Abstract
The role of fibroblast growth factor receptors (FGFR) in normal brain development has been well-documented in transgenic and knock-out mouse models. Changes in FGF and its receptors have also been observed in schizophrenia and related developmental disorders. The current study examines a transgenic th(tk-)/th(tk-) mouse model with FGF receptor signaling disruption targeted to dopamine (DA) neurons, resulting in neurodevelopmental, anatomical, and biochemical alterations similar to those observed in human schizophrenia. We show in th(tk-)/th(tk-) mice that hypoplastic development of DA systems induces serotonergic hyperinnervation of midbrain DA nuclei, demonstrating the co-developmental relationship between DA and 5-HT systems. Behaviorally, th(tk-)/th(tk-) mice displayed impaired sensory gaiting and reduced social interactions correctable by atypical antipsychotics (AAPD) and a specific 5-HT2A antagonist, M100907. The adult onset of neurochemical and behavioral deficits was consistent with the postpubertal time course of psychotic symptoms in schizophrenia and related disorders. The spectrum of abnormalities observed in th(tk-)/th(tk-) mice and the ability of AAPD to correct the behavioral deficits consistent with human psychosis suggests that midbrain 5-HT2A-controlling systems are important loci of therapeutic action. These results may provide further insight into the complex multi-neurotransmitter etiology of neurodevelopmental diseases such autism, bipolar disorder, Asperger’s Syndrome and schizophrenia.
Keywords: FGF receptor-1, midbrain dopamine neurons, 5-HT2A receptor, atypical antipsychotics, sensory gating, social interaction
1. Introduction
The role of fibroblast growth factor receptors (FGFR) in normal brain development has been well-documented in a number of transgenic and knock-out mouse models(Frantz et al., 1994; Saffell et al., 1997; Vaccarino et al., 1999; Pirvola et al., 2002; Shin et al., 2004; Jukkola et al., 2006; Klejbor et al., 2006; Blak et al., 2007; Grothe and Timmer, 2007) as well as by using direct brain gene transfers(Bharali et al., 2005; Stachowiak, 2009).
In humans, altered function of FGFR signaling is associated with developmental defects observed in neurodevelopmental disorders such as schizophrenia and bipolar disorder. Changes in FGF and its receptors FGFR1 have been described in the brains of schizophrenia and bipolar patients suggesting that impaired FGF signaling could underlie abnormal brain development and function associated with these disorders(Gaughran et al., 2006). Furthermore, neuroleptic treatments were shown to increase FGF-2 expression(Ovalle et al., 2001). Consistent with these metabolic findings, a large association study suggested that genetic variation of the FGF receptor increases the risk of developing schizophrenia(O'Donovan et al., 2009). Combined evidence from studies of FGF genes and FGF RNA expression and protein levels suggest that changes in the FGF system contributes to schizophrenia and possibly a wide range of related psychiatric disorders(Terwisscha van Scheltinga et al., 2009).
It is widely accepted that schizophrenia is a neurodevelopmental disease with both genetic and environmental influences and a delayed postpubertal onset(Palmer et al., 2001; Thompson et al., 2004). Multiple genetic links have been reported for schizophrenia in addition to FGFs. Those links include gene products that influence neural development via changes in FGFs, cyclic AMP enzymes, MAPK signaling pathways, or transcription co-activator complexes(De Luca et al., 2003; O'Neill et al., 2004; Millar et al., 2005; O'Donovan et al., 2009). Epigenetic mechanisms (chromatin and DNA modifications) have also been proposed to contribute to the complex patterns of inheritance and etiology of schizophrenia and related diseases(Petronis, 2004; Sharma, 2005). The most parsimonious explanation of the effects of these genes is that they influence a common neurodevelopmental pathway. The recently described Integrative Nuclear FGF Receptor1 (FGFR1) Signaling, or INFS may constitute such a pathway. Central to the INFS mechanism is the release of newly synthesized FGFR1 into the cytosol followed by nuclear translocation(Maher, 1996; Stachowiak et al., 1996; Reilly and Maher, 2001; Myers et al., 2003; Dunham-Ems et al., 2009). The INFS links several signaling mechanisms in which the schizophrenia-linked mutations have been reported to a common transcription co-activator and histone acetylase, CREB Binding Protein (CBP)(Fang et al., 2005; Stachowiak et al., 2007; Dunham-Ems et al., 2009). Transfection of the nuclear form of FGFR1 or its 23 kDa FGF-2 ligand can effectively stimulate neuronal development by brain stem/progenitor cells (Stachowiak et al., 2003; Stachowiak, 2009). Thus, changes in INFS may constitute a common pathological mechanism for the diverse schizophrenia-linked genetic defects.
There is evidence that developmental pathology in human schizophrenia involves DA neurons. Cell bodies of midbrain DA neurons are located in the mesencephalic tegmentum in the substantia nigra compacta (SNc; A9 cell group), which predominantly innervates the dorsal striatum (nigrostriatal system), and in the more medial ventral tegmental area (VTA; A10 cell group), whose projections end in the nucleus accumbens (NAc) (mesolimbic system) or the prefrontal cortex (PFC) (mesocortical system). In patients with schizophrenia and the related Asperger syndrome not subjected to neuroleptic treatments, there is a significant decrease in the volume of the SN area (−21%) and reduction of mean nerve cell volume in the SNc (−15% ) and VTA (−17%)(Bogerts et al., 1983). Altered dendritic morphology(Ikemoto, 2008) and synaptic contacts(Kolomeets and Uranova, 1999) of DA neurons have also been reported in the schizophrenia brain. Maurici suggested an injury to (Na+/K+) ATPase at the SN as a possible pathophysiological mechanism in psychoses(Maurizi, 1984). Changes in SN echogenictiy were found in human patients and this appears to be a trait specific to the disease rather than a result of neuroleptic treatment(Jabs et al., 2001). Some forms of psychosis appear to be associated with an anomaly of the nigrostriatal system(Leonhard, 1999). As stated by Jabs et al(Jabs et al., 2001), these findings urgently require further investigation to illuminate the role of the SN in psychotic disorders.
In this context we have developed a transgenic mouse model(Klejbor et al., 2006), a th(tk-)/th(tk-), which results from hypoplastic development of DA neurons induced by a dominant negative mutant of FGFR1, tyrosine kinase-deleted FGFR1(TK-)(Klejbor et al., 2006). Restriction of mutant expression to catecholamine-neurons containing brain regions(Klejbor et al., 2006) is achieved by fusion with the tyrosine hydroxylase (TH) promoter. Similar to human schizophrenia and related disorders such as Asperger’s Syndrome(Bogerts et al., 1983), there was a reduction of size and density of SNc and VTA TH-immunoreactive neurons(Klejbor et al., 2006). Paradoxically, there were increases of DA and its metabolites in terminal regions of the nigrostriatal DA system, consistent with increased DA transmission. In contrast, there was a reduction of these measures present in the prefrontal cortex. These findings are consistent with human PET studies(Meyer-Lindenberg et al., 2002) and postmortem evidence of increased subcortical DA synthesis in schizophrenia(Toru et al., 1982; Mueller et al., 2004). How DA neuronal hypoplasia could paradoxically lead to DA hypertransmission in the subcortical basal ganglia with concurrent DA hypotransmission in the frontal cortex remains unknown. Behaviorally, the anatomical and chemical changes were coupled with disruption of sensory processing in th(tk-)/th(tk-) mice, correctable by typical antipsychotic drug (TAPD), flupenthixol, a D2 receptor antagonist.
The development of specific neurons is directed by its genetic blueprint along with signals from co-developing cells. Accordingly, genetic or external insults which affect a specific system could also have a broader indirect effect on other neuronal systems forming rewired neuronal networks with new functional properties. These changes could underlie pathologies observed in neurodevelopmental disorders such as autism and adult psychoses. Although in th(tk-)/th(tk-) mice the expression of FGFR1 is targeted by the TH gene promoter specifically to catecholamine producing neurons, it is possible that effects may also be observed in other neuronal systems. One such a system is formed by serotonin producing neurons of the pontine raphe nuclei. Throughout brain development there is a competition between 5-HT and DA for brain target sites and evidence exists that a dynamic relationship between the two systems persists into adulthood. Furthermore, it has been postulated that altered function of 5-HT neurons plays a major role in human psychosis and may underlie the therapeutic effects of atypical antipsychotic drugs (AAPD)(Lieberman et al., 1998).
The current work extends our examination of the th(tk-)/th(tk-) model in two directions. We document that maldevelopment of DA neurons is accompanied by hypertrophic changes in the 5-HT neuronal system and we further elaborate on the consequences of this transgenic manipulation by examining behavioral assays of sensory gating, social interaction and other behavioral deficits. We also document the palliative effects of anti-serotonergic atypical antipsychotic drugs (AAPD) and a specific 5-HT2A antagonist on abnormal sensory gating and social behaviors. The th(tk-)/th(tk-) mouse model offers a new mechanism of action for simultaneous 5-HT2A and D2 antagonism in treating symptoms mimicking those found in schizophrenia and related human psychotic disorders.
2. Methods
2.1 Drugs
Clozapine was obtained from RBI/Sigma (St. Louis, MO). Quetiapine hemifumarate was purchased from Toronto Research Chemicals (Toronto, On, Canada). Clozapine and Quetiapine were dissolved with 5 µl of 20% acetic acid and diluted with phosphate buffered saline (PBS). M100907(Bishop et al., 2004) was dissolved in 0.9% saline. Drug doses were calculated as free base. Clozapine, quetiapine, M100907 or vehicle control were injected intraperitonealy 30 minutes before testing in all behavioral experiments. All injections were given at a volume of 100–200 ul/ 30 g of body weight.
2.2 Animals
Homozygous transgenic th(tk-)/th(tk-) mice were described in(Klejbor et al., 2006). These mice express FGFR1(TK-) fused to rat TH gene promoter (4.5 kb). The progenies were screened for the presence of the transgene by PCR amplification of tail DNA(Klejbor et al., 2006). Mice homozygous for TH-FGFR1(TK-) transgene and control mice (transgene free) lines were derived from parental BCF1 (C57BL/10J/C3H/HeJ) heterozygous mice. The lines showed stable behavioral differences in all generations investigated(Klejbor et al., 2006). Mice used in all specific experiments were selected from multiple litters. Mice (males and females) were singly housed at least four weeks before testing began and throughout testing on a light:dark cycle of 12:12 h with free access to food and water. All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with approval from the University at Buffalo IACUC.
2.3 Prepulse Inhibition (PPI) and Startle Response
Startle response was measured using two chambers (SR-LAB, San Diego Instruments, San Diego, CA) as described in (Klejbor et al., 2006). All PPI test sessions consisted of startle trials (pulse-alone), prepulse trials (prepulse + pulse), and no-stimulus trials (nostim). The pulse-alone trial consisted of a 40 ms 120 dB pulse of broad-band noise. PPI was measured by prepulse + pulse trials that consisted of a 20 msec noise prepulse, 100 msec delay, then a 40 msec 120 dB startle pulse (120 msec onset-to-onset interval). The acoustic prepulse intensities were 4, 8, and 16 dB above the 68 dB background noise (i.e., 72, 76, and 84 dB). The nostim trial consisted of background noise only. The test session began and ended with five presentations of the pulse-alone trial; in between, each acoustic or nostim trial type was presented 10 times in a pseudorandom order. There was an average of 15 sec (range, 12–30 sec) between trials. For drug studies, mice were placed into the startle chambers 30 minutes after each injection, and a 68dB background noise level was presented for a 10 min acclimation period and continued throughout the test session. The amount of PPI was calculated as a percentage score for each acoustic prepulse trial type: % PPI = 100 −{[(startle response for prepulse + pulse)/(startle response for pulse-alone)] × 100}. The magnitude of the acoustic startle response was calculated as the average response to all of the pulse-alone trials, excluding the first and last blocks of five pulse-alone trials presented.
The developmental study (Figure 3A) was performed using different cohorts of 6 control and 6 th(tk-)/th(tk-) mice tested repetitively for PPI at intervals between 2 and 8.5 months of age. Each cohort contained 3 males and 3 females of each genotype. In drug studies, PPI was tested in 3 experimental cohorts (Figure 3B–D). Within each group, all control and transgenic animals were tested with injections of vehicle and each drug dose. Mice were tested twice per week. A vehicle injection was administered before one test session and drug before the second test session. The order of the vehicle and drug injection days were changed each week. Mice were tested once with each drug dose. PPI and startle magnitude on vehicle test days were examined to determine if these measures changed with repeated testing. Since no effects of repeated testing or age were observed the average of the vehicle were used for analysis.
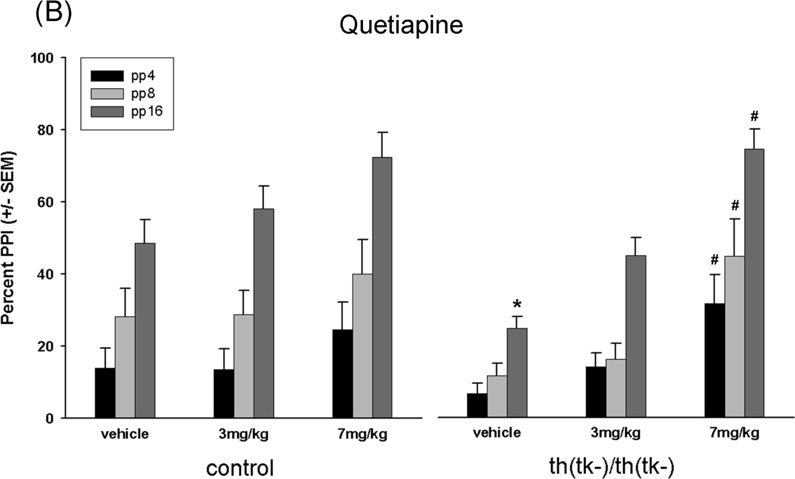
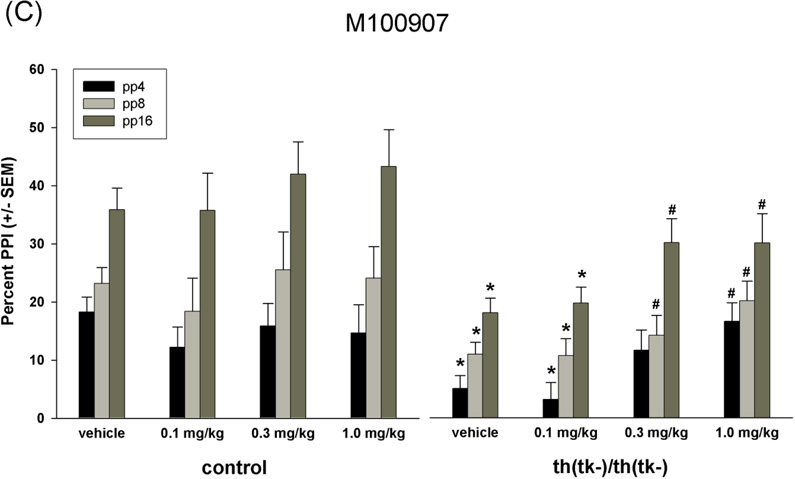
Figure 3.
Percent prepulse inhibition. (A–C) Effects of clozapine, quetiapine and M100907 on PPI in aged-matched adult control and th(tk-)/th(tk-) mice. All animals were repeatedly tested between 7 and 12 months of age as described in Methods. (A) n = 16 control and n = 16 th(tk-)/th(tk-) mice injected with clozapine at 0.5 mg/kg or 3.0 mg/kg, (B) n = 8 control and n = 8 th(tk-)/th(tk-) mice injected with quetiapine at 3.0 mg/kg or 7.0 mg/kg, (C) n = 8 control and n = 11 th(tk-)/th(tk-) mice injected with M100907 at 0.1 mg/kg, 0.3 mg/kg, or 1.0 mg/mg. *Significant difference from control group receiving same treatment at individual stimulus intensity (post hoc; p<0.05 after significant main effect of genotype F value; ANOVA). #Significant difference within genotype from vehicle group at individual stimulus intensity (post hoc; p<0.05 after significant main effect of drug F value; ANOVA). There was a treatment x genotype interaction with clozapine treatment at 3.0 mg/kg (p<0.05) and with M100907 at 0.3 and 1.0 mg/kg (p<0.05). (D) Age-dependent changes in PPI in control (n = 12) and th(tk-)/th(tk-) mice (n=12). *Significant difference between control and th(tk-)/th(tk-) mice at individual stimulus intensity (post hoc; p<0.05 after significant main effect of genotype F value; ANOVA).
Clozapine was injected at doses of 0.5 mg/kg and 3.0 mg/kg in 8 control (5 male and 3 female) and 11 th(tk-)/th(tk-) mice (7 male and 4 female), quetiapine at 3.0 mg/kg and 7.0 mg/kg in 8 control (5 male and 3 female) and 8 th(tk-)/th(tk-) mice (6 male and 2 female) and M100907 at 0.1 mg/kg, 0.3 mg/kg and 1.0 mg/kg in 16 control (12 male and 4 female) and 16 th(tk-)/th(tk-) mice (11 male and 5 female). All animals tested were between 7 and 12 months of age.
2.4 Social behavior, autogrooming, and open field activity
Social behavior was tested using a variant of the resident-intruder paradigm in which a stimulus animal was introduced into the subject’s homecage for three minutes. Prior to testing the subjects’ home cages were not changed for at least four days to allow them to establish the cage as their territory. Female stimulus animals were singly-housed, randomly cycling C57Bl/6Js (Jackson Laboratories, Bar Harbor, ME). Each subject was tested with a different stimulus animal and each stimulus animal was used only once per testing day. Male stimulus animals were singly-housed C57Bl/6Js (Jackson Laboratories, Bar Harbor, ME). Each subject was tested with a different stimulus animal and each stimulus animal was used only once per testing day. A stimulus female was placed into the cage of control and th(tk-)/th(tk-) subjects. After three minutes, the stimulus female was removed. Thirty minutes later a stimulus male was placed into the subject’s homecage. After three minutes, the stimulus animal was removed. All testing occurred in the dark-phase (the active phase) of the light cycle under red-light illumination. The interaction was videotaped from the side using the Nightshot feature on a Sony video camera (DRV-120, Sony Corporation). The behavior of the subject was quantified from the videotape using the Observer Mobile (Noldus Information Technologies, Sterling, VA). The number of bouts observed and the amount of time engaged in the following behaviors was measured: general social contact (contact with the stimulus animal, sniffing (both anogenital and non-anogenital contact), and non-social behavior (autogrooming)). The experimenter scoring the behavior was blind to the genotype and treatment of the subjects. To test open-field activity, the subjects were removed from their home cage and placed alone into a clean Plexiglas open-field testing arena (45cm × 45 cm × 25 cm) for a ten minute testing session, after which they were returned to their home cage. The test was videotaped from above using a SONY TRV-350 Handycam videocamera using the nightshot feature. Movement was analyzed in detail using the Clever Sys. Inc. system.
These behaviors were measured in one cohort of 8 control and 8 th(tk-)/th(tk-) male mice. On testing days, animals were subjected to three experiments: a social behavior test with a female, a social behavior test with a male, and an open-field test. There was a 30-minute interval between each test. Experiments began at 7 months of age for all animals. Mice were initially tested with vehicle injections, following by administration of M100907 at 1.0 mg/kg one week later. After three months the same animals were tested with clozapine injections at 1.0 mg/kg, vehicle injections (one week later), and quetiapine at 7.0 mg/kg (after an additional week). The performance of vehicle injected th(tk-)/th(tk-) mice and nontransgenic controls on all tests remained unchanged between 7 (Figure 4B) and 10 months of age (Figure 4A).
Figure 4.
Effects of (A) M100907 (1.0 mg/kg) and (B) clozapine (1.0 mg/kg) and quetiapine (7.0 mg/kg) on social investigation, autogrooming, and open-field activity in control (n =8) and th(tk-)/th(tk-) mice (n=7). * Significant difference from control group with same drug treatment (ANOVA; p<0.05). #Significant difference within genotype from vehicle treatment (ANOVA; p<0.05).
2.5 Immunocytochemistry and stereology of 5-HT expressing fibers
All adult (12 moth old) mice [6 control and 6 th(tk-)/th(tk-)] were deeply anesthetized (80 mg of Nembutal/kg) and perfused transcardially with 0.9% solution of NaCl with heparin, followed by 4% paraformaldehyde solution in 0.1M phosphate buffer (pH 7.4). The brains were processed and coronal 40-µm-thick sections were cut as described in (Klejbor et al., 2006). The free floating sections were blocked with 10% normal goat serum (NGS) containing 0.3% Triton X-100 for 1 hour and then incubated with anti-5-HT rabbit polyclonal primary antibody (Sigma; 1:1000) for 48 hours in 4oC. After multiple rinses in PBS, sections were incubated for 2–3 hours, at room temperature with the Cy3-conjugated goat anti-rabbit (Jackson ImmunoResearch; diluted 1: 600) appropriate secondary antibodies: The chosen set of brain sections of both experimental as well as control groups underwent negative control with omission of primary antibody. Examined structures included: the ventral tegmental area (VTA) and substantia nigra (SN) its subdivisions: the interfascicular nucleus (IF), the parabrachial pigmentosus nucleus (PBP), the paranigral nucleus PN, the rostral linear raphe nucleus (RLi); compact and reticular parts of the substantia nigra (SNC and SNR, respectively). Thirty sections per each VTA or SN nucleus/mouse (−4.8 to −5.6 mm relative to Bregma (33) were examined with fluorescent microscope (BX-51, Olympus, Japan) and confocal system (Radiance 2100 , Bio-Rad, UK), equipped with Krypton/Argon laser and mounted on the light microscope Eclipse 600 (Nikon, Japan). The confocal laser scanning microscopy images (CLSM) were obtained using x40 and x60 oil immersion objective lenses of N.A.=1.3 and 1.4, respectively. The optimal iris was used for each magnification. For the reconstruction of the image analysis program Laser Sharp 2000 v.4.0. (Bio-Rad; UK) was used. In each case only sections completely stained with fluorescence were taken into account.
Quantitative stereological analysis of the 5-HT fiber densities was performed using the C.A.S.T. Grid system (Olympus, Denmark) as described in(West et al., 1991) . All fibers density measurements were conducted blindly with respect to the genotype.
2.6 5-HT and 5-HIAA assay
The mice [5 controls and 5 th(tk-)/th(tk-)] were analyzed at each age (1 or 12 months). Mice were sacrificed and the analyses were performed as described in(Klejbor et al., 2006). The brain anatomical regions were isolated using punching needles as previously(Klejbor et al., 2006). Tissue samples were obtained bilaterally (striatum, frontal cortex, nucleus accumbens, VTA, and SN). No differences in 5-HT and 5-HIAA levels between hemispheres were observed and the results were combined. The hypothalamus and raphe were isolated and analyzed as single medially located samples. For each mg of tissue collected, 4 to 20 µL of 50 mM perchloric acid containing 100 µM metabisulfite and 500 nM DHBA as an internal standard was added. The samples were injected through a Suppleco Discovery C18 reverse phase 15 cm column with a 2 cm guard column. Detection was done with ESA Coulochem II with ESA computer analysis software.
2.7 Statistical analysis
In PPI experiments, overall repeated measures ANOVAs were used at each drug dose with genotype, drug treatment and prepulse intensity level as the factors. For brevity, the main effects of prepulse intensity (which were always significant) are not discussed. Separate repeated measures ANOVAs were conducted within each genotype using drug treatment as a factor. Post-hoc ANOVAs were used to determine treatment effects at individual prepulse intensities only after a significant main effect of genotype or drug treatment. For other behavioral experiments, means of control and th(tk-)/th(tk-) mice were compared within each trial using a one-way ANOVA followed by a Student-Newman Keuls post-hoc analysis. Means between trials (e.g. behavior after injection with vehicle and behavior after injection with drugs) were compared using a two-way ANOVA using drug and genotype as the factors. A Student-Newman Keuls post-hoc analysis was run if the ANOVA revealed an effect.
The results of the stereological 5-HT fiber counting and 5-HT and 5-HIAA HPLC assays were analyzed using one-way ANOVA followed by LSD post-hoc test.
3. Results
3.1 Postnatal developmental changes in 5-HT/5-HIAA and serotonergic hyperinnervation of the ventral midbrain in th(tk-)/th(tk-) mice
Evidence has shown that there is a co-developmental relationship between DA and 5-HT systems in the brain(Bruno et al., 1987; Rodriguez-Pallares et al., 2003). To determine if the FGFR1(TK-) transgene-induced DA neuronal hypoplasia in th(tk-)/th(tk-) mice leads to alteration of 5-HT neurons, brain regions that shared both DA and 5-HT neurotransmitter systems were analyzed for 5-HT and 5-HIAA. These regions included terminal fields (striatum, frontal cortex, nucleus accumbens, hypothalamus) and the somal origin (VTA, SN, dorsal raphe nucleus) of the two systems. In one month old animals, there were no significant differences between control and th(tk-)/th(tk-) mice in 5-HT and 5-HIAA levels in any brain region examined (Figure 1A,B). However in adult transgenic mice, 5-HT levels were significantly greater (by approximately 70%) in the SN relative to controls (Figure 1A), and in the VTA there was a similar elevation that approached but did not achieve statistical significance. The variability of 5-HT in needle punched samples obtained from the VTA region may reflect heterogeneous distribution of 5-HT terminals in individual nuclei of the VTA (see below). In the VTA but not the SN, 5-HIAA levels were significantly greater in th(tk-)/th(tk-) mice (Figure 1B). The difference in 5-HIAA between VTA and SN may reflect different modes of serotonergic transmission (see discussion). In contrast to midbrain nuclei, 5-HT and 5-HIAA levels were significantly reduced in the pontine raphe of th(tk-)/th(tk-) mice (Figure 1A,B). These differences again were only observed in adult animals. Thus, as with the changes in DA and DA metabolites reported previously(Klejbor et al., 2006), there appears to be a post natal developmental component to the alterations of the serotonergic system in th(tk-)/th(tk-) mice. There were no differences in 5-HT and 5-HIAA levels in the striatum, nucleus accumbens, frontal cortex, or hypothalamus.
Figure 1.
HPLC analyses of 5-HT (A) and 5-HIAA (B) in brain regions of 1 and 12 months old control (n=5) and homozygous th(tk-)/th(tk-) mice (n=5). *Significant differences between the same age control and transgenic mice (ANOVA, LSD p<0.05).
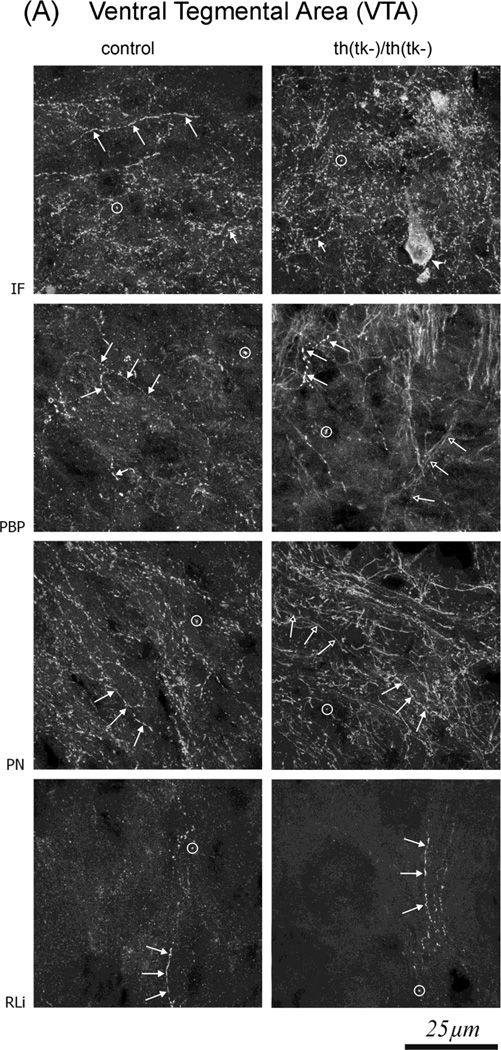
In order to determine the cellular mechanisms underlying the changes in 5-HT and 5-HIAA, innervation of the midbrain nuclei by 5-HT-immunoreactive (ir) fibers was analyzed in adult mice using quantitative immunohistochemistry. In both the VTA (Figure 2A) and SN (Figure 2B), 5-HT-ir fibers were predominantly smooth with small varicosities and thus similar to the D-type produced by the dorsal raphe nucleus(Fibiger and Miller, 1977; van der Kooy and Hattori, 1980). All VTA subnuclei [the interfascicular nucleus (IF), the parabrachial pigmentosus nucleus (PBP), the paranigral nucleus PN and the rostral linear raphe nucleus (RLi)] showed the presence of 5-HT fibers. In the IF and RLi nuclei we found no significant differences in fiber density between control and th(tk-)/th(tk-) mice (Figure 2A, Table 1). In contrast, quantitative analysis showed significant increases in 5-HT fiber density in PBP (2-fold) and in PN (1.5-fold) in th(tk-)/th(tk-) mice compared to controls. In these nuclei we observed long, smooth (thin) fibers without varicosities, present only in the transgenic mice. In the SNr (but not in the SNc), there was a significant (1.7-fold) increase in the density of 5-HT-ir fibers in transgenic mice relative to controls (Figure 2B, Table 1).
Figure 2.
5-HT immunoreactive (5-HT-ir) fluorescent cellular elements neurons in midbrain nuclei of 12 months (PD 360) old mice. Brain sections were stained with anti-5-HT polyclonal Ab and with Cy3 conjugated 2ndary Ab. (A) Examples of 5-HT immunostaining of individual VTA nuclei: parabrachial pigmentosus (PBP), interfascicular (IF), rostral linear raphe (RLi) and paranigral (PN) of 6 month old mice. (B). Examples of 5-HT immunostaining of substantia nigra pars compacta (SNc) and pars reticulate (SNr) are shown . Sections within the row are through the same levels of the SNc or VTA nuclei. The 5-HT-ir fibers innervating the VTA (A) or SN (B) are predominantly smooth with small varicosities and thus similar to the D-type produced by the dorsal raphe nucleus(Fibiger and Miller, 1977; van der Kooy and Hattori, 1980). Only few type M-fibers with short, thick, coarse branches and large spherical varicosities, typically produced by the medial raphe nucleus, were found. The RLi contains single 5-HT-ir fibers that follow a vertical course and relatively small numbers of the 5-HT-ir puncta. The IF has a dense networks of varicose, short, small diameter fibers and a high density of the 5-HT–ir puncta. Also, single 5-HT-ir cells were found in th(tk-)/th(tk-) mice. The PBP nucleus has a dense network of varicose 5-HT-ir fibers that follow an irregular course. In addition in the th(tk-)/th(tk-) but not in controls there are long, smooth (thin) fibers without varicosities. The PN has a dense network of long 5-HT-ir fibers with small varicosities, numerous puncta and smooth fibers without varicosities. In the SNc both 5-HT-ir fibers and puncta are present. (B) There were more 5-HT-ir fibers in the SNr than SNc. In th-tk(-)/th-tk(-) mice the density of 5-HT-IR fibers was increased in PBP and PN VTA nuclei and in SNr. Labels – circle – 5-HT immunoreactive puncta; Long filled arrow – long 5-HT immunoreactive fibers with large and round varicosities; short filled arrow –short 5-HT immunoreactive fibers with ; long empty arrow – long 5-HT immunoreactive fibers without varicosities; filled arrowhead – 5-HT immunoreactive cells. The results of stereological counts of fiber densities are shown in Table 1.
Table 1.
Density of 5-HT terminals in substantia nigra compacta (SNc), reticulata (SNr) and in ventral tegmental area (VTA) nuclei: parabrachial pigmentosus (PBP), interfascicular (IF), rostral linear raphe (RLi) and paranigral (PN) of adult 6 month old mice. Numbers show mean + SEM. Five mice in each group were used.
| Mice group | nucleus | terminal density (No/mm2) |
|---|---|---|
| control | SNc | 2.629+0.742 |
| control | SNr | 3.526+0.420 |
| th(tk-)/th(tk-) | SNc | 2.484+0.052 |
| th(tk-)/th(tk-) | SNr | 5.916+0.257a |
| control | VTAPBP | 2.334+0.296 |
| control | VTAIF | 5.301+0.468 |
| control | VTARLi | 1.151+0.376 |
| control | VTAPN | 4.257+0.358 |
| th(tk-)/th(tk-) | VTAPBP | 5.487+0.624 b |
| th(tk-)/th(tk-) | VTAIF | 5.968+0.467 |
| th(tk-)/th(tk-) | VTARLi | 0.753+0.214 |
| th(tk-)/th(tk-) | VTAPN | 6.067+0.284 a |
Difference between control and th(tk-)/th(tk-) within the same nucleus:
p≤0.005;
p<0.00001 (ANOVA, LSD).
3.2 Deficits in sensory gating appear in adulthood and are reversed by atypical antipsychotic drugs (AAPD) and specific 5-HT2A antagonist M100907
In our previous report we showed that increased subcortical DA output in adult th(tk-)/th(tk-) mice was accompanied by elevated acoustic startle response and reduced PPI, both reversed by typical antipsychotic (TAPD) and DA antagonist α-fluphentixol(Klejbor et al., 2006). In the current study PPI was tested repeatedly in 35 th(tk-)/th(tk-) and 32 nontransgenic control adult mice between 7 and 12 months of age. Vehicle injected th(tk-)/th(tk-) mice across the study (3.59+1.57 at pp4, 13.19+1.66 at pp8, and 29.67+2.77 at pp16) displayed reduced PPI compared to controls (20.62+2.86 at pp4, 31.23+3.24 at pp8, and 51.13+3.25 at pp16) (p<0.001; repeated measures ANOVA). Startle response for vehicle injected animals was on average 49.49%+3.76 higher (p<0.02; one way ANOVA) in th(tk-)/th(tk-) mice.
The effects of atypical antipsychotics (AAPD) clozapine and quetiapine were tested separately in two cohorts (Figure 3A and B, respectively). Clozapine at 3.0 mg/kg and quetiapine at 7.0 mg/kg significantly improved PPI in th(tk-)/th(tk-) while lower doses had no effect. Neither drug affected control mice.
While clozapine and quetiapine block both 5-HT and DA receptors there is evidence that these drugs may also interact with other neurotransmitter receptors(Nasrallah, 2008). If 5-HT hyperinnervation underlies the sensory gating deficits observed in th(tk-)/th(tk-) mice then blockade of 5-HT receptors should reverse PPI deficits in transgenics and have no effect on control mice. Specific 5-HT2A antagonist M100907 significantly improved PPI in th(tk-)/th(tk-) mice at doses of 0.3 mg/kg and 1.0 mg/kg while the performance of control mice were not changed at any dose (Figure 3C). The effects of drugs on magnitude of the startle response are shown in Table 2. In th(tk-)/th(tk-) mice all drugs tested at all doses (except clozapine at 0.5 mg/kg) significantly reduced startle magnitude, while in controls only the highest dose of clozapine (3.0 mg/kg) had an effect.
Table 2.
Average magnitude of the startle response for control and th(tk-)/th(tk-) mice shown as a percentage of the control vehicle amplitude in each separate cohort
| Cohort 1: Clozapine | startle response |
| control vehicle | 100.00+17.22 |
| control 0.5 mg/kg | 107.25+18.58 |
| control 3.0 mg/kg | 52.03+18.13# |
| th(tk-)/th(tk-) vehicle | 134.89+16.09 |
| th(tk-)/th(tk-) 0.5 mg/kg | 111.99+11.23 |
| th(tk-)/th(tk-) 3.0 mg/kg | 91.94+13.67# |
| Cohort 2: Quetiapine | startle response |
| control vehicle | 100.00+ 9.38 |
| control 3.0 mg/kg | 118.36+ 26.77 |
| control 7.0 mg/kg | 89.21+15.98 |
| th(tk-)/th(tk-) vehicle | 135.06+ 9.39 |
| th(tk-)/th(tk-) 3.0 mg/kg | 107.87+13.45# |
| th(tk-)/th(tk-) 7.0 mg/kg | 64.22+14.43# |
| Cohort 3: M100907 | startle response |
| control vehicle | 100.00+14.06 |
| control 0.1 mg/kg | 95.11+13.23 |
| control 0.3 mg/kg | 89.07+12.36 |
| control 1.0 mg/kg | 91.85+10.73 |
| th(tk-)/th(tk-) vehicle | 143.77+14.87 |
| th(tk-)/th(tk-) 0.1 mg/kg | 101.74+8.70# |
| th(tk-)/th(tk-) 0.3 mg/kg | 101.91+10.91# |
| th(tk-)/th(tk-) 1.0 mg/kg | 109.63+9.76# |
Significant main effect within genotype of treatment dose: p<0.05 (ANOVA, LSD).
Given the delayed development of neurochemical changes (Figure 1) we determined the onset of sensory gating deficits in th(tk-)/th(tk-) mice by testing prepulse inhibition (PPI) at time intervals between 2 and 8.5 months of age. There were no significant differences between the genotypes until the 4 month group (Figure 3D). At 6 and 8.5 month groups (Figure 3D) there were significant reductions in PPI in th(tk-)/th(tk-) mice at each prepulse intensity.
3.3. Deficits in social interactions are reversed by the inhibition of 5-HT2A receptors
In addition to impaired sensory gating and hallucinations, which are associated with increased subcortical DA transmission, schizophrenia patients manifest negative symptoms such as social withdrawal and flattened affect. To determine if th(tk-)/th(tk-) mice exhibit deficits that model negative symptoms we analyzed social behavior of socially experienced male th(tk-)/th(tk-) mice with male and female stimulus animals. Vehicle injected transgenics engaged in significantly less stimulus investigation than controls (Figure 4A,B). Other measures of sociability (e.g. anogenital investigation, contact time) had the same pattern and are not shown. By contrast, there were no differences between control and th(tk-)/th(tk-) mice in a non-social behavior, auto-grooming (Figure 4A,B). In the open-field test, the th(tk-)/th(tk-) mice traveled a greater distance in the center as well in the periphery of the arena compared to control mice (Figure 4A,B). Also, th(tk-)/th(tk-) mice spent approximately 70% more time in the center and 10% less time in the periphery than controls (not shown).
Clozapine [3.0 mg/kg, the dose which restored PPI in th(tk-)/th(tk-) mice (Figure 3B)] had no significant effect on social behavior or locomotion, but reduced autogrooming in both genotypes during the pair-test with a male stimulus animal (Figure 4B). Unlike clozapine, quetiapine ameliorates social deficits in patients with schizophrenia(Zhong et al., 2006). In our studies, after quetiapine treatment (7.0 mg/kg) there was no longer a significant genotypic difference in social investigation time (Figure 4B). This was due to a significant increase in time spent interacting with the stimulus animal in transgenic mice after quetiapine treatment, which had no effect in control animals. Auto-grooming and motor activity were unaffected by quetiapine in both genotypes.
Serotonergic hyperinnervation of VTA nuclei suggests that blockade of 5-HT input could normalize mesocortical DA transmission and consequently correct behavioral deficits associated with altered activity of this system. In order to test this prediction we injected mice with the specific 5-HT2A antagonist M100907. M100907 significantly increased the amount of time th(tk-)/th(tk-) mice spent investigating the stimulus animal but had no effect on investigation time in control animals (Figure 4A). Other behavioral measures (autogrooming and open field activity) were unaffected by M100907.
4. Discussion
It is now widely accepted that schizophrenia and related psychotic disorders are associated with developmental aberrations at multiple levels of the neuroaxis including midbrain, cortical and hippocampal remodeling(Bogerts et al., 1983; Weinberger, 1987; Carlsson, 2006). How this abnormal development results in deranged dopaminergic function remains contentious(Weinberger, 1987; Kellendonk et al., 2006). The current th(tk-)/th(tk-) rodent model addresses these considerations by allowing for the expression of a genetic abnormality within a developmental context. The hypoplasia and reduced density of midbrain DA neurons associated with expression of the dominant negative FGFR1(TK-) transgene emphasizes the importance of FGF receptor signaling in DA neurons(Losonczy et al., 1987; Grothe and Timmer, 2007). The magnitude of the observed changes depended upon the dosage of the FGFR1(TK-) transgene(Klejbor et al., 2006).
In a variety of studies the diverse genetic manipulations of FGF/FGFR have been shown to impair development affected neurons in a similar fashion, and their effects have been observed in multiple mice lines and strains (Frantz et al., 1994; Saffell et al., 1997; Vaccarino et al., 1999; Pirvola et al., 2002; Shin et al., 2004; Jukkola et al., 2006; Klejbor et al., 2006; Blak et al., 2007). This firmly establishes that the observed neurodevelopmental and behavioral deficits reflect altered FGFR signaling rather than stochastic genome insertional effects. The essential role of FGFR1 in neuronal development was further documented in vitro using transient transfections of episomal FGFR1(TK-) DNA(Stachowiak et al., 2003). For instance, in human neuronal progenitor cells transfection of FGFR1(TK-) or nucleus targeted FGFR1(SP-/NLS)(TK-) blocked neuronal differentiation (Stachowiak et al., 2003; Fang et al., 2005). In contrast, transfection of the tyrosine kinase containing nuclear form of FGFR1 or nuclear FGFR1 ligand into brain stem cells in vitro(Stachowiak et al., 2003) or in vivo using nanoparticle-mediated gene transfers (Stachowiak, 2009) effectively stimulated neuronal development.
DA neuronal hypoplasia and hyperactivity observed in th(tk-)/th(tk-) mice models changes reported in patients with schizophrenia and Asperger’s Syndrome(Bogerts et al., 1983). Here we showed that FGFR1(TK-)-induced underdevelopment of DA neurons is associated with abnormalities in other neuronal systems, contributing to the phenotype that mimics the complex neurodevelopmental and multi-faceted human schizophrenia disease. Specifically, we show that the hypoplastic development of DA neurons leads to hyperinnervation by the serotonin system and impaired sensory gating which can be reversed by AAPD and a specific 5-HT2A antagonist. The th(tk-)/th(tk-) mice also display social neglect, a deficit typically found in schizophrenia, also alleviated by anti-serotonergic drugs.
Our quantitative anatomical analyses showed that th(tk-)/th(tk-) mice had significantly greater numbers of 5-HT fibers in the PN and PBP nuclei of the VTA, which project principally to the prefrontal cortex and nucleus accumbens, as well as within the SNr region, when compared to the control mice. The morphology of the hyperinnervating serotonergic fibers was consistent with a dorsal raphe origin(Fibiger and Miller, 1977). These axons formed dense networks of 5-HT-immunoreactive fibers with numerous varicosities, some having different form than observed in control mice.
The invasion of 5-HT terminals was corroborated by increased levels of 5-HT in the ventral midbrain regions in th(tk-)/th(tk-) mice. Increased levels of 5-HIAA in the VTA and lack of such an increase in the SN could reflect different modes of 5-HT action - synaptic in the VTA and non-synaptic in the SNc and SNr (in the SNr 5-HT terminals are engaged in a volume transmission whereby in the SNc 5-HT diffuses through extracellular space to reach receptors on nonadjacent cells, including DA neurons(Bunin and Wightman, 1999)). Neither 5-HT nor 5-HIAA was increased in the terminal fields of DA neurons in the striatum, nucleus accumbens or frontal cortex. Thus, DA neurons may be affected by increased 5-HT tone in the midbrain nuclei rather than indirectly via serotonergic control of telencephalic projections into the ventral tegmentum.
The mechanism underlying sprouting of 5-HT terminals into the ventral midbrain is unknown. Concomitant reduction of raphe 5-HT levels suggests that 5-HT neurons may undergo dendritic pruning. It is unlikely that serotonergic hyperinnervation of the ventral midbrain is due to a direct transgenic effect on the 5-HT cells itself because TH promoter fusion targeted FGFR1(TK-) expression to catecholaminergic cells. In neurodevelopment there is a competition between DA and 5-HT neurons for their generation from stem/precursor cells(Rodriguez-Pallares et al., 2003) and 5-HT hyperinnervation is seen in situations where there is a loss of DA cells or DA function(Bruno et al., 1987). Thus, 5-HT hyperinnervation may be a general neurodevelopmental response to hypoplasia of DA cells - a phenomenon that could also occur also in human schizophrenia.
Although DA is of central importance in schizophrenia, evidence also exists that 5-HT has a role in the disorder(Lieberman et al., 1998; Harrison, 1999). Pharmacologically, many hallucinogens are 5-HT2A agonists. Furthermore, so-called atypical antipsychotic drugs (AAPD) have efficacy in treating both the positive and negative symptoms of schizophrenia and are effective in autism(Rausch et al., 2005) through strong 5-HT2A and weaker D2 receptor antagonism(Meltzer et al., 2003; Gray and Roth, 2007). Increased levels of 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) have been reported in human schizophrenia patients (Hansson et al., 1994) and an observed increase in the ratio of 5-HT to DA metabolites predicts a favorable response to clozapine treatment(Pickar et al., 1994). Unmedicated patients with paranoid schizophrenia in the early phase of disease have higher 5-HIAA levels in the CSF than healthy controls (Rimon et al., 1971; Bartfai et al., 1983). Schmidt et al (1993) suggested that schizophrenics who respond to AAPD and not to TAPD may exhibit excessive serotonergic activity(Schmidt et al., 1993). It has also been suggested that first episode subjects with schizophrenia, since they have a strong response to selective 5-HT2A antagonism, have enhanced central serotonin tone as also suggested by increased fenfluramin-induced prolactine response(Abel et al., 1996).
The locus of increased 5-HT activity in brains of schizophrenia patients is unknown. There is a general consensus that 5-HT2A antagonistic therapeutic action of AAPD is through an influence on DA function(Lieberman et al., 1998; Meltzer et al., 2003; Gray and Roth, 2007). 5-HT neurons originating in dorsal and main raphe nuclei innervate DA terminal regions (e.g. prefrontal cortex and striatum). The strongest 5-HT innervation is in the ventral midbrain structures, including the VTA and SN(Moukhles et al., 1997), suggesting that observed changes in CSF 5-HIAA levels in schizophrenia could reflect alterations that occur in 5-HT terminals in this brain region.
To date, very little investigation has been made of 5-HT and its receptors in schizophrenia brainstem because there was little evidence pointing in this direction. Similar to observations of rat brain(Hamada et al., 1998; Cornea-Hebert et al., 1999), intense 5-HT2A receptor immunoreactivity was found in human VTA and SN, and co-localized on TH-immunopositive neurons(Ikemoto et al., 2000). Although the ventral midbrain has the highest density of 5-HT terminals in the brain, no postmortem schizophrenia studies have been published on 5-HT innervation of the DA nuclei. High resolution PET, anatomic MRI definition and PET-MRI registration is currently available for addressing the presence of these 5-HT brainstem changes in schizophrenia(D'Ardenne et al., 2008) and would test the predictions made by the th(tk-)/th(tk-) model.
Reduction in prepulse inhibition (PPI) stemming from excessive subcortical DA or 5-HT neurotransmission has been used as a model of positive schizophrenia symptoms(Geyer and Moghaddam, 2002). Our current study verifies the markedly reduced PPI and increased startle response in th(tk-)/th(tk-) mice compared to control mice of the same genetic background(Klejbor et al., 2006). Although it has been reported that C57BL/6 mice exhibit age-related hearing impairments, there is no evidence that such potential impairments affected the results of our experiments. In control mice there were no significant differences in PPI between ages 2 and 8 months whereas the loss of prepulse hearing over time would artificially reduce PPI. In th(tk-)/th(tk-) mice and not in controls, PPI was improved by AAPD and M100907, indicating that receptor-mediated antagonism was responsible. Also, the transgenic mice exhibited higher startle response magnitudes than controls – not consistent with a hearing impairment.
We previously showed improvements in the behavioral assay of PPI with the TAPD, flupenthixol, a D2 receptor antagonist(Klejbor et al., 2006). We now show a similar correction with AAPD clozapine and quetiapine, 5-HT and D2 receptor antagonists. These drugs have little or no effect on control mice indicating that the changes in PPI and startle are caused by abnormal DA and 5-HT neurotransmission.
The postpubertal onset of positive symptoms in th(tk-)/th(tk-) mice is consistent with findings that human schizophrenia symptoms typically do not become manifest before puberty and may appear late into adulthood(Palmer et al., 2001). In th(tk-)/th(tk-) mice, increases in midbrain 5-HT/5-HIAA contents were found in adult but not prepubertal animals. Likewise, changes in DA and DA metabolites in the telencephalon of adult th(tk-)/th(tk-) mice were absent at 4 weeks of life (our unpublished observations) and diminished at 3 months(Klejbor et al., 2006). Hence, the delayed onset of behavioral symptoms seen in th(tk-)/th(tk-) mice may be related to evolving changes in 5-HT and DA neuronal output, which in turn could reflect developing changes in 5-HT innervation. The latter appears possible given evidence that lesioning of DA neurons in the adult brain may elicit serotonergic sprouting (Revuelta et al., 1999). Alternatively, changes in 5-HT terminals in th(tk-)/th(tk-) mice could occur early in life and the emergence of the biochemical and behavioral phenotype could involve environmental or internal conditions acting upon the abnormal brain circuits, as it has been proposed in human schizophrenia. The th(tk-)/th(tk-) mice allow testing of this challenging hypothesis.
We have now extended the behavioral characterization in th(tk-)/th(tk-) mice by documenting abnormal social interaction. This behavioral deficit does not appear to be a result of general changes in anxiety-like behavior or a simple sensory deficit. In the open-field, th(tk-)/th(tk-) mice travel a greater distance and spend more time in the center of the arena than control mice (Figure 4A and B). Also, th(tk-)/th(tk-) mice spend more time on the open arms of the elevated plus maze than controls (data not shown) revealing that the mice do not exhibit elevated levels of anxiety-like behavior. The th(tk-)/th(tk-) mice exhibit normal habituation/dishabituation to non-social odors and ability to find hidden food (data not shown) indicating that impaired olfaction is unlikely to contribute to reduced social interaction.
In psychosis, impaired social behavior is thought to involve hypofunction of prefrontal cortical and limbic domains, although precise anatomical characterizations have not been extensively reported. Importantly, quetiapine (but not clozapine) improved reduced social interaction in th(tk-)/th(tk-) mice, mimicking human schizophrenia findings. The 5-HT2A antagonist M100907 improved PPI as well social interaction without producing extrapyramidal effects (no changes in motor activity) and without affecting social behaviors or sensory gating of control mice. This suggests that blocking 5-HT2A receptors may a useful therapeutic action against psychotic symptoms. However, effects of AAPD quetiapine, which block both DA and 5-HT receptors, were more pronounced, indicating that optimal therapeutic effects may require antagonism of both types of receptors. So far the use of M100907 in human schizophrenia has produced mixed results(de Paulis, 2001).
5-HT dorsal raphe innervation of DA nuclei has significant effects on DA system function(Fibiger and Miller, 1977; Kelland et al., 1990; Adell and Artigas, 2004). Different responses of SNc and VTA DA neurons to TAPD and AAPD and to 5-HT2A blockade are very well-documented(Hand et al., 1987; Ugedo et al., 1989; Sorensen et al., 1993). The current findings indicate that there is a different hyperinnervation profile of the VTA and SN by 5-HT fibers. There is increased innervation directly within the DA neuron-containing subnuclei of the VTA, but within the SN, the hyperinnervation is in the SNr. This is outside of the main SN DA somatic field and thus may less directly influence DA function. Although DA dendrites could be influenced by 5-HT innervation in the SNr, there also is an indirect influence on DA cells through GABAergic interneurons projecting from the SNr to the SNc. Thus, the 5-HT innervation profile acting on different local circuitries may have significantly different effects overall on VTA and SNc DA function. Although much work remains, one potential DA and 5-HT interactive schema is proposed (Figure 5) in which 5-HT2A blockade may increase cortical DA output and improve social behavior while decreasing DA subcortical output and improving sensory gating.
Figure 5.
Correction of positive and negative symptoms in an FGF/dopamine developmental model of psychosis – why 5-HT2A antagonists are effective therapeutic agents. Serotonergic hyperinnervation results in overstimulation of 5-HT2A receptors in parabrachial pigmentosus and paranigral nuclei of the VTA and in the SNr. In VTA stimulation of 5-HT2A reduces the activity of DA hypoplastic mesocortical neurons leading to hypofrontality and negative symptoms (social disconnection). In contrast, overstimulation of 5-HT2A in SNr may increase the activity of DA SNc neurons causing an excessive DA output by the hypoplastic nigro-striatal system and impaired sensory gating. Activation of the SNc DA neurons may be mediated by GABAergic SNr interneurons.
The th(tk-)/th(tk-) model demonstrates how early changes in DA neuronal populations causes rewiring of other neuronal systems and results in extensive and long-lasting disruptions in behavior similar to those observed in schizophrenia and other psychotic disorders. Brain rewiring may not be limited to DA and 5-HT neuronal systems. It has been reported that 5-HT and DA are important in the development of telencephalic(Diaz et al., 1997; Ohtani et al., 2003; Popolo et al., 2004; Hiramoto et al., 2008) and mesencephalic(Rodriguez-Pallares et al., 2003) structures. Our recent studies indentify abnormalities in the hippocampus and prefrontal cortex of th(tk-)/th(tk-) mice similar to those observed in schizophrenia (unpublished observations). The mechanism of this plasticity and its impact on brain function will be investigated in the future. Through these investigations we may get closer to understanding and correcting the complex biological and environmental features of neurodevelopmental disorders such as autism and schizophrenia.
Acknowledgements
We grateful for funding from University at Buffalo (two IRDF grants) which supported this study and EKS for participating in part as a volunteer.
REFERENCES
- Abel KM, O'Keane V, Murray RM. Enhancement of the prolactin response to d-fenfluramine in drug-naive schizophrenic patients. Br J Psychiatry. 1996;168:57–60. doi: 10.1192/bjp.168.1.57. [DOI] [PubMed] [Google Scholar]
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neuroscience & Biobehavioral Reviews. 2004;28:415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bartfai A, Levander SE, Sedvall G. Smooth pursuit eye movements, clinical symptoms, CSF metabolites, and skin conductance habituation in schizophrenic patients. Biol Psychiatry. 1983;18:971–987. [PubMed] [Google Scholar]
- Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, Bergey EJ, Prasad PN, Stachowiak MK. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci U S A. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Tessmer JL, Ullrich T, Rice KC, Walker PD. Serotonin 5-HT2A receptors underlie increased motor behaviors induced in dopamine-depleted rats by intrastriatal 5-HT2A/2C agonism. J Pharmacol Exp Ther. 2004;310:687–694. doi: 10.1124/jpet.104.066365. [DOI] [PubMed] [Google Scholar]
- Blak AA, Naserke T, Saarimaki-Vire J, Peltopuro P, Giraldo-Velasquez M, Vogt Weisenhorn DM, Prakash N, Sendtner M, Partanen J, Wurst W. Fgfr2 and Fgfr3 are not required for patterning and maintenance of the midbrain and anterior hindbrain. Dev Biol. 2007;303:231–243. doi: 10.1016/j.ydbio.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Hantsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, parkinson patients, and schizophrenics. Biological Psychiatry. 1983;18:951–969. [PubMed] [Google Scholar]
- Bruno JP, Jackson D, Zigmond MJ, Stricker EM. Effect of dopamine-depleting brain lesions in rat pups: role of striatal serotonergic neurons in behavior. Behav Neurosci. 1987;101:806–811. doi: 10.1037//0735-7044.101.6.806. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci. 1999;22:377–382. doi: 10.1016/s0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;(39 Suppl 1):S10–S14. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- De Luca A, Conti E, Grifone N, Amati F, Spalletta G, Caltagirone C, Bonaviri G, Pasini A, Gennarelli M, Stefano B, Berti L, Mittler G, Meisterernst M, Dallapiccola B, Novelli G. Association study between CAG trinucleotide repeats in the PCQAP gene (PC2 glutamine/Q-rich-associated protein) and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;116B:32–35. doi: 10.1002/ajmg.b.10008. [DOI] [PubMed] [Google Scholar]
- de Paulis T. M-100907 (Aventis) Curr Opin Investig Drugs. 2001;2:123–132. [PubMed] [Google Scholar]
- Diaz J, Ridray S, Mignon V, Griffon N, Schwartz JC, Sokoloff P. Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain. J Neurosci. 1997;17:4282–4292. doi: 10.1523/JNEUROSCI.17-11-04282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Lee YW, Stachowiak EK, Pudavar H, Claus P, Prasad PN, Stachowiak MK. Fibroblast growth factor receptor-1 (FGFR1) nuclear dynamics reveal a novel mechanism in transcription control. Mol Biol Cell. 2009;20:2401–2412. doi: 10.1091/mbc.E08-06-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Stachowiak EK, Dunham-Ems SM, Klejbor I, Stachowiak MK. Control of CREB-binding protein signaling by nuclear fibroblast growth factor receptor-1: a novel mechanism of gene regulation. J Biol Chem. 2005;280:28451–28462. doi: 10.1074/jbc.M504400200. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Miller JJ. An anatomical and electrophysiological investigation of the serotonergic projection from the dorsal raphenucleus to the substantia nigra in the rat. Neuroscience. 1977;2:975–987. [Google Scholar]
- Frantz GD, Weimann JM, Levin ME, McConnell SK. Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J Neurosci. 1994;14:5725–5740. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Moghaddam B. Animal models relevant to schizophrenia disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 689–701. [Google Scholar]
- Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12:904–922. doi: 10.1038/sj.mp.4002062. [DOI] [PubMed] [Google Scholar]
- Grothe C, Timmer M. The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Res Rev. 2007;54:80–91. doi: 10.1016/j.brainresrev.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Hamada S, Senzaki K, Hamaguchi-Hamada K, Tabuchi K, Yamamoto H, Yamamoto T, Yoshikawa S, Okano H, Okado N. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Brain Res Mol Brain Res. 1998;54:199–211. doi: 10.1016/s0169-328x(97)00322-7. [DOI] [PubMed] [Google Scholar]
- Hand TH, Hu XT, Wang RY. Differential effects of acute clozapine and haloperidol on the activity of ventral tegmental (A10) and nigrostriatal (A9) dopamine neurons. Brain Research. 1987;415:257–269. doi: 10.1016/0006-8993(87)90207-1. [DOI] [PubMed] [Google Scholar]
- Hansson LO, Waters N, Winblad B, Gottfries CG, Carlsson A. Evidence for biochemical heterogeneity in schizophrenia: a multivariate study of monoaminergic indices in human post-mortal brain tissue. J Neural Transm Gen Sect. 1994;98:217–235. doi: 10.1007/BF01276538. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hiramoto T, Satoh Y, Takishima K, Watanabe Y. Induction of cell migration of neural progenitor cells in vitro by alpha-1 adrenergic receptor and dopamine D1 receptor stimulation. NeuroReport. 2008;19:793–797. doi: 10.1097/WNR.0b013e3282fd1270. [DOI] [PubMed] [Google Scholar]
- Ikemoto K, Nishimura A, Okado N, Mikuni M, Nishi K, Nagatsu I. Human midbrain dopamine neurons express serotonin 2A receptor: an immunohistochemical demonstration. Brain Res. 2000;853:377–380. doi: 10.1016/s0006-8993(99)02237-4. [DOI] [PubMed] [Google Scholar]
- Ikemoto K, Nishimura A, Oda T, Nishi K. Dopamine-related neuronal structures in schizophrenia: Histochemical study using post-mortem brains The World. Journal of Biological Psychiatry. 2008;9:189. [Google Scholar]
- Jabs BE, Berg D, Merschdorf U, Bartsch AJ, Pfuhlmann B. Differences in substantia nigra echogenicity of nosological subtypes within the schizophrenic spectrum. A preliminary transcranial ultrasound study. Neuropsychobiology. 2001;44:183–186. doi: 10.1159/000054940. [DOI] [PubMed] [Google Scholar]
- Jukkola T, Lahti L, Naserke T, Wurst W, Partanen J. FGF regulated gene-expression and neuronal differentiation in the developing midbrain-hindbrain region. Dev Biol. 2006;297:141–157. doi: 10.1016/j.ydbio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Kelland MD, Freeman AS, Chiodo LA. Serotonergic afferent regulation of the basic physiology and pharmacological responsiveness of nigrostriatal dopamine neurons. J. Pharmacol. Exp. Ther. 1990;253:803–811. [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Klejbor I, Myers JM, Hausknecht K, Corso TD, Gambino AS, Morys J, Maher PA, Hard R, Richards J, Stachowiak EK, Stachowiak MK. Fibroblast growth factor receptor signaling affects development and function of dopamine neurons - inhibition results in a schizophrenia-like syndrome in transgenic mice. Journal of Neurochemistry. 2006;97:1243–1258. doi: 10.1111/j.1471-4159.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Uranova NA. Synaptic contacts in schizophrenia: studies using immunocytochemical identification of dopaminergic neurons. Neurosci Behav Physiol. 1999;29:217–221. doi: 10.1007/BF02465329. [DOI] [PubMed] [Google Scholar]
- Leonhard K, Beckmann H, editors. Classification of Endogenous Psychoses and Their Differentiated Etiology. 2nd eddition. Wien, Springer; 1999. [Google Scholar]
- Lieberman JA, Mailman RB, Duncan G, Sikich L, Chakos M, Nichols DE, Kraus JE. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biological Psychiatry. 1998;44:1099–1117. doi: 10.1016/s0006-3223(98)00187-5. [DOI] [PubMed] [Google Scholar]
- Losonczy MF, Davidson M, Davis KL. The dopamine hypothesis of schizophrenia. In: Meltzer HY, editor. Psychopharmacology: the third generation of progress. New York: Raven Press; 1987. pp. 715–726. [Google Scholar]
- Maher PA. Nuclear Translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi CP. Influenza and schizophrenia: a possible connection with the substantia nigra. Med Hypotheses. 1984;15:163–167. doi: 10.1016/0306-9877(84)90122-1. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature Neuroscience. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Moukhles H, Bosler O, Bolam JP, Vallee A, Umbriaco D, Geffard M, Doucet G. Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience. 1997;76:1159–1171. doi: 10.1016/s0306-4522(96)00452-6. [DOI] [PubMed] [Google Scholar]
- Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res. 2004;121:60–69. doi: 10.1016/j.molbrainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Myers JM, Martins GG, Ostrowski J, Stachowiak MK. Nuclear trafficking of FGFR1: a role for the transmembrane domain. J Cell Biochem. 2003;88:1273–1291. doi: 10.1002/jcb.10476. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13:27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- O'Donovan MC, Norton N, Williams H, Peirce T, Moskvina V, Nikolov I, Hamshere M, Carroll L, Georgieva L, Dwyer S, Holmans P, Marchini JL, Spencer CC, Howie B, Leung HT, Giegling I, Hartmann AM, Moller HJ, Morris DW, Shi Y, Feng G, Hoffmann P, Propping P, Vasilescu C, Maier W, Rietschel M, Zammit S, Schumacher J, Quinn EM, Schulze TG, Iwata N, Ikeda M, Darvasi A, Shifman S, He L, Duan J, Sanders AR, Levinson DF, Adolfsson R, Osby U, Terenius L, Jonsson EG, Cichon S, Nothen MM, Gill M, Corvin AP, Rujescu D, Gejman PV, Kirov G, Craddock N, Williams NM, Owen MJ. Analysis of 10 independent samples provides evidence for association between schizophrenia and a SNP flanking fibroblast growth factor receptor 2. Mol Psychiatry. 2009;14:30–36. doi: 10.1038/mp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle S, Zamanillo D, Andreu F, Farre AJ, Guitart X. Fibroblast growth factor-2 is selectively modulated in the rat brain by E-5842, a preferential sigma-1 receptor ligand and putative atypical antipsychotic. Eur J Neurosci. 2001;13:909–915. doi: 10.1046/j.0953-816x.2001.01459.x. [DOI] [PubMed] [Google Scholar]
- Palmer BW, McClure FS, Jeste DV. Schizophrenia in late life: findings challenge traditional concepts. Harv Rev Psychiatry. 2001;9:51–58. doi: 10.1080/10673220127883. [DOI] [PubMed] [Google Scholar]
- Petronis A. The origin of schizophrenia: genetic thesis, epigenetic antithesis, and resolving synthesis. Biol Psychiatry. 2004;55:965–970. doi: 10.1016/j.biopsych.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Pickar D, Owen RR, Jr., Litman RE, Hsiao JK, Su TP. Predictors of clozapine response in schizophrenia. J Clin Psychiatry. 1994;(55 Suppl B):129–132. [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Popolo M, McCarthy DM, Bhide PG. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26:229–244. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch JL, Sirota EL, Londino DL, Johnson ME, Carr BM, Bhatia R, Miller S. Open-label risperidone for Asperger's disorder: negative symptom spectrum response. J Clin Psychiatry. 2005;66:1592–1597. doi: 10.4088/jcp.v66n1216. [DOI] [PubMed] [Google Scholar]
- Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J Cell Biol. 2001;152:1307–1312. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revuelta M, Venero JL, Machado A, Cano J. Serotonin hyperinnervation in the adult rat ventral mesencephalon following unilateral transection of the medial forebrain bundle. Correlation with reactive microglial and astroglial populations. Neuroscience. 1999;91:567–577. doi: 10.1016/s0306-4522(98)00624-1. [DOI] [PubMed] [Google Scholar]
- Rimon R, Roos BE, Rakkolainen V, Alanen Y. The content of 5-hydroxyindoleacetic acid and homovanillic acid in the cerebrospinal fluid of patients with acute schizophrenia. J Psychosom Res. 1971;15:375–378. doi: 10.1016/0022-3999(71)90051-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Guerra MJ, Labandeira-Garcia JL. Elimination of serotonergic cells induces a marked increase in generation of dopaminergic neurons from mesencephalic precursors. European Journal of Neuroscience. 2003;18:2166–2174. doi: 10.1046/j.1460-9568.2003.02949.x. [DOI] [PubMed] [Google Scholar]
- Saffell JL, Williams EJ, Mason IJ, Walsh FS, Doherty P. Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 1997;18:231–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Kehne JH, Carr AA, Fadayel GM, Humphreys TM, Kettler HJ, McCloskey TC, Padich RA, Taylor VL, Sorensen SM. Contribution of serotonin neurotoxins to understanding psychiatric disorders: the role of 5-HT2 receptors in schizophrenia and antipsychotic activity. Int Clin Psychopharmacol. 1993;(8 Suppl 2):25–32. [PubMed] [Google Scholar]
- Sharma RP. Schizophrenia, epigenetics and ligand-activated nuclear receptors: a framework for chromatin therapeutics. Schizophr Res. 2005;72:79–90. doi: 10.1016/j.schres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Shin DM, Korada S, Raballo R, Shashikant CS, Simeone A, Taylor JR, Vaccarino F. Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice. J Neurosci. 2004;24:2247–2258. doi: 10.1523/JNEUROSCI.5285-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. Journal of Pharmacology and Experimental Therapeutics. 1993;266:684–691. [PubMed] [Google Scholar]
- Stachowiak EK, Fang X, Myers J, Dunham S, Stachowiak MK. cAMP-induced differentiation of human neuronal progenitor cells is mediated by nuclear fibroblast growth factor receptor-1 (FGFR1) J Neurochem. 2003;84:1296–1312. doi: 10.1046/j.1471-4159.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Roy I, Lee Y-W, Capacchietti M, Aletta JM, Prasad PN, Stachowiak MK. Targeting novel Integrative Nuclear FGFR1 Signaling by nanoparticle-mediated gene transfer stimulates neurogenesis in adult brain. Integrative Biology. 2009;1:394–403. doi: 10.1039/b902617g. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Maher PA, Joy A, Mordechai E, Stachowiak EK. Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol Biol Cell. 1996;7:1299–1317. doi: 10.1091/mbc.7.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak MK, Maher PA, Stachowiak EK. Integrative Nuclear Signaling in Cell Development-A Role for FGF Receptor-1. DNA Cell Biol. 2007;26:811–826. doi: 10.1089/dna.2007.0664. [DOI] [PubMed] [Google Scholar]
- Terwisscha van Scheltinga AF, Bakker SC, Kahn RS. Fibroblast Growth Factors in Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophr Bull. 2004;30:875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- Toru M, Nishikawa T, Mataga N, Takashima M. Dopamine metabolism increases in post-mortem schizophrenic basal ganglia. J Neural Transm. 1982;54:181–191. doi: 10.1007/BF01254928. [DOI] [PubMed] [Google Scholar]
- Ugedo L, Grenhoff J, Svensson TH. Ritanserin, a 5-HT2 receptor antagonist, activates midbrain dopamine neurons by blocking serotonergic inhibition. Psychopharmacology (Berl) 1989;98:45–50. doi: 10.1007/BF00442004. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Hattori T. Dorsal raphe cells with collateral projections to the caudate-putamen and substantia nigra: a fluorescent retrograde double labeling study in the rat. Brain Research. 1980;186:1–7. doi: 10.1016/0006-8993(80)90250-4. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhong KX, Sweitzer DE, Hamer RM, Lieberman JA. Comparison of quetiapine and risperidone in the treatment of schizophrenia: A randomized, double-blind, flexible-dose, 8-week study. J Clin Psychiatry. 2006;67:1093–1103. doi: 10.4088/jcp.v67n0712. [DOI] [PubMed] [Google Scholar]