Abstract
Background
Cowden’s syndrome is a rare, autosomal dominant disease, caused by mutations in the phosphoinositide 3-kinase and phosphatase and tensin homolog (PTEN) gene. It is associated with hamartomatous polyposis of the gastrointestinal tract, mucocutaneous lesions, and increased risk of developing certain types of cancer. In addition to increased risk of tumour development, mutations in PTEN have also been associated with autoimmunity in both mice and humans. To date, however, an association between Cowden’s syndrome and immune deficiency has been reported in a single patient only.
Methods and Results
Two patients with Cowden’s syndrome and an increased frequency of infections were investigated for possible underlying immunodeficiency. In one patient, hypogammaglobulinaemia with a functional antibody deficiency was identified, whilst the other patient had a persisting CD4+ T cell lymphopenia (with normal antibody production).
Conclusions
Our data indicate that Cowden’s syndrome may be associated with both T cell and B cell immune dysfunction. We recommend that patients with Cowden’s syndrome and an increased frequency of infections are investigated for associated immunodeficiency.
Keywords: Cowden’s syndrome, PTEN, immunodeficiency, antibody deficiency
Introduction
Cowden’s syndrome is a rare, autosomal dominant disease, caused by mutations in the phosphoinositide 3-kinase and phosphatase and tensin homolog (PTEN) gene. It is associated with hamartomatous polyposis of the gastrointestinal tract, mucocutaneous lesions, and increased risk of developing certain types of cancer [OMIM #158350]. In this context, PTEN acts as a tumour suppressor (reported as being the second most commonly mutated tumour suppressor gene in sporadic human cancers [1]). PTEN is a phosphatase, which catalyzes the conversion of phosphatidylinositol(3,4,5)P3 (PIP3) to phosphatidylinositol(4,5)P2, antagonizing the signalling cascades downstream of receptor tyrosine kinases (RTKs) and phosphatidylinositol-3-kinase (PI3K) [2]. Amongst its diverse roles, PI3K activation is involved in the development, activation and differentiation of both T and B lymphocytes [3]. Immune dysregulation, including impaired lymphocyte activation-induced apoptosis, lymphoid hyperplasia, and increased autoimmunity due to defects in T and B cell homeostasis, has been described in mice with targeted heterozygous PTEN mutations [4]. A recent report [5] highlighted a range of autoimmune manifestations and lymphoid hyperplasia in a series of patients with germline PTEN mutations. In addition, mice with a B cell-specific mutation in PTEN were shown to have reduced levels of IgG and IgA, impaired specific antibody production, and defective immunoglobulin class switch recombination (CSR) [6]. To date, however, there have been no reports of antibody deficiency in patients with Cowden’s syndrome, and T cell deficiency has been reported in a single patient only [7].
Case Reports
We report the cases of two young male patients with Cowden’s syndrome, associated with mutations in the PTEN gene, in whom investigation of frequent infections led to the identification of distinct underlying immune abnormalities.
Case 1
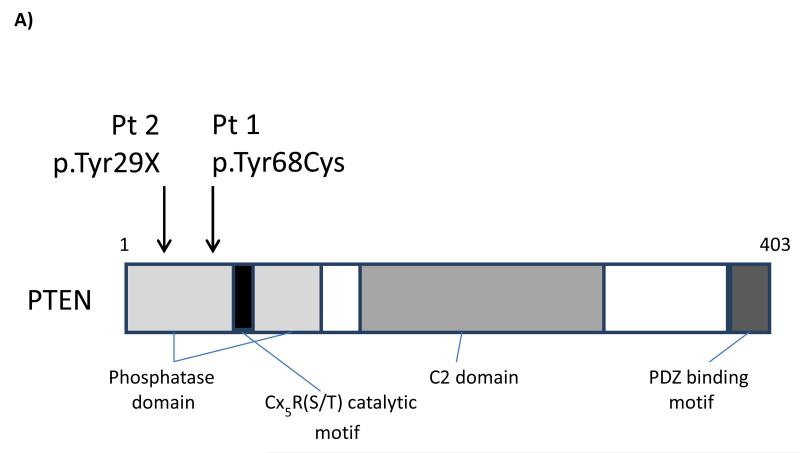
The first case is a 5 year old boy. He was noted to have macrocephaly antenatally, although no cause was identified initially. He was the only child of non-consanguineous parents. It was noted that his father also had macrocephaly. He was referred for a Genetics opinion at 15 months due to increased head circumference. In addition to macrocephaly, with an OFC >99.6th centile, there was evidence of mild developmental delay, but no other obvious features. Genetic testing confirmed the presence of a heterozygous mutation in the PTEN gene (c.203A>G). This mutation, in a highly conserved area in exon 3, encodes an amino acid substitution from tyrosine to cysteine (p.Tyr68Cys) within the phosphatase domain (Figure 1A). A tyrosine to histidine substitution at this position in PTEN has previously been described as abolishing PTEN phosphatase activity [8]. The same mutation was identified in his father. He had suffered from frequent respiratory tract infections from a young age, and had had 3 hospital admissions by the age of 4 years. Immunological investigations at 23 months showed a panhypogammaglobulinaemia (Table 1), with protective levels of specific antibody to tetanus toxoid, but non-protective specific antibodies to H. influenzae type B (Hib), in spite of prior immunization according to the UK vaccination schedule. He was started on prophylactic antibiotics, with a significant improvement in the frequency and severity of respiratory tract infections. In addition, he received a booster dose of the Menitorix vaccine, and a single dose of the unconjugated pneumococcal vaccine, Pneumovax II, to both of which he made good specific antibody responses (Table 1 and Supplementary Table). However, approximately 15 months later, his specific antibodies to Hib and pneumococcus had both fallen to non-protective levels, and his panhypogammaglobulinaemia persisted. His peripheral blood lymphocyte immunophenotypic profile showed normal or slightly elevated numbers of T cells, NK cells and (polyclonal) B cells on two occasions, although he had increased proportions of both CD5+ and CD10+ B cells (Table 1). The results indicate a significant functional impairment of antibody production. Of note, he also had marked tonsillar hyperplasia.
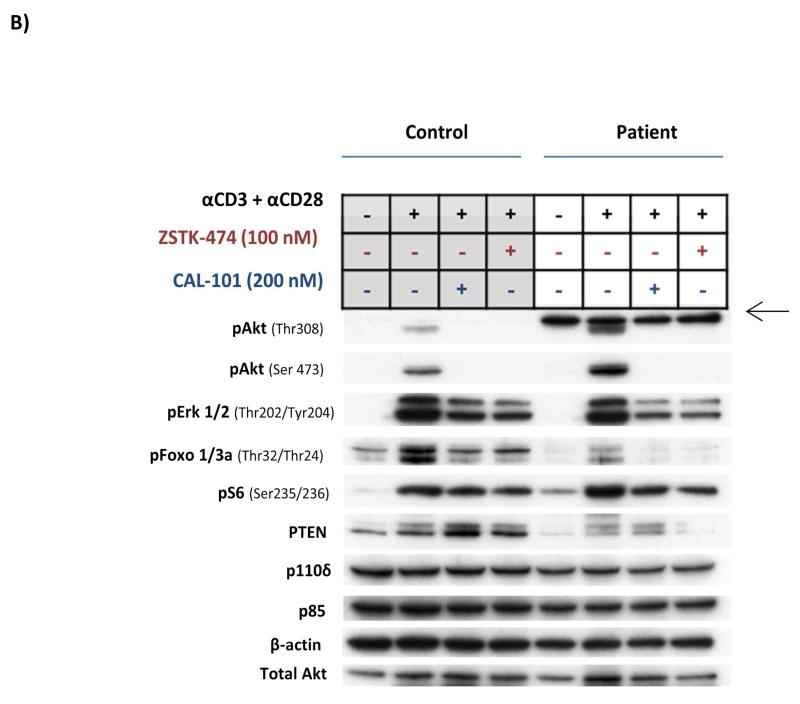
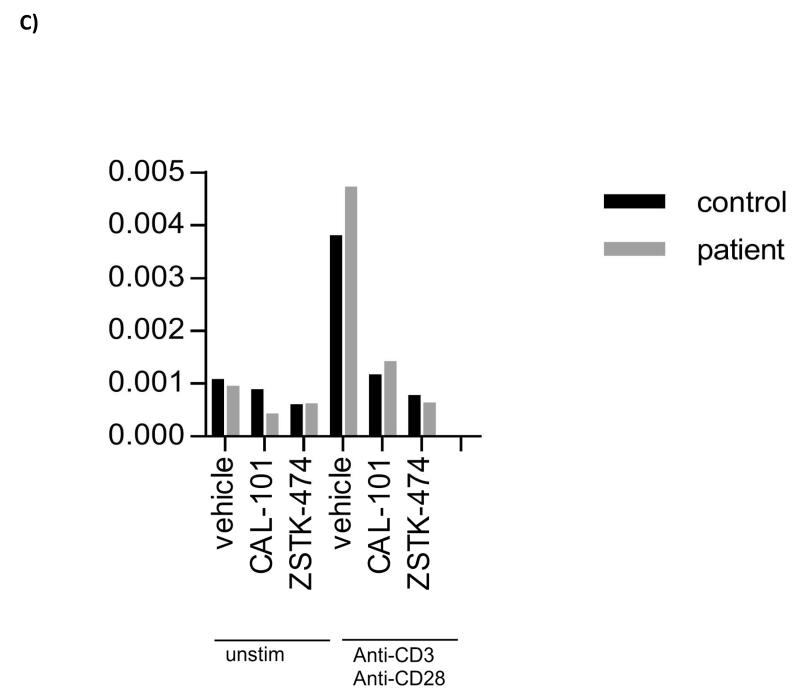
Figure 1.
A) Schematic representation of the PTEN protein, with locations of the mutations identified in Patients 1 and 2 indicated; B) Western blot analysis and C) PIP3 levels in peripheral blood T cells from Patient 1, stimulated in vitro with anti-CD3 and anti-CD28 in the presence or absence of CAL-101 (Idelalisib; PI3Kδ inhibitor) or ZSTK474 (class I PI3K inhibitor). Western blotting and PIP3 analysis were performed as described previously [15,18]. [A consistent, non-specific band with slightly high molecular weight (arrowed in Fig 1B), was seen in the patient’s pAkt Thr308 western blot (but not in the control), which was not inhibited by ZSTK-474 or CAL-101].
Table 1.
Immune parameters in Patients 1 and 2.
|
Patient 1 (@ 23 months)1 |
Patient 1 (post- immunization)2 |
Patient 1 (@ 44 months)3 |
Patient 2 (@ 9 years 2 months)1 |
Patient 2 (post- immunization)2 |
|
|---|---|---|---|---|---|
| IgG | 2.9 (3.1-13.8) | NT | 3.6 (4.9-16.1) | 10.4 (5.4-16.1) | NT |
| IgA | 0.14 (0.3-1.2) | NT | 0.33 (0.4-2.0) | 0.86 (0.7-2.5) | NT |
| IgM | 0.37 (0.5-2.2) | NT | 0.40 (0.5-2.2) | 1.2 (0.5-1.8) | NT |
| Tetanus toxoid 4 | 0.18 | 0.93 | 0.33 | 0.39 | NT |
| H. influenzae B 4 | 0.44 | 8.71 | 0.45 | 0.92 | NT |
| Pneumococcus (% serotypes >0.35 mg/L)5,6 | NT | 100% | 54% | 8% | 100% |
| Absolute lymphocyte count | NT | 8.03 (2.3-5.4) | 2.19 (2.3-5.4) | 1.11 (1.9-3.7) | 1.36 (1.9-3.7) |
| CD3+ T cells (%) | NT | 62.6 (56-75) | 71.3 (56-75) | 63.4 (60-76) | 66.2 (60-76) |
| CD3+ T cells (abs) | NT | 5.03 (1.4-3.7) | 1.56 (1.4-3.7) | 0.70 (1.2-2.6) | 0.90 (1.2-2.6) |
| CD4+ T cells (%) | NT | 31.8 (28-47) | 42.4 (28-47) | 27.6 (31-47) | 26.4 (31-47) |
| CD4+ T cells (abs) | NT | 2.55 (0.7-2.2) | 0.93 (0.7-2.2) | 0.31 (0.65-1.5) | 0.36 (0.65-1.5) |
| CD8+ T cells (%) | NT | 23.1 (16-30) | 22.9 (16-30) | 29 (18-35) | 31.4 (18-35) |
| CD8+ T cells (abs) | NT | 1.85 (0.49-1.3) | 0.50 (0.49-1.3) | 0.32 (0.37-1.1) | 0.43 (0.37-1.1) |
| CD16/56+ NK cells (%) | NT | 7.7 (4-17) | 5.4 (4-17) | 9.4 (4-17) | 13.1 (4-17) |
| CD16/56+ NK cells (abs) | NT | 0.62 (0.13-0.72) | 0.12 (0.13-0.72) | 0.10 (0.1-0.48) | 0.18 (0.1-0.48) |
| CD19+ B cells (%) | NT | 28.9 (14-33) | 22.9 (14-33) | 25.9 (13-27) | 20.4 (13-27) |
| CD19+ B cells (abs) | NT | 2.32 (0.39-1.4) | 0.50 (0.39-1.4) | 0.29 (0.27-0.86) | 0.28 (0.27-0.86) |
| CD19+/CD5+ B cells (%) | NT | NT | 46.8 | 2.3 | 6.1 |
| CD19+/CD10+ B cells (%) | NT | NT | 16.1 | 2.6 | 1.7 |
Serum immunoglobulins are reported in g/L, tetanus toxoid antibodies in IU/ml, and Hib and pneumococcal antibodies in mg/L. Absolute lymphocyte subset counts are reported as number of cells ×109/L. Age related normal ranges, where available, are given in parenthesis below the patient’s values. Abnormally low values (for serum immunoglobulins and lymphocyte subsets) are presented in bold type.
Pre-immunization;
1 month post-immunization with Menitorix and Pneumovax II;
15 months post-immunization;
Protective level for tetanus toxoid = >0.15 IU/ml; protective level for Hib = >1.0 mg/L;
Putative protective serotype specific pneumococcal antibody level = 0.35 mg/L;
Full serotype specific pneumococcal antibody levels are given in the Supplementary Table; NT = not tested.
Analysis of PI3K signalling was undertaken in isolated peripheral blood T cells from this patient. Western blot analysis showed a reduced level of PTEN protein expression (compared with a healthy control), with an increase in Akt and S6 phosphorylation following stimulation with anti-CD3 and anti-CD28, but an unexpected reduction in Foxo phosphorylation (Figure 1B). There was no difference in basal PIP3 levels between the patient’s T cells and those of the control (Figure 1C). Following activation, the patient’s T cells showed slightly higher levels of PIP3 compared with the control, and these responses were completely inhibited by PI3K inhibitors CAL-101 and ZSTK474 (Figure 1C). The results suggest that the mutation had a relatively minor effect on PI3K signalling within the patient’s T cells.
Case 2
The second case is a 10 year old boy who was diagnosed with Cowden’s syndrome at approximately 3 years of age. Genetic analysis revealed a T to A substitution at base 87 (c.87T>A) in one PTEN gene. This mutation encodes a STOP codon in place of a Tyrosine at amino acid position 29 in the PTEN protein (Figure 1A). His parents and a younger sister all tested negative for mutations in the PTEN gene. He had initially presented at 8 months of age, when he was also noted to have macrocephaly (head circumference above the 97th centile). He had suffered from recurrent acute ear and upper respiratory infections from an early age, and had had tympanostomy tubes (grommets) inserted on three occasions, adenoidectomy at 4 ½ years, and tonsillectomy at 8 ½ years. In spite of these interventions, he continued to suffer from recurrent episodes of otitis media. At age 9 years, in view of the ongoing infections, he had his serum immunoglobulin, specific antibody, and lymphocyte subset levels measured. Serum immunoglobulins were normal for age, and he had evidence of satisfactory antibody responses to tetanus toxoid and Hib vaccination in early childhood (Table 1). His specific antibody levels to pneumococcus (against which he had not been immunized) were low, and he was immunized with Pneumovax II, to which he made a normal antibody response (Table 1 and Supplementary Table). However, lymphocyte subset analysis showed a moderate CD4+ T cell lymphopenia and a mild CD8+ T lymphopenia, with normal numbers of NK cells and B cells. On re-testing 6 months later, the CD4+ T cell lymphopenia (both as % of lymphocytes and absolute numbers) persisted (Table 1).
There was no clinical or biochemical evidence of autoimmunity in either patient.
Discussion
We report 2 patients with heterozygous mutations in the PTEN gene in whom there was an increased frequency of (mainly respiratory tract) infections, in whom distinct immunological defects were identified. In addition to its function as a tumour suppressor, PTEN acts as an antagonist of PI3K activity in B and T cell differentiation and homeostasis [3,9,10]. Studies in mice deficient in PTEN have shown altered patterns of B cell differentiation, with expansion of B1 and marginal zone B cells, impaired CSR, and increased autoantibody production [6,11-13], associated with sustained production of PIP3 [14]. Translating these studies to humans with germline PTEN mutations, Heindl et al [5] described an increased prevalence of lymphoid hyperplasia (gastrointestinal, tonsillar and thymic), and of autoimmune disease (Hashimoto’s thyroiditis and autoimmune haemolytic anaemia). They reported no increase in susceptibility to infections, although total lymphocyte counts showed a tendency toward lower lymphocyte numbers [5]. Serum immunoglobulins were reported as being within “lower normal” range (although data presented showed reduced levels of IgG, IgA and IgM respectively in individual patients, in a group of 8 patients having serum immunoglobulins measured; specific antibody responses were not reported) [5]. The two patients reported in our study both presented with an increased frequency of infections in childhood, which prompted investigation of their immune functions. One of the patients showed a panhypogammaglobulinaemia. Specific antibody responses to immunization with the conjugate Hib vaccine, Menitorix, and the unconjugated pneumococcal polysaccharide vaccine, Pneumovax II, were initially normal (at 4 weeks post immunization), but the antibody levels declined rapidly, returning almost to baseline levels by 15 months post immunization, indicating a functional antibody deficiency. Major lymphocyte subpopulations were present in normal numbers in this patient, although there were increased proportions of CD5+ and CD10+ B cell subsets in the blood, a finding that has been noted previously in patients with PTEN mutations [5]. Our second patient had normal serum immunoglobulin levels, protective antibodies to tetanus and Hib, and a normal antibody response to pneumococcal polysaccharide antigen, but had a persistent CD4+ T cell lymphopenia. The two cases illustrate that abnormalities of T cell and B cell development and/or function may be present in patients with Cowden’s syndrome, and may be associated with increased susceptibility to infections.
The difference in immunological phenotypes of our two patients, and their relation to patients with Cowden’s syndrome more generally, are hard to explain. In both cases, the mutations would be predicted to abolish the phosphatase activity of the affected PTEN protein. Studies of PI3K function in patient 1 showed reduced expression of the PTEN protein and increased AKT and S6 phosphorylation (in peripheral blood T cells), but did not show clear differences in either basal or stimulated levels of PIP3 compared with a healthy control. The modest increase in PIPs in T cells from our patient is consistent with the fact that patients with Cowden’s syndrome have one allele of non-mutated PTEN which produces a functional protein, albeit it at reduced levels. Of interest, dominant gain-of-function mutations in PIK3CD, which encodes the p110δ subunit of PI3K, have recently been associated with a clinical syndrome involving antibody deficiency, CD4+ T cell lymphopenia, sinopulmonary infections and lymphoid hyperplasia, associated with increased phosphorylation of Akt kinase [15-17], emphasising the role of PI3K in lymphocyte homeostasis and function. In contrast to our patient with Cowden’s syndrome, patients with the E1021K gain-of-function mutation of PI3Kδ showed a 2-3 fold increase in basal and stimulated PIP3 levels compared with controls [15]. The effects of (heterozygous) PTEN mutations on lymphoid development and function would therefore appear to share some features with gain-of-function mutations in PIK3CD, but with a less severe clinical phenotype.
Immunodeficiency has been reported previously in a single patient with Cowden’s syndrome [7], who presented with recurrent cellulitis and abscesses, and reduced T cell numbers and in vitro T cell proliferative function. This patient had normal levels of serum immunoglobulins. Given the known role of PTEN in regulating PI3K activation, and the function of PI3K in the differentiation and homeostasis of both B and T cells, it is perhaps surprising that immune problems have not been reported more widely in patients with Cowden’s syndrome. Based on our observations, we recommend measuring serum immunoglobulins, specific antibody levels and lymphocyte subpopulations in patients with Cowden’s syndrome who show an increased susceptibility to infections.
Supplementary Material
Supplementary Table. Serotype-specific pneumococcal antibody levels in Patients 1 and 2.
Acknowledgements
We would like to thank Jonathan Clark, Babraham Institute, for help with lipid measurements. Work in KO’s lab was funded by the BBSRC and GSK.
References
- 1.Stokoe D. PTEN. Curr Biol. 2001;11:R502. doi: 10.1016/s0960-9822(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Paluch BE, Wang X, Jiang X. PTEN at a glance. J Cell Sci. 2012;125:4687–92. doi: 10.1242/jcs.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.So L, Fruman DA. PI3K signalling in B- and T-lymphocytes: new developments and therapeutic advances. Biochem J. 442:465–81. doi: 10.1042/BJ20112092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Cristofanno A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon K, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–5. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 5.Heindl M, Handel N, Ngeow J, Kionke J, Wittekind C, Kamprad M, Rensing-Ehl A, Ehl S, Reifenberger J, Loddenkemper C, Maul J, Hoffmeister J, Aretz S, Kiess W, Eng C, Uhlig HH. Autoimmunity, intestinal lymphoid hyperplasia, and defects in mucosal B-cell homeostasis in patients with PTEN hamartoma tumor syndrome. Gastroenterol. 2012;142:1093–6. doi: 10.1053/j.gastro.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki S, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–67. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rushak PJ, Kauh YC, Luscombe HA. Cowden’s disease associated with immunodeficiency. Arch Dermatol. 1981;117:573–5. [PubMed] [Google Scholar]
- 8.Han SY, Kato H, Kato S, Suzuki T, Shibata H, Ishii S, Shiiba K, Matsuno S, Kanamura R, Ishioka C. Functional evaluation of PTEN missense mutations using in vitro phosphoinositide phosphatise assay. Cancer Res. 2000;60:3147–51. [PubMed] [Google Scholar]
- 9.Buckler JL, Liu X, Turka LA. Regulation of T-cell responses by PTEN. Immunol Rev. 2008;224:239–48. doi: 10.1111/j.1600-065X.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol. 2011;23:178–83. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nature Immunol. 2003;4:287–94. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 12.Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class witch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signalling. Immunity. 2006;25:545–57. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Janas ML, Hodson D, Stamataki Z, Hill S, Welch K, Gambardella L, Trotman LC, Pandolfi PP, Vigorito E, Turner M. The effect of deleting p110δ on the phenotype and function of PTEN-deficient B cells. J Immunol. 2008;180:739–46. doi: 10.4049/jimmunol.180.2.739. [DOI] [PubMed] [Google Scholar]
- 14.Browne CD, del Nagro CJ, Cato M, Dengler HS, Rickert RC. Suppression of phsosphatidylinositol 3,4,5-triphosphate production is a key determinant of B cell anergy. Immunity. 2009;31:749–60. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo I, Vadas, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, Blake-Palmer K, Perisic O, Smyth D, Maes M, Fiddler C, Juss J, Cilliers D, Markelj G, Chandra A, Farmer G, Kielkowska A, Clark J, Kracker S, Debre M, Picard C, Pellier I, Jabado N, Morris JA, Barcenas-Morales G, Fischer A, Stephens L, Hawkins P, Barrett JC, Abinun M, Clatworthy M, Durandy A, Doffinger R, Chilvers ER, Cant AJ, Kumararatne D, Okkenhaug K, Williams RL, Condliffe A, Nejentsev S. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–71. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, Avery DT, Moens L, Cannons JL, Biancalana M, Stoddard J, Ouyang W, Frucht DM, Rao VK, Atkinson TP, Agharahimi A, Hussey AA, Folio LR, Olivier KN, Fleisher TA, Pittaluga S, Holland SM, Cohen JI, Oliveira JB, Tangye SG, Schwartzberg PL, Lenardo MJ, Uzel G. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, Agharahimi A, Meuwissen H, Stoddard J, Niemela J, Kuehn H, Rosenzweig SD. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34:272–6. doi: 10.1007/s10875-014-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark J, Anderson KE, Juvin V, Smith TS, Karpe F, Wakelam MJ, Stephens LR, Hawkins PT. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat Methods. 2011;8:267–272. doi: 10.1038/nmeth.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, de la Morena M, Kumararatne D, Harville TO, Hesterberg P, Koleilat M, McGhee S, Perez EE, Raasch J, Scherzer R, Schroeder H, Seroogy C, Huissoon A, Sorenson R, Katial R. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the basic and clinical immunology interest section of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2012;130:S1–24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Paris K, Sorensen RU. Assessment and clinical interpretation of polysaccharide antibody responses. Ann Allergy Asthma Immunol. 2007;99(5):462–464. doi: 10.1016/S1081-1206(10)60572-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table. Serotype-specific pneumococcal antibody levels in Patients 1 and 2.