Abstract
Rodents detect visceral pain in response to noxious levels of rectal distension. However, the mechanoreceptors that innervate the rectum and respond to noxious levels of rectal distension have not been identified. Here, we have identified the mechanoreceptors of capsaicin-sensitive rectal afferents and characterized their properties in response to circumferential stretch of the rectal wall. We have also used the lethal spotted (ls/ls) mouse to determine whether rectal mechanoreceptors that respond to capsaicin and stretch may also develop in an aganglionic rectum that is congenitally devoid of enteric ganglia. In wild type (C57BL/6) mice, graded increases in circumferential stretch applied to isolated rectal segments activated a graded increase in firing of slowly-adapting rectal mechanoreceptors. Identical stimuli applied to the aganglionic rectum of ls/ls mice also activated similar graded increases in firing of stretch-sensitive rectal afferents. In both wild type and aganglionic rectal preparations, focal compression of the serosal surface using von Frey hairs identified mechanosensitive “hot spots,” that were associated with brief bursts of action potentials. Spritzing capsaicin (10 µM) selectively onto each identified mechanosensitive hot spot activated an all or none discharge of action potentials in 32 of 56 identified hot spots in wild type mice and 24 of 62 mechanosensitive hot spots in the aganglionic rectum of ls/ls mice. Each single unit activated by both capsaicin and circumferential stretch responded to low mechanical thresholds (1–2 g stretch). No high threshold rectal afferents were ever recorded in response to circumferential stretch. Anterograde labeling from recorded rectal afferents revealed two populations of capsaicin-sensitive mechanoreceptor that responded to stretch: one population terminated within myenteric ganglia, the other within the circular and longitudinal smooth muscle layers. In the aganglionic rectum of ls/ls mice, only the i.m. mechanoreceptors were identified. Both myenteric and i.m. mechanoreceptors could be identified by their immunoreactivity to the anti-TRPV1 antibody and the vesicular glutamate transporter, Vglut2. Myenteric mechanoreceptors had a unique morphology, consisting of smooth bulbous nodules that ramified within myenteric ganglia. In summary, the rectum of wild type mice is innervated by at least two populations of capsaicin-sensitive rectal mechanoreceptor, both of which respond to low mechanical thresholds within the innocuous range. These findings suggest that the visceral pain pathway activated by rectal distension is likely to involve low threshold rectal mechanoreceptors that are activated within the normal physiological range.
Keywords: nociceptor, sensory nerve, afferent, rectum, mechanoreceptor, rectal nerve
It has been well described in rodents that noxious levels of rectal distension generate a stereotypical pain reflex that typically consists of a series of brisk contractions of the abdominal muscles (Kamp et al., 2003; Arvidsson et al., 2006). This reflex is known as the visceromotor response (VMR). There is substantial and growing evidence that extrinsic spinal afferents, whose cell bodies lie in dorsal root ganglia (DRG), are the most likely class of extrinsic afferent that respond to rectal distension and transmit visceral pain from the rectum to the spinal cord (Furness, 2006; Grundy, 2002, 2002b, 2004; Grundy and Schemann, 2005; Blackshaw et al., 2007; Brookes and Spencer, 2008). However, a major gap in our understanding of how pain is detected and transmitted to the brain following rectal distension is largely due to a lack of knowledge of the properties of the mechanoreceptors that lie in the rectum and how they respond to different levels of rectal distension.
In mammals, there are two distinct spinal afferent nerve pathways that conduct sensory information from the rectum to the spinal cord. These are known as the lumbar splanchnic/lumbar colonic nerves and the pelvic/rectal nerves (Olsson et al., 2006). Both these nerve pathways have been shown to possess between four and five different classes of afferent fiber that respond selectively to a variety of different stimuli (Brierley et al., 2004). However, it is not clear which particular class of afferent fiber is activated by rectal distension to generate the VMR and which nerve pathway carries these pain signals to the spinal cord. To address this issue, we recently performed lesion experiments of the major extrinsic nerve pathways that innervate the rectum, while evoking the VMR repetitively to rectal distension (Brookes and Spencer, 2008). In that study, it was found that lesions applied to the rectal nerves consistently abolished the VMR, whereas in animals where the rectal nerves were left intact, it was found that cutting both the lumbar colonic and hypogastric nerves had no effect on the VMR (Brookes and Spencer, 2008). These results revealed that the visceral pain pathway (VMR) that was directly activated by rectal distension was transmitted predominantly, if not solely, along rectal and pelvic afferents to the spinal cord. Therefore, rectal distension must activate a population of stretch-sensitive rectal afferents that leads directly to the activation of the VMR, via the rectal/pelvic afferent nerve pathway (Brookes and Spencer, 2008).
There is now strong evidence to suggest that activation of capsaicin-sensitive afferents is essential for the transmission of the VMR following distension of the mouse distal colon and rectum. This is supported by a number of key findings. For example, when capsaicin is selectively injected into the distal colon of anesthetized mice, a functional nociceptive pain reflex (abdominal contraction) is triggered (Laird et al., 2001). Furthermore, in transient receptor potential vanilloid 1 (TRPV1) genetic knockout mice (lacking the capsaicin receptor), there is a significantly reduced VMR following rectal distension (Jones et al., 2005). These findings are supported by immunohistochemical studies that have shown the majority (>80%) of spinal afferent DRG neurons, whose nerve endings innervate the mouse distal colon are immunoreactive for the capsaicin receptor, TRPV1 (Robinson et al., 2004). In fact, 82% of thoracolumbar and 50% of lumbosacral DRG neurons in mouse are known to display TRPV1-like immunoreactivity (Brierley et al., 2005). Taken together, there is now general agreement that the detection and transmission of visceral pain following rectal distension are likely to involve the activation of capsaicin-sensitive mechanoreceptors that innervate the rectal wall.
The first functionally identified extrinsic mechanoreceptor shown to innervate the rectum was the rectal intraganglionic laminar ending, or rIGLE, that is found exclusively within myenteric ganglia (Lynn et al., 2003). More recently, however, other studies have now found that extrinsic mechanoreceptors, with morphologies different from rIGLEs, also innervate the rectum, and at anatomical sites different from myenteric ganglia. For example, a population of i.m. rectal mechanoreceptors has been shown to innervate the rectal smooth muscle of piebald lethal mice, that respond to physiological levels of radial stretch and muscle contraction (Spencer et al., 2008). Also, recent studies by the Brookes laboratory have shown that a population of capsaicin-sensitive mechanoreceptors innervates submucosal blood vessels in the small intestine of guinea pigs. This class of mechanoreceptor was found to behave as a high threshold afferent that has mechanotransduction sites comprising varicose branching axons lying on fine blood vessels in the submucosa or mesentery (Song et al., 2006).
In this study, we have characterized in wild type mice, the properties of capsaicin-sensitive rectal afferents that respond directly to rectal distension, and morphologically identified their mechanoreceptors within the rectal wall. We have also used the lethal spotted (ls/ls) mutant mouse to determine whether a population of capsaicin-sensitive rectal afferents innervates an aganglionic rectum (devoid of enteric ganglia), and if so, identify the location and morphology of their mechanoreceptors.
EXPERIMENTAL PROCEDURES
Preparation of tissues
Lethal spotted (ls/ls) mutant mice were bred by intercrossing their heterozygote (ls/lt) siblings obtained from an in house colony, raised at the University of Nevada School of Medicine. By convention, throughout this study, we will refer to homozygote lethal spotted mice as ls/ls. Homozygote ls/ls mice were originally raised on a C57BL/6 strain of mouse. No heterozygote offspring were used in this study. Age-matched C57BL/6 mice (30–45 days of age) were used as experimental controls throughout all studies in this proposal. In general, the vast majority of mice studied were between 30 and 45 days age and were considered to be representative of an adult population. Throughout this study, only male offspring were used for experimentation.
Mice were killed by inhalation anesthetic (Isoflurane, Baxter, USA), followed by cervical dislocation, in accordance with the animal ethics committee at the University of Nevada School of Medicine. All experiments conformed to international guidelines on the ethical use of animals. A minimum number of animals was used to obtain statistically significant data and to reduce suffering. A midline incision was made along the abdominal cavity and the distal colon exposed. The terminal 20 mm of distal colon and rectum (measured from the anal sphincter) was removed from mice while the pelvic ganglia and rectal nerves retained extrinsic neural continuity with the rectum. The entire in vitro preparation was then placed in a Petri dish containing ice cold Krebs solution. A longitudinal incision was made along the entire rectum in both wild type and ls/ls mice. In all preparations, the mucosa, submucosa and submucous plexus were sharp dissected free from the underlying circular muscle. Therefore, in wild type mice, the preparations consisted of circular and longitudinal muscle with the myenteric plexus, while in ls/ls mice, preparations consisted only of circular and longitudinal muscle, with no enteric ganglia present. The same length segment of rectum (10 mm total length from the anus) was studied in in vitro preparations from both ls/ls and wild type mice. In all preparations, the fine rectal nerves always retained neural continuity with the rectum. For the purposes of this study, the rectum was defined as the region of distal large bowel that received a detectable innervation from the rectal nerve trunks that arose from the pelvic ganglia, as described by Olsson et al. (2006). The fine rectal nerves were teased into a separate recording chamber, from which afferent recordings were made. No recordings were made from the pelvic nerve and in fact, the pelvic nerve was not retained in any of our in vitro dissections.
Extracellular afferent recordings
Extrinsic rectal nerves were sharp dissected free of connective tissue to expose discrete nerve trunks. Extracellularly recorded action potentials from single rectal nerve trunks were amplified through an isolated bio-amplifier (ISO-80, World Precision Instruments), then digitized with a powerlab/4sp (AD Instruments) and recorded on an Apple Mac mini personal computer using Chart software (AD Instruments). In general, single rectal nerve trunks did not require further dissection to discriminate and resolve single unit firing. The main chamber containing the preparation was superfused at 6 ml/min with constantly oxygenated Krebs solution at 35±1 °C.
Activation of stretch-sensitive single afferent units
The cut edge of each isolated rectal preparation was attached to an array of miniature hooks made from bent dissecting pins, similar to that first used in the study of Brookes et al. (1999). This rake was attached via fine cotton (7/0) to a cantilever pulley system, whereby weights of increasing weight (1–5 g) could be applied to induce graded levels of circumferential stretch to the rectal wall (see Brierley et al., 2004). During these controlled stretches that were maintained for 20 s duration, extracellular recordings were made from individual rectal nerve trunks, which were maintained in a paraffin oil filled recording chamber. Imposed increases in the circumference of the rectal wall evoked discharges of action potentials in rectal afferents. In preparations where more than three distinct single units responded to circumferential stretch, these recordings were discarded. Only preparations where distinct single units could be discriminated were selected for analysis.
Terminology and classification of rectal afferents
Since recordings have not been made previously from the mouse rectal nerves, we have employed the same classification scheme as developed by Brierley et al. (2004), which first categorized the classes of extrinsic pelvic afferents. Therefore, in this study on rectal afferents, we classify those afferents that respond to circumferential stretch and probing of the serosal surface as muscular afferents, and those afferents that only responded to probing (but not stretch) as serosal afferents. We have retained the terminology of serosal afferents developed by Brierley et al. (2004), even though no extrinsic rectal mechanoreceptors have yet been detected in the serosa (Spencer et al., 2008).
In this study, we have classified a low threshold rectal afferent as an afferent fiber that responded to circumferential stretch at ≤2 g of stretch and a high threshold afferent as >5 g of stretch (see Results).
Identification of mechanosensitive hot spots
To morphologically identify the mechanoreceptors of rectal afferents in the mouse rectum, we utilized the anterograde labeling technique first described by Tassicker et al. (1999). To identify mechanosensitive hot spots of rectal afferent ending, local mechanical distortion of the serosal surface was made using fine calibrated von Frey hairs (Fig. 1A and 1B) (range: 50 mg–500 mg), that typically had tip diameters of <50 µm. By probing the entire serosal surface with von Frey hairs, the receptive fields of rectal afferent endings were identified (see Brierley et al., 2004). Carbon particles were then attached to the tip of the hair and the von Frey hair reapplied to the colon to mark the receptive field of each hot spot as described in Zagorodnyuk and Brookes (2000, 2003).
Fig. 1.
Diagrammatic representation of the preparations used for identifying capsaicin-sensitive rectal mechanoreceptors in wild type and ls/ls mouse rectum. (A) Wild type preparations consisted of circular and longitudinal muscle with myenteric ganglia present. The mucosa and submucosa (with submucosal ganglia) were removed. A von Frey hair was used to identify mechanosensitive hot spots of rectal afferents and a capsaicin-filled micropipette used to spritz capsaicin onto hot spots to identify capsaicin-sensitive mechanoreceptors. A claw made from bent dissection pins was used to apply graded circumferential stretch to the preparation (see Experimental Procedures). (B) Preparations from ls/ls mice lacked all myenteric ganglia, and consisted of circular and longitudinal muscle only. (C) Comparison of muscular afferent mechanosensitivity between wild type and ls/ls mutant mice. Between wild type and mutant ls/ls mice, there was no overall significant difference in the mean firing frequencies of muscular afferents to a maintained 20s circumferential stretch (from n=6 wild type and ls/ls mice).
Immunohistochemistry to characterize mechanosensitive “hot spots”
The technique of anterograde labeling we utilized in the mouse colon was essentially identical to that described for anterograde labeling of pelvic afferents in the guinea-pig rectum (Lynn et al., 2003). The only major difference in techniques was that a 10% biotinamide solution was used and an incubation period of between 15 and 17 h was applied. At the end of the extracellular nerve recording period, one drop of 10% biotinamide (Molecular Probes, Eugene, OR, USA) in an artificial intracellular medium (mM: monopotassium l-glutamate, 150; MgCl2, 7; glucose, 5; EGTA, 1; Hepes, 20; disodium ATP, 5; 0.02% saponin, 1% dimethyl sulfoxide) was placed on the single rectal nerve trunk from which recordings were made in the paraffin oil chamber. The same organ bath was always used for extracellular recordings and anterograde labeling to avoid dislodgement of the marked carbon particles. The Krebs solution was removed from the colonic recording chamber and replaced with a sterile supplemented culture medium (DME/F12 with 10% fetal bovine serum, 1.8 mM CaCl2, 100 i.u. ml−1 penicillin, 100 µg ml−1 streptomycin, 2.5 µg ml−1 amphotericin B, 20 µg ml−1 gentamycin (pH 7.4). The colon chamber was maintained at a constant temperature of 36±1 °C. After 17–20 h, preparations were fixed for 3–4 h in a modified Zamboni’s fixative (15% saturated picric acid and 2% formaldehyde in a 0.1 M phosphate buffer, pH 7.0), cleared in DMSO for 30 min (three times 10 min rinses) and then rinsed in phosphate-buffered saline (PBS: 150 mM NaCl in 10 mM sodium phosphate buffer, pH 7.2). Anterogradely labeled nerve endings in the colon were visualized with streptavidin-CY3 (Alexa-Fluor 488; cat. # S11223; Molecular Probes); at a dilution of 1:1000 for 2 h at room temperature. Nerve endings of anterogradely labeled rectal afferents were analyzed on a Zeiss LSM 510 meta confocal microscope (Germany). In all animals in which strong labeling of neural structures occurred, individual images were taken using a 10× lens (926×926 µm) and montages created using Adobe Photoshop 5.0 software (Adobe Systems Inc., San Jose, CA, USA). Completed montages had dimensions of ~1 cm length by 1 cm width.
Method for quantifying the distance between anterogradely labeled nerve endings and randomly assigned points to marked carbon particles
A montage image of fluorescent afferent nerve fibers was opened in Volumetry G6a (GWH) and an outline was drawn around every carbon particle and every nerve fiber ending. The XY midpoint of each outline was used as the reference position and the distance between each nerve ending and carbon particle was calculated. Then, the distance between each carbon particle to the closest nerve fiber ending (in µm) was determined, as was the number and density of both nerve fiber endings and carbon particles in the field of view. To test whether the distribution of nerve fiber endings and carbon particles was random, for each carbon particle, we inserted 256 randomly positioned points in the montage that represented nerve fiber endings and the distance between these endings and the nearest carbon particle was calculated. Using the montages created, the average distance between the randomly positioned points was obtained. Then, statistical comparison was made between the distance of an identified nerve ending and the closest carbon hot spot, to the distance between identified nerve endings and randomly generated hot spots.
Drugs and solutions
The following drugs were used in the following study: NK1 receptor antagonist (WIN 62,577) (Sigma Chemical Co., MO, USA), NK2 receptor antagonist (Boc-Ala-Ala-D-Trp-Phe-D-Pro-Pro-Nle-NH2) (Bachem California, cat. # H-2794), NK3 receptor antagonist (Trp7, beta-Ala8)-Neurokinin A (4–10) Bachem California, CGRP receptor antagonist (Alpha CGRP (8–37) (human)) (cat. # H-9895, Bachem California Inc.). The capsaicin receptor antagonist, capsazepine was obtained from Sigma Chemical Co.
Immunohistochemical staining
Polyclonal antibodies against the vesicular glutamate transporter, Vglut2 (1:1000; cat. # AB5907) raised in the guinea pig were obtained from Chemicon International, Temecula, CA, USA. Tissues were incubated for 48 h in primary antibody, then for 1 h in secondary antibody (Alexa Fluor 594; 1:100) goat anti-guinea-pig IgG (cat. # A11076) that was obtained from Molecular Probes. Capsaicin immunoreactive nerve endings and fibers were identified using the anti-capsaicin receptor (Ab-1) rat (rabbit), purchased from Calbiochem (cat. # PC420; La Jolla, CA, USA). Alexa Fluor 488 chicken anti-rabbit IgG (cat. # A-21441) obtained from Molecular Probes was used as a secondary antibody to visualize the TRPV1 positive nerve endings. Prior to incubation in TRPV1 antibody, rectal preparations of were fixed with paraformaldehyde (4%) for 4 h, then washed in PBS solution four times for 30 min.
To precisely identify the location of capsaicin-sensitive rectal mechanoreceptors within either the circular or longitudinal muscle layers, we double labeled all anterogradely labeled preparations with the polyclonal antibody against smooth muscle myosin II heavy chain (1:200; Biomedical Technologies Inc., Stoughton, MA, USA). The anti-smooth muscle myosin II IgG raised in rabbit has been shown to only bind smooth muscle myosin II heavy chain in vertebrates. Alexa Fluor 647 goat anti-rabbit IgG (1:100; cat. # A21244) purchased from Molecular Probes was used as secondary antibody to visualize myosin immunoreactivity in the aganglionic ls/ls and wild type mouse rectum. Immunofluorescence for smooth muscle myosin II heavy chain clearly reveals the orientation of the circular muscle fibers from the longitudinal muscle.
RNA isolation and quantitative PCR
DRG from the lumbosacral region of the spinal cord were dissected from eight wild type and eight ls/ls mice, placed in a preservative solution (RNALater; Ambion, Austin, TX, USA) and stored at −20 °C until use. Total RNA extracted in 0.5 ml TRIzol reagent according to the protocol provided by the manufacturer (Invitrogen, Carlsbad, CA, USA) and treated with 1 U/µl DNase I (Promega Corporation, Madison, WI, USA). First-strand cDNA synthesis was performed at 42 °C from 2 µg total RNA using 250 ng random hexamers; 50 mM Tris–HCl (pH 8.3); 75 mM KCl, 3 mM MgCl2; 10 mM dithiothreitol (DTT); 0.125 mM each of deoxy-ATP, deoxythymidine triphosphate (dTTP), deoxyguanidine triphosphate (dGTP) and deoxycytidine triphosphate (dCTP); and 200 U SuperScript II RT. RNAse H (20 U) was added to remove RNA complementary to the cDNA and the reaction diluted 1:5 with nuclease-free water.
Gene-specific oligonucleotide primers yielding an amplicon of 151 bp specific for mouse Trpv1 (NM_001001445) were purchased from SuperArray (Frederick, MD, USA). Primers to amplify 18S ribosomal RNA where as follows: 5′-CACGGCCGGTACAGTGAAA-′3; 5′-AGAGGAGCGAGCGACCAA-′3. All reactions were performed in triplicate in 25 µl total volume containing a 1× concentration of SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 2.5 pmol of each forward and reverse primer and 2–5 µl cDNA. Reactions were amplified in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) located at the Nevada Genomics Center and results analyzed using the manufacturer’s software package. Sample amplification was activated by incubation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min and a subsequent heat dissociation protocol after the last cycle to verify product specificity. Amplification was monitored by measuring the increase in fluorescence caused by SYBR I green binding to double-stranded DNA, resulting in an amplification plot of fluorescence versus cycle number. The threshold cycle (Ct) was defined as the cycle in which fluorescence passes a fixed detection threshold. Standard curves were generated for each target gene using serial dilutions of cDNA from the rectum of wild type mice and by plotting the log of the initial copy number in the standards vs. the Ct value. The Ct values from experimental samples were then used to calculate the amount of specific target genes relative to the standard curve. All samples were normalized to 18S rRNA amplified from the same samples to control for variations in sample quality.
Measurements and statistics
Extracellularly recorded single unit action potentials were discriminated and identified using Chart software (Spike histogram; AD Instruments). Paired or unpaired Student’s t-tests, or one-way analysis of variance (ANOVA) were used appropriate. The use of “n” in the results section refers to the number of animals on which observations were made. P-values <0.05 were used to determine a level of statistical significance.
RESULTS
General observations: spontaneous activity in rectal afferents
In 24 of 51 extracellular recordings from 15 of 16 wild type mice, it was found that a spontaneous discharge of action potentials arose from fine rectal nerve fibers that remained in neural continuity with the isolated rectum. These action potentials (mean firing frequency: 1.6±0.4 Hz; 24 units; n=15) occurred in slack preparations of rectum, that were devoid of all mucosa, submucosa and submucosal plexus. When recordings were made from rectal nerve fibers that innervated the aganglionic rectum of ls/ls mice, 27 of 46 single units were spontaneously active from 14 of 15 mice (mean firing frequency: 1.2±0.4 Hz; n=14). The rectal mechanoreceptors that generated these action potentials in ls/ls mice could not have originated from myenteric ganglia, since no myenteric ganglia are present in the distal 10 mm of rectum from these animals (Ward et al., 2002). There was no significant difference between the mean spontaneous firing frequencies of rectal afferents in wild type or ls/ls mice (P>0.05; unpaired Student’s t-test).
Responses of wild type and ls/ls mouse rectum to circumferential stretch
We were particularly interested whether the rectal afferents that innervated the aganglionic rectum from ls/ls mice would respond to circumferential stretch, despite the absence of all enteric ganglia. It was found that low levels of circumferential stretch (1–2 g) applied to the aganglionic rectum consistently activated low threshold, slowly-adapting rectal mechanoreceptors in all ls/ls mice studied (n=15). In fact, when graded circumferential stretch (1– 5 g) was applied to preparations of aganglionic rectum from ls/ls (n=15) and wild type mice (n=16), there was no overall significant difference in the mean firing frequencies of their stretch-sensitive rectal afferents (Fig. 1C). Despite this, the minimum threshold of circumferential stretch required to activate action potentials in stretch-sensitive rectal afferents from ls/ls mice was significantly higher (2.2±0.2 g: range: 1–3 g; n=14), than for control mice (1.5±0.2 g: range: 1–3 g; n=15; P=0.04, unpaired Student’s t-test). No rapidly adapting or high threshold rectal afferents were ever recorded in either wild type or ls/ls mouse rectum.
Since all stretch-sensitive rectal afferents tested were found to be activated at low mechanical thresholds, we were interested in whether these same afferents would respond as wide dynamic mechanoreceptors, or, would a separate distinct population of high threshold afferents be recruited at higher levels of stretch? To test this, in wild type mice, we applied intense levels of stretch (8 g) to the same preparations that were already found to be activated by low mechanical thresholds (≤2 g). In 10 single units that responded to low levels of stretch (≤2 g), these same units did not fire significantly differently (5 g: 4.3±2.1 Hz; 8 g: 4.7±2.2 Hz) when these same preparations were exposed to high levels (8 g) of stretch (10 units, n=5; P=0.83; paired Student’s t-test). Similar results were obtained in the aganglionic rectum of ls/ls mice. The mean firing frequency of stretch-sensitive rectal afferents that responded to 5 g stretch was 4.7±1.8 Hz and at 8 g was 5.9±1.8 Hz (P=0.21; paired Student’s t-test; 22 units, n=8). This suggests that the low threshold stretch-sensitive rectal afferents in both wild type and ls/ls mice reach their maximal firing frequency at around 5 g of stretch. No distinct high threshold rectal afferents were ever selectively activated by high mechanical thresholds (e.g. 8 g) of stretch (Fig. 2C).
Fig. 2.
Identification of myenteric mechanoreceptors of rectal afferents in wild type mouse rectum. (A–D) The same single unit is activated by probing hot spot 1 (A), 1 g (B), 8 g (C) and stretch and capsaicin (D). The single unit active under these different stimuli is shown on expanded scale during spontaneous firing (Ai) and probing (Ai), 1 g stretch (Bi), 8 g stretch (Ci) and capsaicin (Di). (E) The same unit under all these conditions (Ai–Di) superimposed. (F) A montage of the mechanotransduction site of hot spot 1, where the unit was mechanically activated by probing. The close apposition of the carbon marked hot spot 1 and the anterogradely labeled mechanotransduction sites are apparent. Note, only one hot spot was identified for this unit. Two discrete clusters of nerve endings are associated with the hot spot 1 (see boxes I and G in F) and are shown on expanded scale in G and I, respectively. Both nerve endings terminate in myenteric ganglia as discrete bulbous swellings that arise from single nerve axons. These bulbous endings are immunoreactive to Vglut2, see arrows in H and J. Note, other Vglut2 endings are apparent in H, but they did not originate from the single rectal nerve from which our recordings were made. (K, L) A confocal scan through the longitudinal muscle depth (K) and circular muscle (L). None of these nerve endings can be seen terminating in either muscle layer. Confocal images shown in G, H, I and J were taken on a 100× oil objective.
Identification of functional classes of capsaicin-sensitive rectal afferents in wild type mouse rectum
In total, from 15 wild type mice studied, 56 mechanosensitive units were identified by focal von Frey hair probing of the serosal surface. Of these, 53 of the 56 units (95%) were classified as muscular afferents, since the same unit responded to both probing and stretch, while 3 of the 56 (5%) were serosal afferents that only responded to probing (see Brierley et al., 2004). We sought to determine what proportion of these muscular and serosal afferents, if any, would respond to capsaicin. To test this, we focally spritzed capsaicin selectively onto each mechanosensitive unit underlying marked hot spots (Fig. 1A and 1B). Overall, of the 53 muscular afferents identified above, 29 of the 53 (55%) were capsaicin-sensitive (Fig. 4 and Fig. 5) and all serosal afferents (3 of the 3 identified) were capsaicin-sensitive.
Fig. 4.
Identification of capsaicin-sensitive hot spots of rectal afferent nerve endings that innervate a wild type mouse rectum. (A) Montage showing three discrete hot spots and underlying nerve endings activated by von Frey hair probing of the serosal surface. (B, C) Hot spot 1 was sensitive to probing, but did not respond to capsaicin. (D, E) Hot spot 2 responded to probing and capsaicin. However, the units activated by capsaicin were different from those activated by probing. (F, G) Hot spot 3 responded to probing and capsaicin, where at least two distinct units were activated. One of the units activated by capsaicin (indicated by the black dot) was the same as the single unit that was activated by probing, low (1 g) and high (5 g) degrees of circumferential stretch; and was spontaneously active at rest (see H).
Fig. 5.
Identification of capsaicin-sensitive hot spots of rectal afferent nerve endings that innervate the aganglionic rectum of an ls/ls mouse. (A) A photograph of the aganglionic rectum and the location of the carbon marked hot spots. (B) After anterograde labeling the innervation of the aganglionic rectum and the close apposition of the carbon marked hot spots to nerve endings is apparent. The area represented by the dotted box in A is shown in B. All hot spots (hs1–hs5) responded to probing, but only hot spots 2, 3 and 5 responded to capsaicin. Although capsaicin activated a discharge of action potentials when spritzed onto three of the five hot spots, only the units activated by capsaicin spritzed onto hs5 were found to be the same units as those activated by probing hot spot 5 and circumferential stretch. This response to capsaicin (D) is shown expanded in Fig. 6A. All other capsaicin-activated units belong to axons different from those activated by probing each hot spot.
We then tested how many of the total population of rectal afferents that responded to capsaicin would be classified as muscular afferents, how many would be serosal afferents; and how many would fit neither category. It was found that 34% of all capsaicin-sensitive afferents were muscular afferents, 25% were serosal and 41% were neither muscular nor serosal afferents (Table 1; Fig. 3).
Table 1.
Characteristics of capsaicin-sensitive single units in control C57BL/6 mice
| No. of animals tested |
Total no. of units activated by mechanical probing of serosa |
Total no. of units activated by capsaicin when spritzed onto probing sensitive units |
No. of capsaicin sensitive units that were the same units as those activated by probing |
No. of capsaicin sensitive units that were the same units as those activated by probing and stretch (i.e. muscular afferents) |
No. of units that did not respond to capsaicin but did respond to probing |
No. of capsaicin sensitive muscular afferents that were activated at low mechanical thresholds i.e. ≤2 g stretch |
No. of capsaicin sensitive muscular afferents that were activated at high mechanical thresholds i.e. >5 g |
No. of capsaicin sensitive units that did not respond to stretch |
No. of capsaicin sensitive units that were spontaneously active at rest |
|---|---|---|---|---|---|---|---|---|---|
| 15 | 56 | 32/56 | 19/32 | 11/19 | 24/56 | 11/11 | 0/11 | 21/32 | 23/32 |
Fig. 3.
Functional characterization of capsaicin-sensitive rectal afferents in wild type and ls/ls mouse rectum. In wild type (A) and ls/ls mice (B), the largest proportion of capsaicin-sensitive rectal afferents was neither muscular nor serosal afferents. Although there was a similar number of capsaicin-sensitive muscular afferents in wild type and ls/ls mice, there was a sixfold decrease in the number of capsaicin-sensitive serosal afferents in ls/ls mice compared with wild type animals. (C, D) Approximately half of all muscular afferents in wild type and ls/ls mice were capsaicin-sensitive.
Identification of functional classes of capsaicin-sensitive rectal afferents in the aganglionic rectum from ls/ls mice
We recently showed that a population of i.m. rectal afferents innervated the smooth muscle of the aganglionic rectum in piebald lethal mice; and that the mechanoreceptors of these muscular afferents responded to low levels of circumferential stretch and mechanical probing (Spencer et al., 2008). However, in that study, it was not known whether any of rectal afferents also responded to capsaicin and if so, do they have similar stretch activation properties as rectal afferents in the rectum of wild type mice? In this study, we tested whether the aganglionic rectum of ls/ls mice was innervated by a population of i.m. rectal afferents that respond to both capsaicin and radial stretch.
In ls/ls mice, we found that focally compressing the serosal surface of the aganglionic rectum with von Frey hairs was found to activate 62 mechanosensitive hot spots in 14 mice (Table 2; Fig. 3). Of these, 59 of the 62 (95%) hot spots were found to respond to circumferential stretch (1–2 g) and were therefore classified as muscular afferents (Fig. 3). The remaining 3 of the 62 hot spots (5%) only responded to probing and were therefore classed as serosal afferents (Fig. 3).
Table 2.
Characteristics of capsaicin-sensitive single units in ls/ls mutant mice
| No. of animals tested |
Total no. of units activated by mechanical probing of serosa |
Total no. of units activated by capsaicin when spritzed onto probing sensitive units |
No. of capsaicin- sensitive units that were the same units as those activated by probing |
No. of capsaicin sensitive units that were the same units as those activated by probing and stretch (i.e. muscular afferents) |
No. of units that did not respond to capsaicin but did respond to probing |
No. of capsaicin sensitive muscular afferents that were activated at low mechanical thresholds i.e. ≤2 g stretch |
No. of capsaicin sensitive muscular afferents that were activated at high mechanical thresholds i.e. >5 g stretch |
No. of capsaicin sensitive units that did not respond to stretch |
No. of capsaicin sensitive units that were spontaneously active at rest |
|---|---|---|---|---|---|---|---|---|---|
| 14 | 62 | 24/62 | 7/24 | 6/7 | 38/62 | 6/6 | 0/6 | 13/24 | 16/24 |
We sought to determine what proportion of these muscular and serosal afferents, if any, would respond to capsaicin. To test this, we focally spritzed capsaicin selectively onto each mechanosensitive unit underlying marked hot spots. Overall, of the 59 muscular afferents and three serosal afferents identified in the aganglionic segment, 23 of the 59 muscular afferents responded to capsaicin, while one of the three serosal afferents responded to capsaicin. Importantly, all capsaicin-sensitive muscular afferents were consistently activated by low levels (1–2 g) of stretch (Figs. 2, 4, 5, 6). When 8 g of stretch was applied to capsaicin-sensitive muscular afferents, there was no significant difference in rectal firing frequency (5 g: 2.9±0.9 Hz to 8 g: 3.71±1.1 Hz; P>0.05; n=4).
Fig. 6.
Properties of capsaicin-sensitive rectal muscular afferents that innervate the aganglionic rectum of an ls/ls mouse. (A) The response induced by a capsaicin spritz onto hot spot 5 in Fig. 5D. The two black dots represent the two units activated by capsaicin. Both these units are shown on expanded scale in B and C. Both units 1 and 2 are shown to be activated by capsaicin, low (1 g) and high (5 g) levels of circumferential stretch, probing and were active under spontaneous conditions with no applied stretch. (D, E) Both units 1 and 2 respectively were spontaneously active at rest.
We then tested how many of the total population of rectal afferents that responded to capsaicin in the aganglionic rectum would be classified as muscular afferents, how many would be serosal afferents; and how many would fit neither category. It was found that 25% of all capsaicin-sensitive afferents were muscular afferents, 4% were serosal and 71% were neither muscular nor serosal afferents (Table 2; Fig. 3). Because the mucosa was absent in our preparations, it is possible that some of the muscular afferents may belong to the muscular/mucosal class, as described by Brierley et al. (2004).
Responses to capsaicin are due to activation of the TRPV1 receptor
Capsaicin is known to release neuropeptides such as substance P and CGRP from primary afferent nerve endings. The release of these neuropeptides can itself induce afferent firing in spinal afferents via TRPV1-independent mechanisms. Therefore, it might be argued that the responses to capsaicin seen above were not due to the activation of TRPV1-positive capsaicin-sensitive afferents, but rather the non-specific activation of TRPV1-negative rectal afferents. To test whether responses to topical capsaicin were in fact generated by the direct activation of TRPV1 receptors on rectal afferents, we applied capsazepine (50 µM) to five animals for 30 min prior to spritzing capsaicin onto any hot spots. In these five animals, it was found that no afferent firing was evoked while in the presence of capsazepine. In fact, in the combined presence of antagonists to the NK1 (1 µM), NK2 (1 µM), NK3 (1 µM) and CGRP receptors (10 µM) (see Experimental Procedures), exogenous capsaicin was still able to activate a discharge of action potentials on rectal afferents, adding further support to the data above that capsaicin was directly activating TRPV1 receptors on rectal afferents.
Identification of capsaicin-sensitive mechanoreceptors in wild type mouse rectum
We employed the anterograde labeling technique developed by Tassicker et al. (1999) to identify the capsaicin-sensitive mechanoreceptors that ramified in both wild type and aganglionic rectal preparations. In wild type mice, a significant relationship was identified between carbon marked mechanosensitive hot spots and their underlying labeled nerve endings (see Zagorodnyuk and Brookes, 2000). In fact, it was found that the mean distance between a marked carbon particle and the closest nerve ending was 387.7±103.3 µm (n=8). This distance was significantly less than the mean distance between a carbon marked hot spot and the nearest randomly assigned (computer generated) nerve ending, which was 1528.8±150 µm (P<0.05; n=8). This confirms that the underlying anterogradely labeled nerve endings and closely apposed carbon marked hot spots were not associated by chance.
In light of this correlation, we consistently identified a distinct morphological class of nerve ending within myenteric ganglia of wild type mice that we could functionally correlate as the mechanoreceptors of capsaicin-sensitive rectal muscular afferents (n=6). These mechanoreceptors were identified as smooth bulbous nodules, that were ellipsoidal in shape, and terminated as single or multiple bead-like swellings within myenteric ganglia (Fig. 2G and 2I). Each nodule had measurements in its major and minor axis of 10.8±0.9 µm and 6.1±0.5 µm, respectively (18 endings; n=6 animals). Using confocal microscopy, these nodules could be specifically localized to within myenteric ganglia (Fig. 2H and 2J), with no evidence of any nerve terminal projections into either the circular or longitudinal muscle layers (Fig. 2K and 2L). These myenteric mechanoreceptors were immunoreactive to the TRPV1 antibody (see Fig. 9) and Vglut2 (Fig. 2H and 2J), confirming they were not associated with intrinsic or extrinsic efferent nerve endings (n=4). In total, anterograde labeling from rectal nerve trunks in 37 wild type mice failed to reveal any neuronal structures in myenteric ganglia with morphologies similar to IGLEs, or rIGLEs.
Fig. 9.
Immunohistochemical staining for the TRPV1 receptor in wild type mouse rectum. (A–C) TRPV1 immunoreactivity from three different mice. (A–C) TRPV1-positive nerve endings terminating as discrete bulbous nodules within myenteric ganglia. These bulbous endings are identical to the capsaicin-sensitive myenteric mechanoreceptors that were activated by capsaicin and revealed after anterograde labeling, see Fig. 2G and 2I. Each TRPV1 positive nodule forms an ellipsoidal shape, that can reach up to 10 µm in length in its major axis (long axis).
In addition to myenteric mechanoreceptors, a population of i.m. mechanoreceptors were also identified in both the circular muscle (n=4) or longitudinal muscle (n=2) (Fig. 7). These i.m. mechanoreceptors were indistinguishable between the aganglionic rectum of ls/ls mice (see below) and in wild type mice.
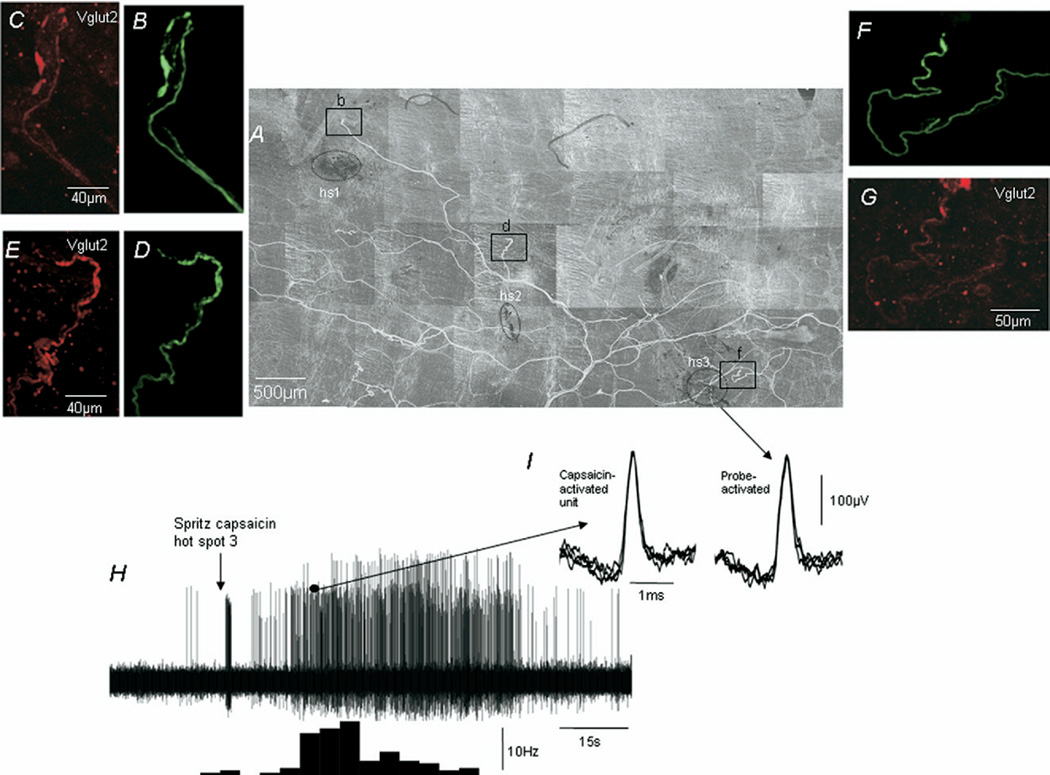
Fig. 7.
Identification of capsaicin-sensitive and insensitive i.m. rectal mechanoreceptors in a wild type mouse rectum. (A) The montage revealed after anterograde labeling of the rectal nerve from which afferent recordings in Fig. 4 were made. Hot spots 1, 2 and 3 (hs1, hs2 and hs3) are indicated and the closely apposed nerve endings are highlighted by boxes b, d and f. The morphology of the nerve endings in boxes b, d and f is shown on expanded scale in B, D and F. Only in hot spot 3 was the same unit activated by capsaicin, probing and stretch. This capsaicin-sensitive i.m. mechanoreceptor is shown in F and is immunoreactive to Vglut2, see G. (H) The response induced after spritzing capsaicin onto hot spot 3. (I) An enlarged scale of the same unit activated by probing hot spot 3 and the activated by capsaicin.
Identification of capsaicin-sensitive mechanoreceptors in the aganglionic rectum of ls/ls mice
Anterograde labeling of rectal afferents in ls/ls mice revealed the identity of the mechanoreceptors of capsaicin-sensitive rectal muscular afferents (Fig. 8). Since these dissected preparations consisted only of smooth muscle, it was unsurprising that i.m. mechanoreceptors were found to be the nerve endings of this particular class of rectal afferent.
Fig. 8.
Identification of i.m. rectal mechanoreceptors in the aganglionic rectum of an ls/ls mouse. (A) A montage of the single anterogradely labeled nerve from which afferent recordings were made and the location of the five distinct mechanotransduction sites marked with carbon. Five mechanosensitive hot spots were identified (see hs1–hs5), all of which ramified within the circular or longitudinal muscle layers. All i.m. mechanoreceptors ramified within the smooth muscle as single or multiple discrete nodules that were immunoreactive to Vglut2, see D, F, H and K, except the endings shown in B and L. Note, only the fine nerve ending shown in C was activated by probing, stretch and capsaicin and is a confirmed i.m. capsaicin-sensitive rectal muscular mechanoreceptor.
Again, the mechanoreceptors of these afferents were significantly closer apposed to carbon-marked hot spots, than randomly assigned (computer generated) hot spots. In fact, in ls/ls mice, the mean distance between a marked carbon particle and the nearest anterogradely labeled nerve ending was 455.3±115.6 µm (n=5), which again was a significantly shorter distance compared with the nearest randomly assigned nerve ending, a distance of 1771.3±396 µm (n=5). I.m. rectal mechanoreceptors of capsaicin-sensitive muscular afferents consisted of single nerve axons (see Fig. 8C, 8E and 8G) that terminated in single or multiple varicose endings within the circular or longitudinal muscle; they were immunoreactive to Vglut2 and TRPV1. These i.m. endings projected from the serosal surface a mean depth of 24 µm (wild type) and 34 µm (ls/ls mouse) into the muscularis.
TRPV1 immunoreactivity in the aganglionic and ganglionic mouse rectum
We used the TRPV1 antibody to identify the morphology of TRPV1 positive nerve endings in the mouse rectum and compare these structures to the capsaicin-sensitive mechanoreceptors identified after anterograde labeling. It was found that myenteric ganglia of the rectum were densely innervated by TRPV1 positive nerve fibers, consistent with previous studies (Ward et al., 2003). Close inspection of the TRPV1 positive nerve endings within myenteric ganglia revealed that each nerve ending terminated in bulbous nodules or swellings. These TRPV1 positive nodules terminated within myenteric ganglia and had dimensions in their major and minor axis of 8.2±4.8 µm and 5.2±0.4 µm (n=4; Fig. 9A–C). These TRPV1 positive nerve endings were identical to the rectal myenteric mechanoreceptors that responded to capsaicin and were identified after anterograde labeling (see Fig. 2G and 2I). There was no significant difference between the size of capsaicin-sensitive myenteric mechanoreceptors identified by anterograde labeling, compared with those identified by the TRPV1 antibody (n=4; P>0.05). I.m. nerve endings could also be readily identified by the TRPV1 antibody. These terminated within the longitudinal and circular muscle layers and were characterized by fine bead-like varicose endings, that could also be identified by Vglut2 immunoreactivity. We were unable to identify any TRPV1 positive structures similar to rIGLEs in guinea-pig rectum (Lynn et al., 2003).
Differences in TRPV1 mRNA expression in lumbosacral DRG
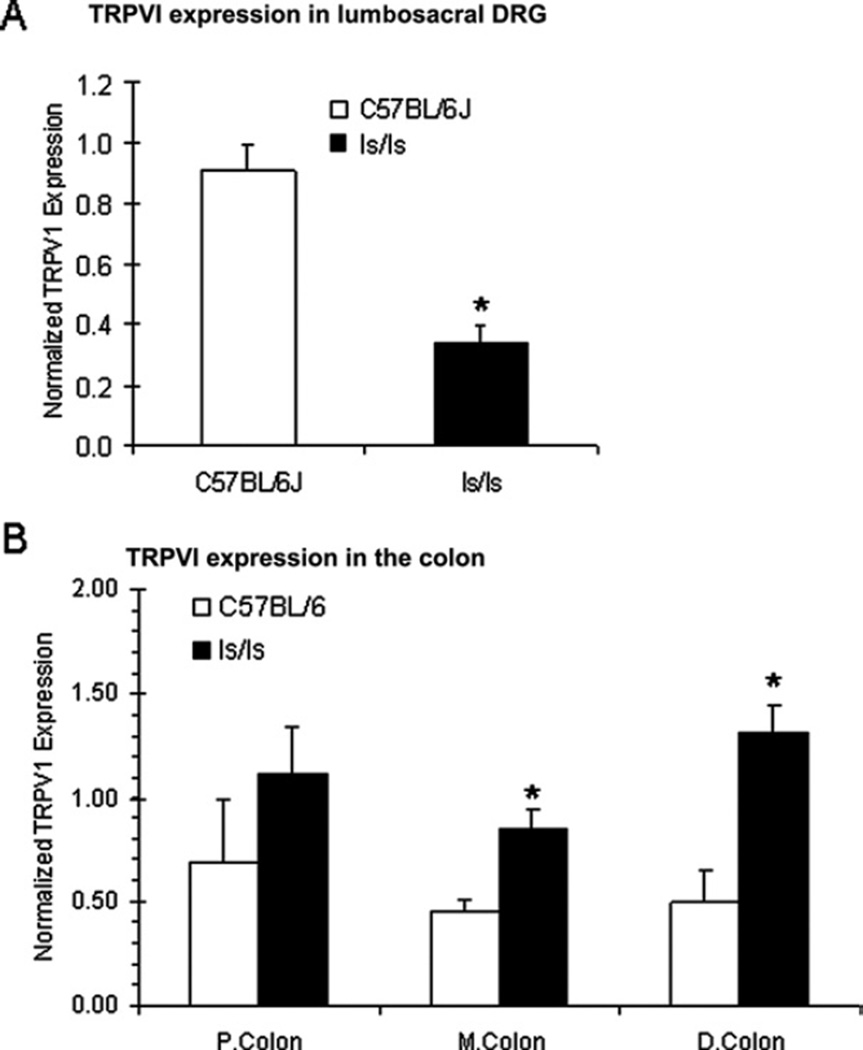
The aganglionic rectum of ls/ls mice is predominantly innervated by spinal afferents whose DRG cell bodies arise from the lumbosacral region of spinal (Payette et al., 1987). In light of this, we sought to determine whether similar levels of TRPV1 were expressed throughout the lumbosacral DRG of ls/ls mice compared with wild type animals. Interestingly, using quantitative RT-PCR, it was found that there was significantly less TRPV1 mRNA in lumbosacral DRG obtained from ls/ls mice, compared with wild type animals (n=8; P<0.05; Fig. 10A). When the entire colon (including the rectum, but excluding the cecum) was sectioned into three equal length segments (e.g. proximal, mid and distal), it was also found that TRPV1 mRNA was also detected throughout each segment of both wild type and ls/ls mice. In fact, TRPV1 was found to be expressed in significantly higher quantities in the mid and distal colon of ls/ls mice compared with wild type animals (Fig. 10B).
Fig. 10.
Quantitative RT-PCR reveals differences in TRPV1 mRNA expression in DRG obtained from the lumbosacral region of spinal cord between wild type and ls/ls mice. (A) In ls/ls mice, TRPV1 mRNA expression was significantly reduced in DRG when compared with wild type animals, from n=8 wild type and ls/ls mice. (B) TRPV1 is also expressed throughout the proximal, mid and distal colon (including rectum). TRPV1 expression was significantly higher in the mid and distal colon of ls/ls mice compared with wild type animals.
DISCUSSION
There is now strong evidence from a variety of laboratories to suggest that in mice, capsaicin-sensitive spinal afferents are involved in the signaling of noxious rectal distension stimuli to the spinal cord (Blackshaw et al., 2007). However, the mechanoreceptors of spinal afferents that lie in the rectal wall and respond to both capsaicin and circumferential stretch had not been identified. In this study, we have morphologically identified two populations of capsaicin-sensitive rectal mechanoreceptor that lie in the rectal wall of mice and which respond directly to circumferential stretch. Previous studies have comprehensively characterized the classes of pelvic afferent in mouse (Brierley et al., 2004, 2005). However, in this study we made recordings from rectal afferents and these sensory fibers may or may not transmit via the pelvic nerves to the spinal cord. Rectal afferents are of particular interest to us, because we have recently shown using lesion studies in vivo that these afferents are primarily, if not solely, responsible for the detection and transmission of noxious rectal distension stimuli that underlies the VMR pain reflex in mouse (Brookes and Spencer, 2008).
Capsaicin-sensitive muscular afferents behave as low threshold mechanoreceptors
A major observation of the current study was that in both wild type and ls/ls mice, all rectal afferents that responded to capsaicin and circumferential stretch were found to respond with action potentials at low mechanical thresholds (1–2 g stretch), suggesting they are likely activated within the normal physiological range. We found no evidence of any high threshold, stretch-sensitive rectal afferents in either wild type or ls/ls mice, consistent with recordings of Brierley et al. (2004) in the mouse pelvic nerve. It is known that at least two classes of spinal afferent respond to distension. One class responds to stretch over a low threshold extending from the physiological range into noxious levels (Gebhart, 2000; Grundy, 2004). These spinal afferents are suggested to contribute to the signaling of visceral pain through some intensity code that recognizes extreme levels of distension or contraction (Grundy, 2004). Other spinal afferents, however, respond only to noxious levels of distension, an extreme example of which is the high threshold mechanoreceptor that fails to respond under normal circumstances (Grundy, 2004). None of our recordings from the mouse rectal nerve revealed any evidence for the existence of high threshold stretch-sensitive afferents. The capsaicin-sensitive rectal muscular afferents we have identified in the wild type and aganglionic rectum fit well into the classification as low threshold afferents that encode largely innocuous levels of stimulation. If muscular afferents are indeed a major class of mechanoreceptor that detect and signal noxious stimuli to the spinal cord, then at least in mice, these rectal mechanoreceptors appear to give rise to an intensity-coded discharge, signaling natural events at low firing rates but are able to signal pain as the stimulus intensifies (see Grundy, 2002a). In support of this notion, a recent study of the mouse bladder found that the low threshold afferent responses were significantly attenuated in TRPV1 genetic knockout animals, while the high threshold afferents were surprisingly unaffected, suggesting that “… TRPV1 may play an important role in normal bladder function” (Daly et al., 2007). Also, in a study of jejunal afferents in the mouse intestine, Rong et al. (2004), showed that the wide dynamic range afferents were significantly affected in the TRPV1 knockout mice, but other classes of afferent, including the high threshold afferents were unaffected.
Since muscular afferents are the only known class of afferent that respond to stretch at this stage, it seems plausible that the capsaicin-sensitive muscular afferents we have identified in the rectal nerve may be highly significant in the transmission of visceral pain triggered by rectal distension. Interestingly, of all muscular afferents identified in both wild type and ls/ls mice, approximately half were potently activated by topical capsaicin. This is consistent with immunohistochemical studies that have shown a high proportion of TRPV1 positive DRG cell bodies in the lumbosacral region of the mouse spinal cord (Robinson et al., 2004). Interestingly, we found that in ls/ls mice, TRPV1 mRNA expression was significantly reduced in DRG of the lumbosacral region, when compared with wild type animals. The functional significance of this result is not clear, but may be related to our recent observation that the VMR is absent when distension is applied to mutant mice with rectal aganglionosis (Spencer, 2005).
Differences in the proportion of capsaicin-sensitive serosal and muscular afferents in wild type and lethal spotted mice
In this study, we have extended our understanding of the rectal muscular afferents that innervate the aganglionic rectum, by identifying whether any of these afferents respond to capsaicin and determining their properties of activation. Interestingly, in ls/ls mice, we found substantially fewer capsaicin-sensitive serosal afferents innervated the aganglionic rectum (4%), compared with 25% in wild type mice. The functional significance of this is unknown. With respect to muscular afferents, there was a similar proportion in wild type and aganglionic rectal preparations 34% compared with 25%, respectively. The observation that the majority of capsaicin-sensitive rectal afferents were neither serosal nor muscular afferents is noteworthy, but their functional role is not clear.
Characteristics of i.m. mechanoreceptors
It was clear that a population of capsaicin-sensitive i.m. mechanoreceptors must have existed in the aganglionic rectal smooth muscle, because there were no other candidates for these mechanoreceptors to innervate (see Fig. 1B). This was confirmed by anterograde labeling which revealed that i.m. mechanoreceptors terminated in either circular or longitudinal smooth muscle layers. Consistent with our previous study on i.m. rectal mechanoreceptors (Spencer et al., 2008), we were unable to identify any mechanoreceptors in the serosa. The capsaicin-sensitive i.m. mechanoreceptors that were identified consisted of single nerve axons that lacked complex specialization, typically terminating within the muscle layers as single or multiple varicose nodules or swellings. Surprisingly, i.m. mechanoreceptors had no obvious preferential orientation within the circular and longitudinal muscle layers, consistent with a previous study on piebald lethal mouse (Spencer et al., 2008). This observation is very different from what is known about i.m. arrays (IMAs) of vagal afferent endings which lie parallel to the longitudinal or circular axis of the smooth muscle layers, although there is no evidence that IMAs behave functionally as sensory endings.
Characteristics of myenteric mechanoreceptors
Myenteric mechanoreceptors were activated by topical capsaicin and circumferential stretch and could be identified after anterograde labeling, or by their immunoreactivity to the TRPV1 antibody. The capsaicin-sensitivity of these mechanoreceptors was prevented by the TRPV1 antagonist, capsazepine. Myenteric mechanoreceptors consisted of single or multiple bulbous nodules that ramified along single nerve axons, in a bead-like fashion and terminated in myenteric ganglia (Fig. 2). Each nodule consistently had smooth surfaces and formed an ellipsoidal shape, which commonly measured up to 10 µm in their long axis (Fig. 2G and 2I). Myenteric mechanoreceptors showed none of characteristics of the leaf-like lamellar endings associated with IGLEs in the upper GI-tract, or rIGLEs in the lower GI-tract. Myenteric mechanoreceptors were readily identified by their immunoreactivity to the TRPV1 antibody and their immunoreactivity to the glutamate transporter, Vglut2, a reliable marker for extrinsic afferents in the GI-tract (Raab and Neuhuber, 2005; Zagorodnyuk and Brookes, 2003). These TRPV1 bulbous nodules were first identified morphologically in the distal colon of guinea pigs and mice in the immunohistochemical study by Ward et al. (2003) but, at that stage, it was not shown that they behaved physiologically as a capsaicin-sensitive low threshold mechanoreceptor.
TRPV1 immunoreactive nerve endings are identical to the capsaicin-sensitive mechanotransduction sites of rectal mechanoreceptors
Our immunohistochemical staining of the mouse rectum with the TRPV1 antibody revealed that most of the VR1 immunoreactive nerve endings ramified within myenteric ganglia, similar to the findings of Ward et al. (2003). In fact, in the study of Ward et al. (2003) it is particularly noteworthy that the VR1 immunoreactive fibers that terminated within myenteric ganglia “… appeared to have an irregular profile, with small bulbous swellings.” We also noted VR1-immunoreactive nerve endings in myenteric ganglia that were morphologically identical to the mechanotransduction sites that we identified as rectal myenteric mechanoreceptors, which responded to topical capsaicin, circumferential stretch and mechanical probing with von Frey hairs. We were unable to identify any structures similar to rIGLEs following immunohistochemical staining for the TRPV1 receptor, consistent with previous studies (Ward et al., 2003). In fact, we were unable in mouse rectum to identify any structures similar to rIGLEs in guinea-pig rectum.
Do the mechanoreceptors of capsaicin-sensitive muscular afferents show similar morphological features to other extrinsic mechanoreceptors identified throughout the gut and other peripheral organs?
In contrast to nociceptors in the somatic nervous system, which are relatively well understood in many regions of the body, very little is known about the properties and morphology of visceral nociceptors that innervate the gastrointestinal tract. In this study, the i.m. mechanoreceptors identified in the rectum had similar morphological characteristics to somatic nociceptors that innervate the dermis and epidermis of the skin (Purves et al., 2001). These somatic nociceptors are often known as “free nerve endings,” because they consist of unspecialized fine nerve endings. This is somewhat analogous to the i.m. mechanoreceptors that we identified within the rectal circular and longitudinal muscle layers. In fact, it is well known that free nerve endings in skin do have unmyelinated terminal branches that ramify widely in the dermis and epidermis, without any obvious nerve terminal structures (Purves et al., 2001). It is also interesting to note that free nerve endings (nociceptors) in skin, which are known to transmit pain to the spinal cord, also have properties of a slowly adapting mechanoreceptor (Purves et al., 2001), similar to both the i.m. and myenteric mechanoreceptors we identified in the wild type mouse rectum.
Recent studies by the Brookes laboratory have functionally and morphologically identified a class of capsaicin-sensitive mechanoreceptor in the small intestine of guinea pigs with properties consistent with high threshold mechanoreceptors. Their mechanotransduction sites consisted of varicose branching axons that were found on blood vessels of the submucosa and mesentery (Song et al., 2006). It is suggested that this novel class of mechanoreceptor represents the specific class of afferent known as the serosal and mesenteric afferent. In our experiments on mouse rectum, the submucosa was always sharp dissected free from our preparations, which precluded investigation of submucosal mechanoreceptors.
CONCLUSIONS
The rectal nerve innervates the rectum of wild type mice with at least two distinct populations of capsaicin-sensitive, slowly-adapting rectal mechanoreceptor: one class that ramifies within the myenteric ganglia, the other an i.m. ending within the circular and longitudinal smooth muscle layers (Fig. 11). Both classes of mechanoreceptor are activated at low mechanical thresholds within the innocuous range. Despite the absence of enteric ganglia, the aganglionic rectum of ls/ls mutant mice also develops a population of capsaicin-sensitive i.m. rectal mechanoreceptors with similar properties to wild type mice. Both myenteric and i.m. mechanoreceptors are likely involved in signaling noxious mechanical stimuli following rectal distension to the spinal cord.
Fig. 11.
Diagrammatic representation of sites of innervation of low threshold, capsaicin-sensitive rectal muscular afferents in the rectum of wild type mice. (A) A cartoon of the extrinsic innervation of the mouse rectum by the rectal nerves that emanate from pelvic ganglia. (B) Rectal mechanoreceptors that responded to low levels of radial stretch and topical capsaicin were found to innervate the myenteric ganglia and both the circular and longitudinal muscle layers. It is possible that the mucosa and submucosa are innervated by capsaicin-sensitive rectal afferents, but were not present in our dissected in vitro preparations.
Acknowledgments
This study was funded by National Institutes of Health (NIH) grants P20 RR-018751 to N.J.S. The RT-PCR experiments were supported by molecular core facilities provided by Dr. William T. Gerthoffer and Dr. Cherie Singer (NIH: P20 RR-018751). We wish to acknowledge the comments of Prof. Simon Brookes and Dr. Vladimir Zagorodnyuk at Flinders University of South Australia and for the teaching of the anterograde labeling technique developed in their laboratory.
Abbreviations
- Ct
threshold cycle
- DRG
dorsal root ganglia
- IMA
i.m. array
- PBS
phosphate-buffered saline
- rIGLE
rectal intraganglionic laminar ending
- TRPV1
transient receptor potential vanilloid 1
- Vglut2
vesicular glutamate transporter
- VMR
visceromotor response
REFERENCES
- Arvidsson S, Larsson M, Larsson H, Lindstrom E, Martinez V. Assessment of visceral pain-related pseudo-affective responses to colorectal distension in mice by intracolonic manometric recordings. J Pain. 2006;7(2):108–118. doi: 10.1016/j.jpain.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and rectal mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127(1):166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and rectal colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Chen BN, Costa M, Humphrey CMS. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. J Physiol. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ. Identification of the pain pathway activated by rectal distension in mice. Proc Aust Soc Neurosci. 2008;18:103. [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system. Oxford: Blackwell Publishing; 2006. pp. 1–274. [Google Scholar]
- Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions the pathobiology of visceral pain. Am J Physiol. 2000;278:G834–G838. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- Grundy D. Towards a reduction of rectal pain? Neurogastroenterol Motil. 2002a;14:217–219. doi: 10.1046/j.1365-2982.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002b;51:2–5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D. What activates visceral afferents? Gut. 2004;53:5–8. doi: 10.1136/gut.2003.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol. 2005;21:176–182. doi: 10.1097/01.mog.0000153315.28327.6e. [DOI] [PubMed] [Google Scholar]
- Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;23:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp EH, Jones RC, 3rd, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol. 2003;284:G434–G444. doi: 10.1152/ajpgi.00324.2002. [DOI] [PubMed] [Google Scholar]
- Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology. 2003;125(3):786–794. doi: 10.1016/s0016-5085(03)01050-3. [DOI] [PubMed] [Google Scholar]
- Olsson C, Chen BN, Jones S, Chataway TK, Costa M, Brookes SJ. Comparison of extrinsic efferent innervation of the guinea-pig distal colon and rectum. J Comp Neurol. 2006;496:787–801. doi: 10.1002/cne.20965. [DOI] [PubMed] [Google Scholar]
- Payette RF, Tennyson VM, Pham TD, Mawe GM, Pomeranz HD, Rothman TP, Gershon MD. Origin and morphology of nerve fibers in the aganglionic colon of the lethal spotted (ls/ls) mutant mouse. J Comp Neurol. 1987;257(2):237–252. doi: 10.1002/cne.902570209. [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, Williams SM. Neuroscience. 2nd edition. Sinauer Associates Inc; 2001. pp. 189–208. [Google Scholar]
- Raab M, Neuhuber WL. Number and distribution of intraganglionic laminar endings in the mouse esophagus as demonstrated with two different immunohistochemical markers. J Histochem Cytochem. 2005;53:1023–1031. doi: 10.1369/jhc.4A6582.2005. [DOI] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labeling. Neurogastroenterol Motil. 2004;16(1):113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild type and TRPV1 knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X-Y, Chen BN, Costa M, Brookes SJ. Identification of mechanically-activated pain pathways from the gut wall. Gastro. 2006;130:A–75. [Google Scholar]
- Spencer N. Impaired extrinsic afferent reflex responses in aganglionic piebald-lethal mouse colon in vivo. Neurogastroenterol Motil. 2005;17:71. abstract 250. [Google Scholar]
- Spencer NJ, Kerrin AO, Zagorodnyuk VP, Hennig GW, Brookes SJ, Muto M. Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. Am J Physiol. 2008 doi: 10.1152/ajpgi.00502.2007. in press. [DOI] [PubMed] [Google Scholar]
- Tassicker BC, Hennig GW, Costa M, Brookes SJ. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tissue Res. 1999;295:437–452. doi: 10.1007/s004410051250. [DOI] [PubMed] [Google Scholar]
- Ward SM, Gershon MD, Keef KD, Bayguinov YR, Nelson C, Sanders KM. Interstitial cells of Cajal and electrical activity in ganglionic and aganglionic colons of mice. Am J Physiol. 2002;283(2):G445–G456. doi: 10.1152/ajpgi.00475.2001. [DOI] [PubMed] [Google Scholar]
- Ward SM, Bayguinov O, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20(16):6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]