Abstract
Histone H3 lysine27-to-methionine (H3K27M) gain-of-function mutations occur in highly aggressive pediatric gliomas. Here, we establish a Drosophila animal model for the pathogenic histone H3K27M mutation and show that its overexpression resembles Polycomb repressive complex 2 (PRC2) loss-of-function phenotypes, causing de-repression of PRC2 target genes and developmental perturbations. Similarly, a H3K9M mutant depletes H3K9 methylation levels and suppresses position-effect variegation in various Drosophila tissues. The histone H3K9 demethylase KDM3B/JHDM2 associates with H3K9M nucleosomes and its overexpression in Drosophila results in loss of H3K9 methylation levels and heterochromatic silencing defects. Here we establish histone lysine-to-methionine mutants as robust in vivo tools for inhibiting methylation pathways that also function as biochemical reagents for capturing site-specific histone-modifying enzymes, thus providing molecular insight into chromatin-signaling pathways.
Histone proteins constitute the core of the eukaryotic chromatin [1]. The SET domain-containing histone methyltransferases such as Trithorax/MLL1/2-COMPASS and Polycomb Repressive Complex 2 (PRC2) methylate lysine residues in the histone H3 amino-terminal tail and are essential for normal development [1]. Establishing direct functions for modified lysine residues in histones has proven difficult due the fact that there are multiple histone gene copies in metazoans [2]. Single allele mutations of histone H3.3 lysine27-to-methionine (H3.3K27M) occur in a subtype of aggressive pediatric brain cancers [3, 4], and act in a dominant manner to deplete H3K27 methylation by inhibiting PRC2 methyltransferase activity [5, 6]. Additional histone H3 lysine-to-methionine mutants possess dominant gain-of-function activities [6], making them attractive tools for in vivo functional studies of histone lysine modifications. Trimethylation of histone H3 lysine 27 (H3K27me3) and lysine 9 (H3K9me3) are associated with distinct forms of transcriptionally silent heterochromatin. Histone H3K27me3 is enriched at so-called “facultative” heterochromatin, whereas H3K9me3 is associated with “constitutive” heterochromatin at telomeres and centromeres [7].
We established wild-type histone H3.3, H3.3K27M and H3.3K9M constructs with a C-terminal FLAG-HA tag [6] for tissue-specific overexpression in Drosophila. Overexpressing H3.3K27M in the posterior compartment of wing imaginal discs driven by engrailed-GAL4 causes a strong reduction in H3K27 methylation levels and derepression of the PRC2 target gene Ultrabithorax (Ubx) (Fig. 1, A to H′, and fig. S1), thus phenocopying knockdown of the catalytic PRC2 subunit E(z) (fig. S2). Increased H3K27 acetylation is observed for H3.3K27M overexpression in Drosophila and mammalian cells and E(z)-RNAi in Drosophila (fig. S3). Genome-wide RNA-seq analysis of H3.3K27M expressing wing discs revealed upregulated RNA transcripts for polycomb targets including Ubx, wingless (wg) and the Polycomb repressive complex 1 subunits Posterior sex combs (Psc) (Fig. 1, I and J, fig. S4, A and B, and table S1). Homeobox (Hox)-containing genes such as engrailed (en) and invected (in) and important signaling pathway components such as cubitus interruptus (ci) were downregulated upon H3.3K27M overexpression (fig. S4, C and D, and table S1). Moreover, flies expressing H3.3K27M under a tissue-specific Distalless-GAL4 driver exhibit gross morphological defects and die around eclosion, resembling E(z)-RNAi under the same driver (fig. S4, E and F, and data not shown).
Fig. 1. H3.3K27M overexpression results in loss of H3K27 methylation and derepression of polycomb target genes.
(A–H) Overexpression of H3.3WT-FLAG-HA or H3.3K27M-FLAG-HA in the posterior compartment of the wing imaginal disc. GFP expression in green marks the posterior domain where the respective histones are overexpressed. Non-apostrophed panels represent merged images that include the GFP signal in green and the respective antibody signal in red. Apostrophed panels only contain the respective antibody signal in red with a white arrow pointing towards the posterior compartment. Overexpression of H3.3WT-FLAG-HA does not result in bulk changes of H3K27me3 (A, A′), H3K27me2 (C, C′) or H3K27me1 (E, E′). Decreased levels of H3K27me3 (B, B′), H3K27me2 (D, D′) and H3K27me1 (F, F′) can be observed when H3.3K27M-FLAG-HA is overexpressed in the posterior compartment of the wing imaginal disc. Ubx remains silenced in wing imaginal discs overexpressing H3.3WT-FLAG-HA (G, G′), however, becomes derepressed when H3.3K27M-FLAG-HA is overexpressed (H, H′). RNA-seq analysis of wing imaginal discs expressing either H3.3WT-FLAG-HA or H3.3K27M-FLAG-HA with a T80-GAL4 driver. Polycomb target genes that are highly enriched for H3K27 trimethylation such as Ultrabithorax (Ubx) (I) and wingless (wg) (J) are derepressed when H3.3K27M-FLAG-HA is overexpressed in wing imaginal discs.
(A, C, E, G) en-GAL4 UAS-2 x EGFP/UAS-H3.3WT-FLAG-HA
(B, D, F, H) en-GAL4 UAS-2 x EGFP/UAS-H3.3K27M-FLAG-HA
(I–J) H3.3WT RNA-seq track: T80-GAL4/UAS-H3.3WT-FLAG-HA
(I–J) H3.3K27M RNA-seq track: T80-GAL4/UAS-H3.3K27M-FLAG-HA
Trimethylation of histone H3 lysine 9 (H3K9me3) by Supressor of variegation 3-9 (Su(var)3-9) proteins is a hallmark of heterochromatin [8]. Histone H3K9me3 serves as a binding substrate for heterochromatin protein 1 (HP1) [9–12], and establishes a transcriptionally repressed state [13–15]. Euchromatic genes that become abnormally juxtaposed to heterochromatic regions are subject to transcriptional silencing through a phenomenon known as position-effect variegation (PEV) [13]. However, less is known about the direct role of H3K9 residue and its methylation in the regulation of gene expression. Indeed, studies in fission yeast point to H3K9 methylation-independent functions for the Su(var)3-9 homolog Clr4 in chromatin silencing [16]. Overexpressing the H3.3K9M mutant in Drosophila wing imaginal discs and mammalian cells globally depletes H3K9 methylation (Fig. 2, A to F, and fig. S5) but does not affect H3K27 methylation (fig. S6). We purified mononucleosomes from wild-type H3.3, H3.3K9M and H3.3K27M overexpressing HEK 293 cells and subjected these samples to MudPIT mass spectrometry (Fig. 2, F and G). Binding of HP1a (CBX5), HP1ß (CBX1) and HP1 (CBX3) were dramatically reduced for H3.3K9M mononucleosomes, as was interaction of the HP1-associated proteins chromatin assembly factor 1a (CHAF1A/p150) [17, 18] and CHAF1B/p60 (Fig. 2G). We found dramatically increased association of the H3K9 demethylase KDM3B and the H3K9/K56 deacetylase SIRT6 with H3K9M mononucleosomes (Fig. 2G).
Fig. 2. H3.3K9M overexpression depletes H3K9 methylation and alters recruitment of HP1 family members and other H3K9-modifying enzymes.
(A–D) H3.3WT-FLAG-HA and H3.3K9M-FLAG-HA were overexpressed in wing imaginal discs as described in Figure 1. Overexpression of H3.3WT-FLAG-HA does not result in significant bulk changes of H3K9me3 (A, A′) and H3K9me2 (C, C′). Decreased levels of H3K9me3 (B, B′) and H3K9me2 (D, D′) can be observed when H3.3K9M-FLAG-HA is overexpressed. (E) Western blotting of whole cell extracts from HEK293 cells expressing H3.3WT-FLAG-HA or H3.3K9M-FLAG-HA. Bulk histone H3K9 trimethylation levels and to some extent H3K9 dimethylation levels are reduced in cells expressing H3.3K9M-FLAG-HA. Dox, Doxycycline induced for 9 days. (F) Western blot of purified mononucleosomes from HEK293 cells expressing H3.3WT-FLAG-HA or H3.3K9M-FLAG-HA. H3K9 dimethylation and H3K9 trimethylation is strongly reduced on H3.3K9M-FLAG-HA containing nucleosomes. (G) MudPIT analysis of mononucleosome-interacting proteins. dNSAF, distributed normalized spectral abundance factor, averaged of 9, 5 and 12 replicates for H3.3WT-FLAG-HA, H3.3K9M-FLAG-HA and H3.3K27M-FLAG-HA, respectively. Green color indicates proteins significantly enriched on H3.3K9M-FLAG-HA containing nucleosomes, red color indicates proteins that are depleted, based on PLGEM signal-to-noise ratios and p-values.
(A, C) en-GAL4 UAS-2 x EGFP/UAS-H3.3WT-FLAG-HA
(B, D) en-GAL4 UAS-2 x EGFP/UAS-H3.3K9M-FLAG-HA
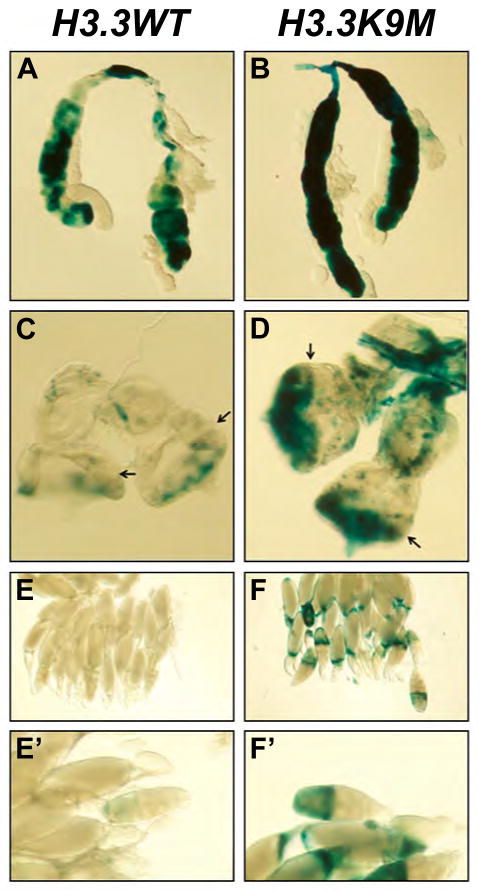
Reduced dosage of Drosophila HP1 and Su(var)3-9 results in suppression of position-effect variegation (PEV) [19–21]. Using a heat shock-inducible lacZ construct inserted within Y-chromosomal heterochromatin [22, 23], overexpression of H3.3K9M results in suppression of PEV in both Drosophila salivary glands and eye-antenna imaginal discs (Fig. 3, A to D). Bulk histone H3K9 methylation levels were decreased in H3.3K9M overexpressing salivary glands (fig. S7). We also assessed the effects of H3.3K9M overexpression on heterochromatic silencing in Drosophila ovaries. The gypsy-lacZ construct is normally silenced in almost all follicle cells but is upregulated upon loss of heterochromatin function [24]. Overexpression of H3.3K9M results in derepression of lacZ (Fig. 3, E to F′), thus the H3K9M mutation disrupts heterochromatic silencing of retroelements.
Fig. 3. Overexpression of H3.3K9M suppresses heterochromatic silencing.
Hsp70-lacZ variegating salivary glands overexpressing H3.3WT-FLAG-HA (A) and H3.3K9M-FLAG-HA (B), stained for lacZ expression using X-gal. Hsp70-lacZ variegating eye-antennal discs overexpressing H3.3WT-FLAG-HA (C) and H3.3K9M-FLAG-HA (D). Arrows in (C) and (D) indicate the position of the morphogenetic furrow. gypsy-lacZ containing ovaries overexpressing H3.3WT-FLAG-HA (E, E′) and H3.3K9M-FLAG-HA (F, F′), stained for lacZ expression using X-gal.
(A, C) Hsp70-LacZ ; UAS-H3.3WT-FLAG-HA/+ ; daughterless-GAL4/+
(B, D) Hsp70-LacZ ; UAS-H3.3K9M-FLAG-HA/+ ; daughterless-GAL4/+
(B) tj-GAL4 gypsy-LacZ/UAS-H3.3WT-FLAG-HA
(C) tj-GAL4 gypsy-LacZ/UAS-H3.3K9M-FLAG-HA
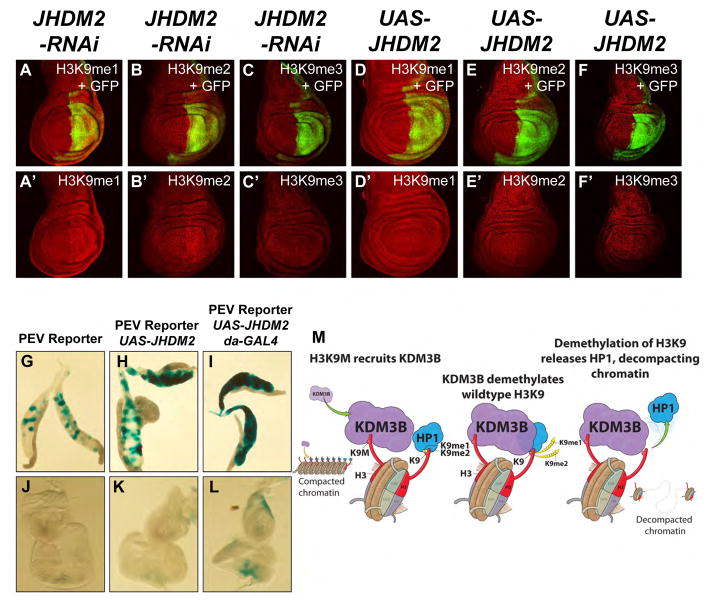
KDM3B was recently characterized as a member of the JumonjiC domain-containing histone demethylases that shows specificity toward H3K9 and is involved gene activation in leukemia cells [25, 26], however, a role for this factor in PEV has not been reported. To test whether increased recruitment of KDM3B by H3K9M nucleosomes might result in decreased H3K9-methylation, we knocked down or over-expressed its Drosophila homolog, JHDM2, in wing imaginal discs. Depletion of JHDM2 results in increased H3K9 methylation levels (Fig. 4, A to C′). Conversely, overexpression depletes H3K9 methylation (Fig. 4, D to F′) and suppresses PEV in both Drosophila salivary glands and eye-antennae imaginal discs (Fig. 4, G to L). KDM3B/JHDM2 and Sirt6 globally affect H3K9 acetylation to a similar degree as H3K9M overexpression (fig. S8 and fig. S9). Sirt6 shows no major effects on PEV in eye-antennae imaginal discs and salivary glands but Sirt6 RNAi results in gypsy-lacZ derepression (fig. S10).
Fig. 4. The H3K9 demethylase KDM3B/JHDM2 interacts with H3K9M and regulates H3K9 methylation levels and heterochromatic silencing.
(A–F) RNAi-mediated knockdown of JHDM2 or JHDM2 overexpression were carried out in wing imaginal discs as described in Figure 1. RNAi-mediated knockdown of JHDM2 results in increased levels of H3K9me1 (A, A′) and H3K9me2 (B, B′) and not H3K9me3 (C, C′). JHDM2 overexpression does not affect H3K9me1 (D, D′) but leads to decreased H3K9me2 (E, E′) and a very weak reduction in H3K9me3 (F, F′). (G–I) Hsp70-lacZ variegating salivary glands with reporter construct (Hsp70-lacZ) only (G), reporter construct and UAS-JHDM2 (H) and reporter construct, UAS-JHDM2 and daughterless-GAL4 driver (I) stained for lacZ expression using X-gal. Compared to salivary glands containing only the reporter construct (G), salivary glands with the reporter construct and UAS-JHDM2 display a weak enhancement in position effect variegation (PEV) (H) possibly due to leakiness of the UAS-JHDM2 construct. PEV is further enhanced when UAS-JHDM2 is expressed under the control of daughterless-GAL4 (I). (J–L) Hsp70-lacZ variegating eye-antennal imaginal discs with reporter construct only (J), reporter construct and UAS-JHDM2 (K) and reporter construct, UAS-JHDM2 and daughterless-GAL4 driver (L) stained for lacZ expression using X-gal. No X-gal staining is observed with the reporter construct only (J). A very weak enhancement is detected with the reporter construct and UAS-JHDM2 combined (K) possibly due to leakiness of the UAS-JHDM2 construct. PEV is further enhanced when UAS-JHDM2 is expressed under the control of daughterless-GAL4 (L) particularly in differentiating areas posterior to the morphogenetic furrow. (M) Model describing the activation of KDM3B/JHDM2 on H3K9M containing nucleosomes.
(A–C) en-GAL4 UAS-2 x EGFP/+ ; UAS-JHDM2-RNAi (TRiP 32975)/+
(D–F) en-GAL4 UAS-2 x EGFP/UAS-JHDM2 (Bloomington 20061)
(G, J) Hsp70-LacZ
(H, K) Hsp70-LacZ ; UAS-JHDM2 (Bloomington 20061)/+
(I, L) Hsp70-LacZ ; UAS-JHDM2 (Bloomington 20061)/+ ; daughterless-GAL4/+
Here we develop an experimental paradigm where histone lysine-to-methionine mutants are implemented to globally modulate histone methylation in vivo. We establish a Drosophila animal model of the H3K27M mutation, which may help elucidate the molecular pathogenesis of pediatric gliomas [3, 4]. We demonstrate that H3K9M globally depletes H3K9 methylation in vivo, disrupts interaction of HP1 proteins and thus suppresses position-effect variegation. To gain mechanistic insight into the molecular function of these mutants, we employed an unbiased proteomic strategy to identify interacting partners. We identified KDM3B/JHDM2 and SIRT6 as potential regulators of H3K9me3 dependent heterochromatic silencing. Indeed Jhdm2 acts as a suppressor of variegation in multiple tissues in our assays, whereas SIRT6 function appears to be restricted to retroelement silencing. Mutations of histone H3.3 lysine36-to-methionine (H3.3K36M) were recently discovered in a sub-type of bone cancer [27]. Thus histone lysine-to-methionine mutations are associated with highly tissue-specific cancer types. Given the importance of heterochromatin in maintaining genomic stability [14, 15] it is plausible that as-yet-uncharacterized H3K9M mutations might occur in some cancers. The system we establish here will provide a powerful tool to inhibit histone lysine modifications at specific residues in vivo and allow to biochemically capture the molecular players involved in chromatin signaling pathways.
Supplemental Materials and Methods
Antibodies
Histone antibodies
Rabbit α-H3 (1791; Abcam) was used at 1:50,000 for Westerns.
Rabbit α-H3K9me1 (Jenuwein laboratory) was used at 1:500 for imaginal disc stainings. Mouse α-H3K9me2 (1220; Abcam) was used at 1:100 for imaginal disc stainings. Rabbit α-H3K9me3 (Jenuwein laboratory) was used at 1:500 for imaginal disc stainings.
Rabbit α-H3K27me1 (07-448; Millipore) was used at 1:400 for imaginal disc stainings. Rabbit α-H3K27me2 (39245; Active Motif) was used at 1:50 for imaginal disc stainings. Rabbit α-H3K27me3 (21467; Shilatifard laboratory) was used at 1:800 for imaginal disc stainings. Rabbit a-H3K27M (BL5381-2.7; Cell Signaling Technology) was used at 1:200 for imaginal disc stainings.
Other antibodies
Rabbit α-E(z) (Müller laboratory) was used at 1:400 for imaginal disc stainings. Rabbit α-HA (805; Santa Cruz) was used at 1:200 for imaginal disc stainings. Mouse α-Ubx (White laboratory) was used at 1:50 for imaginal disc stainings.
Cloning of C-terminally tagged histone H3.3 constructs
The human wild-type H3.3 sequence (on the amino acid level 100% conserved with Drosophila H3.3) followed by a C-terminal FLAG-HA tag as described in [6] was cloned into pUAST-attB via EcoRI/KpnI resulting in pUAST-attB-H3.3-FLAG-HA. ACCATGGCTCGTACAAAGCAGACTGCCCGCAAATCGACCGGTGGTAAAGCACCCAGGAAGCAACTGGCTACAAAAGCCGCTCGCAAGAGTGCGCCCTCTACTGGAGGGGTGAAGAAACCTCATCGTTACAGGCCTGGTACTGTGGCGCTCCGTGAAATTAGACGTTATCAGAAGTCCACTGAACTTCTGATTCGCAAACTTCCCTTCCAGCGTCTGGTGCGAGAAATTGCTCAGGACTTTAAAACAGATCTGCGCTTCCAGAGCGCAGCTATCGGTGCTTTGCAGGAGGCAAGTGAGGCCTATCTGGTTGGCCTTTTTGAAGACACCAACCTGTGTGCTATCCATGCCAAACGTGTAACAATTATGCCAAAAGACATCCAGCTAGCACGCCGCATACGTGGAGAACGTGCTGCTGCAGCAGGTGGCGATTACAAGGATGACGATGACAAGAGTGCCGCAGGTGGCTACCCATACGACGTCCCAGACTACGCTTAA
To create pUAST-attB-H3.3K9M-FLAG-HA and pUAST-attB-H3.3K27M-FLAG-HA mutant constructs, the corresponding mutations were introduced by site directed mutagenesis of pUAST-attB-H3.3-FLAG-HA. To create pUAST-attB-H3.3K9M-FLAG-HA lysine at position 9 (AAA) was mutated to methionine (ATG) and for pUAST-attB-H3.3K27M-FLAG-HA lysine at position 27 (AAG) was mutated to methionine (ATG).
Fly lines
The following fly lines were used: UAS-H3.3WT-FLAG-HA, UAS-H3.3K9M-FLAG-HA, UAS-H3.3K27M-FLAG-HA (this study), en2.4-GAL4 UAS-2xEGFP (Andreas Bergmann), UAS-Sirt6-RNAi (22483, Vienna Drosophila RNAi Center), UAS-Sirt6 (30115, Bloomington), act5c-GAL4/CyO, GAL4-twi UAS-2xEGFP (derived from 4414, Bloomington), GMR35E01-GAL4 (45619; Bloomington), Hsp70-LacZ Tp(3;Y)BL2 [22, 23], tj-GAL4 gypsy-LacZ/CyO [24].
Crosses for overexpression studies in wing imaginal discs and to assess mutant phenotypes in adults were set up and maintained at 29°C. Crosses for overexpression studies in salivary glands, eye-antenna imaginal discs and ovaries were set up and maintained at room temperature (~24°C).
Immunofluorescence labeling of imaginal discs
Antibody labeling of wing imaginal discs was performed as described in [28].
RT PCR Primers
rp49 forward: CCAGTCGGATCGATATGCTAA; rp49 reverse: GTTCGATCCGTAACCGATGT; Sirt6-RNAi forward: GTCATGTTTTGTGGGCTGCA; Sirt6-RNAi reverse: TTGCGCTTGGAACCACTTTG; Sirt6 overexpression forward: CCGGAATTCCTGCAGACGTA; Sirt6 overexpression reverse: TGAGGTTGTGGCCGAAAAGT.
X-Gal staining of salivary glands and imaginal discs
X-Gal staining of salivary glands and imaginal discs was performed as described in [23].
X-Gal staining of ovaries
X-Gal staining of ovaries was performed as described in [29].
Mononucleosome immunoprecipitation
HEK293 TRex FLP-in cells containing Doxycycline inducible histone constructs were generated by the Stowers Institute Tissue Culture Core. Cells were induced with 1ug/ml Doxycycline for 4 days before harvesting. Mononucelosomes were prepared according to a modified protocol available via the Alex Ruthenberg lab (http://ruthenlab.org/web/Protocols.html). For each immunoprecipitation, ~7.5×108 cells were lysed in Buffer A (10mM HEPES, pH7.9, 10mM KCl, 1.5mM MgCl2, 340mM sucrose, 10% glycerol, 0.5mM DTT, 0.5x Protease inhibitor [Sigma #P8340], 0.1% Triton X-100). After cell lysis nuclei were pelleted (1,300xg, 5 minutes), resuspended in Buffer A without Triton X-100 and centrifuged through a sucrose cushion (30% sucrose, 10mM HEPES, pH7.9, 1.5mM MgCl2) at 1,300xg for 12 minutes. Nuclei were then resuspended in Buffer A without Triton X-100 containing 1mM CaCl2 and digested with 24,000U micrococcal nuclease (New England Biolabs, M0247S) per 7.5×108 cells for 15 minutes at 37°C. MNase digestion was stopped by addition of 2mM EGTA, 1mM EDTA, 100mM KCl and 0.05% Triton X-100. Debris was pelleted by centrifugation at 20,000xg for 20 minutes and cleared lysate was incubated with 100ul Anti-FLAG M2 agarose (Sigma, #A2220) for 2 hours at 4°C. Beads were washed 5 times with 5ml wash buffer (50mM HEPES, pH 7.9, 100mM KCl, 10% glycerol, 1mM EDTA, 2mM EGTA, 0.05% Triton X-100) and bound proteins were eluted in 20mM HEPES, pH7.9, 100mM NaCl containing 0.1mg/ml 3x FLAG peptide (Sigma, #F4799). Eluted proteins were digested with Benzonase to remove nucleosomal DNA, precipitated with trichloroacetic acid and subjected to MudPIT mass spectrometry and dNSAF analysis as described previously [30, 31].
Supplementary Material
Acknowledgments
We thank Drs. Thomas Jenuwein, Jürg Müller, Robert White and the Bloomington Stock Center for providing antibodies and fly stocks. We thank the Stowers Molecular Biology core for generating histone point mutants and the Stowers Tissue Culture Core for generating histone mutant cell lines. We thank Stacy Marshall and Wesley Hodges for technical assistance. Histone lysine to methionine studies in Shilatifard’s laboratory was supported in part by a grant from the National Institute of Health CA R01CA089455.
Footnotes
Competing Interest Statement
Antibodies towards H3K27M was provided by Cell Signaling Technology. Ali Shilatifard is a paid member of the Scientific Advisory Board of Cell Signaling Technology.
References
- 1.Shilatifard A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annual review of biochemistry. 2012;81:65–95. doi: 10.1146/annurev-biochem-051710-134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henikoff S, Shilatifard A. Histone modification: cause or cog? Trends in genetics : TIG. 2011;27(10):389–96. doi: 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–31. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 4.Wu G, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–3. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan KM, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–90. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis PW, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–61. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck DB, et al. Chromatin in the nuclear landscape. Cold Spring Harbor symposia on quantitative biology. 2010;75:11–22. doi: 10.1101/sqb.2010.75.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maison C, Almouzni G. HP1 and the dynamics of heterochromatin maintenance. Nature reviews. Molecular cell biology. 2004;5(4):296–304. doi: 10.1038/nrm1355. [DOI] [PubMed] [Google Scholar]
- 9.James TC, Elgin SC. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Molecular and cellular biology. 1986;6(11):3862–72. doi: 10.1128/mcb.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannister AJ, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410(6824):120–4. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama J, et al. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292(5514):110–3. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 12.Lachner M, et al. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410(6824):116–20. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 13.Elgin SC, Reuter G. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harbor perspectives in biology. 2013;5(8):a017780. doi: 10.1101/cshperspect.a017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters AH, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107(3):323–37. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 15.Taddei A, et al. Reversible disruption of pericentric heterochromatin and centromere function by inhibiting deacetylases. Nature cell biology. 2001;3(2):114–20. doi: 10.1038/35055010. [DOI] [PubMed] [Google Scholar]
- 16.Gerace EL, Halic M, Moazed D. The methyltransferase activity of Clr4Suv39h triggers RNAi independently of histone H3K9 methylation. Molecular cell. 2010;39(3):360–72. doi: 10.1016/j.molcel.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murzina N, et al. Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Molecular cell. 1999;4(4):529–40. doi: 10.1016/s1097-2765(00)80204-x. [DOI] [PubMed] [Google Scholar]
- 18.Quivy JP, et al. The HP1-p150/CAF-1 interaction is required for pericentric heterochromatin replication and S-phase progression in mouse cells. Nature structural & molecular biology. 2008;15(9):972–9. doi: 10.1038/nsmb.1470. [DOI] [PubMed] [Google Scholar]
- 19.Eissenberg JC, et al. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(24):9923–7. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eissenberg JC, et al. The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics. 1992;131(2):345–52. doi: 10.1093/genetics/131.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schotta G, et al. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 2002;21(5):1121–31. doi: 10.1093/emboj/21.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu BY, Bishop CP, Eissenberg JC. Developmental timing and tissue specificity of heterochromatin-mediated silencing. EMBO J. 1996;15(6):1323–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Lu BY, Ma J, Eissenberg JC. Developmental regulation of heterochromatin-mediated gene silencing in Drosophila. Development. 1998;125(12):2223–34. doi: 10.1242/dev.125.12.2223. [DOI] [PubMed] [Google Scholar]
- 24.Sarot E, et al. Evidence for a piwi-dependent RNA silencing of the gypsy endogenous retrovirus by the Drosophila melanogaster flamenco gene. Genetics. 2004;166(3):1313–21. doi: 10.1534/genetics.166.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brauchle M, et al. Protein complex interactor analysis and differential activity of KDM3 subfamily members towards H3K9 methylation. PloS one. 2013;8(4):e60549. doi: 10.1371/journal.pone.0060549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, et al. KDM3B is the H3K9 demethylase involved in transcriptional activation of lmo2 in leukemia. Molecular and cellular biology. 2012;32(14):2917–33. doi: 10.1128/MCB.00133-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behjati S, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nature genetics. 2013;45(12):1479–82. doi: 10.1038/ng.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herz HM, et al. Polycomb repressive complex 2-dependent and -independent functions of Jarid2 in transcriptional regulation in Drosophila. Mol Cell Biol. 2012;32(9):1683–93. doi: 10.1128/MCB.06503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handler D, et al. A systematic analysis of Drosophila TUDOR domain-containing proteins identifies Vreteno and the Tdrd12 family as essential primary piRNA pathway factors. EMBO J. 2011;30(19):3977–93. doi: 10.1038/emboj.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson SK, Florens L, Washburn MP. Generation and analysis of multidimensional protein identification technology datasets. Methods in molecular biology. 2009;492:1–20. doi: 10.1007/978-1-59745-493-3_1. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Analytical chemistry. 2010;82(6):2272–81. doi: 10.1021/ac9023999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.