Abstract
BACKGROUND & AIMS
Chronic hepatitis C virus infection activates an intrahepatic immune response, leading to increased expression of interferon (IFN)-stimulated genes and activation of natural killer (NK) cells—the most prevalent innate immune cell in the liver. We investigated whether the elimination of HCV with direct-acting antiviral agents normalizes expression of IFN-stimulated genes and NK cell function.
METHODS
We used multicolor flow cytometry to analyze NK cells from liver and blood of 13 HCV-infected patients who did not respond to treatment with pegylated interferon and ribavirin. Samples were collected before and during IFN-free treatment with daclatasvir and asunaprevir therapy and compared with those from blood of 13 healthy individuals (controls). Serum levels of CXCL10 and CXCL11 were measured by ELISA.
RESULTS
Before treatment, all patients had increased levels of CXCL10 or CXCL11 and a different NK cell phenotype from controls, characterized by increased expression of HLA-DR, NKp46, NKG2A, CD85j, pSTAT1, STAT1, and TNF-related apoptosis-inducing ligand (TRAIL). NK cells from patients also had increased degranulation and decreased production of IFNγ and TNFα compared with NK cells from controls. Nine patients had an end-of-treatment response (undetectable virus) and 4 had virologic breakthrough between weeks 4 and 12 of therapy. A rapid decrease in viremia and level of inflammatory cytokines in all patients was associated with decreased activation of intrahepatic and blood NK cells; it was followed by restoration of a normal NK cell phenotype and function by week 8 in patients with undetectable viremia. This normalized NK cell phenotype was maintained until week 24 (EOT).
CONCLUSIONS
DAA-mediated clearance of HCV is associated with loss of intrahepatic immune activation by IFNα, indicated by decreased levels of CXCL10 and CXCL11 and normalization of NK cell phenotype and function.
Keywords: immune regulation, NS5A inhibitor, NS3 inhibitor, ISG
Introduction
Chronic hepatitis C virus (HCV) infection is a major cause of liver cirrhosis and hepatocellular carcinoma due to the large number of up to 170 million infected people worldwide. The progression of liver disease from hepatitis to fibrosis and cirrhosis may take decades and is caused by a low-level inflammatory response. An interferon-driven innate immune activation is thought to contribute to this process 1.
A characteristic of the innate response in HCV infection is the up-regulation of interferon-stimulated genes (ISGs) that are readily detectable in liver biopsies of chronically infected patients 2. The products of some ISGs, such as CXCL10, are also detectable at increased levels in the systemic circulation 3. This increase in ISG levels is consistent with increased activation of innate immune cells that respond to type I IFN.
NK cells are an important part of this interferon-responsive innate population because they are enriched among lymphocytes in the liver (30%) as compared to the blood (5-20%), and their percentage increases further in viral hepatitis 4. NK cells of patients with chronic HCV infection express higher levels of STAT1 and pSTAT1 than NK cells of healthy controls 5, 6 suggesting that they are activated by type I IFN. STAT1 itself is the product of an ISG and its phosphorylation is an essential part of signaling downstream of the IFNα/β receptor. NK cells of chronically infected patients also display altered expression of activating and inhibitory receptors compared to those of healthy uninfected controls 7,8. The integration of all these signals results in activation of blood and liver NK cells in HCV infection 7 and altered functional phenotype with increased cytotoxicity and decreased production of antiviral cytokines 7, 9. It is currently unknown whether this altered functional profile of NK cells is reversible.
The development of highly effective interferon-free regimens against HCV infection 10, 11 provides a unique opportunity to analyze whether and how fast NK cell activation and liver inflammation resolve when HCV replication is blocked. A normalization of NK cell effector functions would also be of interest in the context of adaptive immune responses because HCV-specific T cells are dysfunctional due to chronic antigen stimulation in HCV infection 12, 13. While a recovery of T cell proliferation was recently reported during treatment of HCV-infected patients with DAAs,14 it remains unknown whether this translates into a full recovery of effector functions. Past IFN-based treatment regimens were not suitable to answer these questions because IFN not only has antiviral, but also immunomodulatory function 15. IFN-based therapies activate the innate immune response followed by the induction of a refractory state 16. They also exacerbate the functional dichotomy of NK cells towards increased cytotoxic effector functions and reduced IFNγ production 17, 18.
Here, we ask whether an IFN-free treatment regimen of daclatasvir (DCV) and asunaprevir (ASV) normalizes innate immune activation and NK cell function. DCV is a potent NS5A replication complex inhibitor with broad genotypic coverage; ASV is an NS3 protease inhibitor active against HCV genotypes 1a and 1b 19. HCV genotype 1b nonresponders to previous PegIFN/RBV therapy were treated with DCV/ASV for 24 weeks. We demonstrate that successful treatment with the DCV/ASV regimen decreases serum levels of the ISG products CXCL10 and CXCL11, and that it decreases STAT1 expression and STAT1 phosphorylation in NK cells. This is associated with changes in the expression of activating and inhibitory receptors on NK cells and a normalization of NK cell function by week 8 of therapy. The results were verified for EOT responders at week 24 indicating that the normalization of NK cell activation and function are maintained in those who clear HCV.
Materials and Methods
Study cohort
NK cells were studied in 13 HCV-infected non-responders to previous PegIFN/RBV therapy prior to and at days 0 and 1, and at weeks 2, 4, 8 and 24 of a 24-week treatment course with 60 mg DCV once daily and 100 mg ASV twice daily (Bristol-Myers Squibb, protocol NCT01888900 on ClinicalTrials.gov). NK cells were studied in 13 uninfected subjects for comparison. Patients underwent paired liver biopsies pretreatment and at either week 2 (n=5) or week 4 (n=8) of therapy. Three patients who later on experienced virologic breakthrough were biopsied at week 2 of therapy, the biopsy of the fourth patient with breakthrough was not studied for NK cell responses. Patients gave written informed consent for research testing under protocols by the institutional review board of NIDDK/NIAMS.
Serological analyses
Serum HCV RNA was quantitated using the Cobas TaqMan real-time PCR (Roche Molecular Diagnostics, Branchburg NJ) with a lower limit of detection of 10 IU/ml and a lower limit of quantification of 25 IU/ml. Serum CXCL10 and CXCL11 were quantitated using the ELISA MAX kit (Biolegend, San Diego, CA) and the Quantikine ELISA kit (R&D Systems, Minneapolis, MN), respectively.
Lymphocyte isolation
Peripheral blood mononuclear cells (PBMCs) were separated from heparin-anticoagulated blood on Ficoll–Histopaque (Mediatech, Manassas, VA) density gradients, washed 3 times with phosphate-buffered saline (PBS, Mediatech), and cryopreserved in 70% fetal bovine serum (FBS, Serum Source International, Charlotte, NC), 20% RPMI1640 (Mediatech) and 10% DMSO (Sigma Aldrich, St. Louis, MO). Liver biopsies were homogenized, washed with PBS and studied the same day as described below.
NK cell analysis
For each patient, cryopreserved PBMC from week 0 to week 8 were thawed and tested on the same day. Healthy donor PBMC were included in each experiment. Because the flow cell of the LSRII flow cytometer was replaced between weeks 8 and 24 of the study protocol, the MFI data from the week 24 (EOT) time point cannot be compared to those from the earlier experiments. The week 24 experiment included PBMC from the week 0 time point and PBMC of healthy donors for comparison.
(i) NK cell frequency and phenotype
Thawed PBMC were stained with ethidium monoazide (EMA), anti-CD19-PeCy5, anti-CD3-AlexaFluor700 (both from BD Biosciences), and with either anti-CD14-V711 (Biolegend) or anti-CD14-PeCy5 (AbD Serotec, Raleigh, NC) to exclude dead cells, T cells, B cells and monocytes. NK cells were identified using anti-CD56-PeCy7 (BD Biosciences). FITC-conjugated antibodies against CD122, CXCR3 (R&D Systems), CD69 and HLA-DR (BD Biosciences), PE-conjugated antibodies against TRAIL, CD300 (BD Biosciences), NKG2A, NKG2D, and NKp44 (Beckman Coulter, Brea, CA), and APC-conjugated antibodies against CD85j (eBiosciences, San Diego, CA), CCR5 (BD Biosciences), NKG2C (R&D Systems), NKp30 and NKp46 (Miltenyi Biotec, Auburn, CA) were added. Liver-infiltrating lymphocytes were stained with anti-TRAIL-PE, anti-CD69-APC/Cy7 (BD Biosciences) and anti-NKp46-APC (Miltenyi Biotec) in addition to EMA and the lineage-specific antibodies described above.
(ii) NK cell degranulation
Thawed PBMC were cultured overnight in RPMI1640 with 10% FBS (Serum Source International), 1% penicillin/streptomycin, 2 mM L-glutamine and 10 mM HEPES (Mediatech). The next day, PBMCs were counted and cultured in the presence of anti-CD107-PE (BD Biosciences) with or without K562 cells (ATCC, Manassas, VA) as described 7 without addition of cytokines, then stained with EMA and lineage-specific antibodies as described above.
(iii) Cytokine production
Thawed PBMC were incubated with or without IL-12 (0.5 ng/ml; R&D Systems) and IL-15 (20 ng/ml; R&D Systems) for 14h, followed by addition of brefeldin A (BD Biosciences) for 4h as described 7. Cells were then washed and stained with EMA and the lineage-specific antibodies described above. Cells were washed again, fixed and permeabilized with the Cytofix/Cytoperm Kit and stained with anti-IFNγ-PE and anti-TNFα-APC (all from BD Biosciences).
(iv) STAT1 / pSTAT1 staining
Thawed PBMC were rested for 2 hours at 37°C, 5% CO2 in RPMI1640 with 10% FBS (Serum Source International), 1% penicillin/streptomycin and 2 mM L-glutamine (Mediatech). After fixation with Cytofix (BD Biosciences) for 10 min at 37°C and 5% CO2 and subsequent centrifugation, cells were permeabilized with BD Phosflow III (BD Biosciences) for 20 min on ice, then washed twice and resuspended in BD Phosflow Buffer (BD Biosciences). All samples were stained with anti-CD56-PE (Beckman Coulter), anti-CD20-PerCP/Cy5.5, anti-CD3-FITC or anti-CD3-APC, and anti-pSTAT1-Alexa488 or anti-STAT1-Alexa647 (all from Biosciences) for 20 min at room temperature.
Samples were analyzed on an LSRII flow cytometer using FacsDiva Version 6.1.3 (BD Biosciences, San Jose, CA) and FlowJo Version 8.8.2 software (Tree Star, Ashland, OR).
Statistical analysis
D'Agostino & Pearson omnibus normality tests, Wilcoxon-signed-rank tests, Mann-Whitney tests or linear regression analyses were performed with GraphPad Prism 5.0a (GraphPad Software, La Jolla, CA). Two-sided p-values <.05 were considered significant.
Results
Effect of DCV/ASV therapy on HCV viremia and liver inflammation
All 13 HCV-infected nonresponders to PegIFN/RBV (Suppl. Table 1) experienced a rapid decrease in serum HCV RNA levels within the first two weeks of DCV/ASV therapy (P=.0038, Fig. 1A). Nine patients developed an EOT response, whereas four had virological breakthrough between weeks 4 and 12 of therapy. Seven out of nine EOT responders achieved a sustained virological response (SVR24); the remaining had not yet reached week 24 post treatment. Serum ALT, CXCL10 and CXCL11 levels decreased significantly within the first eight weeks of DCV/ASV therapy (P=.008, P=.0005 and P=.0007, Fig. 1B-D).
Figure 1. Serum HCV RNA and liver inflammation rapidly decrease with DCV/ASV therapy.
(A) Serum HCV RNA levels of patients who responded to therapy (n=9, filled circles) or had a viral breakthrough and subsequently stopped therapy (n=4, filled squares). A response to DCV/ASV therapy was defined as undetectable viremia at EOT (week 24). ‘Pre’ indicates the time point of the pre-treatment liver biopsy (1 - 4 weeks prior to therapy). L.l.o.q., lower limit of quantitation; ‘t.n.d.’, target not detected.
(B-D) Serum ALT (B), CXCL10 (C) and CXCL11 (D) levels of all patients (n=13) at week 0 and at week 8 of therapy. Filled circles, EOT responders; filled triangles, patients with virological breakthrough. The patient with increased ALT value is patient 12 in Suppl. Table 1.
Effect of DCV/ASV therapy on NK cell activation
The effect of the rapid DAA-mediated decrease in HCV titers on the activation status and function of NK cells was studied by multicolor flow cytometry. Expression of the activation marker HLA-DR, the activating receptor NKp46 and the inhibitory receptors CD85j and NKG2A were higher on NK cells of chronically HCV-infected patients prior to DAA therapy than on those of uninfected controls (Fig. 2). The expression level of HLADR, NKp46, CD85j and NKG2A normalized in patients with undetectable viremia by week 8 of therapy, reaching levels similar to those of NK cells of uninfected controls, and the decrease in expression was greater than in the 3 patients who were viremic at week 8 (Fig. 2). Because the latter were offered a rescue protocol with DCV/ASV/PegIFN/RBV, week 8 was the last time point to compare patients with and without detectable viremia in this study. Week 8 was therefore chosen to document the effect of a DAA-mediated decrease in viremia on NK cell phenotype and function.
Figure 2. NK cell activation decreases within 8 weeks of successful DCV/ASV therapy.
(A-D) Expression of the activation markers HLA-DR (A), NKp46 (B) and the inhibitory receptors CD85j (C) and NKG2A (D) on peripheral blood NK cells of chronic HCV patients prior to treatment (n=13) compared to NK cells of healthy controls (white squares, left graphs). Change in the expression of these NK cell markers in 10 patients who had undetectable viremia (middle graph) compared to 3 patients who were viremic at week 8 (right graphs). Statistical analysis: non-parametric paired Wilcoxon-signed-rank test or unpaired Mann-Whitney test. Median and IQR are shown. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8.
NKp30, NKp44, CD69, NKG2C, NKG2D, CCR5, CD300 and CD122 expression were also assessed. There was a trend of higher NKp30 expression on NK cells of HCV patients compared to those of healthy controls (P=.08), and NKp30 MFI decreased significantly within 8 weeks of DAA therapy (P=.014, not shown). In contrast, the expression level of NKp44, CD69, NKG2C, NKG2D, CCR5, CD300 and CD122 did not differ on NK cells of chronically HCV-infected patients and those of healthy controls and did not change during DCV/ASV therapy (not shown).
Effect of DCV/ASV therapy on NK cell cytotoxicity
NK cells typically exhibit increased cyototoxicity and TRAIL expression but decreased IFNγ production in chronic HCV infection 7, 9. To examine whether the DAA-induced decrease in HCV viremia modulated NK cell effector functions, PBMCs were incubated with major histocompatibility complex class I–negative K562 target cells, and the CD56dim NK cell population was assessed for cell surface expression of CD107a as a readout for degranulation and cytotoxicity. CD56dim NK cells account for 90% of all NK cells in the circulation and represent the fully mature highly cytotoxic NK cell subset 20. The frequency of CD56dim and CD56bright NK cells did not differ between HCV-infected patients and healthy controls (not shown).
The frequency of CD107a+ cells within the CD56dim NK cell population was higher in chronic HCV patients prior to therapy than in uninfected subjects (Fig. 3A, B, left graphs). A significant decrease in frequency and expression level of CD107a+ CD56dim NK cells was observed by week 8 of therapy in patients with undetectable viremia (P=.031 and P=.011, respectively, Fig. 3A, B, middle graphs), and the decrease in the percentage of CD107a+ CD56dim NK cells was greater in patients with undetectable viremia than in those who were viremic at week 8 (P=.014, Fig. 3A, right graph). The same pattern was observed in the total CD56+ NK cell population (Suppl. Fig. 1 A, B).
Figure 3. Increased NK cell cytotoxicity normalizes within 8 weeks of successful DCV/ASV therapy.
(A-E) Frequency CD107a+ cells (A), CD107a expression level (B), frequency of TRAIL+ cells (C), TRAIL expression level (D) and STAT1 expression level (E) in the CD56dim NK cell population of HCV-infected patients prior to treatment (n=13) compared to NK cells of healthy controls (white squares, left graphs). Change in these parameters in 10 patients who had undetectable viremia (middle graphs) compared to 3 patients who were viremic at week 8 (right graphs).
(F) Frequency of pSTAT1+ cells and pSTAT1 expression level in the CD56dim NK cell population of HCV-infected patients prior to treatment (n=13) compared to NK cells of healthy controls (left graphs). Change in these parameters in 10 patients who had undetectable viremia at week 8 (right graphs). Statistical analysis: non-parametric paired Wilcoxon-signed-rank test or unpaired Mann-Whitney test. Median and IQR are shown. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8. MFI, mean fluorescence intensity.
The frequency of TRAIL+ cells and the TRAIL expression level in the CD56dim NK cell subset also decreased significantly during the first week 8 of therapy (P=.012 and P=.004, Fig. 3C, D, left graphs), and the decrease in the frequency of TRAIL+ cells was greater in patients with undetectable viremia at week 8 than in those who had experienced virological breakthrough (P=.014, Fig. 3C, right graph). The decrease in TRAIL expression followed this trend (P=.077, Fig. 3D, right graph). Similar results were observed for the CD56bright NK cell subset (not shown), in which TRAIL is highly expressed 21. Specifically, the median frequency of TRAIL+ cells in the CD56bright NK cell subset decreased from 45.7%, (IQR 23.2-58.5%) prior to therapy to 22.2% (IQR 11.4-47%, P=.027), and median TRAIL expression in CD56bright NK cells decreased from 220 (IQR 166-233) to 164.5 (IQR 32-210, P=.002) during the first 8 weeks of therapy in patients with undetectable viremia (not shown).
Increased NK cell cytotoxicity in chronic HCV infection is thought to be driven by chronic exposure to virus-induced endogenous type I IFN 6, 7, 22. We therefore examined whether the DCV/ASV-induced decrease in HCV titer resulted in decreased expression of STAT1 and its phosphorylated form pSTAT1. The expression level of STAT1, which itself is the product of an ISG, was significantly higher in CD56dim NK cells of chronic HCV patients than in those of uninfected subjects (P=.003) but decreased by week 8 of therapy (P=.02, Fig. 3E). Again, the absolute decrease in STAT1 expression was greater in NK cells of patients with undetectable viremia than in those with viremia at week 8 of therapy (P=.037, Fig. 3E). Likewise, the frequency of pSTAT1-expressing cells in the CD56dim NK cell subset and the pSTAT1 expression level per cell decreased significantly in patients who had undetectable viremia at week 8 of therapy (P=.002 and P=.006, respectively, Fig. 3F).
Effect of DCV/ASV therapy on NK cell cytokine production
To assess the capacity of NK cells to produce IFNγ and TNFα, PBMCs were stimulated with IL-12 and IL-15 in vitro and the CD56bright NK cell subset, which constitutes the main source of NK cell-derived cytokines 22, was studied by flow cytometry. As shown in figure 4A the percentage of IFNγ-producing cells in the CD56bright NK cell subset and the IFNγ expression level were significantly lower in chronic HCV patients compared to uninfected subjects (P=.007 and P=.008, respectively) and increased within the first 8 weeks of therapy in patients with undetectable viremia (P=.008 and P=.002, respectively; Fig. 5A, B). These observations were consistent with changes in the frequency of TNFα+ cells and IFNγ+/TNFα+ cells in the CD56bright NK cell subset (Fig. 4C, D) and were also confirmed in the total NK population (Suppl. Fig. 1C-F).
Figure 4. Decreased IFNγ and TNFα production by NK cells is restored within 8 weeks of successful DCV/ASV therapy.
(A) Frequency of IFNγ+ cells, (B) IFNγ expression level, and frequency of (C) TNFα+ and (D) IFNγ+TNFα+ in the CD56bright NK cell population of chronic HCV patients prior to treatment (n=13) compared to NK cells of healthy controls (white squares, left graphs). Change in these parameters in 10 patients who had undetectable viremia at week 8 (right graphs). Statistical analysis: non-parametric paired Wilcoxon-signed-rank test or unpaired Mann-Whitney test. Median and IQR are shown. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8. MFI, mean fluorescence intensity.
Figure 5. Activation and TRAIL expression of intrahepatic CD56dim NK cells decrease within 2-4 weeks of DCV/ASV therapy in parallel to decreasing viremia and liver inflammation.
(A-B) Comparison of frequency and expression level of CD69+ (A) and NKp46+ (B) cells in peripheral and intrahepatic CD56dim NK cell populations of chronically HCV-infected patients prior to therapy.
(C-D) Frequency and expression level of TRAIL+ (C) and NKp46+ (D) CD56dim NK cells in paired liver biopsies prior to (‘Pre’) and, depending on randomization, either at week 2 or at week 4 of therapy. Horizontal marks identify week 2 biopsies. Statistical analysis: non-parametric paired Wilcoxon-signed-rank test. Filled circles, EOT responders; filled triangle, patient with virological breakthrough after week 8. MFI, mean fluorescence intensity.
Effect of DCV/ASV therapy on intrahepatic NK cells
Next, we examined whether these results extended to the liver. NK cells were studied in paired liver biopsies and blood samples prior to DCV/ASV and, depending on randomization, at week 2 or 4 of DCV/ASV therapy. No patient had experienced a viral breakthrough at the time point of the second biopsy.
The frequency of CD69+ and NKp46+ cells in the CD56dim NK cell population and the MFI of these markers were significantly higher in the liver than in the blood prior to DCV/ASV therapy (Fig. 5A, B), indicating that NK cells were more activated at the site of infection. In contrast, the frequency of TRAIL+ CD56dim NK cells and the TRAIL expression level per cell did not differ between both compartments (data not shown). The HCV titer prior to DCV/ASV therapy correlated with the frequency of TRAIL+ CD56bright NK cells in the pretreatment biopsy (P=.03, r=0.64, data not shown). Accordingly, the frequency of TRAIL+ CD56dim NK cells in the liver and the TRAIL expression level per cell were lower in the on-treatment biopsy (P=.0005 and P=.048, respectively, Fig. 5C) that was taken after a significant decline in viremia had occurred (Fig. 1A). A similar decrease was observed in the frequency and the expression level of TRAIL+ cells in the intrahepatic CD56bright NK cell population (median frequency of 37% prior to treatment compared to 13% at week 2/4, P=.004; median MFI of 113 prior to treatment compared to 73 at week 2/4, P=.027, not shown).
The frequency of NKp46+ cells and the NKp46 expression level in the intrahepatic CD56dim NK cell population decreased as well (P=.027 and P=.006, respectively, Fig. 5D). Collectively, these data indicate a decrease in NK cell activation and normalization of NK cell function in the blood and in the liver during DAA therapy.
NK cell functions normalize in a sequential manner
To investigate how early during DCV/ASV therapy the observed changes in NK cell activation and function occur, we tested PBMC at 24 hours and at 2 weeks of therapy. All patients were included in this analysis because they all exhibited a significant decrease in viral titer during this time period (Fig. 1A). As shown in figure 6A, expression of the activation marker HLA-DR on the total NK cell population decreased within 24h of therapy initiation (P=.005). None of the other cell surface markers (NKp46, NKG2A, CD85j), that were differentially expressed on NK cells of HCV-infected patients and healthy controls, changed during the first two weeks of treatment (not shown). Further, the rapid decrease in HLA-DR expression during the first 24 hours of DCV/ASV therapy was not associated with an immediate restoration of NK cell function. IFNγ production did not significantly improve during the first 24 hours, but normalized by week 2 of therapy (P=.006, Fig. 6B). Markers of NK cell cytotoxicity, such as expression of the degranulation marker CD107 (Fig. 6C) and TRAIL (not shown) did not change during the first 2 weeks of therapy).
Figure 6. NK cell functions normalize in a sequential manner during DCV/ASV therapy.
Changes in HLA-DR expression in all CD56+ NK cells (A) and in the frequency of IFNγ+ CD56bright (B) and CD107+ CD56dim (C) NK cells during the first 24 hours (left graphs) and the first 2 weeks (right graphs) of therapy.
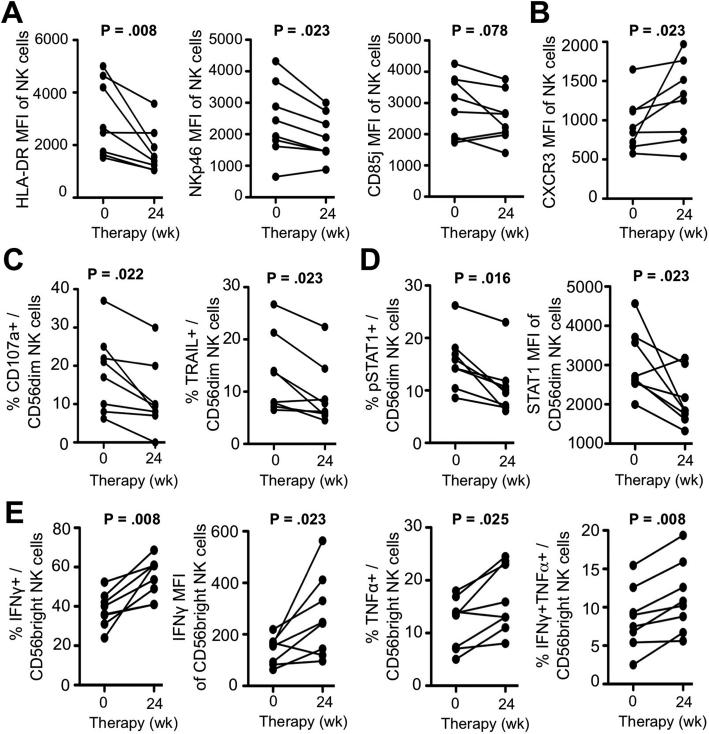
Normalized NK cell function is maintained in EOT responders
Finally, we examined whether the restoration of NK cell phenotype and function were maintained. As shown in figure 7A, HLA-DR, NKp46 and CD85j expression levels on peripheral blood NK cells were lower at week 24 (EOT) compared to week 0 (P=.008, P=.023 and P=.078, respectively). Interestingly, expression of the chemokine receptor CXCR3 significantly increased from week 0 to week 24 (P=.023, Fig.7B). This is consistent with lack of CXCR3 stimulation because the serum concentration of the CXCR3 ligands CXCL10 and CXCL11 had decreased by week 8 of therapy (Fig. 1C,D).
Figure 7. Normalized NK cell phenotype and function are maintained in EOT responders to DCV/ASV therapy.
(A-B) Expression of the activation marker HLA-DR, the activating receptor NKp46, the inhibitory receptor CD85j (A) and the chemokine receptor CXCR3 (B) on peripheral blood CD56+ NK cells of EOT responders at weeks 0 and 24 of therapy.
(C-D) Frequency of CD107a+ and TRAIL+ cells (C) and frequency of pSTAT1+ cells and STAT1 expression level (D) in the CD56dim NK cell population of EOT responders at weeks 0 and 24 of therapy.
(D) in the CD56dim NK cell population of EOT responders at weeks 0 and 24 of therapy.
(E) Frequency of IFNγ+, TNFα+ and IFNγ+TNFα+ cells and IFNγ expression level in the peripheral blood CD56bright NK cell population of EOT responders at weeks 0 and 24 of therapy. Statistical analysis: non-parametric paired Wilcoxon-signed-rank test.
Confirming the week 8 data (Fig. 3), the frequency of CD107+ and TRAIL+ cells in the CD56dim NK cell subset (P=.022 and P=.023, respectively, Fig. 7C) and in the total NK cell population (P=.016 and P=.039, respectively, Suppl. Fig. 2A) were significantly lower at week 24 than prior to therapy. Likewise, the frequency of pSTAT1-expressing cells and the expression level of the ISG STAT1 in the CD56dim NK cell subset had decreased during the 24-week treatment course (P=.016 and P=.023, respectively, Fig. 7D).
The normalization of NK cell cytokine production was confirmed at the week 24 time point, with significantly increased frequencies of IFNγ, TNFα and IFNγ/TNFα producing cells and IFNγ MFI in both the CD56bright (Fig. 7E) and the total NK cell populations (Suppl. Fig. 2C). Collectively, these results indicate that effective removal of HCV by an IFN-free DAA regimen normalizes both phenotype and function of NK cells.
Discussion
NK cells constitute the main innate immune cell population in the liver. Their activation in chronic HCV infection is associated with increased cytotoxic functions, as assessed by TRAIL expression and degranulation, and with decreased production of the antiviral cytokine IFNγ 7, 9. It has previously been suggested that prolonged exposure to low levels of HCV-induced IFNα are responsible for this phenotype 7. However, IFNα protein has rarely and only at exceedingly low levels been detected in patients 23 and chimpanzees 24 with HCV infection. Moreover, a similar NK cell phenotype has also been reported in patients with chronic hepatitis B virus infection 9, a disease which does not induce many ISGs in the liver 25. Interferon free therapy regimens for HCV infection provide a unique opportunity to study the interaction between HCV and the intrahepatic immune system because these regimens rapidly decrease viremia to undetectable levels.
The current study shows that DCV/ASV-mediated HCV clearance is associated with a decrease in NK cell activation and a normalization of NK cell cytotoxic effector functions to levels that observed in uninfected subjects. The rapid normalization of the NK cell phenotype is associated with a decrease in the percentage of pSTAT1-expressing NK cells and a decrease in the expression level of the ISG STAT1 in NK cells. Collectively, these results confirm that type I IFN-mediated NK cell activation via the IFNα/β receptor is indeed responsible for the alteration of NK cell function in chronic HCV infection. Thus, the phenotypic and functional NK cell profile that is observed in chronic HCV infection and exacerbated during IFN-based therapy, is normalized in IFN-free DAA therapy. This is of interest in the context of recent findings in mouse model of chronic hepatitis induced by lymphocytic choriomeningitis virus. Blocking of the IFNα/β receptor increased the number of NK cells and virus-specific CD4 T cells and restored systemic IFNγ levels, thereby inducing virus clearance 26, 27.
Overall, normalization of NK cell phenotype and function followed a hierarchy. While a decrease in the expression level of the activation marker HLA-DR was observed within 24h of DCV/ASV therapy in parallel to a significant decrease in HCV titer, changes in NK cell function were not observed during this time period. The frequency of IFNγ+ NK cells increased significantly by week 2 of therapy, whereas the decrease in the frequency of CD107a+ NK cells became significant only after week 2. Thus, altered cytokine production by NK cells appears to be more readily reversible than altered cytotoxicity. Importantly, all NK cell readouts were indistinguishable from those of healthy uninfected subjects by week 8 of DCV/ASV therapy and confirmed at the week 24 time point in EOT responders.
Because we studied a selected group of patients who were all infected with HCV genotype 1b and nonresponders to previous PegIFN/RBV therapy we performed a detailed characterization of this cohort's NK cell phenotype in comparison to published literature. We confirm earlier studies on increased NKp46 and NKG2A expression on NK cells in HCV infection 7, and in addition report increased expression of the inhibitory receptor CD85j and the activation marker HLA-DR. Increased NKp30 expression in our cohort is consistent with results of de Maria et al. 28, who reported higher NKp30 expression on NK cells of HCV-infected patients than those of uninfected controls. In contrast, Nattermann et al. 29 reported lower NKp30 expression, but in a patient cohort with more diverse HCV genotypes. Importantly, expression of NKp30, NKp46, HLA-DR, NKG2A and CD85j normalized during successful IFN-free DAA therapy in our study.
NKp44 and NKG2C expression did not differ between NK cells of HCV-infected patients and healthy donors in our study, which is consistent with published literature 7, 29. Likewise, NKG2D expression did not differ between NK cells of HCV patients and healthy controls, and there was no change in expression during DAA therapy. This is consistent with reports by de Maria 28 and Nattermann 29 who found no differential expression of NKG2D on NK cells of HCV patients and healthy controls. In contrast, Oliviero 9 reported increased whereas Dessouki 30 reported decreased NKG2D levels in HCV infection. However, the patient cohort studied by Oliviero et al included responders to IFN-based therapy, and the patients were infected with diverse genotypes and had lower HCV viremia and ALT values than in our study. Dessouki et al. studied only the frequency of NKG2D+ NK cells and not the NKG2D expression level.
We propose that the restoration of NK cell function is the result of rapid removal of the HCV and its associated IFN signature, and therefore should also be seen with other direct acting antivirals. It should also extend to other HCV genotypes, as far as they are not associated with increased incidence of breakthrough or relapse. The restoration of NK cell function, in particular the normalization of suppressed IFNγ and TNFα production, may be relevant also for studies on adaptive immune responses. Similar to NK cells, HCV-specific T cells display impaired cytokine production in chronic HCV infection 12, 13. While the proliferation of HCV-specific T cells has recently been reported to improve during antiviral therapy with the DAAs faldaprevir and deleobuvir 14 it has not yet been reported to which extend cytokine production of HCV-specific T cells can be recovered. The current data on recovery of IFNγ and TNFα production by NK cells raise hope that this may also occur for T cells. Restoration of cytokine production, may result in better immune surveillance and prevention of virological relapse, which will be interesting topic to address in future studies.
Supplementary Material
Acknowledgments
The authors thank Bristol-Myers Squibb for providing the medication for this study; Nevitt Morris, Liver Diseases Branch, NIDDK and Thomas Lewis, NIH Department of Transfusion Medicine for support; Heiyoung Park and Jens M Werner for discussion; and all patients for participating in this study.
Financial Support: This study was supported by the NIDDK, NIH intramural research program.
Abbreviations
- ASV
asunaprevir
- DCV
daclatasvir
- DAA
direct acting antivirals
- HCV
hepatitis C virus
- IFNγ
interferon gamma
- NK
natural killer
- PBMCs
peripheral mononuclear cells
- RBV
ribavirin
- TNFα
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: ES and BR designed the immunological study; MG and TJL designed the clinical study. ES, XCL, MK and BR performed experiments. YK, NF and MG enrolled and treated the patients. ES and BR wrote the manuscript, which was critiqued by all authors.
Financial Disclosures and Conflict of Interest Statement: The authors declare that they have no competing interests.
Author names in bold designate shared co-first authors. Doherty DG, O'Farrelly C., Edlich B, Ahlenstiel G, Zabaleta Azpiroz A, Oliviero B, Varchetta S, Ahlenstiel G, Edlich B, Miyagi T, Shimizu S, Teijaro JR, Ng C
References
- 1.Rehermann B. Pathogenesis of chronic viral hepatitis. Differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarasin-Filipowicz M, Oakeley EJ, Duong FH, et al. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A. 2008;105:7034–9. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askarieh G, Alsio A, Pugnale P, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C Virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51:1523–1530. doi: 10.1002/hep.23509. [DOI] [PubMed] [Google Scholar]
- 4.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunological Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 5.Miyagi T, Takehara T, Nishio K, et al. Altered interferon-alpha-signaling in natural killer cells from patients with chronic hepatitis C virus infection. J Hepatol. 2010;53:424–30. doi: 10.1016/j.jhep.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Edlich B, Ahlenstiel G, Zabaleta Azpiroz A, et al. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology. 2012;55:39–48. doi: 10.1002/hep.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlenstiel G, Titerence RH, Koh C, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–35. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest. 2010;40:851–63. doi: 10.1111/j.1365-2362.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- 9.Oliviero B, Varchetta S, Paudice E, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–60. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 10.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. The New Engl J Med. 2013;368:1907–17. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang TJ, Ghany MG. Therapy of hepatitis C-back to the future. N Engl J Med. 2014;370:2043–7. doi: 10.1056/NEJMe1403619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedemeyer H, He XS, Nascimbeni M, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 13.Bengsch B, Seigel B, Ruhl M, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin B, Hennecke N, Lohmann V, et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014;61:1212–9. doi: 10.1016/j.jhep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. Hepatology. 2015;61:712–21. doi: 10.1002/hep.27323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dill MT, Makowska Z, Trincucci G, et al. Pegylated IFN-alpha regulates hepatic gene expression through transient Jak/STAT activation. J Clin Invest. 2014;124:1568–81. doi: 10.1172/JCI70408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlenstiel G, Edlich B, Hogdal LJ, et al. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–9. doi: 10.1053/j.gastro.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliviero B, Mele D, Degasperi E, et al. Natural killer cell dynamic profile is associated with treatment outcome in patients with chronic HCV infection. J Hepatol. 2013;59:38–44. doi: 10.1016/j.jhep.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moretta L, Bottino C, Pende D, et al. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32:1205–1211. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Stegmann KA, Bjorkstrom NK, Veber H, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–97. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 22.Miyagi T, Shimizu S, Tatsumi T, et al. Differential alteration of CD56(bright) and CD56 (dim) natural killer cells in frequency, phenotype, and cytokine response in chronic hepatitis C virus infection. J Gastroenterol. 2011;46:1020–30. doi: 10.1007/s00535-011-0408-8. [DOI] [PubMed] [Google Scholar]
- 23.Meissner EG, Wu D, Osinusi A, et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124:3352–3363. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin EC, Seifert U, Kato T, et al. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–14. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wieland S, Thimme R, Purcell RH, et al. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–74. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson EB, Yamada DH, Elsaesser H, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–7. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teijaro JR, Ng C, Lee AM, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–11. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Maria A, Fogli M, Mazza S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–55. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 29.Nattermann J, Feldmann G, Ahlenstiel G, et al. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–77. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dessouki O, Kamiya Y, Nagahama H, et al. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: Reversion by anti-viral treatment. Biochem Biophys Res Commun. 2010;393:331–7. doi: 10.1016/j.bbrc.2010.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.