Summary
Mutations in the phosphatase PTEN are strongly implicated in autism spectrum disorder (ASD). Here we investigate the function of Pten in cortical GABAergic neurons using conditional mutagenesis in mice. Loss of Pten results in a preferential loss of SST+ interneurons, that increases the ratio of PV/SST interneurons, ectopic PV+ projections in layer I, and increases in inhibition onto glutamatergic cortical neurons. Pten mutant mice exhibit deficits in social behavior and changes in EEG power. Using MGE transplantation, we test for cell-autonomous functional differences between human PTEN wild type (WT) and ASD alleles. The PTEN ASD alleles are hypomorphic in regulating cell size and the PV/SST ratio compared to WT PTEN. This MGE transplantation/complementation assay is efficient and is generally applicable to functionally test ASD alleles in vivo.
Graphical abstract

Introduction
Autism spectrum disorder (ASD) is characterized by deficits in language, social interactions and repetitive behaviors. Some forms of ASD may be caused by an imbalance in excitatory/inhibitory (E/I) tone in the brain (Rubenstein and Merzenich, 2003). An increasing number of ASD genetic risk loci are being identified (Murdoch and State, 2013; Tebbenkamp et al., 2014). Recent data from mouse models, including MeCP2 (Chao et al., 2010), Scn1a (Han et al., 2012), Caspr2 (Peñagarikano et al., 2011), Caspr4 (Karayannis et al., 2014), Shank3 and the mouse strain BTBR (Gogolla et al., 2014), showed defects in GABAergic neurons that contribute to ASD phenotypes by altering E/I balance. This balance may be disrupted by altering the relative numbers, functions and/or connectivity between excitatory neurons and inhibitory interneurons.
In the neocortex, glutamatergic excitatory neurons comprise ~70% of all neurons, and GABAergic inhibitory interneurons account for ~30%. The majority of interneurons arise from the medial and caudal ganglionic eminences (MGE and CGE) of the basal ganglia. Immature interneurons tangentially migrate from the MGE to the cortex, then radially migrate into cortical layers (Anderson et al., 1997). As MGE cells mature, they express specific molecular markers of interneuron subtypes, including somatostatin (SST) and parvalbumin (PV) (Wonders and Anderson, 2006). CGE-derived interneurons express vasoactive intestinal peptide (VIP) and Reelin (that lack SST) (Miyoshi et al., 2010). Furthermore, interneuron subgroups project to different cortical layers and synapse within distinct domains of the neocortex (Huang et al., 2007). Thus, perturbations in different interneuron subgroups can lead to E/I imbalance by a variety of mechanisms.
While most ASD genes individually account for <1% of the genetic risk, some genes, including the phosphatase, PTEN, are more often mutated in ASD (O’Roak et al., 2012). PTEN inhibits PI3K/Akt-signaling after its activation by receptor tyrosine kinases (RTKs) and other receptors (Bourgeron, 2009; Stiles, 2009). The growth-promoting Akt/mTor signaling cascade is also a hub for other ASD candidate genes, including Tsc1&2 (Wiznitzer 2004), and NF1 (Marui et al., 2004; Mbarek et al., 1999).
Pten is broadly expressed in the developing and adult mouse brain, including in glutamatergic and GABAergic neurons (herein and Ljungberg et al., 2009). Pten regulates many developmental processes, including migration (Kölsch et al., 2008), growth and morphogenesis (Kwon et al., 2006), and synaptic dynamics (Fraser et al., 2008; Luikart et al., 2011; Williams et al., 2015). Conditional deletion of Pten in excitatory cortical neurons results in macrocephaly, overgrowth of cortical cell bodies (somas), axons and dendrites; and leads to social interaction deficits; some of these features were rescued by pharmacologic inhibition of the mTor pathway (Kwon et al., 2006; Zhou et al., 2009). However, the role of Pten and other genes in the Akt/mTor pathway are poorly understood in GABAergic cortical interneuron development.
Here, we investigated Pten function during cortical interneuron development. Loss of Pten led to altered distribution of MGE-derived cells, neonatal interneuron death and an overall loss in interneurons. However, preferential loss of SST+ interneurons led to an increased ratio of PV/SST in surviving interneurons in the adult mutant cortex, with ectopic PV+ processes in layer I. Many of these phenotypes were cell autonomous. We also developed a method to virally modify MGE cells before transplantation to study the function of human ASD alleles, and found that PTEN ASD alleles were hypomorphic for multiple phenotypes.
Results
Pten loss in the MGE leads to increased AKT signaling
To determine its role in GABAergic cortical interneuron development, we generated Pten conditional knockouts (cKOs) from the medial ganglionic eminence (MGE) and preoptic area (POA) progenitors by crossing PtenFlox (Suzuki et al., 2001) to Nkx2.1-Cre (Xu et al., 2008) mice. The Ai14 Cre-dependent reporter (Madisen et al., 2010) was used to follow cells that expressed Cre (tdTomato is expressed after Cre-mediated recombination). The Nkx2.1-Cre BAC transgenic drives expression, beginning ~embryonic day (E) 9.5, in most of the ventricular zone (VZ) of the MGE and POA (Figures S1A–S1C). At E12.5, Pten was globally expressed in the brains of WT and PtenFlox/+ mice, including in the MGE (Figures S1D, S1E, S1G and S1H). Efficient loss of Pten protein occurred in early progenitors of the VZ and their progeny in the Nkx2.1-Cre lineage domains (Figures S1C, S1F and S1I).
Since loss of Pten leads to increased phosphorylated Akt (pAkt) and pGSK3beta in neurons (Kwon et al., 2006), we probed E13.5 MGE tissues for pAKT and pGSK3beta. Indeed, pAkt was increased ~3.5 fold at serine473 (p = 0.02) and ~3 fold at threonine308 (p = 0.01) (Figure S1J). Moreover, pGSK3beta at serine9 was increased ~1.6-fold in Pten cKOs (p = 0.03) (Figure S1J). These data demonstrate a role for Pten signaling in regulating the Akt pathway in the embryonic MGE.
Pten loss in GABAergic cortical interneuron progenitors differentially effects SST and PV interneurons
Nkx2.1-Cre+; Pten cKOs survive into adulthood but weighed ~25% less than their WT and PtenFlox/+ littermates at P30 (Figure 1A). In contrast to other Pten mutants (Kwon et al., 2006), the brains of Nkx2.1-Cre+; Pten cKOs were not altered in shape or size (Figure 1A). First, we counted the number of Nkx2.1-Cre-lineage cortical interneurons in the somatosensory cortex. At P30, there was ~53% reduction in the total number of Nkx2.1-Cre-lineage cells (Figures 1B–1G, 1H and Table S1). Next, we assessed the proportion of the two main subgroups of MGE-derived interneurons via expression of somatostatin (SST) and parvalbumin (PV) in the remaining tdTomato+ cells. The % tdTomato+ cells that were SST+ decreased 34% in Pten cKO neocortices (Figures 1B–1G and 1I, p = 0.01). Yet, the % tdTomato+ cells that were PV+ increased 39% (Figures 1E–1G, 1J, p = 0.009). This disproportionate effect on SST+ and PV+ interneuron numbers resulted in a greater PV to SST ratio in the P30 cortex (Figure 1K, p = 0.01), as well as in striatum and hippocampus (Table S1). Moreover, in Pten cKOs, the SST+ neuropil was greatly reduced in layer I, whereas the PV+ neuropil, which normally was restricted to neocortical layers II–VI, ectopically projected into layer I and displayed prominent PV+ puncta (Figures 1E′–1G′).
Figure 1. Nkx2.1-Cre; Pten conditional mutants exhibit reduced interneuron numbers, increased PV/SST ratio and ectopic PV+ processes in layer I.
(A) Images of postnatal day (P) 30 mice and brains (superior and lateral views) from Pten+/+, PtenFlox/+ and PtenFlox/Flox (Nkx2.1-Cre+; Ai14Flox/+) genotypes. Coronal immunofluorescent images of P30 somatosensory cortices show co-expression of tdTomato with SST (B–D) or with PV (E–G). (E′-G′) Higher magnification images of neocortical layer I from images E–G that have been merged with DAPI. Brackets to the right of each panel denote the boundaries of layer I. (H) Quantification of the number of tdTomato+ cells per square millimeter (mm2) in the somatosensory cortex over time. Quantification of the % tdTomato+ cells that express either SST (I) or PV (J). (K) Ratio of tdTomato+/PV+ cells per mm2 over tdTomato+/SST+ cells per mm2 in the neocortex. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01. Scale bars in (G and G′) = 100 μm. See also Figures S1, and S2, and Tables S1 and S2.
Nkx2-1-Cre removed Pten from the stem cells in the VZ. Next, we used DlxI12b-Cre (Potter et al., 2009) to remove Pten from secondary progenitors in the subventricular zone (SVZ) of the entire subpallium. At E15.5, Pten protein was detected in the VZ, but not the SVZ or neuronal layers of the basal ganglia (Figure S2A–S1A″ and S2B–S2B″). At P18, the % tdTomato+ cells that expressed VIP, a marker of CGE-derived interneurons, was unchanged (Figures S2C–S2E and S2L).
Similar to Nkx2.1-Cre cKOs, the % tdTomato+ cells in the DlxI12b-Cre cKOs that expressed SST decreased by 32% (Figures S2F–S2H and S2M, p = 0.004). Moreover, there was a trend for an increased % tdTomato+ cells that expressed PV (increased 24%, but did not reach statistical significance) (Figures S2I–S2K and S2N). Like the Nkx2.1-Cre cKOs, there was a trend for fewer total tdTomato+ cells in the neocortex (Figure S2O). Overall, DlxI12b-Cre cKOs had an increased PV+/SST+ ratio of neocortical interneurons (Figure S2P, p = 0.01). These mutants also had ectopic PV+ projections in neocortical layer I at P18 (Figures S2I′–S2K′). Unfortunately, these mice could not be analyzed at later stages because they die soon after P18 for unknown reasons.
Pten loss in progenitors but not postmitotic interneurons decreased SST+ numbers
Pten loss, beginning in either the VZ (Nkx2.1-Cre) and/or SVZ (DlxI12b-Cre), led to a preferential loss of SST+ interneurons, while PV+ interneurons composed most of the remainder. To test if Pten was required in postmitotic SST+ cells, we used a SST-IRES-Cre mouse line (Taniguchi et al., 2011), which expresses Cre in MGE-derived SST+ cells as they leave the MGE (Figure 2A). Somatosensory cortices were assessed at P30 for SST-IRES-Cre-lineages (tdTomato+). In contrast to Nkx2.1-Cre and DlxI12b-Cre Pten cKOs, SST-IRES-Cre cKOs did not show interneuron loss (Figure 2H), had no change in % tdTomato+ interneuronss that were SST+ or PV+ (Figures 2B–2I) and had no ectopic PV+ neuropil in layer I (Figures 2E–2G). These data demonstrate that the Pten phenotypes observed using Nkx2.1-Cre and DlxI12b-Cre are caused by Pten loss in progenitor cells, which in turn led to changes in the PV/SST ratio.
Figure 2. Normal interneuron numbers and morphology in SST-IRES-Cre; Pten cKOs.
(A) Coronal immunofluorescent image of SST-IRES-Cre+; Ai14Flox/+ showing the pattern of SST-lineage cells (tdtomato+) at embryonic day (E) 15.5. Coronal immunofluorescent images of P30 somatosensory cortices from SST-IRES-Cre+; Ai14Flox/+;Pten+/+, PtenFlox/+ and PtenFlox/Flox show expression of tdTomato with SST (B–D) or with PV (E–G). Brackets to the right of each panel denote the boundaries of layer I. (H) Quantification of the total number of tdTomato+ cells per mm2. Quantification of the % tdTomato+ cells that express SST (I) or PV (J). Data are represented as mean ± SEM. Scale bars in (A and G) = 100 μm. See also Figure S2.
Normal proliferation in Pten cKOs
The reduced numbers of interneurons in the neocortex of Nkx2.1-Cre+; PtenFlox/Flox cKOs could be due to altered proliferation or apoptosis. We evaluated proliferation in the MGE by assessing phospho-histone-3 (PH3) expression and EdU incorporation, measures of M and S phase, respectively. The total number of PH3+ cells within the VZ and SVZ of the Nkx2.1-Cre domain of the MGE, (tdTomato+), was counted at E11.5, E12.5 and E15.5; no changes in PH3+ cells were found (Figures S3A–S3I). Next, we administered a 30 minute pulse of EdU at E11.5, E12.5 and E15.5 and counted the density of EdU+ cells in the MGE’s VZ and SVZ MGE. Lack of Pten had no effect (Figures S3J–S3R), suggesting normal proliferation.
Differential distribution and death of SST+ cells in Pten cKOs
To determine if Pten cKOs showed early changes in cell fate, we assessed the proportion of tdTomato+ cells that expressed SST at E12.5 and E15.5. At E12.5, there were no changes in the number or proportion of SST+ cells assayed using in situ hybridization or antibody staining (data not shown). However, by E15.5, the % of tdTomato+ cells that expressed SST was increased ~45% in the lateral cortex (Figures 3A–3I, p = 0.02), and decreased ~30% in the neocortex (Figures 3A, 3B, 3J–3P, p = 0.006 total, p = 0.002 MZ, p = 0.02 CP), suggesting that SST+ cKO interneurons were biased to occupy the lateral cortex.
Figure 3. Abnormal distribution of SST+ cells and elevated apoptosis in Nkx2.1-Cre; Pten cKOs.
E15.5 coronal immunofluorescent images of Nkx2.1-Cre-lineage cells (tdTomato+) colabeled for SST and DAPI in Pten+/+ (A) and PtenFlox/Flox (E) brains. (B–D and F–H) Higher magnification fluorescent images of the lateral cortex (white boxes in A, E). (I) Quantification of the % tdTomato+ cells that express SST in the lateral cortex. (J–O) Immunofluorescent images of the neocortex (orange boxes in A, E) showing tdTomato+ cells that express SST. (P) Quantification of the % tdTomato+ cells that express SST in the neocortex. (Q–V) Immunofluorescent images of the lateral cortex at P0 showing tdTomato+ cells that express SST and cleaved-caspase-3 (cC3). (W) Quantification of the % tdTomato+ cells that express SST in the lateral cortex at P0. (X) Quantification of the % tdTomato+ cells in the MZ that are cC3+, left bars; tdTomato+/SST+, middle bars; or tdTomato+/SST−, right bars. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars in (E) = 250 μm and (H, O, V) = 100 μm. Abbreviations: MZ (marginal zone), CP (cortical plate), IZ/SVZ (intermediate and subventricular zones).
Next, we assessed if Pten cKO cortical interneurons were susceptible to apoptosis, by testing for expression of cleaved caspase-3 (cC3). While we did not detect changes in apoptosis at E15.5, by E17.5 cC3+ cells were readily detected in ~13% of tdTomato+ marginal zone (MZ) Pten cKO cells, in both the neocortex and lateral cortex (controls had <1%; data not shown). By P0, ~36% of Pten cKO tdTomato+ cells in the MZ of the lateral cortex were cC3+ (Figures 3Q–3V and 3X, p < 0.001).
In the postnatal neocortex, there was progressive loss of MGE-derived (tdTomato+) interneurons in the Pten cKOs: 10% at P0, 47% at P8 and 53% at P30 (Figure 1H). Thus, interneuron apoptosis primarily occurred between E17.5-P8. Interestingly, apoptosis was restricted to the cortical MZ, suggesting that interneuron death may occur due to an inability to integrate into the cortex.
Finally, we asked if SST+ or SST− interneurons were differentially affected by apoptosis, by calculating the % of cC3+/tdTomato+ cells in the MZ that were either SST+ or SST−. Although SST− cells were cC3+, cC3+/tdTomato+ cells in the MZ of Pten cKOs were ~2-fold more likely to be SST+ (Figure 3X, p < 0.001 all conditions). This provided evidence that SST+ interneurons lacking Pten are more susceptible to apoptosis than SST− interneurons. Moreover, at P0, the % tdTomato+ cells that expressed SST in the lateral cortex was reduced 26% (Figure 3W, p = 0.02), consistent with the evidence noted above that this was the period of interneuron death.
Since SST+ and PV+ neurons are the two primary MGE-derived cell types, the tdTomato+/SST− cells are likely to become PV+ cells. Although there is no early marker for PV+ interneurons, there is evidence that the majority of immature MGE cells that express SST will become SST+. (Taniguchi et al., 2011; Vogt et al., 2015). Thus, prenatal expression of SST in the MGE-lineage is correlated with a SST+ fate, and we propose that SST+ embryonic Pten cKO cells are more susceptible to apoptosis than those that are SST− (likely PV+ fate).
Nkx2.1-Cre; Pten cKOs exhibit increased inhibition onto neocortical pyramidal neurons, deficits in social interaction and changes in EEG gamma oscillations
We next examined if Pten loss in interneurons altered the amount of inhibition received by neocortical excitatory neurons. Electrophysiological recordings were made of inhibitory inputs onto excitatory neurons in neocortical layers II/III at P30 (schema, Figure 4A). Despite the loss of half of the MGE-derived interneurons (Figure 1), there was ~2-fold increase in the frequency of spontaneous inhibitory post synaptic currents (IPSCs) onto layer II/III excitatory neurons in Nkx2.1-Cre; PtenFlox/Flox mice (Figure 4C, p = 0.02) but no change in amplitude between groups (Figure 4D). Example IPSCs recordings are shown for Pten+/+ and PtenFlox/Flox (Figure 4B). These data demonstrate that spontaneous inhibitory tone is increased in layers II/III of Pten mutants.
Figure 4. Neocortical principle neurons in Nkx2.1-Cre; Pten cKOs received increased IPSC frequency.
(A) Schema of neocortical recordings performed on principle neurons in somatosensory cortical layers II/III (black) from Nkx2.1-Cre+; Ai14Flox/+ ; Pten+/+, PtenFlox/+ or PtenFlox/Flox slices at P30. Nkx2.1-Cre+ interneurons were tdTomato+ (red). (B) Example traces of inhibitory postsynaptic currents (IPSCs) from Pten+/+ and PtenFlox/Flox slices. Quantification of IPSC frequency onto principle neurons (C) and IPSC amplitude (D). Data are represented as mean ± SEM. *p < 0.05. Abbreviations: (ms) milliseconds, (Hz) hertz, (pA) picoamps.
Next, we performed a series of behavioral assays on Nkx2.1-Cre; Pten cKOs at P30. Pten cKOs were similar to WTs in the open field (Figures 5A–C), and the elevated plus maze (Figures 5D, 5E), suggesting normal levels of exploratory activity, locomotion and anxiety. However, Pten cKOs spent less time with novel mice in a social interaction test (Figure 5F, p = 0.03), and with a novel object (Figure 5I, p = 0.05). Interestingly, Pten cKOs had elevated EEG power during social interaction, with the greatest effect in the gamma frequency range (Figure 5G, p = 0.005). The baseline EEG was also recorded before the social interaction test and exhibited less power in the gamma frequency range in Pten cKOs (Figure 5H, p = 0.04). Overall, these data demonstrated that loss of Pten in cortical interneurons results in abnormal social behavior and altered EEG oscillations, within the gamma frequency band, both at baseline and during a task.
Figure 5. Nkx2.1-Cre; Pten cKOs showed reduced social behavior and altered EEG gamma oscillations.
(A–C) Open field test: quantification of P30 Nkx2.1-Cre+; Pten+/+ (WT) and Ptenflox/flox littermates for time spent in the center of the field (A), distance traveled in the center (B) and in the perimeter (C). (D, E) Elevated plus maze: quantification of the time spent in open arms (D) and the number of entries made (E). (F) Social interaction task: quantification of the amount of social interaction time Nkx2.1-Cre+; Pten+/+ (WT) and Ptenflox/flox littermates spent with a stranger mouse. (G) Social interaction task: quantification of changes in electroencephalogram (EEG) power, log transformed, during periods of social interaction. Graph represents recordings made from prefrontal cortex; significant changes were in the high-γ range (62–90 Hz). (H) Prefrontal EEG, shown as the log transform of the averaged, normalized power spectrum. The graph was analyzed from a period before the social interaction test and the power spectrum from each mouse was normalized by the sum of all values from 0–120 Hz (excluding 58–62 Hz). Nkx2.1-Cre, Ptenflox/flox mice exhibit a decrease in the power of prefrontal baseline high-γ (62–90 Hz) oscillations compared to their WT littermates. (I) Novel object task: just after the social interaction assay, the time each mouse spent with a novel object was measured. (n = 5 mice per genotype, both groups). Data are represented as mean ± SEM. *p < 0.05, **p < 0.01.
Cell autonomous role for Pten in regulating cortical interneuron soma size and interneuron subgroup ratios
To assess the cell autonomous role for Pten on cortical interneuron development, we utilized an MGE cell transplantation assay (Alvarez-Dolado et al., 2006). E12.5 DlxI12b-Cre+; Ai14Flox/+ MGE cells from Pten+/+, PtenFlox/+ or PtenFlox/Flox embryos were transplanted into WT P1 host neocortices and assessed at 35 days post transplant (DPT) (Figure 6A). Transplanted PtenFlox/Flox MGE cells had increased soma area (Figures 6B–6G) compared to both Pten+/+ and PtenFlox/+ (Figure 6H, p < 0.0001, both conditions).
Figure 6. Cortical interneurons derived from transplanted Pten cKO MGE have increased soma size and increased PV+/SST+ ratio.
(A) Schema depicting the transplantation procedure. E12.5 DlxI12b-Cre+; Ai14Flox/+ MGE cells (tdTomato+) that were either Pten+/+, PtenFlox/+ or PtenFlox/Flox were transplanted into WT P1 neocortices and allowed to develop for 35 days post transplant (DPT). Coronal immunoflourescent images of neocortices transplanted with Pten+/+, PtenFlox/+ or PtenFlox/Flox MGE cells show coexpression of tdTomato and SST (B–D) or PV (E–G). Arrows point to coexpressing cells. Quantification of soma size of the tdTomato+ cells (H), and the proportion of tdTomato+ cells that express either SST (I) or PV (J). Data are represented as mean ± SD (H), ± SEM (I, J). (*p < 0.05, **p<0.01, ***p<0.001). Scale bar in (G) = 100 μm. See also Figure S4 and Table S3.
Similar to the cKOs, transplanted Pten cKO MGE cells had a decreased proportion of SST+ cells (Figures 6B–6D and 6I, PtenFlox/+ p = 0.002, PtenFlox/Flox p = 0.0001), and increased PV+ cells (Figures 6E–6G and 6J, p = 0.02). The transplanted MGE cells were also assayed for proteins that are expressed in specific MGE-derived interneuron groups: KV3.1, a channel predominantly expressed in PV+ fast-spiking neurons (Du et al., 1996), as well as Reelin and Npas1 (which are expressed in a subpopulation of SST+ but not PV+, interneurons) (Pesold et al., 1999; Stanco et al., 2014). The % transplanted PtenFlox/Flox MGE cells that expressed KV3.1 was increased (Figures S4A, S4D and S4G, p = 0.001). In conjunction, the % of cells expressing Reelin (Figures S4B, S4E and S4H, p = 0.01) and Npas1 (Figures S4C, S4F and S4I, p = 0.006) were decreased in PtenFlox/Flox MGE transplants, consistent with a reduction of SST+ interneurons.
We next assessed the cell intrinsic physiological properties of the transplanted cells at 45 DPT (Table S3). Despite decreased AP threshold and resting membrane potential in transplanted Pten cKO MGE cells, the majority of cell intrinsic firing properties (i.e. FI slope, adaption ratio and action potential half-width) between WT and Pten cKOs were similar. Together, with the ectopic PV+ processes in neocortical layer I and increased IPSC frequency onto excitatory neurons, these data suggest that Pten cKO cells primarily increased inhibitory tone via an overgrowth mechanism rather than by increased cell intrinsic excitability.
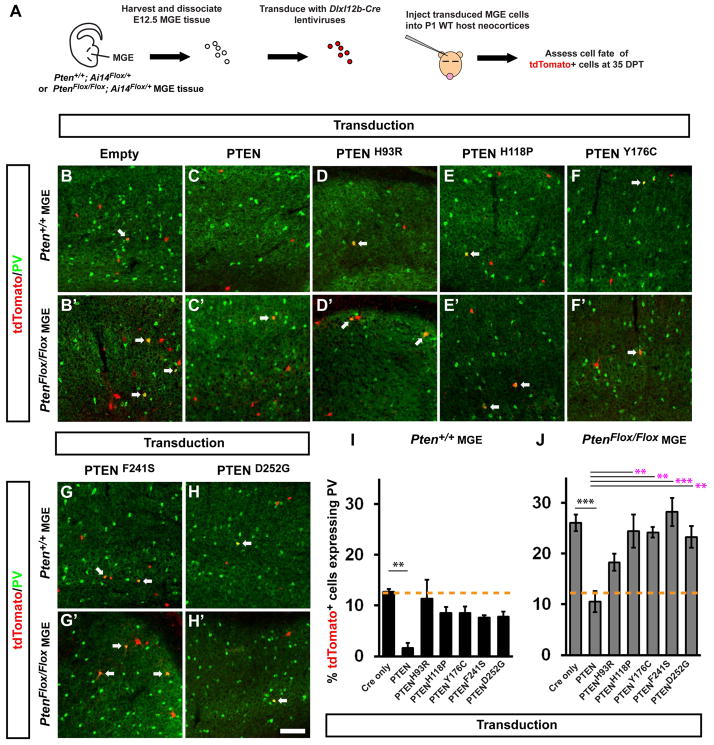
An in vivo complementation assay of PTEN ASD alleles demonstrates functional deficits in cortical interneuron development
Since the increased PV/SST ratio was a prominent phenotype in multiple brain regions (Table S1) and was cell autonomous (Figure 6), it was an ideal phenotype to test if human PTEN ASD alleles were functionally deficient. A major roadblock in ASD research is the lack of efficient screening assays for human alleles found in autism populations, particularly those with the power to test allele function in vivo during brain development. To circumvent this problem, we improved upon methods to introduce genes of interest into MGE cells before transplantation for in vivo development (Vogt et al., 2014) that combine Cre-dependent gene-deletion and activation of a fluorescent reporter in the transduced MGE cells (Vogt et al., 2015) with expression of an allele. In this manner, a WT or ASD allele is expressed while simultaneously deleting the corresponding endogenous gene. First, donor MGE cells that harbor a floxed gene of interest are transduced with a lentivirus, then transplanted into a WT host, allowing the modified cells to develop in vivo. This strategy challenges an ASD allele to complement the phenotypes of the gene deletion. Moreover, by using the DlxI12b enhancer that drives expression in the majority of GABAergic cortical neurons (Potter et al., 2009), we could study the effects of alleles only in GABAergic lineages. To this end, we generated lentiviruses with the DlxI12b enhancer (Arguello et al., 2013; Vogt et al., 2014), followed by a beta-globin minimal promoter driving Cre alone, with WT human PTEN, or with PTEN ASD alleles (Figure S5A). To test the fidelity of this assay, five previously reported PTEN alleles, identified in patients with PTEN-related syndromes and diagnosed with ASD (Butler et al., 2005; Orrico et al., 2009) were chosen for comparison to WT PTEN. Each vector was expressed in HEK293T cells and assayed for Akt activity, via phosphorylation at Ser473 (Figure S5B). Expression of WT PTEN efficiently decreased the level of pAkt. However, none of the PTEN ASD mutants were able to efficiently inactivate Akt, suggesting that they are less functional than WT PTEN.
To assess the function of the PTEN ASD alleles in cortical interneuron development, we transduced MGE cells with the aforementioned viruses before transplantation (schema, Figure 7A). E12.5 MGE cells that were either Ai14Flox/+ (controls) or PtenFlox/Flox; Ai14Flox/+, were transduced with DlxI12b-Cre lentiviruses (Figure S5A). These cells were then transplanted into P1 WT neocortices and developed in vivo until 35 days post transplant (DPT). Cre will result in the expression of tdTomato from the Ai14 locus and will delete endogenous Pten from PtenFlox/Flox MGE cells. Since a PTEN allele is expressed from the same vector, the effect of either overexpression (via transduction of control MGE cells) or complementation (via transduction of PtenFlox/Flox MGE cells) can be assessed.
Figure 7. Complementation assay comparing WT and PTEN ASD alleles reveal reduced function of ASD alleles in regulating PV+ interneuron ratio.
(A) Schema depicting the MGE transplantation and complementation assay. E12.5 control (Pten+/+; Ai14Flox/+) or (PtenFlox/Flox; Ai14Flox/+) MGE cells were transduced with a DlxI12b-Cre-T2a-MCS lentivirus that expresses either Cre only, or Cre with human WT PTEN, or a PTEN ASD allele, from the multiple cloning site (MCS). MGE cells were transplanted into a P1 wild type (WT) neoocortex and assessed at 35 DPT. Coronal immunoflourescent images of neocortices transplanted with transduced control (B–H) or PtenFlox/Flox (B′–H′) MGE cells show expression of tdTomato and PV at 35 DPT. Arrows point to co-expressing cells. Quantification of the % of tdTomato+ control (I) or PtenFlox/Flox (J) transduced-MGE cells that co-express PV. Data are represented as mean ± SEM. (n) = 4, all groups (**p<0.01, ***p<0.001, black asterisks = compared to Cre only lentivirus, magenta asterisks = compared to WT PTEN lentivirus). Scale bar in (H′) = 100 μm. See also Figures S5 and S6.
We first assessed if overexpressing WT or PTEN ASD alleles in control MGE cells changed the numbers of PV+ neurons (Schema, Figure 7A). Interestingly, expression of WT PTEN, but not the PTEN ASD alleles, decreased the proportion of PV+ cells (Figure 7B–7I, p = 0.002), suggesting that PTEN dosage influences this phenotype. Next, we assessed if PTEN ASD alleles could complement the increased % of transplanted cells that are PV+ in Pten cKO MGE cells. Transduction of PtenFlox/Flox MGE cells led to an increased % of PV+ cells compared to control cells with Cre only (Figures 7B, 7B′, 7I, 7J and Table S2), consistent with earlier data (Figures 1 and S2). Complementation with WT PTEN efficiently restored the % of PV+ cells to control levels (Figures 7C′ and 7J, p = 0.0006). Notably, the PTEN ASD mutants were unable to complement the increased % of PV+ cells and many were indistinguishable from Cre alone. Moreover, they differed from WT PTEN (Figures 7C′–7H′ and 7J, p = 0.002 PtenH118P, p = 0.003 PtenY176C, p = 0.0001 PtenF241S, p = 0.005 PtenD252G). While WT and PTEN ASD mutants showed clear distinctions, there was no evidence of a dominant-negative effect induced by PTEN ASD mutants when expressed in control MGE cells. These data showed a role for PTEN dosage in regulating PV+ interneuron ratios and that PTEN ASD alleles are deficient in complementing this phenotype.
The % of transplanted cells that were SST+ were assessed in the same manner (Figures S6A–S6I). SST+ cells were reduced ~15% in PtenFlox/Flox MGE cells compared to control cells transduced with Cre only (S6H–I and Table S2), and the % of PtenFlox/Flox cells that were SST+ increased when complemented with WT PTEN (Figure S6I, p = 0.02). However, some of the PTEN ASD alleles did not complement the decreased % of SST+ cells (Figure S6I, p = 0.02 PTENY176C, p = 0.009 PTEND252G).
Loss of Pten function increases cell size, including the cell bodies (somas) of neurons (Kwon et al., 2006; and Figure 6H). Thus, we compared the function of WT and PTEN ASD alleles with regards to soma area. As expected, deletion of Pten by transduction with Cre led to increased soma areas in PtenFlox/Flox MGE cells (Figure S6K). Next, we quantified soma area after transduction with PTEN ASD alleles; each were less efficient than WT PTEN at preventing the increase in soma area of PtenFlox/Flox MGE cells (Figure S6K, p < 0.0001 for WT PTEN, PTENH118P and PTENY176C, p = 0.04 for PTENF241S and p = 0.0005 for PTEND252G). Finally, we transduced PTEN or the ASD alleles into WT MGE cells, and found no change in soma area with any allele (Figure S6J). Consistent with earlier data (Figure 7I and S6H), these results suggest that the PTEN ASD alleles do not act in a dominant fashion. Together, these data revealed a role for human PTEN ASD alleles in regulating cortical interneuron ratios, cell size, and demonstrated a functional in vivo assay to test the impact each mutant imparts to development.
Discussion
Pten is required to obtain normal numbers of SST+ and PV+ cortical interneurons, the two main subgroups of MGE-derived interneurons. Loss of Pten led to apoptosis that preferentially affected neonatal SST+ interneurons. This resulted in an increased PV+/SST+ ratio, as well as ectopic PV+ projections and a paucity of SST+ axons in cortical layer I. Pten mutants also had a decreased E/I ratio in neocortical layers II/III, and reduced social behaviors. Finally, an MGE transplantation/complementation assay showed that PTEN ASD alleles were hypomorphic in vivo.
Pten regulates the number and ratio of SST and PV interneurons
Pten deletion in MGE progenitors of the VZ (via Nkx2.1-Cre) resulted in a greater reduction in SST+ compared to PV+ interneurons in the neocortex (Table S1). While this phenotype was also seen when Pten was deleted in the SVZ (via DlxI12b-Cre, Figure S2), no interneuron loss was observed using SST-IRES-Cre (Figure 2), which begins to express as these cells become postmitotic, suggesting that Pten regulation of interneuron numbers depends on its function in progenitors. The increased PV/SST ratio was also observed in MGE transplantation assays (Figures 6, 7, S6) consistent with a cell autonomous role for Pten.
The altered PV/SST ratio is likely due to preferential cell death of SST+ cells during late gestation and neonatal ages, rather than a change in cell fate or proliferation. In support of this, we did not observe changes in the proportion of SST+ MGE-derived cells at early embryonic ages (E12.5) or in proliferation (Figure S3). At E15.5, more SST+ interneurons occupied the lateral cortex and were depleted from the neocortex (Figure 3). By P0, there was elevated apoptosis that preferentially affected SST+ neurons in both the lateral and neocortex marginal zones, a time just before the major loss in interneurons were decreased in the neocortex (Figure 1H).
Alterations (increases and decreases) in cortical interneurons have been reported in ASD mouse mutants (Durand et al., 2012; Karayannis et al., 2014; Peñagarikano et al., 2011; Selby et al., 2007; Gogolla et al., 2014, Allegra et al., 2014). Thus, defects in interneuron numbers may be found in some forms of ASD, and the PV/SST ratio should be measured in ASD patients. Moreover, further work should be done in mice to elucidate the mechanisms that control the numbers of PV and SST interneurons. Some insights have been garnered from the analysis of mouse transcription factor mutants that exhibit altered PV/SST ratios, including Dlx1 (Cobos et al., 2005), Lhx6 (Neves et al., 2013), Npas1&3 (Stanco et al., 2014), Olig1 (Silbereis et al., 2014) and Satb1 (Close et al., 2012; Denaxa et al., 2012). While the role of these TFs in neuropsychiatric disorders are obscure, alleles of some of these genes have been associated with disorders (Hamilton et al., 2005, Stanco et al., 2014).
Pten regulates the morphology and connectivity of SST and PV interneurons
Interestingly, PV+ processes ectopically grew into neocortical layer I, suggesting that Pten restrains the spatial distribution and perhaps connectivity of PV+ axons. Loss of Pten in excitatory neurons likewise results in overgrowth of their processes (Kwon et al., 2006). Given that layer I contains the apical dendrites of pyramidal neurons, the excessive PV+ processes could impact the nature and number of inhibitory inputs onto excitatory neurons. Indeed, loss of Pten in MGE-derived GABAergic cortical interneurons resulted in elevated spontaneous inhibitory tone on cortical layer II/III pyramidal neurons (Figure 4), despite the ~50% reduction in MGE-derived interneurons (Figure 1, Table S1). We hypothesize that the overgrowth of PV+ processes in layer I may underlie the increased inhibition. Interestingly, increased inhibitory tone has been observed in other mouse mutants of ASD genes that repress the PI3K/Akt/mTor pathway, including NF1 (Costa et al., 2002; Cui et al., 2008), suggesting that reducing the E/I ratio, through increased inhibition, may be an important mechanism underlying the effects of some ASD genes. Other ASD mouse models have increased E/I (Gogolla et al., 2014); thus various mechanisms that disrupt E/I away from the optimal range, in either direction, may account for cortical dysfunction.
PV+ interneurons are necessary and sufficient for gamma oscillations (Cardin et al., 2009; Sohal et al., 2009), and various (depressed and elevated) alterations have been observed in individuals with ASD (Gandal et al., 2010; Orekhova et al., 2007; Wilson et al., 2007). In Pten cKO mice, we observed reduced gamma rhythms at rest and increased when performing a task (Figure 5). Finally, Pten mutants had deficits in social interaction (Figure 5), supporting the hypothesis that some behavioral and cognitive phenotypes may be caused by E/I imbalance (Rubenstein and Merzenich, 2003).
Loss of Pten in excitatory neurons led to phenotypes that were distinct from those observed from deletion in GABAergic cortical interneurons, including macrocephaly, increased excitation and seizures (Kwon et al., 2006). Moreover, Pten deletion in either neuron type resulted in hyperactivated Akt and hypertrophy, suggesting that while different global phenotypes may be attributed to certain cell types, some cellular phenotypes were common between inhibitory and excitatory neurons (hyperactivated Akt, soma and neurite hypertrophy).
An in vivo complementation assay to screen functional changes in ASD alleles
Due to the many risk alleles found in ASD (Murdoch and State, 2013; Tebbenkamp et al., 2014), we developed an efficient means to screen ASD alleles for functional deficits in vivo. This was performed in cortical interneurons, a cell type implicated in ASD and other neuropsychiatric disorders. A standard way to study an ASD allele is to create a knock-in mouse, which is low-throughput process. Our complementation assay tests cell autonomous effects of ASD alleles in ~two months, and can rapidly investigate molecular, developmental, morphological and electrophysiological parameters in vivo. While this assay requires mouse null mutants, it is now relatively easy to obtain these animals. In addition, the modular design of the lentiviral vector (Figure S5) permits the use of any enhancer or promoter, which could facilitate assaying functions specific to cell-type. Moreover, any gene can be inserted downstream of the Cre cassette. We suggest that this assay will be of great utility for in vivo functional characterization of any mutant allele.
Our assay showed that all five of the tested human PTEN ASD alleles were hypomorphic, based upon their reduced activity to rescue two phenotypes: increased soma area and PV/SST ratio (Figures 7 and S6). Consistent with our results, an assay of these alleles in yeast provided evidence that they had reduced phosphatase function but not as low as PTEN alleles discovered in various cancers (Rodríguez-Escudero et al., 2011). Furthermore, these alleles did not have dominant functions when assayed in WT interneurons (Figures 7 and S6). In sum, our experiments delineate a major role for PTEN in the survival, connectivity and PV/SST ratio of GABAergic cortical interneurons. These findings provide insights into how deficits in PTEN contribute to ASD, and suggests that PI3K/Akt/mTor signaling differentially regulates PV and SST interneuron development.
Experimental procedures
Animals
All mice have been described: Ai14 Cre-reporter (Madisen et al., 2010), Nkx2.1-Cre (Xu et al., 2008), SST-IRES-Cre (Taniguchi et al., 2011), DlxI12b-Cre (Potter et al., 2009), Ptenflox (Suzuki et al., 2001). Mice were initially on a mixed C57BL6/J, CD-1 background. All lines, except SST-IRES-Cre, were backcrossed to CD-1 for at least four generations before analysis. SST-IRES-Cre males were maintained on a C57Bl6/J background. For timed pregnancies, noon on the day of the vaginal plug was counted as embryonic day 0.5. All animal care and procedures were performed according to the University of California at San Francisco Laboratory Animal Research Center guidelines.
Behavior
Open-field test
An individual mouse was placed near the wall-side of 50 × 50 cm open-field arena, and the movement of the mouse was recorded by a video camera for 10 min. The recorded video file was later analyzed with Any-Maze software (San Diego Instruments). Time in the center of the field (a 25 × 25 cm square), and distances travelled in the center and perimeter were measured. The open field arena was cleaned with 70% ethanol and wiped with paper towels between each trial. Investigators were blind to genotype during scoring of videos.
Elevated plus maze test
A mouse was placed at the junction of the open and closed arms, facing the arm opposite to the experimenter, of an apparatus with two open arms without walls (30 × 5 × 0.5 cm) across from each other and perpendicular to two closed arms with walls (30 × 5 × 15 cm) with a center platform (5 × 5 cm), and at a height of 40 cm above the floor. Mouse movement was recorded by video for 10 min and analyzed for the first 5 min. The video file was analyzed, and time and number of entries in the open arms of the apparatus was measured. The elevated plus maze apparatus arms were cleaned with 70% ethanol and wiped with paper towels between each trial. Investigators were blind to genotype during scoring of videos.
Social interaction task
Mice were connected to the EEG preamplifier, and then habituated in their homecage for 15 min before beginning the task. After habituation, we recorded baseline EEG activity for 5 min. Then we introduced an age-matched mouse (3–4 weeks old, same gender) into the homecage of the subject mouse and allowed the freely-moving mice to interact for 5 minutes while continuing to record EEG activity. We recorded EEG using a time-locked video EEG monitoring system (Pinnacle Technology), enabling us to subsequently correlate periods of social interaction (defined as sniffing, close following, and allo-grooming) with specific timepoints in the EEG recording. Investigators were blind to genotype during scoring of videos.
Novel object task
Following the social interaction task, the subject mouse was presented with a novel object (50 mL Falcon tube cap) for 5 minutes and the amount of time spent investigating the object was analyzed. Investigators were blind to genotype during scoring of videos.
Data analysis for EEGs were performed in Matlab (The MathWorks) using custom written software. To analyze changes in power during the baseline period (which occurred just before the social interaction task), a 2-way ANOVA was used and found to be significant for genotype if (p<0.001), frequency if (p<0.001), and genotype × frequency if (p<0.05). Differences between genotypes for each frequency band was tested using Student’s two-tailed, unpaired t-tests. To analyze differences in social interaction-evoked power, we used repeated measures ANOVA with mouse, task condition (baseline vs. social interaction), and genotype × condition (baseline vs. social interaction) as factors.
Electrophysiology
Slice preparation
Slice preparation and intracellular recordings were performed as previously described (Sohal and Huguenard, 2005). Detailed methods can be found in extended experimental procedures.
Lentiviral production
Lentiviral production was performed as previously described (Vogt et al., 2014, Vogt et al., 2015). Detailed methods can be found in extended experimental procedures.
MGE transplantation
MGE transplantations were done as previously described (Vogt et al., 2014, Vogt et al., 2015). A detailed description can be found in extended experimental procedures.
Supplementary Material
Highlights.
Pten regulates the ratio of PV+/SST+ cortical interneurons
Loss of Pten increases PV+ process growth and inhibition
Pten interneuron cKOs have altered social behavior and gamma oscillations
An in vivo screening assay tests human ASD allele function
Acknowledgments
This work was supported by grants to JLRR from: Autism Speaks, Nina Ireland, Weston Havens Foundation, NIMH R01 MH081880, and NIMH R37 MH049428. VSS was supported by the Staglin Family and International Mental Health Research Organization, NIH DP2 MH100011 and NIMH R01 MH100292. KKAC was supported by a NARSAD Young Investigator Award from the Brain and Behavior Foundation. ATL was supported by a Medical Scientist Training Program grant from NIGMS (GM07618).
Footnotes
Author Contributions
DLV, KKAC and ATL performed experiments. All authors conceived experiments and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel Vogt, Email: Daniel.Vogt@ucsf.edu.
John L. R. Rubenstein, Email: John.Rubenstein@ucsf.edu.
References
- Allegra M, Genovesi S, Maggia M, Cenni MC, Zunino G, Sgadò P, Caleo M, Bozzi Y. Altered GABAergic markers, increased binocularity and reduced plasticity in the visual cortex of Engrailed-2 knockout mice. Front Cell Neurosci. 2014;17:163. doi: 10.3389/fncel.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JLR, Alvarez-Buylla A, Baraban SC. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006;26:7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Arguello A, Yang XY, Vogt D, Stanco A, Rubenstein JLR, Cheyette BNR. Dapper Antagonist of Catenin-1 Cooperates with Dishevelled-1 during Postsynaptic Development in Mouse Forebrain GABAergic Interneurons. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0067679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou X-P, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close J, Xu H, De Marco Garcia N, Batista-Brito R, Rossignol E, Rudy B, Fishell G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci. 2012;32:17690–705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–68. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin Regulation of ERK Signaling Modulates GABA Release and Learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, Maes T, Pachnis V. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Rep. 2012;2:1351–62. doi: 10.1016/j.celrep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Patrizi A, Quast KB, Hachigian L, Pavlyuk R, Saxena A, Carninci P, Hensch TK, Fagiolini M. NMDA Receptor Regulation Prevents Regression of Visual Cortical Function in the Absence of Mecp2. Neuron. 2012;76:1078–1090. doi: 10.1016/j.neuron.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ. Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience. 2008;151:476–488. doi: 10.1016/j.neuroscience.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TPL, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiat. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. Sensory Integration in Mouse Insular Cortex Reflects GABA Circuit Maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SP, Woo JM, Carlson EJ, Ghanem N, Ekker M, Rubenstein JL. Analysis of four DLX homeobox genes in autistic probands. BMC Genet. 2005;6:52. doi: 10.1186/1471-2156-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, De la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–686. doi: 10.1038/nrn2188. [DOI] [PubMed] [Google Scholar]
- Karayannis T, Au E, Patel JC, Kruglikov I, Markx S, Delorme R, Héron D, Salomon D, Glessner J, Restituito S, et al. Cntnap4 differentially contributes to GABAergic and dopaminergic synaptic transmission. Nature. 2014;511:236–40. doi: 10.1038/nature13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten Regulates Neuronal Arborization and Social Interaction in Mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G. Dis. Model Mech. 2009;2:389–98. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL. Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci. 2011;31:4345–4354. doi: 10.1523/JNEUROSCI.0061-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui T, Hashimoto O, Nanba E, Kato C, Tochigi M, Umekage T, Ishijima M, Kohda K, Kato N, Sasaki T. Association between the neurofibromatosis-1 (NF1) locus and autism in the Japanese population. Am J Med Genet B. 2004;131:43–47. doi: 10.1002/ajmg.b.20119. [DOI] [PubMed] [Google Scholar]
- Mbarek O, Marouillat S, Martineau J, Barthélémy C, Müh JP, Andres C. Association study of the NF1 gene and autistic disorder. Am J Genet B. 1999;88:729–732. [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJB, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JD, State MW. Recent developments in the genetics of autism spectrum disorders. Curr Opin Genet Dev. 2013;23:310–315. doi: 10.1016/j.gde.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Neves G, Shah MM, Liodis P, Achimastou A, Denaxa M, Roalfe G, Sesay A, Walker MC, Pachnis V. The LIM homeodomain protein Lhx6 regulates maturation of interneurons and network excitability in the mammalian cortex. Cereb Cortex. 2013;23:1811–23. doi: 10.1093/cercor/bhs159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, Elam M. Excess of High Frequency Electroencephalogram Oscillations in Boys with Autism. Biol Psychiat. 2007;62:1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Orrico A, Galli L, Buoni S, Orsi A, Vonella G, Sorrentino V. Novel PTEN mutations in neurodevelopmental disorders and macrocephaly. Clin Genet. 2009;75:195–198. doi: 10.1111/j.1399-0004.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JLR. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 2009;40:167–186. doi: 10.1016/j.mcn.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Escudero I, Oliver MD, Andrés-Pons A, Molina M, Cid VJ, Pulido R. A comprehensive functional analysis of PTEN mutations: Implications in tumor- and autism-related syndromes. Hum Mol Genet. 2011;20:4132–4142. doi: 10.1093/hmg/ddr337. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby L, Zhang C, Sun QQ. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. 2007;412:227–232. doi: 10.1016/j.neulet.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis JC, Nobuta H, Tsai HH, Heine VM, McKinsey GL, Meijer DH, Howard MA, Petryniak MA, Potter GB, Alberta JA, et al. Neuron. 2014;81:574–87. doi: 10.1016/j.neuron.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Huguenard JR. Inhibitory coupling specifically generates emergent gamma oscillations in diverse cell types. PNAS. 2005;102:18638–43. doi: 10.1073/pnas.0509291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanco A, Pla R, Vogt D, Chen Y, Mandal S, Walker J, Hunt RF, Lindtner S, Erdman CA, Pieper AA, et al. NPAS1 Represses the generation of specific subtypes of cortical interneurons. Neuron. 2014;84:940–53. doi: 10.1016/j.neuron.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles BL. Phosphatase and tensin homologue deleted on chromosome 10: Extending its PTENtacles. Int J Biochem Cell B. 2009;41:757–761. doi: 10.1016/j.biocel.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsani D, Fu Y, Lu J, Lin Y, et al. A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbenkamp ATN, Willseya J, State MW, Sestan N. The developmental transcriptome of the human brain: implications for neurodevelopmental disorders. Curr Opin Neurol. 2014;27:149–156. doi: 10.1097/WCO.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Hunt RF, Mandal S, Sandberg M, Silberberg SN, Nagasawa T, Yang Z, Baraban SC, Rubenstein JLR. Lhx6 Directly Regulates Arx and CXCR7 to Determine Cortical Interneuron Fate and Laminar Position. Neuron. 2014;82:350–364. doi: 10.1016/j.neuron.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Wu P-R, Sorrells SF, Arnold C, Alvarez-Buylla A, Rubenstein JLR. Viral-mediated labeling and transplantation of medial ganglionic eminence (MGE) cells for in vivo studies. J Vis Exp. 2015:e52740. doi: 10.3791/52740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, DeSpenza T, Jr, Li M, Gulledge AT, Luikart BW. Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive. J Neurosci. 2015;35:943–59. doi: 10.1523/JNEUROSCI.3144-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and Adolescents with Autism Exhibit Reduced MEG Steady-State Gamma Responses. Biol Psychiat. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiznitzer M. Autism and tuberous sclerosis. J Child Neurol. 2004;19:675–9. doi: 10.1177/08830738040190090701. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon C-H, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.