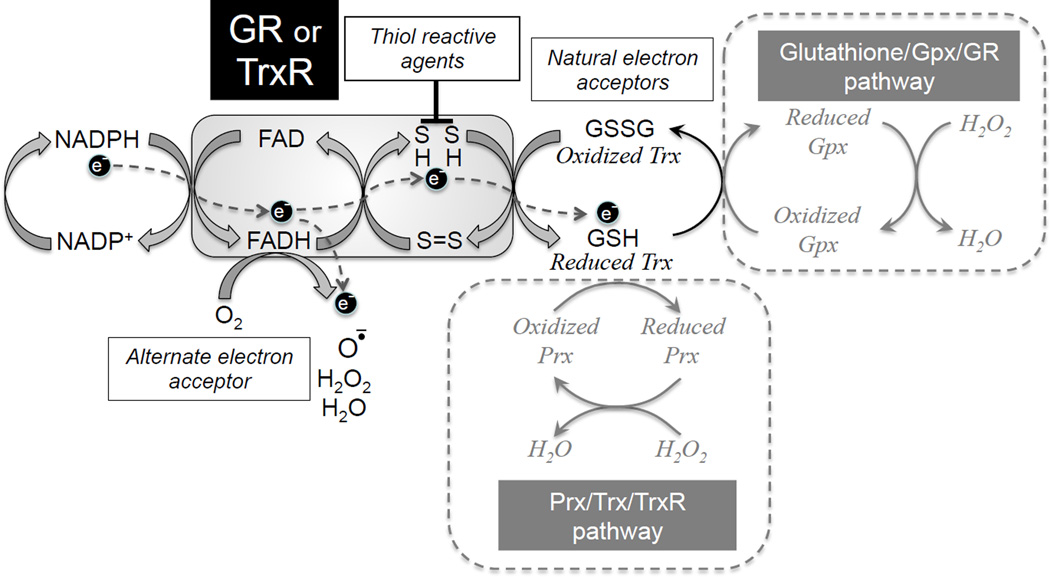
Fig. 1. Proposed mechanism of ROS generation by GR and TrxR2 during reductive stress.
Flow of electrons (e−) as indicated from NADPH to FAD to cysteine-related sulfhydryl groups (S) and then to the natural electron acceptors GSSG for GR, and oxidized Trx for TrxR2. If oxidized GSSG or Trx are in limited supply, the flavin reduction state is increased and electron flow is shunted from FADH2 to O2 as an alternative electron acceptor, with oneelectron reduction producing superoxide, two-electron reduction producing H2O2 or four-electron reduction producing H2O. Similarly, thiol reactive agents which react with SH groups in GR or TrxR2 also prevent electron transfer to the natural electron acceptors, resulting in superoxide, H2O2 and H2O production instead.