Abstract
Objective
We postulated that proteasome inhibition (PI) may be useful in the treatment of SLE by targeting plasmacytoid dendritic cells (pDCs) and plasma cells (PCs), both critical to disease pathogenesis.
Methods
Lupus prone mice were treated with the non-selective PIs carfilzomib and bortezomib, the LMP7-selective immunoproteasome inhibitor ONX 0914, or vehicle control. Tissues were harvested and analyzed by flow cytometry using standard markers. Nephritis was monitored by proteinuria and kidney harvest. Serum anti-dsDNA levels were measured by ELISA and total IgG and dsDNA antibody secreting cells (ASC) by ELIspot. Human PBMCs or mouse bone marrow cells were incubated with TLR agonists and PIs and interferon α measured by ELISA and flow cytometry.
Results
Early treatment of lupus prone mice with the dual targeting PIs carfilzomib or bortezomib or the immunoproteasome specific inhibitor ONX 0914 prevented disease progression, and treatment of mice with established disease dramatically abrogated nephritis. Treatment had profound effects on plasma cells with greater reductions in autoreactive than total IgG ASCs, an effect that became more pronounced with prolonged treatment, and was reflected in decreasing serum autoantibodies. Remarkably, proteasome inhibition efficiently suppressed production of interferon α by toll-like receptor activated pDCs in vitro and in vivo, an effect mediated by both an inhibition of pDC survival and function.
Conclusions
Inhibition of the immunoproteasome is equally efficacious to dual targeting agents in preventing lupus disease progression by targeting two critical pathways in disease pathogenesis, type I interferon activation and autoantibody production by plasma cells.
Keywords: interferon, plasmacytoid dendritic cells, plasma cells, Systemic Lupus Erythematosus, autoimmunity
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by dysregulation in multiple arms of the immune system and the production of hallmark autoantibodies and α interferon. Because this disease continues to be associated with significant morbidity and mortality, there is great interest in the development of new and targeted treatment approaches and correspondingly a better understanding of disease pathogenesis (1). Although several open-label studies of B cell depletion (BCD) as a targeted treatment have demonstrated clinical benefit (2), only a minority of SLE patients have lasting clinical responses (3, 4). Moreover, the recent failure of two large randomized trials of BCD in SLE (5) highlights the need for other therapeutic strategies. In particular, anti-CD20 has variable impact on autoantibodies that are produced by CD20 negative plasma cells. Decreasing autoantibodies may be particularly critical in SLE where they are often directly pathogenic, e.g. antibody induction of cytopenias and antibodies to DNA or DNA- and RNA-binding proteins forming immune complexes that stimulate α interferon.
Bortezomib is a proteasome inhibitor (PI) that effectively kills plasma cells and has demonstrated success in the treatment of multiple myeloma (6-8). Recent promising results suggest that PIs are an effective therapy in a murine model of lupus (9), with relatively selective induction of the unfolded protein response in plasma cells, killing of both long lived and short lived plasma cells, and elimination of autoantibodies. Despite the success of bortezomib in the clinic and in this pre-clinical lupus model, its use in human autoimmune disease is limited because of the development of painful neuropathy in >30% of patients (10). Thus, there is great interest in developing alternative proteasome inhibitors that are less toxic. Carfilzomib is an irreversible proteasome inhibitor that, like bortezomib, primarily inhibits the chymotrypsin-like active sites of the ubiquitously expressed constitutive proteasome and the immunoproteasome, which is found predominantly in immune effector cells (11, 12). In contrast to bortezomib, clinical trials of carfilzomib have shown a low rate of peripheral neuropathy (13). Recently, it has also been shown that the immunoproteasome and not the constitutive proteasome regulates cytokine production in human peripheral blood mononuclear cells (PBMCs) and that selective inhibition of the immunoproteasome with the irreversible inhibitor ONX 0914 (formerly PR-957) is efficacious in both anti-collagen antibody-induced arthritis and collagen-induced arthritis models in mice (14).
Given that bortezomib and other PIs have been shown to block activation of myeloid dendritic cells (mDCs) by Toll-like receptor 4 (TLR) in vitro (15) we postulated that pDC activation via TLR signaling, a pathway that is known to be triggered via immune complex (IC) binding and lead to the production of large quantities of IFN-α, may be similarly blocked by the proteasome inhibitors (16-19). Here, we demonstrate for the first time that proteasome inhibitors target both plasmacytoid dendritic cells producing α interferon and plasma cells producing pathogenic antibodies, creating a powerful synergistic effect in the treatment of SLE.
MATERIALS AND METHODS
Mice and experimental design
NZB/NZWF1, MRL/lpr, and C57BL/6 mice were supplied by Jackson Laboratories. All mice were housed in the animal facility in the University of Rochester Medical Center or Onyx Pharmaceuticals. All experiment protocols were reviewed and approved by the University of Rochester or Onyx Committee on Animal Resources. NZB/NZWF1 mice with established nephritis (24-30 weeks with durable proteinuria ≥2+ proteinuria or 100 mg/dl) (female) and female MRL/lpr mice (10 weeks) were treated with the indicated proteasome inhibitors intravenously via tail vein route, bortezomib (0.50-0.75 mg/kg D1D3), carfilzomib (3-5 mg/kg D1D2), ONX 0914 (10 mg/kg QOD × 3 or 20 mg/kg QW) or vehicle solution. Proteinuria was monitored weekly once or twice with urine dipstick (Uristix by Bayer). Spleen, peripheral blood and bone marrow lymphocytes were collected after sacrifice for flow cytometry and ELISPOT analysis. Kidneys and a portion of spleen were collected for histological analysis.
ELISA assay
Sera from PI treated mice blood samples were collected and frozen prior to quantifying total IgG antibody levels as described (20). Serum was also used for anti-dsDNA analysis following the manufacturer's instructions (Alpha Diagnostic International). Animal identification and treatment information were blinded for all ELISA samples. A proteasome active site ELISA was utilized for proteasome analysis and to monitor inhibition of constitutive and immunoproteasome active sites in PBMC, pDCs and tissue samples from treated animals, and was performed as described previously (21).
Flow cytometry analysis
FITC-CD21, biotin-CD23, PE-CD23, PE-CD1d, APC-B220, FITC-B220, APC-AA4.1,biotin-IgM, FITC-IgM, biotin-CD5 (BD Biosciences) and PE-CD11b (BD Biosciences) were used for B cell subset identification. APC-CD4 (BD Biosciences) and biotin-CD69 (BD Biosciences) were used for T cell analysis. PE-CD11b (BD Biosciences) and FITC-CD11c (BD Biosciences) were used as dendritic cell markers. Plasma cells were identified as k (kappa) light chain+ and CD138+ among the lymphocyte gated cells. Samples were run on a FACSCanto. All antibodies were purchased from eBioscience except where indicated.
Histological analysis
Kidneys of mice from different treatment groups and control mice were fixed in 10% formalin and paraffin embedded or frozen. Kidney sections (4 mm) were stained with hematoxylin and eosin. Pathology was analyzed and scored in a blinded fashion (B.I.G.). Briefly, the severity of glomerular, interstitial, and vascular lesions was determined on a scale of 0 to 4+. Multiple sections at a minimum of two different levels were examined, with each section typically containing >50 glomeruli and >25 blood vessels as described. Spleen sections were frozen and cut into 4 mm sections. Immunohistochemistry slides were stained for FDC-M1 (BD Bioscience), PE-B220, and Biotin-GL7 using a Dako LSAB2 kit. 3-color fluorescent slides were stained for FITC-MOMA (ABD serotec), AMCA-IgM (Vector labs), ALEXA647-IgD or -FITC-B220, BIOTIN-GL7 followed by SA-PE, and APC-CD4 (BD Bioscience) and sections quantitated as described (20).
Detection of antibody-secreting cells by enzyme-linked immunosorbent spot assay
Anti-dsDNA and total IgG secreting cells were detected as previously described (20). Briefly, Millipore were coated with poly–L-lysine (Sigma) and calf thymus DNA (Sigma), blocked with 2% fetal calf serum in PBS, and spleen or BM cell suspensions incubated as serial dilutions starting with 5E5 cells/well overnight at 37 °C. After incubation, plates were washed and incubated with alkaline phosphatase–conjugated goat antibody to mouse IgG (Jackson) for 1 h at RT and detected with Vector Blue Alkaline Phosphatase Substrate Kit III (Burlingame, CA). For assessment of IgG-secreting cells, serial dilutions starting with 1E5 cells/well were incubated in goat anti-mouse IgG coated plates (Southern Biotech). The developed spots were measured by ImmunoSpot 5.0 (Cellular Technology Ltd, Shaker Heights, OH).
Inhibition of IFN-α by proteasome inhibitors and pDC counts
Mouse bone marrow cells were cultured in RPMI complete medium at 0.25 million per 200 ul in 96 flat bottom culture plate over night in the presence of 500 ng/ml CpG (2216, Invitrogen) with or without PIs. Human PMBCs were cultured in serum free media (invitrogen) containing 1ng/mL GM-CSF (Sigma) and 50 U/mL IFN-α (Sigma) at 2.5 × 106 cells/ml with DMSO control or proteasome inhibitor. After 1 h incubation, cells were washed twice with media and cultured in media containing CpG2216. Cells were harvested 24 h after initial treatment. IFN-α production in the culture supernatants was measured by commercial ELISA (Mouse Interferon Alpha ELISA Kit or human ELISA kit PBL interferon source, Piscataway, NJ) following the manufacturer's instructions. In separate experiments mouse cells were harvested and stained for B220, CD11b, CD11c and Ly6C to count pDC numbers by flow cytometry analysis. In some experiments intracellular cytokine staining for IFN-α was performed (Fix & Perm Medium A and B; Invitrogen) with GolgiPlug added during the final 3 h of the culture (BD Bioscience).
RNA analysis
Total RNA was extracted from mouse splenocytes using the Qiagen RNeasy Mini kit (Qiagen, Valenica, CA) and reverse-transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Mx1 expression was defined in duplicate or triplicate real-time quantitative reverse transcriptase-polymerase chain reactions using the Rotor-Gene 3000 thermal cycler (Corbett Life Science, Sydney, New South Wales, Australia) with SYBR Green I (Applied Biosystems, Foster City, CA) for detection of DNA synthesis. Primers were as described (22) with normalization to the β2-microglobulin housekeeping gene and the following cycling conditions: 95°C for 4 minutes followed by 50 cycles of 95°C for 15 seconds, 60°C (annealing temperature) for 60 seconds, and 72°C for 15 seconds.
Statistical analysis
Student's t-test or non-parametric Mann-Whitney U was used for comparison between treatment groups. Chi-squared test was performed on protein survival data. Significance is based on a value of p<0.05.
RESULTS
Novel proteasome inhibitors prevent nephritis progression in Lupus prone mice
To evaluate the ability of carfilzomib and ONX 0914 to prevent lupus nephritis, 10 week-old female MRL/lpr mice were treated for 13 weeks. Both carfilzomib and ONX 0914 inhibited progression of nephritis to a similar level as bortezomib (Fig. 1a left panel and supplemental data). High levels of proteinuria (100 mg/dl) were observed in all the vehicle treated mice by the end of the treatment, whereas less than 20% of treated mice reached this level of proteinuria (Fig. 1a right panel). Similarly, NZB/NZW F1 mice with established nephritis (2+ proteinuria) showed a halt in disease progression (Fig. 1a, right). There was also a significant decrease in the severity of glomerulonephritis (GN) and interstitial inflammation after treatment with ONX 0914 (p=0.03 and 0.003, respectively) or bortezomib (p=0.001 and 0.002, respectively). The impact of carfilzomib was less marked achieving significance only for GN (p=0.05) (Fig 1b). In contrast, the control group displayed severe GN with crescents, necrosis, and mesangial hypercellularity and massive interstitial nephritis (Fig. 1b, left).
Figure 1.
Carfilzomib and ONX 0914 prevent nephritis progression in Lupus prone mice. (a) 10 week-old MRL/lpr mice (n = 10 each group) were treated with bortezomib 0.75 mg/kg D1D3 (closed squares), carfilzomib 3 mg/kg D1D2 (closed triangles), ONX 0914 10 mg/kg QOD (closed circles) or vehicle solution (open circles) for 13 weeks. Significant differences in proteinuria from vehicle treated animals (p<0.05) were observed beginning at 3 weeks for bortezomib, 4 weeks for CFZ, and 2 weeks for ONX 0914. NZB/W mice (proteinuria grade 2+) were treated with carfilzomib (n = 2), ONX 0914 (n = 4) or vehicle solution (n = 6) for 8 weeks (significant differences beginning at 4 weeks for ONX 0914 and 7 weeks for CFZ). (b) Representative kidney sections of NZB/W mice after treatment with 20 mg/ml of ONX 0914 or vehicle solution for 8 weeks. Kidneys were scored from 0 to 4 for glomerulonephritis (GN), interstitial nephritis (IN), and perivascular infiltration (VI) (mean for MRL/lpr mice in A). (c) Serum anti-dsDNA IgG antibody levels and total IgG levels of MRL/lpr mice (significant differences beginning at 7 weeks). Data are shown as mean + s.e.m and are representative of 3 independent experiments and cohorts of treated mice.
Serum anti-dsDNA IgG levels were lowered by carfilzomib and ONX 0914 treatments to a level comparable to that of bortizomib treated mice (Fig 1c). The total IgG levels were also significantly reduced by bortezomib and ONX 0914. Although carfilzomib had effects on total IgG levels early in treatment, this effect became less pronounced over time. This may be because the maximally tolerated dose for carfilzomib in the mouse results in less inhibition of LMP7 (50 – 60%) relative to ONX 0914 and bortezomib (≥80%) (data not shown). Taken together, these data support the hypotheses that proteasome inhibition, including selective inhibition of the immunoproteasome, results in therapeutic improvement in mouse models of SLE.
Elimination of plasma cells and germinal center cells in Lupus prone mice by proteasome inhibition
It has been previously demonstrated that bortezomib decreases plasma cell numbers in the spleen and bone marrow of lupus prone mice (9). Furthermore, we have demonstrated that carfilzomib and ONX 0914 reduce both anti-dsDNA and total IgG levels in the sera of treated animals. Therefore, we measured the impact of proteasome inhibition on plasma cell numbers in spleen and bone marrow of 23-week old MRL/lpr mice after treatment with bortezomib (0.5 mg/kg), carfilzomib (3 mg/kg), ONX 0914 (10 mg/kg), or vehicle solution for 13 weeks. Plasma cells decreased in bortezomib treated animals by 90% and 95% in both spleen and bone marrow, respectively. Carfilzomib and ONX 0914 treatment also decreased plasma cell numbers although not as markedly, by 50% and 65% in spleen and bone marrow, respectively (Fig. 2a). In the NZB/W F1 mice model, splenic plasma cell numbers also decreased approximately 80% after 8 week-treatment of established nephritis with ONX 0914 (20 mg/kg) (Fig. 2d).
Figure 2.
Carfilzomib and ONX 0914 reduce numbers of plasma cells and germinal center cells in Lupus prone mice. (a) 10 week-old MRL/lpr mice were treated for 13 weeks with PIs as in Figure 1 and PC (CD138+, k light chain+) counts defined by flow cytometry in spleen (left) and bone marrow (right). *p=0.0003, **p<0.0001, ***p=0.01, ****p=0.005 (b) The numbers of total IgG ASC (top panels) and anti-dsDNA IgG ASC (bottom panels) from spleen and bone marrow were measured by ELISPOT assays. *p=0.008, **p=0.005, ***p=0.03, ****p=0.01, #p=0.05). (c) Representative spleen sections of normal mice, NZB/W mice treated with vehicle, and NZB/W mice treated with ONX 0914 for 8 weeks (2+protein at start). (d) GC cell (B220+, GL-7+) and PC numbers in spleen of NZB/W mice after 8 weeks of ONX 0914 (n = 3, 20 mg/kg) vs. vehicle solution (n = 3). Representative spleen sections stained with GL7. All data is expressed as mean + s.e.m..
Although flow cytometric analysis is sensitive for assessing numbers of plasma cells, it does not allow analysis of sub-populations of antigen specific antibody secreting cells (ASC). Thus, total IgG and anti-dsDNA IgG ASC numbers were measured by ELISPOT assay in 24 week-old MRL/lpr mice that were treated with bortezomib (0.5 mg/kg), carfilzomib (3 mg/kg), ONX 0914 (10 mg/kg) and vehicle solution for 13 weeks (Fig. 2b). In spleen, the level of decrease in total IgG ASC numbers was highest with bortezomib (74%) followed by carfilzomib (17%) and ONX 0914 (15%). The level of decrease in anti-dsDNA IgG ASC numbers in spleen was even more pronounced: bortezomib (87%), ONX 0914 (44%), and carfilzomib (39%) (Fig. 2b). In bone marrow, the level of decrease in total IgG ASC number was highest with bortezomib (63%) followed by ONX 0914 (44%) and carfilzomib (43%), again with more pronounced declines in anti-dsDNA IgG ASC number [bortezomib (91%), ONX 0914 (79%) and carfilzomib (64%)] (Fig. 2b). Thus, it appears that proteasome inhibition preferentially affects autoantibody secreting plasma cells. The impact on the plasma cell compartment was confirmed by histologic analysis of spleen, where decreases in both numbers and size of IgM and IgG bright plasma cells were observed with proteasome inhibitor treatment (Fig. 2c). Therefore, proteasome inhibition not only eliminates plasma cells but also may impact their antibody secreting capacity or preferentially inhibit high antibody secretors.
Given that lupus prone NZB/W F1 mice develop germinal centers (GC) spontaneously as they age, GC cells (B220+, GL-7+) were enumerated in mouse spleen after 8 weeks of treatment with ONX 0914 (20 mg/kg) and compared to vehicle treated control mice. The GC cells in the spleen of treated mice were 78% lower in numbers compared to the control, and no GC formation was observed histologically (Fig. 2d). Therefore, one explanation for the lower numbers of plasma cells found in spleen and bone marrow after long-term treatment with proteasome inhibition is a reduction in the de novo synthesis of plasma cells in GC reactions.
Another pathway for reduction of the plasma cell compartment is direct killing of preexisting plasma cells. As an initial assessment of the in vivo plasma cell killing by proteasome inhibition, we treated mice for a short-term (one week) during which the de novo synthesis of GC experienced plasma cells should be negligible. Plasma cell numbers and ASCs were enumerated by flow cytometry and ELISPOT analysis as previously described and were reduced (supplemental Fig. 2). As with long-term treatment, the effects of proteasome inhibition on autoreactive ASCs were more marked than for total IgG ASCs (supplemental Fig. 2). Although GC cells from spleen were reduced modestly with ONX 0914 treatment there was no apparent reduction with carfilzomib treatment (data not shown).
Proteasome inhibitors preferentially target active autoantibody secreting cells
To more firmly establish that PIs directly and preferentially kill anti-dsDNA ASCs, we examined their effects on antibody secretion in vitro. When PIs were added in titrating amount to ELISPOT wells, the spot numbers of total IgG ASC did not decrease (Fig. 3a and b). However, the size distribution of spots changed from random to small size spots (Fig. 3a and b), suggesting that more active IgG ASCs are more sensitive to proteasome inhibiton (Fig. 3d, left shift from vehicle). In contrast, both the numbers and size of anti-dsDNA ASC spots decreased as proteasome inhibitor concentrations increased (Fig. 3a and c). The most plausible explanation for this result is that the active anti-dsDNA ASCs are more sensitive to proteasome inhibition, with either preferential killing and/or inhibition of antibody secretion activity.
Figure 3.
PIs abrogate antibody secretion from ASCs in vitro. Spleen cells of NZB/W mice (4+ protein) were incubated overnight with titrating amounts of the indicated PIs during ELISPOT assays. (a) Representative wells of ELISPOT results for IgG (7.8 × 103 cells/well) and anti-dsDNA (250 × 103 cells/well) ASC with bortezomib (31.25 nM), carfilzomib (1 αM), ONX 0914 (2 αM) and control (no PIs) added in vitro. The spot numbers or spot size of IgG (a) or anti-dsDNA IgG secreting cells (b) per 0.1 million spleen cells were normalized to the control (no PI, n=9) and plotted against the titrated amounts of PIs (mean (n=3) + s.e.m). (d). The average spot sizes of IgG ASC ELISPOT were plotted against the average occurrence relative to the highest occurrence with or without PIs.
Proteasome inhibitors abrogate IFN-α production in vitro
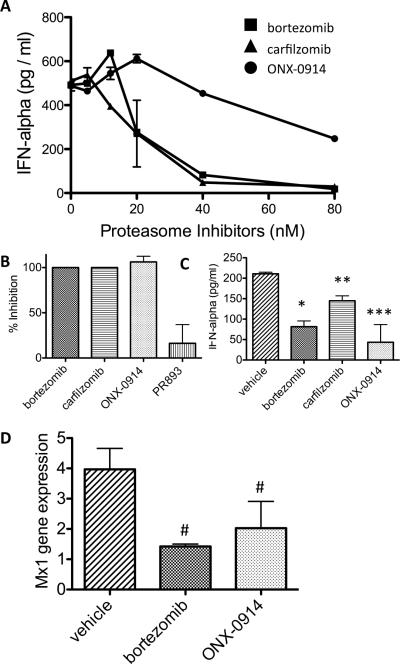
One of the signature pathogenic cytokines found in lupus patients and mice is IFN-α produced in large quantities by pDCs. Given that PIs have been demonstrated to have effects on the production of other pro-inflammatory cytokines by a variety of immune cells, and pDCs effectively become factories for production of IFN-α, we postulated that one of the important effects of proteasome inhibition in lupus may be abrogation of the IFN-α signature. First, we tested if PIs prevent the production of IFN-α by cells stimulated ex vivo via TLR (CpG) activation. Remarkably, all three PIs significantly suppressed the production of IFN-α by mouse BM cells in a concentration dependent manner (Fig. 4a). To evaluate the impact of selective immunoproteasome inhibition, we compared the IFN-α production in CpG stimulated bone marrow cells exposed to ONX 0914 or PR-893 at concentrations resulting in selective inhibition of LMP7 or β5, respectively (Fig. 4b). LMP7 inhibition blocked production of IFN-α by over 90% whereas selective inhibition of β5 did not alter cytokine release. Bortezomib and carfilzomib, which have dual inhibition of β5 and LMP7, also abrogated IFN-α production.
Figure 4.
PIs block IFN-α production in the mouse. (a) C57B6 mouse BM cells were incubated in vitro with titrating amounts of the indicated PIs in the presence of CpG2216 (500 ng/ml) overnight. IFN-α levels in the cell culture supernatants were measured by ELISA. (b) C57B6 mouse BM cells were incubated as above with the indicated PIs (125 nM ONX 0914, 125 nM PR-893, 40 nM bortezomib, or 40 nM carfilzomib) and CpG and IFN-α production assessed. (c) NZB/W mice (proteinuria 3+) were injected in vivo with PIs (bortezomib 0.75 mg/ml D1D3, carfilzomib 5 mg/ml on D1D2, ONX 0914 20 mg/ml on D1D3D5 or control vehicle) for 1 week and again 1 hour before sacrifice and analysis. Extracted BM cells were incubated in vitro overnight in the presence of CpG2216 and IFN-α levels in supernatants measured (mean (n = 3) + s.e.m.). *p=0.01, **p=0.03, ***p=0.06. (d) NZB/W mice were injected in vivo with PIs as above, RNA extracted from spleen cells, and Mx1 expression quantitated by real-time PCR. Data is normalized relative to the housekeeping gene β2-microglobulin (mean n=3 mice per group in duplicate or triplicate + s.e.m.). #p=0.05. Data are representative of 3 independent experiments.
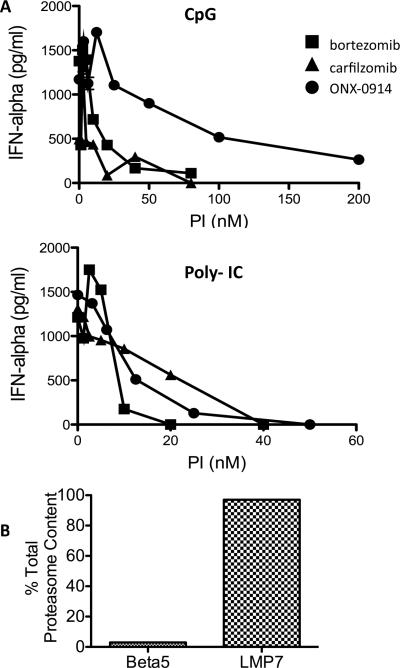
Next, we examined the impact of proteasome inhibition on IFN-α production by human PBMCs. Similar to the mouse studies, LMP7 inhibition blocked cytokine production (Fig. 5a). Of note, this occurred with multiple TLR ligands, including TLR9 (CpG) and TLR3 agonists, at multiple agonist concentrations (Fig. 5a and supplemental data). A similar result was observed with purified pDCs, the cell responsible for the largest production of IFN-α (data not shown). Moreover, analysis of purified pDCs revealed that over 95% of their proteasome activity is mediated by the immunoproteasome (Fig. 5b).
Figure 5.
Immunoproteasome inhibition abrogates IFN-α production by human PBMCs. (a) Human PBMCs were incubated with titrating amounts of the indicated PIs in the presence of CpG2216 (250 ng/ml) or Poly- IC (100 mg/ml) overnight. IFN-α levels in the cell culture supernatants were measured by ELISA. Data are shown from a single donor and are representative of 5 independent experiments with different donors. (b) pDCs were purified from two different human donors, a healthy control and a hemachromatosis patient. Purity of >95% was confirmed by flow cytometry. Proteasome activity was measured as described using both an enzymatic activity assay and an active site ELISA. β5 and LMP7 containing 20S subunit was quantitated in ng/mg protein and expressed as a %total 20S, mean +/− SEM.
Proteasome inhibitors block IFN-α production in vivo
The in vivo effects of proteasome inhibition were assessed using bone marrow cells from treated NZB/W F1 mice activated ex vivo by CpG. Both bortezomib and ONX 0914 reduced IFN-α production by approximately 75%, whereas carfilzomib caused a 40% reduction (Fig. 4c). These results demonstrate that proteasome inhibition can suppress the production of IFN-α in lupus. As further demonstration of the in vivo relevance of this inhibition to the disease process, we observed a significant decrease in the expression of the interferon-inducible gene Mx1 in spleen cells from NZB/W F1 mice treated with the proteasome inhibitors (Fig. 4d).
To further define the mechanisms of this effect, we determined whether pDCs were altered in numbers after in vivo or in vitro treatment with PIs. Notably, the fractions of pDCs in bone marrow were similar after in vivo treatment (Fig. 6a), as were absolute numbers (vehicle treated mice 97.3 × 103±21.5 vs. PI treated mice 98.8 × 103±29.2). However, upon in vitro stimulation there were moderate decreases in pDC numbers in a PI concentration dependent fashion. Notably, IFN producing pDCs appeared to be particularly susceptible to proteasome inhibitor induced cell death (Fig. 6c). Given the presence of residual pDCs at PI concentrations that completely abrogate IFN production, it is likely that proteasome inhibition suppresses pDC function as well via inhibition of IFN production and/or secretion.
Figure 6.
PIs suppress both the survival and function of IFN-α producing pDCs. (a) Flow cytometry analysis of mouse BM cells 1 hour after in vivo injection of PIs as described in the Figure 4c. The mean (n = 2) + s.e.m of %pDCs in total lymphocytes from BM of mice treated with different PIs is shown. (b) Representative flow cytometry analysis of pDCs after 16 hrs incubation in vitro as described in Figure 4 (a). Line graph depicts BM lymphocyte pDC % from in vitro culture incubated with different PIs at titrating concentrations (representative of 3 experiments). (c) C57B6 mouse BM cells were incubated with titrating amounts of the indicated PIs in vitro in the presence of CpG2216 (500 ng/ml) for 5 hours and an additional 3 hours with GolgiPlug. Intracellularly stained IFN-α accumulating pDC and total pDC levels were normalized to the vehicle treated control and plotted against the PI concentrations. Data presented are representative of 3 experiments, and the curves were fitted to the non-linear regression single decay.
DISCUSSION
In this report we present evidence that novel proteasome inhibitors, including selective targeting of the immunoproteasome, are remarkably efficacious in the treatment of murine lupus via a dual inhibition pathogenic IFN-α production and autoreactive plasma cells. Thus, the levels of serum total IgG and anti-dsDNA IgG antibody declined during treatment and correlated with a decrease in plasma cell numbers in spleen and bone marrow even after short-term treatment. Of note, autoreactive plasma cells were more sensitive to immunoproteasome inhibition both in vivo and in vitro. The data presented also show for the first time a unique role for the immunoproteasome in TLR driven interferon α production given that LMP7 (ONX 0914) but not β5 inhibition blocked cytokine production in TLR stimulated murine bone marrow cells and human PBMCs and decreased IFN inducible gene expression in vivo. Overall, these results demonstrate the synergistic effects of proteasome inhibition in lupus, with unique targeting of pathogenic IFN-α production, and provide strong rationale for the clinical development of these agents.
The elimination of plasma cells producing pathogenic autoantibodies observed here may be mediated by multiple pathways, including direct elimination, altered survival, or impaired generation. Given the rapid in vivo effects of proteasome inhibition on the plasma cell compartment and demonstration of in vitro elimination of anti-dsDNA secreting plasma cells, we favor a direct action on PCs as part of the effect. This is in accord with prior data in the literature demonstrating that proteasome inhibition induces apoptosis of multiple myeloma cells (12) and other antibody secreting PCs (9).
Terminally differentiated PCs may be particularly sensitive to the effects of proteasome inhibition since they produce antibodies rapidly with accumulation of misfolded peptides and induction of an unfolded protein response (UPR) (23), which leads to activation of proapoptotic proteins and caspases and ultimately apoptosis (24). The proteasome is the key catalytic machinery that degrades misfolded peptides, and indeed modulation of proteasome expression and activity within the PC compartment may be one mechanism regulating PC survival (25). Our observations that autoreactive PCs were preferentially targeted by proteasome inhibition, as evidenced by the more pronounced reductions of anti-dsDNA antibody secreting cells in vivo in spleen and bone marrow as well as their direct elimination in vitro, may be explained by a higher rate of antibody synthesis and secretion (23). Bortezomib was previously demonstrated to strongly and relatively specifically activate the UPR in PCs in a murine lupus model, with depletion of both long and short-lived PCs (9). Our data extends these observations to novel PIs, including immunoproteasome targeting, an important advance given that the toxicity profile of bortezomib may limit its development in autoimmune diseases.
The observed pronounced decrease in autoreactive PCs with in vivo treatment may in part be because a higher fraction of anti-dsDNA PCs are short-lived and continually generated. One of the key events in early PC differentiation is activation of a transcription factor XBP-1 (26). Proteasome inhibition has been reported to inhibit the auto-phosphorylation of IRE1alpha, leading to inhibition of XBP-1 activation and induction of apoptosis without activation of UPR in myeloma cell lines (27). Therefore, it is tempting to speculate that autoreactive plasmablasts may be highly sensitive to PIs due to the dependency on XBP-1 activity. Proteasome inhibition may also impair PC survival by altering the micro-environmental milieu. It is clear that PCs can have variable life-spans with long lived PCs maintaining protective serum antibodies for years (reviewed in (28)). Some of the factors important for the long life of PCs include IL-6, BAFF, APRIL, and TNF as well as chemokines such as CXCL12 found in bone marrow and inflamed tissues. This milieu may be disrupted in lupus, contributing to the longevity of autoreactive PCs (29). Interestingly, at least two key cytokines contributing to the plasma cell niche, TNF and IL-6, have been demonstrated to be dependent upon LMP7 (21).
A particularly novel aspect of our manuscript is the demonstration that TLR induced IFN-α production is completely abrogated by proteasome inhibition. Evidence supporting a prominent role for Type I interferon activation in SLE includes demonstration of serum elevations among patients with active SLE (30), the more recent demonstration that high IFN levels are a heritable risk factor for SLE (31), induction of autoimmunity with IFN-α treatment of malignancy and hepatitis C (32, 33), and the presence of an IFN-α gene expression signature in human SLE (17, 34). In murine SLE, the demonstration of a Type I IFN signature has been more variable. Thus, in some studies type I IFN has actually been found to exert a protective effect in MRL/lpr mice but a detrimental effect in NZB/W mice (35). Moreover, a prominent Type I IFN signature is found in NZB/W, but is more variable in MRL/lpr mice (36, 37). Although the mechanistic importance of PI targeting of the IFN pathway to the efficacy of these drugs in lupus is strongly supported by our demonstration of a decrease in IFN inducible gene expression in NZB/W mice in vivo, the contribution of this inhibition to the beneficial clinical effects in the MRL/lpr mouse remains unclear.
Plasmacytoid dendritic cells (pDCs) are typically the major source of IFN-α production. In SLE activation occurs via immune complex binding and costimulation of TLRs (TLR-7, -8, or-9) and FcRs on pDCs (38). In our ex vivo bone marrow culture, we confirmed that the majority of IFN-α was indeed produced by pDCs. A transcriptional activator for the IFN-α gene, IRF-7, is induced by constitutively expressed NF-kB. The expression level of IRF-7 also can be upregulated by the NF-kB/p38 MAPK pathway via TLR-9 signaling. IRF-7 activation also requires chloroquine-sensitive machinery upstream of NF-kB/p38 MAPK (39). Therefore, activated NF-kB plays a central role in IFN-α production via IRF-7. It is thus possible that proteasome inhibition blocks IFN-α production by NF-kB inactivation due to inefficient proteasome-dependent degradation of its inhibitor IkB (40). Additionally, as recently suggested PIs may suppress the function of pDCs by disrupting the intracellular trafficking of TLRs and subsequently the nuclear localization of IRF-7 and NF-kB (41). Finally, another mechanism for decreased IFN-α is direct apoptosis of pDCs due to proteasome inhibition. Indeed, pDCs may be particularly sensitive to proteasome inhibition in a similar fashion to plasma cells given the large amounts of IFN-α produced and secreted. Although we observed a moderate decrease in pDCs after in vitro treatment with PIs, this decreased survival does not account for the complete inhibition of IFN-α secretion. Moreover, absolute numbers of pDCs were not altered in vivo, again indicating suppression of both survival and function.
Inhibition of IFN-α likely contributes to the plasma cell effects of proteasome inhibition. Thus, CXCL12 expressed in bone marrow and inflamed tissue is an important factor for plasma cell niches and is enhanced by IFN-α (42). Other important roles for pDC-derived Type I interferon include T-cell independent activation of human B-cell differentiation to plasma cell (43) (44) and activation of IgG secretion in synergy with IL-6 (45). Therefore, it is our speculation that the abrogation of IFN-α production by proteasome inhibition causes more global and indirect benefits on autoantibody regulation.
In conclusion, proteasome inhibition has a beneficial effect in murine lupus with synergistic effects on plasma cells and unique targeting of Type I interferon pathways. The increased therapeutic margin of a selective immunoproteasome inhibitor as well as the LMP7 dependence of IFN-α production provides strong rationale for clinical development in an autoantibody and immune complex driven autoimmune disease such as lupus.
Supplementary Material
Acknowledgments
Dr. Anolik has been supported by the Lupus Research Institute and National Institutes of Health Grants R01AI077674-01A and a grant from Onyx Pharmaceuticals, Inc. Tony Muchamuel, Jing Jiang, Susan Lee, and Christopher Kirk are employed by Onyx Pharmaceuticals, Inc.
Nonstandard abbreviations
- ASC
antibody secreting cell
- APRIL
A proliferation-inducing ligand
- BAFF
B cell activation factor
- BCD
B cell depletion
- BCR
B cell receptor
- IFN
interferon
- PBMC
peripheral blood mononuclear cells
- pDC
plasmacytoid dendritic cell
- PI
proteasome inhibition
- TLR
Toll like receptor
- UPR
unfolded protein response
REFERENCES
- 1.Bongu A, Chang E, Ramsey-Goldman R. Can morbidity and mortality of SLE be improved? Best Practice and Research Clinical Rheumatology. 2002;16:313–332. doi: 10.1053/berh.2001.0228. [DOI] [PubMed] [Google Scholar]
- 2.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis & Rheumatism. 2004;50(8):2580–9. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 3.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56(9):3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 4.Sabahi R, Anolik JH. B-cell-targeted therapy for systemic lupus erythematosus. Drugs. 2006;66(15):1933–48. doi: 10.2165/00003495-200666150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62(1):222–33. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastritis E, Mitsiades CS, Dimopoulos MA, Richardson PG. Management of relapsed and relapsed refractory myeloma. Hematology - Oncology Clinics of North America. 2007;21(6):1175–215. doi: 10.1016/j.hoc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Li Z-W, Chen H, Campbell RA, Bonavida B, Berenson JR. NF-kappaB in the pathogenesis and treatment of multiple myeloma. Current Opinion in Hematology. 2008;15(4):391–9. doi: 10.1097/MOH.0b013e328302c7f4. [DOI] [PubMed] [Google Scholar]
- 8.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clinical Cancer Research. 2008;14(6):1649–57. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 9.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14(7):748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 10.Badros A, Goloubeva O, Dalal JS, Can I, Thompson J, Rapoport AP, et al. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007;110(5):1042–9. doi: 10.1002/cncr.22921. [DOI] [PubMed] [Google Scholar]
- 11.Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Research. 2007;67(13):6383–91. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110(9):3281–90. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vij R, Wang L, Orlowski R, Stewart A, Jagannath S, Lonial S. Carfilzomib a novel proteasome inhibitor for relapsed or refractory multiple myeloma is associated with minimal peripheral neuropathic effects. American Society of Hematology Annual Meeting. 2009 [Google Scholar]
- 14.Muchamuel T, Aujay M, Bennett M, Dajee M, Demo S, Goldstein E, et al. A Novel Inhibitor of the Immunopreoteosome Inhibits IL-23 Production in Vitro and Elicits an Ani-arthritic Effect in Multiple Mouse Models of Rheumatoid Arthritis Arthritis & Rheumatism. 2007 [Google Scholar]
- 15.Nencioni A, Schwarzenberg K, Brauer KM, Schmidt SM, Ballestrero A, Grunebach F, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108(2):551–8. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 16.Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9(9):664–71. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 17.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood.[see comment]. Journal of Experimental Medicine. 2003;197(6):711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174(5):2499–506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 20.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2443–57. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C, et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15(7):781–7. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- 22.Mensah KA, Mathian A, Ma L, Xing L, Ritchlin CT, Schwarz EM. Mediation of nonerosive arthritis in a mouse model of lupus by interferon-alpha-stimulated monocyte differentiation that is nonpermissive of osteoclastogenesis. Arthritis Rheum. 62(4):1127–37. doi: 10.1002/art.27312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67(4):1783–92. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 24.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–4. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 25.Cascio P, Oliva L, Cerruti F, Mariani E, Pasqualetto E, Cenci S, et al. Dampening Ab responses using proteasome inhibitors following in vivo B cell activation. Eur J Immunol. 2008;38(3):658–67. doi: 10.1002/eji.200737743. [DOI] [PubMed] [Google Scholar]
- 26.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 27.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100(17):9946–51. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6(10):741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 29.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199(11):1577–84. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301(1):5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 31.Kariuki SN, Franek BS, Kumar AA, Arrington J, Mikolaitis RA, Utset TO, et al. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res Ther. 12(4):R151. doi: 10.1186/ar3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrenstein MR, McSweeney E, Swane M, Worman CP, Goldstone AH, Isenberg DA. Appearance of anti-DNA antibodies in patients treated with interferon-alpha. Arthritis Rheum. 1993;36(2):279–80. doi: 10.1002/art.1780360224. [DOI] [PubMed] [Google Scholar]
- 33.Kalkner KM, Ronnblom L, Karlsson Parra AK, Bengtsson M, Olsson Y, Oberg K. Antibodies against double-stranded DNA and development of polymyositis during treatment with interferon. Qjm. 1998;91(6):393–9. doi: 10.1093/qjmed/91.6.393. [DOI] [PubMed] [Google Scholar]
- 34.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 35.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173(3):2134–42. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 36.Lu Q, Shen N, Li XM, Chen SL. Genomic view of IFN-alpha response in pre-autoimmune NZB/W and MRL/lpr mice. Genes Immun. 2007;8(7):590–603. doi: 10.1038/sj.gene.6364421. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Karypis G, Hippen KL, Vegoe AL, Ruiz P, Gilkeson GS, et al. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 2006;7(2):156–68. doi: 10.1038/sj.gene.6364286. [DOI] [PubMed] [Google Scholar]
- 38.Ronnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus.[comment]. Journal of Experimental Medicine. 2001;194(12):F59–63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osawa Y, Iho S, Takauji R, Takatsuka H, Yamamoto S, Takahashi T, et al. Collaborative action of NF-kappaB and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J Immunol. 2006;177(7):4841–52. doi: 10.4049/jimmunol.177.7.4841. [DOI] [PubMed] [Google Scholar]
- 40.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–60. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 41.Hirai M, Kadowaki N, Kitawaki T, Fujita H, Takaori-Kondo A, Fukui R, et al. Bortezomib suppresses function and survival of plasmacytoid dendritic cells by targeting intracellular trafficking of Toll-like receptors and endoplasmic reticulum homeostasis. Blood. 117(2):500–9. doi: 10.1182/blood-2010-05-284737. [DOI] [PubMed] [Google Scholar]
- 42.Badr G, Borhis G, Treton D, Richard Y. IFN{alpha} enhances human B-cell chemotaxis by modulating ligand-induced chemokine receptor signaling and internalization. Int Immunol. 2005;17(4):459–67. doi: 10.1093/intimm/dxh227. [DOI] [PubMed] [Google Scholar]
- 43.Giordani L, Sanchez M, Libri I, Quaranta MG, Mattioli B, Viora M. IFN-alpha amplifies human naive B cell TLR-9-mediated activation and Ig production. J Leukoc Biol. 2009;86(2):261–71. doi: 10.1189/jlb.0908560. [DOI] [PubMed] [Google Scholar]
- 44.Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103(8):3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 45.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.