Abstract
Objective
Autism Spectrum Disorder (ASD) is characterized by deficits in social function and the presence of repetitive and restrictive behaviors. Following a previous test of principle, we adopted a quantitative approach to discovering genes contributing to the broader autism phenotype by using social responsiveness as an endophenotype for ASD.
Method
Linkage analyses using scores from the Social Responsiveness Scale (SRS) were performed in 590 families from AGRE, a largely multiplex ASD cohort. Regional and genome-wide association analyses were performed to search for common variants contributing to social responsiveness.
Results
SRS is unimodally distributed in male offspring from multiplex autism families, in contrast with a bimodal distribution observed in females. In correlated analyses differing by SRS respondent, genome-wide significant linkage for social responsiveness was identified at chr8p21.3 (multi-point LOD=4.11; teacher/parent scores) and chr8q24.22 (multi-point LOD=4.54; parent-only scores), respectively. Genome-wide or linkage-directed association analyses did not detect common variants contributing to social responsiveness.
Conclusions
The sex-differential distributions of SRS in multiplex autism families likely reflect mechanisms contributing to the sex ratio for autism observed in the general population and form a quantitative signature of reduced penetrance of inherited liability to ASD among females. The identification of two strong loci for social responsiveness validates the endophenotype approach for the identification of genetic variants contributing to complex traits such as ASD. While causal mutations have yet to be identified, these findings are consistent with segregation of rare genetic variants influencing social responsiveness and underscore the increasingly recognized role of rare inherited variants in the genetic architecture of ASD.
Keywords: linkage, social responsiveness, autism, endophenotype, association
INTRODUCTION
Autism Spectrum Disorder (ASD) is a common neurodevelopmental disorder characterized by deficits in social function and the presence of repetitive and restrictive behaviors. The etiology of ASD is largely unknown, but genetic influences are known to contribute significantly and major genetic risk factors have been identified in a small percentage of patients (1). A majority of the genetic risk variants identified to date are rare, non-heritable mutations that have major effects on disease risk. However, twin and family studies have provided strong evidence for the role of inherited susceptibility factors transmitted via ostensibly unaffected parents (2–4). Thus, identifying forms of transmissible genetic variation in families should have a prominent role in ASD genetic research. Whereas genome-wide association has become a method of choice for the identification of common variants influencing heritability, any contribution of rare inherited variation cannot be reliably identified by this approach. Rare inherited variants that are influential, and that converge on a given risk gene for a significant proportion of cases in a clinical population, should theoretically be identifiable via linkage analysis. Although most prior linkage studies of autism have not yielded strong, consistent signals, subject and phenotype selection may have introduced confounds that we attempt to resolve in the approach described here.
An additional aspect of the contribution of heritable common or rare genetic variation relates to what has been labeled the “broader phenotype” in the first-degree relatives of probands with ASD. The broader phenotype refers to the observation that parents and siblings of probands with autism spectrum disorder often have subthreshold autistic-like impairments in social cognition, behavioral rigidity, or language delay when compared with relatives of non-autistic subjects (5). These intermediate phenotypes, also called “endophenotypes,” considered as separable heritable components of the overall disorder, provide one means of reducing heterogeneity in complex neuropsychiatric diseases such as ASD (6, 7). Quantitative endophenotypes also provide additional power when compared with qualitative phenotypes for linkage analysis (8). This is largely because quantitative measures permit an increase sample size by allowing inclusion of clinically unaffected individuals in the analysis, while at the same time avoiding potentially arbitrary categorical classification of subjects (8, 9).
The social responsiveness scale (SRS) measures heritable, quantitative variation in autistic like social impairments (10, 11). Twin studies and family studies in normal populations and in cohorts with ASD all indicate significant heritability of the SRS (9, 12–14). Based on this, we and others have used the SRS as a quantitative endophenotype for linkage analysis in families with ASD probands (15, 16). However, no genome-wide significant loci have been replicated, consistent with the heterogeneity of ASD and the relatively small sample sizes studied, most consisting of 100–200 families. Furthermore, previous studies have not corrected for sex differences in the distribution of social responsiveness (15, 17, 18), which could attenuate linkage signals. We reasoned that quantitative analysis of social responsiveness with the SRS using high density SNP genotyping with high information content while correcting for sex differences in the distribution of social responsiveness in a relatively large multiplex family sample would be fruitful. Here, we perform multi-point linkage and association analysis using the SRS as a quantitative trait in families from the Autism Genetic Resource Exchange (AGRE). In correlated analyses using SRS scores from teacher or parent respondents and from only parent respondents, we find two loci on chromosome 8 with LOD scores > 4.0, among the highest observed in any linkage study of ASD and consistent with genome-wide significant linkage.
MATERIALS AND METHODS
Samples & biomaterials
Families were drawn from the Autism Genetics Resource Exchange (AGRE) cohort (19). Purified DNA from lymphoblastoid cell lines was obtained from the Rutgers University Cell and DNA Repository (RUCDR; Piscataway, NJ). This study was approved by the Institutional Review Boards at AGRE, UCLA and Washington University.
Phenotypes
Scores from the Social Responsiveness Scale (11) were obtained from the Internet System for Assessing Autistic Children (ISAAC) database in February 2012 (http://agre.autismspeaks.org/site/c.lwLZKnN1LtH/b.5281593/k.8CB5/What_is_ISAAC.htm). When longitudinal scores were available, the earliest assessment was used. For the primary analysis, total raw scores from assessments contributed by teachers were used and assessments contributed by parents were used when teacher scores were not available. The correlation between teacher and parent scores (r2=0.57) is shown in Figure SF1.
Total raw scores from the SRS were adjusted for sex according to normal population means as reported in the SRS manual (11). No adjustment was applied for age, as it was not a significant covariate. Since SRS has not been validated in non-verbal children, we omitted from the analyses individuals with responses to question 9 (“Age of first single words”) from the Autism Diagnostic Interview, Revised (ADI-R) of “993 – had some, lost, not regained” or “994 – milestone not yet reached” (20).
In a correlated analysis, we also examined SRS scores obtained only from parent report. The use of only parent scores decreases sample size (Table ST1) but eliminates any noise introduced by inconsistencies or systematic differences between parent and teacher respondents. While the “parent-only” analysis cannot be considered independent, it explores one of the assumptions made in our initial model, namely that scores contributed by parents and teachers are interchangeable.
Autism diagnoses were derived from a combination of assessment on the Autism Diagnostic Interview, Revised (ADI-R) (20) and/or Autism Diagnostic Observation Schedule (ADOS) (21) and clinician’s best judgment according to standard protocols at AGRE (19). Subjects with Autism, Broad Spectrum, or Not Quite Autism (NQA) were considered “affected” in the qualitative analyses.
SNP genotypes
Raw genotype data for 943 families were drawn from Wang et al (2009) (22) and subjected to independent quality filtering. Additional families were genotyped in the UCLA Neuroscience Genomics Core (UNGC, http://www.semel.ucla.edu/ungc) on the Illumina Omni-1 Quad and Omni-2.5-8 platforms (Illumina, San Diego, CA) according to standard manufacturer protocols. Familial relationships in the combined data set were validated using identity-by-descent (IBD) estimation in PLINK (23). For any monozygotic (MZ) twin pairs or sample duplicates detected during relationship validation, only one individual was retained for downstream analyses. Each data set was subjected to the following quality filters as applied in PLINK: ≤ 5% missing data per person or per SNP; minor allele frequency (MAF) ≥ 1%; Hardy-Weinberg Equilibrium (HWE) p ≥ 10−7. Because SRS scores were available for only a subset of the genotyped cohort, analyses for SRS included 590 families with 1,480 phenotyped individuals.
Genotype imputation was performed separately on each data set using IMPUTE2 and a cosmopolitan reference panel from the 1000 Genomes Project (24–26). Quality filters included: IMPUTE2 quality score (info) ≥0.5; missing data per SNP ≤ 5%; MAF ≥ 1%; HWE p ≥ 10−7. The final data set included 5,814,564 autosomal SNPs passing quality filters.
Linkage analyses
An independent marker set (r2≤0.1) was identified from the genotyped SNP sets using PLINK (option --indep-pairwise) (23). The pruned marker set consisted of 52,354 autosomal SNPs common to all genotyping platforms. Non-parametric multi-point analyses were performed in Merlin (27). Genome-wide multi-point LOD scores can be found in Supplemental Tables ST2 and ST3.
Association analyses
Single-SNP association analyses for SRS as a quantitative trait were performed on imputed genotypes using the --qfam-total option in PLINK (23), which applies linear regression and uses permutation to correct for non-independence between family members. Analyses for autism diagnosis as a qualitative trait were performed using the --tdt option in PLINK, with gene-dropping permutations to correct for non-independence between trios drawn from multiplex families. There were 2,236 affected offspring in 1,250 two-parent families analyzed for association, including trios not used to conduct linkage. Bonferroni correction was applied to p-values derived from association analyses in the linked regions of interest using the number of independent markers in the region, as determined using the --indep-pairwise option in PLINK at r2≤0.5.
RESULTS
Clinical/phenotype characteristics
We identified families from the Autism Genetic Resource Exchange (AGRE) where two or more offspring had been assessed using the Social Responsiveness Scale (SRS) (11). To maximize the cohort of genotyped and phenotyped individuals, we used a combination of teacher-reported and parent-reported SRS scores. The cohort consisted of 590 families including 1,480 phenotyped individuals (Table 1). We examined total raw SRS scores in the offspring and observe a roughly bimodal distribution correlating with ASD diagnosis (Figure 1). Similarly, there are more male offspring with high SRS scores (indicating greater social impairment), consistent with the male sex bias observed in ASD. The distributions using scores from teacher, parent, or mixed teacher/parent respondents are highly similar within each group. The larger number of individuals with minimal social impairment (low SRS scores) reflects ascertainment bias, with unaffected siblings more often receiving assessments from parents than from teachers. We observed significantly higher SRS scores in males than in females from both teacher and parent respondents as previously observed in the literature (11). While there are likely genetic variants contributing to the sex difference in SRS, we adjusted SRS for sex to focus primarily on variants contributing to SRS variation in ASD. Total raw SRS scores were adjusted for sex and transformed to Z-scores using normal population means and standard deviations (11).
Table 1.
Characteristics of 590 families from AGRE with SRS teacher/parent scores.
| SRS | ||||||
|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | ||
|
|
||||||
| Families | 590 | |||||
|
|
||||||
| Phenotyped | 1,480 | |||||
|
|
||||||
| Parents | All | 67 | 36.4 | 25.3 | 5 | 113 |
|
|
||||||
| Fathers | 32 | 35.0 | 24.4 | 6 | 105 | |
|
|
||||||
| Mothers | 35 | 37.7 | 26.3 | 5 | 113 | |
|
|
||||||
| ASD | All | 967 | 101.8 | 33.9 | 3 | 184 |
|
|
||||||
| Males | 761 | 103.2 | 33.5 | 8 | 184 | |
|
|
||||||
| Females | 206 | 96.5 | 35.1 | 3 | 169 | |
|
|
||||||
| Non-ASD Siblings | All | 446 | 27.2 | 27.2 | 0 | 159 |
|
|
||||||
| Males | 198 | 32.2 | 32.0 | 0 | 159 | |
|
|
||||||
| Females | 248 | 23.1 | 22.0 | 0 | 128 | |
Figure 1.

Distribution of total raw SRS scores in offspring from AGRE. SRS scores form a roughly bimodal distribution corresponding to ASD diagnosis. Consistent with the sex bias in ASD, males tend to have higher SRS scores indicating more severe social deficit. Distributions are highly similar regardless of respondent; the larger number of subjects with low SRS scores from parent respondents reflects ascertainment bias, as unaffected children more typically were assessed by parents but not by teachers.
Genome-wide linkage analysis
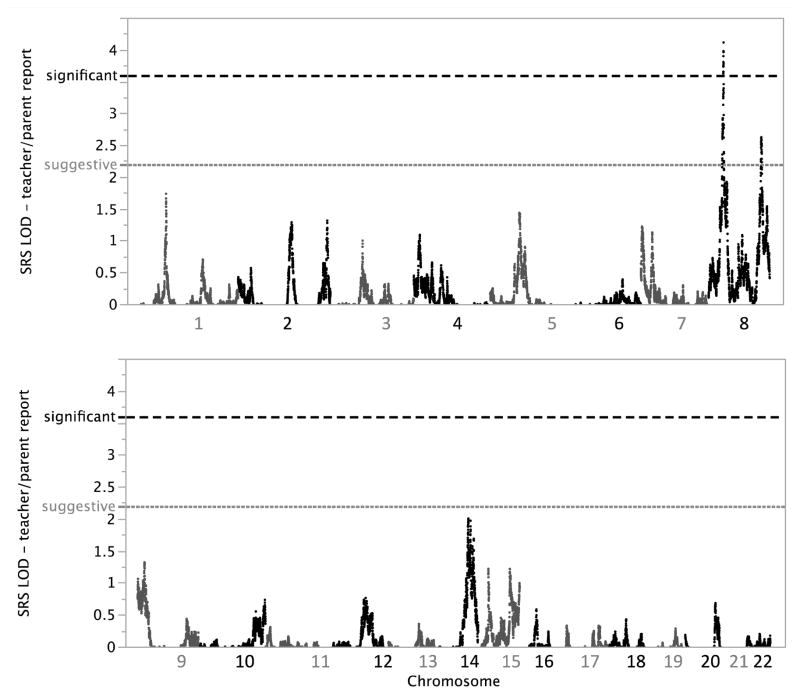
Using an independent set of 52,354 autosomal SNPs pruned for LD (Methods), we performed non-parametric multi-point linkage analyses for SRS as a quantitative trait in 590 families from AGRE (Figure 2). We observed genome-wide significant linkage for SRS on chromosome 8p21.3 centered at rs6587004 (37.6 cM) with a maximum multi-point LOD score of 4.11 (Figure 3A) (28). The two-LOD interval encompassing the linkage peak at chr8p21.3 spans approximately 5.7 cM and 2.9 Mb (rs1316738 to rs17088602). While we report the name and location of the peak SNP for interest, we define a two-LOD drop on either side of the linkage peak as the region of interest in which the causal variant(s) are most likely to be found.
Figure 2.
Non-parametric linkage scan for social responsiveness as a quantitative trait in 590 families from AGRE using teacher/parent reports. Gray and black dotted lines denote thresholds for genome-wide suggestive (LOD≥2.2) and significant (LOD≥3.6) evidence for complex trait linkage, respectively.
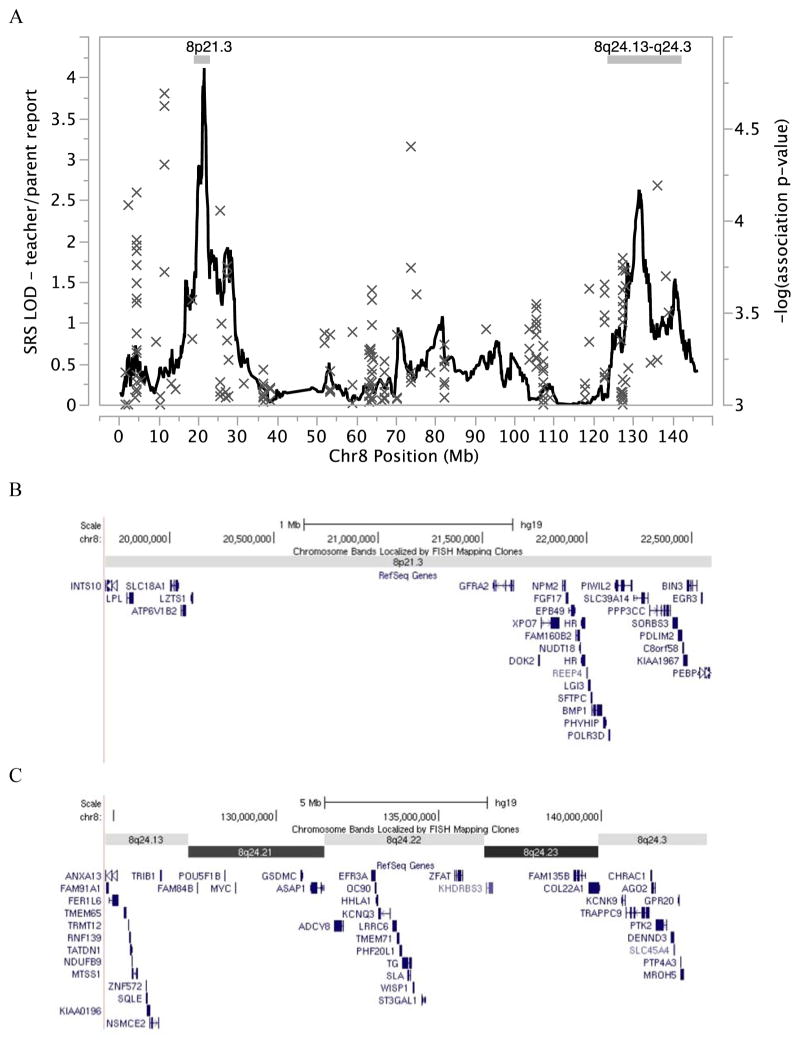
Figure 3.
Linkage and association for SRS on chr8p21.3 and 8q24.22 – teacher/parent report. A) Genome-wide significant evidence for linkage (peak LOD 4.11) is observed at chr8p21.3 and suggestive evidence for linkage (peak LOD 2.63) is observed at chr8q24.22. Gray “x” indicates association signal for SRS as a quantitative trait using imputed SNP genotype data. Bars indicate the 2-LOD regions of interest. B–C) Genes in the 2-LOD region of interest underlying the linkage peaks at chr8p21.3 and chr8q24.22, respectively.
We also observed suggestive evidence for linkage (LOD ≥ 2.2) (28) on chr8q24.22 with multi-point LOD=2.63 (peak SNPs rs2959319 at 139.8 cM and rs6470838 at 140.1 cM) (Figure 3A). The two-LOD interval for the peak at chr8q24.22 spans approximately 35.3 cM and 18.5 Mb (rs10956146 to rs6583595). Linkage signals LOD ≥ 2.2 were not observed elsewhere in the genome (Figure 2).
We considered whether the use of teacher and parent scores interchangeably in our analyzed phenotype may introduce noise to the analysis, and re-analyzed the subset of 536 families in which the SRS score was contributed by a parent (Table ST1). Using parent scores only, we again observed genome-wide significant evidence for linkage at chr8p21.3 (multi-point LOD=3.65, rs6587004 at 37.6 cM) as well as at chr8q24.22 (multi-point LOD=4.54, rs10505568 at 141.0 cM) (Figures SF2, SF3). The significant linkage signal at chr8q24.22 obtained using parent scores overlaps with the suggestive linkage signal observed using teacher/parent scores, as described above. All other LOD scores observed were ≤1.5. Persistence of the linkage signal at chr8p21.3 and enhancement of the previously suggestive signal at 8q24.22 in the analysis limited to parent-reported SRS scores suggests that the observed linkage signals are robust to any phenotypic noise introduced by differences in the individual performing the SRS evaluation, and that biological variants underlying social communication exist in these two regions of chr8.
Genome-wide and linkage-directed association
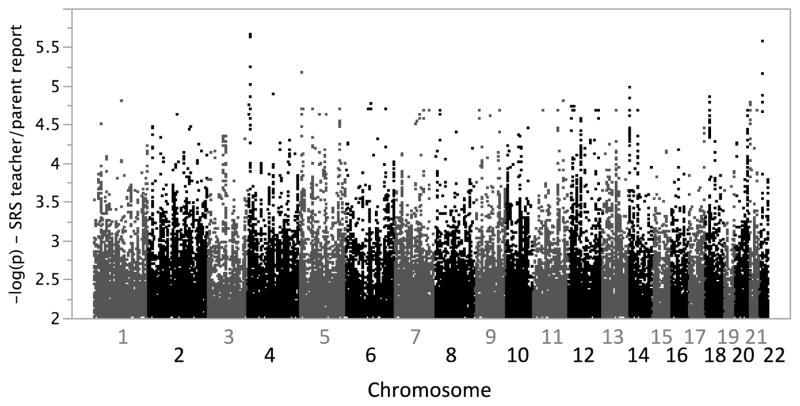
We next undertook quantitative association analyses in an attempt to identify common genetic variants that may contribute to social responsiveness in families from AGRE. A larger cohort of 1,652 nuclear families, including trios that were not informative for linkage analyses, was analyzed for genome-wide association to SRS teacher/parent scores using >5.8 million genotyped and imputed autosomal SNPs. The quantile-quantile plot showed no evidence for score inflation in this family-based analysis (data not shown). The SNP showing strongest association with SRS was rs4689064 on chr4p16.1 (p=2.12×10−6) (Figure 4). This SNP is intronic to TADA2B (transcriptional adaptor 2 (ADA2 homolog); OMIM 608790), which coordinates histone acetyltransferase activity and links activation factors to basal transcription machinery to potentiate transcription. The genomic region has been implicated in a variety of phenotypes, including systemic lupus erythematosus (OMIM 605480), spastic paraplegia (OMIM 612335), and Stargardt disease 4 (OMIM 306786). We also observe modest evidence for association at rs5997325 on chr22q12.1 (p=2.65×10−6) between genes PITPNB (phosphatidylinositol transfer protein, beta) and TTC28 (tetratricopeptide repeat domain 28). This general region has been implicated in multiple disorders such as epilepsy (OMIM 604364), spinal muscular atrophy (OMIM 615048), and myopia (OMIM 608908).
Figure 4.
Genome-wide association results for social responsiveness – teacher/parent report. Quantitative association analyses for SRS were performed using the QFAM option in PLINK.
We specifically examined association signals in the two-LOD intervals underlying the linked regions of interest on chr8p21.3 and chr8q24.22 (Figure 3). For SRS teacher/parent scores, there was no significant evidence for association after correction for multiple testing. Similarly, we examined association scores for autism diagnosis as a qualitative trait and found no significant evidence for association in the two-LOD intervals underlying the linked regions of interest (data not shown). The use of imputed SNP genotypes and a cosmopolitan reference panel from the 1000 Genomes Project (25, 26) greatly enhances genome coverage over the genotyped marker set, hence increases our ability to detect association. The lack of association observed here suggests two likely scenarios: 1) our study remains under-powered to detect common variants of small effect which may contribute to variation in social responsiveness and 2) rare variants may contribute to social responsiveness.
DISCUSSION
We took an endophenotype approach to the identification of genetic loci contributing to autism spectrum disorder (ASD) by searching for genetic factors influencing social responsiveness. Using a combination of teacher-reported and parent-reported scores from the Social Responsiveness Scale (SRS) as a quantitative trait, we extended previous linkage studies performed in the Autism Genetic Resource Exchange (AGRE) cohort to a sample size of 590 families (16). The use of teacher-reports when available is thought to more accurately reflect a child’s social skills in the context of an average group of peers. We note a bimodal distribution of SRS scores among parent-respondents, consistent with both ascertainment bias, where non-autistic children are less likely to have been evaluated by their teachers, and a possible rater contrast effect, where the contrast in social skills between ASD and non-ASD siblings leads parents to underestimate social impairment in the non-ASD sibling and subsequently reduces study power (16).
The observation of a unimodal teacher-report SRS distribution for males suggests a continuum of expression of autistic trait liability across all or most males in this clinically ascertained sample of largely multiplex families. This, and the presence of a bimodal distribution for females in the same cohort, replicates prior reports of contrasting distributions between males and females in multiplex autism families (3, 29). These contrasts support sex differential expression of phenotypic features of social cognition in the setting of familial liability to autism, which may substantially contribute to the gender ratio for autism observed throughout the general population.
Our linkage analysis of 590 families from AGRE identified a novel locus on chr8p21.3 which exceeds the criteria set by Lander & Kruglyak (1995) for genome-wide significant linkage in a complex trait (28) using combined teacher/parent SRS scores. The two-LOD region of interest at chr8p21.3 includes 34 genes (Figure 3B). While the causal variant(s) may reside anywhere in the interval, we note with interest that the peak SNP (rs6587004) lies in an intronic region of GFRA2 (glial cell line-derived neurotrophic factor (GDNF) family receptor alpha-2; OMIM 601956), which encodes a cell-surface receptor for neurotrophic factors involved in neuron survival and differentiation. The region has been implicated in epilepsy (OMIM 612279) and schizophrenia (OMIM 603013) via linkage approaches, although no causal genes have been identified.
Using a subset of 536 families with parent-only SRS reports, we identified a second genome-wide significant linkage peak at chr8q24.22. The peak SNP on chr8q24.22 lies in a gene-poor region between ASAP1 (ArfGAP with SH3 domain, ankyrin repeat and PH domain 1; OMIM 605953) and ADCY8 (adenylate cyclase 8 (brain); OMIM 103070). Among the 68 genes in the linked interval (Figure 3C), we also note with interest KCNQ3, a potassium channel gene implicated in benign neonatal seizures (OMIM 602232); TRAPPC9, a gene encoding a trafficking protein implicated in autosomal recessive mental retardation (OMIM 611966); and KCNK9, a potassium channel gene implicated in Birk-Barel mental retardation dysmorphism syndrome (OMIM 605874). Our region of interest may overlap with a previously reported linkage signal at chr8q24.13 for social responsiveness (Posterior Probability of Linkage = 0.37) (30). Previous studies in the Old Order Amish identified significant evidence for linkage to mental health wellness in this region (OMIM 603663). Re-analysis of the data using a subset of families with parent-reported scores supports our hypothesis that teacher- and parent-reported scores can be combined and analyzed as a single trait.
The use of endophenotypes in psychiatric disease has been debated as a means of increasing power to find genetic variants by focusing on simpler aspects of complex behavioral traits (6–8). Previously, we used another quantitative trait related to delay of spoken language to identify association to the gene CNTNAP2 (31). While previous linkage studies of SRS and ASD in 99 families from AGRE found no overlap between the disorders, we note with interest that the linkage peak for SRS at chr8p21.3 peak (n=590 families) co-localizes with a linkage signal for ASD as a qualitative trait in a substantially larger set of AGRE families (n=1,008 families; multi-point LOD=2.18) (16, 32). Our data support the use of quantitative intermediate traits such as SRS to increase power by reducing heterogeneity in a complex trait such as ASD and identify a highly significant, narrow linked region on chromosome 8p21.3 as well as provide strong evidence for linkage on chromosome 8q24.22.
The genetic heterogeneity of both ASD and social responsiveness are underscored by the lack of consistency between our current findings and prior reports, a common theme in ASD (1, 33). We previously reported a microsatellite linkage scan for teacher-reported SRS in 99 families from AGRE with suggestive evidence for linkage on chromosomes 11 and 17 (16). Suggestive linkage in the subset of families with only male affecteds was observed on chromosome 8 at 100 cM (16); however, the signal does not overlap with the findings reported here. Coon et al (2010) performed linkage analyses for SRS in 64 multiplex families and identified significant non-parametric linkage to chr15q13.3 (LOD 3.64) and suggestive linkage to chr7q31.1-q32.3 (LOD 2.91) (15). In a collection of 70 families segregating ASD with or without language impairment, Bartlett et al (2014) reported strong linkage to SRS as a quantitative trait at chr15q26.2-26.3 and as a dichotomous trait at chr14q32.2-32.33 (34). These studies observed little or no evidence for linkage on chromosome 8p21.3 or 8q24.22; we observe a similar lack of signal in their regions of interest.
We considered whether families contributing to the linkage peak could be distinguished from families showing evidence against linkage. However, we examined genotyping platform, individual and family mean SRS score, age at which the SRS and ADI-R were administered, Vineland adaptive behavior scores, Stanford-Binet IQ full score, and family size and found no significant differences between linked and unlinked families in each of these factors (data not shown).
Association analyses using imputed SNP genotypes both genome-wide and in the linked regions showed little evidence for common variants contributing to SRS. We also saw little evidence for association to ASD in the genomic regions linked to SRS. The lack of common variant signal is unsurprising given the genetic and phenotypic heterogeneity of a behavioral trait related to social cognition. Association studies for ASD in larger cohorts have found only a handful of variants, each of small effect, despite sample sizes several-fold greater than that used here (22, 35, 36).
Together, findings from our study and others are consistent with the increasingly recognized role of rare variants in the genetic architecture of ASD (37). Like ASD, social cognition is a complex behavioral trait, but one that we expect is narrower than the broad diagnosis of ASD. Rapid expansion of the human population may have given rise to rare variants influencing variation in social responsiveness, and ever-larger sample sizes will be needed to identify such variants (38). The brevity and ease of administration of the Social Responsiveness Scale and its correlation with traditional autism diagnostics such as the ADI-R and ADOS make it feasible to recruit larger cohorts for the study of social responsiveness in general as well as valuable as a rapid screening tool for ASD in population-based research (39, 40).
CONCLUSION
The identification of two strong loci for social responsiveness validates the use of quantitative intermediate traits to facilitate identification of genetic variants contributing to complex traits such as ASD. While causal mutations have yet to be identified, these findings underscore the increasingly recognized role of rare inherited variants in the genetic architecture of ASD. Future approaches to the discovery of rare variants conferring susceptibility to ASD, and their convergence on genes that participate in and elucidate mechanisms of causation in autism, will be facilitated by quantitative approaches to linking genetic variation, neural signatures, and behavior in large family studies. Here we have demonstrated the ability to identify susceptibility loci using this approach and have clarified contrasting distributions for quantitative traits in males versus females, which likely reflect protective factors contributing to the observed sex ratios in familial autistic syndromes.
Supplementary Material
Acknowledgments
We thank the patients and families whose participation makes this work possible, as well as Clara M. Lajonchere and Ryan Butler from the Autism Genetic Resource Exchange (AGRE) and Joe DeYoung from the UCLA Neurosciences Genomics Core (UNGC). Thanks also to Rui Luo for her work on genotype imputation and to Lauren Lawrence and Kun Gao for technical assistance. The Autism Genetic Resource Exchange (AGRE) is a program of Autism Speaks and was supported by grant NIMH U24 MH081810 to Clara M. Lajonchere. DMW was supported by NIMH F31 MH093086. This work was supported by ACE Network grants R01 MH081754 and R01 MH100027 as well as ACE Center grants P50 HD055784 and R01 MH071425 to DHG. It was additionally supported by HD042541 and IDDRC Center Grant P30 HD062171 to JNC.
Footnotes
DISCLOSURES
JKL, DMW, RMC and DHG report no competing interests. JNC receives royalties from Western Psychological Services for the commercial distribution of the Social Responsiveness Scale; however, no royalties were generated from research implementation of the scale in the AGRE registry.
References
- 1.Devlin B, Scherer SW. Genetic architecture in autism spectrum disorder. Current opinion in genetics & development. 2012;22(3):229–37. doi: 10.1016/j.gde.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Archives of pediatrics & adolescent medicine. 2009;163(10):907–14. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- 3.Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. The American journal of psychiatry. 2010;167(11):1349–56. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantino JN, Todorov A, Hilton C, Law P, Zhang Y, Molloy E, et al. Autism recurrence in half siblings: strong support for genetic mechanisms of transmission in ASD. Molecular psychiatry. 2013;18(2):137–8. doi: 10.1038/mp.2012.9. [DOI] [PubMed] [Google Scholar]
- 5.Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. The American journal of psychiatry. 1997;154(2):185–90. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. The American journal of psychiatry. 2003;160(4):636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 7.Stoltenberg SF, Burmeister M. Recent progress in psychiatric genetics-some hope but no hype. Human molecular genetics. 2000;9(6):927–35. doi: 10.1093/hmg/9.6.927. [DOI] [PubMed] [Google Scholar]
- 8.Almasy L. The role of phenotype in gene discovery in the whole genome sequencing era. Human genetics. 2012;131(10):1533–40. doi: 10.1007/s00439-012-1191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of general psychiatry. 2003;60(5):524–30. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 10.Constantino JN. The quantitative nature of autistic social impairment. Pediatric research. 2011;69(5 Pt 2):55R–62R. doi: 10.1203/PDR.0b013e318212ec6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constantino JNSRS. Social Responsiveness Scale. Manual. Los Angeles: Western Psychological Services; 2012. [Google Scholar]
- 12.Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. The American journal of psychiatry. 2000;157(12):2043–5. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- 13.Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(4):458–67. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- 14.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biological psychiatry. 2005;57(6):655–60. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Coon H, Villalobos ME, Robison RJ, Camp NJ, Cannon DS, Allen-Brady K, et al. Genome-wide linkage using the Social Responsiveness Scale in Utah autism pedigrees. Molecular autism. 2010;1(1):8. doi: 10.1186/2040-2392-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duvall JA, Lu A, Cantor RM, Todd RD, Constantino JN, Geschwind DH. A quantitative trait locus analysis of social responsiveness in multiplex autism families. The American journal of psychiatry. 2007;164(4):656–62. doi: 10.1176/ajp.2007.164.4.656. [DOI] [PubMed] [Google Scholar]
- 17.Werling DM, Geschwind DH. Understanding sex bias in autism spectrum disorder. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(13):4868–9. doi: 10.1073/pnas.1301602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson EB, Lichtenstein P, Anckarsater H, Happe F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(13):5258–62. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lajonchere CM, Consortium A. Changing the landscape of autism research: the autism genetic resource exchange. Neuron. 2010;68(2):187–91. doi: 10.1016/j.neuron.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutter M, Le Couteur A, Lord C. ADI-R Autism Diagnostic interview Revised. Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 21.Lord C, Rutter M, DiLavore PC, Risi S. ADOS. Autism Diagnostic Observation Schedule. Manual. Los Angeles: Western Psychological Services; 2001. [Google Scholar]
- 22.Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459(7246):528–33. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS genetics. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.1000 Genomes Project [database on the Internet] Available from: http://www.1000genomes.org/
- 26.Genomes Project C. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467(7319):1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nature genetics. 2002;30(1):97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 28.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nature genetics. 1995;11(3):241–7. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 29.Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2009;150B(3):328–34. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piven J, Vieland VJ, Parlier M, Thompson A, O’Conner I, Woodbury-Smith M, et al. A molecular genetic study of autism and related phenotypes in extended pedigrees. Journal of neurodevelopmental disorders. 2013;5(1):30. doi: 10.1186/1866-1955-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American journal of human genetics. 2008;82(1):150–9. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werling DM, Lowe JK, Luo R, Cantor RM, Geschwind DH. Replication of linkage at chromosome 20p13 and identification of suggestive sex-differential risk loci for autism spectrum disorder. Molecular autism. 2014;5(1):13. doi: 10.1186/2040-2392-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature reviews Genetics. 2008;9(5):341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartlett CW, Hou L, Flax JF, Hare A, Cheong SY, Fermano Z, et al. A genome scan for Loci shared by autism spectrum disorder and language impairment. The American journal of psychiatry. 2014;171(1):72–81. doi: 10.1176/appi.ajp.2013.12081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss LA, Arking DE, Daly MJ, Chakravarti A Gene Discovery Project of Johns H, the Autism C. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461(7265):802–8. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, et al. A genome-wide scan for common alleles affecting risk for autism. Human molecular genetics. 2010;19(20):4072–82. doi: 10.1093/hmg/ddq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature reviews Genetics. 2012;13(8):537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(12):1668–76. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- 40.Lee H, Marvin AR, Watson T, Piggot J, Law JK, Law PA, et al. Accuracy of phenotyping of autistic children based on Internet implemented parent report. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2010;153B(6):1119–26. doi: 10.1002/ajmg.b.31103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.