Abstract
Alzheimer’s disease is a progressive, neurodegenerative disorder that develops within the limbic system, spreading radially into anatomically linked brain association areas as the disease progresses. Analysis of temporal-lobe association of neocortex-derived extracellular fluid and cerebrospinal fluid from Alzheimer’s disease patients shows an abundant presence of micro-RNA (miRNA), including the proinflammatory miRNA-146a and miRNA-155. Using a novel and highly sensitive LED-Northern dot-blot focusing technique, we detected the secretion of potentially pathogenic amounts of miRNA-146a and miRNA-155 from stressed human primary neural cells. A conditioned medium containing miRNA-146a and miRNA-155 was found to induce Alzheimer-type gene expression changes in control brain cells. These included downregulation in the expression of an important repressor of the innate immune response, complement factor H (CFH). These effects were neutralized using anti-miRNA strategies. Anti-miRNA-based therapeutics may provide a novel and efficacious treatment to stem the miRNA-mediated spreading of inflammatory signaling involved in Alzheimer’s disease.
Keywords: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, Alzheimer’s disease, digoxigenin, inflammatory neurodegeneration, locked nucleic acids, micro-RNA, miRNA-146a, miRNA-155, spreading
Introduction
Alzheimer’s disease shows a characteristic spreading of inflammatory neuropathology. The disease develops deep in the brain in the hippocampal region and then advances radially into more peripheral neocortical association areas; this pathogenic spreading is progressive and advances with age [1–5]. Cell-to-cell propagation and ‘staging’ have been attributed to various age-related physiological factors such as hypoxia and cerebrovascular factors, brain cell-secreted cytokines, protein aggregates such as tau and Aβ peptides or their fragments, and other small-molecule pathogenic factors including blood-borne neurotoxic elements from the environment [2–8]. These data cumulatively support the hypothesis that the paracrine effects of stressed constituent cells of the human neurovascular unit may contribute toward the ‘spreading events’ characteristic of progressive neurodegenerative disorders [1–3,5,8]. In the current study, we used a novel, highly sensitive, LED-Northern dot-blot technique, and provide four complementary lines of evidence that suggest that micro-RNAs (miRNA), small, highly soluble, 22-nucleotide noncoding RNAs, are involved in the spreading of Alzheimer’s disease. The primary mode of miRNA action is to recognize (by complementarity), and bind to, specific ribonucleotide sequences in the 3′untranslated region (3′-UTR) of target mRNAs, and in doing so downregulate their expression [9–18]. We first show that two proinflammatory miRNAs, miRNA-146a and miRNA-155, are abundant in neocortical extracellular fluid (ECF) and in the cerebrospinal fluid (CSF) of Alzheimer’s disease patients. Next, we show that cytokine tumor necrosis factor-α (TNF-α) and Aβ42-peptide-stressed human neuronal-glial (HNG) cocultures secrete miRNA-146a and miRNA-155, and that a conditioned medium (CM), containing miRNA-146a and miRNA-155, induces inflammatory gene expression in control HNG cells. It is known that upregulated miRNA-146a and miRNA-155 both target and downregulate the expression of complement factor H (CFH), an important regulator and integrator of the innate immune response [9–19]. CFH downregulation is implicated in driving inflammatory degeneration not only in Alzheimer’s disease but also in Down’s syndrome and in rare human prion diseases [10–12]. We further show that anti-miRNA-146a (AM-146a) and anti-miRNA-155 (AM-155) suppress these proliferation effects, suggesting that anti-miRNA (AM) strategies may be useful in the prevention of miRNA-mediated disease spreading.
Materials and methods
Reagents, Alzheimer’s disease tissues, and human neuronal-glial cocultures
The reagents used in these experiments were obtained from independent commercial suppliers and were used without further purification [15–19]. 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC; 161462 Sigma-Aldrich, St Louis, Missouri, USA) was used as described [20–22]. Human recombinant TNF-α (T6674) and Aβ42 peptides (D2534; Sigma-Aldrich) were also used as described previously [15–18]. Alzheimer’s disease and age-matched control human temporal lobe and association neocortical tissue and CSF were obtained from brain and tissue repositories including the Institute for Memory Impairments and Neurological Disorders and the University of California at Irvine; tissues were analyzed for total miRNA using miRNA arrays and LED-Northern blots and CFH abundance using western analysis [8,9,12–14]. All Alzheimer’s disease cases were adult and all brain tissues were from the temporal lobe; the mean (±1 SD) age of the control brain group (N=5) was 72.1±6.5 years and the mean post-mortem interval (PMI; death to brain freezing interval) was 2.2 h; the mean age of the Alzheimer’s disease group (N=5) was 73.2±5.5 years and the mean PMI was 2.15 h. There were no significant differences in the age, sex, or PMI between the Alzheimer and the control tissue groups. HNG cocultures were cultured as described previously and used at 2 weeks of culture [5,10–15].
Extraction of total RNA and protein and quality control
Total RNA and proteins were isolated simultaneously using TRIzol (Invitrogen, Carlsbad, California, USA) [9,12–14]. RNA quality was assessed using an Agilent Bioanalyzer 2100 (Lucent Technologies, Murray Hill, New Jersey, USA; Caliper Technologies, Mountain View California, USA) [15,16]. Typically, 1 µl of total RNA sample was loaded on an RNA chip (6000 Nano Labchip; Caliper Technologies) and analyzed for quality control; RNA integrity numbers values were typically between 8.1 and 9.1 [14–18]. Protein concentrations were determined using the dotMETRIC microassay (sensitivity 0.3 ng protein/ml; Chemicon-Millipore, Billerica, Massachusetts, USA) [12,18].
miRNA array, LED-Northern dot-blot analysis, RT-PCR, and anti-miRNAs
miRNA labeling, hybridization, miRNA arrays, and reverse transcription polymerase chain reaction analysis were performed as described previously [8,9,12–19]. LED-Northern dot-blot analysis was performed using a modified Bio-Dot microfiltration blotting device (LED= LNA, EDC, and DIG; locked nucleic acids – EDC – digoxigenin; detection limit=0.05 fM of a single miRNA species; apparatus #170-6545, BioRad Life Science Research, Hercules, California, USA) [10–18,23]. LED-Northern dot blots are a significant advancement over classical Northern blotting techniques because they utilize LNA-stabilized miRNAs or anti-miRNAs(AMs) covalently linked to a nylon-based membrane matrix (using EDC) and are probed using DIG-labeled small RNAs with fluorescent reporters [19–22]. miRNA and/or AMs, as locked nucleic acid (LNA) oligonucleotides, included a control miRNA-183 (5′-TATGGCACTGGTAGAATTCACT-3′), anti-miRNA-155 (AM-155; 5′-AATTACGATTAGCACTATCCCCA-3′), and a scrambled control AM-155 (AM-155sc; 5′-TTAACATTAGACGATATCCCACC-3′) were purchased from Applied Biosystems/Ambion, Austin, Texas, USA or Exiqon Inc., Woburn, Massachusetts, USA [11,16], and were used at a 5–20 nM ambient concentration that was replenished at every change of ABM (see above; Lonza, Basel, Switzerland) for a total treatment time of 12 and 36 h after the induction of TNF-α + Aβ42 peptide. miRNA-146a and AM-146a sequences and their usage have been described previously [12–17].
Western analysis of CFH and β-actin in Alzheimer ECF and CSF
ECF samples were derived from high-speed supernatants of control and Alzheimer brain homogenates, and CSF was obtained from archived short PMI samples [12,17]. Western immunoblots were performed for the quantification of CFH and β-actin protein in control and Alzheimer’s tissues using human-specific primary antibodies directed against the control protein marker β-actin (3598–100; Sigma-Aldrich) or human CFH (H-7; sc-166613 H-5; sc-166608; Santa Cruz Biotechnologies, Santa Cruz, California, USA) [15–19].
Statistical analysis and data interpretation
All miRNA array and LED-Northern dot blot data were analyzed as described previously [5–7,10–18]. Statistical procedures for protein (ELISA and western analysis) abundance were performed using a two-way factorial analysis of variance (P, ANOVA) using programs and procedures in the SAS language (Statistical Analysis Institute, Cary, North Carolina, USA) [16–19]. Only P-values less than 0.05 (ANOVA) were considered to be statistically significant. Figures were generated using Excel 2011 (Microsoft, Redmond, Washington, USA) and Photoshop CS2 version 9.0.2 (Adobe, San Jose, California, USA).
Results
Upregulation of miRNA-146a and miRNA-155 in Alzheimer’s disease ECF and CSF
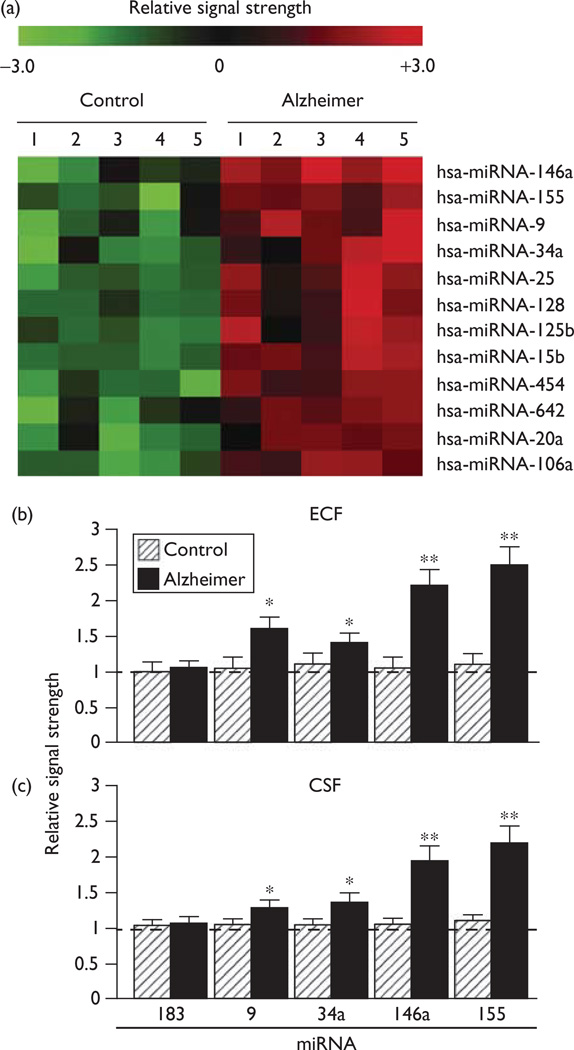
There were no significant differences in age, PMI, or RNA yield or quality between either the control or the Alzheimer brain tissue or CSF groups. Of the 12 different homosapien micro-RNAs (hsa-miRNAs) measured to be significantly upregulated (Fig. 1) in ECF or CSF, miRNA-146a, and miRNA-155 showed the greatest significance in upregulation compared with age-matched controls (P<0.01, ANOVA). At present, we cannot exclude the participation of other human brain-enriched miRNAs or other small-non-coding RNAs that may also contribute toward the proinflammatory neuropathological spreading mechanisms that help define the Alzheimer’s disease process. Interestingly, miRNA-9 and miRNA-125b were also found to be upregulated (P<0.05, ANOVA), and these two miRNAs also have binding sites in the CFH mRNA 3′-UTR (W.J. Lukiw, S. Bhattacharjee; unpublished observations) [24]. In summary, miRNA analysis showed consistent increases in miRNA-146a and miRNA-155 in five short PMI Alzheimer’s disease ECF and CSF samples when compared with five age-matched controls, and the proinflammatory miRNA-146a and miRNA-155 showed the greatest significance (Fig. 1).
Fig. 1.
(a) miRNA contents of human extracellular fluid (ECF) and cerebrospinal fluid (CSF) show an abundant representation of miRNA-146a, miRNA-155, and other Alzheimer’s disease-enriched small-non-coding RNAs (sncRNAs) compared with age-matched controls [10–19]. miRNA array data for the 12 most significantly upregulated miRNAs in control and AD ECF are shown in (a) and quantified in (b); similarly, significantly upregulated miRNAs in control and AD CSF are shown in (c). ECF and CSF miRNA profiles were derived from high-speed supernatants of previously characterized age-matched control and AD brains [17–19]. PMIs for age-matched control or AD human brain tissues and CSF were all less than 2.2 h; all tissues were from the superior temporal lobe neocortex; the study group (controls N=5; AD N=5) of tissues showed no significant differences in age (72.1±6.5 vs. 73.2±5.5 years, P<0.85), PMI (mean 2.1±2.1 vs. 2.2±1.4 h, P<0.95), RNA A260/280 indices (2.10±0.5 vs. 2.05±0.4, P<0.95), or RNA 28S/18S (1.5 vs. 1.45, P<0.92) in age-matched control and AD, respectively. RNA integrity numbers (RIN) ranged between 8.1 and 9. No significant differences were found in the total RNA yield between the control and the AD groups; the dashed horizontal line at 1.0 indicates the control miRNA-183 signal in the ECF or CSF for ease of comparison. miRNAs were analyzed by LC Sciences (Houston Texas, USA) using miRNA array panels containing 1898 individual human miRNA targets; interestingly, miRNA-9, miRNA-125b, miRNA-146a, and miRNA-155 all have binding sites within the CFH mRNA 3′-UTR. AD, Alzheimer’s disease; ANOVA, analysis of variance; CFH, complement factor H; miRNA, micro-RNA; PMI, postmortem interval. *P<0.05; **P<0.01 (ANOVA) [10–18].
Upregulation of miRNA-146a and miRNA-155 in stressed human neuronal-glial primary cocultures
We next studied the cytokine TNF-α-mediated and Aβ42 peptide-mediated induction of proinflammatory miRNAs in a 2-week-old primary culture of HNG cells (Fig. 2a) using a LED-Northern technique (Fig. 2b). Using 5S RNA and miRNA-183 in the same sample as an internal control, the level of miRNA-146a was found to increase 2.4- and 4.2-fold over zero time controls 12 and 36 h after stress treatment, and miRNA-155 was found to increase 3.8- and 5.1-fold over zero time controls 12 and 36 h after stress treatment, and these results were highly significant (Fig. 2c). Bioinformatics analysis indicated that miRNA-146a and miRNA-155 have overlapping target recognition sites within the CFH mRNA 3′-UTR; both miRNA-146a and miRNA-155 are known to downregulate CFH expression through an miRNA-CFH mRNA 3′-UTR mechanism (Fig. 2d) [10–15,24].
Fig. 2.
(a) HNG cells 2 weeks in culture; ×20; cells are triple stained using antibodies to glial fibrillary acidic protein (GFAP), a glial-specific marker (green fluorescence; λmax=556 nm), with βTUBIII, a neuron-specific marker (red; λmax = 702 nm), and Hoescht 33258 to highlight cellular nuclei (blue; λmax=461 nm) [12–15]; (b) representation of a typical result, this panel depicts LED-Northern dot blots of miRNA-146a and miRNA-155 in control and stressed HNG cells using unchanging 5S RNA and miRNA-183 as internal control markers [10]; a ‘halo’ around each dot appears to be associated with increased sensitivity of the LED Northern protocol (unpublished observations). (c) graphed results of 5S RNA, miRNA-183, miRNA-146a, and miRNA-155 in stressed HNG cells indicating relative levels; the dashed horizontal line at 1.0 indicates 5S RNA control levels for ease of comparison (loaded at 1/20th the concentration of the miRNAs); N=5; *P<0.05; **P<0.01 (ANOVA); (d) overlapping miRNA-155 and miRNA-146a high-affinity binding sites in the 232 nucleotide CFH mRNA 3′-UTR (Genbank BC142688); an energy of association for the miRNA–mRNA of less than −22 kcal/mol defines an exceptionally stable miRNA–mRNA interaction and a potential CFH mRNA 3′-UTR miRNA-regulatory control (MiRC) region 5′-TTTAGTATTAA-3′(white letters on a black background) [24]; ‘|’ indicates a full hydrogen bond between the miRNA and CFH mRNA 3′-UTR and ‘:’ indicates a partial hydrogen bond; vertical black arrows show an miRNA-146a–miRNA-155 interaction domain in the CFH mRNA 3′-UTR (highlighted in gray; see text) [16,17,24]. ANOVA, analysis of variance; CFH, complement factor H; HNG, human neuronal-glial; miRNA, micro-RNA.
Downregulation of CFH protein expression in neuronal-glial primary cocultures after treatment with a conditioned medium
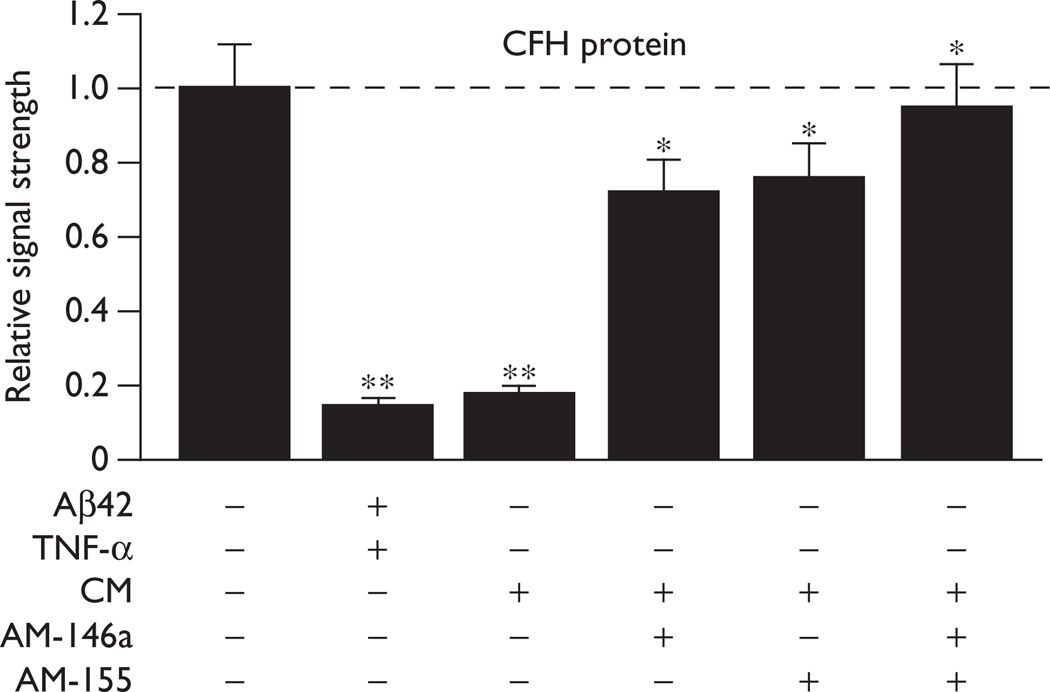
We next examined the effects of this miRNA-146a and miRNA-155-containing CM from 36-h stressed primary cultures of HNG cells (Fig. 2c) on CFH expression in control HNG cells. Importantly, the CM was filtered to remove TNF-α and Aβ42 peptide, and major constituents of the CM included miRNA-146a and miRNA-155 (data not shown). Incubation of control HNG cells with either Aβ42 + TNF-α or this ‘pathogenic’ CM extract downregulated a known miRNA-146a and miRNA-155 mRNA target (Fig. 2d), CFH, to 0.15- and 0.18-fold the control values after 36 h (Fig. 3; three leftmost panels). This effect was suppressed by adding AM-146a or miRNA-155 (AM-155) to the neuronal-glial cells after the addition of the CM, restoring CFH expression to between 72 and 76% of the control (Fig. 3). AM-146a and AM-155 together restored CFH protein levels to 94% of the control. These observations indicated that miRNAs within the CM were able to induce CFH deficits known to lead to proinflammatory gene expression changes in human brain cells, an effect that was blocked using AM strategies (Fig. 3). ECF and CSF from Alzheimer’s disease patients have also been shown to induce proinflammatory gene expression programs in neuronal-glial cells, including the specific upregulation of miRNA-146a and downregulation of CFH [10–18] (W.J. Lukiw, S. Bhattacharjee; unpublished observations). In sum, the results suggest that miRNAs carried by the CSF or ECF in vivo or CM in vitro promote the propagation of innate immune and pathogenic proinflammatory signaling known to drive the Alzheimer’s disease process.
Fig. 3.
Results of a Western analysis of complement factor H (CFH) protein levels in control and stressed HNG cells in primary culture, and rescue to homeostatic levels by AM-146a and AM-155; a dashed horizontal line at 1.0 indicates the control CFH protein levels for ease of comparison; stress increases both miRNA-146a and miRNA-155, thus reducing the expression of CFH [9–19]; conditioned medium (CM) contained only trace levels of TNF-α and the Aβ42 peptide; the major small RNA constituents of the CM were miRNA-146a and miRNA-155 (data not shown) [14–16]; AM-146a and AM-155, either separately or together, reverse this effect and restore CFH back to homeostatic levels; N=5; ANOVA, analysis of variance; HNG, human neuronal-glial; miRNA, micro-RNA; TNF-α, tumor necrosis factor-α. *P<0.05; **P<0.01 (ANOVA).
Discussion
The spreading mechanism for Alzheimer’s disease inflammatory signaling throughout the higher function regions of the human brain is not well understood. Our results contribute four new observations to our understanding of the progressive and propagating nature of Alzheimer’s disease inflammatory neuropathology: (a) that potentially pathogenic miRNAs are abundant in Alzheimer’s disease ECF and CSF; (b) that the growth medium of stressed primary HNG cells contains elevated miRNA levels; (c) that the miRNA-containing CM from stressed primary HNG cells can downregulate known proinflammatory regulatory glycoproteins such as CFH; and (d) that AM strategies can suppress the phenomenon of miRNA-mediated inflammatory spreading.
The initial work in this report indicates that aged, Alzheimer’s disease-affected human brain cells appear to be exporting substantial quantities of miRNAs into the ECF and CSF, and that the complexities of these miRNAs – including the inflammation-relevant miRNA-146a and miRNA-155 – are known contributors to altered innate immune responses and inflammatory neuropathology [10–19]. The presence of stable miRNAs in Alzheimer’s disease, ECF, and CSF at high enough concentrations to transmit inflammatory signaling suggests that miRNA bioactivities may extend well outside of the cell cytoplasm to further mediate homeostatic or pathogenic interneural and cell–cell communication [24–26]. Extracellular microvesicles and exosomes containing miRNAs suggest one plausible disease-proliferating mechanism [25]. Whether these externalized miRNAs are completely contained within these vesicles or exosomes or are secreted as free oligonucleotides is open to speculation [24–26]. Complex miRNA secondary and tertiary structures and/or miRNA-binding proteins may prolong the half-life of miRNA [18,25]. Using advanced LED-Northern type assays, it will be interesting to test whether other human neurological diseases with a progressive, age-related nature may use pathogenic miRNAs, in a paracrine manner, to spread pathological signaling throughout neighboring neural cells and throughout the CNS and other cell and tissue systems. Finally, it will be of interest to determine whether lower amounts of TNF-α and Aβ42 peptide or ECF, CSF, or CM preparations over longer incubation periods can induce similar pathogenic miRNA generation, what individual brain cell types might be contributing to this multiple pathogenic miRNA output, and whether different brain cell types secrete a different repertoire of pathogenic miRNAs [13–17]. This knowledge may be profitably utilized with the aid of AM and related pharmacological approaches that have not yet been considered in the effective management of brain disease progression.
Conclusion
This study provides evidence (a) that the potentially pathogenic and proinflammatory miRNA-146a and miRNA-155 are abundant in Alzheimer’s disease ECF and CSF; (b) that these miRNAs are secreted by human brain cells stressed with factors known to be elevated in Alzheimer’s disease brain; (c) that a conditioned medium (CM) containing miRNA-146a and miRNA-155 will induce inflammatory gene expression in control brain cells, an effect that is mediated in part by the downregulation of the important immune system regulator CFH; and (d) that these effects are suppressed using AM. AM strategies may therefore be useful in the prevention of miRNA-mediated pathogenic spreading not only in Alzheimer’s disease but perhaps also in other progressive, inflammatory degenerations of the human nervous system.
Acknowledgments
Thanks are extended to Drs E. Head, D.R.C. McLachlan, W. Poon, G. Tejada, and T. Saing for human brain tissues or extracts, to Drs P. Dua, C. Eicken, and C. Hebel for the miRNA array work and initial data interpretation, and to D. Guillot and A.I. Pogue for expert technical and bioinformatics assistance. Additional human brain tissues were provided by the Memory Impairments and Neurological Disorders (MIND) Institute and the University of California, Irvine Alzheimer’s Disease Research Center (UCI-ADRC; NIA P50 AG16573). Research on miRNA in the Lukiw laboratory was supported through Translational Research Initiative Grants from LSUHSC, Alzheimer Association Investigator-Initiated Research Grant IIRG-09-131729, and NIA Grants AG18031 and AG038834. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
These studies were presented in part at the ‘Eighth Annual microRNA in Human Disease and Development’ conference, Cambridge Massachusetts, USA symposium 12–13 March 2012.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cui JG, Hill JM, Zhao Y, Lukiw WJ. Expression of inflammatory genes in the primary visual cortex of late-stage Alzheimer’s disease. Neuroreport. 2007;18:115–159. doi: 10.1097/WNR.0b013e32801198bc. [DOI] [PubMed] [Google Scholar]
- 2.Bancher C, Braak H, Fischer P, Jellinger KA. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neurosci Lett. 1993;162:179–182. doi: 10.1016/0304-3940(93)90590-h. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, et al. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23:S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Lim HS, Masliah E, Lee HJ. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res. 2011;70:339–348. doi: 10.1016/j.neures.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Cui JG, Lukiw WJ. Natural secretory products of human neural and microvessel endothelial cells: implications in pathogenic ‘spreading’ and Alzheimer’s disease. Mol Neurobiol. 2006;34:181–192. doi: 10.1385/MN:34:3:181. [DOI] [PubMed] [Google Scholar]
- 6.McLachlan DR, Lukiw WJ, Kruck TP. New evidence for an active role of aluminum in Alzheimer’s disease. Can J Neurol Sci. 1989;16:490–497. doi: 10.1017/s0317167100029826. [DOI] [PubMed] [Google Scholar]
- 7.Lukiw WJ. Evidence supporting a biological role for aluminum in chromatin compaction and epigenetics. J Inorg Biochem. 2010;104:1010–1012. [PubMed] [Google Scholar]
- 8.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 9.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kB-dependent induction of miRNA-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YY, Alexandrov PN, Pogue AI, Zhao Y, Bhattacharjee S, Lukiw WJ. miRNA-155 upregulation and complement factor H deficits in Down’s syndrome. Neuroreport. 2012;23:168–173. doi: 10.1097/WNR.0b013e32834f4eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukiw WJ, Dua P, Pogue AI, Eicken C, Hill JM. Up-regulation of micro RNA-146a (miRNA-146a), a marker for inflammatory neurodegeneration, in sporadic Creutzfeldt-Jakob disease (sCJD) and Gerstmann-Straussler-Scheinker (GSS) syndrome. J Toxicol Environ Health A. 2011;74:1460–1468. doi: 10.1080/15287394.2011.618973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ. Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem. 2010;285:38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YY, Cui JG, Dua P, Pogue AI, Bhattacharjee S, Lukiw WJ. Differential expression of miRNA-146a-regulated inflammatory genes in human primary neural, astroglial and microglial cells. Neurosci Lett. 2011;499:109–113. doi: 10.1016/j.neulet.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogue AI, Percy ME, Cui JG, Li YY, Bhattacharjee S, Hill JM, et al. Up-regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal sulfate-stressed human astroglial primary cell cultures. J Inorg Biochem. 2011;105:1434–1437. doi: 10.1016/j.jinorgbio.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukiw WJ. NF-kB-regulated micro RNAs (miRNAs) in primary human brain and retinal cells. Exp Neurol. 2011;235:484–490. doi: 10.1016/j.expneurol.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higaki S, Gebhardt BM, Lukiw WJ, Thompson HW, Hill JM. Effect of immunosuppression on gene expression in the HSV-1 latently infected mouse trigeminal ganglion. Invest Ophthalmol Vis Sci. 2002;43:1862–1869. [PubMed] [Google Scholar]
- 17.Lukiw WJ, Zhao Y, Cui JG. An NF-kB-sensitive miRNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J Biol Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Cui JG, Kuroda H, Chandrasekharan NV, Pelaez RP, Simmons DL, Lukiw WJ. Cyclooxygenase-3 gene expression in Alzheimer hippocampus and in stressed human neural cells. Neurochem Res. 2004;29:1731–1737. doi: 10.1023/b:nere.0000035809.70905.8a. [DOI] [PubMed] [Google Scholar]
- 20.Pall GS, Hamilton AJ. Improved northern blot method for enhanced detection of small RNA. Nat Protoc. 2008;3:1077–1084. doi: 10.1038/nprot.2008.67. [DOI] [PubMed] [Google Scholar]
- 21.Pall GS, Codony-Servat C, Byrne J, Ritchie L, Hamilton A. Carbodiimide-mediated cross-linking of RNA to nylon membranes improves the detection of siRNA, miRNA and piRNA by northern blot. Nucleic Acids Res. 2007;35:e60. doi: 10.1093/nar/gkm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SW, Li Z, Moore PS, Monaghan AP, Chang Y, Nichols M, John B. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic Acids Res. 2010;38:e98. doi: 10.1093/nar/gkp1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukiw Walter J, Alexandrov PN, Zhao Y, Hill JM, Bhattacharjee S. J Biol Chem. 2012 (manuscript submitted). [Google Scholar]
- 24.Lukiw WJ, Bhattacharjee S, Dua P, Alexandrov PN. Common micro RNAs (miRNAs) target complement factor H (CFH) regulation in Alzheimer’s disease (AD) and in age-related macular degeneration (AMD) Int J Biochem Mol Biol. 2012;3:105–116. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]