Abstract
Background
The aim of this study was to explore the value of NeuroGam software in diagnosis of epilepsy by 99Tcm-ethyl cysteinate dimer (ECD) brain imaging.
Material/Methods
NeuroGam was used to analyze 52 cases of clinically proven epilepsy by 99Tcm-ECD brain imaging. The results were compared with EEG and MRI, and the positive rates and localization to epileptic foci were analyzed.
Results
NeuroGam analysis showed that 42 of 52 epilepsy cases were abnormal. 99Tcm-ECD brain imaging revealed a positive rate of 80.8% (42/52), with 36 out of 42 patients (85.7%) clearly showing an abnormal area. Both were higher than that of brain perfusion SPECT, with a consistency of 64.5% (34/52) using these 2 methods. Decreased regional cerebral blood flow (rCBF) was observed in frontal (18), temporal (20), and parietal lobes (2). Decreased rCBF was seen in frontal and temporal lobes in 4 out of 36 patients, and in temporal and parietal lobes of 2 out of 36 patients. NeuroGam further showed that the abnormal area was located in a different functional area of the brain. EEG abnormalities were detected in 29 out of 52 patients (55.8%) with 16 cases (55.2%) clearly showing an abnormal area. MRI abnormalities were detected in 17 out of 43 cases (39.5%), including 9 cases (52.9%) clearly showing an abnormal area. The consistency of NeuroGam software analysis, and EEG and MRI were 48.1% (25/52) and 34.9% (15/43), respectively.
Conclusions
NeuroGam software analysis offers a higher sensitivity in detecting epilepsy than EEG or MRI. It is a powerful tool in 99Tcm-ECD brain imaging.
MeSH Keywords: Epilepsy, Absence; Tomography, Emission-Computed; Tomography, X-Ray Computed
Background
Epilepsy is a clinical syndrome caused by abnormal discharge in the cerebral cortex. In addition to the risk of accidents, the frequent recurrence of seizures may easily lead to mental illnesses [1]. Preoperative evaluation and localization of epileptic foci depends on the regional distribution of lesions, especially in surgery for refractory epilepsy [2]. Therefore, it is of great significance to locate and delineate epileptic foci to facilitate clinical diagnosis and treatment [3].
Traditionally, epileptic foci were localized mainly based on a combination of clinical observations, electroencephalography (EEG), and anatomical imaging of brain. However, the challenges include limitations associated with the inherent structure and complexity of scalp EEG signals, and the spatial resolution of the electrode array [4].
The efficiency of 99mTc-ethylcysteinate dimer (99mTc-ECD) single-photon emission computed tomography (SPECT) has largely been demonstrated in detecting perfusion abnormalities and identifying epileptic foci [5]. However, this method, which entails visual observation and experience of nuclear medicine physicians, is associated with strong subjectivity, poor repeatability, and bias [6].
Software tools for 3D voxel-by-voxel analysis of brain perfusion SPECT have been developed, such as the statistical parametric mapping (SPM), the 3-dimensional stereotactic surface projection (3D-SSP), and the NeuroGam software [7–9]. These tools provide the possibility of brain perfusion analysis in strictly defined cortical functional areas such as the Brodmann (Br) areas [10]. The aim of this study was to explore the value of NeuroGam software analysis in epilepsy diagnosis and localization using 99Tcm-ethylcysteinate dimer (ECD) brain imaging.
Material and Methods
Population
A total of 52 patients diagnosed with epilepsy at the Department of Neurology of the 3rd Hospital of Hebei Medical University (Hebei province, China) were included in this study. The diagnosis and the seizure types of the 52 patients with epilepsy were in accordance with the criteria of the International League Against Epilepsy (ILAE) [11]. Pregnant women, patients with a history of psychiatric disorders, and patients with history, signs, or findings of other neurological disorders were excluded. The cases included 15 cases of generalized tonic-clonic seizure (GTCS) and 37 cases of partial seizure that included 8 cases of simple partial seizure, 12 cases of complex partial seizure, and 17 cases of GTCS secondary to partial seizure (Table 1).
Table 1.
Participants’ clinical demographics.
| Epilepsy patients | Control group | ||
|---|---|---|---|
| Number of cases | 52 | 12 | |
| Age years | 37.2±13.4 (13~58) | 32.4±11.7 (19~52) | |
| Sex (M/F) | 25/27 | 7/5 | |
| Seizure types (n) | GTCS (15) | ||
| Partial seizure (37) | Simple partial seizure (8) | ||
| Complex partial seizure (12) | |||
| GTCS secondary to partial seizure (17) | |||
GTCS – generalized tonic-clonic seizure.
All 52 patients underwent EEG examination, and 43 also underwent MRI. The control group was composed of healthy individuals without neuropsychiatric disorders or other serious systemic diseases. The study was approved by the regional Ethics Committee at the 3rd Hospital of Hebei Medical University, and written informed consent was obtained from all participants. The demographic and clinical characteristics of the participants are listed in Table 1.
SPECT studies
Single-photon emission computed tomography (SPECT) studies were performed in interictal stage after ≥24 h of no seizure activity. EEG examination and MRI were performed in 2 weeks. Brain perfusion single-photon emission computed tomography (SPECT) imaging was performed 30 min following intravenous injection of 555 MBq of 99mTc-ECD (99mTc, HTA Co., Ltd.; ECD, Beijing Shihong Pharma Centre, China) in a dimly lit and quiet room with the patients lying supine with eyes closed. A 20% window centered on the 140 keV photo peak of 99mTc was used. Projection data were obtained using a 2-head SPECT (Infinia Vc Hawkeye, GE, USA) with low-energy-high-resolution parallel hole collimators (128×128 matrix, 60 projections over 360° rotation, steps of 6° for 30 s per projection, zoom factor 1.5). Images were reconstructed by filtered back projection with a Butterworth filter of cutoff frequency 0.5 and order 10. Attenuation was corrected using Chang’s method (attenuation coefficient, 0.12/cm). No attenuation correction was applied.
Image analysis by visual observation
The SPECT images were visually inspected by 2 experienced nuclear medicine physicians who were blinded to patients’ clinical data. Based on the transaxial plane, and referring to the coronal and sagittal plane, positive lesions were confirmed by decreased or absent radioactive uptake, in consecutive views (≥2 in the same plane) in more than 2 planes [12].
Image analysis using NeuroGam software
The reconstructed data of images were elaborated by NeuroGam software (GE Medical System, Segami Corp., Columbia, MD, USA), which is a statistical software for automated diagnosis of brain perfusion SPECT images. This software uses an affine anatomical co-registration by blocks of data defined in the Talairach space. It can be used to investigate regional cerebral blood flow (rCBF) objectively and easily in the cerebral lobes of left and right hemispheres, and especially the predefined Brodmann functional areas (Br).
The 3-dimensional (3D) volume of the brain in each study was reoriented according to a defined line, which was rendered horizontal automatically. It fits the inferior pole of the occipital lobe and the inferior edge of the frontal lobe. Raw data for lateral deviations were corrected by defining a line above the interhemispheric fissure and automatically orienting this line in the vertical plane. The vertical anterior commissure line (AC) and the posterior commissure line (PC) were defined for the intermediate level of the pons and anterior plane of the temporal lobes. The analytical volume was limited in the lateral, superior, and inferior planes of the brain.
After anatomical standardization and voxel normalization to cerebellum mean values, the Talairach technique normalized the brain volume to facilitate a voxel-by-voxel comparison of the ECD uptake in the brain cortex with a normal database of subjects also corrected volumetrically. Analysis of individual study utilized a computer program to create a z-score map, which calculated z-scores using the following equation [13]:
The z-score maps are displayed either in the standard cuts or by overlay on a 3D anatomical topographic representation by means of a specific color scale. This is equivalent to presenting the individual data items in terms of standard deviation from a normal age-matched database. Abnormal areas are defined as those with decreased uptake (below 2 standard deviations of the normal mean uptake in area >50% pixels) [14].
EEG and MRI studies
All patients underwent routine EEG examination, and each recording of EEG was obtained digitally using an electrode positioned on the scalp according to international 10–20 system. The EEG was interpreted by a consultant neurologist, trained and experienced in electro-physiologic studies. Spike waves, sharp waves, spike (or sharp) slow-wave complex, and multi-spike waves were considered abnormal waveforms. MRI of the brain in the axial, sagittal, and (or) coronal planes was performed in 43 patients with an Avanto 1.5-T scanner (Siemens, Germany). The diagnosis of epilepsy was confirmed by 2 MRI specialists working together. Diagnostic criteria included the presence of temporal lobe atrophy, hippocampal sclerosis, gliosis, and other local abnormal signals [15]. A patient with lesional MRI findings was considered MRI-positive.
Statistical analysis
Continuous data are expressed as mean ± standard deviation. Rate comparison was done by chi-square test, and multiple comparisons of rates were analyzed. Analyses were conducted using the SPSS statistical software (version 17.0), considering P<0.05 as the level of significance.
Results
SPECT rCBF imaging results in interictal stage by visual analysis
Thirty-one cases of 52 patients with epilepsy showed abnormal results with 99Tcm-ECD brain perfusion imaging; the positive rate was 59.6% (31/52). Abnormal lesions of 7 cases were not localized clearly due to decreased or scattered distribution of the images of rCBF. The abnormal lesions of 24 cases were localized in 31 patients [77.4% (24/31)]. In these 24 cases, 22 cases showed partial decrease in rCBF, including 2 cases in the frontal lobe and 20 in the temporal lobe; 2 cases showed partially increased rCBF, including 1 case involving the temporal lobe and 1 case involving the parietal lobe.
NeuroGam software analysis
Forty-two out of 52 patients [80.8% (42/52)] with epilepsy showed abnormal 99Tcm-ECD brain perfusion imaging. The abnormal lesions of 6 cases were not clear due to generally decreased or scattered distribution of images of rCBF. Abnormal lesions of 42 cases were localized in 36 patients [85.7% (36/42)]. Qualitative and semi-quantitative analyses were performed in these 36 patients by the NeuroGam software.
In these 36 cases, 34 showed partially decreased rCBF, including 10 cases involving the frontal lobe, 18 temporal lobes, and 2 parietal lobes. The abnormal lesions were displayed further by the NeuroGam software in the following Br areas: 4 cases involving Br 44, 6 in Br 46, 13 in Br 22, 5 in Br 38, and 2 cases in Br 7. Four out of 34 patients showed partially decreased rCBF in multiple lobes, including the frontal and temporal lobes, and also displayed 4 cases in Br 44, 3 cases in Br 22, and 1 case in Br 38. Two cases showed partially increased rCBF in the temporal (Br 21) and parietal lobes (Br 40).
Z-scores in the rCBF-decreased frontal (Br 44, 46), temporal (Br 22, 38) and parietal (Br 7) lobes, were −3.2±0.9, −3.4±1.0, and −3.3±0.8, respectively. Z-scores in the rCBF-increased temporal (Br 21) and parietal (Br 40) lobes were 3.0±0.7 and 3.1±0.9, respectively (Table 2).
Table 2.
The Z value of decreased rCBF in the cerebral lobes of epilepsy patients and controls.
| Frontal lobe | Temporal lobe | Parietal lobe | Occipital lobe | Insular lobe | |
|---|---|---|---|---|---|
| Control group (n=12) | −1.1±0.3 | −1.0±0.6 | −1.2±0.7 | −1.0±0.4 | −0.9±0.7 |
| Epilepsy group (n=34) | −3.2±0.9 | −3.4±1.0 | −3.3±0.8 | −0.8±0.6 | −1.0±0.2 |
In the control group, the images of 99Tcm-ECD rCBF were normal, the cerebral cortex radioactivity was equally distributed, and the z-scores were in the range of 2 standard deviations.
EEG and MRI results
Twenty-nine cases of 52 patients with epilepsy showed abnormal waveform of EEG, with a positive rate of 55.8% (29/52). Abnormal EEG pattern was marked by diffuse slow waves in 13 cases, local slow waves in 10 cases, and records of epilepsy wave, such as the spike, sharp, or spike slow waves in 6 cases. The abnormal lesions of these 29 cases were localized in 16 patients [55.2% (16/29)]. Seventeen cases of 43 patients with epilepsy showed abnormal MRI [39.5% (17/43)]. The abnormal lesions of 17 cases were localized in 9 patients [52.9% (9/17)], including 4 cases in the temporal lobe, 3 cases in the frontal lobe, and 1 each in the parietal and occipital lobes.
Comparative analysis
The positive rate and the rate of localization using the NeuroGam software analysis were 80.0% and 85.7%, respectively, which were higher than those by visual analysis in 99Tcm-ECD brain perfusion imaging. Out of all 52 cases, 24 cases that were abnormal in 99Tcm-ECD brain perfusion imaging were identified as abnormal areas by the NeuroGam software analysis, which was consistent with visual analysis. Seven cases that were positive but not abnormal were all confirmed positive and 4 cases were determined as abnormal areas; 21 cases that were negative showed positive and were determined as abnormal areas in 8 cases, positive but not abnormal in 3 cases, and the remaining 10 cases were negative. The complete consistency of the results by these 2 methods was 65.4% (34/52).
The positive rates using 3 methods of the NeuroGam software analysis, EEG, and MRI were statistically significant (χ2=17.217, P=0.000). Multiple comparison of the NeuroGam software analysis with EEG and MRI was statistically significant, (χ2=7.501, P=0.006, χ2=17.004, P=0.000). In the diagnosis of abnormal areas, the NeuroGam software analysis showed 85.7%, higher than EEG (55.2%) and MRI (52.9%). In terms of consistency of the findings, the NeuroGam software analysis showed 48.1% (25/52) and 34.9% (15/43), compared with EEG and MRI, respectively (Table 3).
Table 3.
Consistency of results with NeuroGam software analysis and EEG and MRI.
| Number of cases | Exact consistency | Partial consistency | Complete inconsistency | |
|---|---|---|---|---|
| NeuroGam software analysis and EEG | 52 | 20 (38.5%) | 5 (9.6%) | 27 (51.9%) |
| NeuroGam software analysis and MRI | 43 | 11 (25.6%) | 4 (9.3%) | 28 (65.1%) |
Case sample
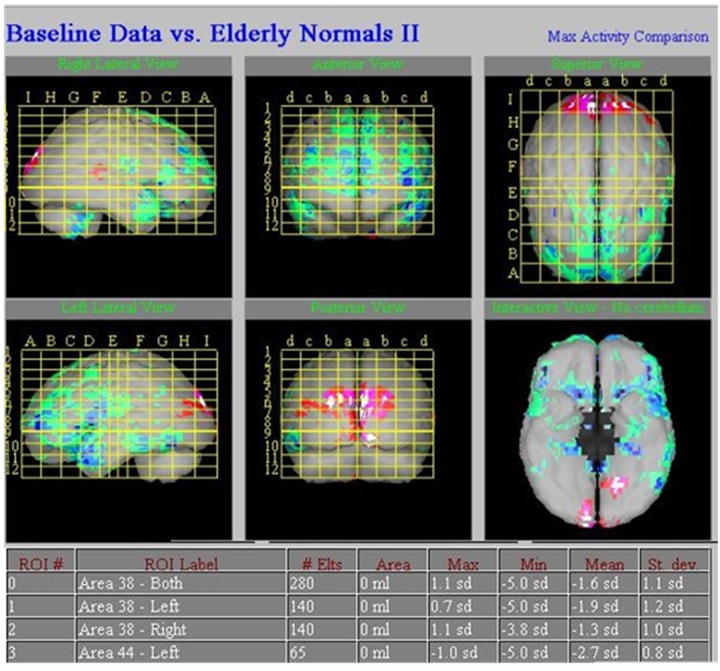
A 43-year-old female patient suffered loss of consciousness, with limb spasm persisting for more than 15 years. The symptoms of epilepsy seizure presented up to 12 times daily, and antiepileptic drugs caused no significant improvement. EEG waves were in the normal physiological range and no abnormal waves were detected. No abnormalities were seen in MRI. Decreased perfusion was observed in the left frontal lobe by 99Tcm-ECD brain perfusion imaging. The NeuroGam software analysis revealed the decrease areas of rCBF in the left frontal lobe (Br 44) and bilateral temporal lobe (Br 38). A semi-quantitative analysis of the areas with decreased rCBF, which was compared with the regional database by z-score, revealed a decrease of 2.7 standard deviations in the left frontal lobe (Br 44) and a decrease of 1.9 and 1.3 standard deviations in bilateral temporal lobe (Br 38) (Figures 1, 2).
Figure 1.
Typical images of perfusion defects in a 43-year-old female patient with epilepsy. NeuroGam software analysis revealed areas of decreased rCBF in the left frontal lobe (Br 44) and bilateral temporal lobe (Br 38).
Figure 2.
Typical images of respective perfusion defects in a 43-year-old female patient with epilepsy are shown. Semi-quantitative analysis of the decreased areas of rCBF, which was compared with the regional database by z-score, showed a decrease of 2.7 standard deviations in the left frontal lobe (Br 44) and a decrease of 1.9 and 1.3 standard deviations in bilateral temporal lobe (Br 38).
Discussion
Changes in brain metabolism and blood perfusion often occur simultaneously, so the decrease or increase of rCBF is the pathophysiological basis in diagnostic localization of epilepsy lesions in SPECT [16]. Although SPECT has the potential to localize the ictal-onset zone accurately, it cannot be widely applied in routine clinical examinations due to extremely short ictal phase and difficulty in capture, or decreased drug induction rate and security [16]. The positive rate of localizing epileptic foci by SPECT imaging in the interictal stage is about 50~80%, which is higher than that of EEG and MRI [17], but limitations associated with low sensitivity and specificity and inaccurate positioning still persist.
According to our results, 42 cases with abnormal rCBF were found by the NeuroGam software analysis and the positive rate was 80.8%, which is significantly higher than the EEG (55.8%), MRI (39.5%) and is consistent with the previous studies [17]. The results suggest a higher sensitivity in highlighting the epileptic foci in interictal stage using this software. Decreased rCBF was the most typical change of the epileptic foci in interictal stage, although the underlying mechanism is not clear. It was generally believed that it may be related to the lack of neurons and cortical atrophy [18]. Two cases of 42 patients, which showed increased rCBF in interictal stage, were considered to be related with frequent epileptic discharge, continued increase of rCBF after seizure, and the subclinical epileptic seizure, which may reflect a change in neuronal activity precipitating the transition from the interictal to the ictal state [19]. Six cases, which showed diffuse decreased rCBF and could not be positioned clearly, should be further analyzed combined with the clinical data.
The ability of 99Tcm-ECD brain perfusion imaging by SPECT in diagnosis of epilepsy had been established[20] as a basis of locating the epileptic foci for brain functional partition and semi-quantitative analysis by using the NeuroGam software. Due to rapid extracerebral washout, better background-to-brain ratios were evident with 99Tcm-ECD by comparing with another radiopharmaceutical: hexamethyl propylene amine oxime (HMPAO); it may therefore allow subtle focal changes in cortical uptake to be more readily detected because of their greater contrast against the background activity in the white matter and the extracerebral tissues [21]. Some authors have suggested that the grey-white differentiation in uptake may be greater with 99Tcm-ECD than with 99Tcm-HMPAO SPECT. 99Tcm-ECD has also been shown to be an optimal tracer for seizure localization, since it has a longer decomposition time that may make it easier to obtain true ictal studies. Its use was associated with shorter injection latencies and a higher proportion of ictal injections than with unstabilized 99Tcm-HMPAO, thereby enhancing the sensitivity and specificity of localizing seizures in intractable partial epilepsy [22].
The results of this study indicate good consistency of the abnormal areas displayed by the NeuroGam software analysis with the visual analysis in 99Tcm-ECD SPECT imaging. The complete consistency of the results by these 2 methods was 65.4% (34/52). In addition, semi-quantitative analysis, represented by Z value, could be used to compare individual and healthy population databases provided by the software, and clearly display and localize the epileptic foci.
The results of NeuroGam software analysis showed that the rCBF abnormal areas accounted for 54.8% (23/42) of the temporal lobe, which is consistent with the literature [17]. The rCBF abnormal areas outside of the temporal lobe were mainly located in the frontal lobe in 33.3% (14/42) and the parietal lobe in 7.1% (3/42). Four cases showed multilobe abnormalities located in the frontal and temporal lobes. Multiple lobe abnormalities are possibly related to neuronal connections, or multiple epileptic foci in the same patient [23], or may be associated with the NeuroGam software itself. Multiple lesions increase the difficulty in diagnostic localization of epileptic foci. By combining with the clinical results, MRI, EEG, and SPECT in ictal phase, the software may display a few limitations in locating epileptic foci.
In this study, NeuroGam software analysis assisted in 36 out of 42 cases in definitively mapping abnormal brain images, with a positioning rate of 85.7%, which is higher than EEG (55.2%) and MRI (52.9%). NeuroGam software analysis displayed and located lesions accurately in 11 out of 16 patients diagnosed by EEG and in 5 out of 9 patients by MRI. In addition, NeuroGam software analysis was capable of positioning diagnosis in 10 and 5 cases that were difficult to locate by EEG and MRI. MRI is based on abnormal structural and signal changes associated with the disease, while epilepsy is often associated with changes in function and metabolism. Therefore, NeuroGam software analysis may have a higher value in locating epileptic foci than conventional MRI. But the consistent rate of abnormal areas analyzed by NeuroGam software compared with EEG and MRI were only 48.1% (25/52) and 34.9% (15/43), respectively. This result suggests the need for comprehensive clinical data to locate epileptic foci. The accuracy of NeuroGam software analysis requires further in-depth research. It is believed that the accuracy also can be further improved by combining the advantages of the electrical activity in EEG and the anatomical structure in MRI.
NeuroGam software analysis, a method based on pixel analysis, has the advantage of not being affected by researchers and eliminating the differences between observers, and can detect the tiny decrease in blood perfusion that cannot be distinguished by visual analysis. The software has the synthetic ability to research the brain function intuitively and through quantitative analysis. The method is more objective and has good repeatability than the traditional visual methods such as cerebral blood flow perfusion imaging [24]. It is similar to SPM, which is currently used worldwide for brain functional imaging. However, the SPM process is complex and is based on the Matlab platform. Processing images by SPM still need MRIcro Java and Talairach and a series of auxiliary software, restricting its use [25].
NeuroGam software analysis has no technical limitations. The analytical method is simple and its use is likely to increase [14]. The software can directly enable statistical analysis of the original data at the voxel level. Using specific image correction, differences in patients’ cerebral blood flow distribution are shown in the Talairach standard graph as 3D reconstruction, and analyzed visually and qualitatively. In addition, the software offers a healthy population database of reference data with age- and gender-matched patients for comparison (results expressed in standard deviations), and enables semi-quantitative analysis on each leaf and different Brodmann areas of the brain cortex.
The software can be used for both individual clinical analysis and large sample study. It not only offers differential analysis of individuals and normal groups in comparing brain perfusion, but also provides images for subtraction analysis in the same individual, and therefore is better than SPM [24]. NeuroGam software analysis is especially suitable for daily clinical practice, and enables clinical application in locating epileptic foci. As an alternate diagnostic option in epilepsy, it may be more powerful compared with traditional methods.
Conclusions
In conclusion, our results suggest that the combined use of visual and NeuroGam software analysis improves the diagnostic yield of 99Tcm-ECD brain imaging in patients with epilepsy in interictal stage. NeuroGam software analysis offers a higher sensitivity in detecting epilepsy than EEG or MRI, and may contribute to a more detailed and objective evaluation of rCBF in patients with epilepsy. It is a powerful tool in 99Tcm-ECD brain imaging in locating epileptic foci. The results of the present study were derived from a small cohort of patients, and need to be replicated in a larger-sample population.
Footnotes
Source of support: Departmental sources
References
- 1.Rapinesi C, Del Casale A, Serata D, et al. Epilepsy and brain injury: a case report of a dramatic neuropsychiatric vicious circle. Brain Inj. 2013;27(7–8):940–43. doi: 10.3109/02699052.2013.775489. [DOI] [PubMed] [Google Scholar]
- 2.Desai A, Bekelis K, Thadani VM, et al. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54(2):341–50. doi: 10.1111/j.1528-1167.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 3.Maehara T. Neuroimaging of epilepsy. Neuropathology. 2007;27(6):585–93. doi: 10.1111/j.1440-1789.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 4.Haneef Z, Chen DK. Functional neuro-imaging as a pre-surgical tool in epilepsy. Ann Indian Acad Neurol. 2014;17(Suppl 1):S56–64. doi: 10.4103/0972-2327.128659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Paesschen W. Ictal SPECT. Epilepsia. 2004;45(Suppl 4):35–40. doi: 10.1111/j.0013-9580.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- 6.Shorvon SD. A history of neuroimaging in epilepsy 1909–2009. Epilepsia. 2009;50(Suppl 3):39–49. doi: 10.1111/j.1528-1167.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujitani S, Matsuda K, Nakamura F, et al. Statistical parametric mapping of interictal 123I-iomazenil SPECT in temporal lobe epilepsy surgery. Epilepsy Res. 2013;106(1–2):173–80. doi: 10.1016/j.eplepsyres.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Masuda H, Honma J, et al. Ictal SPECT analyzed by three-dimensional stereotactic surface projection in frontal lobe epilepsy patients. Epilepsy Res. 2006;68(2):95–102. doi: 10.1016/j.eplepsyres.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Paschali A, Constantoyannis C, Angelatou F, Vassilakos P. Perfusion brain SPECT in assessing motor improvement after deep brain stimulation in Parkinson’s disease. Acta Neurochir. 2013;155(3):497–505. doi: 10.1007/s00701-012-1610-z. [DOI] [PubMed] [Google Scholar]
- 10.Valotassiou V, Papatriantafyllou J, Sifakis N, et al. Brain perfusion SPECT with Brodmann areas analysis in differentiating frontotemporal dementia subtypes. Curr Alzheimer Res. 2014;11(10):941–54. doi: 10.2174/1567205011666141107125104. [DOI] [PubMed] [Google Scholar]
- 11.Kerr M, Linehan C, Thompson R, et al. A White Paper on the medical and social needs of people with epilepsy and intellectual disability: the Task Force on Intellectual Disabilities and Epilepsy of the International League Against Epilepsy. Epilepsia. 2014;55(12):1902–6. doi: 10.1111/epi.12848. [DOI] [PubMed] [Google Scholar]
- 12.Berkovic S, Newton M, Rowe C. Epilepsy Surgery. New York: Raven Press; 1991. Localization of epileptic foci using SPECT. [Google Scholar]
- 13.Paschali A, Messinis L, Lyros E, et al. Neuropsychological functions and rCBF SPECT in Parkinson’s disease patients considered candidates for deep brain stimulation. Eur J Nucl Med Mol Imaging. 2009;36(11):1851–58. doi: 10.1007/s00259-009-1168-z. [DOI] [PubMed] [Google Scholar]
- 14.Paschali A, Messinis L, Kargiotis O, et al. SPECT neuroimaging and neuropsychological functions in different stages of Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2010;37(6):1128–40. doi: 10.1007/s00259-010-1381-9. [DOI] [PubMed] [Google Scholar]
- 15.Doelken MT, Richter G, Stefan H, et al. Multimodal coregistration in patients with temporal lobe epilepsy – results of different imaging modalities in lateralization of the affected hemisphere in MR imaging positive and negative subgroups. Am J Neuroradiol. 2007;28(3):449–54. [PMC free article] [PubMed] [Google Scholar]
- 16.Jayalakshmi S, Sudhakar P, Panigrahi M. Role of single photon emission computed tomography in epilepsy. Int J Mol Imaging. 2011;2011:803920. doi: 10.1155/2011/803920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.la Fougere C, Rominger A, Forster S, et al. PET and SPECT in epilepsy: a critical review. Epilepsy Behav. 2009;15(1):50–55. doi: 10.1016/j.yebeh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Jefferys JG. Advances in understanding basic mechanisms of epilepsy and seizures. Seizure. 2010;19(10):638–46. doi: 10.1016/j.seizure.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner C, Serles W, Leutmezer F, et al. Preictal SPECT in temporal lobe epilepsy: regional cerebral blood flow is increased prior to electroencephalography-seizure onset. J Nucl Med. 1998;39(6):978–82. [PubMed] [Google Scholar]
- 20.Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Semin Nucl Med. 2008;38(4):227–39. doi: 10.1053/j.semnuclmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Asenbaum S, Brucke T, Pirker W, et al. Imaging of cerebral blood flow with technetium-99m-HMPAO and technetium-99m-ECD: a comparison. J Nucl Med. 1998;39(4):613–18. [PubMed] [Google Scholar]
- 22.O’Brien TJ, Brinkmann BH, Mullan BP, et al. Comparative study of 99mTc-ECD and 99mTc-HMPAO for peri-ictal SPECT: qualitative and quantitative analysis. J Neurol Neurosurg Psychiatry. 1999;66(3):331–39. doi: 10.1136/jnnp.66.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamatogi Y, Ohtahara S. Multiple independent spike foci and epilepsy, with special reference to a new epileptic syndrome of “severe epilepsy with multiple independent spike foci”. Epilepsy Res. 2006;70(Suppl 1):S96–104. doi: 10.1016/j.eplepsyres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Tranfaglia C, Palumbo B, Siepi D, et al. Semi-quantitative analysis of perfusion of Brodmann areas in the differential diagnosis of cognitive impairment in Alzheimer’s disease, fronto-temporal dementia and mild cognitive impairment. Hell J Nucl Med. 2009;12(2):110–14. [PubMed] [Google Scholar]
- 25.Schrouff J, Rosa MJ, Rondina JM, et al. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics. 2013;11(3):319–37. doi: 10.1007/s12021-013-9178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]