Abstract
Synapses are formed by interneuronal connections that permit a neuronal cell to pass an electrical or chemical signal to another cell. This passage usually gets damaged or lost in most of the neurodegenerative diseases. It is widely believed that the synaptic dysfunction and synapse loss contribute to the cognitive deficits in patients with Alzheimer’s disease (AD). Although pathological hallmarks of AD are senile plaques, neurofibrillary tangles, and neuronal degeneration which are associated with increased oxidative stress, synaptic loss is an early event in the pathogenesis of AD. The involvement of major kinases such as mitogen-activated protein kinase (MAPK), extracellular receptor kinase (ERK), calmodulin-dependent protein kinase (CaMKII), glycogen synthase-3β (GSK-3β), cAMP response element-binding protein (CREB), and calcineurin is dynamically associated with oxidative stress-mediated abnormal hyperphosphorylation of tau and suggests that alteration of these kinases could exclusively be involved in the pathogenesis of AD. N-methyl-D-aspartate (NMDA) receptor (NMDAR) activation and beta amyloid (Aβ) toxicity alter the synapse function, which is also associated with protein phosphatase (PP) inhibition and tau hyperphosphorylation (two main events of AD). However, the involvement of oxidative stress in synapse dysfunction is poorly understood. Oxidative stress and free radical generation in the brain along with excitotoxicity leads to neuronal cell death. It is inferred from several studies that excitotoxicity, free radical generation, and altered synaptic function encouraged by oxidative stress are associated with AD pathology. NMDARs maintain neuronal excitability, Ca2+ influx, and memory formation through mechanisms of synaptic plasticity. Recently, we have reported the mechanism of the synapse redox stress associated with NMDARs altered expression. We suggest that oxidative stress mediated through NMDAR and their interaction with other molecules might be a driving force for tau hyperphosphorylation and synapse dysfunction. Thus, understanding the oxidative stress mechanism and degenerating synapses is crucial for the development of therapeutic strategies designed to prevent AD pathogenesis.
Keywords: NMDA receptor, Oxidative stress, Kinases, Tau protein, Synaptic function, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD), the most common neurodegenerative disorder, is characterized by deposition of amyloid-beta plaques (Aβ), neurofibrillary tangles (NFTs), and hyperphosphorylated tau (a microtubule binding protein) [1]. It has been reported that impairment of amyloid precursor protein (APP) metabolism in AD leads to increased production of Aβ. A high level of Aβ production is directly correlated with other critical events such as formation of tangles, neuron loss, synapse loss, and neurotransmission dysfunction [2] (Fig. 1). Interestingly, these changes are associated with N-methyl-D-aspartate receptor (NMDA) receptor activation and oxidative stress which ultimately results in AD pathology. Besides, Aβ is also reported to trigger NMDA-mediated Ca2+ influx, excitotoxicity, and stress-related signaling pathways in neurons which may exacerbate aging-related increases in oxidative stress, impaired energy metabolism, and defective Ca2+ homeostasis [3]. The NMDA receptors (NMDARs) are cationic channels gated by the neurotransmitter glutamate having critical roles in excitatory synaptic transmission, plasticity, as well as in excitotoxicity in the central nervous system (CNS). The activation of NMDAR glutamate release leads to massive Ca2+ fluxes into the postsynaptic cells. Previous reports suggest that oligomeric Aβ-induced Ca2+ influx occurs through postsynaptic NMDAR. Furthermore, this can lead to excessive formation of reactive oxygen species (ROS) and oxidative stress [4]. Synapses are formed by connections between two neurons that allow a neuronal cell to pass a signal to another cell. This channel usually gets damaged or lost in most neurodegenerative diseases (Fig. 2). Accumulating evidence suggests that dysfunction and loss of synaptic connections may be an important early event underlying AD progression. Insightful synapse degeneration in AD is characterized by the worsening of cognitive function, synapse loss, and neuronal cell death [5]. Synaptic function and plasticity have also been extensively studied in the transgenic mouse models that show abnormal synaptic transmission and impaired long-term potentiation (LTP) which are often well associated with Aβ plaque formation [6]. Neurodegenerative disorders are characterized by progressive cell loss in specific neuronal populations and mechanisms that have been put forward to account for AD with aging including inflammation and oxidative stress [7, 8]. Recently, Rai et al. [9] also proved that NMDAR activation, excessive Ca2+ fluxes, and free radical generation are associated with synaptic dysfunction and tau phosphorylation [10]. Excessive amounts of glutamate are associated with intense transient influx of Ca2+, leading to mitochondrial functional impairments characterized by activation of the permeability transition pores in the inner mitochondrial membrane, cytochrome c release and depletion of ATP, and simultaneous formation of ROS [11]. In addition, an increase in cytoplasmic Ca2+ triggers intracellular cascades which lead to increased levels of ROS and oxidative stress [12]. Analysis of AD brains revealed that the extensive synapse loss is strongly correlated with cognitive impairment [13]. Cognitive function in AD patients is also closely interrelated to the density of presynaptic glutamatergic neurons and postsynaptic neurotoxicity [8, 9, 14]. A previous report by Arendt [15] suggests that synaptic decline occurs early in disease progression and neuronal death alone is not sufficient for disease progression. Cooper and Bear [16] have reported that synapses are selectively removed prior to cell death and dementia in AD patient may therefore be attributed to progressive reduction in synaptic integrity [17]. Thus, proper synaptic function is crucial for learning and memory. Therefore, from previous observations, it seems that proper NMDAR and synapse function are necessary for learning and memory, and any improper in NMDAR and synapse function may lead to progression of AD pathogenesis. In this review, we connect the link between NMDAR-mediated oxidative stress, Ca2+ dysregulation, and kinases on synapse function.
Fig. 1.

Comparative changes in healthy and diseased neuron implicated in AD pathogenesis. The abnormal function of Aβ activates several stress-related kinases that results in damaged nucleus and mitochondria in diseased neurons in AD pathogenesis
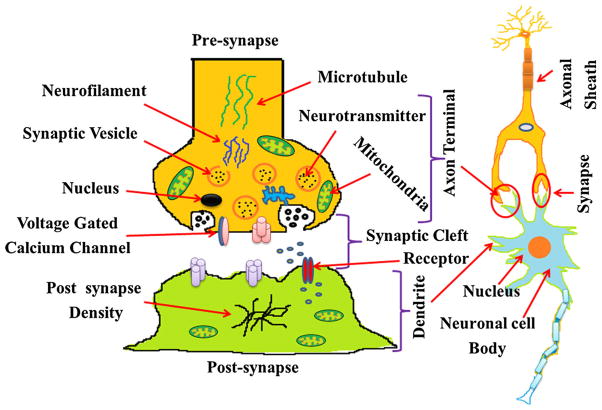
Fig. 2.
Structure of synapse: Synapse is a specialized communication junction between two cells comprised of two major units: a presynaptic cell (usually a neuron) that sends out a signal and a postsynaptic cell that receives the signal. Neurotransmitter molecules diffuse across the synaptic cleft and bind to their specific receptors on the postsynaptic cell. This recreates the action potential in the postsynaptic cell. This channel usually gets damaged or lost in neurodegenerative diseases
NMDA Receptor-Mediated Oxidative Stress
NMDA type of glutamate receptor (NMDAR) plays an important role in learning and memory formation and also considered crucial for brain development and function in the central nervous system. Activation of NMDAR leads to cytosolic free intracellular Ca2+ increase [10] required for LTP and long-term depression (LTD) [18] and, more likely, for synaptic plasticity [19]. NMDA receptor subunit such as NR1, NR2A, and NR2B maintains the synaptic plasticity and neuronal function. The NR2A subunit is involved in the induction of LTP, whereas the NR2B subunit contributes to the formation of LTD and, thus, memory function. Elevation of cytosolic free Ca2+ leads to derangement of many intracellular processes that normally regulate Ca2+ sequestration and energy metabolism [7]. Modulations of Ca2+, glutamate, and NMDAR also induce some other biochemical mechanisms such as oxidative stress which further compromise with cell death [8]. Oxidative stress is one of the most important mechanisms involved in toxic events observed in neuronal cells in different neurodegenerative disorders as measured by free radical generation and lipid peroxidation [20]. Under normal conditions, ROS act as signaling molecules in many physiological processes including redox homeostasis and cellular signal transduction [21]. By activating proteins such as tyrosine kinases, mitogen-activated protein kinases and ROS are important mediators of signal transduction pathways [22]. Increased production of cellular ROS and oxidative stress has been reported to induce autophagy, a homeostatic process that enables cells to degrade cytoplasmic proteins and organelles [23]. Nitric oxide (NO) released by NO synthase may induce synaptic changes by causing neurotoxicity. Recent studies suggest that NO may be acting as a neuronal messenger in the central nervous system and is involved in the pathophysiology of neurodegenerative disorders [24]. Liu et al. [25] suggested that generation of reactive nitrogen species (RNS) and ROS triggers oxidative stress and eventually leads to neuronal damage. Thus, the increased free radical generation may ultimately lead to synaptic dysfunction which is an important pathophysiological component of AD [26]. Moreover, Kamat et al. [10] also suggest that oxidative stress causes cognitive deficiency, neurofibrillary tangle (NFT)-like pathological changes, and oxidative stress as seen in AD pathology via tau hyperphosphorylation. AD includes a variety of risk factors such as extracellular deposition of β-amyloid, accumulation of intracellular neurofibrillary tangles, oxidative neuronal damage, and inflammatory cascades [27]. Therefore, NMDAR activation, free radical generation, apoptosis, and their consequence on synapse function may lead to AD progression.
NMDA Receptor-Mediated Apoptotic Cell Death
Excitotoxic cell death (ECD) is a characteristic of mammalian brains in several types of neuronal apoptosis. A key event in ECD is a massive increase in intracellular Ca2+ by overstimulation of NMDAR. Excitotoxicity is defined as a toxic process characterized by a sustained stimulation of excitatory amino acid receptors mainly involving NMDARs [28, 29]. Different toxic events derived from excitotoxicity have been characterized in experimental models including upregulation of detrimental signaling pathways, disrupted Ca2+ homeostasis, and ROS/RNS with further oxidative/nitrosative stress ultimately leading to cell death [9]. Apoptosis plays a significant role in cell death during neurodegenerative disorders such as AD [30]. The activation of caspases, a major apoptotic pathway, is also characterized by mitochondrial dysfunction with the release of cytochrome c and activation of caspase-9 and, subsequently, of caspase-3 [7]. Lines of evidence suggest that caspase-3 activations were also found in the brain of AD patients [31]. Most neurons in the mammalian central nervous system possess receptors for apoptosis, the excitatory neurotransmitter glutamate. Overactivation of glutamate receptors can induce apoptosis by a mechanism involving Ca2+ influx [32]. Neuronal cell death is also triggered in response to increased oxidative stress, in which free radicals such as the superoxide anion radical and the hydroxyl radical damage cellular lipids, proteins, and nucleic acids [33]. So, collective information suggests that NMDAR-mediated oxidative stress and neuronal apoptosis directly or indirectly influences synapse function.
Biochemical Mechanism of Apoptosis and Synaptic Dysfunction
Biochemical mechanisms involved in apoptosis can be activated at synapse which can alter synaptic function and promote degeneration of synapses [34]. Apoptosis can be induced in synaptosome preparations and neuritis of cultured brain neurons by insults that induce apoptosis in intact neurons. Caspase-mediated cleavage of synaptic proteins may control the process of neuronal apoptosis. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunits are selectively degraded in hippocampal neurons after exposure to an apoptotic dose of glutamate, resulting in decreased Ca2+ influx and, thereby, preventing excitotoxicity [32]. Apoptotic pathways may also function in synaptic plasticity, particularly under conditions of stress and injury. The elevation of caspase-3 activity correlated temporally with memory impairment, reduced spine density and size, altered excitatory synaptic transmission, and enhanced LTD. Remarkably, pharmacologic inhibition of caspase-3 ameliorated the synaptic transmission, spine size, and memory deficits in these AD transgenic mice [35]. Increased caspase-3 activity is also reported in human AD brain, and elevated levels of caspase-3 are observed in the postsynaptic density fraction of AD brain [36]. Inhibition of caspase-3 activity is beneficial to reverse cognitive decline in APP mice possibly due to a requirement for caspase-3 activity in normal synaptic function [37, 38]. Thus, apoptotic executes by caspase-3 activation may contribute to neuronal apoptosis and synapse dysfunction and further provoke AD progression.
Mitochondria as a Regulator of Apoptosis and Oxidative Stress
Since most neurodegenerative disorders are associated with mitochondrial abnormalities, AD is associated with similar fashion of mitochondrial dysfunction. The mitochondria play a critical role in the regulation of apoptosis which is implicated in the aging process. Age-related mitochondrial oxidative stress may contribute to apoptosis [39]. The mitochondria are significantly reduced in various types of cells and its dysfunction is one of the causative pathophysiology of AD [40]. The most regular defect in mitochondrial electron transport enzymes in AD is a deficiency in cytochrome c oxidase which leads to an increase in ROS production, a reduction in energy stores, and disturbance in energy metabolism [41]. Indeed, ROS generation is an important mechanism accounting for cellular injury in many neurodegenerative disorders [42]. Such selective oxidative modification may cause the cells to be more vulnerable to apoptotic inducers [43]. Thus, the mitochondria appear to influence the aging process via modifying the regulatory machinery of apoptosis and that the mitochondria have a central role in aging-related neurodegenerative diseases like AD [7]. Consistently, oxidative stress-induced accumulation of Aβ-protein in AD causes lysosome membrane degradation and ultimately leads to neuronal cell death [44]. The mitochondria damaged by oxidative stress in pyramidal neurons of AD are subjected to neurodegeneration [45]. Thus, inhibition of the mitochondrial complexes leads to diminished ATP production and resulted in impaired energy metabolism. Several lines of evidence support that NO impairs mitochondrial/cellular respiration and other functions by inhibiting the activities of several key enzymes, particularly cytochrome c oxidase, and thereby causing ATP depletion [46]. In cultured neuroblastoma cells, overexpression of tau results in mitochondria with decreased ATP levels and increased susceptibility to oxidative stress [47]. Furthermore, the activity and composition of mitochondrial enzymes are disrupted in the mouse model of tauopathy [48]. Thus, tau may also directly influence mitochondrial function. It appears that mitochondria-mediated oxidative stress influences tau function, and the abnormal function of tau also influences the mitochondria. Hence, the mitochondria and tau influence each other’s function, which is profoundly associated with AD pathogenesis.
Molecular Mechanism of Oxidative Stress-Mediated Tau Phosphorylation
Inactivation of protein phosphatase 1/protein phosphatase 2A (PP1/PP2A) via oxidative stress has been shown in vitro and in vivo to be involved in hyperphosphorylation of tau and prolonged phosphorylation of extracellular receptor kinase (ERK) 1/2 [49]. Thus, it is intriguing to postulate that oxidative stress-mediated PP1 and PP2A inhibition in AD may account for enhanced ERK1/2 activity and subsequent tau hyperphosphorylation and neurofibrillary tangle formation. Li et al. [50] suggested that a decrease in PP2A activity causes the activation of ERK1/2, MEK1/2, and several other kinases and the abnormal hyperphosphorylation of tau both via an increase in its phosphorylation and a decrease in its dephosphorylation in AD brain. Stress-activated protein kinase (SAPK) and p38 MAPKs are members of the complex superfamily of MAP serine/threonine protein kinases. This superfamily also includes the ERKs (also referred to as MAPKs), which are typically activated by mitogens, c-Jun N-terminal kinase (JNK)/SAPK, and p38 mitogen-activated protein kinase (MAPK), which are known as stress-activated kinases [51]. This can be attributed to the fact that the activities of these enzymes are stimulated by a variety of exogenous and endogenous stress-inducing stimuli including ROS and oxidative stress (Fig. 3). The kinases that phosphorylate tau can be activated by NMDA-mediated oxidative stress such as hyperactivation of Cdk5 signaling pathway, MAPK, and several stress-activated protein kinases [52]. Thus, induction of this protein by JNK/SAPK could serve as a potential marker for pathologies associated with chronic oxidative stress. Among them, cyclic AMP-dependent protein kinase (PKA) and calcium-calmodulin-dependent protein kinase II (CaMKII) are associated with NMDAR remodeling. The phosphorylation of tau by these kinases inhibits the ability of tau to promote microtubule assembly and facilitates the polymerization of tau into paired helical filament (PHF) [53]. Protein phosphatase mediates the regulation of protein kinase C during long-term depression in the adult hippocampus in vivo. The neural substrates of learning and memory are thought to involve long-term changes in synaptic function, including LTD of synaptic strength. All these studies signify that inhibition of phosphates activates the number of kinases which actively participate in the activation of oxidative stress. Activation of kinases also stimulates the NMDAR and CaMKII remodeling. Moreover, inhibition of phosphatase also causes tau hyperphosphorylation, a critical event of AD pathology. Thus, phosphatase inhibition, kinase activation, NMDAR remodeling, and oxidative stress are strongly correlated with each other and, hence, influence synaptic function.
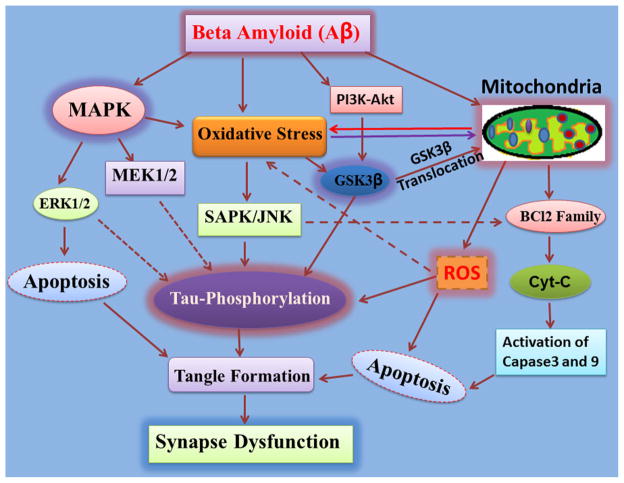
Fig. 3.
Synapse dysfunction: as a consequence of MAPK and mitochondrial oxidative stress-mediated tau hyperphosphorylation. β-Amyloid activates several stress-related kinases that causes oxidative stress. The phosphorylation/activation of ERK1/2 and MEK1/2 via MAPK results in apoptosis. Consequently, abnormal hyperphosphorylation of tau leads to synapse dysfunction. Aβ also results in mitochondrial dysfunction by affecting the prosurvival protein Bcl-2. The mitochondrial-mediated caspase pathway gets triggered by the Bcl-2 family of proteins, then cytochrome c release, and finally, apoptosis. This ultimately leads to synapse dysfunction
Modulation of NMDA Receptor and Kinases on Oxidative Stress
NMDA receptors influence death and survival pathways for synapse function. Activation of NMDAR induces the ERKs, which promote a signaling cascade important for neuronal survival. Thus, the synaptic NMDAR activates ERK, promoting cell survival [54], whereas the extra synaptic pool of NMDARs trigger mitochondrial membrane potential breakdown, as well as cell body and dendritic damage [55]. Moreover, activation of synaptic NMDAR leads to activation of the cAMP response element-binding protein (CREB), a transcription factor also related to cell survival pathways [56, 57], and phosphorylates CREB [58]. Several studies have shown that oligomeric Aβ induces partial blockade of NMDAR currents, which leads to reduction of calcium influx that limits CaMKII function [59, 60]. In fact, oligomeric Aβ-mediated LTP impairment is believed to involve a decrease in the activation of MAPK, CaMKII, and Akt/protein kinase B, but not protein kinases A and C [61, 62]. Aβ has also been shown to induce synaptic depression by activating mGluRs, which triggers a series of downstream molecular events involving MAPK and calcineurin, which ultimately promotes internalization of AMPA receptors and synapse collapse [63]. Kinases that are important for synaptic function such as PKC, PKA, and CaMKII have reduced basal activity and stimulation-induced activation is impaired [64]. Phosphatase activity contributes to changes in phosphorylation state of bcl-2 family member protein BAD, and the CREB and dephosphorylation of BAD and CREB is associated with impaired memory [65]. This regulatory mechanism of Aβ influences NMDAR and CaMKII function and impairs learning and memory function. Abnormal function of Aβ also activates several stress-related kinases that cause oxidative stress and apoptosis, which are heavily implicated in AD pathogenesis (Fig. 4). In the later stage, all these disparities result into neuronal dysfunction and synaptic loss.
Fig. 4.
Molecular mechanism of tau phosphorylation mediated synaptic dysfunction: High level of Aβ production is directly correlated with critical event for synaptic dysfunction, i.e., formation of tangles. Excitotoxic cell death (ECD) is an event due to increased intracellular Ca2+ by overstimulation of NMDA receptor. Overstimulation of NMDAR leads to upregulation of detrimental signaling pathways, disrupting Ca2+ homeostasis and oxidative/nitrosative stress ultimately toward apoptosis. The mitochondrial oxidative stress and the release of cytochrome c, activation of caspase-9, and subsequently of caspase-3 cause neuronal damage. Mitochondria-mediated oxidative stress influences tau function resulting in the hyperphosphorylation of tau which governs the major synaptic dysfunction by forming tangles
Glycogen Synthase Kinase-3β
Glycogen synthase kinase-3 (GSK-3) is a pivotal molecule in the development of AD. Inhibition of GSK-3 reduces the production of Aβ peptides in amyloid plaques and the hyperphosphorylation of tau protein in neurofibrillary tangles [66]. GSK-3beta is involved in the formation of PHF-tau, which is an integral component of the NFT deposits that disrupt neuronal function and a marker of neurodegeneration in AD [10, 67]. GSK-3 also phosphorylates and inhibits cAMP responsive element-binding protein [68], a universal modulator of memory and intermediate molecule of the NMDAR pathway. Moreover, GSK-3β promotes actin and tubulin assembly [69], processes required for synaptic reorganization during memory formation. Additionally, within the brain, MAPK inactivates GSK-3β by direct phosphorylation at its C-terminus [70]. Dephosphorylation of GSK-3 at inhibitory sites is coordinated by PP1, PP2A, and protein phosphatase 2B (PP2B, calcineurin) [71]. PP1 preferentially acts as a phosphatase for GSK-3β, while PP2A favors GSK-3α [72, 73]. On the other hand, the overexpression of GSK-3β inhibits PP2A, which may serve as a negative feedback mechanism for GSK-3β activity [74]. GSK-3β activity is negatively regulated by several signal transduction cascades that protect neurons against apoptosis suggesting the interesting possibility that activation of GSK-3β may contribute to neuronal apoptosis [75]. In response to oxidant stress, GSK-3β translocates to the mitochondria in a kinase activity-dependent manner and enhances production of cytotoxic ROS from mitochondria. This study identifies GSK-3β, a kinase known to participate in oxidative stress, cell death, and neurodegeneration, as a fundamental element in the downregulation of the antioxidant cell defense [76]. Collectively, it seems that Aβ activates GSK-3β which induces oxidative stress, hyperphosphorylation of tau, NFT formation, neuronal death, and synaptic loss that can induce memory deficits.
NMDA Receptor and Calcineurin (PP2B) Activity
Since intracellular Ca2+ coming from several sources and mostly through glutamate-mediated contribute to calcineurin (CaN) activation and may be preferentially increased by NMDAR activity in animals [77]. Furthermore, mild oxidative stress is thought to increase CaN activity through the release of Ca2+ from intracellular stores [78] or a decrease in effectiveness of inhibitory proteins [79]. This would suggest that the release of Ca2+ from intracellular stores could decrease NMDAR function through CaN activation and increased oxidative stress that can influence synapse function [80]. Chen et al. [81] have reported that β-amyloid reduces NMDAR function and impairs LTP through enhanced CaN activity. As the disease progression starts, increased expression of CaN inhibitory proteins may shift the balance of Ca2+-activated kinases/phosphatases. Therefore, calcineurin alteration promotes tau phosphorylation, neurodegeneration, tangle formation [82, 83] and, subsequently, synapse dysfunction.
Phosphatase Inhibition, NMDA Receptor, Tau Hyperphosphorylation, and Synapse Function
Protein phosphatase inhibition leads to the induction of synaptic plasticity in the form of LTP involved in memory formation. Inhibition of protein phosphatases reduces postsynaptic signaling as a major mechanism for basal synaptic transmission and memory formation [84]. It has been reported that inhibition of protein phosphatases increases tau phosphorylation and initiates neuronal cell death which includes altered Ca2+ homeostasis and glutamate excitotoxicity that alter the memory formation [85, 86]. Previous reports suggested that accumulation and mislocalization of hyperphosphorylated tau in the somatodendritic compartment of neurons in AD disrupts glutamate receptor trafficking and synaptic function [87–89]. Not many available reports show how tau hyperphosphorylation and aggregation contributes to the synaptic deficits and neuronal death in AD. Hoover et al. [88] suggest that abnormal tau phosphorylation may also affect postsynaptic receptor targeting and display disrupted targeting of excitatory glutamate receptor and dendritic spines (Fig. 5). Impaired NMDAR can lead to dendritic spine dysfunction and removal of dendritic spine [90]. Tau protein also binds to a postsynaptic protein complex which includes PSD-95, the scaffold for synaptic NMDARs which is a critical regulator of synaptic plasticity [91]. Endogenous tau is typically present in dendrites in the postsynapse, where it interacts with the PSD-95/NMDAR complex [92]. Furthermore, microinjection of human tau into the presynaptic terminal of the squid axon has blocked synaptic transmission possibly by preventing proper docking of synaptic vesicles [93]. Therefore, tau may play an important role in regulating synaptic function and targeting neurotransmitter receptors to the synapse [94]. Zhang et al. [95, 96] also showed that glutamate release during stimulus and intracellular Ca2+ release from presynaptic terminals led to abnormal synapse function. These reports suggest that presynaptic dysfunction might be an early component of synaptic dysfunction in AD. Thus, lines of evidence supported that the tau protein is actively engaged in synapse dysfunction and associated with NMDAR function. In conclusion, the glutamate-induced excitotoxicity and synaptic dysfunction could be an excellent target for the therapy of AD.
Fig. 5.
Beta amyloid-mediated synapse loss: Deposition of amyloid plaques (Aβ) is characterized in Alzheimer’s disease (AD) which affects NMDAR resulting in dendritic damage caused due to mitochondrial oxidative stress as a consequence of excitotoxicity. Aβ also trigger NMDA mediated Ca2+ influx which results in dendritic spine toxicity mediated by CREB and hyperphosphorylation of Tau. Ca2+ influx can also lead to mitochondrial oxidative stress along with caspase-3-mediated apoptosis and tau hyperphosphorylation and ultimately leads to synapse loss
NMDA Receptor and Synapse Function
NMDARs are located in neuronal cell membranes at synaptic and extrasynaptic locations, where they are believed to mediate distinct physiological and pathological processes. NMDARs are glutamate-gated ion channels that are highly permeable to Ca2+ and Ca2+ influx through NMDAR. This is essential for synaptogenesis. Synaptic remodeling leads to long-lasting changes in synaptic efficacy such as long-term potentiation and long-term depression [97]. The NMDARs are involved in cellular mechanism for learning and memory function [9]. NMDARs are involved in a wide array of biological processes which are crucial for brain development and function [98]. NMDAR activation might also play an important role in extracellular adenosine regulation, with important consequences for the regulation of excitatory synaptic transmission, plasticity, and excitotoxicity [99]. NMDAR activation also led to influx of Ca2+ through a ligand- and voltage-sensitive calcium channel [100], which triggered significant advances in understanding the cellular cascades initiated as a result of tetanic stimulation. The glutamatergic hypothesis of AD states that glutamate-related excitotoxic mechanisms involving the NMDAR lead to neurodegeneration and cell death [101]. The activation of glutamate receptors has also been found to induce the release of glutamate and induce a massive accumulation of Ca2+. This influx of Ca2+ contributes to an alteration of cell function, leading to cell death either through free radicals or through overload of the mitochondria, resulting in free radical formation, caspase activation, and the release of apoptosis-inducing factors [7, 102]. Therefore, synaptic stimulation through NMDARs is important for learning and memory functions, but excess glutamate can over-stimulate these receptors resulting in excitotoxicity and neurodegeneration.
Glutamate and Aβ-Mediated Ca2+ Dysregulation and Synapse Function
Glutamate plays an essential role in learning and memory by triggering NMDAR to allow a controlled amount of calcium into a nerve cell. Ca2+ helps to create the chemical environment required for information storage. Excess glutamate, on the other hand, overstimulates NMDARs so that they allow even more calcium into nerve cells and the process leads to disruption and death of the cells [20]. It has been reported that inhibition of protein phosphatases increases tau phosphorylation and initiates neuronal cell death which include altered Ca2+ homeostasis and glutamate excitotoxicity [86]. In contrast to most disease models, aged animals exhibit neurologic changes that usually include synaptic dysfunction and Ca2+ dysregulation [103]. Aβ plaques, a pathological feature of AD, induce extracellular accumulation of glutamate and intracellular deposition of calcium ion Ca2+. In vivo Ca2+ imaging studies corroborate the idea that different subsets of neurons in AD transgenic mice can be found exclusively near Aβ plaques and appeared to result from a relative decrease in synaptic inhibition [104]. In vivo imaging of aged APP transgenic mouse brain shows elevated intracellular Ca2+ and aberrant Ca2+ homeostasis in a subset of neurites in close proximity of Aβ plaques [105]. The abnormal Ca2+ handling of neurons affected by Aβ is associated with loss of dendritic spines and neuritic dystrophy, mediated in part by the Ca2+-dependent protein phosphatase calcineurin [106]. It is notable that calcineurin is also required for apoptosis and LTD, as well as for Aβ-induced spine loss and endocytosis of NMDARs [107]. Thus, a large buildup of glutamate can occur and induce a massive accumulation of Ca2+, leading to apoptosis. It has also been noted that Aβ plaques increase neurons’ vulnerability to excitotoxicity and loss of synaptic protein.
NMDA Receptor, CaMKII, and CREB
The transcription factor CREB plays an essential role in the maintenance of LTP. CREB signaling has been recently involved in several brain pathological conditions including cognitive and neurodegenerative disorders. Altered hippocampal-dependent synaptic plasticity and memory mediates synapse loss through the CREB signaling pathway [108]. The activation of CREB by phosphorylation is triggered in neurons by a wide variety of signaling processes, from increases in intracellular Ca2+ through activation of voltage- or ligand-gated channels to changes in cAMP levels. The phosphorylation of CREB by kinases from several signaling pathways may be a mechanism for memory formation [109]. Activation of synaptic NMDARs toward CaMKII, which may also phosphorylate and activate CREB in neurons, is associated with increased Ca2+ [110]. Interestingly, the increase in nuclear Ca2+ can also activate CREB, indicating that nuclear kinases may have a direct role in the modulation of CREB activity [111]. Loss of excitatory synapses in the hippocampus induced by Aβ requires functional NMDARs for proper synapse maintenance [61]. Aβ also influences CREB activation, which is crucial for the maintenance of LTP. LTP plays a crucial role in memory formation. Vitolo et al. [112] showed that Aβ decreases the activity of CREB and thus reduces the expression of genes encoding proteins that are essential for LTP. Another study found that excessive activation of extrasynaptic NR2B-containing NMDARs, which leads to downregulation of CREB, underlies oligomeric Aβ-mediated LTP inhibition [113]. Thus, it may be inferred from the above reports that Aβ also modulates CaMKII, CREB, and NMDAR essential for maintenance of LTP.
Therapeutic Strategies
The therapies applied till date for the treatment of AD include the following: antibody vaccination and immunization therapies; gene therapy; enzyme-based therapies such as β-secretase inhibitors, -secretase inhibitors and modulators, and cholinesterase inhibitors; receptor-based therapies such as NMDA receptor antagonists, AMPA receptor modulators, peroxisome proliferator-activated receptor agonists, M1 muscarinic agonists, receptor for advanced glycation end product-related mechanisms, ad nicotine acetylcholine receptor agonists; redox stress-based therapies such as antioxidants and anti-inflammatory and neuroprotective approaches; and tau-related therapies. Moreover, several other therapies were also designed to treat AD by targeting cholesterol biosynthesis, astrocyte-modulating agents, homocysteine-lowering strategies, and caspase inhibitors. Most of the abovementioned therapies also passed through clinical trials but results were not successful so far. Among all the therapies which are currently in the market, only two classes of drugs are available for commercial purpose such as the cholinesterase inhibitor and NMDA receptor antagonist. However, these classes of drugs are also not successful either to give complete remedy from AD. There may be some significant gaps which are necessary to understand and improve the current therapies.
Significant Gap in Research
Oxidative stress-induced damage in the brain is associated with aging and is usually involved in the development of AD pathology in a clinical set of condition. Some observational studies have suggested that antioxidant therapy could overcome the disease progression of AD. Much research has been carried out with antioxidant therapy, but antioxidant randomized clinical trials in AD have had mixed results. Oxidative stress is a well-established pathophysiological factor in AD but till now the use of antioxidants in the prevention or therapy gave conflicting results. Some of the reports have shown antioxidant therapy for the betterment of AD pathology (Table 1), but still therapy is not so promising. In our opinion, antioxidant therapy alone is not looking so hopeful. We should use some more specific targets with combinational therapy rather than a single antioxidant such as NMDAR antagonist, neuronal kinases, and antioxidant as well as flavonoids.
Table 1.
List of pharmacologically active antioxidants, flavonoids, and vitamins used in AD pathology
| S. nos. | Antioxidants/flavanoids | References |
|---|---|---|
| 1 | Vitamin C and vitamin E | [114, 115] |
| 2 | Vitamin C | [116] |
| 3 | Vitamin B12 | [117, 118] |
| 4 | Vitamin E | [119–123] |
| 5 | α-Lipoic acid, CoQ10 | [124, 125] |
| 6 | Selenium and vitamin E | [126] |
| 7 | MnSOD | [127] |
| 8 | Curcumin | [128, 129] |
| 9 | Memory XL (folic acid, vitamin B12, vitamin E, acetyl-L-carnitine, SAM, NAC) | [130, 131] |
| 10 | Ebenone/idebenone | [132–134] |
| 11 | Estrogen | [135, 136] |
| 12 | Colostrinin | [137–140] |
| 13 | Epigallocatechine gallate (EGCG) | [141] |
| 14 | Resveratrol | [142, 143] |
| 15 | Pramipexole | [144] |
| 16 | Latrepirdine | [145, 146] |
| 17 | Ubiquinone, vitamin E, or lipoic acid | [147, 148] |
| 18 | Lipoic acid | [149] |
| 19 | Silibinin/quercitin | [150–152] |
| 19 | Melatonin | [153–155] |
| 20 | Caffeine | [156] |
| 21 | Selegiline (L-deprenyl) | [119] |
| 22 | Ginkgo biloba | [157–159] |
| 23 | Gugulipid | [160, 161] |
Future Direction
In conclusion, through this review, we have tried to give our perspective on the wide variety of interaction between NMDAR-mediated oxidative stresses with the etiology of Alzheimer’s disease. NMDAR-mediated oxidative stress mechanisms are likely to play an important role in the synapse dysfunction in the pathogenesis of AD. Moreover, mitochondrial-mediated oxidative stress and apoptosis are also suggested to be contributing factors in AD pathogenesis. Furthermore, oxidative stress-mediated kinase and tau phosphorylation provides a connection of synapse dysfunction in AD. As we are not getting complete remedies from antioxidant therapy or known NMDAR antagonist drug used for AD pathology, should we go for combinational therapy? Or are there so many intermediate molecules between NMDAR to neurodegeneration? Should we go for target intermediate molecules? Therefore, understanding the role of oxidative stress-associated molecule and kinases in synapse dysfunction during AD pathogenesis may also lead to the development of mechanism-based therapeutics and better constructive strategies.
Acknowledgments
This work was supported in part by the Council of Scientific and Industrial Research (CSIR), India and the National Institutes of Health, USA.
Abbreviations
- AD
Alzheimer’s disease
- CNS
Central nervous system
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- NMDA receptor
N-methyl-D-aspartate receptor
- Aβ
Beta amyloid
- APP
Amyloid precursor protein
- CaMKII
Calmodulin-dependent protein kinase
- NFT
Neurofibrillary tangle
- NO
Nitric oxide
- PP
Protein phosphatase
- MAPK
Mitogen-activated protein kinase
- PKA
Protein kinase
- ERK
Extracellular receptor kinase
- ECD
Excitotoxic cell death
- LTD
Depression
- LTP
Long-term potentiation
- CREB
cAMP response element-binding protein
- GSK-3β
Glycogen synthase-3β
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Pradip K. Kamat, Email: pkkama01@louisville.edu, pradeepkamat2004@gmail.com, Division of Physiology and Biophysics, School of Medicine, University of Louisville, Louisville, KY 40202, USA
Anuradha Kalani, Division of Physiology and Biophysics, School of Medicine, University of Louisville, Louisville, KY 40202, USA.
Shivika Rai, Division of Pharmacology, Central Drug Research Institute (CDRI), P.O. Box 173, Lucknow 226001, UP, India.
Supriya Swarnkar, Scripp Institute, Jupiter, FL 33458, USA.
Santoshkumar Tota, Division of Pharmacology, Central Drug Research Institute (CDRI), P.O. Box 173, Lucknow 226001, UP, India.
Chandishwar Nath, Division of Pharmacology, Central Drug Research Institute (CDRI), P.O. Box 173, Lucknow 226001, UP, India.
Neetu Tyagi, Division of Physiology and Biophysics, School of Medicine, University of Louisville, Louisville, KY 40202, USA.
References
- 1.Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry. 2007;78:461–464. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Yang L, Zheng H. Role of APP and Abeta in synaptic physiology. Curr Alzheimer Res. 2012;9:217–226. doi: 10.2174/156720512799361691. [DOI] [PubMed] [Google Scholar]
- 3.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 5.Rai S, Kamat PK, Nath C, Shukla R. Glial activation and post-synaptic neurotoxicity: the key events in streptozotocin (ICV) induced memory impairment in rats. Pharmacol Biochem Behav. 2014;117:104–117. doi: 10.1016/j.pbb.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, Wu T, Hamto P, Devidze N, Yu GQ, Palop JJ, Noebels JL, Mucke L. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamat PK, Tota S, Shukla R, Ali S, Najmi AK, Nath C. Mitochondrial dysfunction: a crucial event in okadaic acid (ICV) induced memory impairment and apoptotic cell death in rat brain. Pharmacol Biochem Behav. 2012;100:311–319. doi: 10.1016/j.pbb.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Kamat PK, Rai S, Swarnkar S, Shukla R, Nath C. Mechanism of synapse redox stress in okadaic acid (ICV) induced memory impairment: role of NMDA receptor. Neurochem Int. 2014 doi: 10.1016/j.neuint.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Rai S, Kamat PK, Nath C, Shukla R. A study on neuroinflammation and NMDA receptor function in STZ (ICV) induced memory impaired rats. J Neuroimmunol. 2013;254:1–9. doi: 10.1016/j.jneuroim.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Kamat PK, Rai S, Swarnkar S, Shukla R, Ali S, Najmi AK, Nath C. Okadaic acid-induced tau phosphorylation in rat brain: role of NMDA receptor. Neuroscience. 2013;238:97–113. doi: 10.1016/j.neuroscience.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls DG. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol. 2002;34:1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- 12.Farooqui AA, Yi Ong W, Lu XR, Halliwell B, Horrocks LA. Neurochemical consequences of kainate-induced toxicity in brain: involvement of arachidonic acid release and prevention of toxicity by phospholipase A(2) inhibitors. Brain Res Brain Res Rev. 2001;38:61–78. doi: 10.1016/s0169-328x(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 13.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell KF, Bennett DA, Cuello AC. Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment. J Neurosci. 2007;27:10810–10817. doi: 10.1523/JNEUROSCI.3269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 16.Cooper LN, Bear MF. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat Rev Neurosci. 2011;13:798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- 17.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 18.Fetterolf F, Foster KA. Regulation of long-term plasticity induction by the channel and C-terminal domains of GluN2 subunits. Mol Neurobiol. 2011;44:71–82. doi: 10.1007/s12035-011-8190-4. [DOI] [PubMed] [Google Scholar]
- 19.Lau CG, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Murphy J, Bennett MV, Zukin RS. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem Soc Trans. 2009;37:1369–1374. doi: 10.1042/BST0371369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamat PK, Tota S, Saxena G, Shukla R, Nath C. Okadaic acid (ICV) induced memory impairment in rats: a suitable experimental model to test anti-dementia activity. Brain Res. 2010;1309:66–74. doi: 10.1016/j.brainres.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Droge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp Gerontol. 2002;37:1333–1345. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 22.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 23.Kissova I, Plamondon LT, Brisson L, Priault M, Renouf V, Schaeffer J, Camougrand N, Manon S. Evaluation of the roles of apoptosis, autophagy, and mitophagy in the loss of plating efficiency induced by Bax expression in yeast. J Biol Chem. 2006;281:36187–36197. doi: 10.1074/jbc.M607444200. [DOI] [PubMed] [Google Scholar]
- 24.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Liu J, Sun D, Alcock NW, Wen J. Spinal cord injury increases iron levels: catalytic production of hydroxyl radicals. Free Radic Biol Med. 2003;3:64–71. doi: 10.1016/s0891-5849(02)01184-x. [DOI] [PubMed] [Google Scholar]
- 26.Sachdeva R, Babbar R, Puri V, Agarwal S, Krishana B. Correlation between cognitive functions and nitric oxide levels in patients with dementia. Clin EEG Neurosci. 2011;42:190–194. doi: 10.1177/155005941104200309. [DOI] [PubMed] [Google Scholar]
- 27.Chopra K, Misra S, Kuhad A. Neurobiological aspects of Alzheimer’s disease. Expert Opin Ther Targets. 2011;15:535–555. doi: 10.1517/14728222.2011.557363. [DOI] [PubMed] [Google Scholar]
- 28.Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol. 2005;94:4281–4289. doi: 10.1152/jn.00661.2005. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls DG, Johnson-Cadwell L, Vesce S, Jekabsons M, Yadava N. Bioenergetics of mitochondria in cultured neurons and their role in glutamate excitotoxicity. J Neurosci Res. 2007;85:3206–3212. doi: 10.1002/jnr.21290. [DOI] [PubMed] [Google Scholar]
- 30.Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- 31.Engidawork E, Gulesserian T, Yoo BC, Cairns N, Lubec G. Alteration of caspases and apoptosis-related proteins in brains of patients with Alzheimer’s disease. Biochem Biophys Res Commun. 2001;281:84–93. doi: 10.1006/bbrc.2001.4306. [DOI] [PubMed] [Google Scholar]
- 32.Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- 34.Mattson MP, Duan W. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- 35.D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 36.Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, Trojanowski JQ, Arnold SE. Caspase-3 is enriched in post-synaptic densities and increased in Alzheimer’s disease. Am J Pathol. 2008;173:1488–1495. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Abeta(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 38.Xie H, Guan J, Borrelli LA, Xu J, Serrano-Pozo A, Bacskai BJ. Mitochondrial alterations near amyloid plaques in an Alzheimer’s disease mouse model. J Neurosci. 2013;33:17042–17051. doi: 10.1523/JNEUROSCI.1836-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi R, Perkins G. Dynamics of mitochondrial structure during apoptosis and the enigma of Opa1. Biochim Biophys Acta. 2009;1787:963–972. doi: 10.1016/j.bbabio.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardoso SM, Rego AC, Penacho N, Oliveira CR. Apoptotic cell death induced by hydrogen peroxide in NT2 parental and mitochondrial DNA depleted cells. Neurochem Int. 2004;45:693–698. doi: 10.1016/j.neuint.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YQ, Herman B. Expression and modification of ARC (apoptosis repressor with a CARD domain) is distinctly regulated by oxidative stress in cancer cells. J Cell Biochem. 2008;104:818–825. doi: 10.1002/jcb.21666. [DOI] [PubMed] [Google Scholar]
- 44.Zhang XD, Wang Y, Wu JC, Lin F, Han R, Han F, Fukunaga K, Qin ZH. Down-regulation of Bcl-2 enhances autophagy activation and cell death induced by mitochondrial dysfunction in rat striatum. J Neurosci Res. 2009;87:3600–3610. doi: 10.1002/jnr.22152. [DOI] [PubMed] [Google Scholar]
- 45.Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2009;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui H, Chen B, Chicoine LG, Nelin LD. Overexpression of cationic amino acid transporter-1 increases nitric oxide production in hypoxic human pulmonary microvascular endothelial cells. Clin Exp Pharmacol Physiol. 2011;38:796–803. doi: 10.1111/j.1440-1681.2011.05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumont M, Stack C, Elipenahli C, Jainuddin S, Gerges M, Starkova NN, Yang L, Starkov AA, Beal F. Behavioral deficit, oxidative stress, and mitochondrial dysfunction precede tau pathology in P301S transgenic mice. FASEB J. 2011;25:4063–4072. doi: 10.1096/fj.11-186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho AK, Terriff DL, Price DM, Wloka MT, Chik CL. The role of inducible repressor proteins in the adrenergic induction of arylalkylamine-N-acetyltransferase and mitogen-activated protein kinase phosphatase-1 in rat pinealocytes. Endocrinology. 2007;148:743–751. doi: 10.1210/en.2006-1154. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Schulz E, Wenzel P, Munzel T, Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhandari A, Holmes CP, Szardenings AK. Alpha, alpha-difluoro-beta-ketophosphonates as potent inhibitors of protein tyrosine phosphatase 1B. Bioorg Med Chem Lett. 2004;14:4301–4306. doi: 10.1016/j.bmcl.2004.05.082. [DOI] [PubMed] [Google Scholar]
- 51.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuentes F, Zimmer D, Atienza M, Schottenfeld J, Penkala I, Bale T, Bence KK, Arregui CO. Protein tyrosine phosphatase PTP1B is involved in hippocampal synapse formation and learning. PLoS ONE. 2012;7:e41536. doi: 10.1371/journal.pone.0041536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans DB, Rank KB, Bhattacharya K, Thomsen DR, Gurney ME, Sharma SK. Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau’s ability to promote microtubule assembly. J Biol Chem. 2000;275:24977–24983. doi: 10.1074/jbc.M000808200. [DOI] [PubMed] [Google Scholar]
- 54.Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- 56.Kaufman AM, Milnerwood AJ, Sepers MD, Coquinco A, She K, Wang L, Lee H, Craig AM, Cynader M, Raymond LA. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci. 2012;32:3992–4003. doi: 10.1523/JNEUROSCI.4129-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Q, Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology. 2013;74:69–75. doi: 10.1016/j.neuropharm.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez M, Lamas M. NMDA receptor mediates proliferation and CREB phosphorylation in postnatal Muller glia-derived retinal progenitors. Mol Vis. 2009;15:713–721. [PMC free article] [PubMed] [Google Scholar]
- 59.Gomperts SN, Carroll R, Malenka RC, Nicoll RA. Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. J Neurosci. 2000;20:2229–2237. doi: 10.1523/JNEUROSCI.20-06-02229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 61.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Townsend M, Mehta T, Selkoe DJ. Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Rowan MJ, Anwyl R. Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci. 2004;24:6049–6056. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. J Neurophysiol. 2004;91:2437–2444. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- 65.Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 67.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansen T, Rehfeld JF, Nielsen FC. GSK-3beta reduces cAMP-induced cholecystokinin gene expression by inhibiting CREB binding. Neuroreport. 2004;15:841–845. doi: 10.1097/00001756-200404090-00021. [DOI] [PubMed] [Google Scholar]
- 69.Koivisto L, Hakkinen L, Matsumoto K, McCulloch CA, Yamada KM, Larjava H. Glycogen synthase kinase-3 regulates cytoskeleton and translocation of Rac1 in long cellular extensions of human keratinocytes. Exp Cell Res. 2004;293:68–80. doi: 10.1016/j.yexcr.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 70.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 72.Hernandez F, Gomez de Barreda E, Fuster-Matanzo A, Lucas JJ, Avila J. GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol. 2010;223:322–325. doi: 10.1016/j.expneurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu GP, Zhang Y, Yao XQ, Zhang CE, Fang J, Wang Q, Wang JZ. Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol Aging. 2008;29:1348–1358. doi: 10.1016/j.neurobiolaging.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 2008;105:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 77.Jouvenceau A, Dutar P. A role for the protein phosphatase 2B in altered hippocampal synaptic plasticity in the aged rat. J Physiol Paris. 2006;99:154–161. doi: 10.1016/j.jphysparis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Kamsler A, Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol Neurobiol. 2004;29:167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- 79.Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- 82.Ermak G, Morgan TE, Davies KJ. Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer’s disease. J Biol Chem. 2001;276:38787–38794. doi: 10.1074/jbc.M102829200. [DOI] [PubMed] [Google Scholar]
- 83.Cook CN, Hejna MJ, Magnuson DJ, Lee JM. Expression of calcipressin1, an inhibitor of the phosphatase calcineurin, is altered with aging and Alzheimer’s disease. J Alzheimers Dis. 2005;8:63–73. doi: 10.3233/jad-2005-8108. [DOI] [PubMed] [Google Scholar]
- 84.Koss DJ, Hindley KP, Riedel G, Platt B. Modulation of hippocampal calcium signalling and plasticity by serine/threonine protein phosphatases. J Neurochem. 2007;102:1009–1023. doi: 10.1111/j.1471-4159.2007.04579.x. [DOI] [PubMed] [Google Scholar]
- 85.Lu Y, Rosenberg PA. NMDA receptor-mediated extracellular adenosine accumulation is blocked by phosphatase 1/2A inhibitors. Brain Res. 2007;1155:116–124. doi: 10.1016/j.brainres.2007.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Butterfield DA, Pocernich CB. The glutamatergic system and Alzheimer’s disease: therapeutic implications. CNS Drugs. 2003;17:641–652. doi: 10.2165/00023210-200317090-00004. [DOI] [PubMed] [Google Scholar]
- 87.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 88.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2012;68:1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li YY, Cui JG, Hill JM, Bhattacharjee S, Zhao Y, Lukiw WJ. Increased expression of miRNA-146a in Alzheimer’s disease transgenic mouse models. Neurosci Lett. 2011;487:94–98. doi: 10.1016/j.neulet.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McKinney RA. Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. J Physiol. 2010;588:107–116. doi: 10.1113/jphysiol.2009.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 92.Mondragon-Rodriguez S, Trillaud-Doppia E, Dudilot A, Bourgeois C, Lauzon M, Leclerc N, Boehm J. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. J Biol Chem. 2012;287:32040–32053. doi: 10.1074/jbc.M112.401240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreno H, Choi S, Yu E, Brusco J, Avila J, Moreira JE, Sugimori M, Llinas RR. Blocking effects of human tau on squid giant synapse transmission and its prevention by T-817 MA. Front Synaptic Neurosci. 2011;3:3. doi: 10.3389/fnsyn.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ittner LM, Gotz J. Amyloid-beta and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 95.Zhang L, Li YH, Meng K, Xie W. The expressions of AMPAR/GluR2 in hippocampal CA1 area of rats before and after late-phase long-term potentiation reversal. Sheng Li Xue Bao. 2010;62:23–29. [PubMed] [Google Scholar]
- 96.Zhang H, Wu CY, Wang W, Harrington MA. Interneuronal synapses formed by motor neurons appear to be glutamatergic. Neuroreport. 2011;22:809–813. doi: 10.1097/WNR.0b013e32834b6d5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez-Otano I, Ehlers MD. Learning from NMDA receptor trafficking: clues to the development and maturation of glutamatergic synapses. Neurosignals. 2004;13:175–189. doi: 10.1159/000077524. [DOI] [PubMed] [Google Scholar]
- 99.Desilva TM, Kinney HC, Borenstein NS, Trachtenberg FL, Irwin N, Volpe JJ, Rosenberg PA. The glutamate transporter EAAT2 is transiently expressed in developing human cerebral white matter. J Comp Neurol. 2007;501:879–890. doi: 10.1002/cne.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ascher P, Nowak L. Early biophysics of the NMDA receptor channel. J Physiol. 2009;587:4563–4564. doi: 10.1113/jphysiol.2009.178640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bleich S, Sperling W, Wiltfang J, Maler JM, Kornhuber J. Excitatory neurotransmission in alcoholism. Fortschr Neurol Psychiatr. 2003;71:S36–S44. doi: 10.1055/s-2003-40504. [DOI] [PubMed] [Google Scholar]
- 102.Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 103.Foster TC, Kumar A. Calcium dysregulation in the aging brain. Neuroscientist. 2002;8:297–301. doi: 10.1177/107385840200800404. [DOI] [PubMed] [Google Scholar]
- 104.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 105.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 107.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Souza MA, Magni DV, Guerra GP, Oliveira MS, Furian AF, Pereira L, Marquez SV, Ferreira J, Fighera MR, Royes LF. Involvement of hippocampal CAMKII/CREB signaling in the spatial memory retention induced by creatine. Amino Acids. 2012;43:2491–2503. doi: 10.1007/s00726-012-1329-4. [DOI] [PubMed] [Google Scholar]
- 109.Vierci G, Oliveira CS, Perera LR, Bornia N, Leal RB, Rossi FM. CREB is modulated in the mouse superior colliculus in developmental and experimentally-induced models of plasticity. Int J Dev Neurosci. 2013;31:46–52. doi: 10.1016/j.ijdevneu.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 111.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 112.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/ CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li XY, Zhan XR, Liu XM, Wang XC. CREB is a regulatory target for the protein kinase Akt/PKB in the differentiation of pancreatic ductal cells into islet beta-cells mediated by hepatocyte growth factor. Biochem Biophys Res Commun. 2011;404:711–716. doi: 10.1016/j.bbrc.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 114.Arlt S, Müller-Thomsen TM, Beisiegel U, Kontush A. Effect of one-year vitamin C- and E-supplementation on cerebrospinal fluid oxidation parameters and clinical course in Alzheimer’s disease. Neurochem Res. 2012;37:2706–2714. doi: 10.1007/s11064-012-0860-8. [DOI] [PubMed] [Google Scholar]
- 115.Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bagi Z, Cseko C, Toth E, Koller A. Oxidative stress-induced dysregulation of arteriolar wall shear stress and blood pressure in hyperhomocysteinemia is prevented by chronic vitamin C treatment. Am J Physiol Heart Circ Physiol. 2003;285:2277–2283. doi: 10.1152/ajpheart.00448.2003. [DOI] [PubMed] [Google Scholar]
- 117.Nadeau A, Roberge AG. Effects of vitamin B12 supplementation on choline acetyltransferase activity in cat brain. Int J Vitam Nutr Res. 1988;58:402–406. [PubMed] [Google Scholar]
- 118.Ikeda T, Yamamoto K, Takahashi K. Treatment of Alzheimer-type dementia with intravenous methylcobalamin. Clin Ther. 1992;14:426–437. [PubMed] [Google Scholar]
- 119.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 120.Sung S, Yao Y, Uryu K, et al. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 121.Nakashima H, Ishihara T, Yokota O, et al. Effects of α-tocopherol on an animal model of tauopathies. Free Radic Biol Med. 2004;37:176–186. doi: 10.1016/j.freeradbiomed.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 122.Dias-Santagata D, Fulga TA, Duttaroy A, Feany MB. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J Clin Investig. 2007;117:236–245. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pavlik VN, Doody RS, Rountree SD, Darby EJ. Vitamin E use is associated with improved survival in an Alzheimer’s disease cohort. Dement Geriatr Cogn Disord. 2009;28:536–540. doi: 10.1159/000255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bolognesi ML, Bergamini C, Fato R, Oiry J, Vasseur JJ, Smietana M. Synthesis of new lipoic acid conjugates and evaluation of their free radical scavenging and neuroprotective activities. Chem Biol Drug Des. 2014;83:688–696. doi: 10.1111/cbdd.12282. [DOI] [PubMed] [Google Scholar]
- 125.Sancheti H, Kanamori K, Patil I, Díaz Brinton R, Ross BD, Cadenas E. Reversal of metabolic deficits by lipoic acid in a triple transgenic mouse model of Alzheimer’s disease: a 13C NMR study. J Cereb Blood Flow Metab. 2014;34:288–296. doi: 10.1038/jcbfm.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kryscio RJ, Abner EL, Schmitt FA, et al. A randomized controlled Alzheimer’s disease prevention trial’s evolution into an exposure trial: the PREADViSE trial. J Nutr Health Aging. 2013;17:72–75. doi: 10.1007/s12603-012-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Esposito L, Raber J, Kekonius L, et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rajasekar N, Dwivedi S, Tota SK, Kamat PK, Hanif K, Nath C, Shukla R. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur J Pharmacol. 2013;715:381–394. doi: 10.1016/j.ejphar.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 129.Ringman M, Cole GM, Tend E. Oral curcumin for the treatment of mild-to-moderate Alzheimer’s disease: tolerability and clinical and biomarker efficacy results of a placebo-controlled 24-week study. Proceedings of the International Conference of Alzheimer’s disease; Chicago. 2008. abstract. [Google Scholar]
- 130.Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a vitamin/nutriceutical formulation for moderate-stage to later-stage Alzheimer’s disease: a placebo-controlled pilot study. Am J Alzheimers Dis Other Demen. 2009;24:27–33. doi: 10.1177/1533317508325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chan A, Remington R, Kotyla E, Lepore A, Zemianek J, Shea TB. A vitamin/nutriceutical formulation improves memory and cognitive performance in community-dwelling adults without dementia. J Nutr Health Aging. 2010;14:224–230. doi: 10.1007/s12603-010-0054-5. [DOI] [PubMed] [Google Scholar]
- 132.Gutzmann H, Hadler D. Sustained efficacy and safety of idebenone in the treatment of Alzheimer’s disease: update on a two-year double-blind multicentre study. J Neural Transm Suppl. 1998;54:301–310. doi: 10.1007/978-3-7091-7508-8_30. [DOI] [PubMed] [Google Scholar]
- 133.Thal LJ, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, Weiner MFF, Zamrini E, Thomas RG. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology. 2003;61:1498–1502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- 134.Weyer G, Babej-Dölle RM, Hadler D, Hoffmann S, Hermann WM. A controlled study of 2 doses of idebenone in the treatment of Alzheimer’s disease. Neuropsychobiology. 1997;36:73–82. doi: 10.1159/000119366. [DOI] [PubMed] [Google Scholar]
- 135.Mulnard RA, Cotman CW, Kawas C, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. J Am Med Assoc. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 136.Singh S, Thakur MK. Gonadal steroids do not affect apolipoprotein E expression in aging mouse cerebral cortex. Cell Mol Neurobiol. 2011;31:401–405. doi: 10.1007/s10571-010-9631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boldogh I, Liebenthal D, Hughes TK, et al. Modulation of 4HNE-mediated signaling by proline-rich peptides from ovine colostrum. J Mol Neurosci. 2003;20:125–134. doi: 10.1385/JMN:20:2:125. [DOI] [PubMed] [Google Scholar]
- 138.Zabłocka A, Janusz M. Effect of the proline-rich polypeptide complex/colostrinin™ on the enzymatic antioxidant system. Arch Immunol Ther Exp. 2012;60:383–390. doi: 10.1007/s00005-012-0187-9. [DOI] [PubMed] [Google Scholar]
- 139.Leszek J, Inglot AD, Janusz M, et al. Colostrinin proline-rich polypeptide complex fromovine colostrum—a long-term study of its efficacy in Alzheimer’s disease. Med Sci Monit. 2002;8:I93–I96. [PubMed] [Google Scholar]
- 140.Bilikiewicz A, Gaus W. Colostrinin1 (a naturally occurring proline-rich, polypeptide mixture) in the treatment of Alzheimer’s disease. J Alzheimers Dis. 2004;6:17–26. doi: 10.3233/jad-2004-6103. [DOI] [PubMed] [Google Scholar]
- 141.Jia N, Han K, Kong JJ, Zhang XM, Sha S, Ren GR, Cao YP. (−)-Epigallocatechin-3-gallate alleviates spatial memory impairment in APP/PS1 mice by restoring IRS-1 signaling defects in the hippocampus. Mol Cell Biochem. 2013;380:211–218. doi: 10.1007/s11010-013-1675-x. [DOI] [PubMed] [Google Scholar]
- 142.Li SY, Wang XB, Kong LY. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur J Med Chem. 2014;71:36–45. doi: 10.1016/j.ejmech.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 143.Lu C, Guo Y, Yan J, Luo Z, Luo HB, Yan M, Huang L, Li X. Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer’s disease. J Med Chem. 2013;56:5843–5859. doi: 10.1021/jm400567s. [DOI] [PubMed] [Google Scholar]
- 144.Jones RW. Dimebon disappointment. Alzheimers Res Ther. 2010;2:25. doi: 10.1186/alzrt49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Steele JW, Gandy S. Latrepirdine (Dimebon®), a potential Alzheimer therapeutic, regulates autophagy and neuropathology in an Alzheimer mouse model. Autophagy. 2013;9:617–818. doi: 10.4161/auto.23487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bharadwaj PR, Bates KA, Porter T, Teimouri E, Perry G, Steele JW, Gandy S, Groth D, Martins RN, Verdile G. Latrepirdine: molecular mechanisms underlying potential therapeutic roles in Alzheimer’s and other neurodegenerative diseases. Transl Psychiatry. 2013;3:e332. doi: 10.1038/tp.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf-Wagner S, Waichunas D, Bumgarner L, Bourdette D, Silbert L, Kaye J. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J Alzheimers Dis. 2014;38:111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hardas SS, Sultana R, Clark AM, Beckett TL, Szweda LI, Murphy MP, Butterfield DA. Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. Redox Biol. 2013;1:80–85. doi: 10.1016/j.redox.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quinn JF, Bussiere JR, Hammond RS, et al. Chronic dietary α-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 150.Lu P, Mamiya T, Lu LL, et al. Silibinin prevents amyloid b peptide-induced memory impairment and oxidative stress in mice. Br J Pharmacol. 2009;157:1270–1277. doi: 10.1111/j.1476-5381.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tota S, Kamat PK, Shukla R, Nath C. Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment. Behav Brain Res. 2011;221:207–215. doi: 10.1016/j.bbr.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 152.Tota S, Awasthi H, Kamat PK, Nath C, Hanif K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav Brain Res. 2010;209:73–79. doi: 10.1016/j.bbr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 153.Feng Z, Zhang JT. Protective effect of melatonin on β-amyloid-induced apoptosis in rat astroglioma c6 cells and its mechanism. Free Radic Biol Med. 2004;37:1790–1801. doi: 10.1016/j.freeradbiomed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 154.Matsubara E, Bryant-Thomas T, Quinto JP, et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- 155.Saxena G, Bharti S, Kamat PK, Sharma S, Nath C. Melatonin alleviates memory deficits and neuronal degeneration induced by intracerebroventricular administration of streptozotocin in rats. Pharmacol Biochem Behav. 2010;94:397–403. doi: 10.1016/j.pbb.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 156.Prasanthi JRP, Dasari B, Marwarha G, et al. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic Biol Med. 2010;49:1212–1220. doi: 10.1016/j.freeradbiomed.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Andrieu S, Ousset PJ, Coley N, Ouzid M, Mathiex-Fortunet H, Vellas B The GuidAge study Group. GuidAge study: a 5-year double-blind, randomized trial of EGb761 for the prevention of Alzheimer’s disease in elderly subjects with memory complaints rationale, design and baseline data. Curr Alzheimer Res. 2008;5:406–415. doi: 10.2174/156720508785132271. [DOI] [PubMed] [Google Scholar]
- 158.Vellas B, Coley N, Ousset PJ, et al. Long-term use of standardized ginkgo biloba extract for the prevention of Alzheimer’ disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11:851–859. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- 159.Snitz BE, O’Meara ES, Carlson MC, Arnold AM, Ives DG, Rapp SR, Saxton J, Lopez OL, Dunn LO, Sink KM, DeKosky ST Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saxena G, Singh SP, Pal R, Singh S, Pratap R, Nath C. Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav. 2007;86:797–805. doi: 10.1016/j.pbb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 161.Niranjan R, Kamat PK, Nath C, Shukla R. Evaluation of guggulipid and nimesulide on production of inflammatory mediators and GFAP expression in LPS stimulated rat astrocytoma, cell line (C6) J Ethnopharmacol. 2010;127:625–630. doi: 10.1016/j.jep.2009.12.012. [DOI] [PubMed] [Google Scholar]