Abstract
Background
Synaptic contacts between neurons and their targets are dynamic entities that can change depending on developmental and functional states of the pre- and postsynaptic cell. However, the molecular factors involved in this plasticity have remained largely unknown. We have demonstrated previously that the Drosophila tumor suppressor gene, discs-large (dlg), is expressed at neuromuscular synapses, and is required for normal synapse structure. A family of dlg homologues is also expressed at mammalian synapses, where they interact with the N-methyl-D-aspartate receptor and ion channels. Here, we provide the first demonstration of the involvement of dlg in structural synaptic plasticity during postsynaptic target growth.
Results
We used a temperature-sensitive dlg allele to demonstrate that there are two stages, late embryogenesis and larval stages, at which dlg is necessary for normal formation of synapses. These stages are coincident with dynamic DLG expression at presynaptic sites in the late embryo, and at postsynaptic regions in the larva. Ultrastructural and confocal analyses reveal that Drosophila neuromuscular junctions undergo a dramatic expansion of the postsynaptic apparatus, which is paralleled by target muscle growth. We show that this process of postsynaptic expansion is partially blocked in dlg mutants.
Conclusions
Our results demonstrate that dlg is required during synapse maturation. We show that dlg is involved in the determination of postsynaptic size during target muscle growth. Because motorneuron targets in the larva are continuously growing, synaptic contacts are structurally plastic, undergoing continuous expansion. We conclude that dlg plays an important role in this form of structural synaptic plasticity.
Background
The efficacy of a presynaptic signal depends on a number of factors, including the number and arrangement of pre-and postsynaptic ion channels and receptors, the availability of synaptic vesicles, and the structure of the pre- and postsynaptic surface. These synaptic properties are not static, and change during development and plasticity [1,2]. For example, changes in synapse structure are thought to underlie the long-lasting increase in synaptic efficacy during long-term potentiation (LTP) [2,3]. During development of the insect neuromuscular junction, a continuous increase in the size of individual synaptic boutons as well as their number accompanies the ongoing growth of the target muscle cell [4].
The mechanisms that determine the development, maintenance and plasticity of synaptic structure have been addressed in several systems by identifying components of the pre- and postsynaptic apparatus [5]. The list of synaptic molecules that may function in synapse development is large, and includes basal lamina proteins such as agrin and S-laminin, cell-adhesion molecules (CAMs) such as fasciclins and neural-CAM, growth factors such as neurotrophins, vesicle proteins such as synapsins, second messengers such as nitric oxide, and postsynaptic receptors [6–10]. In most cases, however, the role of these molecules in determining synapse structure is unknown.
A set of proteins localized to pre- and/or postsynaptic sites are members of the MAGUK family (membrane-associated guanylate kinase homologues) [11]. MAGUKs are multi-domain proteins characterized by the presence of one to several PDZ (PSD-95/DLG/ZO-1) domains (also named Drosophila homology regions; DHRs), SH3 (src-homology region 3), and guanylate kinase (GUK) domains. Recent studies show that the second PDZ domain of PSD-95/SAP-90 binds to the cytoplasmic tail of the N-methyl-D-aspartate (NMDA) receptor, a receptor involved in synaptic plasticity [12]. SH3 domains bind to a proline-rich motif found in several transduction proteins including a GTPase-binding protein [13] and dynamin [14]. The GUK domain has significant similarity to other guanylate kinases, but lacks a conserved three-amino-acid ATP-binding motif required for the conversion of GMP into GDP [11].
Some MAGUKs are found exclusively in the brain, whereas others are found in epithelial cell junctions [15–20]. Both the rat synaptic protein, PSD-95/SAP-90, and the mammalian tight junction protein, ZO-1, bind or co-purify with spectrin, a protein associated with the cytoskeleton [16,21]. The erythrocyte cell junction-like complex contains a MAGUK, p55, which has a binding site for the cytoskeleton-associated protein, band 4.1 [22]. These cytoskeletal interactions, as well as the presence of an enzymatic or nucleotide-binding domain, suggest that MAGUKs may be involved in a transduction cascade that regulates the formation and/or structure of cell junctions.
The product of the Drosophila tumor suppressor gene, discs-large (dlg), is a member of the MAGUK family. In epithelial tissues, the DLG protein is expressed at septate junctions, and in the nervous system at central and peripheral synaptic regions [15,19]. Mutations in dlg lead to the formation of neoplastic tumors in epithelial and neural tissues, to abnormal development of septate and synaptic junctions, and to the loss of cellular polarity in epithelial tissues [15,19,23]. These results show that dlg functions in contact-dependent differentiation of cells, and in the proper development of cell junctions.
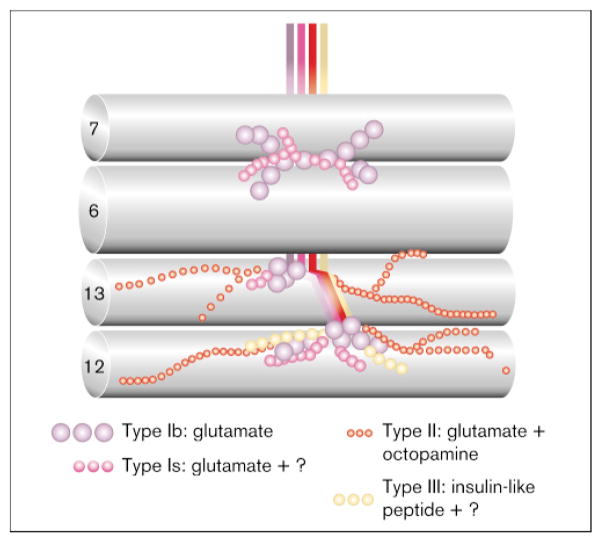
We have demonstrated previously that, at the fly larval neuromuscular junction, dlg is expressed primarily at one type of synaptic bouton. The body wall muscles of Drosophila larvae are innervated by three classes of structurally different neuromuscular endings [24–26] (Fig. 1): type I boutons innervate all muscle fibers and are responsible for excitatory junctional potentials mediated by glutamate [27]; type II boutons contain octopamine and glutamate and innervate all but eight body wall muscles [28]; and a third class of boutons contains peptides, such as proctolin [29], insulin-like peptide [4] or leucokinin I [30], and innervate discrete populations of muscle fibers in a segment-specific fashion (it is not known whether this class of motorneuron contains glutamate in addition to peptides). We demonstrated that most of the DLG immunoreactivity is found postsynaptically, associated with a characteristic type I bouton postsynaptic specialization, the subsynaptic reticulum (SSR) [19]. The SSR is a highly convoluted system of postsynaptic membranes that surrounds the presynaptic type I bouton. The functional significance of this structure is not known, but it has been proposed to be the site where glutamate receptors cluster, and where glutamate uptake is accomplished [19]. Some DLG immunoreactivity is also associated with the presynaptic membrane. Both type I bouton subtypes, Is (small) and Ib (big), show anti-DLG immunoreactivity, although immunoreactivity at type Ib is significantly more intense, possibly reflecting a larger SSR at type Ib boutons [25,26].
Figure 1.
The body wall muscles of Drosophila larva are innervated by synaptic boutons with different structure, position on the muscle cells, and neurotransmitter content. Depicted are the longitudinal muscle fibers 6, 7, 12 and 13, which are innervated by different complements of motorneurons. Type I motorneurons are different for each of the four muscle cells shown. A single type II motorneuron innervates muscles 12 and 13.
Mutations in the dlg locus result in a poorly developed and much simpler SSR [19], supporting the model that dlg is involved in the development of synaptic structure. Interestingly, PSD-95/SAP-90, a closely related vertebrate dlg homolog, which shares 58 % overall identity with the DLG-A gene product, is localized at the postsynaptic density in the hippocampus [16] and at presynaptic spines in the cerebellum [17]. It is possible that PSD-95/SAP-90 has a role similar to dlg in the brain. However, a functional similarity remains to be determined.
The study of Lahey et al. [19] demonstrated that dlg is important for the normal development of a glutamatergic synapse. However, whether there are critical stages at which dlg is required, or whether dlg is involved in the formation or maintenance of synapses, is unknown. Here, we have used a temperature-sensitive allele of dlg to examine these questions. In addition, we determined the developmental pattern of DLG expression, and its correlation to the ontogeny of postsynaptic structure. Our results indicate that there are two stages at which dlg is necessary for normal development of synapses. These stages correlate with the expression of DLG at type I synapses in wild-type, and with the appearance of the abnormal postsynaptic structure in dlg mutants. Our results support the role of dlg as an important component of the cascade of events during development of synapse structure.
Results
Dynamic dlg expression at type I boutons
Our previous studies demonstrated that DLG is expressed at both the pre- and postsynaptic membrane during the last stage of larval development [19]. Moreover, in the presence of abnormal dlg, postsynaptic structure was disrupted. To understand the function of dlg at glutamatergic synapses, we first examined the developmental pattern of DLG expression during synaptogenesis and synapse maturation (Figs 2,3). During embryonic stages 15, 16, and early-to-mid stage 17, DLG was expressed lightly in most neurons (data not shown). During this period, many muscles became innervated (or contacted by motorneurons), but little or no label was found at boutons or postsynaptic muscles (Fig. 2a,b). In contrast, during late stage 17, about 2 hours before hatching, DLG immunoreactivity was localized primarily in the axons and at type I boutons (Fig. 2c). Double staining of these late stage 17 preparations with the nerve-specific antibody, anti-horse radish peroxidase (HRP) [31], and with anti-DLG antibodies revealed that DLG was present inside type I boutons (Fig. 2c, arrowhead). However, the developing bouton border and their filopodia were devoid of DLG immunoreactivity (Fig. 2c, arrow).
Figure 2.
Developmental expression of DLG at type Ib boutons in wild-type. (a,b) type Ib boutons at muscles 12 and 13 in the mid-stage-17 embryo, about 4 h before hatching, double-stained with (a) anti-HRP, and (b) with anti-DLG antibodies. Note that at this stage little DLG label is observed. Arrow in (a) points to filopodia observed in the newly formed boutons at this stage. (c) Type Ib boutons at muscles 12 and 13 in the late-stage-17 embryo, about 2 h before hatching, double-stained with anti-DLG (green) and anti-HRP (red) antibodies. Regions of antigen colocalization appear yellow in the micrographs. Note that DLG immunoreactivity is present inside type I boutons (arrowhead) and is absent from the developing bouton border and filopodia (arrow). (d) Type Ib boutons at muscles 12 and 13 in the early first instar. Note that the DLG label (arrow) is present at the edge of the bouton (yellow), and at the postsynaptic muscle (green; arrow). (e) Wandering third instar type Ib boutons at muscle 12 double-labeled with anti-DLG and anti HRP antibodies. The anti-HRP and anti-DLG label colocalize at the border of the bouton (yellow), and the DLG label extends to the postsynaptic muscle (green). Is: type Is boutons; Ib: type Ib boutons; III: type III boutons; II: type II boutons. Bar = 1 μm in (a–d) and 3 μm in (e).
Figure 3.
Wild-type DLG expression at muscles 6 and 7 during larval stages: (a) in early first instar (0 h); (b) during mid-to-late first instar (about 12 h after hatching); (c) in the second instar stage (about 36 h after hatching); and (d) in the third instar wandering stage (about 96 h after hatching). Arrows point to the DLG label surrounding boutons. Bar = 1.5 μm.
First instar larvae were studied at two periods — within 20 minutes of hatching (early first instar), and at about 8–12 hours after hatching (mid first instar). In contrast to late embryo, DLG immunoreactivity was concentrated at the border of synaptic boutons in early first instar larvae (Figs 2d and 3a). Double-labeling experiments with anti-HRP and with anti-DLG antibodies suggested that staining at the bouton borders represented DLG expression at both pre- and postsynaptic sites (see below). At this stage, anti-HRP antibodies are known to stain axons and presynaptic boutons [32]. However, we found that DLG immunoreactivity co-localized only partially with HRP immunoreactivity at the edge of the bouton (yellow label in Fig. 2d), and that it extended into the muscle junctional area, which was devoid of HRP immunoreactivity (green label and arrow in Fig. 3d). At this stage, the DLG label appeared as a crescent-shaped area, at the border of each bouton, and was undetectable in the axon (Fig. 5a).
Figure 5.
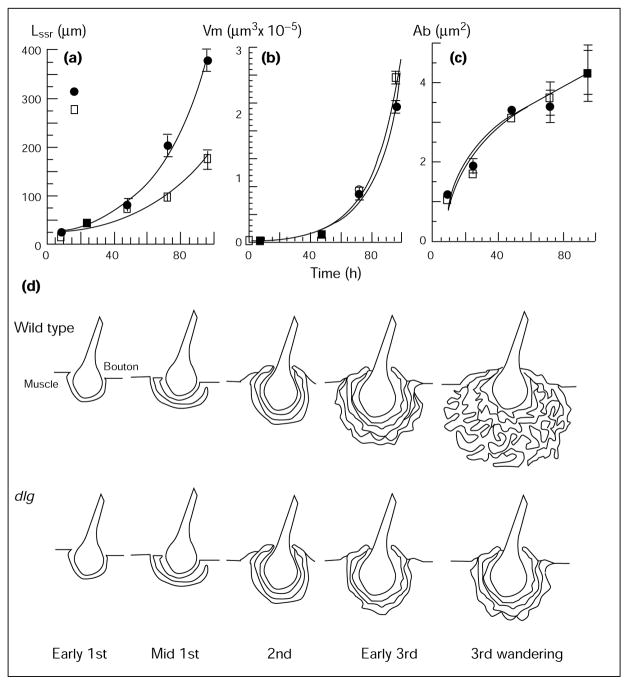
Morphometric analysis of wild-type and dlgv59/Df boutons through development. (a) length of the SSR (LSSR); (b) volume of the muscle cell (Vm), (c) area of the bouton (Ab). Time axis is measured in hours after hatching. (d) Schematic representation of SSR development during larval stages in wild-type and dlg mutants.
During later larval stages, DLG immunoreactivity became progressively localized around the entire periphery of the bouton (Figs 2e and 3b–d). As with the first instar, double-labeling experiments indicated that some of the anti-DLG label colocalized with anti-HRP-immunoreactivity at the bouton border. However, DLG immunoreactivity became increasingly extensive in the postsynaptic junctional muscle region. This observation is consistent with our previous immunoelectron microscopic study showing that anti-DLG immunoreactivity is concentrated in the SSR, and less prominently at the presynaptic membrane [19].
The results described above indicate that DLG expression is dynamic, being concentrated first in axons and presynaptic boutons, and later at presynaptic boutons and postsynaptic junctional sites. These findings suggest that DLG may have a function first in the motorneuron, well after synaptogenesis, and later in the muscle for the proper formation of the SSR. We therefore examined the late-embryonic and post-embryonic development of type I synapses in wild-type and in dlg mutants.
The SSR is formed during the first larval instar, and increases in complexity during late larval stages
The most prominent phenotype of dlg type I synapses was an abnormal SSR appearance during the last larval stage [19]. We therefore looked at the development of the SSR at the electron microscopic (EM) level in wild-type and in dlg mutants, to determine whether structural abnormalities in the mutant were the result of disrupted development or disrupted maintenance of type I synapses.
Transmission EM examination of semi-serial thin sections through type I boutons at larval stages demonstrated that the SSR began to develop during the first larval instar, from the junctional muscle membrane. At this stage, the postsynaptic membrane was very simple and unfolded, forming a depression or gutter over which lay the bouton (Fig. 4a). The presynaptic bouton at this stage was smaller than third-instar type I boutons. However, like the third-instar bouton, it was circular to oval in cross-section, and was filled with 40 nm clear synaptic vesicles. Moreover, the presumed active zones, which appeared as dense ‘T’ shaped structures associated with a cluster of synaptic vesicles, were already visible (small arrow in Fig. 4a).
Figure 4.

Development of the SSR at type Ib boutons in wild-type and in dlgv59/Df mutant samples. (a) First instar wild-type, (b) second instar wild-type, and (c) early third instar wild-type Ib bouton. (d) First instar dlgv59/Df, (e) second instar dlgv59/Df, (f) early third instar dlgv59/Df type Ib bouton. Abbreviations: bl, basal lamina; b, presynaptic bouton; m, muscle; t, trachea. Short arrow = active zones, long arrow = SSR membrane. Bar = 0.22 μm in (a,d), and 0.44 μm in (b,c,e,f).
During mid-first instar stage (8–12 hours after hatching), the junctional muscle membrane began to invaginate at several regions, forming long finger-like structures — the initial folds of the SSR — which partially surrounded type I boutons (data not shown).
During the second instar stage, the SSR became more extensive, having an increased number (about 3–4) of membrane layers around the bouton (Fig. 4b). In contrast to later stages, however, these membrane layers were unfolded and concentric to the presynaptic membrane. In addition, many boutons were still localized on the surface of the muscle and were not completely surrounded by the SSR. Complete surrounding of boutons by the SSR became apparent during the late second instar stage.
By the early third instar stage, the SSR began to acquire its final, wandering third instar semblance. Many layers of membranes now surrounded the boutons, and these layers began to fold, losing their parallel arrangement with the presynaptic membrane, and acquiring the elaborate appearance of third instar type Ib SSR (Fig. 4c). Figure 5d shows a diagrammatic representation of these developmental changes at the SSR.
To quantify the increase in membrane surface during the larval stages, the SSR cross-section at the bouton midline was traced for each bouton, scanned into a computer, and the SSR membrane length and the presynaptic bouton cross-sectional area measured (Fig. 5a,c). We found that the SSR cross-sectional length increased in an exponential fashion, following equation 1:
| (1) |
where LSSR is the mean SSR length (μ), t is the time after hatching (hours), and R is the correlation coefficient for equation 1 (Fig. 5a). Comparisons of the SSR length with the muscle volume indicated that both followed an exponential time course (Fig. 5b). The change of muscle volume through the larval stages could be described by equation 2 (Fig. 5b):
| (2) |
where Vm is the volume of the muscle (μm3). The time constant of Vm, however, was 60 % larger than the SSR length–time constant. These results indicate that the increase in SSR length may be related to the dramatic increase in muscle size (154-fold in volume from first to third instar) during larval development. It has been shown previously that the number and size of boutons also increase exponentially during this stage [4]. Although a cause–effect mechanism cannot be established at this time, these results show that neuromuscular junctions expand continuously during the larval period as the target muscles grow. This neuromuscular junction expansion is the result of the growth of presynaptic endings (increase in number and size of boutons [4]) and, as we show here, of an increase in the postsynaptic surface.
The SSR does not expand normally in dlg mutants
To determine when the dlg mutant phenotype was first apparent during development, we performed similar ultra-structural and morphometric analyses of dlgv59/Df mutants (Figs 4,5). In these dlg/Df mutants, the SSR expanded more slowly than in wild-type (LSSR(t) = 18.8 × e0.02t; R = 0.98), never reaching the final length of wild-type SSR.
For example, during the third instar, SSR membranes in dlg/Df mutants had an unfolded appearance that resembled wild-type second instar SSR (Fig. 4d–f). Similarly, the SSR cross-sectional length was significantly smaller (p < 0.001) during late second to third instar larval stages (Fig. 5b,c).This difference could not be accounted for by a difference in bouton area or muscle volume, which were similar to wild-type (Fig. 5b,c). These observations are summarized in a diagrammatic form in Figure 5d. These results also demonstrate that the signaling mechanisms that regulate the size of the muscle junctional membrane during target muscle growth are altered in the mutants.
DLG is required in embryos and in mid-to-late larvae for development of normal synaptic structure
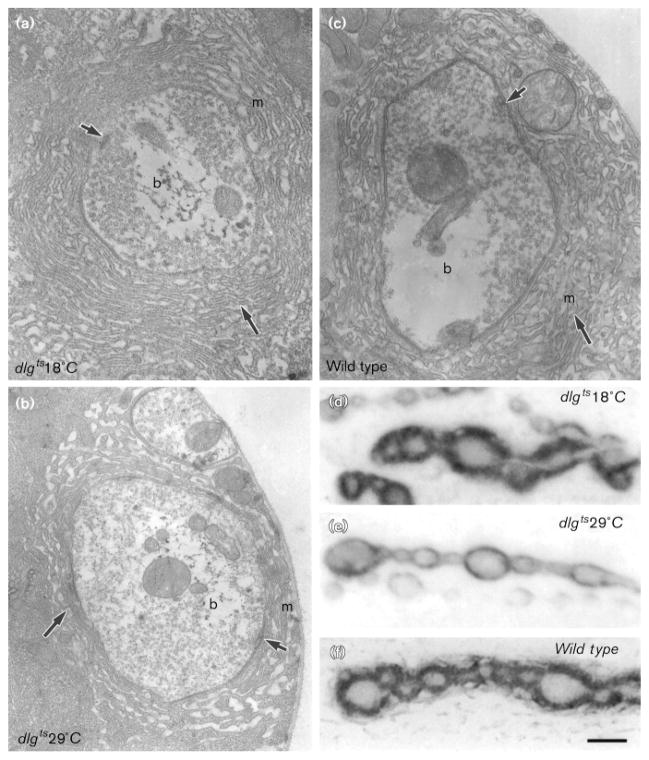
The expression of DLG in the presynaptic bouton during late embryogenesis and in both the pre- and postsynaptic cell during larval stages suggested that dlg may function throughout synapse maturation. To determine when dlg is required for proper development of type I boutons, we examined a temperature-sensitive allele of dlg [23]. At the permissive temperature (18 °C), dlg ts/Df behaved as an hypomorph; for example, dlg ts/Df mutants still developed imaginal disc tumors, but these tumors were very small. At this permissive temperature, type I boutons were similar to those in wild-type at both the light and electron microscopical level (Fig. 6a,c,d,f). In wild-type, the mean cross-sectional SSR length was 370.9 ± 22 μm, as compared with 364.9 ± 19 μm in dlg ts/Df at permissive temperature.
Figure 6.
Type Ib boutons in dlgts/Df reared at permissive and restrictive temperature. (a–c) electron micrographs of third instar type Ib boutons in (a) dlgts/Df at 18 °C, (b) dlgts/Df at 29 °C, and (c) wild-type. Note the less extensive SSR in dlgts/Df at 29 °C as compared to dlgts/Df at 18 °C or wild-type. Short arrows: active zone; longer arrow: SSR; b: presynaptic bouton; m: postsynaptic muscle. (d–f) anti-DLG immunoreactivity at muscle 6 type Ib boutons in (d) dlgts/Df at 18 °C, (e) dlgts/Df at 29 °C, and (f) in wild-type. Notice that anti-DLG immunoreactivity in dlgts/Df at 18 °C is similar to wild-type, and that its extent in dlgts/Df at 29 °C boutons is significantly reduced. Bar = 0.44 μm in (a–c), and 5 μm in (d–f).
At the restrictive temperature (29 °C), dlg ts/Df was similar to the genetically null allele, dlgm52/Df, or to dlgv59/Df. Tumors in the imaginal discs and brain were very large, and the SSR at type I boutons was poorly developed (Fig. 6b,e). In dlg ts/Df reared at restrictive temperature, SSR length was 169.2 ± 19 μm, which was similar to dlgv59/Df (172 ± 13 μm).
The reduction in SSR length and complexity, determined by the ultrastructural studies, was paralleled by changes at the light microscopical level that could be easily observed using anti-DLG immunocytochemistry. Previous immunoelectron microscopical studies revealed that anti-DLG antibodies strongly stained the SSR and, to a lesser extent, the presynaptic membrane [19]. In dlg ts/Df at 18 °C, as in wild-type, DLG immunoreactivity, observed at the light- microscopical level, appeared as a thick doughnut-shaped structure that surrounded type I boutons (Fig. 6d,f). This structure was present, but clearly thinner in dlg ts/Df reared at 29 °C, as it was in dlgv59/Df (Fig. 6e; [19]). These results show that the structural changes in dlg ts/Df mutants can be detected at the light microscopical level using anti-DLG antibodies.
We then shifted dlg ts/Df animals and wild-type controls to 29 °C at different stages of embryonic and larval development to determine whether there were critical stages when DLG was required for normal development of the SSR. The morphology of type I boutons, visualized by anti-DLG immunocytochemistry, was then examined at the wandering third instar stage, and blindly scored by four observers according to the criteria described in the Materials and methods section.
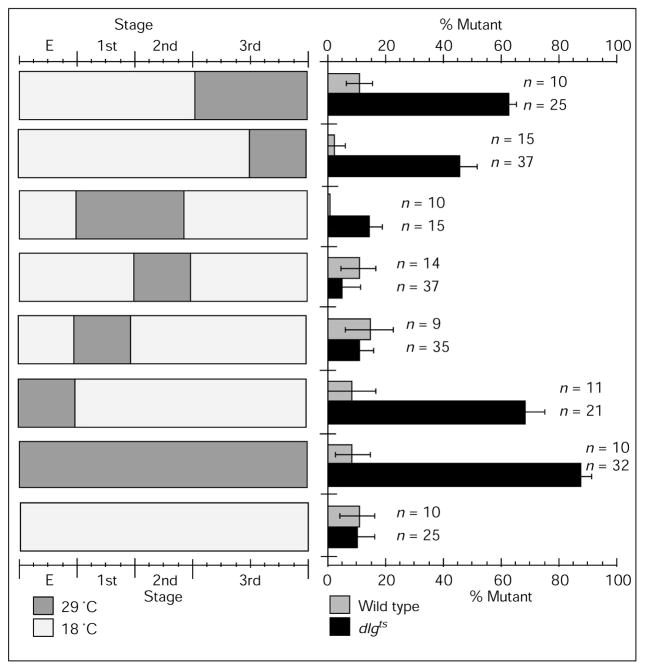
Figure 7 shows the percentage of samples with a mutant phenotype at type I synapses in animals subjected to different temperature-shift paradigms. There were two distinct stages where low DLG levels affected type I bouton structure in the third instar stage. The most dramatic effect of dlg suppression was during embryonic stages —most dlg ts/Df larvae pulsed during the embryonic stage died before reaching wandering third instar stage, and about 85 % of the surviving animals of this group displayed a mutant phenotype at third-instar type I boutons (Fig. 7). This effect was not due to temperature changes, because about 93 % of wild-type controls had a wild-type phenotype at type I synapses. Interestingly, a 29 °C temperature shift during the first instar, immediately after embryogenesis, was without effect in dlg ts/Df larvae. We conclude from these experiments that dlg is required during embryogenesis, possibly after the period when synapses are first formed.
Figure 7.
Percentage of samples with a mutant phenotype at type Ib boutons in dlgts/Df mutants pulsed to restrictive temperature at different stages of embryonic and larval development. Number of wild-type and mutant samples for each temperature pulse paradigm are shown next to each bar. The approximate duration of each temperature pulse (tp, in days) is indicated below. The onset and duration of temperature pulses was determined by examining larval molts (see Materials and methods). E, embryonic stage (tp ~ 40 h in dlgts/Df and controls); 1st, first instar (tp ~ 3 days in dlgts/Df, and 2 days in controls); 2nd, second instar (tp ~ 4 days in dlgts/Df, and 3 days in controls); 3rd, third instar (tp ~ 6 days in dlgts/Df, and 4 days in controls). Second bar from the top, wandering third instar (tp ~ 4 days in dlgts/Df, and 2 days in controls).
A second, though less dramatic, requirement of dlg was found during larval stages: the later during larval development that the temperature was shifted to 29 °C, the higher the percentage of samples with a mutant phenotype. Maximum effect was obtained during mid second to third larval instar, resulting in about 60 % of samples with a mutant phenotype.
The findings that DLG was required during embryonic stages for normal development of the third instar type Ib bouton suggested that dlg may be important at earlier stages of muscle innervation. To investigate whether abnormal DLG would result in an abnormal differentiation of synaptic boutons at embryonic stages, we examined the development of innervation at muscles 6, 7, 12 and 13, in wild-type, dlgv59/Df and dlgm52/Df from stages 15–17 (Fig. 8). For these studies, we used two markers of growing axons and synaptic boutons — anti-Fasciclin II and anti-HRP. No differences were found either in pathway that axons follow, or in growth-cone morphology, or in the timing at which growth cones reach their target and start to differentiate into synaptic boutons. These results were not surprising, as studies of DLG expression had shown that dlg is not expressed at the presynaptic boutons until late embryogenesis, a few hours before the beginning of the larval period.
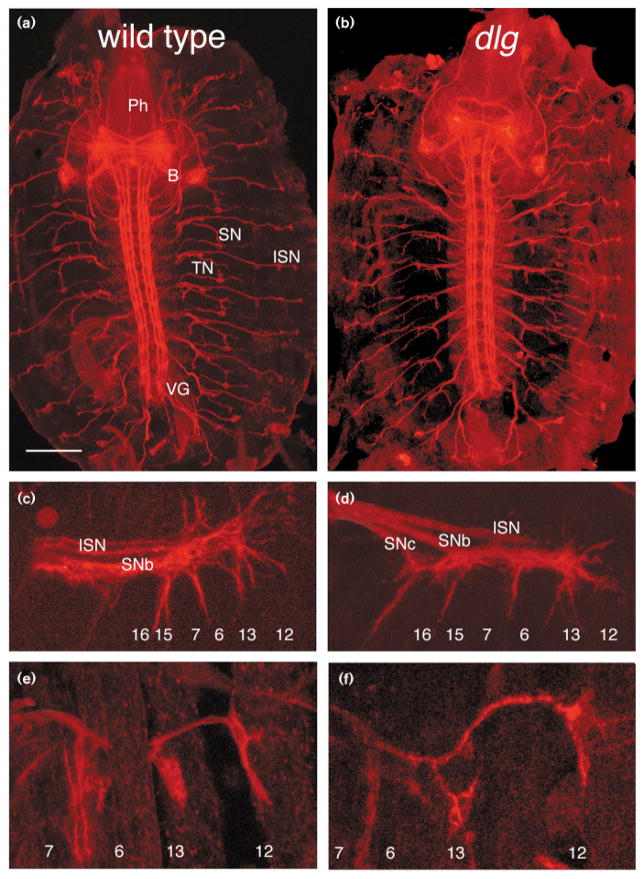
Figure 8.
Development of innervation during the embryonic period in dlg mutants. (a,b) Stage-16 embryo fillets stained with anti-fasciclin II antibody showing that pathway of motor axons in dlg mutant (b) is indistinguishable from that in wild-type controls (a). (c,d) High-magnification view of stage-16 growth cones stained with anti-Fasciclin II antibody, as they interact with muscles 6, 7, 12 and 13 in wild-type (c) and a dlgv59/Df mutant embryo (d). The approximate location of muscle fibers is indicated in each figure. (e,f) High-magnification view of growth cones and synaptic boutons at muscles 6, 7, 12 and 13, in stage-17 wild-type (e) and dlgv59/Df (f) embryos stained with anti-Fasciclin II antibodies. Abbreviations: ISN, intersegmental nerve; SN, segmental nerve; TN, transverse nerve; VG, ventral ganglion; B, brain; Ph, pharyngeal apparatus. Bar = 116 μm in (a,b), and 13 μm in (c–f).
Discussion
In this paper, we provide evidence that dlg is required for the proper regulation of synapse structure during development. Our studies, using the temperature-sensitive allele dlgHF321, demonstrate that there are two stages during development — embryogenesis and larval stages — when dlg function is required for the normal formation of third instar type I bouton postsynaptic specializations.
The embryonic neural requirement for dlg suggests that it has an important role during the period of synapse maturation. Drosophila larval muscles are polyinnervated by several motorneurons that display different transmitters and structural features (Fig. 1). Although different types of synaptic boutons may innervate the same muscle cell, often in proximity to each other, postsynaptic specializations are structurally different for each bouton type. For example, type Ib boutons are completely surrounded by the muscle cell, which forms a highly elaborate SSR, whereas type II or type III boutons lie on depressions of the muscle surface, and an SSR fails to form. These observations suggest that there must be a differential signaling mechanism by which the presynaptic cell elicits a local structural response in the postsynaptic cell. At least some of the bouton types (types I and II) start to develop by late embryogenesis ([4,28,32]; and B.G. and V.B., unpublished observations), indicating that the decision of the muscle cells to form different postsynaptic specializations must occur during this stage.
Although the interpretation of the embryonic critical period is speculative at this point, our results indicate that dlg may function during the developmental period at which the decision to form a specific type of synaptic specialization is made. Our ultrastructural analysis reveals that the SSR does not begin to form until the first larval instar, and therefore it is likely that the cascade of events leading to the formation of the SSR occurs during late embryonic stages, just before the first instar stage. Consistent with this possibility is the fact that DLG is not observed at the neuromuscular junction until a few hours before the first instar. Moreover, we were unable to detect any morphological alterations in pathfinding, timing of motorneuron to muscle interaction, differentiation of synaptic boutons from growth cones, or expression of the cell-adhesion molecule fasciclin II. However, it is possible that properties of the motor axon not detected with our techniques may be affected during this stage, such as the organization of presynaptic channels, or other molecules important for synaptic function.
A second period in which DLG was required was during larval stages. Our results show that pulses carried out during late larval instars were more effective in affecting SSR development than pulses in early larval stages. This was not the result of the duration of the temperature pulse, as a pulse of about 3 days during late third instar stage was more effective than a 4-day pulse during the first and second instar stages. Ultrastructural examination of type Ib boutons during development reveals that mid-second instar to wandering third instar stage is a period of dramatic SSR expansion. Moreover, it is at this period that the mutant phenotype in dlg mutant SSR becomes apparent, suggesting that dlg is required for expansion of the SSR.
The lack of effect when shifting to restrictive temperature during first and second instar stages may reflect a lesser dlg requirement at these stages. Another possibility is that the turnover of DLG protein may be such that enough normal DLG is present from previous stages to support SSR development. Alternatively, type Ib boutons from larvae pulsed during the first or second instar stages could recover at later stages.
We do not believe that the changes in SSR structure are due to changes in motorneuron cell fate. All abdominal muscles examined appear to be innervated by the normal complement of neuronal types, as determined by light and electron microscopical analysis of synaptic bouton types in dlg mutants ([19] and this report). We have examined the distribution and ultrastructure of type I, II, and III boutons in dlg mutants ([19]; B.G. and V.B., unpublished observations). Except for the changes in SSR structure at type I boutons, all other morphological aspects of the different bouton types, such as vesicle types and distribution, shape of the presynaptic density and overall shape of the presynaptic boutons, appear normal in the mutant. These observations do not support a change in motorneuron cell fate, but rather a defective interaction between type I boutons and the postsynaptic muscle cell.
That dlg is required after synaptogenesis is also suggested by our immunocytochemical studies showing a dynamic pattern of dlg expression at type I neurons and muscles. Prior to hatching, but well after motorneurons first contact muscle fibers, DLG appears in the presynaptic bouton. After hatching, DLG becomes most prominent at the bouton border, and progressively more extensive in the postsynaptic muscle. Dynamic patterns of protein expression have been observed for a number of developmentally relevant molecules such as fasciclins and connectin [33–35]. Unlike those two proteins, however, which show dynamic patterns of expression before and during pathfinding and synaptogenesis, dlg expression at nerve terminals occurs several hours after initial synaptic contact. This later expression may reflect a function of dlg in synapse differentiation (or maturation) rather than in the initial stages of target recognition and synaptogenesis.
The developmental regulation of dlg expression, together with the finding that dlg is required in the embryonic and larval stages for normal development of synapse structure, suggest that dlg may function in two different, but related processes. The first process may be the determination of synapse structure in the initial stages of synapse maturation, and the second the expansion of neuromuscular junctions as the muscles grow. Alteration in either process would result, as we observe here, in abnormal development of synapse structure.
The germ-line clone studies of Perrimon [23] demonstrated that there are both maternal and zygotic dlg expressions. In the absence of both components, embryos do not complete embryonic development, and display profound defects in morphogenesis and neurogenesis. In the presence of maternal but the absence of zygotic dlg, embryogenesis can be completed, but mutant larvae show defects in dividing cells, in the formation of epithelial and neural junctions, and in pupariation [15,19,23]. Here, we only examined effects of zygotic dlg suppression, and it is possible, therefore, that dlg function in embryonic synapse development is underestimated due to the presence of maternal dlg. It is clear from our studies in temperature-sensitive mutants, however, that zygotic dlg function is required during embryogenesis for proper formation of type I boutons and full viability.
Our ultrastructural studies reveal that the SSR begins to develop during the larval stages from the junctional muscle region. Morphometric analysis of the SSR indicates that it increases exponentially with time, and that this increase correlates with an increase in muscle volume during larval development. This observation has several interesting implications. First, it suggests that there must be a continuous signaling mechanism in the muscle cell that regulates the size of the postsynaptic junctional membrane, in a fashion that is correlated to changes in muscle volume. Second, these observations strengthen the idea that synapses are dynamic, plastic structures, that change continuously during development depending on many factors. A similar conclusion has been obtained by examining the growth of presynaptic boutons through development —both the number and the size of synaptic boutons increase during development, possibly as an additional mechanism to match pre- and postsynaptic surfaces ([4] and this report). Similarly, we have shown previously that levels of electrical activity regulate the number of synaptic boutons [36].
In dlg mutants, the morphology of the SSR is abnormal ([19] and this report), being less extensive and less complex. Our developmental analysis demonstrates that the process of SSR expansion during muscle growth is abnormal in the mutant, suggesting an involvement of dlg in the signal transduction cascade that regulates the arrangement of the postsynaptic junctional cytoskeleton and membrane during target cell growth. In wild-type, the increase in volume of muscle cells is accompanied by an increase in postsynaptic surface, presumably as one of the mechanisms by which the same presynaptic motorneuron can drive a constantly growing muscle cell throughout development. In dlg mutants, this regulatory process is altered, and the SSR does not develop properly.
The deduced amino-acid sequence of DLG predicts a number of domains that may be involved in cytoskeletal interactions and regulation of GMP/GTP metabolism (see [11] for discussion). The current evidence suggests that MAGUKs are associated with junctions or junction-like complexes at the intracellular side of the membrane, and that they interact with cytoskeletal components such as spectrin and band 4.1. These observations are consistent with the idea that dlg may be involved in the cytoskeletal and membrane rearrangements underlying the dynamics of the postsynaptic apparatus during synapse maturation and growth. Interestingly, it has been reported recently that PSD-95/SAP90 binds to an amino-acid motif (tSXV) present in the carboxy-terminal region of an NMDA receptor subunit, through the PDZ2 domain [12]. NMDA receptors have been implicated in LTP in the mammalian brain. Moreover, many studies suggest that there are changes in structural properties of synapses following LTP [37].
Several types of channels, receptors, and cell-surface molecules such as glutamate receptors, the Shaker potassium channel, and fasciclin II contain an tSXV motif [12,38]. Therefore, they may establish direct interactions with DLG. DLG may provide an interface between extracellular signals and a number of intracellular signaling cascades both during development and during the normal operation of the synapse.
Materials and methods
Fly stocks
Flies were reared using standard procedures at 25 °C unless otherwise indicated. The following stocks were used for these studies: dlgHF321 (referred to as dlgts), a temperature-sensitive allele of dlg [23,39]; dlgv59, a mutant allele that deletes a third of the guanylate kinase domain [15]; and Df(1) N71 (referred to as Df), a deficiency that uncovers the dlg locus. Mutant dlg stocks were maintained over the balancer FM7 and a Y chromosome containing a wild-type copy of dlg. The deficiency stock was maintained using a duplication, Dp(1:2)v[65b] (referred to as Dp), containing a wild-type copy of dlg in the second chromosome. All experiments were performed on dlg/Df mutants, obtained by mating dlg/FM7 females to Df;Dp/+ males. The female dlg/Df progeny can be identified by the presence of tumors in the imaginal discs and CNS. As wild type, the strain Canton-S (CS) was used.
Developmental studies and immunocytochemistry
Larvae were staged using the morphology of anterior spiracles and mouth hooks, and their age and length. Early first instars were dissected within 20 min after hatching. Studies in embryonic stages, before cuticle formation, were done using the fillet preparation [32]. To distinguish mutant samples from heterozygotes, embryos were double-stained with anti-DLG antibodies and either anti-Fasciclin II monoclonal antibodies (kindly provided by C.S. Goodman), or anti-HRP. Mutant embryos could be clearly identified by the lack of DLG-immunoreactivity in epithelial cell borders (cuticle-secreting cells and salivary gland cells) which stain brightly in wild-type. Studies in late embryos, after cuticle formation, were performed by manual dechorionation and devitelinization of embryos, followed by dissection of body walls as in larval stages [4]. Immunocytochemistry and confocal microscopy were performed as in [19]. For developmental measurements of muscle volume, body wall muscle preparations from each stage were stained with FITC-conjugated phalloidin, and volume of muscle 7 (Vm) was estimated assuming an elliptic muscle cross-section, and by measuring muscle thickness (a), muscle length (L), and muscle width (b) at the confocal microscope, according to the equation Vm = L2π√(a2 + b2)/2.
Electron microscopy
Samples were prepared for TEM as in [25]. For studies in first and second larval instars, samples were fixed for 30 min to 2 h in fresh Trump’s fixative. Longer fixation times before osmium treatment at these stages resulted in poor preservation of membranes. All studies were done on the ventral longitudinal muscles 6, 7, 12 and 13 of the second or third abdominal segments using semi-serial sections. To distinguish dlgv59/Df from dlgv59/FM7 females before the third instar larval stage (when visualization of imaginal disc tumors is difficult), the intestine from each sample was dissected out, and separately stained for anti-DLG immunocytochemistry. While intestine epithelia from dlgv59/FM7 displayed immunoreactivity at cellular borders, intestine epithelia from dlgv59/Df were devoid of immunoreactivity, allowing precise identification of dlgv59/Df samples.
For morphometric analysis of boutons, EM negatives were printed at 30 000 × final magnification, and the subsynaptic reticulum (SSR) traced. SSR drawings were scanned into a computer, and analyzed as in [19] using the program NIH Image 1.57. The number of wild-type samples and type Ib boutons used for the EM morphometric analysis was: first instar, 2 samples (16 boutons); second instar, 6 samples (26 boutons); early third instar, 2 samples (13 boutons); wandering third instar, 3 samples (10 boutons). The number of dlgv59/Df mutant samples was: first instar, 2 samples (16 boutons); second instar, 4 samples (28 boutons); early third instar, 2 samples (13 boutons); wandering third instar, 3 samples (10 boutons). Results were expressed as mean ± S.E.M.
Temperature pulses
Broods (from the parental cross dlgts/FM7 × Df/Y; Dp/+) were maintained in an 18 °C incubator, and shifted to a 29 °C incubator at different stages of development. Identical temperature pulses were performed in wild-type. As temperature pulse shifts affect the length of each larval stage, and because there is some variability in absolute developmental times, we used the molts as markers for transition between instars. To determine molting stages, plates containing the larvae were examined for the presence of shed mouth-hook parts and larvae in active molting behavior. In general, the duration of development (from egg laying to wandering third instar) at 29 °C was 4 ± 2 days for dlgts/Df, and 4 ± 1 days in controls. At 18 °C it was 15 ± 3 days in dlgts/Df, and 10 ± 2 days in controls. The duration of development in all the temperature paradigms was 13 ± 1 days for dlgts/Df, and 10 ± 1 days for controls. For temperature pulses involving embryonic stages, parents were placed at 29 °C for 5 h prior to transferring to a new food vial for examination of the progeny. dlgts/Df females were identified by dissecting the larvae and looking for the presence of tumors in the imaginal discs. We found that, even at 18 °C, dlgts/Df had small but visible tumors in the imaginal discs. For analysis of boutons, images collected by confocal microscopy were blindly and independently scored by 4 naive observers according to the following criteria. Observers were first presented with 5 different representative examples of wild-type and dlg/Df type Ib bouton strings. Then they were instructed to score the experimental and control type Ib in a scale of 1–3 with regard to two characteristics of type Ib boutons which are affected in the mutants: the thickness of the bouton rim stained by anti-DLG antibodies; and the presence of perisynaptic network. Samples with a score of 3 or less were considered as ‘wild type’ and samples with a score of more than 3 as ‘mutant’. The percentage of mutant samples in each experimental and control group was expressed as the average percentage found by each observer ± S.E.M.
Acknowledgments
We wish to thank Elizabeth Connor, Susan Cumberledge, Randall Phillis, and James Trimarchi for helpful comments and discussions. We also thank the Electron Microscopy and Image Analysis Facility at the University of Massachusetts. This work was supported by NIH grant NS30072 and by a Sloan Fellowship to V.B.
References
- 1.Lnenicka GA, Murphey RK. The refinement of invertebrate synapses during development. J Neurobiol. 1989;20:339–355. doi: 10.1002/neu.480200507. [DOI] [PubMed] [Google Scholar]
- 2.Weiler IJ, Hawrylak N, Greenough WT. Morphogenesis in memory formation: synaptic and cellular mechanisms. Behav Brain Res. 1995;66:1–6. doi: 10.1016/0166-4328(94)00116-w. [DOI] [PubMed] [Google Scholar]
- 3.Genisman Y, deToledo-Morrell F, Heller RE, Rossi M, Parshall RF. Structural synaptic correlate of long-term potentiation: formation of axospinous synapses with multiple, completely partitioned transmission zones. Hippocampus. 1993;3:435–446. doi: 10.1002/hipo.450030405. [DOI] [PubMed] [Google Scholar]
- 4.Gorczyca MG, Augart C, Budnik V. Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J Neurosci. 1993;13:3692–3704. doi: 10.1523/JNEUROSCI.13-09-03692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Neuron. 1993;10:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 6.Lohof AM, Ip NY, Poo M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 7.Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 8.Roskams JA, Bredt DS, Dawson TM, Ronnett GV. Nitric oxide mediates the formation of synaptic connections in developing and regenerating olfactory receptor neurons. Neuron. 1994;13:289–299. doi: 10.1016/0896-6273(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 9.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 10.Rutishauser U, Landmesser L. Polysialic acid on the surface of axons regulates patterns of normal and activity-dependent innervation. Trends Neurosci. 1991;14:528–532. doi: 10.1016/0166-2236(91)90006-g. [DOI] [PubMed] [Google Scholar]
- 11.Woods DF, Bryant PJ. ZO-1, DLGA and PSD-95/SAP-90: homologous proteins in tight, septate and synaptic cell junctions. Mech Dev. 1993;44:85–89. doi: 10.1016/0925-4773(93)90059-7. [DOI] [PubMed] [Google Scholar]
- 12.Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 13.Cicchetti P, Mayer BJ, Thiel G, Baltimore D. Identification of a protein that binds to the SH3 region of Abl and is similar to Bcr and GAP-rho. Science. 1992;257:803–806. doi: 10.1126/science.1379745. [DOI] [PubMed] [Google Scholar]
- 14.Booker GW, Gout I, Downing AK, Driscoll PC, Boyd J, Waterfield MD, Campbell ID. Solution structure and ligand-binding site of the SH3 domain of p85α subunit of phosphatidylinositol 3-kinase. Cell. 1993;73:813–822. doi: 10.1016/0092-8674(93)90259-s. [DOI] [PubMed] [Google Scholar]
- 15.Woods DF, Bryant PJ. The disc-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 16.Cho K-O, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila Disc-Large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 17.Kistner U, Wenzel BM, Veh RW, Cases-Langhoff C, Garner AM, Appeltauer U, et al. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J Biol Chem. 1993;268:4580–4583. [PubMed] [Google Scholar]
- 18.Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila disc-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahey T, Gorczyca M, Jia X, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller BM, Kistner U, Veh RW, Casses-Langhoff C, Becker B, Gundelfinger ED, Garner CC. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila Discs-Large tumor suppressor protein. J Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Citi S. The molecular organization of tight junctions. J Cell Biol. 1993;121:485–489. doi: 10.1083/jcb.121.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marfatia SM, Lue RA, Branton D, Chishti AH. Identification of the protein 4. 1 binding interface on Glycophorin C and p55, a homologue of the Drosophila discs-large tumor suppressor protein. J Biol Chem. 1995;270:715–719. doi: 10.1074/jbc.270.2.715. [DOI] [PubMed] [Google Scholar]
- 23.Perrimon N. The maternal effect of lethal(1) discs-large-1: a recessive oncogene of Drosophila melanogaster. Dev Biol. 1988;127:392–407. doi: 10.1016/0012-1606(88)90326-0. [DOI] [PubMed] [Google Scholar]
- 24.Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci. 1989a;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia X, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild-type and mutants with increased excitability. J Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atwood H, Govind CK, Wu C-F. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- 27.Jan LY, Jan YN. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976;262:215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson MS, Halpern ME, Keshishian H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: characterization of fiber-specific neuromuscular endings. J Neurosci. 1988;8:242–255. doi: 10.1523/JNEUROSCI.08-01-00242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantera R, Nässel DR. Segmental peptidergic innervation of abdominal targets in larval and adult dipteran insects revealed with an antiserum against leucokinin I. Cell Tissue Res. 1992;269:459–471. doi: 10.1007/BF00353901. [DOI] [PubMed] [Google Scholar]
- 31.Jan LY, Jan YN. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and grasshopper embryos. Proc Natl Acad Sci USA. 1982;72:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansen J, Halpern ME, Keshishian H. Axonal guidance and the development of muscle fiber-specific innervation in Drosophila embryos. J Neurosci. 1989b;9:4318–4332. doi: 10.1523/JNEUROSCI.09-12-04318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman CS, Grenningloh G, Bieber AJ. Molecular genetics of neural cell adhesion molecules in Drosophila. In: Letourneau PC, Kater SB, Macagno ER, editors. The Nerve Growth Cone. New York: Raven Press; 1992. pp. 283–303. [Google Scholar]
- 34.Keshishian H, Chiba A. Neuromuscular development in Drosophila: insights from single neurons and single genes. Trends Neurosci. 1993;16:278–283. doi: 10.1016/0166-2236(93)90182-l. [DOI] [PubMed] [Google Scholar]
- 35.Meadows LA, Gell D, Broadie K, Gould AP, White RAH. The cell adhesion molecule, connectin, and the development of the Drosophila neuromuscular system. J Cell Sci. 1994;107:321–328. doi: 10.1242/jcs.107.1.321. [DOI] [PubMed] [Google Scholar]
- 36.Budnik V, Zhong Y, Wu C-F. Morphological plasticity of motor axon terminals in Drosophila mutant with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiler IJ, Hawrylak N, Greenough W. Morphogenesis in memory formation: synaptic and cellular mechanisms. Behav Brain Res. 1995;66:1–6. doi: 10.1016/0166-4328(94)00116-w. [DOI] [PubMed] [Google Scholar]
- 38.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 39.Woods DF, Bryant PJ. Molecular cloning of the lethal(1) discs-large-1 oncogene of Drosophila. Dev Biol. 1989;134:222–235. doi: 10.1016/0012-1606(89)90092-4. [DOI] [PubMed] [Google Scholar]