Abstract
Glioblastomas continue to rank among the most lethal primary human tumors. Despite treatment with the most rigorous surgical interventions along with the most optimal chemotherapeutic and radiation regimens, the median survival is just 12–15 months for patients with glioblastoma. Among the histological hallmarks of glioblastoma, necrosis has been demonstrated to be a powerful predictor of poor patient prognosis. Over the years, there have been many advances in our understanding of the molecular mechanisms underlying glioblastoma formation, yet the mechanisms that lead to tumor necrosis remain unclear. One pathway that may lead to necrosis in glioblastoma involves the neurotransmitter, glutamate, which has been shown to accumulate in the peritumoral fluid as a result of decreased cellular uptake by glioblastoma cells. This accumulation leads to subsequent glutamate excitotoxicity and probable necrosis through a massive elevation of intracellular Ca2+ and reduction in cellular ATP levels. We propose that a pathway involving tumor necrosis factor-α (TNF-α), astrocyte-elevated gene-1 (AEG-1), and nuclear factor-kappa B (NF-κB) leads to decreased glutamate uptake through coordinated downregulation of the excitatory amino acid transporter 2 (EAAT2), the glutamate transporter responsible for the majority of glutamate uptake in the human brain. In addition, we suggest that AEG-1 signaling, loss of phosphatase and tensin homolog (PTEN), and ionotropic glutamate receptor activity lead to AKT pathway activation, which results in nutrient overconsumption and necrosis. Together, these pathways provide a new perspective on glioblastoma necrosis involving the process of glutamate excitotoxicity. Future research should address the components of these molecular pathways in order to better understand the mechanism of necrosis in glioblastoma and to begin to develop targeted therapies that may improve patient prognosis in the future.
Keywords: Glioblastoma, necrosis, NF-kappaB, Tumor necrosis factor alpha, glutamate uptake
Grading of Astrocytomas
Adult brain tumors of glial origin are divided into astrocytomas, oligodendrogliomas, and oligoastrocytomas based on several key histopathological features. In addition, these tumors are classified clinically as benign World Health Organization (WHO) grade I (pilocytic) astrocytoma and grade II (diffuse) astrocytoma and malignant WHO grade III (anaplastic) astrocytoma and grade IV astrocytoma (glioblastoma), the most malignant form. Low-grade diffuse astrocytomas follow long clinical courses if detected early; however, the more malignant Grade III gliomas carry a five-year survival of 29.4%, and glioblastomas carry a five-year survival of just 3.4%.1
These tumors are also classified according to several molecular markers that are present in tumor tissue obtained from these patients. Currently, most molecular diagnostic analyses focus on mutations in cell cycle regulators, such as inactivating p53 mutations that are found in more than 50% of Grade II astrocytomas,2 or chromosomal abnormalities that are present in astrocytomas,3 oligodendrogliomas,4 or oligoastrocytomas.5 Though these molecular findings do carry prognostic significance in some cases, it is likely that future molecular genotypic analysis of astrocytomas will include a wider array of cellular markers, namely factors that contribute more directly to the highly malignant features of these tumors, such as tumor necrosis, which leads to poor patient prognosis.
Physiological Mechanism of Necrosis in Glioblastomas
Necrosis is a hallmark feature of glioblastoma,6 with some studies suggesting its presence in over 85% of cases.7–11 The pathway of necrosis in glioblastoma begins with acute cellular ATP depletion as a result of electron transport chain collapse and subsequent decreased oxidative phosphorylation (Figure 1). This lack of ATP leads to failure of ATP-dependent ion channels and pumps, which initiates a massive cell volume increase through Na+ influx. The increased intracellular Na+ concentration results in activation of the Na+-K+-ATPase, which further depletes cellular ATP stores.12 This depletion leads to the opening of non-selective Ca2+ channels, resulting in elevated intracellular Ca2+ levels and activation of the Ca2+-ATPase with eventual mitochondrial depolarization.12 In addition, with severe ATP depletion, ionic homeostasis is no longer maintained by K+ efflux, leading to further Na+ and water influx that precipitate cellular swelling and collapse. As the cell membrane ruptures, the contents of the cell are released into the extracellular space, and the final stage of necrosis involves protease activation and a localized inflammatory response.
Figure 1. Mechanism of glutamate excitotoxicity-induced necrosis in glioblastoma.
Glutamate accumulation as a result of decreased functional EAAT2 activity triggers ionotropic glutamate receptor activation, which causes an increase in the [Ca2+]i. At the same time, elevated levels of extracellular glutamate inhibit system activity. This inhibition leads to decreased intracellular cysteine and subsequent impairment of glutathione production, culminating in the inability to neutralize reactive oxygen species. Reactive oxygen species cause membrane oxidation and ATP-dependent Ca2+ release from the endoplasmic reticulum (ER), leading to mitochondrial damage and further ATP depletion. With severe reduction in ATP levels, Na+ and water enter the cell and precipitate a massive cell volume increase. K+ efflux fails to maintain ionic homeostasis, which results in further increases in Na+ and water influx. As a result, the cell swells, and its plasma membrane ruptures, leading to cellular collapse. In the final stage, the leakage of intracellular contents results in the activation of extracellular proteases, which induces inflammation and eventual necrosis. NMDA-R, N-methyl D-aspartic acid receptor; AMPA-R, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; EAAT2, excitatory amino acid transporter 2; ROOH, reactive oxygen species; ROH, neutralized reactive oxygen species.
The Role of Glutamate Uptake and Release in Glioblastoma
Glutamate is the predominant excitatory neurotransmitter in the mammalian CNS and is essential for normal neuronal function and neurotransmission. However, accumulation of glutamate in the extracellular space as a result of various pathological processes in the brain can lead to glutamate excitotoxicity,13, 14 a phenomenon associated with many human diseases, including schizophrenia,15 amyotrophic lateral sclerosis, for review, see16 epilepsy, for review, see17 and glioblastoma. for review, see18
The extracellular concentration of glutamate must be tightly regulated in order to avoid neurotoxic effects. Because passive diffusion is limited in its ability to remove glutamate from the synaptic cleft19 and because glutamate cannot be metabolized by extracellular enzymes, the principal mechanism for the maintenance of low extracellular glutamate is through Na+-dependent glutamate transporters that are anchored within the cell membranes of astrocytes and neurons. These excitatory amino acid transporters (EAATs) are driven by the electrochemical gradients across the plasma membrane and require Na+ for glutamate binding and K+ for net transport. EAAT2, the predominant isoform expressed in astrocytes and the form responsible for the majority of glutamate uptake in the human brain, is a hexamer transmembrane protein whose downregulation leads to glutamate excitotoxicity. reviewed in19
The effect of EAAT2 downregulation is that glioma cells are unable to control the extracellular glutamate concentration. Previous studies show that glioma glutamate uptake is decreased compared to normal astrocytes,20 and whereas normal astrocytes rapidly deplete glutamate from enriched media, the extracellular concentration of glutamate in the media of glioma cultures increases above baseline levels.20 In vivo studies using spectroscopic MRI confirm that the peritumoral fluid surrounding high-grade oligodendrogliomas contains increased levels of glutamate.21 Moreover, microdialysis studies demonstrate that necrotic tissue in glioblastomas also shows elevated levels of glutamate compared to viable non-necrotic tumor tissue.22 While these authors speculate that high extracellular levels of glutamate may be a result of cellular destruction in necrotic tissue, glutamate may be the major cause rather than the result of necrosis. For example, studies have shown that glutamate accumulation leads to microglial activation in the vicinity of the tumor, which would further enhance the inflammatory response associated with glioma necrosis.23 Additionally, EAAT2 downregulation leads to a loss of ischemic tolerance by astrocytes,24 which in the setting of glioblastoma, would promote necrosis in hyperproliferative areas.
Though EAAT2 regulates extracellular glutamate through reuptake, the release of glutamate is regulated by the Na+-independent system , which exchanges intracellular glutamate for extracellular cystine. Glutamate release through the activity of system is normally handled through reuptake by EAAT2 to decrease the extracellular glutamate concentration. However, in glioblastomas that express high levels of system 18 and low levels of EAAT2,20 glutamate progressively accumulates in the extracellular space and precipitates local excitotoxicity of neurons and likely tumor cells as well.
Pathway Leading to Glutamate-Mediated Necrosis in Glioblastoma
The mechanism of glutamate-mediated necrosis in glioblastoma remains unclear, but several key components of the glutamate uptake pathway have been implicated in certain physiological aspects of necrotic cell death. It has been proposed that altered EAAT2 RNA splicing events in glioblastoma lead to a lack of EAAT2 functional diversity and consequent glutamate transporter dysregulation.25 While tumor cells exhibit decreased EAAT2 expression, neurons demonstrate further reduced expression of EAAT2,19 which may lead to preferential glutamate accumulation in tumor cells.20 Glutamate accumulation then triggers increased glutamate release through system , which further enhances the glutamate excitotoxic milieu.
There are two pathways that lead to cell death as a result of glutamate excitotoxicity. One pathway involves activation of ionotropic glutamate receptors, followed by alterations in calcium homeostasis and eventual cell death from reactive oxygen species damage26 (Figure 1). It has been suggested that this pathway leads to necrosis. reviewed in27 The second pathway involves glutamate-mediated inhibition of system , which results in deficient intracellular cystine28 (Figure 1). This deficiency prevents the intracellular reduction of cystine to cysteine, with a resulting impairment in glutathione production. Affected cells accumulate reactive oxygen radicals, which cannot be neutralized. These radicals increase Ca2+ release from intracellular stores, such as the endoplasmic reticulum, and cause massive ATP depletion, membrane oxidation, and necrosis. Though it has been proposed that this type of oxidative glutamate excitotoxicity leads to a form of active apoptotic cell death with morphological features of necrosis,26 including swollen intracellular organelles and loss of mitochondrial cristae with normal nuclear morphology,26 these experiments were conducted in neurons and not tumor cells, which may exhibit entirely different characteristics upon glutamate toxicity. In addition, apoptosis and programmed cell death are not necessarily synonymous, given that various signaling cascades of the necrotic pathway have been shown to be executed in a systematic, regulated manner.29 Thus, necrosis may be an active rather than a passive form of cell death that requires the coordinated functioning of specific cellular programs. In the case of glutamate excitotoxicity, it has also been shown that apoptotic pathway activation can lead to EAAT2 cleavage and resulting impairment in functional glutamate uptake,30 which could then precipitate necrotic cell death through either of these pathways. However, in the end, it may be the morphological and functional changes associated with necrosis, rather than the underlying molecular pathway, that more profoundly impact patient prognosis.
Necrosis and Glioblastoma Expansion
Unlike other soft-tissue masses which have unlimited physical growth capacity, the expansion of brain tumors is restricted by the skull. Therefore, brain tumors must kill surrounding neurons and supporting cells in order to expand. However, there is still debate over the mechanism of this growth. The traditional explanation of glutamate excitotoxicity in the setting of glioblastoma is that this process leads to necrosis of surrounding neurons, which allows more space for tumor growth. However, it has not been determined that glioblastoma cells themselves are not resistant to this excitotoxic environment and would not also undergo tumor necrosis as a result of excitotoxicity. Therefore, whether glutamate transporter downregulation occurs solely on the advancing, infiltrative tumor borders or whether this downregulation is uniform throughout the tumor mass will determine the functional significance of this downregulation. Future studies must focus on comparing the spatial and structural relationship of EAAT2 loss to tumor growth in order to determine the effect of localized excitotoxicity on glioblastoma expansion.
Molecular Pathways Involved in Glutamate-Mediated Necrosis in Glioblastoma
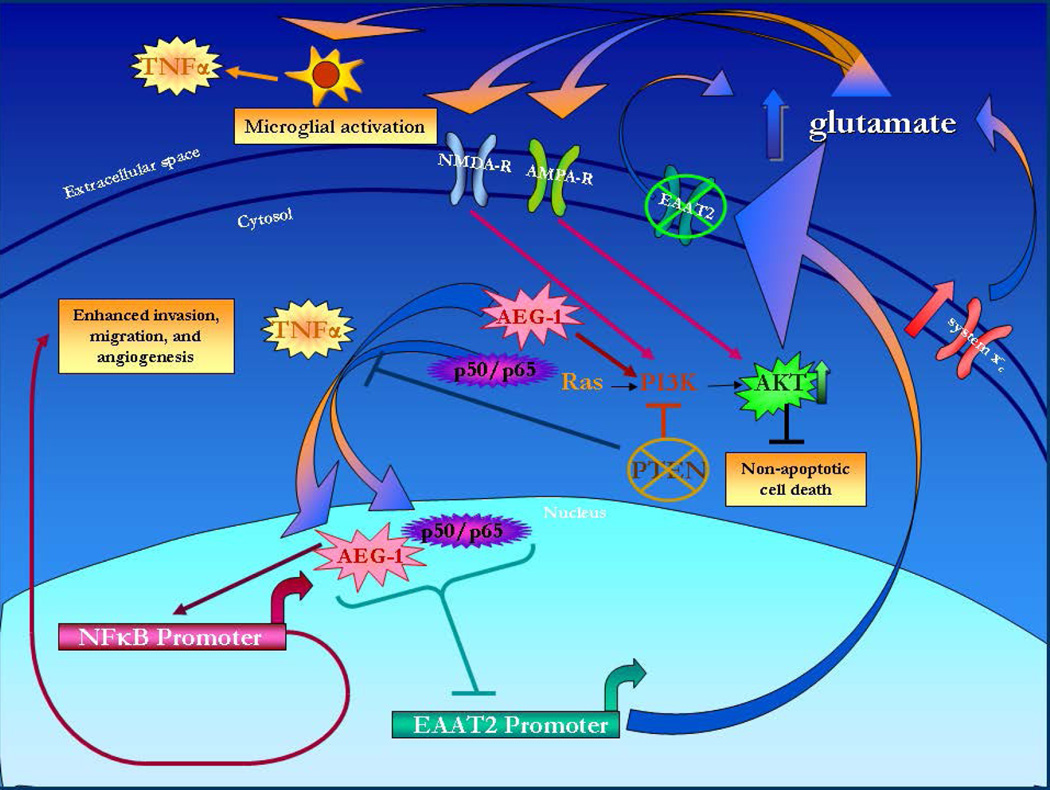
Based on the key pathways that are activated in glioblastoma, our hypothesis is that activation of the pathway involving TNFα, NF-κB, and AEG-1 diminishes glutamate uptake and causes subsequent excitotoxicity (Figure 2). Furthermore, PTEN mutations along with elevated levels of extracellular glutamate lead to AKT pathway activation that precipitates glioblastoma necrosis (Figure 2).
Figure 2. Molecular pathway leading to extracellular glutamate accumulation in glioblastoma.
AEG-1, astrocyte-elevated gene 1; TNF-α, tumor necrosis factor-alpha; PI3K, phosphatidylinositol-3-kinase; PTEN, phosphatase and tensin homolog; NMDA-R, N-methyl D-aspartic acid receptor; AMPA-R, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; EAAT2, excitatory amino acid transporter 2; NF-κB, nuclear factor-kappa B.
Tumor necrosis factor α (TNFα), astrocyte elevated gene-1 (AEG-1), and nuclear factor-kappa B (NF-κB)
Despite advances in understanding apoptotic signaling in glioblastoma, the impact of TNFα production on glioblastoma pathogenesis remains controversial. Though TNFα can activate pathways involved in tumor migration and proliferation, other downstream pathways of TNFα lead to apoptosis. For example, it has been shown that TNFα release is decreased in glioblastoma compared to Grade II astrocytomas,31 which would limit apoptosis in these advanced tumors. On the other hand, other studies have indicated that TNFα is overexpressed in glioblastomas32 and that this overexpression is associated with increased necrosis and decreased survival,33 possibly through enhanced procoagulation in these tumors.34 Therefore, it is the balance between these pro-survival and pro-apoptotic pathways that determines malignant potential.
Along with TNFα expression analysis in glioblastoma, many studies have focused on the link between TNFα and glutamate excitotoxicity. TNFα inhibits glutamate uptake in primary astrocytes,35–37 downregulates glutamine synthetase, the enzyme responsible for converting glutamate to glutamine,38, 39 activates AMPA receptors,40 and downregulates the glutamate transporter, EAAT2, responsible for regulating the extracellular concentration of glutamate.41, 42 Furthermore, TNFα enhances calcium-dependent glutamate release43 and increases the intracellular concentration of calcium in astrocytes, which could trigger increased glutamate release and further toxicity.
Hypoxia also plays a role in TNFα-induced EAAT2 downregulation. As tumor cells continue to proliferate, they must utilize cellular pathways to meet their increasing metabolic demands. However, as nutrient supply diminishes, tumor cells undergo starvation and eventual necrosis. In response to hypoxia, C6 glioma cells demonstrate an initial increase in glutamate transporter expression, but this expression is not sufficient to reconstitute functional transporter activity or ameliorate hypoxia-induced ATP depletion and mitochondrial damage.44 Moreover, after prolonged periods of hypoxia, functional glutamate uptake declines,45 further exacerbating the glutamate-excitotoxic environment. A possible mechanism of EAAT2 downregulation may involve TNFα, which has been shown to specifically downregulate EAAT2 in astrocytes in response to hypoxia.46 Thus, tumor cells demonstrating enhanced TNFα expression would experience loss of EAAT2 and an increase in extracellular glutamate; unable to recover from hypoxia, these cells would undergo eventual necrosis.
Recent studies show that TNFα induces expression of astrocyte-elevated gene-1 (AEG-1), which is overexpressed in more than 95% of human brain tumors.47 AEG-1 overexpression enhances colony growth of HeLa cells in soft agar,48 while AEG-1 knockdown decreases colony-forming ability in immortalized human adult astrocytes that stably express Ha-Ras.49 These results indicate that the increased expression of AEG-1 observed in glioblastoma may provide these tumors with a significant growth potential and may enhance glioblastoma formation.
In order to maintain their accelerated growth, glioblastomas must develop a rich blood supply to fulfill their significant demand for nutrients, and they must also overcome apoptotic signals. AEG-1 helps to facilitate this accelerated growth by preventing apoptotic cell death in serum starvation conditions. AEG-1 overexpression significantly prevents serum starvation-induced cell death in normal as well as immortalized fetal astrocytes in an AKT-dependent manner.50 Additionally, AEG-1 knockdown reduces cell viability under serum starvation conditions in immortalized adult astrocytes, indicating that these transformed cell types require AEG-1 to survive serum starvation.50 Together, these results indicate that AEG-1 overexpression in glioblastoma is required for cells to survive periods of serum starvation that would limit growth through nutrient deprivation and through activation of pro-apoptotic pathways. Though AEG-1 may be necessary for initial tumor propagation in the absence of extensive angiogenesis, this unimpeded growth may also induce necrosis by coercing tumor growth in the absence of a sufficient nutrient supply. By preventing nutrient deprivation-induced apoptosis, AEG-1 provides these tumors with seemingly infinite growth potential, and therefore, AEG-1 may represent a useful target for glioblastoma therapy in the future.
AEG-1 also interacts with and activates the promoter for the transcription factor, nuclear factor-kappa B (NF-κB).48 NF-κB is a heterodimer consisting of p65 and p50 and is present in its inactive state in the cytoplasm, where it is bound to the inhibitor of kappa B-α (IκB-α). Upon activation by a variety of molecular factors, NF-κB translocates to the nucleus and regulates the transcription of many genes, some of which are involved in oncogenesis. AEG-1 activates the NF-κB pathway by facilitating IκB-α degradation and by increasing binding of the transcriptional activator p50/p65 complex in the nucleus.48 AEG-1 also binds to and activates the NF-κB promoter.51 In addition, AEG-1, along with p65, has been shown to translocate to the nucleus upon TNFα treatment, where these two proteins then interact.48 In the nucleus, AEG-1 binds to p65 and enhances activation of NF-κB -targeted genes.51 Importantly, NF-κB activation is prominent in high-grade compared to low-grade astrocytomas,52 which indicates a possible function for NF-κB in tumor progression.
AEG-1 has also been shown to downregulate the promoter for the glutamate transporter, EAAT2,47 which is already underactive in a methylated state in glioma cell lines.53 AEG-1 overexpression by glioblastomas could affect glutamate homeostasis and lead to a glutamate excitotoxic environment, which would result in widespread cell death in the peri-tumoral area. In addition, TNFα reduces EAAT2 mRNA and protein expression in PHFA in an NF-κB - dependent manner,42 suggesting a common pathway for TNFα and AEG-1 in NF-κB-mediated EAAT2 downregulation. Thus, the culmination of AEG-1 signaling in glioblastoma involves the direct activation of NF-κB and the subsequent downregulation of EAAT2. Along with its role in AKT activation through the PI3K pathway, AEG-1 stimulates unregulated growth in the absence of an adequate metabolic supply, and these pathways together would lead to tumor necrosis.
Phosphatase and tensin homolog (PTEN) and AKT
The tumor suppressor antagonist of the PI3K/AKT/mTOR pathway, phosphatase and tensin homolog (PTEN), is frequently mutated in astrocytic tumors.54 Importantly, these mutations are more common in glioblastomas than low-grade astrocytomas, mostly as a result of PTEN loss due to LOH.55 Recently, it was shown that PTEN haploinsufficiency accelerates the formation of glioblastomas from low-grade astrocytomas,56 indicating a potential role of PTEN in astrocytoma progression. In addition, PTEN is found to be mutated more commonly in primary glioblastomas, in which there is traditionally a greater extent of necrosis, than secondary glioblastomas,57 demonstrating a potential necrogenic role for PTEN in glioblastoma.58 PTEN also inhibits TNFα-induced NF-κB activation,59 which prevents the induction of anti-apoptotic genes by NF-κB. In the setting of PTEN loss, NF-κB activation would promote survival of tumor cells and lead to glioblastoma progression.
PTEN interacts with the phosphatidylinositol-3-kinase (PI3K) pathway by directly antagonizing the formation of phosphatidylinositol 3,4,5-triphosphate (PIP3) by PI3K, which leads to decreased AKT phosphorylation and activity. This reduction in AKT activity leads to tumor suppression through cell cycle checkpoint activation and eventual apoptosis. In glioblastoma, PTEN loss results in AKT elevation,60 a process that enhances the malignant characteristics of transformed cells. For example, AKT activation in conjunction with but not in place of Ras can convert anaplastic astrocytoma to glioblastoma in a human astrocyte model, resulting in many characteristic features of glioblastoma, including diverse cellular morphology and areas of necrosis.61 Thus, AKT activation may be a necessary step in the progression of glioblastoma. Furthermore, this transformation to a more malignant phenotype may potentially implicate AKT in the development of necrosis, a hallmark feature of glioblastoma.
AKT activation also occurs through the action of glutamate, which has differential effects on the AKT pathway to precipitate tumor necrosis. Glutamate has been shown to activate AKT through the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor in glial cells62 and glioblastoma cells,63 a process that may lead to invasive growth and anaplasia in tumor cells. In addition to AMPA receptor involvement, N-methyl-D-aspartic acid (NMDA) receptor stimulation leads to AKT activation in a PI3K-dependent manner,64 and this activation results in cell death which is necrotic in nature.65 Though one pathway leading from glutamate excitotoxicity to tumor necrosis seems to involve AKT activation, glutamate can also induce loss of AKT phosphorylation and kinase activity, which leads to glutamate excitotoxicity as well.66 For example, it has been shown that NMDA toxicity in astrocytes requires inhibition of the PI3K pathway, and this toxicity is reversed by metabotropic glutamate receptor activation.67 In this model, it is likely the downstream induction of other pro-survival signaling molecules that mediates neuroprotection, though. AKT activation has also been shown to be protective from glutamate excitotoxicity in oligodendrocytes,68 but this toxicity is apoptotic, not necrotic, in nature. Nonetheless, this finding suggests a role for AKT in survival from excitotoxicity-induced apoptosis, which would confer a more aggressive phenotype to malignant tumor cells. Together, these observations suggest that the result of glutamate activity on AKT-mediated necrosis in glioblastoma is dependent on the activation of and signaling through specific glutamate receptor subtypes as well as the involvement of specific downstream effectors.
Though the mechanism of AKT-mediated necrosis in glioblastoma remains unclear, it has been shown that AKT activation prevents non-apoptotic programmed cell death with morphological features of necrosis in glioblastoma cells.69 In the setting of PTEN loss, AKT activation may confer a more malignant phenotype to tumor cells, which may lead to enhanced tumor growth and the over-consumption of available nutrients, a process that has been linked to tumor necrosis.6 On the other hand, glutamate-mediated AKT inhibition could result in necrotic-like cell death in glioblastoma cells. Thus, it is the balance between AKT activation and inhibition that may determine the extent of necrosis in glioblastoma.
Conclusions
Necrosis is an important prognostic factor in the setting of glioblastoma. While some studies have assessed patient survival as a function of necrosis, there are few studies addressing the molecular mechanisms underlying necrosis formation in glioblastoma. Considering its role in excitotoxic cell death, glutamate seems to be a key factor in the necrosis cascade in glioblastoma. Not only does glutamate directly cause cell death through perturbations in Ca2+ homeostasis, but glutamate also interacts with AKT to promote glioblastoma progression and tumor necrosis. In addition, various cell signaling molecules, including TNFα, AEG-1, and NF-κB, cause coordinated accumulation of glutamate through reuptake inhibition to promote local excitotoxicity and necrosis. Therefore, glutamate may be a central mediator of necrosis in glioblastoma, and future research should focus on the application of various glutamate antagonists to assess the therapeutic potential of glutamate modulation in the treatment of glioblastoma.
ACKNOWLEDGMENTS
The authors wish to thank past and present members of the Department of Neuroscience/Center for Neurovirology for their support and sharing of ideas and reagents. We also thank C. Schriver for editorial assistance. This work was made possible by grants awarded by NIH to KK.
ABBREVIATIONS
- CNS
central nervous system
- TNF-α
tumor necrosis factor-α
- AEG-1
astrocyte-elevated gene-1
- NF-κB
nuclear factor-kappa B
- EAAT2
excitatory amino acid transporter 2
- PTEN
phosphatase and tensin homolog
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-D-aspartic acid
REFERENCES
- 1.Central Brain Tumor Registry of the United States: Statistical Report: Primary Brain Tumors in the United States 1998–2002. Hinsdale IL: Central Brain Tumor Registry of the United States; 2006. www.cbtrus.org. [Google Scholar]
- 2.Okamoto Y, Di Patre PL, Burkhard C, Horstman S, Jourde B, Fahey M, et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108:49–56. doi: 10.1007/s00401-004-0861-z. [DOI] [PubMed] [Google Scholar]
- 3.Schröck E, Blume C, Meffert MC, du Manoir S, Bershc W, Kiessling M, et al. Recurrent gain of chromosome arm 7q in low-grade astrocytic tumors studied by comparative genomic hybridization. Genes Chromosomes Cancer. 1996;15:199–205. doi: 10.1002/(SICI)1098-2264(199604)15:4<199::AID-GCC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Reifenberger G, Louis DN. Oligodendroglioma: toward molecular definitions in diagnostic neuro-oncology. J Neuropathol Exp Neurol. 2003;62:111–126. doi: 10.1093/jnen/62.2.111. [DOI] [PubMed] [Google Scholar]
- 5.Maintz D, Fiedler K, Koopmann J, Rollbrocker B, Nechev S, Lenartz D, Stangl AP, Louis DN, Schramm J, Wiestler OD, von Deimling A. Molecular genetic evidence for subtypes of oligoastrocytomas. J Neuropathol Exp Neurol. 1997;56:1098–1104. doi: 10.1097/00005072-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 7.Barker FG, 2nd, Davis RL, Chang SM, Prados MD. Necrosis as a prognostic factor in glioblastoma multiforme. Cancer. 1996;77:1161–1166. doi: 10.1002/(sici)1097-0142(19960315)77:6<1161::aid-cncr24>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Homma T, Fukushima T, Vaccarella S, Yonekawa Y, Di Patre PL, Franceschi S, Ohgaki H. Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol. 2006;65:846–854. doi: 10.1097/01.jnen.0000235118.75182.94. [DOI] [PubMed] [Google Scholar]
- 9.Miller CR, Dunham CP, Scheithauer BW, Perry A. Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol. 2006;24:5419–5426. doi: 10.1200/JCO.2006.08.1497. [DOI] [PubMed] [Google Scholar]
- 10.Pierallini A, Bonamini M, Pantano P, Palmeggiani F, Raguso M, Osti MF, et al. Radiological assessment of necrosis in glioblastoma: variability and prognostic value. Neuroradiology. 1998;40:150–153. doi: 10.1007/s002340050556. [DOI] [PubMed] [Google Scholar]
- 11.Smith SF, Simpson JM, Brewer JA, Sekhon LH, Biggs MT, Cook RJ, Little NS. The presence of necrosis and/or microvascular proliferation does not influence survival of patients with anaplastic oligodendroglial tumours: review of 98 patients. J Neurooncol. 2006;80:75–82. doi: 10.1007/s11060-006-9158-5. [DOI] [PubMed] [Google Scholar]
- 12.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 13.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 15.McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophr Res. 1991;4:209–231. doi: 10.1016/0920-9964(91)90034-o. [DOI] [PubMed] [Google Scholar]
- 16.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 17.Ludolph AC, Meyer T, Riepe MW. The role of excitotoxicity in ALS--what is the evidence? J Neurol. 2000;247(Suppl 1):I7–I16. doi: 10.1007/s004150050552. [DOI] [PubMed] [Google Scholar]
- 18.Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J Neurochem. 2008;105:287–295. doi: 10.1111/j.1471-4159.2008.05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 20.Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rijpkema M, Schuuring J, van der Meulen Y, van der Graaf M, Bernsen H, Boerman R, van der Kogel A, Heerschap A. Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed. 2003;16:12–18. doi: 10.1002/nbm.807. [DOI] [PubMed] [Google Scholar]
- 22.Roslin M, Henriksson R, Bergström P, Ungerstedt U, Bergenheim AT. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61:151–160. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- 23.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) Upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 25.Münch C, Penndorf A, Schwalenstöcker B, Troost D, Ludolph AC, Ince P, Meyer T. Impaired RNA splicing of 5'-regulatory sequences of the astroglial glutamate transporter EAAT2 in human astrocytoma. J Neurol Neurosurg Psychiatry. 2001;71:675–678. doi: 10.1136/jnnp.71.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi DW. Calcium and excitotoxic neuronal injury. Ann N Y Acad Sci. 1994;747:162–171. doi: 10.1111/j.1749-6632.1994.tb44407.x. [DOI] [PubMed] [Google Scholar]
- 28.Cho Y, Bannai S. Uptake of glutamate and cysteine in C-6 glioma cells and in cultured astrocytes. J Neurochem. 1990;55:2091–2097. doi: 10.1111/j.1471-4159.1990.tb05800.x. [DOI] [PubMed] [Google Scholar]
- 29.Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003;283:1–16. doi: 10.1016/s0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 30.Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Jr, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281:14076–14084. doi: 10.1074/jbc.M600653200. [DOI] [PubMed] [Google Scholar]
- 31.Mabrouk GM, Ali EM, El-Rehany MA, El-Samoly HM. TGF-beta1, TNF-alpha and cytochrome c in human astrocytic tumors: a short-term follow up and correlation with survival. Clin Biochem. 2007;40:255–260. doi: 10.1016/j.clinbiochem.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Roessler K, Suchanek G, Breitschopf H, Kitz K, Matula C, Lassmann H, Koos WT. Detection of tumor necrosis factor-alpha protein and messenger RNA in human glial brain tumors: comparison of immunohistochemistry with in situ hybridization using molecular probes. J Neurosurg. 1995;83:291–297. doi: 10.3171/jns.1995.83.2.0291. [DOI] [PubMed] [Google Scholar]
- 33.Raza SM, Fuller GN, Rhee CH, Huang S, Hess K, Zhang W, Sawaya R. Identification of necrosis-associated genes in glioblastoma by cDNA microarray analysis. Clin Cancer Res. 2004;10(1 Pt 1):212–221. doi: 10.1158/1078-0432.ccr-0155-3. [DOI] [PubMed] [Google Scholar]
- 34.Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R. Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery. 2002;51:2–12. doi: 10.1097/00006123-200207000-00002. discussion 12-3. [DOI] [PubMed] [Google Scholar]
- 35.Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, Gelbard HA. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 36.Szymocha R, Akaoka H, Dutuit M, Malcus C, Didier-Bazes M, Belin MF, Giraudon P. Human T-cell lymphotropic virus type 1-infected T lymphocytes impair catabolism and uptake of glutamate by astrocytes via Tax-1 and tumor necrosis factor alpha. J Virol. 2000;74:6433–6441. doi: 10.1128/jvi.74.14.6433-6441.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Chao CC, Hu S. Tumor necrosis factor-alpha potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev Neurosci. 1994;16:172–179. doi: 10.1159/000112104. [DOI] [PubMed] [Google Scholar]
- 39.Huang TL, O'Banion MK. Interleukin-1 beta and tumor necrosis factor-alpha suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem. 1998;71:1436–1442. doi: 10.1046/j.1471-4159.1998.71041436.x. [DOI] [PubMed] [Google Scholar]
- 40.Gelbard HA, Dzenko KA, DiLoreto D, del Cerro C, del Cerro M, Epstein LG. Neurotoxic effects of tumor necrosis factor alpha in primary human neuronal cultures are mediated by activation of the glutamate AMPA receptor subtype: implications for AIDS neuropathogenesis. Dev Neurosci. 1993;15:417–422. doi: 10.1159/000111367. [DOI] [PubMed] [Google Scholar]
- 41.Carmen J, Rothstein JD, Kerr DA. Tumor necrosis factor-alpha modulates glutamate transport in the CNS and is a critical determinant of outcome from viral encephalomyelitis. Brain Res. 2009;1263:143–154. doi: 10.1016/j.brainres.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proc Natl Acad Sci U S A. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett. 2008;432:232–236. doi: 10.1016/j.neulet.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 44.Hsu L, Rockenstein E, Mallory M, Hashimoto M, Masliah E. Altered expression of glutamate transporters under hypoxic conditions in vitro. J Neurosci Res. 2001;64:193–202. doi: 10.1002/jnr.1065. [DOI] [PubMed] [Google Scholar]
- 45.Ohashi M, Amano S, Hazama F, Handa J. Hypoxic effects on glutamate uptake in cultured glial cells. Acta Pathol Jpn. 1993;43:154–159. doi: 10.1111/j.1440-1827.1993.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 46.Boycott HE, Wilkinson JA, Boyle JP, Pearson HA, Peers C. Differential involvement of TNF alpha in hypoxic suppression of astrocyte glutamate transporters. Glia. 2008;56:998–1004. doi: 10.1002/glia.20673. [DOI] [PubMed] [Google Scholar]
- 47.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Emdad L, Sarkar D, Su ZZ, Randolph A, Boukerche H, Valerie K, Fisher PB. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- 49.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–1484. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 52.Angileri FF, Aguennouz M, Conti A, et al. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112:2258–2266. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- 53.Zschocke J, Allritz C, Engele J, Rein T. DNA methylation dependent silencing of the human glutamate transporter EAAT2 gene in glial cells. Glia. 2007;55:663–674. doi: 10.1002/glia.20497. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 55.Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 56.Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68:3286–3294. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koul D, Takada Y, Shen R, Aggarwal BB, Yung WK. PTEN enhances TNF-induced apoptosis through modulation of nuclear factor-kappaB signaling pathway in human glioma cells. Biochem Biophys Res Commun. 2006;350:463–471. doi: 10.1016/j.bbrc.2006.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 61.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- 62.Morales M, González-Mejía ME, Bernabé A, Hernández-Kelly LC, Ortega A. Glutamate activates protein kinase B (PKB/Akt) through AMPA receptors in cultured Bergmann glia cells. Neurochem Res. 2006;31:423–429. doi: 10.1007/s11064-005-9034-2. [DOI] [PubMed] [Google Scholar]
- 63.Ishiuchi S, Yoshida Y, Sugawara K, Aihara M, Ohtani T, Watanabe T, et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J Neurosci. 2007;27:7987–8001. doi: 10.1523/JNEUROSCI.2180-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perkinton MS, Ip JK, Wood GL, Crossthwaite AJ, Williams RJ. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signalling to MAP kinase (Erk1/2), Akt/PKB and CREB in striatal neurones. J Neurochem. 2002;80:239–254. doi: 10.1046/j.0022-3042.2001.00699.x. [DOI] [PubMed] [Google Scholar]
- 65.Portera-Cailliau C, Price DL, Martin LJ. Non-NMDA and NMDA receptor-mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: Further evidence for an apoptosis-necrosis continuum. J. Comp. Neurol. 1997;378:88–104. [PubMed] [Google Scholar]
- 66.Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci U S A. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D'Onofrio M, Cuomo L, Battaglia G, Ngomba RT, Storto M, Kingston AE, et al. Neuroprotection mediated by glial group-II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J Neurochem. 2001;78:435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- 68.Ness JK, Wood TL. Insulin-like growth factor I, but not neurotrophin-3, sustains Akt activation and provides long-term protection of immature oligodendrocytes from glutamate-mediated apoptosis. Mol Cell Neurosci. 2002;20:476–488. doi: 10.1006/mcne.2002.1149. [DOI] [PubMed] [Google Scholar]
- 69.Mochizuki T, Asai A, Saito N, Tanaka S, Katagiri H, Asano T, et al. Akt protein kinase inhibits non-apoptotic programmed cell death induced by ceramide. J Biol Chem. 2002;277:2790–2797. doi: 10.1074/jbc.M106361200. [DOI] [PubMed] [Google Scholar]