Abstract
Neuroligins are postsynaptic cell-adhesion molecules that bind to presynaptic neurexins. Although the general synaptic role of neuroligins is undisputed, their specific functions at a synapse remain unclear, even controversial. Moreover, many neuroligin gene mutations were associated with autism, but the pathophysiological relevance of these mutations is often unknown, and their mechanisms of action uninvestigated. Here, we examine the synaptic effects of an autism-associated neuroligin-4 substitution (called R704C) which mutates a cytoplasmic arginine residue that is conserved in all neuroligins. We show that the R704C mutation, when introduced into neuroligin-3, enhances the interaction between neuroligin-3 and AMPA-receptors, increases AMPA-receptor internalization, and decreases postsynaptic AMPA-receptor levels. When introduced into neuroligin-4, conversely, the R704C mutation unexpectedly elevated AMPA-receptor mediated synaptic responses. These results suggest a general functional link between neuroligins and AMPA-receptors, indicate that both neuroligin-3 and -4 act at excitatory synapses but perform surprisingly distinct functions, and demonstrate that the R704C mutation significantly impairs the normal function of neuroligin-4, thereby validating its pathogenicity.
INTRODUCTION
Neuroligins are postsynaptic cell-adhesion molecules that bind to presynaptic neurexins. 1 Mammalian neurons co-express four highly homologous neuroligins (neuroligin-1 to -4, referred to as NL1 to NL4) that function differentially in excitatory and inhibitory synapses. 2–10 Several missense mutations as well as deletions were described in human NLGN1 and NLGN3 genes primarily in patients with autism, while more than 50 nonsense and missense mutations and deletions were reported in the NLGN4 gene. 1,11–16 Most disease-associated neuroligin mutations likely disrupt expression or folding of the affected neuroligin, suggesting a loss-of-function mechanism of pathogenesis. Some putative neuroligin mutations, however, are not predicted loss-of–function alleles, and may be polymorphisms without functional consequence (i.e., not be pathogenic), or alternatively mediate partial loss-of-function or even gain-of-function effects. This is especially true for an autism-associated arginine to cysteine substitution at position 704 (R704C) of NL4, the only known mutation in a cytoplasmic residue of a neuroligin, 16 which affects a residue that is highly conserved in all neuroligins (Fig. S1).
Most gene mutations found in autism and schizophrenia are too rare to achieve statistical significance, making it difficult to assess whether these mutations are pathophysiologically relevant. One approach to address this problem is to examine whether a particular mutation changes the function of the protein that is encoded by the affected gene. Towards this end, we earlier produced NL3 knockin mice carrying the R704C substitution.17 We targeted NL3 instead of NL4 in these mice because NL4 is poorly conserved in mice and expressed at low levels, and because the R704 residue is highly conserved in all neuroligins (Fig. S1). As analyzed in acute slices, the NL3-R704C mutation caused a profound synaptic transmission phenotype.17 However, the mechanism of action of the mutation and its effects on NL4 instead of NL3 remained unresolved, motivating us to reexamine the R704C mutation mechanistically in the present study. Our data indicate that the R704C mutation selectively affects postsynaptic AMPAR trafficking, suggesting a surprising link between AMPARs and neuroligins that is confirmed by the co-immunoprecipitation of NL3 with AMPARs. Moreover, our results suggest that the normal function of NL4 also operates in excitatory synapses, is significantly altered by the R704C substitution, and differs dramatically from that of NL3 despite their high degree of sequence homology. These results suggest that the R704C substitution in NL4 is pathogenic and impairs cognitive functions at least in part by altering AMPAR trafficking.
MATERIALS AND METHODS
Mouse husbandry
NL3 R704C KI mice (Figs. 1 and 2) were maintained and genotyped as described. 17 All mouse work was approved by Stanford University Administrative Panel on Laboratory Animal Care (APLAC).
Figure 1. NL3 R704C-knockin selectively impairs AMPAR-mediated synaptic responses in cultured hippocampal neurons.
A-C, R704C-knockin impairs AMPAR-mediated excitatory postsynaptic currents (EPSCs; A) but not NMDAR-mediated EPSCs (B) or GABAAR-mediated inhibitory postsynaptic currents (IPSCs; C). Analyses were performed in hippocampal neurons cultured from newborn littermate WT and NL3 R704C-knockin mice (left, representative traces; right, summary graphs).
D & E, R704C-knockin decreases the frequency and amplitude of excitatory (D; mEPSCs) but not of inhibitory (E; mIPSCs) spontaneous postsynaptic currents. Recordings were performed in hippocampal neurons cultured from newborn littermate WT and NL3 R704C-knockin mice in the presence of tetrodotoxin (1 µM; left, representative traces; right, summary graphs of the mini amplitude [top] and frequency [bottom]).
Data shown are means ± SEM; statistical significance was assessed by Student’s t-test (**, p<0.01; ***, p<0.001). Numbers of neurons/independent cultures analyzed are shown in the bars. For additional data, see Fig. S2.
Figure 2. NL3 R704C-knockin decreases surface AMPARs and increases AMPAR endocytosis.
A, R704C mutation decreases the size but not the density of surface GluA1-receptor puncta, as visualized by immunocytochemistry 24 in non-permeabilized hippocampal neurons cultured from littermate WT and NL3 R704C-knockin mice (left, representative images; right, summary graphs of the GluA1 puncta density and size).
B, R704C knockin mutation decreases the size of AMPA-induced EPSCs in cultured hippocampal neurons (left, representative traces; middle, cumulative probability plots and summary graph of the AMPA-induced EPSC charge; right, same for the EPSC amplitude).
C, Measurements of GluA1-receptor internalization by a two-stage labeling protocol that separately visualizes surface (green) and internalized GluA1-receptors (red). 24 Representative images are shown on the left, and a summary graph of the fraction of internalized GluA1-receptor on the right.
Data shown are means ± SEM; statistical significance was assessed by Student’s t-test (*, p<0.05; ***, p<0.001). Numbers of independent cultures (A) or of neurons/independent cultures analyzed (B and C) are shown in the bars.
Primary neuronal cultures
Newborn mice (postnatal day P0-P2) were used to derive primary mixed cultures of hippocampal and olfactory bulb neurons as described (for detailed procedures, see supplementary information).18,19
Plasmids constructs, virus productions, and infection and transfection of cultured neurons
Neuroligin constructs (empty for control vectors) were cloned after the human synapsin promoter and followed by an IRES-EGFP expression cassette for fluorescence visualization in expressing cells (Fig. S3). Lentiviruses were produced using transfected HEK293 cells, 20 and used as described. 21 Neuronal transfections were performed using the Ca2+/phosphate method (for detailed protocols, see supplementary information). 22
Electrophysiology
Whole-cell voltage-clamp recordings were performed essentially as described, using pharmocological blockers to isolate AMPAR-, NMDAR-, and GABAAR-mediated synaptic currents and extracellular stimulation to elicit evoked responses. 19,23
Immunocytochemistry
Immunostaining of AMPAR surface levels and AMPAR endocytosis (Fig. 2) was performed by sequential applications of rat, mouse or/and rabbit antibodies (for details, see Fig. S5 and ref. 24). Cell-surface binding assays were performed in HEK293 cells expressing HA-tagged NL3-WT or NL3-R704C (Fig. S5). 25,26
Immunoprecipitation and immunoblotting
Co-immunoprecipitations of AMPARs with NL3 were performed using HA-antibodies and cultured cortical neurons infected with lentiviruses encoding HA-tagged NL3-WT or NL3-R704C. Samples were analyzed by immunoblotting using fluorescently labeled anti-GLUA2 antibody with synaptophysin as an internal control (Fig. 4).
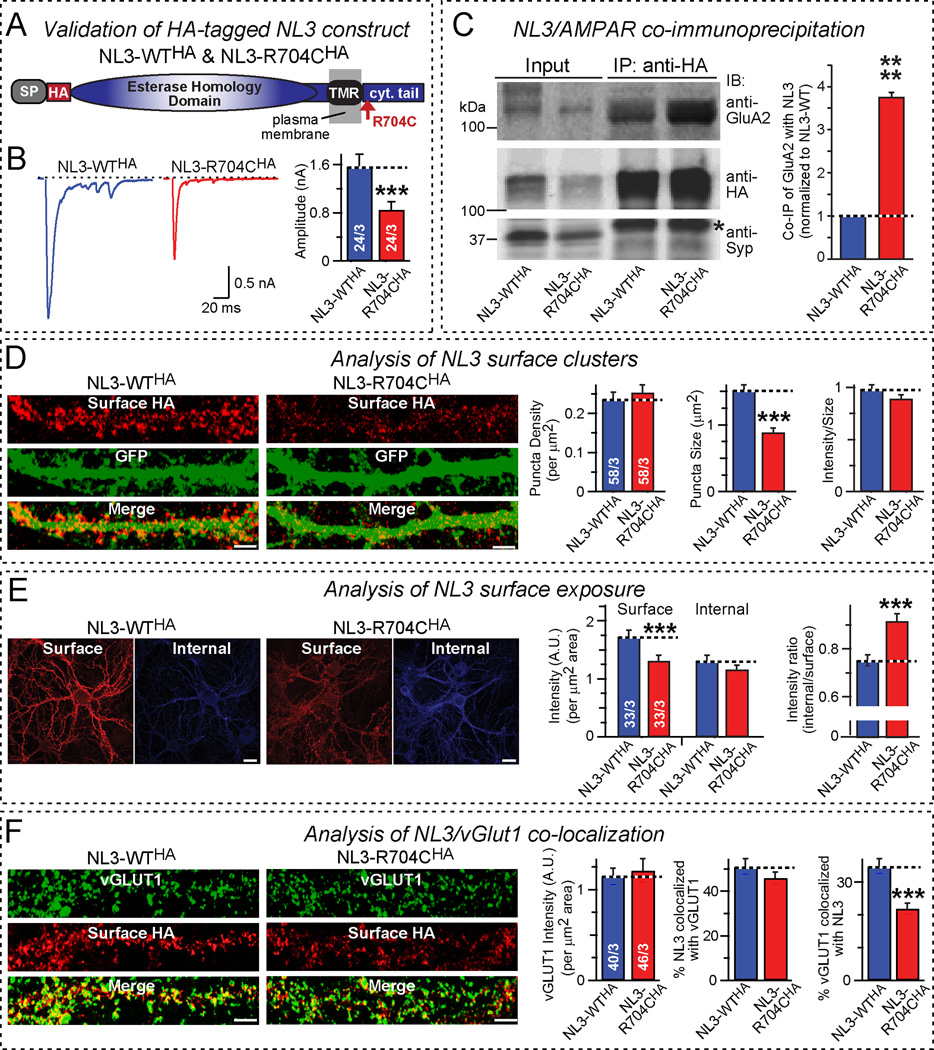
Figure 4. R704C mutation increases NL3 interaction with AMPARs and decreases NL3 surface expression.
A, Domain structures of HA-tagged NL3 proteins used for analyzing the effects of the R704C mutation on NL3/AMPAR interactions and on NL3 surface expression.
B, Lentiviral expression of HA-tagged NL3-R704C decreases AMPAR-mediated EPSCs in cultured hippocampal neurons similar to untagged NL3-R704C (left, representative traces; right, summary graph of EPSC amplitudes).
C, NL3 co-immunoprecipitates with AMPARs: R704C mutation enhances co-immunoprecipitation. Proteins were solubilized with Triton X-100 from hippocampal neurons infected with lentiviruses expressing HA-tagged NL3-WT and NL3-R704C, and NL3 was immunoprecipitated with HA antibodies. Input fractions and immunoprecipitates were immunoblotted for the AMPAR GluA2 subunit, for HA (to detect NL3), and for synaptophysin (Syp) as a negative control (left, representative blot [asterisk = IgG heavy chain]; right, quantification of the relative levels of GluA2 in the immunoprecipitates normalized for that in the WT NL3 sample; n=3 independent experiments).
D, R704C mutation decreases the size but not density or staining intensity of postsynaptic NL3 clusters. Non-permeabilized neurons were probed with an antibody to HA to selectively label surface NL3 after lentiviral co-expression of HA-tagged NL3-WT or NL3-R704C with EGFP (left, representative images [red, HA labeling; green, EGFP fluorescence]; scale bars = 5 µm; right, summary graphs of the density [left], the size [middle], and the HA-labeling intensity [right] of HA-positive postsynaptic puncta).
E, R704C mutation shifts NL3 localization from the surface to the cell interior. HA-tagged surface NL3 was immunostained in non-permeabilized neurons co-expressing NL3-WT or NL3-R704C with EGFP with an HA antibody under conditions that saturate surface-exposed HA epitopes. Neurons were then permeabilized and probed with a second HA antibody to label internal HA-tagged NL3, and the ratio of internal to surface NL3 was calculated; note that absolute surface and internal NL3 values cannot be compared because different primary and secondary antibodies were used (left, representative images; right, summary graphs of the surface and internal signal for HA-tagged NL3 (left), and of the ratio of internal and surface-exposed NL3 (right); scale bars = 15 µm). For a detailed protocol, see Fig. S5.
F, R704C mutation decreases the synaptic NL3 levels, as monitored by co-localization with the synaptic vesicle protein vGLUT1. Surface HA-NL3 was immunostained without permeabilization as in D & E, then vGLUT1 was immunostained after permeabilization (left, representative images, right, summary graphs showing: total vGLUT1 intensity [left], percentage of NL3 co-localization with vGLUT1 [middle], and percentage of vGLUT1 co-localized with NL3 [right]; scale bars = 5 µm).
Data shown are means ± SEM; statistical significance was assessed by Student’s t-test (***, p<0.001; ****, p<0.0001). Numbers of neurons/ independent cultures analyzed are shown in the bars.
RESULTS
The R704C substitution in NL3 decreases synaptic AMPAR levels and increases AMPAR endocytosis
We had shown earlier that the NL3 R704C-knockin mutation caused a selective decrease in AMPAR- but not NMDAR-mediated synaptic responses.17 To explore the functional importance and mechanism underlying this observation, we analyzed hippocampal neurons cultured from littermate WT and NL3-R704C knockin mice. We confirmed a specific, ~60% decrease in AMPAR-mediated evoked EPSCs without a change in NMDAR-mediated EPSCs or in GABAAR-mediated evoked IPSCs (Figs. 1A–1C; Table S1). To explore whether this phenotype reflected a loss in postsynaptic AMPARs, we measured spontaneous ‘mini’ mEPSCs (which are mediated by AMPARs) and mIPSCs (GABAAR-mediated). Consistent with the decrease in AMPAR-mediated evoked EPSCs, we observed a significant decrease in mEPSC amplitude (~25% decrease) and frequency (~60% decrease), but no change in mIPSC amplitude or frequency (Figs. 1D and 1E).
The decreased mEPSC amplitude in NL3 R704C-mutant synapses indicates that the postsynaptic concentration of AMPARs per synapse may be decreased, whereas the decreased mEPSC frequency could be due to either a silencing of a subset of synapses, an undersampling of small mEPSCs, or a reduction in synapse numbers. We measured paired-pulse ratios (PPRs), which are indirect measurements of the presynaptic release probability of excitatory and inhibitory synapses, but failed to uncover a change, arguing against a presynaptic effect (Fig. S2). To directly monitor postsynaptic AMPARs, we quantified the surface levels of AMPARs by immunocytochemistry of non-permeabilized neurons using GluA1 antibodies (Fig. 2A). 24 In addition, we electrophysiologically recorded AMPAR EPSCs following an acute application of AMPA, an AMPAR agonist (Fig. 2B). Both methods revealed a large decrease (~40–60%) in surface AMPAR levels in NL3-R704C knockin neurons, suggesting that the R704C substitution decreased postsynaptic AMPAR levels.
To test how the NL3-R704C decreases surface AMPAR levels, we measured the internalization of the AMPAR subunit GluA1. To this end, we labeled live neurons with an antibody to the extracellular GluA1 sequences for 5 min, washed the cells, and incubated them for another 15 min before fixing the neurons and separately staining them for surface AMPARs and internalized AMPARs. 24 We detected a ~50% increase in GluA1 internalization in NL3-R704C knockin neurons, suggesting that the R704C substitution in NL3 accelerates the endocytosis of surface AMPARs (Fig. 2C).
R704C-mutant NL3 acts in a dominant and cell-autonomous fashion
The NL3-R704C knockin produces a different, more severe phenotype (Figs. 1 and 2) than the NL3 knockout, 27–29 suggesting a possible gain-of-function effect. To explore this hypothesis, we tested whether expression of R704C-mutant NL3 in wild-type neurons produces a phenotype that mimics the R704C knockin mutation.
We first examined the synaptic effects of infecting cultured olfactory bulb neurons with a control lentivirus or with lentiviruses expressing wild-type or R704C-mutant NL3 (Fig. S3). Olfactory bulb neurons were chosen because of their well-defined neuronal composition, 18 and lentiviral infection because it accomplishes homogeneous gene delivery to all neurons in culture. 24 We observed the same selective impairment in AMPAR-mediated synaptic responses in olfactory bulb neurons expressing R704C-mutant NL3 (but not in neurons expressing NL3-WT) as in hippocampal neurons containing the NL3 R704C-knockin mutation (Fig. 3A). Since wild-type neurons express endogenous NL3-WT, R704C-mutant NL3 must act as a dominant negative in this experiment.
Figure 3. Expression of NL3-R704C in wild-type neurons phenocopies NL3 R704C-knockin mutation.
A, Measurements of AMPAR- and NMDAR-mediated EPSCs and of IPSCs in WT olfactory bulb neurons that were globally infected with a control lentivirus or lentiviruses expressing NL3-WT or NL3-R704C as indicated (left, representative traces; right, summary graphs). After lentiviral infection, all neurons express the lentivirally encoded proteins.
B, Same as A, except that recordings were performed in sparsely transfected neurons, such that a single transfected neuron containing the control or NL3 expression plasmid is surrounded by non-transfected neurons.
C, Measurements of spontaneous AMPAR-mediated miniature EPSCs (mEPSCs; top) or of miniature IPSCs (mIPSCs; bottom) in sparsely transfected olfactory bulb neurons containing a control plasmid or NL3 expression plasmids as indicated.
Data shown are means ± SEM; statistical significance was assessed by Student’s t-test (*, p<0.05; **, p<0.01; ***, p<0.001). Numbers of analyzed neurons/ independent cultures are shown in the bars. For additional data, see Figs. S3 and S4.
We next inquired if the R704C phenotype is due to a cell-autonomous mechanism. We sparsely transfected olfactory bulb neurons with the plasmids used for lentiviral infection experiments (control, NL3-WT, and NL3-R704C), and measured synaptic responses in the transfected neurons (Figs. 3B and S3). Again, we observed in NL3-R704C expressing neurons a selective AMPAR-impairment similar to that of NL3-R704 knockin neurons (Figs. 3B, 3C, and S4). Thus, R704C-mutant NL3 alters AMPAR trafficking by a postsynaptic, cell-autonomous, and dominant-negative mechanism that is identical between NL3 R704C-knockin neurons and wild-type neurons with exogenously expressed NL3-R704C. Unexpectedly, neurons expressing exogenous NL3-WT did not have an increase in AMPAR- or NMDAR-mediated EPSCs compared to control neurons. Since expression of WT- NL3 and NL3-R704C causes a similarly large increase in synapse numbers, 17 the fact that NL3-WT does not increase EPSCs suggests that the morphologically observed newly induced synapses are non-functional.
The R704C-mutation enhances the interaction of NL3 with AMPARs
How does the NL3-R704C mutation decrease surface AMPAR levels and increase AMPAR endocytosis? We hypothesized that NL3 may be associated with AMPARs in neurons, and that the R704C mutation changes this association. To test this hypothesis, we used NL3-WT and R704C-NL3 containing an extracellular HA epitope, allowing us to monitor and immunoprecipitate NL3 (Fig. 4A and S5A). We first confirmed in hippocampal neurons that lentiviral expression of HA-tagged NL3-R704C produces the same phenotypes as the R704C knockin in hippocampal neurons and as non-tagged NL3-R704C in olfactory bulb neurons (Fig. 4B; Table S1). We then examined whether AMPARs are co-immunoprecipitated with HA-tagged NL3, using synaptophysin as a negative control (Fig. 4C). In these experiments, we monitored AMPARs with antibodies to the GluA2 AMPAR subunit, and also asked whether NL3-R704C changes the co-immunoprecipitation of AMPARs. We detected significant co-immunoprecipitation of GluA2 with NL3-WT that was dramatically enhanced by the R704C mutation (Fig. 4C). These experiments suggest that NL3 is directly or indirectly associated with AMPARs, possibly via common binding to a scaffolding protein such as PSD-95, 30 and that the R704C-mutation enhances this association.
The most plausible explanation for the observed effects of the R704C mutation in NL3 – the decrease in AMPAR surface levels and the increase in AMPAR endocytosis (Fig. 2) – is that the R704C-mediated increase in the interaction of NL3 with AMPARs is accompanied by accelerated NL3 endocytosis, thereby enhancing internalization of bound AMPARs. To test this hypothesis, we measured the density and size of NL3-containing surface puncta in hippocampal neurons expressing HA-tagged NL3-WT or NL3-R704C (Figs. S5B and S5C). We found that the density of postsynaptic NL3-containing puncta (like that of AMPAR puncta; Fig. 2A) was unchanged by the R704C mutation, but that the size of the puncta, a reflection of the amount of NL3 present in a synapse under our assay conditions, was dramatically decreased (Figs. 4D). We then measured the relative distribution of NL3-WT and NL3-R704C between the cell surface and cell interior by immunocytochemistry, and observed a relative loss of NL3-R704C from the cell surface (Figs. 4E and S5B). The R704C mutation in NL3 did not change the density of vGLUT1-positive puncta, measured to monitor excitatory synapse numbers (Figs. 4F). NL3-vGLUT1 co-localization experiments, however, confirmed that the R704C mutation caused significant loss of NL3 from excitatory synapses (Figs. 4F). Viewed together, these experiments suggest that the R704C-mutation causes NL3 both to bind more strongly to AMPARs (directly or indirectly) and to undergo more endocytosis, thereby depleting AMPARs from postsynaptic specializations.
R704C mutation in NL4 enhances AMPAR-mediated synaptic responses
We initially analyzed the R704C mutation in NL3 despite the fact that this autism-relevant mutation was observed in NL4 because we wanted to examine this mutation using a knockin approach without exogenous expression of mutant proteins. 17,23 We could not do such a knockin for mouse NL4 because mouse NL4 is expressed at only low levels, and most importantly, its sequence is not yet present in the current mouse genome sequence. 4 However, the current finding that the NL3-R704C knockin phenotype is fully reproduced by expression of exogenous NL3-R704C in wild-type neurons – as long as it is compared to the NL3-WT (Fig. 3) – indicates that the effects of the R704C mutation on NL4 can be examined by expression of R704C-mutant NL4 in wild-type neurons.
We infected cultured hippocampal neurons with lentiviruses expressing either EGFP only (control), or both EGFP and NL4-WT or NL4-R704C. We then measured evoked AMPAR- and NMDAR-mediated EPSCs and GABAAR-mediated IPSCs in these neurons (Fig. 5). Strikingly, we observed that overexpression of NL4-WT – consistent with earlier results 10 – caused a decrease in both AMPAR- (~38%) and NMDAR-mediated EPSCs (~45%) without affecting IPSCs or the PPR (Figs. 5A, and S6). In contrast, overexpression of NL4-R704C produced an increase in AMPAR- and NMDAR-mediated responses (~66% and ~100%, respectively), compared to NL4-WT (Fig. 5A). Since NL4 expression also increases synapse numbers, 10 it seems to induce formation of non-functional synapses and additionally to inactivate existing synapses, while the R704C mutation reverses this inactivating effect of NL4. The selective effects of exogenously expressed NL4 on excitatory synapses in all parameters analyzed and the distinct changes induced in these effects by the R704C mutation strongly suggests that NL4 normally also acts in excitatory synapses, and that the R704C mutation impairs NL4 function.
Figure 5. NL4 R704C-mutation abolishes the suppression of excitatory glutamatergic synaptic responses by NL4-WT.
A, Suppression of excitatory synaptic responses by NL4-WT in hippocampal neurons is abolished by the NL4 R704C-mutation. Neurons were infected with a control lentivirus or lentiviruses expressing WT or R704C-mutant NL4, and AMPAR- (top) and NMDAR-mediated evoked EPSCs (middle) as well as GABAAR-mediated IPSCs (bottom) were analyzed (left, representative traces; right, summary graphs of postsynaptic current amplitudes.
B, NL4-R704C increases the total surface levels of AMPARs, whereas NL4-WT has no effect. EPSCs induced by locally puffing AMPA (50 µM for 100 ms) onto a neuron were measured in lentivirally infected hippocampal neurons (left, representative traces; right, summary graphs of the integrated EPSC charge transfer).
C, R704C mutation does not affect NL4 surface localization (same experiment as Figs. 4D and 4E, except HA-tagged WT-NL4 and R704C-NL4 were probed). Left panels depict representative images (scale bars = 5 µm), and right panels summary graphs of the density and size of surface HA-positive puncta (left and middle graphs, respectively), and of the intensity ratio of internal vs. surface HA-immunofluorescence (right graph).
D, R704C mutation does not affect NL4 synaptic localization (same experiment as Fig. 4F, but for HA-tagged NL4-WT and NL4-R704C). Left panels depict representative images (scale bars = 2 µm), and right panels summary graphs of the vGLUT1 intensity (left), percentage of NL4 co-localization with vGLUT1 (middle), and percentage of vGLUT1 co-localization with NL4 (right).
Data shown in summary graphs are means ± SEM; statistical significance was assessed by Student’s t-test (*, p<0.05; **, p<0.01; ***, p<0.001). Numbers of neurons/independent cultures analyzed are shown in the bars.
To confirm that the NL4-R704C-induced changes reflect changes in surface AMPARs, we measured the effect of NL4-WT and NL4-R704C on the response to AMPA puffed onto the neurons (Fig. 5B). We found that expression of WT-NL4 had no significant effect on the total AMPAR-induced current, which is due to both synaptic and extrasynaptic AMPARs. Since in the same NL4-WT-expressing neurons the synaptic AMPAR-mediated responses are decreased, this result suggests that WT-NL4 may not change the total surface AMPAR levels, but cause their redistribution out of synapses. Strikingly but consistent with the synaptic physiology, we found that expression of R704C-NL4 increased the AMPA-induced current in neurons expressing NL4 (Fig. 5B). Thus, in NL4 the R704C mutation appears to truly increase surface AMPAR levels.
Because the R704C-mutants of NL3 and NL4 produced opposing effects on AMPARs in neurons, we hypothesized that the R704C mutation may act via different cellular mechanisms in an isoform–specific manner. To test this hypothesis, we extracellularly tagged NL4-WT and NL4-R704C with HA-epitopes (similar to NL3 in Fig. 4A), and probed if the R704C mutation affected the synaptic distribution of NL4. Interestingly, we did not detect a significant difference in the relative surface expression of NL4-WT and NL4-R704C (Fig. 5C) or in their synaptic localization (assessed via their co-localization with vGluT1; Fig. 5D). These data suggest that unlike in NL3, the R704C mutation in NL4 does not cause increased internalization of NL4 and may thereby stabilize surface AMPARs.
DISCUSSION
In the present study, we have examined the molecular mechanisms by which the R704C point mutation (which alters an arginine residue that is conserved in the cytoplasmic tail of all neuroligins) may cause significant imbalance between excitatory and inhibitory synaptic transmission, and thereby contribute to autism etiologies. Our data allow four principal conclusions.
First, we showed that the NL3-R704C substitution specifically decreased neuronal surface AMPAR levels, and that this decrease is due, at least in part, to an increase in AMPAR endocytosis. This conclusion is based on genetic knockin experiments (Figs. 1 and 2) as well as expression experiments in wild-type neurons (Figs. 3 and 4), using both lentivirally infected neurons (a condition in which all neurons express the wild-type or mutant NL3; Fig. 3A) and sparsely transfected neurons (a condition that involves competition between transfected and non-transfected neurons; Figs. 3B and 3C). From these two different expression methods, we established that the NL3-R704C mutation reduces AMPAR surface levels by a gain-of-function, cell-autonomous mechanism (Fig. 3).
Second, we found that NL3 interacts with AMPARs and that this interaction is enhanced by the R704C mutation. This finding not only links neuroligins to AMPARs, but also suggests a mechanistic explanation for the cell-autonomous, dominant nature of the R704C phenotype: By binding better to AMPARs, NL3-R704C can displace NL3-WT from AMPARs; by being endocytosed more rapidly, NL3-R704C then enhances the endocytosis of AMPARs (Figs. 2 and 4).
Third, we observed that expression of WT-NL4 caused a decrease in both AMPAR- and NMDAR-mediated EPSCs whereas it had no effect on IPSCs (Fig. 5A). This observation suggested that NL4 normally functions, at least in part, in excitatory neurons, but that its function is different from that of NL3.
Fourth and finally, we showed that the R704C-mutation also strongly affected the function of NL4, but in a dramatically different fashion than that of NL3. Unlike in NL3, the R704C mutation in NL4 did not enhance NL4 endocytosis, and caused a relative increase in postsynaptic AMPAR levels compared to NL4-WT, suggesting that the effects of the R704C-mutation on AMPAR levels are dependent on the specific neuroligin isoform into which it is introduced.
Implications
Our observations have potentially important implications. First, they strengthen the notion that different neuroligins, despite extensive homology spanning their entire sequences, have distinct functions. At the same time, the fact that the R704C substitution in both NL3 and NL4produces a functional change in AMPARs, although in opposite directions, indicates that neuroligins are governed by similar mechanistic principles consistent with their homology.
Second, our results suggest that NL4 like NL3acts in excitatory synapses, but differs functionally from NL3. NL4 is likely also present at glycinergic synapses, 7 and may like NL3 function in both excitatory and inhibitory synapses.
Third, our observations demonstrate that by altering NL4 function, the R704C mutation is likely pathogenic, and may act by enhancing synaptic transmission at least in a subset of excitatory synapses.
Fourth, our data suggest that neuroligins – at least NL3 and NL4 – physiologically interact with AMPARs as part of their function in conferring particular properties onto synapses.
How is it possible that the R704C mutation has distinct effects in NL3 vs. NL4, especially if it acts via enhancing an interaction of neuroligins with AMPARs? Our data show that the R704C mutation increases the internalization of NL3 but not of NL4, which may explain its distinct effects on NL3 and NL4 (summarized in Fig. S7), but does not account for the different effects of these neuroligins on AMPARs in general. Although our data thus do not provide a definitive final analysis of all neuroligin function, they do support the overall notion that neuroligins are complex transducers of a presynaptic signal into a postsynaptic regulatory effect that differentially operates in various synapse types.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jeesun Kim, Bing Wu, and Yoon Seok Kim for their help during the project. This paper was supported by grants from the NIH (R37-MH052804 to T.C.S.; MH092931 to M.W.), an NIH K99 award to J.A. (MH103531-01), and a postdoctoral grant award to S.C. (Stanford Institute for Chemical Biology, ChEM-H112878).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPLEMENTARY INFORMATION
Supplementary information is available at Molecular Psychiatry’s website.
REFERENCES
- 1.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichtchenko K, et al. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 3.Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 4.Bolliger MF, et al. Unusually rapid evolution of Neuroligin-4 in mice. Proc Natl Acad Sci U S A. 2008;105:6421–6426. doi: 10.1073/pnas.0801383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 7.Hoon M, et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci U S A. 2011;108:3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takacs VT, Freund TF, Nyiri G. Neuroligin 2 is expressed in synapses established by cholinergic cells in the mouse brain. PLoS One. 2013;8:e72450. doi: 10.1371/journal.pone.0072450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007;26:1738–1748. doi: 10.1111/j.1460-9568.2007.05842.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, et al. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci. 2009;29:10843–10854. doi: 10.1523/JNEUROSCI.1248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamain S, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laumonnier F, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008;16:614–618. doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- 14.Talebizadeh Z, et al. Novel splice isoforms for NLGN3 and NLGN4 with possible implications in autism. J Med Genet. 2006;43:e21. doi: 10.1136/jmg.2005.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, et al. Variations analysis of NLGN3 and NLGN4X gene in Chinese autism patients. Mol Biol Rep. 2014;41:4133–4140. doi: 10.1007/s11033-014-3284-5. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, et al. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry. 2005;10:329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- 17.Etherton MR, Tabuchi K, Sharma M, Ko J, Sudhof TC. An autism-associated point mutation in the neuroligin cytoplasmic tail selectively impairs AMPA receptor-mediated synaptic transmission in hippocampus. EMBO J. 2011;30:2908–2919. doi: 10.1038/emboj.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao P, Maximov A, Sudhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maximov A, Pang ZP, Tervo DG, Sudhof TC. Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J Neurosci Methods. 2007;161:75–87. doi: 10.1016/j.jneumeth.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Ho A, et al. Genetic analysis of Mint/X11 proteins: essential presynaptic functions of a neuronal adaptor protein family. J Neurosci. 2006;26:13089–13101. doi: 10.1523/JNEUROSCI.2855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaeser PS, et al. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 2009;64:227–239. doi: 10.1016/j.neuron.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson GR, et al. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc Natl Acad Sci U S A. 2012;109:18120–18125. doi: 10.1073/pnas.1216398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chanda S, Marro S, Wernig M, Sudhof TC. Neurons generated by direct conversion of fibroblasts reproduce synaptic phenotype caused by autism-associated neuroligin-3 mutation. Proc Natl Acad Sci U S A. 2013;110:16622–16627. doi: 10.1073/pnas.1316240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Sudhof TC. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell. 2013;154:75–88. doi: 10.1016/j.cell.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uemura T, Mishina M. The amino-terminal domain of glutamate receptor delta2 triggers presynaptic differentiation. Biochem Biophys Res Commun. 2008;377:1315–1319. doi: 10.1016/j.bbrc.2008.10.170. [DOI] [PubMed] [Google Scholar]
- 26.Uemura T, et al. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etherton M, et al. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci U S A. 2011;108:13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothwell PE, et al. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irie M, et al. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.