Abstract
Purpose
We identified a discrete population of stem cell-like tumor cells expressing 5 essential transcription factors required to reprogram pluripotency in prostate tumor cell lines and primary prostate cancer tissue.
Materials and Methods
DU145 and PC3 human prostate cancer cell lines (ATCC®), tumor tissue from patients with prostate cancer and normal prostate tissue were evaluated for the reprogramming factors OCT3/4 (Cell Signaling Technology®), SOX2, Klf4 (Santa Cruz Biotechnology, Santa Cruz, California), Nanog (BioLegend®) and c-Myc (Cell Signaling) by semiquantitative reverse transcriptase-polymerase chain reaction, histological and immunohistochemical analysis. Stem cell-like tumor cells were enriched by flow cytometric cell sorting using E-cadherin (R&D Systems®) as a surface marker, and soft agar, spheroid and tumorigenicity assays to confirm cancer stem cell-like characteristics.
Results
mRNA expression of transcription factors OCT3/4 and SOX2 highly correlated in primary prostate tumor tissue samples. The number of OCT3/4 or SOX2 expressing cells was significantly increased in prostate cancer tissue compared to that in normal prostate or benign prostate hyperplasia tissue (p <0.05). When isolated from the DU145 and PC3 prostate cancer cell lines by flow cytometry, stem cell-like tumor cells expressing high OCT3/4 and SOX2 levels showed high tumorigenicity in immunodeficient mice. In vivo growth of the parental DU145 and PC3 prostate cancer cell lines was inhibited by short hairpin RNA knockdown of OCT3/4 or SOX2.
Conclusions
Data suggest that prostate tumor cells expressing pluripotent stem cell transcription factors are highly tumorigenic. Identifying such cells and their importance in prostate cancer growth could provide opportunities for novel targeting strategies for prostate cancer therapy.
Keywords: prostate, prostatic neoplasms, pluripotent stem cells, transcription factors, gene expression
Prostate cancer is the most common and second most lethal cancer in males in Western society.1 The origin of prostate cancer remains unclear and recent identification of prostate cancer stem cells led to the speculation that this minor fraction of tumor cells may initiate malignant transformation. Such tumor heterogeneity was previously attributed to the growth of multiple genetic subclones created by somatic mutation or variation in the tumor microenvironment.2 An alternative concept postulates that most tumors originate from common progenitor cells or their immediate progeny and give rise to heterogeneous cell populations as a result of differentiation.3 This hypothesis suggests that tumors contain a distinct cellular compartment that retains stem cell characteristics, namely multipotency, self-renewal and proliferation potential, which in turn drive oncogenesis and tumor progression.4 However, despite growing evidence for the existence of tumor initiating cells the stem cell properties of tumor initiating cells are still largely unresolved.5,6 Recent studies suggest that cancer cells have common molecular signatures that are similar to those of pluripotent embryonic stem cells.7,8 The core transcription factors of this stemness signature, including OCT3/4, SOX2, Nanog, c-Myc and Klf4, have been used to successfully reprogram differentiated somatic cells into pluripotent stem cells.9 We examined the hypothesis that reprogrammed pluripotent stem cell transcription factors may have pivotal roles in the maintenance of stem cell-like tumor initiating cells in prostate cancer cases.
MATERIALS AND METHODS
Human Cell Lines and Tissues
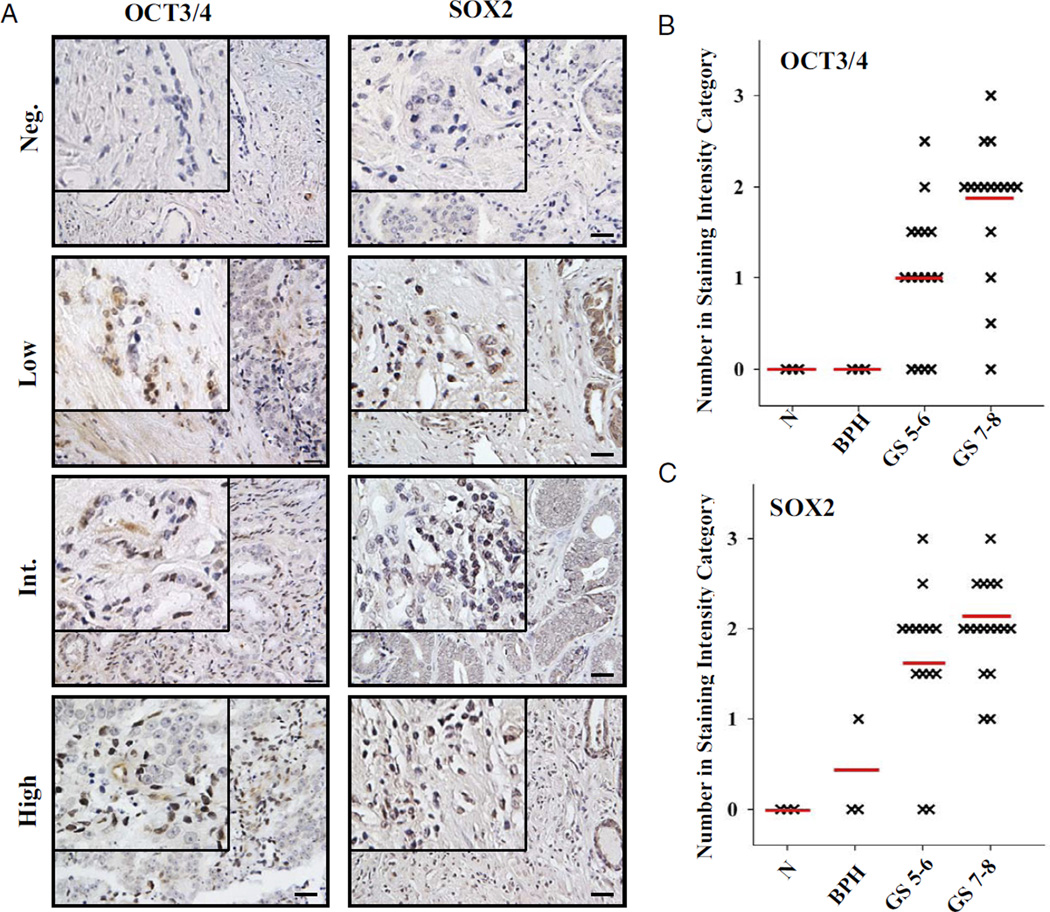
The human prostate cancer cell lines DU145 and PC3, and ES (National Stem Cell Bank, WiCell®) were cultured as recommended. Spheroid cultures were generated as described. PCR arrays (OriGene, Rockville, Maryland) contained cDNA from 48 prostate samples of men 48 to 87 years old with GS 5–9 (fig. 1). A normal prostate pool (Clontech™) comprised RNA from 32 white men 21 to 50 years old who died suddenly and were pathologically confirmed to have a normal prostate. Seven other samples were obtained from the Department of Urology at our institution under a human subject protocol with institutional review board approval. Informed consent was provided for specimen use according to the Declaration of Helsinki. Clinical diagnoses were confirmed at the Department of Pathology at our institution. Tissue arrays (Cybrdi, Rockville, Maryland) contained 40 duplicate prostate tissues, including cancerous, BPH and adjacent normal tissue, from men 20 to 82 years old with GS 5–8 (fig. 2).
Figure 1.
Representative pluripotent stem cell gene expression results in 3 independent experiments in prostate cancer tissue. A to E, semiquantitative RT-PCR using TissueScan prostate tissue panels shows normal prostate (N), PSs and ESs as controls. Band intensity was calculated using AlphaEase. Transcript levels were normalized to β-actin and are shown as relative units standardized to normal tissue pool. Statistical significance was considered at p <0.05. Asterisk indicates statistically different from normal. Double dagger indicates statistically different from ES. F, relative SOX2 mRNA levels vs relative OCT3/4 mRNA levels in tissue samples (Spearman correlation coefficient 0.4730, p <0.0001). Other possible transcription level combinations did not attain significance.
Figure 2.
Immunohistochemical staining for OCT3/4 and SOX2 in human prostate cancer tissues. A, representative results of at least 2 independent experiments reveal immunostaining for OCT3/4 and SOX2 using prostate tissue arrays. Neg., negative. Int., intermediate. Brown areas indicate positive nuclear staining. Reduced from ×20. Scale bar represents 70 µm. Insets, reduced from ×40. B and C, classification of different GS samples based on staining intensity. GS 5–6 and 7–8 tissues were significantly different than normal (N) and BPH tissue (2-tailed Mann-Whitney rank test p <0.05). Red lines indicate mean.
Immunofluorescence
Cells were grown on glass coverslips, fixed in 4% paraformaldehyde (Sigma®) and permeabilized with 0.2% Triton X-100/phosphate buffered saline. Cells were blocked with 10% goat serum/0.05% Triton X-100/phosphate buffered saline before incubation with primary antibodies, including anti-E-cadherin, anti-OCT3/4 (Santa Cruz Biotechnology), anti-SOX2 (Abcam®) and anti-β-catenin (BD Bio-sciences), overnight at 4C. Slides were washed, incubated with Alexa Fluor® 594 and/or 488 conjugated secondary antibodies and mounted using Vectashield® containing DAPI to counterstain nuclei. Cells were examined using an Axiophot™ microscope.
Histological and Immunohistochemical Analysis
Tissue arrays were stained with antibody to OCT3/4 (Abcam) or SOX2 (R&D Systems). To assess nuclear staining an arbitrary system was used by 3 independent reviewers blinded to sample identity. A total of 20 random fields were examined and the overall percent of positive nuclear staining was histologically scored on a scale of negative, low—less than 5%, intermediate—5% to 25%, high—26% to 50%, very high—51% to 75% and extremely high—76% to 100%.
Western Blotting
Equal amounts of total cellular extracts were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. OCT3/4, c-Myc, β-actin (Cell Signaling Technology), SOX2, Klf4, Nanog or E-cadherin were detected.
Isolation of RNA and RT-PCR Analysis
Semiquantitative RT-PCR was done using a TissueScan Prostate Cancer II panel (OriGene) of first strand cDNA from 48 human prostate samples and 7 tissue samples from patients with prostate cancer. ES cells, PSs and normal human prostate RNA served as controls. Products were analyzed for OCT4A pseudogenes, as described by Panagopoulos et al.10 Relative transcript levels were quantitated using AlphaEase® and normalized to β-actin in each case.
Flow Cytometry and Cell Sorting
OCT3/4 served as a marker to identify tumor cells, which were then stained with primary antibodies for E-cadherin, PODXL, SSEA1, SSEA4 (R&D Systems), CD44, CD9, CD24 (BD Pharmingen™), integrin-α2β1 (Abcam), ESA (BioMeda™) and CD133 (Miltenyi Biotech, Bergisch Glad-bach, Germany), and analyzed by flow activated cell sorting. For E-cadherin subpopulation sorting only the top 5% to 10% most brightly stained cells were selected for the positive population and only the bottom 5% to 10% most dimly stained cells were selected for the negative population.
Mouse Xenograft Models
Male C.B-17/IcrHsd SCID mice (Harlan™) were used under an institutional animal care approved protocol. Tumor cells were injected subcutaneously into the back of 5 mice per group. Tumors were measured with calipers weekly for the first 3 weeks and twice weekly thereafter. Tumor volume was calculated using the formula, 0.5ab2, where b represents the smaller of the 2 perpendicular indexes. Mice were sacrificed when moribund or when subcutaneous tumors were 15 mm in diameter.
shRNA OCT3/4 and SOX2 Knockdown
Plasmid vectors (OriGene) encoding OCT3/4 or SOX2 were used. For transfection 1.5 × 105 DU145 or PC3 cells per well were seeded in 6-well plates in medium without antibiotics the day before the experiment. Cells were washed and transfected using Lipofectamine™. Transfected cells were selected using puromycin, pooled and single cell cloned before Western blot analysis using 100 µg protein. Transfection efficiency was 75% to 80% and 25% to 30% of cloned cells were positive.
Prostate Spheroid Culture and Soft Agar Assay
Prostate spheroid cultures were made from DU145 and PC3 cells, and primary prostate tumor tissue according to the method of Shi et al.11 Flow sorted cells were seeded at 2,000 cells per well in a 6-well plate in 0.3% agarose (Sigma) in growth medium overlaid on a base of 0.6% agarose. Cultures were fed every 4 or 5 days for 2 to 3 weeks until the colonies were well formed. Colonies were stained with 0.5 mg/ml crystal violet, washed with 30% methanol, photographed and counted.
Statistical Analysis
For statistical analysis ANOVA was used to determine differences among normal, prostate spheroid and embryonic stem cell cultures (fig. 1). When differences were detected, we applied Newman-Keuls multiple comparison testing to determine statistical significance. The Spearman coefficient of rank correlation was also used to test for associations between the different transcription factors for patient data using Prism®, version 4. To compare OCT3/4 and SOX2 expression in tissue samples the 2-tailed Mann-Whitney rank test was applied with significance considered at p <0.05 (fig. 2).
RESULTS
Reprogramming Factors
Expression in prostate tumor cells
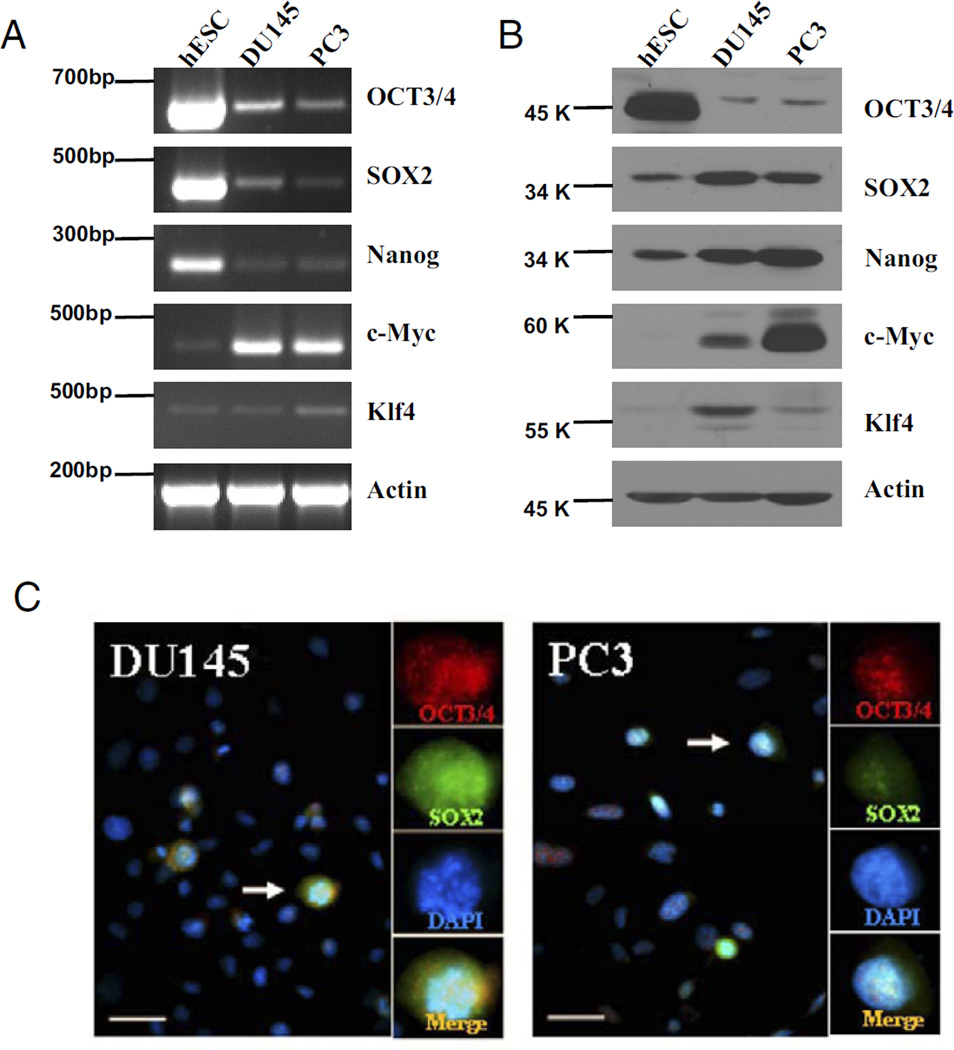
Semiquantitative RT-PCR was done to evaluate mRNA expression of the reprogramming factors OCT3/4, SOX2, Nanog, c-Myc and Klf4 in prostate cancer cell lines and ES cells. DU145 and PC3 cells expressed detectable levels of mRNA for OCT3/4, SOX2, Nanog, c-Myc and Klf4 (fig. 3, A). OCT 3/4 transcripts were confirmed and shown not to be those of the related pseudogenes. Compared to ES cells these cells showed relatively low OCT3/4, SOX2 and Nanog levels but expressed high levels of the oncogene c-Myc and the context dependent oncogene Klf4.
Figure 3.
Representative results show identification of stem cell-like tumor cells with pluripotent stem cell reprogramming factors in 3 independent experiments in prostate cancer cell lines. A and B, RT-PCR or Western blot detected OCT3/4, SOX2, Nanog, c-Myc and Klf4 expression in DU145 and PC3 cell lines with human embryonic stem cells (hESC) as control. Data were normalized to β-actin. C, DU145 and PC3 cells were immunostained for OCT3/4 (red areas), SOX2 (green areas) and DAPI (blue areas). Arrow indicates merged OCT3/4 and SOX2 staining (Merge). Scale bar represents 20 µm. Reduced from ×40.
Western blot analysis revealed lower OCT3/4 expression, and higher c-Myc and Klf4 expression in DU145 and PC3 cells than in ES cells (fig. 3, B). In contrast to RT-PCR, Western blot analysis of SOX2 and Nanog revealed similar or higher expression in these tumor cell lines than in ES cells. Double staining against OCT3/4 and SOX2 revealed that only a discrete population of tumor cells, representing about 5% to 10% of total cells, stained positive for each in the prostate tumor cell lines (fig. 3, C).
Detection in primary prostate tumor tissue
RT-PCR was applied to tumor tissue samples. Primary PSs and ES cells served as positive controls. All 5 transcription factors were increased in PS vs normal preparations (fig. 1, A to E). Statistical analysis showed that PS and ES cells were significantly different from normal (p <0.05) except for Klf4, for which ES did not attain significance.
mRNA transcripts for OCT3/4, SOX2, Nanog, c-Myc and Klf4 were increased in 28 of the 55 patient prostate cancer samples (more than 50%) compared to normal specimens. All possible combinations of transcription factors showed that significance was achieved only between OCT3/4 and SOX2 (Spearman correlation coefficient 0.4730, p <0.0001), suggesting a possible functional link between OCT3/4 and SOX2 in prostate cancer cases (fig. 1, F).
Immunohistochemical staining intensity of OCT3/4 and SOX2 was evaluated using a tissue microarray (fig. 2, A). Nuclear OCT3/4 and SOX2 staining was observed in 27 of 35 (77%) and 29 of 35 prostate tumor tissues (82%), respectively, with little to no staining in BPH and normal samples. The extent of nuclear positive staining in prostate tumor samples varied widely. Thus, data were stratified into 4 staining categories, including negative, low—less than 5%, intermediate—5% to 25% or high—26% to 50%. No samples showed more than 50% nuclear staining. Figure 2, A shows representative nuclear staining patterns. The number of OCT3/4 or SOX2 expressing cells was significantly lower in normal prostate and BPH samples than in prostate tumor tissues (p <0.05, fig. 2, B and C). In prostate tumor tissue samples an increasing number of OCT3/4 and SOX2 expressing cells was evident with increasing GS.
Prostate Stem Cell-Like Tumor Cells
Isolation
Potential cell surface markers (CD9, CD24, CD44, CD133, E-cadherin, ESA, PODXL, SSEA1, SSEA4 and integrin-α2β1) of prostate stem cell-like tumor cells for cell sorting were screened in DU145 and PC3 cells. OCT3/4 served as a marker to identify tumor cells with highly increased stem cell reprogramming factors. Results showed that OCT3/4 nuclear positive cells were exclusively located in colonies demonstrating classic morphology as malignant holoclones12 with high expression of the epithelial marker E-cadherin.13 Most OCT3/4 positive cells in the prostate tumor cell lines had high surface expression of E-cadherin (fig. 4, A). E-cadherin low or negative colonies contained few OCT3/4 positive tumor cells. PC3, which has decreased surface E-cadherin expression due to deletion of the α-catenin gene,14–16 also showed colocalized nuclear OCT3/4 staining with cytoplasmic E-cadherin staining. All other surface markers evaluated were detected but did not colocalize with OCT3/4 staining (data not shown).
Figure 4.
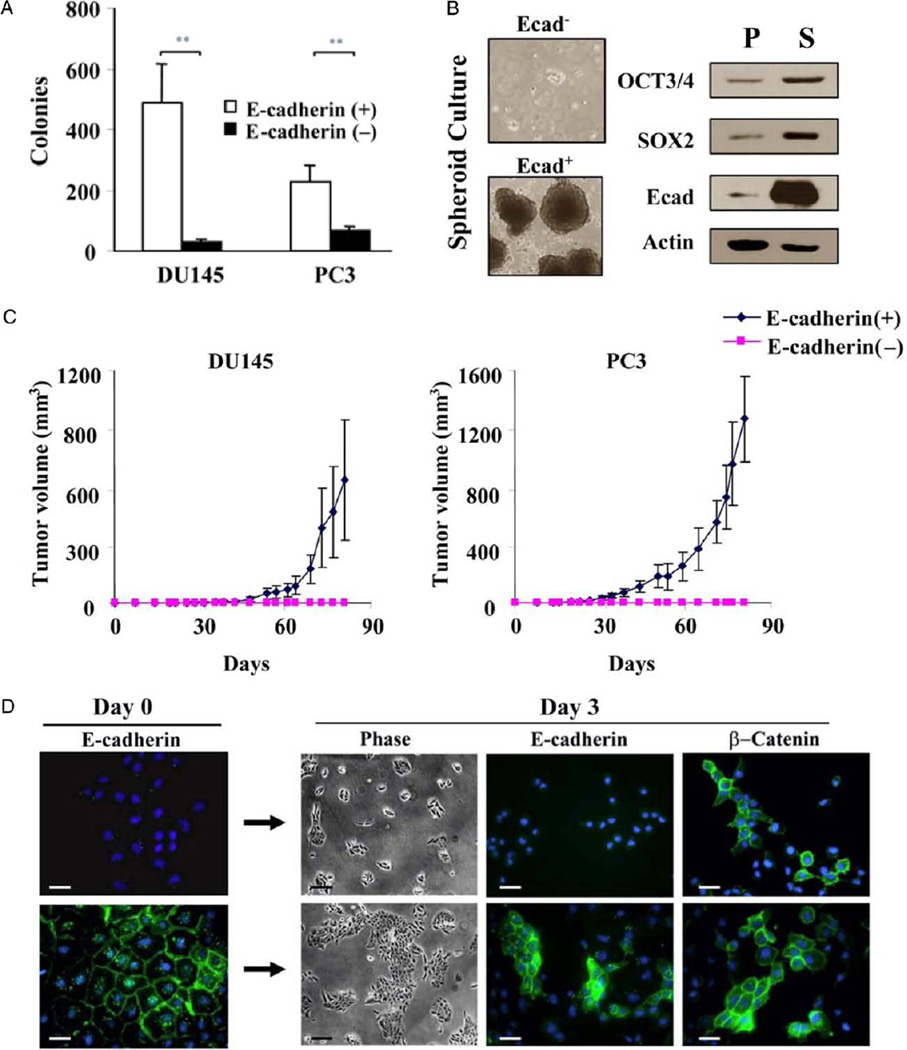
Isolation of stem cell-like prostate tumor cells. A, to identify surface markers for isolating stem cell-like cells from prostate cell lines DU145 and PC3 cells were immunostained for OCT3/4 (red areas), E-cadherin (Ecad) (green areas) and DAPI (blue areas). OCT3/4 and E-cadherin staining was also merged (arrow). Reduced from ×40. Scale bar represents 20 µm. B and C, for phenotypic analysis of DU145 and PC3 cells using double staining with E-cadherin and CD44 or integrin-α2β1 cells were gated on E-cadherin+ (green curves) or E-cadherin− (blue curves) population. FITC, fluorescein isothiocyanate. D and E, flow cytometry (FCS) analysis of DU145 and PC3 cells reveals E-cadherin (E-cad) expression. Isotype matched controls were used to set analysis gates for E-cadherin cell sorting. F, representative results of 3 independent experiments show that RT-PCR identified OCT3/4, SOX2, Nanog, c-Myc and Klf4 expression in E-cadherin+ and E-cadherin− cells isolated from DU145 and PC3 cells. Data were normalized to β-actin.
E-cadherin positive cells showed OCT3/4 positive staining, and high CD44 and integrin-α2β1 expression (fig. 4, B and C). Putative stem cell-like populations were isolated from each prostate cancer cell line by flow cytometry based on the E-cadherin expression profiles. In these studies 17% of DU145 cells and 5.5% of PC3 cells were positive for E-cadherin based on the isotype control (fig. 4, D and E). Highly purified cell subpopulations were obtained by isolating the top 5% to 10% of cells that highly expressed E-cadherin and the bottom 5% to 10% without E-cadherin expression. To confirm the enrichment of stem cell-like tumor cells after cell sorting the gene expression of pluripotent stem cell reprogramming factors in the E-cadherin+ and E-cadherin− populations was examined at the mRNA level (fig. 4, F). Only E-cadherin+ cells expressed all 5 essential pluripotent stem cell reprogramming factors, indicating that E-cadherin can serve as a distinct surface marker to isolate prostate tumor initiating cells.
Characterization of E-cadherin+ cells
Flow cytometry sorted E-cadherin+ cells formed more clones than their E-cadherin− counterparts (p <0.01, fig. 5, A). This difference was not due to the adhesion properties conferred by E-cadherin in positive cells since approximately equal numbers of E-cadherin+ and E-cadherin− cells attached upon initial plating. Sorted E-cadherin+ and E-cadherin− DU145 tumor cells were also cultured in serum-free medium containing epithelial growth factor and basic fibroblast growth factor under low attachment conditions. Results showed that only E-cadherin+ cells had the ability to form prostate spheroids (fig. 5, B). Western blot analysis of the spheroid culture generated from these E-cadherin+ cells further revealed increased levels of the stem cell reprogramming factors OCT3/4 and SOX2 compared to levels in the unsorted parental DU145 cell line (fig. 5, B).
Figure 5.
Functional analysis of stem cell-like prostate tumor cells. A, colony forming assay using sorted E-cadherin+ and E-cadherin− cells shows mean ± SD of 2 independent experiments. Asterisks indicate p <0.01. B, spheroid culture assay in E-cadherin (Ecad) sorted cells. Western blot of unsorted parental line (P) and E-cadherin+ spheroids (S) demonstrates OCT3/4, SOX2 and E-cadherin protein levels. Data were normalized to β-actin. Reduced from ×5. C, tumorigenic assay of sorted E-cadherin+ and E-cadherin− cells reveals representative mean ± SD tumor volume in 5 SCID mice per group in 2 independent experiments. D, immunostaining of E-cadherin− and E-cadherin+ DU145 cells with E-cadherin or β-catenin antibodies during 3-day differentiation. Phase contrast microscopy reduced from ×5 (scale bar indicates 100 µm). Fluorescence microscopy reduced from ×40 (scale bar indicates 20 µm).
To examine their tumorigenic potential flow cytometry sorted stem cell-like DU145 and PC3 prostate cancer cells were injected into SCID mice (fig. 5, C). Mice injected with E-cadherin+ stem cell-like tumor cells showed solid tumors within 30 days after cell inoculation (1 × 105 DU145 and 1 × 103 PC3). Mice injected with the same number of E-cadherin− nonstem cell-like cells of either prostate cancer cell line failed to show tumors during 80 days of observation.
To assess self-renewal capacity E-cadherin+ and E-cadherin− DU145 cells were evaluated by immunofluorescence analysis using E-cadherin and β-catenin antibodies (fig. 5, D). After 3 days in culture each population was positive for β-catenin but E-cadherin− cells proliferated slowly and remained negative for E-cadherin. In contrast, the E-cadherin+ cell population was highly proliferative and produced E-cadherin+ and E-cadherin− subpopulations, suggesting that asymmetrical division occurred and the E-cadherin+ cell population was enriched with stem cells.
In Vivo Tumorigenicity Inhibition
DU145 or PC3 prostate cancer cells were transfected with plasmids encoding shRNAs targeting OCT3/4 or SOX2, or with shRNA control plasmids. OCT3/4 and SOX2 shRNA sequences dramatically decreased the expression of their respective protein (fig. 6, A and B). Equal numbers of OCT3/4-shRNA, SOX2-shRNA or control shRNA transfected DU145 or PC3 cells were then inoculated into SCID mice. Cells infected with control shRNA plasmids showed detectable tumor growth within 30 days but mice injected with cells infected with OCT3/4 shRNA or SOX2 shRNA failed to show detectable tumors during 10 weeks of observation (fig. 6, C and D).
Figure 6.
OCT3/4 or SOX2 knockdown tumorigenicity in DU145 and PC3 cells. A and B, Western blot shows decreased OCT3/4 or SOX2 protein levels in human DU145 and PC3 prostate cancer cells transfected with shRNA. Unsorted DU145 (1 × 105) or PC3 (3 × 105) cells were subcutaneously injected in SCID mice. C and D, mean ± SD tumor volume after OCT 3/4, SOX2 or control shRNA treatment.
DISCUSSION
A major tenet of the cancer stem cell theory is that a discrete subset of cells in a tumor has the capability of self-renewal and multipotency, which gives rise to a heterogeneous population of cancer cells.4 Our results show that all 5 critical pluripotent stem cell transcription factors required to reprogram differentiated somatic cells (OCT3/4, SOX2, Nanog, c-Myc and Klf4) were identified in prostate tumor cell lines as well as in primary prostate tumor tissue. Enriched prostate stem cell-like tumor cells showed the holoclonal phenotype, known to contain self-renewing tumor initiating cells.12 These cells underwent asymmetrical division, preferentially formed prostate spheroids and had high proliferative capacity in vitro. This discrete stem cell-like tumor cell population had strong tumorigenicity in SCID mice. Consistent with other reports identifying prostate tumor initiating cells,12,17,18 these results confirm the existence of stem cell-like tumor cells in prostate cancer cell lines and to our knowledge identify such cells for the first time in primary tumor tissue from patients.
Based on the marker used for isolation several candidate populations of prostate stem/progenitor cells have been reported, including cells that preferentially express the surface molecules CD44, integrin-α2β1 or CD133.19–21 In DU145 and PC3 cells CD44 expression is exceedingly high at about 90% and about 100%, respectively, making it difficult to isolate the stem cell population using only this marker. Thus, investigators have typically used CD44 combined with other markers, including CD24, CD133 and integrin-α2β1, although with mixed results.19–21
In our study E-cadherin, which showed distinguishable expression in the DU145 and PC3 cell lines (about 17% and about 5.5%, respectively), served as a solitary, reliable, discrete marker to isolate the stem-like cell population from prostate cancer cell lines. This E-cadherin+ population also showed high expression of CD44 and integrin-α2β1, another 2 surface markers associated with purified stem cell populations, compared to that of the E-cadherin− population.
The cell adhesion molecule E-cadherin has an important role in maintaining the undifferentiated stage of ES cancer stem cells.22 It is down-regulated through EMT. E-cadherin down-regulation is thought to correlate with highly invasive tumors and poor prognosis in prostate cancer cases23,24 but several studies have failed to support this notion.25–28 For example, high E-cadherin expression was observed in prostate carcinoma bone metastasis, suggesting the transient nature of EMT.25–28 Other studies indicate that malignant prostate tumor cells up-regulate E-cadherin upon contact with host cells at the metastatic site.28 These data suggest that tumor cells only transiently down-regulate E-cadherin for invasion and E-cadherin is re-expressed after metastatic seeding.29 Other groups reported that E-cadherin is highly expressed in stem cell enriched holoclonal carcinoma cells13 and tumor spheres.30 Our findings are consistent with the mentioned evidence that E-cadherin+ cells in prostate tumor cell lines may have incomplete EMT and represent a stem cell-like subpopulation. Complete EMT cells (E-cad-herin− cells) may eventually lose self-renewal and proliferative capacity.
CONCLUSIONS
Our findings strongly support the existence of stem cell-like tumor initiating cells in prostate cancer pre-clinical models and in patient prostate cancer tissue. Two factors (SOX2 and OCT3/4) correlated closely when assessed by mRNA expression. Cells enriched for these factors formed macroscopic tumors when injected into SCID mice. Treatment with shRNA against SOX2 or OCT3/4 prevented tumor growth in vivo. The observation that a pluripotent stem cell transcriptional balance is important for survival of these tumor initiating cells and the identification of such cells in the tumors of patients with prostate cancer could prove highly relevant to the future design of novel targeted therapy for prostate cancer.
Supplementary Material
ACKNOWLEDGMENTS
Drs. Nao Terada and Steve Dalton provided advice and critiqued the manuscript.
Study received institutional review board, and institutional animal care and use committee approval.
Supported by startup funds from the Department of Urology, University of Florida (ZS), American Cancer Society (ZS) and Ocala Royal Dames for Cancer Research (ZS), McKnight Brain Institute (DAS) and National Institutes of Health/ National Heart, Lung and Blood Institute Grant HL70143 (DAS).
Abbreviations and Acronyms
- BPH
benign prostate hyperplasia
- DAPI
4,6-diamidino-2-phenylindole
- EMT
epithelial-to-mesenchymal transition
- ES
human embryonic stem cell line H9
- GS
Gleason score
- PS
primary prostate tumor spheroid cultured cell
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SCID
severe combined immunodeficiency
- sh
short hairpin
Footnotes
Financial interest and/or other relationship with CureVac.
Supplementary material for this article can be obtained at http://www.urology.ufl.edu/JOU0510.pdf.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 3.Lawson DA, Xin L, Lukacs R, et al. Prostate stem cells and prostate cancer. Cold Spring Harb Symp Quant Biol. 2005;70:187. doi: 10.1101/sqb.2005.70.003. [DOI] [PubMed] [Google Scholar]
- 4.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 5.Rossi DJ, Weissman IL. Pten, tumorigenesis, and stem cell self-renewal. Cell. 2006;125:229. doi: 10.1016/j.cell.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Widschwendter M, Fiegl H, Egle D, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Porath I, Thomson MV, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong DJ, Liu H, Ridky TW, et al. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibro-blasts by defined factors. Cell. 2007;131:861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Panagopoulos I, Moller E, Isaksson M, et al. A PCR/restriction digestion assay for the detection of the transcript variants 1 and 2 of POU5F1. Genes Chromosomes Cancer. 2008;47:521–529. doi: 10.1002/gcc.20555. [DOI] [PubMed] [Google Scholar]
- 11.Shi X, Gipp J, Bushman W. Anchorage-independent culture maintains prostate stem cells. Dev Biol. 2007;312:396. doi: 10.1016/j.ydbio.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Chen X, Calhoun-Davis T, et al. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 2008;68:1820. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 13.Locke M, Heywood M, Fawell S, et al. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65:8944. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 14.Morton RA, Ewing CM, Nagafuchi A, et al. Reduction of E-cadherin levels and deletion of the α-catenin gene in human prostate cancer cells. Cancer Res. 1993;53:3585. [PubMed] [Google Scholar]
- 15.Ewing CM, Ru N, Morton RA, et al. Chromosome 5 suppresses tumorigenicity of PC3 prostate cancer cells: correlation with re-expression of α-catenin and restoration of E-cadherin function. Cancer Res. 1995;55:4813. [PubMed] [Google Scholar]
- 16.Inge LJ, Rajasekaran SA, Wolle D, et al. α-Catenin overrides src-dependent activation of β-catenin oncogenic signaling. Mol Cancer Ther. 2008;7:1386. doi: 10.1158/1535-7163.MCT-07-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 18.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 19.Dubrovska AS, Kim RJ, Salamone JR, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106:268. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurt EM, Kawasaki BT, Klarmann GJ, et al. CD44+ CD24− prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrawala LT, Calhoun-Davis R, Schneider-Broussard, et al. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 22.Eastham AM, Spencer H, Soncin F, et al. Epitheial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 23.Umbas R, Schalken JA, Aalders TW, et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52:5104. [PubMed] [Google Scholar]
- 24.Ikonen T, Matikainen M, Mononen N, et al. Association of E-cadherin germ-line alterations with prostate cancer. Clin Cancer Res. 2001;7:3465. [PubMed] [Google Scholar]
- 25.Rubin MA, Mucci NR, Figurski J, et al. Cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum Pathol. 2001;32:690. doi: 10.1053/hupa.2001.25902. [DOI] [PubMed] [Google Scholar]
- 26.Saha B, Arase A, Imam SS, et al. Overexpression of E-cadherin and β-catenin proteins in metastatic prostate cancer cells in bone. Prostate. 2008;68:78. doi: 10.1002/pros.20670. [DOI] [PubMed] [Google Scholar]
- 27.Tsukino H, Kuroda Y, Imai H, et al. Lack of evidence for the association of E-cadherin gene polymorphism with increased risk or progression of prostate cancer. Urol Int. 2004;72:203. doi: 10.1159/000077115. [DOI] [PubMed] [Google Scholar]
- 28.Yates CC, Shepard CR, Stolz DB, et al. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer. 2007;96:1246. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaffer CL, Brennan JP, Slavin JL, et al. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 30.Lang SH, Sharrard RM, Stark M, et al. Prostate epithelial cell lines form spheroids with evidence of glandular differentiation in three dimensional Matrigel cultures. Br J Cancer. 2001;85:590. doi: 10.1054/bjoc.2001.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.