Summary
Many tumors become addicted to autophagy for survival, suggesting inhibition of autophagy as a potential broadly-applicable cancer therapy. ULK1/Atg1 is the only serine/threonine kinase in the core autophagy pathway and thus represents an excellent drug target. Despite recent advances in the understanding of ULK1 activation by nutrient deprivation, how ULK1 promotes autophagy remains poorly understood. Here, we screened degenerate peptide libraries to deduce the optimal ULK1 substrate motif and discovered fifteen phosphorylation sites in core autophagy proteins that were verified as in vivo ULK1 targets. We utilized these ULK1 substrates to perform a cell-based screen to identify and characterize a potent ULK1 small molecule inhibitor. The compound SBI-0206965 is a highly selective ULK1 kinase inhibitor in vitro and suppressed ULK1-mediated phosphorylation events in cells, regulating autophagy and cell survival. SBI-0206965 greatly synergized with mTOR inhibitors to kill tumor cells, providing a strong rationale for their combined use in the clinic.
Introduction
Autophagy is a central cellular mechanism for the elimination of damaged proteins, protein complexes, and organelles. This evolutionarily conserved process plays a crucial role in the cellular response to nutrient deprivation as well as other stresses, in addition to being required for proper cellular and tissue homeostasis during embryonic development and defense against pathogens. Defects in autophagy pathways have been associated with a number of human pathologies, including infectious diseases, neurodegenerative disorders, and cancer (Green and Levine, 2014). In spite of these highly conserved fundamental cellular functions, the molecular and biochemical details of how autophagy is initiated for different cargoes as well as the coordination of steps starting with autophagosome induction to ultimate fusion with the lysosome remain poorly understood.
Pioneering studies in budding yeast first defined 36 core genes required for autophagy (Atg), most of which are conserved in mammals (Tsukada and Ohsumi, 1993). One of the most upstream components of the pathway in yeast is the ATG1 gene, which is notable for being the only core autophagy gene to encode a serine/threonine kinase. Atg1 forms a complex with multiple regulatory subunits, including Atg13 and Atg17. In mammals, there are two Atg1 homologs, ULK1 and ULK2, which similarly bind to an Atg13 homolog and an Atg17-like protein, FIP200 (Chan, 2009). The ULK1 kinase complex is activated in response to nutrient deprivation and serves as a critical initiator of starvation-induced autophagy. Whether the ULK1 complex is needed for bulk steady-state autophagy that some cell types undergo remains unclear. Moreover, it has been reported that certain forms of selective autophagy proceed without involvement of the ULK1 complex (Cheong et al., 2011), presumably at least in part via direct signaling to the downstream Vps34/Beclin1 complex.
The requirement for ULK1 in autophagy initiation has been most extensively studied in the context of nutrient deprivation. The mechanistic target of rapamycin complex 1 (mTORC1) is a serine/threonine kinase complex that is inhibited by a wide-variety of cellular stresses and as such serves as a central integrator that coordinates cell growth and catabolism under nutrient replete conditions. Studies in S. cerevisiae, D. melanogaster, and mammalian cells have demonstrated that mTOR acutely inhibits Atg1/ULK1 function through phosphorylation of Atg1/ULK1 and/or some of its associated regulatory subunits (Chan, 2009) Beyond this inhibitory event, ULK1 also receives positive inputs from the cellular energy sensor AMP-activated protein kinase (AMPK), which is activated in response to cellular stresses that lower intracellular ATP levels, such as is caused by glucose or oxygen deprivation as well as mitochondrial insults (Egan et al., 2011b). While AMPK activates ULK1 by phosphorylating at least four sites (Egan et al., 2011a), mTORC1 inactivates ULK1 by phosphorylating a single site, Ser757 (Kang et al., 2013; Kim et al., 2011). As several physiological stresses result in both AMPK activation and mTOR inhibition, ULK1 activation proceeds through positive signals from AMPK and loss of inhibitory signals from mTORC1 (Egan et al., 2011a). It is notable however that mTORC1 is inactivated by many stresses beyond just those affecting AMPK, and the data to date suggest that pharmacological suppression of mTORC1 is sufficient to induce ULK1 kinase activity (Russell et al., 2013).
While mTOR inhibitors have been proposed as general anti-cancer agents due to the requirement of mTOR signaling for cell growth in most tumor settings, it is notable that mTOR inhibitors are largely cytostatic in clinical use (Li et al., 2014). Autophagy is a well-established cell survival mechanism that promotes ATP generation by recycling metabolites when nutrients are low. Given that mTOR inhibition will lead to ULK1-dependent autophagy, it has been hypothesized that ULK1 activation and the subsequent induction of autophagy mediates some of the pro-cell survival effects that occur in response to these drugs (Jiang et al., 2015). As such, combining ULK1 inhibition with mTOR inhibition would be predicted to convert this cytostatic outcome to a cytotoxic one.
Given that autophagy can promote cell survival following a variety of cellular stresses, including in tumor cells that experience considerable ongoing metabolic stress, several labs have investigated whether autophagy inhibition, through genetic and pharmacologic means, holds utility for the treatment of cancer (Guo et al., 2013; Jiang et al., 2015; White, 2015), and recent data have suggested that lung cancers in particular may be selectively dependent on autophagy (Karsli-Uzunbas et al., 2014). However, it is clear that the role of autophagy in tumor initiation, progression, and resistance to treatment is complex and context specific. It has been suggested that at the early stages of tumor formation the enhancement of autophagic flux may be therapeutically beneficial by clearing mutant or misfolded proteins and alleviating cellular stress (White, 2015). Conversely, once a solid tumor is established and “rewiring” of metabolic signaling pathways has occurred in the nutrient deprived tumor core, autophagy may be upregulated to allow for cell survival under nutrient-poor conditions, posing a barrier to treatment (Kroemer, 2015). To date, most clinical efforts have focused on using the general autophagy inhibitors chloroquine and hydroxychloroquine, either as standalone agents or in combination with other anti-cancer therapeutics such as mTOR inhibitors for the aforementioned reasons (Rangwala et al., 2014). However, the development of potent and selective autophagy inhibitors has remained largely elusive, in part because the majority of the core autophagy proteins that have been screened for small molecules lie far downstream in the pathway and are not readily druggable enzymes. As ULK1 is a potentially druggable serine/threonine kinase representing one of the first biochemical steps of autophagy, it therefore represents an attractive target for modulation by small molecules (Jiang et al., 2015). Using our identified in vivo ULK1-dependent phosphorylation events, we report here the discovery and characterization of SBI-0206965, a potent and specific small molecule ULK1 kinase inhibitor. We demonstrate the ability of this compound to suppress ULK1 downstream phosphorylation events in cells and reveal therapeutic potential for this agent in combination with mTOR inhibitors.
RESULTS
Determination of the ULK1 kinase Consensus Phosphorylation Site
To identify additional substrates of ULK1 that may be important for the control of autophagy, we identified an optimal ULK1 phosphorylation consensus motif using arrayed degenerate peptide libraries, as we have previously performed for AMPK and AMPK-related kinases (Goodwin et al., 2014; Gwinn et al., 2008). To generate active ULK1 for these experiments, epitope-tagged ULK1 was co-expressed with its subunits FIP200 and Atg13 in HEK-293T cells and peptide eluted from affinity resin. The purified ULK1 complex exhibited robust kinase activity towards a known substrate, Atg13, in a dose-responsive fashion (Figure S1A). We used the purified ULK1 complex to screen a peptide library to determine its preferred sequence surrounding the phosphorylation site (Figure 1A). The results obtained with ULK1 correlate well with recent data on the peptide substrate specificity of the budding yeast ortholog of ULK1, Atg1 (Papinski et al., 2014). Unlike other Ser/Thr kinases (Miller et al., 2008; Turk, 2008) that phosphorylate sites near charged residues or proline, ULK1 had an unusual preference for hydrophobic residues at multiple positions surrounding the phosphorylation site. In particular, ULK1 strongly preferred a Leu or Met residue at position -3, while both aliphatic and aromatic hydrophobic residues were selected in the +1 and +2 positions. In addition, ULK1 strongly prefers Ser over Thr as the phosphoacceptor residue (Figure 1B). A consensus peptide substrate (ULKtide) that incorporated residues selected at each position flanking the phosphorylation site was efficiently phosphorylated by ULK1 in vitro. Starting with this peptide, we substituted key residues in ULKtide and measured the ability of our purified ULK1 complex to utilize these mutant peptides as substrates in an in vitro kinase assay. These experiments confirmed the importance of the -3, +1, and +2 positions for maximal phosphorylation efficiency (Figure 1C).
Figure 1. Determination of the optimal ULK1 consensus phosphorylation motif.

(A) A positional scanning peptide library (PSPL) approach was used to define the optimal ULK1 consensus phosphorylation motif. A spatially arrayed PSPL was subjected to in vitro phosphorylation using active ULK1 and radiolabeled ATP. Each peptide contained a fixed residue at one of nine positions relative to the centrally fixed phosphoacceptor (an equal mix of Ser and Thr), with the remaining positions being equimolar mixtures of the 17 amino acids (excluding Ser, Thr, and Cys). Aliquots of each reaction were spotted onto an avidin membrane, which was washed, dried, and exposed to a phosphor-storage screen to quantify radiolabel incorporation into each peptide. The heat map was generated in Microsoft Excel using normalized and log transformed signals corresponding to peptides with the indicated amino acid at the indicated position.
(B) Scaled sequence logo showing ULK1 sequence specificity obtained from the PSPL data. ULK1 prefers hydrophobic residues at the –3, +1, and +2 positions. The logo was generated using EnoLogos, with the height of the letter being proportional to the signal for the corresponding amino acid at the indicated position.
(C) Rates of ULK1 phosphorylation for ULKtide variants with individual amino acid substitutions are shown (normalized to unmodified ULKtide peptide). ULKtide was estimated to have a 15 μM Km. Peptide phosphorylation was assayed at a 5 μM concentration using a radiolabeled kinase assay. Incorporation of γ-32P-ATP into these peptides was determined by a phosphocellulose filter-binding assay.
Identification of ULK1 substrates
A position-specific scoring matrix based on ULK1 peptide substrate specificity (Figure 1B) was used to bioinformatically search the human proteome for sites closely matching the ULK1 substrate consensus motif (Obenauer et al., 2003). We chose to focus first on those candidate substrates with highly conserved roles in autophagy, as a subset of core autophagy pathway components bears multiple ULK1 consensus sites. To define ULK1 phosphorylation sites in vivo, we took advantage of the fact that ULK1 is constitutively active when overexpressed in HEK-293T cells. We therefore compared global phosphorylation events on epitope-tagged candidate targets when co-expressed with wild-type (WT) ULK1 or a kinase-dead (KD) version. Using mass spectrometry, we identified phosphopeptides derived from each candidate protein under these conditions. These experiments revealed that in a subset of proteins bearing multiple ULK1 consensus sites, peptides from these candidate substrates were isolated that were highly phosphorylated in the presence of co-expressed WT but not KD ULK1. We first focused on the components of the ULK1 kinase complex itself, including FIP200, Atg13, and Atg101 (Figure 2 and Figure S2).
Figure 2. Identification of novel ULK1-dependent phosphorylation sites in vivo.
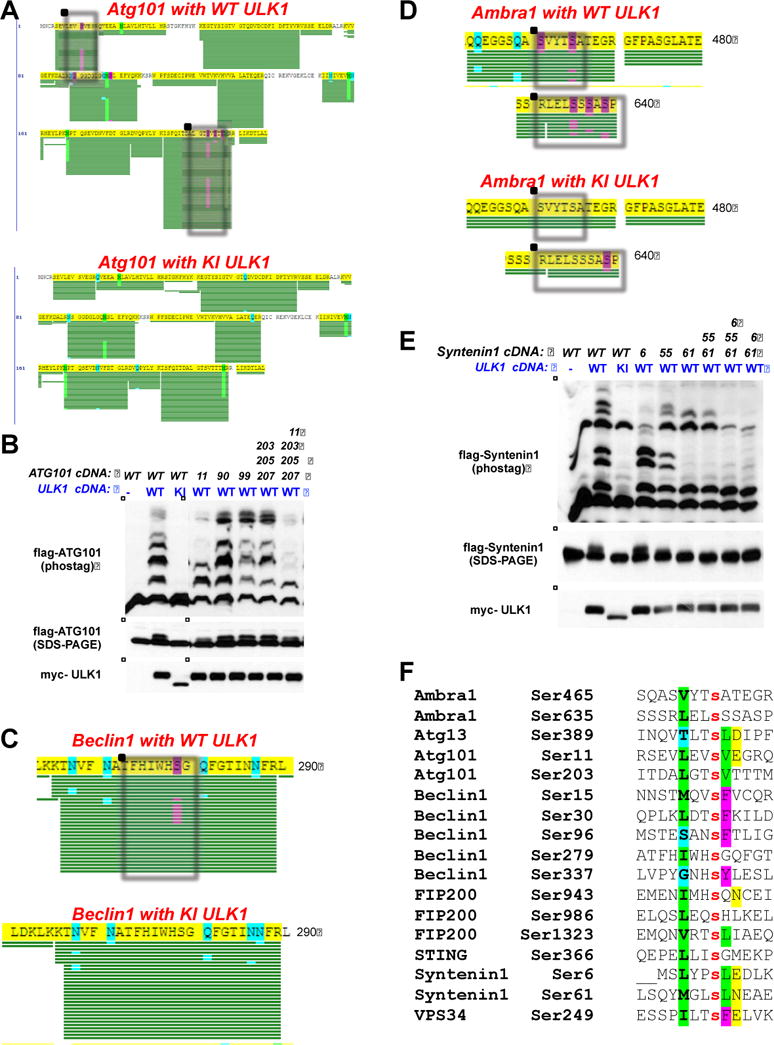
(A) Myc-tagged wild-type ULK1 (WT ULK1; top) or Myc-tagged kinase-inactive ULK1 (KI ULK1; bottom) was transfected into HEK-293T cells along with wild-type Flag-tagged Atg101 (Flag-Atg101) and immunoprecipitated with M2 agarose. The immunoprecipitate was run out on an SDS-PAGE gel, stained with coomassie, and the band corresponding to Flag-Atg101 was cut out, isolated, and subjected to tryptic digest and LC/MS/MS analysis. The phosphorylated sites that conform to the optimal ULK1 phosphorylation motif that were identified by this analysis are boxed. Green bars indicate peptide coverage, and purple highlights indicate phosphorylation events.
(B) WT ULK1 or KI ULK1 was transfected into HEK-293T cells along with Flag-Atg101 or Flag-Atg101 serine-to-alanine point-mutants. The specific mutants used in this analysis are indicated by the position(s) of the substituted amino acid (top). Cellular lysates were isolated 24 hours post-transfection, run out on an SDS-PAGE gel containing the Phos-Tag reagent (middle) or a standard SDS-PAGE gel lacking the Phos-Tag reagent (bottom), and transferred to PVDF membranes, which were subsequently immunoblotted with the indicated antibodies.
(C) Same as 2A except using wild-type Flag-tagged Beclin1 as a substrate.
(D) Same as 2A except using wild-type Flag-tagged Ambra1 as a substrate.
(E) Same as 2B except using wild-type Flag-tagged Syntenin-1 (Flag-Syntenin-1) or Flag-tagged Syntenin-1 serine-to-alanine point mutants. The specific mutants used in this analysis are indicated by the position(s) of the substituted amino acid (top). Cellular lysates were isolated 24 hours post-transfection, run out on an SDS-PAGE gel containing the Phos-Tag reagent (middle) or a standard SDS-PAGE gel lacking the Phos-Tag reagent (bottom), and transferred to PVDF membranes, which were subsequently immunoblotted with the indicated antibodies.
(F) Alignment of all novel ULK1 phosphorylation sites identified in this analysis, alongside the STING phosphorylation site which was previously reported as a ULK1 site (Konno et al., 2013). Phosphorylation sites that contain residues conforming to the optimal ULK1 phosphorylation motif at the –3 (green), +1 (green), and +2 (yellow) positions are highlighted.
Atg101 was first identified in a mass spectrometry analysis of ULK1 interactors and found to encode a highly conserved integral component of the ULK1/FIP200/Atg13 complex in mammalian cell immunoprecipitations (Hosokawa et al., 2009; Mercer et al., 2009). Atg101 binds directly to Atg13 and is critical for Atg13 stabilization and its resultant stimulation of ULK1 kinase activity. To map potential ULK1-dependent phosphorylation events in Atg101, we co-expressed Flag-tagged Atg101 with WT or KD ULK1 and performed MS/MS analysis of total peptides in the Flag-Atg101 immunoprecipitates to map total phosphorylation sites in Atg101 under the two conditions. We observed that two specific serine sites (Ser11, Ser203) within human Atg101 were stoichiometrically phosphorylated in cells co-expressing WT but not KD ULK1 (Figure 2A). Notably. these two ULK1-dependent phosphorylation sites conform well to the optimal ULK1 substrate motif, in particular having hydrophobic residues at the -3 and +1 positions, which suggests that they may be direct ULK1 substrates in vivo. To further explore ULK1 phosphorylation of Atg101 in cells, we examined the migration pattern of Atg101 during SDS-PAGE in gels containing the Phos-Tag reagent, a phosphate binding dinuclear metal complex that specifically retards the mobility of phosphoproteins (Kinoshita et al., 2006). Comparing the mobility pattern of Atg101 on a Phos-Tag gel revealed a robust mobility change suggestive of phosphorylation (Figure 2B) when co-overexpressed in HEK-293T cells with WT ULK1. Mutation of Atg101 Ser11 abolished much of the mobility change, and this was further enhanced by additional mutation of Ser203 and nearby sites, which corroborated their identification as potential ULK1-dependent sites by mass spectrometry. We next performed a similar analysis of FIP200 and Atg13 phosphorylation events, and we discovered multiple serine sites in FIP200 and Atg13 bearing the ULK1 substrate consensus whose phosphorylation was induced by overexpressed WT ULK1 in cells (Figure S2A, S2B).
Next, we examined components of the Beclin1/Vps34 complex, which lies directly downstream of the ULK1 complex in autophagy initiation (Kim and Guan, 2015). Similar to Atg101, mass spectrometry identified sites of phosphorylation in Beclin1 induced by co-expression of WT but not KD ULK1 in HEK-293T cells, and those sites most extensively phosphorylated in this context matched the ULK1 optimal substrate sequence, suggesting that Beclin1 may also be a direct ULK1 substrate (Figure 2C). Further analysis identified additional serine residues in Beclin1 that conform to the optimal ULK1 phosphorylation motif that were not observed by mass spectrometry analysis, and some of these contributed to Beclin1 mobility changes on Phos-Tag gels (Figure S2C). One of these sites, Ser15, was recently identified as a site of ULK1 phosphorylation and was reported to play a conserved role in autophagy induction (Russell et al., 2013). We found that mutation of at least three of these serine residues (Ser15, Ser30, Ser337) was required to prevent the observed mobility shift on Phos-Tag gels (Figure S2C). Examination of another component of the Beclin1 complex, Ambra1, also revealed multiple ULK1-dependent phosphorylation events in cells, suggesting that many components of the Beclin/Vps34 complex may be targeted by ULK1 (Figure 2D). Finally, we examined a known ULK1 interacting protein, Syntenin-1, which was also reported as an ULK1 substrate (Rajesh et al., 2011). Here, we found that the previously reported in vitro phosphorylation site, Ser6, along with a second site, Ser61, are jointly responsible for altered mobility of Syntenin-1 in the presence of ULK1 in vivo (Figure 2E). Collectively, the majority of the candidate ULK1 phosphorylation sites we identified across these substrates contain hydrophobic residues in the -3 position (Figure 2F), consistent with our identified optimal ULK1 substrate sequence (Figure 1B) and with another site recently reported in another ULK1 substrate, STING (Konno et al., 2013). In contrast with the preferred peptide sequence, hydrophobic residues were not over-represented at the +2 position, possibly because short sequence motifs bearing three hydrophobic residues in close proximity might be inaccessible to phosphorylation by kinases.
In contrast to the majority of the substrates we examined that contained between two to four ULK1-dependent phosphorylation sites, one protein, the lipid kinase Vps34, apparently contained only a single ULK1 phosphorylation site (Figure 2F). The highly conserved Ser249 of Vps34 was stoichiometrically phosphorylated in HEK-293T cells when co-expressed with WT but not KD ULK1 (Figure 3A, B). Mutation of Ser249 alone was sufficient to abolish phosphorylation of Vps34 by ULK1 in vitro (Figure 3C) and prevented a ULK1-induced mobility shift on a standard SDS-PAGE gel upon co-expression in cells (Figure 3D).
Figure 3. Vps34 Ser249 is a novel ULK1 phosphorylation site in vivo.

(A) Either Myc-tagged wild-type ULK1 (WT ULK1; bottom) or Myc-tagged kinase-inactive ULK1 (KI ULK1; top) was transfected into HEK-293T cells along with wild-type Flag-tagged Vps34 (WT Vps34) and immunoprecipitated with M2 agarose. The immunoprecipitate was run out on an SDS-PAGE gel, stained with coomassie, and the band corresponding to WT Vps34 was cut out, isolated, and subjected to tryptic digest and LC/MS/MS analysis. Green bars indicate peptide coverage, and purple highlights indicate phosphorylation events. Arrow indicates serine 249.
(B) Clustal alignment of Vps34 serine 249 across species shows that it is conserved throughout evolution and conforms to the optimal ULK1 phosphorylation motif.
(C) An in vitro kinase assay was performed using Flag-tagged WT Vps34 (Vps34 WT) or a Flag-tagged serine-to-alanine point mutant Vps34 (Vps34 S249A) as substrates for either WT ULK1 or KI ULK1. The in vitro kinase assay was performed in the presence of radiolabeled γ-32P-ATP (top). Vps34 WT, Vps34 S249A, WT ULK1, and KI ULK1 were produced in HEK-293T cells (bottom).
(D). Vps34 WT or Vps34 S249A and WT ULK1 or KI ULK1 were transfected into HEK-293T cells. Cellular lysates were isolated 24 hours post-transfection, run out on an SDS-PAGE gel, and transferred to PVDF membranes, which were subsequently probed with the indicated antibodies. Arrow indicates a mobility shift representative of phosphorylation that only occurs with the Vps34 WT and WT ULK1 combination.
(E). HEK-293T cells were transfected with Vps34 WT or Vps34 S249A and WT ULK1, wild-type Myctagged ULK2 (ULK2), or wild-type Myc-tagged ULK3 (ULK3). Cellular lysates were isolated 24 hours post-transfection and immunoblotted with the indicated antibodies.
We explored the potential function of Vps34 phosphorylation by ULK1 by introducing nonphosphorylatable (Ser249Ala) or phospho-mimetic (Ser249Asp) mutants into conditional Vps34-floxed murine embryonic fibroblasts (MEFs) (Jaber et al., 2012). After first corroborating the requirement of Vps34 for proper autophagy and ultimate cell viability (Figure S3A, data not shown), we tested the effects of these mutants in five assays of Vps34 function in autophagy. However, Ser249 in Vps34 was not required under the conditions we examined (Figure S3B–E). Given that ULK1 appears to phosphorylate multiple sites in Beclin1 and Ambra1 at the same time that it is phosphorylating Ser249 of Vps34, the data suggest that the sum effects of ULK1 on the different Beclin1/Vps34 subcomplexes is a highly regulated series of events requiring further study.
We next developed a phospho-specific antibody to Vps34 Ser249, whose signal was increased when ULK1 or ULK2, but not ULK3, was co-expressed with wild-type Vps34 but not a Ser249Ala Vps34 mutant in HEK-293T cells (Figure 3E).
Identification and characterization of a small molecule inhibitor of ULK1
To further examine how ULK1 regulates autophagy, we sought to identify small molecule inhibitors of the kinase. Starting with a focal adhesion kinase (FAK) inhibitor that showed potent cross-reactivity towards ULK1, we used a target-based reverse pharmacology approach to screen a focused library of pyrimidine analogues to identify small molecule ULK1 inhibitors. We performed in cellulo screening of the most promising 100 analogues from the in vitro kinase screening, testing their dose-dependent ability to inhibit the phosphorylation of Vps34 Ser249 by co-expressed ULK1 in HEK-293T cells. Structure-activity relationship (SAR) data were used to drive medicinal chemistry efforts that led to the ULK1 inhibitor tool compound SBI-0206965 (Figure 4A). Dose-response analysis of SBI-0206965 revealed an in vitro IC50 of 108 nM for ULK1 kinase activity and 711 nM for the highly related kinase ULK2 (Figure 4B). We found SBI-0206965 (hereafter referred to as “6965”) inhibited Ser249 phosphorylation of overexpressed Vps34 when used at ~5 μM (Figure 4C). To ensure that 6965 was not specifically selective towards phosphorylation of Vps34 Ser249, we examined its ability to inhibit the phosphorylation of other ULK1 substrates we had identified. After validating a commercial antibody that could recognize a ULK1 phosphorylation site in Beclin1 (Ser15) when Beclin1 is overexpressed (Figure S4A), we found that 6965 inhibited Beclin1 Ser15 and Vps34 Ser249 phosphorylation to comparable extents in HEK-293T cells (Figure 4D). Furthermore, 6965 treatment collapsed the bandshift that Syntenin-1 and Atg13 undergo when co-expressed with wild-type ULK1 (Figure S4B, S4C). Unfortunately none of these existing phospho-specific antibodies against the ULK1 sites in Beclin1 or Vps34 are sensitive enough to recognize the endogenous proteins in a total cell lysate.
Figure 4. In cellulo screen identifies SBI-0206965 as a potent ULK1 kinase inhibitor against its downstream substrate phosphorylation sites.

(A) Chemical structure of SBI-0206965 (“6965”), the lead ULK1 competitive kinase inhibitor.
(B) The IC50 value for 6965 against wild-type ULK1 and ULK2 was determined using an in vitro kinase assay. Wild-type ULK1 (left) and wild-type ULK2 (right) were assayed using 10 μM MBP in the presence of 30 μM radiolabeled γ-32P-ATP. 6965 was tested in triplicate in a 10-dose IC50 mode with 3-fold serial dilution and a starting dose of 1 μM.
(C). Human embryonic kidney cells (HEK-293T) were transfected with wild-type (WT) or kinase inactive (KI) Myc-tagged ULK1 and wild-type Flag-tagged Vps34 (WT Vps34). 24 hours post-transfection, cells were treated with a panel of putative ULK1 competitive inhibitors in a dose response manner (1, 10, 50 μM). Cellular lysates were isolated after 1 hour of treatment and immunoblotted with the indicated antibodies. Representative results for SBI-0206965 are shown.
(D) HEK-293T cells were transfected with WT or KI ULK1 and WT Vps34 (left) or wild-type Flag-tagged Beclin1 (WT Beclin1; right). 24 hours post-transfection, cells were treated with 6965 (10 μM) or DMSO. Cellular lysates were isolated after 1 hour of treatment and immunoblotted with the indicated antibodies.
(E) Wild-type or Ulk1/Ulk2 double knockout mouse embryonic fibroblasts (MEFs) (Cheong et al., 2011) were treated with fresh media (Dulbecco’s modified Eagle medium [DMEM] containing 10% fetal bovine serum [FBS]) containing 1 μM INK128, 1 μM AZD8055, or DMSO or with starvation media (Earle’s balanced salt solution [EBSS]) in the presence or absence of 10 μM 6965. Cellular lysates were isolated after 1 hour of treatment and immunoblotted with the indicated antibodies.
We next examined whether 6965 would inhibit endogenous ULK1 activity. To activate endogenous ULK1, we treated MEFs with either amino acid starvation media (EBSS) or the mTOR ATP-competitive inhibitors INK128 or AZD8055 (Chresta et al., 2010; Hsieh et al., 2012). In wild-type MEFs, we observed a mobility shift in endogenous Beclin1 and Atg13 in response to EBSS starvation media or the mTOR catalytic inhibitors, which was abolished in Ulk1/2-deficient MEFs (Figure 4E). The EBSS and mTOR-inhibitor induced mobility shift in Beclin1 and Atg13 was inhibited by 6965 co-treatment in wild-type MEFs (Figure 4E). Notably, neither Beclin1 nor Atg13 underwent a mobility shift upon treatment with either EBSS or the mTOR catalytic inhibitors in Ulk1/2-deficient MEFs, and no further decrease in their basal mobility was observed with 6965 co-treatment. We hypothesize that the mobility shifts observed in endogenous Beclin1 and Atg13 induced by mTOR inhibitors and starvation media reflect phosphorylation of endogenous Beclin1/Atg13 by endogenous ULK1/2 as they only occur in wild-type but not Ulk1/2-deficient MEFs.
SBI-0206965 is a highly selective kinase inhibitor
We next examined the specificity of 6965 using the DiscoveRx KINOMEscan panel of 456 purified human kinases in a competition-binding assay (Fabian et al., 2005; Karaman et al., 2008). By this analysis, 6965 was very selective, only inhibiting 10 out of 456 kinases >95% when tested at 10 μM (Figure 5A, 5B). The S(35) selectivity index of 6965 was 0.123, as measured by the percentage of the kinome inhibited below 35% of the control at this concentration (Figure S5A, S5B) [S(35) = (number of kinases with %Ctrl <35)/(number of kinases tested)]. This makes the selectivity profile of 6965 comparable to several kinase inhibitors currently used in clinical oncology, including Imatinib and Lapatinib (Figure S5B). Notably, by this assay, all the kinases bound by SBI-0206965 with an affinity comparable to ULK1 (FAK, Src, Abl, and Jak3) were tyrosine kinases (Figure 5C). Moreover, the only known functional ULK1 homolog in mammals, ULK2, was inhibited almost as well as ULK1 in this assay (Figure 5C), consistent with measurements obtained from in vitro kinase assays (Figure 4B).
Figure 5. SBI-0206965 is a highly selective kinase inhibitor.

(A) The kinase selectivity profile for SBI-0206965 was determined using the DiscoveRx KINOMEscan profiling service (http://www.discoverx.com/). Briefly, 6965 was screened at a 1 μM dose for its ability to impair binding of a panel of 456 kinases to substrate in an in vitro binding assay. Scores for the primary screen hits are reported as a percent of the DMSO control (% Control). Lower scores reflect stronger inhibitory effects of 6965 on the target kinase.
(B) A TREEspot interaction map (http://www.discoverx.com/tools-resources/interaction-maps) was generated to visually represent the selectivity profile for 6965 against the panel of kinases tested in 5A. Kinases whose binding was inhibited by 6965 are marked with red circles, with larger circles indicating stronger inhibitory effects. Kinases tested in this analysis are arrayed according to their phylogenetic groupings in the human kinome.
(C) The list of the kinases whose binding was found to be inhibited by 6965 to less than 4% of the DMSO negative control in the screen described in 5A. Kinases whose binding was inhibited to less than 1% of the DMSO negative control are highlighted in red.
(D) In vitro kinase assays were performed for the kinases highlighted in 5C. These assays were performed in the presence of 6965 in a dose response manner to identify the IC50 value for 6965 for each of these individual kinases. Kinases whose IC50 value was less than 1-fold difference than ULK1 are highlighted in yellow. Of the remaining kinases, those kinases whose IC50 value was less than what was identified for ULK2 are highlighted in brown. In vitro kinase assays performed by Reaction Biology (http://www.reactionbiology.com/).
(E) Immunoflourescence imaging of A549 cells treated in the presence or absence of 5 μM 6965 for 2 hours followed by 4 μM of the mTOR catalytic inhibitor AZD8055 for 24 hours. Autophagic vacuoles were detected using the Cyto-ID autophagy detection kit (Chan et al., 2012) and are visualized in green while cell nuclei were counterstained by DAPI and are visualized in blue.
(F) PC3 human prostate cells that stably express a construct encoding LC3 fused to green fluorescent protein (GFP-LC3) were transfected with siRNAs against the top 18 kinases whose binding was shown to be inhibited by 6965 (Figure 5A). 48 hours after RNAi transfection, the cells were treated with 1 μM of the catalytic mTOR inhibitors AZD8055 or INK128 for 4 hours and assessed for the presence of GFP-LC3 puncta (see Experimental Procedures). The average number of GFP-LC3 puncta and standard deviation for each siRNA and drug treatment are shown. Control immunoblots demonstrating siRNA efficiency in Figure S5E. [“SRC” = c-Src; “c-Src kinase” = CSK]
(G) Representative immunofluorescence images for the data shown in 5F. GFP-LC3 puncta are visualized in green and cell nuclei, which were counterstained with DAPI, are visualized in blue.
We subsequently measured the selectivity of SBI-0206965 against its top binding kinases using radiolabel kinase assays in vitro at varying inhibitor concentrations. Here, we tested the ten kinases whose binding was most suppressed by 6965 by the DiscoverRx ATP binding assay (from Figure 5C). Of these kinases, only FAK and FLT3 showed an IC50 comparable to ULK1 when measured by an in vitro kinase assay (Figure 5D), and 6965 inhibited Src, Jak3, and ULK2 five- to ten-fold less potently than ULK1 and FAK. Consistent with its efficacy of FAK inhibition in vitro, we found that at 10 μM, 6965 reduced endogenous FAK signaling in HEK293T cells (as judged by FAK and Paxillin phosphorylation) to an extent comparable to its inhibition of ULK1 signaling, albeit when ULK1 was overexpressed (Figure S5C). To examine the selectivity of 6965 more rigorously, we examined its impact on endogenous ULK1 and FAK signaling, as well as a number of other critical growth pathways, in wild-type MEFs that were starved or treated with mTOR inhibitors to endogenously activate ULK1 (Figure 4E, S5D). Notably, in MEFs, 6965 did not impair endogenous FAK, AMPK, mTOR, Akt, or Erk signaling at 10 μM, a dose at which it is capable of preventing a mobility shift in Atg13 that occurs in response to mTOR inhibition (Figure 4E, S5D). The suppression of endogenous ULK1 signaling was also monitored by a very recently developed phospho-specific antibody to one of the ULK1 sites in Atg13 (Ser318, which is Ser355 in human Atg13 isoform1) (Figure S5D). In fact, at 10 μM 6965 only modestly reduced FAK and Src signaling (as judged by FAK and Paxillin phosphorylation) and increased both AMPK and Erk-dependent signaling with increasing doses (Figure S5D). Thus, 6965 appears quite selective for ULK1 in vitro and in vivo, with a secondary selectivity towards FAK.
SBI-0206965 suppresses autophagy induced by mTOR inhibition, and this is phenocopied by ULK1 siRNA
To test the ability of 6965 to block autophagy and cell survival, initial studies were performed in A549 lung cancer cells, which are highly sensitive to mTOR inhibition (Sun et al., 2005). We observed that the catalytic ATP-competitive mTOR kinase inhibitor AZD8055 induced robust autophagy as visualized by accumulation of the Cyto-ID autophagy dye (Chan et al., 2012), and this effect was strongly suppressed by treatment with 5 μM 6965 (Figure 5E). Next, we genetically assessed the requirement for ULK1 versus other kinases inhibited by 6965 to induce autophagy after pharmacological mTOR inhibition. A robust high-throughput microscopy method for quantifying GFP-LC3 puncta was established using the PC3 prostate-cancer cell line stably expressing a GFP-LC3 construct (Degtyarev et al., 2008). Using this assay, we performed a focused RNAi analysis of the top 20 kinases identified in the DiscovRx screen as best binding to 6965. Quantitative measurement on wells of cells transfected with control siRNAs revealed a consistent 2-fold induction in GFP-LC3 puncta formation after treatment with either of the mTOR catalytic inhibitors INK128 or AZD8055 (Figure 5F,G). Strikingly, of the 18 kinases tested, only one kinase, ULK1, nearly fully abolished the LC3 puncta induced by the mTOR inhibitors (Figure 5F; Figure S5E). The ability of ULK1 siRNA to nearly fully ablate the autophagic response induced by mTOR inhibition suggests that, in this cell line at least, ULK1 is essential for stimulating autophagy in response to mTOR suppression.
SBI-0206965 prevents ULK1-dependent cell survival following nutrient deprivation
A well-established function of autophagy is to promote cell survival under conditions of nutrient deprivation. For example, ATG5-deficient immortal, nontumorigenic baby mouse kidney epithelial cells display no growth defects when cultured under normal media conditions; however when such cells are placed into starvation media, they undergo apoptosis at a greatly accelerated rate compared to controls (Guo et al., 2011). Similarly, we previously demonstrated that RNAi against ULK1 and ULK2 phenocopied RNAi against ATG5 with regards to the loss of cell viability under nutrient deprived conditions (Egan et al., 2011b). To examine whether our small molecule ULK1 inhibitor would similarly affect cell survival under nutrient deprived conditions, we treated MEFs with 6965 in the context of normal media, amino acid-deprived media, or glucose-deprived media. After 18 hours of amino acid deprivation, 15% of the vehicle-treated MEFs were positive for both 7-AAD and AnnexinV, indicative of end-stage apoptosis (Figure 6A), versus 42% of the 6965-treated cells. Similar effects were also seen in glucose-deprived MEFs, where 6965 also promoted enhanced cell death (Figure S6A). An immunoblot timecourse analysis of amino-acid starved cells revealed that active cleaved caspase-3 and the cleavage of its target PARP was only robustly observed in starved, 6965 co-treated cells (Figure 6B). Interestingly, the immunoblot analysis revealed that 6965 treatment induced the loss of ULK1 and Atg13 protein, but only in nutrient-deprived and not nutrient-replete conditions. Perhaps only in this context when ULK1 is activated does the direct binding of 6965 stimulate ULK1 turnover. We also directly compared 6965 to chloroquine (CQ), a lysosomotropic agent that acts as a general autophagy inhibitor (Kroemer, 2015), in their ability to drive apoptosis under starvation conditions. While both 6965 and CQ induced cell death only under starvation conditions, it was notable that 10 μM 6965 was more effective than 20 μM CQ at rapidly inducing apoptosis in this context (Figure S6B). Control immunoblotting revealed that 20 μM CQ was effective at blocking autophagic flux, preventing the turnover of the Atg8 family member LC3 as well as the autophagy cargo receptor p62/SQSTM1 (Figure S6C, 6B). The degradation of both ULK1 and Atg13 was again specifically observed under starvation conditions in cell treated with 6965 (Figure S6C). Of note, administration of CQ or bafilomycin A1, but not the proteasome inhibitor MG132, abrogated the degradation of both ULK1 and Atg13 indicating that their own turnover is lysosomal in nature (Figure S6D).
Figure 6. SBI-0206965 synergizes with starvation and mTOR inhibition to induce an enhanced apoptotic response.

(A) Wild-type mouse embryonic fibroblasts (MEFs) were treated with fresh media (Dulbecco’s modified Eagle medium [DMEM] containing 10% fetal bovine serum [FBS]) or starvation media (Earle’s balanced salt solution [EBSS]) and 10 μM 6965 or DMSO for 18 hours. Cells were collected, stained with 7-AAD and PE-AnnexinV, and quantified for apoptosis by fluorescence-activated cell sorting (FACS) analysis. Red numbers indicate the percentage of 7-AAD/PE-AnnexinV doublepositive cells, representative of end-stage apoptosis and death.
(B) Western analysis of wild-type MEFs treated as in 6A for 1, 6, and 18 hours. Cellular lysates were immunoblotted with the indicated antibodies. Chloroquine (CQ) at 20 μM serves as a positive control for autophagy inhibition.
(C) Same as 6A except using a lung cancer cell line derived from a genetically engineered mouse model of lung cancer driven by mutant Kras and deletion of p53. Red numbers indicate the percentage of AnnexinV-positive cells.
(D) Same as 6A except using the human glioblastoma cell line U87MG. Red numbers indicate the percentage of AnnexinV-positive cells.
Beyond the general role of autophagy that occurs in many cell types as a response mechanism to nutrient deprivation, there has also been considerable interest in the role of autophagy as a survival mechanism for tumor cells faced with nutrient limitations due to poor vascularization as well as metabolic stress resulting from chemotherapies or targeted therapeutics (White, 2015). We examined whether 6965 would selectively promote apoptosis under conditions in which autophagy is actively engaged in tumor cells similar to what we observed in MEFs. Indeed, 6965 promoted apoptosis (AnnexinV+ cells) particularly in the nutrient-starved state in human U87MG glioblastoma cells and in murine lung carcinoma cells derived from a genetically engineered mouse model of lung cancer driven by mutant Kras and loss of p53 (Figure 6C,6D). Notably, these murine lung cancer cells have been previously shown to be particularly dependent on autophagy (Guo et al., 2013).
The small molecule ULK1 inhibitor SBI-0206965 converts the cytostatic response to catalytic mTOR inhibitors into a cytotoxic response
Given that mTOR inhibition induces ULK1 activation (Figure 4E and S5C) and ULK1 siRNA or 6965 suppresses autophagy induced by mTOR inhibitors (Figure 5E–G), we examined whether 6965 would reduce cell survival in the face of mTOR inhibition. A549 cells were treated with a constant dose of AZD8055 (1 μM), which induces cytostatic growth arrest, and escalating doses of 6965. At a 5 μM dose, 6965 triggered apoptosis in 23% of cells when combined with AZD8055 versus 10% of cells in the absence of AZD8055 and 7% of cells treated with AZD8055 alone (Figure 7A). The induction of AnnexinV+ apoptotic cells was heightened at 10 μM dosing of 6965. As observed in MEFs with nutrient deprivation combined with 6965, immunoblot analysis revealed that only the combination of 6965 with mTOR inhibitors triggered caspase activation in A549 cells, paralleling the FACS analysis of cell death (Figure S7A). Moreover, degradation of total ULK1 and Atg13 was observed as before (Figure 6B, S6C): only in the presence of the autophagy activating stimulus (AZD8055) and 6965 (Figure S7A).
Figure 7. SBI-0206965 converts the cytostatic effects of three different mTOR inhibitors into a cytotoxic apoptotic response in lung cancer cells.

(A) A549 human lung cancer cells were treated with the catalytic mTOR inhibitor AZD8055 (1 μM) or DMSO and increasing doses of 6965. Cells were treated for 72 hours and then collected, stained wit PE-AnnexinV, and quantified by FACS analysis. Red numbers indicate the percentage of AnnexinV-positive cells, representing cells actively undergoing apoptosis.
(B) A549 cells were treated with DMSO, 1 μM INK128, 500 nM rapamycin, or 1 μM AZD8055 in the presence or absence of 10 μM 6965 or 10 μM chloroquine (CQ). Cells were treated for 48 hours and then collected, stained with PE-AnnexinV, and quantified by FACS analysis.
(C) Western analysis of A549 cells treated as in 7B. Cellular lysates were immunoblotted with the indicated antibodies.
(D) Model: mTOR inhibitors trigger autophagy by preventing mTOR-dependent inhibitory phosphorylation events on autophagy regulators, including the serine/threonine kinase ULK1. Thus mTOR inhibition triggers autophagy, which promotes cell survival. Co-administration of an mTOR inhibitor with a ULK1 catalytic inhibitor results in a loss of autophagy induction and prevents the cell survival effect resulting from mTOR inhibition, converting a cytostatic response into a cytotoxic response.
To examine whether 6965 would broadly synergize with other mTOR inhibitors, we examined the ability of rapamycin and a second mTOR catalytic inhibitor, INK128 (MLN0128), to synergize with 6965 (Figure 7B). Similar to AZD8055, both rapamycin and INK128 exhibited a mild apoptotic response on their own in A549 cells but significantly enhanced this apoptotic response when combined with 6965 (Figure 7B). Notably, the extent of AnnexinV+ apoptotic cells induced by the three different mTOR inhibitors was mirrored by the proportion of Atg13 protein loss and the extent to which Beclin1 mobility was impaired (Figure 7C). This also appeared to reflect the potency of mTOR inhibition, as assessed by 4EBP1 mobility shift and loss of the direct mTOR phosphorylation of ULK1 on Ser757 (Figure 7C). A model for the selectivity of different mTOR substrates for inhibition by rapamycin or catalytic mTOR inhibitors proposed that substrate quality may dictate how easily a substrate phosphorylation site is blocked by mTOR inhibition (Kang et al., 2013). From this analysis, ULK1 was identified an excellent mTOR substrate in vitro, similar to 4EBP1, and required full mTOR catalytic inhibition to reduce its phosphorylation. Hence, stronger mTOR inhibition, provided by catalytic mTOR inhibitors, is expected to be superior to rapamycin with regards to blocking phosphorylation of ULK1 at the Ser757 mTOR inhibitory site (Kang et al., 2013). Indeed, the extent of apoptosis induced by mTOR inhibitors combined with 6965 thus parallels the ability of these three mTOR drugs to inhibit ULK1 phosphorylation and to induce degradation of the ULK1/Atg13 complex in the presence of 6965. Furthermore, equimolar 6965 was more potent than chloroquine at inducing apoptosis in combination with all three mTOR drugs (Figure 7B, C).
Discussion
Given that the most upstream component of the conserved autophagy cascade encodes the only serine/threonine kinase in the cascade, it has been assumed that ULK1-dependent phosphorylation of other components of the pathway must instruct and provide proper temporal and spatial cues for autophagy initiation. Here, we shed additional light on the complexities involved in the autophagic process by identifying multiple ULK1-dependent phosphorylation sites in Beclin1, Ambra1, Atg101, Syntenin-1, and Vps34. Recent reports that AMPK phosphorylates Beclin1 and Vps34 on distinct sites from these ULK1 sites (Kim et al., 2013) and that the stress-induced MK2/MK3 kinases also target additional sites in Beclin1 (Wei et al., 2015) indicates that much work remains to fully dissect the context-specific, temporal, and spatial differences in the regulation of Vps34/Beclin1 complexes. The induction of ULK1 activity following catalytic mTOR inhibition alone (Figure 4E and S5C) is consistent with amino acid deprivation induction of ULK1 kinase activity, which does not appear to involve AMPK. mTORC1 phosphorylates and inhibits ULK1 via at least one well-established serine site in ULK1, Ser757 (Kang et al., 2013; Kim et al., 2011) and the extent to which different mTOR inhibitors blocked phosphorylation of Ser757 correlated with the ability of 6965 to promote apoptosis. Given that ULK1 is inhibited by mTORC1, further delineation of ULK1 substrate phosphorylation sites may yield valuable biomarkers for mTOR inhibitors, as the attendant increase in ULK1-dependent substrate phosphorylation would be predicted to reflect the relative intensity and effective duration of mTOR inhibition. This appears to be the case as revealed by endogenous phospho-Atg13 levels in MEFs following starvation or mTOR inhibitor treatment (Figure S5D).
The fact that ULK1 is the only conserved serine/threonine kinase in the autophagy cascade makes it a very attractive target for therapeutic development. Our finding that ULK1 and its binding partner Atg13 are selectively degraded by combining 6965 with starvation or with mTOR inhibitors but not by any of these treatments alone indicates that specifically the active pool of ULK1 kinase complexes may be sensitive to 6965-induced lysosomal degradation. These results provide additional biomarkers for ULK1 inhibition in vivo, as they suggest that ULK1 or Atg13 will be degraded when effectively inhibited by on-target small molecule inhibitors in those cells that rely on ULK1 for survival. Notably, when comparing three mTOR inhibitors for their ability to synergize with 6965 to induce cell death in A549 cells, the extent of apoptosis was paralleled best by loss of Atg13 protein, which in turn mirrored the level of caspase-cleaved PARP. Given the number of ongoing clinical trials combining CQ and CQ analogs with existing cancer therapies, which include mTOR inhibitors (Rangwala et al., 2014), it also worth noting that equimolar 6965 induced more apoptosis than CQ when combined with mTOR inhibitors (Figure 7B,C).
Recently, the crystal structure of ATP-competitive kinase inhibitors bound to ULK1 was reported, marking the first report of the ULK1 atomic structure (Lazarus et al., 2015). However, the small molecule inhibitors developed in this study were non-selective, inhibiting tens of kinases >95% which made them impossible to utilize for cell culture studies (Lazarus et al., 2015). Our study here on 6965 therefore reports one of the first tool compounds that inhibits ULK1 with exquisite selectivity and is suitable for cell studies for the autophagy field. Future efforts will explore the use of this compound and its derivatives in animal models of cancer and other diseases.
mTOR inhibitors are being widely tested in clinical trials for oncology, and rapamycin analogues are the approved standard of care for advanced renal cell carcinoma and other solid tumors (Benjamin et al., 2011). From a therapeutic perspective, an exciting prediction is that ULK1 inhibition will convert the cytostatic response to mTOR inhibition to a cytotoxic response due to loss of autophagic maintenance of cell survival (Figure 7D). We directly tested this hypothesis and found that it holds true in A549 non-small cell lung cancer cells treated with three different mTOR inhibitors (Figure 7). An important step moving forward will be to delineate those tumors that are most likely to show benefit from ULK1 inhibitors like 6965. In hamartoma syndromes, such as tuberous sclerosis complex and Peutz-Jeghers syndrome, inactivating mutations in the tumor suppressors TSC1, TSC2, PTEN, and LKB1/STK11 result in mTOR hyperactivation, and these mutations are targetable by mTOR inhibitors (Bissler et al., 2008). One would hypothesize that the tumors most sensitive to mTOR inhibitors and hence those perhaps most likely to benefit from dual mTOR/ULK1 inhibition will be those tumors bearing mutations in genes encoding components of the core mTOR signaling machinery itself (Grabiner et al., 2014). Another possibility is that combining a ULK1 inhibitor such as 6965 with mTOR inhibitors may allow for lower dosing schedules of catalytic mTOR inhibitors. ULK1 inhibition therefore represents an exciting new modality to avoid therapeutic resistance in the many patients currently treated with mTOR inhibitors. The additionally identified ULK1-dependent phosphorylation sites, functional assays, and ULK1 inhibitor tool compound identified in this study will help catalyze further exploration of the roles of this highly conserved autophagy kinase in cell biology and in therapeutic possibilities for a wide variety of diseases.
EXPERIMENTAL PROCEDURES
Antibodies and reagents
Cell Signaling Antibodies used: Atg13 (#6940), Beclin1 (#3495), PARP (#9542), 4E-BP1 (#9452), phospho-ULK1 S757 (#6888), Ulk1 (#8054), p70 S6K (#9202), phospho-S6 S235/236 (#4858), Myc tag (#2278), LC3B (#3868), Vps34 (#4263), phospho-AMPK T172 (#2535), phospho-ACC S79 (#3661), phospho-Paxillin Y118 (#2541), and PathScan Multiplex Western Cocktail I (#5301). Phospho-Vps34 Ser249 antibody was developed in collaboration with Gary Kasof at Cell Signaling Technology. Sigma antibodies used: ULK1 (A7481), β-actin (A5541), and Flag tag polyclonal (F7425). Gabarap antibody from Abgent (PM037). Phospho-FAK Y397 from Abcam (ab4803). FAK from Epitomics (2146-1). p62 SQSTM1 antibody from Progen (03-GPP62-C). Phospho-Beclin1 S15 from Abbiotec (254515). Phospho-Atg13 S318 (human S355) from Rockland (600-401-C49S). EBSS (14155-063) and glucose-free media (11966-025) from Gibco/Life Technologies. Chloroquine (C6628) and bafilomycin A1 (B1793) from Sigma. AZD-8055 (A-1008) from Active Biochem. AnnexinV-PE Apoptosis Detection Kit from BD Biosciences. Phos-tag™ AAL-107 from NARD (#304-93521). Ad5-CMV-Cre was purchased from the University of Iowa viral vector core.
Supplementary Material
Highlights.
ULK1 phosphorylates multiple autophagy components, including VPS34 on Ser249
SBI-0206965 is a highly-selective ULK1 kinase inhibitor that blocks autophagy
SBI-0206965 combined with starvation or mTOR inhibition leads to ULK1 degradation
SBI-0206965 synergizes with mTOR inhibition to induce cell death
Acknowledgments
We thank Dr. Gary Kasof at Cell Signaling Technology for developing the Phospho-VPS34 Ser249 antibody in conjunction with D.F.E. and R.J.S. We thank Drs. Craig Thompson (MSKCC) for the Ulk1/2 MEFs (Cheong et al., 2011) and Dr. Wei-Xing Zong (SUNY StonyBrook) for the Vps34-floxed MEFs (Jaber et al., 2012). We thank Pedro Aza-Blanc in the Functional Genomics core at the Sanford Burnham Medical Research Institute for assistance with the GFP-LC3 quantitation. D.F.E. was supported by the predoctoral T32 CMG training grant to UCSD/Salk. M.G.H.C. was supported by a postdoctoral fellowship from the American Cancer Society (122862-PF-12-258-01-TBG). This work is supported in part by National Institutes of Health grants R01CA188694 to N.D.P.C., R01GM104047 to B.E.T., and R01CA172229 and DoD grant W81XWH-13-1-0043 to R.J.S. Work in the laboratory of R.J.S. was also supported in part through the Howard Hughes Medical Institute, the Salk CCSG P30 CA014195, and the Leona M. and Harry B. Helmsley Charitable Trust grant #2012-PG-MED002.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information contains seven supplemental figures and figure legends and Extended Experimental Procedures.
AUTHOR CONTRIBUTIONS
DFE generated ULK1 for peptide library screening in Fig S1A, performed analysis of all ULK1 substrates and in depth analysis of Vps34 Ser249 mutant function, comprising all experiments in Figures 2 and 3 except where noted. Experiments in Figures 4, 6, and 7 were performed by DFE and MGHC. DFE performed the in cellulo screen of ULK1 inhibitors that led up to Figure 4C, with compounds synthesized by MV and RPD, with assistance from DJS and PT in the lab of NDPC. HJL in the lab of BET performed the peptide library screening in Figure 1A, and CMJ performed the follow-up peptide analysis in Figure 1C, 1D. JMA performed all mass spectrometry analysis contained in Figure 2. HZ performed experiments for Figure 5E. JR and PAB performed the RNAi screen of kinases in GFP-LC3 PC3 cells for Figure 5F, 5G. Data in Figure 1 were analyzed by CJM, DFE, HJL, RJS, and BET. Data in Figures 2 and 3 were analyzed by DFE and RJS. Data in Figure 4 and 5 were analyzed by DFE, MGHC, RJS, MV, DJS, PT, and NDPC. Data in Figures 6 and 7 were analyzed by DFE, MGHC, and RJS. RJS and NDPC designed the project and guided experiments and analysis of data, with input from all authors. DFE, MGHC, and RJS wrote the manuscript with input from BET, DJS, PT, and NDPC.
References
- Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EY. mTORC1 phosphorylates the ULK1-mAtg13-FIP200 autophagy regulatory complex. Sci Signal. 2009;2:e51. doi: 10.1126/scisignal.284pe51. [DOI] [PubMed] [Google Scholar]
- Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, Kuksin D, Lin B, Qiu J. A novel image-based cytometry method for autophagy detection in living cells. Autophagy. 2012;8:1371–1382. doi: 10.4161/auto.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011a;7 doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011b;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabiner BC, Nardi V, Birsoy K, Possemato R, Shen K, Sinha S, Jordan A, Beck AH, Sabatini DM. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4:554–563. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–1219. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–979. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]
- Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Overholtzer M, Thompson CB. Autophagy in cellular metabolism and cancer. J Clin Invest. 2015;125:47–54. doi: 10.1172/JCI73942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341:1236566. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;5:749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155:688–698. doi: 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest. 2015;125:1–4. doi: 10.1172/JCI78652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MB, Novotny CJ, Shokat KM. Structure of the human autophagy initiating kinase ULK1 in complex with potent inhibitors. ACS chemical biology. 2015;10:257–261. doi: 10.1021/cb500835z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009;5:649–662. doi: 10.4161/auto.5.5.8249. [DOI] [PubMed] [Google Scholar]
- Miller ML, Jensen LJ, Diella F, Jorgensen C, Tinti M, Li L, Hsiung M, Parker SA, Bordeaux J, Sicheritz-Ponten T, et al. Linear motif atlas for phosphorylation-dependent signaling. Sci Signal. 2008;1:ra2. doi: 10.1126/scisignal.1159433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic acids research. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papinski D, Schuschnig M, Reiter W, Wilhelm L, Barnes CA, Maiolica A, Hansmann I, Pfaffenwimmer T, Kijanska M, Stoffel I, et al. Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Mol Cell. 2014;53:471–483. doi: 10.1016/j.molcel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, et al. Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10:1391–1402. doi: 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Turk BE. Understanding and exploiting substrate recognition by protein kinases. Curr Opin Chem Biol. 2008;12:4–10. doi: 10.1016/j.cbpa.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


