Abstract
Decades of studies have shown evolutionarily conserved molecular networks consisting of transcriptional factors, diffusing growth factors, and signaling pathways that regulate proper lung development. Recently, microRNAs (miRNAs), small, noncoding regulatory RNAs, have been integrated into these networks. Significant advances have been made in characterizing the developmental stage– or cell type–specific miRNAs during lung development by using approaches such as genome-wide profiling and in situ hybridization. Results from gain- or loss-of-function studies revealed pivotal roles of protein components of the miRNA pathway and individual miRNAs in regulating proliferation, apoptosis, differentiation, and morphogenesis during lung development. Aberrant expression or functions of these components have been associated with pulmonary disorders, suggesting their involvement in pathogenesis of these diseases. Moreover, genetically modified mice generated in these studies have become useful models of human lung diseases. Challenges in this field include characterization of collective function and responsible targets of miRNAs specifically expressed during lung development, and translation of these basic findings into clinically relevant information for better understanding of human diseases. The goal of this review is to discuss the recent progress on the understanding of how the miRNA pathway regulates lung development, how dysregulation of miRNA activities contributes to pathogenesis of related pulmonary diseases, and to identify relevant questions and future directions.
Keywords: miRNA, protein components of the miRNA pathway, post-transcriptional regulation, lung development and pulmonary diseases
Lung development begins with the specification of trachea and lung progenitors in the foregut endoderm. This is followed by endoderm budding and branching, and formation of tree-like conducting airways. At the end of this branching process, primitive saccules are formed at the distal end of epithelial tubules, along with the expansion of the lumen and the appearance of alveolar type I and type II cells. These primitive saccules then undergo alveologenesis to form numerous smaller, mature alveolar units for gas exchange. In the trachea and conducting airways, the epithelial progenitors differentiate into diverse cell types, including ciliated, secretory, neuroendocrine, and basal cells. As the epithelial tubules branch and differentiate, mesenchymal derivatives also develop to form structures, such as the airway smooth muscle, the pulmonary and bronchial vascular systems, and interstitial components of alveoli.
Molecular regulatory networks, consisting of fibroblast growth factors (FGF), Sonic Hedgehog (Shh), bone morphogenetic proteins (BMPs), transforming growth factor (TGF), retinoic acid, wingless-type MMTV integration site family (Wnt), Notch, and various transcription factors, have been implicated in regulating the branching and differentiation of both lung epithelial and mesenchymal components (1–8). The mesenchymal–epithelial interactions mediated by diffusible growth factors play instrumental roles in coordinating the proper branching and differentiation (9, 10). Defects in these pathways lead to aberrant lung development that has been associated with human diseases, such as familial pulmonary hypertension, pediatric cancers, asthma, pulmonary fibrosis, and chronic obstructive pulmonary disease (11–16). Recent studies have shown that the miRNA pathway is an essential part of the complex regulatory networks that regulate lung development. Here, we summarize recent findings on roles of the protein components of the miRNA pathway and individual miRNAs in lung development, and in the pathogenesis of related pulmonary disorders. Due to space limitations, topics such as miRNAs in lung infection, immune response, and inflammation are not discussed. In addition, some miRNAs that have significant roles in pathogenesis of particular pulmonary diseases, such as idiopathic pulmonary fibrosis (IPF), asthma, lung cancer, or pulmonary hypertension, are not discussed, and can be found in several recent, excellent review articles (17–28).
Overview of the miRNA Pathway
miRNAs are endogenous, small, noncoding RNAs that regulate gene expression post-transcriptionally, and play important roles in diverse biological processes, including development, metabolism, cell differentiation, and proliferation (29–34). Studies have revealed a large number of miRNAs in the mammalian genome, which may target over 60% of the protein-coding mRNAs. According to their genomic location, miRNAs are classified as intergenic or intragenic. Intragenic miRNAs include intronic miRNAs and exonic miRNAs that reside within the introns or exons of protein-coding genes, respectively (35). Recent studies showed that the transcription of most intronic/exonic miRNAs and their host genes are coregulated (36, 37). A significant number of miRNAs are encoded by polycistronic or bicistronic transcriptional units that generate multiple miRNAs (38). Similar to protein-coding genes, expression, processing, and function of a specific miRNA are under the control of transcriptional factors, epigenetic modification, the protein components of miRNA pathway, cellular stresses, and growth factors (39–46).
For the majority of miRNAs, the following common pathway mediated by a set of protein components is required for biogenesis and function (Figure 1 and Table 1). Primary miRNAs (pri-miRNAs) are transcribed by RNA polymerase II, which can encode single or multiple miRNAs (47, 48). Pri-miRNAs are processed by the endonuclease, drosha, ribonuclease type III (Drosha), and its cofactor, DiGeorge syndrome critical region 8 (DGCR8) into stem-loop intermediate miRNA precursors (pre-miRNAs) with imperfect base-paired stem (Figure 1). pre-miRNAs are then transported into the cytoplasm through Exportin 5/Ran, where the loop is removed by another RNase III endonuclease, dicer 1, ribonuclease type III (Dicer1), and its associated protein, TAR (the human immunodeficiency virus transactivation response element) RNA binding protein 2, to form double-stranded miRNA:miRNA* (the star strand) duplex (Figure 1) (49, 50). This is followed by the interaction of Argonaute (Agos) proteins with a mature miRNA and the formation of miRNA-induced silencing complex (miRISC), which orient a miRNA for binding to its complementary site, generally located within the 3′ untranslated region (UTR) of a target mRNA (Figure 1). miRISCs are usually aggregated and concentrated in cytoplasmic granules named GW/p-bodies, involved in miRNA-mediated translation suppression, or target mRNA cleavage or deadenylation and degradation. It is still unclear whether translational suppression and deadenylation/degradation are coupled or independent events. Translational suppression by miRNA can happen without trinucleotide repeat containing 6 (Tnrc6) family members in Drosophila S2 cells (51). However, Tnrc6 is required for deadenylation and degradation. Extensive biochemical studies support the current model, in which Tnrc6 is directly recruited by Ago/miRNA complex. Tnrc6 then binds directly to poly (A)–binding protein and recruits deadenylase complexes to the 3′UTR of target mRNA (52). This leads to deadenylation, and eventually decapping and mRNA degradation (Figure 1) (52).
Figure 1.
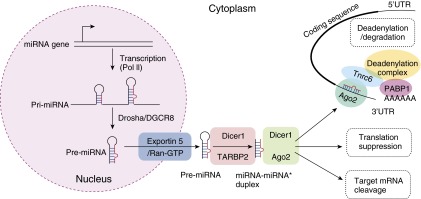
The miRNA biogenesis and function. miRNA genes are transcribed by RNA polymerase II (Pol II) to form long primary miRNAs (pri-miRNAs). pri-miRNA is processed by RNase III Drosha and DiGeorge syndrome critical region 8 (DGCR8), yielding a hairpin-shaped miRNA precursor (pre-miRNA) with the length of roughly 70 nucleotides. The pre-miRNA is exported from the nucleus into the cytoplasm by Exportin 5/Ran-GTP (GTP binding RAN, member RAS oncogene family). In cytoplasm, pre-miRNA is further processed by Dicer1, an RNase III enzyme, resulting in an imperfect miRNA:miRNA* (the star strand) duplex. The mature miRNA forms miRNA-induced silencing complex (miRISC) together with Argonaute (Ago). Mature miRNA then guides miRISC to find its complementary binding sites, usually within the 3′ untranslated region (UTR) of target mRNA, where additional proteins, such as trinucleotide repeat containing 6 (Tnrc6) and deadenylation complexes, are recruited. This eventually leads to translational repression or deadenylation and mRNA degradation. PABP1, poly (A)–binding protein 1; TARBP2, TAR (HIV transactivation response element) RNA binding protein 2.
Table 1.
The Protein Components of the miRNA Pathway
| |
|
|
Mutant Mice |
Lung Phenotype |
|---|---|---|---|---|
| Gene Symbol | Description | Function | Author, Yr (Ref. No.) | Author, Yr (Ref. No.) |
| Drosha | Drosha, RNase type III | pri-miRNA processing | Zhdanov et al., 2011 (149) | Not examined |
| DGCR8 | DiGeorge syndrome critical region gene 8 | pri-miRNA processing | Wang et al., 2007 (150) | Not examined |
| Xpo5 | Exportin5 | Exporting pre-miRNA from nuclei to cytoplasm | Not available | Not examined |
| Ran | RAN, member RAS oncogene family | Exporting pre-miRNA from nuclei to cytoplasm | Not available | Not examined |
| Dicer1 | Dicer1, RNase type III | miRNA processing | Harris et al., 2006 (64) | Harris et al., 2006 (64) |
| Tarbp2 | TAR (HIV) RNA binding protein 2 | miRNA processing | Not available | Not examined |
| Ago1 | Argonaute RISC catalytic subunit 1 | miRNA-induced silencing complex | Wang et al., 2012 (62) | Not examined |
| Ago2 | Argonaute RISC catalytic subunit 2 | miRNA-induced silencing complex | Liu, 2004 (34) | Not examined |
| Ago3 | Argonaute RISC catalytic subunit 3 | miRNA-induced silencing complex | Van Stry, et al., 2012 (151) | Not examined |
| Ago4 | Argonaute RISC catalytic subunit 4 | miRNA-induced silencing complex | Qi et al., 2006 (152) | Not examined |
| Tnrc6a | Trinucleotide repeat containing 6a | miRNA-induced silencing complex | Jiang et al., 2012 (63) | Not examined |
| Tnrc6b | Trinucleotide repeat containing 6b | miRNA-induced silencing complex | Not available | Not examined |
| Tnrc6c | Trinucleotide repeat containing 6c | miRNA-induced silencing complex | Not available | Not examined |
Definition of abbreviations: Ago, Argonaute; DGCR8, DiGeorge syndrome critical region 8; Dicer1, dicer 1, RNase type III; Drosha, drosha, RNase type III; HIV, human immunodeficiency virus; miRNA, microRNA; pre-miRNA, miRNA precursor; pri-miRNA, primary miRNA; RAN, ras-related nuclear protein; RAS, rat sarcoma viral oncogene; RISC, RNA-induced silencing complex; TAR, HIV transactivation response element; Tnrc, trinucleotide repeat containing; Xpo, exportin.
Most mammalian miRNAs form imperfect base pairs with complementary binding sites within 3′UTR of target mRNAs. Although the “seed sequence” (nucleotides 2–8 of miRNA) is essential for target recognition (53), pairing of other regions also have modest impact for miRNA:mRNA binding (53). Algorithms based on a combined score of seed pairing and the influences of other regions in miRNA-target recognition, together with the preferential evolution conservation, have been used to predict miRNA targets. Predicted miRNA target databases, such as Targetscan (http://www.targetscan.org/), are the first place to look for potential targets. However, false-positive or negative predictions do exist, as the miRNA:mRNA interaction can be influenced by binding site accessibility, RNA secondary structure, and proximity of sites for other miRNAs or RNA binding proteins (53).
In general, each mammalian miRNA has a large number of predicted targets (averaging ∼ 300) (53). Results from in vitro and in vivo studies showed that the effect of miRNA on the protein synthesis of a single mRNA target is modest; therefore, miRNAs are often considered to be fine tuners. Although the impact for individual targets is modest, miRNAs can significantly influence growth, differentiation, metabolism, and apoptosis by regulating the expression of multiple targets that function at different steps of the same biological process (29, 54). In addition, miRNA might target distinct pathways and biological processes in different cell types. 3′UTR reporter assay is the main approach for proving whether the suppression of target expression by miRNA depends on 3′UTR binding sites. However, due to the overexpression of both miRNAs and 3′UTR luciferase reporters in this assay, some validated targets in vitro might not represent bona fide responsible targets in vivo. Additional studies of expression of miRNA and its targets in vivo are required to establish this regulatory relationship. Therefore, identification of responsible targets or downstream pathways of miRNAs remains a major challenge.
Signaling pathways important for lung development can modulate the stability and activity of miRNA processing complexes (41, 43, 55). In several recent reports, the Hippo signaling pathway has been shown to play a significant role in regulating miRNA biogenesis in a cell density–dependent manner. In cells of low density, Yamaguchi sarcoma virus oncogen (Yes)-associated protein (Yap), the downstream component of the Hippo pathway, mainly localizes in the nucleus, where it binds and sequesters p72 (DEAD [Asp-Glu-Ala-Asp] box helicase 17). With the increase of cell density, Yap translocates to the cytoplasm and releases p72, which then forms complexes with Drosha and promotes the production of miRNAs (46). Interestingly, in another study, nuclear transcriptional coactivator Tafazzin (TAZ), which is abundant at low cell density, is required for efficient pre-miRNA processing (45). The Hippo pathway plays significant roles in development and diseases (56). In mouse lung, disruption of Yap leads to early branching defect (57). Moreover, Yap is required for maintenance of adult airway progenitor cells (58). Deregulation of Hippo pathway has been associated with different cancers, including lung cancer (59–61). It is plausible that, both in development and diseases, Hippo pathway might influence cell behavior, partially by modulating miRNA activities.
Expression of subsets of miRNAs either for promotion or inhibition of cell growth are selectively regulated by phosphorylating TAR RNA binding protein 2, the partner of Dicer1 in mammalian cells by mitogen-activated protein kinase (MAPK)/extracellular signal–regulated kinase (ERK), which can be activated by serum or growth factors, such as FGFs (43). These indicate that signaling pathways regulate the miRNA machinery to achieve specific biological responses. In addition, multiple Agos and Tnrc6 family members are known to play a role within the miRISC at the effective step of the miRNA pathway. Reports showed that they are differentially expressed in developing tissues, such as skin and yolk sac (62, 63). In the case of skin, the proportion of miRNAs associated with Ago family members correlates with the abundance of each Ago protein (62). This indicates the activities of miRNAs can be regulated by the expression or modification of these protein components.
miRNAs and Protein Components of the miRNA Pathway in Lung Endoderm
Dicer1 in Mouse Lung Development
An important role of the miRNA pathway in lung development was first shown by the conditional deletion of Dicer1 in the endoderm of primary lung bud (64). At Embryonic Day 15.5 (15.5 d post coitum), the mutant lungs showed the formation of a large epithelial sac within each lobe instead of tree-like airway structures, likely due to significant reduction of branching activities in early stages of development (64). Epithelial cells in these mutant lungs continued to grow; however, aberrant cell death was observed in the epithelium, where Dicer1 was inactivated (64). Although Dicer1 was deleted in the epithelium, expression of FGF10 in the mesenchyme was up-regulated. This likely leads to changes in the expression of other genes, such as BMP4 and Sprouty. These observations suggest that genes downstream of the miRNA pathway in lung epithelium play critical roles in epithelial–mesenchymal interactions essential for proper lung morphogenesis (64).
DICER1 in Lung Cancers
Significantly, germline mutation of DICER1 is associated with human pleuropulmonary blastoma (PPB), a rare, inherited pediatric lung tumor (65). Recently, DICER1 mutations have been associated with cystic nephroma, medulloepithelioma, and Sertoli-Leydig cell tumors (65–67). The early stages of PPB feature cystic expansion of airways lined by benign-appearing epithelium. This is followed by the malignant transformation of mesenchymal cells adjacent to abnormal epithelium, and the formation of sarcoma (65). These phenotypes resemble the epithelial sac and mesenchymal expansion observed in Dicer1 conditional mutant mice (64). Although most patients with PPB harbor a heterozygous germline mutation of DICER1, expression of DICER1 protein was undetectable in segments of epithelium of PPB tumors, likely due to additional somatic mutation. However, heterozygous DICER1 expression was retained in the malignant mesenchyme. This combination of heterozygous germline mutation together with segmental loss of DICER1 in epithelium is different as compared with endoderm deletion of Dicer1 in mutant mice. This likely explains why PPB lungs are functional at birth, as compared with mouse lungs of Dicer1 mutant. Together, these results suggest that loss of DICER1 in the epithelium of human developing lungs alters the expression of diffusible factors that are important for epithelial–mesenchymal interactions, which leads to abnormal growth of mesenchymal cells in PPB. Although findings from mouse genetic studies shed light on the pathogenesis of PPB, more sophisticated genetic manipulation is required to potentially generate an animal model for PPB.
Heterozygous deletion of Dicer1 in airway epithelium enhances tumor development in a mouse model of K-Ras–induced lung cancer (68), strongly suggesting that Dicer1 functions as a haploinsufficient tumor suppressor (69). This is further supported by observations of reduced expression of DICER1 and other miRNA pathway components, and a global down-regulation of miRNAs in human tumor tissues, including those of lung cancers (70–72).
miR-17-92 Clusters in the Proliferation of Epithelial Progenitors
miRNAs from the miR-17-92 cluster and its two homologous clusters (miR106a-363 and miR-106b-25) are highly expressed during early stages of lung development, and decrease in late stages or adult lungs (73). Expression of miR-17-92 can be detected in both mesenchyme and epithelium. Overexpression of miR-17-92 in distal lung endoderm under the control of the mouse surfactant protein C (Sftpc) promoter resulted in increased proliferation and expansion of the distal progenitor population. However, the number of club and ciliated cells was reduced, together with an abnormality in the formation of terminal sacs in Embryonic Day–18.5 lungs (73). One of the direct targets of miR-17-92 in distal lung epithelial cells is Retinoblastoma-like 2 (Rbl2) (73), and down-regulation of Rbl2 in transgenic mice at least partially contributes to the increased epithelial proliferation (Figure 2). In another report, miR-17-20–106a was shown to regulate epithelial branching by suppressing signal transducer and activator of transcription 3 (Stat3) and mitogen-activated protein kinase 14 (MAPK14), the downstream components of FGF10-FGFR2 signaling. Inhibition of miR-17 paralogs leads to the upregulation of Stat3 and MAPK14 in embryonic lung epithelium cultured with FGF10 (74). Furthermore, miR-17-92 has been shown to target CCAAT/enhancer binding protein and GATA binding protein 6 in a newly isolated human lung progenitor population (E-cadherin/leucine-rich repeat containing G protein-coupled receptor 6 positive) to maintain the balance between differentiation and self-renewal (75).
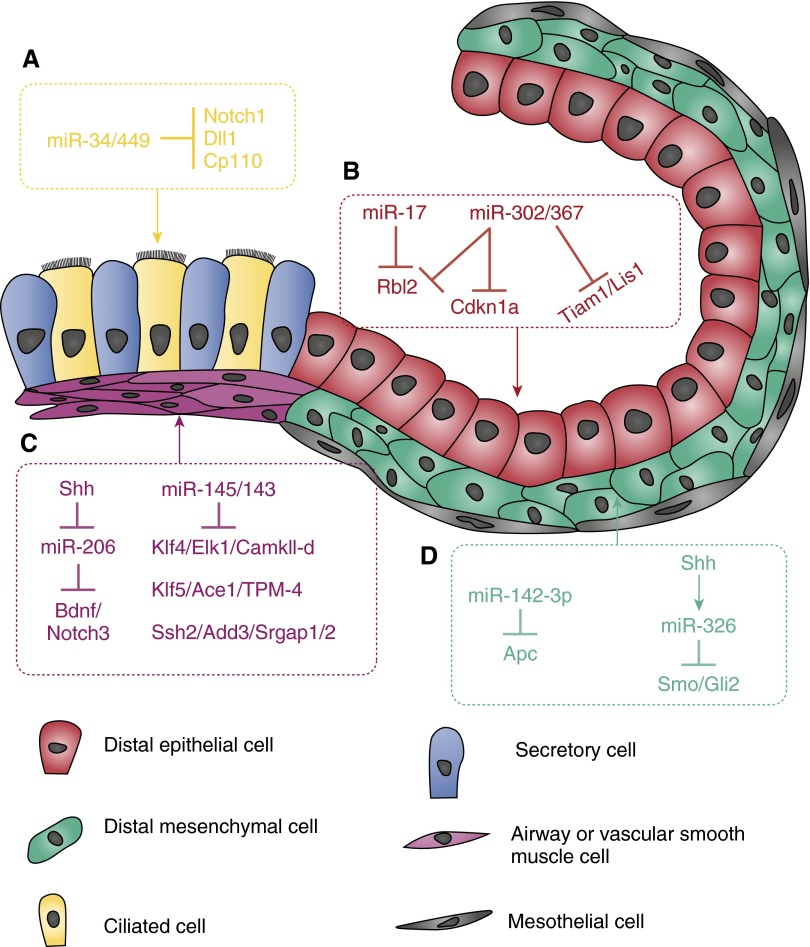
Figure 2.
The function and targets of miRNAs in different cell types of developing mouse lungs. (A) miR-34/449 promotes ciliogenesis by targeting Notch1, δ-like 1 (Dll1), and centriolar coiled coil protein 110 (Cp110). (B) Highly expressed in epithelium of early developing lungs, miR-17 and miR-302/367 promote proliferation by suppressing retinoblastoma-like (Rbl) 2 and cyclin-dependent kinase inhibitor 1A (Cdkn1a); miR-302/267 also regulates cell polarity by targeting T-cell lymphoma invasion and metastasis 1 (Tiam1) and platelet-activating factor acetylhydrolase 1b, regulatory subunit 1 (Lis1 or Pafah1b1). (C) Sonic Hedgehog (Shh) up-regulates expression of brain-derived neurotrophic factor (Bdnf) and Notch3 by suppressing miR-206, resulting in increased smooth muscle cell (SMC) proliferation and innervation; miR-145/143 promotes SMC differentiation by down-regulating Klf4 and many other genes associated with synthetic phenotype. (D) miR-142-3p and miR-326 regulate mesenchymal cell proliferation and differentiation by modulating wingless-type MMTV integration site family and Shh pathways, respectively.
Mice deficient for miR-17-92 are smaller and die shortly after birth. The most significant phenotype was severe hypoplastic lungs, as compared with other organs, such as heart. However, no specific branching or other developmental abnormality was found in these lungs (76). Moreover, results from mice with double or triple deletion of miR-17-92, miR-106b-25, and miR-106a-363 clusters showed that they cooperate functionally in regulating embryonic growth and apoptosis (76). This proves a role for miR-17-92 and its paralog clusters in promoting proliferation and growth of embryonic lungs as well as other organs in vivo. Due to its ubiquitous expression in both developing epithelium and mesenchyme, conditional knockout mice are required to dissect their activities in different cell types of embryonic lungs.
miR-17-92 Clusters in Lung Cancer
Dysregulation of developmental programs is associated with tumorigenesis. Extensive studies have shown an oncogenic role for miR-17-92 in the pathogenesis of different human cancers, including lung cancer (77, 78). Amplification and overexpression of miR-17-92 cluster is observed in lung cancer cells (77, 79, 80). In tumors, expression of miR-17-92 cluster is induced by oncogenes, such as v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (NYMC) and E2F family members (81). It promotes cell growth by targeting genes critical for growth arrest, such as cyclin-dependent kinase inhibitor 1A and retinoblastoma 1-related genes (82). Furthermore, studies have shown that inhibition of miR-17-5p and miR-20a with antisense oligonucleotides induce apoptosis selectively in lung cancer cells overexpressing miR-17-92 (83). Although the proliferation activities of miR-17-92 clusters in development are consistent with their oncogenic roles in tumors, the prevalence of miR-17-92 cluster amplification/up-regulation in lung cancers is still unclear. Because miR-106a-363 and miR-106b-25 knockout animals are viable and grow to adulthood without obvious phenotype, they are useful to test whether deletion of miR-106a-363 and miR-106b-25 in vivo will slow the initiation or progression of K-Ras–induced lung cancer.
miR-302/367 Cluster in the Proliferation and Apical–Basal Polarity of Lung Endoderm
miR-302/367 cluster is identified as a direct target of Gata6 in mouse lung endoderm (84). Similar to the miR-17-92 cluster, levels of miR-302/367 are high at early stages of lung development, but decline rapidly as development proceeds (84). Overexpression of miR-302/367 driven by a human SFTPC promoter leads to a thickening of distal epithelium and reduction in sacculation (84). In the airways of these transgenic mice, numbers of both Sox2-positive and Sox9-positive progenitor cells are significantly increased, whereas markers for both distal and proximal airways, including CC10 and Sftpc, are dramatically reduced (84). This was further supported by the reduced proliferation, together with increased differentiation, in transgenic mice, in which miR-302/367 activities were reduced by the expression of a miR-302/367 sponge, which consisted of multiple miR-302/367 binding sites (84). In the lung endoderm, miR-302/367 promotes the proliferation of undifferentiated progenitor cells, partially by targeting Rbl2 and cyclin-dependent kinase inhibitor 1A (Figure 2). miR-302/367 also regulates apical–basal polarity of lung epithelium by suppressing T-cell lymphoma invasion and metastasis 1 and platelet-activating factor acetylhydrolase, isoform 1b, subunit 1 (Lis1 or Pafah1b1) (Figure 2) (84). Little is known about the miR-302/367 cluster in human lung diseases. A recent report showed that high miR-367 is associated with unfavorable prognosis in resected non–small cell lung cancer (85).
miR-34/449 in Ciliogenesis of Airway Epithelium
miR-449a/b/c (referred to as miR-449) is transcribed from a polycistronic cluster. miR-449 is highly expressed in tissues with multiciliated cells, such as lungs (86–88). Its expression is significantly induced in the process of differentiation of both human airway mucociliary epithelial cells (HAECs) grown on an air–liquid interface and mucociliary epidermis of Xenopus embryos (88, 89). In both systems, expression of miR-449 is selectively enriched in ciliated cells. Loss or knockdown of miR-449 expression in both HAECs and Xenopus embryonic epidermis leads to significant reduction of ciliated cells. miR-449 promotes ciliogenesis by directly targeting Notch1 and δ-like 1 (Figure 2) (88). Conversely, miR-449 null mice developed normally, without gross defects. One of the explanations for the lack of phenotype is the compensatory role of miR-34 family members that share identical seed sequence and similar expression pattern with miR-449 (90, 91). miR-34 family members are highly expressed in lungs and also increased in the process of ciliogenesis of HAECs (88, 91). In miR-449 null testis, levels of miR-34 are up-regulated, suggesting a compensatory expression and function (90). Most recently, their redundant and significant roles in ciliogenesis were reported in mice deficient for all miR-34/449. Deletion of all three loci of miR-34/449 in mice resulted in a significant decrease in cilia length and cilia number, due to defective basal body maturation and apical docking. Derepression of Cp110, a centriolar protein that negatively regulates primary cilia assembly, partially contribute to the abnormal ciliogenesis in miR-34/449 null mice (Figure 2) (92).
Interestingly, levels of all miR-34/449 family members are significantly reduced in HAECs of patients with asthma and after IL13 stimulation (93). It is known that high Notch activity in airway epithelium is associated with increased numbers of mucous cells together with reduced numbers of ciliated cells (94). Furthermore, treatment of adult HAECs with Notch antagonists resulted in increased ciliated cells and decreased numbers of mucous cells (94). In addition, Notch antagonists blocked IL13-induced mucous metaplasia. Together, this suggests that reduced miR-34/449 in asthmatic airway epithelium might lead to increased Notch activity, which might contribute to the increased number of mucous cells (93).
miR-34/449 Function as a Tumor Suppressor
miR-34 is known to be a direct target of p53 in blocking cell proliferation and inducing apoptosis (40). Lost or reduced expression of miR-34 or hypermethylation of its promoter have been observed in multiple cancers, including both small cell and non–small cell lung cancers (95–97). Overexpression of miR-34 in lung cancer cells or animal models leads to inhibition of the initiation or progression of cancer or sensitization to radiotherapy (98, 99). Interestingly, the p53 pathway remains intact in miR-34 null mice. This is likely due to the redundancy of miR-449 or other p53 downstream effectors, such as Cdkn1a and BCL2-associated X protein that are working in parallel with miR-34 (91). Indeed, miR-34a deficiency strongly promotes tumorigenesis in a K-Ras–induced lung cancer when p53 is haploinsufficient, strongly suggesting a tumor suppressor activity for miR-34 (100). Unlike miR-34, expression of miR-449 is induced by the cell cycle regulatory transcription factor, E2F1, a positive regulator for cell proliferation (101). It has been suggested that miR-449 functions as a negative feedback regulator of E2F1 to avoid excessive E2F1-induced proliferation (101). Expression of miR-449 is down-regulated in non–small cell lung cancers (102). Similar to miR-34, overexpression of miR-449 leads to growth arrest of lung cancer cells by targeting myelocytomatosis oncogene and met proto-oncogene (102, 103). Although the activity of miR-449 in ciliogenesis is mainly assigned to the suppression of Notch or centriolar protein, Cp110 (Figure 2) (88), miR-449/34 might play an important role in promoting cell cycle exit, which is essential to initiate the differentiation of airway epithelial cells. It is unknown whether miR-449 deficiency alone or in combination with miR-34 deletion will promote lung tumorigenesis, similar to what has been observed in miR-34 null mice.
miR-4423 in Ciliogenesis of Human Airway Epithelium and Lung Cancer
Recently, a primate-specific miRNA, miR-4423, was found to be selectively expressed in human mucociliary epithelium. Similar to miR-449, overexpression of miR-4423 promotes ciliogenesis. Intriguingly, the seed sequence and predicted mRNA targets of miR-4423 overlap with those of miR-449/miR-34. However, whether miR-449/34 and miR-4423 synergistically regulate ciliogenesis of HAECs remains unknown. The expression of miR-4423 is also significantly reduced in a large fraction of lung cancers, including both squamous carcinomas and adenocarcinomas, relative to matched adjacent normal tissues. In addition, overexpression of miR-4423 in several lung cancer cell lines reduces their anchorage-independent growth and the size of tumors formed in xenograft model (104). The finding of primate-specific miRNAs, or large-animal–specific response or function of miR-124 (105), presents a need to develop additional model systems other than murine models.
miRNAs in Embryonic Lung Mesenchyme
miR-326 as Part of the Shh Signaling Pathway in Developing Lung Mesenchyme
In a genome-wide profiling to identify miRNAs associated with the Shh pathway in early developing lungs, expression of both miR-326 and its host gene, Arrestin β1, are found to be selectively enriched in embryonic mesenchymal cells, and are specifically regulated by Shh activity (106). Furthermore, functional analysis showed that miR-326 acts as a negative modulator for Shh signaling by directly targeting smoothened and GLI-Kruppel family member 2. This suggests a miR-326–mediated negative feedback loop in modulating Shh pathway during lung development (Figure 2) (106). In brain tumor progenitor cells, miR-326 was also shown to modulate Shh signaling by targeting smoothened (107, 108).
Recent studies showed a reactivation of the Shh pathway in bleomycin-induced fibrotic lungs and in human IPF lungs (109–111). This might lead to aberrant epithelial–fibroblast interaction and induction of fibroblast cell proliferation. In a separate report, miR-326 was shown to regulate TGFb1, E26 avian leukemia oncogene 1, 5′ domain, SMAD family member 3, and matrix metallopeptidase 9 in fibroblast cells and in a bleomycin-induced pulmonary fibrosis (112). In addition, levels of miR-326 were found to be significantly lower in IPF lung tissues as compared with control (112). This contradicts findings in early developing lung mesenchyme in which high Shh activity led to up-regulation of miR-326; however, it will be advantageous to investigate how miR-326 is down-regulated, and whether this relates to reactivation of Shh in human IPF lungs.
miR-142-3p Modulates Wnt Signaling in Embryonic Lung Mesenchyme
In another array profiling of miRNAs selectively enriched in epithelium or mesenchyme of early developing lungs, miR-142-3p was found to be selectively expressed in mesenchyme (113). Disruption miR-142-3p expression in cultured embryonic lungs using vivo-morpholino, an octa-guanidinium dendrimer–conjugated morpholino, resulted in reduced branching and ectopic expression of α-smooth muscle actin in distal lung mesenchyme. miR-142-3p influences smooth muscle cell (SMC) progenitor proliferation and differentiation by directly regulating adenomatous polyposis coli, a negative regulator of Wnt signaling (Figure 2) (113).
miRNAs and Protein Components of the miRNA Pathway in SMCs
Dicer1, Drosha, and DGCR8 in Proliferation and Differentiation of Vascular SMCs
Although the role of the miRNA pathway in the development of vascular SMCs (vSMCs) has been extensively examined in systemic arteries, such as aortas, little is known regarding its activity in airway SMCs and pulmonary vSMCs, two components of lung mesenchyme important for development and diseases. SMCs from different organs or structures share core regulatory networks; however, they are regulated by distinct environmental signals during development, and they differ in their expression of ion channels, hormone receptors, and cell-signaling pathways. Here, we review recent findings on miRNAs in vSMCs, and discuss the need to study whether airway SMCs and pulmonary vSMCs are regulated by the same or distinct mechanisms.
The important role of the miRNA pathway in vSMCs was demonstrated by the deletion of Dicer1 specifically in vSMCs. Deletion of Dicer1 by transgelin (SM22a)-Cre resulted in reduced proliferation and disrupted differentiation of vSMCs, leading to abnormal vessel wall formation and extensive hemorrhage (114, 115). SM22α-Cre is mainly expressed in vSMCs, with little or no expression in visceral SMCs. This prevents the analysis of the role of Dicer in SMCs of other organs, such as smooth muscle of lungs. Recently, Dicer1 was deleted by an inducible myosin, heavy polypeptide 11–CreERT2 in 3- to 4-week-old mice that resulted in significant lower blood pressure due to reduced expression of contractile proteins and loss of vascular contraction function (116). Myosin, heavy polypeptide 11–CreERT2 is expressed in visceral SMCs, and deletion of Dicer1 in intestinal SMCs leads to a relaxed state of gut smooth muscle (116). These observations were supported by similar defects in vSMC development in animals in which Drosha or DGCR8, two other key components in pri-miRNA processing, are specifically deleted in SMCs (117, 118). However, how deletion of Dicer1, Drosha, or DGCR8 affects airway SMCs or pulmonary vSMCs is unknown.
The defects of protein components of the miRNA pathway in SMCs of pulmonary diseases, such as asthma and pulmonary hypertension, are still poorly understood. However, there is evidence that hypoxia, which contributes to the pathogenesis of various human lung diseases, including pulmonary artery hypertension, can enhance the overall activity of the miRNA pathway in pulmonary arterial SMCs (PASMCs). Hypoxia induces the prolyl-hydroxylation and accumulation of Ago2, a key component of miRISC (119). In addition, TGF/BMP signaling regulates the expression of miR-21 and a subset of miRNAs post-transcriptionally by modulating the activity of pri-miRNA processing machinery in SMCs (41). Together, these results strongly suggest that the expression or function of protein components of the miRNA pathway can be modulated in association with developmental or disease conditions.
miR-145/143 in Regulating SMC Differentiation
Several individual miRNAs are shown to play pivotal roles in regulating vSMC differentiation. miR-145 and miR-143 are evolutionary conserved and transcribed from a polycistronic transcription unit without homology to each other (120). In adult mice, expression of the miR-145/143 cluster is regulated by serum response factor (Srf) and myocardin, and selectively enriched in SMCs, including bronchial SMCs and pulmonary vSMCs (120). The smooth muscle layers of arteries were noticeably thinner in miR-145 single-knockout or miR-145/143 double-knockout mice, likely due to a reduction in cell size (120). Results from several mouse genetic or disease models showed that loss of miR-143/miR-145 leads to disarray of actin stress fibers, diminished migratory activity of SMCs, and significantly compromised vSMC contraction (42, 120–122). Results from cell culture and tissues of knockout mice showed that genes involved in cytoskeleton organization (Ssh2, Add3, and Srgap1/2), podosome formation and migration (PDGF-R, Pkc and Fascin), and SMC proliferation and differentiation (Klf4/5, Elk1, Ace1, Tpm-4 and CamkII-δ) are under the control of miR-145 and miR-143 (42, 120–122), thus suggesting a synergetic role of these two miRNAs in regulating proper SMC phenotype (Figure 2). These defects in SMC differentiation and proliferation within vessel walls led to the development of hypotension in mutant mice. In cultured vSMCs, expression of miR-145/143 is also transcriptionally activated by TGF-β and BMP4 that promotes a contractile phenotype by targeting Klf4 (123). Interestingly, the phenotype of miR-143/miR-145 null mice is much milder as compared with that of SMC-specific deletion of Dicer1, suggesting that additional miRNA must be required for proper differentiation of vSMCs in vivo (114, 116).
miR-145/143 in Pulmonary Hypertension and Fibrosis
A significant up-regulation of miR-145/143 in response to hypoxia was observed both in the lung and right ventricle, but not in other organs (121). In response to chronic hypoxia, miR-145 knockout animals displayed no increase in systolic right ventricular pressure and right ventricular hypertrophy, unlike wild-type mice that showed significant increase of right ventricular pressure and right ventricular hypertrophy as expected (121). In addition, knockdown of miR-145, but not miR-143, leads to a milder response to chronic hypoxia, suggesting a specific role of miR-145 in this process (121). In human tissue, miR-145 was significantly elevated in both idiopathic or heritable pulmonary arterial hypertension (iPAH or hPAH) conditions as compared with controls. Levels of miR-145 in PASMCs isolated from patients with PAH with bone morphogenetic protein receptor, type II mutation is much higher compared with control PAMSCs from normal subjects. In addition, knockdown of bone morphogenetic protein receptor, type II in normal PAMSCs resulted in significant up-regulation of both miR-145 and miR-143. Together, these data suggest that miR-145 is downstream of BMP signaling in PAMSCs, and may represent a novel target for PAH treatment (121).
Although miR-145/143 expression is enriched in SMCs, miR-145 is shown to promote myofibroblast differentiation. In contrast, miR-145 deficiency diminished TGF-β1–induced α-smooth muscle actin expression. miR-145 expression is also increased in lungs of patients with IPF as compared with healthy control lungs. miR-145 deficiency is protective against bleomycin-induced pulmonary fibrosis (124). Overexpression of miR-145/143 also can inhibit the growth of lung adenocarcinoma cells, likely due to the misexpression of an miRNA that normally does not present in epithelial-derived cells (125–127). miR-145/143 is also abundantly expressed in airway SMCs. However, it is unknown whether airway SMCs are properly differentiated in miR-145/143 null mice or whether miR-145/143 null mice are protected from the development of asthma in response to ovalbumin challenge.
miR-133 in SMC Differentiation
miR-1 and miR-133 play crucial roles in the differentiation and function of skeletal and cardiac muscle. In vivo, miR-1 and miR-133 synergistically specify cardiomyocytes by suppressing myocardin during the development of heart (128–130). In cultured SMCs, miR-1 also suppressed SMC differentiation by targeting myocardin; however, the level of miR-1 in vSMCs in vivo is negligible (131, 132). In contrast, miR-133 is expressed in vSMCs in vivo and is regulated by platelet-derived growth factor–induced activation of MAPK/extracellular signal–regulated kinase 1/2. Overexpression of miR-133 by adenovirus significantly reduced vSMC proliferation, whereas knockdown of miR-133 by anti–miR-133 increased growth (132). miR-133 influences the proliferation and migration of vSMCs by targeting transacting transcription factor-1 and moesin. Furthermore, miR-133 plays a significant role in vSMC proliferation and phenotype switch after balloon injury of carotid arteries in vivo. These results suggest that miR-133 is a key regulator of vSMC phenotype (132). The role of miR-133 in airway SMCs and pulmonary vSMCs remains unknown.
miR-206 in the Innervation of Airway SMCs
miR-206 is important for regeneration of neuromuscular synapses after acute nerve injury of skeletal muscle (133). In mouse developing lungs, brain-derived neurotrophic factor (Bdnf) is required for airway smooth muscle innervation. Interestingly, Bdnf is directly targeted by miR-206, the expression of which is significantly suppressed by Shh activation (Figure 2). This suggested that the Shh pathway increases Bdnf expression post-transcriptionally by suppressing miR-206 (127). This is further supported by increased expression of Bdnf and innervation in embryonic lungs of miR-206 null mice (134). Little is known about the expression and function of miR-206 in human or animal asthmatic airway SMCs. It is also unknown whether miR-206 null mice will respond to ovalbumin challenge differently. These are important and interesting questions for future investigation.
miR-206 in the Proliferation and Differentiation of vSMCs
miR-206 directly targets Notch3, which plays a critical role in the development of the pulmonary vessel walls (Figure 2). Levels of miR-206 are significantly reduced in PASMCs isolated from hypoxia-induced PAH mice, which negatively correlates with increased levels of Notch3. Reduction of miR-206 in human PASMCs causes increased proliferation and reduced apoptosis, which are key histopathological features of PAH. These results suggest that miR-206 is a potential regulator of proliferation, apoptosis, and differentiation of PASMCs (135). Although the role of miR-206 in SMC proliferation has not been examined in early developing lungs, reduced expression of miR-206 by Shh might not only be responsible for the increased innervation of SMCs, but also for the promotion of SMC proliferation. Despite this progress, it is unknown whether miR-206 null mice will develop PAH with or without challenge, such as hypoxia.
Future Directions
It is clear that miRNAs play critical roles in regulating the formation and function of lungs. Moreover, expression and activities of miRNAs can be modulated by signaling pathways or transcriptional factors, and by the expression and modification of the protein components of the miRNA pathway. Despite all this progress, the work on miRNAs in lung development and disease has just begun. Genome-wide profiling has identified a significant number of developmental stage- or cell type–specific miRNAs (Figure 2) (73, 74, 106, 113, 136), and also miRNAs associated with pathogenesis of different pulmonary disorders (105, 137–148). However, only a handful of them have been functionally analyzed in vivo, largely due to the unavailability of genetically modified mice. Analysis of functions and responsible targets of these miRNAs likely will reveal unexpected insights into mechanisms of lung development and diseases. Among the protein component of the miRNA pathway (Table 1), Dicer1 is the only one whose function has been examined in mouse lung endoderm in vivo. Characterizations of the expression, modification, and functions of other protein components during lung development, or in response to injuries and stresses associated with pulmonary disorders, will potentially reveal novel activities of the miRNA pathway in these processes.
Acknowledgments
Acknowledgments
The authors apologize to the many researchers whose work was not cited in this review due to space limitations. They thank Wellington Cardoso, Wei Xu, Maria Stupnikov, and Munemasa Mori for discussions and comments on the manuscript. They thank Maria Stupnikov for the artwork of figures. Work in Jining Lü’s laboratory was supported by grants from the National Heart, Lung, and Blood Institute.
Footnotes
This work was supported by National Institutes of Health grants P01 HL47049 and R01 HL081800 (J.L.).
Originally Published in Press as DOI: 10.1165/rcmb.2014-0232RT on September 11, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, et al. Lung organogenesis. Curr Top Dev Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:558–563. doi: 10.1513/pats.200905-031RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrisey EE, Cardoso WV, Lane RH, Rabinovitch M, Abman SH, Ai X, Albertine KH, Bland RD, Chapman HA, Checkley W, et al. Molecular determinants of lung development. Ann Am Thorac Soc. 2013;10:S12–S16. doi: 10.1513/AnnalsATS.201207-036OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domyan ET, Sun X. Patterning and plasticity in development of the respiratory lineage. Dev Dyn. 2011;240:477–485. doi: 10.1002/dvdy.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 8.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 9.Chuang PT, McMahon AP. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 2003;13:86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 10.Hogan BL, Yingling JM. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr Opin Genet Dev. 1998;8:481–486. doi: 10.1016/s0959-437x(98)80121-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Marquez H, Kim YK, Qian J, Shao F, Fine A, Cruikshank WW, Quadro L, Cardoso WV. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest. 2014;124:801–811. doi: 10.1172/JCI70291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hind M, Gilthorpe A, Stinchcombe S, Maden M. Retinoid induction of alveolar regeneration: from mice to man? Thorax. 2009;64:451–457. doi: 10.1136/thx.2008.105437. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovitch M. Pathobiology of pulmonary hypertension. Annu Rev Pathol. 2007;2:369–399. doi: 10.1146/annurev.pathol.2.010506.092033. [DOI] [PubMed] [Google Scholar]
- 14.Warburton D, Shi W, Xu B. TGF-β–Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol. 2013;304:L83–L85. doi: 10.1152/ajplung.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrisey EE. Wnt signaling and pulmonary fibrosis. Am J Pathol. 2003;162:1393–1397. doi: 10.1016/S0002-9440(10)64271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Xiao L, Sun L, Liu F. Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res. 2012;61:337–346. doi: 10.33549/physiolres.932289. [DOI] [PubMed] [Google Scholar]
- 17.Ariel D, Upadhyay D. The role and regulation of microRNAs in asthma. Curr Opin Allergy Clin Immunol. 2012;12:49–52. doi: 10.1097/ACI.0b013e32834ecb7f. [DOI] [PubMed] [Google Scholar]
- 18.Greene CM, Gaughan KP. microRNAs in asthma: potential therapeutic targets. Curr Opin Pulm Med. 2013;19:66–72. doi: 10.1097/MCP.0b013e32835a5bc8. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X. The emerging role of microRNAs in asthma. Mol Cell Biochem. 2011;353:35–40. doi: 10.1007/s11010-011-0771-z. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277:2015–2021. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang H, Hata A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol. 2012;19:224–231. doi: 10.1097/MOH.0b013e3283523e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin PY, Yu SL, Yang PC. MicroRNA in lung cancer. Br J Cancer. 2010;103:1144–1148. doi: 10.1038/sj.bjc.6605901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald RA, Hata A, MacLean MR, Morrell NW, Baker AH. MicroRNA and vascular remodelling in acute vascular injury and pulmonary vascular remodelling. Cardiovasc Res. 2012;93:594–604. doi: 10.1093/cvr/cvr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oglesby IK, McElvaney NG, Greene CM. MicroRNAs in inflammatory lung disease—master regulators or target practice? Respir Res. 2010;11:148. doi: 10.1186/1465-9921-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Roy S, Sen CK. MiRNA in innate immune responses: novel players in wound inflammation. Physiol Genomics. 2011;43:557–565. doi: 10.1152/physiolgenomics.00160.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sessa R, Hata A. Role of microRNAs in lung development and pulmonary diseases. Pulm Circ. 2013;3:315–328. doi: 10.4103/2045-8932.114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomankova T, Petrek M, Kriegova E. Involvement of microRNAs in physiological and pathological processes in the lung. Respir Res. 2010;11:159. doi: 10.1186/1465-9921-11-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 30.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 32.Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579:5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 35.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 36.Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 39.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 40.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–122. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M, Yu Q. miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev. 2009;23:2388–2393. doi: 10.1101/gad.1819009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaulk SG, Lattanzi VJ, Hiemer SE, Fahlman RP, Varelas X. The Hippo pathway effectors TAZ/YAP regulate dicer expression and microRNA biogenesis through Let-7. J Biol Chem. 2014;289:1886–1891. doi: 10.1074/jbc.C113.529362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori M, Triboulet R, Mohseni M, Schlegelmilch K, Shrestha K, Camargo FD, Gregory RI. Hippo signaling regulates microprocessor and links cell-density–dependent miRNA biogenesis to cancer. Cell. 2014;156:893–906. doi: 10.1016/j.cell.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 50.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu PH, Isaji M, Carthew RW. Functionally diverse microRNA effector complexes are regulated by extracellular signaling. Mol Cell. 2013;52:113–123. doi: 10.1016/j.molcel.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun JE, Huntzinger E, Izaurralde E. The role of GW182 proteins in miRNA-mediated gene silencing. Adv Exp Med Biol. 2013;768:147–163. doi: 10.1007/978-1-4614-5107-5_9. [DOI] [PubMed] [Google Scholar]
- 53.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell. 2010;39:373–384. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 57.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 60.Visser S, Yang X. LATS tumor suppressor: a new governor of cellular homeostasis. Cell Cycle. 2010;9:3892–3903. doi: 10.4161/cc.9.19.13386. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Z, Hao Y, Liu N, Raptis L, Tsao MS, Yang X. TAZ is a novel oncogene in non–small cell lung cancer. Oncogene. 2011;30:2181–2186. doi: 10.1038/onc.2010.606. [DOI] [PubMed] [Google Scholar]
- 62.Wang D, Zhang Z, O’Loughlin E, Lee T, Houel S, O’Carroll D, Tarakhovsky A, Ahn NG, Yi R. Quantitative functions of Argonaute proteins in mammalian development. Genes Dev. 2012;26:693–704. doi: 10.1101/gad.182758.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Z, Yu N, Kuang P, Chen M, Shao F, Martin G, Chui DH, Cardoso WV, Ai X, Lü J. Trinucleotide repeat containing 6a (Tnrc6a)–mediated microRNA function is required for development of yolk sac endoderm. J Biol Chem. 2012;287:5979–5987. doi: 10.1074/jbc.M111.297937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultze-Florey RE, Graf N, Vorwerk P, Koscielniak E, Schneider DT, Kratz CP. DICER1 syndrome: a new cancer syndrome. Klin Padiatr. 2013;225:177–178. doi: 10.1055/s-0033-1337976. [DOI] [PubMed] [Google Scholar]
- 67.Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, Barfoot R, Burke A, Chisholm J, Hewitt M, et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011;48:273–278. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 68.Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, Kirsch DG, Golub TR, Jacks T. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009;23:2700–2704. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, Marine JC. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ. 2010;17:633–641. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karube Y, Tanaka H, Osada H, Tomida S, Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S, Mitsudomi T, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–115. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 73.Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W, Parnigotto PP, et al. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-cadherin distribution. Dev Biol. 2009;333:238–250. doi: 10.1016/j.ydbio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oeztuerk-Winder F, Guinot A, Ochalek A, Ventura JJ. Regulation of human lung alveolar multipotent cells by a novel p38α MAPK/miR-17-92 axis. EMBO J. 2012;31:3431–3441. doi: 10.1038/emboj.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olive V, Li Q, He L. mir-17-92: a polycistronic oncomir with pleiotropic functions. Immunol Rev. 2013;253:158–166. doi: 10.1111/imr.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 80.Osada H, Takahashi T. let-7 and miR-17-92: small-sized major players in lung cancer development. Cancer Sci. 2011;102:9–17. doi: 10.1111/j.1349-7006.2010.01707.x. [DOI] [PubMed] [Google Scholar]
- 81.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc–regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 82.Inomata M, Tagawa H, Guo YM, Kameoka Y, Takahashi N, Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- 83.Matsubara H, Takeuchi T, Nishikawa E, Yanagisawa K, Hayashita Y, Ebi H, Yamada H, Suzuki M, Nagino M, Nimura Y, et al. Apoptosis induction by antisense oligonucleotides against miR-17-5p and miR-20a in lung cancers overexpressing miR-17-92. Oncogene. 2007;26:6099–6105. doi: 10.1038/sj.onc.1210425. [DOI] [PubMed] [Google Scholar]
- 84.Tian Y, Zhang Y, Hurd L, Hannenhalli S, Liu F, Lu MM, Morrisey EE. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development. 2011;138:1235–1245. doi: 10.1242/dev.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campayo M, Navarro A, Viñolas N, Diaz T, Tejero R, Gimferrer JM, Molins L, Cabanas ML, Ramirez J, Monzo M, et al. Low miR-145 and high miR-367 are associated with unfavourable prognosis in resected nonsmall cell lung cancer. Eur Respir J. 2013;41:1172–1178. doi: 10.1183/09031936.00048712. [DOI] [PubMed] [Google Scholar]
- 86.Lizé M, Herr C, Klimke A, Bals R, Dobbelstein M. MicroRNA-449a levels increase by several orders of magnitude during mucociliary differentiation of airway epithelia. Cell Cycle. 2010;9:4579–4583. doi: 10.4161/cc.9.22.13870. [DOI] [PubMed] [Google Scholar]
- 87.Lizé M, Klimke A, Dobbelstein M. MicroRNA-449 in cell fate determination. Cell Cycle. 2011;10:2874–2882. doi: 10.4161/cc.10.17.17181. [DOI] [PubMed] [Google Scholar]
- 88.Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, et al. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol. 2011;13:693–699. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- 89.Martinez-Anton A, Sokolowska M, Kern S, Davis AS, Alsaaty S, Taubenberger JK, Sun J, Cai R, Danner RL, Eberlein M, et al. Changes in microRNA and mRNA expression with differentiation of human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2013;49:384–395. doi: 10.1165/rcmb.2012-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bao J, Li D, Wang L, Wu J, Hu Y, Wang Z, Chen Y, Cao X, Jiang C, Yan W, et al. MicroRNA-449 and microRNA-34b/c function redundantly in murine testes by targeting E2F transcription factor-retinoblastoma protein (E2F-pRb) pathway. J Biol Chem. 2012;287:21686–21698. doi: 10.1074/jbc.M111.328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Concepcion CP, Han YC, Mu P, Bonetti C, Yao E, D’Andrea A, Vidigal JA, Maughan WP, Ogrodowski P, Ventura A. Intact p53-dependent responses in miR-34–deficient mice. PLoS Genet. 2012;8:e1002797. doi: 10.1371/journal.pgen.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song R, Walentek P, Sponer N, Klimke A, Lee JS, Dixon G, Harland R, Wan Y, Lishko P, Lize M, et al. miR-34/449 miRNAs are required for motile ciliogenesis by repressing cp110. Nature. 2014;510:115–120. doi: 10.1038/nature13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C, Barczak AJ, et al. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186:965–974. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 96.Gallardo E, Navarro A, Viñolas N, Marrades RM, Diaz T, Gel B, Quera A, Bandres E, Garcia-Foncillas J, Ramirez J, et al. miR-34a as a prognostic marker of relapse in surgically resected non–small-cell lung cancer. Carcinogenesis. 2009;30:1903–1909. doi: 10.1093/carcin/bgp219. [DOI] [PubMed] [Google Scholar]
- 97.Tanaka N, Toyooka S, Soh J, Kubo T, Yamamoto H, Maki Y, Muraoka T, Shien K, Furukawa M, Ueno T, et al. Frequent methylation and oncogenic role of microRNA-34b/c in small-cell lung cancer. Lung Cancer. 2012;76:32–38. doi: 10.1016/j.lungcan.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 98.Balça-Silva J, Sousa Neves S, Gonçalves AC, Abrantes AM, Casalta-Lopes J, Botelho MF, Sarmento-Ribeiro AB, Silva HC. Effect of miR-34b overexpression on the radiosensitivity of non–small cell lung cancer cell lines. Anticancer Res. 2012;32:1603–1609. [PubMed] [Google Scholar]
- 99.Kasinski AL, Slack FJ. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-Ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res. 2012;72:5576–5587. doi: 10.1158/0008-5472.CAN-12-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okada N, Lin CP, Ribeiro MC, Biton A, Lai G, He X, Bu P, Vogel H, Jablons DM, Keller AC, et al. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes Dev. 2014;28:438–450. doi: 10.1101/gad.233585.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lizé M, Pilarski S, Dobbelstein M. E2F1-inducible microRNA 449a/b suppresses cell proliferation and promotes apoptosis. Cell Death Differ. 2010;17:452–458. doi: 10.1038/cdd.2009.188. [DOI] [PubMed] [Google Scholar]
- 102.Luo W, Huang B, Li Z, Li H, Sun L, Zhang Q, Qiu X, Wang E. MicroRNA-449a is downregulated in non–small cell lung cancer and inhibits migration and invasion by targeting c-Met. PLoS ONE. 2013;8:e64759. doi: 10.1371/journal.pone.0064759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miao LJ, Huang SF, Sun ZT, Gao ZY, Zhang RX, Liu Y, Wang J. MiR-449c targets c-Myc and inhibits NSCLC cell progression. FEBS Lett. 2013;587:1359–1365. doi: 10.1016/j.febslet.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Perdomo C, Campbell JD, Gerrein J, Tellez CS, Garrison CB, Walser TC, Drizik E, Si H, Gower AC, Vick J, et al. MicroRNA 4423 is a primate-specific regulator of airway epithelial cell differentiation and lung carcinogenesis. Proc Natl Acad Sci USA. 2013;110:18946–18951. doi: 10.1073/pnas.1220319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang Z, Cushing L, Ai X, Lü J. miR-326 is downstream of Sonic Hedgehog signaling and regulates the expression of Gli2 and Smoothened. Am J Respir Cell Mol Biol. 2014;51:273–283. doi: 10.1165/rcmb.2013-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferretti E, De Smaele E, Miele E, Laneve P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E, Screpanti I, et al. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J. 2008;27:2616–2627. doi: 10.1038/emboj.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Babashah S, Sadeghizadeh M, Hajifathali A, Tavirani MR, Zomorod MS, Ghadiani M, Soleimani M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int J Cancer. 2013;133:579–589. doi: 10.1002/ijc.28043. [DOI] [PubMed] [Google Scholar]
- 109.Liu L, Kugler MC, Loomis CA, Samdani R, Zhao Z, Chen GJ, Brandt JP, Brownell I, Joyner AL, Rom WN, et al. Hedgehog signaling in neonatal and adult lung. Am J Respir Cell Mol Biol. 2013;48:703–710. doi: 10.1165/rcmb.2012-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moshai EF, Wémeau-Stervinou L, Cigna N, Brayer S, Sommé JM, Crestani B, Mailleux AA. Targeting the Hedgehog–glioma pathway inhibits bleomycin-induced lung fibrosis in mice. Am J Respir Cell Mol Biol. 2014;51:11–25. doi: 10.1165/rcmb.2013-0154OC. [DOI] [PubMed] [Google Scholar]
- 111.Bolaños AL, Milla CM, Lira JC, Ramírez R, Checa M, Barrera L, García-Alvarez J, Carbajal V, Becerril C, Gaxiola M, et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L978–L990. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 112.Das S, Kumar M, Negi V, Pattnaik B, Prakash YS, Agrawal A, Ghosh B. MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50:882–892. doi: 10.1165/rcmb.2013-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carraro G, Shrestha A, Rostkovius J, Contreras A, Chao CM, El Agha E, Mackenzie B, Dilai S, Guidolin D, Taketo MM, et al. miR-142-3p balances proliferation and differentiation of mesenchymal cells during lung development. Development. 2014;141:1272–1281. doi: 10.1242/dev.105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 2010;30:1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan Y, Balazs L, Tigyi G, Yue J. Conditional deletion of Dicer in vascular smooth muscle cells leads to the developmental delay and embryonic mortality. Biochem Biophys Res Commun. 2011;408:369–374. doi: 10.1016/j.bbrc.2011.02.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernández-Hernando C, Offermanns S, Miano JM, Sessa WC. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PLoS One. 2011;6:e18869. doi: 10.1371/journal.pone.0018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Z, Wu J, Yang C, Fan P, Balazs L, Jiao Y, Lu M, Gu W, Li C, Pfeffer LM, et al. DiGeorge syndrome critical region 8 (DGCR8) protein–mediated microRNA biogenesis is essential for vascular smooth muscle cell development in mice. J Biol Chem. 2012;287:19018–19028. doi: 10.1074/jbc.M112.351791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan P, Chen Z, Tian P, Liu W, Jiao Y, Xue Y, Bhattacharya A, Wu J, Lu M, Guo Y, et al. miRNA biogenesis enzyme Drosha is required for vascular smooth muscle cell survival. PLoS One. 2013;8:e60888. doi: 10.1371/journal.pone.0060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu C, So J, Davis-Dusenbery BN, Qi HH, Bloch DB, Shi Y, Lagna G, Hata A. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol. 2011;31:4760–4774. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111:290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 122.Boettger T, Beetz N, Kostin S, Schneider J, Krüger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang S, Cui H, Xie N, Icyuz M, Banerjee S, Antony VB, Abraham E, Thannickal VJ, Liu G. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 2013;27:2382–2391. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cho WC, Chow AS, Au JS. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol. 2011;8:125–131. doi: 10.4161/rna.8.1.14259. [DOI] [PubMed] [Google Scholar]
- 126.Yin R, Zhang S, Wu Y, Fan X, Jiang F, Zhang Z, Feng D, Guo X, Xu L. microRNA-145 suppresses lung adenocarcinoma-initiating cell proliferation by targeting OCT4. Oncol Rep. 2011;25:1747–1754. doi: 10.3892/or.2011.1252. [DOI] [PubMed] [Google Scholar]
- 127.Chen Z, Zeng H, Guo Y, Liu P, Pan H, Deng A, Hu J. miRNA-145 inhibits non–small cell lung cancer cell proliferation by targeting c-Myc. J Exp Clin Cancer Res. 2010;29:151. doi: 10.1186/1756-9966-29-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 130.Wystub K, Besser J, Bachmann A, Boettger T, Braun T. miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development. PLoS Genet. 2013;9:e1003793. doi: 10.1371/journal.pgen.1003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen J, Yin H, Jiang Y, Radhakrishnan SK, Huang ZP, Li J, Shi Z, Kilsdonk EP, Gui Y, Wang DZ, et al. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol. 2011;31:368–375. doi: 10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 133.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci. 2011;31:15407–15415. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jalali S, Ramanathan GK, Parthasarathy PT, Aljubran S, Galam L, Yunus A, Garcia S, Cox RR, Jr, Lockey RF, Kolliputi N. Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS ONE. 2012;7:e46808. doi: 10.1371/journal.pone.0046808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bhaskaran M, Wang Y, Zhang H, Weng T, Baviskar P, Guo Y, Gou D, Liu L. MicroRNA-127 modulates fetal lung development. Physiol Genomics. 2009;37:268–278. doi: 10.1152/physiolgenomics.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:1239–1247. doi: 10.1165/rcmb.2010-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schlosser K, White RJ, Stewart DJ. miR-26a linked to pulmonary hypertension by global assessment of circulating extracellular microRNAs. Am J Respir Crit Care Med. 2013;188:1472–1475. doi: 10.1164/rccm.201308-1403LE. [DOI] [PubMed] [Google Scholar]
- 141.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 142.Collison A, Herbert C, Siegle JS, Mattes J, Foster PS, Kumar RK. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm Med. 2011;11:29. doi: 10.1186/1471-2466-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Honeyman L, Bazett M, Tomko TG, Haston CK. MicroRNA profiling implicates the insulin-like growth factor pathway in bleomycin-induced pulmonary fibrosis in mice. Fibrogenesis Tissue Repair. 2013;6:16. doi: 10.1186/1755-1536-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu F, Qin HB, Xu B, Zhou H, Zhao DY. Profiling of miRNAs in pediatric asthma: upregulation of miRNA-221 and miRNA-485–3p. Mol Med Rep. 2012;6:1178–1182. doi: 10.3892/mmr.2012.1030. [DOI] [PubMed] [Google Scholar]
- 145.Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics. 2011;43:479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garbacki N, Di Valentin E, Huynh-Thu VA, Geurts P, Irrthum A, Crahay C, Arnould T, Deroanne C, Piette J, Cataldo D, et al. MicroRNAs profiling in murine models of acute and chronic asthma: a relationship with mRNAs targets. PLoS One. 2011;6:e16509. doi: 10.1371/journal.pone.0016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF, Lindsay MA. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One. 2009;4:e5889. doi: 10.1371/journal.pone.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ezzie ME, Crawford M, Cho JH, Orellana R, Zhang S, Gelinas R, Batte K, Yu L, Nuovo G, Galas D, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67:122–131. doi: 10.1136/thoraxjnl-2011-200089. [DOI] [PubMed] [Google Scholar]
- 149.Zhdanova O, Srivastava S, Di L, Li Z, Tchelebi L, Dworkin S, Johnstone DB, Zavadil J, Chong MM, Littman DR, et al. The inducible deletion of Drosha and microRNAs in mature podocytes results in a collapsing glomerulopathy. Kidney Int. 2011;80:719–730. doi: 10.1038/ki.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van Stry M, Oguin TH, III, Cheloufi S, Vogel P, Watanabe M, Pillai MR, Dash P, Thomas PG, Hannon GJ, Bix M. Enhanced susceptibility of Ago1/3 double-null mice to influenza A virus infection. J Virol. 2012;86:4151–4157. doi: 10.1128/JVI.05303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qi Y, He X, Wang XJ, Kohany O, Jurka J, Hannon GJ. Distinct catalytic and non-catalytic roles of ARGONAUTE4 in RNA-directed DNA methylation. Nature. 2006;443:1008–1012. doi: 10.1038/nature05198. [DOI] [PubMed] [Google Scholar]