Trust (WT091663MA). The rest of the authors declare that they have no relevant conflicts of interest.
Methods
Reagents
PGD2 and LTE4 were purchased from Enzo Life Science (Farmingdale, NY). TM30089 was supplied by ChemieTek (Indianapolis, Ind). The human CD4+ T Cell Isolation Kit II, anti-human CRTH2 MicroBead Kits, T-cell activation/expansion kits, and anti-human CRTH2 antibody were from Miltenyi Biotec (Bergisch Gladbach, Germany). X-VIVO 15 medium was purchased from Lonza. AIM V medium was purchased from Invitrogen (Carlsbad, Calif). HBSS was from Gibco (Carlsbad, Calif). Ficoll-Paque Plus was supplied by GE Healthcare (Pittsburgh, Pa). Lymphoprep was purchased from Axis-Shield UK (Dundee, United Kingdom). The RNeasy Mini kit and Omniscript Reverse Transcription kit were supplied from Qiagen. Real-time qPCR Master Mix and probes were from Roche. Primers were synthesized by Eurofins MWG Operon (Ebersberg, Germany). Human rIL-8, anti–IL-8, and anti-CD54 (ICAM-1) antibodies were purchased from R&D Systems. Anti-CD31 (PECAM-1) antibody was from Thermo Fisher Scientific (Waltham, Mass). Anti-human GM-CSF, CD3, CD4, CCR6, CCR7, CD45RO, IL-4, IL-5, IL-8, IL-13, and IFN-γ antibodies and Annexin V–allophycocyanin were obtained from BioLegend (San Diego, Calif). Anti-human CD16, IL-22, and GATA3 antibodies and the viability dye eFluor 780 were from eBioscience (San Diego, Calif). Anti-human RORγt antibody was from BD Biosciences. Human rIL-2, human rIL-4, and human rGM-CSF were from PeproTech (Rocky Hill, NJ). Other chemicals were from Sigma-Aldrich (St Louis, Mo).
TH2 cell preparation
Human CD4+CRTH2+ TH2 cells were prepared, as described in our previous reports.E1 Briefly, PBMCs were isolated from buffy coats by using Ficoll-Paque Plus density gradient centrifugation, followed by CD4+ cell purification with the MACS CD4+ T Cell Isolation Kit II. After a 7-day culture in AIM V medium containing 10% human serum, 50 U/mL rhIL-2, and 100 ng/mL rhIL-4, CRTH2+ cells were isolated from the CD4+ culture by means of positive selection with the anti-human CRTH2 MicroBead Kit. The harvested CD4+/CRTH2+ cells were treated as TH2 cells and further amplified in X-VIVO 15 medium containing 10% human serum and 50 U/mL rhIL-2 before use. For ex vivo TH2 cells, CRTH2+ cell isolation was directly conducted after CD4+ cell purification without culture.
Flow cytometric analysis for TH2 cell phenotype
For surface marker staining, TH2 cells or PBMCs from fresh blood were fluorescently labeled with antibodies to CD3, CD4, CRTH2, CCR6, CCR7, CD45RA, and CD45RO in PBS containing 0.2% BSA and 2 mmol/L EDTA and then fixed with 2% formaldehyde. Transcription factor staining was conducted in permeabilization buffer with 0.05% saponin-containing antibodies to GATA3 and RORγt. For analysis of intracellular cytokines, TH2 cells were stimulated with PMA (5 ng/mL) and ionomycin (500 ng/mL) for 4 to 5 hours. Brefeldin A (5 μg/mL) was added at 30 minutes after starting stimulation. Then the cells were stained in a permeabilization buffer containing antibodies to IFN-γ, IL-4, IL-5, IL-8, IL-13, and IL-22. Nonstimulated cells were used as a negative control, and dead cells were excluded by using the viability dye eFluor 780. After staining, the cells were acquired with an LSR II Flow Cytometer (BD Biosciences).
Western blotting
TH2 cells were treated for 15 minutes. Cell lysis and Western blotting were performed, as described previously.E2 Briefly, the cells were solubilized in lysis buffer (20 mmol/L Tris-HCl [pH 7.4], 250 mmol/L sucrose, 1 mmol/L EDTA, 1 mmol/L ethyleneglycol-bis-[β-aminoethylether]-N,N,N′,N′-tetraacetic acid, 1 mmol/L sodium orthovanadate, 10 mmol/L sodium glycerophosphate, 50 mmol/L sodium fluoride, 5 mmol/L sodium pyrophosphate, 0.1% 2-mercaptoethanol, protease inhibitor mixture, and 1% Triton X-100). The samples were fractionated by using SDS-PAGE and then electrophoretically transferred to a nitrocellulose membrane and probed with antibodies, as indicated in the Results section. The intensity of immunopositive bands was quantified by using ImageJ software (National Institutes of Health, Bethesda, Md).
Quantification of cell aggregates
Briefly, cell clumps were identified by using the Otsu method of thresholding. The intensity and size of identified objects were measured. Results are reported as integrated intensity.
Fig E1.
Analysis of cell phenotype. A and B, Expanded TH2 cells were CD3+CD4+CRTH2+CD45RO+GATA3+CCR6−CCR7−CD45RA−RORγt− effector memory cells (Fig E1, A), which produce the type II cytokines IL-8 and IL-22 after stimulation with PMA (5 ng/mL) and ionomycin (500 ng/mL; Fig E1, B), as detected by means of flow cytometry. Blue lines represent staining of indicated cell markers, and red lines represent unstained controls. C, The same cytokine profile was observed in freshly isolated ex vivo TH2 cells by using qPCR (mRNA) and Luminex (protein) assays (high background IL-8 levels in the unstimulated sample precluded accurate analysis of this cytokine in the Luminex assay; n = 6 for Fig E1, A and B; n = 2 for mRNA and n = 1 for protein in Fig E1, C).
Fig E2.
Network diagram of genes depicting pathways involved in activation of TH2 cells induced by PGD2 and LTE4 based on microarray data. A, PI3K pathway. B, Apoptosis pathway. Red color shows gene upregulation, and green color shows downregulation.
Fig E3.
Phosphorylation of Akt in TH2 cells after treatment with PGD2 and LTE4 in the presence or absence of TM30089 and montelukast. The intensity of the bands for phospho-Akt was quantified after normalization with the bands for total Akt.
Fig E4.
Effect of TM30089 and montelukast on transcriptional regulation of cytokine genes induced by PGD2 and LTE4 in TH2 cells determined by using qPCR. The control sample was treated as 1-fold (n = 1).
Fig E5.

Effect of IL-8 on neutrophil migration. A, Neutrophil migration to various concentrations of rhIL-8 in chemotaxis assay. B, Effect of increasing concentration of anti–IL-8 antibody on neutrophil migration induced by 50 nmol/L rhIL-8.
Fig E6.
Effect of GM-CSF on the decrease in CD16 levels in neutrophils, a biomarker of apoptosis, induced by serum deprivation. A, Decrease of CD16high neutrophils with the time of serum deprivation. B, Inhibitory effect of various concentration of rhGM-CSF on the decrease of CD16high neutrophils induced by serum deprivation for 12 hours. C, The inhibitory effect of rhGM-CSF (1 ng/mL) was reversed by anti–GM-CSF antibody in a dose-dependent manner. IC50, Inhibitory concentration of 50%.
Table E1.
Primers and probes used for qPCR
| Gene | Primer | Probe no. |
|---|---|---|
| IL3 | 5′-TTGCCTTTGCTGGACTTCA-3′ 5′-CTGTTGAATGCCTCCAGGTT-3′ |
60 |
| IL8 | 5′-AGACAGCAGAGCACACAAGC-3′ 5′-ATGGTTCCTTCCGGTGGT-3′ |
72 |
| IL9 | 5′-CTTCCTCATCAACAAGATGCAG-3′ 5′-AGAGACAACTGGTCACATTAGCAC-3′ |
59 |
| IL21 | 5′-AGGAAACCACCTTCCACAAA-3′ 5′-GAATCACATGAAGGGCATGTT-3′ |
7 |
| IL22 | 5′-CAACAGGCTAAGCACATGTCA-3′ 5′-ACTGTGTCCTTCAGCTTTTGC-3′ |
6 |
| CSF1 | 5′-GCAAGAACTGCAACAACAGC-3′ 5′-ATCAGGCTTGGTCACCACAT-3′ |
19 |
| GMCSF | 5′-TCTCAGAAATGTTTGACCTCCA-3′ 5′-GCCCTTGAGCTTGGTGAG-3′ |
1 |
| GAPDH | 5′-AGCCACATCGCTCAGACAC-3′ 5′-GCCCAATACGACCAAATCC-3′ |
60 |
CSF1, Macrophage colony-stimulating factor 1.
Table E2.
List of cytokines, chemokines, their receptors, and CD molecule genes regulated by PGD2, LTE4, or their combination in TH2 cells detected by means of microarray∗
| Upregulation |
Downregulation |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Sample treatment |
Gene | Sample treatment |
||||
| PGD2 | LTE4 | PGD2 + LTE4 | PGD2 | LTE4 | PGD2 + LTE4 | ||
| IL1A | + | + | ++ | IL1F7 | − | ||
| IL3 | ++ | ++ | +++ | IL15 | − | − | − |
| IL4 | + | ++ | IL28B | − | |||
| IL5 | +++ | + | +++ | IL29 | − | ||
| IL8 | ++ | ++ | +++ | CCL4L1 | − | ||
| IL9 | ++ | CCL28 | − | − | |||
| IL13 | ++ | + | +++ | CXCL16 | − | − | |
| IL16 | + | + | + | IL6R | − | ||
| IL22 | + | IL7R | − | − | − | ||
| IL26 | +++ | +++ | IL10RB | − | |||
| IL4I1 | + | IL11RA | − | − | |||
| ILF2 | + | IL15RA | − | ||||
| CSF1 | ++ | + | +++ | CCR2 | − | − | − |
| CSF2 | +++ | ++ | +++ | CCR3 | − | − | − |
| LTA | + | ++ | CCR7 | − | |||
| TNF | + | ++ | CXCR4 | − | |||
| TNFSF8 | ++ | + | ++ | CD3D | − | − | |
| TNFSF10 | + | + | + | CD40 | − | ||
| TNFSF11 | + | + | ++ | CD47 | − | ||
| TNFSF14 | + | + | ++ | CD48 | − | ||
| CCL1 | + | ++ | +++ | CD79B | − | − | |
| CCL3 | ++ | +++ | CD80 | − | |||
| CCL3L1 | ++ | +++ | CD86 | − | |||
| CCL3L3 | ++ | +++ | CD99L2 | − | |||
| CCL4L2 | ++ | +++ | CD200R1 | − | − | ||
| CCL5 | + | GPR44 | − | − − | |||
| CCL15 | + | + | |||||
| IFNGR2 | + | + | ++ | ||||
| IL1RAP | + | + | |||||
| IL1RL1 | +++ | + | +++ | ||||
| IL1RL2 | + | ||||||
| IL2RB | + | + | |||||
| IL2RG | + | + | |||||
| IL4R | + | + | |||||
| IL17RA | + | + | |||||
| IL17RB | + | ||||||
| IL18R1 | + | + | + | ||||
| IL18RAP | ++ | ++ | +++ | ||||
| IL21R | + | ++ | |||||
| IL27RA | + | + | + | ||||
| IL28RA | + | ||||||
| CCR4 | ++ | + | |||||
| CXCR3 | + | ||||||
| CXCR5 | + | ||||||
| CXCR6 | + | ||||||
| TNFRSF8 | ++ | ++ | |||||
| TNFRSF9 | +++ | ++ | +++ | ||||
| CD2BP2 | + | + | |||||
| CD5L | + | ||||||
| CD9 | + | + | + | ||||
| CD28 | + | + | |||||
| CD40LG | + | ++ | |||||
| CD44 | + | + | |||||
| CD52 | + | ||||||
| CD53 | + | + | |||||
| CD55 | + | ||||||
| CD58 | + | + | |||||
| CD59 | + | ||||||
| CD69 | ++ | ++ | +++ | ||||
| CD70 | + | ||||||
| CD81 | + | ||||||
| CD82 | + | + | + | ||||
| CD83 | + | ++ | |||||
| CD84 | + | + | |||||
| CD109 | + | ++ | |||||
| CD151 | + | ||||||
| CD200 | + | ||||||
| CD226 | + | + | |||||
| CD276 | + | + | |||||
CSF, Macrophage colony-stimulating factor.
++ or − −, Fold change of 3 or greater; +++, fold change of 6 or greater.
Concentrations of PGD2 and LTE4 were 100 and 50 nmol/L, respectively.
Table E3.
List of cytokine genes regulated by PGD2, LTE4, or their combination in TH2 cells detected by means of PCR array∗
| Upregulation |
Downregulation |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Sample treatment |
Gene | Sample treatment |
||||
| PGD2 | LTE4 | PGD2 + LTE4 | PGD2 | LTE4 | PGD2 + LTE4 | ||
| IL3 | + | ++ | +++ | BMP4 | − | − | − |
| IL5 | ++ | ++ | +++ | BMP8B | − | − | − |
| IL8 | ++ | + | +++ | GDF9 | − | − | − |
| IL9 | + | + | +++ | IFNA5 | − | − | − |
| IL10 | + | + | ++ | IFNK | − − | − | − |
| IL13 | + | + | ++ | IL15 | − | − | − |
| IL21 | + | ++ | +++ | IL17C | − | − | − |
| IL22 | + | ++ | +++ | IL18 | − | − | − |
| CSF1 | + | + | ++ | IL24 | − | − | − |
| CSF2 | ++ | ++ | +++ | NODAL | − − | − | − |
| FASLG | ++ | + | ++ | TGFB3 | − | − | − |
| LTA | + | + | ++ | TNFSF13B | − − | − | − − |
| PDGFA | ++ | + | ++ | ||||
| TNF | + | + | + | ||||
| TNFSF8 | + | + | ++ | ||||
| TNFSF10 | + | + | + | ||||
| TNFSF11 | ++ | + | ++ | ||||
| TNFSF14 | + | + | + | ||||
CSF, Macrophage colony-stimulating factor.
++ or − −, Fold change of 3 or greater; +++, fold change of 6 or greater.
Concentrations of PGD2 and LTE4 were 100 and 50 nmol/L, respectively.
References
- 1.Schleimer R.P., Fox C.C., Naclerio R.M., Plaut M., Creticos P.S., Togias A.G. Role of human basophils and mast cells in the pathogenesis of allergic diseases. J Allergy Clin Immunol. 1985;76:369–374. doi: 10.1016/0091-6749(85)90656-6. [DOI] [PubMed] [Google Scholar]
- 2.Philipps G.D., Holgate S.T. Interaction of inhaled LTC4 with histamine and PGD2 on airway caliber in asthma. J Appl Physiol. 1989;66:304–312. doi: 10.1152/jappl.1989.66.1.304. [DOI] [PubMed] [Google Scholar]
- 3.Sampson S.E., Sampson A.P., Costello J.F. Effect of inhaled prostaglandin D2 in normal and atopic subjects, and of pretreatment with leukotriene D4. Thorax. 1997;52:513–518. doi: 10.1136/thx.52.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujitani Y., Kanaoka Y., Aritake K., Uodome N., Okazaki-Hatake K., Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J Immunol. 2000;168:443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- 5.Pettipher R., Hansel T.T., Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6:313–325. doi: 10.1038/nrd2266. [DOI] [PubMed] [Google Scholar]
- 6.Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagata K., Hirai H. The second PGD2 receptor CRTH2: structure, properties, and functions in leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:169–177. doi: 10.1016/s0952-3278(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 8.Mjösberg J.M., Trifari S., Crellin N.K., Peters C.P., van Drunen C.M., Piet B. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 9.Xue L., Salimi M., Panse I., Mjösberg J.M., McKenzie A.N., Spits H. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka K., Hirai H., Takano S., Nakamura M., Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun. 2004;316:1009–1014. doi: 10.1016/j.bbrc.2004.02.151. [DOI] [PubMed] [Google Scholar]
- 11.Xue L., Gyles S.L., Wettey F.R., Gazi L., Townsend E., Hunter M.G. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 12.Xue L., Barrow A., Pettipher R. Novel function of CRTH2 in preventing apoptosis of human Th2 cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:7580–7586. doi: 10.4049/jimmunol.0804090. [DOI] [PubMed] [Google Scholar]
- 13.Lynch K.R., O'Neill G.P., Liu Q., Im D.S., Sawyer N., Metters K.M. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 14.Heise C.E., O'Dowd B.F., Figueroa D.J., Sawyer N., Nguyen T., Im D.S. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.C., Hsu F.I., Barrett N.A., Friend D.S., Grenningloh R., Ho I.C. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 16.Parmentier C.N., Fuerst E., McDonald J., Bowen H., Lee T.H., Pease J.E. Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1. J Allergy Clin Immunol. 2012;129:1136–1142. doi: 10.1016/j.jaci.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 17.Xue L., Barrow A., Fleming V.M., Hunter M.G., Ogg G., Klenerman P. Leukotriene E4 activates human Th2 cells for exaggerated proinflammatory cytokine production in response to prostaglandin D2. J Immunol. 2012;188:694–702. doi: 10.4049/jimmunol.1102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood L.G., Baines K.J., Fu J., Scott H.A., Gibson P.G. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142:86–93. doi: 10.1378/chest.11-1838. [DOI] [PubMed] [Google Scholar]
- 19.Moore W.C., Hastie A.T., Li X., Li H., Busse W.W., Jarjour N.N. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–1563.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reich M., Liefeld T., Gould J., Lerner J., Tamayo P., Mesirov J.P. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 21.Jones T.R., Carpenter A.E., Lamprecht M.R., Moffat J., Silver S.J., Grenier J.K. Scoring diverse cellular morphologies in image based screens with iterative feedback and machine learning. Proc Natl Acad Sci U S A. 2009;106:1826–1831. doi: 10.1073/pnas.0808843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue L., Gyles S.L., Barrow A.M., Pettipher R. Inhibition of PI3K and calcineurin suppresses chemoattractant receptor-like molecule expressed on Th2 cells (CRTH2)-dependent responses of Th2 lymphocytes to prostaglandin D2. Biochem Pharmacol. 2007;73:843–853. doi: 10.1016/j.bcp.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Ford P.M., Ford S.E., Gibson J. Evaluation of antigen-induced buffy coat leucocyte aggregation as a simple test of allergic reactivity. Int Arch Allergy Appl Immunol. 1977;53:56–61. doi: 10.1159/000231731. [DOI] [PubMed] [Google Scholar]
- 24.Dransfield I., Buckle A.M., Savill J.S., McDowall A., Haslett C., Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. 1994;153:1254–1263. [PubMed] [Google Scholar]
- 25.Robinson D.S. Th2 cytokines in allergic diseases. Br Med Bull. 2000;56:956–968. doi: 10.1258/0007142001903625. [DOI] [PubMed] [Google Scholar]
- 26.Taylor G.W., Taylor I., Black P., Maltby N.H., Turner N., Fuller R.W. Urinary leukotriene E4 after antigen challenge and in acute asthma and allergic rhinitis. Lancet. 1989;1:584–588. doi: 10.1016/s0140-6736(89)91611-5. [DOI] [PubMed] [Google Scholar]
- 27.Fajt M.L., Gelhaus S.L., Freeman B., Uvalle C.E., Trudeau J.B., Holguin F. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paruchuri S., Jiang Y., Feng C., Francis S.A., Plutzky J., Boyce J.A. Leukotriene E4 activates peroxisome proliferator-activated receptor gamma and induces prostaglandin D2 generation by human mast cells. J Biol Chem. 2008;283:16477–16487. doi: 10.1074/jbc.M705822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y.H., Voo K.S., Liu B., Chen C.Y., Uygungil B., Spoede W. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himmel M.E., Crome S.Q., Ivison S., Piccirillo C., Steiner T.S., Levings M.K. Human CD4+ FOXP3+ regulatory T cells produce CXCL8 and recruit neutrophils. Eur J Immunol. 2011;41:306–312. doi: 10.1002/eji.201040459. [DOI] [PubMed] [Google Scholar]
- 31.Sarra M., Cupi M.L., Pallone F., Monteleone G. Interleukin-21 in immune and allergic diseases. Inflamm Allergy Drug Targets. 2012;11:313–319. doi: 10.2174/187152812800959040. [DOI] [PubMed] [Google Scholar]
- 32.Nograles K.E., Zaba L.C., Shemer A., Fuentes-Duculan J., Cardinale I., Kikuchi T. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Yang J., Gao Y.D., Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151:297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 34.Smith W.B., Guida L., Sun Q., Korpelainen E.I., van den Heuvel C., Gillis D. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood. 1995;86:3938–3944. [PubMed] [Google Scholar]
- 35.Yamashita N., Tashimo H., Ishida H., Kaneko F., Nakano J., Kato H. Attenuation of airway hyperresponsiveness in a murine asthma model by neutralization of granulocyte-macrophage colony-stimulating factor (GM-CSF) Cell Immunol. 2002;219:92–97. doi: 10.1016/s0008-8749(02)00565-8. [DOI] [PubMed] [Google Scholar]
- 36.Green R.H., Brightling C.E., Woltmann G., Parker D., Wardlaw A.J., Pavord I.D. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikuchi S., Nagata M., Kikuchi I., Hagiwara K., Kanazawa M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int Arch Allergy Immunol. 2005;137(suppl 1):7–11. doi: 10.1159/000085425. [DOI] [PubMed] [Google Scholar]
- 38.Takeshita K., Yamasaki T., Nagao K., Sugimoto H., Shichijo M., Gantner F. CRTH2 is a prominent effector in contact hypersensitivity-induced neutrophil inflammation. Int Immunol. 2004;16:947–959. doi: 10.1093/intimm/dxh096. [DOI] [PubMed] [Google Scholar]
- 39.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 40.Maekawa A., Kanaoka Y., Xing W., Austen K.F. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamedova L., Capra V., Accomazzo M.R., Gao Z.G., Ferrario S., Fumagalli M. CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol. 2005;71:115–125. doi: 10.1016/j.bcp.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanaoka Y., Maekawa A., Austen K.F. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem. 2013;288:10967–10972. doi: 10.1074/jbc.C113.453704. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Xue L., Gyles S.L., Wettey F.R., Gazi L., Townsend E., Hunter M.G. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- Xue L., Gyles S.L., Barrow A.M., Pettipher R. Inhibition of PI3K and calcineurin suppresses chemoattractant receptor-like molecule expressed on Th2 cells (CRTH2)-dependent responses of Th2 lymphocytes to prostaglandin D2. Biochem Pharmacol. 2007;73:843–853. doi: 10.1016/j.bcp.2006.11.021. [DOI] [PubMed] [Google Scholar]