Abstract
d-Serine, a co-agonist at the NMDA receptor (NMDAR), is synthesized from l-serine by the enzyme serine racemase (SR), which is heavily expressed in the forebrain. Although SR was originally reported to be localized exclusively to astrocytes, recent conditional knock out results demonstrate that little SR is expressed in forebrain astrocytes. As a consequence, the cellular location of its product, d-serine, in the brain is also uncertain. Immunocytochemistry now indicates that SR is expressed primarily in forebrain glutamatergic neurons with the remainder in GABAergic interneurons. We utilized SR deficient (SR−/−) mice, which have <15 % of normal d-serine levels, to validate and optimize a d-serine immunohistochemical method. Nearly all of the d-serine in neocortex and hippocampus (HP) is found in neurons, with virtually no d-serine co-localizing with two astrocyte markers. Interestingly, only a subset of the d-serine positive neurons contained SR in the neocortex and HP. Greater than half of the d-serine positive neurons were GABAergic interneurons, with a majority of these neurons containing parvalbumin and/or somatostatin. Only ~25–40 % of interneurons expressed SR in the neocortex and HP. Finally, we demonstrate in human post-mortem neocortex that SR is found in both excitatory and inhibitory neurons, but not in S100β-containing astrocytes. In sum, these findings conclusively demonstrate that the majority of d-serine is both synthesized and stored in neurons. It will be important to determine the functional significance for the separation of synthesis and storage of d-serine in neurons, as well as the presence of this NMDAR co-agonist in GABAergic interneurons.
Keywords: NMDA receptor, Parvalbumin, Somatostatin, CAMKIIα, Hippocampus, Cortex
Introduction
d-Amino acids are now well established as mediators and modulators of neuronal activity in mammals (Boehning and Snyder 2003; Wolosker et al. 2008). Activation of the N-methyl-d-aspartate receptor (NMDAR) requires the binding of either glycine or d-serine at the glycine modulatory site (GMS) on the GluN1 subunit (Kishi and Macklis 2004). d-Serine is enriched in corticolimbic regions of the brain, where its localization closely parallels that of NMDARs (Schell et al. 1995). Therefore, d-serine is thought to be the primary NMDAR co-agonist in the forebrain because of its high concentration in this region and because elimination of synaptic d-serine reduces NMDAR-mediated currents (Mothet et al. 2000; Schell et al. 1995). Recent evidence also suggests that d-serine is the primary co-agonist for synaptic, but not extra-synaptic NMDARs in the hippocampus (HP) (Papouin et al. 2012), via non-vesicular release through alanine–serine–cysteine-1 (Asc-1) transporters (Rosenberg et al. 2013).
The cloning and characterization of serine racemase (SR) demonstrated that d-serine is synthesized endogenously in the mammalian brain through the conversion of l- to d-serine (Wolosker et al. 1999). Deletion of the SR gene reduces cortical d-serine levels by ~85 % (Basu et al. 2009). Initial in vitro studies and immunohistochemical studies suggested that SR was present mainly in astrocytes, which, therefore, were the major source of d-serine in the brain (Mothet et al. 2005; Schell et al. 1995; Wolosker et al. 1999). However, recent immunohistochemical studies have suggested a more prominent neuronal expression of SR (Ding et al. 2011; Miya et al. 2008; Ehmsen et al. 2013). Furthermore, using mice with conditional SR deletions either in excitatory forebrain neurons (nSR−/−) or astrocytes (aSR−/−), we have demonstrated that the majority (~65 %) of SR is expressed in forebrain excitatory neurons, particularly in the neocortex and HP, and that 15 % or less is expressed in astrocytes (Benneyworth et al. 2012). Notably, the cellular localization of SR has not been demonstrated in human brain.
The cellular location of d-serine itself also is not well characterized. Some immunohistochemical studies, both from brain tissue and cell culture, suggest a primary localization in astrocytes (Fossat et al. 2012; Schell et al. 1995; Martineau et al. 2013), while other studies in rats found that d-serine was located primarily in neurons (Kartvelishvily et al. 2006; Curcio et al. 2013). However, the specificity of d-serine immunostaining in brain tissue has yet to be established. Thus, we utilized SR−/− mice as a negative control to optimize d-serine staining and used this new protocol to accurately quantify the cellular localization of d-serine, as well as SR, in the rodent brain. Finally, we demonstrate the cellular location of SR in the human brain, which comports with the findings in mice.
Materials and Methods
Animals
Constitutive SR−/− mice (Basu et al. 2009) were generated as previously described. SR ± sires and dams were bred to produce wild-type (WT), as well as SR−/− offspring. Adult male mice (3–5 months old) were used for all experiments. Animals were group housed in polycarbonate cages and maintained on a 12:12 h light/dark cycle in a temperature (22 °C) and humidity-controlled vivarium. Animals were given access to food and water ad libitum. All animal procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee.
Drugs
SR−/− mice received a single subcutaneous (s.c.) injection of d-serine (300 mg/kg; Sigma-Aldrich, St. Louis, MO) at a volume of 5 ml/kg dissolved in 0.9 % sodium chloride. Mice were perfused 2 h following the injection.
Antibody Characterization
See Table 1 for a complete list of antibodies and working concentrations. The rabbit polyclonal antibody to d-serine (cat # ab6472; Abcam) was characterized in this manuscript by immunohistochemistry using SR−/− mice.
Table 1.
List of antibodies used for immunohistochemistry and immunofluorescence
| Antibody | Antigen/immunogen | Species/dilution | Source/cat no. |
|---|---|---|---|
| Mouse | |||
| Primary | |||
| d-Serine (colorimetric) | Synthetic d-Serine conjugated to bovine serum albumin (BSA) | Rabbit polyclonal 1:60K | Abcam ab6472 |
| d-Serine (immunofluorescence) | Synthetic d-serine conjugated to bovine serum albumin (BSA) | Rabbit polyclonal 1:15K | Abcam ab6472 |
| GAD67 (clone 1G10.2) | Recombinant GAD67 protein | Mouse 1:500 | Millipore MAB5406 |
| GFAP | Preparation of full-length human recombinant GFAP expressed in bacteria and highly purified | Rabbit polyclonal 1:500 | Abcam ab7260 |
| GFAP (clone GA5) | Purified GFAP from porcine spinal cord | Mouse 1:10K | Millipore MAB360 |
| NeuN | Purified cell nuclei from mouse brain | Mouse 1:1,000 | Millipore MAB377 |
| Parvalbumin (clone PARV-19) | Frog muscle parvalbumin | Mouse 1:10K | Sigma P3088 |
| S100β (clone SH-B1) | Bovine brain S100β | Mouse 1:5K | Sigma S2532 |
| Serine racemase (clone A-17; immunofluorescence) | Raised against N-terminal amino acids 1–50 of mouse serine racemase | Goat polyclonal 1:50 | SCBT sc-5751 |
| Serine racemase (colorimetric) | C-terminal amino acids 191–340 of human serine racemase | Rabbit polyclonal 1:1,000 | SCBT sc-48741 |
| Somatostatin (clone YC7) | Synthetic peptide corresponding to amino acids 1–14 of cyclic somatostatin conjugated to bovine thyroglobulin using carbodiimide | Rat monoclonal 1:200 | Millipore MAB354 |
| Secondary | |||
| Biotinylated goat anti-rabbit | 1:1,000 | Vector BA-1000 | |
| Goat anti-mouse AlexaFluor 555 IgG (H+L) | 1:500, 1:3K | Life Technologies A-21422 | |
| Goat anti-mouse AlexaFluor 647 IgG (H+L) | 1:1,000 | Life Technologies A-21235 | |
| Goat anti-rat AlexaFluor 555 IgG (H+L) | 1:500 | Life Technologies A-21434 | |
| Rabbit anti-goat AlexaFluor 555 IgG (H+L) | 1:500 | Life Technologies A-21428 | |
| Streptavidin AlexaFluor-488 | 1:1,000 | Life Technologies S-32354 | |
| Human | |||
| Primary | |||
| CAMKIIa (6G9) | Partially purified full length native rat protein | Mouse 1:300 | Novus Biologicals NB100-1983 |
| Parvalbumin (clone PARV-19) | Frog muscle parvalbumin | Mouse 1:5K | Sigma Aldrich, P3088 |
| S100β (clone SH-B1) | Bovine brain S100β | Mouse 1:2,500 | Sigma Aldrich S2532 |
| Serine racemase (immunofluorescence) | C-terminal amino acids 191–340 of human serine racemase | Rabbit polyclonal 1:50 | SCBT sc-48741 |
| Secondary | |||
| Biotinylated goat anti-rabbit | 1:250 | Vector Laboratories BA-1000 | |
| Goat anti-mouse Alexa Fluor 647 IgG (H+L) | 1:250 | Life Technologies A-21235 | |
| Streptavidin Alexa Fluor-488 | 1:2,500 | Life Technologies S-32354 |
Amino Acid–BSA Conjugation
l- or d-Serine (100 mM) was dissolved in 1 ml of a 1.5 M sodium acetate solution (pH 8). Then, 500 μl of a 0.5 M glutaraldehyde (25 % = 2.5 M stock) solution was added for 5 min, with stirring. The mixture containing activated l-serine was mixed with 1 ml of a 1.5 M acetate solution (pH 8) containing 15 mg of BSA and was left to react for 10 min with stirring. 500 μl of a 0.1 M sodium borohydride solution was then added for 5 min with stirring. The conjugate was subject to overnight dialysis (Spectrum Laboratories; 12–14 kDa MWCO; Before use, the dialysis membrane was boiled several minutes in a large excess of 10 mM sodium bicarbonate, followed by several minutes in boiling 10 mM sodium EDTA. This was repeated and the membrane was washed several times in distilled water) at 4 °C in 0.1 M sodium phosphate buffer (pH 7.6). Insoluble material was then removed by centrifugation at 10,000×g for 15 min.
d-Serine Immunohistochemistry
Tissue for immunohistochemical studies was obtained from transcardially perfused mice. Animals were deeply anesthetized with sodium pentobarbital (180 mg/kg, i.p.) and perfused with 0.1 M phosphate-buffered saline followed by a fixative of 3 % glutaraldehyde (25 % stock; Fisher Scientific), 1 % paraformaldehyde (16 % stock; Electron Microscopy Sciences), 0.2 % sodium metabisulfite (Sigma Aldrich), and 10 U/ml Heparin salt (Sigma Aldrich). Brains were post-fixed for 24 h in fixative and cryoprotected in 30 % sucrose. Immunohistochemistry was performed on 20 μm free-floating sagittal or coronal sections. Sections were treated with freshly made 0.2 % sodium metabisulfite (Sigma Aldrich) and 0.5 % sodium borohydride (Sigma Aldrich) for 10 min (to reduce the free aldehydes). Sections were treated for 60 min with blocking solution (0.02 M Tris-buffered saline (TBS) containing 10 % normal goat serum and 0.1 % Triton X-100). d-Serine was localized using a primary antibody of rabbit origin (1:60K) diluted in blocking solution including l-serine–glutaraldehyde–BSA conjugate (10–200-fold dilution of 100 mM dialyzed stock). Incubation in primary antibody was done for ~40 h at 4 °C. Sections were incubated with biotinylated secondary antibody (1:1,000) for 90 min and Elite ABC reagent (1:100 dilution of each reagent in TBS w/0.1 % Triton X-100, ABC Elite kit, Vector Laboratories) for 60 min. Colorimetric detection was performed with 3,3-diaminobenzidine (0.02 %) enhanced with nickel (II) sulfate (0.08 %) in 0.1 M phosphate buffer containing 0.01 % hydrogen peroxide. In between each incubation step, sections were washed 3 times for 10 min each in 0.02 M TBS [except prior to the metabisulfite/borohydride incubation and colorimetric detection when only 0.1 M phosphate buffer (PB) was used]. Experimental and corresponding control samples were processed in parallel. Immunostaining was visualized on a Ziess Axioskop microscope using StereoInvestigator software (MBF Bioscience; Welliston, VT) to capture the digital images under constant conditions for subjects of each comparison. For the 20× brain region images, multiple overlapping images were brought into register using Photoshop CS5 (Adobe Systems Inc., San Jose, CA) to create the resulting collage images.
d-Serine Immunofluorescence
Brain sections were treated with Schiff’s Reagent (Sigma Aldrich) for 20 min at room temperature with shaking, followed by washing with 0.1 M hydrochloric acid containing 0.5 % sodium metabisulfite for 10 min at room temperature. The sections were then incubated 4× with freshly made 0.2 % sodium metabisulfite and 1.0 % sodium borohydride for 15 min each wash, in order to decolorize the sections. Sections were washed 3× in TBS and then incubated for 60 min with blocking solution (10 % normal goat serum and 0.1 % Triton X-100). d-Serine was localized using a primary antibody of rabbit origin diluted in blocking solution containing a 10-fold dilution of 100 mM l-serine–glutaraldehyde–BSA conjugate. For neuronal co-localization, sections were also incubated with mouse anti-NeuN. For astrocyte co-localization, sections were incubated with mouse anti-GFAP or mouse anti-S100β. Mouse anti-GAD67, mouse anti-parvalbumin, and rat antisomatostatin were used to label GABAergic neurons. Incubation in primary antibodies was done overnight with agitation at room temperature. Sections were washed 3× in TBS and then incubated with biotinylated secondary antibody for 90 min at room temperature. After washing 3× in TBS, sections were incubated in the dark with streptavidin Alexa Fluor-488 and species appropriate secondary Alexa Fluor-555 IgG (H+L) (1:500 or 1:3,000 for GFAP) or Alexa Fluor-647 IgG (H+L) for 90 min at room temperature. After washing, sections were mounted on slides, allowed to dry for at least 10 min, and coverslipped using ProLong Gold Antifade Reagent (Life Technologies). Immunofluorescence was visualized on a Zeiss Axio ImagerM2 equipped with an Axiocam MRm camera and Apotome 2.0. Immunofluorescent images appearing in figures were obtained using a Zeiss Axio ImagerM2 at 40×. Psuedocoloring was performed in StereoInvestigator. In PowerPoint 2011, the images were adjusted for contrast and brightness and were cropped to improve the display of the regions of interest.
Quantification of d-Serine Localization
d-Serine positive cells were counted in the prefrontal cortex (PFC), primary somatosensory barrel field cortex (S1), and HP. From each mouse (n = 3), cells were counted from 4 to 5 sections per region that spanned across the rostral-caudal axis according to Paxinos and Franklin, 2001 (PFC 2.34 to 1.54, Cg1, PrL, IL; S1 0.38 to −1.94; HP −1.34 to −2.5), and were counterbalanced for hemisphere. For each section, all the d-serine positive cells were counted in the defined region at 20× and it was then determined whether these same cells co-localized with the other cellular marker. The determination for whether a cell was positive or not for each marker was made in the same focal plane. Due to the almost complete lack of d-serine staining in astrocytes, only one section per brain region per mouse (n = 3) was used to quantify d-serine co-localization with S100β and GFAP. Furthermore, due to the restricted expression of GFAP in the cortex, d-serine co-localization was only assessed in the areas of defined cortex that contained GFAP positive cells. Regions of interest and cell counts were performed using StereoInvestigator (MBF Bioscience; Williston, VT).
Quantification of SR Localization and Density
Due to the high density of SR positive cells, all of the PV and SOM cells were first counted in the HP [not including stratum pyramidale and the granule cell layer (GCL)], PFC, and S1 (n = 3 mice; 4–5 sections per region). It was then determined whether these PV and/or SOM cells co-localized with SR. The density of SR cells in the analyzed PFC and S1 sections (4–5 sections per region) was stereologically estimated at 20× using the Optical Fractionator module of StereoInvestigator (MBF Bioscience, Williston, VT). The chosen grid size (PFC 250 × 250 μm; S1 400 × 400 μm) and counting frame (100 × 100 μm) estimated densities with high precision (coefficient of error <0.07). The density of PV and SOM cells was calculated by dividing the total number of counted cells by the area of the analyzed region.
Human Post-Mortem Immunofluorescence
Formalin-fixed primary motor cortex (BA4) brain tissue from 3 male control cases (AN18870, AN10180, AN16218) was provided by the Harvard Brain Tissue Resource Center that is supported in part by PHS Grant number R24-MH068855. Case AN18870 was 58-years old, died of cancer with a post-mortem interval (PMI) of 20.5 h, and the brain tissue was in formalin for 1.3 years. Case AN10180 was 73-years old with a PMI of 24 h, and the brain tissue was in formalin for 1.3 years. Case AN16218 was 72-years old with a PMI of 27.7 h, and the brain tissue was in formalin for 3.3 years. Tissue blocks were cryoprotected (0.1 M PB, 33 % glycerol, 33 % ethylene glycol) for 14 d at 4 °C and then transferred to −20 °C. Brain tissue was cut at a thickness of 40 μm on a freezing microtome and sections were stored in cryoprotectant at −20 °C. All solutions were made in 0.1 M PBS with 0.5 % TritonX-100 (washing solution) except where noted. The tissue sections were washed 3× for 5 min between all steps. Tissue sections were washed and then incubated in a citric buffer (0.1 M citric acid, 0.2 M Na2HPO4 made in water) antigen unmasking solution. The sections were placed into citric buffer that was heated to 80 °C and then allowed to cool for 20 min without agitation. Sections were washed, incubated in a water solution containing 0.3 % H2O2 and 10 % methanol, and washed again. The tissue was blocked at room temperature in 2 % BSA and then placed in blocking solution containing the primary antibodies for two nights at 4 °C. Sections were washed and placed in a 1 % BSA solution containing biotinylated secondary antibody for 2 h at room temperature. Following washing, the sections were placed in a 1 % BSA solution containing a streptavidin–fluorophore conjugated antibody and a species appropriate fluorophore conjugated secondary antibody for 4 h at room temperature. Lipofuchsin autofluorescence was quenched by placing the tissue in a cupric sulfate (0.5 M sodium acetate, 0.001 M cupric III sulfate in water) solution for 10 min at room temperature. After washing in 0.1 M PB, sections were mounted on slides, allowed to dry for at least 10 min, and cover slipped using ProLong Gold Antifade Reagent (Life Technologies).
Results
Optimized Immunostaining Protocol for d-Serine in Brain Tissue
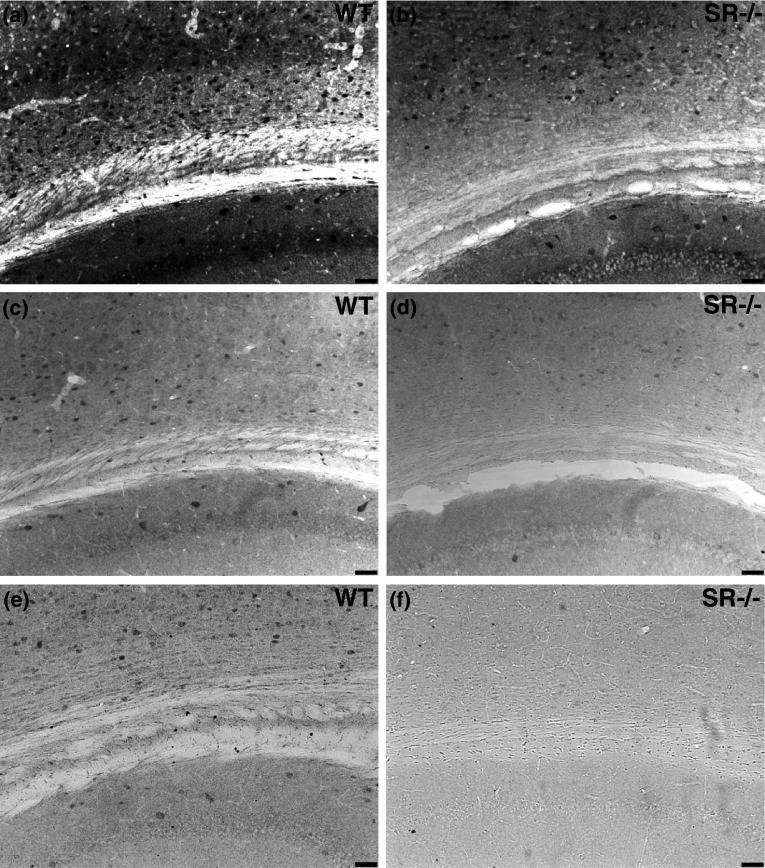
We first wanted to validate the specificity of the d-serine antibody for immunohistochemistry using brain tissue from our constitutive SR knockout mouse (SR−/−), the cortex of which has <15 % of the d-serine levels of wild-type (WT) mice. Our initial validation was based on a protocol used to demonstrate that d-serine was found in neurons (Kartvelishvily et al. 2006). This protocol used glutaraldehyde as the main fixative, as the antibody was created using a d-serine–BSA glutaraldehyde conjugate (d-ser–BSA), and included a 0.1 mM l-ser–glutaraldehyde conjugate to block the antibody from non-specifically binding l-serine in the tissue sections. However, when 200 (Fig. 1a, b) or 20-fold (Fig. 1c, d) dilutions of a dialyzed 100 mM l-serine–BSA conjugate were used, there was intense cellular staining in SR−/− tissue, although less intense than staining in WT tissue. Therefore, we increased the concentration of the conjugate in the primary antibody incubation to determine if the staining in SR−/− mice was due to non-specific l-serine binding. A 10-fold dilution of 100 mM l-serine–BSA allowed for detection of d-serine in WT tissue (Fig. 1e) and prevented staining in SR−/− tissue (Fig. 1f). Furthermore, fixation conditions, which did not include glutaraldehyde (4 % paraformaldehyde) fixation and l-serine–BSA blocking during the primary antibody incubation, resulted in identical staining across WT and SR−/− subjects (data not shown).
Fig. 1.
Optimized conditions for d-serine immunohistochemistry in adult rodent brain. Sagittal brain sections (×10) from a wild-type (WT; a, c, e) and SR−/− b, d, f mouse incubated with d-serine antibody and increasing concentrations of l-ser–BSA. Low concentrations of a, b 200-fold and c, d 20-fold dilutions of 100 mM l-ser–BSA produce intense cellular staining in WT and SR−/− tissue. e, f A 10-fold dilution of 100 mM l-ser–BSA is required to block non-specific binding of the d-serine primary antibody to l-serine in the glutaraldehyde fixed mouse brain tissue, as demonstrated by the lack of cellular d-serine staining in SR−/− tissue. Scale bars represent 50 μm. l -ser–BSA l-serine–bovine serum albumin glutaraldehyde conjugate
Distribution of d-Serine in the Adult Brain
With this new validated immunostaining protocol, we next wanted to assess the distribution of d-serine across brain regions. In the several cortical brain regions that we investigated (Fig. 2a–d), primary somatosensory barrel field cortex (S1), primary motor cortex (M1), medial prefrontal cortex (mPFC), and primary visual cortex (V1), d-serine was evenly distributed across cortical laminae. In the HP (Fig. 2e), there was dark staining in the ventral molecular layer (Mol) of the dentate gyrus (DG) and cellular staining in the hilus (Hi), stratum radiatum (s.r.), stratum oriens (s.o.), and lacunosum moleculare (l.m.). Interestingly, there was very limited cellular staining in the stratum pyramidale (s.p.) or GCL, which is consistent with what was observed in prior studies even when l-serine–glutaraldehyde conjugate was excluded from the primary antibody incubation (Schell et al. 1997; Schell et al. 1995; Wolosker et al. 1999).
Fig. 2.
Regional distribution of d-serine in the adult brain. Sagittal brain sections from wild-type (WT) mice demonstrating the distribution of d-serine positive cells in the a S1, b M1, c mPFC, d V1, and e hippocampus. There is an even distribution of d-serine positive cells across layers I through VI of the cortical laminae. In the hippocampus, cellular d-serine staining occurs in the Hi, s.o., s.r., and Mol layers. However, there was almost no cellular staining in the GCL or in the s.p. Hi hilus, GCL granule cell layer, M1 primary motor cortex, mPFC medial prefrontal cortex, Mol molecular layer, S1 primary somatosensory cortex, s.o., stratum oriens, s.p. stratum pyramidale, s.r. stratum radiatum, V1 primary visual cortex. Each panel represents tiled ×20 images
d-Serine is Primarily Expressed in Neurons
Due to the uncertainty of whether d-serine is stored in astrocytes or neurons, immunofluorescence was used to determine the cellular location of d-serine. First, we validated our d-serine immunofluorescence protocol and demonstrated the absence of d-serine staining in: WT tissue lacking primary antibody (Fig. 3a), SR−/− mouse brain (Fig. 3b, c), and in WT cerebellum (Fig. 3d–f). Streptavidin-conjugated fluorophores must be used as avidin conjugated antibodies produce “cellular-like” non-specific staining in glutaraldehyde fixed tissue in the absence of primary antibody (data not shown). We found that in S1 and HP (Fig. 3g–i) subfields that nearly all (Table 2; 83–96 %) of the d-serine positive cells co-localized with the neuronal marker, NeuN. In the mPFC and S1, d-serine cells are evenly distributed across laminae, although in S1 there appears to be fewer cells in layer I and enrichment in layer IV. In the dorsal and ventral HP, d-serine cells were concentrated in the s.o and s.r. of CA1/CA3 and in the Hi and Mol of the DG, as well as subiculum. There were, however, sporadic d-serine positive cells located in s.p. and GCL.
Fig. 3.
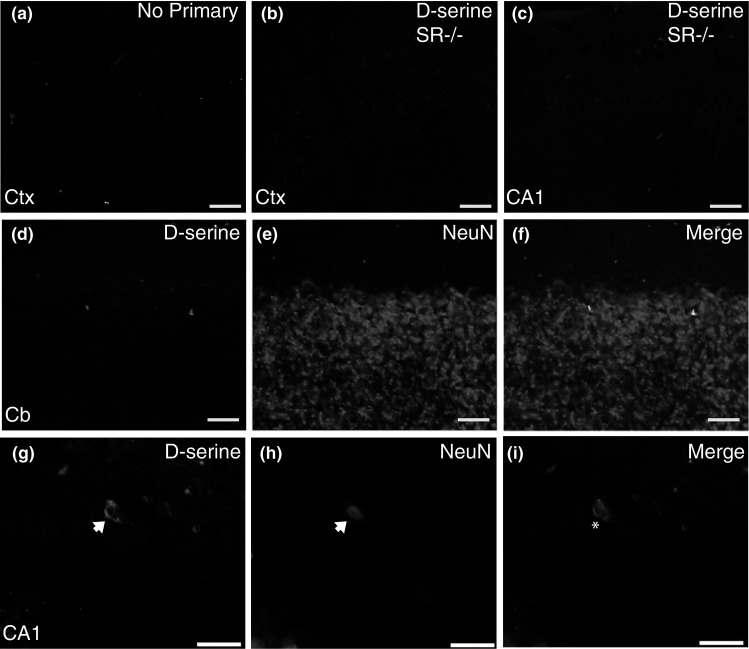
d-serine is found mainly in neurons of the adult brain. Representative coronal brain sections (×20) from adult WT and SR−/− mice. There is no immunofluorescent d-serine signal when a primary antibody, but not l-serine–BSA conjugate, is omitted. There is also no immunofluorescent d-serine signal in SR−/−. b cortex and c hippocampus using the d-serine primary antibody. d–i shows double immunofluorescent labeling of d-serine d, g with the neuronal marker, neuronal nuclei (NeuN; e, h) in Cb, and hippocampus (CA1). Merged images are shown in panels (f, Cb; i, CA1; d-serine, green; NeuN, magenta). There are no d-serine positive cells in Cb, while in S1 and CA1, d-serine positive cells almost exclusively co-localize with NeuN. Arrows indicate d-serine positive cells. Asterisks indicate DS/NeuN overlap. CA1 cornu ammonis, Cb cerebellum, Ctx cortex. Scale bars represent 50 μm in a–f and 25 μm in g–i (Color figure online)
Table 2.
d-serine is almost exclusively localized to neurons in the hippocampus and cortex
| PFC | S1 | DG | CA3 | CA1 | |
|---|---|---|---|---|---|
| NeuN (n = 3 mice) | |||||
| % Co-localization with d-serine | 88 ± 1.4 | 84 ± 6.1 | 83 ± 2.3 | 83 ± 3.3 | 96 ± 0.9 |
| Average # d-serine cells counted/mouse | 537 | 691 | 116 | 297 | 381 |
| S100β (n = 3 mice) | |||||
| % Co-localization with d-serine | 4 ± 1.1 | 1 ± 0.6 | 6 ± 1.1 | 5 ± 3.8 | 4 ± 2.3 |
| Average # d-serine cells counted/mouse | 50 | 149 | 27 | 44 | 35 |
| GFAP (n = 3 mice) | |||||
| % Co-localization with d-serine | 0 | 0 | 0 | 3 ± 2.1 | 1.4 ± 0.7 |
| Average # d-serine cells counted/mouse | 43 | 152 | 7 | 31 | 46 |
| SR (n = 3 mice) | |||||
| % Co-localization with d-serine | 56 ± 4.2 | 52 ± 4.7 | 23 ± 7.1 | 41 ± 6.3 | 37 ± 0.9 |
| Average # d-serine cells counted/mouse | 47 | 389 | 43 | 50 | 120 |
The percent co-localization of d-serine positive cells with NeuN (neurons), S100β (astrocyte), GFAP (astrocyte), and SR was quantified from 3 WT mice in the PFC, S1, and hippocampus (DG; CA3; CA1). The percent co-localization is represented as the average ± SEM
CA1/3 cornu ammonis, DG dentate gyrus, GFAP glial fibrillary acidic protein, PFC prefrontal cortex, NeuN neuronal nuclei, SR serine racemase
In contrast to what was found with NeuN, there was negligible co-localization (Table 2; 0–6 %) of d-serine in all of the brain regions examined with two astrocyte markers, S100β (Fig. 4a–c) and GFAP (Fig. 4d–f). S100β labeled a greater proportion of astrocytes and was more evenly distributed throughout the brain than GFAP (data not shown). The lack of GFAP staining in intermediate cortical layers was due neither to glutaraldehyde fixation nor primary antibody (data not shown).
Fig. 4.
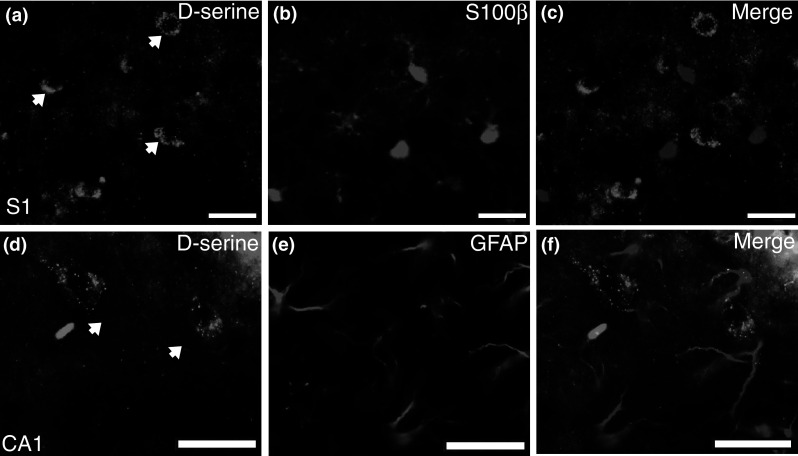
d-Serine is not found in S100β or GFAP-containing astrocytes of the adult brain. Representative coronal brain sections from adult WT mice. d-Serine positive cells (arrows) in a S1 and (d; CA1) hippocampus do not overlap with the astrocyte markers b S100β or e GFAP. Merged images are shown in panels (c, S1; f, CA1; d-serine, green; S100β, and GFAP, magenta). CA1 cornu ammonis, S1 primary somatosensory cortex. Scale bars represent 25 μm (Color figure online)
We next wanted to demonstrate that our protocol does not bias against astrocytic staining. Therefore, we peripherally injected SR−/− mice with 300 mg/kg d-serine, a dose that restores cortical d-serine levels (Balu et al. 2013), and then stained for d-serine, since nearly all of the amino acid would have to pass through astrocytes from the bloodstream into the brain. In SR−/− mice perfused 2 h after injection, there was d-serine staining in both astrocytes, primarily perivascular (Fig. 5a–c), and in neurons (Fig. 5d–f).
Fig. 5.
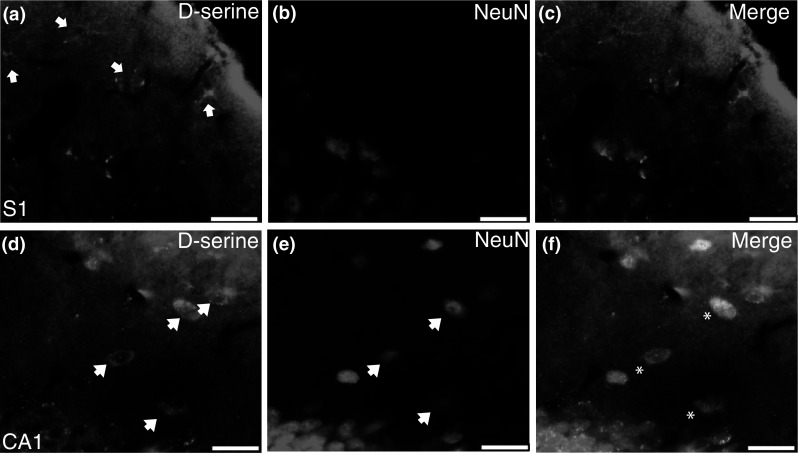
d-Serine can be visualized in astrocytes and neurons of SR−/− mice injected with d-serine (300 mg/kg). Representative coronal brain sections (×40) from adult SR−/− mice perfused 2 h after d-serine injection. When perfused 2 h after injection, clear d-serine staining was found in both astrocytes and neurons in SR−/− mice. d-serine was found both in astrocytes (a; S1; arrows), with some of them unsheathing blood vessels, as well as in neurons (d; CA1; arrows). Merged images are shown in (c, S1; f, CA1; d-serine, green; NeuN, magenta). Asterisks indicate DS/SR overlap. CA1 cornu ammonis, S1 primary somatosensory cortex. Scale bars represent 25 μm (Color figure online)
d-Serine is Found in Some, But Not All SR-Expressing Neurons
To date, no studies have examined the co-expression of SR and d-serine in brain tissue. The SR antibody used here was previously validated in SR−/− mice (Miya et al. 2008). Under the current fixation conditions, the antibody recognized SR and produced a pattern of expression consistent with the literature, in that there was high forebrain expression with little to no hindbrain expression and no labeling in SR−/− mice (data not shown). As shown in Table 2, only ~50 % of d-serine positive cells expressed SR in the PFC and S1 (Fig. 6a–c). The amount of co-localization was even lower in the HP, ranging from 23 to 41 %. The DS/SR cells in the HP were located outside of the s.p. and GCL.
Fig. 6.

d-Serine partially co-localizes with serine racemase in the adult brain. Representative coronal brain sections (×40) from adult WT mice. A portion of d-serine positive cells (arrows) in a S1 overlap with the SR (b; arrows). Merged images are shown in panels (c; d-serine, green; SR, magenta). Asterisks indicate DS/SR overlap. S1 primary somatosensory cortex. Scale bars represent 25 μm (Color figure online)
d-Serine is Abundantly Found in GABAergic Neurons
The location and morphology of many of the d-serine-containing neurons, particularly in the HP, suggested that they were gamma amino butyric acid (GABA) interneurons. We first used an antibody against the 67 kDa isoform of the enzyme glutamic acid decarboxylase (GAD67), which is responsible for producing the majority of cortical GABA. Approximately 50–60 % of d-serine positive neurons co-localized with GAD67 in the PFC and S1 (Fig. 7a–c), while there was approximately 75 % co-localization in the HP (Fig. 7d–f). GAD67/DS neurons were found in the deeper cortical layers (IV–VI) in S1. In the hippocampal formation, a majority of the GAD67/DS cells were found in s.o., s.r., and Mol, with fewer cells located in l.m. and Hi. There were also GAD67/DS neurons in s.p. and at the base of the GCL.
Fig. 7.
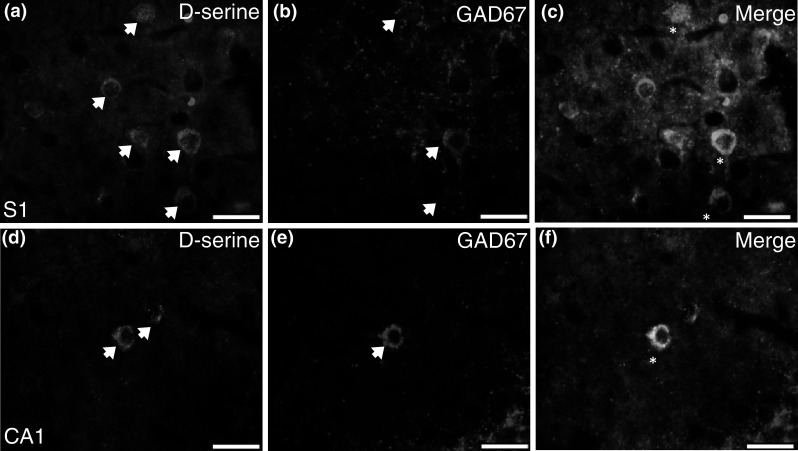
d-Serine co-localizes with GAD67 in the adult brain. Representative coronal brain sections (×40) from adult WT and SR−/− mice. A portion of d-serine positive cells (arrows) in a S1 and (d; CA1) hippocampus overlap with GAD67 (b, e; arrows). Merged images are shown in panels (c, S1; f, CA1; d-serine, green; GAD67, magenta). Asterisks indicate DS/GAD67 overlap. CA1 cornu ammonis, GAD67 glutamic acid decarboxylase of 67 kDa, S1 primary somatosensory cortex. Scale bars represent 25 μm (Color figure online)
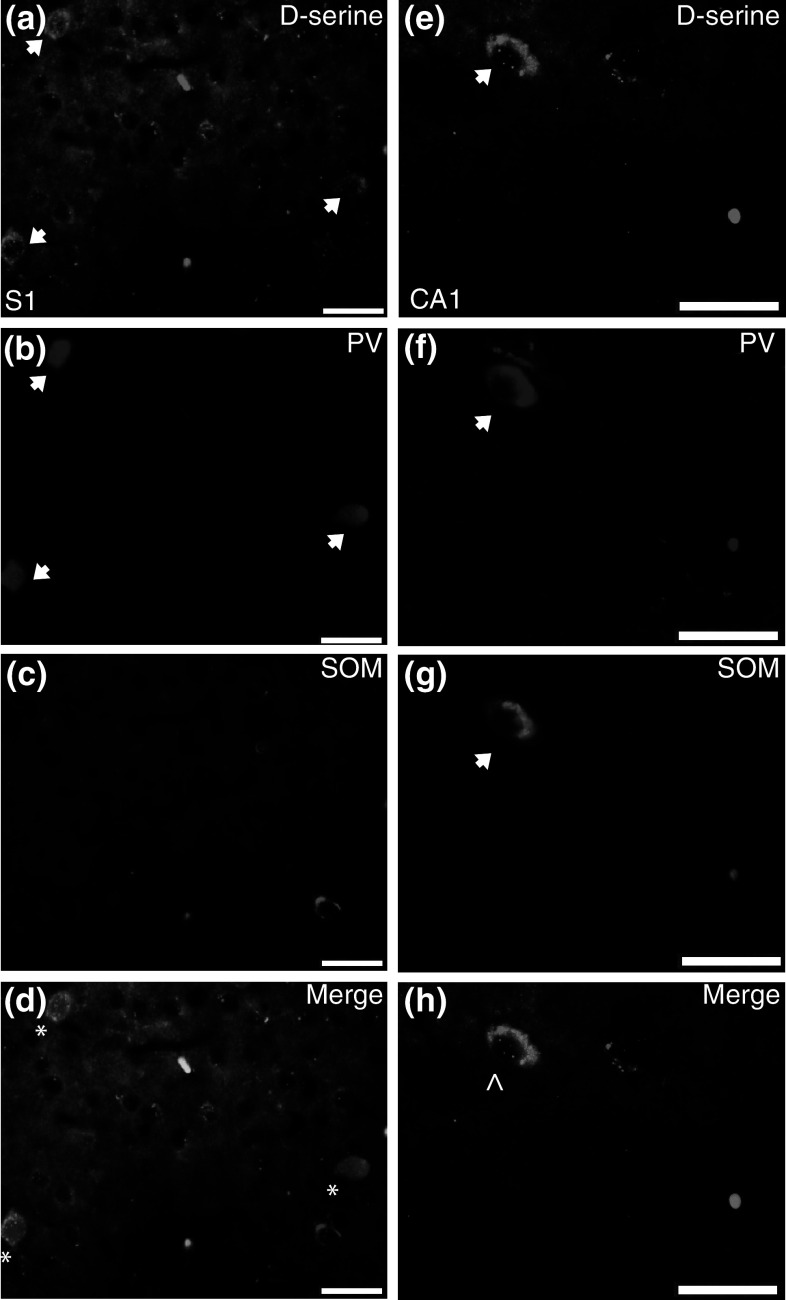
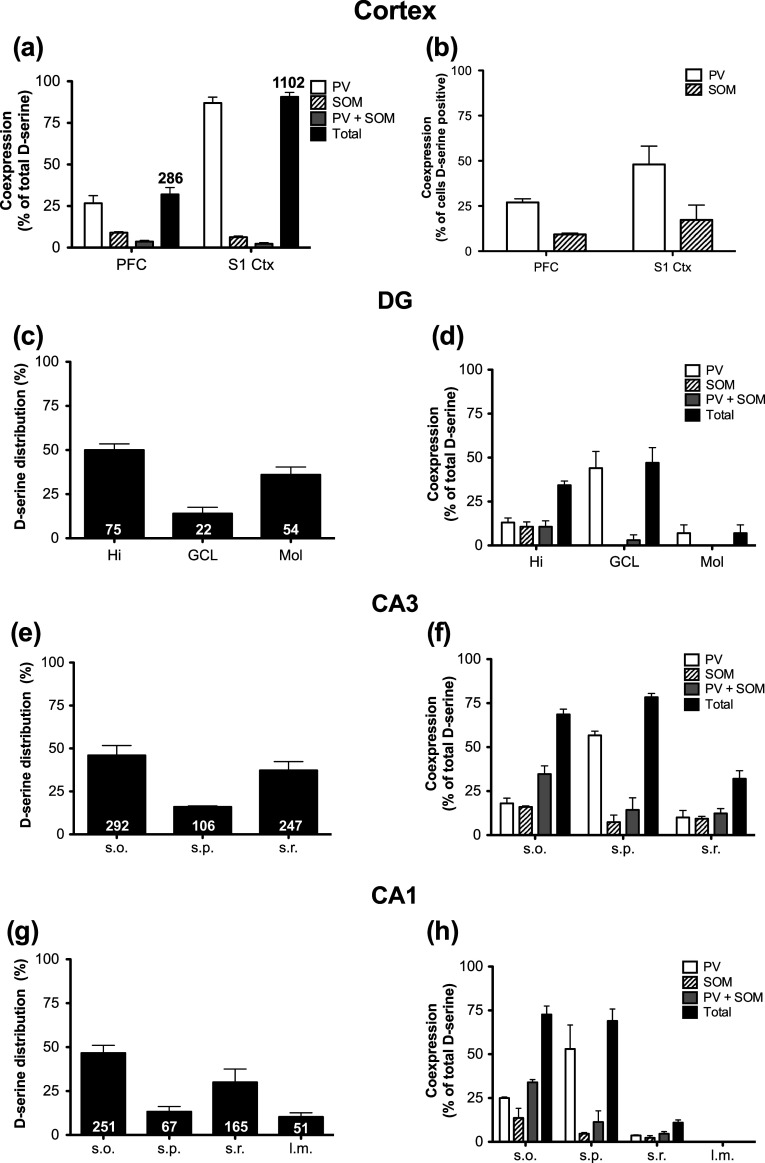
There are many types of GABAergic neurons that are classified according to various characteristics, including morphology, electrophysiology, calcium-binding protein expression, and co-localized neuropeptides (DeFelipe et al. 2013). We used parvalbumin (calcium-binding protein; PV) and somatostatin (neuropeptide; SOM) to identify in which population(s) of GABA neurons d-serine is localized (Fig. 8). Triple labeling for d-serine, PV, and SOM revealed that in the PFC and S1, respectively, 30 and 90 % of d-serine positive neurons expressed PV and/or SOM (Fig. 9a). Consistent with previous reports, there was virtually no overlap between PV and SOM in the cortex (Rudy et al. 2011; Xu et al. 2010). The lower percentage of d-serine cells containing PV or SOM in the PFC was because there are fewer PV and SOM GABAergic neurons located in the PFC. In addition, the higher overlap of d-serine with PV or SOM in S1 cortex as compared to GAD67 reflects difficulty in determining co-localization due to intense GAD67 neuropil staining in the intermediate cortical layers. When PV and SOM, respectively, were used as the primary markers in the PFC, 30 and 10 % of these neurons contained d-serine, while in S1, 50 % of PV and 17 % of SOM neurons had d-serine (Fig. 9b).
Fig. 8.
d-Serine co-localizes to parvalbumin and somatostatin positive inhibitory neurons. Representative coronal brain sections from adult WT mice. A portion of d-serine positive neurons (arrows) in a S1 and (e; CA1) hippocampus overlap with PV (b, f; arrows) and/or SOM (c, g; arrow). Merged images are shown in panels (d, S1; h, CA1; d-serine, green; PV, blue; SOM, magenta). Asterisks indicate DS/PV overlap and (^) indicates DS/PV/SOM overlap. CA1 cornu ammonis, PV parvalbumin, SOM somatostatin, S1 primary somatosensory cortex. Scale bars represent 25 μm (Color figure online)
Fig. 9.
Quantification of d-serine-containing parvalbumin and somatostatin neurons in the neocortex and hippocampus. a Percentage of d-serine positive cells that express PV (open bars), SOM (striped), or PV+SOM (gray bars) in the PFC and S1. Total (black bars) represents the total percentage of d-serine positive cells that express PV, SOM, and PV+SOM. b Percentage of PV (open bars) or SOM (striped bars) positive neurons that contain d-serine in the PFC and S1. Distribution of d-serine positive cells in each subfield of the hippocampus (c, DG; e, CA3; and g; CA1). The numbers in each bar represent the total number of counted cells from three animals. Percentage of d-serine positive cells that express PV (open bars), SOM (striped), or PV+SOM (gray bars) in each subfield of the hippocampus (d, DG; f, CA3; and h; CA1). Total (black bars) represents the total percentage of d-serine positive cells that express PV, SOM, and PV+SOM. CA1 cornu ammonis, DG dentate gyrus, Hi hilus, GCL granule cell layer, Mol molecular layer, PV parvalbumin, PFC prefrontal cortex, SOM somatostatin, s.o. stratum oriens, s.p. stratum pyramidale; s.r. stratum radiatum; S1 primary somatosensory cortex. All values represent the mean ± SEM (Color figure online)
Due to the discrete localization of GABAergic neuron populations to different layers in the HP (Klausberger 2009; Houser 2007), we assessed the co-expression of d-serine within the different layers of each subfield. First, we found that a majority of d-serine cells were located in the Hi and Mol of the DG, as well as in the s.o. and s.r. of CA3 and CA1 (Fig. 9c, e, g). In the Hi, GCL, and Mol 35, 47, and 7 %, respectively, of d-serine cells contained PV and/or SOM (Fig. 9d). In s.o. and s.p. of CA3 and CA1, 75 % of d-serine cells expressed PV and/or SOM, while only 30 and 11 % were localized with these GABAergic markers in s.r. of CA3 and CA1, respectively (Fig. 9f, h). The low percentages of d-serine overlap with PV/SOM in the s.r. and l.m. were due to the lack of PV/SOM-expressing neurons in these laminae, which is in agreement with the literature (Klausberger 2009).
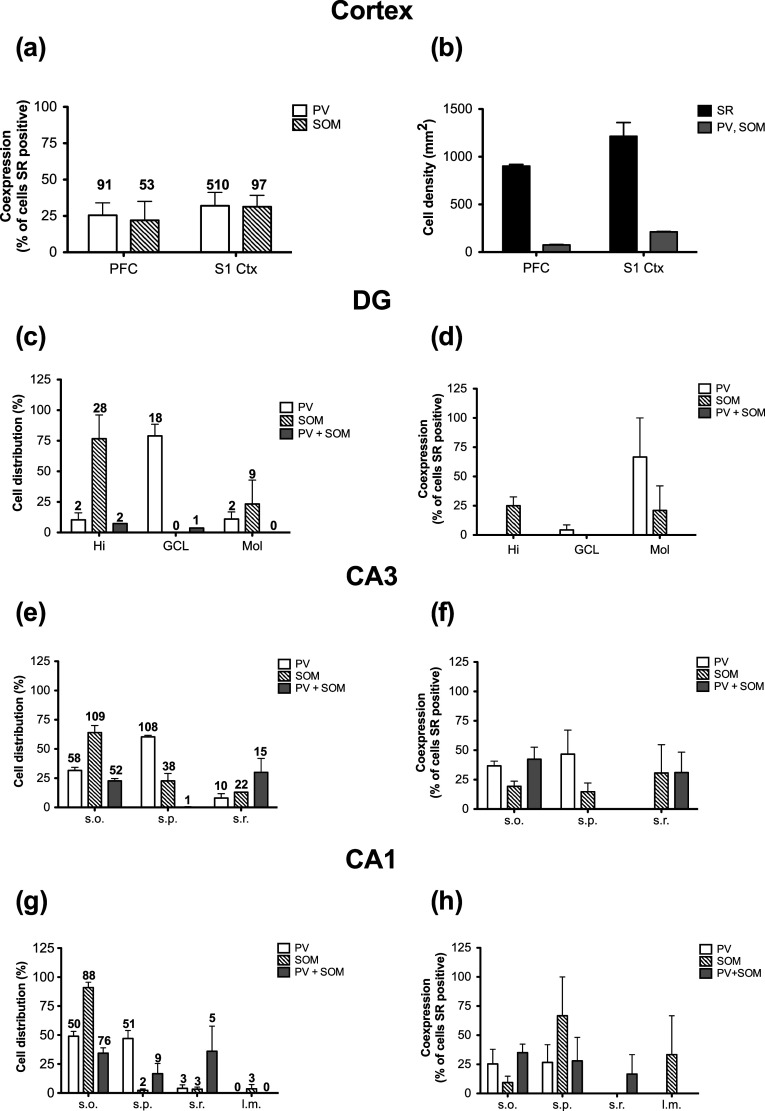
Inhibitory Neurons Express SR
Given the abundant expression of d-serine in cortical and hippocampal GABAergic neurons, we examined whether these same cells also express SR. Although we found that 25–30 % of PV and SOM neurons contain SR (Fig. 10a), they represent only a small subpopulation of the total number of SR-containing neurons. The estimated density of all SR neurons in the PFC and S1 is 900–1,200 cells/mm2, while the total number of PV and SOM cells is only 75–200 (Fig. 10b). These numbers are in agreement with the estimate that inhibitory neurons constitute ~10–20 % of all neurons in the cortex (Rudy et al. 2011). In the HP, SR expression was very high in excitatory neurons of the GCL and in the s.p. of CA1 and CA3 (data not shown), as previously reported. We counted PV and SOM neurons first and then determined what percentage of these cells also expressed SR. Most of the PV and/or SOM neurons were located in the Hi and GCL of the DG, and in s.o. and s.p. of CA3 and CA1 (Fig. 10c, e, g). The percentage of SR-expressing PV and SOM cells varied across subfield and lamina. In the DG, co-localization (25 %) was mainly restricted to SOM neurons in the Hi and Mol (Fig. 10d). In CA3 and CA1, 30 % of PV and/or SOM neurons expressed SR in s.o. and s.p. (Fig. 10f, h). There was also some co-localization in s.r and l.m., but the number of PV and SOM neurons in these layers was much lower than in s.o. and s.p.
Fig. 10.
Quantification of serine racemase expressing parvalbumin and somatostatin neurons in the neocortex and hippocampus. a Percentage of parvalbumin PV (open bars) and somatostatin SOM (striped bars) positive neurons that express SR in the PFC and S1. The numbers above each bar represent the total number of counted cells from three animals. b The estimated density of all SR (black bars), as well as PV or SOM (PV, SOM; gray bars) neurons in the PFC and S1. Distribution of PV (open bars), SOM (striped), and PV+SOM (gray bars) neurons in each subfield of the hippocampus (c, DG; e, CA3; and g; CA1). The numbers above each bar represents the total number of counted cells from three animals. Percentage of PV (open bars), SOM (striped), and PV+SOM (gray bars) positive neurons that express SR in each subfield of the hippocampus (d, DG; f, CA3; and h; CA1). CA1 cornu ammonis, DG dentate gyrus, Hi hilus, GCL granule cell layer, Mol molecular layer, PV parvalbumin, PFC prefrontal cortex, SOM somatostatin; s.o. stratum oriens, s.p. stratum pyramidale, s.r. stratum radiatum, S1 primary somatosensory cortex, SR serine racemase. All values represent the mean ± SEM
Excitatory and Inhibitory Neurons Express SR in Human Cortex
Finally, we wanted to determine the cellular localization of SR in the human brain. We tested three different SR antibodies in paraformaldehyde-fixed human primary motor cortical (BA4) tissue and found that only the antibody directed against human SR was effective for immunohistochemistry (Fig. 11a). As immunostaining with this antibody has never been published, we validated its specificity for SR in WT and SR−/− tissue (Fig. 11b, c). There was intense SR staining in pyramidal neurons, as well as in other neuronal cell types. Therefore, we used immunofluorescence to determine whether SR is expressed in neurons (calmodulin kinase II alpha (CAMKIIα), excitatory neurons; PV, inhibitory neurons) or astrocytes (S100β). We found that SR was expressed in both excitatory (Fig. 12a–c) and inhibitory (Fig. 12d–f) neurons, but not in S100β-containing astrocytes (Fig. 12g–i).
Fig. 11.
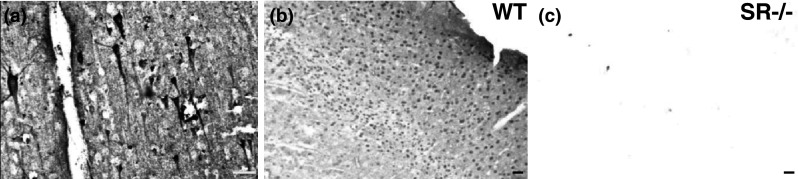
Validation of serine racemase antibody used for human post-mortem immunofluorescence. (a, BA4) Human primary motor cortex incubated with a rabbit anti-(SR) antibody showed intense staining of pyramidal neurons (×20). This antibody was then validated using b WT and c SR−/− brain tissue. Sagittal brain sections from SR−/− mice (×10) did not show any SR staining. SR serine racemase. Scale bars represent 50 μm
Fig. 12.
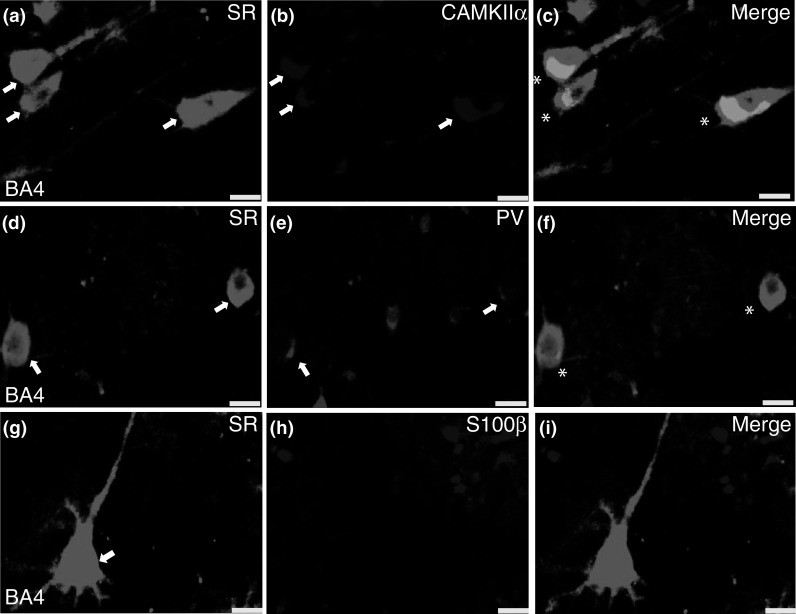
Serine racemase is expressed in neurons in human post-mortem cortex. Representative coronal brain sections of human primary motor cortex (BA4; ×40). SR (a, d, g; arrows) were expressed mainly by excitatory neurons (b; CAMKIIα; arrows) and a subset of GABAergic neurons (e; PV; arrows), but not S100β-containing astrocytes h. Merged images are shown in panels (c, f, i; SR, green; CAMKIIα, PV, and S100β, magenta). CAMKIIα calcium-calmodulin kinase II alpha, PV parvalbumin, SR serine racemase. Scale bars represent 25 μm
Discussion
Although SR was initially thought to be an astrocytic enzyme, it is now known that it is located almost exclusively in neurons in the forebrain (Benneyworth et al. 2012). However, the cellular location of d-serine in the brain still remains unresolved. Thus, we utilized SR−/− mice to validate a d-serine immunohistochemistry protocol and determined that d-serine is almost exclusively stored in neurons, particularly GABAergic neurons in the neocortex and HP. We also showed that SR and d-serine co-localized within the same cell, although there was a discrepancy between the sites of d-serine synthesis and storage. Finally, we demonstrate for the first time that SR is found in both excitatory and inhibitory neurons, but not astrocytes, in human post-mortem neocortex.
We utilized brain tissue from SR−/− mice, which have <15 % of normal d-serine levels, as a negative control to determine the specificity of our immunohistochemical protocol. We established that the brains need to be fixed with glutaraldehyde and a high concentration of l-serine–BSA conjugate must be included during the primary antibody incubation to block binding of the d-serine antibody to tissue l-serine. The first studies (Schell et al. 1997; Schell et al. 1995; Wolosker et al. 1999) showing astrocytic d-serine localization used a d-serine antibody generated by Schell et al. (1995) and did not use the l-serine blocking conjugate, while more recent studies (Fossat et al. 2012; Martineau et al. 2013) used a GemacBio antibody and paraformaldehyde as the primary fixative, and did not include the l-serine–BSA blocking conjugate. The different staining patterns obtained for d-serine between our study and previous studies are likely due to differences in fixation and the inclusion of high concentrations of blocking l-serine–BSA. The d-serine antibody is derived from immunizing rabbits with glutaraldehyde conjugated d-serine–BSA. Thus, glutaraldehyde needs to be the fixative as it is part of the epitope recognized by the d-serine antibody. In addition, paraformaldehyde is not very effective in retaining amino acids inside cells, which are rapidly released during fixation (Aprison 1990). The source of the antibody does not likely contribute to the staining differences, as neuronal d-serine localization was found using the Schell et al. 1995 and MoBitec (same antibody sold by GemacBio and Abcam) antibodies (Kartvelishvily et al. 2006; Ehmsen et al. 2013) along with 0.1–0.2 mM l-serine glutaraldehyde conjugates. It is also possible that low amounts of d-serine in small astrocytic processes or other cell populations might be below the sensitivity of our immunohistochemistry protocol.
Interestingly, we found that a majority of cortical d-serine-containing neurons were in fact GABAergic, as determined by co-localization with the enzyme GAD67. Because there are numerous subtypes of GABAergic interneurons, we further characterized which type of inhibitory neurons contained d-serine based on the presence of the calcium-binding protein PV and neuropeptide SOM. In the mouse S1 cortex, PV and SOM: label distinct cell populations, are found almost exclusively in layers II–VI, and account for approximately 60–70 % of all GABA neurons (Rudy et al. 2011; Xu et al. 2010). We found that in S1 cortex, 87 % of the d-serine positive cells contained PV and 6 % contained SOM. Although we could not discriminate between the classes of PV neurons with our staining, we hypothesize that basket cells would be the predominant d-serine-containing population, since their axons target proximal dendrites of pyramidal neurons, where NMDARs would be present. Basket cells are likely the dominant inhibitory system in the cortex and have been implicated in maintaining fast (gamma frequency) cortical rhythms (Cardin et al. 2009) and controlling excitation/inhibition balance. Therefore, if basket cells could also release d-serine, this would be another mechanism by which an important population of GABAeric neurons could regulate network activity by enhancing their NMDAR responsiveness to pyramidal neuron afferents. SOM expression in the neocortex has been most associated with Martinotti cells which have axons that innervate dendrites of pyramidal cells. Future studies will be needed to determine whether inhibitory neurons can in fact release d-serine, a process that has been shown to occur in the HP via neuronal Asc-1, a high affinity transporter of d-serine (Rosenberg et al. 2013).
Similar to the cortex, d-serine positive cells in the HP were also predominantly GABAergic. Immunostaining with PV and SOM highlighted the subfield and laminar-specific differences across interneuron populations. In the DG, a majority of d-serine positive cells that expressed PV were located in the GCL and to a lesser extent the Hi, while SOM-containing d-serine neurons were found almost exclusively in the Hi. PV cells in the DG are predominantly basket and chandelier cells that target excitatory granule cells. SOM neurons are all virtually located in the Hi and synapse primarily with granule cell dendrites in the outer molecular layer where perforant path (pp) fibers terminate, ideally positioning these neurons to modulate the influence of the pp on dentate granule cell activity (Houser 2007). In s.o. of CA3 and CA1, the majority of d-serine neurons expressed PV and/or SOM, while in s.p. almost all were only PV positive. Pyramidal cells in CA1 receive 92 % of their GABAergic input onto dendrites (Megias et al. 2001). The inhibitory PV and/or SOM-containing neurons in s.o. project to the dendrites of CA1 pyramidal cells (Klausberger 2009). These d-serine-containing inhibitory neurons are in a prime location to regulate hippocampal activity in all components of the circuit. The ability of these inhibitory neurons to also release d-serine in an activity-dependent manner would add another layer of regulation. Future studies will be needed to understand the functional consequences of d-serine-containing GABA neurons in the HP.
SR is the enzyme that catalyzes the racemization of l-serine to d-serine. However, we found that only 50 % of d-serine positive cells expressed SR in the cortex. In the HP, the co-expression varied depending on the subfield and lamina, but was generally around 25–40 %. In addition to racemization, SR also catalyzes the α–β elimination of water from both l- and d-serine to generate pyruvate and ammonia, with the latter reaction prevailing over the former (Foltyn et al. 2005). Under physiological conditions, SR would consume both enantiomers by α–β elimination and prevent the accumulation of d-serine (Wolosker 2011). Thus, it has been proposed that SR and d-serine need to be compartmentalized, with neuronal SR producing the majority of d-serine that is then transported to astrocytes for activity-dependent release (Wolosker 2011). Our results support this hypothesis with the important exception that d-serine is not being shuttled between neurons and astrocytes, but rather d-serine is being shuttled between neurons, consistent with recent electrophysiological findings (Rosenberg et al. 2013). In S1 cortex and HP, nearly all of the d-serine were found in PV and/or SOM neurons, while only 25 % of these inhibitory neurons expressed SR. This percentage of inhibitory neurons represents a small percentage (2 % in cortex) of the total SR neuron population, which is comprised mostly of excitatory neurons. Presumably this d-serine transport would occur via Asc-1, which is expressed exclusively on neurons and is localized to cell bodies and dendritic fields (Helboe et al. 2003).
Finally, we determined whether what we observed in mice with regard to SR localization in the brain translated to humans. We found that similar to mice, SR was primarily expressed in excitatory neurons and to a lesser extent in PV+inhibitory neurons, but not in S100β-containing astrocytes from human post-mortem BA4 cortex. To our knowledge, only one other study has performed SR immunohistochemistry in human post-mortem tissue (Verrall et al. 2007). This study found hippocampal and cortical SR expression to be greater in white matter than in gray matter, which is inconsistent with what is observed in rodents and what we found in human cortex. Our studies differed in the SR antibody used, tissue storage, as well as in the sectioning, antigen unmasking, and staining of tissue. Future work examining other brain regions and using additional cell-specific markers will more fully establish the location of SR throughout the human brain.
In summary, our findings demonstrate that d-serine and SR are located almost exclusively in neurons in the neocortex and HP of the adult mouse. In addition, the co-expression of SR with excitatory and inhibitory neurons in the human cortex suggests that SR is a predominantly neuronal enzyme in the mammalian brain. Most of the d-serine is found in GABAergic neurons, particularly those containing PV and SOM. This finding could have major implications on how GABAergic neurons regulate forebrain activity, especially given the important role PV neurons play in controlling gamma oscillations (Cardin et al. 2009). Furthermore, our findings could provide a possible link between reduced NMDAR activation and GABAergic dysfunction, which are thought to underlie such disorders as schizophrenia (Balu and Coyle 2011).
Acknowledgments
We would like to thank Drs. Sabina Berretta, Sue Andersen, and Ole Isaacson for the generous use of their microscopes. We would also like to thank Harry Pantazopoulos, Rebecca Shivangi Misra, Anita Bechtholt, Jesse McLean, and John Muschamp for technical advice, and Jiamin Feng for animal colony maintenance and genotyping. We thank Francine Benes and the staff of the Harvard Brain Tissue Resource Center that is supported in part by PHS grant number R24-MH068855 for providing all the tissues, as well as the study subjects and their families. An Andrew P. Merrill Research Fellowship and Phyllis & Jerome Lyle Rappaport Mental Health Research Scholars Award (DTB), as well as the National Institutes of Health grants R01MH05190 and P50MH0G0450 (JTC) supported this work.
Conflict of interest
JTC has served as a consultant for EnVivo, and Abbvie in the last 2 years. A patent owned by Massachusetts General Hospital for the use of d-serine as a treatment for serious mental illness could yield royalties for Dr. Coyle. The remaining authors have no conflict of interest.
Footnotes
Darrick T. Balu and Shunsuke Takagi contributed equally to this work.
References
- Aprison MH (1990) The discovery of the neurotransmitter role of glycine. In: Ottersen OP, Storm-Mathisen J (eds) Glycine neurotransmission. Wiley, New York, pp 1–23 [Google Scholar]
- Balu DT, Coyle JT (2011) Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neurosci Biobehav Rev 35(3):848–870. doi:10.1016/j.neubiorev.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT (2013) Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci USA 110(26):E2400–2409. doi:10.1073/pnas.1304308110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT (2009) Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 14(7):719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT (2012) Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol. doi:10.1007/s10571-012-9808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Snyder SH (2003) Novel neural modulators. Annu Rev Neurosci 26:105–131. doi:10.1146/annurev.neuro.26.041002.131047 [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459(7247):663–667. doi:10.1038/nature08002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio L, Podda MV, Leone L, Piacentini R, Mastrodonato A, Cappelletti P, Sacchi S, Pollegioni L, Grassi C, D’Ascenzo M (2013) Reduced d-serine levels in the nucleus accumbens of cocaine-treated rats hinder the induction of NMDA receptor-dependent synaptic plasticity. Brain. doi:10.1093/brain/awt036 [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R, Ascoli GA (2013) New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14(3):202–216. doi:10.1038/nrn3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Ma N, Nagahama M, Yamada K, Semba R (2011) Localization of d-serine and serine racemase in neurons and neuroglias in mouse brain. Neurol Sci 32(2):263–267. doi:10.1007/s10072-010-0422-2 [DOI] [PubMed] [Google Scholar]
- Ehmsen JT, Ma TM, Sason H, Rosenberg D, Ogo T, Furuya S, Snyder SH, Wolosker H (2013) d-Serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J Neurosci 33(30):12464–12469. doi:10.1523/JNEUROSCI.4914-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltyn VN, Bendikov I, De Miranda J, Panizzutti R, Dumin E, Shleper M, Li P, Toney MD, Kartvelishvily E, Wolosker H (2005) Serine racemase modulates intracellular d-serine levels through an alpha, beta-elimination activity. J Biol Chem 280(3):1754–1763. doi:10.1074/jbc.M405726200 [DOI] [PubMed] [Google Scholar]
- Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L, Millan MJ, Oliet SH, Mothet JP (2012) Glial d-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex 22(3):595–606. doi:10.1093/cercor/bhr130 [DOI] [PubMed] [Google Scholar]
- Helboe L, Egebjerg J, Moller M, Thomsen C (2003) Distribution and pharmacology of alanine–serine–cysteine transporter 1 (asc-1) in rodent brain. Eur J Neurosci 18(8):2227–2238 [DOI] [PubMed] [Google Scholar]
- Houser CR (2007) Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res 163:217–232. doi:10.1016/S0079-6123(07)63013-1 [DOI] [PubMed] [Google Scholar]
- Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H (2006) Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J Biol Chem 281(20):14151–14162. doi:10.1074/jbc.M512927200 [DOI] [PubMed] [Google Scholar]
- Kishi N, Macklis JD (2004) MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci 27(3):306–321. doi:10.1016/j.mcn.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Klausberger T (2009) GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci 30(6):947–957. doi:10.1111/j.1460-9568.2009.06913.x [DOI] [PubMed] [Google Scholar]
- Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, Klingauf J, Sweedler JV, Jahn R, Mothet JP (2013) Storage and uptake of d-serine into astrocytic synaptic-like vesicles specify gliotransmission. J Neurosci 33(8):3413–3423. doi:10.1523/JNEUROSCI.3497-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI (2001) Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102(3):527–540 [DOI] [PubMed] [Google Scholar]
- Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H (2008) Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol 510(6):641–654. doi:10.1002/cne.21822 [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH (2000) d-serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 97(9):4926–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G (2005) Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci USA 102(15):5606–5611. doi:10.1073/pnas.0408483102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH (2012) Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150(3):633–646. doi:10.1016/j.cell.2012.06.029 [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, Foltyn VN, Inoue R, Mori H, Billard JM, Wolosker H (2013) Neuronal d-serine and glycine release via the asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci 33(8):3533–3544. doi:10.1523/JNEUROSCI.3836-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011) Three groups of interneurons account for nearly 100 % of neocortical GABAergic neurons. Dev Neurobiol 71(1):45–61. doi:10.1002/dneu.20853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH (1995) d-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA 92(9):3948–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Brady RO Jr, Molliver ME, Snyder SH (1997) d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci 17(5):1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW (2007) d-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci 26(6):1657–1669. doi:10.1111/j.1460-9568.2007.05769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H (2011) Serine racemase and the serine shuttle between neurons and astrocytes. Biochim Biophys Acta 1814(11):1558–1566. doi:10.1016/j.bbapap.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH (1999) Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 96(23):13409–13414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Dumin E, Balan L, Foltyn VN (2008) d-Amino acids in the brain: d-serine in neurotransmission and neurodegeneration. FEBS J 275(14):3514–3526. doi:10.1111/j.1742-4658.2008.06515.x [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM (2010) Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518(3):389–404. doi:10.1002/cne.22229 [DOI] [PMC free article] [PubMed] [Google Scholar]