Abstract
Myeloid derived suppressor cells (MDSCs) are an immunosuppressive population of immature myeloid cells found in advanced stage cancer patients and mouse tumor models. Production of inducible nitric oxide synthase (iNOS) and arginase, as well as other suppressive mechanisms, allow MDSCs to suppress T cell-mediated tumor clearance and foster tumor progression. Using an unbiased global gene expression approach in conditional p120-catenin knockout mice (L2-cre;p120ctnf/f), a model of oral-esophageal cancer, we have identified CD38 as playing a vital role in MDSC biology, previously unknown. CD38 belongs to the ADP-ribosyl cyclase family and possesses both ectoenzyme and receptor functions. It has been described to function in lymphoid and early myeloid cell differentiation, cell activation and neutrophil chemotaxis. We find that CD38 expression in MDSCs is evident in other mouse tumor models of esophageal carcinogenesis, and CD38high MDSCs are more immature than MDSCs lacking CD38 expression, suggesting a potential role for CD38 in the maturation halt found in MDSC populations. CD38high MDSCs also possess a greater capacity to suppress activated T cells, and promote tumor growth to a greater degree than CD38low MDSCs, likely as a result of increased iNOS production. Additionally, we have identified novel tumor-derived factors, specifically IL-6, IGFBP-3 and CXCL16, which induce CD38 expression by MDSCs ex vivo. Finally, we have detected an expansion of CD38-positive MDSCs in peripheral blood of advanced stage cancer patients and validated targeting CD38 in vivo as a novel approach to cancer therapy.
Keywords: CD38, myeloid derived suppressor cells, immature myeloid cells, iNOS, NFκB
Introduction
The immune system (both innate and adaptive) plays an essential role in limiting tumor growth, and therefore, tumor progression requires escape from immune surveillance. One mechanism that allows for tumor escape is the activation and expansion of immunosuppressive cell populations, including but not limited to, regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) (1), the latter also referred to as immature myeloid cells (IMCs). Certain therapeutics have demonstrated potential efficacy against MDSCs (2); however, the need for more selective anti-MDSC therapeutics remains.
MDSCs have been observed in a number of mouse tumor models and represent a heterogeneous population of immature monocytes and granulocytes that are identified by their CD11b+Gr-1+ phenotype in mice (3). In human disease, the first immature myeloid cell population with immunosuppressive capacity was described in head and neck cancer (4), and since then MDSCs have been documented in cancers of the esophagus, stomach, pancreas, lung, kidney, colon, skin, prostate, and breast (5–10). The immunophenotype of human MDSCs varies (11), however, their immunosuppressive mechanisms match those found in murine CD11b+Gr-1+ MDSC populations.
MDSCs induce immune suppression primarily through inhibition of T cell-mediated tumor clearance (3), but can also promote inhibition of NK cells (12) and activation of Tregs (13). Arginase-1 and inducible nitric oxide synthase-2 (iNOS) provide the bulk of enzymatic activity required for MDSCs to suppress T cell proliferation and activation (3). Arginase-1 deprives T cells of arginine by converting it into urea and L-ornithine, thereby reducing expression of CD3ζ chain, which renders T cells unable to respond to activation signals (14). iNOS inhibits T cell function by a variety of mechanisms, including inhibition of JAK3/STAT5 signaling (15), MHC Class II expression (16) and induction of apoptosis (17).
CD38 expression is a common characteristic to several immunosuppressive cell types. Foxp3+CD25+CD4+ Tregs expressing high CD38 levels possess a greater immunosuppressive activity than CD38low Tregs (18). CD38+CD8+ T cells suppress proliferation of CD4+ effector T cells, which requires IFNγ secretion and cell-to-cell contact (19). Similarly, CD19+CD24highCD38high B cells inhibit differentiation of T helper 1 cells in an IL-10 dependent manner, and their dysfunction may play a role in autoimmune disorders such as systemic lupus erythematosus (20).
CD38 is a member of the ribosyl cyclase family and is expressed on the surface of diverse immune cells, including B cells, T cells, NK cells and myeloid cells (21). CD38 possesses independent ectoenzyme and receptor functions. As an ectozyme, CD38 catalyzes synthesis and hydrolysis of cyclic ADP-ribose (cADPR), converting NAD+ to ADP-ribose (ADPR), as well as cADPR into ADPR (21,22). Furthermore, at acidic pH, CD38 catalyzes synthesis and hydrolysis of nicotinic acid adenine dinucleotide phosphate (NAADP) (21,22). Both reactions are essential for calcium signaling, specifically for mobilization of intracellular Ca2+ (22). Receptor activity of CD38 has been documented in multiple immune cell types, where it is dependent on localization to the lipid rafts and association with professional signaling complexes (21).
In both mouse and human myeloid cells, ligation of CD38 receptor leads to suppressed growth and survival resulting in loss of the most differentiated immune populations (23). In this study we have identified CD38 as a novel marker for MDSCs that possess greater immunosuppressive capacity, thereby promoting tumor growth in vivo. We have identified a mechanistic role for CD38 in promoting expansion of the monocytic MDSC population, as well as in regulating expression of the effector molecule iNOS by these cells. Additionally, we have established for the first time that several cytokines, specifically IFNγ, TNFα, IGFBP-3, CXCL16 and IL-6, are capable of inducing CD38 expression in MDSCs. Finally, we have demonstrated that administration of an anti-CD38 monoclonal antibody slows disease progression in tumor-bearing mice. As we have detected an expansion of CD38-positive MDSC-like population in peripheral blood of advanced-stage cancer patients, this study introduces the concept of anti-CD38 monoclonal antibody therapy for potential treatment of certain solid tumors.
Materials and Methods
Cell lines
AKR and HNM007 mouse ESCC tumor lines have been described previously (Opitz & Harada 2002; Takaoka et al. 2004). Cells were maintained in DMEM + 10% FBS and passaged or harvested at ~80% confluency. We have propagated cells from frozen stocks of the original vials that were authenticated by short tandem repeat analysis for highly polymorphic microsatellites FES/FPS, vWA31, D22S417, D10S526 and D5S592 so as to validate the identity of cells by comparing cells from the earliest stocks and those grown >8–12 passages. All cell lines have been tested for mycoplasma contamination on a regular basis.
Generation of MDSCs
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania. The L2-Cre;p120ctnf/f mouse model of oral-esophageal cancer was described previously (24). The mouse ESCC tumor lines have been described previously (25)(26). 2.5x105 AKR or HNM007cells/animal were injected subcutaneously into C57BL/6J or Cd38−/− mice (gift from Dr. Eduardo Chini). Subcutaneous tumor-bearing mice were aged until tumors reached a volume of 0.8cm3. Spleens and bone marrow were harvested upon euthanasia for MDSC isolation.
Flow cytometry and cell sorting (FACS)
Single cell suspensions were prepared from mouse bone marrow or spleen by mechanical disruption. For NFκB and iNOS staining, cells were fixed (BD Cytofix) and permeabilized with methanol. Peripheral blood from previously untreated, advanced stage HNC patients was obtained with informed consent under University of Pennsylvania IRB protocol #417200 or Philadelphia VA Medical Center protocol #01090. Patients’ peripheral blood mononuclear cells (PBMC) were separated using gradient centrifugation.
T cell suppression
CD11b+Gr-1+, CD11b+Gr-1+CD38low, and CD11b+Gr-1+CD38high cell populations were sorted by FACS. Antigen-specific CD8+ T cell suppression was tested as described previously (24). OVA-peptide was used to stimulate proliferation of OT-1 T cells.
RNA microarray and qPCR
RNA was isolated from FACS-sorted MDSCs to perform microarray (GeneChip Mouse Exon 1.0 ST Array, Affymetrix). Ingenuity Pathway Analysis software was used for data analysis. cDNA was generated using oligo-dT primers and Superscript II Reverse Transcriptase. qPCR was performed using validated SYBR Green primers and ABI7000 (Applied Biosystems).
Ex vivo MDSC differentiation
Generation of MDSCs from bone marrow has been described previously (27). Cytokine concentrations used: 0.1 ng/ml (GM-CSF and IL-4), 10 ng/ml (TNFα and IFNγ), and 100 ng/ml (IL-6, CXCL16 and IGFBP-3). HNM007 or AKR conditioned media (CM) were used at 50% v/v. Anti-CD38 or IgG2a isotype control antibodies were used at 10ug/ml.
Colony formation and cell recovery assays
Isolation of MDSCs from tumor-bearing L2-cre;p120−/− mice by magnetic cell sorting was described previously (24). 200,000 cells were cultured in MethoCult medium (Stem Cell Technologies). Anti-CD38 or IgG2a isotype control antibodies were used at 10ug/mL. Colonies were counted after 7 days. For recovery assays, 5x105 MDSCs were seeded in complete RPMI 1640 medium supplemented with antibodies; cells were quantified by Trypan Blue exclusion using a Countess automated cell counter (Invitrogen).
Cytokine array
Media from ex vivo differentiation cultures were collected and snap-frozen after 1 or 5 days of culture. Mouse cytokine array C3 kit (Raybiotech) was used according to the manufacturer’s protocol. Results were quantified using the ImageJ protein array analyzer and normalized to positive controls.
ESCC/MDSC co-transplantation and anti-CD38 therapeutic study
C57BL/6J recipient mice from Jackson Labs were injected subcutaneously with a mixture of 2.5x105 syngeneic HNM007 tumor cells with either 2.5x105 CD38low or CD38hi MDSCs obtained from HNM007 tumor-bearing C57BL/6J mice. Recipient mice injected with 2.5x105 syngeneic HNM007 tumor cells alone served as controls. For antibody treatment experiments, anti-CD38 monoclonal antibody or IgG2a isotype control antibody were administered intraperitoneally every 48 hours starting on day 5 post-injection. Measurements were taken every 2–3 days once tumors became palpable.
Histology
Subcutaneous tumors were fixed in buffered formalin solution, paraffin-embedded and stained with hematoxylin and eosin (H&E). Antigen-specific staining was performed as described previously (24).
Statistical analysis
The Student’s t test was used to determine whether there is significant difference between two experimental groups (p≤0.05 was considered statistically significant).
Additional details (qPCR primers, antibodies and detailed ex vivo differentiation protocol) can be found in Supplementary Materials and Methods.
Results
Myeloid-derived suppressor cells from tumor-bearing L2-Cre;p120f/f mice exhibit elevated CD38 expression
We have previously demonstrated that MDSCs play a fundamental role in tumor initiation and progression in a spontaneous genetic mouse model of ESCC (L2-Cre;p120f/f; referred to hereafter as p120−/−) (24). Here we sought to identify genes associated with an immature myeloid phenotype that contribute to the tumor promoting activities of MDSCs, thereby providing a platform to elucidate underlying molecular mechanisms. To that end, we performed microarray analysis of splenic MDSCs from 6–8 month old tumor-bearing p120−/− mice and age-matched littermate controls (Supplementary Fig.1; GEO accession number GSE71706). Among the 964 genes showing differential expression between the two groups (Figure 1A), we identified Cd38 (ranked fifth highest among all genes tested (Supplementary Table 1)) as a candidate gene of interest, as it has roles in both innate and adaptive immunity in mice and humans, including, but not limited to chemotaxis of murine and human neutrophils (28,29), early myeloid differentiation (23) and lymphoid cell activation (30). We validated Cd38 mRNA and protein expression in MDSCs from tumor-bearing mice, compared to those isolated from control mice (Fig. 1B-D). We also observed increased CD38 in splenic MDSCs isolated from L2-IL1β mice, a model of Barrett’s esophagus and esophageal adenocarcinoma (31) (Supplementary Fig. 2).
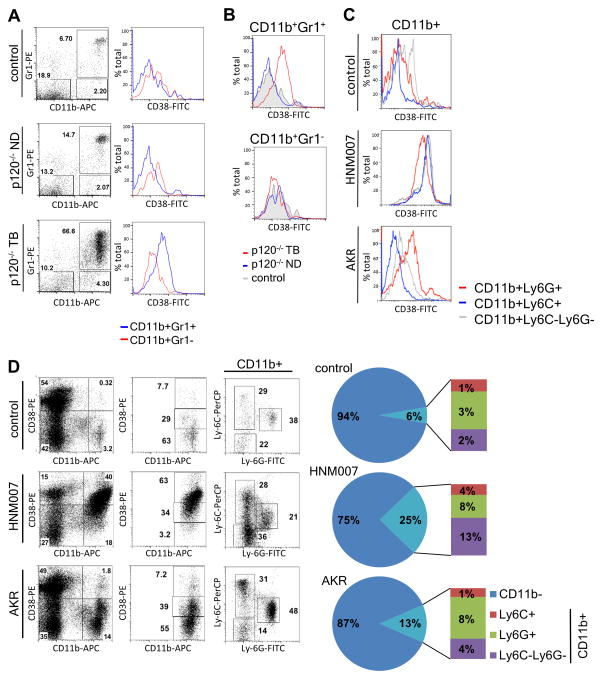
Figure 1. CD38 is significantly upregulated in CD11b+Gr-1+ cells from tumor-bearing p120−/− mice.
(A) Heatmap illustrating the results of microarray analysis performed using CD11b+Gr-1+ cells from the spleens of 6 tumor-bearing p120−/− mice and 3 pooled samples from healthy littermate controls (n=9). Increased expression of the Cd38 gene and protein in CD11b+Gr-1+ cells from tumor-bearing mice was confirmed by (B) qPCR (*p=0.007) and (C) FACS (n= 3; *p=0.009). (D) Frequencies of CD38+ cells (*p=0.003).
CD38 expression correlates with ESCC progression and expansion of monocytic MDSC population
To determine the kinetics of CD38 expression in MDSCs, we analyzed splenic CD11b+Gr-1+ populations from non-diseased (8 weeks) and tumor-bearing (6–8 months) p120−/− mice, as well as control mice. CD11b+Gr-1+ cells were slightly more abundant in spleens of non-diseased p120−/− mice and markedly elevated in spleens of tumor-bearing p120−/− mice, compared to control mice (Fig 2A). CD38 expression was markedly increased only in splenic MDSCs from tumor-bearing p120−/− mice (Fig. 2B), while a more mature subset of myeloid cells (CD11b+Gr-1−) exhibited no change in CD38 levels (Fig 2B).
Figure 2. CD38 expression increases in monocytic myeloid cells with disease progression.
(A) Splenocytes from healthy control, non-diseased (ND) p120−/− and tumor-bearing (TB) p120−/− mice were analyzed by FACS for CD38 expression on myeloid cell populations. (B) Histograms comparing CD38-FITC fluorescence levels on two cell subsets from control, p120−/− non-diseased and p120−/− tumor-bearing mice. (C) Splenocytes from control non-diseased and AKR or HNM007 subcutaneous tumor-bearing C57BL/6 mice analyzed by FACS. Histograms compare CD38 expression levels in listed subpopulations from control and tumor-bearing mice. (D) Splenocytes from control and tumor-bearing mice were analyzed by FACS for distribution of CD11b, Ly-6C, Ly-6G and CD38 antigens. Pie charts demonstrate the frequencies of lymphoid (CD11b−) and myeloid (CD11b+) cell populations in spleens of control and tumor-bearing mice with the myeloid population further broken down into Ly-6C+, Ly-6G+ and Ly-6C−Ly-6G− subsets (n=3 per group).
We next tested two murine ESCC cell lines (AKR (25) and HNM007 (26)) for their ability to generate MDSCs in vivo using a syngeneic transplant model. We observed dramatically increased CD38 levels in all myeloid populations from spleens of HNM007 tumor-bearing mice, yet in AKR tumor-bearing mice CD38 levels were overall lower (Fig. 2C, Supplementary Fig. 3). Interestingly, while both cell lines induced expansion of myeloid populations in spleens of tumor-bearing mice, it was significantly more pronounced (p=0.0009) in HNM007 tumor-bearing mice (Fig. 2D). Furthermore, we observed differences in distribution of granulocytic and monocytic MDSCs (G-MDSC and M-MDSC, respectively), as well as mature monocytes (Fig. 2D). G-MDSCs (CD11b+Ly-6G+) were less abundant (p=0.02) in HNM007 tumor-bearing mice, compared to AKR. There also was a trend of M-MDSC (CD11b+Ly-6C+) expansion, accompanied by a significant increase in mature monocytes (CD11b+Ly-6C−Ly-6G−) in HNM007, compared to AKR tumor-bearing and control mice (p=0.02). These findings suggest that CD38 may be relevant to M-MDSC expansion in tumor-bearing mice.
CD38high MDSCs possess greater immunosuppressive and tumor-promoting capacity than CD38low MDSCs
Since the CD38high MDSC population expands in tumor-bearing mice, we hypothesized that CD38high MDSCs possess greater immunosuppressive potential than CD38low MDSCs. To test this, we sorted CD38high and CD38low MDSCs from HNM007 tumor-bearing mice and assessed their capacity to suppress OT-1 T cell growth following antigen stimulation. CD38high MDSCs demonstrated significantly greater T cell suppressive capacity, compared to their CD38low counterparts (Fig. 3A), at 2:1 OT-1 to MDSC ratio, while a trend of increased suppression was observed at 1:1 and 4:1 ratios.
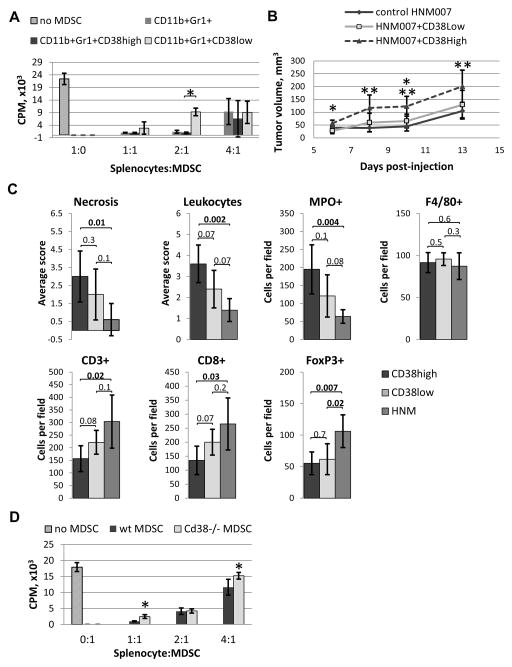
Figure 3. CD38high MDSCs are more immunosuppressive and promote tumor growth more efficiently than the CD38low MDSCs.
(A) CD38high and CD38low MDSCs from tumor-bearing p120−/− mice were used in a T cell suppression assay (n=3; *p=0.0007). (B) C57BL/6 mice were injected with HNM007 cells in combination with MDSCs (CD38High or CD38Low) or alone (n=5 per group). Tumor volumes were compared between the CD38High and CD38Low groups (*p=0.004 and 0.03), and between CD38High and control HNM007 tumors (** p=0.01, 0.003 and 0.01). (C) Quantification of immunohistochemical analysis of tumor sections from (B). (D) Splenic MDSCs from HNM007 tumor-bearing Cd38−/− or wt mice were used in a T cell suppression assay (*p=0.003 and 0.04).
Next we evaluated the impact of co-injection of CD38high MDSCs with HNM007 cells on tumor growth. Tumor volumes in CD38high group were significantly larger than CD38low tumors on days 6 and 10 (Fig. 3B), and larger than control HNM007 tumors on days 8, 10 and 13 (Fig. 3B). No difference in size was detected between the CD38low and control HNM007 tumors. Furthermore, we found morphological differences between the tumors from different experimental groups. CD38high-injected tumors were characterized by increased necrosis, inflammatory infiltrate (mostly by MPO+ neutrophils), and reduced number of CD3+ T cells (including CD8+ cytotoxic T cells), compared to controls (Fig. 3C, Supplementary Fig. 4). Surprisingly, control tumors had more Tregs (FoxP3+), compared to both CD38high and CD38low co-injected tumors. These results suggest that CD38high MDSCs may possess greater tumor-promoting capacity than CD38low MDSCs in vivo.
Next we investigated whether CD38 is required for the immunosuppressive function of MDSCs by analyzing the capacity of MDSCs from Cd38−/− and Cd38+/+ (wt) mice bearing HNM007 tumors to suppress OT-1 T cell proliferation. Interestingly, Cd38−/− MDSCs exhibited significantly reduced immunosuppressive capacity at 1:1 and 4:1 OT-1 to MDSC ratios (Fig. 3E).
CD38high MDSCs are phenotypically different from the CD38low subset
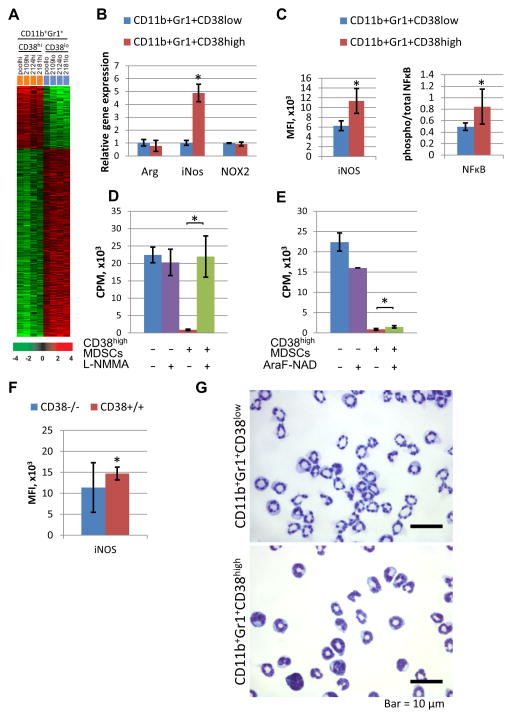
Next we analyzed CD38high and CD38low splenic MDSCs from tumor-bearing p120−/− mice via microarray (Supplementary Fig.5; GEO accession number GSE71706) and detected differential expression of 498 genes (Fig. 4A, Supplementary Table 2). Among genes with the greatest increase in expression, was inducible nitric oxide synthase (iNos). qPCR analysis further revealed that iNos expression was significantly elevated in CD38high MDSCs compared to CD38low MDSCs, while expression of arginase 1 (Arg1) and NADPH oxidase subunit (Nox2) , two additional mediators of MDSC suppressive function, was comparable in these subpopulations (Fig. 4B). iNOS protein expression was also validated in CD38high MDSCs (Fig 4C, Supplementary Fig. 5B). Since iNos is a target of NFκB transactivation (32), we evaluated the levels of total and phosphorylated NFκB in CD38high and CD38low MDSCs and found increased phosphoNFκB-to-totalNFκB ratio in the CD38high population (Fig. 4C, Supplementary Fig. 5B). To test whether iNOS contributes to the increased immunosuppressive capacity of CD38high MDSCs, we used an iNOS inhibitor (L-NMMA), and found that it completely abrogated OT-1 T cell suppression mediated by CD38high MDSCs (Fig. 4D). Finally, the CD38 inhibitor AraF-NAD (33) partially rescued OT-1 T cell proliferation (Fig. 4E), suggesting that CD38 enzymatic activity is required for immunosuppressive capacity of CD38high MDSCs. Furthermore, iNOS expression was decreased in MDSCs isolated from the spleens of HNM007 tumor-bearing Cd38−/− mice (Fig. 4F, Supplementary Fig. 5C).
Figure 4. CD38high MDSCs are phenotypically different from the CD38low subset.
(A) Heatmap illustrating the results of microarray analysis of CD38high and CD38low CD11b+Gr-1+ cells from spleens of 4 tumor-bearing p120−/− mice. (B) qPCR analysis of iNos, Arg1 and Nox2 gene expression (*p=9x10−8). (C) Quantification of iNOS expression and NFκB activation (calculated as a ratio of phosphorylated/total p65 protein), measured by FACS (*p=10−5 and 0.007, respectively; MFI=mean fluorescence intensity). (D) iNOS inhibitor (L-NMMA) and (E) CD38 inhibitor (AraF-NAD) were tested in a T cell suppression assay (*p=0.004 and 0.04, respectively). (F) Expression levels of iNOS in splenic MDSCs from tumor-bearing Cd38−/− or wt mice, measured by FACS (*p=0.05). (G) Cytospin preparations of CD38high and CD38low MDSCs.
Morphological assessment of sorted CD38low and CD38high MDSCs revealed that the CD38high population consists of more immature cells, such as promyelocytes (~10%), myelocytes (5–10%), metamyeloctyes (5–10%), and band cells (~70%), whereas the CD38low population consists of band cells (<10%) and mature neutrophils (>90%) (Fig. 4G), demonstrating that CD38high MDSCs are morphologically more immature than CD38low MDSCs.
IFNγ, TNFα, CXCL16, IGFBP-3 and IL-6 induce CD38 expression
Since we found that MDSCs from HNM007 tumor-bearing mice have increased CD38 expression, compared to AKR tumors (Fig. 2C), we sought to understand signaling pathways underlying this phenotype. We performed ex vivo bone marrow differentiation assays using GM-CSF, IL-4 (both required for CD11b+Gr-1+ cell generation from bone marrow progenitors (27)) and conditioned media (CM) from either HNM007 or AKR cells. Only HNM007 CM induced CD38 expression (Fig. 5A). Since IFNγ and TNFγ are key components of the pro-inflammatory milieu and are known activators of CD38 transcription (34), we used these cytokines in ex vivo differentiation assays. Interestingly, both factors, individually or in combination, induced CD38 expression in CD11b+Gr-1+ cells (Fig. 5A). A cytokine array using CM from ex vivo differentiation experiments revealed several factors, including CXCL16 and IGFBP-3 that were present at higher levels in HNM007 cultures as compared to AKR cultures (Fig.5B). In addition, the pro-inflammatory cytokine IL-6, a predicted activator of CD38 transcription (34), was elevated in HNM007 cultures, albeit not as dramatically as CXCL16 or IGFBP-3 (Fig. 5B). Next we investigated the capacity of recombinant IL-6, CXCL16 and IGFBP-3 to increase CD38 expression ex vivo. Interestingly, addition of IL-6, CXCL16 and IGFBP-3 in combination induced a moderate, yet significant, increase in CD38 expression in AKR CM cultures (Fig. 5C).
Figure 5. IFNγ, TNFα, IGFBP-3, CXCL16 and IL-6 induce CD38 expression and impair myeloid cell differentiation.
(A) CD38 expression in CD11b+Gr-1+ cells from ex vivo differentiation cultures was tested by FACS (MFI=mean fluorescence intensity; n=3; * p=0.0001, **p=2.5x10−5). (B) Cytokine array performed with media from ex vivo differentiation cultures (24 or 120-hour). Each cytokine tested in duplicate. Difference in normalized expression between HNM007 and AKR groups is shown. (C) Ex vivo differentiation as in (A) with the addition of cytokines to the AKR conditioned media (n=3; *p=0.05, ** p=0.005). (E) In mice, tumor progression leads to MDSC expansion. Tumor progression leads to amplified signals (such as cytokines) reaching MDSCs, which induces a differentiation halt and expansion of CD38high monocytic MDSCs with enhanced immunosuppressive capacity (mediated by iNOS, which produces nitric oxide (NO)).
Cross-linking of CD38 by an agonistic antibody impairs expansion and survival of CD11b+Gr-1+ cells in vitro and suppresses tumor growth in vivo
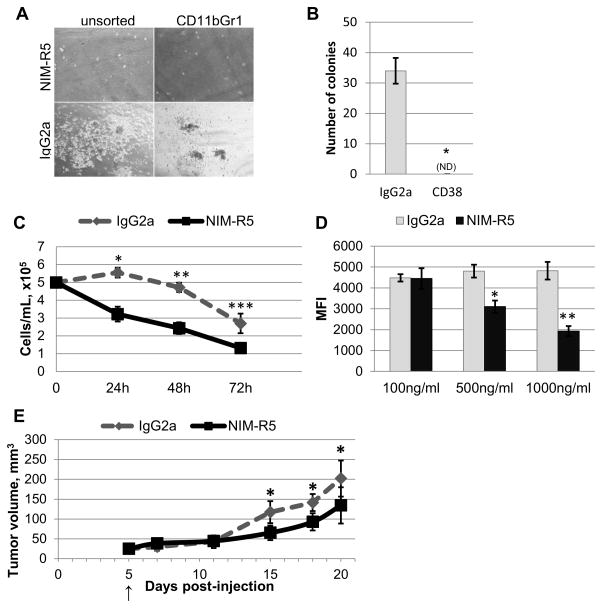
To test whether cross-linking of CD38 with a monoclonal antibody has an effect on MDSC function(s), MDSCs from spleens of tumor-bearing p120−/− mice were cultured in methylcellulose-based medium in the presence of an anti-CD38 monoclonal antibody (NIM-R5) or isotype control (IgG2a). Addition of anti-CD38 antibody inhibited growth of colonies from splenic MDSCs, and the effect of anti- CD38 antibody remained unchanged regardless of whether splenocytes were pre-sorted (Fig. 6A and 6B), demonstrating that the anti-CD38 antibody inhibits MDSC proliferation and survival in vitro. In suspension culture, sorted MDSCs survive only a few days, but their survival was further reduced in the presence of anti-CD38 antibody (Fig. 6C). We also tested whether CD38 cross-linking inhibits accumulation of CD11b+Gr-1+CD38high cells ex vivo in the presence of HNM007 CM. Using an additional anti-CD38 antibody (clone 90), we observed a dose-dependent decrease in CD38 expression within the CD11b+Gr-1+ population (Fig. 6D). Given that the proportion of CD11b+Gr-1+ cells within the culture remained consistent (25–30%; data not shown), these data demonstrate that the CD11b+Gr-1+CD38high population is likely depleted as a result of CD38 cross-linking. Lastly, anti-CD38 antibody treatment resulted in decreased tumor growth rate in vivo in a subcutaneous HNM007 transplant ESCC model (Fig. 6E). In aggregate, these data demonstrate the importance of CD38 for MDSC-mediated ESCC progression and suggest targeting CD38 as an approach to ESCC therapy.
Figure 6. Cross-linking of CD38 by an agonistic antibody impairs expansion and survival of CD11b+Gr-1+ cells in vitro and suppresses tumor growth in vivo.
(A) Representative images from methylcellulose cultures of CD11b+Gr-1+ cells treated with anti-CD38 monoclonal antibody (NIMR-5) or isotype control (IgG2a), after 5 days of culture. (B) Number of colonies formed following 7 days of culture (n=3; *p=4x10−5). (C) CD11b+Gr-1+ cells were cultured in RPMI with anti-CD38 or isotype control antibody, and counted at indicated time points. (n=6 per group; *p=5x10−7, **p=2x10−7, ***p=0.0005). (D) Ex vivo differentiation performed with HNM007 conditioned medium and anti-CD38 agonist (NIM-R5) or isotype control (IgG2a) antibody. CD38 expression (using the clone 90 antibody) on the surface of CD45+7-AAD−CD11b+Gr-1+ cells was measured by FACS (n=3 per group; * p=0.003, ** p=0.0005). (E) HNM007 tumor growth kinetics in C57BL/6 mice treated with anti-CD38 (NIM-R5) or isotype control (IgG2a) antibody (arrow marks the start of treatment; n=6 per group; *p=0.005, 0.005 and 0.04).
CD38 is expressed on human MDSC-like cell population that is expanded in peripheral blood of advanced-stage cancer patients
To determine whether our findings may be relevant to human cancers, we analyzed CD38 expression in the low-density CD15hiCD33lo population of PBMCs from advanced stage head and neck cancer and non-small cell lung cancer patients and healthy donors. In contrast to our observations in mice, we found that CD38 expression levels were unchanged in CD15hiCD33lo PBMCs from cancer patients, compared to healthy donors (Supplementary Fig.8). However, this population was significantly expanded from 0.5% of total PBMCs in healthy donors to up to 17% in cancer patients (Fig.7).
Figure 7. CD38+ MDSC-like population is expanded in the peripheral blood of advanced-stage cancer patients.
Histograms depict frequencies of low density CD38+CD15highCD33low cells in peripheral blood mononuclear cells (PBMC) from head and neck (HNC) and non-small cell lung (NSCLC) cancer patients and healthy donors.
Discussion
Using spontaneous genetic and syngeneic transplant tumor models, as well as an ex vivo differentiation model, we have established for the first time that tumor-derived signals drive expansion of monocytic MDSCs by inducing CD38 expression. Expansion of the CD11b+Gr-1+CD38high cell population occurs after initial splenic MDSC accumulation is evident, which likely indicates a requirement of threshold levels of tumor-derived signals for induction of CD38 by MDSCs (Fig. 5D). Interestingly, two different ESCC cell lines exhibited differential capacities to induce expansion of CD38high MDSCs, thereby suggesting that the tumor cells are responsible for promoting CD38 expression on MDSCs. Based upon our ex vivo studies, the tumor-derived signals may act directly on immature myeloid cell populations present in hematopoietic tissues to promote CD38 expression. Furthermore, our data suggest that the tumor-derived signals do not promote enhanced proliferation of CD38high MDSCs (RB1 pathway was activated in CD38high MDSCs (Supplementary Fig. 6)), but provide these cells with increased survival potential.
Herein, we demonstrate that CD38high MDSCs are halted at an earlier differentiation stage compared to CD38low MDSCs. CD38 ligation can contribute directly to the differentiation halt (23), which suggests that CD38 signaling may contribute to the maintenance of undifferentiated state observed in CD38high MDSCs. Although CD38 has been demonstrated to bind CD31 (21), we do not know if this interaction contributes to the observed properties of CD38high MDSCs.
CD38high MDSCs express elevated iNOS levels compared to CD38low MDSCs, and iNOS is required for T cell suppression by CD38high MDSCs. Interestingly, CD38 can induce iNOS upregulation in murine activated microglia (resident monocytes of the brain) (35). Furthermore, Cd38−/− mice produce less tumor-associated microglia in a syngeneic transplant model of glioma (36). Strikingly, we have found that in Cd38−/− mice, subcutaneous ESCC tumors induce a less pronounced expansion of M-MDSCs, regardless of the cell line used to generate tumors (Supplementary Fig. 7). These findings support the premise that CD38 promotes expansion of M-MDSCs, as well as elevated iNOS expression. We also observed increased NFκB activation in CD11b+Gr-1+CD38high cells. This is consistent with observations made in murine B cells, where CD38 ligation activates NFκB (37). Furthermore, NFκB-mediated activation of iNOS has been described in LPS-stimulated macrophages (38), highlighting the possibility that increased NFκB activation in CD38high MDSCs may contribute to increased iNOS expression observed in these cells.
Several factors are likely to be responsible for activating CD38 expression, including IFNγ, TNFα (34), as well as IL-6, IGFBP-3 and CXCL16. We have demonstrated that IFNγ and TNFα induce bone marrow-derived CD11b+Gr-1+ cells to express CD38 ex vivo. As both IFNγ and TNFα are often produced during chronic inflammation, they may be the primary inducers of CD38 expression (Fig. 5D). In fact, TNFα inhibition can impair immunosuppressive capacity of MDSCs and induce differentiation in a murine model of chronic inflammation, while MDSCs from Tnf−/− mice have reduced iNOS levels (39).
Our finding of a CXCL16 and IGFBP-3-mediated response in MDSCs has not been described previously. However, CXCL16 expression can be promoted by IFNγ and TNFα (40), the two most potent inducers of CD38 expression in our ex vivo system. Interestingly, IGFBP-3 has been shown to increase intracellular Ca2+ levels in vitro (41). Ca2+ signaling, which can be mediated by ectoenzymatic activity of CD38 (34), is important for multiple immunomodulatory processes (42,43). Therefore, it is possible that in MDSCs IGFBP-3 can be modulating Ca2+ mobilization by increasing CD38 expression.
IL-6 is a major regulator of STAT3 signaling, which is essential for establishment of immunosuppressive microenvironment within the tumor (44). In MDSCs, STAT3 activation enhances production of the S100A8/A9 pro-inflammatory proteins, which also contribute to maintenance of a low differentiation or immature state (45). These data are in agreement with our observation that IL-6 can promote CD38 expression on MDSCs generated ex vivo, since CD38high MDSCs are less differentiated than CD38low MDSCs (Fig. 4B).
Herein, we demonstrate the efficacy of anti-CD38 monoclonal antibody treatment in vitro and in vivo. Moreover, we report CD38 expression by human MDSCs; therefore, anti-CD38 therapy may represent a novel approach to targeting this immunosuppressive population in cancer treatment strategies. Furthermore, since CD38high Tregs possess enhanced suppressive potential compared to CD38low Tregs (18,19), anti-CD38 therapy may present the advantage of targeting several immunosuppressive cell types at the same time. Recently, an anti-CD38 monoclonal antibody (Daratumumab) was shown to be efficient in treatment of multiple myeloma in pre-clinical studies (46). A similar approach may induce ablation of MDSCs in patients with advanced stage solid cancers, and thus, may be suitable as an adjuvant to conventional therapies. The expression pattern of CD38 in a broad range of cell types can raise a concern about potential adverse effects of anti-CD38 therapy (47), however, early clinical studies of Daratumumab in multiple myeloma have demonstrated an acceptable safety profile, suggesting that an appropriate dosage and treatment schedule allow for minimizing of the effects of targeting CD38 in normal tissue (48).
MDSCs contribute to the T cell suppression repertoire found in cancer, which merits further investigation as a prospective therapeutic target (49). In this study, we have identified CD38 as being suitable for potential MDSC targeting and useful in identification of potently immunosuppressive MDSC populations. Thus, anti-CD38 monoclonal antibody therapy (46) may hold potential for targeting CD38-expressing MDSCs (50) in patients with certain types of cancer.
Supplementary Material
Acknowledgments
We are grateful to the Center for Molecular Studies in Digestive and Liver Diseases (NIH P30-DK050306), the Molecular Pathology and Imaging Core (J. Katz, A. Bedenbaugh, D. Budo, and R. Hasan), the Molecular Biology/Gene Expression Core (G. Wu and S. Keilbaugh), the Transgenic and Chimeric Mouse Core, the Cell Culture Core, the Penn Microarray and Flow Cytometry and Cell Sorting Facilities, and CHOP Pathology Core Laboratories. We also thank Ann Tierney for assistance with statistical analyses, Dr. Gregory Beatty for analysis of cytospin preparations, and members of the Rustgi and Singhal laboratories for discussions.
Funding: This work was supported by the National Institutes of Health/NCI grant P01-CA098101 (AKR, TW, TK), National Institutes of Health/NCI grant U01-CA14305603 (AKR), National Institutes of Health/NIDDK (T32-DK007066) (TW), National Institutes of Health (F32-CA162719) (TW), National Institutes of Health/NIDDK Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306), American Cancer Society (RP-10-033-01-CCE), National Institutes of Health NIH/NIDCR (K08-DE022842) (DB), National Institutes of Health (Transformative R01-CA163256-01) (SS), Italian Ministry of Education, University and Research (Progetto PRIN and FIRB) (FM) and by the Fondazione Ricerca in Medicina Sperimentale (FIRMS) (FM).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Waldron TJ, Quatromoni GJ, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells: Targets for therapy. Oncoimmunology Landes Bioscience. 2013;2:e24117. doi: 10.4161/onci.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. 2009/02/07 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 5.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 7.Liu C-Y, Wang Y-M, Wang C-L, Feng P-H, Ko H-W, Liu Y-H, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14 /CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 9.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 10.Vuk-Pavlović S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate. 2010;70:443–55. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–7. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 13.Huang B, Pan P-Y, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr- 1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, et al. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171:1232–9. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 15.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–34. [PubMed] [Google Scholar]
- 16.Harari O, Liao JK. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des. 2004;10:893–8. doi: 10.2174/1381612043452893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, et al. Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev. 2002;188:97–113. doi: 10.1034/j.1600-065x.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 18.Patton DT, Wilson MD, Rowan WC, Soond DR, Okkenhaug K. The PI3K p110δ regulates expression of CD38 on regulatory T cells. PLoS One. 2011;6:e17359. doi: 10.1371/journal.pone.0017359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahri R, Bollinger A, Bollinger T, Orinska Z, Bulfone-Paus S. Ectonucleotidase CD38 demarcates regulatory, memory-like CD8+ T cells with IFN-γ-mediated suppressor activities. PLoS One. 2012;7:e45234. doi: 10.1371/journal.pone.0045234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–86. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 22.Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem. 2012;287:31633–40. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todisco E, Suzuki T, Srivannaboon K, Coustan-Smith E, Raimondi SC, Behm FG, et al. CD38 ligation inhibits normal and leukemic myelopoiesis. Blood. 2000;95:535–42. 2000/01/11 ed. [PubMed] [Google Scholar]
- 24.Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19:470–83. doi: 10.1016/j.ccr.2011.02.007. 2011/04/13 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opitz O, Harada H. A mouse model of human oral-esophageal cancer. J …. 2002;110:761–9. doi: 10.1172/JCI15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaoka M, Harada H, Deramaudt TB, Oyama K, Andl CD, Johnstone CN, et al. Ha-Ras(G12V) induces senescence in primary and immortalized human esophageal keratinocytes with p53 dysfunction. Oncogene Nature Publishing Group. 2004;23:6760–8. doi: 10.1038/sj.onc.1207923. [DOI] [PubMed] [Google Scholar]
- 27.Youn J-I, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partida-Sánchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–16. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 29.Partida-Sanchez S, Iribarren P, Moreno-Garcia ME, Gao J-L, Murphy PM, Oppenheimer N, et al. Chemotaxis and Calcium Responses of Phagocytes to Formyl Peptide Receptor Ligands Is Differentially Regulated by Cyclic ADP Ribose. J Immunol. 2004;172:1896–906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- 30.Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N. CD38 and chronic lymphocytic leukemia: a decade later. Blood. 2011;118:3470–8. doi: 10.1182/blood-2011-06-275610. 2011/07/19 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quante M, Bhagat G, Abrams Ja, Marache F, Good P, Lee MD, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. Elsevier Inc. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–53. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Muller-Steffner HM, Malver O, Hosie L, Oppenheimer NJ, Schuber F. Slow-binding inhibition of NAD+ glycohydrolase by arabino analogues of beta-NAD. J Biol Chem. 1992;267:9606–11. [PubMed] [Google Scholar]
- 34.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–86. doi: 10.1152/physrev.00035.2007. 2008/07/16 ed. [DOI] [PubMed] [Google Scholar]
- 35.Mayo L, Jacob-Hirsch J, Amariglio N, Rechavi G, Moutin M-J, Lund FE, et al. Dual Role of CD38 in Microglial Activation and Activation-Induced Cell Death. J Immunol. 2008;181:92–103. doi: 10.4049/jimmunol.181.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy A, Blacher E, Vaknine H, Lund FE, Stein R, Mayo L. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neuro Oncol. 2012;14:1037–49. doi: 10.1093/neuonc/nos121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaku H, Horikawa K, Obata Y, Kato I, Okamoto H, Sakaguchi N, et al. NF-kappaB is required for CD38-mediated induction of C(gamma)1 germline transcripts in murine B lymphocytes. Int Immunol. 2002;14:1055–64. doi: 10.1093/intimm/dxf072. [DOI] [PubMed] [Google Scholar]
- 38.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 39.Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38:541–54. doi: 10.1016/j.immuni.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–72. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 41.Seurin D, Lombet A, Babajko S, Godeau F, Ricort J-M. Insulin-like growth factor binding proteins increase intracellular calcium levels in two different cell lines. PLoS One. 2013;8:e59323. doi: 10.1371/journal.pone.0059323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sochorová K, Budinský V, Rozková D, Tobiasová Z, Dusilová-Sulková S, Spísek R, et al. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on dendritic cells and inhibit induction of antigen-specific T cells. Clin Immunol. 2009;133:69–77. doi: 10.1016/j.clim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Vukcevic M, Zorzato F, Spagnoli G, Treves S. Frequent calcium oscillations lead to NFAT activation in human immature dendritic cells. J Biol Chem. 2010;285:16003–11. doi: 10.1074/jbc.M109.066704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Weers M, Tai Y-T, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson GT. CD38 as a therapeutic target. Mol Med. 2006;12:345–6. doi: 10.2119/2006-00082.Stevenson. 2007/03/24 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laubach JP, Tai Y-T, Richardson PG, Anderson KC. Daratumumab granted breakthrough drug status. Expert Opin Investig Drugs. 2014;23:445–52. doi: 10.1517/13543784.2014.889681. [DOI] [PubMed] [Google Scholar]
- 49.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 50.Chillemi A, Zaccarello G, Quarona V, Ferracin M, Ghimenti C, Massaia M, et al. Anti-CD38 antibody therapy: windows of opportunity yielded by the functional characteristics of the target molecule. Mol Med. 2013;19:99–108. doi: 10.2119/molmed.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.