Abstract
In the past few years, there have been a large number of genes identified that contribute to the lifetime risk of Parkinson's disease (PD). Some genes follow a Mendelian inheritance pattern, but others are risk factors for apparently sporadic PD. Here, we will focus on those genes nominated by genome-wide association studies (GWAS) in sporadic PD, with a particular emphasis on genes that overlap between familial and sporadic disease such as those encoding a-synuclein (SNCA), tau (MAPT), and leucine-rich repeat kinase 2 (LRRK2). We will advance the view that there are likely relationships between these genes that map not only to neuronal processes, but also to neuroinflammation. We will particularly discuss evidence for a role of PD proteins in microglial activation and regulation of the autophagy-lysosome system that is dependent on microtubule transport in neurons. Thus, there are at least two non-mutually exclusive pathways that include both non-cell-autonomous and cell-autonomous mechanisms in the PD brain. Collectively, these data have highlighted the amount of progress made in understanding PD and suggest ways forward to further dissect this disorder.
Introduction
It is nearly 200 years since James Parkinson famously described the symptoms of his eponymous disorder (1). Since then, our understanding of Parkinson's disease (PD) has developed hugely. We now recognize that many of the classic motor symptoms such as bradykinesia stem from the loss of dopaminergic innervation of the striatum due to the progressive loss of dopamine neurons in the substantia nigra pars compacta (SNpc). Reflecting this, dopamine replacement therapy remains the primary treatment option. Postmortem analysis has shown that neuron loss can also occur in other brain regions and also revealed the presence of Lewy bodies. These and related structures, Lewy neurites, have become the pathologically defining feature of PD and represents insoluble and aggregated proteins, particularly α-synuclein, packed into intracellular inclusions. However, how these inclusion bodies form and indeed the pathogenesis of PD remain a mystery.
The epidemic of post-encephalitic parkinsonism in the early twentieth century and the cases resulting from inadvertent exposure to MPTP suggested that PD could be due to environmental factors (2). It was not until 1996 that genetics was given serious consideration as a causative factor with the first reporting of a family showing Mendelian segregation of PD. Using linkage analysis, a genetic factor was mapped to chromosome 4 and the locus labeled PARK1 (3). Soon after, an A53T mutation was found in the SNCA gene that encodes the α-synuclein protein (4) leading to a surge in interest in PD genomics that continues to the current time. Nineteen loci that segregate with familial forms of the disease have been reported (Table 1), collectively suggesting that monogenic PD accounts for 5–10% of all PD cases. This aspect of causation in PD has been extensively reviewed elsewhere (5,6)
Table 1.
PARK loci and identified genes that segregate with familial forms of PD
| PARK loci | Gene | Confirmed/putative | Inheritance | Function |
|---|---|---|---|---|
| PARK1/4 | SNCA | Confirmed | AD | Protein aggregation/prion-like-transmission/synaptic function |
| PARK2 | PARKIN | Confirmed | AR | Mitochondrial maintenance/mitophagy/ubiquitin-proteasome |
| PARK3 | Unknown | Putative | AD | Unknown |
| PARK5 | UCH-L1 | Putative | AD | Ubiquitin hydrolase |
| PARK6 | PINK1 | Confirmed | AR | Mitochondrial function/mitophagy |
| PARK7 | DJ-1 | Confirmed | AR | Mitochondrial function/cell stress response |
| PARK8 | LRRK2 | Confirmed | AD | Protein and membrane trafficking/neurite structure/lysosomal autophagy/synaptic function |
| PARK9 | ATP13A2 | Confirmed | AR | Lysosomal autophagy/mitochondrial function |
| PARK10 | Unknown | Confirmed | Risk factor | Unknown |
| PARK11 | GIGYF2 | Putative | AD | Tyrosine kinase receptor signaling/insulin like growth factor pathway |
| PARK12 | Unknown | Confirmed | Risk factor | Unknown |
| PARK13 | HTRA2 | Putative | AD | Mitochondrial function |
| PARK14 | PLA2G6 | Confirmed | AR | Mitochondrial function |
| PARK15 | FBX07 | Confirmed | AR | Mitochondrial maintenance/mitophagy/ubiquitin-proteasome |
| PARK16 | RAB7L1 | Confirmed | Risk factor | Protein and membrane trafficking/lysosomal autophagy |
| PARK17 | VPS35 | Confirmed | AD | Lysosomal autophagy/endocytosis |
| PARK18 | EIF4G1 | Putative | AD | Protein translation |
| PARK19 | DNAJC6 | Confirmed | AR | Synaptic function/endocytosis |
| PARK20 | SYNJ1 | Confirmed | AR | Synaptic function/endocytosis |
Perhaps more disruptively, genetic studies centered on idiopathic cases have successfully identified genetic risk factors under the common disease common variant (CDCV) model. In this review, we will focus on how these genetic findings have shaped our understanding of sporadic PD pathophysiology and draw attention to a number of interesting pathways that result from these analyses.
Genome-wide Association Studies in PD
The CDCV hypothesis suggests that there is a cumulative effect of multiple genetic variants, each of low penetrance, to the risk of an apparently idiopathic disease, such as PD. By looking at ∼1 million polymorphisms in matched case and control cohorts, genome-wide association studies (GWAS) use linkage disequilibrium to nominate genetic regions that impart moderate risk of disease and are present at appreciable frequency in the population (7). In 2009, two independent groups working on a Caucasian and Japanese population sample set reported between them five loci associated with PD (8,9). These included loci containing the genes SNCA, MAPT, LRRK2, BST1 as well as the locus PARK16. Collectively, these studies support the CDCV hypothesis in sporadic PD. Additionally, as SNCA and LRRK2 are genes for Mendelian forms of parkinsonism, GWAS provide strong support for the concept that sporadic PD is mechanistically related to inherited disease.
Further analysis suggested that polymorphisms in the MAPT and BST1 loci were exclusive to European and Japanese populations respectively, highlighting the heterogeneity between disparate ethnic groups. For the MAPT locus, the H2 haplotype that confers risks for disease in Caucasians is absent in Asian populations. BST1 did reach genome-wide significance in a European ancestry population in a PD meta-analysis (10). By combining five Caucasian-based PD GWAS data sets, six previously nominated risk loci including BST1 were confirmed whereas a further five loci were identified for the first time. The total population-attributed risk (PAR) of these 11 loci was determined to be ∼60% (10). These results demonstrate how meta-analyses can be used to find common variants that make small contributions to disease pathogenesis by increasing the resolution of genetic risk (11). The latest and most extensive meta-analysis, incorporating all European ancestry data sets, looked at over 7.8 million variants in a discovery set of >13 000 cases and 95 000 controls. (12). Following validation in the replication set, Nalls et al. found 28 risk variants in 24 loci that are associated with PD (Table 2). Six of these loci were novel whereas four others (GBA, GAK-DGKQ, SNCA and the HLA region) were shown to contain two independent risk variants.
Table 2.
Risk factors for idiopathic PD identified through meta-analysis of GWAS data sets
| Nominated gene | SNP | Chromosome location | P-value | Odds ratio | Implicated pathway(s) |
|---|---|---|---|---|---|
| SNCA | rs356182 | 4q21.1 | 4.16 × 10−73 | 0.76 | Protein aggregation/prion-like-transmission/synaptic function |
| MAPT | rs17649553 | 17q21.1 | 2.37 × 10–48 | 0.769 | Protein aggregation/neurite structure/axonal transport |
| GAK | rs34311866 | 4p16 | 1.02 × 10–43 | 0.786 | Protein and membrane trafficking/lysosomal autophagy |
| GBA | rs35749011 | 1q21 | 1.37 × 10−29 | 1.824 | Lysosomal autophagy/immune response/metabolic pathways |
| MCCC1 | rs12637471 | 3q27 | 2.14 × 10−21 | 0.842 | 3-methylcrotonoyl-CoA carboxylase subunit/leucine breakdown/metabolic pathways |
| ACMSD | rs6430538 | 2q21.3 | 9.13 × 10−20 | 0.875 | Metal ion binding/metabolic pathways |
| STK39 | rs1474055 | 2q24.3 | 1.15 × 10−20 | 1.214 | Immune response/cell stress response/protein kinase binding |
| BST1 | rs11724635 | 4p15 | 9.44 × 10–18 | 1.126 | Immune response |
| RAB7L1 | rs823118 | 1p32 | 1.66 × 10−16 | 1.122 | Protein and membrane trafficking/lysosomal autophagy |
| LRRK2 | rs76904798 | 12q12 | 5.24 × 10–14 | 1.155 | Protein and membrane trafficking/neurite structure/lysosomal autophagy/synaptic function |
| INPP5F | rs117896735 | 10q26.11 | 4.34 × 10–13 | 1.624 | Phosphoric ester hydrolase activity |
| MIR4697 | rs329648 | 11q25 | 9.83 × 10–12 | 1.105 | Non-protein coding |
| RIT2 | rs12456492 | 18q12.3 | 7.74 × 10–12 | 0.904 | GTP-binding/synaptic function/calmodulin binding |
| CCDC62 | rs11060180 | 12q24.31 | 6.02 × 10–12 | 1.105 | Cancer/nuclear receptor coactivator |
| STX1B | rs14235 | 16p11.2 | 2.43 × 10–12 | 1.103 | Synaptic function/exocytosis |
| HLA-DQB1 | rs9275326 | 6p21.3 | 1.19 × 10–12 | 0.826 | Immune response |
| GPNMB | rs199347 | 7p15 | 1.18 × 10–12 | 1.11 | Integrin binding/heparin binding/cancer |
| GCH1 | rs11158026 | 14q22.1-q22.2 | 5.85 × 10–11 | 0.904 | Synaptic function/dopamine synthesis |
| DDRGK1 | rs8118008* | 20p13 | 3.04 × 10–11 | 1.111 | Protein binding |
| SCARB2 | rs6812193 | 4q21.1 | 2.95 × 10–11 | 0.907 | Lysosomal autophagy/GCase receptor |
| VPS13C | rs2414739 | 15q22.2 | 1.23 × 10–11 | 1.113 | Endocytosis/protein and membrane trafficking |
| SIPA1L2 | rs10797576 | 1q42.2 | 4.87 × 10−10 | 1.131 | GTPase activator activity |
| FGF20 | rs591323 | 8p22 | 6.68 × 10–8 | 0.916 | Growth factor activity |
| SREBF1/RAI1 | rs11868035 | 17p11.2 | 5.98 × 10–5 | 0.939 | Chromatin binding/cholesterol and steroid metabolic processes |
It is critical to note at this point that the single-nucleotide polymorphisms (SNPs) reported to be associated with PD most are unlikely to be the actual functional variant but rather are in linkage disequilibrium with a polymorphism that does affect risk. This means that although many loci are assigned gene names, the true causative gene(s) remains formally ambiguous. The improved resolution offered by exome and whole genome sequencing technologies may help resolve this issue in the future. Also important to note is that most GWAS-nominated variants do not change amino acid sequences of the encoded proteins and, therefore, likely work by alterations in gene expression. Expression quantitative trait mapping (eQTL) has therefore been useful in prioritizing candidate genes (13).
With these caveats, there are some loci for which there are exceptionally strong single candidates. For example, within the reproducibly associated region on Chr4, the SNCA gene is particularly strong given its prior genetic and pathological association with PD. To date, over 800 SNPs within the SNCA gene have been reported with nearly half showing a significant association with sporadic PD (http://pdgene.org/view?gene=SNCA). Collectively, these variants may have a substantive effect as a PAR of 12% for the SNCA region has been reported (14). Mechanistically, polymorphisms in the SNCA, particularly around the 3′ untranslated region, may affect RNA processing and ultimately protein expression (8). Additionally, polymorphisms within the microsatellite region Rep1 located in the promoter region ∼10 kb upstream from the SNCA gene start site alters susceptibility to PD (15). Expansion of Rep1, a polymorphic mixed-dinucleotide repeat, has been shown to increase α-synuclein expression in human brains (16,17) and transgenic mouse models (18). Increased expression of α-synuclein is known to cause parkinsonism in a dose-dependent manner as demonstrated by individuals harboring SNCA copy number variant (19,20). As duplication and triplication, respectively, result in a 50 and 100% increase in a-synuclein expression, it is feasible that chronic increased α-synuclein expression in the order of as little as 10% may increase the risk of developing PD in the range of that nominated by GWAS (21).
The second significant variant to come out of the meta-analysis maps to the MAPT locus on chromosome 17, with the top SNP being rs17649553. The PAR attributed to this locus is 10%, second only to SNCA (14). Although MAPT has been associated with Progressive Supranuclear Palsy (PSP) (22), another parkinsonism disorder, its identification as the second highest-ranked risk factor for PD was somewhat surprising. In contrast to the association of the H1 haplotype with PSP (23), for PD it is the H2 haplotype. This is consistent with the lack of association between MAPT haplotype and PD in Japanese samples (9), as discussed earlier. The MAPT locus is large, containing several genes and the H1/H2 haplotypes do not recombine, meaning that it is plausible that another gene rather than MAPT is associated with PD. However, given that MAPT mutations are associated with frontotemporal dementia with parkinsonism and that tau pathology is sometimes observed upon postmortem analysis of idiopathic PD or some familial mutations (24,25), MAPT remains the most logical candidate for PD risk in this locus. Whether the variants affect expression of MAPT, as is likely for SNCA, or other aspects of gene regulation such as splicing, is not yet clear.
Along with SNCA and MAPT, a third gene that had been associated with inherited PD, LRRK2, was also identified in the original PD GWAS (8,9). Interestingly, the associated SNPs were located in the 5′ region of the LRRK2 locus, again suggesting effects on transcript processing and expression. LRRK2 is a kinase, and neuronal toxicity associated with increased LRRK2 kinase activity has been documented (26,27). The G2019S mutations, which elevates kinase activity, account for ∼1% of idiopathic PD cases in Caucasian populations (28), probably due to incomplete, age-dependent penetrance (29). Therefore, although not proven, it is possible that increased LRRK2 expression, leading indirectly to a modest overall risk by increasing net kinase activity, might be relevant to disease risk. In contrast to SNCA and MAPT, there may be risk associated with variants that change amino acids, likely to be rare individually but cumulatively important in a large gene such as LRRK2. For example, in Asian populations, four exonic variants (G2019S, G2385R, R1628P and A419V) were found to be risk factors associated with PD susceptibility in a LRRK2 centered meta-analysis (30).
Numerically, GBA could be argued to be the most exciting risk factor gene identified as mutations in this gene are more common than SNCA or LRRK2 (31). Additionally, variants in the GBA locus confer an odds ratio of 1.84 for disease risk, the highest of all PD-associated genes. Supporting a general link to α-synuclein pathology, GBA is also a risk factor for dementia with Lewy bodies at an even higher odds ratio of 8.28 (32). GBA encodes a lysosomal hydrolase, glucocerebrosidase (GCase), which converts the lipid glucocerebroside into glucose and ceramide. Loss of GBA function results in abnormal lipid accumulation and in an autosomal recessive manner presents as the lysosomal storage disorder Gaucher disease characterized by the presence of glucocerebroside-laden lysosomes in macrophages (33). Over 300 GBA mutations have been reported with N370S and L444P being the most common. Both Gaucher patients and heterozygous carriers have an increased likelihood of developing PD with 2–10% of PD patients, depending on ethnicity, harboring GBA mutation (34). Carriers of a single GBA mutation have an odds ratio of 5.4 for developing PD (35). Some mutations, like E326K, appear to be exclusive to PD patients (36). A heterozygous E471G mutation in SCARB2 has also been reported as a modifier for Gaucher disease (37). Interestingly, SNP rs6812193, located upstream of SCARB2 gene and in the locus shared by FAM47E, is a reported risk factor for idiopathic PD (12,38). SCARB2 encodes for lysosomal integral membrane protein type 2 (LIMP-2), a receptor for GCase (39). Therefore, collectively, these data show that GCase and associated genes represent a strong risk factor for PD likely due to a partial loss of function.
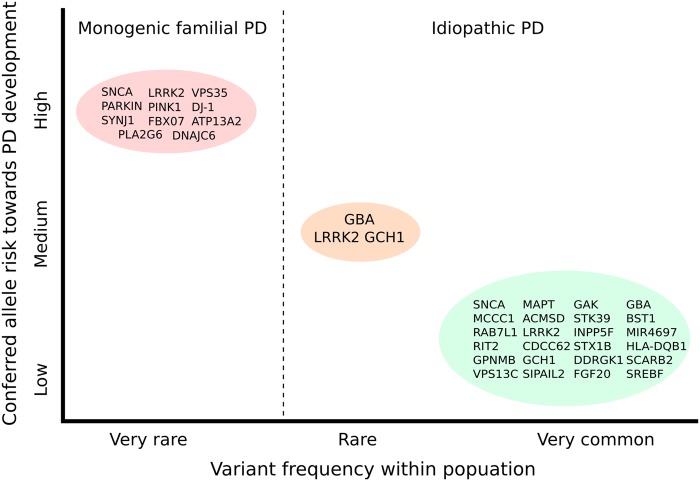
As discussed earlier, as well as loci including Mendelian genes, there are an additional 20 loci that confer a variable amount of risk to PD development (Fig. 1). To go through each of them would become rather repetitive and, at many loci, there are no candidates that can be strongly prioritized. However, we will briefly discuss some of the loci that have been replicated across studies.
Figure 1.
Schematic overview of frequency and conferred risk of genes and genetic loci associated with the development of familial and idiopathic PD/parkinsonism. Genes listed in the red circle represent those identified through analysis of familial cases and give rise to monogenic forms of PD. The green circle contains genes in loci nominated as risk factors for idiopathic PD following meta-analysis of all European GWAS data sets. Lastly, the orange circle highlights a number of high-risk variants with reported odds ratios of >5. Adapted from (131).
The HLA locus, specifically the HLA-DQB1 allele, was reported to be associated with PD prior to GWAS (40). However, the first SNP in this region to reach genome-wide significance was situated in HLA-DRA (41). Subsequent associations were reported in HLA-DRB5 (10) and HLA-DRB1 (42). The most recent meta-analysis returned the HLA-DQB1 allele as a risk factor for PD (12). The reasons for the uncertainty around which exact allele influences risk is likely due to the HLA region being extremely polymorphic and also displaying a high density of closely located genes and complicated patterns of linkage disequilibrium (43). It is also plausible that multiple HLA loci are associated with PD (44). Of note, the HLA region harbors multiple risk alleles that alter susceptibility to Multiple Sclerosis (45). Nonetheless, the data suggest that one or more genes in the HLA region influence PD risk.
The PARK16 locus contains a risk factor with a relatively strong effect, with an OR of 1.3 for rs957211 in the original Japanese GWAS (9). Reflecting the locus designation, rather than a single named gene, there are three candidate genes in a single linkage disequilibrium block (NUCKS1, RAB7L1 and SLC41A1) with two additional genes nearby that might also be candidates (SLC45A3 and PM20D1/FLJ32569). Therefore, unlike the loci that also contain Mendelian genes, there is no clear single candidate in this region. Using an eQTL approach might slightly narrow the number of candidates, but there is signal at both NUCKS1 and RAB7L1, and some associations between cytosine methylation and risk allele (12). Therefore, for PARK16, there is no clear single gene that explains disease risk, and the locus may be complex with epistatic interactions between more than one gene.
Similar situations occur at other GWAS loci for PD. On chr4p, there is a risk locus including the gene encoding cyclin G-associated kinase, GAK, and Diacylglycerol Kinase, DGKQ, that are all reasonable candidate genes. To date, no evidence has been presented showing that any of the genes have eQTL or other associations. The one candidate that might be most logical is GAK, in that mutations in a homologous gene, DNAJC6, that encodes for auxilin are associated with an early onset form of parkinsonism (46). Along similar lines, there is signal from GWAS around the gene encoding GTP cyclohydrolase (GCH1), a key enzyme in the synthesis of dopamine that is a known cause of dopa-responsive dystonia (47). GCH1 is reportedly a substantive risk factor for PD with heterozygous variants conferring an odds ratio of 7.5 for PD development (48). Enrichment of GCH1 variants in PD patients has been confirmed in a second smaller study (49).
Implicated Molecular Pathways
These results show that even sporadic PD has a significant genetic component, but that this is oligogenic in nature and each locus imparts a small to modest contribution to lifetime risk of disease. Although there is ambiguity about which gene is the functional effector at many loci, it is reasonable to infer that there are one or two genes per locus and that they should share some underlying biological connections. It is noteworthy that for familial PD, factors that govern mitochondrial functioning and clearance are given considerable weight, especially in the recessive diseases related to PINK1/parkin. For sporadic PD, the complement system and innate immunity are highlighted, along with aspects of regulation of vesicular transport. There are likely even relationships between these major themes, as protein degradation and its regulation is a component of several groups of these genes. We will therefore discuss which of these pathways to parkinsonism are the most promising in the context of tying together multiple nominated PD risk genes.
Neuroinflammation and the innate immune system
Neuroinflammation and the complement system provide an interesting convergence point for a number of genetic risk factors of PD. In particular, pathway analysis point to leucocyte/lymphocyte activity and cytokine mediated signaling (50). At least half of the genes identified as risk factors for PD in individuals of European ancestry have been associated with the complement cascade system in some capacity: SNCA, MAPT, GBA, STK39, LRRK2, HLA, GPNM8, GCH1, DDRGK1, SCARB2, FGF20 and SREBF1 [reviewed in (51)].
The idea that neuroinflammation and the innate immune system contribute to PD etiology predates GWAS and includes observations such as post-encephalitic parkinsonism that clearly has an inflammatory basis (52). Neuroinflammation is reported to induce α-synuclein aggregation and dopamine neuronal loss in mice following administration of avian influenza virus H5N1 (53). Clinically, analysis of CSF and postmortem brain reveal an increase in pro-inflammatory cytokines (IFNγ, TNFα, etc.) in PD (54) whereas the prolonged use of ibuprofen, a non-steroidal anti-inflammatory drug, has been suggested to lower the risk of developing PD (55).
Microglia are the resident innate immune cells of the brain, providing immune surveillance and releasing cocktails of pro-inflammatory cytokines, chemokines, reactive oxygen and nitrogen species and growth factors after activation (52). Microglial can remain active years after injury (56), and a high density of activated microglia has been observed in the SN of PD brains (57). Given the delicate nature of dopaminergic neurons, it is conceivable that they are sensitive to a chronic inflammatory environment. Supporting this concept, degeneration of dopaminergic neurons with sparing of GABAergic and seretonergic neurons was observed in the nigrostriatal system of rats following intranigral injection of lipopolysaccharides (LPS) (58).
The association of the HLA locus with PD (12,41) provides genetic support of the involvement of the complement system in PD pathogenesis. Genes within the HLA region encode for the major histocompatibility (MHC) class of proteins that bind peptide fragments and present them to T cells. Microglia act as antigen presenting cells and have been found to have higher MHC expression in the PD brains compared with controls (57). Similarly, elevated MHC class II (MHCII) antigen expression was also observed in the SN and striatum of mice using toxin models of dopamine depletion (59). MHCII is encoded by HLA-DRA, the original allele identified in GWAS studies (41).
α-Synuclein is also linked to neuroinflammation. The presence of aggregated α-synuclein in mice is sufficient to induce MHCII expression in microglia (60,61). Transgenic mice overexpressing human α-synuclein have elevated levels of activated microglia in the brain (61). This is phenocopied following LPS administration into the SN of mice overexpressing α-synuclein or pathogenic variants compared with α-synuclein knockout mice (62). In the transgenic mice, dopaminergic neuronal death occurs alongside the presence of aggregated, insoluble nitrated α-synuclein.
In the context of the immune system, α-synuclein acts as a danger-associated molecular pattern (DAMP) and is capable of stimulating toll-like receptors (TLR) (51). Excess neuronal α-synuclein can undergo exocytosis into the microenvironment (63) where it is cleared by neighboring cells, thereby preventing the degradation system in one cell from becoming overburdened. This process is not exclusive to physiological α-synuclein and can be hijacked by pathogenic variants (64–66). Misfolded and fibrillar forms of α-synuclein have been shown to activate microglia (67) via TLR2 (68) and TLR4 (69). TLR4 has specifically been shown to mediate cell death in a murine MPTP model of PD (70) whilst polymorphisms in the TLR4 gene were recently proposed to alter the risk of developing sporadic PD in Han Chinese population (71), although this result has not been replicated at the time of writing. Collectively, these studies demonstrate that α-synuclein instigates the production of inflammatory cytokines and ROS that may then contribute to the neuronal loss witnessed in PD (51).
At present, the role of MAPT in neuroinflammation with regards to PD is poorly understood. However, given the presence of tau pathology in some cases of PD, inferences can be made from other tauopathies, particularly Alzheimer's disease (AD). Activated microglia are often observed alongside tau deposits in the brains of human AD patients and mouse models (72–74). In this instance, neuroinflammation is reported to precede tau pathology (74), meaning that tau aggregation may be consequential to the actions of inflammatory mediators on neurons. In line with this concept, cytokines such as IL-1, IL-6 and nitric oxide are reported to increase tau phosphorylation in primary neuronal cells (75,76). Whether MAPT acts in part by modulating neuroinflammation in the PD brain remains to be clarified.
LRRK2 also has established and clear-cut links to inflammation and immunity. LRRK2 has moderate homology to the receptor-interacting protein kinases, a family of kinases with known roles in immunity (77). Variants at the LRRK2 locus were nominated as conferring increased risk of Crohn's disease (78) and leprosy infection (79). Both monocytes and B-lymphocytes have high basal expression of LRRK2, which is increased following exposure to gamma interferon (IFNγ) (80). Microglia from mice also has expression of LRRK2 that can be increased via LPS treatment (81). LPS treatment induces phosphorylation of LRRK2 at s910 and s935, thereby altering its interaction with 14-3-3 proteins and eliciting a shift in subcellular localization (82,83). Microglia and macrophages are involved in xenophagy, a selective autophagic process centered on the clearance of pathogens. Thus, any disruption of this heavily regulated system is likely to impact the clearance of non-host entities. In line with this, LRRK2 knockdown in macrophages has been shown to impair LPS initiated -autophagy (83) and clearance of Salmonella typhimurium (80).
Associations with immunity, neuroinflammation and the recessive PD genes have also been reported. Similar to LRRK2, Parkin has been associated with leprosy infection as polymorphisms in the promoter region increase vulnerability to Mycobacterium leprae (84). An increased susceptibility to Mycobacterium tuberculosis infection has also been observed in Parkin knockout mice (85). LPS administration to Parkin deficient mice results in increased production of microglia derived TNFα, IL-6 and iNOS (86) and increased neuronal loss (87) compared with wild-type controls. Similarly, analysis of brain homogenates from LPS-treated Pink1 knockout mice showed higher levels of IL-1B, IL-12 and TNFα in comparison with wild type (88). Striatal gene expression profiling in Pink1 null mice revealed an aberrant expression of genes that regulate innate immunity (88). Furthermore, in a study of brain inflammation, acutely prepared cortical slices to mimic injury from Pink1 null mice displayed increased NFκB signaling along with higher levels of TNFα, IL-6 and IL-1B compared with wild-type controls (89).
Emerging evidence suggests mitochondria can contribute to innate immune pathways [reviewed in (90)], providing a further avenue for Parkin and Pink1 to indirectly affect inflammatory signaling. Eukaryotic mitochondria are endosymbiants that have bacterial origins (91) and share a number of similarities such as a nuclear independent circular genome with non-methylated CpG. motifs (92) and N-formylated proteins (91). Similar to α-synuclein, mitochondria are endogenous DAMPs that can act through TLR9 (93) to elicit an immune response, as shown by intravenous administration of a mitochondrial DAMP cocktail inducing inflammation in the lungs and liver of rats (94). Both Parkin and Pink1 act in synergy to maintain mitochondrial integrity in drosophila (95) and mouse (96) models. Therefore, one can link multiple forms of genetically inherited parkinsonism to the regulation of innate immunity in response to a number of extrinsic or intrinsic agents.
Less is certain about what possible role the remaining candidate genes for PD risk play in the complement cascade. BST1 encodes for the leucocyte surface protein CD157 and may facilitate pre-B-cell growth, being up-regulated in bone marrow cells from patients diagnosed with rheumatoid arthritis (97). STK39 and its protein Ste20-related proline/alanine rich-kinase (SPAK) have been shown to alter intestinal inflammatory levels in mouse models of colitis (98,99). Similarly, FGF20 is also associated with colitis and has been shown to have therapeutic benefits in mice (100). DDRGK1 has been shown to regulate the activity of the transcription factor NFκB (101). GPNMB has been shown to be increase expression of the matrix metalloprotease-3, a proteinase implicated in extracellular matrix degradation, in BV2 cells following LPS activation (102). SREBF1 meanwhile may regulate innate immune responses through its actions on lipid metabolism (103). SCARB2 is a known receptor for entrovirus 71 (EV71) (104) and finally RIT2 may modulate INF-γ signaling (105).
It is therefore possible to map multiple candidates from the GWAS-derived linkage peaks to aspects of innate immunity. Whether neuroinflammation is the only pathway is more difficult to assess. Some of the genes nominated by GWAS for PD are not classic immune genes. For example, expression of MAPT is largely restricted to neurons, suggesting that there may be other pathways that are restricted to these cells (Fig. 2). Next, we will discuss an alternate set of pathways that may include cell-autonomous mechanisms restricted to neurons.
Figure 2.
Molecular processes involved in PD pathogenesis as highlighted by genetic findings. Using genes recently nominated as risk factors for idiopathic PD along with those responsible for familial PD, it is possible to extrapolate a number of cellular processes that may underlie disease development. Each large gray circle represents a biological process and details the genes linked to it. Genes listed in italics represent nominated risk factors for idiopathic PD, identified through GWAS, whereas those in normal font are associated with familial PD. An asterisk denotes that the gene is linked to both forms of the disorder. Some genes like SNCA and LRRK2 are associated with multiple processes. While the majority of cellular pathways contribute to both familial and sporadic forms of the disease, neuroinflammations likely plays a more prominent role the latter. Conversely, mitochondrial dysfunction shows a greater association with familial PD. Adapted from (132).
Membrane trafficking and protein clearance
A second molecular pathway that is implicated in PD involves vesicular trafficking and degradation of proteins and organelles by the autophagy-lysosome pathway. These aspects are interconnected as organelles like the endoplasmic reticulum (ER), Golgi apparatus, small vesicles and lytic compartments collectively form the endomembrane system. Neurons are highly polarized cells with the soma being fundamentally different from axons and synaptic terminals. The neuronal cell body contains the nucleus, where gene expression is controlled, and the largest amounts of ER, Golgi apparatus and lysosomes which are major sites of manufacture and clearance of proteins. Synapses are specialized for neurotransmitter release and reuptake. An extensive network of microtubule tracks that constitutes the cellular cytoskeletal network connects the two regions of the neuron. Given this arrangement, it is clear that communication between different compartments of the cell is important and likely regulated in neurons in different ways from other cell types that are less specialized.
Tau, the protein product of the MAPT gene, is fundamental to the maintenance of the cytoskeletal network and governs axonal transport along this system through interactions with the motor proteins Kinesin and Dynein (106). Tau isoforms with more repeat (4R) regions exhibit stronger binding to microtubules than those with fewer (3R). Such shorter isoforms of tau inhibit axonal transport by motor proteins (107). The H1 haplotype of tau reportedly increases the 4R:3R isoform ratio (108). As discussed earlier, a possible mechanism by which MAPT risk alleles might affect PD pathogenesis is via altered splicing, which would therefore be a neuronal autonomous mechanism.
Tau is also heavily regulated by phosphorylation, which tends to decrease its propensity for microtubule association. Interestingly, higher levels of S396 phosphorylated tau were observed in synapse-enriched fractions from PD samples (109). α-Synuclein has been reported to promote tau phosphorylation via GSK3β at S396 and other residues (110,111). LRRK2 mutations are reportedly capable of giving rise to tangle pathology (25), and both LRRK2 and tau have been implicated in neurite morphology (112,113). Recently, LRRK2 was shown to directly increase tau phosphorylation at several epitopes both in vitro and in vivo (114). In particular, transgenic mice expressing the hyper kinase active G2019S LRRK2 protein showed evidence of increased tau phosphorylation (115). Like α-synuclein, LRRK2 may also utilize GSK3β to phosphorylate tau (116). Finally, LRRK2 can interact directly with microtubules via specific isoforms of β-tubulin (117). Therefore, two major genes for PD that are also GWAS risk candidates can be linked to the regulation of tau, with presumed attendant effects on axonal communication between cell body and synapse.
One functional consequence of impairment to axonal trafficking is on the retrieval and clearance of proteins and organelles. Additionally, gathering evidence suggests that LRRK2 is a direct regulator of autophagy. We and others have demonstrated that LRRK2 physically interacts with another candidate gene, RAB7L1 (118,119). We have also nominated, from an unbiased screen, GAK as a direct LRRK2 interactor. Together, LRRK2, Rab7L1 and GAK form a complex that promotes the clearance of Golgi-derived vesicles via the lysosomal autophagy pathway, a phenomena that can be promoted by LRRK2 pathogenic mutations (118). Extending this further, VPS35 has been implicated in the same functional pathway with overexpression capable of rescuing endolysosomal and Golgi apparatus defects caused by PD linked mutations in LRRK2 (119). Auxilin, encoded by DNAJC6 and expressed exclusively in neurons, may also contribute to this pathway as it has been shown to facilitate clathrin-mediated trafficking between Golgi and lysosomes (46). Collectively, these observations support the hypothesis that multiple PD genes and risk factors can be mapped to a linked pathway related to the early events in vesicular transport and initiation of autophagy.
There are several additional observations suggesting that lysosomal function, which is required to turnover of proteins and organelles by autophagy, is impacted in PD. For example, postmortem examination of sporadic PD brains reveals a decrease in number of lysosomal associated proteins including cathepsin D, LAMP-1 and LAMP-2A (120). In animal models, impairment of autophagy via knockout of autophagy-related 7 (ATG7) in the SN lead to the buildup of presynaptic α-synuclein, striatal dopamine depletion and motor deficits (121).
The clearest genetic indication that the lysosomal autophagy pathway plays a role in PD comes from ATP13A2 and GBA. ATP13A2 (PARK9) encodes for a lysosomal ATPase, and pathogenic mutations are associated with Kufor-Rakeb syndrome, a complex juvenile-onset form of parkinsonism (122). Loss of ATP13A2 function has been shown to result in a lysosomal deficiency and deterioration marked by impaired lysosomal acidification, instability of lysosomal membrane and decreased clearance of autophagosomes (123). In human fibroblasts and mouse primary cortical neurons, deficiency of ATP13A2 has also been reported to result in α-synuclein accumulation (124) whereas in yeast, worms and neuronal models, overexpression of ATP13A2 was protective against α-synuclein induced toxicity (125). Additionally, ATP13A2 expression was observed to be elevated in surviving dopaminergic neurons in human PD brains (122,126).
GBA is also associated with increased intracellular α-synuclein as observed in the brains of idiopathic PD individuals (127). Transgenic mice carrying the D409V mutation showed an age-dependent accumulation of α-synuclein (128). Similarly, reduced GBA expression in mouse neurons or expression of pathogenic N370S in iPSCs also leads to α-synuclein accumulation and the presence of oligomers (129). In the same system, α-synuclein was interestingly shown to inhibit vesicular GCase trafficking from the ER-Golgi complex to lysosomes. Thus, it is possible that any above normal α-synuclein expression brought about by genetic variations may instigate a positive feedback loop where lysosomal function is impaired leading to further elevation of intracellular α-synuclein levels. This would ultimately give rise to α-synuclein oligomer formation. In PC12 cells, pharmacological induction of autophagy or restoration of GCase shuttling to lysosomes using either rapamycin or isofagomine, respectively, is capable of abating α-synuclein accumulation in the presence of overexpressed mutant GBA (128). Likewise, any functional impairment of LIMP-2 that may arise from changes in the SCARB2 locus may also inhibit GCase trafficking and resulting in a similar outcome.
These diverse examples suggest that the autophagy-lysosome system can, when dysregulated, lead to PD. Here, we tie them into neuronal-specific processes by considering that neurons have an especially high requirement for adequate and accurate regulation of vesicle transport via the microtubule system that depends on tau. Thus, in this view of the PD risk genes, we see a more neuronal pathway for pathogenesis.
Concluding Remarks
While genetic analysis of PD risk in both familial and sporadic cases has provided us with a wealth of information, in a sense we currently have jigsaw puzzle pieces without knowing the full picture that we are trying to reconstruct. However, it is clear that there are specific sets of interactions between implicated genes for some forms of PD. For example, in recessive parkinsonism, there are strong ties between Parkin and Pink1 (130), whereas we have proposed a link between Lrrk2, Rab7l1 and Gak by virtue of the three proteins being in the same complex (118). As discussed earlier, there are ways to extend these analyses out further to include other genes for PD and related disorders.
By working through the examples given here, we propose two generic pathways that might contribute to PD pathogenesis and, interestingly, these may indicate both cell-autonomous and non-cell-autonomous mechanisms. Neuroinflammation would involve both neurons and microglia (and potentially other immunologically active cells) whereas the autophagy-lysosome system, dependent as it is on microtubules, would lead to more restricted damage pathways. These are not meant to be mutually exclusive competing hypotheses as it is clear that some proteins, α-synuclein in particular, might be involved in both synaptic function (hence neuronal) but also in triggering neuroinflammation via TLR signaling. The autophagy pathway is likely important in both neurons and non-neuronal cells, further blurring these clean lines. Overall, it is our view at this time that PD pathways include both neuronal and non-neuronal cells and that the risk of PD is complex because both of these cell types can be impacted.
Thus far, GWAS has proved to be a powerful and successful tool for identifying common genetic variants associated with increased risk of PD development.
However, the danger with this approach is that because the GWAS linkage peaks are broad, several of the nominated candidates in the paragraph above may not actually be the causal genes. Where a given biological process is a broad category, the probability of overlap by chance alone is raised. Clarification of the actual functional gene at each locus is therefore required before re-assessing biological pathways.
Both eQTL mapping and epigenetic changes have been observed for a number of GWAS loci that might clarify some regions (12), but these might be further refined by improved technical approaches such as replacing current array data sets with RNA-Seq or by adding in new data sets. The examples of protein complex identification above (118) show how useful unbiased, ideally genome-wide functional screens can be. One could imagine adding other proteomics approaches, both in human brain and as screening tools for interactions, to refine candidates. Finally, siRNA-based screens against PD-relevant phenotypes, which might include immune regulation, autophagy or microtubule transport, could provide additional resolution at specific loci.
One of the biggest remaining challenges to the field will be to move from candidate genes to actual functional variants. A limitation in assessing the CDCV hypothesis is that we usually do not have full genomic coverage, relying instead on assayable proxy markers for genetic variation. Re-sequencing in high depth the PD loci, perhaps in combination with exome (or whole genome) sequencing, will likely yield resolution at some loci. It will still be important to test candidate variants in functional assays, again highlighting the importance of having validated assays that are specific for PD relevant processes.
Overall, there has been a huge and surprising acceleration in our understanding of pathways leading to parkinsonism in the past decade. We hope that this pace will continue in the next few years and start to impact how we view, and eventually treat, this devastating disorder.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Aging.
Acknowledgments
Conflict of Interest statement. None declared.
References
- 1.Parkinson J. (2002) An essay on the shaking palsy. J. Neuropsychiatry Clin. Neurosci., 14, 223–236. [DOI] [PubMed] [Google Scholar]
- 2.Trinh J., Farrer M. (2013) Advances in the genetics of Parkinson disease. Nat. Rev. Neurol., 9, 445–454. [DOI] [PubMed] [Google Scholar]
- 3.Polymeropoulos M.H., Higgins J.J., Golbe L.I., Johnson W.G., Ide S.E., Di Iorio G., Sanges G., Stenroos E.S., Pho L.T., Schaffer A.A. et al. (1996) Mapping of a gene for Parkinson's disease to chromosome 4q21-q23. Science, 274, 1197–1199. [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R. et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science, 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- 5.Corti O., Lesage S., Brice A. (2011) What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol. Rev., 91, 1161–1218. [DOI] [PubMed] [Google Scholar]
- 6.Farrer M.J. (2006) Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet., 7, 306–318. [DOI] [PubMed] [Google Scholar]
- 7.Lin M.K., Farrer M.J. (2014) Genetics and genomics of Parkinson's disease. Genome Med., 6, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon-Sanchez J., Schulte C., Bras J.M., Sharma M., Gibbs J.R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S.W., Hernandez D.G. et al. (2009) Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet., 41, 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A. et al. (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet., 41, 1303–1307. [DOI] [PubMed] [Google Scholar]
- 10.International Parkinson Disease Genomics Consortium Nalls M.A., Plagnol V., Hernandez D.G., Sharma M., Sheerin U.-M., Saad M., Simón-Sánchez J., Schulte C., Lesage S. et al. (2011) Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet, 377, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer C.C.A., Su Z., Donnelly P., Marchini J. (2009) Designing genome-wide association studies: sample size, power, imputation, and the choice of genotyping chip. PLoS Genet., 5, e1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalls M.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M., DeStefano A.L., Kara E., Bras J., Sharma M. et al. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet., 46, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez D.G., Nalls M.A., Moore M., Chong S., Dillman A., Trabzuni D., Gibbs J.R., Ryten M., Arepalli S., Weale M.E. et al. (2012) Integration of GWAS SNPs and tissue specific expression profiling reveal discrete eQTLs for human traits in blood and brain. Neurobiol. Dis., 47, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards T.L., Scott W.K., Almonte C., Burt A., Powell E.H., Beecham G.W., Wang L., Züchner S., Konidari I., Wang G. et al. (2010) Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet., 74, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maraganore D.M., de Andrade M., Elbaz A. et al. (2006) Collaborative analysis of α-synuclein gene promoter variability and Parkinson disease. JAMA, 296, 661–670. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs J., Tichopad A., Golub Y., Munz M., Schweitzer K.J., Wolf B., Berg D., Mueller J.C., Gasser T. (2008) Genetic variability in the SNCA gene influences α-synuclein levels in the blood and brain. FASEB J., 22, 1327–1334. [DOI] [PubMed] [Google Scholar]
- 17.Linnertz C., Saucier L., Ge D., Cronin K.D., Burke J.R., Browndyke J.N., Hulette C.M., Welsh-Bohmer K.A., Chiba-Falek O. (2009) Genetic regulation of alpha-synuclein mRNA expression in various human brain tissues. PloS One, 4, e7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cronin K.D., Ge D., Manninger P., Linnertz C., Rossoshek A., Orrison B.M., Bernard D.J., El-Agnaf O.M.A., Schlossmacher M.G., Nussbaum R.L. et al. (2009) Expansion of the Parkinson disease-associated SNCA-Rep1 allele upregulates human α-synuclein in transgenic mouse brain. Hum. Mol. Genet., 18, 3274–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrer M., Kachergus J., Forno L., Lincoln S., Wang D.-S., Hulihan M., Maraganore D., Gwinn-Hardy K., Wszolek Z., Dickson D. et al. (2004) Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann. Neurol., 55, 174–179. [DOI] [PubMed] [Google Scholar]
- 20.Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R. et al. (2003) Alpha-synuclein locus triplication causes Parkinson's disease. Science, 302, 841. [DOI] [PubMed] [Google Scholar]
- 21.Hardy J. (2010) Genetic Analysis of pathways to Parkinson disease. Neuron, 68, 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höglinger G.U., Melhem N.M., Dickson D.W., Sleiman P.M.A., Wang L.-S., Klei L., Rademakers R., de Silva R., Litvan I., Riley D.E. et al. (2011) Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet., 43, 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandrovcova J., Pittman A.M., Malzer E., Abou-Sleiman P.M., Lees A.J., Wood N.W., de Silva R. (2009) Association of MAPT haplotype-tagging SNPs with sporadic Parkinson's disease. Neurobiol. Aging, 30, 1477–1482. [DOI] [PubMed] [Google Scholar]
- 24.Rajput A., Dickson D.W., Robinson C.A., Ross O.A., Dächsel J.C., Lincoln S.J., Cobb S.A., Rajput M.L., Farrer M.J. (2006) Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology, 67, 1506–1508. [DOI] [PubMed] [Google Scholar]
- 25.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B. et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron, 44, 601–607. [DOI] [PubMed] [Google Scholar]
- 26.Smith W.W., Pei Z., Jiang H., Dawson V.L., Dawson T.M., Ross C.A. (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci., 9, 1231–1233. [DOI] [PubMed] [Google Scholar]
- 27.West A.B., Moore D.J., Biskup S., Bugayenko A., Smith W.W., Ross C.A., Dawson V.L., Dawson T.M. (2005) Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl Acad. Sci. USA, 102, 16842–16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeraully T., Tan E.K. (2012) Genetic variants in Sporadic Parkinson's disease: East vs West. Parkinsonism Relat. Disord., 18, Supplement 1, S63–S65. [DOI] [PubMed] [Google Scholar]
- 29.Kachergus J., Mata I.F., Hulihan M., Taylor J.P., Lincoln S., Aasly J., Gibson J.M., Ross O.A., Lynch T., Wiley J. et al. (2005) Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet., 76, 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X., Tang K.-F., Li Y., Xiong Y.-Y., Shen L., Wei Z.-Y., Zhou K.-J., Niu J.-M., Han X., Yang L. et al. (2012) Quantitative assessment of the effect of LRRK2 exonic variants on the risk of Parkinson's disease: A meta-analysis. Parkinsonism Relat. Disord., 18, 722–730. [DOI] [PubMed] [Google Scholar]
- 31.Schapira A.H.V. (2015) Glucocerebrosidase and Parkinson disease: Recent advances. Mol. Cell Neurosci., 66, Part A, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nalls M.A., Duran R., Lopez G. et al. (2013) A multicenter study of glucocerebrosidase mutations in dementia with lewy bodies. JAMA Neurol., 70, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang N.-Y., Lee Y.-N., Lee H.-J., Kim Y.S., Lee S.-J. (2013) Glucocerebrosidase, a new player changing the old rules in Lewy body diseases. Biol. Chem., 394, 807–818. [DOI] [PubMed] [Google Scholar]
- 34.Sidransky E., Lopez G. (2012) The link between the GBA gene and parkinsonism. Lancet Neurol., 11, 986–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R., Bar-Shira A., Berg D., Bras J., Brice A. et al. (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med., 361, 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duran R., Mencacci N.E., Angeli A.V., Shoai M., Deas E., Houlden H., Mehta A., Hughes D., Cox T.M., Deegan P. et al. (2013) The glucocerobrosidase E326 K variant predisposes to Parkinson's disease, but does not cause Gaucher's disease. Mov. Disord., 28, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velayati A., DePaolo J., Gupta N., Choi J.H., Moaven N., Westbroek W., Goker-Alpan O., Goldin E., Stubblefield B.K., Kolodny E. et al. (2011) A mutation in SCARB2 is a modifier in Gaucher disease. Hum. Mutat., 32, 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Do C.B., Tung J.Y., Dorfman E., Kiefer A.K., Drabant E.M., Francke U., Mountain J.L., Goldman S.M., Tanner C.M., Langston J.W. et al. (2011) Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet., 7, e1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reczek D., Schwake M., Schröder J., Hughes H., Blanz J., Jin X., Brondyk W., Van Patten S., Edmunds T., Saftig P. (2007) LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell, 131, 770–783. [DOI] [PubMed] [Google Scholar]
- 40.Lampe J.B., Gossrau G., Herting B., Kempe A., Sommer U., Füssel M., Weber M., Koch R., Reichmann H. (2003) HLA typing and Parkinson's disease. Eur. Neurol., 50, 64–68. [DOI] [PubMed] [Google Scholar]
- 41.Hamza T.H., Zabetian C.P., Tenesa A., Laederach A., Montimurro J., Yearout D., Kay D.M., Doheny K.F., Paschall J., Pugh E. et al. (2010) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat. Genet., 42, 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed I., Tamouza R., Delord M., Krishnamoorthy R., Tzourio C., Mulot C., Nacfer M., Lambert J.-C., Beaune P., Laurent-Puig P. et al. (2012) Association between Parkinson's disease and the HLA-DRB1 locus. Mov. Disord. Off. J. Mov. Disord. Soc., 27, 1104–1110. [DOI] [PubMed] [Google Scholar]
- 43.Wissemann W.T., Hill-Burns E.M., Zabetian C.P., Factor S.A., Patsopoulos N., Hoglund B., Holcomb C., Donahue R.J., Thomson G., Erlich H. et al. (2013) Association of Parkinson disease with structural and regulatory variants in the HLA region. Am. J. Hum. Genet., 93, 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill-Burns E.M., Factor S.A., Zabetian C.P., Thomson G., Payami H. (2011) Evidence for more than one Parkinson's disease-associated variant within the HLA region. PLoS ONE, 6, e27109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patsopoulos N.A., Barcellos L.F., Hintzen R.Q., Schaefer C., van Duijn C.M., Noble J.A., Raj T., Gourraud P.-A., Stranger B.E., Oksenberg J. et al. (2013) Fine-mapping the genetic association of the major histocompatibility complex in multiple sclerosis: HLA and non-HLA effects. PLoS Genet., 9, e1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edvardson S., Cinnamon Y., Ta-Shma A., Shaag A., Yim Y.-I., Zenvirt S., Jalas C., Lesage S., Brice A., Taraboulos A. et al. (2012) A Deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS ONE, 7, e36458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinberger D., Weber Y., Korinthenberg R., Deuschl G., Benecke R., Martinius J., Müller U. (1998) High penetrance and pronounced variation in expressivity of GCH1 mutations in five families with dopa-responsive dystonia. Ann. Neurol., 43, 634–639. [DOI] [PubMed] [Google Scholar]
- 48.Mencacci N.E., Isaias I.U., Reich M.M., Ganos C., Plagnol V., Polke J.M., Bras J., Hersheson J., Stamelou M., Pittman A.M. et al. (2014) Parkinson's disease in GTP cyclohydrolase 1 mutation carriers. Brain J. Neurol., 137, 2480–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guella I., Sherman H.E., Appel-Cresswell S., Rajput A., Rajput A.H., Farrer M.J. (2015) Parkinsonism in GTP cyclohydrolase 1 mutation carriers. Brain, 138, e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmans P., Moskvina V., Jones L., Sharma M., Vedernikov A., Buchel F., Sadd M., Bras J.M., Bettella F. et al. , (ipdgc), T.I.P.D.G.C., (2013) A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson's disease. Hum. Mol. Genet., 22, 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dzamko N., Geczy C.L., Halliday G.M.. Inflammation is genetically implicated in Parkinson's disease. Neuroscience. doi:10.1016/j.neuroscience.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 52.Tansey M.G., Goldberg M.S. (2010) Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis., 37, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang H., Boltz D., Sturm-Ramirez K., Shepherd K.R., Jiao Y., Webster R., Smeyne R.J. (2009) Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl Acad. Sci., 106, 14063–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reale M., Greig N.H., Kamal M.A. (2009) Peripheral chemo-cytokine profiles in Alzheimer's and Parkinson's diseases. Mini Rev. Med. Chem., 9, 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noyce A.J., Bestwick J.P., Silveira-Moriyama L., Hawkes C.H., Giovannoni G., Lees A.J., Schrag A. (2012) Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann. Neurol., 72, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Block M.L., Zecca L., Hong J.-S. (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci., 8, 57–69. [DOI] [PubMed] [Google Scholar]
- 57.McGeer P.L., Itagaki S., Boyes B.E., McGeer E.G. (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology, 38, 1285–1291. [DOI] [PubMed] [Google Scholar]
- 58.Liu M., Bing G. (2011) Lipopolysaccharide animal models for Parkinson's disease. Park. Dis., 2011, e327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurkowska-Jastrzebska I., Wrońska A., Kohutnicka M., Członkowski A., Członkowska A. (1999) MHC class II positive microglia and lymphocytic infiltration are present in the substantia nigra and striatum in mouse model of Parkinson's disease. Acta Neurobiol. Exp. (Warsz.), 59, 1–8. [DOI] [PubMed] [Google Scholar]
- 60.Harms A.S., Cao S., Rowse A.L., Thome A.D., Li X., Mangieri L.R., Cron R.Q., Shacka J.J., Raman C., Standaert D.G. (2013) MHCII is required for α-synuclein-induced activation of microglia, CD4T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci., 33, 9592–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theodore S., Cao S., McLean P.J., Standaert D.G. (2008) Targeted overexpression of human α-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol., 67, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao H.-M., Kotzbauer P.T., Uryu K., Leight S., Trojanowski J.Q., Lee V.M.-Y. (2008) Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. J. Neurosci., 28, 7687–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee H.-J., Patel S., Lee S.-J. (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci., 25, 6016–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee H.-J., Suk J.-E., Bae E.-J., Lee S.-J. (2008) Clearance and deposition of extracellular α-synuclein aggregates in microglia. Biochem. Biophys. Res. Commun., 372, 423–428. [DOI] [PubMed] [Google Scholar]
- 65.Lee H.-J., Suk J.-E., Bae E.-J., Lee J.-H., Paik S.R., Lee S.-J. (2008) Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int. J. Biochem. Cell Biol., 40, 1835–1849. [DOI] [PubMed] [Google Scholar]
- 66.Desplats P., Lee H.-J., Bae E.-J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.-J. (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci., 106, 13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Béraud D., Hathaway H.A., Trecki J., Chasovskikh S., Johnson D.A., Johnson J.A., Federoff H.J., Shimoji M., Mhyre T.R., Maguire-Zeiss K.A. (2012) Microglial activation and antioxidant responses induced by the Parkinson's disease protein α-synuclein. J. Neuroimmune Pharmacol., 8, 94–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim C., Ho D.-H., Suk J.-E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H.-J. et al. (2013) Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun., 4, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. (2013) Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia, 61, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noelker C., Morel L., Lescot T., Osterloh A., Alvarez-Fischer D., Breloer M., Henze C., Depboylu C., Skrzydelski D., Michel P.P. et al. (2013) Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci. Rep., 3, 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao J., Han X., Xue L., Zhu K., Liu H., Xie A. (2015) Association of TLR4 gene polymorphisms with sporadic Parkinson's disease in a Han Chinese population. Neurol. Sci., doi:10.1007/s10072-015-2227-9. [DOI] [PubMed] [Google Scholar]
- 72.Overmyer M., Helisalmi S., Soininen H., Laakso M., Riekkinen P., Alafuzoff I. (1999) Reactive microglia in aging and dementia: an immunohistochemical study of postmortem human brain tissue. Acta Neuropathol. (Berl.), 97, 383–392. [DOI] [PubMed] [Google Scholar]
- 73.Sasaki A., Kawarabayashi T., Murakami T., Matsubara E., Ikeda M., Hagiwara H., Westaway D., George-Hyslop P.S., Shoji M., Nakazato Y. (2008) Microglial activation in brain lesions with tau deposits: comparison of human tauopathies and tau transgenic mice TgTauP301L. Brain Res., 1214, 159–168. [DOI] [PubMed] [Google Scholar]
- 74.Yoshiyama Y., Higuchi M., Zhang B., Huang S.-M., Iwata N., Saido T.C., Maeda J., Suhara T., Trojanowski J.Q., Lee V.M.-Y. (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron, 53, 337–351. [DOI] [PubMed] [Google Scholar]
- 75.Li Y., Liu L., Barger S.W., Griffin W.S.T. (2003) Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci. Off. J. Soc. Neurosci., 23, 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saez T.E., Pehar M., Vargas M., Barbeito L., Maccioni R.B. (2004) Astrocytic nitric oxide triggers tau hyperphosphorylation in hippocampal neurons. Vivo Athens Greece, 18, 275–280. [PubMed] [Google Scholar]
- 77.Zhang D., Lin J., Han J. (2010) Receptor-interacting protein (RIP) kinase family. Cell Mol. Immunol., 7, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrett J.C., Hansoul S., Nicolae D.L., Cho J.H., Duerr R.H., Rioux J.D., Brant S.R., Silverberg M.S., Taylor K.D., Barmada M.M. et al. (2008) Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat. Genet., 40, 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang F.-R., Huang W., Chen S.-M., Sun L.-D., Liu H., Li Y., Cui Y., Yan X.-X., Yang H.-T., Yang R.-D. et al. (2009) Genomewide association study of leprosy. N. Engl. J. Med., 361, 2609–2618. [DOI] [PubMed] [Google Scholar]
- 80.Gardet A., Benita Y., Li C., Sands B.E., Ballester I., Stevens C., Korzenik J.R., Rioux J.D., Daly M.J., Xavier R.J. et al. (2010) LRRK2 Is involved in the IFN-γ response and host response to pathogens. J. Immunol., 185, 5577–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moehle M.S., Webber P.J., Tse T., Sukar N., Standaert D.G., DeSilva T.M., Cowell R.M., West A.B. (2012) LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci., 32, 1602–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dzamko N., Inesta-Vaquera F., Zhang J., Xie C., Cai H., Arthur S., Tan L., Choi H., Gray N., Cohen P. et al. (2012) The IkappaB kinase family phosphorylates the Parkinson's disease kinase LRRK2 at Ser935 and Ser910 during Toll-like receptor signaling. PLoS ONE, 7, e39132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schapansky J., Nardozzi J.D., Felizia F., LaVoie M.J. (2014) Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum. Mol. Genet., 23, 4201–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mira M.T., Alcaïs A., Van Thuc N., Moraes M.O., Di Flumeri C., Hong Thai V., Chi Phuong M., Thu Huong N., Ngoc Ba N., Xuan Khoa P. et al. (2004) Susceptibility to leprosy is associated with PARK2 and PACRG. Nature, 427, 636–640. [DOI] [PubMed] [Google Scholar]
- 85.Manzanillo P.S., Ayres J.S., Watson R.O., Collins A.C., Souza G., Rae C.S., Schneider D.S., Nakamura K., Shiloh M.U., Cox J.S. (2013) The ubiquitin ligase Parkin mediates resistance to intracellular pathogens. Nature, 501, 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran T.A., Nguyen A.D., Chang J., Goldberg M.S., Lee J.-K., Tansey M.G. (2011) Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-Kappa B. PLoS ONE, 6, e23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frank-Cannon T.C., Tran T., Ruhn K.A., Martinez T.N., Hong J., Marvin M., Hartley M., Treviño I., O'Brien D.E., Casey B. et al. (2008) Parkin deficiency increases vulnerability to inflammation-related Nigral degeneration. J. Neurosci., 28, 10825–10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akundi R.S., Huang Z., Eason J., Pandya J.D., Zhi L., Cass W.A., Sullivan P.G., Büeler H. (2011) Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS ONE, 6, e16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J., Byun J.-W., Choi I., Kim B., Jeong H.-K., Jou I., Joe E. (2013) PINK1 deficiency enhances inflammatory cytokine release from acutely prepared brain slices. Exp. Neurobiol., 22, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.West A.P., Shadel G.S., Ghosh S. (2011) Mitochondria in innate immune responses. Nat. Rev. Immunol., 11, 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sagan L. (1967) On the origin of mitosing cells. J. Theor. Biol., 14, 225–IN6. [DOI] [PubMed] [Google Scholar]
- 92.Cardon L.R., Burge C., Clayton D.A., Karlin S. (1994) Pervasive CpG suppression in animal mitochondrial genomes. Proc. Natl Acad. Sci. USA, 91, 3799–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S. (2011) TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature, 472, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature, 464, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Greene J.C., Whitworth A.J., Kuo I., Andrews L.A., Feany M.B., Pallanck L.J. (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl Acad. Sci., 100, 4078–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palacino J.J., Sagi D., Goldberg M.S., Krauss S., Motz C., Wacker M., Klose J., Shen J. (2004) Mitochondrial dysfunction and oxidative damage in Parkin-deficient Mice. J. Biol. Chem., 279, 18614–18622. [DOI] [PubMed] [Google Scholar]
- 97.Quarona V., Zaccarello G., Chillemi A., Brunetti E., Singh V.K., Ferrero E., Funaro A., Horenstein A.L., Malavasi F. (2013) CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin. Cytom., 84B, 207–217. [DOI] [PubMed] [Google Scholar]
- 98.Yan Y., Laroui H., Ingersoll S.A., Ayyadurai S., Charania M., Yang S., Dalmasso G., Obertone T.S., Nguyen H., Sitaraman S.V. et al. (2011) Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice. J. Immunol. Baltim. Md 1950, 187, 1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y., Viennois E., Xiao B., Baker M.T., Yang S., Okoro I., Yan Y. (2013) Knockout of Ste20-like proline/alanine-rich kinase (SPAK) attenuates intestinal inflammation in mice. Am. J. Pathol., 182, 1617–1628. [DOI] [PubMed] [Google Scholar]
- 100.Jeffers M., McDonald W.F., Chillakuru R.A., Yang M., Nakase H., Deegler L.L., Sylander E.D., Rittman B., Bendele A., Sartor R.B. et al. (2002) A novel human fibroblast growth factor treats experimental intestinal inflammation. Gastroenterology, 123, 1151–1162. [DOI] [PubMed] [Google Scholar]
- 101.Xi P., Ding D., Zhou J., Wang M., Cong Y.-S. (2013) DDRGK1 regulates NF-κB activity by modulating IκBα stability. PloS One, 8, e64231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi F., Duan S., Cui J., Yan X., Li H., Wang Y., Chen F., Zhang L., Liu J., Xie X. (2014) Induction of matrix metalloproteinase-3 (MMP-3) expression in the microglia by lipopolysaccharide (LPS) via upregulation of glycoprotein nonmetastatic melanoma B (GPNMB) expression. J. Mol. Neurosci. MN, 54, 234–242. [DOI] [PubMed] [Google Scholar]
- 103.Jeon T.-I., Osborne T.F. (2012) SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. TEM, 23, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamayoshi S., Yamashita Y., Li J., Hanagata N., Minowa T., Takemura T., Koike S. (2009) Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat. Med., 15, 798–801. [DOI] [PubMed] [Google Scholar]
- 105.Liscovitch N., French L. (2014) Differential co-expression between α-Synuclein and IFN-γ signaling genes across development and in Parkinson's disease. PLoS ONE, 9, e115029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lei P., Ayton S., Finkelstein D.I., Adlard P.A., Masters C.L., Bush A.I. (2010) Tau protein: Relevance to Parkinson's disease. Int. J. Biochem. Cell Biol., 42, 1775–1778. [DOI] [PubMed] [Google Scholar]
- 107.Dixit R., Ross J.L., Goldman Y.E., Holzbaur E.L.F. (2008) Differential regulation of dynein and Kinesin motor proteins by tau. Science, 319, 1086–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Majounie E., Cross W., Newsway V., Dillman A., Vandrovcova J., Morris C.M., Nalls M.A., Ferrucci L., Owen M.J., O'Donovan M.C. et al. (2013) Variation in tau isoform expression in different brain regions and disease states. Neurobiol. Aging, 34, 1922.e7–1922.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muntané G., Dalfó E., Martinez A., Ferrer I. (2008) Phosphorylation of tau and α-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer's disease, and in Parkinson's disease and related α-synucleinopathies. Neuroscience, 152, 913–923. [DOI] [PubMed] [Google Scholar]
- 110.Duka T., Rusnak M., Drolet R.E., Duka V., Wersinger C., Goudreau J.L., Sidhu A. (2006) Alpha-synuclein induces hyperphosphorylation of Tau in the MPTP model of parkinsonism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol., 20, 2302–2312. [DOI] [PubMed] [Google Scholar]
- 111.Duka T., Duka V., Joyce J.N., Sidhu A. (2009) Alpha-synuclein contributes to GSK-3beta-catalyzed tau phosphorylation in Parkinson's disease models. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol., 23, 2820–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu C.W., Lee G., Jay D.G. (1999) Tau is required for neurite outgrowth and growth cone motility of chick sensory neurons. Cell Motil. Cytoskeleton, 43, 232–242. [DOI] [PubMed] [Google Scholar]
- 113.MacLeod D., Dowman J., Hammond R., Leete T., Inoue K., Abeliovich A. (2006) The familial parkinsonism gene LRRK2 regulates neurite process morphology. Neuron, 52, 587–593. [DOI] [PubMed] [Google Scholar]
- 114.Bailey R.M., Covy J.P., Melrose H.L., Rousseau L., Watkinson R., Knight J., Miles S., Farrer M.J., Dickson D.W., Giasson B.I. et al. (2013) LRRK2 phosphorylates novel tau epitopes and promotes tauopathy. Acta Neuropathol. (Berl.), 126, 809–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melrose H.L., Dächsel J.C., Behrouz B., Lincoln S.J., Yue M., Hinkle K.M., Kent C.B., Korvatska E., Taylor J.P., Witten L. et al. (2010) Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol. Dis., 40, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawakami F., Shimada N., Ohta E., Kagiya G., Kawashima R., Maekawa T., Maruyama H., Ichikawa T. (2014) Leucine-rich repeat kinase 2 regulates tau phosphorylation through direct activation of glycogen synthase kinase-3β. FEBS J., 281, 3–13. [DOI] [PubMed] [Google Scholar]
- 117.Law B.M.H., Spain V.A., Leinster V.H.L., Chia R., Beilina A., Cho H.J., Taymans J.-M., Urban M.K., Sancho R.M., Blanca Ramírez M. et al. (2014) A direct interaction between leucine-rich repeat kinase 2 and specific β-tubulin isoforms regulates tubulin acetylation. J. Biol. Chem., 289, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beilina A., Rudenko I.N., Kaganovich A., Civiero L., Chau H., Kalia S.K., Kalia L.V., Lobbestael E., Chia R., Ndukwe K. et al. (2014) Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl Acad. Sci. USA, 111, 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacLeod D.A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B.D., MacCabe B.D., Marder K.S., Honig L.S., Clark L.N. et al. (2013) RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron, 77, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alvarez-Erviti L., Rodriguez-Oroz M.C., Cooper J.M., Caballero C., Ferrer I., Obeso J.A., Schapira A.H.V. (2010) Chaperone-mediated autophagy markers in Parkinson disease brains. Arch. Neurol., 67, 1464–1472. [DOI] [PubMed] [Google Scholar]
- 121.Ahmed I., Liang Y., Schools S., Dawson V.L., Dawson T.M., Savitt J.M. (2012) Development and characterization of a new Parkinson's disease model resulting from impaired autophagy. J. Neurosci. Off. J. Soc. Neurosci., 32, 16503–16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramirez A., Heimbach A., Gründemann J., Stiller B., Hampshire D., Cid L.P., Goebel I., Mubaidin A.F., Wriekat A.-L., Roeper J. et al. (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet., 38, 1184–1191. [DOI] [PubMed] [Google Scholar]
- 123.Dehay B., Ramirez A., Martinez-Vicente M., Perier C., Canron M.-H., Doudnikoff E., Vital A., Vila M., Klein C., Bezard E. (2012) Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc. Natl Acad. Sci. USA, 109, 9611–9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Usenovic M., Tresse E., Mazzulli J.R., Taylor J.P., Krainc D. (2012) Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J. Neurosci. Off. J. Soc. Neurosci., 32, 4240–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gitler A.D., Chesi A., Geddie M.L., Strathearn K.E., Hamamichi S., Hill K.J., Caldwell K.A., Caldwell G.A., Cooper A.A., Rochet J.-C. et al. (2009) Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet., 41, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramonet D., Podhajska A., Stafa K., Sonnay S., Trancikova A., Tsika E., Pletnikova O., Troncoso J.C., Glauser L., Moore D.J. (2012) PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum. Mol. Genet., 21, 1725–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murphy K.E., Gysbers A.M., Abbott S.K., Tayebi N., Kim W.S., Sidransky E., Cooper A., Garner B., Halliday G.M. (2014) Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson's disease. Brain, 137, 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cullen V., Sardi S.P., Ng J., Xu Y.-H., Sun Y., Tomlinson J.J., Kolodziej P., Kahn I., Saftig P., Woulfe J. et al. (2011) Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann. Neurol., 69, 940–953. [DOI] [PubMed] [Google Scholar]
- 129.Mazzulli J.R., Xu Y.-H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. (2011) Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell, 146, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clark I.E., Dodson M.W., Jiang C., Cao J.H., Huh J.R., Seol J.H., Yoo S.J., Hay B.A., Guo M. (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with Parkin. Nature, 441, 1162–1166. [DOI] [PubMed] [Google Scholar]
- 131.McCarthy M.I., Abecasis G.R., Cardon L.R., Goldstein D.B., Little J., Ioannidis J.P.A., Hirschhorn J.N. (2008) Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet., 9, 356–369. [DOI] [PubMed] [Google Scholar]
- 132.Kalia L.V., Lang A.E. (2015) Parkinson's disease. Lancet, doi:10.1016/S0140-6736(14)61393-3. [Google Scholar]