Abstract
Bidirectional communication between the immune system and the brain is essential for mounting the appropriate immunological, physiological, and behavioral responses to immune activation. Aging, however, may impair this important bi-directional interaction. In support of this notion, peripheral infection in the elderly is associated with an increased frequency of behavioral and cognitive complications. Recent findings in animal models of aging and neurodegenerative disease indicate that microglia, innate immune cells of the brain, become primed or reactive. Understanding age- and disease-associated alterations in microglia is important because glia (microglia and astrocytes) play an integral role in propagating inflammatory signals that are initiated in the periphery. In this capacity, brain glia produce inflammatory cytokines that target neuronal substrates and elicit a sickness-behavior syndrome that is normally beneficial to the host organism. Increased reactivity of microglia sets the stage for an exaggerated neuroinflammatory cytokine response following activation of the peripheral innate immune system, which may underlie subsequent long-lasting behavioral and cognitive deficits. In support of this premise, recent findings indicate that stimulation of the peripheral immune system in aged rodents causes exaggerated neuroinflammation that is paralleled by cognitive impairment, prolonged sickness, and depressive-like complications. Therefore, the purpose of this review is to discuss the new evidence that age-associated priming of microglia could play a pathophysiological role in exaggerated behavioral and cognitive sequelae to peripheral infection.
Immune-to-brain communication and the induction of the sickness response
Immune-to-brain communication is important because the brain is responsible for critical components of the immune response (e.g., fever, sickness behavior, and immunomodulation). This immune-to-brain communication is mediated, in part, by inflammatory cytokines (reviewed by Dantzer et al. 2008). During a peripheral infection, activation of peripheral innate immune cells leads to secretion of inflammatory cytokines including interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNFα; Bluthe et al. 1991, 1995). This signal is communicated across the blood–brain barrier (BBB) where it induces brain glia to produce the same inflammatory cytokines. Within the brain, these cytokines and their corresponding secondary mediators, including prostaglandins (Ericsson et al. 1997; Marty et al. 2008) and nitric oxide (Konsman et al. 1999), target neuronal substrates and initiate the brain response to peripheral infection.
In order for the peripheral cytokine signal to reach the brain, the signal must overcome the obstacle of the BBB (Bechmann et al. 2007). This is accomplished through several distinct pathways. First, cytokines in the blood can readily gain access into the brain by diffusion through a few, relatively porous regions of the BBB adjacent to the circumventricular organs (CVOs; Lacroix et al. 1998; Laflamme et al. 1999). In this manner, cytokines can interact directly with microglia and astrocytes of the glial limitans. A second key pathway involves interactions between cytokines and endothelial cells in the brain. These endothelial cells have IL-1 receptors and transmit the cytokine signal into the brain (Ching et al. 2007) by either transporting IL-1β to the abluminal side (Quan and Banks 2007) or by producing IL-1β de novo for secretion on the abluminal side. A third pathway involves direct neural activation by cytokines in the periphery via the vagus nerve (Goehler et al. 1998, 2006; Konsman et al. 2000; Marvel et al. 2004) and catecholaminergic circuits of the sympathetic nervous system (Johnson et al. 2008). The degree to which each pathway is activated depends on the stimulus, but all pathways are likely to contribute to immune-to-brain signaling. These immune-to-brain signaling pathways are reviewed in greater detail elsewhere (Nguyen et al. 2002; Quan and Banks 2007; Dantzer et al. 2008).
Once this cytokine-mediated signal reaches the brain, the inflammatory signal is propagated by astrocytes, microglia, and perivascular macrophages to elicit the physiological and behavioral symptoms of sickness, including fever, increased sleep, reduced appetite, lethargy, and decreased social interaction (Kent et al. 1996; Konsman et al. 2002). The physiological responses are aimed at attenuating replication of pathogens (e.g. fever and reduced plasma iron concentrations) and relocating resources to the breakdown of carbohydrate, fat, and protein (Hypothalamic-pituitary adrenal [HPA] axis and acute phase; Kluger 1978; Kluger and Rothenburg 1979). These pathways help to meet the increased metabolic demand required for clearance of the pathogen. At the same time, the sickness behaviors induced by cytokines conserve energy by suppressing appetite, libido, and social interaction, and by increasing time spent sleeping. Thus, the cytokine-mediated behavioral and physiological responses to infection initiate a reorganization of the host's priorities.
The physiological and behavioral aspects of the sickness response are highly evolutionarily conserved and adaptive (Maier and Watkins 1998). Stereotyped patterns of sickness are exhibited by mammals, birds (Johnson et al. 1993), reptiles (Merchant et al. 2008), and invertebrates (Adamo 2006). Even caterpillars show reduced feeding behavior after LPS injection (Adamo et al. 2007). This adaptive nature of sickness behavior is further supported by work showing that disruption of sickness responses, including the febrile response and suppression of the consumption of food, negatively impacted the survival of the host (Murray and Murray 1979; Hart 1988). Moreover, social withdrawal and other anti-social behaviors are an effective way to conserve energy and limit the spread of infection (Maier and Watkins 1998). An additional adaptive feature of the sickness response is that it can be modulated by environmental factors such as mating (Weil et al. 2006b) and protecting offspring (Aubert et al. 1997; Weil et al. 2006a).
In summary, this cytokine-meditated sickness response is transient, evolutionarily conserved, and beneficial to the host organism in fighting infection. Circumstances that alter the immune response, such as aging, stress, and disease, may cause a maladaptive sickness response that leads to behavioral and cognitive complications. The aim of the balance of this review is to discuss the impact of age-related changes in microglia and their role in exaggerated sickness responses and subsequent long-lasting complications.
Microglia, innate immune cells of the central nervous system (CNS)
Brain glia, astrocytes (or astroglia) and microglia, are integral to cytokine-mediated sickness behavior (Bluthe et al. 1994; Nguyen et al. 2002). Astrocytes are derived from neuronal progenitors and have many diverse functions, including acting as a neurotransmitter sink, maintaining the integrity of the BBB, providing structural support, releasing neurotrophic factors, and engaging in synaptic transmission. Microglia are bone marrow-derived myeloid lineage cells (Priller et al. 2001), which are interspersed throughout the CNS, and represent approximately 15% of the total CNS cell population. In early development, myeloid cells migrate from the bone marrow to the brain parenchyma and terminally differentiate into microglia. Microglial turnover in the brain is limited (Carson et al. 2006); however, microglia replacement from the bone marrow is detected in CNS infection, prion disease, and Alzheimer's Disease (Simard and Rivest 2004; Bechmann et al. 2005; Mildner et al. 2007). In the absence of inflammatory stimuli, microglia display a ramified morphology with small cell bodies and many long, thin processes. In this resting, or “surveying,” state microglia are actively sampling their microenvironment by pulsing their projections (Davalos et al. 2005; Nimmerjahn et al. 2005). When microglia are activated, they become de-ramified with enlarged cell bodies and retracted, condensed processes. Activated microglia perform macrophage-like activities including scavenging, phagocytosis, antigen presentation, and inflammatory cytokine production (Hanisch and Kettenmann 2007).
Microglia and astrocytes work together to propagate and modulate neuroinflammatory responses. Active glia produce both inflammatory and anti-inflammatory cytokines. A myriad of reports indicate that the pro-inflammatory cytokines including IL-1β, IL-6, and TNFα are essential for induction and the maintenance of sickness behavior (reviewed by Dantzer 2001). These cytokine signals also trigger the release of secondary inflammatory mediators including prostaglandins and nitric oxide (Ericsson et al. 1997; Guan et al. 1997; Konsman et al. 1999; Marty et al. 2008). In regard to the temporal response of glia, microglia are activated first and then recruit astrocytes to further propagate inflammatory signals (Hwang et al. 2006). These neuroinflammatory processes are normally transient, with microglia returning to a resting state as the immune stimulus is resolved.
Evidence of an altered glial population in the aged brain
The topic of age-related changes in glia in the brain was reviewed as early as 1985 (Vernadakis 1985). In the past 15 years, researchers have continued to uncover evidence of age-associated alterations in glia. For example, inflammatory markers, consistent with microglia and astrocyte activation, were detected in the brain of aged rats (Perry et al. 1993). Increased markers of microglial activation were also detected in older primates (Sheffield and Berman 1998) and elderly humans (Streit and Sparks 1997). Similar glial changes were also detected in models of neurodegeneration including prion disease (Betmouni et al. 1996) and Alzheimer's disease (McGeer et al. 1987). These initial reports, in conjunction with more recent studies indicate that there is an elevated inflammatory profile in the normal, non-diseased aging brain consisting of increased expression of major histocompatibility complex (MHC) II (Perry et al. 1993; Ogura et al. 1994; Streit and Sparks 1997; Sheffield and Berman 1998; Morgan et al. 1999; Nicolle et al. 2001; Streit et al. 2004; Godbout et al. 2005; Frank et al. 2006; Henry et al. 2008b), the scavenger receptors (CD68; Godbout et al. 2005; Wong et al. 2005), Cd11b and Cd11c integrins (Perry et al. 1993; Stichel and Luebbert 2007), and toll-like receptors (TLRs; Letiembre et al. 2007). Furthermore, the expression of glial fibrillary acidic protein (GFAP), an astrocyte marker that is up-regulated with activation, was increased in several of these same rodent models of aging (Morgan et al. 1999; Lee et al. 2000; Godbout et al. 2005). In addition to up-regulation of activation markers, microglia from non-diseased, aged brains also show changes in morphology. Recent studies using the ionized calcium-binding adaptor protein-1 (Iba1), a protein expressed on the surface of microglia (Imai et al. 1996; Ohsawa et al. 2004), showed that microglia of aged dogs and gerbils exhibited thickened and de-ramified processes compared with younger adults (Choi et al. 2007; Hwang et al. 2008). Along with these age-associated changes in the expression and morphology of glia, modest increases in inflammatory cytokines, including IL-1β and IL-6 have been detected in the brain of aged rodents (Murray and Lynch 1998; Ye and Johnson 1999; Maher et al. 2004; Sierra et al. 2007; Chen et al. 2008; Richwine et al. 2008a). A final point is that the numbers of resident astrocytes and microglia are not increased in the brain with age (Long et al. 1998), indicating that markers of reactivity are increased in existing astrocyte and microglia populations (Long et al. 1998; Morgan et al. 1999).
Immune consequences of a reactive glial population
A potential immune consequence of a reactive glial profile in the brain is an exaggerated neuroinflammatory cytokine response after activation of the innate immune system (Perry et al. 2003; Huang et al. 2008). As described above, markers of glial reactivity are detected in rodent models of aging and neurodegenerative disease. In rodent models of neurodegeneration, such as amyotrophic lateral sclerosis, Alzheimer's disease, and prion disease, peripheral immune activation with lipopolysaccharide (LPS) caused amplified neuroinflammation and accelerated progression of the disease (Sly et al. 2001; Combrinck et al. 2002; Nguyen et al. 2004; Cunningham et al. 2005; Kamer et al. 2008). These findings are reviewed in greater detail elsewhere (Perry et al. 2007; Kamer et al. 2008) and have contributed to the hypothesis that the increased reactive glial profiles detected in the non-diseased, aged brain may also predispose older individuals to exaggerated neuroinflammatory responses to peripheral immune activation (Godbout and Johnson 2006; Perry et al. 2007; Dilger and Johnson 2008; Fig. 1). This hypothesis may be of particular relevance because systemic infection is associated with an increased frequency of behavioral and cognitive complications in the elderly (Penninx et al. 2003; Evans et al. 2005). This hypothesis is difficult to test in humans but several groups have begun to explore this premise using rodent models of aging.
Fig. 1.
Microglial priming in the aged brain. This figure illustrates that aging is associated with increased reactive or primed microglia in the CNS. Primed microglia express a subset of activation markers, but exhibit minimal pro-inflammatory cytokine release in the absence of immune stimulus. When these primed microglia are activated, they are activated to a greater extent than are non-primed microglia. Therefore, the inflammatory cytokine response initiated by microglia is amplified and prolonged in the aged brain.
In support of the aging and glial reactivity hypothesis, peripheral injection of LPS caused an exaggerated neuroinflammatory cytokine response (IL-1β and IL-6) and prolonged sickness behavior in aged (22–24 m) BALB/c mice compared to young adults (Godbout et al. 2005). Several subsequent studies showed that peripheral LPS caused exaggerated levels of IL-1β expression in the hippocampus of aged mice compared to young adults (Chen et al. 2008; Henry et al. 2008; Richwine et al. 2008). Elevated levels of IL-1β and TNFα mRNA expression were detected 24 and 72 h after LPS injection (Richwine et al. 2008). Moreover, in a rat model of aging where increased reactive glia (i.e., increased MHC II expression) were present (Frank et al. 2006), peripheral injection of Escherichia coli promoted higher levels of IL-1β in the hippocampus of aged rats compared to young adults (Barrientos et al. 2006). The levels of IL-1β were prolonged in the hippocampus of older rats infected with E. coli (Barrientos et al. 2009). Furthermore, these aged rats showed an altered and prolonged febrile response (Barrientos, et al. 2009).
It is important to mention that while peripheral LPS or E. coli caused higher levels of IL-1β in the brain of aged rodents compared to young adults, plasma levels of IL-1β were not amplified (Godbout et al. 2005; Barrientos et al. 2006, 2009). The disconnection between peripheral IL-1β induction and brain IL-1β has been interpreted to suggest that amplification of the cytokine response occurs within the brain. In support of this idea, intraperitoneal LPS injection markedly increased neuronal activity (as measured by c-fos staining) in several CVOs and in the nucleus of the solitary tract in the brainstem of aged mice compared to younger adults after LPS injection (Gaykema et al. 2007). Because peripheral immune signals are interpreted in CVOs and in the brainstem (Goehler et al. 1998; Marvel et al. 2004; Goehler et al. 2006), these data indicate that the immune signal from the periphery is amplified within the aged brain. Moreover, the amplified neuroinflammatory response was detected in several brain areas. For instance, the exaggerated inflammatory response in the aged brain was evident in samples from the whole brain (Godbout et al. 2005), hippocampus (Abraham and Johnson 2009; Chen et al. 2008; Henry et al. 2008; Richwine et al. 2008), cortex (Henry et al. 2008), and hypothalamus (Abraham and Johnson 2009).
The idea that the cytokine signal from the periphery becomes amplified within the brain is supported by several studies. For example, central activation of the innate immune system by intracerebroventricular (i.c.v.) administration of LPS caused amplified mRNA expression of inflammatory cytokines, IL-1β, IL-6, and TNFα, and prolonged sickness behavior in aged (22–24 m) mice (Huang et al. 2008). Moreover, repeated i.c.v. injection of the HIV-associated protein, gp120, caused a prolonged sickness response paralleled by elevated expression of IL-1β and IL-6 mRNA in the hippocampus of aged mice compared to young adults (Abraham et al. 2008). Consistent with these data, other reports indicate that older mice were more sensitive to septic shock induced by i.c.v. administration of LPS (Chorinchath et al. 1996) and had elevated TNFα production in the brain and plasma after challenge with LPS compared to young adult controls (Kalehua et al. 2000). Work using primary, mixed glial cultures and coronal brain sections established from the brain of aged rodents also supports the hypothesis of an amplified neuroinflammatory response in the aged brain. In both cases, these culture systems were hyper-responsive to LPS stimulation and produced more inflammatory cytokines (IL-1β and IL-6) compared to cultures established from the brains of young adults (Ye and Johnson 2001; Xie et al. 2003). Collectively, these data support the notion that cells within the brain are hyper-responsive to peripheral or central immune activation.
Based on the findings discussed above, many authors have suggested that primed or reactive microglia play a significant role in the exaggerated neuroinflammatory response in aged mice following innate immune activation. Studies with minocycline, an anti-inflammatory agent and inhibitor of microglial activation (Nikodemova et al. 2007), support the notion that microglia contribute to age-associated neuroinflammation. For example, in aged rats minocycline reduced the age-associated increase in brain MHC II and CD86 expression and attenuated the age-related impairments in long-term potentiation (Griffin et al. 2006). Moreover, minocycline pretreatment in aged BALB/c mice normalized the LPS-induced exaggerated mRNA expression of neuroinflammatory markers, including IL-1β, toll-like receptor (TLR)-2, and indoleamine 2, 3 dioxygenase (IDO), to the levels of young adults injected with LPS (Henry et al. 2008). It is important to note that while minocycline attenuated IL-1β in the brain, it had no affect on plasma levels of IL-1β (Henry et al. 2008). Another report showed that peripheral LPS injection caused amplified intracellular expression of IL-1β and IL-10 in aged microglia compared to young adults (Henry et al. 2008b). In this study, microglia-specific mRNA levels of TLR2, IDO, IL-10, and IL-1β were also increased in microglia of aged mice compared to those of young adults (Henry et al. 2009). These mRNA results are similar to those from a study using transgenic p.7.2fms-EGFP mice in which microglia/macrophage were labeled with a green fluorescent protein. In this study, microglia were sorted by GFP expression and microglia from aged transgenic mice had the highest mRNA induction of both IL-1β and IL-10 after peripheral LPS injection compared to young adult mice (Sierra et al. 2007). A final key point is that work by Henry et al. (2008a,b) demonstrated that the reactive (MHC II+) microglia of aged mice had the most prominent IL-1β induction following immune challenge compared to non-reactive (MHC II–) microglia (Henry et al. 2009). Taken together, these findings indicate that peripheral LPS challenge caused microglial hyperactivity specifically in reactive (MHC II+) microglia (Fig. 1). These data support the hypothesis that reactive microglia play a key role in exaggerated neuroinflammation in the aged following innate immune activation.
Behavioral and cognitive complications associated with amplified neuroinflammation
The induction of cytokine-mediated sickness behavior is a necessary and beneficial response to systemic infection. Nonetheless, amplified or prolonged neuroinflammation can lead to a maladaptive sickness response (Fig. 2). As mentioned in a previous section, intraperitoneal injection of LPS caused protracted IL-1β and IL-6 expression in the brain of aged BALB/c mice that was paralleled by a prolonged sickness response (Godbout et al. 2005). In this study older mice showed protracted anorexia, lethargy, and social withdrawal. These mice also had marked reduction in body weight compared to young adults. Consistent with these findings, i.c.v injection of either LPS or GP120 also caused prolonged sickness response in aged mice (Abraham et al. 2008; Huang et al. 2008). Moreover, a recent study showed that the prolonged LPS-induced sickness behavior in aged mice was reversed by attenuating IL-1β expression with i.c.v. infusion of IL-1 receptor antagonist (IL-1RA; Abraham and Johnson 2009). Thus, there is a direct relationship between amplified brain cytokine response and protracted sickness behavior in the aged.
Fig. 2.
Potential complications associated with exaggerated neuroinflammation following peripheral immune challenge. Enhanced neuroinflammation after stimulation of the central or peripheral innate immune system is associated with amplified activation of microglia, which can lead to a number of behavioral, cognitive, and neuronal complications.
Increased cytokine production in the aged brain after immune challenge is also associated with impaired cognitive function. For example, injection of LPS caused an amplified cytokine response in the hippocampus of older mice that was paralleled by impaired hippocampal-dependent spatial memory (Chen et al. 2008). Moreover, infection by E. coli led to prolonged production of IL-1β in the hippocampus of aged rats (Barrientos et al. 2009) and impaired long-term contextual memory (Barrientos et al. 2006). In a model of prion disease where increased microglia priming was detected, intraperitoneal injection of LPS led to delirium-like behavior in mice with pre-symptomatic prion disease compared to non-diseased controls (Cunningham et al. 2008). The mechanisms by which cytokines cause cognitive complications are unclear, but a potential explanation is that neuroinflammatory pathways can impact neuronal plasticity (e.g. neurogenesis, long-term potentiation, and dendritic restructuring). For example, in aged mice where neuroinflammation was prolonged, increased dendritic atrophy was detected in the CA1 region of the hippocampus (Richwine et al. 2008). In summary, prolonged neuroinflammation can impair hippocampal-dependent memory and increase delirium-like behaviors.
Another aspect of the maladaptive sickness response is the induction of depressive-like symptoms. This is relevant because there is a significant connection between inflammation and depression in humans and rodents (Raison et al. 2006; Dantzer et al. 2008). For example, in animal models, situations that promoted pro-inflammatory cytokine production by the brain (e.g. IL-1β, IL-6, or TNFα), such as systemic LPS challenge (Frenois et al. 2007), chronic infection with Bacilli Calmette-Guerin (BCG; Moreau et al. 2005), stroke (Craft and DeVries 2006), or psychological stress (Chourbaji et al. 2006), were also associated with depressive-like behavior. In regard to aging, peripheral stimulation of the innate immune system with LPS caused prolonged depressive-like behavior in aged mice (72 h post LPS injection) with increased resignation behavior in the forced-swim and tail-suspension tests. This depressive-like behavior was evident even after the acute effects of LPS on lethargy and food intake were resolved (Godbout et al. 2008).
The underlying cause of this transition from sickness to prolonged depression is unclear. There appears, however, to be a relationship between induction of indoleamine 2,3 diogenase (IDO) and depressive-like symptoms (reviewed by Dantzer et al. 2008). IDO is a ubiquitous enzyme that, in the brain, is expressed by macrophages and microglia. IDO is induced by inflammatory cytokines, including IL-1β, IFNγ, and TNFα. Active IDO converts tryptophan (TRP) into kynurenine (L-KYN), which can be subsequently processed into neuroactive mediators, including 3’hydroxy-kynurenine (3’HK) and quinolinic acid (QUIN; Stone and Darlington 2002). In rodents, systemic immune activation and ischemic brain injury were associated with increased IDO-dependent metabolism of TRP and elevated levels of KYN and QUIN in the brain (Saito et al. 1993a, b; Lestage et al. 2002). Work by O’Connor et al. (2008) showed that that inhibition of IDO by 1-methyl tryptophan (1-MT) inhibited acute depression associated with LPS injection in adult mice. This same report indicated that, in the absence of immune stimulation, peripheral administration of L-KYN mimicked the acute depressive-like effects of LPS in adult mice (O'Connor et al. 2008). Thus, IDO metabolism of TRP into KYN may be the key pathway involved in inflammation-related depression. In a mouse model of aging, intraperitoneal injection of LPS caused prolonged expression and activity of IDO in the brain (Godbout et al. 2008) and higher induction of microglia-specific IDO mRNA in aged mice compared to young adults (Henry et al. 2008b). Thus, amplified microglial activation in the aged brain may lead to significant activation of the IDO pathway.
In summary, the production of inflammatory cytokines (i.e. IL-1β, IL-6, and TNF) by microglia plays an important role in the induction and maintenance of sickness behavior. The data discussed above indicate that reactive glia produce higher levels of these cytokines for a longer duration compared to younger adults. These data indicate that an amplified and prolonged glial response in the aged brain results in a maladaptive response to sickness (Fig. 2).
Possible mechanisms underlying microglial priming and reactivity
Altered glial profiles are detected in animal models of both neurodegeneration and aging. In the prion model of neurodegeneration, the neuronal death and associated inflammation is the most likely cause of microglial priming (Perry et al. 2007). In the aging models, the cause of the priming is unclear. Age-associated priming is likely associated with the inflammatory status of the brain. For example, it is well documented that basal levels of oxidative stress and inflammation are increased in the aged brain. These findings are consistent with the “free radical hypothesis of aging” in which oxidative damage to cell membranes and intracellular proteins increases as a function of age (Beckman and Ames 1998). Supporting evidence for this premise is derived from studies of aging rodents using the caloric restriction (CR) model. In the CR model, total calories are restricted by 40% from weaning onward while dietary intake of essential nutrients is maintained. In this model, life span is extended by 30–50% and this extension is thought to be related to increased DNA repair, increased metabolic efficiency, and reduced oxidative stress (Lee et al. 1999). Restricting caloric intake reversed the age-related expression of the oxidative and inflammatory markers and enhanced the expression of growth and trophic factors (Lee et al. 2000). CR also reduced other markers of glial reactivity, including GFAP and CD68 scavenger receptor, in the brain of older rodents (Morgan et al. 1999; Wong et al. 2005). Thus, a major contributing factor of age-related microglial reactivity may be associated with increased oxidative stress and inflammation over a lifetime. Independent of the underlying cause of priming, glial reactivity is associated with an exaggerated and prolonged neuroinflammatory response following stimulation of the innate immune system.
Microglia priming and reactivity in the aged brain may also be associated with impairments in normal regulation be of glial cells. It is necessary that microglia activation be tightly regulated to prevent disruption of brain homeostasis caused by excess neuroinflammation. Anti-inflammatory cytokines such as IL-10, TGFβ, and IL-1RA help to resolve inflammatory signals in the brain (Shull et al. 1992; Bluthe et al. 1999; Heyen et al. 2000). Moreover, other anti-inflammatory pathways, including glucocorticoids and catecholamines, also attenuate neuroinflammation (Dello Russo et al. 2004; Simard and Rivest 2007). Given how vital these pathways are in regulating activation of glia and neuroinflammation, it is plausible that one or more of these pathways are altered during normal aging, leading to increased microglial reactivity and maladaptive responses to immune stimulation. For instance, recent studies indicate that there is decreased glucocorticoid receptor expression in astrocytes in the hippocampus of older rats (Kasckow et al. 2009). These deficits may be associated with impaired negative feedback of glucocorticoids in the aged brain (Mizoguchi et al. 2009).
Age-related deficits in microglial regulation by chemokines, neurotransmitters, and neuropeptides may also contribute to microglia priming and impaired microglial responses (Fig. 3). For instance, CD200 is a member of the immunoglobulin superfamily and is constitutively expressed on the surface of neurons. The corresponding CD200 receptor (CD200R) is expressed on microglia (Hoek et al. 2000; Lyons et al. 2007) and loss of this receptor impairs microglial regulation. (Hoek et al. 2000). This is relevant because there was less CD200R on the surface of microglia from aged rats compared to those from younger adults and this reduction was associated with increased MHC II expression (Lyons et al. 2007). These data are consistent with those from a previous study showing that levels of CD200R mRNA were lower in brain of older rats (Frank et al. 2006). Furthermore, subcutaneous injection of a mimetic of the neural cell adhesion molecule (NCAM) enhanced CD200 expression in aged mice, reduced the age-related increase in CD86, and attenuated expression of pro-inflammatory cytokines in the brain following LPS injection (Downer et al. 2008). Another neuronal regulator of microglia, the chemokine fractalkine (CX3CL1), may also play a role in age-related microglial dysregulation. For example, CX3CR1-deficient mice (CX3CR1–/– mice) exhibited enhanced microglial activation after repeated intraperitoneal LPS injection, which was also associated with microglial-mediated neurotoxicity (Cardona et al. 2006).
Fig. 3.
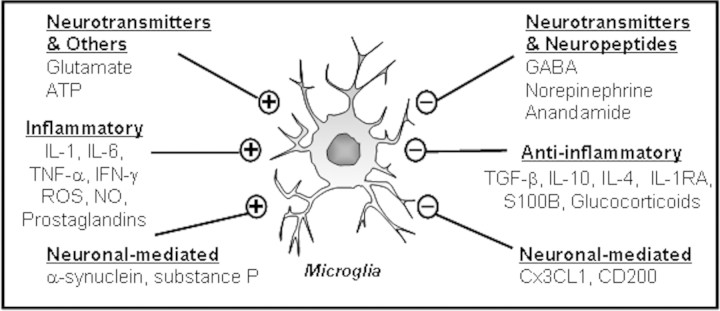
Microglial regulatory systems. There are several systems involved in microglial regulation. This figure illustrates factors that either activate (+) or (–) inhibit microglia. It is plausible that one or more of these pathways are altered in aging, leading to enhanced microglial priming or reactivity.
Recent studies show that microglia also express receptors for neurotransmitters (Pocock and Kettenmann 2007). GABA, an inhibitory neurotransmitter, attenuated IL-1β production by interacting with GABAB receptors on microglia (Kuhn et al. 2004). Moreover, reduced numbers of somatostatin-expressing GABA-ergic interneurons in the hippocampus of aged rats were associated with increased mRNA expression of IL-1β and TNFα in the hippocampus (Gavilan et al. 2007). Microglial activation is also modulated by neuropeptides including the endogenous cannabinoid (CB) anandamide. CB receptors are present on microglia (Cabral and Marciano-Cabral 2005) and stimulation of CB1/2 receptors with the selective agonist, WIN-55212-2, reduces microglial activation and memory deficits in aged rats (Marchalant et al. 2008). Collectively, these data show that impairment of normal mechanisms of microglial regulation in the aged brain may underlie the enhanced neuroinflammation and neurobehavioral consequences concomitant with immune activation in the aged.
Conclusions
The present review discusses findings that indicate that brain glia become more reactive during aging. Moreover, several studies support the premise that activation of this reactive glial population leads to an amplified and prolonged neuroinflammatory response which, in turn, promotes a maladaptive sickness response. Although is it is unclear how glial reactivity increases with age, it is clear that a better understanding of the pathways of microglial regulation is needed. It is plausible that changes in microglia or in microglial regulation also occur in human aging and are involved in neurobehavioral impairments associated with inflammation such as delirium, depression, and accelerated progression of neurological disease. New techniques of positron emission tomography offer a minimally invasive screening of neuroinflammatory processes in the human brain (Venneti et al. 2008). These techniques are being used for potential early diagnosis of Alzheimer's disease, but may also help increase our understanding of other neuroinflammatory complications associated with aging.
Funding
This work is supported by the National Institutes of Health (grant R21-MH077817 to J.P.G); National Institutes of Health (T32-AI-05-5411 Training Grant to C.J.H); participation in the 2009 Society of Integrated and Comparative Biology (SCIB) symposium was supported by NSF grant 0849163 (to J.P.G).
Footnotes
From the symposium “Psychoneuroimmunology Meets Integrative Biology” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2009, at Boston, Massachusetts.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–21. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2008;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo SA. Comparative psychoneuroimmunology: evidence from the insects. Behav Cogn Neurosci Rev. 2006;5:128–40. doi: 10.1177/1534582306289580. [DOI] [PubMed] [Google Scholar]
- Adamo SA, Fidler TL, Forestell CA. Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain Behav Immun. 2007;21:292–300. doi: 10.1016/j.bbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11:107–18. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Watkins RL, Rudy JW, Maier SF. Brain Behav Immun. [in press] 2009. Characterization of the sickness response in young and aging rats following E. coli infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–32. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simburger E, Naftolin F, Dirnagl U, Nitsch R, Priller J. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. Faseb J. 2005;19:647–49. doi: 10.1096/fj.04-2599fje. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Betmouni S, Perry VH, Gordon JL. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74:1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Beaudu C, Kelley KW, Dantzer R. Differential effects of IL-1ra on sickness behavior and weight loss induced by IL-1 in rats. Brain Res. 1995;677:171–76. doi: 10.1016/0006-8993(95)00194-u. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioral effects of lipopolysaccharide in rats. Psychoneuroendocrino. 1999;24:301–11. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur J pharmacol. 1991;209:281–83. doi: 10.1016/0014-2999(91)90184-r. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Pawlowski M, Suarez S, Parnet P, Pittman Q, Kelley KW, Dantzer R. Synergy between tumor necrosis factor alpha and interleukin-1 in the induction of sickness behavior in mice. Psychoneuroendocrino. 1994;19:197–207. doi: 10.1016/0306-4530(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Marciano-Cabral F. Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. J Leukoc Biol. 2005;78:1192–97. doi: 10.1189/jlb.0405216. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–11. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, Jaeger LB, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–86. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, Lee HS, Lee IS. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J Vet Med Sci. 2007;69:1131–36. doi: 10.1292/jvms.69.1131. [DOI] [PubMed] [Google Scholar]
- Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–30. [PubMed] [Google Scholar]
- Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, Schwaninger M, Gass P. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–94. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Craft TK, DeVries AC. Role of IL-1 in poststroke depressive-like behavior in mice. Biol Psychiat. 2006;60:812–18. doi: 10.1016/j.biopsych.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biological Psychiat. 2009;65:304–12. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann NY Acad Sci. 2001;933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–58. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1beta production. J Neuroinflamm. 2004;1:9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–39. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer EJ, Cowley TR, Lyons A, Mills KH, Berezin V, Bock E, Lynch MA. Neurobiol Aging. 2008. A novel anti-inflammatory role of NCAM-derived mimetic peptide, FGL. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–79. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiat. 2005;58:175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–22. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrino. 2007;32:516–31. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilan MP, Revilla E, Pintado C, Castano A, Vizuete ML, Moreno-Gonzalez I, Baglietto-Vargas D, Sanchez-Varo R, Vitorica J, Gutierrez A, Ruano D. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem. 2007;103:984–96. doi: 10.1111/j.1471-4159.2007.04787.x. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Balachandran MK, Godbout JP, Johnson RW, Goehler LE. Enhanced neuronal activation in central autonomic network nuclei in aged mice following acute peripheral immune challenge. Auton Neurosci. 2007;131:137–42. doi: 10.1016/j.autneu.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24:521–38. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O’Connor J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacol. 2008;33:2341–51. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience. 2006;140:1415–34. doi: 10.1016/j.neuroscience.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 1998;804:306–10. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–72. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Guan Z, Baier LD, Morrison AR. p38 mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1beta. J Biol Chem. 1997;272:8083–89. doi: 10.1074/jbc.272.12.8083. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav R. 1988;12:123–37. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflamm. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–17. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyen JR, Ye S, Finck BN, Johnson RW. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kappaB. Brain Res Mol Brain Res. 2000;77:138–47. doi: 10.1016/s0169-328x(00)00042-5. [DOI] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–71. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–53. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IK, Lee CH, Li H, Yoo KY, Choi JH, Kim DW, Kim DW, Suh HW, Won MH. Comparison of ionized calcium-binding adapter molecule 1 immunoreactivity of the hippocampal dentate gyrus and CA1 region in adult and aged dogs. Neurochem Res. 2008;33:1309–15. doi: 10.1007/s11064-007-9584-6. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006;21:457–67. doi: 10.1016/j.nbd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Bioph Res Co. 1996;224:855–62. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H, 3rd, Fleshner M. Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain Behav Immun. 2008;22:1078–86. doi: 10.1016/j.bbi.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, Curtis SE, Dantzer R, Bahr JM, Kelley KW. Sickness behavior in birds caused by peripheral or central injection of endotoxin. Physiol Behav. 1993;53:343–48. doi: 10.1016/0031-9384(93)90215-2. [DOI] [PubMed] [Google Scholar]
- Kalehua AN, Taub DD, Baskar PV, Hengemihle J, Munoz J, Trambadia M, Speer DL, De Simoni MG, Ingram DK. Aged mice exhibit greater mortality concomitant to increased brain and plasma TNF-alpha levels following intracerebroventricular injection of lipopolysaccharide. Gerontology. 2000;46:115–28. doi: 10.1159/000022146. [DOI] [PubMed] [Google Scholar]
- Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, de Leon MJ. Alzheimer's disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. J Alzheimers Dis. 2008;13:437–49. doi: 10.3233/jad-2008-13408. [DOI] [PubMed] [Google Scholar]
- Kasckow J, Xiao C, Herman JP. Exp Gerontol. 2009. Glial glucocorticoid receptors in aged Fisher 344 (F344) and F344/Brown Norway rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bret-Dibat JL, Kelley KW, Dantzer R. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav R. 1996;20:171–75. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. The evolution and adaptive value of fever. Am Sci. 1978;66:38–43. [PubMed] [Google Scholar]
- Kluger MJ, Rothenburg BA. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science. 1979;203:374–76. doi: 10.1126/science.760197. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–48. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4434–46. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–59. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Kuhn SA, van Landeghem FK, Zacharias R, Farber K, Rappert A, Pavlovic S, Hoffmann A, Nolte C, Kettenmann H. Microglia express GABA(B) receptors to modulate interleukin release. Mol Cell Neurosci. 2004;25:312–22. doi: 10.1016/j.mcn.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Feinstein D, Rivest S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 1998;8:625–40. doi: 10.1111/j.1750-3639.1998.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. 1999;19:10923–30. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–3. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–7. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–54. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–13. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher FO, Martin DS, Lynch MA. Increased IL-1beta in cortex of aged rats is accompanied by downregulation of ERK and PI-3 kinase. Neurobiol Aging. 2004;25:795–806. doi: 10.1016/j.neurobiolaging.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Cerbai F, Brothers HM, Wenk GL. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. Neurobiol Aging. 2008;29:1894–1901. doi: 10.1016/j.neurobiolaging.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty V, El Hachmane M, Amedee T. Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta. Eur J Neurosci. 2008;27:3132–50. doi: 10.1111/j.1460-9568.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei. Brain Behav Immun. 2004;18:123–34. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- Merchant M, Fleury L, Rutherford R, Paulissen M. Effects of bacterial lipopolysaccharide on thermoregulation in green anole lizards (Anolis carolinensis) Vet Immunol Immunopathol. 2008;125:176–81. doi: 10.1016/j.vetimm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2 + monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–53. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ikeda R, Shoji H, Tanaka Y, Maruyama W, Tabira T. Aging attenuates glucocorticoid negative feedback in rat brain. Neuroscience. 2009;159:259–70. doi: 10.1016/j.neuroscience.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192:537–44. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–99. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J Biol Chem. 1998;273:12161–68. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr. 1979;32:593–96. doi: 10.1093/ajcn/32.3.593. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, D’Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–9. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–31. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC) {alpha}/betaII. J Biol Chem. 2007;282:15208–16. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Mol Psychiatr. 2008. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–26. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem. 2004;88:844–56. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiat. 2003;54:566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–67. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–12. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–35. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–61. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–35. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrino. 2008;33:1369–77. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Saito K, Crowley JS, Markey SP, Heyes MP. A mechanism for increased quinolinic acid formation following acute systemic immune stimulation. J Biol Chem. 1993;268:15496–503. [PubMed] [Google Scholar]
- Saito K, Nowak TS, Jr, Suyama K, Quearry BJ, Saito M, Crowley JS, Markey SP, Heyes MP. Kynurenine pathway enzymes in brain: responses to ischemic brain injury versus systemic immune activation. J Neurochem. 1993;61:2061–70. doi: 10.1111/j.1471-4159.1993.tb07443.x. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–99. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. Faseb J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Neuroprotective effects of resident microglia following acute brain injury. J Comp Neurol. 2007;504:716–29. doi: 10.1002/cne.21469. [DOI] [PubMed] [Google Scholar]
- Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, Chin JE. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res Bull. 2001;56:581–88. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28:1507–21. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–12. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75:130–38. doi: 10.1007/s001090050097. [DOI] [PubMed] [Google Scholar]
- Venneti S, Wiley CA, Kofler J. J Neuroimmune Pharmacol. 2008. Imaging microglial activation during neuroinflammation and Alzheimer's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernadakis A. The aging brain. Clin Geriatr Med. 1985;1:61–94. [PubMed] [Google Scholar]
- Weil ZM, Bowers SL, Dow ER, Nelson RJ. Maternal aggression persists following lipopolysaccharide-induced activation of the immune system. Physiol Behav. 2006;87:694–99. doi: 10.1016/j.physbeh.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Bowers SL, Pyter LM, Nelson RJ. Social interactions alter proinflammatory cytokine gene expression and behavior following endotoxin administration. Brain Behav Immun. 2006;20:72–79. doi: 10.1016/j.bbi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, de Beer MC, de Villiers WJ, Finch CE. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neurosci Lett. 2005;390:76–80. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182:135–41. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–92. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]




