Abstract
An increasing recognition has emerged of the complexities of the global health agenda—specifically, the collision of infections and noncommunicable diseases and the dual burden of over- and undernutrition. Of particular practical concern are both 1) the need for a better understanding of the bidirectional relations between nutritional status and the development and function of the immune and inflammatory response and 2) the specific impact of the inflammatory response on the selection, use, and interpretation of nutrient biomarkers. The goal of the Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE) is to provide guidance for those users represented by the global food and nutrition enterprise. These include researchers (bench and clinical), clinicians providing care/treatment, those developing and evaluating programs/interventions at scale, and those responsible for generating evidence-based policy. The INSPIRE process included convening 5 thematic working groups (WGs) charged with developing summary reports around the following issues: 1) basic overview of the interactions between nutrition, immune function, and the inflammatory response; 2) examination of the evidence regarding the impact of nutrition on immune function and inflammation; 3) evaluation of the impact of inflammation and clinical conditions (acute and chronic) on nutrition; 4) examination of existing and potential new approaches to account for the impact of inflammation on biomarker interpretation and use; and 5) the presentation of new approaches to the study of these relations. Each WG was tasked with synthesizing a summary of the evidence for each of these topics and delineating the remaining gaps in our knowledge. This review consists of a summary of the INSPIRE workshop and the WG deliberations.
Keywords: nutrition biomarkers, inflammation and nutrition, immune function and nutrients, BOND and inflammation/infection, assessment of micronutrient biomarkers
PART I: EXECUTIVE SUMMARY OF THE INSPIRE PROJECT
A. Introduction
The recent publication of the 2010 Global Burden of Disease (GBD)17 analyses (1) and the launch of the new series on maternal and child nutrition (2) highlight the current trends in global health, particularly as they affect the “1000 day” period of pregnancy through an infant’s first 2 y of life. As itemized in Text Box 1, the following is apparent:
Text Box 1 . Trends highlighted in the GBD 2010 (1).
● Although the proportion of overall disease burden attributable to childhood underweight has more than halved between 1990 and 2010, it remains the eighth risk factor worldwide.
● High BMI has increased globally to become the sixth greatest risk factor worldwide.
● Among children <5 y old, childhood underweight was still the leading risk factor worldwide in 2010, followed by nonexclusive or discontinued breastfeeding and household air pollution from solid fuels.
● High blood pressure, high BMI, and high fasting blood glucose have all increased significantly in terms of their impact on global health between the 1990 and 2010 GBD analyses.
● Deaths from NCDs increased by ∼8 million between 1990 and 2010, accounting for 2 of every 3 deaths (34.5 million) worldwide in 2010. Of these, 8 million people died of cancer (38% more than in 1990), 12.9 million died of ischemic heart disease and stroke collectively [1 in 4 worldwide compared with 1 in 5 in 1990; 1.3 million deaths were due to diabetes, twice as many as in 1990 (1)].
Additional global health data
● More than 35 million people now live with HIV/AIDS; 3.3 million of them are under the age of 15 (3).
● In 2012, 2.3 million people were newly infected with HIV; 260,000 were under the age of 15.
● In 2012, 1.6 million people died of AIDS; 210,000 of them were under the age of 15 (4).
● In 2012, malaria caused an estimated 627,000 deaths (with an uncertainty range of 473,000–789,000), mostly among African children (5).
● In 2012, there were ∼207 million cases of malaria with ∼80% of cases limited to 17 countries, most prominently in Africa.
● The tuberculosis death rate decreased by 45% between 1990 and 2012 (6). Nevertheless, in 2012 there were ∼8.6 million new cases of tuberculosis and 1.3 million deaths (7).
● More than 95% of tuberculosis deaths occur in LMICs, and it is among the top 3 causes of death for women aged 15 to 44 (7).
● Diarrhea kills 2195 children every day—more than AIDS, malaria, and measles combined (8).
● Diarrheal diseases account for 1 in 10 child deaths worldwide (∼760,000/y), making diarrhea the second leading cause of death among children under the age of 5 (9).
● Great strides have been made in reducing the impact of undernutrition on child health, but stunting and undernutrition remain major targets for the global health community.
● An alarming trend has emerged in the global prevalence of overweight and obesity (10) across the life course.
● This trend has extended into low-/middle-income countries (LMICs), historically the focus of efforts to address undernutrition.
● The prevalence of noncommunicable diseases (NCDs), cardiovascular diseases (CVDs), cancers, chronic respiratory diseases, and diabetes is increasing globally and prominently in LMICs.
● Communicable infectious diseases (CIDs), including HIV, malaria, tuberculosis, and diarrheal disease, remain daunting public health concerns, again hitting LMICs hardest.
● In most settings, a collision is occurring of CIDs, NCDs, food insecurity, and malnutrition within the same population and, in many settings, in the same individuals [malnutrition = over- and/or undernutrition, reflecting an imbalance of total energy, macronutrients (protein, fat, carbohydrate), vitamins, and/or minerals].
This collision, including the dual burden of over- and undernutrition, is just beginning to be addressed in any meaningful manner (11-14). All of these trends have significant implications both in terms of their impact on the population and the health systems designed to combat them. How to assess this complex scenario is one of the great challenges confronting the global health community.
From a biological perspective, 2 common threads run through all of these problems: the importance of nutrition and the role of inflammation. For any of these global health concerns, a strong nutrition component exists both in terms of the role of nutrition in susceptibility to and prevention and treatment of the disease, as well as an outcome of the condition, i.e., the impact of each of these on nutritional endpoints (intake, status). Inflammation is also recognized as a common feature of both CIDs and NCDs (15, 16). Moreover, we have learned that nutrition and inflammation also share a bidirectional relation such that each affects the other in ways that we are just beginning to appreciate (17). For the purpose of this review (see Supplemental Figure 1 for a table of contents), important definitions and terminologies are provided in Text Box 2.
Text Box 2 . Definitions and terminology.
Acute phase response (APR):
● An innate body defense that triggers a sequence of physiologic changes in response to a myriad of stressors, including microbial invasion, tissue injury, immunologic reactions, and inflammatory processes (18–20).
● Begins when activated macrophages, primed by IFN- γ, release a complex network of cytokines such as TNF-α, IL-1, and IL-6. Cytokines then stimulate hepatocytes in the liver to produce the acute phase proteins (see Working Group 1) (21–23).
Acute phase proteins:
● More than 200 plasma proteins are modulated by the APR (18).
● Functions include anti-proteinase activity, coagulation properties, transport functions, immune response modulation, and/or miscellaneous enzymatic activity to restore the delicate homeostatic balance disturbed by trauma or infection
● Classification:
○ positive or negative, reflecting their respective increase or decrease in response to the APR
○ early, intermediate, or late, reflecting differential time course and differential magnitude of change in response to the APR
○ type 1, stimulated by TNF and/or IL-1, or type 2, stimulated by IL-6
Acute inflammation:
● A physiologic response to infection or tissue injury that is usually self-limiting and resolves rapidly, allowing the body to remain healthy and maintain homeostasis.
Chronic inflammation:
● Inflammatory responses that fail to regulate themselves become chronic and contribute to the continuation of the “disease” condition in the body; conditions of chronic inflammation include NCDs (cancer, CVD, diabetes, obesity, autoimmune diseases).
Clinical inflammation:
● Based on whether the individual has clear symptoms of the inciting cause of inflammation (e.g., clinical disease or trauma), for example, fever. It can be either acute (e.g., active malaria) or chronic (e.g., latent infection with tuberculosis).
Subclinical inflammation:
● Has 2 phases and is characterized as follows:
○ initial phase: there is a short (24–48 h) incubation period during which the pathogen can multiply or invade tissues; this initial phase may or may not be followed by clinical symptoms
○ second phase: occurs during convalescence after an acute illness
● Inflammation may be covert if the infection is minor or the body’s immunity is particularly effective in preventing the disease and able to isolate the cause of the infection, preventing clinical inflammation.
In both phases, subclinical inflammation may only be detected biochemically (24). The biochemical changes in inflammatory biomarkers during subclinical inflammation strongly relate to alterations in many nutrient biomarkers. Hence, detecting the presence of subclinical inflammation using inflammatory biomarkers in apparently healthy people is important in population studies.
Our ability to delineate a clear role for nutrition in any disease is complicated by the impact of inflammation. This effect can occur as a result of the role of a given nutrient biomarker as an “acute phase protein” rising or falling in response to inflammation, or as a result of a direct impact of inflammation on nutrient absorption or homeostasis. Our understanding of the biology of any of these conditions, CIDs or NCDs, and the role of either nutrition or inflammation, demands a better appreciation of these complex interactions. Once understood, the challenge then becomes the determination of effective strategies to account for them for both individuals and populations.
Of particular concern to those attempting to determine the nature of the nutrition and health relation is the fact that both acute and chronic inflammation may have a direct impact on the selection, use, and interpretation of the most commonly used biomarkers of nutrient status, function, or effect. This has resulted in attempts to adjudicate this impact via approaches to account for the influence of inflammation (25). However, the complexity of this interaction continues to pose a dilemma for clinicians trying to determine the nutritional needs of their patients as well as for those planning or evaluating programs to ameliorate malnutrition at a population level. This conundrum has become a prominent driver of efforts to discover, develop, and implement new biomarkers of nutrition, including the efforts of such leading technical agencies as the CDC and the WHO charged with providing advice to those conducting surveys at regional or national levels (26).
B. Birth of INSPIRE
The challenges outlined above have also become obstacles in global policy to address specific conditions. A prominent example is the situation arising from concerns about the safety and effectiveness of interventions to prevent and treat iron deficiency (ID) in malaria-endemic areas (27). Historical concerns about iron in the context of infection were heightened in response to a study (28) reporting that universal iron supplementation during early childhood increased the risk of severe morbidity and mortality from malaria and other infections, particularly when supplements were given to individuals who were not iron-deficient. In response to these concerns, the WHO/CDC amended existing guidance with regard to use of iron supplements to include the caveat that, in areas of endemic malaria, iron status should be assessed before interventions (26). Aside from obvious challenges of invasive blood sampling and capacity needs for such a large undertaking, the predominant method for assessing iron nutrition, serum ferritin measurement, is affected by the presence of inflammation.
In response to the specific issues around the iron and malaria question, a collaboration was initiated between the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the US NIH and the Bill and Melinda Gates Foundation to address the following objectives: 1) identification and clarification of biological mechanisms affecting iron metabolism (in both host and infectious organisms) in malaria-endemic areas and 2) development of biomarkers/indicators to assess iron status in women, infants, and young children, including biomarkers of exposure, status, and function that can be used in the context of inflammation/infection.
In addition, the NICHD has also partnered with the Bill and Melinda Gates Foundation and others to initiate the Biomarkers of Nutrition for Development (BOND) Program (29). The goal of the BOND program is to service the food and nutrition community by providing evidence-based advice about the best choice of biomarkers for specific uses and to address key gaps in our knowledge about how best to assess the role of nutrition in health. Phase I of the BOND program included an evaluation of 6 nutrients— zinc, iron, folic acid, vitamin B-12, vitamin A, and iodine—chosen for their public health importance and because they represent the range of issues confronting those who are addressing the role of nutrition in research, clinical care, or program development/evaluation.
Consequent to the deliberations of the expert panels constituted under both projects, the mounting evidence outlined above, and input from the larger community, it became clear that the issue of inflammation and nutrient assessment was a cross-cutting critical gap that needed to be addressed. In response to this challenge, the “Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE)” Project was initiated.
C. Project description
The overarching goal of the INSPIRE project is to review the evidence with regard to the relations between nutrition, immune function, and the inflammatory response. The intent is to make that information available for the development of principles and guidance that could be universally applied to the discovery, development, and use of current and new biomarkers. The guiding principles are intended to inform and support the efforts of individuals/organizations with an interest in the field, irrespective of their level of expertise or nature of their activities. Specifically, the INSPIRE project was designed to do the following:
● Review what is known about the impact of inflammation (acute or chronic) on selection, use, and interpretation of biomarkers specifically and nutrition more broadly, including basic biology to explain the nature of nutrition/inflammation interactions, implications for the use and interpretation of biomarkers of nutritional status, function, and effect including monitoring and evaluation of interventions, and implications for biomarker discovery and development.
● Translate the extant knowledge: develop a document to be published and posted on the BOND website (30) that contains a set of principles to inform and guide the community with regard to how to account for the impact of inflammation on selection, use, and interpretation of biomarker data.
● Develop a research agenda based on the outcomes of 1 and 2 above to address current gaps in our understanding of the nutrition/inflammation relations.
I. Structure.
The INSPIRE organizational structure consists of the NICHD Secretariat and a Scientific Steering Committee (SSC) (Supplemental Table 1). The SSC was established to provide scientific oversight and guidance for the working groups (WGs) and to help develop the products of INSPIRE. To achieve the project goals, the SSC identified 4 overarching themes, and 5 WGs were formed to address elements of these themes (Text Box 3).
Text Box 3 . INSPIRE WG themes.
WG theme 1: overview of the role of nutrition in immune function and the inflammatory response
WG theme 2: specific relations between nutrients, immune function, and inflammatory response—impact of nutrition on the immune response
WG theme 3: specific relations between nutrients, immune function, and inflammatory response—impact of the immune response on nutrition
WG theme 4: translating evidence to practice—approaches to addressing the nutrition and inflammation relations
WG theme 5: methodologies and new technologies including the potential use of systems biology and new “-omics” technologies for discovery of new biomarkers
II. Process.
The WGs consisted of 4 to 6 members and a chair, chosen on the basis of input from the SSC. WG members are listed in Supplemental Table 1. These WGs met via teleconference 2 to 3 times to further refine the suggested outline and to begin the process of drafting the WG reports. A content-focused INSPIRE workshop was convened in Bethesda, MD, 28–30 November 2012. This provided WG members with the opportunity to invite others in areas identified as relevant to the WG deliberations as well as experts to provide additional insights about specific issues in need of in-depth coverage. To address overlapping and cross-cutting issues, the WGs were encouraged to closely collaborate in order to avoid redundancy and to maintain a logical train of thought across the WGs. The objectives of the workshop were as follows: 1) to present preliminary results of WG deliberations, 2) to discuss cross-cutting issues to avoid overlap, and 3) to exchange ideas and obtain experts’ perspectives on specific topics identified by the WG.
Deliberations from the INSPIRE workshop supported the process of developing the final WG summary reports. Discussions reinforced the notion of this effort as a progression of thought from an understanding of the basic biology of inflammation/infection and nutrition and culminating in suggestions about how best to account for these interactions in making clinical and programmatic decisions based on the interpretation of extant data. Finally, attention was paid to the identification of new technologies and science that might be applied to further our understanding of these relations.
These proceedings include a brief summary of the INSPIRE workshop, manuscripts provided by invited speakers, and the final WG reports. The summaries of deliberations of WGs 1–4 are presented in detail below. Highlights of WG 5 are found in the text below after the description of that section of the workshop.
III. Summary of the INSPIRE workshop.
The INSPIRE workshop opened with an overview provided by the SSC co-chairs, Drs. Catharine Ross and Simin Meydani, and the NICHD Secretariat, Dr. Daniel Raiten. The first session included presentations by Stephen Hursting who reviewed the current knowledge with regard to the relation of inflammation and chronic disease (31). This was followed by a review of immunology, mucosal immunobiology, innate immunity, and response to pathogens provided by Dr. Kelsall’s review which included a coverage of key elements of the immune response including the biology and factors affecting the induction of IgA (32), an introduction to various aspects of the role of the gut microbiota in immune function (33, 34), and a brief discussion of inflammatory bowel disease as an exemplar of how these factors may interact in a clinically relevant manner (35). See Supplemental Figure 2 for the complete meeting agenda.
Each session that followed focused on the INSPIRE themes. The theme 1 session began with an overview of “the role of nutrition in immune function and the inflammatory response” by Dr. Harry Dawson who emphasized several key elements to consider including the following: 1) contributory factors to the inability to translate research findings into clinical practice, 2) the importance of the choice or choices of species used to model human responses, 3) challenges associated with defining nutrient status in the context of the APR and its impact on selection and interpretation of biomarkers of nutrient status, and 4) recognition that the effect of nutrition on specific immune response pathways is related to many factors, including the severity of deficiency, presence of other deficiencies, presence of other infections, age, genetics, etc. Dr. Kate Claycombe followed with an overview of the role of nutrition in the genetics and epigenetics of inflammation (36).
The next set of content sessions focused on the bidirectional relations between nutrition and inflammation. Dr. Zulfiqar Bhutta began the discussion with an overview of nutrition, immune function, inflammation, and infant health from a global perspective (37) followed by an overview of the current understanding of the relations between nutrition, the microbiome, gastrointestinal immunity, and inflammation (38). In addition to providing coverage of the extant knowledge regarding these relationships, Dr. Duffy (38) highlighted some seminal questions for future attention including 1) can functional “meta-omics” approaches lead to informed understanding of microbial roles in digestion and metabolism, 2) will understanding how foods shape the microbiota lead to healthier lifestyles across generations, and, 3) what roles will the biological action of small molecules produced by probiotic strains play in health and disease?
The following session addressed the converse of the relation, i.e., the impact of inflammation on nutrition. The opening presentation was provided by Dr. Patrick van Rheenen, who covered the clinical utility of currently available biomarkers of gut wall integrity. He provided insights about the general relations between malnutrition, gastrointestinal integrity, and inflammation. This was followed by a discussion of the utility of 3 specific biomarkers in that context: measurement of citrulline as a reflection of intestinal integrity (39, 40); intestinal FA binding protein (I-FABP) (41), also a marker of gastrointestinal integrity; and fecal calprotectin, a potential early signal for intestinal inflammation (42).
Clinical implications of the relations between nutrition and metabolic correlates of inflammation were the subjects of the presentation by Dr. Liza Makowski (43). Dr. Makowski and colleagues explored the interactions between oxidative stress, inflammation, and such clinical outcomes as obesity and diabetes. Their article is a summary of their work indicating that certain obesogenic dietary patterns drive a tissue-specific increase in oxidative damage that may affect functionality. Their animal models offer potential mechanisms by which oxidative stress via activation of inflammatory responses can affect insulin signaling, glucose intolerance, and diabetes (43).
As outlined above, the goals of the INSPIRE initiative are to present the extant knowledge with regard to what we know about the relations between nutrition, immune function, and inflammation but also to evaluate best ways to translate that information into practical application. The session on translation included 2 presentations that addressed the latter.
In the field of clinical nutrition, much attention has been generated by the observations linking inflammation to performance and the interpretation of available biomarkers used to assess of specific nutrients (25). One of the leaders in the effort to address this conundrum is Dr. David Thurnham who provided an overview of the state of the science and strategies for addressing this complex dilemma in nutrition (25). The second talk in this session was a discussion of mathematical/statistical approaches to addressing inflammation/nutrition provided by Dr. Ravi Varadhan.
One of the highlights of all of the presentations was a discussion of the exciting new developments in approaches to studying these complex interactions. The final session of the workshop included presentations that highlighted some of these new innovative approaches. Beginning with an overview by Ben van Ommen of how best to apply systems biology to the question of nutrition and inflammation, the session concluded with coverage of the current understanding of proteome dynamics presented by Dr. Mark Hellerstein. These presentations and the subsequent deliberations were seminal in stimulating discussions about the application of these new technologies to the study of nutrition and inflammation and to reinforce the notion that the evaluation of the role of nutrition must be conducted in a more physiologic context that moves away from the “siloed” single-nutrient approach that has predominated the field to date. The deliberations of the last WG included listing a series of core questions that might be addressed by using a “systems biology” approach outlined in Text Box 4.
Text Box 4 . Core questions that “systems biology” could address.
● How can “systems biology” be used to better understand the role of single and multiple micronutrients in such outcomes as growth, neurological function, or immunocompetence?
● How can a better understanding of these roles be exploited to identify sensitive and specific biomarkers of nutrient exposure, status, and function and the effect of nutritional interventions to address these functional domains?
● Would knowledge gained from this improved understanding have equal value/utility for clinical and population-based applications?
● Within a given biological system, what biomarkers might be identified and exploited to assess the function role and effect of micronutrient insufficiency/excess?
● What is the mechanism by which single- or multiple-micronutrient deficiency/excess affects body function, metabolism, and particularly the innate or adaptive immune responses?
● Why do some micronutrients (either singly or in combination) have beneficial effects whereas others do not?
● With regard to the immune system/nutrition interface, how can the role of micronutrients in immune systems be exploited to explain the impact of inflammation and the immune response on nutrition and vice versa?
● What is the appropriate level of nutrient supplementation to support healthy immune and inflammatory responses?
To move this agenda forward, this WG described a 2-step process that includes the following:
● Research phase focusing on the development of the systems “model” that can utilize existing and emerging approaches including various “-omics” approaches (proteomics, genomics, metabolomics) and computational systems approaches [the WG highlighted the need to explore relations at various levels of complexity and integration (e.g., intracellular, local tissues, and systemic, including signaling processes] and can develop a complex bioinformatics infrastructure. The essential elements of this infrastructure consist of 3 essential components:
○ a knowledge database storing the interactions and pathways of interest, with an ability to apply bioinformatics and visualizing tools for analyzing data at the network level,
○ specific metadata attached to each analyzed sample describing the subject and his or her health status for investigating large human data sets, and
○ a way of integrating all relevant data sets for subsequent meta-analyses.
● Translation phase:
○ Move the result of research to application to support nutritional practice and health care. The overarching goal is the delivery of a relatively limited set of quantifiable variables in accessible body fluids that are either key regulators of the mechanisms involved or biomarkers of the studied processes.
○ Ideally, the novelty and advantage of the systems approach are that it takes into consideration the complex context within which nutrition and health outcomes must be evaluated and treated (i.e., it includes consideration of all relevant aspects of the individual including nutrient status, health status, environmental factors, etc.).
○ Such models and diagnostics subsequently need to be applied in practical health care and dietary advice settings, both in developed and developing countries.
The challenge will be to develop and implement infrastructures and on-site facilities, including point-of-care applications that allow the use of all relevant diagnostics and biomarkers for the translation of the systems research performed in a high-tech laboratory setting and ultimately a field operation setting. In addition to the requisite diagnostic tools that evolve from the research, it will be critical to have local infrastructure, including essential technical capacity and resources to support implementation at both point-of-care and eventually population levels to support the creation and implementation and evaluation of evidence-based public health programs.
PART II: WG REPORT SUMMARIES
The following are reports summarizing the deliberations of the 4 WGs. The INSPIRE Secretariat drafted these reports on the basis of the deliberations of each of the WGs, with the goal of presenting a progression of thought beginning with an overview of the basic biology describing the relations between nutrition, immune function, and inflammation (WG 1), followed by overviews of the bidirectional nature of these relations [i.e., the impact of nutrition on immune function/inflammation (WG 2) and the impact of inflammation, both acute and chronic, on nutrition (WG 3)], and concluding with a focus on the translation of the extant knowledge to develop some potential approaches to addressing these relations specifically as they pertain to clinical/population-based assessment (WG 4). Accompanying each summary is a list of research priorities identified by the WGs. The INSPIRE organizers and Secretariat gratefully acknowledge the efforts and input of the WGs in the development of these reports.
A. WG theme 1: overview of immune function, inflammation, role of nutrition, and considerations for further study
Objective: The objective was to review the basic biology that characterizes the relations between nutrition, immune function, and inflammation and the relative strengths and weaknesses of current approaches to research. WG 1 summary and conclusions are provided in Text Box 5.
Text Box 5 . WG 1 summary and conclusions.
● A large body of literature documents the interactions between nutrition and the immune and/or inflammatory response.
● The nexus of much of this interaction is within the constellation of immunologic/neuroendocrinologic responses to stress referred to as the “APR.”
● The APR has profound consequences that affect both metabolism and nutritional homeostasis and the ability to assess nutrient status vis-á-vis concentrations of biomarkers mobilized during an APR.
● Specific immune and inflammatory pathways that are affected by single nutrients remain largely undefined, which limits the ability to translate the evidence into clinical use.
● Factors contributing to this inability are reviewed and include the following:
○ choice or choices of species used to model human responses,
○ difficulty in defining human nutrient status in clinical settings due to confounding effects of the APR,
○ the concurrent presence of multiple nutrient deficiencies, and
○ an emerging appreciation of interactions with the microbiome.
● This review includes information on the role of specific case study nutrients (vitamin A, vitamin D, zinc, iron, selenium, folic acid) in dependent pathways.
Conclusions: Some useful consensus exists between animal and human models as well as human-specific pathways. Suggestions are offered for a focused research agenda that includes an expanded exploration of the effects of genetics and epigenetics in these relations.
I. Introduction.
Much is known about the intimate and inextricable role of nutrition in immunity and/or inflammation (44); however, few studies describe nutrient-mediated immunomodulation in humans. This review contains an overview of several key elements of the nutrition-inflammation nexus including the following: an examination of the roles of specific micronutrients and their interactions in dependent pathways, the potential effects of genetics and epigenetics in determining the effects of nutrition on immunity and inflammation, and coalescing all of this knowledge into a description of the nature and impact of the APR. This overview is intended to provide context for the subsequent discussions by the other WGs involved in the INSPIRE review and concludes with a summary of suggested research priorities. The case study nutrients covered in this review are vitamin A, iron, zinc, vitamin D, folic acid, and selenium and were chosen on the basis of their public health importance and because they provide the most relevant evidence with regard to their known effect on immunity.
II. Innate immune and barrier function.
Innate immunity, also referred to as nonspecific immunity, is the first line of defense against invading pathogens and consists of anatomic barriers and humoral and cellular components (Text Box 6).
Text Box 6 . Components of the innate immune system.
● Anatomic barriers include the following:
○ skin
○ mouth and nose
○ gastrointestinal tract
○ respiratory tract
○ eyes
● Inflammation: stimulated by infection/injury involving the release of chemical mediators to initiate the APR, etc.
● Physiologic barriers include the following:
○ temperature
○ pH
○ oxygen tension
○ production of proteins such as lysozyme, complement and antimicrobial components of the APR such as C-reactive protein (CRP), serum amyloid A (SAA), α1-acid glycoprotein (AGP), cathelicidins, and defensins
● Complement system: responses that support both innate and adaptive/cellular immunity and consists of >30 membrane-bound and soluble plasma proteins, is activated in a cascade-like manner, and is responsible for the detection and removal of foreign entities/pathogens or marking them for attack (45, 46)
● Cellular components of the innate immune response:
○ mast cells
○ phagocytes, including macrophages, neutrophils, dendritic cells
○ basophils and eosinophils
○ NK cells
○ γδ T cells
Tables 1 and 2 summarize data from animal and human models, respectively, with regard to the impact of specific nutrients on aspects of innate immunity. The key points from animal studies include the following:
TABLE 1.
Strength of evidence: impact of micronutrient deficiencies on nonspecific or innate immunity (animal studies)1
| PEM deficiency | Vitamin A deficiency | Vitamin D deficiency | Zinc deficiency | Iron deficiency | Folate deficiency | Selenium deficiency | |
| Epithelial barrier function | ++++ | ++++ | — | +++ | + | + | + |
| Physiologic barriers | ++++ | ++ | ++ | + | + | — | + |
| Macrophage function | ++++ | ++ | +++ | + | + | + | +++ |
| Neutrophil function | +++ | +++ | + | ++ | ++ | ++ | +++ |
| NK cell function | ++ | +++ | + | ++ | ++ | ++ | −/+ |
| NK T cell function | + | ++ | +++ | ? | ? | ? | ? |
PEM, protein-energy malnutrition; −/+, mixed evidence; +, weak evidence; ++, partial evidence; +++, good evidence; ++++, strong evidence; ?, no studies.
TABLE 2.
Strength of evidence: impact of micronutrient deficiencies on nonspecific or innate immunity (human studies)1
| PEM deficiency | Vitamin A deficiency | Vitamin D deficiency | Zinc deficiency | Iron deficiency | Folate deficiency | Selenium deficiency | |
| Epithelial barrier function | ++++ | ++++ | — | +++ | + | ++ | ? |
| Physiologic barriers | ++++ | ++ | + | + | + | — | ? |
| Macrophage function | ++++ | + | + | + | + | ? | ? |
| Neutrophil function | +++ | ++ | + | ++ | +++ | ++ | ? |
| NK cell function | + | + | + | ++ | — | ? | −/+ |
| NK T cell function | ? | + | ? | ? | ? | ? | ? |
PEM, protein-energy malnutrition; −/+, mixed evidence; +, weak evidence; ++, partial evidence; +++, good evidence; ++++, strong evidence; ?, no studies.
● Protein-energy malnutrition (PEM) has its largest effect in reducing barrier function, both epithelial (47–52) and physiologic (53–55), followed by the function of macrophages (56–59), neutrophils (60–65), and to a lesser extent, NK cell activity (66).
● Vitamin A and zinc deficiencies have effects similar to PEM (67–77), with the exception that NK cell (but not macrophage) function is reduced by VA deficiency (VAD) (78, 79). Physiologic barrier function is not markedly affected by either vitamin A or zinc.
● Vitamin D deficiency (VDD) has modest effects on innate immunity, with the greatest effect seen on macrophage function (80–86).
● ID primarily affects neutrophil function with less significant effects on macrophage bacteriocidal function (see “Potential Points of Interaction between Micronutrients Involved in Dependent Pathways” below).
The key points from human studies include the following:
● PEM has the largest effect on aspects of innate immunity than do any of the nutrients covered. PEM reduces epithelial (87–92) and physiologic (93–97) barrier function as well as the function of macrophages (95, 98) and neutrophils (62, 99–101). NK cell activity is also affected to a lesser extent (66).
● VAD is primarily manifested through compromised epithelial barrier function (67, 102–105) with a minimal effect on NK cell activity (105–107).
● Zinc deficiency (ZnD) primarily compromises epithelial barrier (108), macrophage (95), and neutrophil function (109–111).
● VDD in humans has a modest effect on innate immunity, with the exception of macrophage function and production of cathelicidins and defensins (112).
● ID primarily affects neutrophil function.
As a result of increased interest in the activities of vitamin D beyond the traditional focus on bone/calcium, an important new understanding of vitamin D’s role in immune function has emerged. Liu et al. (113) described a vitamin D–dependent pathogen-sensing axis in human macrophages that highlights the importance of vitamin D in innate immunity. They also described a potential disparity in this response that could explain differences in susceptibility to infections on the basis of racial/genetic background. In essence, they described an innate immune response involving activation of Toll-like receptors (TLRs) to trigger direct antimicrobial activity against intracellular bacteria that is mediated by NO in murine models but not in humans. In this newly observed model in human macrophages, TLR activation upregulates the expression of the vitamin D receptor (VDR) and the Vitamin D-1-hydroxylase genes, leading to induction of the antimicrobial peptide cathelicidin and killing of intracellular bacteria such as Mycobacterium tuberculosis. This is a primate-specific response (114) and is greatly influenced by cytokines such as IL-4 and IFN-γ. Initially, these observations were restricted to monocytes and macrophages; however, recent studies showed that cathelicidin antimicrobial peptide (CAMP) is induced by vitamin D in keratinocytes and respiratory and bladder epithelial cells (113). These data are derived from cell culture models. In vivo data are emerging that support vitamin D–dependent induction of cathelicidin in skin and peripheral blood monocytes by administration of large amounts of vitamin D to humans (115).
III. Acquired immune function: animal and human studies.
The basic characteristics of the acquired immune system are outlined in Text Box 7.
Text Box 7 . Acquired or adaptive immunity.
● Is not inherited
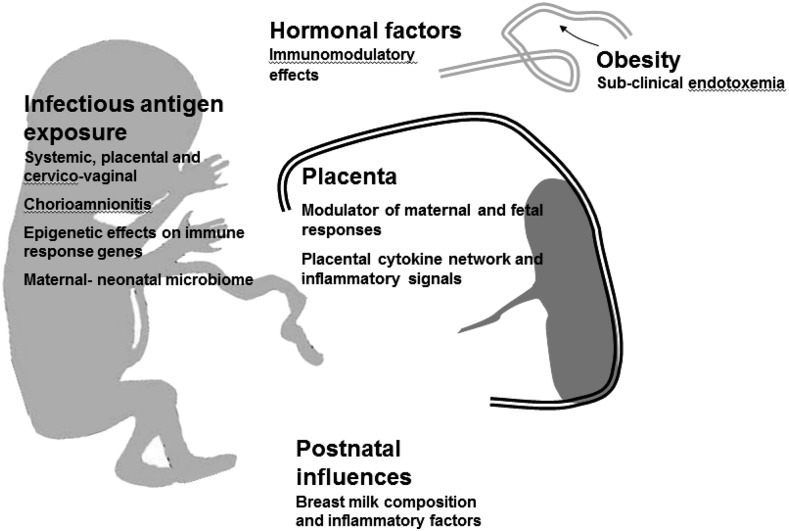
● Can be active, resulting from the development of antibodies in response to an antigen, as from exposure to an infectious disease or through vaccination, or passive, resulting from the transmission of antibodies, as from mother to fetus through the placenta, via breastfeeding or by the injection of antiserum
● Components of the acquired immune system include T and B lymphocytes that are derived from the same hematopoietic stem cells in bone marrow and morphologically indistinguishable until after they are activated
Antigen-presenting cells
To distinguish between “self” and a foreign or deranged cell, certain cells, e.g., dendritic cells, B cells, and to a lesser extent, macrophages, are equipped with special “costimulatory" features that are recognized by receptors on T cells
B cells
● Function in the humoral immune response
● Are the source of immunoglobulins or antibodies, the function of which is to identify and neutralize foreign objects: the 5 types of antibodies are IgA, IgD, IgE, IgG, and IgM
T cells
● T precursors (or progenitors) migrate from the bone marrow to the thymus where they are called thymocytes and where they develop into T cells
● Cytotoxic T cells [TCs, killer T cells, or cytotoxic T lymphocyte (CTLs)] induce the death of cells that are infected with viruses (and other pathogens) or are otherwise damaged or dysfunctional
● Helper T cells [cluster of differentiation (CD) 4+ lymphocytes] are not cytotoxic but facilitate those processes via expression of T cell receptors (TCRs) that recognize antigens; CD4 cells require a smaller stimulus for activation than cytotoxic T cells
● The activation of a naive helper T cell causes it to release cytokines, which influence the activity of many cell types, including the antigen-presenting cells that activated it
● Helper T cells can provide extra signals that “help" activate cytotoxic cells
a. NF-κB signaling and induction and maintenance of T-regulatory cells.
NF-κB function and mechanism of activation are described in Text Box 8.
Text Box 8 . NF-κB function and mechanism of activation.
Function
● Serves as a major transcription factor that coordinates the innate and adaptive immune response
● NF-κB binding is necessary, but not sufficient, to regulate the expression of several thousand genes including the proinflammatory cytokines IL-1β, TNF, and IL-6 (116)
Mechanism
● NF-κB is activated upon ligation of the T cell receptor, B cell receptor, or one of the many TLRs as well as in response to proinflammatory cytokines, mitogens, growth factors, and oxidants
● Activation of NF-κB is a multistep process involving the following:
○ degradation of inhibitory subunit [most commonly nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha(IκBα)], leading to
○ “activation” of a 2-protein complex [most commonly p50 Nuclear factor NF-kappa-B (NFKB1)/p65 rel avian reticuloendotheliosis (RELA)].
This complex migrates to the nucleus and binds to DNA in the promoter region of genes, and initiates transcription.
i. Nutrients and NF-κB signaling.
Several hundred mediators of NF-κB signaling pathways have been described in the literature (117), including almost all essential nutrients and >100 bioactive food components. Some specific effects of nutrients include the following:
● Vitamin A: increased NF-κB occurs during experimental VAD in mice (118) that is normalized by all trans retinoic acid (ATRA) administration. Mechanistic evidence for in vitro vitamin A–inhibited basal and LPS, TNF-induced NF-κB activation (119) includes
○ retinoic acid receptor (RAR) α–mediated reduction in p65 protein (118) and
○ ligand-dependent cytoplasmic sequestration of p50/p65 by retinoid X receptor (RXR) (120).
● Vitamin D: mechanisms of vitamin D–mediated inhibition of NF-κB signaling include:
○ inhibition of v-rel avian reticuloendotheliosis viral oncogene homolog (Rel) transcription by competitive binding of VDR/RXR binding to the Rel promoter (121),
○ induction of IκBα (122) and inhibition of IκBα degradation (123), and
○ hyperactivation of NF-κB occurs in VDR knockout mice (124).
● Zinc: it can inhibit or stimulate NF-κB signaling in vitro depending on the cell type and stimulus used (125). The following zinc effects have been reported:
○ Hyperactivation of NF-κB occurs in zinc-deficient mice in response to sepsis (126).
○ In humans, the generation of TNF by peripheral blood mononuclear cells (PBMCs) in response to LPS is reduced by short-term zinc deprivation.
○ Bioinformantics analysis indicated that NF-κB binding to its cognate element is reduced by ZnD (127).
○ IL2 gene expression is decreased in zinc-deficient humans (128). Interestingly, this relation and its correction are proposed as a potential biomarker for ZnD in a manner analogous to some of the classic enzyme stimulation assay for vitamins (vitamin B-6, thiamin, and riboflavin). However, due to the lack of specificity (i.e., IL-2 expression is affected by several other nutrients), this is unlikely to assume a significant role as a specific zinc biomarker.
● Vitamin E: inhibition of NF-κB by such antioxidants as vitamin E has been shown to occur via multiple mechanisms (129).
b. Induction and maintenance of T-regulatory cells.
T-regulatory (Treg) cells play an integral role in self-regulatory processes through their involvement in both shutting down immune responses after successful elimination of pathogens and in preventing autoimmunity (130, 131). Members of the Treg cell family are numerous, but the best-characterized are those that express CD4, CD25, and forkhead box P3 (FoxP3). Again, specific nutrients have been implicated in various aspects of Treg activity.
i. Role of vitamin A in Treg activity.
Mouse gut dendritic cells generate ATRA in response to specific stimuli such as TLR2 ligands (132). This locally generated ATRA imparts gut homing specificity to T and B cells. ATRA is also essential for the generation of gut-homing FoxP3-expressing Treg cells (133). The generation of ATRA was originally thought to be restricted to gut dendritic cells. However, recent data indicate that other cytokines, such as IL-3 or IL-4, stimulate such other cells as macrophages (134), basophils (135), and stellate cells (136) to produce ATRA, leading to the generation of FoxP3-expressing Treg cells in tissues such as lung and liver.
The induction and maintenance of Treg cells by ATRA is extremely complex, and >200 studies, most using mice, have been published to date. Certain aspects of this process are species-specific. For example, the FoxP3 protein, the master regulatory transcription factor in determining Treg differentiation, is transiently induced upon human, but not mouse, T cell activation (137). The mouse FoxP3 gene contains 3 functional retinoic acid response elements (RAREs), 2 in the promoter region and 1 in the enhancer region (138). Although treatment of human T cells with ATRA or with an RAR-α agonist induces histone acetylation at the FoxP3 gene promoter and FoxP3 expression (139), demonstration of an RARE in human FoxP3 has not been reported to date. Last, only 18% of the nearly 5000 predicted FoxP3 DNA-binding sites are conserved between species (140).
ii. Role of vitamin D in Treg activity.
The mouse and human FOXP3 promoters also contain functional vitamin D response elements (VDREs) (141, 142). Increased FOXP3 expression has been reported after in vitro treatment of human and mouse T cells with 1,25-dihydroxyvitamin D [1,25(OH)2D]. The situation in vivo may be more complex, because mice with a T cell–targeted knockdown of the VDR manifested no change in the frequency or function of peripheral FoxP3+CD4+ T cells. These observations suggest a dominant influence on Treg induction by non–T cells (143). In vivo data for vitamin A– and vitamin D–dependent induction of Tregs in humans are emerging but are extremely difficult to establish methodologically because of the likely need to obtain biopsied materials (144–146).
iii. Role of other nutrients in Treg activity.
In addition to vitamins A and D, other nutrients may affect the induction of Tregs. Tregs in mice express high levels of the folate receptor (147), and gut Treg induction depends on dietary folate (148). The zinc finger domains of FOXP3 are required for its function, and approximately one-half of the transcription factors that regulate FOXP3 contain zinc. However, zinc-deficient animals have normal numbers of Tregs (149).
c. Nutrients and the acquired immune system.
As outlined in Table 3 and documented in Supplemental Tables 2–8, studies in animal models have revealed the following:
TABLE 3.
Strength of evidence: impact of micronutrient deficiencies on cell-mediated and humoral immunity (animal studies)1
| PEM deficiency | Vitamin A deficiency | Vitamin D deficiency | Zinc deficiency | Iron deficiency | Folate deficiency | Selenium deficiency | |
| Antigen-presenting cell function | ++ | ++ | ++ | ++ | ++ | ? | ? |
| T cell function | ++++ | +++ | ++ | +++ | ++ | ++ | +++ |
| B cell function | ++ | +++ | + | +++ | + | ? | ++ |
PEM, protein-energy malnutrition, −/+, mixed evidence; +, weak evidence; ++, partial evidence; +++, good evidence; ++++, strong evidence; ?, no studies.
● PEM and deficiencies of vitamin A, zinc, or iron have a robust, albeit variable effect in reducing antigen-presenting cell (APC) function (150–160).
● PEM and vitamin A, zinc, and iron deficiencies exert their greatest effect on reducing CD4+ T cell function (79, 161, 162). Proliferation is often reduced, and polarization to a particular antigen is reduced/altered (163–165).
● VAD causes reduced trafficking of T and B cells to tissues (158, 166, 167).
● CD8+ T cell function is the least studied of the major components of acquired immunity.
● VDD has a modest effect on cell-mediated and humoral immunity with the greatest effect seen on APC and T cell function (85, 168–173). In particular, NK T cell development in mice is critically dependent on vitamin D (173).
The data from human studies, presented in Table 4, reveal the following:
TABLE 4.
Strength of evidence: impact of micronutrient deficiencies on cell-mediated and humoral immunity (human studies)1
| PEM deficiency | Vitamin A deficiency | Vitamin D deficiency | Zinc deficiency | Iron deficiency | Folate deficiency | Selenium deficiency | |
| Antigen presenting cell function | + | ? | ? | ? | ? | ? | ? |
| T cell function | +++ | ++ | + | ++ | ++ | ++ | ++ |
| B cell function | ++ | ++ | + | + | + | ? | ++ |
PEM, protein-energy malnutrition; +, weak evidence; ++, partial evidence; +++, good evidence; ?, no studies.
● Only PEM has evidence attesting to an effect in reducing human APC function (174).
● PEM and vitamin A and zinc deficiencies have their largest effect in reducing CD4+ T cell function (98). There are suggestions of reduced proliferation for all 3 and modest evidence of alteration in polarization for VAD and supplementation (106).
● VDD has a relatively minor effect on cell-mediated and humoral immunity in humans, with the greatest effect seen on APC and T cell function (175, 176).
● ID primarily affects CD4+ T cell function, with a lesser effect on B cell function and no conclusive evidence with regard to its impact on APCs (177–181).
● Megaloblastic anemia due to folic acid deficiency has been associated with depressed cell-mediated immunity (182).
Parenthetically, the implications of the effect of PEM, vitamin A, zinc, and iron on CD4+ T cell counts are important in the context of interpreting the impact of nutritional interventions in conditions such as HIV in which CD4+ T cell counts are used as a primary outcome. Because we appreciate that nutrient deficiency in itself affects CD4+ T cell count, we must design studies that can distinguish between a disease (e.g., HIV pathogenesis or treatment) effect and the impacts of malnutrition or its amelioration. For example, a study that evaluates the impact of a single or multiple micronutrient intervention on HIV might include an HIV-negative control to be able to distinguish between an HIV-specific effect and the impact of ameliorating nutritional insufficiency. This distinction has obvious biological and programmatic/public health implications.
Among the limitations of the extant evidence is that most of the research to date has focused on the effect of single-nutrient deficiencies on immune response. Few studies examined the simultaneous association of multiple nutrients, or their status, with immune function (183, 184). The design of such studies in humans is challenging for several reasons, including
● our relatively limited understanding of the nature of micronutrient interactions within biological systems,
● the complexities of the health context in low- to middle-income settings where multiple-micronutrient insufficiency may be common but often coexists with multiple other conditions contributing to both acute and chronic inflammation, and
● low prevalence of multiple-micronutrient deficiencies in otherwise healthy participants in developed countries; when they do exist in certain subgroups such as adolescents, certain racial groups, or the elderly, confounders such as age, disease, etc., further complicate experimental design and data interpretation.
d. Potential points of interaction between micronutrients involved in dependent pathways.
Several points of interaction among the various micronutrients seem likely and are outlined in the accompanying Text Box 9. For the purpose of this overview, the general molecular actions of 4 micronutrients (vitamins A and D, zinc, folic acid) will be the primary focus, including their specific points of convergence on the regulation of 2 major regulators of inflammation: NF-κB activity and the induction, and maintenance, of Treg cells.
Text Box 9 . Potential pathways of intersection for micronutrients.
● Myelomonocytic cell differentiation (vitamins A and D)
● Macrophage polarization (vitamins A and D, zinc, folic acid)
● B and T cell proliferation (zinc, iron, folic acid, others)
● T cell apoptosis (zinc, iron, vitamin A, vitamin E, others)
● Regulation of NF-κB activity (vitamins A, D, and E; zinc; iron; many others)
● Induction, and maintenance, of Treg cells (vitamins A and D, zinc, folic acid)
● Regulation of gene expression and/or activity of protein kinase C, cyclins, and cyclin-dependent kinases (e.g., as seen with iron): such defects in one or more of these factors may affect or modulate lymphocyte responses to stimuli
IV. Role of specific micronutrients in the immune system.
Tables 1–4 summarize the extant knowledge with regard to the role of PEM and micronutrient deficiencies in immune function (more details are shown in Supplemental Tables 2–8). Particular attention was devoted to studies that identified mechanisms at the cellular or molecular level. The following is a brief summary highlighting key aspects of these relations. Particular attention is given to those aspects for which congruity exists between findings from animal and human models to inform the discussion of well-characterized human-specific pathways.
a. Mechanisms of vitamin A effect on the immune system.
Most of the effects of vitamin A on immune or inflammatory responses can be explained via binding of the vitamin A metabolite, ATRA, to 1 of 3 zinc-finger proteins containing members of the nuclear receptor superfamily, RAR-α, RAR-β, and RAR-γ (185). These function as ligand-dependent transcriptional regulators by binding, usually as heterodimers to RXR-α, -β, and -γ and to RARE in target genes. Apo (unliganded) RAR can also occupy regulatory elements at their target genes and repress their expression. Ligand-dependent repression of gene expression by RAR can also occur. Recent estimates, in various human cell lines, indicate that RARs are constitutively bound to ∼500 genomic sites, and ATRA treatment induces RAR binding to 500–600 DNA sites (186).
The number of genes actually regulated in this manner is unknown. It has been >10 y since the last large-scale survey was done. At that time, the estimated number of genes directly regulated by retinoic acid was very large (187), whereas the number of direct targets was fewer. Although new data are needed to further elucidate the magnitude of these relations, it is clear that vitamin A has multiple genetically mediated effects on the immune response via the activities of ATRA.
b. Mechanisms of vitamin D effect on the immune system.
Although some effects of vitamin D are mediated independently of VDR, the large majority of the activity of vitamin D can be explained via binding of the vitamin D metabolite [1,25(OH)2D] to a single, zinc-finger containing, ligand-activated transcription factor, VDR. The heterodimeric partner, RXR, is required for transcriptional activity. The VDR/RXR complex binds to specific sites within the promoter region of genes known as VDRE and activates transcription (188).
The presumed number of genes containing a VDRE and/or regulated by VDR/RXR is very large. Recent human data suggest that VDR can bind to >2700 sites in the genome and directly regulates >200 genes (189). Like vitamin A, vitamin D can affect immune function through this genetic interaction. Because vitamins A and D share a nuclear receptor (RXR), compete for coactivators and corepressors, and reciprocally regulate the expression of their receptors, a wide variety of combined effects on gene regulation likely occurs (190–192).
c. Mechanisms of the zinc effect on the immune system.
The molecular actions of zinc appear to be more complex than those of vitamins A and D. In humans, the zinc-containing proteome contains <3000 members (193). More than 10% of this proteome encodes enzymes, including alcohol and aldehyde dehydrogenases, involved in vitamin A metabolism (193). Zinc is also necessary to maintain the structural integrity of proteins such as zinc-finger containing transcription factors (194). Approximately 50% of all transcription factors, including the vast majority of transcription factors involved in leukocyte lineage commitment (T cell vs. B cell commitment, CD4 vs. CD8 lineage commitment, Treg commitment) and effector function, contain zinc (195). Furthermore, 477 members of the poly-zinc-finger (poly-ZF) family of putative transcriptional repressors in humans (330 in mice) involved in epigenetic gene silencing contain zinc (195).
The ability of free zinc to act as an intracellular signaling molecule, similar to calcium ion (Ca2+), was recently described (196). An appreciation of the molecular complexities of zinc is just emerging. It will be essential to determine what the hierarchy of functional pathway maintenance is during ZnD.
d. Mechanisms of folic acid effects on the immune system.
The molecular actions of folic acid are also extraordinarily complex. Folic acid, along with other nutrients involved in one-carbon metabolism (e.g., methylcobalamin, choline, betaine, and methionine), is required for the synthesis of DNA and S-adenosylmethionine (SAM)–dependent methylation of DNA and histones. The number of specific sites [specifically sites on DNA where cytosine and guanine are separated by a phosphate; referred to as cytosine-phosphate-guanine (CpG) sites] in the genome that are potential targets for methylation is staggering. The recent Encyclopedia of DNA Elements (ENCODE) project has identified 1.2 million putative CpG methylation sites in the human genome (8.6% of nonrepetitive genomic CpGs) (197). The methylation pattern is unique to each cell type. The number of genes regulated by histone methylation is similar to that regulated by DNA methylation, and the pattern of histone methylation is unique to each cell type (198). The roles of folate in methylation-dependent DNA modifications will be covered in greater detail in the following section on epigenetics.
V. Genetics and epigenetics of inflammation: role of nutrition.
Genetic variations/polymorphisms and copy number variation have been shown to have potent effects on immune function and inflammation (199). These observations were extended into studies exploring the heterogeneity of responses resulting from nutrition intervention trials.
The copy numbers of a gene typically vary as a result of duplication of the portion of the chromosome that contains the gene. This accounts for ∼12% of variation found in the human genome (200). In addition to established associations between genetic variations of inflammatory cytokine genes and increased circulating levels, other genetic variation such as changes in copy number can modulate inflammation. To our knowledge, there are no studies that examined the effect of copy number variation on the effect of nutrition on immune function or inflammation, but this could be a fertile area for future research.
a. Epigenetics.
Understanding the role that epigenetics plays in determining the plasticity and heterogeneity of human immune responses is essential for planning and interpreting data generated by nutrition intervention trials. Basic principles regarding epigenetics and immunity/inflammation are highlighted in the accompanying Text Box 10. In the following section, we will consider the role of nutritionally regulated epigenetic control of genes involved in immunity and/or inflammation.
Text Box 10 . Basic principles of epigenetics.
● Epigenetic modifications that lead to gene expression alterations involve transmitting heritable modifications without DNA base pair changes.
● Epigenetics play a role in determining immune responses to various organisms.
● Epigenetic changes can persist postmitotically or transgenerationally.
● DNA methylation is considered to be the most permanent form of epigenetic regulation and is associated with transgenerational effects, including genomic imprinting.
● Major epigenetic mechanisms include the following:
○ Methylation of CpG islands found in the regulatory regions of DNA. The number of CpG sites in the genome that are potential targets for methylation is very large (197).
-
○ Post-translational modification of nucleosomal histone proteins. Examples of histone modifications include:
■methylation of histone lysine and arginine
■ histone phosphorylation
■ histone ubiquitination
■ histone acetylation activity
○ Generation of noncoding RNA, such as long intergenic noncoding ribonucleic acids (lincRNA) (201) or microRNA (miRNA), that affect mRNA translatability or stability
Aside from the role of miRNA in immune development and function, and despite the surging interest in epigenetics in the past decade, surprising little is known about how immunity or inflammation is modulated via epigenetic mechanisms. The majority of epigenetic studies were conducted with the use of T cells and macrophages. Factors that control T cell development such as IL-2 are regulated via promoter demethylation in T cells (202). Naive CD4+ T cells differentiate into specific and stable T-helper (Th) cell phenotypes (including, but not limited to, Th1, Th2, Th17, and Tregs). Chromatin remodeling allows increased binding of specific factors to the regulatory regions of IFNG in Th1 cells or IL4 (203) in Th2 cells.
Few studies have addressed how nutrients play a regulatory role in altering immune function or inflammation via epigenetic pathways. A small but rapidly growing literature suggests that non-nutrient, bioactive food components may regulate epigenetic pathways. Claycombe et al. discuss these in a companion article to this supplement “Epigenetics of inflammation and immune dysfunction: the role of nutrition” (36). The following is a summary of the current knowledge about nutrient control of epigenetic events in general with a focus on immune or inflammatory responses.
b. Role of specific nutrients in genetics/epigenetics.
i. Role of vitamin A in epigenetic processes and the genetics of immune function.
Cell culture models and a very small number of human clinical studies indicate that nutritional factors including vitamins A and D affect numerous genes involved in chromatin remodeling or processes that are involved in epigenetic regulation.
Hypomethylation of hepatic DNA occurs in response to vitamin A treatment in rats (204), and hypermethylation of long interspersed element (LINE) 1 is found in peripheral blood white cells of vitamin A–deficient children (205). Genomewide DNA methylation assays demonstrated several hundred differentially methylated CpG sites between ATRA-treated human embryonic stem cells and neuroblastoma cells (206). The mechanisms for these phenomena are unknown, but ATRA induces glycine N-methyltransferase, an enzyme that regulates the provision of methyl groups. Because this mechanism has the potential to alter the global abundance of methyl donors, and because vitamin A has general actions on cell differentiation, it is unknown whether these effects are indirect or whether specific genes are targeted for epigenetic regulation by vitamin A in vivo. Emerging evidence suggests that both mechanisms are operative.
Substantial in vitro evidence exists for a role for ATRA in epigenetic regulation of stem cell or tumor cell differentiation via specific targeting of genes (Text Box 11). Evidence for effects of vitamin A on epigenetic regulation of immune function is emerging.
Text Box 11 . Epigenetic impact of ATRA.
● DNA sequences found within genes involved in the regulation of morphogenesis (known as “homeobox transcription factors”) are prototypical ATRA-regulated genes.
● ATRA causes the recruitment of the histone H3-K27 demethylase lysine (K)-specific demethylase 6A KDM6A/UTX to the promoters of the homeobox transcription factors HOXA13 and HOXB13 in human NTERA-2 clone D1 Cell Line human (NT2/D1) teratocarcinoma cells (207, 208), leading to a loss of H3-K27 methylated histone and gene transcription.
● The histone H3-K27 demethylase KDM6B/JMJD3is an ATRA-inducible gene in mouse neural stem cells, human HeLa cells, and human U2OS osteosarcoma cells (209).
● Treatment of neural stem cells with ATRA induced recruitment of KDM6B/JMJD3 to the promoter region of the homeobox transcription factor distal-less homeobox (5Dlx5) (209).
● ATRA-treatment was accompanied by binding of methylated H3-K4 to the promoter region and demethylated H3-K27 to the enhancer region of the RET gene in human neuroblastoma cell line (SK-N-BE) (210).
Because ATRA has potent growth inhibitory and differentiation actions on a wide range of leukemic cell lines, the majority of studies examined the epigenetic effects of ATRA on the differentiation of myeloid leukemia cells. Few studies have been conducted in primary cells. RARα physically associates with the H3-K4 histone methyltransferase-5 (MLL5) and promoter region of CCAAT/enhancer binding protein ε (C/EBPe), enhancing neutrophilic differentiation of the acute promyelocytic leukemia cell line HL-60 (211). Upregulation of several miRNAs occurs during ATRA-induced differentiation of the acute promyelocytic leukemia nuclear bodies (NB4) (212).
The generation of the bioactive form of vitamin A is under tight control. Studies in mice suggest that cytochrome P450 (CYP) 26A1, an enzyme involved in numerous aspects of drug metabolism, is also involved in ATRA catabolism. It is induced by ATRA and subject to a high level of epigenetic regulation. Promoter methylation may play a limited role in Cyp26a1 gene silencing in the absence of ATRA (213) but may account for lack of ATRA induction of Cyp26a1 in ATRA-resistant cells (214). In addition, high constitutive levels of methylated H3-K27 are associated with the promoter of the mouse Cyp26A gene in mouse F9 teratocarcinoma cells that are lost after treatment with ATRA (214).
Simultaneously, increased recruitment of acetylated H3-K9 and H3-K14 to the Cyp26A promoter occurs in response to ATRA treatment (214). Upon ATRA withdrawal, H3-K27 histone methylation occurs, which leads to polycomb repressive complex 2–mediated gene silencing of Cyp26a1 in mouse embryonic stem cells (215). The potential implications of these types of interactions on drug safety and efficacy has been discussed (216) and will be covered in further detail by WG 2.
ii. Role of vitamin D in epigenetic processes and the genetics of immune function.
Evidence is emerging for vitamin D in epigenetic regulation of gene expression. Some of this evidence is derived from models of tumor cell differentiation in vitro. KDM6B/JMJD3 histone demethylase is induced by vitamin D, but not ATRA, in human colon cancer cells (217) and has been implicated in the regulation of cyclin-dependent kinase inhibitor 1A (CDKN1A)/p21CIP1 by 1,25(OH)2D in normal prostate cells (218). Histone acetylation controls cathelicidin gene expression by vitamin D in keratinocytes (219). Vitamin D induces the expression of miRNA in different cell types. Plasma miRNA profiles have been determined in subjects given high-dose vitamin D, but the within-subject heterogeneity and small number of subjects precluded any determination of definitive vitamin D–regulated miRNA (220).
The essentiality of vitamin D in regulating invariant invariant NK T cell development in mice is thought to be via epigenetic mechanisms (173). There is other evidence for a transgenerational effect of vitamin D on immunity. Adult mice exposed to in utero VDD developed milder and delayed experimental autoimmune encephalomyelitis, but adult mice exposed to perinatal VDD developed more severe and earlier onset experimental autoimmune encephalomyelitis (221).
Like vitamin A, the generation and degradation of the bioactive form of vitamin D is under tight control. The promoter region of CYP27B1, the enzyme responsible for 1,25(OH)2D generation, is demethylated, and histones associated with the promoter deacetylated in response to vitamin D treatment in mouse kidney proximal tubule-derived mouse cortical tubular cells and the human embryonic kidney–derived cell line 293F (222). The induction of CYP24A1, the enzyme responsible for 1,25-dihydroxyvitamin D3 catabolism, by VDR is dependent on the expression of the histone demethylase KDM6B/JMJD3 (223). Inflammation can also affect the generation of 1,25-dihydroxyvitamin D3 via epigenetic mechanisms. The miRNA miR-125b, a positive regulator of inflammation in macrophages (224), negatively regulates VDR and CYP24A1 (224, 225). A greater association of KDM6B/JMJD3 to the Cyp24A1promoter gene and induction of Cyp24A1 mRNA occurs in response to LPS in mouse macrophages (217). These data suggest that inflammation leads to decreased VDR activity and increased vitamin D catabolism.
iii. Role of zinc in epigenetic processes and the genetics of immune function.
In addition to the zinc-dependent enzymes already mentioned, all class of enzymes involved in epigenetic regulation depend on zinc to varying degrees. All DNA methyltransferases, the Su(var)3–9, Enhancer of Zeste, Trithorax (SET) domain–containing histone methyltransferase family, and the great majority of histone demethylases are zinc-dependent (226). Similarly, the human [MOZ (monocytic leukaemia zinc finger protein), yeast Ybf2 (renamed Sas3, for Something about silencing 3) and Sas2 and mammalian TIP60 (HIV Tat-interacting protein 60 kDa)] (MYST) family of histone acetyltransferases and all members of the histone deacetylase superfamily, except for the sirtuins, are zinc-dependent.
The mechanism or mechanisms behind these phenomena are unknown. A recent study used miRNA profiling to identify miRNAs that were responsive to zinc depletion and repletion in humans (127). Other examples of some of the evidence with regard to the impact of zinc status on epigenetic regulation of gene expression are highlighted in Text Box 12.
Text Box 12 . Impact of zinc status.
● ZnD induces global DNA hypomethylation in vivo (227).
● In vivo evidence exists for an epigenetic component related to the immunosuppressive effects of high and low concentrations of zinc.
● Gestational zinc deprivation in mice leads to immunodeficiency that can be observed through 3 subsequent generations (228).
● Zinc supplementation of zinc-repleted pregnant rats suppresses immune functions in their offspring (229).
● Prenatal zinc supplementation of zinc-adequate rats also adversely affects immunity in offspring (230).
iv. Folic acid and methylation events related to immunity.
A methyl-donor–deficient diet fed to pregnant sheep led to immunologic changes, an increased APR (serum haptoglobin) and serum IgG to exogenously administered ovalbumin in male (but not female) lambs at 1 y postgestation (231).
v. Vitamin E.
Polymorphisms at cytokine genes may determine the effect of vitamin E on cytokine production in the elderly (232). Having a G allele at the position −174 of the IL6 gene results in increased IL-6 in the circulation, and changes from G to C decrease IL6 gene expression (233). In a similar manner, G to A substitution at the −308 position of the tumor necrosis factor α (TNFA) gene has been shown to increase TNF-α secretion (234) and a C-A substitution at position −863 in the TNF gene is associated with decreased circulating TNF concentrations (235). In addition, single nucleotide polymorphisms in the TNF gene and TNF secretion from PBMCs of elderly subjects who are supplemented with vitamin E were tested. Results showed that participants with the A/A and A/G genotypes at TNF 308G > A who were treated with vitamin E had significantly lower TNF production (232).
Variants in the genes encoding TNFA, IL10, and glutathione S-transferase pi 1 (GSTP1) influence the effect of α-tocopherol on inflammatory cell responses in healthy men (236). GSTP1 is one of the members of the glutathione S-transferase supergene family that has been suggested to play a role in the pathogenesis of cancer (237). By using PBMCs from healthy men who were treated with LPS, another study showed that anti-inflammatory effects of vitamin E supplementation (e.g., decreased IL-6 secretion) depends on having the GG genotype at position 313 of the GSTP1 gene (236).
Clearly much has been learned, at the basic biological level, about the processes of genetic and epigenetic regulation of immune function and inflammation. The clinical implications of these processes are just emerging, as is our understanding of the roles that individual or multiple micronutrients might play. The research agenda engendered by these enticing relations is discussed below.
VI. The APR and the study of nutrition-inflammation interactions.
The application of our basic understanding of the immune system and its implications for health are best understood by the phenomena that occur in response to many stressors, including microbial invasion, tissue injury, immunologic reactions, and inflammatory processes. The array of immunologic, neuroendocrine, and neurobiological responses is collectively referred to as the APR (238). Because of a diversity of stimuli, differential kinetics and magnitude, and other factors that can affect the APR, establishing a single unifying definition has been difficult. Because conditions that might contribute to an APR within given study designs/descriptions have not been consistently provided, the ability to gain a full appreciation of the impact of the APR on nutrition has been challenging; however, some general concepts are understood. General characteristics and manifestations of the APR are described in Text Box 13.
Text Box 13 . Characteristics of the APR.
● A coordinated response between neuroendocrine and immune systems
● Allows for controlled inflammatory response to support tissue repair and immune competence in response to a particular stressor
● The hypothalamic-pituitary-adrenal (HPA) axis and sympathetic/adrenomedullary system then provide rapid and adaptive counter-regulatory mechanisms critical for host survival by ensuring energy substrate mobilization, hemodynamic stability, and restoration of homeostasis to avoid the deleterious effects of prolonged inflammation (239)
● Corticosteroids and catecholamines are the major stress hormones that induce an APR; glucagon, growth hormone, and renin also contribute to a lesser extent
Mechanism of the neuroendocrine-inflammatory APR
● The APR is initiated by inflammatory cytokines (TNF, IL-1β, and IL-6), resulting in the stimulation of corticotropin (CRH) to increase corticosteroid and catecholamine production (240).
● The HPA exerts inhibitory effects on the innate immune system and inflammatory response, including a decrease in inflammatory cytokine production and modification of leukocyte trafficking and function (239).
● These stress hormones suppress Th1 cytokine production and stimulate Th2 cytokine production.
● A selective shift from a proinflammatory to an anti-inflammatory state occurs mediated by adaptive changes in Th1 and Th2 cytokine production.
● Glucocorticoids, norepinephrine, and epinephrine inhibit IL-1, TNF, IFN-γ, and IL-12 production and upregulate IL-10, IL-4, and TGF-β production (241).
● Glucocorticoids also inhibit chemokine secretion, including monocyte chemoattractant protein (MCP) 1 and IL-8 (242), and proliferation and differentiation of all immune cells, including dendritic cells and macrophages.
● Glucocorticoids and norepinephrine similarly inhibit the actions of NF-κB cells to suppress proinflammatory cytokine production (243).
● The sympathetic nervous system (SNS) innervates the main sites of the immune system and postganglionic fibers terminate in primary and secondary lymphoid organs.
● Epinephrine systemically regulates the immune system, whereas norepinephrine receptors are selectively expressed on Th1 cells (244).
Aside from the metabolic impact of the APR on substrate utilization and nutrient homeostasis, of particular relevance to the impact of the APR on nutrition are the changes in numerous plasma protein concentrations. Plasma concentrations of >200 proteins change in response to acute inflammation and are referred to as APPs (245) (the APP classification scheme can be found in Text Box 14).
Text Box 14 . Different classification schemes of APPs.
● Positive or negative: reflecting their respective increase or decrease in response to the APR
● Early, intermediate, or late: reflecting differential time course and differential magnitude of change in response to the APR
● Type 1 (stimulated by TNF and/or IL-1β, enhanced by IL-6) or type 2 (stimulated by IL-6) (246)
● A change of ∼25% in plasma concentration of a protein in response to inflammation has been suggested as the simplest criterion to define an APP (247).
Our ability to further explore the APR and characterize its impact is contingent on the models used to explore these relations. We learned what cytokines induce specific APPs, the function of specific APPs, and that the magnitude and duration of the APR can be species-specific. For example, CRP is a major positive APP in humans, pigs, and dogs, whereas SAA, serum amyloid P (SAP), and haptoglobin are the major APPs in mice (245). Human, but not rodent, CRP activates the classical pathway of complement in serum (248). Thus, care must be taken when choosing the species to model the human APR.
a. Nutritional/metabolic implications of the APR.
The APR and accompanying changes in APP concentrations can result in profound metabolic consequences with significant nutritional implications (Text Box 15).
Text Box 15 . Metabolic/nutritional impact of the APR.
● Decreased nutrient intake (anorexia, fever)
● Impaired nutrient metabolism including decreased nutrient digestion, accelerated endogenous losses of nutrients, and changes in nutrient transport and regulation)
The functional basis of these changes includes the following:
● Direct antipathogen activities (e.g., increased CRP, complement)
● Host-induced stressors that inhibit pathogens (e.g., fever and iron sequestration)
● Reallocation of resources to establish an effective immune response (e.g., mobilization of amino acids from muscle for use by hepatocytes and leukocytes)
Although evidence exists for extrahepatic synthesis of APPs, the majority are derived from hepatocytes in higher order vertebrates. Table 5 shows a survey of the most commonly encountered human APPs along with their putative functions. Recent evidence from proteomic studies in stress-challenged mice and humans suggests the existence of several hundred more proteins that might meet this definition (249, 250).
TABLE 5.
Function of the major acute phase reactants1
| Function | |
| Positive | |
| α1-Acid glycoprotein (AGP/ORM) | Opsonin |
| α1-Antitrypsin | Serpin |
| α1-Glycoprotein (SAP) | Opsonin |
| α2-Macroglobulin | Serpin |
| Angiogenin | Angiogenesis |
| Ceruloplasmin2 | Fe oxidation, Cu transport |
| Complement component 3 | Opsonin |
| Complement component 4 binding protein | Opsonin |
| Complement factor B | Opsonin |
| CRP | Opsonin |
| Ferritin2 | Fe sequestration |
| Fibrinogen | Coagulation |
| Gc globulin (vitamin D binding protein)2 | Complement activation, vitamin D transport |
| Haptoglobin2 | Fe sequestration |
| Hemopexin2 | Fe sequestration |
| Hepcidin antimicrobial peptide2 | Fe sequestration |
| Histidine-rich glycoprotein | Hemostasis |
| Lactoferrin2 | Fe sequestration |
| LPS binding protein | Bacterial pattern recognition receptor |
| Mannose-binding lectin (protein C) 2 | Complement activation |
| Metallothionein2 | Zn transport |
| Serum amyloid A1 | Chemoattractant |
| Serum amyloid A2 | Unknown |
| Transcobalamin?2 | Vitamin B-12 transport |
| von Willebrand factor | Hemostasis |
| Negative | |
| Albumin2 | Oncotic pressure, lipid transport |
| α2-HS-glycoprotein | Ca2+ transport? |
| Apolipoprotein A-I2 | Lipid metabolism |
| Retinol binding protein 42 | Retinol transport |
| Selenoprotein P2 | Se transport |
| Thyroxine-binding globulin | Thyroid hormone transport |
| Transcortin | Glucorticoid transport |
| Transferrin2 | Fe transport |
| Transthyretin2 | Thyroid hormone transport |
AGP, alpha 1 acid glycoprotein; CRP, C-reactive protein; Gc, group-specific component globulin; HS, Heremans-Schmid; ORM, orosomucoid; SAP, serum amyloid P; ?, possible.
Involved in nutrient transport.
b. Roles and impact of APPs.
Approximately 50% of the major APPs are involved in nutrient transport or regulation of nutrient status. This challenges the ability to distinguish between changes in nutrient status consequent to exposure vs. the response of a nutrient-related APP to an APR. It might also compromise efforts to implement and monitor an intervention effectively in a patient experiencing an APR, because the metabolic responses to the APR may, by their nature (i.e., a physiologic override of normal metabolic/nutritional processes), be intransigent to usual approaches to mitigation (e.g., use of a dietary supplement to increase concentrations).
Conversely, it is also the case that certain aspects of the APR are blunted in severely malnourished children (251–253). Another important consideration in this context is the impact of chronic stress on the APR and consequent metabolic problems. The latter scenario is exemplified by what we have learned about the impact of HIV infection on the inflammatory response and subsequent changes in metabolism that might account for some of the chronic untoward complications of that disease and its treatment (254–256). These types of disease-mediated chronic relations will be discussed in further detail by WG 3.
In addition to the broad impact of the APR cascade, APPs also play specific roles within the human body and are outlined in Text Box 16.
Text Box 16 . Putative roles of APPs.
● Effector molecules
● Regulating inflammation
● Nutrient transport
● Redistribution of substrates for the synthesis of positive APPs and maintenance of plasma oncotic pressure are 2 nonspecific mechanisms proposed for the change.
● Nutrient sequestration limiting access to pathogens or to limit oxidative damage:
○ iron
○ zinc
For example, vitamin D binding protein has multiple functions:
○ it binds 95% of vitamin D in plasma,
○ acts as a macrophage-activating factor and neutrophil chemoattractant, and
○ participates in the classical and alternative complement activation pathways (115).
○ During an APR, it circulates in the plasma at molar concentrations 20-fold higher than the total amount of bound vitamin D metabolites (258), indicating that its immunologic role may dominate its transport function during the APR.
In many ways, the response to stresses such as infections may be seen as paradoxical (i.e., having a negative impact on both host and pathogen). This view is particularly evidenced by the nutritional responses to infection (e.g., nutrient sequestration, anorexia) (257). To understand how this paradox is manifested and how it is intended to defend the host, it is useful to look at what is known about specific nutrients in this context.
Sequestration of nutrients to limit the growth and function of pathogens has been proposed as one reason for the redistribution of some nutrients during the APR. This is most evident for iron (259) but may also occur with zinc (260). Another intriguing possibility is that nutrient deprivation increases the level of stress on all host cells, causing those that are subsequently stressed by intracellular pathogens or trauma to die (257). Similarly, to limit host tissue damage, sequestration of pro-oxidant nutrients and a change in the antioxidant balance in favor of the host in response to reactive oxygen species (ROS) produced during the immune response have been proposed (18). Last, compartmentalization of nutrients during inflammation may create a permissive environment for inflammation to occur via temporary removal of nutrients (e.g., vitamin A, zinc) that negatively regulate NF-κB activity (118, 126).
c. Case study: vitamin A and the APR.
Rather than a full discussion of each of the putative APP mechanisms, a case study exploration of the various proposed scenarios using vitamin A as an example will be illuminating. Although vitamin A is absolutely required for mounting an effective immune response, it is tempting to speculate that temporarily inhibiting plasma delivery of vitamin A to tissues is beneficial to the host by creating a favorable environment for inflammation. The accompanying Text Box 17 outlines potential mechanisms that might describe how this limitation might occur and evidence for how this may manifest in the context of infection.
Text Box 17 . Possible mechanisms to explain vitamin A’s role in immune response.
● Vitamin A, via conversion to ATRA, can inhibit NF-κB activation (118).
● ATRA can inhibit macrophage responses to proinflammatory stimuli in vitro and in vivo (119).
● Vitamin A/ATRA is necessary for immune tolerance via Treg induction in in vitro and in vivo animal models (133).
Confirmation of vitamin A’s role is provided by 4 types of observations:
● Elimination of infections sometimes leads to a relatively rapid rebound in plasma retinol without nutritional intervention (23).
● Repletion can be more difficult during infections (261, 262).
● Acute, high-dose repletion can sometimes increase pathology (e.g., respiratory infections) (263, 264).
● Host-pathogen competition for vitamin A: examples include helminth parasites, such as Ascaris and Onchocerca, which express a range of retinol-binding proteins, retinol dehydrogenases, and RARs and may utilize retinol/retinoic acid for growth and development (265–267).
Several RARs have also been found in the long terminal repeat of the HIV virus, and replication of HIV is stimulated or inhibited by ATRA (268–270).
Several strategies have been designed to assess nutrient status in the face of the APR, including the use of a single APR to identify subjects with and without an active APR, reporting nutrient concentrations and prevalence rates of deficiency in those with and without elevated APPs, or using multiple APPs to adjust nutrient values for phase or severity of infection (271, 272). Each of these strategies may be useful, but they are limited, and identification of biomarkers of nutrient status that are not sensitive or responsive to inflammation remains an important avenue of research. This will be addressed extensively by WG 4. The application of genomic metabolomic and/or proteomic approaches to this problem may be useful (127, 273, 274).
VII. Conclusions.
Considerable progress has been made toward understanding the mechanistic roles of specific nutrients in the function of leukocytes of rodent models and in human cell lines. In particular, the discovery of cell signaling networks by which nutrients regulate the differentiation and phenotype of regulatory leukocytes has been an important development. The translation of these mechanistic results in ways that improve outcomes in human diseases has been limited. Aside from the obvious inability to control the experimental environment, the complex nutritional context of at-risk human populations presents a daunting challenge. For example, although research with rodents typically examines deficiencies of a single nutrient in a diet in which all other nutrient amounts are optimal, human diets may commonly be lacking in multiple nutrients and simultaneously have certain nutrient excesses. Consequently, a key research priority is the need to examine interactions between essential nutrients with the immune and related systems to determine if they have additive, synergistic, facilitating, or unpredictable effects relative to an individual’s nutritional status.
In human populations, genetic and epigenetic differences likely account for important variations in the response of the immune system to nutrient fortification. Reliance on inbred mouse species housed in highly controlled environments may not be the most relevant model for understanding the implication of these genetic interactions in humans. New approaches for model systems that more closely duplicate the dietary, genetic, and hygienic realities of human populations should be considered.
VIII. Research Priorities.
Recent estimates indicate that rats, mice, and hamsters account for ∼80% of the animals used in all laboratory experimentation (275). Although some of these data reflect mandatory safety testing of cosmetic products—and discipline-specific uses have not been compiled—the proportion of rodents used in nutrition and immunologic research is likely to be similar. A survey of the literature (provided here as Tables 1–4) indicates that the vast majority of evidence for effects of micronutrients on the immune system comes from animal models. There are compelling reasons to use rodent models including well-defined genetics, a vast array of genetically altered animals, ease of care, control of diet, and a high reproductive rate. However, there are substantial differences in the structure, regulation, and responsiveness of the immune system, particularly in mechanisms related to inflammation, between humans and rodents that should not be ignored (276–278) when trying to extrapolate findings made in rodents to humans. Clearly, identifying and establishing better models that can provide more translatable findings to humans is a high research priority (279).
Another high-priority research objective is the development of better biomarkers of nutritional status that can be applied to diverse healthy populations as well as those with infections or inflammation. Current efforts are limited by the confounding effects of the APR on the selection and interpretation of nutrient biomarkers. Conversely, there are the impacts of PEM and single- and multiple-micronutrient malnutrition on the APR. Most mechanistic studies use single-micronutrient deficiencies and attempt to control for decreases in food intake when possible. In fact, single-micronutrient deficiencies are rare and often coexist with PEM in resource-limited settings. To the limited degree that they have been studied in experimental models, multiple deficiencies can often have an additive or synergistic effect on the immune response. Similarly, multiple infections found in resource-limited settings are likely to affect nutrient requirements to a greater degree than single infections.
If an individual is malnourished (over- or undernourished) in calories or nutrients, his/her exposure to bioactive food components or drugs will be quite different from that of those individuals who are nutritionally adequate. In addition to the well-studied effects of vitamins and minerals on immune function, bioactive compounds in foods such as l-theanine, flavonoids/polyphenols such as epigallocatechin gallate, curcumin, and isothiocyanates such as phenethyl isothiocyanate and sulforaphane also affect immune or inflammatory responses when fed to animals (280). A limited number of studies show that these compounds are active in humans as well (280–282).
The interaction of the effects of micronutrient status with the gut microbiota is an emerging area of research interest (283). The composition of the gut microbiota is affected by nutritional status and nutrition/eating habits. Overnutrition affects the gut microbiota, and a large number of studies have shown that caloric/fat intake influences the ratio of different phyla of bacteria within the intestinal tract (283). Humans and animals with high fat intakes tend to have more Firmicutes, a phyllum of bacteria that may actually enhance fat absorption (284). With regard to undernutrition, a limited number of studies have shown that PEM is permissive for more pathogenic bacteria (90, 285). It is not clear whether malnutrition sets up conditions that are favorable for pathogenic bacterial growth and/or whether impaired immune surveillance leads to outgrowth of noncommensal bacteria. Recent studies indicate that some microbiomes actually contribute to malnutrition (91). A limited number of other studies also suggest that vitamin A (286), selenium (287), iron (288), and zinc deficiencies (289) may affect the microbiome. Text Box 18 includes a list of research priorities highlighted in this review.
Text Box 18 . Research priorities.
● Identifying/establishing models to better translate findings to humans
● Improved understanding of the interactions of micronutrient status with the gut microbiota
● Understanding the cost/benefits of accounting for the influence of the APR on interpretation and use of biomarkers of nutrient status
● Determining the hierarchy of cells and molecules for possession of deficient nutrients in order to maintain their function
● Determining potential points of interaction of nutrients with pleotropic effects on immune function
● Understanding the role of genetic variation on the responses to micronutrients
● Improved understanding about the potential impact of micronutrient interventions on immune function, particularly in individuals in whom activation of elements of the immune system has already occurred
● Understanding how nutrients metabolism may be affected by inflammation via epigenetic pathways
● Understanding how nutrients may play a regulatory role in altering immune function or inflammation via epigenetic pathways
B. WG theme 2: specific relations between nutrients, immune function, and inflammatory response—impact of nutrition on the immune response
Objective: Review the extant data to evaluate the impact of malnutrition on immune function and the inflammatory response. WG 2 summary and conclusions can be found in Text Box 19.
Text Box 19 . WG 2 summary and conclusions.
● The review uses data on 3 micronutrients (vitamin A, zinc, and iron), anthropometrically defined undernutrition (stunting, wasting, and underweight), and obesity to evaluate the effect on the following:
○ immune function,
○ recovery of immune function in response to nutritional interventions,
○ related health outcomes (e.g., risk of infection), and
○ markers of inflammation.
● Malnutrition in all forms impairs both innate and adaptive immunity, resulting in changes that can impair resistance to or recovery from infections.
● Supplementation can have benefits (e.g., reducing mortality and some types of morbidity in the case of vitamin A and zinc) but may also increase risk of adverse outcomes in selected populations as has been seen for both vitamin A and iron.
● Adverse outcomes may result from supplementation to nondeficient individuals in population-based programs, but the underlying mechanisms accounting for both benefit and potential adverse effects require further research in order to target intervention programs appropriately.
● Conclusion: nutrition for optimal health and immune function appears to require balance, without stunting, wasting, and underweight on one hand, and without obesity on the other hand. Similarly, micronutrient deficiencies impair immunity, but supplementation of at least some micronutrients to nondeficient individuals may cause harm under some circumstances.
I. Introduction.
The evidence with regard to the impacts of malnutrition, including both under- and overnutrition, on health across the life cycle is incontrovertible (1). As outlined by WG 1 and summarized in Table 6, many of the effects of undernutrition are mediated through the immune system, including changes in host defenses that affect resistance to or recovery from infections. In addition, overnutrition may induce chronic inflammation that increases the risk of both infectious and NCDs. Furthermore, effects can be lasting, even across generations, predisposing undernourished children in utero to the metabolic syndrome and related diseases later in life (322, 323).
TABLE 6.
Effects of micronutrient supplementation on immune function and vaccine responses (human studies)1
| Type of malnutrition and type of immune function | Effects of supplementation on immune system (reference) |
| Vitamin A | |
| Epithelial barrier function | ● High-dose VAS above 6 mo of age decreases risk of death from infections such as measles and diarrheal diseases (290) |
| ● VAS in the context of HIV infection or lower respiratory tract infections has been associated with adverse outcomes (105, 263, 290–292) | |
| ● Improved barrier function (105) | |
| ● Abnormal dual-sugar (lactulose, mannitol) intestinal permeability inversely correlated with serum retinol (293) and partially normalized by supplementation in VA-deficient individuals in some studies (294) but not others (295, 296) | |
| ● Increased symptomatology after high-dose VAS during acute lower respiratory tract infections reported in some studies (105) may indicate restored mucus production | |
| Innate physiologic barriers | ● VAS did not affect CRP, SAA, and/or AGP concentrations among children with subclinical or clinical VAD (297–299) |
| ● CRP and AGP did not change in men with low VA stores given VA (300) | |
| Macrophage function | ● Increased monocyte numbers (301) |
| ● Decreased TNF-α concentrations in children (301) but the opposite in men (106) | |
| ● Decreased IFN-γ concentrations in children (302) but not in pregnant women (303) | |
| ● Increased IL-10 concentrations in children (105, 301) but no or an opposite effect in adults (106, 303, 304) | |
| Neutrophil function | ● Neutrophil phagocytosis of latex beads increased in VA-deficient children given a single dose of VA (305) |
| ● Neutrophil phagocytosis of E. coli did not change in men with low VA stores given VA (300) | |
| NK cell function | ● An increase in NK cells in HIV-infected children supplemented with VA (105) |
| ● NK cell numbers did not change in men with low VA given supplemental VA; however, there was a positive correlation between VA stores and peripheral blood NK cells (300) | |
| T cell function | ● Increased lymphocyte counts, particularly CD4 counts (105) |
| ● CD4:CD8 ratios were increased or unchanged by supplementation (306) | |
| ● Increase in naive CD4 T cells after supplementation; higher memory CD8, CD45RO (307) | |
| ● Higher mitogen-induced IL-2, IL-4, and TNF in response to supplementation (106) | |
| B cell function | ● Supplementation had no overall effect on measles vaccine response (308) and a negative response in individuals with baseline high titers (103) |
| ● No overall effect on oral polio vaccination | |
| ● Supplemented children increased IgG1 responses to DTP, minor IgG3 response, no change in IgG2 and IgG4 (309) | |
| ● No overall effect on BCG vaccine in VA-supplemented children, lower response in VA-supplemented boys at 2 mo (310) | |
| ● Increased antibody responses to some vaccines (measles vaccine by 9 mo of age, oral polio vaccine, type 1, hepatitis B vaccine, rabies vaccine) (103) | |
| ● Short-term diminished cellular response to PPD in boys (103) and hospitalized children (105) | |
| Zinc | |
| Epithelial barrier function | ● Zn supplementation in children led to an improvement in the lactulose:mannitol excretion ratio in some (311) but not other (312, 313) studies; ratio may improve in infected children without Zn deficiency given supplemental Zn (314) |
| Innate physiologic barriers | ● Zn-supplemented children had higher serum complement C3 (315) |
| NK cell function | ● Children with diarrhea supplemented with Zn supplementation do not exhibit an increase in NK cells (316) |
| ● Increased NK cell activity in the elderly (317) | |
| T cell function | ● Increased CD4+ cell count in AIDS patients (318) |
| ● Increased thymocyte count in thymus (319) | |
| ● Increased thymulin activity (320) | |
| ● No consistent effect of Zn supplementation on DTH to TB antigen [reviewed in (103)] | |
| B cell function | ● No effect of Zn supplementation of mothers on BCG response in children (321) |
AGP, α1-acid glycoprotein; BCG, bacille Calmette-Guérin; CD, cluster of differentiation; CD45RO, cluster of differentiation antigen 45 RO; CRP, C-reactive protein; DTH, delayed-type hypersensitivity; DTP, diphtheria tetanus-pertussis; PPD, purified protein derivative; SAA, serum amyloid A; TB, tuberculosis; VA, vitamin A; VAD, vitamin A deficiency; VAS, vitamin A supplementation.
This review includes examples of undernutrition, including undernutrition defined by poor growth (i.e., underweight, stunting, and wasting) and deficiencies of specific micronutrients (vitamin A, zinc, and iron) as well as overnutrition, focusing on obesity. For each example, a geographic and life cycle perspective is offered along with a description of the clinical signs and biochemical markers that define each condition. The principal aim will be to describe the impact of malnutrition on the immune system and on the related risk of disease. The primary data sources for this review are human studies, including results from nutrition intervention trials that were either preventive (i.e., trying to prevent malnutrition and related disease risk) or therapeutic (i.e., trying to treat a particular disease, such as pneumonia, in subjects at risk of deficiency). Data from animal studies that offer unique insights were also considered. A final goal is to address how malnutrition may affect commonly used biomarkers of inflammation.
II. Vitamin A.
The biology and biomarkers used for assessing vitamin A were recently reviewed (324), and are discussed in more detail in WG 3. A brief overview of the basics of vitamin A is provided in Text Box 20.
Text Box 20 . Basics of vitamin A: biology and assessment.
● Primary causes of VAD include:
○ poor intake/food insecurity [the limited availability/accessibility of either animal source (preformed) vitamin A or alternative sources of vitamin A] and
○ poor absorption (e.g., fat malabsorption).
● A high infectious disease burden can lead to decreased appetite, decreased absorption, liver or kidney disease, and other factors affecting loss of retinol binding protein (RBP), and depletion of body stores through excessive metabolism and increased excretion of vitamin A (325).
● Vitamin A is stored in the liver, and the most widely used biomarkers in population studies are serum retinol and serum RBP (324).
● Retinol is transported through the serum by RBP in an ∼1:1 molar ratio; thus, these markers correlate closely with one another.
● Serum retinol and RBP also correlate with liver vitamin A stores in subjects with or at risk of clinical deficiency.
● RBP is also a negative APP; thus, inflammation and infection have a negative effect on serum retinol and RBP concentrations (324).
● More quantitative assessment techniques include the following:
○ Dose-response tests that indicate the adequacy of liver stores and isotope-dilution tests that reflect the amount of vitamin A stored in the liver. Both are labor intensive and expensive compared with serum retinol and RBP (324).
○ Conjunctival impression cytology has been used as a marker for vitamin A status but has not been widely adopted (324).
● More accurate and stable biomarkers of VAD are needed.
a. Epidemiology of VAD.
The challenge of obtaining vitamin A, a fat-soluble vitamin, is great in many settings because preformed vitamin A is found primarily in animal-source foods including liver, eggs, and whole milk. Although it can also be obtained through precursors (β-carotene and other provitamin A carotenoids), which are found in orange-yellow vegetables and fruit and green leafy vegetables, the conversion of carotenoids to vitaminA is variable. Text Box 20 provides a summary of causes contributing to VAD.
On the basis of serum retinol concentrations <0.70 μmol/L (or 20 μg/dL), 122 countries are characterized as having moderate to severe VAD problems in preschool-aged children, whereas 88 countries fulfill this criterion with regard to pregnant women; it is estimated that a total of 190 million children and 19 million women are affected (5). The regions that are most affected by VAD are Africa and Southeast Asia (325). Preschool-aged children and pregnant women face the highest risk, reflecting high nutritional demands and high infectious disease burden (325). The principal clinical manifestation of VAD is xerophthalmia in various levels of severity, ranging from night blindness and Bitot’s spots to corneal scarring and blindness. Other consequences of VAD are anemia and a higher risk of child mortality from infectious diseases (325).
b. Effects of VAD/vitamin A supplementation on immune function and disease risk.
As discussed by WG 1, VAD and vitamin A supplementation affect a range of innate and adaptive immune functions. Overall, VAD has been associated with decreased barrier function and skewing toward a Th1 response. Several human studies documented that VAD is associated with increased Th1-driven delayed-type hypersensitivity (DTH) responses and reduced Th2-driven antibody responses to vaccines; vitamin A supplementation reverses these responses (104, 105, 326). An animal study indicated that a high vitamin A intake may be linked to asthma (164), an association that remains to be studied in humans. Vitamin A supplementation has mostly been reported to have reciprocal effects of VAD (Table 6).
The important effects of vitamin A on immune function are reflected in the many community and clinical studies that showed that vitamin A affects mortality and morbidity. Large randomized trials led to meta-analyses concluding that prophylactic vitamin A supplementation of children between 6 mo and 5 y of age reduces overall mortality by 23–30% (290, 327–328) (Table 7). Trials in infants <6 mo of age produced conflicting results. No study found a beneficial effect between 1 and 5 mo of age, and the effect in neonates is still under debate; whereas some studies suggested a beneficial effect in South Asia and in boys, the results have been heterogeneous (332). One possible explanation for the apparent lack of benefit of vitamin A supplementation below 6 mo of age is that infants in such studies are protected from infection by maternally derived immune factors (e.g., serum IgG, factors in breast milk) to a greater degree than are older infants. Thus, the benefits of correcting VAD on survival are less pronounced.
TABLE 7.
Effects of interventions (preventive and therapeutic) on clinical outcomes (human studies)
| Type of malnutrition | Effect of preventative intervention on clinical outcomes (reference) | Effect of therapeutic intervention on clinical outcomes (reference) |
| Vitamin A | ● Decreased overall mortality in children between 6 mo and 5 y of age (290, 327–328) | ● Decreased severity of measles infection (105, 329) |
| ● No effect on overall mortality in children between 1 and 5 mo of age (330) | ● No effect or even increased severity of pneumonia (105, 263, 331) | |
| ● Conflicting evidence with regard to effect on overall mortality in neonates (332) | ● Beneficial effect of low daily doses vs. high doses on incidence and duration of respiratory infections (333) | |
| ● Reduced incidence of measles infection (105) in infant boys but not infant girls (334) | ||
| ● Reduced incidence and severity of diarrhea severity for some pathogens (105, 335, 336) | ||
| ● Reduced incidence and severity of malaria (105) | ||
| ● Potentially increased incidence and severity of respiratory infections (105) | ||
| ● Increased mother-to-child transmission of HIV (105) | ||
| Zinc | ● Decreased morbidity: diarrhea, pneumonia, and malaria episodes (337–339) | ● Decreased mortality: diarrhea (340) |
| ● Decreased incidence of opportunistic infections in AIDS patients (341) | ● Decreased morbidity: fewer diarrhea episodes (340) | |
| ● Fewer treatment failures in serious infections of suspected bacterial etiology in young infants (342) | ||
| ● Conflicting evidence of impact in the treatment of pneumonia (343–345) | ||
| ● Reduced duration of cold symptoms (346); increased duration of fever and inflammatory responses in treatment of gram-negative catheter sepsis in adults and childhood pneumonia (347, 348) | ||
| Iron | ● Increased risk of malaria and of death in malaria-endemic areas (349, 350) | ● No clear benefits |
| ● Risk may be greater in nondeficient individuals |
Disease-specific studies have likewise not shown uniformly beneficial effects. Although vitamin A may have a preventive effect on the incidence or severity of diarrhea and measles in community trials, it has had little or the opposite effect on respiratory infections (105). The same is seen in studies of vitamin A as a therapeutic agent (Table 7).
More in-depth mechanistic studies have provided clues to these conflicting results. For instance, neonatal vitamin A supplementation had a different effect on in vitro whole-blood cytokine responses depending on the sex and the vaccination status of the children (301). Vitamin A supplementation of 6- to 15-mo-old children differentially modified the association between gut chemokine and cytokine responses and duration of infections, depending on the infectious pathogen (351). Also, although it is clear that inflammation is associated with decreased serum retinol and RBP concentrations, the role of vitamin A supplementation on inflammation and its markers is less clear. Vitamin A supplementation of children <5 y of age increased their APP, CRP, and AGP values in response to vomiting and diarrhea but not in response to cough and fever (297) (Table 8).
TABLE 8.
Effects of malnutrition (or supplementation) on commonly used markers of inflammation (evidence from human studies)1
| Marker (reference) |
||||||
| Type of malnutrition | CRP | AGP | IL-6 | TNF-α | IL-10 | IL-4 |
| Vitamin A | ↑2,3 ↓2,4 (297) | ↑2,3 ↓2,4 (297) | — | ↑2 (106, 303) | ↓ (106) | ↑2 (106) |
| Zinc | — | — | — | ↓ (352–358) | — | — |
| Iron | — | — | ↓ (354) | ↓ (359) | — | ↓ (360) |
| Underweight, stunting, or wasting (PEM) | ↓ (361) | — | ↓ (98) | ↓ (98) | — | ↔ (98) |
| Obesity | ↑ (362–368) | ↑ (362–368) | ↑ (362–368) | ↑ (362–368) | — | — |
AGP, α1-acid glycoprotein; CRP, C-reactive protein; PEM, protein-energy malnutrition; ↓, decrease, ↑, increase; ↔, variable.
Supplementation.
In children with diarrhea and vomiting.
In children with cough and fever.
Clearly, VAD is associated with critical impairment of immune function. However, the effects of vitamin A supplementation on the immune system may lead to variable clinical outcomes, depending on the nature of the immune response needed to combat the infecting pathogens. For this reason, vitamin A supplementation may be a double-edged sword. For some—and on the basis of the clinical trials, presumably most—infectious diseases, vitamin A supplementation may be beneficial; in other situations it may be harmful (369, 370). Important effect modifiers may be population vitamin A status, age, sex, vaccination status, season, pathogen prevalence, disease type, and disease burden. The latter is also reflected in differences in effect over region and season (332, 370).
III. Zinc.
The basics of zinc biology and biomarkers were recently reviewed (371) and are discussed in more detail by WG 4. A brief summary of zinc assessment methods is found in the accompanying Text Box 21.
Text Box 21 . Basics of zinc: clinical effects and assessment.
● The most common effects of severe ZnD in humans include growth retardation, delayed sexual maturation, skin lesions, anorexia, alopecia, poor cognitive development, and impaired immune function (372, 373).
● The most common causes of ZnD are dietary insufficiency, limited nutrient bioavailability from local diets, and excretion during recurrent episodes of infection.
● Plasma, urinary, and hair zinc are reliable biomarkers of zinc status in healthy populations.
● Their use in deficient populations is problematic (374).
● Plasma zinc concentrations are most often used to assess zinc status at the population level and have a cutoff of <0.65 μg/dL to define ZnD in children (375).
● Plasma zinc is influenced by factors unrelated to zinc status, including inflammation and recent dietary intake, making it a poor indicator of individual zinc status (376).
Estimates of the childhood deaths attributable to zinc deficiency are based on a population-level modeling. The population-attributable fractions for ZnD for diarrhea, pneumonia, and malaria are calculated by estimating the reduction in mortality if the risk of ZnD was reduced by a theoretical minimum in children <5 y old (377).
a. Epidemiology of ZnD.
ZnD is a global problem that contributes significantly to disease morbidity and mortality, particularly in low-income countries (372, 378). Although severe ZnD is rare, mild to moderate deficiency is common throughout the world (372) and is a predisposing factor for a range of infections, particularly diarrhea (379). ZnD is estimated to account for 450,000 deaths annually, >4% of deaths among young children, and 1% of all deaths in children globally (378).
The estimation of zinc availability in the diet in combination with childhood stunting rates to generate a likelihood of deficiency remains the accepted method for assessing population-based ZnD. The estimation of zinc availability in the diet in combination with childhood stunting rates to generate a likelihood of deficiency has been used by the International Zinc Consultative Group (380) and remains the accepted method for assessing population-based ZnD. On the basis of estimates of dietary zinc availability and the prevalence of childhood stunting, it is estimated that ∼15–30% or 1–2 billion of the world’s population is zinc deficient (378, 381). The prevalence of ZnD is greatest in South and Southeast Asia, sub-Saharan Africa, and Central America (372, 382). Mild to moderate ZnD is thought to be common in low-income populations, especially in populations with a low consumption of zinc-rich animal-source foods and high intakes of foods rich in phytates, which inhibit zinc absorption (383). Children <5 y of age, pregnant women, and the elderly comprise the groups at high risk of deficiency. Within those groups, premature and small-for-gestational-age infants and preschool-aged children, predominantly those 6–23 mo of age are at greatest risk (372, 379). Infants are particularly vulnerable because the zinc content of human milk declines sharply in the postpartum period regardless of maternal zinc status (372).
b. Effects of ZnD or supplementation on immune function and disease risk.
As discussed by WG 1, zinc is known to play a critical role in the integrity, function, and maintenance of host defense systems (384, 352, 353) (summarized in Text Box 22).
Text Box 22 . Summary of zinc effects on immune system and markers of inflammation.
● In experimental studies in human volunteers, ZnD was associated with decreased thymulin activity, limited proinflammatory cytokine responses, decreased CD4/CD8 T-lymphocyte ratios, and impaired NK cell lytic activity (320).
● Similar deficits were observed in in vitro and animal studies along with leucopenia, thymic atrophy, altered antibody diversity, and compromised B lymphocyte development and antibody production, particularly of IgG subclasses (353, 354).
● Macrophage function is also adversely affected, suppressing opsonization and dysregulation of intracellular killing, cytokine production, and phagocytosis in deficient animals (354, 355, 385).
● Supplementation has been shown to restore or improve host barrier defenses and innate and adaptive immune function in experimental studies in animals and humans and in patients with altered zinc metabolism (379, 353) (Table 6).
● The impact of zinc status on Th17 or Treg cells is not known.
● Studies with animal models have shown that ZnD has impacts on inflammatory markers including a shift from Th1- to Th2-type cytokine responses, in which the expressions of the Th1 cytokines IFN-γ and IL-2 and the proinflammatory cytokine TNF are downregulated (352, 354, 355) (Table 8).
● Zinc supplementation can restore production of proinflammatory cytokines (353).
● Although inflammation is associated with decreased plasma zinc concentrations (356, 357), data from one recent study suggest that zinc status has no impact on serum CRP concentrations (358).
Furthermore, interactions of ZnD and environmental enteropathy (EE) that plague children in developing areas may well be synergistically interactive, each worsening the other and their potential impact on impaired child development (386). Human and animal studies showed that mild ZnD can impair multiple mediators of host immunity, ranging from physical barriers of the skin to innate and acquired immunity, increasing the risk of severe infection (387, 354). Globally, these effects translate to 10% of diarrheal diseases, 16% of acute lower respiratory infections, and 18% of malaria episodes being attributed to ZnD (378). Of the 450,000 deaths attributed to ZnD, >50% result from diarrhea (378). In addition, poor zinc status has been implicated in ∼7% and 10% of pneumonia and malaria deaths, respectively (378).
Neither ZnD nor zinc supplementation has an appreciable effect on responses to bacille Calmette-Guérin (301), diphtheria and tetanus toxoid (388), rabies (389), influenza (390), Haemophilus influenza type b (321), and pneumococcal vaccines (389, 391). Zinc supplementation, however, may improve antibody responses to cholera vaccination in children (392). It should be noted that the influence of zinc status on vaccination has not been well studied. Most of the evidence is drawn from secondary analyses or small observational studies. Few account for the effects of age, sex, degree of deficiency, supplement dose, and duration of dosing.
Results of preventive trials in children living in areas of endemic ZnD showed that zinc supplementation significantly reduced the incidence of pneumonia and persistent diarrhea (379, 337, 338) (Table 7). Zinc prophylaxis in children is associated with a reduction in diarrhea mortality of 13% and pneumonia mortality of 15%; however, despite the implied association of zinc status with malaria mortality (199), zinc prophylaxis does not appear to affect deaths from malaria (339), raising questions about both the value of current methods of zinc assessment and the specific role of zinc in malaria. Significant reductions in acute and persistent diarrhea morbidity were also observed in zinc-supplemented children in therapeutic trials (340). On the basis of these results, the WHO recommended in 2004 that children be given oral zinc for 10–14 d with oral rehydration salts for the treatment of diarrhea (393).
Evidence of whether or not zinc supplementation provides a similar therapeutic benefit to children with severe pneumonia is conflicting (343–345). However, results from a recent trial in India indicate that zinc adjunctive therapy can reduce treatment failures in infants aged 7–120 d with severe infections of suspected bacterial etiology (342). On the other hand, some data suggest that supplementation during the clinical phase of gram-negative infections may exaggerate APRs and prolong hospitalization (347, 348, 394). The clinical and immunologic effects of therapeutic zinc supplementation on the outcome of infection are likely to depend on age, zinc dosage and frequency, degree of deficiency, region, and infectious agent. Further mechanistic studies are needed to optimize zinc therapy and to define populations most likely to benefit.
IV. Iron.
The biology of iron and the available assessment tools have been reviewed extensively (27, 395) and are discussed by WG 4. Text Box 23 summarizes some of the basics.
Text Box 23 . Basics of iron: clinical effects and assessment.
● The principal clinical manifestation of ID is microcytic, hypochromic anemia resulting from ID erythropoiesis.
● Iron is an important component of hemoglobin, hence the effect of deficiency on erythropoiesis, but it is also a key component of myoglobin as well as many enzymes that affect a wide range of physiologic processes.
● In addition to anemia, ID can cause other, often “subclinical,” physiologic deficits in muscle function, energy metabolism, cognitive function, and immune function (396).
● Assessment of iron status is challenged in the context of infection and inflammation due to the innate iron homeostatic process, including changes in iron absorption and subsequent changes in circulating iron and iron transport proteins, the primary biomarkers of iron status.
● ID is diagnosed by using a combination of indicators of iron nutrition, including serum iron, percentage of saturation of transferrin (the principal serum transport protein for iron) with iron, and serum concentration of ferritin, the soluble serum form of the iron storage protein primarily found in the liver and other tissues where iron is stored (397).
● A growing consensus exists for the use of the ratio of serum ferritin to soluble transferrin receptor as the best indicator of iron status because it is less susceptible to the effects of inflammation on biomarker performance and interpretation (27).
● Other indicators such as hepcidin and zinc protoporphyrin (ZPP) are discussed by WG 4.
a. Epidemiology of ID.
Nutritional ID and ID-anemia are highly prevalent, with the WHO estimating that 16.6% of the world’s population was affected in 2000, with prevalence rates being highest (>20%) in sub-Saharan Africa, India, other countries in Asia, the Pacific Islands, and the Middle East (398). ID typically results from a combination of dietary inadequacy and high physiologic need resulting from growth in infants and children and from the demands of the fetus during pregnancy. Menstrual blood loss is another condition that increases iron requirements and hence the risk of deficiency (397). Thus, infancy and early childhood, pregnancy, and childbearing years are the principal life stages in which ID is a risk (399, 400).
b. Effects of ID on immune function and disease risk.
As outlined by WG 1 and summarized in Text Box 24, ID affects key aspects of innate immunity.
Text Box 24 . Summary of the impact of iron on immune function and inflammation.
● ID affects the function of phagocytes such as neutrophils and macrophages (359, 401).
● As a transition metal and because of its oxidation-reduction potential, iron is an important component of the active site of enzymes such as NADPH oxidase and myeloperoxidase, which are important in producing the oxidative burst involved in killing microorganisms ingested by phagocytes.
● Although phagocytic function is not impaired by ID, neutrophils and macrophages from iron-deficient individuals have an impaired ability to generate an oxidative burst and produce hypochlorous acid, key components of bacterial killing (402, 403).
● Defects in bacterial killing by neutrophils have also been documented in ID and recover within 2 wk of initiation of iron supplementation (404).
● Production of proinflammatory cytokines such as TNF and IL-6 by innate immune cells is also reduced by ID (359).
● In the adaptive immune system, lymphocyte function is impaired by ID because proliferation requires the activity of iron-containing enzymes including ribonucleotide reductase. ID inhibits the proliferation of T lymphocytes and, to a lesser extent, B lymphocytes (359).
● Thymic function and the number of T cells in the periphery are also diminished by ID (401, 405), but supplementation has a variable effect on restoring the number of peripheral T cells (103). ID was associated with lower production of the Th2 cytokine IL-4 and higher production of the Th1 cytokine IFN-γ (360).
A recent review found that ID in humans does not affect antibody responses to vaccination (including responses to T cell–dependent antigens), although the data are limited and impaired responses were noted in animal studies (103). The contribution of ID to morbidity and mortality from infectious diseases worldwide is less than that attributed to VAD and ZnD (406).
A critical element of the interaction of iron with infection is the restriction of iron availability to invading pathogens (which, like host cells, require iron for growth). This is a major part of the innate defense of mammals against microorganisms that have specific mechanisms for acquiring iron in low-iron environments, such as host tissues (259, 407). In brief, the acute phase peptide hepcidin is synthesized in the liver in response to infection or inflammation (IL-6 plays a specific role in inducing its transcription) and it blocks the normal flow of iron from the gut, senescent erythrocytes, or tissue iron stores via transferrin to the bone marrow or other tissues in need of iron. This activity of hepcidin markedly decreases serum iron concentrations, which restricts iron availability to extracellular pathogens and may also affect iron availability to intracellular pathogens (e.g., malaria parasites) that depend on transferrin-mediated iron acquisition. This host response prompts the inference that iron supplementation during infection may be detrimental, a topic that is covered in greater detail by WG 3. Although results are equivocal for most infections, it is clear that iron supplementation in areas where malaria is endemic can have adverse effects, perhaps by enhancing survival of the parasite but possibly via other mechanisms as well, including promotion of iron-catalyzed oxidative damage or facilitation of concurrent bacterial infections by opportunistic, invasive pathogens such as Salmonella (28, 259, 349, 350).
V. Undernutrition.
For the purposes of this discussion and consistent with the work of WG 1, undernutrition will be referred to as PEM to distinguish it from single- or multiple-micronutrient deficiencies. The primary manifestations of PEM covered are underweight (low weight-for-age), stunting (low height-for-age), and wasting (low weight-for-height).
a. Epidemiology of underweight, stunting, and wasting.
The prevalence of under-5 underweight, stunting, and wasting due to undernutrition, although diminished due to aggressive public health interventions, remains high (1). The most vulnerable remain in low-income countries. Regional differences are seen, with underweight being most prevalent in Asia and eastern Africa, stunting in eastern and middle Africa as well as in South Central Asia, and wasting in South Central Asia. Severe wasting (<3 SDs) is often used as a trigger for nutritional rehabilitation programs and is most common in South Central Asia and middle Africa. The causes of PEM are diverse, but in these affected regions early childhood infections are typically associated with poor water and sanitation, poverty, food insecurity, and inadequate health care (408). In particular, a low availability of micronutrient-rich, animal-source foods is associated with the development of malnutrition (406). Repeated episodes of infection leading to impaired absorptive function also likely contribute to the development of undernutrition and may compound the problem of inadequate food intake (323).
PEM can also develop in adults and is typically defined as a BMI (in kg/m2) <18.5. Older adults are at particular risk of malnutrition, with the prevalence of low BMI increasing 4-fold above 75 y of age compared with those aged 45–64 y in the United States (409). Older adults also tend to have lower lean body mass and higher adiposity than younger adults due to loss of muscle mass with aging (410). The development of malnutrition in the elderly is complex and may include poverty, decreased ability to prepare food, depression, or the development of illness, but a common feature is a decrease in appetite or ”physiologic anorexia” (322).
b. Effects of PEM on immune function and disease risk.
The relation between PEM and host resistance to infections is bidirectional. This relation is magnified at either end of the life course, but particularly in the first 2 y of life in children living in areas with heavy pathogen exposures. Infections can precipitate poor nutrition via several mechanisms (406, 411), and antibiotic use during refeeding from malnutrition can enhance recovery, suggesting that an untreated (and undiagnosed) bacterial infection was inhibiting recovery (412). In addition, the frequency, severity, and risk of mortality from diarrhea, pneumonia, malaria, and other infections are highest in malnourished children due, at least in part, to impaired host defenses. Moreover, PEM indirectly affects the risk of infection and perhaps even NCDs via its role as the core of several “vicious cycles of poverty” (322, 397).
The effects of PEM occur through both its effects on immune and inflammatory function as well as on host protective barriers and development (2). The putative mechanisms by which PEM affects immune function and inflammation were covered by WG 1.
Acquired or cell-mediated immunity (CMI) is impaired by anthropometrically defined undernutrition. Infants and children with underweight, wasting, and stunting are typically also at risk of multiple-micronutrient malnutrition deficiencies; thus, it can be difficult to specifically associate particular immune deficiencies with these conditions. CMI is also specifically impaired in elderly adults with PEM. Serum antibody responses to vaccination also seem to be impaired in the elderly (256) unlike in younger malnourished subjects. Some additional, relevant aspects of the impact and risks associated with PEM are summarized in Text Box 25.
Text Box 25 . Summary of relevant effects of PEM on immune function/inflammation.
● PEM is associated with significant deficits in the function of the thymus, accounting, at least in part, for the decreased numbers of T cells seen in secondary lymphoid tissues (e.g., lymph nodes) and blood (413, 414).
● The impact on the T cell compartment appears to have a negative effect on some vaccine responses (e.g., DTH) and response to bacille Calmette-Guérin (BCG) vaccine that depend particularly on T cells (103).
● DTH responses to common environmental antigens are also impaired by PEM and, in turn, are associated with an increased risk of infection (415).
● Although the DTH response is sensitive to PEM, such associations are not seen when analyzing serum antibody responses to vaccination (103).
● The impact of PEM may be greater on CD4 Th than on cytotoxic CD8 T cells, as indicated by a decreased ratio of CD4 to CD8 cells (98).
● Responses by Th1 cells, which produce IFN-α, are also diminished (413).
● Secretory IgA responses do seem to be impaired by PEM (416), perhaps due to defects in IgA secretion across epithelial surfaces (a feature of IgA and IgM responses to mucosal infections) rather than to defects in antibody production (98) that could affect all antibody classes.
● The adverse effect of PEM on CMI could be caused by deficits in energy or specific micronutrients (e.g., vitamins, amino acids) having direct effects on the thymus or peripheral T cells (417).
● Other indirect effects may result from the host’s physiologic response to PEM, including endocrine-mediated effects (e.g., increased corticosterone concentrations, decreased leptin concentrations as a result of decreased fat mass) (98, 413).
● Intestinal permeability is increased in severe malnutrition (263, 264) and could increase the risk of invasive bacterial disease.
● Although many aspects of neutrophil function (e.g., phagocytosis) can remain normal in undernourished patients, defects have been seen in bacterial killing (260, 265) as well as in the killing ability of NK cells (260).
● Complement activity can also be impaired in PEM, although the effect is heterogeneous (266).
● Increased blood glucocorticoid concentration has also been seen in PEM (257, 260)
● Impaired innate immunity likely contributes to the increased risk of bacteremia in children with PEM (267).
● Inflammatory responses mediated by innate immune cells are also impaired, because PEM decreases the production of 3 important proinflammatory cytokines that mediate the induction of the APR, IL-1α, TNF, and IL-6 (260).
● Lower IL-1α production is also associated with a lower fever response in malnourished children with bacterial infections (268).
● Providing high-quality protein during nutritional rehabilitation restores the APP response (269) by an as yet to be determined mechanism.
Although acquired immunity, including CMI, is most consistently affected by PEM, some aspects of innate immunity may also be affected in underweight, wasted, and stunted individuals, with effects being more pronounced in more severely malnourished patients, (e.g., those 3 SD below the expected mean, or with a clinical presentation of Kwashiorkor or Marasmus). Relevant aspects of the impact of PEM on the innate immune systems are highlighted in Text Box 25.
VI. Obesity.
a. Epidemiology of obesity.
According to the WHO (418) and the recent GBD analysis (1), the global prevalence of overweight/obesity (BMI >30) is increasing at alarming rates, even in LMICs. Of particular concern is the dramatic increase in overweight/obesity in children <5 y of age.
Biochemical markers of obesity include high serum leptin, low adiponectin, and elevated inflammatory makers including CRP, TNF, and IL-6 (362–365). Insulin resistance and type 2 diabetes are often comorbidities with obesity. Coincident with the increase in obesity rates has been a concurrent increase in those comorbid NCDs, including hypertension, diabetes, CVD risk, and cancer (1). It has been suggested that the development of insulin resistance and diabetes may be due to the state of chronic low-grade inflammation found in obesity (366, 367).
b. Effects of obesity on immune function and disease risk.
Obesity and its concomitants (e.g., insulin resistance) have been associated with impaired immune/inflammatory responses (419, 420). A summary of the reputed effects of obesity on immune function/inflammation and disease risk are found in Text Box 26.
Text Box 26 . Summary of effects of obesity on immune function/inflammation.
● Lowered numbers of circulating leukocytes and impaired proliferation of mitogen-stimulated lymphocytes (368)
● Increased thymic aging and reduction in the diversity of the T cell repertoire (421)
○ Changes in NK cell numbers (422) and skewing toward a Treg and Th2 T cell phenotype (423) have also been reported, with increases in serum concentrations of TNF, IL-6, IL-1β, CRP, and leptin (362–368).
● Obese patients have longer stays in intensive care units and have an increased risk of dying after hospitalization (424, 425).
● Obesity is an independent risk factor for infections after surgery (426–428).
● Obesity is also an independent risk factor for a worse outcome after infection with pandemic influenza A (H1N1) (429–433).
● Obese subjects have a greater risk of hospitalization with respiratory infections during the influenza season than do nonobese individuals (434).
● Associations have been reported between higher BMI/obesity in children and a greater risk of respiratory illness (435) as well as a greater severity of respiratory syncytial infection (436). However, other studies found no increased risk of respiratory illness in obese subjects (437, 438).
● Higher BMI also been associated with a greater risk of periodontal infections (439), Staphylococcus aureus nasal carriage (440), gastric infection by Helicobacter pylori (441), and herpes simplex virus type 1 infection diagnosed via serum antibody (442).
● Obesity (adiposity) is associated with inflammation via the impact on macrophages (443).
With respect to vaccinations, there are several studies reporting impaired responses in obese adults and children including the following:
● An impaired response was found to hepatitis B vaccination (444, 445).
● A decreased anti-tetanus antibody response after tetanus vaccination was observed in obese compared with healthy-weight children (446).
● Trivalent influenza vaccination was associated with a lower antibody titer 1 y after vaccination, and impaired CD8 responses were observed against vaccine strains in obese individuals (447).
In support of the human studies, animal models of diet-induced obesity clearly demonstrated immune impairment and increased inflammation. Closely mimicking human obesity, mice with diet-induced obesity developed insulin resistance, elevated leptin, and elevated lipid TGs leading to fatty liver and increased inflammatory markers (448–450). Compared with lean mice, diet-induced obese mice had greater morbidity and mortality from primary and secondary influenza infections (451, 452), impaired resistance to Leishmania major infection (453), and increased mortality from S. aureus–induced sepsis (454).
VII. Conclusions.
Nutrients affect the immune system in a variety of ways (455). We reviewed how malnutrition—due to VAD, ZnD, ID, stunting, wasting, underweight, and obesity—affect immune function. Malnutrition in all of these forms is uniformly associated with impaired immune function, and mechanistic studies reveal effects on both the innate and adaptive immune systems. Not surprisingly, malnutrition is also associated with increased susceptibility to infectious diseases and mortality.
In mechanistic studies there is evidence that treatment of malnutrition restores immune function; and population-based intervention trials, in some cases, showed decreased morbidity and mortality with supplementation. However, some population studies also raise concerns; particularly for micronutrient supplementation there are indications of lack of effects or adverse outcomes of supplementation in some situations. As discussed above, adverse outcomes were seen during vitamin A and iron supplementation programs, and the mechanisms underlying these adverse effects may involve altered host resistance.
The nutritional state for optimal immune function appears to require balance, without stunting, wasting, and underweight on one hand, and without obesity on the other hand. Similarly, micronutrient deficiencies impair immunity, but excesses of at least some micronutrients may cause harm under some circumstances. More research is needed to refine our understanding of the mechanisms of and solutions to these circumstances.
VIII. Research priorities.
A list of research priorities is provided in Text Box 27. For all of the nutritional scenarios covered in the present review, it seems clear that more studies are needed to optimize the use of supplementation in order to maximize benefit and minimize risk.
Text Box 27 . Research priorities.
● Accurate and stable biomarkers of VAD
● Effect of vitamin A supplementation on inflammation and inflammatory markers
● Mechanisms behind the variable clinical outcomes seen as a result of the impact of vitamin A on the different immune responses
● Effect of vitamin A supplementation on neonates and infants <6 mo
● Influence of zinc status on vaccine response
● Other confounders in the zinc–vaccine response association (age, sex, degree of zinc deficiency, etc.)
● Impact of therapeutic vs. preventive use of zinc supplementation for different clinical outcomes
● Mechanistic studies to optimize zinc therapy and to define populations most likely to benefit
● Impact of zinc status on Th17 or Treg cells
C. WG theme 3: specific relations between nutrients, immune function, and inflammatory response—impact of the immune response on nutrition and impact of inflammation/infection on nutrition
Objective: To evaluate the evidence with regard to the impact of inflammation on nutrition/nutrient status across the continuum of inflammatory conditions (from acute infection to chronic NCD states). Please see Text Box 28 for WG 3 summary and conclusions.
Text Box 28 . WG 3 summary and conclusions.
● A paradigm is presented that incorporates an appreciation of the complex global health scenario and the importance of considering the intimate and inextricable interactions between inflammation and nutrition and their impact on health status assessment.
● The multiple effects of inflammation on both inflammatory and nutritional biomarkers are presented in a variety of conditions that exemplify these effects, including various infections and NCDs (e.g., obesity, obstructive lung diseases), and from a life cycle stage perspective (pregnancy and infancy).
● Attention is given to the important role of the gastrointestinal barrier and the gastrointestinal ecology including the emerging appreciation of the importance of the microbiome.
● Additional coverage includes the immunologic and nutritional manifestations of derangements in this ecology commonly referring to as “environmental enteropathy.”
● The conditions covered illustrate both the complexity of host profiling and the need for identifying priority biomarkers that reflect status, function, and effect of interventions in individuals and at the population level.
● Context-specific examples are considered in relation to gut integrity and secondary effects of drug exposures.
● Infections and undernutrition coexist in underprivileged individuals and populations and constitute a 2-way causal interrelation, the “malnutrition-infection cycle,” whereby undernutrition generally increases the risk and severity of infection and infections and/or their treatment may impair food intake and affect nutritional status.
● Infection and inflammation influence nutritional status and biomarker selection and interpretation in multiple ways including the following:
○ altered intakes through effects on appetite
○ altered absorption through effects on mucosal integrity
○ increased caloric requirements through altered metabolism and host immune responses
● Six nutrients are considered in detail (iron; vitamins A, D, and B-12; zinc; and folate), and summary statements of extant evidence about the roles that these nutrients play in inflammation are presented.
● Research priorities are highlighted ranging from basic science to epidemiologic profiling and the identification of risk categories.
I. Introduction.
This review follows and builds on the information presented by WGs 1 and 2 with a particular focus on the impact of acute and chronic inflammatory conditions on nutrition and, wherever possible, on nutrient biomarker selection, use, and interpretation. Commonly used inflammation and nutritional biomarkers are reported by WG 4. Of particular concern is the impact of the inflammatory APR in terms of both relevant aspects of its pathophysiology and its effects on biomarker selection and interpretation. In addition, the more generalized “acute stress response” will be discussed because it also contributes to the response to disease. The review begins with an overview of the current understanding of the clinical implications of the APR and the distinct but related acute stress response. This is followed by a summary of the critical role of gastrointestinal ecology. After an outline of general features of the infection-nutrition interaction, 6 case study nutrients are reviewed in order to explore these relations. Several case study clinical contexts are presented including the following: infectious disease exposures and EE, reflecting the implication of derangements in intestinal integrity; chronic and NCDs; pregnancy and infancy; and a brief discussion of drug-nutrient interactions. To set the stage for this discussion, the following sections will briefly address 2 distinct but related aspects of the human response to stress, the inflammatory APR and the acute stress response.
II. Global health and the inflammatory APR.
As described in detail by WG 1, an APR is a stereotypic, generalized host reaction, caused by a cascade of cytokines released by activated phagocytic cells (238). However, recent research suggests that different stimuli eliciting an APR may not only have different effects on the magnitude of the responses but also lead to the qualitatively different responses highlighted by WG 1. A range of medical, physiologic, and environmental conditions may precipitate an APR (Table 9).
TABLE 9.
Conditions that may precipitate an APR1
| Medical |
|||
| Diseases | Accidents | Physiologic | Environmental |
| Infection | Trauma | Gestation | Indoor cooking with biomass fuel |
| Noninfectious inflammatory diseases (diabetes, cancer) | Burns | Parturition | Pollution (toxin exposures) |
| Infarction and hemorrhage | Latrogenic (surgery, drugs, radiation) | Vaccination | Climate change (temperature/weather extremes) |
APR, acute phase response.
In low-income countries, infectious diseases continue to be major causes of the APR, although some infections may downregulate or impair the ability to mount an APR. Moreover, as highlighted in the GBD study (1), NCDs, including obesity, are assuming a greater role in the health picture in these same resource-constrained settings. Thus, both the infectious and NCD burden is considerable, especially among the poor, due to lack of safe water and sanitation, inadequate vaccination coverage, poor health care access and delivery, and the combination of food insecurity and limited dietary diversity leading to poor nutrition. Hence, elevated serum APPs occur not only among those with acute clinical infection but also among asymptomatic individuals, reflecting subclinical, residual, unrecognized infection or chronic NCDs. In view of the increasing importance of obesity, the latter is considered as a specific inflammatory state in a later section of this review (section V.b.).
In addition to diseases, the environment has also assumed a more prominent role as a factor contributing to poor health and the manifestations of the APR. One exposure that has assumed increasing importance to the global health community is the impact of household fossil fuel use for indoor cook stoves (456). Dutta et al. (457) reported systemic inflammation (evidenced by increased serum IL-6, IL-8, TNF, and CRP) among rural Indian women cooking with biomass fuels compared with those cooking with a clean fuel such as liquefied petroleum gas.
III. The acute stress response.
In addition to the APR, the body also has an exquisite system for responding to stress, broadly referred to as the acute stress response. The acute stress response, also known as the “fight or flight response,” was initially described by Cannon (458) and represents a cascade of events involving the SNS and the HPA. The stress response is distinct from the APR in that the latter is an immune/inflammatory response triggered by cytokine response to a stimulus, whereas the former is a more generalized metabolic response to stress involving multiple systems, most prominently the neuroendocrine axis. The components and physiologic implications of the APR are described by WG 1. The characteristics of the acute stress response and its links to the inflammatory response including the intimate interaction with the neuroendocrine system, mediated largely via the HPA and the sympathetic/adrenomedullary system, are described in Text Box 29.
Text Box 29 . Evidence for the link of the acute stress response and inflammation.
● Synergistic coupling of the HPA axis and SNS may be required for host anti-inflammatory actions.
● CRH receptor knockout mice have an impaired stress response (459).
● When the HPA axis is interrupted by adrenalectomy, mice transform into more inflammation-susceptible hosts and demonstrate increased mortality when exposed to viral infections; this susceptibility is diminished when glucocorticoid replacement is given (460).
● In sympathectomized mice, macrophage stimulation with LPS results in increased production of TNF (239, 461).
● A defective HPA or sympathetic/adrenomedullary response may account for altered pathophysiology seen in several autoimmune diseases, such as rheumatoid arthritis. In rheumatoid arthritis, the local inflammatory response is intensified and there is a predominant shift toward Th1 cytokine production (IL-12, TNF), which may result from an attenuated response of the HPA axis (241).
● Lewis rats, possessing a hypoactive HPA axis, are more prone to Th1-shifted states, such as arthritis, when exposed to pathogens, such as streptococcal cell wall (462).
● After patients underwent insulin tolerance testing, plasma corticotropin and cortisol concentratons were reported to be lower in patients with rheumatoid arthritis vs. healthy controls (463).
● In addition, patients with rheumatoid arthritis may have a reduction in sympathetic nerve fibers, which diminishes the anti-inflammatory response from the sympathetic/adrenomedullary system (241).
● Patients with sustained inflammatory disease may have blunted HPA or SNS responses.
a. Evidence that chronic stress can be harmful to the host.
The link between immune function/inflammation and the acute stress response has clinical implications that have just begun to be explored. Although the acute stress response effects are temporary, such that catabolism and immunosuppression are initially beneficial, over a prolonged time period these effects can be damaging. As a result, the host could be more vulnerable to infections, neoplastic disease, and metabolic derangements. General causes and implications of chronic inflammation were reviewed by WG 4.
The impact of this type of chronic stress can result in an increase in glucocorticoids, thereby antagonizing growth hormone actions on fat tissue. Chronic episodes of stress may lead to an attenuation of growth hormone actions to induce lipolysis, which could contribute to increased visceral adiposity. In addition, enhanced glucocorticoid secretion can lead to insulin resistance and profound hyperinsulinemia (464).
Experimental basic science showed that in monosodium l-glutamate (MSG)–induced hypothalamus-damaged and hyperadipose rats, exposure to LPS resulted in HPA hyperactivity likely due to leptin resistance, hyperinsulinemia (which may precede insulin resistance), and hypertriglyceridemia (465). In addition, catecholamines can induce production of the proinflammatory cytokine IL-6 locally at the myocardium (466); IL-6 stimulates hepatic production of CRP, which can contribute to progression of atherosclerosis. Persistent elevations in catecholamines can alter hemodynamics and lead to endothelial injury. The injured endothelium releases chemokines that attract monocytes, lymphocytes, and glucocorticoids, which could facilitate the early stages of atherosclerosis through induction of vascular cellular adhesion molecules (467).
The cascade of events associated with HIV infection exemplifies the potential detrimental effects of a chronic acute stress response. HIV-infected monocytes and macrophages are stimulated to produce IL-1, TNF, and IFN-α, which can increase CRH release and thus potentially affect immunomodulation of cytokine production (254). Taken together, the chronic activation of glucocorticoids and catecholamines can cause changes in the inflammatory cytokine milieu to selectively alter the pattern of cytokine secretion from Th1 to Th2. This may be linked in part to the increased predominance of visceral adiposity (255), insulin resistance (256), and atherosclerosis (468) in HIV-infected patients.
IV. Overview of clinical implications of infection.
The impact of infection on nutrition is incontrovertible. Text Box 30 outlines the general pathways by which this impact is manifested.
Text Box 30 . Effects of infections on nutritional status and validity of biomarkers.
● Infection may affect nutritional status directly as a result of poor appetite, local lesions, or other manifestations causing pain (e.g., oral or esophageal ulcers impairing food intake, diarrhea impairing absorption and increasing excretion of nutrients).
● The APR mediated through its cardinal manifestations may result in:
○ fever via increases in resting energy expenditure,
○ anorexia via reduction in the intake of energy and nutrients, and
○ impaired absorption and increased utilization and excretion of nutrients.
● Infection and the APR also affect nutrition indirectly via the role of some nutrient biomarkers as acute phase reactants (e.g., ferritin), thereby influencing their validity if used to assess specific micronutrient status. These effects are more severe with generalized, frequent, or recurrent infections.
Infections and undernutrition constitute a 2-way causal interrelation, the “malnutrition-infection cycle,” whereby undernutrition generally increases risk and severity of infection and infections and/or their treatment may impair food intake and nutritional status. This scenario is played on a global scale and puts infants and children <5 y at particular risk (408). The historic approach with regard to nutrition is to develop and roll out intervention programs to address particular aspects of this situation (e.g., universal single/multiple-micronutrient interventions and/or vaccines). However, evidence continues to emerge that, in the absence of a full appreciation of the complexities of a given health context and the biology of nutrition within that context, nutritional interventions to circumvent this cycle may have deleterious effects. The elements of this complexity are highlighted in Text Box 31.
Text Box 31 . Factors that might affect nutritional interventions.
● Baseline nutritional intake and status of the individual/population including the following:
○ impact of infection/inflammation on biomarkers used to assess status
○ deficient vs. replete: a particular consideration for “universal”/countrywide intervention programs
● Stage of the life cycle:
○ infants: breastfeeding
○ children: complementary feeding
○ pregnancy/lactation
● Bioavailability, safety, and efficacy of proposed dose, form, composition, and administration route of the intervention, particularly within the specific conditions of the region/cultural setting
● Appreciation of the nature of the concurrent infection or infections and the body’s response to infection/inflammation as outlined by WGs 1 and 2
● The nature of the biological relation between a given nutrient and an infectious organism (e.g., malaria)
● The impact of other therapeutic interventions (e.g., drugs, vaccines)
The complexities of this cycle are illustrated by the results of a large study in a highly malarious area in which daily iron supplementation in young children increased infectious adverse events, especially in those considered iron replete (28). Another example is the observation that vitamin A supplementation, which generally lowers mortality in children, increased the risk of mother-to-child HIV transmission in pregnant and postpartum HIV-infected women in Tanzania (469), although not in South Africa (470) or Malawi (471). In infants of HIV-positive mothers, postpartum maternal and neonatal vitamin A supplementation may hasten progression to death in breastfed children who are PCR negative at 6 wk (291). The following sections cover specific examples of the infection-malnutrition cycle.
Having laid out the basic concepts underlying the potential interactions between inflammation, infection, and nutrition, it is useful to explore how these interactions affect specific aspects of nutrition. To explore this aspect of the inflammation/nutrition relation, 6 specific nutrients—iron, zinc, vitamin A, folate, vitamin B-12, and vitamin D—are examined. The following 8 questions were used to frame this discussion:
● What is the role of [nutrient] in the basic biology of immunity/inflammation? (This was extensively addressed by WGs 1 and 2.)
● What is the evidence that [nutrient] status or intervention affects inflammation/infection? (This is informed by materials presented by WG 2.)
Is there evidence that inflammation/infection affects [nutrient] status?
● What mechanisms are responsible for altering [nutrient] status?
● Is there evidence that different infections or types and sources of inflammation have differential effects on [nutrient] status?
● Is there evidence that host genetic effects modulate the effects of infection/inflammation on [nutrient] status?
● What are the implications for host [nutrient] status?
● What are the implications for biomarker assessment of [nutrient] status?
The responses to these questions are outlined for each of the 6 nutrients in Supplemental Table 9 and Supplemental Figure 3).
a. Exposure to specific and multiple infections through the life course.
It is not uncommon for individuals in low-income countries to have multiple, coexisting infections and NCDs. The scenario is best exemplified by the comorbidities associated with HIV infection (472). Although HIV is most commonly used as the example of comorbidity, the recent analysis of the GBD clearly indicates that there are various colliding epidemics of infectious diseases and NCDs occurring particularly in low-/middle-income settings (1). Other examples of comorbid conditions include latent infections (e.g., mycobacteria, cytomegalovirus), chronic helminth infections (e.g., geohelminths, schistosomes, filariae) maintained by regular exposure, chronic viral infections (HIV; hepatitis A, B, and C), with frequent, superimposed acute respiratory, gastrointestinal, and urogenital tract infections. In childhood, diarrhea may occur 6–8 times/y.
The inflammatory drivers are multiple, especially because infection exposure occurs from birth onward. These patterns of comorbidity may be complex. Helminth infections are often established in early childhood, and the prevalence and intensity peaks in childhood, with persistent infection exposure through adulthood. Along with effects such as EE discussed below, these infections give rise to APRs during the tissue-phase of the primary infection or during reinfection, whereas stable, low-intensity infections may not be associated with elevated levels of acute phase reactants.
The chronicity of effect depends on the nature of the infection, including its transmission patterns. Some examples include the following:
● In holoendemic malaria areas with year-round transmission, a large proportion of the population, including pregnant women, may experience asymptomatic parasitemia, concurrent elevation of serum APRs, and anemia of chronic inflammation.
● Overt tuberculosis, which affects 11 million people/y, causes a massive APR; but even latent tuberculosis, which affects one-third of the world’s population, has been associated with a slightly increased APR (H Friis, University of Copenhagen, personal communication, 2012).
● HIV continues to be a significant problem, with >34 million people now living with HIV/AIDS; 3.4 million of them are <15 y old (473). Current knowledge with regard to their nutritional needs was recently reviewed (474). Comorbid conditions (both infectious and NCDs) continue to be a common concomitant of HIV infection and may impair ability to produce an APR, thereby further increasing the risk of common and opportunistic infections that drive an APR.
The use and interpretation of nutritional biomarkers must take into account these age-specific and potentially multiple effects of infection. This also highlights the importance of clear clinical definitions of subject categories in studies of nutritional biomarkers.
b. Chronic malaria and parasitic infections.
In 2010, there were 219 million cases of malaria, with ∼80% of cases limited to 17 countries, most prominently in Africa with an estimated 660,000 deaths (with an uncertainty range of 490,000–836,000), mostly among African children (475). Not only does this represent a significant global health concern but its unique characteristics offer specific insights into the nature and implications of chronic exposure.
Many children, adults, and pregnant women living under conditions of perennial malaria transmission remain susceptible to malaria parasitemia. This is often asymptomatic due to the partial acquisition of malarial immunity. Chronic parasitemias lasting several months are common in such individuals unless treated with antimalarial drugs. Age differences in patterns of malaria immunity relate to this chronicity of exposure. Hepatosplenomegaly occurs with persistent infections and a chronic inflammatory state develops, often with hypergammaglobulinemia, which is attributable to reticuloendothelial and lymphoid hyperplasia. In a minority of individuals this may result in hyperreactive malaria splenomegaly with enormous splenic enlargement (476). Chronic exposures to other parasitic infections (e.g., schistosomiasis) may also cause hepatosplenomegaly, and these coexposures may further exacerbate chronic inflammation. Pathologic hepatic disease in childhood can lead to anemia, RBC production failure, and growth impairment. The association between hepatosplenomegaly, secondary to these chronic exposures, and cytokine responses has been little studied (477). Proinflammatory mechanisms are important, but there is little research relating these to nutritional correlates and biomarkers and their potential interactions (478). The following sections describe what is known about the impact of infection, NCDs, and certain physiologic states on nutrition with specific examples provided within each category.
c. Intestinal barrier integrity.
Any discussion of the interaction between infection, nutrition, and health must include an appreciation of the expanding understanding of the critical roles played by the gastrointestinal environment. The importance of gastrointestinal integrity is demonstrated by numerous reports documenting a relation between increased intestinal permeability and poor growth in low-income countries (479–481). In addition, the gastrointestinal environment, including the critical influence of the gut microbiome, plays an integral role as a barrier protecting the host from invasion by pathogenic organisms found in our environment. Key features of and approaches for assessing gastrointestinal function/integrity are highlighted in Text Box 32.
Text Box 32 . Key features of the gastrointestinal barrier.
● The barrier between the indigenous community of bacteria comprising the “gut microbiome,” residing in the gastrointestinal lumen and the sterile bloodstream, is formed by a layer of epithelial cells closely interconnected by tight junctions. This barrier plays a significant role in protecting the hosts from a myriad of potential adverse environmental exposures (482).
● These cells produce an antimicrobial layer composed of defensins, immunoglobulins, and mucins.
● Underneath the epithelium lies a complex network of communicating dendritic and immune cells that prevent leakage of luminal contents into the systemic circulation.
● The microbiome itself forms an ecological barrier that deters pathogens. Disruption of this intestinal barrier increases gut permeability and enables luminal bacteria and microbe-associated products to enter the bloodstream, a process called “bacterial translocation” (68, 483, 484).
● Bacterial translocation has been implicated in numerous infectious [e.g., HIV (485) or malaria (486)] and noninfectious [e.g., liver (487)] diseases.
Approaches to assessing loss of gastrointestinal barrier integrity or function
● Dual sugar absorption test:
○ This test is based on the premise that large sugar molecules such as lactulose do not cross the intestinal barrier in the healthy patients, whereas smaller molecules such as mannitol do.
○ Loss of gut wall integrity causes lactulose to leak into the circulation and results in an increase in the urinary lactulose-to-mannitol ratio.
○ Because of its laborious nature the dual sugar absorption test is unsuitable for monitoring purposes in clinical practice in resource-poor settings.
● Citrulline test for functional enterocyte mass:
○ Citrulline is a nonessential amino acid that is produced from glutamine in small intestinal enterocytes. Loss of enterocytes results in declining circulations of citrulline in the blood and is shown in cytotoxic treatment–induced mucositis (40).
○ Serum concentrations of citrulline ≥20 μmol/L predict success of small bowel nutritional rehabilitation in short bowel syndrome, villous atrophy syndrome, and chemotherapy-induced mucositis.
○ The utility of this test to assess recovery of enterocyte mass in rehabilitated malnourished patients to allow transition to community care has not been established.
○ The presence of intestinal inflammation does not affect plasma citrulline concentrations.
● Calprotectin:
○ Calprotectin is a cytosolic protein with antimicrobial activity that is released from neutrophils in the gastrointestinal tract and is found in high concentrations in the stool of patients with intestinal inflammation (e.g., Crohn disease and ulcerative colitis).
○ Healthy infants and toddlers have higher stool amounts than do school-aged children, teenagers, and adults. This finding is consistent with the observation that the gut microbiome functionally matures in the first few years of life (488).
○ Calprotectin amounts may be elevated in the stool of malnourished children with EE, although this has not yet been examined.
● Several other biomarkers have been identified, which may have utility in assessing gut inflammation (489):
○ lactoferrin
○ the enzyme biomarker, M2-PK, an early marker for colorectal polyps
○ S100A12 (a genetic marker that might be associated with neutrophil function and inflammation)
Although much has been learned about the interactions between the gut microbiome, immune system, and nutrition (283), the association of measurements of inflammatory markers of gut wall integrity with nutritional profiles has not been studied extensively. This is an important research gap because enteric infections are common causes of child morbidity and mortality, particularly in low-resource settings; thus, identification of relevant biomarkers for assessing risk is a priority.
d. Environmental enteropathy.
EE, also called tropical enteropathy, is a chronic subclinical condition caused by persistent fecal-oral contamination that results in colonization of the small intestine, destruction of intestinal villi, and inflammation (490, 491). The relations between EE, malnutrition, and development are complex and include impaired cognition and behavioral development in infants and young children consequent to the loss of essential nutrients integral to brain development (492, 493). EE has been implicated as a cause of stunting or chronic malnutrition; as much as 40% of stunting among under-5 children is attributed to EE in sub-Saharan Africa (494). Not only are growth and development adversely affected by EE, it also causes impaired immunologic responses to oral vaccines (495).
The epidemiology of EE is not well described, because diagnostic criteria are lacking, as are reliable point-of-care biomarkers. The intestinal permeability test using lactulose and mannitol has been used extensively to assess the integrity of intestinal barrier function, but it is limited due to its sensitivity and specificity. Candidate biomarkers currently being tested include neutrophil myeloperoxidase, fecal neopterin, citrulline, Regeneration gene 1A, Regeneration gene 1B, antibody to bacterial cell wall LPS core oligosaccharide (EndoCab antibody), and zonulin (see above section on intestinal barrier integrity) (491).
Immunologic manifestations of EE are outlined in Text Box 33.
Text Box 33 . Immunologic/inflammatory impacts of EE.
● Mechanistically, the manifestations of EE are the result of a persistent cell-mediated inflammatory reaction in the mucosa, which leads to increased rates of cell destruction (496).
● Lamina propria T cell subpopulations (CD3, CD4, and CD8) are higher than normal, but CD8:CD4 values are normal.
● Cells expressing the CD25 activation marker are more commonly observed.
● Intraepithelial lymphocytes are common and contain perforin granules that reflect cytotoxic capability and indicate that the mucosa is under severe distress.
● The mucosa of children with EE shows increased numbers of both pro- and anti-inflammatory cytokine-producing cells, although this increase is smaller in children with malnutrition.
● Cells producing anti-inflammatory cytokines are markedly reduced in severely malnourished children, giving an upper hand to proinflammatory cytokine–producing cells (497).
People living in communities in poor conditions of hygiene and sanitation are not equally affected by EE, which implies the need to discover underlying factors, such as genetic predisposition, amount of exposure to pathogen, role of pre-existing malnutrition, as well as the role of immune system (490). Characterization of EE in at-risk individuals is clearly important for the interpretation of nutritional biomarkers.
V. Chronic and noncommunicable diseases.
As highlighted in the recent review of the GBD (1), there are several alarming global trends that occur with regard to NCDs.
● The prevalence of high BMIs has increased globally to become the sixth greatest risk worldwide.
● 1.3 million deaths were due to diabetes in 2010, twice as many as in 1990.
● Deaths from NCDs (diabetes, CVD, cancer) increased by ∼8 million between 1990 and 2010, accounting for 2 of every 3 deaths (34.5 million) worldwide in 2010.
Each of the major NCDs has been shown to have a strong inflammatory component, including cancer (498–500), CVD (501–503), and diabetes (504–506). For the purposes of this review, WG 3 chose to focus on obesity and chronic obstructive pulmonary disease (COPD) as examples of the inflammation, nutrition, and NCD nexus.
a. The double burden of malnutrition.
Developed nations in the 1980s saw an upsurge in overweight and obesity among adolescents. Now, LMICs are facing the same problem of an increasing prevalence of overweight/obesity. In addition, these same settings are facing the added trend of overweight and obesity coexisting with undernutrition (associated with deficits of calories, specific single or multiple nutrients) in the same individuals, households, or communities—a condition called the double burden of malnutrition (6). This double burden is occurring in many settings, but recent data from Bangladesh offer a graphic example of the problem. In Bangladesh, where childhood stunting prevalence is one of the highest in the world (41% with height-for-age z scores ≤2, and 24% of women chronically malnourished with a BMI <18.5), overweight and obesity affect 17% women of childbearing age (507).
Among the challenges presented by the double burden are how best to develop and roll out programs to address either under- or overnutrition in settings in which both coexist. Clinically, the concern becomes how best to assess not only nutritional status broadly but how best to address potential interactions of over- and undernutrition with inflammation (i.e., with the APR). As will be outlined below, obesity (and its comorbidities) has an array of effects on the inflammatory response and vice versa. Add to that the impact of anomalies in the status of single or multiple micronutrients, and one can envision a complex scenario that can affect clinical accuracy and the development of effective interventions. Clearly, this complexity outlines an urgent research agenda.
The role and impact of selected nutrients on immune function and the inflammatory response were covered by WGs 1 and 2 and are covered in additional detail in Supplemental Table 9. The following is a brief discussion of the relations between overweight/obesity individuals and inflammation.
b. Overweight and obesity are chronic inflammatory states.
Overweight and obesity are inflammatory in nature because adipose tissue releases a number of inflammatory mediators (508). The main source of the mediators is believed to be macrophages that infiltrate adipose tissue, although adipocytes also contribute to the inflammatory process. Chronic low-grade inflammation is also a risk factor for type 2 diabetes and the metabolic syndrome (a complex of symptoms that include a combination of obesity, hypertension, impaired fasting glucose, or dyslipidemia). In obese subjects, compared with those with normal weight, there is an upregulation of systemic indicators of inflammation such as total leukocyte count, serum APRs, proinflammatory cytokines and chemokines, soluble adhesion molecules, and prothrombotic mediators. Although there may be an overlap between obese and nonobese states, obese individuals may have a severalfold increase in serum concentrations of TNF, IL-6, MCP-1, IFN-γ–induced protein 10, IL-18, macrophage migration inhibitory factor (MIF), and regulated on activation, normal T cell expressed and secreted (RANTES).
These chronic inflammatory mediators occurring in overweight and obesity are considered to be involved in insulin resistance and the metabolic syndrome. Adipose tissue is in fact a large endocrine organ secreting the proinflammatory hormone leptin and the hormones visfatin and resistin, which cause insulin resistance (509–511). In addition, it produces APRs, cytokines, chemokines, growth factors, components of the alternative complement system, and RBP. Ectopic deposition of adipose tissue may also occur in the absence of obesity and causes inflammation. This is particularly important in causing cardiac pathologies if the deposit is in the heart. This perhaps underlies the “thin-fat” hypothesis of increased atherosclerotic heart disease among Indians who have increased visceral fat deposition that may not be associated with obesity (512). The concentration of inflammatory mediators in obesity is reduced with increased physical activity (513, 514), although whether the reduction is due to physical activity itself or to reduced body weight needs to be clarified.
c. Inflammation and nutrition in obstructive lung diseases.
Persistent inflammation seen in many chronic diseases is associated with increased morbidity and mortality (515–518). Moreover, therapies aimed at reducing inflammation were found to lower overall mortality among healthy people with elevated CRP (519), reduce progression of atherosclerosis in patients with coronary artery disease (520), and improve exercise capacity (521) in patients with COPD. The topic of how inflammation affects nutrition in chronic NCDs or vice versa, however, is less well studied. In this section, obstructive lung disease (OLD) is discussed as an example of the bidirectional relation that may exist between inflammation and nutrition in chronic diseases, in order to identify potential gaps in our understanding (characteristics of OLD are shown in Text Box 34). In Figure 1, a schematic is provided of the bidirectionality of this relation identifying potential nutritional contributors and outcomes. A range of nutritional disturbances is considered, including obesity, weight loss, muscle wasting, adipose tissue accumulation, and specifically, micronutrients and vitamins A and D (Figure 1).
Text Box 34 . Characteristics of OLD.
OLDs are a group of chronic respiratory conditions characterized by:
The spectrum of OLD ranges from:
● reversible airway disease, more commonly seen in asthma, to
● irreversible airway disease associated with emphysema and more commonly seen in COPD (523).
Inflammation occurs both locally in the lungs but also in the systemic circulation (524). In both asthma and COPD, local inflammation leads to airflow limitation either through:
● hypertrophy of mucous glands,
● excessive secretions, or
● bronchial smooth muscle hyperactivity.
In chronic OLD, the inflammation leads to either airway remodeling (525) or destruction of lung parenchyma. Chronic lung inflammation in COPD is followed by increased systemic inflammation, weight loss, and low muscle mass (526).
FIGURE 1.
Bidirectional relation between inflammation and nutrition in obstructive lung diseases. Various nutritional contributors and outcomes are associated with systemic inflammation resulting from lung inflammation in obstructive lung disease. Original to manuscript produced with permission from William Checkley. CRP, C-reactive protein.
In chronic conditions such as asthma, inflammation and nutritional disturbances are often related to epiphenomena such as environmental exposures to tobacco smoke, air pollution, or airborne allergens. Evidence that inflammation leads to worse nutrition is reported in studies linking increased TNF production (527–530) and higher resting energy expenditure (531) with being underweight in patients with COPD. Moreover, the systemic inflammation may also be directly responsible for muscle wasting and cachexia seen in some patients with COPD (532–535). It was also reported that high-sensitivity CRP was a marker for impaired energy metabolism, lower functional capacity, and a lower St. George’s Respiratory Questionnaire score in patients with COPD (536). Previous studies showed that a low fat-free mass assessed by bioelectrical impedance analysis is an independent predictor of mortality irrespective of fat mass (534).
More recently, a placebo-controlled randomized trial in patients with COPD found that nutritional supplementation, in combination with a short course of anabolic steroids vs. nutritional supplementation alone, enhanced gain in fat-free mass and respiratory muscle function in depleted patients with COPD over an 8-wk period (537); however, a post hoc analysis did not identify a survival benefit from the intervention (538). Hence, more evidence is needed to determine if the prevention of weight loss in patients with COPD is a modifiable endpoint with nutritional interventions, and if such interventions lead to a measureable survival benefit.
i. Obesity and OLD/asthma.
The importance of obesity in asthma has received increasing attention over the past decade, in part because of the striking increase in the prevalence of global obesity (550–552) change (539–541). Potential mechanisms to explain the impact of obesity on asthma are outlined in Text Box 35.
Text Box 35 . Obesity and OLD/asthma: potential mechanisms.
● Reduced lung volumes (542)
● Greater airway responsiveness (543)
● Increased local and systemic inflammation that may be associated with an exaggerated response to environmental triggers (541)
● An increase in abdominal fat mass associated with a chronic elevation of the circulating concentrations of inflammatory mediators including CRP and pro- and anti-inflammatory cytokines and chemokines, leading to a state of low-grade inflammation (508 )
Research in a multitude of settings with the use of various study designs in children and adults has identified an association between obesity and asthma (544–551). However, there is insufficient evidence to state whether obese children have a greater risk of more severe asthma (541).
In terms of potential interventions, a few small-scale, randomized studies examined the role of weight loss in adults (552–554) but none have been conducted in children. A recent systematic review found weak evidence to support weight reduction as an intervention to improve asthma outcomes. Better evidence on the relation between weight loss and asthma outcomes will be provided by the BE WELL (Breathe Easier through Weight Loss Lifestyle) intervention, a randomized clinical trial of the efficacy of an evidence-based, comprehensive, behavioral weight-loss intervention in obese adults aged 18–70 y with suboptimally controlled, persistent asthma (555).
ii. Specific micronutrients and OLD.
The role of micronutrient deficiencies in the etiology of OLD has also gained considerable attention over the past 2 decades (556). Multiple observational studies found micronutrient deficiencies to be associated with either asthma (557–565) or COPD (566, 567), although randomized supplementation trials yielded inconsistent results (558, 561, 562). Plausible mechanisms and extant evidence for 2 micronutrients, vitamins A and D, are presented in Text Box 36.
Text Box 36 . Case studies: micronutrients and OLD/asthma.
Vitamin A
● Vitamin A is necessary for optimal lung epithelial cell differentiation and lung development (68, 568, 569).
● Animal studies have shown that VAD promotes airway hyper-responsiveness and alters the mechanical properties of the lung parenchyma (570).
● Multiple observational studies found that VAD is associated with a higher risk of asthma (571–575). However, no randomized trials have been conducted to support a causal relation.
● In a follow-up of 5430 children randomly assigned to receive vitamin A supplementation early in life (i.e., maternal supplementation or during the first 6 mo of infancy), supplementation with vitamin A was not associated with a decreased risk of asthma or lower forced expiratory volume in 1 s to forced vital capacity (FEV1:FVC) ratios at 9–23 y of age (576).
● Observational studies identified an inverse association between asthma risk and vitamin A status, which may be explained by reduction in serum retinol due to inflammation. So it is not clear whether this is a nutritional effect or a secondary consequence of inflammation.
Vitamin D
● Poor prenatal vitamin D status has been implicated in increased asthma risk and severity in the offspring, although evidence to support an important relation remains controversial (577).
● VDD may impair lung development and function (578–580) and may weaken immunologic defenses against respiratory infections, which could trigger asthma exacerbations (581).
● Low vitamin D concentrations may increase risk of allergic sensitization leading to abnormalities in the allergic immune response (582).
● Several studies reported an association between VDD and increased asthma risk (581, 583–590).
● Evidence exists for a link between lower maternal vitamin D intake and higher risk of wheezing, allergic rhinitis, and eczema in childhood (591–595).
● In a multicenter study in the United States, vitamin D insufficiency (<30 μg/L) was predictive of severe asthma exacerbations (586).
● No randomized trials have been published on the effects of vitamin D supplementation to prevent asthma or to reduce morbidity.
● Five ongoing clinical trials may provide further insights into the role of vitamin D in the chronic inflammation related to asthma (596).
Inflammation and nutritional disturbances observed in OLD could also be epiphenomena of environmental exposures that result in allergic inflammatory responses. For example, cigarette smoke, ozone, and nitrogen oxides (primarily NO and NO2) are all rich in free radicals (597), which may not only affect overall antioxidant micronutrient concentrations but also increase local inflammatory responses in the lung. Allergic inflammation in the lungs may also occur in response to traffic-related pollutants as evidenced by observations of positive associations between traffic, ambient pollutant exposures, and both asthma and allergy outcomes in asthmatic children (598–605).
The existence of plausible mechanisms to explain possible interactions between specific or multiple micronutrients in the absence of clinical trial data to support such relations suggests either poor design or lack of attention to sound principles of nutritional assessment in such trials (e.g., the need for accurate baseline data to establish status before interventions or to explain subject status). A need also exists for better clinical characterization, including nutrient-specific biomarkers, that accounts for the potential impact of inflammation in order to address these relations. Better data are needed with regard to the role of nutrients in OLD, both in terms of etiology/risk and as potential outcomes (primary: nutrient malnutrition; secondary: metabolic derangements leading to loss of appetite, changes in body composition) of the OLD process.
VI. Pre- and postnatal conditions, outcomes, and inflammation.
Pregnancy inflammation (infectious or noninfectious) is often associated with increased risk of adverse pregnancy outcomes, and subclinical endotoxemia has been associated with systemic and adipose tissue inflammation in pregravid obese women (606). The features of noninfectious inflammation in pregnancy are highlighted in Text Box 37.
Text Box 37 . Features of noninfectious inflammatory conditions of pregnancy.
● Normal pregnancy may increase serum α1-antichymotrypsin (ACT) (587, 588, 607), and parturition induces an APR (589, 590, 608), with serum ACT concentrations occurring at levels similar to those found in patients with tuberculosis (609).
● Macrophage infiltration of adipose tissue and the placenta is characteristic of established obesity.
● In pregnancy, obesity is associated with an exaggerated inflammatory response with increased macrophage recruitment to the placenta resulting in elevated proinflammatory cytokine production (606).
● Macrophage-associated cytokines linked to insulin resistance include MCP-1, macrophage inflammatory protein 1α (MIP-1α), IL-1, IL-6, and IL-18 (610).
● Obese women with gestational diabetes mellitus release more leptin in response to cytokine stimulation than do those without diabetes (611, 612).
● Pregnancy inflammation (infectious or noninfectious) is often associated with increased risk of adverse pregnancy outcomes, and subclinical endotoxemia has been associated with systemic and adipose tissue inflammation in pregravid obese women (606).
● Binding of pathogenic microbes to TLRs, which are widely expressed at the maternal-fetal interface, commonly results in the production of cytokines and antimicrobial factors via a common signaling pathway during pregnancy (613).
● TLRs have also been linked with their expression in the placenta in cases of macrophage-driven inflammation in obesity and intrauterine infection, subsequent chorioamnionitis, and preterm delivery (613, 614).
● An association between in utero markers of inflammation and increased risk of preterm birth has been reported (615, 616).
● A “leaky” placenta has been associated with the pathophysiologic defects associated with inflammation resulting from obesity and/or oxidative stress associated with diabetes (617). These defects include increased vascular endothelial growth factor (VEGF), vascular leakage of albumin, and altered junctional adhesion molecules.
● Changes in placental transfer of lipids (FAs) and cytokines have been noted in obese pregnant women and in those with diabetes (618).
● This “leakage” is one of the suspected side effects of gestational diabetes mellitus and pregestational diabetes.
a. Oxidative stress in pregnancy and in prematurity.
Tissue lipid peroxidation is increased in healthy pregnancy, but gestational increases in lipid peroxidation by-products are aggravated in overweight, obese, and diabetic pregnant women, and oxidative stress is an important consequence of pregnancy obesity that can directly affect micronutrient status. Oxidative stress associated with increased ROS and/or reactive nitrogen species has also been linked to poor reproductive outcomes (e.g., idiopathic infertility, poor semen quality, and low-quality oocytes) (619) as well as poor implantation and increased endometriosis via placental ischemia/hypoxia-mediated defects (620). Thus, it has been suggested that early-onset pre-eclampsia, spontaneous miscarriage, and intrauterine growth restriction may represent a spectrum of placental oxidative stress–related disorders (620). The role of oxidative stress biomarkers (F2-isoprostanes, oxidized low density lipoprotein, malondialdehyde, hyperhomocysteinemia, NO, peroxynitrites, 8-oxodeoxyguanosine, reduced glutathione/oxidized glutathione, 4-hydroxy-2-nonenal, tyrosine nitration) and their nutritional modification in relation to pregnancy pathogenesis requires further investigation (619).
There is good evidence that oxidative stress is associated with preterm birth complications due to an underdeveloped and highly stressed antioxidant system (621, 622). Because the activity of key antioxidant enzymes only matures in the last weeks of gestation, preterm infants have a developmentally immature antioxidant system showing generally low activity of superoxide dismutase, catalase, and glutathione peroxidase enzyme activities, thus predisposing to oxidative stress. Moreover, preterm infants demonstrate a relatively high activity of the xanthine/xanthine oxidase system that can aggravate oxidative stress via production of superoxide anion. Membrane α-tocopherol concentrations and plasma and tissue carotenoid concentrations are lower in premature infants than in full-term infants due to lack of placental transfer of lipophilic antioxidants during the late gestation (623). There are also indications of problems with the intestinal absorption of fat-soluble antioxidant micronutrients, such as tocopherol, in premature infants, which might relate to the immaturity of the intestine and limited absorption of these vitamins. Because of the current conflicting findings, further research is needed to establish whether preterm or term human milk, both of which contain a range of hydrophilic and lipophilic antioxidants, provides better protection against oxidative stress than formulas fortified with a few antioxidants.
Preterm oxidative stress and metabolic programming of the fetus may propel the fetus toward obesity and insulin resistance.Maternal obesity also contributes to an environment in which the embryo and fetus may be exposed to oxidative stress, because the expression of genes related to oxidative stress are elevated in the placenta of obese women. Lipid peroxides, oxysterols, and oxidized lipoproteins can act as ligands for nuclear receptors such as liver X receptor, which is involved in important metabolic pathways, and lead to metabolic programming of the offspring toward insulin resistance and future obesity (624, 625). Pregnancy obesity is also reported to impair fetal iron status, which may be linked to hepcidin homeostasis (626).
b. Acute and chronic infection in pregnancy.
It has been well established that pregnant women are more susceptible to some infections, and for this reason they represent a special population group (627). Systemic immune responses in pregnancy are modulated rather than suppressed (628) and are influenced by placental cytokine networks (Figure 2); these changes include both proinflammatory and anti-inflammatory profiles (613). In addition the adaptive immune response is downregulated whereas innate immunity is enhanced. As a result, pregnant women are more susceptible to parasitic infections and to severe bacterial and viral infections. This leads to distinct inflammatory pathways with maternal, placental, and fetal consequences (630). Specific pathways are described in the following sections, indicating biomarkers that are induced or altered by inflammation, and indicating relations with nutritional status and biomarker profiling.
FIGURE 2.
Maternal and fetal effects with infectious and noninfectious inflammation. Factors such as infections, hormonal factors, or maternal obesity affect the systemic immune system during pregnancy. Maternal and fetal immune responses are modulated by placental pro- and anti-inflammatory cytokine networks. Adapted from reference 629 with permission.
Important outcomes of pregnancy infection are preterm birth and low birth weight, both of which have important nutritional characteristics that effect biomarker profiles. These adverse pregnancy outcomes may result from acute infection late in pregnancy or from chronic infection with persistent low-grade inflammation from early gestation. Bacterial vaginosis and chorioamnionitis may be nutritionally modulated, and maternal iron status could be important, with the mucosal immune biomarker lactoferrin in influencing infection profiles (629).
Other chronic pregnancy infections, such as soil-transmitted helminths, contribute to maternal nutritional anemias, which are associated with similar adverse pregnancy outcomes (631–633). Low-grade bacteremia and/or cytokines generated within oral tissues have also been associated with increased risk of preterm birth and neonatal morbidity (634). In the context of low-grade inflammation, regardless of cause, extent, and location, there is evidence for an impact on gastrointestinal morphology and function, which can have nutritional implications (635). Thus, the effects of both acute and chronic infections on nutritional status raise the possibility that nutritional interventions, in addition to infection control, could modulate pregnancy outcomes, especially in developing countries where an underlying prevalence of undernutrition is common and where exposure to pregnancy infections such as malaria is often high (632). Nevertheless, few studies have examined how pregnancy inflammation/infection itself directly modulates nutritional status or biomarker profiles of the mother or the infant. Even the utility of CRP as an indicator of inflammation/infection during pregnancy has been questioned (636). Studies usually describe how infection and inflammation are associated with nutritional deficiencies [e.g., iron, vitamin B-12, folate, and vitamin D for bacterial vaginosis (637–639) and anemia or obesity for stillbirths (640)].
c. Fetal and infant infection.
The immunologic responses by the maternal, placental, and/or fetal immune systems can affect fetal development (641) and perinatal survival. The question of whether these adverse effects are due to a direct effect of the pathogen (viral, bacterial) or are secondary through maternal responses to inflammation/cytokines requires further investigation. Some investigators consider that impaired offspring TLR function is associated with increased susceptibility to infections and to preterm labor (613).
Several infections are known to affect fetal growth. An important example in humans is Plasmodium falciparum malaria, which, after placental parasite sequestration, induces specific maternal endocrine, immunologic, and hematologic responses that are associated with fetal growth restriction and preterm birth (642). Infants born to these mothers exposed to malaria in pregnancy have increased infant anemia and mortality risk (643). Another example encompasses the range of gastrointestinal nematodes, which are mostly chronic maternal infections, and which have been associated with stunting in the offspring (644). At least in an animal model, these infections in pregnancy may lead to offspring stunting by causing inflammation, and this has been associated with increased cortisol concentrations and altered cytokine profiles (645).
Infants of mothers with pregnancy inflammation and/or infection have altered systemic immune exposures. A fetal inflammatory response syndrome is described in which infants born after placental infection have elevated inflammatory cytokine profiles (628, 646), despite absence of cultivable microorganisms. Constituents in breast milk help protect the infant from enteric infections (e.g., lactoferrin), whereas inflammatory factors, such as in subclinical and clinical mastitis and which influence postnatal infection transmission via breast milk (e.g., HIV), may directly influence infant mucosal immunity as well as the infant’s microbiome. This is an area for further research.
d. Mastitis.
Mastitis, an inflammatory condition of the breast tissues, may or may not be accompanied by infection (647, 648).Milk stasis and infection are the 2 majors causes of mastitis (647, 649), but the concept of mastitis as used in contemporary empirical studies remains inconsistent, perhaps due to the disparate views of its etiology (650). The details of the inflammatory response have been best described in cows (651).
In humans, subclinical mastitis (SCM) is usually defined by the absence of external inflammatory symptoms and one or more of the following indicators: reduced milk secretion, increased sodium-to-potassium (Na+:K+) ratio, elevated IL-8 concentrations, elevated milk leukocyte or somatic cell count, and/or a high bacterial count in a milk sample. Other ancillary tests include the following: milk microbiological cultures, N-acetyl-β-d-glucosaminidase activity, pH, lactose content, electrical conductivity, flow measurements, and quantification of APPs (648, 652). Some suggest that somatic cell and IL-8 counts are more informative indicators of mammary inflammation than sodium content (653). Others suggest microbial identification techniques based on pyrosequencing of the 16S ribosomal RNA gene to obtain a description of the human-milk microbiome (654), as a useful diagnostic tool to assess pathogen persistence (655). Metagenomic and transcriptomic analysis of milk samples from healthy and mastitis-affected women are currently underway. Preliminary results indicate that human mastitis may be characterized by a mammary bacterial dysbiosis, a process in which the population of mastitis agents increases whereas other bacteria, normal or commensal mammary microbiota, decrease (648). How these responses relate to, or result from, maternal nutritional factors is little studied.
Mastitis enhances vertical mother-infant transmission of infections, particularly HIV (656). Prompt diagnosis and treatment prevents lactation failure, recurrent mastitis, and breast abscess (655), but the source of entry of pathogenic bacteria remains uncertain. In contrast to mastitis, systemic inflammation, although unconfirmed, may be the cause of SCM. SCM may affect the health of the newborn (657), because it may alter milk composition through changes in intramammary inflammation and leakage, changes in the metabolic activity of the mammary epithelial cells, and inflammation-mediated changes in nutrient transport (648, 652).
Mastitis has been associated with oxidative stress (658) and changes in cytokine gene and protein expression associated with adaptive immunity (659). In some conditions, supplementation with vitamins A, D, and E and copper and selenium was protective against mastitis (652), but others reported in HIV-infected women that antioxidant micronutrient supplementation did not lower (660), and might even increase the risk of SCM (661).
e. Polymorphisms and epigenetic effects associated with infection, inflammation, oxidative stress, and pregnancy outcomes.
Polymorphisms in the TLR genes have been associated with both increased susceptibility to infections, in particular bacterial vaginosis, and with preterm labor (613). Some superoxide dismutase and catalase polymorphisms have been related to the development of prematurity complications. It was suggested that single nucleotide polymorphisms involved in the generation of ROS and reactive nitrogen species, or free radical scavenging proteins, might be used to identify preterm infants who might benefit from specific antioxidant prevention strategies against free radical–mediated diseases (662). More recently, the emerging role of oxidant molecules in fetal programming was reviewed (663). It is now recognized that genomewide association studies only explain a small proportion of the known heritability of phenotypes. Future studies based on higher definition exome chips that can identify highly penetrant but rare variants, using full genome sequencing to better examine regulatory regions, and studies of genone-epigenome interactions, will better illuminate these issues.
Basic principles of epigenetics were highlighted by WG 1 (Text Box 10). More specifically, in pregnancy, the epigenetic effects of maternal infection, oxidative stress, and nutrition exposures on fetal immune response genes have been little studied (663). Nutritional interactions may be critical because oxidative stress has been associated with epigenesis (663). Changes in methylation patterns can be directed or stochastic in nature. Animal experiments clearly show that changes in maternal methyl-donor supply (choline, methionine, folate, and vitamin B-12) can influence the offspring phenotype (664). Emerging data from humans are confirming such associations and, importantly, indicate that maternal nutritional exposures before and during the earliest stages of pregnancy can be critical (665, 666). Folic acid supplements in pregnancy have been associated with a slightly increased risk of wheeze and lower respiratory infection in children up to 18 mo, which may relate to methyl donors in the maternal diet during pregnancy that influence respiratory health in children, consistent with epigenetic mechanisms (667).
Similarly, the consequences of cigarette smoking in pregnancy on these important aspects of early development may have an epigenetic basis (668), and may influence nutrition in children, including risk of obesity and stunting (669).The periods of gestational susceptibility of the epigenome need to be defined precisely, particularly in relation to peak gestational periods of exposure or infection risk. An example of an infectious disease exposure is the well-established peak P. falciparum malaria prevalence at 13–16 wk gestation in primigravidae living in high malaria transmission areas (670).
f. Profiling nutritional and inflammatory biomarkers in mothers and infants in relation to clinical outcomes.
Profiling of nutritional and inflammatory biomarkers in mothers and neonates at birth should be emphasized to allow identification, through longitudinal studies, of baseline characteristics related to subsequent infant immunologic responsiveness, infection risk, nutritional status, and vaccine efficacy. In particular, there is a need for a better understanding of mechanisms affecting iron acquisition of pathogens causing pregnancy and perinatal infections, because this could have implications for maternal iron supplementation (629). Two recent epidemiologic studies showed that iron status predicted malaria risk in very young children. Tanzanian infants recruited at birth and who developed ID during follow-up had significantly decreased odds of subsequent parasitemia (23% decrease; P < 0.001) and subsequent severe malaria (38% decrease; P = 0.04) (671). ID was also associated with 60% lower all-cause mortality (P = 0.04) and 66% lower malaria-associated mortality (P = 0.11) (671). Likewise, malaria risk was predicted by iron status in Malawian preschool-aged children, suggesting that ID protects against malaria parasitemia and clinical malaria in young children (672). Children with ID at baseline had a lower incidence of malaria parasitemia and clinical malaria during a year of follow-up [adjusted HRs (95% CI): 0.55 (0.41, 0.74) and 0.49 (0.33, 0.73), respectively]. Whether these infection risks are definable using birth/neonatal nutritional and/or inflammatory profiling is uncertain. The extent to which invasive bacterial disease is present may also be a risk and unclear (673).
In pregnant women, host iron status may affect maternal and/or neonatal infection risk, potentially contributing to neonatal death (629). Iron acquisition mechanisms of pathogens causing stillbirth, preterm birth, and congenital infection are described but not understood in relation to host iron status. There is in vitro evidence that iron availability influences the severity and chronicity of infections that cause these clinical outcomes. However, in vivo, the risk is unknown because relevant studies of maternal iron supplementation did not assess infection risk (629). Caution with maternal iron supplementation may be indicated in iron-replete women who have high infection exposure, but the challenge in distinguishing iron-replete and iron-deficient women remains. The investigation of infection risk in relation to iron status in mothers and infants and the relevance of nutritional biomarker profiling is certainly an area in need of further research.
VII. Inflammation in specific conditions: drug exposures.
a. Nutrition and pharmacology: overview.
In the face of pandemic infection and the explosion of NCDs, the use of available single- and multiple-drug regimens will be expected to increase in all settings. Similarly, efforts to ameliorate hunger and more specifically single- and multiple-micronutrient insufficiencies continue to be a high global health priority. The potential clash of these public health interventions demands attention. Numerous examples of the potential for adverse effects of nutrient-drug interactions have been explored previously in the context of conditions such as cancer (674) and HIV (216, 675).
The role of nutrition in all aspects of pharmacology (pharmacokinetics and pharmacodynamics) was been outlined previously (216). Essentially, nutrients and drugs share common pathways and mechanisms of absorption, metabolism, transport, and elimination. Consequently, the potential exists for either of these to affect the other. The implications of these potential interactions are significant when one appreciates the magnitude and complexity of the global health reality, i.e., that major infectious diseases and NCDs are treated with the available single- (and more often multiple-) drug regimens. As noted throughout this report, these conditions continue to be highly prevalent in LMICs, subject in many cases to the impact of food insecurity and all aspects of the malnutrition continuum, including exposure to large-scale, high-dose micronutrient interventions. Thus, a need exists to recognize the potential for adverse outcomes consequent to the bidirectional relation between nutrients and drugs.
Aside from the well-established mechanisms of interactions outlined in Text Box 38, recent studies have highlighted another route, via genetic induction of key enzymes within critical pathways of drug metabolism.
Text Box 38 . Potential mechanisms of nutrient-drug interactions.
● Ingestion:
○ Both drugs and disease can cause changes in appetite and nutrient intake; resultant malnutrition can affect drug efficacy.
● Absorption:
○ Drugs and foods can have a mechanical effect, via binding or adsorption, that can influence the absorptive processes resulting in an increase or decrease in drug and nutrient absorption.
○ Some drugs can affect gastrointestinal motility, thereby increasing or decreasing absorption of nutrients.
○ Chemical factors, in particular the pH of the stomach contents and the influence of foods therein, can affect the subsequent absorption of drugs.
● Metabolism:
○ Mixed function oxidase (MFO)/CYP isozymes and conjugase systems convert drugs and nutrients into their active and excretory forms and are nutrient/cofactor dependent. Therefore, specific nutrient deficiencies or excesses can affect these systems and either increase or decrease activity.
○ Certain drugs can increase the activity of the MFO systems required to convert nutrient precursors into their active forms. Non-nutritive bioactive components in foods, supplements, and complementary and alternative medicine therapies (e.g., St. John’s wort, flavonoids) can induce MFO activity, thereby affecting drug metabolism.
● Functional utilization/activation of dependent systems:
○ Epigenetics: impact of nutrients and/or drugs on genetics/phenotypic expression
● Distribution: the utilization of both drugs and nutrients depends on body composition (obesity is a factor as is weight loss), availability, and functional integrity of transport proteins and receptor integrity and intracellular metabolic machinery.
For example, 1,25(OH)2D was observed to regulate genes responsible for the production of enzymes (including CYP3A4) responsible for detoxification in the intestine (676). This study, which was followed by numerous others, highlights an important and underappreciated role for vitamin D in drug metabolism through the induction of gene expression of key drug-metabolizing enzymes. This phenomenon should be evaluated closely, particularly in light of the expanding interest in vitamin D supplementation to boost immune function and for use in numerous conditions including HIV and other infections. Several of these types of interactions, including a similar enzyme-nutrient interaction involving vitamin A, were highlighted by WG 1.
The following is a brief coverage of specific aspects of these relationss as pertains to inflammation, nutrition, and health.
b. Steroids.
Numerous studies in humans have documented effects on inflammation of various drug exposures, although how these changes translate into effects on nutritional status or nutrient status or assessment (i.e., biomarker performance or interpretation) is less well defined. A basic categorization into steroidal and nonsteroidal compounds might be used for considering the influence of drug exposures. One of the most extensively documented compounds is the human glucocorticoid cortisol (or hydrocortisone). Various synthetic glucocorticoids are used in therapy and affect the immune system, with specific clinical consequences. . Glucocorticoids antagonize TNF-stimulated lipolysis and reduce resistance to the antilipolytic effect of insulin in human adipocytes (677). The extent to which this occurs may reduce the potentially deleterious effects of excess lipolysis and contribute to fat deposition in obesity, as well as inducing appetite leading to weight gain. Other effects include the inhibition of prostaglandin synthesis at the level of phospholipase A2 and cyclo-oxygenase isomerase, potentiating the anti-inflammatory effect (678). Glucocorticoids act nonselectively and may impair normal anabolism, leading to negative nitrogen balance, altered amino acid profiles, and muscle breakdown.
c. Nonsteroidal compounds.
Several anti-inflammatory drugs fall into this category, including aspirin, statins, and flavones, all of which may have an adjunctive effect on chronic inflammation. Aspirin can attenuate lipid-induced insulin resistance in healthy men, unrelated to changes in inflammatory markers (679). Aspirin is also commonly associated with gastrointestinal bleeding, potentially leading to mucosal injury and ulceration (680), and oxidative stress associated with released iron and inflammation, a scenario that can contribute to altered nutrient/micronutrient absorption and should be considered in clinically assessing nutritional status.
Reduced inflammation is also an effect of statins, which, in the NHANES 1999–2004, were associated with lower CRP and white blood cell (WBC) counts (681). These multiple effects indicate that drug exposures should be considered in the assessment of nutritional homeostasis. A detailed review of these effects is required to improve understanding of the consequences of the potentially wide variety of drug exposures on nutritional biomarkers in various clinical situations.
VIII. Conclusions.
Assessing micronutrient status in the presence of inflammation is difficult due to changes in APPs associated with infection and pathophysiologic changes in the host. These may involve changes in rates of synthesis and breakdown, plasma volume changes and hemodilution, and tissue sequestration. Improved understanding of the impact of inflammation on nutritional status is essential to correctly assess micronutrient status and to target those with the greatest need.
In this review a framework was presented to enable a structured approach to these assessments. It is essential to have an understanding of acute and chronic stress responses to inflammation and to translate impact in specific risk groups and among those living under different exposure conditions. The variability between infectious and noninfectious exposures is considered. Where possible, a generic approach has been adopted, although specific examples are used to illustrate particular host response pathways. The paradigm of multiple infection exposure is emphasized, and various templates were used to summarize the multiple effects of inflammation on both inflammatory and nutritional biomarkers in a variety of conditions, with an emphasis on obesity, pregnancy and infancy, and chronic infections. These illustrate both the complexity of host profiling and the need for identifying priority biomarkers, and those with functional significance on clinical outcomes. Context-specific examples were considered in relation to gut integrity and to the secondary effects of drug exposures. Nutrients considered in detail include iron, vitamin A, vitamin D, vitamin B-12, zinc, and folate (Supplemental Table 9). Summary statements of evidence that these nutrients play a role in inflammation were presented. The biochemical and functional consequences for the host and implications for biomarker assessment were considered.
IX. Research priorities.
Research priorities are highlighted in Text Box 39 and range from basic science to epidemiologic profiling and the identification of risk categories.
Text Box 39 . Research priorities.
Inflammation in specific conditions
● Elucidation of host mechanisms that influence iron acquisition by pathogens, particularly in the context of pregnancy and congenital and perinatal infections
● Nutritional correlates of proinflammatory states secondary to parasitic infection
● Long-term trials of nutritional supplementation, with or without anabolic steroids, on mortality outcomes in COPD
● Data to determine if reduction in inflammation reduces muscle wasting and all-cause mortality
● Trials of micronutrient supplementation in later life to determine effects on asthma exacerbations or severity
● An improved understanding of the pathogenic mechanisms related to environmental exposures may help identify the relevance of these to potential nutritional therapies
● Evaluation of preterm or term human milk, containing a range of hydrophilic and lipophilic antioxidants, for protection against oxidative stress in infants compared with formulas fortified with a few antioxidants
● Systematic review of effects of drug exposures on biomarkers of nutritional status
● Maternal and infant microbiome profiles and mucosal immunity
Inflammation in pregnancy and nutritional modulation of immune responsiveness
● Investigation of epigenetic influences on fetal immune responses and relation to nutritional exposures
● Critical periods of susceptibility to the epigenome need to be identified in relation to peak gestational periods of exposure (e.g., gestational timing of period of undernutrition) or for infection; e.g., for P. falciparum malaria peak prevalence at 13–16 wk gestation in high-transmission areas)
● Longitudinal studies in mothers and infants are required to identify the outcomes of these maternal exposures in their children in terms of clinical outcomes and inflammatory and nutritional biomarkers
Characterization of nutritional and inflammatory biomarkers of risk
● Characterization at birth of nutritional and inflammatory biomarkers to identify, through longitudinal studies, their relation with subsequent infant immunologic responsiveness, infection risk, nutritional status, and vaccine efficacy (this approach requires descriptive noninterventional studies and a focus on risk profiling in the first 2 y of life)
● Tests of development and cognition are relevant as additional assessment categories
● Characterization of inflammatory factors in subclinical and clinical mastitis that may influence postnatal infection transmission via breast milk (e.g., HIV) and that may directly influence infant mucosal immunity as well as the infant microbiome
● The role of oxidative stress biomarkers (F2-isoprostanes, oxidized LDL, malondialdehyde, hyperhomocysteinemia, NO, peroxynitrites, 8-oxodeoxyguanosine, reduced glutathione/oxidized glutathione, 4-hydroxy-2-nonenal, tyrosine nitration) and their nutritional modification in relation to pregnancy pathogenesis
● Evaluation of serum citrulline cutoffs in severely undernourished children as a recovery biomarker of the enterocyte cell mass and of fecal calprotectin as a biomarker of EE (calprotectin and other proxy markers should also be further assessed in less severely undernourished children)
d. WG theme 4: translating evidence to practice—approaches to addressing the nutrition and inflammation relations
Objective: 1) Identify clinically and programmatically relevant biomarkers of inflammation in the context of acute inflammation/infection and chronic inflammation/NCDs; 2) explore the impact of inflammation on nutrient biomarkers and summarize key features and challenges in nutrient biomarker performance and interpretation and use in the context of acute vs. chronic inflammation and individuals vs. populations; 3) identify approaches and evaluate options to account for the impact of inflammation on biomarker selection, use, and interpretation in clinical settings/points of care and population- level surveys. WG 4 summary and conclusions can be found in Text Box 40.
Text Box 40 . WG 4 summary and conclusions.
● For the evaluation of the available options to address the effect of inflammation on nutrition, a number of working definitions were presented: APR, APP, and categories of inflammation (e.g., acute, chronic, clinical, and subclinical inflammation).
● The WG also described the relative strengths and weaknesses of commonly used biomarkers of inflammation including WBC count, CRP, AGP, erythrocyte sedimentation rate (ESR), albumin, procalcitonin (PCT), TNF, and IL-6.
● Differences between acute and chronic inflammation were delineated.
● The WG outlined currently available options to account for the potential impact of inflammation on nutrient biomarker use and interpretation:
○ Inclusion of CRP and/or AGP measurements as adjuncts to nutritional assessments. However, consensus has been elusive with regard to how best to use these inflammatory markers in data analysis and interpretation.
○ Use of cytokines: although there has been interest, this approach has been found to be generally less useful in this context, primarily because cytokines have a substantially shorter half-life than do CRP or AGP. As a consequence, concentrations of cytokines may have returned to normal while the effect of subclinical inflammation on nutrient biomarkers continues.
○ Identification of biomarkers that are relatively less affected by inflammation (e.g., soluble transferrin receptor vs. ferritin). However, this approach is often a challenge due to higher cost, assay difficulty, and factors that may affect these markers (e.g., transferrin receptor is increased in malaria).
● The WG explored aspects of these interactions on biomarkers of specific nutrients including iron, vitamin A, hemoglobin, zinc, iodine, folate, and vitamins B-6, B-12, C, and D.
WG recommendations:
One or more biomarkers of inflammation should be measured in assessments of micronutrient status in populations with a known or suspected moderate-to-high prevalence of inflammation. At a minimum, CRP should be measured because of its feasibility and the availability of data on its relation to nutrient biomarkers. The ideal cutoff for CRP needs further evaluation and is likely lower than the 5 or 10 mg/L used in the literature.
● The addition of AGP as a measure of longer-term inflammation is probably useful in most settings and should be measured with CRP if resources are available, with a cutoff of 1 g/L. CRP and AGP cutoffs may also differ by demographic group (e.g., age, sex) and by nutrient biomarker, which needs to be investigated.
● With regard to available adjustment options for inflammation, the WG concluded the following:
○ In settings with considerable prevalence of inflammation, the exclusion approach may bias prevalence estimates and reduce precision and should therefore not be used. The correction factor approach is being readily used and is relatively simple to apply to data sets. However, it may also bias prevalence estimates and may not be applicable in all settings, where regression modeling may provide an alternate preferred approach.
Conclusion: Until more work is done to achieve consensus on approaches to account for inflammation, the WG recommended presenting data on the prevalence of micronutrient deficiencies in the population overall as well as among those with inflammation and among those with no inflammation.
I. Introduction.
As reviewed by WGs 1–3, our understanding of the interrelations between nutrition, immune function, and the acute and chronic inflammatory response continues to evolve. Coincident with the emergence of new knowledge is the need to translate this evidence to clinical and public health practice. In this section, the clinically and programmatically relevant biomarkers of inflammation, the impact of inflammation on nutrient biomarkers, and potential approaches to account for the impact of inflammation on nutrient biomarker interpretation will be addressed. The vernacular with regard to immune function and inflammation is complex, and many of the core concepts were presented in the previous sections (WGs 1–3).
II. APR and APPs.
The production of APPs is induced and regulated by the cytokines in response to tissue damage/stress. The types of APPs are covered in more detail by WG 1. Table 10 summarizes the response of several positive and negative APPs to inflammation. Compared with cytokines that have very short half-lives, APPs remain longer in the blood and can therefore be matched with changes in nutrient biomarker concentrations. The characteristic changes in APPs resulting from the APR have primarily been evaluated in healthy adults undergoing elective surgeries (24, 374, 685, 686).
TABLE 10.
Acute phase proteins in response to an inflammatory event1
| Acute phase protein | Acronym | Normal range | Amount of response to inflammation | Time to maximum response |
| Positive | ||||
| C-reactive protein | CRP | 0.001–10 mg/L | 20- to 1000-fold | 24–48 h |
| α1-Acid glycoprotein | AGP | 0.6–1.0 g/L | 2–5 times | 4–5 d |
| α1-Antichymotrypsin | ACT | 0.2–0.6 g/L | 2–5 times | 24–48 h |
| Ceruloplasmin | 0.3–0.4 g/L | 30–60% | 4–5 d | |
| Haptoglobin | 0.5–2.6 g/L | — | — | |
| Fibrinogen | 1.9–3.3 g/L | 30–60% | 24–48 h | |
| Serum amyloid A | SAA | ∼0.01 g/L | 20- to 1000-fold | 24–48 h |
| White blood cells | WBCs | >11,000/μL (adults) | — | 10 h |
| Erythrocyte sedimentation rate | ESR | Age <50 y: | Up to 100-fold | |
| Men, 0–15 mm/h | ||||
| Women, 0–20 mm/h | ||||
| Age ≥50 y: | ||||
| Men, 0–20 mm/h | ||||
| Women, 0–30 mm/h | ||||
| Procalcitonin | PCT | ≤0.5 μg/L | 200-fold | 6–24 h |
| Negative | ||||
| Transferrin | 2.0–3.0 g/L | −30% to 60% | 24–48 h | |
| Albumin | Alb | 35–50 g/L | −30% to 60% | 1–2 wk |
| Prealbumin (transthyretin) | PAB | 140–450 mg/L (depending on age) | −30% to 60% | 24–48 h |
| Retinol binding protein | RBP | >0.7 μmol/L | −26% to 40% | 24–48 h |
III. Acute vs. chronic inflammation.
An inflammatory response can be caused by infections and noninfectious entities, such as trauma, autoimmune disease, and chronic disease. The causes of inflammation can be characterized as acute (lasting days to weeks) or chronic (lasting months to years). There may be exceptions to these categories of inflammation, because certain diseases or conditions do not fit into a single category.
Acute inflammation is characterized by the following features:
● It begins within seconds of a cellular insult by such harmful stimuli as bacteria, viruses, parasites, toxins, or trauma.
● It may initially be covert or subclinical before clinical symptoms appear.
● Macrophages or blood monocytes (24) are the cells most commonly associated with initiating the APR cascade.
● Activated macrophages release cytokines (e.g., IL-1 and TNF), which then trigger the next series of reactions.
● These reactions take place locally and act primarily on the microcirculation at the site of “injury” in a nonspecific response.
● Locally, stroma cells (e.g., fibroblasts and endothelial cells) are activated to cause the release of a second wave of cytokines that include IL-6, as well as additional IL-1 and TNF. These cytokines magnify the homoeostatic stimulus and potentially prime all cells in the body with the potential to initiate and propagate this homeostatic response.
Chronic or low-grade inflammation is a critical element of NCDs, including the pathogenesis of atherosclerosis and insulin resistance (687, 688), and is characterized by a 2- to 3-fold elevation in systemic plasma concentrations of several cytokines including TNF and IL-6 as well as CRP (689). Why inflammation does not resolve with a return to homeostasis is not yet understood. Chronic inflammation may be due to loss of a barrier function, a continued APR to a normal nonthreatening stimulus, or infiltration of high numbers of inflammatory cells into areas where they are not normally found (17). The overproduction of inflammatory mediators may amplify the inflammatory response [e.g., oxidants, cytokines, chemokines, eicosanoids, or matrix metalloproteinases (17)] and contribute to the chronicity of inflammation.
IV. Implications of chronic inflammation.
The recognition of the profound impact of inflammatory states on health outcomes is just beginning to be recognized. As outlined in Text Box 41, the emerging evidence with regard to the role of chronic inflammation in the elderly offers a good case study of the potential implications of these relations.
Text Box 41 . Implications of low-grade/chronic inflammation: case study—elderly.
● Many older adults exhibit a low-grade proinflammatory state characterized by increased concentrations of cytokines and APPs (690).
● Potential causes include the following (691):
○ increased production of ROS,
○ development of disease-specific pathological processes,
○ increased fat mass,
○ decreased sex hormones, and
○ increased senescent cells
● Chronic infections may also produce low-grade elevation of many of these same mediators.
● Epidemiologic studies have used inflammatory markers such as IL-1, IL-6, and TNF and their receptors to examine the associations between adverse health outcomes in older adults and the chronic proinflammatory state.
● Although serum cytokines were once thought to be simple markers of inflammatory processes, increasing evidence suggests that some of these markers, including IL-6 and TNF, can have a powerful pathophysiologic impact on cells and tissues over time (498, 692, 693).
● Studies have shown significant associations between these markers and reduced physical function (694–698), cognitive function (699–704), incident mobility disability (705–707), and dementia (708, 709–712).
Despite the increasing awareness of the association between inflammatory markers and adverse health outcomes in older adults, the integration of these inflammatory markers into clinical practice has been limited. Possible reasons for this include a lack of clear mechanistic understanding, a plethora of inflammatory mediators that can now be measured, and the inconsistent relations of these markers with age-related outcomes. It has been argued that the main impediments to the clinical application of inflammatory mediators are their profusion and the inconsistency of their relation to outcomes (708, 713).
V. Clinically/programmatically relevant biomarkers of inflammation.
This section highlights specific biomarkers of inflammation that are most commonly used in clinical and public health settings. These biomarkers may be used to reflect one or more categories of inflammation, namely acute, subclinical, or chronic inflammation. Although serum markers of inflammation are often used to reflect systemic inflammation, they are, in general, not specific for diagnosing individual clinical conditions. However, inflammatory markers are occasionally used clinically for nonspecific diagnosis of serious underlying disease to mark treatment response and to predict clinical outcomes (714). An extensive review of inflammatory biomarkers used in research settings is outside the scope of this review. The most commonly used inflammatory biomarkers, their ranges, and their uses are summarized in Table 11. The following sections provide some additional details.
TABLE 11.
Summary of commonly used biomarkers of inflammation1
| Biomarker of inflammation | Normal range | Settings where used | Clinical vs. population use | Use in resource-limited settings | Comments |
| WBC | 4–11,000/μL | Acute inflammation (usually infection) | Clinical | Y | Varies by age |
| CRP | 0.001–10 mg/L | Acute, subclinical, chronic | Clinical, population | Y | |
| AGP | 0.6–1.0 g/L | Subclinical | Population | Y | |
| ESR | 0–30 mm/h | Acute, subclinical, chronic | Clinical | Y | Increases with age and higher in females |
| Albumin | 30–50 g/L | Acute inflammation (usually infection) | Clinical | N | Decreased during pregnancy |
| PCT | ≤0.5 μg/L | Acute inflammation (usually infection) | Clinical | N | |
| IL-6 | — | Chronic | Population | N | Use in aging |
| TNF-αR1 | — | Chronic | Population | N | Use in aging |
AGP, α1-acid glycoprotein; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; N, no; PCT, procalcitonin; TNF-αR1, TNF-α receptor 1; WBC, white blood cell; Y, yes.
a. WBCs.
Leukocytosis, an increase in the number of WBCs, has long been recognized as a nonspecific marker of systemic infection, tissue damage, allergic reaction, malignancy, or physiologic processes. Leukocytosis is classified according to the component of WBCs that contribute to an increase in the total number of WBCs; types include increased neutrophil count, lymphocyte count, monocyte count, eosinophilic granulocyte count, basophilic granulocyte count, or blastocyte (immature) cells. WBC count is routinely used clinically as an initial screening marker in patients with fever. Although most bacterial infections cause neutrophilia (≥10,000/mm3), the WBC and neutrophil counts alone are not sensitive or specific enough to accurately predict bacterial infection. Automated cell counter analyzers, common in many clinical laboratories, provide precise information on WBC counts as part of a complete blood count profile using only a small volume of freshly collected anticoagulated whole blood. Cutoff values for elevated WBC counts are age-specific and vary greatly (>11,000/μL in adults).
b. CRP.
CRP is an APP produced in response to acute injury, infection, or other inflammatory stimuli. Because CRP concentration increases within 4–6 h of an insult and is a measure of underlying systemic inflammation, it has numerous clinical uses including detecting bacterial infection, autoimmune disease, and malignancy, as well as measuring response to treatment (714). CRP may also remain elevated in the presence of a continued, chronic inflammatory process [e.g., atherosclerosis, rheumatoid arthritis, and obesity (715)].
Clinically, 5 mg/L originally defined the limit of detection of the method of analysis, and 10 mg/L was accepted as the upper level of the normal range. To identify subclinical inflammation in apparently healthy people in population studies and in research, 5 mg/L has generally become accepted as the upper limit of the normal range (716). CRP is used (in combination with other risk factors) to define risk groups for CVD.The American Heart Association and the CDC defined CVD risk groups as follows: low-risk CRP, <1.0 mg/L; average risk, 1.0–3.0 mg/L, and high risk, >3.0 mg/L (717).
Small differences in normal CRP concentrations have been found relative to race, age, and sex (718–720); however, because CRP responses to inflammation are usually large, and these differences are small, they received little attention until the introduction of the new high-sensitivity CRP assay that allows measurement to concentrations of 0.3 mg/L, compared with 3 mg/L for the modern conventional assay. Population data from NHANES 1999–2002 indicate that concentrations of CRP are higher among women than amoung men (median: 2.7 vs. 1.6 mg/L, respectively) and increase with age [median: 1.4 mg/L (20 – 29 y) vs. 2.7 mg/L (>80 y)] but vary less across categories of race or ethnic background (719). Later work by Cartier et al. (720 found that if CRP concentrations are adjusted for subcutaneous adipose tissue, the sex differences disappear, suggesting the greater subcutaneous fat in women to be responsible for the higher CRP concentrations.
The preanalytical stability of CRP in serum or plasma is excellent (11 d at room temperature, 2 mo refrigerated, 3 y frozen at −20°C) (721). No significant difference has been observed between samples collected either while fasting or nonfasting (722) and diurnal variation of CRP is negligible (723). Most CRP assays provide comparable results (724, 725), probably due to the availability of international reference materials and external quality assessment (EQA) programs to monitor the degree of variability and bias in laboratory assays (e.g., UK National EQA Service, US College of American Pathologists).
c. AGP.
AGP is a slower reacting positive APP (see Table 10) (24). At birth, concentrations are low and increase slowly, reaching adult values by 10 mo of age (726).
The methods of analysis are similar to those for CRP. There is a certified human serum reference material [ERM-DA470/IFCC (727)] for calibration and since 2008 it has been possible to standardize methods against this. There is an EQA scheme available, which is run by Randox Laboratories Ltd. UK (728).
AGP is not commonly used in clinical situations but is used for assessing longer-term (>5 d) or subclinical inflammation in population studies. With the onset of infection or trauma, changes in plasma nutrient concentrations closely parallel those of CRP. As clinical symptoms disappear, CRP concentrations fall sharply, but nutrient concentrations (e.g., iron, vitamin A) do not respond as rapidly. Therefore, AGP, which remains elevated longer than CRP and may take 3–5 d to reach a plateau, is often used to monitor inflammation during this time period (24).
The combination of CRP and AGP has been used to detect those who have only recently been infected and who are not yet showing clinical evidence of disease (increased CRP only) and those who have recovered and are convalescing (increased AGP with or without an increased CRP). However, as mentioned earlier, the changes in CRP and AGP have been described on the basis of acute tissue injury secondary to surgical procedures, so it is unclear how CRP and AGP act in response to an infectious agent (24).
d. ESR.
The ESR is defined as the rate at which erythrocytes suspended in plasma settle when placed in a vertical tube (mm/h) and is a nonspecific measure of inflammation that primarily reflects plasma viscosity. It is often assessed in low-resource settings with the use of an upright tube, but automated analyzers are also available. Despite its wide use, ESR has a number of disadvantages compared with other biomarkers (e.g., CRP), including slow changes in response to treatment and false elevation secondary to anemia. ESR is probably most useful clinically when markedly elevated (>100 mm/h), indicating infection or neoplasm (729).
e. Albumin.
Albumin is most commonly used as a marker of nutritional status (adequate protein intake) and also acts as a negative APP. The complex mechanisms responsible for serum albumin control render the interpretation of serum albumin concentrations challenging (730). Unlike prealbumin or transthyretin, which has a short half-life (∼2 d) and therefore reflects recent dietary intake, albumin has a long half-life (∼20 d); however, albumin is affected by several other factors and therefore has little value in the assessment of monitoring of nutritional status (731). Distribution ranges have been shown to differ on the basis of age and sex (732). A normal concentration is 3.5–5 g/dL (732).
f. PCT.
PCT is the prohormone of calcitonin. It has been identified as a biomarker of inflammation with potential for distinguishing bacterial from viral infections and correlates well with clinical severity (733, 734). Compared with either CRP or WBCs, PCT has been shown to be a better marker for identifying patients with invasive bacterial infections (735). In healthy individuals, circulating concentratons of PCT are generally very low but can increase by thousands-fold within 4 to 6 h in response to systemic infection (peaking as early as 12 to 24 h after onset). By contrast, CRP concentrations increase slowly during the first 6–12 h, peaking 48 to 72 h after infection onset. PCT also shows a dose-response relation, with higher concentrations correlating with increased severity of infection (735). The cutoff point for PCT varies widely and is most commonly ≥0.5 μg/L but can be as low as ≥0.12 μg/L (736). Multiple methods for PCT measurement are available from serum or plasma, including rapid, manual, and automated immunometric assays. The choice of assay and cutoffs depends on the intended clinical use (737).
g. TNF-α.
The origin of TNF in chronic inflammation is mainly the infiltrated macrophages in adipose tissue (738). Concentrations of TNF are 7.5 higher in obese than in lean subjects (739). Increased concentrations have also been observed in smokers; in patients with type 2 diabetes, metabolic syndrome, and atherosclerosis; and in aging (739–741). TNF promotes endothelial activation, increasing vascular permeability; decreases insulin sensitivity; and contributes to the progression of atherosclerosis (742–744). Plasma concentrations of TNF predict the risk of myocardial infarction (745). The most common available methods for TNF measurement are ELISA and radioisotope-labeled immune assays. Cutoffs are not standardized, and the reference range can vary from 0.55 to 2.53 pg/mL (746).
h. IL-6.
IL-6 has been classified as both a pro- and anti-inflammatory cytokine (747). It inhibits TNF production (748) and also stimulates the production of IL-1 receptor antagonist, IL-10, and soluble TNF receptors, which have anti-inflammatory effects (747, 749). IL-6 is released from contracting muscles during exercise, inducing lipolysis and fat oxidation and is involved in glucose homeostasis (689). The role of IL-6 in insulin resistance remains controversial. IL-6 can be measured by ELISA. Although IL-6 precedes the increase in CRP after exposure to bacterial infection, it has a very short half-life. Cutoff points vary in the literature from 25 to 150 ng/L (750, 751).
i. Conclusions.
In summary, numerous biomarkers of inflammation are available to detect acute, subclinical, and chronic inflammation in both clinical and public health settings. Table 11 summarizes the most commonly used biomarkers of inflammation. Text Box 42 contains a summary and some suggestions with regard to the available biomarkers of inflammation.
Text Box 42 . Summary of common biomarkers of inflammation.
● CRP remains the most commonly used biomarker of inflammation, both in clinical settings and to evaluate inflammation in populations.
● Several portable assays for CRP are available, which makes it a useful biomarker in resource-poor settings.
● Biomarkers such as PCT offer potentially more sensitive options compared with CRP and may be useful clinically to distinguish between bacterial and viral infections.
● In populations, AGP when measured together with CRP may be useful to detect subclinical inflammation.
● The use of ESR is not recommended in population settings, because several exogenous factors can affect values, and numerous population-specific cutoffs are therefore needed.
● Cytokines, such as TNF and IL-6, may have utility in detecting chronic inflammation in certain populations, such as the elderly. However, their routine clinical or programmatic use in populations has yet to be defined.
● Defining cutoffs for inflammatory biomarkers is an urgent research priority, in particular for clinical practice.
● These cutoffs may depend on the setting and nature of the target population as well as how they are used to account for the effect of inflammation on nutrition biomarkers.
VI. Nutrition and inflammation: specific nutrient perspectives.
a. Overview.
The myriad of causative factors and types of inflammation that need to be considered in the context of nutritional assessment were covered in the previous WG reviews and are briefly summarized in Text Box 43. Similarly, basic concepts with regard to the nature of the nutrient-inflammation relations were also covered previously by the INSPIRE WG reviews. The following sections cover the key features and challenges to interpretation presented by the inflammatory response of biomarkers of specific nutrients. The nutrients included below are those that have been addressed in the previous WG reviews, with a particular focus on the 6 nutrients that were covered under the BOND project: iron, zinc, iodine, vitamin A, folate, and vitamin B-12. These nutrients were selected for their public health importance and because the relations described represent the range of issues that the community needs to address when choosing and interpreting a nutrient or nutrients for clinical or population surveillance purposes. Vitamin D and hemoglobin are also covered because of their obvious importance to global health. The emphasis on these nutrients does not imply that similar effects are absent with other nutrients.
Text Box 43 . Specific signals that inflammation may need to be considered in the context of nutrient assessment.
● The type of inflammation (acute vs. chronic, clinical vs. subclinical, and etiologies of inflammation) may also be important to consider when evaluating the impact of inflammation on nutrition biomarkers.
● Acute inflammation is most commonly indicated by the presence of the following:
○ systemic bacterial infections,
○ tissue injury, and
○ allergies caused by food and environmental exposures.
● Conditions most often associated with the presence of chronic inflammation include the following:
○ gastrointestional disturbances such as celiac and inflammatory bowel diseases and environmental enteritis,
○ obesity,
○ arthritis,
○ asthma, and
○ NCDs including diabetes, cancer, and CVD.
● Other individual factors, such as age, socioeconomic status, diet, and breastfeeding may influence the APR and therefore the pattern of inflammation.
● Specific environmental conditions may predict the presence of inflammation, including conditions found in resource-constrained settings/LMICs such as poor sanitation, poor water quality, and food safety concerns.
In postpartum women, APPs remain elevated for some weeks after delivery.
b. Iron.
ID remains the most common micronutrient deficiency in the world, but the distinction of nutritional ID is often obscured by inflammation because the normal control of iron metabolism is disrupted by the primary mediators of the APR (i.e., TNF and IL-1) (752).
As outlined in Text Box 44 and reviewed in detail elsewhere [(753) and BOND iron review], a myriad of biomarkers exist that can be applied to the assessment of iron nutrition.
Text Box 44 . Currently available indicators of iron status.
Iron stores
● Bone marrow iron
● Ferritin
● Body iron stores or transferrin receptor (TfR):ferritin ratio
Iron supply
● Serum/plasma iron
● Total iron-binding capacity
● Transferrin saturation
● EP or ZPP
● Reticulocyte hemoglobin concentration
Functional iron deficit
● sTfR
● Hemoglobin
● Hematocrit (or packed cell volume)
● MCV
● Mean cell hemoglobin
● RBC distribution width
Experimental biomarkers
● Hepcidin
● Non–transferrin-bound iron
The BOND iron review can be found on the BOND website (30). The complexity of iron assessment led the WHO/CDC technical consultation on the assessment of iron status at the population level to compare the theoretical advantages for population-based surveys of the following 5 indicators of iron status: hemoglobin, serum ferritin, soluble transferrin receptor (sTfR), ZPP/erythrocyte protoporphyrin (EP), and mean cell volume (MCV) (26). The WHO/CDC consultation recommended serum ferritin as the single best indicator in areas where inflammation is less common and measuring sTfR in areas where inflammation is prevalent.
Hepcidin is a promising iron biomarker, because it is suppressed during ID and anemia but stimulated by inflammation, infection, and iron overload (754). Although hepcidin may be a sensitive marker for iron utilization and absorption, few data are available on hepcidin concentration and its relation to established iron markers in population studies. The relative strengths/weaknesses of other biomarkers of iron were previously reviewed (753).
Links between inflammation and iron biomarkers: current guidance.
With the use of data obtained from NHANES for women 20–49 y of age, the following associations were reported (755):
● A weak positive association was seen between body iron and CRP (body iron 0.7 mg/kg higher when CRP is >5 mg/L)
● A strong positive association between ferritin and CRP (24% higher ferritin concentrations when CRP is >5 mg/L)
● A weak positive association between sTfR and CRP (4% higher sTfR concentrations when CRP is >5 mg/L) (755)
Although the first 2 associations remained significant after controlling for certain covariates, the association for sTfR was no longer significant after controlling for age, sex, race-ethnicity, smoking, supplement use, fasting, and estimated glomerular filtration rate (eGFR). Of note, this population had a low prevalence of elevated CRP (∼20%) and ID (10–19%); thus, these relations may be not be directly applicable to populations with a high prevalence of these conditions. Text Box 45 contains a summary of suggested approaches to iron assessment for populations.
Text Box 45 . Approaches to iron assessment for populations.
● The recommendation of the WHO/CDC consultative group for population-based iron assessment was a combination of the following:
○ hemoglobin (with due recognition that there are other causes of anemia besides ID),
○ serum ferritin and sTfR, and, where possible,
○ the serum concentration of an APP (e.g., CRP or AGP) (26).
● In places where there is little inflammation, serum ferritin concentrations are recommended as the best indicator of ID.
● If infectious diseases are seasonal, the survey should be done in the season of lowest prevalence.
● Another approach to adjust concentrations of ferritin for the presence of inflammation is to use correction factors based on CRP and AGP measurements, as proposed by Thurnham et al. (272).
Anemia: hemoglobin.
Anemia remains a major and complex global health problem affecting one-quarter of the world’s population, most commonly women and preschool-aged children (756). One of the Global Targets for 2015 by WHO member states is a 50% reduction in the prevalence of anemia among women of reproductive age (757). The causes of anemia include inadequate dietary intake of micronutrients, most prominently iron, increased bodily demand for iron, inherited hemoglobinopathies, and infections (758). Hemoglobin is the main biochemical indicator of anemia and can be easily measured in surveys and in clinical settings by using a portable hemoglobinometer, such as the HemoCue (Radiometer Group) (759).
Although the relation between current or recent illness and anemia, the so-called “anemia of infection,” has been well characterized (760–763), the relation between biomarkers of inflammation and the prevalence of anemia is less studied. Several studies reported statistically significant relations between APP and anemia as shown in Text Box 46.
Text Box 46 . Significant relations between APP and anemia.
● In a national cross-sectional survey in Papua, New Guinea, preschool-aged children, the prevalence of anemia was higher among those with elevated CRP, AGP, or both. Similarly, anemia was higher in the incubation and early convalescence stages of inflammation compared to children with no inflammation (764). There was a stronger association between elevated CRP and anemia than between AGP and anemia. Children with inflammation had a prevalence of anemia ∼1.5 to 1.7 times higher than those without inflammation.
● A national cross-sectional survey in Nicaragua of preschool-aged children found that children with an elevated AGP were 1.4 times more likely to have anemia, controlling for age and mother’s educational level (765).
● In Marshallese children aged 1–5 y anemia prevalence was significantly higher among those with an elevated CRP or AGP than in those with a normal CRP/AGP (766).
● In a study in HIV-positive post-partum Zimbabwean women, those with an elevated AGP had significantly lower hemoglobin concentrations than those without elevated AGP (767).
● Nonmalarial inflammation (defined as elevated CRP and/or AGP without malaria) was the third most important risk factor for anemia in preschool-aged Kenyan children, after malaria and ID, controlling for multiple nutritional and demographic factors (768).
● Wieringa et al. (357) reported that infants with an elevated AGP had a slightly higher prevalence of anemia, although this was not statistically significant, and there was no association between elevated CRP and anemia.
● Gamble et al. (769) found no association in preschool-aged children (ages 1–5 y) between anemia and a combined marker of inflammation based on TNF-α and AGP.
The current evidence suggests an association between APP and anemia, although the strength of the association appears to be less than that found between APP and other nutrient-specific biomarkers (e.g., serum ferritin). In addition, there may be other factors that may influence the strength of the association of APPs and anemia within a population, such as the overall prevalence of both anemia and inflammation. Although expert groups have provided guidance on adjusting hemoglobin concentrations for altitude and cigarette smoking, no such guidance has been provided on how to account for APPs.
A need exists for more research on the association between inflammation and anemia. In the interim, some authors have recommended that cross-sectional surveys that collect information on anemia and markers of inflammation present the prevalence of anemia both among those with and without inflammation, in addition to the overall prevalence of anemia and the prevalence of inflammation, in order to allow for comparisons between populations (764, 765).
c. Vitamin A.
VAD remains a significant public health problem, primarily in preschool-aged children and pregnant women (770). The epidemiology of VAD was discussed in the WG 2 review and indicators of vitamin A have been reviewed extensively (BOND review) (30). Text Box 47 provides a summary of currently available biomarkers of vitamin A.
Text Box 47 . Currently available indicators of vitamin A.
● Night blindness: primary functional biomarker
● Serum retinol:
○ Retinol in serum measured by HPLC is the most widely used and accepted indicator for vitamin A status.
○ Concentrations of retinol <0.7 μmol/L in children aged 6–72 mo indicates VAD (770, 771).
○ Retinol is homeostatically regulated and is not a good indicator of status when there are adequate liver stores of vitamin A (770).
● RBP:
○ RBP is more stable than retinol.
○ It can be measured by using an ELISA technique, which is a much simpler method than HPLC (772).
○ RBP typically uses the same cutoff as for retinol, although population-specific cutoffs may be advisable becausee the RBP:retinol ratio is consistently <1:1 (773).
○ Because it correlates well with retinol, RBP measurements can be used to estimate vitamin A status.
● Breast-milk retinol (cutoff of ≤1.05 μmol or 8 μg/g milk fat) may reflect recent consumption rather than long-term status (324).
● Relative dose response (RDR) (cutoff of ≥20%) (771)
● Modified relative dose response (MRDR; cutoff ratio of ≥0.06)
○ Both RDR and MRDR are susceptible to inflammation.
○ MRDR and RDR tend to be used more in research situations rather than in population studies (771).
● Retinol isotope dilution: although not widely used clinically or programmatically, this is the most sensitive biomarker of vitamin A status to liver reserves of vitamin A.
Influence of inflammation on vitamin A assessment.
As outlined by WGs 1 and 2 and above, RBP is a negative APP (i.e., RBP decreases in the presence of an APR). Because RBP is the carrier protein for retinol, retinol concentrations also decrease in the presence of inflammation. Consequently, inflammation affects the interpretation of serum retinol and RBP.
Thurnham et al. (716) carried out a meta-analysis to calculate factors to adjust serum retinol concentrations for the presence of inflammation using CRP and AGP to define the stage of inflammation. In the presence of elevated CRP concentrations (>5 mg/L), serum retinol concentrations were 13% lower; in the presence of elevated CRP and AGP (>1 g/L), serum retinol concentrations were depressed by 24%, and elevated AGP concentrations alone decreased serum retinol concentrations by 11% compared with the normal (presumably uninflamed) group. Recently, the same factors were used to adjust RBP concentrations because of the good correlation between RBP and retinol. In NHANES data from adults, very similar results were obtained because there was a weak negative association between serum retinol and CRP (∼10% lower concentrations of serum retinol when CRP was >5 mg/L) (755). This association was true for crude data as well as after controlling for age, sex, race/ethnicity, smoking, supplement use, fasting, and eGFR.
Although this proposed adjustment seems to work under conditions of nonspecific inflammation, this approach may not be applicable in all settings. For example, in a recent study from a malaria-endemic area, concentrations of RBP, after adjustment for inflammation, showed residual differences of −0.09 μmol/L between apparently healthy preschool-aged children with and without Plasmodium parasites. This seems to indicate that the meta-analysis correction for inflammation may not completely adjust concentrations when malaria, as indicated by malaria parasitemia, is reported (774).
Because human milk has been suggested as a potentially useful biomarker of vitamin A status of women and, by extrapolation, of their children and potentially other groups within the population (324), it is useful to explore the potential impact of inflammation on the use of breast-milk vitamin A concentrations and interpretation. To date, limited evidence suggests that human-milk vitamin A is not susceptible to the influence of inflammation (775). However, recent data from a subset of women recruited as part of a national survey in Cameroon indicate that human-milk vitamin A concentrations (casual samples at ≥1 mo postpartum) were associated with CRP, and this association seemed to be explained by a decrease in plasma RBP (776). The overall relation between inflammation, specific biomarkers such as CRP and human-milk vitamin A remains unresolved. In part, the ability to more definitively delineate this relation will be contingent on comparisons between populations of low vs. high prevalence of VAD.
d. Zinc.
The importance of zinc to health and, more specifically, the immune system and the APR was described by WGs 1 and 2 and reviewed recently (371). With specific regard to zinc assessment, the current view of the international nutrition community as represented by the International Zinc Nutrition Consultative Group and WHO is that serum or plasma zinc concentration is the primary indicator for use in the assessment of zinc status of a population (375). Potential factors influencing the interpretation of serum/plasma zinc concentrations include age, sex, time of day of the blood collection, and the fasting status of the individual (371). Cutoffs have therefore been established for different age and sex groups and blood sampling conditions (777). In addition to plasma/serum zinc, a number of other biomarkers (listed in Text Box 48) have been identified and are at various stages of viability for either clinical or population-based use. With the exception of plasma/serum zinc, most are not widely used either clinically or for population-based surveillance.
Text Box 48 . Currently available zinc biomarkers/indicators.
Plasma/serum zinc
● Most widely used
● May reflect recent meals rather than usual dietary intake
● Affected by inflammation
Potential alternatives
● Hair zinc
○ Potential alternative to plasma/serum zinc because it appears to be unaffected by inflammation
○ Offers technical challenges (sample collection, contamination, etc.)
● Urinary zinc
Emerging biomarkers
● Nail zinc
● Zinc-dependent proteins (e.g., metallothioniene)
● Oxidative stress and DNA integrity
● Zinc kinetics
● Functional biomarkers (e.g., taste acuity, neurobehavioral function)
Not useful
● Erythrocyte and leukocyte zinc (778)
● Zinc-dependent enzymes
Influence of inflammation on zinc assessment.
As far as the potential impact of inflammation, it has been reported that zinc is shifted from the bone marrow into the liver during the APR (779). As a consequence, plasma/serum zinc concentrations are decreased and do not reflect zinc intakes in individuals experiencing infections/acute inflammation. The degree to which inflammation reduces serum/plasma zinc concentrations and approaches to account for this effect are unclear. In a multisite cohort study in children in Africa, for every increment in CRP concentrations by 1.0 mg/L, plasma zinc was 1.0 μmol/L lower (780). To date, a biomarker has not been identified that differentiates dietary ZnD from a response to infection/inflammation (374, 781). One potential alternative might be hair zinc concentrations, which were observed to be stable during acute infections in a few studies (782).
e. Folate.
The role of folate in public health has been reviewed extensively (783). Of the currently available biomarkers, the most commonly used are serum folate, RBC folate, and plasma homocysteine concentrations. Text Box 49 provides a list of available biomarkers for assessing folate.
Text Box 49 . Currently available folate biomarkers/indicators.
● Serum folate
● RBC folate
● Plasma total homocysteine
● Urinary folate/folic acid
● Urinary and serum p-aminobenzoylglutamate (pABG) and p-acetamidobenzoylglutamte (apABG); these are catabolites of folate oxidative metabolism.
Influence of inflammation on folate assessment.
The reported relation between markers of inflammation and folate biomarkers has been inconsistent in the literature. Weak associations between CRP, AGP, and serum or erythrocyte folate have been reported but not consistently. Among adults in NHANES, there was a weak negative association between serum folate and CRP (∼5% lower concentrations of serum folate when CRP is >5 mg/L) and a weak positive association between RBC folate and CRP (∼5% higher concentrations when CRP is >5 mg/L) (755). Plasma total homocysteine, which is elevated in deficiencies of folate, vitamin B-12, riboflavin, and vitamin B-6, may also be elevated in chronic inflammation states (784).
f. Vitamin B-12.
The role of vitamin B-12 in health and disease was previously reviewed (785, 786). The available biomarkers of vitamin B-12 were also recently reviewed (785) and are summarized in the Text Box 50.
Text Box 50 . Currently available vitamin B-12 biomarkers/indicators.
● Serum/plasma total vitamin B-12
● Serum holotranscobalamin
● Serum/plasma methylmalonic acid (MMA)
● Plasma total homocysteine
Influence of inflammation on vitamin B-12 assessment.
None of the vitamin B-12 specific biomarkers, serum vitamin B-12, holotranscobalamin, and MMA, are correlated with CRP or AGP, even in populations with a high prevalence of acute infection (787). Comments on total homocysteine are the same as for folate in the preceding section.
g. Iodine.
Iodine is a trace element required by the thyroid gland to produce thyroxine and tri-iodothyronine, thyroid hormones necessary for multiple processes related to normal growth and development. The biology including a review of all available biomarkers (Text Box 51) of iodine was recently completed (788).
Text Box 51 . Currently available iodine biomarkers/indicators.
● Urinary iodine concentration
● Thyrotropin
● Thyroglobulin
● Tri-iodothyronine and tetra-iodothyronine
● Goiter
Influence of inflammation on iodine assessment.
Limited data exist to suggest an association between inflammation and urinary iodine. In NHANES (adults), a weak positive association was observed between urine iodine and CRP (∼8% higher concentrations when CRP is >5 mg/L) for crude data, but the association was no longer significant after controlling for age, sex, race/ethnicity, smoking, supplement use, fasting, eGFR, and urine creatinine (755). Illness, including infection, sepsis, trauma, and chronic diseases, can cause alterations in tri-iodothyronine, tetra-iodothyronine, and thyrotropin levels, even in individuals with normal underlying thyroid function (789).
h. Vitamin D.
VDD is common in much of the world. The risk or prevalence is higher in dark-skinned people, infants/young children, pregnant women, the elderly, and in women who are covered for religious reasons as well as in northern latitudes, particularly after winter. As outlined by WGs 1 and 2, an expanding body of evidence attests to the importance of vitamin D to immune function and the inflammatory response, with potential therapeutic applications for acute and chronic infectious and inflammatory diseases (790). Of the currently available biomarkers (Text Box 52), 25-Hydroxy vitamin D [25(OH)D] has been accepted as the most reliable indicator, particularly of exposure (791).
Text Box 52 . Currently available vitamin D biomarkers/indicators.
● 25(OH)D:
○ generally recognized as the best currently available biomarker
○ reflects intake
○ functional relevance not clearly established
● Functional biomarkers:
○ parathyroid hormone
○ calcium absorption
○ bone resorption
○ bone mineral density
Influence of inflammation on vitamin D assessment.
Although considerable evidence exists to associate concentrations of 25(OH)D with various inflammatory states and CRP (792), a cause or effect relation has not been established. Data on the influence of inflammation on biomarkers of vitamin D status are limited. Among adults from NHANES, there was a weak negative association between serum 25(OH)D and CRP (∼5% lower concentrations when CRP is >5 mg/L). This was true for crude data as well as after controlling for age, sex, race/ethnicity, smoking, supplement use, fasting, and eGFR (755).
i. Summary of impact of inflammation on nutrient biomarkers.
The impact of inflammation on nutrient biomarkers is well described for several key nutrients, such as iron and vitamin A. For many nutrients (e.g., vitamin D or iodine), there appears to be an association between their biomarkers and markers of inflammation. However, it is not at all clear whether this reflects a cause or effect relation. More study is needed to refine our understanding of these relations. In the interim, because of the ubiquitous nature of acute/chronic inflammation and its effects on nutrient biomarkers, WG 4 suggested that the routine assessment of inflammatory status be included along with nutrient assessment. This will enable a more reliable and valid interpretation of the results of micronutrient status assessment of both patients and populations.
The WG recognized that the inflammatory response may be associated with higher or lower nutrient biomarker concentrations (i.e., it will be difficult to determine a cause or effect relation). In addition, the magnitude of effect in different settings and how to account for the effects of individual factors remain important research questions. Table 12 summarizes the effects of inflammation on some of the key nutrient biomarkers used in both clinical and public health settings. Additional details on the assessment, cutoffs, and interpretation of nutrient biomarkers are provided in the BOND reports found on the BOND website (30).
TABLE 12.
Summary of key nutrient biomarkers1
| Nutrient | Commonly used biomarker/indicators | Magnitude and direction of inflammation effect | Settings where used | Notes |
| Iron | Ferritin | +++ | Clinical, research, population | |
| sTfR | + | Research, population | sTfR assays are not yet standardized and compare poorly | |
| Hemoglobin | Clinical | |||
| Body iron | + | Research, population | ||
| Ratio of TfR:ferritin | + | Research | ||
| TfR index | + | Research, clinical | ||
| ZPP | + | Clinical, population | ||
| Hepcidin | 0 | Research | ||
| Vitamin A | Retinol | − | Clinical, research, population | |
| RBP | − | Research, population | ||
| Breast-milk retinol | Research | |||
| Retinol dose response test | Research | |||
| Zinc | Serum/plasma zinc | − | Clinical, population | Not reliable in clinical settings if patient inflamed |
| Folate | Erythrocyte folate | + | Clinical, population | |
| Plasma or serum folate | − | Clinical, population | ||
| Vitamin B-12 | Serum/plasma total cobalamin | 0 | Clinical, population | |
| Serum holotranscobalamin | Research | Superior diagnostic performance of holotranscobalamin is controversial | ||
| Plasma/urine MMA | 0 | Clinical, population | MMA assay is more expensive | |
| Plasma total homocysteine | + | Clinical | Elevated in both folate and vitamin B-12 deficiency | |
| Iodine | Urinary iodine | 0 | Population | No known association with inflammation |
| Vitamin D | 25(OH)D | − | Clinical, population | |
| Vitamin B-6 | Plasma pyridoxal 5-phosphate | − | Research, population | |
| Vitamin C | Serum ascorbic acid | − | Research, population | Freshly prepared serum needs to be promptly acidified with meta-phosphoric acid and frozen to stabilize ascorbic acid |
MMA, methylmalonic acid; RBP, retinol binding protein; sTfR, soluble transferrin receptor; TfR, transferrin receptor; ZPP, zinc protoporphyrin; 0, no change; 25(OH)D, 25-hydroxyvitamin D; −, decrease; +, increase; +++, major increase.
VII. How to account for the potential impact of inflammation on biomarker selection, use, and interpretation.
As outlined above and by WGs 1–3, the relation between inflammation and nutrient biomarkers has a significant impact on the selection, use, and interpretation of nutritional biomarkers for assessment of micronutrient biomarkers. The complications caused by these relations can lead to incorrect diagnosis in individuals, as well as over- or underestimation of the prevalence of micronutrient deficiencies in a population; furthermore, inflammation may affect the ability to accurately monitor and evaluate the impact of nutritional interventions (357).
Although no current standardized and universally accepted method or methods for accounting for inflammation exist, several approaches have been proposed. The suggested approaches may vary by nutrient biomarkers as well as in the setting used (e.g., clinical care vs. public health or population surveys). As discussed previously, the issue of bias is important in comparing approaches to account for the impact of inflammation on nutrient biomarkers. For example, individuals who have a higher prevalence of inflammation may also have a higher prevalence of micronutrient deficiencies. The following sections will explore specific aspects of nutrient assessment from the perspective of clinical/point of care vs. population assessment, followed by an overview of the relative strengths and weaknesses of proposed solutions to this conundrum.
a. Considerations for clinical settings/point of care.
As reviewed by WG 3, infection and inflammation can affect individual nutritional status through several mechanisms, including suppression of appetite, altered nutrient absorption and excretion, increased caloric requirements through altered metabolism, and nutrient-drug interactions. In a study that estimated the magnitude of the effect of inflammation on plasma micronutrient concentrations of hospitalized patients, for each micronutrient the change in plasma concentrations varied markedly from patient to patient (793). The authors concluded that, in the presence of moderate or severe systemic inflammatory response (CRP >20 mg/L), plasma concentrations of selenium, zinc, and vitamins A, B-6, C, and D were clinically uninterpretable. However, it was possible to interpret these micronutrients if the systemic inflammatory response was less severe: for zinc when CRP is <20 mg/L, for selenium and vitamins A and D when CRP is <10 mg/L, and for vitamins B-6 and C when CRP is <5 mg/L. In clinical settings, it may be best to re-evaluate the nutritional status of individuals when the inflammation has resolved. However, certain chronic disease conditions, such as short gut syndrome, feature a high prevalence of subclinical inflammation as well as rapid transit and malabsorption of nutrients, and thus a high risk of multiple-micronutrient deficiencies (794). Similarly, children in resource-poor settings who are exposed to frequent infections with associated anorexia are at risk of long-standing malnutrition of clinical significance. In such settings, regular, frequent monitoring of micronutrient status is needed and should be assessed with measurements of APPs, such as CRP.
b. Considerations for population-level surveys.
Population-level estimates of micronutrient status are important for the implementation, monitoring, and evaluation of nutrition programs, as well as for comparisons between countries. Population-level interventions to address micronutrient deficiencies focus on the prevention of deficiency and shifting the population distribution of a biomarker (or reducing overall prevalence) rather than treating high-risk individuals. The effects of inflammation on population-level estimates of nutrition status are significant and can lead to distorted estimates of micronutrient deficiencies, either overestimating or underestimating the true prevalence, if inflammation is not accounted for in the analysis (357). Furthermore, the extent of the effect likely depends on the prevalence of inflammation and prevalence of the deficiency in the population.
Examples of where accounting for the effect of inflammation on micronutrient prevalence estimates would be helpful include the following:
● A country assesses the prevalence of a micronutrient deficiency, finds the prevalence is high, and implements a population-level intervention program, such as fortification, micronutrient powders, or supplementation. Another survey is performed at a later time to see if the prevalence of the micronutrient deficiency has decreased. If the prevalence of APPs differs between the 2 surveys, this could potentially mask an apparently effective intervention program or make an intervention appear to be successful when in fact it was not.
● In comparing countries or geographic areas within a country, decisions may be made to start intervention programs and/or allocate resources in areas with a higher prevalence of a micronutrient deficiency. If the level of inflammation differs between geographic areas, this may affect the prevalence of the micronutrient deficiency and result in inappropriate program interventions and/or allocation of resources.
Before deciding on approaches to account for inflammation, issues related to biomarker assessment must first be addressed, as follows:
● To assess the prevalence of micronutrient deficiencies, the methods used to collect, store, transport, and analyze the specimens must be performed with high quality. There are generally expert groups that provide guidance on the laboratory tests available, their advantages and disadvantages, laboratory quality assurance programs, and cutoff values to identify abnormal concentrations (e.g., WHO expert groups, International Council for Control of Iodine Deficiency, BOND).
● The quality of CRP and AGP measurements is also important, as is ensuring the quality of laboratory methods. One area that is in need of further investigation is to assess the relation between APPs and micronutrient biomarkers, each on their continuous scale (which may need to be transformed), and determine if there are clear cutoffs for the APP or if alternative methods to account for the APPs that do not rely on cutoffs are needed. Currently, one cutoff is used for CRP and/or for AGP and applied equally to all micronutrient biomarkers thought to be affected by inflammation, but this approach would benefit from further investigation.
● Some surveys measure only CRP, some only AGP, and some collect both. Strategies on how to deal with a variety of approaches based on these APPs from existing surveys are needed. If resources are available, measuring both CRP and AGP is preferred. If limited, either CRP or AGP should be measured, although further investigation is needed to determine, for each nutrient biomarker, whether CRP alone, AGP alone, or some combination of the 2 can account for inflammation.
c. Review of proposed approaches.
Potential approaches to account for inflammation will be discussed in detail and include ignoring inflammation, exclusion of individuals with inflammation, adjustment of the biomarker cutoff values, correction factors, statistical approaches, and others. Again, the approach will depend on whether applied in a clinical or public health setting and the context or underlying prevalence of inflammation.
i. Ignore inflammation.
This approach is used if APPs are not measured or APPs are measured but ignored in the analysis of nutritional status. Although this approach is currently used for some biomarkers of micronutrient status, it may result in an under- or overestimate of the prevalence of that micronutrient depending on the biomarker and the context. For the context issue, this would depend on the prevalence of the elevated APP and the strength of the association between the APP and the micronutrient biomarker. If the prevalence of the elevated APP is low, such as in many developed countries, then ignoring the prevalence of an elevated APP would likely have a minimal effect on the prevalence of the micronutrient deficiency. In populations with a high prevalence of elevated APPs, this could have a large impact on the prevalence of the micronutrient deficiency. In comparing 2 cross-sectional surveys within a country, it is often assumed that the prevalence of an elevated APP tends to change little within a country. However, there is little information available about how the prevalence of elevated APPs varies within a country over time. In countries with a low prevalence of elevated APPs this is probably less of an issue. However, in countries with higher prevalence of elevated APPs, one might expect that the prevalence of elevated APPs may have seasonal variability due to the effect of infectious diseases and perhaps other factors that affect APP concentrations, such as programs to treat infectious diseases. For example, in 4 cross-sectional surveys conducted in preschool-aged children in the same 60 villages in Kenya in 2007–2010, the prevalence of elevated CRP varied from 10.5% to 48.2% (795).
ii. Exclusion.
In this approach, those with an elevated APP are excluded from the analysis. An important issue is the cutoff used to define an elevated APP. For example, although a cutoff for CRP of >5 mg/L is typically used by investigators to define subclinical inflammation in nutrition surveys (716), a lower cutoff of >3 mg/L is recommended to define cardiovascular risk (796). Furthermore, it is not clear whether APP cutoff values should vary by nutrition biomarkers. The effect of the exclusion approach can be quite variable depending of the prevalence of the elevated APP and the strength of the association between the APP and micronutrient biomarker. In some countries, the prevalence of elevated APPs can be very low and therefore excluding them would have little effect on the prevalence of micronutrient deficiencies. However, in some areas, the prevalence of elevated APPs can be quite high, up to 60% or higher. Excluding those with inflammation can affect precision and, most likely, validity. For precision, in areas with a high prevalence of elevated APPs, the exclusion approach can dramatically lower the sample size and therefore reduce the precision of the prevalence estimate of the micronutrient deficiency. Concerning validity, individuals with an elevated APP may be systematically different from those with a normal APP. In some settings, it is likely that those with an elevated APP are of lower socioeconomic status (SES), and by excluding them, the estimate of the prevalence of micronutrient deficiencies would be biased. For example, in Laos, the prevalence of elevated CRP in children was significantly different according to maternal education (797). Among preschool-aged children in Papua, New Guinea, those with an elevated CRP, AGP, or both were younger and more likely to be stunted or underweight (764).
iii. Use of different micronutrient deficiency cutoff values for those with and without an elevated APP.
In this approach, one cutoff value for micronutrient deficiency is used for those without an elevated APP and a different cutoff used for those with an elevated APP. For example, for serum ferritin, the cutoff for preschool-aged children is <12 μg/L; however, if the child is known to have inflammation, the cutoff value of <30 μg/L has been suggested (798). This is problematic because it does not account for varying degrees of inflammation (799). Furthermore, having different cutoffs may be confusing, because this does not provide a distinct international standard. One issue with this approach is that the cutoff values may differ by target group and even perhaps the context of the survey—for example, one performed in an area with a high level of malaria vs. a survey performed in an area without malaria—or may differ between developed and developing countries. Therefore, for this approach to be useful, context-specific cutoffs may be needed.
iv. Four-level inflammation correction factor approach.
This approach uses concentrations of CRP >5 mg/L and AGP >1 g/L to create the following 4 inflammation categories (272):
● Reference: normal CRP and AGP concentrations
● Incubation: CRP concentration is elevated but AGP is normal
● Early convalescence: both CRP and AGP are elevated
● Late convalescence: CRP is normal but AGP is elevated
A number of correction factors can then be derived on the basis of these 4 categories. For example, the ratio of geometric means of reference group (neither CRP nor AGP is increased) to those of the respective inflammation groups is calculated. The correction factor approach groups people with subclinical inflammation into “risk” categories depending on their relation with the time of initial infection and the period of clinical disease. This approach is suitable where the subjects are apparently healthy, that is, they show no evidence of symptoms requiring medical intervention and only demonstrate subclinical inflammation. For serum ferritin, correction factors calculated as part of a meta-analysis were also proposed (272). Overall, for the studies included in the meta-analyses, inflammation increased ferritin by ∼30% and was associated with a 14% (95% CI: 7%, 21%) underestimation of ID. When applying the meta-analysis correction factors in an area endemic for malaria, concentrations of ferritin, after adjustment for inflammation, showed a residual difference of +12 μg/L between apparently healthy preschool-aged children with and without Plasmodium parasites. This observation indicated that the meta-analysis correction for inflammation might not completely adjust concentrations when malaria is reported (774). The correction factor approach has raised awareness of the effect of APPs on biomarkers of micronutrient status and has been applied frequently. Advantages of this approach are that it is easy to apply and is preferred in many situations to ignoring inflammation or exclusion. The disadvantages of this approach include the following: 1) the survey must have both CRP and AGP, 2) it does not account for possible effect modifiers and confounders (e.g., age, sex, SES), 3) it is applied assuming the current cutoffs for CRP and AGP are applicable to all biomarkers of micronutrient status, and 4) the net effect of the correction factor is that it results in prevalence estimates of micronutrient status similar to the exclusion approach when internal correction factors are applied. Compared with the exclusion approach, the 4-category inflammation model has better precision but is still subject to the potential biases that may occur when the exclusion approach is applied.
v. Direct standardization.
The direct standardization approach is commonly applied to comparing mortality statistics between geographic areas with different age distributions. A similar approach could be applied to the prevalence of micronutrient deficiencies by standardizing on the prevalence of elevated APPs. As with mortality statistics, there could be different standardized weights depending on whether the country is a developing or developed country. In addition, there may be different weights depending on the target group. The approach would be to calculate the prevalence of micronutrient deficiency in those with and without inflammation, then calculate a weighted prevalence estimate by using a “standard” prevalence estimate of inflammation (e.g., 30%). This approach does not correct for inflammation per se but allows for a more valid comparison of surveys over time within a geographic area or in the comparison of different geographic areas. That is, if the prevalence of inflammation was made to be the same in all surveys (taking into account development status and target group), a fair comparison could be made.
vi. Regression modeling.
In the analysis of survey data, the relation between the micronutrient biomarker and APPs could be investigated through the use of linear regression. Linear regression is a statistical approach to model the relation between a scale dependent variable y and one or more explanatory variables denoted x. There may be a need to transform the micronutrient biomarker, so that it has an approximately normal distribution (800). A nonlinear impact of APPs on micronutrient biomarkers can also be accommodated by using generalized additive models (801). Regression modeling could also take into account potential interaction (i.e., the relation between the micronutrient biomarker and the APP is modified by a third factor) and confounding. The regression model could include, for example, covariates such as age, sex, anthropometry, and SES. Covariates can play 2 different roles in the regression models: they could predict the true nutrient concentration (i.e., nutrient concentrations under no inflammation) and they could modify the effect of inflammation on nutrient concentration. The modeling process would yield an adjustment equation for the micronutrient biomarker and would be applied to those with the elevated APP in order to remove the impact of inflammation. For example, to calculate an adjusted ferritin for the effects of CRP, one would use the slope of CRP from the regression as follows: adjusted ferritin = ferritin – CRP × slope.
For anemia, a regression approach is commonly used to adjust individual hemoglobin values to account for altitude (802). This approach is used at the individual level to categorize an individual as anemic or not anemic and at the population level to estimate the prevalence of anemia adjusted for altitude.
Advantages to the regression approach are that it can account for the impact of covariates, and, unlike the correction factor approach, it adjusts for the impact of inflammation in a continuous manner, i.e., the higher the APP, the greater the net correction. It also does not rely on the use of specific cutoffs for APPs. If this approach is promising, it may be possible to derive an equation that would allow for the correction of various micronutrient biomarkers across all surveys, similar to correcting hemoglobin for altitude. A potential challenge is that the relations between CRP and/or AGP and different nutritional biomarkers are nonlinear. AGP increases only slowly after the initial trauma; therefore, AGP is rarely increased between the time of infection and the appearance of clinical signs. Thus, elevated AGP concentrations are only found during convalescence and probably show a reasonably stable (positive or negative) relation with the biomarker concentration. In the case of CRP, the concentration of this protein increases rapidly after infection and biomarker concentrations change in parallel (e.g., ferritin positively, retinol and RBP negatively). During convalescence, the relation is dampened—CRP decreases rapidly whereas some nutritional biomarkers return to normal more gradually. Thus, although AGP may lend itself to regression modeling techniques, the relation between CRP and micronutrients is more complicated because, depending on the point in time relative to the infection, the strength of the association might vary.
Kung’u et al. (803) used regression modeling to adjust for the influence of APR on iron status indicators. A recent article (804) developed a regression approach to account for inflammation and compared it with the correction factor approach. This study found a similar prevalence of ID by using the 2 approaches. However, these regression approaches did not model the impact of covariates. The regression model formulation and the underlying assumptions for valid adjustment are not clear in these approaches. More work is needed to develop statistical approaches that rigorously model the relation between micronutrient markers and APPs, and the impact of the covariates on them. Such models would possess well-characterized inferential properties (e.g., bias, SE) and transparent assumptions.
To further evaluate approaches to account for inflammation, the CDC, the Global Alliance for Improved Nutrition, and the NICHD have formed a collaborative research group called Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia, which is conducting secondary analysis from nationally and regionally representative nutrition surveys (805).
VIII. Conclusions and research priorities.
In summary, although several approaches to account for inflammation have been proposed in the literature, there is currently no consensus on a single preferred method (Table 13). Nevertheless, the effect of inflammation on nutrient biomarkers cannot be ignored, and we recommend that at a minimum one or more biomarkers of inflammation be measured in assessments of nutritional status in populations with a known or suspected moderate-to-high prevalence of inflammation (as measured by elevated APPs). Although there is no current consensus on which inflammatory biomarker to use, we recommend at a minimum measuring CRP, because it is feasible to measure and data are available on its relation to nutrient biomarkers. The addition of AGP as a measure of longer-term inflammation is probably useful in most settings and should be measured with CRP if resources are available The ideal cutoffs for CRP and AGP need further investigation and may not be the same in all settings and for all nutrition biomarkers.
TABLE 13.
Summary of approaches to account for inflammation1
| Approach | Description | Settings where useful | Pros | Cons | Notes |
| Ignore inflammation | APPs not measured or nothing done to account for APPs | 1) Nutrient biomarkers that have no association with inflammation, 2) settings with low prevalence of inflammation | Easy | Biased estimate of nutrient status | May be ok when comparing 2 surveys in the same setting if concentration of APP is stable |
| Exclusion | Remove individuals with elevated APPs | Settings with low prevalence of inflammation | Easy | 1) Relies on concrete APP cutoffs, 2) may lose sample size (precision), 3) may introduce bias (validity) | Need standard cutoffs for APPs; exclude based on which APP? (CRP ± AGP) |
| Change nutrient biomarker cutoff | Use different cutoff for nutrient indicator for those with inflammation (e.g., ferritin <30 ug/L) | Single population with fixed effects | Easy | 1) Does not account for varying degrees of inflammation, 2) cutoffs may differ by target group | Cutoffs need to be defined |
| Four-level inflammation correction factors | Use CRP and AGP to create 4 categories (no inflammation, incubation, early convalescence, and late convalescence) | No demographic differences between those with and without subclinical inflammation | Relatively easy | 1) Need both CRP and AGP, 2) does not account for potential confounders and effect modifiers, 3) produces similar estimates as exclusion approach | Individual country CF vs. meta-analysis CF |
| Direct standardization | Apply weights to prevalence estimates | Need to weight by target group, setting, etc. | May allow for comparisons between surveys | ||
| Regression modeling | Linear regression taking into account potential interaction and confounding to produce corrected micronutrient concentration to produce corrected micronutrient concentration that accounts for inflammation | Covariates are measured | Accounts for continuous effect of inflammation on biomarkers | Difficult |
AGP, α1-acid glycoprotein; APP, acute phase protein; CF, correction factor; CRP, C-reactive protein.
In settings with a considerable prevalence of inflammation, the exclusion approach may result in biased prevalence estimates and will reduce precision. The correction factor approach is being more readily used and is relatively simple to apply to data sets. However, it may not be applicable in all settings. Further investigation into regression modeling appears to be warranted, because it is less subject to bias and allows for continuous correction of inflammation, even at levels below traditionally used cutoffs.
Until more work is done to achieve consensus on approaches to account for inflammation (see research priorities in Text Box 53), we recommend presenting data on the prevalence of micronutrient deficiencies in the population overall, and presented separately for those with inflammation and those without inflammation.
Text Box 53 . Research priorities.
● What biomarkers of inflammation should be used in different settings? There is a need to evaluate the utility of composite/aggregate markers of inflammation. In addition, better biomarkers of chronic inflammation and organ/tissue-specific biomarkers need to be developed. Research on the strength of the association between each micronutrient biomarker and CRP and AGP is needed. Some micronutrient biomarkers may change in response to inflammation more like CRP, some more like AGP, and others somewhere in between. What APP cutoffs should be used?
● Investigate the optimal APP cutoff value for each micronutrient biomarker. For each micronutrient biomarker, investigate various cutoff values for CRP and AGP that have the strongest relation with the micronutrient biomarker. This could be through receiver-operator characteristic (ROC) approaches or other approaches. It is possible that for each biomarker of micronutrient status, there could be a different cutoff value of CRP and/or AGP.
● Do the characteristic patterns of change in APPs differ according to inflammation etiologies (e.g., trauma, infection, subclinical inflammation)?
● Are the type, level, and duration of inflammation important?
● Are there settings where inflammation can be ignored?
● What are potential confounders of inflammation in different settings (age, sex, SES, stunting, malaria, HIV, tuberculosis, etc.)? In assessing the association between micronutrient biomarkers and APPs, it is important to determine if the association is modified or confounded by other factors, such as age, SES, infections, and malnutrition. Ignoring effect modification and confounding could lead to less than optimal ways to account for APPs in assessing micronutrient status.
● What is the role of inflammation on the nutrient content of breast milk?
● Longitudinal studies of otherwise healthy, inflamed individuals (e.g., vaccine response)
● Is a modeling approach feasible and valid to address the inflammation and nutrition association?
● Longitudinal collection of inflammatory and nutrient biomarkers and morbidity data with or without an accompanying nutrient intervention
● Standardization of inflammatory biomarkers
Supplementary Material
Acknowledgments
The INSPIRE Consultative Group includes the following collaborators: Namanjeet Ahluwalia, Tahmeed Ahmed, Lindsay Allen, Melinda Beck, Helene Blanchard, Eleanor Brindle, Kenneth Brown, Philip Calder, William Checkley, Kate Claycombe, Christian Coles, Fabian Esamai, Henrik Friis, Elizabeth Gardner, Steve Grinspoon, Richard Guerrant, Marc Hellerstein, Janet Hunt, Janet King, Kirk Klasing, Bas Kramer, Kristine Koski, Christine Northrop-Clewes, Christine Pfeiffer, Andrew Prentice, Manuel Ramirez-Zea, Patrick van Rheenan, Lothar Rink, Suman Srinivasa, Christine Stabell Benn, Charles Stephensen, Parminder Suchdev, Kevin Sullivan, David Thurnham, Ravi Varadhan, Andre van der Ven, Jeremy Walston, and Jeff Xia. The Scientific Steering Committee includes the following contributors: Bernard Brabin, Ian Darnton-Hill, Cindy Davis, Rafael Flores-Ayala, Gilman Grave, Janet Hunt, Klaus Karmer, Simin Meydani, Andrew Prentice, Daniel Raiten, Catherine Ross, Pamela Starke-Reed, Charles Stephensen, Ben Van Ommen.
Footnotes
Abbreviations used: ACT, α1-antichymotrypsin; AGP, α1-acid glycoprotein; apABG, p-acetamidobenzoylglutamte; APC, antigen-presenting cell; APP, acute phase protein; APR, acute phase response; ATRA, all trans retinoic acid; BOND, Biomarkers of Nutrition for Development; CAMP, cathelicidin antimicrobial peptide; C/EBPe, CCAAT/enhancer binding protein ɛ CD, cluster of differentiation; CDKN1A, cyclin-dependent kinase inhibitor 1A; CID, communicable infectious disease; CMI, cell-mediated immunity; COPD, chronic obstructive pulmonary disease; CpG, cytosine-phosphate-guanine; CRH, corticotropin; CRP, C-reactive protein; CYP, cytochrome P450; CYP, cytochrome P450; CVD, cardiovascular disease; Dlx5, distal-less homeobox 5; DTH, delayed-type hypersensitivity; EE, environmental enteropathy; eGFR, estimated glomerular filtration rate; EQA, external quality assessment; EP, erythrocyte protoporphyrin; ESR, erythrocyte sedimentation rate; FoxP3, forkhead box P3; GBD, Global Burden of Disease; GSTP1, glutathione S-transferase pi 1; HOX, homeobox; HPA, hypothalamic-pituitary-adrenal; ID, iron deficiency; I-FABP, intestinal fatty acid binding protein; IFNG, interferon γ INSPIRE, Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; KDM6B/JMJD3, lysine (K)-specific demethylase 6B; LINE, long interspersed element; LMIC, low-/middle-income country; MCP, monocyte chemoattractant protein; MFO, mixed function oxidase; MIF, migration inhibitory factor; MIP-1α, macrophage inflammatory protein 1α miRNA microRNA; MLL5, methyltransferase 5; MMA, methylmalonic acid; MRDR, modified relative dose response; NCD, noncommunicable disease; NFKB1, Nuclear factor NF kappa B-p105 subunit; NICHD, National Institute of Child Health and Human Development; OLD, obstructive lung disease; pABG, p-aminobenzoylglutamate; PBMC, peripheral blood mononuclear cell; PCT, procalcitonin; PEM, protein-energy malnutrition; poly-ZF, poly-zinc-finger; RA, retinoic acid; RAR, retinoic acid receptor; RARE, retinoic acid response element; RBP, retinol binding protein; RDR, relative dose response; REL, v-rel avian reticuloendotheliosis viral oncogene homolog; RELA, v-rel avian reticuloendotheliosis; RET, ret proto-oncogene; ROS, reactive oxygen species; RXR, retinoid × receptor; SAA, serum amyloid A; SAP, serum amyloid P; SCM, subclinical mastitis; SES, socioeconomic status; SET, Su(var)3–9, Enhancer of Zeste, Trithorax; SNS, sympathetic nervous system; SSC, Scientific Steering Committee; sTfR, soluble transferrin receptor; Th, T-helper; TLR, Toll-Like receptor; TNFA, tumor necrosis factor α Treg, T-regulatory; VAD, vitamin A deficiency; VDD, vitamin D deficiency; VDR, vitamin D receptor; VDRE, vitamin D response element; VEGF, vascular endothelial growth factor; WBC, white blood cell; WG, working group; ZnD, zinc deficiency; ZPP, zinc protoporphyrin; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet. Maternal and child nutrition; 2013 [cited 2014 Mar 25]. Available from: http://www.thelancet.com/series/maternal-and-child-nutrition.
- 3.World Health Organization. HIV/AIDS. Global Health Observatory (GHO); c2015 [cited 2014 Jun 20]. Available from: http://www.who.int/gho/hiv/en/.
- 4.American Foundation for Aids Research. Statistics: worldwide; c2015 [cited 2014 Jun 20]. Available from: http://www.amfar.org/about-hiv-and-aids/facts-and-stats/statistics–worldwide/.
- 5.World Health Organization. Factsheet on the World Malaria Report 2013; 2013 [cited 2014 Jun 20]. Available from: http://www.who.int/malaria/media/world_malaria_report_2013/en/.
- 6.World Health Organization. How many TB cases and deaths are there? Global Health Observatory (GBO); c2015 [cited 2014 Jun 20]. Available from: http://www.who.int/gho/tb/epidemic/cases_deaths/en/.
- 7.World Health OrganizationTuberculosis; c2015 [cited 2014 Jun 20]. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/.
- 8.Centers for Disease Control and Prevention. Global diarrhea burden: global water, sanitation and hygiene; 2013 [cited 2014 Jun 20]. Available from: http://www.cdc.gov/healthywater/global/diarrhea-burden.html.
- 9.World Health Organization Diarrhoeal disease; c2015 [cited 2014 Jun 20]. Available from: http://www.who.int/mediacentre/factsheets/fs330/en/.
- 10.World Health Organization. Obesity. Global Health Observatory (GHO); c2015 [cited 2014 Jun 22]. Available from: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/.
- 11.Mayosi BM, Lawn JE, van Niekerk A, Bradshaw D, Abdool Karim SS, Coovadia HM. Health in South Africa: changes and challenges since 2009. Lancet 2012;380:2029–43. [DOI] [PubMed] [Google Scholar]
- 12.Creswell J, Raviglione M, Ottmani S, Migliori GB, Uplekar M, Blanc L, Sotgiu G, Lonnroth K. Tuberculosis and noncommunicable diseases: neglected links and missed opportunities. Eur Respir J 2011;37:1269–82. [DOI] [PubMed] [Google Scholar]
- 13.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 14.Garmendia ML, Corvalan C, Uauy R. Addressing malnutrition while avoiding obesity: minding the balance. Eur J Clin Nutr 2013;67:513–7. [DOI] [PubMed] [Google Scholar]
- 15.Camps J, Garcia-Heredia A. Introduction: oxidation and inflammation, a molecular link between non-communicable diseases. Adv Exp Med Biol 2014;824:1–4. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Aguilera A, Rull A, Rodriguez-Gallego E, Riera-Borrull M, Luciano-Mateo F, Camps J, Menendez JA, Joven J. Mitochondrial dysfunction: a basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediators Inflamm 2013; 2013:135698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, Folkerts G, Friedmann PS, Frost GS, Guarner F, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr 2009;101(Suppl 1):S1–45. [DOI] [PubMed] [Google Scholar]
- 18.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 19.Gordon AH, Koy A. The acute phase response to injury and infection: the roles of interleukin 1 and other mediators. Amsterdam: Elsevier; 1985. [Google Scholar]
- 20.Gruys E, Toussaint MJM, Landman WJM, Tivapasi M, Chamanza R, van Veen L. Infection, inflammation and stress inhibit growth: mechanisms and non-specific assessment of the processes by acute phase proteins. In: Wensing T, editor. Production diseases in farm animals. 10th annual conference, 1998. Wageningen (The Netherlands): Wageningen Press; 1999. p. 72–87.
- 21.Andus T, Geiger T, Hirano T, Kishimoto T, Heinrich PC. Action of recombinant human interleukin 6, interleukin 1 beta and tumor necrosis factor alpha on the mRNA induction of acute-phase proteins. Eur J Immunol 1988;18:739–46. [DOI] [PubMed] [Google Scholar]
- 22.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 1994;368:339–42. [DOI] [PubMed] [Google Scholar]
- 23.Thorn CF, Lu ZY, Whitehead AS. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scand J Immunol 2004;59:152–8. [DOI] [PubMed] [Google Scholar]
- 24.Thurnham D, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. WHO report: priorities in the assessment of vitamin A and iron status in populations. Geneva (Switzerland): World Health Organization; 2012. Available from: http://www.who.int/nutrition/publications/micronutrients/background_paper4_report_assessment_vitAandIron_status.pdf.
- 25.Thurnham DI, Northrop-Clewes CA, Knowles J. The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J Nutr 2015;145:1137S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization; Centers for Disease Control and Prevention. Assessing the iron status of populations: including literature reviews. Report of a Joint WHO/CDC Technical Consultation on the Assessment of Iron Status at the Population Level 2nd edition. Geneva (Switzerland): WHO; 2007. [Google Scholar]
- 27.Raiten DJ, Namaste S, Brabin B. Considerations for the safe and effective use of iron interventions in areas of malaria burden—executive summary. Int J Vitam Nutr Res 2011;81:57–71. [DOI] [PubMed] [Google Scholar]
- 28.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006;367:133–43. [DOI] [PubMed] [Google Scholar]
- 29.Raiten DJ, Namaste S, Brabin B, Combs G Jr, L'Abbe MR, Wasantwisut E, Darnton-Hill I. Executive summary—Biomarkers of Nutrition for Development: building a consensus. Am J Clin Nutr 2011;94(Suppl):633S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Eunice Kennedy Shriver National Institute of Child Health and Human Development. Biomarkers of Nutrition for Development (BOND) Program [cited 2014 Jul 7]. Available from: https://www.nichd.nih.gov/global_nutrition/programs/bond/Pages/index.aspx.
- 31.Lashinger LM, Ford NA, Hursting SD. Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J Nutr 2014;144:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol 2008;1:11–22. [DOI] [PubMed] [Google Scholar]
- 33.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012;336:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 36.Claycombe KJ, Brissette CA, Ghribi O. Epigenetics of inflammation, maternal infection, and nutrition. J Nutr 2015;145:1109S–15S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salam RA, Das JK, Bhutta ZA. Current issues and priorities in childhood nutrition, growth, and infections. J Nutr 2015;145:1116S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy LC, Raiten DJ, Hubbard VS, Starke-Reed P. Progress and challenges in developing metabolic footprints from diet in human gut microbial co-metabolism. J Nutr 2015;145:1123S–30S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crenn P, De Truchis P, Neveux N, Galperine T, Cynober L, Melchior JC. Plasma citrulline is a biomarker of enterocyte mass and an indicator of parenteral nutrition in HIV-infected patients. Am J Clin Nutr 2009;90:587–94. [DOI] [PubMed] [Google Scholar]
- 40.Lutgens LC, Blijlevens NM, Deutz NE, Donnelly JP, Lambin P, de Pauw BE. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer 2005;103:191–9. [DOI] [PubMed] [Google Scholar]
- 41.Vreugdenhil AC, Wolters VM, Adriaanse MP, Van den Neucker AM, van Bijnen AA, Houwen R, Buurman WA. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand J Gastroenterol 2011;46:1435–41. [DOI] [PubMed] [Google Scholar]
- 42.van Rheenen PF. Role of fecal calprotectin testing to predict relapse in teenagers with inflammatory bowel disease who report full disease control. Inflamm Bowel Dis 2012;18:2018–25. [DOI] [PubMed] [Google Scholar]
- 43.Johnson AR, Makowski L. Nutrition and metabolic correlates of obesity and inflammation: clinical considerations. J Nutr 2015;145:1131S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calder PC, Ahluwalia N, Albers R, Bosco N, Bourdet-Sicard R, Haller D, Holgate ST, Jonsson LS, Latulippe ME, Marcos A, et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br J Nutr 2013;109(Suppl 1):S1–34. [DOI] [PubMed] [Google Scholar]
- 45.Kolev M, Le Friec G, Kemper C. The role of complement in CD4(+) T cell homeostasis and effector functions. Semin Immunol 2013;25:12–9. [DOI] [PubMed] [Google Scholar]
- 46.Köhl J. Self, non-self, and danger: a complementary view. Adv Exp Med Biol 2006;586:71–94. [DOI] [PubMed] [Google Scholar]
- 47.Deitch EA. Bacterial translocation: the influence of dietary variables. Gut 1994;35(1 Suppl):S23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012;487:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katayama M, Xu D, Specian RD, Deitch EA. Role of bacterial adherence and the mucus barrier on bacterial translocation: effects of protein malnutrition and endotoxin in rats. Ann Surg 1997;225:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schäffer MR, Tantry U, Ahrendt GM, Wasserkrug HL, Barbul A. Acute protein-calorie malnutrition impairs wound healing: a possible role of decreased wound nitric oxide synthesis. J Am Coll Surg 1997;184:37–43. [PubMed] [Google Scholar]
- 51.Butzner JD, Butler DG, Miniats OP, Hamilton JR. Impact of chronic protein-calorie malnutrition on small intestinal repair after acute viral enteritis: a study in gnotobiotic piglets. Pediatr Res 1985;19:476–81. [DOI] [PubMed] [Google Scholar]
- 52.Zijlstra RT, Donovan SM, Odle J, Gelberg HB, Petschow BW, Gaskins HR. Protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus. J Nutr 1997;127:1118–27. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto M, Fujisawa Y, Nishioka K. Physiologic role of the complement system in host defense, disease, and malnutrition. Nutrition 1998;14:391–8. [DOI] [PubMed] [Google Scholar]
- 54.Jennings G, Bourgeois C, Elia M. The magnitude of the acute phase protein response is attenuated by protein deficiency in rats. J Nutr 1992;122:1325–31. [DOI] [PubMed] [Google Scholar]
- 55.Jennings G, Cruickshank AM, Shenkin A, Wight DG, Elia M. Effect of aseptic abscesses in protein-deficient rats on the relationship between interleukin-6 and the acute-phase protein, alpha 2-macroglobulin. Clin Sci (Lond) 1992;83:731–5. [DOI] [PubMed] [Google Scholar]
- 56.Redmond HP, Leon P, Lieberman MD, Hofmann K, Shou J, Reynolds JV, Goldfine J, Johnston RB Jr, Daly JM. Impaired macrophage function in severe protein-energy malnutrition. Arch Surg 1991;126:192–6. [DOI] [PubMed] [Google Scholar]
- 57.Fock RA, Vinolo MA, de Moura Sa Rocha V, de Sa Rocha LC, Borelli P. Protein-energy malnutrition decreases the expression of TLR-4/MD-2 and CD14 receptors in peritoneal macrophages and reduces the synthesis of TNF-alpha in response to lipopolysaccharide (LPS) in mice. Cytokine 2007;40:105–14. [DOI] [PubMed] [Google Scholar]
- 58.Stapleton PP, Barry LK, Freeman TA, Mestre JR, Daly JM. Decreased peritoneal macrophage NF-kappaB translocation to the nucleus in protein energy malnutrition—a role for the glucocorticoid response? Clin Nutr 2004;23:177–82. [DOI] [PubMed] [Google Scholar]
- 59.McCarter MD, Naama HA, Shou J, Kwi LX, Evoy DA, Calvano SE, Daly JM. Altered macrophage intracellular signaling induced by protein-calorie malnutrition. Cell Immunol 1998;183:131–6. [DOI] [PubMed] [Google Scholar]
- 60.Vinolo MA, Crisma AR, Nakajima K, Rogero MM, Fock RA, Borelli P. Malnourished mice display an impaired hematologic response to granulocyte colony-stimulating factor administration. Nutr Res 2008;28:791–7. [DOI] [PubMed] [Google Scholar]
- 61.Harris MC, Douglas SD, Lee JC, Ziegler MM, Gerdes JS, Polin RA. Diminished polymorphonuclear leukocyte adherence and chemotaxis following protein-calorie malnutrition in newborn rats. Pediatr Res 1987;21:542–6. [DOI] [PubMed] [Google Scholar]
- 62.Keusch GT, Urrutia JJ, Guerrero O, Castaneda G, Smith H Jr. Serum opsonic activity in acute protein-energy malnutrition. Bull World Health Organ 1981;59:923–9. [PMC free article] [PubMed] [Google Scholar]
- 63.Suda AK, Mathur M, Deo K, Deo MG. Kinetics of mobilization of neutrophils and their marrow pool in protein-calorie deficiency. Blood 1976;48:865–75. [PubMed] [Google Scholar]
- 64.Schopfer K, Douglas SD. Neutrophil function in children with kwashiorkor. J Lab Clin Med 1976;88:450–61. [PubMed] [Google Scholar]
- 65.Silva SV, Garcia-Souza EP, Moura AS, Barja-Fidalgo C. Maternal protein restriction during early lactation induces changes on neutrophil activation and TNF-alpha production of adult offspring. Inflammation 2010;33:65–75. [DOI] [PubMed] [Google Scholar]
- 66.Ritz BW, Gardner EM. Malnutrition and energy restriction differentially affect viral immunity. J Nutr 2006;136:1141–4. [DOI] [PubMed] [Google Scholar]
- 67.Heath ML, Sidbury R. Cutaneous manifestations of nutritional deficiency. Curr Opin Pediatr 2006;18:417–22. [DOI] [PubMed] [Google Scholar]
- 68.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med 1925;42:753–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolbach SB, Howe PR. Epithelial repair in recovery from vitamin A deficiency—an experimental study. J Exp Med 1933;57:511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madjid B, Sirisinha S, Lamb AJ. The effect of vitamin A and protein deficiency on complement levels in rats. Proc Soc Exp Biol Med 1978;158:92–5. [DOI] [PubMed] [Google Scholar]
- 71.Wiedermann U, Chen XJ, Enerback L, Hanson LA, Kahu H, Dahlgren UI. Vitamin A deficiency increases inflammatory responses. Scand J Immunol 1996;44:578–84. [DOI] [PubMed] [Google Scholar]
- 72.Bohannon DE, Kiorpes TC, Wolf G. The response of the acute-phase plasma protein alpha2-macroglobulin to vitamin A deficiency in the rat. J Nutr 1979;109:1189–94. [DOI] [PubMed] [Google Scholar]
- 73.Twining SS, Schulte DP, Wilson PM, Fish BL, Moulder JE. Vitamin A deficiency alters rat neutrophil function. J Nutr 1997;127:558–65. [DOI] [PubMed] [Google Scholar]
- 74.Kawamura T, Ogawa Y, Nakamura Y, Nakamizo S, Ohta Y, Nakano H, Kabashima K, Katayama I, Koizumi S, Kodama T, et al. Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency. J Clin Invest 2012;122:722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duggan C, Gannon J, Walker WA. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr 2002;75:789–808. [DOI] [PubMed] [Google Scholar]
- 76.Gong H, Takami Y, Amemiya T, Tozu M, Ohashi Y. Ocular surface in Zn-deficient rats. Ophthalmic Res 2004;36:129–38. [DOI] [PubMed] [Google Scholar]
- 77.Fraker PJ, Zwickl CM, Luecke RW. Delayed type hypersensitivity in zinc deficient adult mice: impairment and restoration of responsivity to dinitrofluorobenzene. J Nutr 1982;112:309–13. [DOI] [PubMed] [Google Scholar]
- 78.Nauss KM, Phua CC, Ambrogi L, Newberne PM. Immunological changes during progressive stages of vitamin A deficiency in the rat. J Nutr 1985;115:909–18. [DOI] [PubMed] [Google Scholar]
- 79.Dawson HD, Ross AC. Chronic marginal vitamin A status affects the distribution and function of T cells and natural T cells in aging Lewis rats. J Nutr 1999;129:1782–90. [DOI] [PubMed] [Google Scholar]
- 80.Abu-Amer Y, Bar-Shavit Z. Impaired bone marrow-derived macrophage differentiation in vitamin D deficiency. Cell Immunol 1993;151:356–68. [DOI] [PubMed] [Google Scholar]
- 81.Bar-Shavit Z, Noff D, Edelstein S, Meyer M, Shibolet S, Goldman R. 1,25-Dihydroxyvitamin D3 and the regulation of macrophage function. Calcif Tissue Int 1981;33:673–6. [DOI] [PubMed] [Google Scholar]
- 82.Gavison R, Bar-Shavit Z. Impaired macrophage activation in vitamin D3 deficiency: differential in vitro effects of 1,25-dihydroxyvitamin D3 on mouse peritoneal macrophage functions. J Immunol 1989;143:3686–90. [PubMed] [Google Scholar]
- 83.Kankova M, Luini W, Pedrazzoni M, Riganti F, Sironi M, Bottazzi B, Mantovani A, Vecchi A. Impairment of cytokine production in mice fed a vitamin D3-deficient diet. Immunology 1991;73:466–71. [PMC free article] [PubMed] [Google Scholar]
- 84.Yang S, Smith C, Prahl JM, Luo X, DeLuca HF. Vitamin D deficiency suppresses cell-mediated immunity in vivo. Arch Biochem Biophys 1993;303:98–106. [DOI] [PubMed] [Google Scholar]
- 85.Mathieu C, Van Etten E, Gysemans C, Decallonne B, Kato S, Laureys J, Depovere J, Valckx D, Verstuyf A, Bouillon R. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J Bone Miner Res 2001;16:2057–65. [DOI] [PubMed] [Google Scholar]
- 86.Gysemans C, van Etten E, Overbergh L, Giulietti A, Eelen G, Waer M, Verstuyf A, Bouillon R, Mathieu C. Unaltered diabetes presentation in NOD mice lacking the vitamin D receptor. Diabetes 2008;57:269–75. [DOI] [PubMed] [Google Scholar]
- 87.Shiner M, Redmond AO, Hansen JD. The jejunal mucosa in protein-energy malnutrition: a clinical, histological, and ultrastructural study. Exp Mol Pathol 1973;19:61–78. [DOI] [PubMed] [Google Scholar]
- 88.Brewster DR, Manary MJ, Menzies IS, O'Loughlin EV, Henry RL. Intestinal permeability in kwashiorkor. Arch Dis Child 1997;76:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Welsh FK, Farmery SM, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV. Gut barrier function in malnourished patients. Gut 1998;42:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omoike IU, Abiodun PO. Upper small intestinal microflora in diarrhea and malnutrition in Nigerian children. J Pediatr Gastroenterol Nutr 1989;9:314–21. [DOI] [PubMed] [Google Scholar]
- 91.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013;339:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Little MO. Nutrition and skin ulcers. Curr Opin Clin Nutr Metab Care 2013;16:39–49. [DOI] [PubMed] [Google Scholar]
- 93.Keusch GT, Torun B, Johnston RB Jr, Urrutia JJ. Impairment of hemolytic complement activation by both classical and alternative pathways in serum from patients with kwashiorkor. J Pediatr 1984;105:434–6. [DOI] [PubMed] [Google Scholar]
- 94.Haller L, Zubler RH, Lambert PH. Plasma levels of complement components and complement haemolytic activity in protein-energy malnutrition. Clin Exp Immunol 1978;34:248–52. [PMC free article] [PubMed] [Google Scholar]
- 95.Hughes S, Kelly P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol 2006;28:577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doherty JF, Golden MH, Raynes JG, Griffin GE, McAdam KP. Acute-phase protein response is impaired in severely malnourished children. Clin Sci (Lond) 1993;84:169–75. [DOI] [PubMed] [Google Scholar]
- 97.Durán-Chávez C, Sánchez-Herrera G, Cañedo-Solares I, Pérez-Ortíz B. C-reactive protein in severely malnourished children. Nutr Res 1994;14:967–75. [Google Scholar]
- 98.Woodward B. Protein, calories, and immune defenses. Nutr Rev 1998;56:S84–92. [DOI] [PubMed] [Google Scholar]
- 99.Gershwin ME, Beach RS, Hurley LS. Nutrition and immunity. Orlando (FL): Academic Press; 1985. [Google Scholar]
- 100.Nayak KC, Sethi AS, Aggarwal TD, Chadda VS, Kumar KK. Bactericidal power of neutrophils in protein calorie malnutrition. Indian J Pediatr 1989;56:371–7. [DOI] [PubMed] [Google Scholar]
- 101.Schopfer K, Douglas SD. Fine structural studies of peripheral blood leucocytes from children with kwashiorkor: morphological and functional properties. Br J Haematol 1976;32:573–7. [DOI] [PubMed] [Google Scholar]
- 102.Rao V, Friend J, Thoft RA, Underwood BA, Reddy PR. Conjunctival goblet cells and mitotic rate in children with retinol deficiency and measles. Arch Ophthalmol 1987;105:378–80. [DOI] [PubMed] [Google Scholar]
- 103.Savy M, Edmond K, Fine PE, Hall A, Hennig BJ, Moore SE, Mulholland K, Schaible U, Prentice AM. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr 2009;139(Suppl):2154S–218S. [DOI] [PubMed] [Google Scholar]
- 104.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 105.Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev 2005;18:446–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahmad SM, Haskell MJ, Raqib R, Stephensen CB. Vitamin A status is associated with T-cell responses in Bangladeshi men. Br J Nutr 2009;102:797–802. [DOI] [PubMed] [Google Scholar]
- 107.Jason J, Archibald LK, Nwanyanwu OC, Sowell AL, Buchanan I, Larned J, Bell M, Kazembe PN, Dobbie H, Jarvis WR. Vitamin A levels and immunity in humans. Clin Diagn Lab Immunol 2002;9:616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab 2013;62(Suppl 1):19–29. [DOI] [PubMed] [Google Scholar]
- 109.Businco L, Menghi AM, Rossi P, D'Amelio R, Galli E. Zinc-dependent chemotactic defect in an infant with acrodermatitis. Arch Dis Child 1980;55:966–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Honzík T, Magner M, Janda A, Bartunkova J, Polaskova S, Zeman J. Prolonged impairment of polymorphonuclear cells functions in one infant with transient zinc deficiency: a case report. Prague Med Rep 2008;109:184–93. [PubMed] [Google Scholar]
- 111.Weston WL, Huff JC, Humbert JR, Hambidge KM, Neldner KH, Walravens PA. Zinc correction of defective chemotaxis in acrodermatitis enteropathica. Arch Dermatol 1977;113:422–5. [PubMed] [Google Scholar]
- 112.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 2009;182:4289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770–3. [DOI] [PubMed] [Google Scholar]
- 114.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics 2009;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, Kanada K, Yamasaki K, Alexandrescu D, Gallo RL. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol 2008;122:829–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boston University Biology; c2015. NF-kB target genes [cited 2013 Nov 14]. Available from: http://www.bu.edu/nf-kb/gene-resources/target-genes/.
- 117.Boston University Biology; c2015. NF-kB transcription factors [cited 2013 Nov 14]. Available from: http://www.bu.edu/nf-kb.
- 118.Austenaa LM, Carlsen H, Hollung K, Blomhoff HK, Blomhoff R. Retinoic acid dampens LPS-induced NF-kappaB activity: results from human monoblasts and in vivo imaging of NF-kappaB reporter mice. J Nutr Biochem 2009;20:726–34. [DOI] [PubMed] [Google Scholar]
- 119.Chen Q, Ma Y, Ross AC. Opposing cytokine-specific effects of all trans-retinoic acid on the activation and expression of signal transducer and activator of transcription (STAT)-1 in THP-1 cells. Immunology 2002;107:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem 1999;274:7674–80. [DOI] [PubMed] [Google Scholar]
- 121.Dong X, Lutz W, Schroeder TM, Bachman LA, Westendorf JJ, Kumar R, Griffin MD. Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter. Proc Natl Acad Sci USA 2005;102:16007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 2010;184:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Y, Kong J, Sun T, Li G, Szeto FL, Liu W, Deb DK, Wang Y, Zhao Q, Thadhani R, et al. 1,25-Dihydroxyvitamin D(3) suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kappaB activation. Arch Biochem Biophys 2011;507:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol 2010;177:686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients 2012;4:676–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, Knoell DL. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2010;298:L744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci USA 2011;108:20970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prasad AS, Bao B, Beck FW, Sarkar FH. Correction of interleukin-2 gene expression by in vitro zinc addition to mononuclear cells from zinc-deficient human subjects: a specific test for zinc deficiency in humans. Transl Res 2006;148:325–33. [DOI] [PubMed] [Google Scholar]
- 129.Glauert HP. Vitamin E and NF-kappaB activation: a review. Vitam Horm 2007;76:135–53. [DOI] [PubMed] [Google Scholar]
- 130.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- 131.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531–62. [DOI] [PubMed] [Google Scholar]
- 132.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol 2008;9:981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ross AC. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr 2012;96:1166S–72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Broadhurst MJ, Leung JM, Lim KC, Girgis NM, Gundra UM, Fallon PG, Premenko-Lanier M, McKerrow JH, McCune JM, Loke P. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS Pathog 2012;8:e1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spiegl N, Didichenko S, McCaffery P, Langen H, Dahinden CA. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood 2008;112:3762–71. [DOI] [PubMed] [Google Scholar]
- 136.Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B, Grakoui A. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol 2013;190:2009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol 2006;24:209–26. [DOI] [PubMed] [Google Scholar]
- 138.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity 2010;33:313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol 2007;179:3724–33. [DOI] [PubMed] [Google Scholar]
- 140.Sadlon TJ, Wilkinson BG, Pederson S, Brown CY, Bresatz S, Gargett T, Melville EL, Peng K, D'Andrea RJ, Glonek GG, et al. Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J Immunol 2010;185:1071–81. [DOI] [PubMed] [Google Scholar]
- 141.Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, et al. 1,25-Dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol 2011;31:3653–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, Lee SH, Kim WU, Kang I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol 2012;188:5276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol 2011;41:822–32. [DOI] [PubMed] [Google Scholar]
- 144.Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, Quigley EM, Kiely B, Akdis CA, O'Mahony L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 2012;61:354–66. [DOI] [PubMed] [Google Scholar]
- 145.Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M, Roder S, Rolle-Kampczyk U, von Bergen M, Olek S, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 2013;68:220–8. [DOI] [PubMed] [Google Scholar]
- 146.Mottaghi A, Salehi E, Keshvarz A, Sezavar H, Saboor-Yaraghi AA. The influence of vitamin A supplementation on Foxp3 and TGF-beta gene expression in atherosclerotic patients. J Nutrigenet Nutrigenomics 2012;5:314–26. [DOI] [PubMed] [Google Scholar]
- 147.Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity 2007;27:145–59. [DOI] [PubMed] [Google Scholar]
- 148.Kinoshita M, Kayama H, Kusu T, Yamaguchi T, Kunisawa J, Kiyono H, Sakaguchi S, Takeda K. Dietary folic acid promotes survival of Foxp3+ regulatory T cells in the colon. J Immunol 2012;189:2869–78. [DOI] [PubMed] [Google Scholar]
- 149.Takahashi H, Nakazawa M, Takahashi K, Aihara M, Minami M, Hirasawa T, Ikezawa Z. Effects of zinc deficient diet on development of atopic dermatitis-like eruptions in DS-Nh mice. J Dermatol Sci 2008;50:31–9. [DOI] [PubMed] [Google Scholar]
- 150.Abe M, Akbar F, Matsuura B, Horiike N, Onji M. Defective antigen-presenting capacity of murine dendritic cells during starvation. Nutrition 2003;19:265–9. [DOI] [PubMed] [Google Scholar]
- 151.Conzen SD, Janeway CA. Defective antigen presentation in chronically protein-deprived mice. Immunology 1988;63:683–9. [PMC free article] [PubMed] [Google Scholar]
- 152.Konyer JE, Hillyer LM, Woodward B. Splenic dendritic cell populations of the weanling mouse involute proportionately with total nucleated cell numbers throughout acute protein and energy deficiencies except in the most advanced stages of nitrogen-to-energy imbalance. Nutr Res 2003;23:921–31. [Google Scholar]
- 153.Niiya T, Akbar SM, Yoshida O, Miyake T, Matsuura B, Murakami H, Abe M, Hiasa Y, Onji M. Impaired dendritic cell function resulting from chronic undernutrition disrupts the antigen-specific immune response in mice. J Nutr 2007;137:671–5. [DOI] [PubMed] [Google Scholar]
- 154.Redmond HP, Gallagher HJ, Shou J, Daly JM. Antigen presentation in protein-energy malnutrition. Cell Immunol 1995;163:80–7. [DOI] [PubMed] [Google Scholar]
- 155.Zhang X, Hillyer LM, Woodward BD. The capacity of noninflammatory (steady-state) dendritic cells to present antigen in the primary response is preserved in acutely protein- or energy-deficient weanling mice. J Nutr 2002;132:2748–56. [DOI] [PubMed] [Google Scholar]
- 156.Chang SY, Cha HR, Chang JH, Ko HJ, Yang H, Malissen B, Iwata M, Kweon MN. Lack of retinoic acid leads to increased langerin-expressing dendritic cells in gut-associated lymphoid tissues. Gastroenterology 2010;138(4):1468–78, 1478e1–6. [DOI] [PubMed] [Google Scholar]
- 157.Duriancik DM, Hoag KA. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8(+)Gr-1(+) memory T lymphocytes in C57BL/6J mice. Cell Immunol 2010;265:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 2010;33:71–83. [DOI] [PubMed] [Google Scholar]
- 159.Vlasova AN, Chattha KS, Kandasamy S, Siegismund CS, Saif LJ. Prenatally acquired vitamin A deficiency alters innate immune responses to human rotavirus in a gnotobiotic pig model. J Immunol 2013;190:4742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang Y, Yuan Y, Tao Y, Wang W. Effects of vitamin A deficiency on mucosal immunity and response to intestinal infection in rats. Nutrition 2011;27:227–32. [DOI] [PubMed] [Google Scholar]
- 161.Ahmed F, Jones DB, Jackson AA. The interaction of vitamin A deficiency and rotavirus infection in the mouse. Br J Nutr 1990;63:363–73. [DOI] [PubMed] [Google Scholar]
- 162.Zhao Z, Ross AC. Retinoic acid repletion restores the number of leukocytes and their subsets and stimulates natural cytotoxicity in vitamin A-deficient rats. J Nutr 1995;125:2064–73. [DOI] [PubMed] [Google Scholar]
- 163.Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology 2009;137:1391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol 2008;180:1834–42. [DOI] [PubMed] [Google Scholar]
- 165.Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr 2004;134:2660–6. [DOI] [PubMed] [Google Scholar]
- 166.Friedman A, Sklan D. Impaired T lymphocyte immune response in vitamin A depleted rats and chicks. Br J Nutr 1989;62:439–49. [DOI] [PubMed] [Google Scholar]
- 167.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006;314:1157–60. [DOI] [PubMed] [Google Scholar]
- 168.Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol 2011;23:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Goldstein BD, Kurt RA. Dietary vitamin D3 restriction influences tumor growth, but not the ability to generate an antigen-specific immune response in OTII transgenic mice. Immunol Invest 2009;38:365–82. [DOI] [PubMed] [Google Scholar]
- 170.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA 2001;98:6800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nguyen NL, Chen K, McAleer J, Kolls JK. Vitamin D regulation of OX40 ligand in immune responses to Aspergillus fumigatus. Infect Immun 2013;81:1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.O'Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest 2002;109:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci USA 2008;105:5207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hughes SM, Amadi B, Mwiya M, Nkamba H, Tomkins A, Goldblatt D. Dendritic cell anergy results from endotoxemia in severe malnutrition. J Immunol 2009;183:2818–26. [DOI] [PubMed] [Google Scholar]
- 175.Yener E, Coker C, Cura A, Keskinoglu A, Mir S. Lymphocyte subpopulations in children with vitamin D deficient rickets. Acta Paediatr Jpn 1995;37:500–2. [DOI] [PubMed] [Google Scholar]
- 176.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 2010;11:344–9. [DOI] [PubMed] [Google Scholar]
- 177.Ahluwalia N, Sun J, Krause D, Mastro A, Handte G. Immune function is impaired in iron-deficient, homebound, older women. Am J Clin Nutr 2004;79:516–21. [DOI] [PubMed] [Google Scholar]
- 178.Bagchi K, Mohanram M, Reddy V. Humoral immune response in children with iron-deficiency anaemia. BMJ 1980;280:1249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dhur A, Galan P, Hercberg S. Iron status, immune capacity and resistance to infections. Comp Biochem Physiol A Comp Physiol 1989;94(1):11–9. [DOI] [PubMed] [Google Scholar]
- 180.Mullick S, Rusia U, Sikka M, Faridi MA. Impact of iron deficiency anaemia on T lymphocytes and their subsets in children. Indian J Med Res 2006;124:647–54. [PubMed] [Google Scholar]
- 181.Santos PC, Falcao RP. Decreased lymphocyte subsets and K-cell activity in iron deficiency anemia. Acta Haematol 1990;84:118–21. [DOI] [PubMed] [Google Scholar]
- 182.Gross RL, Reid JV, Newberne PM, Burgess B, Marston R, Hift W. Depressed cell-mediated immunity in megaloblastic anemia due to folic acid deficiency. Am J Clin Nutr 1975;28:225–32. [DOI] [PubMed] [Google Scholar]
- 183.Molls RR, Ahluwalia N, Mastro AM, Smiciklas-Wright H, Handte GC. Nutritional status predicts primary subclasses of T cells and the lymphocyte proliferation response in healthy older women. J Nutr 2005;135:2644–50. [DOI] [PubMed] [Google Scholar]
- 184.Payette H, Rola-Pleszczynski M, Ghadirian P. Nutrition factors in relation to cellular and regulatory immune variables in a free-living elderly population. Am J Clin Nutr 1990;52:927–32. [DOI] [PubMed] [Google Scholar]
- 185.Repa JJ, Hanson KK, Clagett-Dame M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc Natl Acad Sci USA 1993;90:7293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mahony S, Mazzoni EO, McCuine S, Young RA, Wichterle H, Gifford DK. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol 2011;12:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 2002;43:1773–808. [DOI] [PubMed] [Google Scholar]
- 188.Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, Selznick SH, Whitfield GK. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol 1997;154( Suppl):S57–73. [PubMed] [Google Scholar]
- 189.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2010;20:1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schräder M, Bendik I, Becker-Andre M, Carlberg C. Interaction between retinoic acid and vitamin D signaling pathways. J Biol Chem 1993;268:17830–6. [PubMed] [Google Scholar]
- 191.Koszewski NJ, Herberth J, Malluche HH. Retinoic acid receptor gamma 2 interactions with vitamin D response elements. J Steroid Biochem Mol Biol 2010;120:200–7. [DOI] [PubMed] [Google Scholar]
- 192.Bastie JN, Balitrand N, Guillemot I, Chomienne C, Delva L. Cooperative action of 1alpha,25-dihydroxyvitamin D3 and retinoic acid in NB4 acute promyelocytic leukemia cell differentiation is transcriptionally controlled. Exp Cell Res 2005;310:319–30. [DOI] [PubMed] [Google Scholar]
- 193.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 2013;4:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laity JH, Lee BM, Wright PE. Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol 2001;11:39–46. [DOI] [PubMed] [Google Scholar]
- 195.Emerson RO, Thomas JH. Adaptive evolution in zinc finger transcription factors. PLoS Genet 2009;5:e1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, et al. Zinc is a novel intracellular second messenger. J Cell Biol 2007;177:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Encode Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012;489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yip KY, Cheng C, Bhardwaj N, Brown JB, Leng J, Kundaje A, Rozowsky J, Birney E, Bickel P, Snyder M, et al. Classification of human genomic regions based on experimentally determined binding sites of more than 100 transcription-related factors. Genome Biol 2012;13:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, et al. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 2005;37:1234–41. [DOI] [PubMed] [Google Scholar]
- 200.Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu Rev Med 2010;61:437–55. [DOI] [PubMed] [Google Scholar]
- 201.Lee JT. Epigenetic regulation by long noncoding RNAs. Science 2012;338:1435–9. [DOI] [PubMed] [Google Scholar]
- 202.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol 2003;4:235–40. [DOI] [PubMed] [Google Scholar]
- 203.Morinobu A, Kanno Y, O'Shea JJ. Discrete roles for histone acetylation in human T helper 1 cell-specific gene expression. J Biol Chem 2004;279:40640–6. [DOI] [PubMed] [Google Scholar]
- 204.Rowling MJ, McMullen MH, Schalinske KL. Vitamin A and its derivatives induce hepatic glycine N-methyltransferase and hypomethylation of DNA in rats. J Nutr 2002;132:365–9. [DOI] [PubMed] [Google Scholar]
- 205.Perng W, Rozek LS, Mora-Plazas M, Duchin O, Marin C, Forero Y, Baylin A, Villamor E. Micronutrient status and global DNA methylation in school-age children. Epigenetics 2012;7:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheong HS, Lee HC, Park BL, Kim H, Jang MJ, Han YM, Kim SY, Kim YS, Shin HD. Epigenetic modification of retinoic acid-treated human embryonic stem cells. BMB Rep 2010;43:830–5. [DOI] [PubMed] [Google Scholar]
- 207.Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 2007;449:731–4. [DOI] [PubMed] [Google Scholar]
- 208.Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 2007;318:447–50. [DOI] [PubMed] [Google Scholar]
- 209.Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, Hermanson O, Rosenfeld MG. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature 2007;450:415–9. [DOI] [PubMed] [Google Scholar]
- 210.Angrisano T, Sacchetti S, Natale F, Cerrato A, Pero R, Keller S, Peluso S, Perillo B, Avvedimento VE, Fusco A, et al. Chromatin and DNA methylation dynamics during retinoic acid-induced RET gene transcriptional activation in neuroblastoma cells. Nucleic Acids Res 2011;39:1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fujiki R, Chikanishi T, Hashiba W, Ito H, Takada I, Roeder RG, Kitagawa H, Kato S. GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature 2014;505:455–9. [DOI] [PubMed] [Google Scholar]
- 212.Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder H, et al. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene 2007;26:4148–57. [DOI] [PubMed] [Google Scholar]
- 213.Pozzi S, Rossetti S, Bistulfi G, Sacchi N. RAR-mediated epigenetic control of the cytochrome P450 Cyp26a1 in embryocarcinoma cells. Oncogene 2006;25:1400–7. [DOI] [PubMed] [Google Scholar]
- 214.Kashyap V, Gudas LJ. Epigenetic regulatory mechanisms distinguish retinoic acid-mediated transcriptional responses in stem cells and fibroblasts. J Biol Chem 2010;285:14534–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yuan W, Wu T, Fu H, Dai C, Wu H, Liu N, Li X, Xu M, Zhang Z, Niu T, et al. Dense chromatin activates Polycomb repressive complex 2 to regulate H3 lysine 27 methylation. Science 2012;337:971–5. [DOI] [PubMed] [Google Scholar]
- 216.Raiten DJ. Nutrition and pharmacology: general principles and implications for HIV. Am J Clin Nutr 2011;94(Suppl):1697S–702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pereira F, Barbachano A, Singh PK, Campbell MJ, Munoz A, Larriba MJ. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle 2012;11:1081–9. [DOI] [PubMed] [Google Scholar]
- 218.Thorne JL, Maguire O, Doig CL, Battaglia S, Fehr L, Sucheston LE, Heinaniemi M, O'Neill LP, McCabe CJ, Turner BM, et al. Epigenetic control of a VDR-governed feed-forward loop that regulates p21(waf1/cip1) expression and function in non-malignant prostate cells. Nucleic Acids Res 2011;39:2045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Antal AS, Dombrowski Y, Koglin S, Ruzicka T, Schauber J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Dermatoendocrinol 2011;3:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jorde R, Svartberg J, Joakimsen RM, Coucheron DH. Plasma profile of microRNA after supplementation with high doses of vitamin D3 for 12 months. BMC Res Notes 2012;5:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fernandes de Abreu DA, Landel V, Feron F. Seasonal, gestational and postnatal influences on multiple sclerosis: the beneficial role of a vitamin D supplementation during early life. J Neurol Sci 2011;311:64–8. [DOI] [PubMed] [Google Scholar]
- 222.Kim MS, Fujiki R, Kitagawa H, Kato S. 1alpha,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol Cell Endocrinol 2007;265–266:168–73. [DOI] [PubMed] [Google Scholar]
- 223.Pereira F, Barbachano A, Silva J, Bonilla F, Campbell MJ, Munoz A, Larriba MJ. KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum Mol Genet 2011;20:4655–65. [DOI] [PubMed] [Google Scholar]
- 224.Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD, O'Connell RM, Baltimore D. MicroRNA-125b potentiates macrophage activation. J Immunol 2011;187:5062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Komagata S, Nakajima M, Takagi S, Mohri T, Taniya T, Yokoi T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol Pharmacol 2009;76:702–9. [DOI] [PubMed] [Google Scholar]
- 226.Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol 2012;13:297–311. [DOI] [PubMed] [Google Scholar]
- 227.Wallwork JC, Duerre JA. Effect of zinc deficiency on methionine metabolism, methylation reactions and protein synthesis in isolated perfused rat liver. J Nutr 1985;115:252–62. [DOI] [PubMed] [Google Scholar]
- 228.Beach RS, Gershwin ME, Hurley LS. Gestational zinc deprivation in mice: persistence of immunodeficiency for three generations. Science 1982;218:469–71. [DOI] [PubMed] [Google Scholar]
- 229.Raqib R, Hossain MB, Kelleher SL, Stephensen CB, Lonnerdal B. Zinc supplementation of pregnant rats with adequate zinc nutriture suppresses immune functions in their offspring. J Nutr 2007;137:1037–42. [DOI] [PubMed] [Google Scholar]
- 230.Sharkar MT, Jou MY, Hossain MB, Lonnerdal B, Stephensen CB, Raqib R. Prenatal zinc supplementation of zinc-adequate rats adversely affects immunity in offspring. J Nutr 2011;141:1559–64. [DOI] [PubMed] [Google Scholar]
- 231.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA 2007;104:19351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Belisle SE, Leka LS, Delgado-Lista J, Jacques PF, Ordovas JM, Meydani SN. Polymorphisms at cytokine genes may determine the effect of vitamin E on cytokine production in the elderly. J Nutr 2009;139:1855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 1998;102:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brown K, Luddington R, Baglin T. A common polymorphism in the tumour necrosis factor-alpha gene associated with high TNF levels is not a risk factor for venous thromboembolism. Br J Haematol 1998;101:480–2. [DOI] [PubMed] [Google Scholar]
- 235.Skoog T, van't Hooft FM, Kallin B, Jovinge S, Boquist S, Nilsson J, Eriksson P, Hamsten A. A common functional polymorphism (C→A substitution at position -863) in the promoter region of the tumour necrosis factor-alpha (TNF-alpha) gene associated with reduced circulating levels of TNF-alpha. Hum Mol Genet 1999;8:1443–9. [DOI] [PubMed] [Google Scholar]
- 236.England A, Valdes AM, Slater-Jefferies JL, Gill R, Howell WM, Calder PC, Grimble RF. Variants in the genes encoding TNF-alpha, IL-10, and GSTP1 influence the effect of alpha-tocopherol on inflammatory cell responses in healthy men. Am J Clin Nutr 2012;95:1461–7. [DOI] [PubMed] [Google Scholar]
- 237.Zhao M, Lewis R, Gustafson DR, Wen WQ, Cerhan JR, Zheng W. No apparent association of GSTP1 A(313)G polymorphism with breast cancer risk among postmenopausal Iowa women. Cancer Epidemiol Biomarkers Prev 2001;10:1301–2. [PubMed] [Google Scholar]
- 238.Dinarello CA. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med 1984;311:1413–8. [DOI] [PubMed] [Google Scholar]
- 239.Molina PE. Neurobiology of the stress response: contribution of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock 2005;24:3–10. [DOI] [PubMed] [Google Scholar]
- 240.Haddad JJ, Saade NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol 2002;133:1–19. [DOI] [PubMed] [Google Scholar]
- 241.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 2002;966:290–303. [DOI] [PubMed] [Google Scholar]
- 242.Miyamasu M, Misaki Y, Izumi S, Takaishi T, Morita Y, Nakamura H, Matsushima K, Kasahara T, Hirai K. Glucocorticoids inhibit chemokine generation by human eosinophils. J Allergy Clin Immunol 1998;101:75–83. [DOI] [PubMed] [Google Scholar]
- 243.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol 2006;6:318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Correa SG, Maccioni M, Rivero VE, Iribarren P, Sotomayor CE, Riera CM. Cytokines and the immune-neuroendocrine network: what did we learn from infection and autoimmunity? Cytokine Growth Factor Rev 2007;18:125–34. [DOI] [PubMed] [Google Scholar]
- 245.Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comp Med 2009;59:517–26. [PMC free article] [PubMed] [Google Scholar]
- 246.Petersen HH, Nielsen JP, Heegaard PM. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res 2004;35:163–87. [DOI] [PubMed] [Google Scholar]
- 247.Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci 1982;389:406–18. [DOI] [PubMed] [Google Scholar]
- 248.Suresh MV, Singh SK, Ferguson DA Jr, Agrawal A. Role of the property of C-reactive protein to activate the classical pathway of complement in protecting mice from pneumococcal infection. J Immunol 2006;176:4369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wait R, Chiesa G, Parolini C, Miller I, Begum S, Brambilla D, Galluccio L, Ballerio R, Eberini I, Gianazza E. Reference maps of mouse serum acute-phase proteins: changes with LPS-induced inflammation and apolipoprotein A-I and A-II transgenes. Proteomics 2005;5:4245–53. [DOI] [PubMed] [Google Scholar]
- 250.Qian WJ, Jacobs JM, Camp DG II, Monroe ME, Moore RJ, Gritsenko MA, Calvano SE, Lowry SF, Xiao W, Moldawer LL, et al. Comparative proteome analyses of human plasma following in vivo lipopolysaccharide administration using multidimensional separations coupled with tandem mass spectrometry. Proteomics 2005;5:572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Morlese JF, Forrester T, Jahoor F. Acute-phase protein response to infection in severe malnutrition. Am J Physiol 1998;275:E112–7. [DOI] [PubMed] [Google Scholar]
- 252.Reid M, Badaloo A, Forrester T, Morlese JF, Heird WC, Jahoor F. The acute-phase protein response to infection in edematous and nonedematous protein-energy malnutrition. Am J Clin Nutr 2002;76:1409–15. [DOI] [PubMed] [Google Scholar]
- 253.Manary MJ, Yarasheski KE, Berger R, Abrams ET, Hart CA, Broadhead RL. Whole-body leucine kinetics and the acute phase response during acute infection in marasmic Malawian children. Pediatr Res 2004;55:940–6. [DOI] [PubMed] [Google Scholar]
- 254.Grinspoon SK, Bilezikian JP. HIV disease and the endocrine system. N Engl J Med 1992;327:1360–5. [DOI] [PubMed] [Google Scholar]
- 255.Johnson JA, Albu JB, Engelson ES, Fried SK, Inada Y, Ionescu G, Kotler DP. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am J Physiol Endocrinol Metab 2004;286:E261–71. [DOI] [PubMed] [Google Scholar]
- 256.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010;33:2244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.LeGrand EK, Alcock J. Turning up the heat: immune brinksmanship in the acute-phase response. Q Rev Biol 2012;87:3–18. [DOI] [PubMed] [Google Scholar]
- 258.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest 1984;74:1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science 2012;338:768–72. [DOI] [PubMed] [Google Scholar]
- 260.Cassat JE, Skaar EP. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin Immunopathol 2012;34:215–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rahman MM, Mahalanabis D, Alvarez JO, Wahed MA, Islam MA, Habte D, Khaled MA. Acute respiratory infections prevent improvement of vitamin A status in young infants supplemented with vitamin A. J Nutr 1996;126:628–33. [DOI] [PubMed] [Google Scholar]
- 262.Stephensen CB, Franchi LM, Hernandez H, Campos M, Colarossi A, Gilman RH, Alvarez JO. Assessment of vitamin A status with the relative-dose-response test in Peruvian children recovering from pneumonia. Am J Clin Nutr 2002;76:1351–7. [DOI] [PubMed] [Google Scholar]
- 263.Stephensen CB, Franchi LM, Hernandez H, Campos M, Gilman RH, Alvarez JO. Adverse effects of high-dose vitamin A supplements in children hospitalized with pneumonia. Pediatrics 1998;101:E3. [DOI] [PubMed] [Google Scholar]
- 264.Forrester JE, Sztam KA. Micronutrients in HIV/AIDS: is there evidence to change the WHO 2003 recommendations? Am J Clin Nutr 2011;94:1683S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hurst RJ, Else KJ. Retinoic acid signalling in gastrointestinal parasite infections: lessons from mouse models. Parasite Immunol 2012;34:351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Meenan NA, Ball G, Bromek K, Uhrin D, Cooper A, Kennedy MW, Smith BO. Solution structure of a repeated unit of the ABA-1 nematode polyprotein allergen of Ascaris reveals a novel fold and two discrete lipid-binding sites. PLoS Negl Trop Dis 2011;5:e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang J, Czech B, Crunk A, Wallace A, Mitreva M, Hannon GJ, Davis RE. Deep small RNA sequencing from the nematode Ascaris reveals conservation, functional diversification, and novel developmental profiles. Genome Res 2011;21:1462–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ladias JA. Convergence of multiple nuclear receptor signaling pathways onto the long terminal repeat of human immunodeficiency virus-1. J Biol Chem 1994;269:5944–51. [PubMed] [Google Scholar]
- 269.Kiefer HL, Hanley TM, Marcello JE, Karthik AG, Viglianti GA. Retinoic acid inhibition of chromatin remodeling at the human immunodeficiency virus type 1 promoter: uncoupling of histone acetylation and chromatin remodeling. J Biol Chem 2004;279:43604–13. [DOI] [PubMed] [Google Scholar]
- 270.Hanley TM, Kiefer HL, Schnitzler AC, Marcello JE, Viglianti GA. Retinoid-dependent restriction of human immunodeficiency virus type 1 replication in monocytes/macrophages. J Virol 2004;78:2819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Thurnham DI, Mburu AS, Mwaniki DL, De Wagt A. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc 2005;64:502–9. [DOI] [PubMed] [Google Scholar]
- 272.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 273.Kussmann M, Panchaud A, Affolter M. Proteomics in nutrition: status quo and outlook for biomarkers and bioactives. J Proteome Res 2010;9:4876–87. [DOI] [PubMed] [Google Scholar]
- 274.Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr 2012;32:183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.European Commission, Council of the European Union, European Parliament. Sixth Report on the Statistics on the Number of Animals used for Experimental and other Scientific Purposes in the Member States of the European Union. Brussels (Belgium): Office for Official Publications of the European Communities; 2010. [Google Scholar]
- 276.Haley PJ. Species differences in the structure and function of the immune system. Toxicology 2003;188:49–71. [DOI] [PubMed] [Google Scholar]
- 277.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731–8. [DOI] [PubMed] [Google Scholar]
- 278.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 2013;110:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ross AC. Use of laboratory studies for the design, explanation, and validation of human micronutrient intervention studies. J Nutr 2012;142(Suppl):157S–60S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 2009;14:141–53. [PubMed] [Google Scholar]
- 281.Kamath AB, Wang L, Das H, Li L, Reinhold VN, Bukowski JF. Antigens in tea-beverage prime human Vgamma 2Vdelta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc Natl Acad Sci USA 2003;100:6009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Izzi V, Masuelli L, Tresoldi I, Sacchetti P, Modesti A, Galvano F, Bei R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front Biosci (Landmark Ed) 2012;17:2396–418. [DOI] [PubMed] [Google Scholar]
- 283.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 2011;474:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 285.Monira S, Nakamura S, Gotoh K, Izutsu K, Watanabe H, Alam NH, Endtz HP, Cravioto A, Ali SI, Nakaya T, et al. Gut microbiota of healthy and malnourished children in bangladesh. Front Microbiol 2011;2:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol 2010;184:6799–806. [DOI] [PubMed] [Google Scholar]
- 287.Kasaikina MV, Kravtsova MA, Lee BC, Seravalli J, Peterson DA, Walter J, Legge R, Benson AK, Hatfield DL, Gladyshev VN. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J 2011;25:2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zimmermann MB, Chassard C, Rohner F, N'Goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. Am J Clin Nutr 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 289.Shore A, Nieves C, Maki A, Ukanova M, Mai V, Cousins R, Langkamp-Henken B. Effects of zinc deficiency on colonic microbiota. FASEB J 2010;24 (Meeting Abstr Suppl Ib242). [Google Scholar]
- 290.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, Ward BJ, Nathoo KJ, Malaba LC, Zijenah LS, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis 2006;193:860–71. [DOI] [PubMed] [Google Scholar]
- 292.Bresee JS, Fischer M, Dowell SF, Johnston BD, Biggs VM, Levine RS, Lingappa JR, Keyserling HL, Petersen KM, Bak JR, et al. Vitamin A therapy for children with respiratory syncytial virus infection: a multicenter trial in the United States. Pediatr Infect Dis J 1996;15:777–82. [DOI] [PubMed] [Google Scholar]
- 293.Quadro L, Gamble MV, Vogel S, Lima AA, Piantedosi R, Moore SR, Colantuoni V, Gottesman ME, Guerrant RL, Blaner WS. Retinol and retinol-binding protein: gut integrity and circulating immunoglobulins. J Infect Dis 2000;182(Suppl 1):S97–102. [DOI] [PubMed] [Google Scholar]
- 294.Thurnham DI, Northrop-Clewes CA, McCullough FS, Das BS, Lunn PG. Innate immunity, gut integrity, and vitamin A in Gambian and Indian infants. J Infect Dis 2000;182(Suppl 1):S23–8. [DOI] [PubMed] [Google Scholar]
- 295.Filteau SM, Rollins NC, Coutsoudis A, Sullivan KR, Willumsen JF, Tomkins AM. The effect of antenatal vitamin A and beta-carotene supplementation on gut integrity of infants of HIV-infected South African women. J Pediatr Gastroenterol Nutr 2001;32:464–70. [DOI] [PubMed] [Google Scholar]
- 296.Lima AA, Soares AM, Lima NL, Mota RM, Maciel BL, Kvalsund MP, Barrett LJ, Fitzgerald RP, Blaner WS, Guerrant RL. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr 2010;50:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Filteau SM, Morris SS, Raynes JG, Arthur P, Ross DA, Kirkwood BR, Tomkins AM, Gyapong JO. Vitamin A supplementation, morbidity, and serum acute-phase proteins in young Ghanaian children. Am J Clin Nutr 1995;62:434–8. [DOI] [PubMed] [Google Scholar]
- 298.Coutsoudis A, Kiepiela P, Coovadia HM, Broughton M. Vitamin A supplementation enhances specific IgG antibody levels and total lymphocyte numbers while improving morbidity in measles. Pediatr Infect Dis J 1992;11:203–9. [DOI] [PubMed] [Google Scholar]
- 299.Semba RD, Muhilal, West KP Jr., Natadisastra G, Eisinger W, Lan Y, Sommer A. Hyporetinolemia and acute phase proteins in children with and without xerophthalmia. Am J Clin Nutr 2000;72:146–53. [DOI] [PubMed] [Google Scholar]
- 300.Ahmad SM, Haskell MJ, Raqib R, Stephensen CB. Markers of innate immune function are associated with vitamin a stores in men. J Nutr 2009;139:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Jørgensen MJ, Fisker AB, Sartono E, Andersen A, Erikstrup C, Lisse IM, Yazdanbakhsh M, Aaby P, Benn CS. The effect of at-birth vitamin A supplementation on differential leucocyte counts and in vitro cytokine production: an immunological study nested within a randomised trial in Guinea-Bissau. Br J Nutr 2013;109:467–77. [DOI] [PubMed] [Google Scholar]
- 302.Wieringa FT, Dijkhuizen MA, West CE, van der Ven-Jongekrijg J, van der Meer JW, Muhilal. Reduced production of immunoregulatory cytokines in vitamin A- and zinc-deficient Indonesian infants. Eur J Clin Nutr 2004;58:1498–504. [DOI] [PubMed] [Google Scholar]
- 303.Cox SE, Arthur P, Kirkwood BR, Yeboah-Antwi K, Riley EM. Vitamin A supplementation increases ratios of proinflammatory to anti-inflammatory cytokine responses in pregnancy and lactation. Clin Exp Immunol 2006;144:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bessler H, Wyshelesky G, Osovsky M, Prober V, Sirota L. A comparison of the effect of vitamin A on cytokine secretion by mononuclear cells of preterm newborns and adults. Neonatology 2007;91:196–202. [DOI] [PubMed] [Google Scholar]
- 305.Jimenez C, Leets I, Puche R, Anzola E, Montilla R, Parra C, Aguilera A, Garcia-Casal MN. A single dose of vitamin A improves haemoglobin concentration, retinol status and phagocytic function of neutrophils in preschool children. Br J Nutr 2010;103:798–802. [DOI] [PubMed] [Google Scholar]
- 306.Benn CS, Lisse IM, Bale C, Michaelsen KF, Olsen J, Hedegaard K, Aaby P. No strong long-term effect of vitamin A supplementation in infancy on CD4 and CD8 T-cell subsets: a community study from Guinea-Bissau, West Africa. Ann Trop Paediatr 2000;20:259–64. [DOI] [PubMed] [Google Scholar]
- 307.Semba RD, Muhilal, Ward BJ, Griffin DE, Scott AL, Natadisastra G, West KP Jr., Sommer A. Abnormal T-cell subset proportions in vitamin-A-deficient children. Lancets 1993;341:5–8. [DOI] [PubMed] [Google Scholar]
- 308.Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin A treatment. Int J Epidemiol 2010;39(Suppl 1):i48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Semba RD, Muhilal, Scott AL, Natadisastra G, West KP Jr., Sommer A. Effect of vitamin A supplementation on immunoglobulin G subclass responses to tetanus toxoid in children. Clin Diagn Lab Immunol 1994;1:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Benn CS, Fisker AB, Napirna BM, Roth A, Diness BR, Lausch KR, Ravn H, Yazdanbakhsh M, Rodrigues A, Whittle H, et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ 2010;340:c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Alam AN, Sarker SA, Wahed MA, Khatun M, Rahaman MM. Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut 1994;35:1707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn PG, Northrop-Clewes CA, Hoare S, Cole TJ, Horan SJ, Longman SC, et al. A trial of zinc supplementation in young rural Gambian children. Br J Nutr 1993;69:243–55. [DOI] [PubMed] [Google Scholar]
- 313.Wessells KR, Hess SY, Rouamba N, Ouedraogo ZP, Kellogg M, Goto R, Duggan C, Ouedraogo JB, Brown KH. Associations between intestinal mucosal function and changes in plasma zinc concentration following zinc supplementation. J Pediatr Gastroenterol Nutr 2013;57:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Roy SK, Behrens RH, Haider R, Akramuzzaman SM, Mahalanabis D, Wahed MA, Tomkins AM. Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Pediatr Gastroenterol Nutr 1992;15:289–96. [DOI] [PubMed] [Google Scholar]
- 315.Sheikh A, Shamsuzzaman S, Ahmad SM, Nasrin D, Nahar S, Alam MM, Al Tarique A, Begum YA, Qadri SS, Chowdhury MI, et al. Zinc influences innate immune responses in children with enterotoxigenic Escherichia coli-induced diarrhea. J Nutr 2010;140:1049–56. [DOI] [PubMed] [Google Scholar]
- 316.Sazawal S, Black RE, Bhan MK, Jalla S, Sinha A, Bhandari N. Efficacy of zinc supplementation in reducing the incidence and prevalence of acute diarrhea—a community-based, double-blind, controlled trial. Am J Clin Nutr 1997;66:413–8. [DOI] [PubMed] [Google Scholar]
- 317.Mariani E, Neri S, Cattini L, Mocchegiani E, Malavolta M, Dedoussis GV, Kanoni S, Rink L, Jajte J, Facchini A. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: interactive influence of +647 MT1a and -174 IL-6 polymorphic alleles. Exp Gerontol 2008;43:462–71. [DOI] [PubMed] [Google Scholar]
- 318.Isa L, Lucchini A, Lodi S, Giachetti M. Blood zinc status and zinc treatment in human immunodeficiency virus-infected patients. Int J Clin Lab Res 1992;22:45–7. [DOI] [PubMed] [Google Scholar]
- 319.Mocchegiani E, Santarelli L, Costarelli L, Cipriano C, Muti E, Giacconi R, Malavolta M. Plasticity of neuroendocrine-thymus interactions during ontogeny and ageing: role of zinc and arginine. Ageing Res Rev 2006;5:281–309. [DOI] [PubMed] [Google Scholar]
- 320.Prasad AS. Zinc: mechanisms of host defense. J Nutr 2007;137:1345–9. [DOI] [PubMed] [Google Scholar]
- 321.Osendarp SJ, Fuchs GJ, van Raaij JM, Mahmud H, Tofail F, Black RE, Prabhakar H, Santosham M. The effect of zinc supplementation during pregnancy on immune response to Hib and BCG vaccines in Bangladesh. J Trop Pediatr 2006;52:316–23. [DOI] [PubMed] [Google Scholar]
- 322.Chapman IM. Weight loss in older persons. Med Clin North Am 2011;95:579–93. [DOI] [PubMed] [Google Scholar]
- 323.Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr 2003;133:316S–21S. [DOI] [PubMed] [Google Scholar]
- 324.Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94(Suppl):658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.World Health Organization. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency. Geneva (Switzerland): World Health Organization; 2009.
- 326.Ross AC. Vitamin A supplementation and retinoic acid treatment in the regulation of antibody responses in vivo. Vitam Horm 2007;75:197–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Fawzi WW, Chalmers TC, Herrera G, Mosteller F, Vitamin A. Supplementation and child mortality: a meta-analysis. JAMA 1993;269:898–903. [PubMed] [Google Scholar]
- 328.Beaton GH, Martorell R, McCabe G, L’abbe KA, Edmonston B, Ross AC. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. Toronto: University of Toronto; 1993. [Google Scholar]
- 329.Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2005;4:CD001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gogia S, Sachdev HS. Neonatal vitamin A supplementation for prevention of mortality and morbidity in infancy: systematic review of randomised controlled trials. BMJ 2009;338: b919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ni J, Wei J, Wu T. Vitamin A for non-measles pneumonia in children. Cochrane Database Syst Rev 2005;3:CD003700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Gogia S, Sachdev HS. Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less. Cochrane Database Syst Rev 2011;10:CD007480. [DOI] [PubMed] [Google Scholar]
- 333.Donnen P, Sylla A, Dramaix M, Sall G, Kuakuvi N, Hennart P. Effect of daily low dose of vitamin A compared with single high dose on morbidity and mortality of hospitalized mainly malnourished children in senegal: a randomized controlled clinical trial. Eur J Clin Nutr 2007;61:1393–9. [DOI] [PubMed] [Google Scholar]
- 334.Diness BR, Martins CL, Balé C, Garly M-L, Ravn H, Rodrigues A, Whittle H, Aaby P, Benn CS. The effect of high-dose vitamin A supplementation at birth on measles incidence during the first 12 months of life in boys and girls: an unplanned study within a randomised trial. Br J Nutr 2011;105:1819–22. [DOI] [PubMed] [Google Scholar]
- 335.Long KZ, García C, Santos Jose I, Rosado Jorge L, Hertzmark E, DuPont Herbert L, Ko G, Vitamin A. Supplementation has divergent effects on norovirus infections and clinical symptoms among Mexican children. J Infect Dis 2007;196:978–85. [DOI] [PubMed] [Google Scholar]
- 336.Diness BR, Christoffersen D, Pedersen UB, Rodrigues A, Fischer TK, Andersen A, Whittle H, Yazdanbakhsh M, Aaby P, Benn CS. The effect of high-dose vitamin A supplementation given with bacille Calmette-Guerin vaccine at birth on infant rotavirus infection and diarrhea: a randomized prospective study from Guinea-Bissau. J Infect Dis 2010;202:S243–51. [DOI] [PubMed] [Google Scholar]
- 337.Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, et al. ; Zinc Investigators’ Collaborative Group. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr 1999;135:689–97. [DOI] [PubMed] [Google Scholar]
- 338.Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, Diener-West M, Faruque AS, Black RE. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: randomised controlled trial. Lancet 2005;366:999–1004. [DOI] [PubMed] [Google Scholar]
- 339.Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, Jhass A, Rudan I, Campbell H, Black RE, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11(Suppl 3):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 2000;72:1516–22. [DOI] [PubMed] [Google Scholar]
- 341.Mocchegiani E, Muzzioli M. Therapeutic application of zinc in human immunodeficiency virus against opportunistic infections. J Nutr 2000;130(5, Suppl):1424S–31S. [DOI] [PubMed] [Google Scholar]
- 342.Bhatnagar S, Wadhwa N, Aneja S, Lodha R, Kabra SK, Natchu UC, Sommerfelt H, Dutta AK, Chandra J, Rath B, et al. Zinc as adjunct treatment in infants aged between 7 and 120 days with probable serious bacterial infection: a randomised, double-blind, placebo-controlled trial. Lancet 2012;379:2072–8. [DOI] [PubMed] [Google Scholar]
- 343.Basnet S, Shrestha PS, Sharma A, Mathisen M, Prasai R, Bhandari N, Adhikari RK, Sommerfelt H, Valentiner-Branth P, Strand TA. A randomized controlled trial of zinc as adjuvant therapy for severe pneumonia in young children. Pediatrics 2012;129:701–8. [DOI] [PubMed] [Google Scholar]
- 344.Bose A, Coles CL, Gunavathi JH, Moses P, Raghupathy P, Kirubakaran C, Black RE, Brooks WA, Santosham M. Efficacy of zinc in the treatment of severe pneumonia in hospitalized children <2 y old. Am J Clin Nutr 2006;83:1089–96. [DOI] [PubMed] [Google Scholar]
- 345.Brooks WA, Yunus M, Santosham M, Wahed MA, Nahar K, Yeasmin S, Black RE. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet 2004;363:1683–8. [DOI] [PubMed] [Google Scholar]
- 346.Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev 2011;2:CD001364. [DOI] [PubMed] [Google Scholar]
- 347.Braunschweig CL, Sowers M, Kovacevich DS, Hill GM, August DA. Parenteral zinc supplementation in adult humans during the acute phase response increases the febrile response. J Nutr 1997;127:70–4. [DOI] [PubMed] [Google Scholar]
- 348.Coles CL, Bose A, Moses PD, Mathew L, Agarwal I, Mammen T, Santosham M. Infectious etiology modifies the treatment effect of zinc in severe pneumonia. Am J Clin Nutr 2007;86:397–403. [DOI] [PubMed] [Google Scholar]
- 349.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr 2001;131(Suppl): 616S–33S; discussion 633S–5S. [DOI] [PubMed] [Google Scholar]
- 350.Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev 2011;10:CD006589. [DOI] [PubMed] [Google Scholar]
- 351.Long KZ, Santos JI, Rosado JL, Estrada-Garcia T, Haas M, Al Mamun A, DuPont HL, Nanthakumar NN. Vitamin A supplementation modifies the association between mucosal innate and adaptive immune responses and resolution of enteric pathogen infections. Am J Clin Nutr 2011;93:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Fraker PJ, King LE, Laakko T, Vollmer TL. The dynamic link between the integrity of the immune system and zinc status. J Nutr 2000;130(5, Suppl):1399S–1406S. [DOI] [PubMed] [Google Scholar]
- 353.Prasad AS. Zinc and immunity. Mol Cell Biochem 1998;188:63–9. [PubMed] [Google Scholar]
- 354.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998;68(2, Suppl):447S–63S. [DOI] [PubMed] [Google Scholar]
- 355.Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis 2000;182(Suppl 1):S62–8. [DOI] [PubMed] [Google Scholar]
- 356.Mburu AS, Thurnham DI, Mwaniki DL, Muniu EM, Alumasa FM. The influence of inflammation on plasma zinc concentration in apparently healthy, HIV+ Kenyan adults and zinc responses after a multi-micronutrient supplement. Eur J Clin Nutr 2010;64:510–7. [DOI] [PubMed] [Google Scholar]
- 357.Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA, Muhilal . Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr 2002;132:3061–6. [DOI] [PubMed] [Google Scholar]
- 358.Bui VQ, Stein AD, DiGirolamo AM, Ramakrishnan U, Flores-Ayala RC, Ramirez-Zea M, Grant FK, Villalpando S, Martorell R. Associations between serum C-reactive protein and serum zinc, ferritin, and copper in Guatemalan school children. Biol Trace Elem Res 2012;148:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Cherayil BJ. Iron and immunity: immunological consequences of iron deficiency and overload. Arch Immunol Ther Exp (Warsz) 2010;58:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Jason J, Archibald LK, Nwanyanwu OC, Bell M, Jensen RJ, Gunter E, Buchanan I, Larned J, Kazembe PN, Dobbie H, et al. The effects of iron deficiency on lymphocyte cytokine production and activation: preservation of hepatic iron but not at all cost. Clin Exp Immunol 2001;126:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Manary MJ, Yarasheski KE, Smith S, Abrams ET, Hart CA. Protein quantity, not protein quality, accelerates whole-body leucine kinetics and the acute-phase response during acute infection in marasmic Malawian children. Br J Nutr 2004;92:589–95. [DOI] [PubMed] [Google Scholar]
- 362.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004;110:1564–71. [DOI] [PubMed] [Google Scholar]
- 363.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 2009;6:399–409. [DOI] [PubMed] [Google Scholar]
- 364.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004;92:347–55. [DOI] [PubMed] [Google Scholar]
- 365.Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc 2009;68:370–7. [DOI] [PubMed] [Google Scholar]
- 366.Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med 2009;8:55–60. [PubMed] [Google Scholar]
- 367.Ye J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep 2011;11:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, Fagoaga OR. Influence of obesity on immune function. J Am Diet Assoc 1999;99:294–9. [DOI] [PubMed] [Google Scholar]
- 369.Iannotti LL, Trehan I, Manary MJ. Review of the safety and efficacy of vitamin A supplementation in the treatment of children with severe acute malnutrition. Nutr J 2013;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Benn CS, Diness BR, Roth A, Nante E, Fisker AB, Lisse IM, Yazdanbakhsh M, Whittle H, Rodrigues A, Aaby P. Effect of 50 000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ 2008;336:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr 2011;94(Suppl):679S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Caulfield LE, Richard SA, Rivera JA, Musgrove P, Black RE. Stunting, wasting, and micronutrient deficiency disorders. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease control priorities in developing countries. 2nd edition. Washington (DC): World Bank; 2006, p. 561–567. [PubMed] [Google Scholar]
- 373.Prasad AS. Zinc: an overview. Nutrition 1995;11(1, Suppl):93–9. [PubMed] [Google Scholar]
- 374.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr 2009;89(Suppl):2040S–51S. [DOI] [PubMed] [Google Scholar]
- 375.de Benoist B, Darnton-Hill I, Davidsson L, Fontaine O, Hotz C. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG Interagency Meeting on Zinc Status Indicators. Food Nutr Bull 2007;28(3, Suppl):S480–4. [DOI] [PubMed] [Google Scholar]
- 376.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull 2007;28(3, Suppl)S403–29. [DOI] [PubMed] [Google Scholar]
- 377.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ; Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347–60. [DOI] [PubMed] [Google Scholar]
- 378.Fischer Walker CL, Ezzati M, Black RE. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur J Clin Nutr 2009;63:591–7. [DOI] [PubMed] [Google Scholar]
- 379.Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr 2004;24:255–75. [DOI] [PubMed] [Google Scholar]
- 380.Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lonnerdal B, Ruel MT, Sandtrom B, Wasantwisut E, Hotz C, et al; International Zinc Nutrition Consultative Group. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25(1, Suppl 2)S99–203. International Zinc Nutrition Consultative Group (IZiNCG) Technical Document No. 1. [PubMed]
- 381.Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012;7:e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Boy E, Mannar V, Pandav C, de Benoist B, Viteri F, Fontaine O, Hotz C. Achievements, challenges, and promising new approaches in vitamin and mineral deficiency control. Nutr Rev 2009;67(Suppl 1):S24–30. [DOI] [PubMed] [Google Scholar]
- 383.Sandstead HH. Zinc deficiency: a public health problem? Am J Dis Child 1991;145:853–9. [DOI] [PubMed] [Google Scholar]
- 384.Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr 2003;133(5, Suppl 1):1485S–9S. [DOI] [PubMed] [Google Scholar]
- 385.Rink L, Gabriel P. Extracellular and immunological actions of zinc. Biometals 2001;14:367–83. [DOI] [PubMed] [Google Scholar]
- 386.Lindenmayer GW, Stoltzfus RJ, Prendergast AJ. Interactions between zinc deficiency and environmental enteropathy in developing countries. Adv Nutr 2014;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Ibs KH, Rink L. Zinc-altered immune function. J Nutr 2003;133(5, Suppl 1):1452S–6S. [DOI] [PubMed] [Google Scholar]
- 388.Brüssow H, Sidoti J, Dirren H, Freire WB. Effect of malnutrition in Ecuadorian children on titers of serum antibodies to various microbial antigens. Clin Diagn Lab Immunol 1995;2:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Moore SE, Goldblatt D, Bates CJ, Prentice AM. Impact of nutritional status on antibody responses to different vaccines in undernourished Gambian children. Acta Paediatr 2003;92:170–6. [DOI] [PubMed] [Google Scholar]
- 390.Gardner EM, Bernstein ED, Popoff KA, Abrutyn E, Gross P, Murasko DM. Immune response to influenza vaccine in healthy elderly: lack of association with plasma beta-carotene, retinol, alpha-tocopherol, or zinc. Mech Ageing Dev 2000;117:29–45. [DOI] [PubMed] [Google Scholar]
- 391.Osendarp SJ, Prabhakar H, Fuchs GJ, van Raaij JM, Mahmud H, Tofail F, Santosham M, Black RE. Immunization with the heptavalent pneumococcal conjugate vaccine in Bangladeshi infants and effects of zinc supplementation. Vaccine 2007;25:3347–54. [DOI] [PubMed] [Google Scholar]
- 392.Albert MJ, Qadri F, Wahed MA, Ahmed T, Rahman AS, Ahmed F, Bhuiyan NA, Zaman K, Baqui AH, Clemens JD, et al. Supplementation with zinc, but not vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. J Infect Dis 2003;187:909–13. [DOI] [PubMed] [Google Scholar]
- 393.World Health Organization; United Nation's Children's Fund. Joint statement: clinical management of acute diarrhoea. Geneva (Switzerland): World Health Organization; 2004 [cited 2014 Jun 20]. Available from: http://www.childinfo.org/files/ENAcute_Diarrhoea_reprint.pdf.
- 394.Krones C, Klosterhalfen B, Fackeldey V, Junge K, Rosch R, Schwab R, Stumpf M, Klinge U, Schumpelick V. Deleterious effect of zinc in a pig model of acute endotoxemia. J Invest Surg 2004;17:249–56. [DOI] [PubMed] [Google Scholar]
- 395.Lynch S. Case studies: iron. Am J Clin Nutr 2011;94:673S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001;131(Suppl):568S–80S. [DOI] [PubMed] [Google Scholar]
- 397.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010;116:4754–61. [DOI] [PubMed] [Google Scholar]
- 398.Rastogi T, Mathers C. Global burden of iron deficiency anaemia in the year 2000. Geneva (Switzerland): World Health Organization; 2002. Available from: http://www.who.int/healthinfo/statistics/bod_irondeficiencyanaemia.pdf.
- 399.Cameron BM, Neufeld LM. Estimating the prevalence of iron deficiency in the first two years of life: technical and measurement issues. Nutr Rev 2011;69(Suppl 1):S49–56. [DOI] [PubMed] [Google Scholar]
- 400.Coad J, Conlon C. Iron deficiency in women: assessment, causes and consequences. Curr Opin Clin Nutr Metab Care 2011;14:625–34. [DOI] [PubMed] [Google Scholar]
- 406.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371:243–60. [DOI] [PubMed] [Google Scholar]
- 401.Kuvibidila S, Baliga BS. Role of iron in immunity and infection. In: Calder PC, Field CJ, Gill HS, editors. Nutrition and immune function. New York: CAB International; 2002, p. 209, 426. [Google Scholar]
- 402.Paino IM, Miranda JC, Marzocchi-Machado CM, Cesarino EJ, de Castro FA, de Souza AM. Phagocytosis, oxidative burst, and produced reactive species are affected by iron deficiency anemia and anemia of chronic diseases in elderly. Biol Trace Elem Res 2009;129:116–25. [DOI] [PubMed] [Google Scholar]
- 403.Kurtoglu E, Ugur A, Baltaci AK, Mogolkoc R, Undar L. Activity of neutrophil NADPH oxidase in iron-deficient anemia. Biol Trace Elem Res 2003;96:109–15. [DOI] [PubMed] [Google Scholar]
- 404.Walter T, Arredondo S, Arevalo M, Stekel A. Effect of iron therapy on phagocytosis and bactericidal activity in neutrophils of iron-deficient infants. Am J Clin Nutr 1986;44:877–82. [DOI] [PubMed] [Google Scholar]
- 405.Muñoz C, Rios E, Olivos J, Brunser O, Olivares M. Iron, copper and immunocompetence. Br J Nutr 2007;98(Suppl 1):S24–8. [DOI] [PubMed] [Google Scholar]
- 407.Doherty CP. Host-pathogen interactions: the role of iron. J Nutr 2007;137:1341–4. [DOI] [PubMed] [Google Scholar]
- 408.Bhutta ZA, Salam RA. Global nutrition epidemiology and trends. Ann Nutr Metab 2012;61(Suppl 1):19–27. [DOI] [PubMed] [Google Scholar]
- 409.Fryar CD, Ogden CL. Prevalence of underweight among adults aged 20 years and over: United States, 1960–1962 through 2007–2010. NCHS Health E-Stats. Atlanta (GA): Centers for Disease Control and Prevention; 2012. [cited 2013 Nov 14]. Available from: http://www.cdc.gov/nchs/data/hestat/underweight_adult_07_10/underweight_adult_07_10.htm.
- 410.Roubenoff R. The pathophysiology of wasting in the elderly. J Nutr 1999 129(1, Suppl):256S–9S. [DOI] [PubMed] [Google Scholar]
- 411.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Trehan I, Maleta KM, Manary MJ. Antibiotics for uncomplicated severe malnutrition. N Engl J Med 2013;368:2436–7. [DOI] [PubMed] [Google Scholar]
- 413.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol 2005;115:1119–28. [DOI] [PubMed] [Google Scholar]
- 414.Savino W, Dardenne M. Nutritional imbalances and infections affect the thymus: consequences on T-cell-mediated immune responses. Proc Nutr Soc 2010;69:636–43. [DOI] [PubMed] [Google Scholar]
- 415.Koster FT, Palmer DL, Chakraborty J, Jackson T, Curlin GC. Cellular immune competence and diarrheal morbidity in malnourished Bangladeshi children: a prospective field study. Am J Clin Nutr 1987;46:115–20. [DOI] [PubMed] [Google Scholar]
- 416.Watson RR, McMurray DN, Martin P, Reyes MA. Effect of age, malnutrition and renutrition on free secretory component and IgA in secretions. Am J Clin Nutr 1985;42:281–8. [DOI] [PubMed] [Google Scholar]
- 417.Keusch GT. Host defense mechanisms in protein energy malnutrition. Adv Exp Med Biol 1981;135:183–209. [DOI] [PubMed] [Google Scholar]
- 418.World Health Organization. Obesity and overweight. Media centre. Geneva (Switzerland): World Health Organization; 2013. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 419.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism 2013;62:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Tarantino G, Scalera A, Finelli C. Liver-spleen axis: intersection between immunity, infections and metabolism. World J Gastroenterol 2013;19:3534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood 2009;114:3803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Guo H, Xu B, Gao L, Sun X, Qu X, Li X, Liu S, Feng J, Wang J, Tang Y, et al. High frequency of activated natural killer and natural killer T-cells in patients with new onset of type 2 diabetes mellitus. Exp Biol Med (Maywood) 2012;237:556–62. [DOI] [PubMed] [Google Scholar]
- 423.van der Weerd K, Dik WA, Schrijver B, Schweitzer DH, Langerak AW, Drexhage HA, Kiewiet RM, van Aken MO, van Huisstede A, van Dongen JJ, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes 2012;61:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Bercault N, Boulain T, Kuteifan K, Wolf M, Runge I, Fleury JC. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med 2004;32:998–1003. [DOI] [PubMed] [Google Scholar]
- 425.Bochicchio GV, Joshi M, Bochicchio K, Nehman S, Tracy JK, Scalea TM. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg 2006;203:533–8. [DOI] [PubMed] [Google Scholar]
- 426.Dossett LA, Dageforde LA, Swenson BR, Metzger R, Bonatti H, Sawyer RG, May AK. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect (Larchmt) 2009;10:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Dowsey MM, Choong PF. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res 2008;466:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Vilar-Compte D, Mohar A, Sandoval S, de la Rosa M, Gordillo P, Volkow P. Surgical site infections at the National Cancer Institute in Mexico: a case-control study. Am J Infect Control 2000;28:14–20. [DOI] [PubMed] [Google Scholar]
- 429.Hanslik T, Boelle PY, Flahault A. Preliminary estimation of risk factors for admission to intensive care units and for death in patients infected with A(H1N1)2009 influenza virus, France, 2009–2010. PLoS Curr 2010;2:RRN1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011;52:301–12. [DOI] [PubMed] [Google Scholar]
- 431.Santa-Olalla Peralta P, Cortes-Garcia M, Vicente-Herrero M, Castrillo-Villamandos C, Arias-Bohigas P, Pachon-del Amo I, Sierra-Moros MJ. Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April-December 2009. Euro Surveill 2010;15. [DOI] [PubMed] [Google Scholar]
- 432.Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, Belay B, Jain S, Cox C, Kamimoto L, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE 2010;5:e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Díaz E, Rodriguez A, Martin-Loeches I, Lorente L, del Mar Martin M, Pozo JC, Montejo JC, Estella A, Arenzana A, Rello J. Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest 2011;139:382–6. [DOI] [PubMed] [Google Scholar]
- 434.Kwong JC, Campitelli MA, Rosella LC. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis 2011;53:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Jedrychowski W, Maugeri U, Flak E, Mroz E, Bianchi I. Predisposition to acute respiratory infections among overweight preadolescent children: an epidemiologic study in Poland. Public Health 1998;112:189–95. [DOI] [PubMed] [Google Scholar]
- 436.Akiyama N, Segawa T, Ida H, Mezawa H, Noya M, Tamez S, Urashima M. Bimodal effects of obesity ratio on disease duration of respiratory syncytial virus infection in children. Allergol Int 2011;60:305–8. [DOI] [PubMed] [Google Scholar]
- 437.Brandt M, Harder K, Walluscheck KP, Schottler J, Rahimi A, Moller F, Cremer J. Severe obesity does not adversely affect perioperative mortality and morbidity in coronary artery bypass surgery. Eur J Cardiothorac Surg 2001;19:662–6. [DOI] [PubMed] [Google Scholar]
- 438.Dossett LA, Heffernan D, Lightfoot M, Collier B, Diaz JJ, Sawyer RG, May AK. Obesity and pulmonary complications in critically injured adults. Chest 2008;134:974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ylöstalo P, Suominen-Taipale L, Reunanen A, Knuuttila M. Association between body weight and periodontal infection. J Clin Periodontol 2008;35:297–304. [DOI] [PubMed] [Google Scholar]
- 440.Herwaldt LA, Cullen JJ, French P, Hu J, Pfaller MA, Wenzel RP, Perl TM. Preoperative risk factors for nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol 2004;25:481–4. [DOI] [PubMed] [Google Scholar]
- 441.Perdichizzi G, Bottari M, Pallio S, Fera MT, Carbone M, Barresi G. Gastric infection by Helicobacter pylori and antral gastritis in hyperglycemic obese and in diabetic subjects. New Microbiol 1996;19:149–54. [PubMed] [Google Scholar]
- 442.Karjala Z, Neal D, Rohrer J. Association between HSV1 seropositivity and obesity: data from the National Health and Nutritional Examination Survey, 2007–2008. PLoS ONE 2011;6:e19092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc 2011;70:408–17. [DOI] [PubMed] [Google Scholar]
- 444.Weber DJ, Rutala WA, Samsa GP, Bradshaw SE, Lemon SM. Impaired immunogenicity of hepatitis B vaccine in obese persons. N Engl J Med 1986;314:1393. [DOI] [PubMed] [Google Scholar]
- 445.Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA 1985;254:3187–9. [PubMed] [Google Scholar]
- 446.Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM. Reduced tetanus antibody titers in overweight children. Autoimmunity 2006;39:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36:1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.O'Rourke RW, White AE, Metcalf MD, Winters BR, Diggs BS, Zhu X, Marks DL. Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism 2012;61:1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011;11:738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol 1992;262:R1025–32. [DOI] [PubMed] [Google Scholar]
- 451.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol 2010;184:3127–33. [DOI] [PubMed] [Google Scholar]
- 452.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr 2007;137:1236–43. [DOI] [PubMed] [Google Scholar]
- 453.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med 2007;204:441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Strandberg L, Verdrengh M, Enge M, Andersson N, Amu S, Onnheim K, Benrick A, Brisslert M, Bylund J, Bokarewa M, et al. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS ONE 2009;4:e7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Klasing KC. Nutrition and the immune system. Br Poult Sci 2007;48:525–37. [DOI] [PubMed] [Google Scholar]
- 456.Rylance J, Gordon SB, Naeher LP, Patel A, Balmes JR, Adetona O, Rogalsky DK, Martin WJ II. Household air pollution: a call for studies into biomarkers of exposure and predictors of respiratory disease. Am J Physiol Lung Cell Mol Physiol 2013;304:L571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Dutta A, Ray MR, Banerjee A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol Appl Pharmacol 2012;261:255–62. [DOI] [PubMed] [Google Scholar]
- 458.Cannon WB. Bodily changes in pain, hunger, fear, and rage. New York: Appleton-Century-Crofts; 1929. [Google Scholar]
- 459.Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 1998;20:1093–102. [DOI] [PubMed] [Google Scholar]
- 460.Ruzek MC, Pearce BD, Miller AH, Biron CA. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol 1999;162:3527–33. [PubMed] [Google Scholar]
- 461.Chelmicka-Schorr E, Kwasniewski MN, Czlonkowska A. Sympathetic nervous system and macrophage function. Ann N Y Acad Sci 1992;650:40–5. [DOI] [PubMed] [Google Scholar]
- 462.Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, Wilder RL. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci USA 1989;86:2374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Eijsbouts AM, van den Hoogen FH, Laan RF, Hermus AR, Sweep CG, van de Putte LB. Hypothalamic-pituitary-adrenal axis activity in patients with rheumatoid arthritis. Clin Exp Rheumatol 2005;23:658–64. [PubMed] [Google Scholar]
- 464.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord 2000;24(Suppl 2):S50–5. [DOI] [PubMed] [Google Scholar]
- 465.Castrogiovanni D, Gaillard RC, Giovambattista A, Spinedi E. Neuroendocrine, metabolic, and immune functions during the acute phase response of inflammatory stress in monosodium L-glutamate-damaged, hyperadipose male rat. Neuroendocrinology 2008;88:227–34. [DOI] [PubMed] [Google Scholar]
- 466.Murray DR, Prabhu SD, Chandrasekar B. Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation 2000;101:2338–41. [DOI] [PubMed] [Google Scholar]
- 467.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun 2002;16:622–53. [DOI] [PubMed] [Google Scholar]
- 468.Dolan SE, Hadigan C, Killilea KM, Sullivan MP, Hemphill L, Lees RS, Schoenfeld D, Grinspoon S. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr 2005;39:44–54. [DOI] [PubMed] [Google Scholar]
- 469.Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, Manji K, Kapiga S, Mwakagile D, Essex M, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS 2002;16:1935–44. [DOI] [PubMed] [Google Scholar]
- 470.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM; South African Vitamin A Study Group. Randomized trial testing the effect of vitamin A supplementation on pregnancy outcomes and early mother-to-child HIV-1 transmission in Durban, South Africa. AIDS 1999;13:1517–24. [DOI] [PubMed] [Google Scholar]
- 471.Kumwenda N, Miotti PG, Taha TE, Broadhead R, Biggar RJ, Jackson JB, Melikian G, Semba RD. Antenatal vitamin A supplementation increases birth weight and decreases anemia among infants born to human immunodeficiency virus-infected women in Malawi. Clin Infect Dis 2002;35:618–24. [DOI] [PubMed] [Google Scholar]
- 472.Haregu TN, Oldenburg B, Setswe G, Elliott J, Vajira N. Epidemiology of comorbidity of HIV/AIDS and non-communicable diseases in developing countries: a systematic review. J Global Health Care Systems 2012;2(1):1–12. [Google Scholar]
- 473.World Health Organization; UNAIDS; UNICEF. Global HIV/AIDS response: epidemic update and health sector progress towards universal access. UNAIDS; 2011 [cited 2014 Jun 20]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20111130_ua_report_en.pdf.
- 474.Raiten DJ, Mulligan K, Papathakis P, Wanke C. Executive summary–nutritional care of HIV-infected adolescents and adults, including pregnant and lactating women: what do we know, what can we do, and where do we go from here? Am J Clin Nutr 2011;94(Suppl):1667S–76S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.World Health Organization. Malaria. Media centre 2013[cited 2013 Oct 30]. Available from: http://www.who.int/mediacentre/factsheets/fs094/en/.
- 476.Facer CA, Crane GG. Hyperreactive malarious splenomegaly. Lancet 1991;338:115–6. [DOI] [PubMed] [Google Scholar]
- 477.Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW. Hepatosplenomegaly associated with chronic malaria exposure: evidence for a pro-inflammatory mechanism exacerbated by schistosomiasis. Parasite Immunol 2009;31:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Coutinho HM, Leenstra T, Acosta LP, Su L, Jarilla B, Jiz MA, Langdon GC, Olveda RM, McGarvey ST, Kurtis JD, et al. Pro-inflammatory cytokines and C-reactive protein are associated with undernutrition in the context of Schistosoma japonicum infection. Am J Trop Med Hyg 2006;75:720–6. [PubMed] [Google Scholar]
- 479.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr 2003;133:1332–8. [DOI] [PubMed] [Google Scholar]
- 480.Hossain MI, Nahar B, Hamadani JD, Ahmed T, Roy AK, Brown KH. Intestinal mucosal permeability of severely underweight and nonmalnourished Bangladeshi children and effects of nutritional rehabilitation. J Pediatr Gastroenterol Nutr 2010;51:638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 1991;338:907–10. [DOI] [PubMed] [Google Scholar]
- 482.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol 2013;4:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Steffen EK, Berg RD, Deitch EA. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis 1988;157:1032–8. [DOI] [PubMed] [Google Scholar]
- 484.Snider GL, Falling LJ, Rennard SI. Respiratory medicine. Philadelphia: WB Saunders; 1994. p. 1331–97. [Google Scholar]
- 485.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev 2013;26:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Chau JY, Tiffany CM, Nimishakavi S, Lawrence JA, Pakpour N, Mooney JP, Lokken KL, Caughey GH, Tsolis RM, Luckhart S. Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal salmonella bacteremia. Infect Immun 2013;81:3515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Schnabl B. Linking intestinal homeostasis and liver disease. Curr Opin Gastroenterol 2013;29:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Sherwood RA. Faecal markers of gastrointestinal inflammation. J Clin Pathol 2012;65:981–5. [DOI] [PubMed] [Google Scholar]
- 490.Korpe PS, Petri WA Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med 2012;18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 2012;86:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Bartelt LA, Lima AA, Kosek M, Penataro Yori P, Lee G, Guerrant RL. "Barriers" to child development and human potential: the case for including the "neglected enteric protozoa" (NEP) and other enteropathy-associated pathogens in the NTDs. PLoS Negl Trop Dis 2013;7:e2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev 2006;64(5 Pt 2):S24–33; discussion S72–91. [DOI] [PubMed] [Google Scholar]
- 494.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009;374:1032–5. [DOI] [PubMed] [Google Scholar]
- 495.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Lunn PG. The impact of infection and nutrition on gut function and growth in childhood. Proc Nutr Soc 2000;59:147–54. [DOI] [PubMed] [Google Scholar]
- 497.Campbell DI, Murch SH, Lunn PG, Eli M, Sullivan PB, Mendy J. Disruption of the epithelial mitotic cycle in tropical enteropathy and malnutrition. J Pediatr Gastroenterol Nutr 1999;8:550. [Google Scholar]
- 498.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Palsson-McDermott EM, O'Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. BioEssays 2013;35:965–73. [DOI] [PubMed] [Google Scholar]
- 500.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res 2006;4:221–33. [DOI] [PubMed] [Google Scholar]
- 501.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 502.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Gómez-Guerrero C, Mallavia B, Egido J. Targeting inflammation in cardiovascular diseases. still a neglected field? Cardiovasc Ther 2012;30:e189–97. [DOI] [PubMed] [Google Scholar]
- 504.Luft VC, Schmidt MI, Pankow JS, Couper D, Ballantyne CM, Young JH, Duncan BB. Chronic inflammation role in the obesity-diabetes association: a case-cohort study. Diabetol Metab Syndr 2013;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Schmidt MI, Duncan BB. Diabesity: an inflammatory metabolic condition. Clin Chem Lab Med 2003;41:1120–30. [DOI] [PubMed] [Google Scholar]
- 506.Duncan BB, Schmidt MI. The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol Ther 2006;8:7–17. [DOI] [PubMed] [Google Scholar]
- 507.National Institute of Population Research and Training. Bangladesh demographic and health survey 2011. Dhaka (Bangladesh); 2011 [cited 2014 Jun 20]. Available from: http://www.measuredhs.com/pubs/pdf/PR15/PR15.pdf.
- 508.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jonsson LS, Kolb H, Lansink M, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011;106(Suppl 3):S5–78. [DOI] [PubMed] [Google Scholar]
- 509.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2007;318:565. [DOI] [PubMed] [Google Scholar]
- 510.McTernan PG, Kusminski CM, Kumar S. Resistin. Curr Opin Lipidol 2006;17:170–5. [DOI] [PubMed] [Google Scholar]
- 511.Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul 2009;43:157–68. [PubMed] [Google Scholar]
- 512.Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr 2004;134:205–10. [DOI] [PubMed] [Google Scholar]
- 513.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011;11:607–15. [DOI] [PubMed] [Google Scholar]
- 514.Bergman D. The endocrinology of exercise. Intern Emerg Med 2013;8(Suppl 1):S17–21. [DOI] [PubMed] [Google Scholar]
- 515.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:250–5. [DOI] [PubMed] [Google Scholar]
- 516.de Mutsert R, Grootendorst DC, Axelsson J, Boeschoten EW, Krediet RT, Dekker FW. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant 2008;23:2957–64. [DOI] [PubMed] [Google Scholar]
- 517.Wärnberg J, Gomez-Martinez S, Romeo J, Diaz LE, Marcos A. Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci 2009;1153:164–75. [DOI] [PubMed] [Google Scholar]
- 518.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 520.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O'Shaughnessy C, Ganz P. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005;352:29–38. [DOI] [PubMed] [Google Scholar]
- 521.Lee TM, Lin MS, Chang NC. Usefulness of C-reactive protein and interleukin-6 as predictors of outcomes in patients with chronic obstructive pulmonary disease receiving pravastatin. Am J Cardiol 2008;101:530–5. [DOI] [PubMed] [Google Scholar]
- 522.Ryu JH, Scanlon PD. Obstructive lung diseases: COPD, asthma, and many imitators. Mayo Clin Proc 2001;76:1144–53. [DOI] [PubMed] [Google Scholar]
- 523.Vrugt B, Aalbers R. Inflammation and bronchial hyperresponsiveness in allergic asthma and chronic obstructive pulmonary disease. Respir Med 1993;87(Suppl B):3–7. [DOI] [PubMed] [Google Scholar]
- 524.Wouters EF, Reynaert NL, Dentener MA, Vernooy JH. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: is there a connection? Proc Am Thorac Soc 2009;6:638–47. [DOI] [PubMed] [Google Scholar]
- 525.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–45. [DOI] [PubMed] [Google Scholar]
- 526.van den Borst B, Gosker HR, Schols AM. Central fat and peripheral muscle: partners in crime in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:8–13. [DOI] [PubMed] [Google Scholar]
- 527.de Godoy I, Donahoe M, Calhoun WJ, Mancino J, Rogers RM. Elevated TNF-alpha production by peripheral blood monocytes of weight-losing COPD patients. Am J Respir Crit Care Med 1996;153:633–7. [DOI] [PubMed] [Google Scholar]
- 528.Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1994;150:1453–5. [DOI] [PubMed] [Google Scholar]
- 529.Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, Griffiths TL, Shale DJ. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1414–8. [DOI] [PubMed] [Google Scholar]
- 530.Nguyen LT, Bedu M, Caillaud D, Beaufrere B, Beaujon G, Vasson M, Coudert J, Ritz P. Increased resting energy expenditure is related to plasma TNF-alpha concentration in stable COPD patients. Clin Nutr 1999;18:269–74. [DOI] [PubMed] [Google Scholar]
- 531.Cohen RI, Marzouk K, Berkoski P, O'Donnell CP, Polotsky VY, Scharf SM. Body composition and resting energy expenditure in clinically stable, non-weight-losing patients with severe emphysema. Chest 2003;124:1365–72. [DOI] [PubMed] [Google Scholar]
- 532.Engelen MP, Schols AM, Baken WC, Wesseling GJ, Wouters EF. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J 1994;7:1793–7. [DOI] [PubMed] [Google Scholar]
- 533.King DA, Cordova F, Scharf SM. Nutritional aspects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005;82:53–9. [DOI] [PubMed] [Google Scholar]
- 535.Wouters EF, Schols AM. Prevalence and pathophysiology of nutritional depletion in chronic obstructive pulmonary disease. Respir Med 1993;87(Suppl B):45–7. [DOI] [PubMed] [Google Scholar]
- 536.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 2006;61:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease: a placebo-controlled randomized trial. Am J Respir Crit Care Med 1995;152:1268–74. [DOI] [PubMed] [Google Scholar]
- 538.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1791–7. [DOI] [PubMed] [Google Scholar]
- 539.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med 2006;174:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Chinn S, Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax 2001;56:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Lang JE. Obesity, nutrition, and asthma in children. Pediatr Allergy Immunol Pulmonol 2012;25:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Castro-Rodríguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med 2001;163:1344–9. [DOI] [PubMed] [Google Scholar]
- 545.Ford ES. Asthma, body mass index, and C-reactive protein among US adults. J Asthma 2003;40:733–9. [DOI] [PubMed] [Google Scholar]
- 546.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, Avol E, Peters JM. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol 2003;158:406–15. [DOI] [PubMed] [Google Scholar]
- 547.Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med 2004;170:78–85. [DOI] [PubMed] [Google Scholar]
- 548.Michelson PH, Williams LW, Benjamin DK, Barnato AE. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Ann Allergy Asthma Immunol 2009;103:381–5. [DOI] [PubMed] [Google Scholar]
- 549.Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol 2004;160:969–76. [DOI] [PubMed] [Google Scholar]
- 550.Robinson CL, Baumann LM, Romero K, Combe JM, Gomez A, Gilman RH, Cabrera L, Gonzalvez G, Hansel NN, Wise RA, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 2011;66:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Stanley AH, Demissie K, Rhoads GG. Asthma development with obesity exposure: observations from the cohort of the National Health and Nutrition Evaluation Survey Epidemiologic Follow-up Study (NHEFS). J Asthma 2005;42:97–9. [DOI] [PubMed] [Google Scholar]
- 542.Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol 1995;79:1199–205. [DOI] [PubMed] [Google Scholar]
- 543.Litonjua AA, Sparrow D, Celedon JC, DeMolles D, Weiss ST. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the Normative Aging Study. Thorax 2002;57:581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Moreira A, Bonini M, Garcia-Larsen V, Bonini S, Del Giacco SR, Agache I, Fonseca J, Papadopoulos NG, Carlsen KH, Delgado L, et al. Weight loss interventions in asthma: EAACI Evidence-Based Clinical Practice Guideline (Part I). Allergy 2013;68:425–39. [DOI] [PubMed] [Google Scholar]
- 553.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, Wood LG. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy 2013;43:36–49. [DOI] [PubMed] [Google Scholar]
- 554.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000;320:827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Ma J, Strub P, Camargo CA Jr, Xiao L, Ayala E, Gardner CD, Buist AS, Haskell WL, Lavori PW, Wilson SR. The Breathe Easier through Weight Loss Lifestyle (BE WELL) Intervention: a randomized controlled trial. BMC Pulm Med 2010;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev 2001;23:268–87. [DOI] [PubMed] [Google Scholar]
- 557.Bodner C, Godden D, Brown K, Little J, Ross S, Seaton A; Aberdeen WHEASE Study Group. Antioxidant intake and adult-onset wheeze: a case-control study. Eur Respir J 1999;13:22–30. [DOI] [PubMed] [Google Scholar]
- 558.Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, Britton J. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy 2003;33:1355–9. [DOI] [PubMed] [Google Scholar]
- 559.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 1999;354:482–3. [DOI] [PubMed] [Google Scholar]
- 560.Misso NL, Brooks-Wildhaber J, Ray S, Vally H, Thompson PJ. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur Respir J 2005;26:257–64. [DOI] [PubMed] [Google Scholar]
- 561.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax 2004;59:652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Tellez-Rojo MM, Moreno-Macias H, Reyes-Ruiz NI, del Rio-Navarro BE, Ruiz-Navarro MX, Hatch G, Slade R, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med 2002;166:703–9. [DOI] [PubMed] [Google Scholar]
- 563.Schünemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, Klocke RA, Trevisan M. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med 2001;163:1246–55. [DOI] [PubMed] [Google Scholar]
- 564.Schwartz J, Weiss ST. Dietary factors and their relation to respiratory symptoms: the Second National Health and Nutrition Examination Survey. Am J Epidemiol 1990;132:67–76. [DOI] [PubMed] [Google Scholar]
- 565.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med 1995;151:1401–8. [DOI] [PubMed] [Google Scholar]
- 566.Britton JR, Pavord ID, Richards KA, Knox AJ, Wisniewski AF, Lewis SA, Tattersfield AE, Weiss ST. Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med 1995;151:1383–7. [DOI] [PubMed] [Google Scholar]
- 567.Schwartz J, Weiss ST. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I). Am J Clin Nutr 1994;59:110–4. [DOI] [PubMed] [Google Scholar]
- 568.Sharma HS, Misra UK. Postnatal distribution of vitamin A in liver, lung, heart and brain of the rat in relation to maternal vitamin A status. Biol Neonate 1986;50:345–50. [DOI] [PubMed] [Google Scholar]
- 569.Zachman RD. Role of vitamin A in lung development. J Nutr 1995;125(6, Suppl):1634S–8S. [DOI] [PubMed] [Google Scholar]
- 570.McGowan SE, Smith J, Holmes AJ, Smith LA, Businga TR, Madsen MT, Kopp UC, Kline JN. Vitamin A deficiency promotes bronchial hyperreactivity in rats by altering muscarinic M(2) receptor function. Am J Physiol Lung Cell Mol Physiol 2002;282:L1031–9. [DOI] [PubMed] [Google Scholar]
- 571.Allen S, Britton JR, Leonardi-Bee JA. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax 2009;64:610–9. [DOI] [PubMed] [Google Scholar]
- 572.Arora P, Kumar V, Batra S. Vitamin A status in children with asthma. Pediatr Allergy Immunol 2002;13:223–6. [DOI] [PubMed] [Google Scholar]
- 573.Mizuno Y, Furusho T, Yoshida A, Nakamura H, Matsuura T, Eto Y. Serum vitamin A concentrations in asthmatic children in Japan. Pediatr Int 2006;48:261–4. [DOI] [PubMed] [Google Scholar]
- 574.Morabia A, Menkes MJ, Comstock GW, Tockman MS. Serum retinol and airway obstruction. Am J Epidemiol 1990;132:77–82. [DOI] [PubMed] [Google Scholar]
- 575.Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, Della Vecchia R, D'Orazio N. Plasma lycopene and antioxidant vitamins in asthma: the PLAVA study. J Asthma 2007;44:429–32. [DOI] [PubMed] [Google Scholar]
- 576.Checkley W, West KP Jr, Wise RA, Wu L, LeClerq SC, Khatry S, Katz J, Christian P, Tielsch JM, Sommer A. Supplementation with vitamin A early in life and subsequent risk of asthma. Eur Respir J 2011;38:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Shaheen SO. Vitamin D deficiency and the asthma epidemic. Thorax 2008;63:293. [DOI] [PubMed] [Google Scholar]
- 578.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest 2005;128:3792–8. [DOI] [PubMed] [Google Scholar]
- 579.Bossé Y, Maghni K, Hudson TJ. 1alpha,25-Dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics 2007;29:161–8. [DOI] [PubMed] [Google Scholar]
- 580.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med 2011;183:1336–43. [DOI] [PubMed] [Google Scholar]
- 581.Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, Zhang G, Sly PD, Holt PG. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J 2011;38:1320–7. [DOI] [PubMed] [Google Scholar]
- 582.Hollams EM. Vitamin D and atopy and asthma phenotypes in children. Curr Opin Allergy Clin Immunol 2012;12:228–34. [DOI] [PubMed] [Google Scholar]
- 583.Bener A, Ehlayel MS, Tulic MK, Hamid Q. Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol 2012;157:168–75. [DOI] [PubMed] [Google Scholar]
- 584.Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, Forno E, Kelly R, Paul K, Sylvia J, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 2012;186:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009;179:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, Weiss ST, Litonjua AA. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010;126(1):52–8, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, Boner AL. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J 2011;37:1366–70. [DOI] [PubMed] [Google Scholar]
- 588.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr 2011;158:437–41. [DOI] [PubMed] [Google Scholar]
- 589.Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, Teach SJ. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr 2010;156:948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, Litonjua AA, Gao J, Gao X. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration 2011;81:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Camargo CA Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011;127:e180–7. [DOI] [PubMed] [Google Scholar]
- 592.Camargo CA Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007;85:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, Simell O, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy 2009;39:875–82. [DOI] [PubMed] [Google Scholar]
- 594.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J 2010;35:1228–34. [DOI] [PubMed] [Google Scholar]
- 595.Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med 2012;186:508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin D and asthma. Am J Respir Crit Care Med 2012;185:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.McGowan SE, Hunninghake GW. Neutrophils and emphysema. N Engl J Med 1989;321:968–70. [DOI] [PubMed] [Google Scholar]
- 598.Baumann LM, Robinson CL, Combe JM, Gomez A, Romero K, Gilman RH, Cabrera L, Hansel NN, Wise RA, Breysse PN, et al. Effects of distance from a heavily transited avenue on asthma and atopy in a periurban shantytown in Lima, Peru. J Allergy Clin Immunol 2011;127:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Kim JJ, Huen K, Adams S, Smorodinsky S, Hoats A, Malig B, Lipsett M, Ostro B. Residential traffic and children's respiratory health. Environ Health Perspect 2008;116:1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect 2006;114:766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med 2008;177:1331–7. [DOI] [PubMed] [Google Scholar]
- 602.Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, Wilson K, Villareal M, Burkle J, Lockey J. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol 2005;116:279–84. [DOI] [PubMed] [Google Scholar]
- 603.Vedal S, Petkau J, White R, Blair J. Acute effects of ambient inhalable particles in asthmatic and nonasthmatic children. Am J Respir Crit Care Med 1998;157:1034–43. [DOI] [PubMed] [Google Scholar]
- 604.Venn AJ, Lewis SA, Cooper M, Hubbard R, Britton J. Living near a main road and the risk of wheezing illness in children. Am J Respir Crit Care Med 2001;164:2177–80. [DOI] [PubMed] [Google Scholar]
- 605.Yu O, Sheppard L, Lumley T, Koenig JQ, Shapiro GG. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect 2000;108:1209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, et al. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011;19:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Friis H, Gomo E, Koestel P, Ndhlovu P, Nyazema N, Krarup H, Michaelsen KF. HIV and other predictors of serum beta-carotene and retinol in pregnancy: a cross-sectional study in Zimbabwe. Am J Clin Nutr 2001;73:1058–65. [DOI] [PubMed] [Google Scholar]
- 608.Friis H, Gomo E, Mashange W, Nyazema N, Kostel P, Wieringa F, Krarup H. The acute phase response to parturition: a cross-sectional study in Zimbabwe. Afr J Reprod Health 2009;13:61–8. [PubMed] [Google Scholar]
- 609.Friis H, Range N, Braendgaard Kristensen C, Kaestel P, Changalucha J, Malenganisho W, Krarup H, Magnussen P, Bengaard Andersen A. Acute- phase response and iron status markers among pulmonary tuberculosis patients: a cross-sectional study in Mwanza, Tanzania. Br J Nutr 2009;102:310–7. [DOI] [PubMed] [Google Scholar]
- 610.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Lappas M, Permezel M, Rice GE. Release of proinflammatory cytokines and 8-isoprostane from placenta, adipose tissue, and skeletal muscle from normal pregnant women and women with gestational diabetes mellitus. J Clin Endocrinol Metab 2004;89:5627–33. [DOI] [PubMed] [Google Scholar]
- 612.Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol 2005;186:457–65. [DOI] [PubMed] [Google Scholar]
- 613.Koga K, Aldo PB, Mor G. Toll-like receptors and pregnancy: trophoblast as modulators of the immune response. J Obstet Gynaecol Res 2009;35:191–202. [DOI] [PubMed] [Google Scholar]
- 614.Frei R, Steinle J, Birchler T, Loeliger S, Roduit C, Steinhoff D, Seibl R, Buchner K, Seger R, Reith W, et al. MHC class II molecules enhance Toll-like receptor mediated innate immune responses. PLoS ONE 2010;5:e8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Cobo T, Palacio M, Martinez-Terron M, Navarro-Sastre A, Bosch J, Filella X, Gratacos E. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol 2011;205(2):126, e1–8. [DOI] [PubMed] [Google Scholar]
- 616.McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci USA 2012;109(Suppl 2):17281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Leach L, Taylor A, Sciota F. Vascular dysfunction in the diabetic placenta: causes and consequences. J Anat 2009;215:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Henry SL, Barzel B, Wood-Bradley RJ, Burke SL, Head GA, Armitage JA. Developmental origins of obesity-related hypertension. Clin Exp Pharmacol Physiol 2012;39:799–806. [DOI] [PubMed] [Google Scholar]
- 619.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Berti C, Biesalski HK, Gartner R, Lapillonne A, Pietrzik K, Poston L, Redman C, Koletzko B, Cetin I. Micronutrients in pregnancy: current knowledge and unresolved questions. Clin Nutr 2011;30:689–701. [DOI] [PubMed] [Google Scholar]
- 621.Knuppel RA, Hassan MI, McDermott JJ, Tucker M, Morrison JC. Oxidative stress and antioxidants: preterm birth and preterm infants. In: Morrison JC, editor. Preterm birth—mother and child. Rijeka (Croatia): InTech; 2012. p. 125–50. [Google Scholar]
- 622.Ochoa JJ, Ramirez-Tortosa MC, Quiles JL, Palomino N, Robles R, Mataix J, Huertas JR. Oxidative stress in erythrocytes from premature and full-term infants during their first 72 h of life. Free Radic Res 2003;37:317–22. [DOI] [PubMed] [Google Scholar]
- 623.Böhles H. Antioxidative vitamins in prematurely and maturely born infants. Int J Vitam Nutr Res 1997;67:321–8. [PubMed] [Google Scholar]
- 624.Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, Freeman DJ. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 2010;119:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes 2010;59:3058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is hepcidin the link? J Perinatol 2013;33:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Brabin BJ. Epidemiology of infection in pregnancy. Rev Infect Dis 1985;7:579–603. [DOI] [PubMed] [Google Scholar]
- 628.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010;63:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Dotters-Katz S, Kuller J, Heine RP. Parasitic infections in pregnancy. Obstet Gynecol Surv 2011;66:515–25. [DOI] [PubMed] [Google Scholar]
- 629.Brabin L, Brabin BJ, Gies S. Influence of iron status on risk of maternal or neonatal infection and on neonatal mortality with an emphasis on developing countries. Nutr Rev 2013;71:528–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochrane Database Syst Rev 2009;2:CD005547. [DOI] [PubMed] [Google Scholar]
- 632.Haider BA, Yakoob MY, Bhutta ZA. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health 2011;11(Suppl 3):S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Imhoff-Kunsch B, Briggs V. Antihelminthics in pregnancy and maternal, newborn and child health. Paediatr Perinat Epidemiol 2012;26(Suppl 1):223–38. [DOI] [PubMed] [Google Scholar]
- 634.Matevosyan NR. Periodontal disease and perinatal outcomes. Arch Gynecol Obstet 2011;283:675–86. [DOI] [PubMed] [Google Scholar]
- 635.Peuhkuri K, Vapaatalo H, Korpela R. Even low-grade inflammation impacts on small intestinal function. World J Gastroenterol 2010;16:1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.van de Laar R, van der Ham DP, Oei SG, Willekes C, Weiner CP, Mol BW. Accuracy of C-reactive protein determination in predicting chorioamnionitis and neonatal infection in pregnant women with premature rupture of membranes: a systematic review. Eur J Obstet Gynecol Reprod Biol 2009;147:124–9. [DOI] [PubMed] [Google Scholar]
- 637.Dunlop AL, Taylor RN, Tangpricha V, Fortunato S, Menon R. Maternal vitamin D, folate, and polyunsaturated fatty acid status and bacterial vaginosis during pregnancy. Infect Dis Obstet Gynecol 2011;2011:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, Klebanoff MA. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr 2007;137:2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Verstraelen H, Delanghe J, Roelens K, Blot S, Claeys G, Temmerman M. Subclinical iron deficiency is a strong predictor of bacterial vaginosis in early pregnancy. BMC Infect Dis 2005;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Di Mario S, Say L, Lincetto O. Risk factors for stillbirth in developing countries: a systematic review of the literature. Sex Transm Dis 2007;34(7, Suppl):S11–21. [DOI] [PubMed] [Google Scholar]
- 641.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev 2002;8:30–8. [DOI] [PubMed] [Google Scholar]
- 642.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007;7:93–104. [DOI] [PubMed] [Google Scholar]
- 643.le Cessie S, Verhoeff FH, Mengistie G, Kazembe P, Broadhead R, Brabin BJ. Changes in haemoglobin levels in infants in Malawi: effect of low birth weight and fetal anaemia. Arch Dis Child Fetal Neonatal Ed 2002;86:F182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Koski KG, Scott ME. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu Rev Nutr 2001;21:297–321. [DOI] [PubMed] [Google Scholar]
- 645.Odiere MR, Scott ME, Leroux LP, Dzierszinski FS, Koski KG. Maternal protein deficiency during a gastrointestinal nematode infection alters developmental profile of lymphocyte populations and selected cytokines in neonatal mice. J Nutr 2013;143:100–7. [DOI] [PubMed] [Google Scholar]
- 646.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 2010;63:73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.World Health Organization. Mastitis: causes and management. Geneva (Switzerland): World Health Organization; 2000. [Google Scholar]
- 648.Contreras GA, Rodriguez JM. Mastitis: comparative etiology and epidemiology. J Mammary Gland Biol Neoplasia 2011;16:339–56. [DOI] [PubMed] [Google Scholar]
- 649.Michie C, Lockie F, Lynn W. The challenge of mastitis. Arch Dis Child 2003;88:818–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Kvist LJ. Toward a clarification of the concept of mastitis as used in empirical studies of breast inflammation during lactation. J Hum Lact 2010;26:53–9. [DOI] [PubMed] [Google Scholar]
- 651.Schukken YH, Gunther J, Fitzpatrick J, Fontaine MC, Goetze L, Holst O, Leigh J, Petzl W, Schuberth HJ, Sipka A, et al. Host-response patterns of intramammary infections in dairy cows. Vet Immunol Immunopathol 2011;144:270–89. [DOI] [PubMed] [Google Scholar]
- 652.Semba RD, Neville MC. Breast-feeding, mastitis, and HIV transmission: nutritional implications. Nutr Rev 1999;57:146–53. [DOI] [PubMed] [Google Scholar]
- 653.Hunt KM, Williams JE, Shafii B, Hunt MK, Behre R, Ting R, McGuire MK, McGuire MA. Mastitis is associated with increased free fatty acids, somatic cell count, and interleukin-8 concentrations in human milk. Breastfeed Med 2013;8:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011;6:e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Sordillo LM. New concepts in the causes and control of mastitis. J Mammary Gland Biol Neoplasia 2011;16:271–3. [DOI] [PubMed] [Google Scholar]
- 656.Semba RD. Mastits and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci 2000;918:156–62. [DOI] [PubMed] [Google Scholar]
- 657.Wockel A, Abou-Dakn M, Beggel A, Arck P. Inflammatory breast diseases during lactation: health effects on the newborn—a literature review. Mediators Inflamm 2008;2008:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Heinrichs AJ, Costello SS, Jones CM. Control of heifer mastitis by nutrition. Vet Microbiol 2009;134:172–6. [DOI] [PubMed] [Google Scholar]
- 659.Nelson CD, Reinhardt TA, Lippolis JD, Sacco RE, Nonnecke BJ. Vitamin D signaling in the bovine immune system: a model for understanding human vitamin D requirements. Nutrients 2012;4:181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Nussenblatt V, Lema V, Kumwenda N, Broadhead R, Neville MC, Taha TE, Semba R. Epidemiology and microbiology of subclinical mastitis among HIV-infected women in Malawi. Int J STD AIDS 2005;16:227–32. [DOI] [PubMed] [Google Scholar]
- 661.Arsenault JE, Aboud S, Manji KP, Fawzi WW, Villamor E. Vitamin supplementation increases risk of subclinical mastitis in HIV-infected women. J Nutr 2010;140:1788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Giusti B, Vestrini A, Poggi C, Magi A, Pasquini E, Abbate R, Dani C. Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants. Free Radic Res 2012;46:1130–9. [DOI] [PubMed] [Google Scholar]
- 663.Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy 2012;2012:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Waterland RA. Do maternal methyl supplements in mice affect DNA methylation of offspring? J Nutr 2003;133:238. [DOI] [PubMed] [Google Scholar]
- 665.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 2008;105:17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Khulan B, Cooper WN, Skinner BM, Bauer J, Owens S, Prentice AM, Belteki G, Constancia M, Dunger D, Affara NA. Periconceptional maternal micronutrient supplementation is associated with widespread gender related changes in the epigenome: a study of a unique resource in the Gambia. Hum Mol Genet 2012;21:2086–101. [DOI] [PubMed] [Google Scholar]
- 667.Håberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child 2009;94:180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Prescott SL. Effects of early cigarette smoke exposure on early immune development and respiratory disease. Paediatr Respir Rev 2008;9:3–9. [DOI] [PubMed] [Google Scholar]
- 669.Koshy G, Delpisheh A, Brabin BJ. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur J Public Health 2011;21:286–91. [DOI] [PubMed] [Google Scholar]
- 670.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ 1983;61:1005–16. [PMC free article] [PubMed] [Google Scholar]
- 671.Gwamaka M, Kurtis JD, Sorensen BE, Holte S, Morrison R, Mutabingwa TK, Fried M, Duffy PE. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis 2012;54:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Jonker FA, Calis JC, van Hensbroek MB, Phiri K, Geskus RB, Brabin BJ, Leenstra T. Iron status predicts malaria risk in Malawian preschool children. PLoS ONE 2012;7:e42670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Maltha J, Jacobs J. Iron deficiency and malaria mortality: possible implication of invasive bacterial diseases. Clin Infect Dis 2012;55:748. [DOI] [PubMed] [Google Scholar]
- 674.Giovannucci E, Chan AT. Role of vitamin and mineral supplementation and aspirin use in cancer survivors. J Clin Oncol 2010;28:4081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Raiten DJ, Grinspoon S, Arpadi S. Nutritional considerations in the use of ART in resource-limited settings. Geneva (Switzerland): World Health Organization; 2005 [cited 2014 Jun 20]. Available from: http://www.who.int/nutrition/topics/Paper%20Number%206%20-%20Nutritional%20-%20ART.pdf.
- 676.Kutuzova GD, DeLuca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol 2007;218:37–44. [DOI] [PubMed] [Google Scholar]
- 677.Lee MJ, Fried SK. Glucocorticoids antagonize tumor necrosis factor-alpha-stimulated lipolysis and resistance to the antilipolytic effect of insulin in human adipocytes. Am J Physiol Endocrinol Metab 2012;303:E1126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Goppelt-Struebe M, Wolter D, Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol 1989;98:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Möhlig M, Freudenberg M, Bobbert T, Ristow M, Rochlitz H, Weickert MO, Pfeiffer AF, Spranger J. Acetylsalicylic acid improves lipid-induced insulin resistance in healthy men. J Clin Endocrinol Metab 2006;91:964–7. [DOI] [PubMed] [Google Scholar]
- 680.Rainsford KD, Stetsko PI, Sirko SP, Debski S. Gastrointestinal mucosal injury following repeated daily oral administration of conventional formulations of indometacin and other non-steroidal anti-inflammatory drugs to pigs: a model for human gastrointestinal disease. J Pharm Pharmacol 2003;55:661–8. [DOI] [PubMed] [Google Scholar]
- 681.Yoon SS, Dillon CF, Carroll M, Illoh K, Ostchega Y. Effects of statins on serum inflammatory markers: the U.S. National Health and Nutrition Examination Survey 1999–2004. J Atheroscler Thromb 2010;17:1176–82. [DOI] [PubMed] [Google Scholar]
- 682.Koj A. Definition and classification of acute-phase proteins. In: Gordon AH, Koj A, editors. The acute-phase response to injury and infection. 1st ed. Amsterdam: Elsevier Science; 1985, p. 139–44. [Google Scholar]
- 683.Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B 2005;6:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Kaysen GA, Dubin JA, Muller HG, Mitch WE, Levin NW, Hemo Group. Levels of alpha1 acid glycoprotein and ceruloplasmin predict future albumin levels in hemodialysis patients. Kidney Int 2001;60:2360–6. [DOI] [PubMed] [Google Scholar]
- 685.Myers MA, Fleck A. Observations on the delay in onset of the acute phase protein response. Br J Exp Pathol 1988;69:169–76. [PMC free article] [PubMed] [Google Scholar]
- 686.Fleck A, Myers MA. Diagnostic and prognostic significance of acute phase proteins. In: Gordon AH, Koj A, editors. The acute phase response to injury and infection. 1st ed. Amsterdam: Elsevier Science; 1985. p. 249–71. [Google Scholar]
- 687.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74. [DOI] [PubMed] [Google Scholar]
- 688.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7. [DOI] [PubMed] [Google Scholar]
- 689.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 2005;98:1154–62. [DOI] [PubMed] [Google Scholar]
- 690.Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci 1998;53:M20–6. [DOI] [PubMed] [Google Scholar]
- 691.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol 2004;39:687–99. [DOI] [PubMed] [Google Scholar]
- 692.Duerrschmid C, Crawford JR, Reineke E, Taffet GE, Trial J, Entman ML, Haudek SB. TNF receptor 1 signaling is critically involved in mediating angiotensin-II-induced cardiac fibrosis. J Mol Cell Cardiol 2013;57:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA, Parise G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am J Physiol Cell Physiol 2013;304:C717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Kuller LH. Serum levels of IL-6 and development of disability in older persons. J Am Geriatr Soc 1999;47:755–6. [DOI] [PubMed] [Google Scholar]
- 695.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci 2000;55:M709–15. [DOI] [PubMed] [Google Scholar]
- 696.Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 2002;50:638–44. [DOI] [PubMed] [Google Scholar]
- 697.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L, Harris TB, Guralnik JM, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2004;59:242–8. [DOI] [PubMed] [Google Scholar]
- 698.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci 2009;64:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 2002;59:371–8. [DOI] [PubMed] [Google Scholar]
- 700.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 2003;61:76–80. [DOI] [PubMed] [Google Scholar]
- 701.Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, Wauters A, Maes M, Jolles J, Steinbusch HW, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol 2003;134:142–50. [DOI] [PubMed] [Google Scholar]
- 702.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci 2004;59:268–74. [DOI] [PubMed] [Google Scholar]
- 703.Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol A Biol Sci Med Sci 2008;63:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci 2010;1207:155–62. [DOI] [PubMed] [Google Scholar]
- 705.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci 1997;52:M201–8. [DOI] [PubMed] [Google Scholar]
- 706.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999;47:639–46. [DOI] [PubMed] [Google Scholar]
- 707.Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, Nevitt M, Visser M, Harris T, Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc 2004;52:1105–13. [DOI] [PubMed] [Google Scholar]
- 708.Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. J Gerontol A Biol Sci Med Sci 2013;68:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol 2002;52:168–74. [DOI] [PubMed] [Google Scholar]
- 710.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch Neurol 2004;61:668–72. [DOI] [PubMed] [Google Scholar]
- 711.Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, Mariani E, Licastro F, Patterson C. Blood inflammatory markers and risk of dementia: the Conselice Study of Brain Aging. Neurobiol Aging 2007;28:1810–20. [DOI] [PubMed] [Google Scholar]
- 712.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology 2007;68:1902–8. [DOI] [PubMed] [Google Scholar]
- 713.Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis 2011;2:175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Watson J, Round A, Hamilton W. Raised inflammatory markers. BMJ 2012;344:e454. [DOI] [PubMed] [Google Scholar]
- 715.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013;14:232–44. [DOI] [PubMed] [Google Scholar]
- 716.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 717.Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: application to clinical and public health practice: report from the Laboratory Science Discussion Group. Circulation 2004;110:e545–9. [DOI] [PubMed] [Google Scholar]
- 718.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH Jr, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol 2005;46:464–9. [DOI] [PubMed] [Google Scholar]
- 719.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med 2005;352:1611–3. [DOI] [PubMed] [Google Scholar]
- 720.Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, Despres JP. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am J Clin Nutr 2009;89:1307–14. [DOI] [PubMed] [Google Scholar]
- 721.World Health Organization. Use of anticoagulants in diagnostic laboratory investigations. Geneva (Switzerland): WHO; 2002 [cited 2014 Jun 2014]. Available from: http://whqlibdoc.who.int/hq/2002/WHO_DIL_LAB_99.1_Rev.2.pdf.
- 722.Ledue TB, Rifai N. High sensitivity immunoassays for C-reactive protein: promises and pitfalls. Clin Chem Lab Med 2001;39:1171–6. [DOI] [PubMed] [Google Scholar]
- 723.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem 2001;47:426–30. [PubMed] [Google Scholar]
- 724.Zegers I, Charoud-Got I, Ryzchon M, Trapmann S, Emons H, Schimmer H. Certification of C-reactive protein in reference material ERM-DA472/IFCC: certified reference material ERM-DA472/IFCC. Luxembourg: European Commission, Joint Research Centre, IRMM; 2009. [Google Scholar]
- 725.Goodall SR. Advances in plasma protein standardization. Ann Clin Biochem 1997;34:582–7. [DOI] [PubMed] [Google Scholar]
- 726.Sann L, Bienvenu J, Lahet C, Divry P, Cotte J, Bethenod M. Serum orosomucoid concentration in newborn infants. Eur J Pediatr 1981;136:181–5. [DOI] [PubMed] [Google Scholar]
- 727.Zegers I, Schreiber W, Sheldon J, Blirup-Jensen S, Munoz A, Merlini G, et al. Certification of proteins in the human serum: certified reference material ERM - DA470k/IFCC. Luxembourg: Office for Official Publications of the European Communities; 2008. [cited 2013 Nov 14]. Available from: http://www.clinchem.org/content/56/12/1880.full.pdf.
- 728.Randox Laboratories [Internet]. [cited 2014 Feb 27]. Available from: http://www.riqas.com/.
- 729.Fincher RM, Page MI. Clinical significance of extreme elevation of the erythrocyte sedimentation rate. Arch Intern Med 1986;146:1581–3. [PubMed] [Google Scholar]
- 730.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432–7. [DOI] [PubMed] [Google Scholar]
- 731.Shenkin A, Cederblad G, Elia M, Isaksson B; International Federation of Clinical Chemistry. Laboratory assessment of protein-energy status. Clin Chim Acta 1996;253:S5–59. [DOI] [PubMed] [Google Scholar]
- 732.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal 1999;13:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993;341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med 2008;9:407–13. [DOI] [PubMed] [Google Scholar]
- 735.Yo CH, Hsieh PS, Lee SH, Wu JY, Chang SS, Tasi KC, Lee CC. Comparison of the test characteristics of procalcitonin to C-reactive protein and leukocytosis for the detection of serious bacterial infections in children presenting with fever without source: a systematic review and meta-analysis. Ann Emerg Med 2012;60:591–600. [DOI] [PubMed] [Google Scholar]
- 736.Maniaci V, Dauber A, Weiss S, Nylen E, Becker KL, Bachur R. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics 2008;122:701–10. [DOI] [PubMed] [Google Scholar]
- 737.Christ-Crain M, Muller B. Procalcitonin in bacterial infections–hype, hope, more or less? Swiss Med Wkly 2005;135:451–60. [DOI] [PubMed] [Google Scholar]
- 738.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 2001;60:349–56. [DOI] [PubMed] [Google Scholar]
- 739.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 1995;95:2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol 2000;121:255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Paolisso G, Rizzo MR, Mazziotti G, Tagliamonte MR, Gambardella A, Rotondi M, Carella C, Giugliano D, Varricchio M, D'Onofrio F. Advancing age and insulin resistance: role of plasma tumor necrosis factor-alpha. Am J Physiol 1998;275:E294–9. [DOI] [PubMed] [Google Scholar]
- 742.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- 743.Jovinge S, Hamsten A, Tornvall P, Proudler A, Bavenholm P, Ericsson CG, Godsland I, de Faire U, Nilsson J. Evidence for a role of tumor necrosis factor alpha in disturbances of triglyceride and glucose metabolism predisposing to coronary heart disease. Metabolism 1998;47:113–8. [DOI] [PubMed] [Google Scholar]
- 744.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem 1997;272:971–6. [DOI] [PubMed] [Google Scholar]
- 745.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 746.Todd J, Simpson P, Estis J, Torres V, Wub AH. Reference range and short- and long-term biological variation of interleukin (IL)-6, IL-17A and tissue necrosis factor-alpha using high sensitivity assays. Cytokine 2013;64:660–5. [DOI] [PubMed] [Google Scholar]
- 747.Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today 1997;18:428–32. [DOI] [PubMed] [Google Scholar]
- 748.Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med 1994;179:1529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 2003;285:E433–7. [DOI] [PubMed] [Google Scholar]
- 750.Whiteside TL. Cytokine measurements and interpretation of cytokine assays in human disease. J Clin Immunol 1994;14:327–39. [DOI] [PubMed] [Google Scholar]
- 751.Messer J, Eyer D, Donato L, Gallati H, Matis J, Simeoni U. Evaluation of interleukin-6 and soluble receptors of tumor necrosis factor for early diagnosis of neonatal infection. J Pediatr 1996;129:574–80. [DOI] [PubMed] [Google Scholar]
- 752.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response—lessons from malaria and human immunodeficiency virus. Ann Clin Biochem 2008;45:18–32. [DOI] [PubMed] [Google Scholar]
- 753.Raiten D, , Namaste S, Brabin B, , editors, editors. doi: 10.1024/0300-9831/a000051. Considerations for the safe and effective use of iron interventions in areas of malaria burden: full technical report 2009 [cited 2014 Jun 20]. Available from: https://www.nichd.nih.gov/global_nutrition/programs/iron_and_malaria/Documents/Iron_Malaria_Tech_Report_2012.pdf. [DOI] [PubMed]
- 754.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med 2011;62:347–60. [DOI] [PubMed] [Google Scholar]
- 755.Haynes BM, Pfeiffer CM, Sternberg MR, Schleicher RL. Selected physiologic variables are weakly to moderately associated with 29 biomarkers of diet and nutrition, NHANES 2003–2006. J Nutr 2013;143(Suppl):1001S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 2009;12:444–54. [DOI] [PubMed] [Google Scholar]
- 757.World Health Organization. Global targets 2025 [cited 2014 Mar 19]. Available from: http://www.who.int/nutrition/topics/nutrition_globaltargets2025/en/.
- 758.World Health Organization; United Nations Children's Fund. Focusing on anaemia: towards an integrated approach for effective anaemia control. Geneva (Switzerland): WHO; 2004 [cited 2014 June 20]. Available from: http://www.who.int/nutrition/publications/micronutrients/WHOandUNICEF_statement_anaemia/en/.
- 759.HemoCue; 2014. [cited 2014 Jun 20]. Available from: http://www.hemocue.us/.
- 760.Hassan K, Sullivan KM, Yip R, Woodruff BA. Factors associated with anemia in refugee children. J Nutr 1997;127:2194–8. [DOI] [PubMed] [Google Scholar]
- 761.Reeves JD, Yip R, Kiley VA, Dallman PR. Iron deficiency in infants: the influence of mild antecedent infection. J Pediatr 1984;105:874–9. [DOI] [PubMed] [Google Scholar]
- 762.Thurnham DI. Infection and the etiology of anemia. In: Kraemer K, editor. Nutritional anemia. Zurich, Switzerland: Sight and Life Press; 2007, p. 31–2. [Google Scholar]
- 763.Yip R, Dallman PR. The roles of inflammation and iron deficiency as causes of anemia. Am J Clin Nutr 1988;48:1295–300. [DOI] [PubMed] [Google Scholar]
- 764.Shinoda N, Sullivan KM, Tripp K, Erhardt JG, Haynes BM, Temple VJ, Woodruff B. Relationship between markers of inflammation and anaemia in children of Papua New Guinea. Public Health Nutr 2013;16:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Sullivan KM, Venugopalan B, Jefferds ME, Boy E, Bonilla J, Sandino I, Halleslevens P. Association of elevated alpha(1)-acid glycoprotein (AGP) and the prevalence of anemia in Nicaraguan preschool children. Food Nutr Bull 2012;33:137–41. [DOI] [PubMed] [Google Scholar]
- 766.Maqsood M, Dancheck B, Gamble MV, Palafox NA, Ricks MO, Briand K, Semba RD. Vitamin A deficiency and inflammatory markers among preschool children in the Republic of the Marshall Islands. Nutr J 2004;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Rawat R, Stoltzfus RJ, Ntozini R, Mutasa K, Iliff PJ, Humphrey JH. Influence of inflammation as measured by alpha-1-acid glycoprotein on iron status indicators among HIV-positive postpartum Zimbabwean women. Eur J Clin Nutr 2009;63:787–93. [DOI] [PubMed] [Google Scholar]
- 768.Foote EM, Sullivan KM, Ruth LJ, Oremo J, Sadumah I, Williams TN, Suchdev PS. Determinants of anemia among preschool children in rural, western Kenya. Am J Trop Med Hyg 2013;88:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Gamble MV, Palafox NA, Dancheck B, Ricks MO, Briand K, Semba RD. Relationship of vitamin A deficiency, iron deficiency, and inflammation to anemia among preschool children in the Republic of the Marshall Islands. Eur J Clin Nutr 2004;58:1396–401. [DOI] [PubMed] [Google Scholar]
- 770.World Health Organization. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO global database on vitamin A deficiency. Geneva (Switzerland): WHO; 2009. [cited 2013 Nov 14]. Available from: http://whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf.
- 771.World Health Organization. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes. Geneva (Switzerland): WHO; 1996. [cited 2013 Nov 14]. Available from: http://www.who.int/nutrition/publications/micronutrients/vitamin_a_deficiency/WHO_NUT_96.10/en/index.html.
- 772.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 2004;134:3127–32. [DOI] [PubMed] [Google Scholar]
- 773.Engle-Stone R, Haskell MJ, Ndjebayi AO, Nankap M, Erhardt JG, Gimou MM, Brown KH. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. J Nutr 2011;141:2233–41. [DOI] [PubMed] [Google Scholar]
- 774.Rohner F, Northrop-Clewes C, Tschannen AB, Bosso PE, Kouassi-Gohou V, Erhardt JG, Bui M, Zimmermann MB, Mascie-Taylor CN. Prevalence and public health relevance of micronutrient deficiencies and undernutrition in pre-school children and women of reproductive age in Cote d'Ivoire, West Africa. Public Health Nutr 2014;17:2016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Dancheck B, Nussenblatt V, Ricks MO, Kumwenda N, Neville MC, Moncrief DT, Taha TE, Semba RD. Breast milk retinol concentrations are not associated with systemic inflammation among breast-feeding women in Malawi. J Nutr 2005;135:223–6. [DOI] [PubMed] [Google Scholar]
- 776.Engle-Stone R, Haskell MJ, Nankap M, Ndjebayi AO, Brown KH. Breast milk retinol and plasma retinol-binding protein concentrations provide similar estimates of vitamin A deficiency prevalence and identify similar risk groups among women in Cameroon but breast milk retinol underestimates the prevalence of deficiency among young children. J Nutr 2014;144:209–17. [DOI] [PubMed] [Google Scholar]
- 777.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980). Am J Clin Nutr 2003;78:756–64. [DOI] [PubMed] [Google Scholar]
- 778.Peretz A, Neve J, Jeghers O, Leclercq N, Praet JP, Vertongen F, Famaey JP. Interest of zinc determination in leucocyte fractions for the assessment of marginal zinc status. Clin Chim Acta 1991;203:35–46. [DOI] [PubMed] [Google Scholar]
- 779.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 2005;102:6843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Duggan C, MacLeod WB, Krebs NF, Westcott JL, Fawzi WW, Premji ZG, Mwanakasale V, Simon JL, Yeboah-Antwi K, Hamer DH. Plasma zinc concentrations are depressed during the acute phase response in children with falciparum malaria. J Nutr 2005;135:802–7. [DOI] [PubMed] [Google Scholar]
- 781.King JC. Does zinc absorption reflect zinc status? Int J Vitam Nutr Res 2010;80:300–6. [DOI] [PubMed] [Google Scholar]
- 782.Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr 2008;99(Suppl 3):S14–23. [DOI] [PubMed] [Google Scholar]
- 783.Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr 2011;94(Suppl):666S–72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Wu JT. Circulating homocysteine is an inflammation marker and a risk factor of life-threatening inflammatory diseases. J Biomed Lab Sci 2007;19:107–11. [Google Scholar]
- 785.Rush EC, Katre P, Yajnik CS. Vitamin B12: one carbon metabolism, fetal growth and programming for chronic disease. Eur J Clin Nutr 2014;68:2–7. [DOI] [PubMed] [Google Scholar]
- 786.Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev 2004;62(6 Pt 2):S3–12; discussion S13. [DOI] [PubMed] [Google Scholar]
- 787.Folsom AR, Desvarieux M, Nieto FJ, Boland LL, Ballantyne CM, Chambless LE. B vitamin status and inflammatory markers. Atherosclerosis 2003;169:169–74. [DOI] [PubMed] [Google Scholar]
- 788.Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, Raghavan R, Raiten R. Biomarkers of Nutrition for Development—iodine review. J Nutr 2014;144(Suppl):1322S–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol 2010;205:1–13. [DOI] [PubMed] [Google Scholar]
- 790.Khoo AL, Chai L, Koenen H, Joosten I, Netea M, van der Ven A. Translating the role of vitamin D3 in infectious diseases. Crit Rev Microbiol 2012;38:122–35. [DOI] [PubMed] [Google Scholar]
- 791.Cashman KD, Kiely M. EURRECA—Estimating vitamin D requirements for deriving dietary reference values. Crit Rev Food Sci Nutr 2013;53:1097–109. [DOI] [PubMed] [Google Scholar]
- 792.Querfeld U. Vitamin D and inflammation. Pediatr Nephrol 2013;28:605–10. [DOI] [PubMed] [Google Scholar]
- 793.Duncan A, Talwar D, McMillan DC, Stefanowicz F, O'Reilly DS. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr 2012;95:64–71. [DOI] [PubMed] [Google Scholar]
- 794.Yang CF, Duro D, Zurakowski D, Lee M, Jaksic T, Duggan C. High prevalence of multiple micronutrient deficiencies in children with intestinal failure: a longitudinal study. J Pediatr 2011;159(1):39–44, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Suchdev PS, Shah A, Jefferds ME, Eleveld A, Patel M, Stein AD, Macdonald B, Ruth L. Sustainability of market-based community distribution of Sprinkles in western Kenya. Matern Child Nutr 2013;9(Suppl 1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Ridker PM. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Cardiology patient page. Circulation 2003;108:e81–5. [DOI] [PubMed] [Google Scholar]
- 797.Knowles J, Thurnham DI, Phengdy B, Houamboun K, Philavong K, Keomoungkhone I, Keovilay K. Impact of inflammation on the biomarkers of iron status in a cross-sectional survey of Lao women and children. Br J Nutr 2013;110:2285–97. [DOI] [PubMed] [Google Scholar]
- 798.Abraham K, Muller C, Gruters A, Wahn U, Schweigert FJ. Minimal inflammation, acute phase response and avoidance of misclassification of vitamin A and iron status in infants—importance of a high-sensitivity C-reactive protein (CRP) assay. Int J Vitam Nutr Res 2003;73:423–30. [DOI] [PubMed] [Google Scholar]
- 799.Witte DL. Can serum ferritin be effectively interpreted in the presence of the acute-phase response? Clin Chem 1991;37:484–5. [PubMed] [Google Scholar]
- 800.Carroll RJ, Ruppert D. Transformation and weighting in regression. New York: Chapman and Hall; 1988. [Google Scholar]
- 801.Hastie T, Tibshirani R. Generalized additive models. New York: Chapman and Hall; 1990. [DOI] [PubMed] [Google Scholar]
- 802.Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Haemoglobin adjustments to define anaemia. Trop Med Int Health 2008;13:1267–71. [DOI] [PubMed] [Google Scholar]
- 803.Kung'u JK, Wright VJ, Haji HJ, Ramsan M, Goodman D, Tielsch JM, Bickle QD, Raynes JG, Stoltzfus RJ. Adjusting for the acute phase response is essential to interpret iron status indicators among young Zanzibari children prone to chronic malaria and helminth infections. J Nutr 2009;139:2124–31. [DOI] [PubMed] [Google Scholar]
- 804.Engle-Stone R, Nankap M, Ndjebayi AO, Erhardt JG, Brown KH. Plasma ferritin and soluble transferrin receptor concentrations and body iron stores identify similar risk factors for iron deficiency but result in different estimates of the national prevalence of iron deficiency and iron-deficiency anemia among women and children in Cameroon. J Nutr 2013;143:369–77. [DOI] [PubMed] [Google Scholar]
- 805.Namaste S, Suchdev P, Grant A. Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia (BRINDA): project overview. FASEB J 2013;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.