Abstract
We sought to compare the risk of end stage renal disease (ESRD), ischemic heart event (IHE), congestive heart failure (CHF), cerebrovascular accident (CVA), and all-cause mortality among 470,386 individuals with resistant and nonresistant hypertension (non-RH). Resistant hypertension (60,327 individuals) was sub-categorized into 2 groups; 23,104 patients with cRH (controlled on 4 or more medicines) and 37,223 patients with uRH (uncontrolled on 3 or more medicines) in a 5 year retrospective cohort study. Cox proportional hazard modeling was used to estimate hazard ratios adjusting for age, gender, race, body mass index, chronic kidney disease (CKD), and co-morbidities. Resistant hypertension (cRH and uRH) compared to non-RH, had multivariable adjusted hazard ratios (95% confidence intervals) of 1.32 (1.27–1.37), 1.24 (1.20–1.28), 1.46 (1.40–1.52), 1.14 (1.10–1.19), and 1.06 (1.03–1.08) for ESRD, IHE, CHF, CVA, and mortality, respectively. Comparison of uRH to cRH had hazard ratios of 1.25 (1.18–1.33), 1.04 (0.99–1.10), 0.94 (0.89–1.01), 1.23 (1.14–1.31), and 1.01 (0.97–1.05) for ESRD, IHE, CHF, CVA, and mortality, respectively. Males and Hispanics had greater risk for ESRD within all 3 cohorts. Resistant hypertension had greater risk for ESRD, IHE, CHF, CVA, and mortality. The risk of ESRD and CVA and were 25% and 23% greater, respectively, in uRH compared to cRH supporting the linkage between blood pressure and both outcomes.
Keywords: Resistant Hypertension, Cardiovascular outcomes, Renal outcomes, Mortality Outcomes
Introduction
Patients with difficult to control or resistant hypertension are encountered by many clinicians and yet there remain many unknown aspects about this population. The prognosis and comparative outcomes in resistant hypertension deserve better understanding as it would provide valuable insights into the management of this population. Less is known about individuals with refractory hypertension who never achieve blood pressure control despite multiple antihypertensive medicines. These knowledge gaps may be due in part from the fact that resistant hypertension itself is difficult to identify due to patient, physician, and confounders inherent to the health care environment such as heterogeneity of care (1–3). In addition, those with resistant hypertension represent a relatively small subpopulation accounting for 10–15% of those with hypertension (4–9). Longitudinal observations in resistant hypertension are relatively few and the comparisons of outcomes with non-resistant hypertension have been limited in various ways (10–14).
The descriptive studies on resistant hypertension have reported high rates of vascular disease and end organ damage (9, 15, 16). Other studies have shown high rates of cardiovascular morbidity and mortality (10, 12, 14) particularly among persons with pre-existing ischemic heart disease and chronic kidney disease (CKD) (17–19). Past studies have compared the resistant hypertension population among specialized populations such as those with pre-existing ischemic heart disease, incident resistant hypertension, or small cohorts. Overall, these studies have shown a greater risk for cardiovascular and mortality outcomes in persons with resistant hypertension compared to those with non-resistant hypertension (11, 17, 18, 20, 21). The assumption is that the resistant hypertension population has an adverse physiology and is therefore, at greater risk for morbidity and mortality. These risks may be in addition to those conferred by higher blood pressures or their associated adverse effects.
We previously described a resistant hypertension cohort within a large ethnically diverse hypertension population using an electronic health record (EHR) based approach (9). This was one of the largest and most diverse populations with resistant hypertension assembled to date and utilized a single health care system with reliable clinical encounter information and medication use. With this cohort and passive follow-up through a comprehensive EHR, we sought to evaluate and compare the risk of renal, cardiovascular, and mortality outcomes among individuals identified with controlled resistant hypertension, uncontrolled resistant hypertension, and non-resistant hypertension.
Results
Cohort Characteristics
A total of 470,386 individuals were identified for the study cohort (Figure 1) as previously described (9). The mean age (SD) was 65 (11) years with 45% males (Table 1). The race/ethnic composition was 43% white, 21% Hispanic, 13% black, and 8% Asian. The mean blood pressure of the entire study cohort was 133/75 mmHg. Resistant hypertension was identified in 60,327 (12.8%) individuals of whom 23,104 met our criteria for cRH and 37,223 met our criteria for uRH. Thus, uRH accounted for 61.7% of the resistant hypertension population and 7.9% of all hypertensive individuals.
Figure 1.
Among approximately 2.4 million adult KPSC members, 470,386 individuals were identified with hypertension. Resistant hypertension was identified in 60,327 (12.8%) with 4.9% controlled resistant hypertension (cRH) and 7.9% uncontrolled resistant hypertension (uRH).
Table 1.
Characteristics by Hypertension Category
| Characteristics | All | non-RH | cRH | uRH | P value |
|---|---|---|---|---|---|
|
|
|||||
| N(%) | 470,386 (100) | 410,059 (87.2) | 23,104 (4.9) | 37,223 (7.9) | P<0.001 |
| Age, median (Q1, Q3) | 64 (56, 73) | 63 (56, 72) | 70 (62, 77) | 68 (60, 76) | P<0.001 |
| Female, % | 55 | 55 | 46 | 56 | P<0.001 |
| Race, % | |||||
| White | 43 | 42 | 49 | 43 | P<0.001 |
| Black | 13 | 12 | 18 | 19 | P<0.001 |
| Hispanic | 21 | 21 | 17 | 19 | P<0.001 |
| Asian/Pacific | 8 | 8 | 7 | 6 | P<0.001 |
| Other | 16 | 17 | 10 | 12 | P<0.001 |
| SBP, median (Q1, Q3) | 132 (122, 143) | 131 (121, 140) | 125 (116, 132) | 150 (144, 161) | P<0.001 |
| DBP, median (Q1, Q3) | 75 (67, 82) | 75 (68, 82) | 67 (60, 74) | 79 (71, 87) | P<0.001 |
| BMI ≥ 30, % | 43 | 42 | 48 | 50 | P<0.001 |
| Median Creatinine (Q1, Q3), mg/dl | 1.0 (0.8, 1.1) | 0.9 (0.8, 1.1) | 1.1 (0.9, 1.4) | 1.0 (0.9, 1.3) | P<0.001 |
| Median eGFR (Q1, Q3), ml/min/1.73m2 | 74 (59, 88) | 75 (61, 89) | 60 (45, 76) | 65 (49, 81) | P<0.001 |
| Chronic Kidney Disease, % | 26 | 24 | 49 | 42 | P<0.001 |
| Diabetes mellitus, % | 32 | 30 | 52 | 45 | P<0.001 |
| Ischemic Heart Disease, % | 25 | 22 | 49 | 36 | P<0.001 |
| Cerebrovascular Disease, % | 10 | 9 | 19 | 15 | P<0.001 |
RH(resistant hypertension); cRH (BP<140/90 on 4 or more medicine); uRH (SBP>/=140/90 on 3 or more medicines)
SBP (systolic blood pressure); DBP (diastolic blood pressure); BMI (Body mass index)
Compared to the non-RH population, the RH population had a greater prevalence of comorbid conditions including DM (48% vs 30%), CKD (45% vs 24%), ischemic heart disease (41% vs 22%), and cerebrovascular disease (16% vs 9%) (P<0.001 for all). cRH and uRH were similar in age, BMI, race/ethnicity composition and comorbidities. Blood pressures were highest in uRH (154/79 mmHg) compared to both cRH (123/67 mmHg) and non-RH (132/75 mmHg).
Antihypertensive Drug Usage
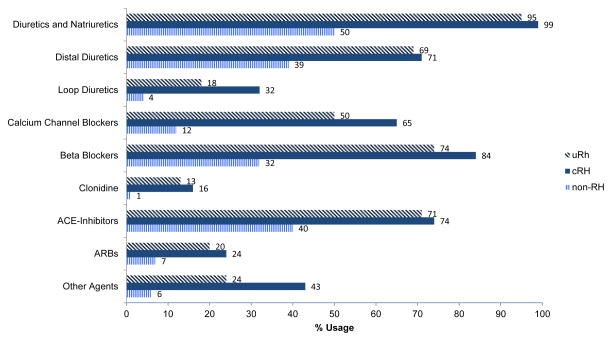
Diuretics were the most frequently prescribed class of medications in all 3 groups (Figure 2). Individuals with cRH were more likely to be prescribed an angiotensin converting enzyme inhibitor or angiotensin receptor blocker (93%) compared to the non-RH (47%) and uRH populations (88%).
Figure 2.
The use of antihypertensive medication classes among the study cohort within each hypertension group: non-resistant hypertension (non-RH), controlled resistant hypertension (cRH), and uncontrolled resistant hypertension (uRH). Diuretics and renin angiotensin system blockers were the most frequently prescribed.
Outcomes
Overall 114,364 events occurred amongst the cohort and 18.5% experienced at least one event (Table 2). Mortality occurred in 43,580 (9.3%) individuals. In addition, there were 26,894 IHE, 12,306 CHF, 16,799 CVA, and 14,785 incident ESRD events (Table 2). The resistant hypertension population had greater proportions of those who reached any of the outcomes (31.0 vs 16.7%) compared to the non-RH population (P<0.001). The total number of all outcomes combined for each group was 87,217 in non-RH, 12,039 in cRH, and 15,218 in uRH. The rate of any outcome was highest in cRH (34.8%) followed by uRH (28.7%) and non-RH (16.7%).
Table 2.
Outcome/Event rates for the Study Cohort and by Hypertension Category
| Outcome | All HTN Subjects N=470,386 |
non-RH N=410,059 |
cRH N=23,104 |
uRH N=37,223 |
|---|---|---|---|---|
| Ischemic Heart Event, % (n) | 5.72 (26,894) | 5.09 (20,889) | 11.57 (2,674) | 8.95 (3,331) |
| Congestive Heart Failure, % (n) | 2.62 (12,306) | 2.01 (8,257) | 8.63 (1,993) | 5.52 (2,056) |
| Cerebrovascular Event, % (n) | 3.57 (16,799) | 3.24 (13,270) | 5.79 (1,337) | 5.89 (2,192) |
| End Stage Renal Disease, % (n) | 3.14 (14,785) | 2.52 (10,353) | 8.12 (1,877) | 6.86 (2,555) |
| Death, % (n) | 9.26 (43,580) | 8.37 (34,338) | 18.00 (4,158) | 13.66 (5,084) |
| Individuals with any Event, % (n) | 18.5 (87,034) | 16.7 (68,337) | 34.8 (8,029) | 28.7 (10,668) |
Non-RH (non-resistant hypertension)
RH (resistant hypertension); cRH (BP<140/90 on 4 or more medicine); uRH (SBP>/=140/90 on 3 or more medicines)
After adjustment for age, gender, race, BMI, CKD, Charlson comorbidity index, and the comorbidities of DM, ischemic heart disease, CHF, CKD, and cerebrovascular disease, the resistant hypertension (cRH and uRH) population compared to non-RH population, had HRs (95% CI) of 1.24 (1.20–1.28), 1.46 (1.40–1.52), 1.14 (1.10–1.19), 1.32 (1.27–1.37), and 1.06 (1.03–1.08) for IHE, CHF, CVA, ESRD, and all-cause mortality, respectively (Table 3, Figure 3). Unadjusted event free survival was superior for non-RH compared to resistant hypertension for all the outcomes studied (Figure 4).
Table 3.
Comparative Risk for Outcomes among Different Hypertension Categories
| Outcome (Mean follow up) | Ischemic Heart Event (4.20) | Congestive Heart Failure (4.28) | Cerebrovascular Event (4.26) | End Stage Renal Disease (4.25) | Mortality (4.33) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) | |
| RH (cRH+uRH) vs non-RH | 1.98 (1.92, 2.03) | 1.24 (1.20,1.28) | 3.36 (3.23, 3.49) | 1.46 (1.40, 1.52) | 1.81 (1.74, 1.88) | 1.14 (1.10, 1.19) | 2.94 (2.84, 3.04) | 1.32 (1.27, 1.37) | 1.80 (1.76, 1.85) | 1.06 (1.03, 1.08) |
| cRH vs non-RH | 2.33 (2.23, 2.42) | 1.21 (1.16, 1.26) | 4.37 (4.16, 4.59) | 1.51 (1.43, 1.59) | 1.80 (1.70, 1.90) | 1.01 (0.95, 1.07) | 3.26 (3.11, 3.43) | 1.16 (1.10, 1.22) | 2.13 (2.06, 2.20) | 1.05 (1.02, 1.09) |
| uRH vs non-RH | 1.77 (1.70, 1.83) | 1.26 (1.21, 1.31) | 2.74 (2.61, 2.89) | 1.42 (1.35, 1.50) | 1.81 (1.73, 1.90) | 1.24 (1.18, 1.30) | 2.74 (2.62, 2.86) | 1.45 (1.39, 1.52) | 1.60 (1.55, 1.65) | 1.06 (1.03, 1.09) |
| cRH vs uRH | 1.32 (1.25, 1.39) | 0.96 (0.91, 1.01) | 1.59 (1.49, 1.69) | 1.06 (0.99, 1.12) | 0.99 (0.93, 1.06) | 0.81 (0.76, 0.88) | 1.19 (0.99, 1.27) | 0.80 (0.75, 0.85) | 1.33 (1.28, 1.39) | 0.99 (0.95, 1.03) |
Models were adjusted for age, race, gender, BMI, eGFR, Charlson Comorbidity Index, and co-morbidities including CKD and DM
RH(resistant hypertension); cRH (BP<140/90 on 4 or more medicine); uRH (SBP>/=140/90 on 3 or more medicines)
SBP (systolic blood pressure); DBP (diastolic blood pressure); BMI (Body mass index)
Figure 3.
Multivariable adjusted hazard ratio (95% confidence interval) for ischemic heart event, congestive heart failure, cerebrovascular accident, end stage renal disease, and all-cause mortality in subjects with: (a) RH (cRH + uRH) in comparison to those with non-RH (b) cRH vs non-RH (c) uRH vs non-RH and (d) uRH vs cRH.
Figure 4.
Kaplan Meier survival curves for the primary endpoints (a) ischemic heart event (b) cerebrovascular accident (c) congestive heart failure (d) end stage renal disease (e) all-cause mortality and (f) combined events in patients with non-resistant hypertension (non-RH) and resistant hypertension (RH) which includes both uncontrolled (uRH) and controlled resistant hypertension (cRH).
Controlled Resistant Hypertension (cRH) vs Non-Resistant Hypertension (non-RH)
The multivariable adjusted HRs (95% CI) for individuals with cRH compared to their counterparts with non-RH were 1.21 (1.16–1.26), 1.51 (1.43–1.59), 1.01(0.95–1.07), 1.16 (1.10–1.22), and 1.05 (1.02–1.09 for IHE, CHF, CVA, ESRD, and all-cause mortality, respectively (Table 3, Figure 3).
Uncontrolled Resistant Hypertension (uRH) vs Non-Resistant Hypertension (non-RH)
In both crude and adjusted models, individuals with uRH were at greater risk for all outcomes compared to non-RH (Table 3, Figure 3). Multivariable adjusted HRs (95% CIs) in those with uRH vs. those with non-RH were 1.26 (1.21–1.31), 1.42(1.35–1.50), 1.24(1.18–1.30), 1.45 (1.39–1.52), and 1.06 (1.03–1.09) for IHE, CHF, CVA, ESRD, and all-cause mortality, respectively (Table 3, Figure 3).
Uncontrolled Resistant Hypertension (uRH) vs Controlled Resistant Hypertension (cRH)
Compared to cRH, individuals with uRH were at greater risk for CVA (HR, 1.23; 95% CI, 1.14–1.31) and ESRD (HR, 1.25; 95% CI, 1.18–1.33). Risk for IHE (HR, 1.04; 95% CI, 0.99–1.10), CHF (HR, 0.95; 95% CI, 0.89–1.01) and all-cause mortality (HR, 1.01; 95% CI, 0.97–1.05), were similar between those with uRH and cRH (Table 3, Figure 3).
Intragroup Comparisons within non-RH, cRH, and uRH
Multivariable adjusted HR’s for ESRD demonstrated similar trends within non-RH, cRH, and uRH (Table 4). Males, Hispanics, DM, ischemic heart disease, and cerebrovascular disease were all associated with higher risk for ESRD. Similar to past observations, older age (>/=60 years) and BMI>/=30 were associated with paradoxically lower risk for ESRD. Older age increased risk for mortality. For IHE, CHF, and CVA outcomes, older age, male gender, CKD, DM, pre-existing ischemic heart disease and cerebrovascular disease were associated with increased HR’s within all 3 hypertension cohorts (Appendix Table 2).
Table 4.
Intragroup Comparisons for ESRD and Mortality Outcomes
| Non-RH | ||||
|---|---|---|---|---|
| ESRD | 95% CI | Mortality | 95% CI | |
|
|
||||
| Age >=60 vs. 18–59 | 0.64 | (0.61, 0.68) | 2.72 | (2.62, 2.82) |
| Male vs Female | 1.47 | (1.40, 1.54) | 1.23 | (1.20, 1.26) |
| White | 1.00 | 1.00 | ||
| Black vs. White | 1.44 | (1.34, 1.54) | 0.95 | (0.92, 0.99) |
| Hispanic vs. White | 1.45 | (1.37, 1.53) | 0.76 | (0.73, 0.78) |
| Asian vs. White | 1.17 | (1.08, 1.28) | 0.53 | (0.51, 0.56) |
| Other vs. White | 0.36 | (0.32, 0.41) | 0.44 | (0.42, 0.46) |
| BMI >=30 vs. <30 | 0.81 | (0.77, 0.85) | 0.59 | (0.57, 0.60) |
| Chronic Kidney Disease Yes vs. No | 5.25 | (4.98, 5.53) | 1.85 | (1.80, 1.89) |
| Diabetes mellitus Yes vs. No | 2.01 | (1.91, 2.10) | 1.30 | (1.27, 1.34) |
| Ischemic Heart Disease Yes vs. No | 2.17 | (2.05, 2.29) | 1.34 | (1.31, 1.38) |
| Cerebrovascular Disease Yes vs. No | 1.13 | (1.06, 1.20) | 1.57 | (1.53, 1.62) |
| cRH | ||||
|---|---|---|---|---|
| ESRD | 95% CI | Mortality | 95% CI | |
|
|
||||
| Age >=60 vs. 18–59 | 0.57 | (0.50, 0.66) | 1.75 | (1.55, 1.97) |
| Male vs Female | 1.31 | (1.17, 1.45) | 1.17 | (1.09, 1.24) |
| White | 1.00 | 1.00 | ||
| Black vs. White | 1.58 | (1.38, 1.80) | 0.86 | (0.79, 0.94) |
| Hispanic vs. White | 1.63 | (1.42, 1.86) | 0.86 | (0.78, 0.94) |
| Asian vs. White | 1.50 | (1.25, 1.81) | 0.61 | (0.53, 0.70) |
| Other vs. White | 0.43 | (0.31, 0.61) | 0.61 | (0.52, 0.72) |
| BMI >=30 vs. <30 | 0.76 | (0.68, 0.85) | 0.60 | (0.56, 0.64) |
| Chronic Kidney Disease Yes vs. No | 5.18 | (4.49, 5.96) | 1.64 | (1.53, 1.75) |
| Diabetes mellitus Yes vs. No | 1.97 | (1.75, 2.21) | 1.30 | (1.22, 1.39) |
| Ischemic Heart Disease Yes vs. No | 1.47 | (1.30, 1.67) | 1.31 | (1.21, 1.42) |
| Cerebrovascular Disease Yes vs. No | 1.13 | (1.00, 1.27) | 1.41 | (1.32, 1.52) |
| uRH | ||||
|---|---|---|---|---|
| ESRD | 95% CI | Mortality | 95% CI | |
|
|
||||
| Age >=60 vs. 18–59 | 0.41 | (0.37, 0.45) | 1.77 | (1.60, 1.95) |
| Male vs Female | 1.27 | (1.17, 1.39) | 1.10 | (1.04, 1.16) |
| White | 1.00 | 1.00 | ||
| Black vs. White | 1.59 | (1.42, 1.78) | 0.87 | (0.80, 0.94) |
| Hispanic vs. White | 1.66 | (1.48, 1.85) | 0.79 | (0.73, 0.85) |
| Asian vs. White | 1.57 | (1.34, 1.86) | 0.59 | (0.52, 0.68) |
| Other vs. White | 0.36 | (0.27, 0.48) | 0.50 | (0.43, 0.57) |
| BMI >=30 vs. <30 | 0.78 | (0.71, 0.85) | 0.60 | (0.56, 0.64) |
| Chronic Kidney Disease Yes vs. No | 8.05 | (7.12, 9.11) | 1.88 | (1.77, 2.00) |
| Diabetes mellitus Yes vs. No | 2.74 | (2.47, 3.05) | 1.32 | (1.24, 1.40) |
| Ischemic Heart Disease Yes vs. No | 1.54 | (1.40, 1.70) | 1.34 | (1.25, 1.43) |
| Cerebrovascular Disease Yes vs. No | 1.11 | (1.00, 1.23) | 1.47 | (1.38, 1.57) |
RH(resistant hypertension); cRH (BP<140/90 on 4 or more medicine); uRH (SBP>/=140/90 on 3 or more medicines)
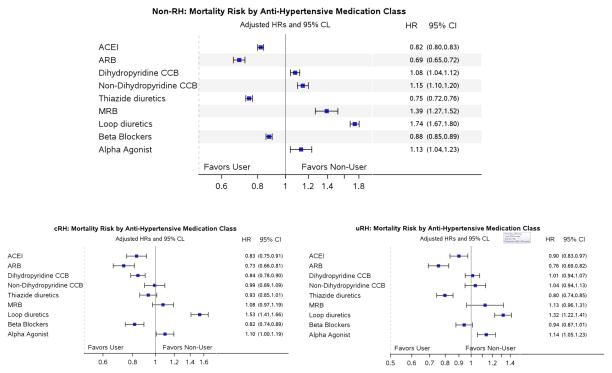
The non-RH population had the strongest association with medication use and outcomes. Within non-RH, use of both ACEI and thiazide type diuretics were associated with lower risk for IHE, CHF, CVA, and mortality (figure 5, Appendix Table 3). Use of ACEI, ARB, and thiazide type diuretics were all associated with lower mortality risk within all 3 cohorts.
Figure 5.
For mortality, use of ACEI, ARB, and thiazide type diuretics were all associated with lower risk amongst the different hypertension sub groups.
Discussion
In this study, we found that the risk for cardiovascular, renal, and all-cause mortality outcomes were increased in individuals with cRH and uRH compared to those with non-RH. The resistant hypertension population as a whole had greater risk for IHE, CHF, CVA, and ESRD compared to those with non-RH. There was a modest 6% increased mortality risk as well. The uRH subpopulation of resistant hypertension, which had the highest blood pressures, demonstrated the greatest risk for the outcomes studied. The uRH population compared to their counterparts with cRH experienced over a 20% increased risk for CVA and ESRD underscoring the importance of blood pressure control. Within non-RH, cRH, and uRH, there was a consistently increased risk for ESRD in males, Hispanics, and those with co-morbidities of CKD, DM, ischemic heart disease, and cerebrovascular disease. Older age was observed to have lower risk for ESRD across all hypertension groups and was likely related to the competing risk of death which has been well described (22). Similarly, the obesity paradox was observed in our cohort which has also been described (23). Overall, our findings were drawn from a cohort of patients with hypertension that had relatively high blood pressure control rates in which the mean blood pressure was 132/75 mmHg.
Our study cohort and findings represent real world outcomes and comparisons among a relatively large and diverse population of resistant and non-resistant hypertension. Compared to observations from more homogeneous populations from Europe, China, or the United States Veterans administration (15, 24, 25), our population was more representative. We also believe that our findings have generalizability in observing these different hypertension populations compared to more controlled (artificial) environments such as clinical trials. Our clinical practice environment with a standardized approach minimizes some of the limitations with past observations such as fragmented data, fragmented populations, and heterogeneous practice patterns. The observations from our study were derived from a single integrated health system where all hypertensive individuals were exposed to a comparable treatment environment including medications. KPSC has a relatively homogeneous hypertension management program (26). Nevertheless, the comparative outcomes reported represent findings from a real world clinical practice environment where decisions are made based on provider perceptions. We also feel that the mixture of size, diversity, and length of follow up our cohort is a strength of our study.
Resistant hypertension represents an outlier among the general hypertension population. Clinically they are more difficult to treat and control than non-RH. Though imperfect, we used an operational definition of resistant hypertension that is based on the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), the American Heart Association (27, 28), and the European Society of Hypertension/European Society of Cardiology (29) guidelines. The criteria using 3 medicines have been used to define and study resistant hypertension for over 30 years (30). The majority (up to 85%) of hypertensive individuals is treated with 1 or 2 medicines (31–33). We have previously shown that treated hypertensive individuals within KPSC averaged 1.97 anti-hypertensive medicines (34) and others have described similar numbers (33). Thus, patients prescribed 3 or more medicines are outliers of the hypertension population and represent a different sub population such as what we are attempting to describe. We did not require the use of a diuretic as defining criteria because we were primarily interested in identifying those with difficult to treat hypertension. A subset of patients who may have been intolerant of diuretics but required 3 or more medications were also included in our resistant hypertension study cohort. Overall, 97% of the resistant hypertension population (cRH and uRH) were on a diuretic. Within resistant hypertension, we also wanted to account for the effects of blood pressure and blood pressure control. Though we did not fully evaluate of the effects of blood pressure across different ranges, we did further sub categorize resistant hypertension as controlled and uncontrolled based on the systolic blood pressure 140mmHg systolic blood pressure cutoff.
Blood pressure is an important component within resistant hypertension just as it is for general hypertension. Recently, the blood pressure goals and targets have come into question since aggressive blood pressures have not translated into improved outcomes (35–38). However, the data and national guidelines continue to support minimum targets of blood pressures under 150 mmHg systolic (35) and perhaps lower for stroke risk reduction (39). Within our study, the 14% higher risk of CVA in resistant hypertension was no longer observed when resistant hypertension was controlled.
The resistant hypertension population is presumed to have an adverse physiology which is prone to worsened outcomes. Among the general hypertension population, there are very few comparative studies on resistant hypertension compared to non-RH. Thus measureable risks and prognosis are not well known. A short term study on incidence of resistant hypertension suggested that individuals with resistant hypertension had a 47% higher risk of cardiovascular events (10). Two studies that evaluated hypertensive patients identified with coronary artery disease found that patients with resistant hypertension experienced worsened cardiovascular and mortality events compared to those with non-RH (17, 18). Among CKD patients, those with resistant hypertension have been reported to experience a two-fold greater risk for both cardiovascular events and ESRD compared to those without resistant hypertension (19). Overall, there are even fewer studies that have evaluated CKD and ESRD outcomes in resistant hypertension. Our findings within a large diverse general hypertension population further demonstrate that individuals with resistant hypertension are at greater risk for renal failure in addition to cardiovascular outcomes and mortality.
In our study, we sought to further categorize resistant hypertension based on blood pressure control and presumed response to medications. Our assumption was that those with difficult to control blood pressures but who eventually attained control (<140/90mm Hg) may be different to those are not controlled (cRH vs uRH). We found similar risks for IHE, CHF, and mortality in cRH and uRH. However, our findings do suggest that CVA and ESRD risk may be reduced by controlling blood pressure in uRH. Somewhat unexpectedly, we observed that the cRH had greater number of adverse events compared to uRH (Table 2). This may speak to the fact that the cRH population represented the sickest population with the most co-morbidities including CKD, DM. IHD, and CVA (Table 1). The lowest blood pressure was in the cRH cohort ((123/67 mmHg) and may be reflective of a weakened physiologic state. One example is the fact that the adjusted risk for CHF was greater in cRH vs uRH suggestive of inotropic compromise and inability to maintain higher blood pressures. Additionally, the greater burden of co morbidities particularly vascular disease through the years may result in irreversible remodeling of the vascular beds. Thus they may require relatively higher blood pressures to maintain systemic perfusion and adequate delivery of nutrients throughout the body. To that end, the cRH population may have been over treated whereas uRH patients had blood pressures conducive to better perfusion. Potential over treatment of hypertension and the existence of a U shaped curve for blood pressure has been observed in populations with CKD and cardiovascular disease (36, 38, 40). The question of where the ideal blood pressures should be, especially in those with chronic disease burdens, remains largely unanswered.
Few comparisons have been made between controlled and uncontrolled subpopulations of resistant hypertension as was performed in our study (41). The subpopulation of those with uRH is even less understood. This population, which is difficult to treat and in whom blood pressure is never controlled, may represent a different biological phenotype. In our study, we used a lower threshold of 3 or more antihypertensive medicines to define uRH. Using this definition, we found that 7.9% of the hypertensive cohort had uRH and these individuals were prone to worsened outcomes, particularly CVA compared to cRH. The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study used a more rigid definition of uRH of 5 or more medicines without control and found a rate of 0.5% among hypertensive individuals.
The prognosis and comparative outcomes in resistant hypertension deserve better understanding. Determining the risks and survival in these subpopulations can help shape treatment strategies of the hypertension itself and also co-morbidities. Medication classes and rationale behind their use may have to be more defined in order to account for the greater risk of certain outcomes in the resistant hypertension population. Comorbidities such as hyperlipidemia or DM may need to be managed more aggressively. Conversely, if survival is shorter in resistant hypertension then we may need to consider certain comorbidities with poor prognoses as competing outcomes
The relevance of current national hypertension guidelines may need to be reconsidered among resistant hypertension in order to achieve better blood pressure control. The resistant hypertension population may benefit from more individualized care rather than broad recommendations. For instance, the black population was over represented in our RH cohort compared to non-RH. While KPSC uses a JNC based medication treatment guideline, the efficacy of diuretics and calcium channel blockers in the black population has been well described (42, 43). Greater emphasis on these medications in blacks may lower the proportion of resistant hypertension in this group. Another example is to assess the physiology of hypertension by patients’ response to treatment. A study on resistant hypertension that treated after classifying uncontrolled blood pressures as either volume or vasoconstrictive hypertension led to management strategies that achieved greater blood pressure reduction (44).
Potential Limitations
There are several potential limitations to our study that may confound the findings. We identified the study cohort from a 2 year window and followed individuals up to 5 years. In contrast to real world phenomenon where blood pressures vary over time, we somewhat arbitrarily identified a cohort as resistant hypertension. The assumption was that once individuals were identified as resistant hypertension that they would remain so throughout the observation period. Conversely, those with non-RH may have gone on to develop resistant hypertension. We also did not have information on the duration of hypertension. Due to our definition, the resistant hypertension population would have had to had some duration of follow up to have medicine titrated to at least 3 medicines introducing an immortal time bias. Non-RH individuals could have been identified based on only 2 visits. Therefore, the resistant hypertension population was more likely to have pre-existing vascular damage that may not have been identified at the start of the observation period compared with the non-RH population. By studying hypertension categories, we are somewhat trivializing the impact of blood pressure on our outcomes studied since the step wise risk of cardiovascular outcomes with higher blood pressures has been well described (45, 46). In addition, we cannot account for heterogeneous practice patterns. Certain patients may be deemed sicker by clinicians and received more comprehensive care such as in our cRH population. They may have been seen more frequently and also had better health maintenance care. The fact that there was only a modest (6%) mortality risk in the resistant hypertension population compared to the general hypertension population may further suggest this confounding.
Conclusion
Our comparative outcomes demonstrated that the resistant hypertension population was at greater risk for IHE, CHF, CVA, ESRD, and mortality. The uRH subgroup of resistant hypertension appears to demonstrate the greatest risk particularly for CVA and ESRD. The higher risks of resistant hypertension compared to non-RH for IHE and for CHF appear to remain non-modifiable, whereas the higher risk of CVA and ESRD were reduced in our observational study in those with cRH. Our findings underscore the hypothesis that resistant hypertension has a more adverse physiology and deserves better understanding in order to better manage this population.
Methods
Study Population
A retrospective, longitudinal cohort study of Kaiser Permanente Southern California (KPSC) members was performed between January 1, 2006 and December 31, 2010. The KPSC health care system is a prepaid integrated health plan providing comprehensive care to over 3.6 million members throughout Southern California, from Bakersfield to San Diego at 13 medical centers and over 200 satellite clinics. As of December 2010, there were over 2.4 million adult members within KPSC. The patient population is ethnically and socioeconomically diverse, reflecting the general population of Southern California (47). All KPSC members have similar benefits and access to health care services, clinic visits, procedures, and copays for medications. Complete health care encounters are tracked using a common EHR from which all study information was extracted.
Many of the details of the study population has been previously described (9). Briefly, individuals age 18 years and older with hypertension and a documented blood pressure measurement were identified in the time period between January 1, 2006 and December 31, 2007. Individuals were followed until they experienced any outcome or until the end of observation (December 31, 2010). Hypertension was identified by inpatient and outpatient International Classification of Diseases, Ninth Revision (ICD-9) codes specific to hypertension (401.xx, 402.xx, 403.xx, 404.xx, 405.xx). Inclusion into the study cohort required a minimum of 2 separately dated ICD-9 codes for hypertension. The accuracy of ICD-9 coding for the diagnosis of hypertension has been previously validated (48). The date of the second ICD-9 hypertension code was used as the index date. Blood pressure values closest in date to the index date were used. In those encounters with multiple blood pressure measurements, the lowest value was used for analysis to minimize white coat hypertension. Individuals who did not have a blood pressure measurement or those who were diagnosed with secondary hypertension (renovascular disease, adrenal disorders, Cushing’s syndrome, aortic coarctation, and secondary hypertension not specified) were excluded from the study cohort. Sleep apnea was not excluded as it is often coexistent with hypertension and not necessarily a causative factor.
Data Collection and Laboratory Measurements
All laboratory data, vital sign assessments (including blood pressure measurements), and diagnostic and procedure codes are collected in the EHR as part of routine clinical care encounters. Comorbidities, including diabetes mellitus (DM), ischemic heart disease, congestive heart failure (CHF), and cerebrovascular disease, were assessed based on inpatient and outpatient ICD-9 diagnoses coding. The Deyo adaption of the Charlson Comorbidity Index was also determined using ICD-9 diagnosis codes from inpatient and outpatient encounters as an overall measure of disease burden (49). Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate of less than 60 mL/min per 1.73m2 estimated from serum creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration Equation (50). Data on hospitalizations and diagnoses that occurred outside the healthcare system were available through administrative claims records.
Assessment of Medication Use
Antihypertensive medication data were retrieved from the KPSC pharmacy dispensing records (9). Prescription orders, pharmacy fills, and refills are tracked for KPSC members with pharmacy benefits. Individuals were determined to be on an antihypertensive medication if it was prescribed and dispensed for 7 or more days supply within the observation period at any time on or after the initial diagnosis of hypertension. Medications that were prescribed and filled for less than 7 days were not considered.
Antihypertensive medication classes included angiotensin-converting enzyme inhibitors (ACEI), alpha blockers, angiotensin receptor blockers (ARB), beta blockers, dihydropyridine and nondihydropyridine calcium-channel blockers, central acting agents, thiazide and loop type diuretics, potassium sparing diuretics, mineralocorticoid receptor antagonists, centrally acting alpha agonists, and direct renin inhibitors. Single pill combination drugs were classified into their different respective components. The total number of blood pressure medications was defined by the number of different antihypertensive medications taken by each subject and may have included multiple medications from the same drug class.
KPSC Hypertension Treatment Guideline
KPSC has a standardized hypertension management program which includes continuous processes to standardize blood pressure measurements. KPSC publishes and advocates an internally derived hypertension algorithm which is followed by the majority of physicians (26, 34). For patients with systolic blood pressure 140–159 and diastolic blood pressure 90–99 mmHg, the guideline suggests initiating treatment with either a diuretic alone or an angiotensin converting enzyme inhibitor (ACEI)/diuretic combination. The ACEI/diuretic combination was suggested for all patients with systolic blood pressure ≥160 and/or diastolic blood pressure ≥100 mmHg. If blood pressure was still not in control with full doses, the third medication was a beta blocker to full dose. If blood pressure was still not controlled, the fourth medication was a calcium channel blocker.
Definitions of Resistant Hypertension and Sub Categories
Individuals were classified as having resistant hypertension if their systolic blood pressure was ≥140mmHg and/or their diastolic blood pressure was ≥90mmHg while prescribed 3 or more different antihypertensive medications concomitantly; or prescribed 4 or more medications concomitantly regardless of blood pressure control. All other hypertensive individuals were categorized as non-resistant hypertension (non-RH). These criteria were based on based on the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), the American Heart Association (27, 28), and the European Society of Hypertension/European Society of Cardiology (29) guidelines. Resistant hypertension individuals who were on 4 or more antihypertensive medicines with controlled blood pressure (<140/90 mmHg) were classified as controlled resistant hypertension (cRH). Among those with resistant hypertension, individuals were classified as having uncontrolled resistant hypertension (uRH) if their systolic blood pressure was ≥140mmHg or their diastolic blood pressure was ≥90mmHg while on 3 or more antihypertensive medications.
Outcomes
The primary outcomes evaluated were ischemic heart event (IHE), congestive heart failure (CHF), cerebrovascular accident (CVA), incident end-stage renal disease (ESRD), and all-cause mortality as separate competing outcomes. Any hospitalization with the primary or secondary diagnoses of IHE and CHF were used to identify these outcomes. IHE was defined as any code for myocardial infarction, angina, coronary occlusion, myocardial necrosis, and any procedure codes for percutaneous intervention or coronary artery bypass grafting (Appendix Table 1). CHF was defined as heart failure and cardiomyopathy. CVA was defined as stroke, central nervous system bleed, or cerebrovascular disease/accident not otherwise specified. ESRD was defined as treatment with dialysis or renal transplantation. The ESRD population is reliably captured within an internal KPSC database established primarily for billing purposes. Mortality information for the cohort was obtained from several complementary sources, including KPSC administrative sources, state vital statistics, and Social Security Administration death files. For the latter two sources, a probabilistic match was made based on name, address, birth date, Social Security Number (when available) and other demographic information. Because data from these latter sources may be delayed, the date of December 31, 2010 was used to censor follow-up. Each event was followed separately without competing outcomes. Thus, individuals were followed until the occurrence of that particular event, disenrollment from the health plan, end of the study period (December 31, 2010), or death. Follow-up was not censored when another event occurred with the exception of death.
Statistical Analyses
The demographic characteristics and comorbidities in cRH, uRH, and non-RH were evaluated and compared. χ2 test was used for categorical variables and the nonparametric Kruskal-Wallis test for continuous variables.
The primary analysis was to compare risk for IHE, CHF, CVA, ESRD, and all-cause mortality among non-RH, RH, cRH, and uRH. Event rates and Kaplan-Meier survival curves were constructed to describe event free survival in the 4 groups. Cox proportional hazards regression modeling was used to estimate hazard ratios (HR) for each outcome separately. Multivariable HRs were calculated with adjustment for potential confounders including age group (18–59 or >=60), sex, race/ethnicity (black or non-black), body mass index (BMI, <30 or >=30), Charlson comorbidity index (CCI), and pre-existing comorbidities including DM, CKD, ischemic heart disease, CHF, and cerebrovascular disease. The multivariable Cox models were constructed to estimate HRs for four pairwise comparisons: 1) resistant hypertension vs. non-RH; 2) cRH vs non-RH; 3) uRH vs. non-RH; and 4) uRH vs cRH.
Intragroup comparisons were made within non-RH, cRH, and uRH to evaluate risk for outcomes among different age group (18–59 or >=60), gender, BMI (<30 or >=30), race/ethnicity, and co-morbidities. The effects of different medications on IHE, CHF, CVA, ESRD, and all-cause mortality were also assessed. Within non-RH, cRH, and uRH, multivariable HR’s were calculated for each outcome based on use of specific medication classes.
All statistical analyses were generated using SAS statistical software (version 9.2; SAS Institute, Inc., Cary, NC). Results with P< 0.05 were considered statistically significant. The study protocol was reviewed and approved by the KPSC Institutional Review Board and was exempt from informed consent.
Acknowledgments
Financial Support: This study was partially funded and supported by Kaiser Permanente Southern California Regional Research. Support was also provided by a research grant from the National Institute of Health grants K24-DK091419 to K.K.Z.
Appendix
Table 1.
ICD-9 codes used to capture outcomes/events during hospitalizations
|
Table 2.
Intragroup Comparisons
| non-RH | ||||
|---|---|---|---|---|
| Ischemic Heart Event (95% CI) | Congestive Heart Failure (95% CI) | Cerebrovascular Event (95% CI) | ||
|
|
||||
| Age >=60 vs. 18–59 | 1.21 (1.17, 1.25) | 1.74 (1.63, 1.85) | 1.99 (1.90, 2.09) | |
| Male vs Female | 1.80 (1.75, 1.86) | 1.11 (1.06, 1.16) | 1.10 (1.06, 1.14) | |
| White | 1.00 | 1.00 | 1.00 | |
| Black vs. White | 0.79 (0.75, 0.83) | 1.21 (1.14, 1.30) | 1.19 (1.13, 1.25) | |
| Hispanic vs. White | 0.89 (0.86, 0.92) | 0.92 (0.87, 0.98) | 0.90 (0.86, 0.95) | |
| Asian vs. White | 0.94 (0.89, 0.99) | 0.76 (0.69, 0.84) | 0.75 (0.70, 0.81) | |
| Other vs. White | 0.57 (0.54, 0.60) | 0.64 (0.58, 0.70) | 0.45 (0.42, 0.49) | |
| BMI >=30 vs. <30 | 0.96 (0.93, 0.99) | 0.90 (0.86, 0.94) | 0.80 (0.77, 0.83) | |
| Chronic Kidney Disease Yes vs. No | 1.20 (1.16, 1.24) | 2.00 (1.90, 2.10) | 1.38 (1.33, 1.44) | |
| Diabetes mellitus Yes vs. No | 1.36 (1.32, 1.40) | 1.61 (1.53, 1.68) | 1.30 (1.25, 1.35) | |
| Ischemic Heart Disease Yes vs. No | 3.46 (3.35, 3.56) | 1.89 (1.79, 2.00) | 1.34 (1.29, 1.40) | |
| Cerebrovascular Disease Yes vs. No | 1.27 (1.23, 1.32) | 1.31 (1.24, 1.38) | 3.32 (3.19, 3.46) | |
| cRH | ||||
|---|---|---|---|---|
| Ischemic Heart Event (95% CI) | Congestive Heart Failure (95% CI) | Cerebrovascular Event (95% CI) | ||
|
|
||||
| Age >=60 vs. 18–59 | 1.10 (0.98, 1.24) | 1.36 (1.17, 1.59) | 1.95 (1.59, 2.39) | |
| Male vs Female | 1.41 (1.30, 1.54) | 1.07 (0.98, 1.18) | 1.00 (0.89, 1.12) | |
| White | 1.00 | 1.00 | 1.00 | |
| Black vs. White | 0.75 (0.67, 0.84) | 1.04 (0.93, 1.18) | 1.07 (0.92, 1.23) | |
| Hispanic vs. White | 0.90 (0.81, 1.01) | 0.95 (0.84, 1.08) | 0.96 (0.82, 1.12) | |
| Asian vs. White | 0.93 (0.80, 1.09) | 0.70 (0.57, 0.87) | 0.69 (0.54, 0.88) | |
| Other vs. White | 0.81 (0.68, 0.97) | 0.74 (0.58, 0.94) | 0.59 (0.44, 0.78) | |
| BMI >=30 vs. <30 | 0.96 (0.89, 1.05) | 0.80 (0.72, 0.87) | 0.74 (0.66, 0.83) | |
| Chronic Kidney Disease Yes vs. No | 1.19 (1.09, 1.29) | 1.62 (1.47, 1.80) | 1.39 (1.23, 1.56) | |
| Diabetes mellitus Yes vs. No | 1.40 (1.29, 1.52) | 1.48 (1.34, 1.63) | 1.29 (1.15, 1.45) | |
| Ischemic Heart Disease Yes vs. No | 3.23 (2.92, 3.59) | 1.63 (1.44, 1.84) | 1.36 (1.19, 1.55) | |
| Cerebrovascular Disease Yes vs. No | 1.24 (1.14, 1.36) | 1.28 (1.16, 1.41) | 2.63 (2.34, 2.95) | |
| uRH | ||||
|---|---|---|---|---|
| Ischemic Heart Event (95% CI) | Congestive Heart Failure (95% CI) | Cerebrovascular Event (95% CI) | ||
|
|
||||
| Age >=60 vs. 18–59 | 1.08 (0.98, 1.19) | 1.24 (1.08, 1.41) | 1.76 (1.54, 2.02) | |
| Male vs Female | 1.37 (1.28, 1.47) | 1.04 (0.96, 1.14) | 0.95 (0.87, 1.03) | |
| White | 1.00 | 1.00 | 1.00 | |
| Black vs. White | 0.78 (0.71, 0.86) | 1.08 (0.97, 1.21) | 1.11 (0.99, 1.24) | |
| Hispanic vs. White | 0.92 (0.84, 1.01) | 0.88 (0.78, 0.99) | 0.90 (0.80, 1.01) | |
| Asian vs. White | 0.97 (0.84, 1.12) | 0.79 (0.64, 0.96) | 0.68 (0.56, 0.83) | |
| Other vs. White | 0.70 (0.60, 0.81) | 0.62 (0.50, 0.77) | 0.54 (0.44, 0.66) | |
| BMI >=30 vs. <30 | 0.94 (0.88, 1.01) | 0.89 (0.82, 0.98) | 0.78 (0.72, 0.86) | |
| Chronic Kidney Disease Yes vs. No | 1.37 (1.27, 1.47) | 2.00 (1.81, 2.20) | 1.40 (1.28, 1.53) | |
| Diabetes mellitus Yes vs. No | 1.53 (1.42, 1.65) | 1.76 (1.60, 1.94) | 1.19 (1.09, 1.30) | |
| Ischemic Heart Disease Yes vs. No | 2.82 (2.60, 3.06) | 1.62 (1.46, 1.80) | 1.35 (1.22, 1.48) | |
| Cerebrovascular Disease Yes vs. No | 1.24 (1.14, 1.35) | 1.21 (1.09, 1.34) | 2.57 (2.34, 2.82) | |
RH(resistant hypertension); cRH (BP<140/90 on 4 or more medicine); uRH (SBP>/=140/90 on 3 or more medicines)
Table 3A.
Medication Usage and Risk of Outcomes within Hypertension Categories
| Outcome: Ischemic Heart Event
| |||
|---|---|---|---|
| non-RH | cRH | uRH | |
| Medication Usage (Yes vs No) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| ACEI | 0.96 (0.93, 0.99) | 0.94 (0.83, 1.07) | 0.97 (0.88, 1.07) |
| ARB | 1.03 (0.98, 1.09) | 1.03 (0.91, 1.17) | 1.07 (0.97, 1.19) |
| Thiazide Diuretic | 0.79 (0.76, 0.81) | 0.91 (0.82, 1.01) | 0.95 (0.87, 1.03) |
| Loop Diuretic | 1.00 (0.95, 1.06) | 1.01 (0.91, 1.12) | 1.06 (0.97, 1.16) |
| Beta Blockers | 1.14 (1.10, 1.17) | 1.11 (0.98, 1.25) | 1.10 (1.00, 1.20) |
| Dihydropyridine CCB | 1.14 (1.09, 1.20) | 0.90 (0.83, 0.98) | 1.15 (1.07, 1.24) |
| Non-Dihydropyridine CCB | 1.13 (1.06, 1.20) | 0.99 (0.82, 1.01) | 1.08 (0.97, 1.20) |
| Alpha Agonist | 1.08 (0.96, 1.22) | 0.98 (0.88, 1.10) | 1.20 (1.09, 1.31) |
| Mineralocorticoid Receptor Blocker | 0.89 (0.76, 1.05) | 0.78 (0.68, 0.91) | 0.88 (0.97, 1.16) |
Table 3B.
| Outcome: Congestive Heart Failure
| |||
|---|---|---|---|
| non-RH | cRH | uRH | |
| Medication Usage (Yes vs No) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| ACEI | 0.90 (0.86, 0.95) | 1.09 (0.95, 1.26) | 1.01 (0.90, 1.14) |
| ARB | 0.95 (0.88, 1.02) | 0.95 (0.82, 1.10) | 0.95 (0.83, 1.08) |
| Thiazide Diuretic | 0.71 (0.67, 0.75) | 0.99 (0.88, 1.11) | 0.86 (0.88, 0.96) |
| Loop Diuretic | 2.12 (2.00, 2.25) | 1.96 (1.74, 2.22) | 1.56 (1.40, 1.73) |
| Beta Blockers | 0.99 (0.94, 1.03) | 0.99 (0.86, 1.14) | 1.02 (0.91, 1.14) |
| Dihydropyridine CCB | 1.20 (1.12, 1.29) | 0.83 (0.75, 0.91) | 1.21 (1.10, 1.33) |
| Non-Dihydropyridine CCB | 1.15 (1.06, 1.25) | 0.98 (0.88, 1.11) | 1.20 (1.05, 1.37) |
| Alpha Agonist | 1.07 (0.90, 1.27) | 1.05 (0.92, 1.20) | 1.16 (1.03, 1.30) |
| Mineralocorticoid Receptor Blocker | 1.34 (1.16, 1.56) | 1.10 (0.97, 1.26) | 1.18 (0.96, 1.46) |
Table 3C.
| Outcome: Cerebrovascular Accident
| |||
|---|---|---|---|
| non-RH | cRH | uRH | |
| Medication Usage (Yes vs No) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| ACEI | 0.91 (0.88, 0.94) | 1.15 (0.96, 1.37) | 0.91 (0.81, 1.02) |
| ARB | 0.82 (0.77, 0.88) | 1.07 (0.89, 1.28) | 0.91 (0.80, 1.04) |
| Thiazide Diuretic | 0.76 (0.73, 0.79) | 1.00 (0.87, 1.16) | 0.90 (0.81, 1.00) |
| Loop Diuretic | 0.99 (0.92, 1.06) | 1.04 (0.90, 1.21) | 0.97 (0.86, 1.09) |
| Beta Blockers | 1.02 (0.98, 1.05) | 0.94 (0.80, 1.10) | 0.95 (0.86, 1.06) |
| Dihydropyridine CCB | 1.07 (1.01, 1.14) | 1.02 (0.90, 1.15) | 1.00 (0.91, 1.10) |
| Non-Dihydropyridine CCB | 1.13 (1.05, 1.21) | 1.07 (0.87, 1.16) | 1.03 (0.90, 1.00) |
| Alpha Agonist | 1.09 (0.95, 1.25) | 1.05 (0.91, 1.22) | 1.06 (0.94, 1.20) |
| Mineralocorticoid Receptor Blocker | 1.01 (0.84, 1.23) | 0.94 (0.80, 1.10) | 0.96 (0.72, 1.28) |
Table 3D.
| Outcome: End Stage Renal Disease
| |||
|---|---|---|---|
| non-RH | cRH | uRH | |
| Medication Usage (Yes vs No) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| ACEI | 0.77 (0.73, 0.81) | 0.80 (0.69, 0.94) | 0.89 (0.79, 1.00) |
| ARB | 0.92 (0.85, 1.00) | 1.03 (0.88, 1.21) | 1.05 (0.92, 1.19) |
| Thiazide Diuretic | 0.62 (0.59, 0.66) | 0.85 (0.74, 0.97) | 0.72 (0.64, 0.80) |
| Loop Diuretic | 1.71 (1.60, 1.83) | 1.78 (1.55, 2.04) | 1.55 (1.39, 1.72) |
| Beta Blockers | 0.95 (0.90, 0.99) | 1.05 (0.90, 1.22) | 1.02 (0.92, 1.14) |
| Dihydropyridine CCB | 1.46 (1.36, 1.57) | 1.39 (1.24, 1.56) | 1.53 (1.40, 1.69) |
| Non-Dihydropyridine CCB | 1.40 (1.29, 1.53) | 1.43 (1.22, 1.68) | 1.30 (1.14, 1.49) |
| Alpha Agonist | 1.55 (1.33, 1.80) | 1.50 (1.32, 1.70) | 1.50 (1.35, 1.67) |
| Mineralocorticoid Receptor Blocker | 1.19 (1.00, 1.41) | 0.75 (0.62, 0.89) | 0.75 (0.59, 0.97) |
Table 3E.
| Outcome: Mortality
| |||
|---|---|---|---|
| non-RH | cRH | uRH | |
| Medication Usage (Yes vs No) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| ACEI | 0.82 (0.80, 0.84) | 0.83 (0.76, 0.92) | 0.90 (0.83, 0.97) |
| ARB | 0.69 (0.66, 0.72) | 0.73 (0.66, 0.81) | 0.76 (0.69, 0.83) |
| Thiazide Diuretic | 0.75 (0.73, 0.77) | 0.93 (0.86, 1.01) | 0.80 (0.74, 0.86) |
| Loop Diuretic | 1.74 (1.68, 1.80) | 1.53 (1.41, 1.67) | 1.32 (1.22, 1.41) |
| Beta Blockers | 0.88 (0.86, 0.90) | 0.82 (0.75, 0.89) | 0.94 (0.88, 1.01) |
| Dihydropyridine CCB | 1.08 (1.04, 1.12) | 0.84 (0.79, 0.90) | 1.01 (0.95, 1.08) |
| Non-Dihydropyridine CCB | 1.15 (1.10, 1.20) | 0.99 (0.90, 1.09) | 1.04 (0.95, 1.13) |
| Alpha Agonist | 1.13 (1.04, 1.23) | 1.10 (1.01, 1.20) | 1.14 (1.05, 1.23) |
| Mineralocorticoid Receptor Blocker | 1.39 (1.27, 1.52) | 1.08 (0.97, 1.19) | 1.13 (0.96, 1.32) |
Footnotes
Disclosure
No other authors have any conflicts of interest relevant to this manuscript.
References
- 1.Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339(27):1957–63. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 2.Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47(3):345–51. doi: 10.1161/01.HYP.0000200702.76436.4b. [DOI] [PubMed] [Google Scholar]
- 3.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–34. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 4.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. Jama. 2010;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 5.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124(9):1046–58. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC, Jr, Crowley K, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34(16):1204–14. doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 7.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57(6):1076–80. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 8.Sarafidis PA. Epidemiology of resistant hypertension. J Clin Hypertens (Greenwich) 2011;13(7):523–8. doi: 10.1111/j.1751-7176.2011.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sim JJ, Bhandari SK, Shi J, Liu IL, Calhoun DA, McGlynn EA, et al. Characteristics of Resistant Hypertension in a Large, Ethnically Diverse Hypertension Population of an Integrated Health System. Mayo Clinic proceedings Mayo Clinic. 2013;88(10):1099–107. doi: 10.1016/j.mayocp.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–42. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaksson H, ÖStergren J. Prognosis in therapy-resistant hypertension. Journal of Internal Medicine. 1994;236(6):643–9. doi: 10.1111/j.1365-2796.1994.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 12.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340–6. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 13.Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49(4):839–45. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 14.Muxfeldt ES, Cardoso CR, Dias VB, Nascimento AC, Salles GF. Prognostic impact of the ambulatory arterial stiffness index in resistant hypertension. J Hypertens. 2010;28(7):1547–53. doi: 10.1097/HJH.0b013e328339f9e5. [DOI] [PubMed] [Google Scholar]
- 15.Cuspidi C, Macca G, Sampieri L, Michev I, Salerno M, Fusi V, et al. High prevalence of cardiac and extracardiac target organ damage in refractory hypertension. J Hypertens. 2001;19(11):2063–70. doi: 10.1097/00004872-200111000-00018. [DOI] [PubMed] [Google Scholar]
- 16.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 17.Bangalore S, Fayyad R, Laskey R, Demicco DA, Deedwania P, Kostis JB, et al. Prevalence, predictors, and outcomes in treatment-resistant hypertension in patients with coronary disease. The American journal of medicine. 2014;127(1):71–81. e1. doi: 10.1016/j.amjmed.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Gong Y, Handberg E, Messerli FH, Bakris GL, Ahmed A, et al. Predictors and outcomes of resistant hypertension among patients with coronary artery disease and hypertension. J Hypertens. 2014;32(3):635–43. doi: 10.1097/HJH.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Nicola L, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. Journal of the American College of Cardiology. 2013;61(24):2461–7. doi: 10.1016/j.jacc.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 20.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and Prognosis of Resistant Hypertension in Hypertensive Patients. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18(11):1422–8. doi: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 22.O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–65. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. The Lancet. 2006;368(9536):666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 24.Jin CN, Liu M, Sun JP, Fang F, Wen YN, Yu CM, et al. The prevalence and prognosis of resistant hypertension in patients with heart failure. PloS one. 2014;9(12):e114958. doi: 10.1371/journal.pone.0114958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a veterans affairs population. J Clin Hypertens (Greenwich) 2014;16(10):741–5. doi: 10.1111/jch.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim JJ, Handler J, Jacobsen SJ, Kanter MH. Systemic Implementation Strategies to Improve Hypertension: The Kaiser Permanente Southern California Experience. Can J Cardiol. 2014;30(5):544–52. doi: 10.1016/j.cjca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Cifkova R, Erdine S, Fagard R, Farsang C, Heagerty AM, Kiowski W, et al. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J Hypertens. 2003;21(10):1779–86. doi: 10.1097/01.hjh.0000084773.37215.1b. [DOI] [PubMed] [Google Scholar]
- 30.Gifford JRAYW, Tarazi RC. Resistant Hypertension: Diagnosis and Management. Annals of Internal Medicine. 1978;88(5):661–5. doi: 10.7326/0003-4819-88-5-661. [DOI] [PubMed] [Google Scholar]
- 31.Fung V, Huang J, Brand R, Newhouse JP, Hsu J. Hypertension treatment in a medicare population: adherence and systolic blood pressure control. Clin Ther. 2007;29(5):972–84. doi: 10.1016/j.clinthera.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–14. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 33.Banegas JR, Navarro-Vidal B, Ruilope LM, de la Cruz JJ, Lopez-Garcia E, Rodriguez-Artalejo F, et al. Trends in hypertension control among the older population of Spain from 2000 to 2001 to 2008 to 2010: role of frequency and intensity of drug treatment. Circulation Cardiovascular quality and outcomes. 2015;8(1):67–76. doi: 10.1161/CIRCOUTCOMES.114.001191. [DOI] [PubMed] [Google Scholar]
- 34.Sim JJ, Bhandari SK, Shi J, Kalantar-Zadeh K, Rasgon SA, Sealey JE, et al. Plasma Renin Activity (PRA) Levels and Antihypertensive Drug Use in a Large Healthcare System. Am J Hypertens. 2012;25(3):379–88. doi: 10.1038/ajh.2011.216. [DOI] [PubMed] [Google Scholar]
- 35.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 36.Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, et al. J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31(23):2897–908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- 37.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ. Impact of Achieved Blood Pressures on Mortality Risk and End-Stage Renal Disease Among a Large, Diverse Hypertension Population. Journal of the American College of Cardiology. 2014;64(6):588–97. doi: 10.1016/j.jacc.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Group SPSS, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–15. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, et al. Blood pressure and mortality in u.s. Veterans with chronic kidney disease: a cohort study. Annals of internal medicine. 2013;159(4):233–42. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calhoun DA, Booth JN, 3rd, Oparil S, Irvin MR, Shimbo D, Lackland DT, et al. Refractory hypertension: determination of prevalence, risk factors, and comorbidities in a large, population-based cohort. Hypertension. 2014;63(3):451–8. doi: 10.1161/HYPERTENSIONAHA.113.02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328(13):914–21. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 43.Wright JT, Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293(13):1595–608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 44.Egan BM, Basile JN, Rehman SU, Davis PB, Grob CH, 3rd, Riehle JF, et al. Plasma Renin test guided drug treatment algorithm for correcting patients with treated but uncontrolled hypertension: a randomized controlled trial. Am J Hypertens. 2009;22(7):792–801. doi: 10.1038/ajh.2009.63. [DOI] [PubMed] [Google Scholar]
- 45.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345(18):1291–7. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 46.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 47.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhandari SK, Pashayan S, Liu IL, Rasgon SA, Kujubu DA, Tom TY, et al. 25-hydroxyvitamin D levels and hypertension rates. Journal of clinical hypertension. 2011;13(3):170–7. doi: 10.1111/j.1751-7176.2010.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 50.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]