Abstract
Oligomeric forms of alpha-synuclein and beta-amyloid are toxic protein variants thought to contribute to the onset and progression of Parkinson’s (PD) and Alzheimer’s (AD) diseases, respectively. Detection of toxic variants in human cerebrospinal fluid (CSF) and blood has great promise to facilitate early and accurate diagnoses of these devastating diseases. Two hurdles that have impeded the use of these protein variants as biomarkers are the availability of reagents that can bind the different variants and a sensitive assay to detect their very low concentrations. We previously isolated antibody based reagents that selectively bind two different oligomeric variants of alpha-synuclein and two of beta-amyloid and developed a phage-based capture ELISA with sub-femtomolar sensitivity to quantify their presence. Here we used these reagents to show that these oligomeric alpha-synuclein variants are preferentially present in the PD brain tissue, CSF and sera and that the oligomeric beta-amyloid variants are preferentially present in the AD brain tissue, CSF and sera. Some AD samples also had alpha-synuclein pathology and some PD samples also had beta-amyloid pathology, and very intriguingly, these PD cases also had a history of dementia. Detection of different oligomeric alpha-synuclein and beta-amyloid species is an effective method to identify tissue, CSF and sera from PD and AD samples, respectively and samples which also contained early stages of other protein pathologies, indicating their potential value as blood based biomarkers for neurodegenerative diseases.
Keywords: Phage Capture ELISA, Single-Chain Variable Fragments, Brain Tissue, Serum, Cerebrospinal Fluid
Graphical Abstract

Introduction
Protein aggregation is a common feature in the progression of many neurological disorders including AD and PD (Crews & Masliah, 2010; Tsigelny et al., 2008; Guerrero-Muñoz et al., 2014). In diseased brain, monomeric proteins can assemble into different aggregates including smaller oligomers and larger protofibrillar and fibrillar assemblies (Guerrero-Muñoz et al., 2014). While fibrils are the primary pathological diagnostic feature, recent research suggests that oligomers are the most toxic species (Guerrero-Muñoz et al., 2014). Various alpha-synuclein oligomers were shown to play toxic roles in PD, (Cox et al., 2014; Chaari et al., 2013; Halliday & McCann, 2008; Lynch et al., 2008; Emadi et al., 2007; Emadi et al., 2009; Sierks et al., 2011; Wang et al., 2010) a disorder resulting in resting tremors, posture instability and rigidity (Dauer & Przedborski, 2003). Alpha-synuclein is produced in healthy neurons as a monomeric 14 kDa protein, however, in diseased tissue it assembles into large fibrillar aggregates that are the primary constituent of Lewy bodies (LB) and Lewy neurites, the pathological hallmarks of PD (Spillantini et al., 1997; Spillantini et al., 1998). There is also a reduction in dopaminergic neurons in the substantia nigra pars compacta in PD brains (Martin & Teismann, 2009). Alternatively, aggregation of beta-amyloid and tau are implicated in AD, a neurodegenerative disease that causes cognitive deficits and altered behavior (Reitz & Mayeux, 2014). While beta-amyloid and tau fibrils are respectively the primary constituents of the amyloid plaques and neurofibrillary tangles (Hoozemans et al., 2006), various small oligomers of both have been implicated in the onset and progression of AD (Sinjoanu et al., 2008; Crimins et al., 2013; Wischik et al., 2014; Pooler et al., 2014; Tian et al., 2013; Zhou et al., 2012; Salvadores et al., 2014; Klein et al., 2001; Park et al., 2013; Yang et al., 2013). Relative concentrations of beta-amyloid 42 and 40, and phosphorylated to total tau are currently used as AD biomarkers. The beta-amyloid 42 form and hyperphosphorylated tau variants are both more prone to aggregation (Moreth et al., 2013; Di Carlo et al., 2012). Selective detection of specific toxic oligomeric protein variants may improve diagnosis of these neurodegenerative diseases.
We developed an in vitro atomic force microscopy (AFM) based biopanning protocol that enables us to generate single chain variable domain antibody fragments (scFvs) from phage display libraries that selectively bind disease related protein variants (Williams et al., 2015; Kasturirangan et al., 2013). We previously isolated the 10H and D5 scFvs which recognize two morphologically distinct alpha-synuclein oligomers and selectively bind post-mortem human PD brain tissue (Emadi et al., 2007; Emadi et al., 2009). We also previously isolated the A4 and C6T scFvs which recognize morphologically distinct beta-amyloid oligomers and selectively bind post-mortem human AD brain tissue (Zameer et al., 2008; Kasturirangan et al., 2012; Kasturirangan et al., 2013). Here we use these scFvs in conjunction with a previously developed phage capture ELISA to assess oligomeric protein content in human samples (Williams et al., 2014). The protein variant selective scFvs are used to capture the target antigen and a phage displayed version of a second scFv that is protein specific, but not morphology specific is used as the detection antibody. This assay provides two separate methods to validate the identity of the target antigen, first the specificity of the capture scFv which binds only a specific variant of the target antigen and second the detection antibody which binds a different epitope of the target antigen. In our study we employed this capture ELISA to characterize brain tissue, CSF and sera samples from post-mortem, pathologically confirmed AD, PD and age-matched cognitively normal controls to demonstrate the potential value of specific oligomeric protein variants as blood based biomarkers for neurodegenerative diseases.
Material and Methods
Human Samples
Post-mortem human brain tissue, sera and CSF samples from patients with pathologically confirmed PD or AD, as well as age-matched cognitively normal controls (ND) were provided by Dr. Thomas Beach, director of the Brain and Body Donation Program at Banner/Sun Health Research Institute (Beach et al., 2008; Beach et al., 2015). Samples were obtained from deceased elderly subjects who had volunteered for the Banner Sun Health Research Institute Brain and Body Donation Program (BBDP; www.brainandbodydonationprogram.org). All enrolled subjects signed an Institutional Review Board-approved informed allowing both clinical assessments during life and several options for brain and/or bodily organ donation after death. Brain tissue samples from nine different PD, six AD and five ND cases were obtained. Table 1 summarizes the characteristics of the utilized human subjects. Classification of PD samples is based on the unified staging system for Lewy body disorders (Beach et al., 2009). Sera samples from eight of the nine PD, all six AD and four of the five ND cases, and CSF samples from six of the nine PD, all six AD and four of the five ND cases were also available for analysis.
Table 1.
Patient Cohort Information
| Samples | Gender | Age | PMI | Neurological Diagnosis (Yrs) | Dementia (Yrs) | Plaque Density | Braak Score | Unified LB Stage | Pathology Summary |
|---|---|---|---|---|---|---|---|---|---|
| PD 1 | F | 80 | 7.5 | 11 | Moderate | III | lll. Brainstem/Limbic | Parkinson’s disease; Microscopic changes of Alzheimer’s disease but insufficient for diagnosis. | |
| PD 2 | F | 82 | 2.75 | 16 | Moderate | IV | lll. Brainstem/Limbic | Parkinson disease; Moderate microscopic lesions of Alzheimer’s disease (moderate plaques). | |
| PD 3 | M | 85 | 2.16 | 6 | Moderate | IV | llb. Limbic Predominant | Parkinson’s disease; Microscopic changes of Alzheimer’s disease. | |
| PD 4 | M | 72 | 10 | 17 | Sparse | II | llb. Limbic Predominant | Parkinson’s disease | |
| PD 5 | M | 70 | 1.83 | 12 | 3 | Zero | III | lll. Brainstem/Limbic | Parkinson Disease; Dementia (history) |
| PD 6 | F | 73 | 2.16 | 29 | 6 | Sparse | III | lV. Neocortical | Parkinson’s disease; Dementia (history) |
| PD 7 | M | 75 | 2.25 | 21 | 1 | Zero | III | lV. Neocortical | Parkinson’s disease; Dementia (history) |
| PD 8 | M | 77 | 4 | 11 | Sparse | III | lV. Neocortical | Parkinson’s disease | |
| PD 9 | F | 78 | 3.5 | 16 | 6 | Sparse | III | lV. Neocortical | Parkinson’s disease; Dementia (clinical history) |
| AD 1 | F | 82 | 4.5 | 9 | 9 | Moderate | IV | 0. No Lewy bodies | Alzheimer’s disease |
| AD 2 | F | 93 | 1.4 | 4 | 4 | Moderate | IV | 0. No Lewy bodies | Alzheimer’s Disease |
| AD 3 | M | 94 | 3 | 14 | 14 | Moderate | IV | 0. No Lewy bodies | Alzheimer’s Disease |
| AD 4 | M | 79 | 3.92 | 7 | 7 | Frequent | V | 0. No Lewy bodies | Alzheimer’s disease |
| AD 5 | F | 96 | 3 | 21 | 5 | Frequent | VI | 0. No Lewy bodies | Alzheimer’s disease |
| AD 6 | F | 87 | 3 | 5 | 5 | Frequent | V | 0. No Lewy bodies | Alzheimer’s disease |
| ND 1 | F | 87 | 2.83 | Zero | II | 0. No Lewy bodies | Control | ||
| ND 2 | F | 91 | 2 | Zero | II | 0. No Lewy bodies | Control; Entorhinal cortex neurofibrillary tangles consistent with normal aging. | ||
| ND 3 | F | 83 | 4.83 | Moderate | II | 0. No Lewy bodies | Control; Microscopic changes of Alzheimer’s disease but insufficient for diagnosis. | ||
| ND 4 | M | 89 | 2.5 | Moderate | II | 0. No Lewy bodies | Control; Microscopic changes of Alzheimer’s disease but insufficient for diagnosis. | ||
| ND 5 | M | 86 | 2.75 | Moderate | II | 0. No Lewy bodies | Control; Microscopic changes of Alzheimer’s disease but insufficient for diagnosis. |
Brain Tissue Homogenization
Brain tissue from the middle temporal gyrus (MTG) was homogenized for immunoassay assessment as previously described (Williams et al., 2014). Briefly, each sample was chopped and resuspended in homogenization buffer containing 50 mM Tris and 5mM Ethylenediaminetetraacetic acid (EDTA) at pH 7.0. Sonication was then completed at 50% amplitude with 10 seconds pulse on and 15 seconds pulse off for a total of five minutes. After centrifugation at 13,000 rpm for 20 minutes, the supernatants were stored at −80°C for future use.
ScFvs
The 10H and D5 scFvs were previously isolated against two morphologically distinct alpha-synuclein oligomeric variants (Emadi et al., 2007; Emadi et al., 2009), where D5 bound in vitro generated alpha-synuclein aggregates of molecular weights 29 kDa and 56 kDa corresponding to dimers and tetramers (Emadi et al., 2007) and 10H bound in vitro generated aggregates of molecular weights 42 kDa and ~80 kDa corresponding to trimers and hexamers (Emadi et al., 2009). The corresponding molecular weights of the oligomeric alpha-synuclein variants bound by 10H and D5 are illustrated in supplementary figure 1A (adapted from Emadi et al., 2007; Emadi et al., 2009; Kasturirangan et al., 2013; Xin et al., 2015). These scFvs did not show cross-reactivity with in vitro generated oligomeric beta-amyloid aggregates (Emadi et al., 2009), but do selectively block cytotoxicity of PD brain homogenates compared to control brain homogenates (Xin et al., 2015). A third anti-alpha-synuclein scFv, D10, was previously shown to bind multiple morphologies of alpha-synuclein including monomeric, oligomeric and fibrillar forms (Emadi et al., 2009; Zhou et al., 2004) and was used here as the detection antibody in the capture ELISA (Supplementary Fig. 1A). Since 10H and D5 recognize different oligomeric alpha-synuclein species the alpha-synuclein complexes formed using 10H in conjunction with D10 and D5 with D10 should differ. Therefore, analysis of each human sample with both scFvs increases the likelihood of identifying patients with oligomeric alpha-synuclein pathology especially since the level of each oligomeric alpha-synuclein species at the time of testing may vary from person to person and dependent on the stage of the disease.
The A4 and C6T scFvs were previously isolated against morphologically distinct oligomeric variants of beta-amyloid, A4 binding an in vitro generated aggregate and C6T an AD brain derived aggregate (Zameer et al., 2008; Kasturirangan et al., 2012; Kasturirangan et al., 2013). In indirect ELISAs both A4 phage and scFv bind oligomeric beta-amyloid but not monomeric or fibrillar structures (Zameer et al., 2008). A4 also protected human neuroblastoma cells against neuronal toxicity induced by oligomeric beta-amyloid (Zameer et al., 2008). Western blot analysis using supernatant from hAPP overexpressing 7PA2 cells showed C6T binding to an ~ 11 kDa protein. When brain tissue from control and triple transgenic AD mice were probed with C6T, it preferentially reacted with the triple transgenic AD mice (Kasturirangan et al., 2013). Comparison of A4 and C6T’s binding specificity via AFM show that A4 selectively binds synthetically derived beta-amyloid oligomers while C6T selectively binds AD brain derived beta-amyloid oligomers (Supplementary Fig. 1B – adapted from Kasturirangan et al., 2013). These results highlighted the difference in A4 and C6T’s target antigen. The H1v2 scFv which recognizes all morphologies of beta-amyloid (Yuan et al., 2006) was used as the detection reagent. Since A4 and C6T bind different oligomeric beta-amyloid variants (Kasturirangan et al., 2013) use of both scFvs with H1v2 should increase the likelihood of identifying samples containing oligomeric beta-amyloid, similar to above rationale for identifying oligomeric alpha-synuclein with 10H and D5.
ScFv Production
To produce the scFvs, HB2151 cells containing the scFv plasmids were cultured overnight in 2xYT, 0.1% glucose and ampicillin at 37°C with shaking. 10 ml of this overnight culture was added to 1 liter of fresh media and incubated at 37°C with shaking until OD600 was 0.8. After the addition of Isopropyl β-D-1-thiogalactopyranoside (IPTG) the flask was transferred to 30°C. The following day, the supernatant was concentrated using a tangential flow filter (10 kDa filter (Millipore)). The purified scFv was then isolated by means of Fast Protein Liquid Chromatography (FPLC) via a protein A-Sepharose column (GE healthcare, NJ). C6T was purified via nickel NTA sepharose beads (Qiagen, CA) and imidazole elution as previously described Kasturirangan et al., 2013. Following dialysis, the scFvs were stored at −20°C. SDS-PAGE gel and Western blot analysis was used to confirm purity and presence of the antibodies. Antibody concentrations were calculated using Bicinchoninic Acid (BCA) assay (Pierce, USA).
Phage Production
The capture ELISA protocol employed here utilizes the morphology specific scFvs as the capture antibody and a protein specific scFv as a detection antibody. The protein specific scFv is expressed on a phage particle where the phage coat proteins can be biotinylated to greatly amplify the signal (Williams et al., 2014). Here we used a phage displayed version of the D10 scFv which recognizes all morphologies of alpha-synuclein (Emadi et al., 2009; Zhou et al., 2004) for detection of alpha-synuclein aggregates and the H1v2 scFv which recognizes all morphologies of beta-amyloid (Yuan et al., 2006) for detection of beta-amyloid aggregates. The D10 and H1v2 phage particles were produced as previously described (MRC Laboratory of Molecular Biology and the MRC Centre for Protein Engineering (Cambridge, UK) - http://www.lifesciences.sourcebioscience.com/media/143421/tomlinsonij.pdf). Basically an overnight culture of the TG1 cells containing the scFv plasmid of interest was diluted 1/100 and cultured in 2xYT containing 100 ug/ml ampicillin and 1% glucose until OD600 was between 0.4 and 0.6. After a 30 minute incubation with 2×1011 of KM13 helper phage or hyperphage (Progen, Germany), the culture was spun and resuspended in 2xYT containing 100 ug/ml ampicillin, 50 ug/ml kanamycin and 0.1% glucose. The cells were cultured at 30°C overnight and the following day centrifuged for 30 minutes at 3000×g. The supernatant was combined with PEG/NaCl and incubated for 1 hour on ice. The solution was again centrifuged at 3000×g for 30 minutes and the pellet saved. The pellet was resuspended in PBS and incubated on ice for 1 hour. Following this incubation step, the solution was centrifuged at 11,600×g for 10 minutes and the supernatant stored at −80°C.
The concentration of the phage was estimated using the BCA assay. Once produced, the phages were biotinylated using the EZ-Link Pentylamine-Biotinylation kit (Thermo Scientific, USA) essentially as described (Williams et al., 2014).
Phage Capture ELISA
The phage capture ELISA was performed as previously described (Williams et al., 2014). Basically the capture morphology specific scFvs (D5 and 10H for alpha-synuclein and A4 and C6T for beta-amyloid) (Emadi et al., 2007; Emadi et al., 2009; Zameer et al., 2008; Kasturirangan et al., 2012; Kasturirangan et al., 2013) were bound to the wells of a high binding ELISA plate (Costar) for 30–60 minutes at 37°C. After three washes with phosphate-buffered saline with 0.1% Tween-20 (PBST), the remaining unbound sites were blocked with 2% milk. Following three washes, the antigen was added. Brain tissue was diluted to 100 ug/ml and sera and CSF samples were diluted 1/100 v/v. The plates were again washed three times and incubated with 200 ng/ml of 40 mmol carboxyl biotinylated detection phage. The plates were then washed four times and incubated with a 1/1000 dilution of avidin-HRP (Sigma-Aldrich, USA). After four more washes the binding intensities were detected using the SuperSignal ELISA Femto Maximum Sensitivity Substrate (Thermo Scientific, USA) kit. The signals intensities were quantified using a Wallac Victor2 microplate reader following a 1 and 20 minute development period.
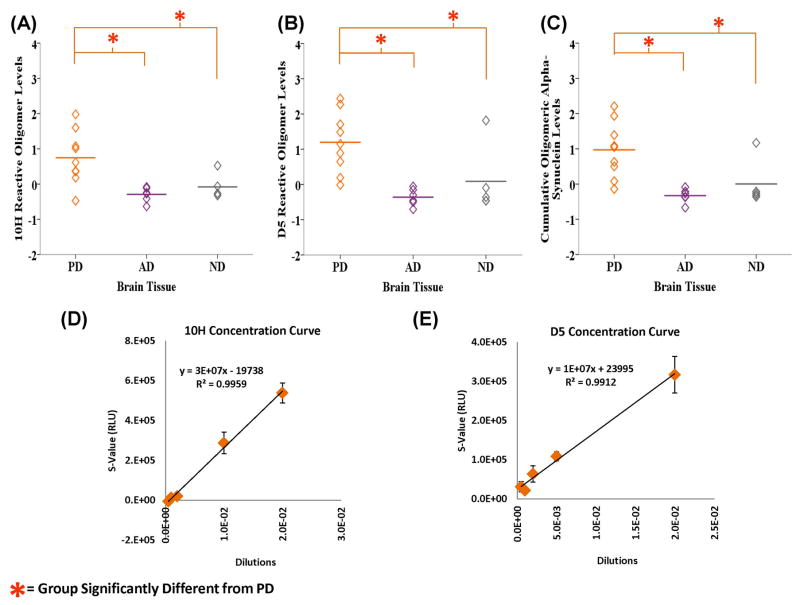
Since oligomeric proteins are generally very “sticky”, sample collection and storage conditions need to be controlled since this can influence repetition of results. Most of the ELISA trials were conducted more than once depending on sample availability. A representative example of sample reproducibility is shown in supplementary figure 2 which displays 10H’s reactivity with brain tissue. The 10H and D5 calibration curves in figures 1D and 1E, respectively, is also based on the average of three trials. The R2 value is fairly high with 0.9959 for 10H and 0.9912 for D5 and the error bars quite small at most data points.
Figure 1.
Statistical Analysis
The raw ELISA data for each sample was divided by the PBS control to obtain a signal ratio. The mean plus two standard deviations (SD) was calculated for the control group and that value subtracted from each sample to determine positive cases (except for the CSF tested with A4 and C6T where 1.5 SD was utilized). These results were plotted on graphs generated via the IBM SPSS Statistics 22 program. Significant differences between the PD, AD and ND groups for the four test scFvs were evaluated using one-way ANOVA and LSD Post-Hoc analyses made available through SPSS. Results were significant at P < 0.05. The mean and standard deviations for each group were also reported. The other graphs and tables were created using Microsoft Excel 2010. The results in Supplementary Figure 2 are based on dividing the test samples by the mean plus two standard deviations of the control group.
For the data presented in Tables 2 and 3, each sample signal ratio was divided by the mean plus two standard deviations (SD) of the controls. Sample signals greater than two SDs from the controls are indicated by a “+” sign, with additional “+” signs used for each additional two SD increase in the signal from controls. The sensitivity and specificity for the PD versus ND cases and AD versus ND cases were calculated with the help of MedCalc for the brain tissue, CSF and sera results. Power analyses was carried out using the G*Power 3.1.4 analysis tool to determine the number of samples required in future studies to obtain 80% power. A one-tail “A Priori” t-test examining the difference between two independent means was calculated for each scFv using an alpha level of 0.05, power at 0.8, an allocation ratio of 1 and the effect size computed using the mean and standard deviations of the test and control groups from the current study.
Table 2.
Oligomeric Alpha-Synuclein Levels in Human Brain Tissue, CSF and Sera Samples
| Samples | Brain Tissue | CSF | Sera | |||
|---|---|---|---|---|---|---|
| 10H | D5 | 10H | D5 | 10H | D5 | |
| PD 1 | + | |||||
| PD 2 | + | +++ | + | ++++ | ||
| PD 3 | ++ | ++ | + | ++ | ++++ | |
| PD 4 | +++ | +++++ | ||||
| PD 5 | ++++++ | ++++++ | + | |||
| PD 6 | ++ | +++ | + | + | +++ | |
| PD 7 | ++++++ | ++++++ | + | + | ||
| PD 8 | +++++ | +++++ | + | + | + | ++++ |
| PD 9 | +++++ | ++++ | ++ | + | ++++++ | |
| AD 1 | + | |||||
| AD 2 | ||||||
| AD 3 | + | ++ | ||||
| AD 4 | ||||||
| AD 5 | ||||||
| AD 6 | + | ++ | ||||
| ND 1 | ||||||
| ND 2 | +++ | ++++++ | ||||
| ND 3 | ||||||
| ND 4 | ||||||
| ND 5 | ||||||
Table 3.
Oligomeric Beta-Amyloid Levels in Human Brain Tissue, CSF and Sera Samples
| Samples | Brain Tissue | CSF | Sera | |||
|---|---|---|---|---|---|---|
| A4 | C6T | A4 | C6T | A4 | C6T | |
| PD 1 | +++++ | |||||
| PD 2 | ||||||
| PD 3 | +++ | |||||
| PD 4 | ||||||
| PD 5 | ++++++ | |||||
| PD 6 | + | |||||
| PD 7 | + | |||||
| PD 8 | + | |||||
| PD 9 | ||||||
| AD 1 | ++++++ | + | +++ | ++ | ||
| AD 2 | ++++ | + | + | ++++ | ++ | |
| AD 3 | + | + | ++ | |||
| AD 4 | ++ | + | ++++++ | |||
| AD 5 | + | + | +++ | |||
| AD 6 | ++++++ | ++ | +++++ | |||
| ND 1 | ||||||
| ND 2 | ||||||
| ND 3 | ||||||
| ND 4 | ||||||
| ND 5 | ||||||
Results
Human Brain Tissue Samples
Oligomeric Alpha-Synuclein
Homogenized human brain tissue samples from PD, AD and ND cases were analyzed for the presence of two different toxic oligomeric alpha-synuclein aggregates using the 10H and D5 scFvs. We ascertained significant differences between the groups using one-way ANOVA and LSD post hoc analyses. One-way ANOVA analyses revealed that the levels of 10H (F2, 16 = 8.157, P = 0.004) and D5 (F2, 16 = 14.488, P = 0.000) reactive oligomeric alpha-synuclein were both statistically different between the groups (Figs. 1A, B). LSD post-hoc analysis indicated that the PD group (M = 0.746, SD = 0.753) was statistically different from both the AD (M = −0.294, SD = 0.205, P = 0.002) and ND groups (M = −0.231, SD = 0.116, P = 0.009) with 10H. Similarly the PD group (M = 1.196, SD = 0.860) reacted significantly more with D5 compared to the AD (M = −0.361, SD = 0.238, P = 0.001) and control groups (M = −0.345, SD = 0.173, P = 0.019). Mean cumulative oligomeric alpha-synuclein level (the sum of 10H and D5) was also significantly different between the groups (F2, 16 = 11.928, P = 0.001) and LSD post hoc analyses indicated that the difference was between the PD (M = 0.971, SD = 0.792) and AD groups (M = −0.328, SD = 0.197, P = 0.001) and PD and control (ND) groups (M = −0.288, SD = 0.066, P = 0.002) (Fig. 1C). Since it is extremely difficult to stably purify individual oligomeric protein variants to homogeneity, it is not feasible to perform calibration curves to determine the concentration of the different oligomeric protein species present in different samples. As an alternative, we determined dilution curves from a known sample as a means to calculate relative concentrations. Dilutions of one of the PD brain tissue homogenate were used to determine the linear calibration curves for the 10H (Fig. 1D) and D5 (Fig. 1E) oligomeric alpha-synuclein variants with a good linear fit as determined by R-squared values.
Oligomeric Beta-Amyloid
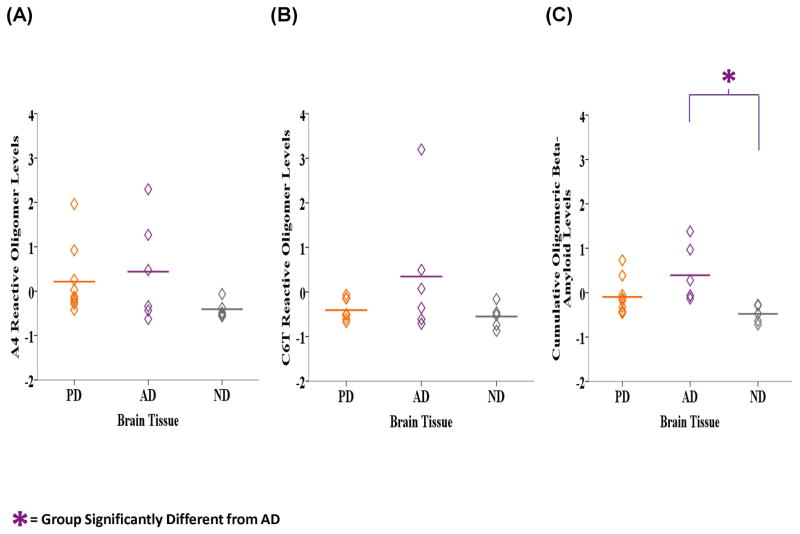
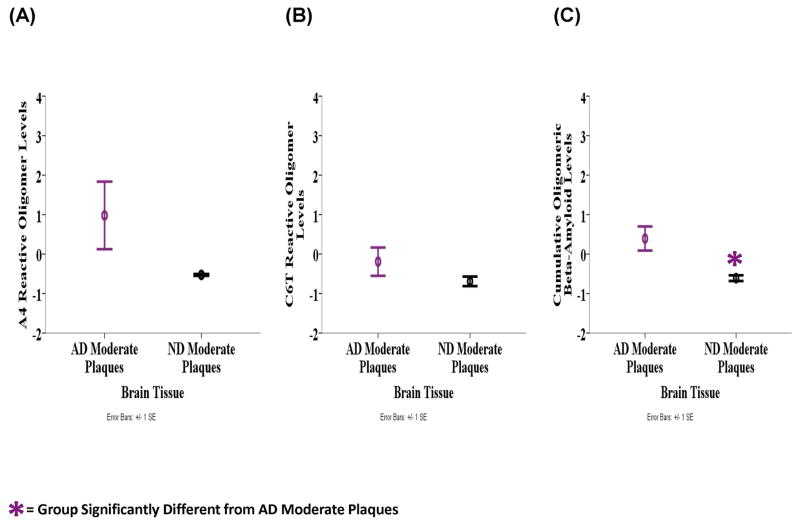
Similarly, we analyzed the homogenized human brain tissue samples for the presence of toxic oligomeric beta-amyloid aggregates using the A4 and C6T scFvs. The mean level of A4 reactive oligomeric beta-amyloid variants was highest in the AD samples (M = 0.439, SD = 1.152), lower in the PD samples (M = 0.214, SD = 0.768) and lowest in the ND samples (M = −0.406, SD = 0.203) (Fig. 2A). Similarly with C6T the AD samples (M = 0.347, SD = 1.466) were most reactive, followed by the PD (M = −0.407, SD = 0.227) and then ND groups (M = −0.550, SD = 0.275) (Fig, 2B). The mean cumulative level of A4 and C6T reactive oligomeric beta-amyloid variants was significantly different between the groups (F2, 17 = 5.125, P = 0.018) (Fig. 2C). LSD post-hoc analyses revealed that the AD tissue samples (M = 0.393, SD = 0.635) were significantly more reactive compared to the ND samples (M = −0.478, SD = 0.201, P = 0.006) and almost compared to the PD samples (M = −0.097, SD = 0.406, P = 0.056). The AD brain samples contained three cases with moderate plaque loads and three with high plaque loads, while the ND samples also contained three cases with moderate plaques and two with either no or only light plaques. We compared the three AD brain samples with moderate plaque loads to the three ND brain samples with moderate plaque loads to determine if the presence of oligomeric beta-amyloid could discriminate between these two groups with similar amyloid plaque loads. A4 reactive oligomeric beta-amyloid levels were higher in the AD samples (M = 0.977, SD = 1.482) compared to the ND samples (M = −0.528, SD = 0.041) (Fig. 3A). Similarly with C6T, the AD samples (M = −0.192, SD = 0.620) were more reactive compared to the ND samples (M = −0.691, SD = 0.211) (Fig. 3B). The cumulative level of A4 and C6T reactive oligomeric beta-amyloid was significantly higher in the AD samples (M = 0.393, SD = 0.528) compared to the ND group (M = −0.609, SD = 0.125) (F1, 4 = 10.246, P = 0.033) (Fig. 3C).
Figure 2.
Figure 3.
Human CSF Samples
Oligomeric Alpha-Synuclein
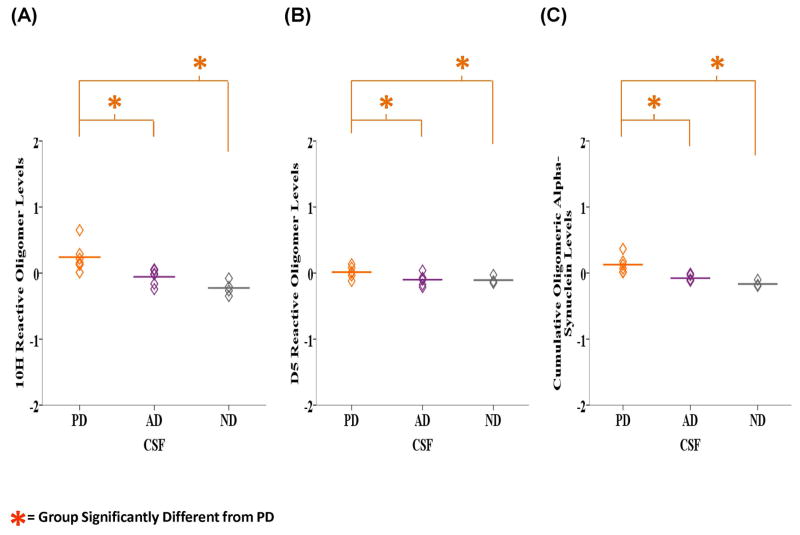
Next, we analyzed the available corresponding CSF samples from the PD, AD and ND cases described above for the presence of 10H and D5 reactive oligomeric alpha-synuclein. Likewise, the groups were evaluated using one-way ANOVA and LSD post hoc analyses for any resulting statistically significant differences. ANOVA analysis indicated that there was a significant difference in the levels of 10H (F2, 13 = 10.407, P = 0.002), D5 (F2, 13 = 3.799, P = 0.05) and cumulative 10H and D5 (F2, 13 = 14.637, P = 0.000) reactive oligomeric alpha-synuclein between the groups (Figs. 4A, B and C). LSD post-hoc analyses revealed that the PD samples (M = 0.241, SD = 0.221) contained significantly higher 10H reactive oligomeric alpha-synuclein compared to AD (M = −0.057, SD = 0.120, P = 0.008) and ND samples (M = −0.226, SD = 0.113, P = 0.001). Similarly, with D5 the PD samples (M = 0.014, SD = 0.090) were more reactive than the AD (M = −0.010, SD = 0.088, P = 0.032) and ND samples (M = −0.107, SD = 0.054, P = 0.040). The mean cumulative 10H and D5 reactive oligomeric alpha-synuclein level was also significantly higher in the PD samples (M = 0.127, SD = 0.133) compared to the AD (M = −0.078, SD = 0.046, P = 0.002) and ND (M = −0.166, SD = 0.043, P = 0.000) cases.
Figure 4.
Oligomeric Beta-Amyloid
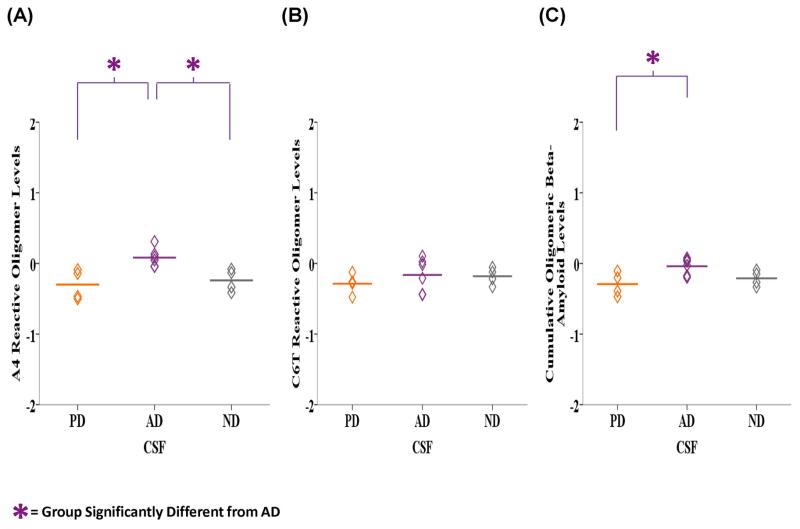
We analyzed the CSF samples for presence of A4 and C6T reactive oligomeric beta-amyloid. Due to the limited CSF availability, we were only able to assay 4 of the PD samples. The level of A4 reactive oligomeric beta-amyloid was significantly different between the groups (F2, 11 = 7.905, P = 0.007) (Fig. 5A). LSD post-hoc analyses showed significantly higher reactivity with the AD samples (M = 0.083, SD = 0.132) compared to both the PD (M = −0.299, SD = 0.214, P = 0.004) and ND (M = −0.240, SD = 0.160, P = 0.012) samples. With C6T, the average level was higher in the AD (M = −0.163, SD = 0.238) compared to the PD (M = −0.286, SD = 0.145) and ND samples (M = −0.181, SD = 0.121) (Fig. 5B). The cumulative A4 and C6T reactive oligomeric beta-amyloid levels were also significantly different between the groups (F2, 11 = 4.839, P = 0.031) (Fig. 5C). The AD samples (M = −0.040, SD = 0.118) reacted significantly more than the PD samples (M = −0.293, SD = 0.168, P = 0.013) and almost significantly greater than the ND samples (M = −0.211, SD = 0.110, P = 0.070).
Figure 5.
Human Sera Samples
Oligomeric Alpha-Synuclein
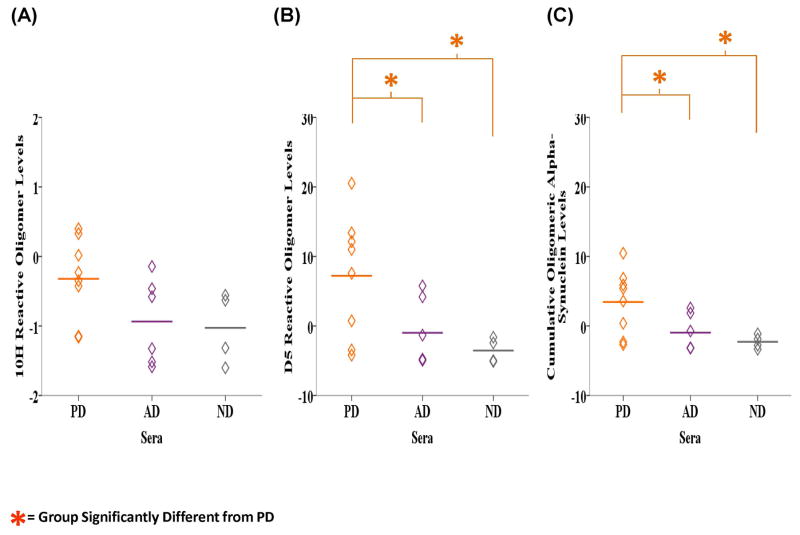
Finally, we analyzed the corresponding PD, AD and ND sera samples for the presence of oligomeric alpha-synuclein using the 10H and D5 scFvs. Detection in sera constitutes a less invasive diagnostic process making sera the preferred test medium in our detection system. The average level of 10H reactive oligomeric alpha-synuclein in the sera samples were higher in the PD samples (M = −0.322, SD = 0.593) compared to the AD (M = −0.937, SD = 0.615) and ND samples (M = −1.027, SD = 0.513) (Fig. 6A). ANOVA analysis showed a significant difference in the levels of D5 (F2, 15 = 4.431, P = 0.031) and cumulative 10H and D5 (F2, 15 = 4.475, P = 0.030) reactive oligomeric alpha-synuclein between the groups (Figs. 6B and C). The levels of D5 reactive oligomeric alpha-synuclein were significantly higher in the PD samples (M = 7.217, SD = 8.783) compared to AD (M = −0.984, SD = 4.840, P = 0.038) and ND (M = −3.540, SD = 1.770, P = 0.019) samples based on LSD post-hoc analyses. The same was true for the cumulative levels of 10H and D5 reactive oligomeric alpha-synuclein in the PD samples (M = 3.448, SD = 4.638) compared to the AD (M = −0.960, SD = 2.652, P = 0.036) and ND cases (M = −2.283, SD = 0.980, P = 0.019).
Figure 6.
Oligomeric Beta-Amyloid
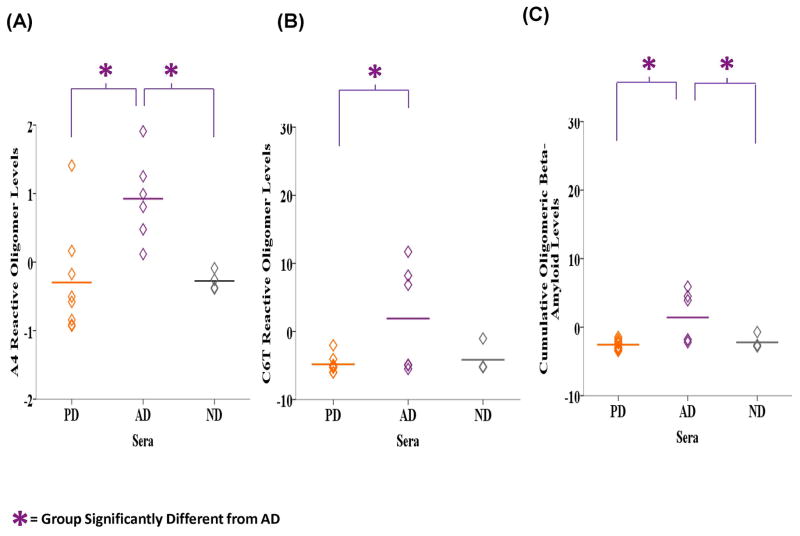
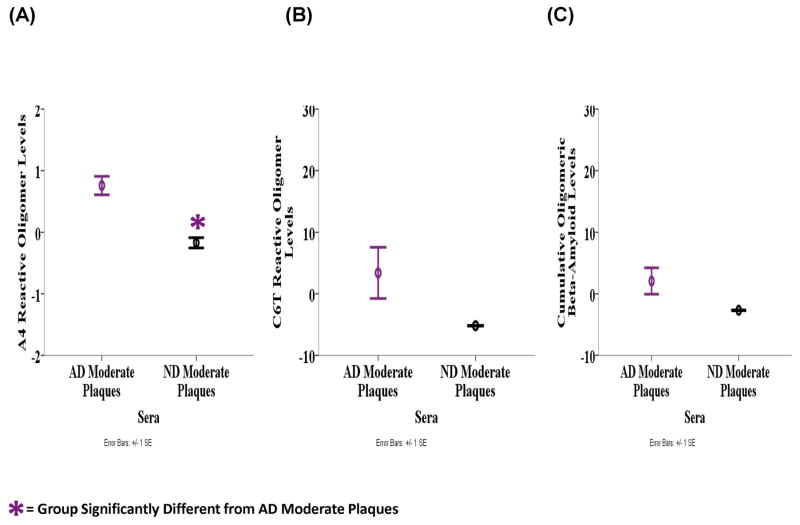
Oligomeric beta-amyloid levels in sera were also evaluated and on average, the level of A4 reactive oligomeric beta-amyloid in the sera samples was significantly different between the AD, PD and ND groups based on ANOVA analyses (F2, 15 = 6.992, P = 0.007) (Fig. 7A). LSD post-hoc analyses revealed that the AD samples (M = 0.926, SD = 0.624) contained higher levels of A4 reactive oligomeric beta-amyloid compared to the PD (M = −0.297, SD = 0.787, P = 0.003) and ND (M = −0.276, SD = 0.138, P = 0.012) samples. The average C6T reactivity was significantly different between the groups (F2, 15 = 3.843, P = 0.045) where the AD samples (M = 1.899, SD = 7.854) contain significantly higher oligomeric beta-amyloid level compared to PD samples (M = −4.833, SD = 1.293, P = 0.018) and almost significantly more than the ND samples (M = −4.167, SD = 2.084, P = 0.065) (Fig. 7B). Similarly, the mean cumulative level of A4 and C6T reactive oligomeric beta-amyloid in the sera samples were significantly different between the groups (F2, 15 = 5.838, P = 0.013) (Fig. 7C). Based on LSD post-hoc analyses the AD cases (M = 1.412, SD = 3.746) had significantly higher levels compared to PD (M = −2.565, SD = 0.744, P = 0.005) and ND (M = −2.222, SD = 1.008, P = 0.025) cases. If we just compare oligomeric beta-amyloid levels in the three AD sera samples with moderate plaques to the three ND samples with moderate plaques, levels of A4 reactive oligomeric beta-amyloid was significantly higher in the AD group (M = 0.758, SD = 0.260) compared to the ND group (M = −0.173, SD = 0.118) (F1, 3 = 20.943, P = 0.020) (Fig. 8A), while C6T reactive oligomeric beta-amyloid level was also higher in the AD group (M = 3.394, SD = 7.192) compared to ND group (M = −5.186, SD = 0.057) (Fig. 8B). The mean cumulative A4 and C6T reactive oligomeric beta-amyloid level was also higher in the AD sera cases with moderate plaques (M = 2.076, SD = 3.713) compared to the ND sera cases with moderate plaques (M = −2.679, SD = 0.088) (Figs. 8C).
Figure 7.
Figure 8.
Comparison of Cumulative Oligomeric Alpha-Synuclein and Beta-Amyloid Levels
The levels of 10H and D5 reactive oligomeric alpha-synuclein and A4 and C6T oligomeric beta-amyloid for each individual human brain tissue, CSF and sera samples are shown in Tables 2 and 3, respectively. The average signal ratio to PBS for each sample was divided by the mean +2SD of the ND group. Higher oligomeric protein levels are indicated with “+” signs as follows: “+” is an oligomeric protein level greater than the mean +2SD, “++” is an oligomeric level greater than the mean +4SD, and each additional “+” represents an oligomeric protein level that is an additional 2SD greater than the mean.
Increased level of 10H reactive oligomeric alpha-synuclein was detected in the brain tissue of 8 of the 9 PD cases and 1 ND case (Table 2). The D5 scFv also reacted with 8 of the 9 PD samples and the same ND case that reacted with 10H. Impressively, all 9 PD cases were selected between 10H and D5 since the one PD case not recognized by 10H was identified by D5 and vice versa. In CSF, 10H selected all the tested PD cases, while D5 recognized 4 of 6 PD samples. Therefore, between 10H and D5 all PD cases were identified. Lastly, utilizing sera, 10H reacted with 3 of the 8 PD cases, while D5 selected 6 of the 8 PD cases. Overall, the levels of 10H and D5 reactive oligomeric alpha-synuclein showed a strong preference for the PD samples compared to the AD and control samples.
In brain tissue, A4 reactive oligomeric beta-amyloid was detected in 3 of the AD cases and 4 of the PD samples, while C6T also selected 3 of the AD cases (Table 3). Together, the A4 and C6T scFvs selected all 6 AD samples since the three cases identified by A4 were different from the three recognized by C6T. For CSF A4 had reactivity with 4 of the AD cases, C6T with two and together A4 and C6T selected 5 of the 6 AD samples. In sera, A4 reactive oligomeric beta-amyloid was identified in all 6 AD samples. C6T reactive oligomeric beta-amyloid was present in 3 AD cases. Therefore, A4 and C6T identified all 6 AD sera samples.
Mixed Protein Pathology
Using the different anti-oligomeric scFvs, we could distinguish AD, PD and ND cases equally well using brain tissue, CSF and sera samples supporting the value of specific protein variants in CSF and/or sera as biomarkers for different neurodegenerative diseases. Further analysis of the CSF and sera samples tested here indicates mixed pathology present in several cases, where some AD samples also displayed reactivity with our anti-oligomeric alpha-synuclein scFvs and some of the PD samples also displayed reactivity with our anti-oligomeric beta-amyloid scFv. The anti-synuclein 10H and D5 scFvs were collectively reactive with 3 CSF and 2 sera AD samples (Table 2). The two sera AD samples that showed positive alpha-synuclein reactivity also showed positive reactivity in the corresponding CSF samples. Similarly, when analyzing for oligomeric beta-amyloid, two PD sera samples showed reactivity (Table 3). These results indicate that multiple protein pathologies associated with different neurodegenerative diseases could be present in one individual.
Sensitivity and Specificity
To determine the sensitivity and specificity of the 10H and D5 scFvs for the PD group we counted a case as positive if the sample displayed reactivity with 10H and/or D5 and compared them to reactivity with the ND samples (Table 4). Analyzing brain tissue samples, all 9 PD cases were selected along with 1 ND yielding 100% sensitivity and 80% specificity. With CSF samples, all 6 PD cases and none of the ND cases were positive making the sensitivity and specificity both 100%. Finally, with sera samples 6 of the 8 PD cases and none of the ND cases were positive yielding 75% sensitivity and 100% specificity. The AD cases were analyzed in a similar manner based on positive reaction with A4 and/or C6T compared to the controls (Table 4). Analyzing brain tissue samples, all 6 AD and none of the ND samples were positive yielding 100% sensitivity and specificity. With CSF samples 5 of the 6 AD and none of the ND samples were positive yielding 83% sensitivity and 100% specificity. Finally, utilizing sera samples all 6 AD and none of the ND cases were selected, again yielding 100% sensitivity and specificity.
Table 4.
Sensitivity and Specificity
| Sensitivity | Specificity | |
|---|---|---|
| PD Brain Tissue | 100 | 80 |
| PD CSF | 100 | 100 |
| PD Sera | 75 | 100 |
| AD Brain Tissue | 100 | 100 |
| AD CSF | 83 | 100 |
| AD Sera | 100 | 100 |
Discussion
Oligomeric variants of the alpha-synuclein and beta-amyloid proteins have been implicated in the underlying pathology of PD and AD, respectively (Cox et al., 2014; Chaari et al., 2013; Halliday & McCann, 2008; Lynch et al., 2008; Emadi et al., 2007; Emadi et al., 2009; Sierks et al., 2011; Sinjoanu et al., 2008; Wang et al., 2010; Crimins et al., 2013; Wischik et al., 2014; Pooler et al., 2014; Tian et al., 2013; Zhou et al., 2012; Salvadores et al., 2014; Klein et al., 2001; Park et al., 2013; Yang et al., 2013). Because of their important roles in these devastating diseases, reagents that can detect specific toxic oligomeric protein variants in human samples have potential value as tools to facilitate diagnosis of these diseases and to study mechanisms of disease progression. We developed biopanning technology that enables us to isolate scFvs reactive with specific protein variants and a capture ELISA protocol that allows sensitive sub-femtomolar detection (Williams et al., 2014; Williams et al., 2015). The scFvs 10H and D5 were previously characterized for specificity to different oligomeric variants of alpha-synuclein. 10H recognized in vitro generated aggregates of molecular weights 42 kDa and ~80 kDa whereas D5 reacted with in vitro generated alpha-synuclein aggregates of molecular weights 29 kDa and 56 kDa (Emadi et al., 2007; Emadi et al., 2009) (Supplementary Fig. 1A). Detection of multiple oligomeric protein morphologies increases the probability of detecting oligomeric alpha-synuclein pathology in any given sample. Similarly the A4 and C6T scFvs bind different oligomeric variants of beta-amyloid where A4 binds an in vitro generated beta-amyloid oligomeric species while C6T binds an AD brain derived beta-amyloid oligomeric species (Supplementary Fig. 1B). In this study we used all four of these morphology specific scFvs with our phage capture ELISA to analyze post-mortem human brain tissue, CSF and sera samples from PD, AD and ND cases to identify samples with oligomeric alpha-synuclein and/or beta-amyloid pathology in order to distinguish PD from controls, AD from controls and PD or AD cases with mixed protein pathology. Using these morphology specific scFvs as the capture antibody and a protein specific scFv (D10 for alpha-synuclein, H1v2 for beta-amyloid) as a detection antibody provides two different means to ensure that we are selectively detecting alpha-synuclein or beta-amyloid aggregates. First the capture scFvs were chosen because of their selectivity for the target protein aggregate and because the scFvs do not show cross reactivity with other protein aggregates (Emadi et al., 2007; Emadi et al., 2009; Kasturirangan et al., 2013; Zameer et al., 2008; Kasturirangan et al., 2012). Second, the detection antibody is protein specific, selectively binding either the alpha-synuclein (D10) or beta-amyloid (H1v2) aggregate already in complex with the capture scFv.
The levels of 10H and/or D5 reactive oligomeric alpha-synuclein were statistically greater for the PD samples compared to the AD and ND groups (Table 2) using brain homogenates (Figs. 1A, 1B and 1C), CSF (Figs. 4A, B and C) and sera samples (Figs. 6A, B and C). Jointly, 10H and D5 selected all the PD brain tissue and CSF samples and 6 out of 8 PD sera samples. To determine the sensitivity and specificity of the D5 and 10H scFvs collectively for PD, the results obtained with the PD samples were compared to the respective ND samples (Table 4). With brain tissue our sensitivity was 100% and specificity 80% (one ND case was selected). The sensitivity and specificity were both 100% with CSF and 75% and 100%, respectively, with sera. Collectively, these results demonstrate that detection of specific oligomeric alpha-synuclein variants is a powerful tool to distinguish PD from control samples. Oligomeric alpha-synuclein has been previously detected in the plasma of PD subjects using undiluted samples (El-Agnaf et al., 2006) providing precedent for our identification of oligomeric alpha-synuclein in media such as CSF and sera. Because of the sub-femtomolar sensitivity of our capture ELISA (Williams et al., 2014), we could dilute the CSF and sera samples 1 in 100 and still obtain significant signal differences. A simple ELISA protocol that can selectively identify PD patients from sera samples has great promise as it would alleviate painful sample procurement and could facilitate a more accurate and widespread diagnosis.
Levels of A4 and C6T reactive oligomeric beta-amyloid clearly distinguish AD brain tissue homogenates (Figs. 2A, B and C), CSF (Figs. 5A, B and C) and sera samples (Figs. 7A, B and C) from the controls and most PD samples. In CSF and sera the levels of A4 reactive oligomers were statistically greater in the AD cases compared to both the PD and ND groups. Cumulative A4 and C6T oligomeric beta-amyloid levels selected all six AD brain tissue samples, five of six CSF samples and all six sera samples (Table 3). Sensitivity and specificity of the A4 and C6T scFvs collectively for AD compared to ND samples was also determined. Analysis of both brain tissue and sera samples yielded sensitivity and specificity values of 100% for AD compared to ND and analysis of CSF samples yielded a sensitivity of 83% and specificity of 100% (Table 4). These results demonstrate support for the application of our oligomeric beta-amyloid scFvs as potential diagnostic biomarkers for AD pathology. Furthermore, when comparing the levels of oligomeric beta-amyloid in AD cases having moderate plaque loads to cognitively normal cases also possessing moderate plaque loads, we could readily distinguish between these two groups using either brain tissue or more impressively sera samples. These results demonstrate that it may be possible to distinguish between cognitively normal and AD patients with similar plaque loads using simple blood tests. Additionally, the results provide further evidence for a strong correlation between the presence of specific oligomeric variants of beta-amyloid and AD dementia rather than between plaque loads and dementia.
Oligomeric beta-amyloid variants have been implicated in the progression of AD (Wisniewski & Goñi, 2014; van Helmond et al., 2009; Resende et al., 2008; Klaver et al., 2011) and we show here that the presence of selected oligomeric beta-amyloid variants in sera samples can be used to differentiate AD cases from controls. Similarly oligomeric alpha-synuclein has been implicated in PD (Cox et al., 2014; Chaari et al., 2013; Halliday & McCann, 2008; Lynch et al., 2008; Emadi et al., 2007; Emadi et al., 2009; Sierks et al., 2011; Wang et al., 2010) and we also show that the presence of selected oligomeric alpha-synuclein variants in CSF or sera can be used to differentiate PD cases from controls. The presence of oligomeric beta-amyloid however was also detected in two PD sera samples and oligomeric alpha-synuclein was present in three AD CSF and two AD sera samples (Tables 2 and 3). The presence of PD pathology in some AD cases and AD pathology in some PD cases is to be expected since there is significant overlap between the different neurodegenerative diseases. Interestingly, two of the AD samples contained oligomeric alpha-synuclein in both CSF and sera samples (Table 2) suggesting that early stage alpha-synuclein pathology may be developing in these samples. The presence of alpha-synuclein positive lewy bodies were reported in 22% of familial AD cases and 50–60% of sporadic AD cases particularly in the amygdala (Wirths & Bayer, 2003; Lippa et al., 1998; Hamilton, 2000; Swirski et al., 2014) so the presence of oligomeric alpha-synuclein should be expected in a substantial percentage of the AD samples. In a parallel fashion, dementia affects nearly one third of Parkinson’s patients (Martí et al., 2007) and there is an increase in cortical Aβ plaque loads in Parkinson’s disease with dementia (PDD) patients (Irwin et al., 2013). Therefore a significant percentage of PD cases should show beta-amyloid pathology as reflected here by the presence of A4 reactive oligomeric beta-amyloid in sera. Interestingly, the two PD samples with high oligomeric beta-amyloid levels in serum also had a history of dementia based on post-mortem pathological analysis. None of the AD samples analyzed here indicated the presence of alpha-synuclein containing Lewy bodies based on the unified staging system for Lewy body disorders (Beach et al., 2009). However, the presence of oligomeric alpha-synuclein in two of the AD samples is consistent with the incidence of alpha-synuclein pathology in AD cases and suggests that the presence of oligomeric alpha-synuclein may be potential early stage biomarkers for alpha synuclein pathology, an analysis which will need additional studies using larger sample sizes.
When analyzing oligomeric alpha-synuclein and beta-amyloid content in our samples, we utilized two scFvs for each protein target to increase the likelihood of binding disease related variants of the targeted proteins. If a sample does not contain protein variants recognized by one scFv it may contain variants recognized by the second scFv. Additionally, calculation of cumulative levels of multiple protein variants, for example total 10H and D5 reactive oligomers may increase distinction between healthy and affected individuals since 10H and D5 recognize different protein variants (Emadi et al., 2007; Emadi et al., 2009). The cumulative level is based on tallying the results from each scFv tested separately rather than adding both scFvs to a single well because higher concentrations of each scFv can be immobilized when analyzing separately, increasing the sensitivity of the assay. Overall, the anti-alpha-synuclein scFvs reacted strongly with the PD samples while the anti-beta-amyloid scFvs reacted strongly with the AD samples, though occasionally selected AD samples reacted with the oligomeric alpha-synuclein scFvs and selected PD samples reacted with the oligomeric beta-amyloid scFv. These results suggest the possibility that personalized treatment plans may be required to treat different patients. For example, if a PD patient also showed high beta-amyloid reactivity, a personalized treatment plan could be created to target both the alpha-synuclein and beta-amyloid aggregates, whereas a PD patient with only alpha-synuclein would benefit from a different therapy targeting only the identified alpha-synuclein variants. This customized therapeutic approach may enable more efficient and effective treatment strategies to be administered based primarily on the personalized results of such diagnostic tests.
Some caution should be taken in interpreting results obtained with brain tissue samples. Here all brain tissue samples were obtained from the MTG. Since both AD and PD progress from one region in the brain to another, but in different patterns, future evaluation of multiple brain regions from both AD and PD subjects may provide further insight into how protein pathologies progress in these diseases. It should also be pointed out that while we could readily distinguish AD, PD and ND samples in the cases studied here, we expect the result will be even better with more cases taken during disease progression. The different small soluble oligomeric variants of beta-amyloid and alpha-synuclein that are detected by the scFvs we used here are likely preferentially generated during earlier stages of disease progression and therefore present at lower concentrations during later stages. Since all the samples tested here were from end-stage post-mortem pathologically verified PD and AD cases which have well formed fibrillar alpha-synuclein or beta-amyloid aggregates respectively, we expect that higher concentrations of the relevant oligomeric protein species may be obtained in samples taken during early stages of disease progression. Also, the average age of our control cases is 87 years, where the chances of any of the cases having an undiagnosed early stage neurodegenerative disease are relatively high, and therefore the signals we obtained with the PD and AD cases could be much higher if younger controls were utilized to determine baseline measurements. Table 1 provides some evidence for such undiagnosed pathology since three out of five control cases had microscopic changes of AD in their pathology which was insufficient for diagnosis, but which may result in the control samples producing higher signals in the ELISA studies. However, even with the post-mortem end-stage samples used here and the aged control cases, we could still readily distinguish between the PD, AD and ND cases.
The statistically significant data obtained in this study with our four scFvs and small sample size indicates merit for future studies with larger sample sizes. Such significant findings in a larger sample size will help to further validate the potential diagnostic capability of our scFvs with this phage ELISA technology. We therefore performed power analysis to determine the sample size needed in a future cohort using the results with sera since diagnostics with sera would be the most wide spread application. The power was set to 0.8, the alpha level 0.05 and the effect size calculated using the mean and standard deviations from the current study. Analyzing the data obtained when using the 10H scFv, a total of 18 PD and ND samples would be needed. We utilized a total of 12 samples (8 PD and 4 ND) in the current study and obtained a p-value of 0.068. Analysis using the D5 scFv would require 12 total samples to get sufficient power which is expected since our current sample size of 12 samples produced statistically significant results. Similarly analysis using the A4 scFv requires 6 samples to get sufficient power and we used 10 (6 AD and 4 ND) in the current sample and obtained statistically significant results. Power analysis for the C6T scFv indicated we would need 24 samples. The small sample size requirements for these studies to obtain sufficient power reflects the proficiency of the oligomeric morphology selective scFvs at distinguishing between diseased and healthy individuals. While power analysis of the data indicates we only require relatively small sample sizes, we intend to use substantially larger sample sizes in future studies to validate these results. We also plan to analyze ante-mortem sera samples to determine the potential use of specific aggregated beta-amyloid and alpha-synuclein variants as early blood based biomarkers for AD and PD. Analysis of longitudinal ante-mortem samples may provide valuable information on how concentration levels of select protein variants change during the course of different neurodegenerative diseases, and provide additional insight into potential toxic mechanisms associated with these diseases. Overall, in this study we utilized a panel of four scFvs that recognize two different oligomeric variants of alpha-synuclein and two different oligomeric beta-amyloid variants to characterize human post-mortem brain tissue, CSF and sera samples of PD, AD and cognitively normal control cases. The presence of the oligomeric alpha-synuclein variants readily identified PD brain tissue, CSF and sera samples while the presence of oligomeric beta-amyloid variants readily identified AD brain tissue, CSF and sera samples. In select cases the presence of both variants indicated PD cases with dementia or AD cases with alpha-synuclein pathology. These results indicate the promise of using selected protein variants as biomarkers in CSF or sera for individual diagnoses of specific neurodegenerative disorders and the promise for developing personalized treatment plans.
Supplementary Material
Acknowledgments
We would like to thank Ricky Pham and Now Bahar Alam for their contributions to this study. We would also like to thank Dr. Geidy E. Serrano at Banner Sun Health Research Institute for her assistance with patient information. We are grateful to the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of post-mortem human samples. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. This research was partially supported by grants from the Michael J Fox Foundation for Parkinson’s Research and from DOD, Grant Number: W81XWH-12-1-0583.
Abbreviations
- AD
Alzheimer’s disease
- AFM
Atomic Force Microscopy
- BCA
Bicinchoninic Acid
- CSF
Cerebrospinal fluid
- EDTA
Ethylenediaminetetraacetic acid
- FPLC
Fast Protein Liquid Chromatography
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- LB
Lewy bodies
- M
Mean
- MTG
Middle Temporal Gyrus
- ND
Controls
- PBST
Phosphate-Buffered Saline with Tween-20
- PD
Parkinson’s disease
- PDD
Parkinson’s disease dementia
- PMI
Post-Mortem Interval
- scFv
Single Chain Variable Fragment
- SD
Standard Deviation
Footnotes
The authors have no conflict of interest to declare.
Contributor Information
Stephanie M. Williams, Email: swilli4@asu.edu.
Philip Schulz, Email: Philip.Schulz@asu.edu.
Michael R. Sierks, Email: sierks@asu.edu.
References
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, 3rd, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35:354–389. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: Description and Eexperience, 1987–2007. Cell Tissue Banking. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaari A, Hoarau-Véchot J, Ladjimi M. Applying chaperones to protein-misfolding disorders: Molecular chaperones against α-synuclein in Parkinson’s disease. Int J Biol Macromol. 2013;60:196–205. doi: 10.1016/j.ijbiomac.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Cox D, Carver JA, Ecroyd H. Preventing α-synuclein aggregation: The role of the small heat-shock molecular chaperone proteins. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842:1830–1843. doi: 10.1016/j.bbadis.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010;19:R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires-Jones TL. The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer’s disease. Ageing Research Reviews. 2013;12:757–763. doi: 10.1016/j.arr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s Disease: Mechanisms and Models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Di Carlo M, Giacomazza D, San Biagio PL. Alzheimer’s disease: biological aspects, therapeutic perspectives and diagnostic tools. Journal of Physics: Condensed Matter. 2012;24:244102. doi: 10.1088/0953-8984/24/24/244102. [DOI] [PubMed] [Google Scholar]
- El-Agnaf OMA, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of α-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. The FASEB Journal. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR. Isolation of a Human Single Chain Antibody Fragment Against Oligomeric α-Synuclein that Inhibits Aggregation and Prevents α-Synuclein-induced Toxicity. J Mol Biol. 2007;368:1132–1144. doi: 10.1016/j.jmb.2007.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi S, Kasturirangan S, Wang MS, Schulz P, Sierks MR. Detecting Morphologically Distinct Oligomeric Forms of α-Synuclein. J Biol Chem. 2009;284:11048–11058. doi: 10.1074/jbc.M806559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Muñoz MJ, Castillo-Carranza DL, Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol. 2014;88:468–478. doi: 10.1016/j.bcp.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Halliday GM, McCann H. Human-based studies on α-synuclein deposition and relationship to Parkinson’s disease symptoms. Exp Neurol. 2008;209:12–21. doi: 10.1016/j.expneurol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Hamilton RL. Lewy Bodies in Alzheimer’s Disease: A Neuropathological Review of 145 Cases Using α-Synuclein Immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJM, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int J Dev Neurosci. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Lee VMY, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nature reviews Neuroscience. 2013;14:626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturirangan S, Li L, Emadi S, Boddapati S, Schulz P, Sierks MR. Nanobody specific for oligomeric beta-amyloid stabilizes nontoxic form. Neurobiol Aging. 2012;33:1320–1328. doi: 10.1016/j.neurobiolaging.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Kasturirangan S, Reasoner T, Schulz P, Boddapati S, Emadi S, Valla J, Sierks MR. Isolation and characterization of antibody fragments selective for specific protein morphologies from nanogram antigen samples. Biotechnol Prog. 2013;29:463–471. doi: 10.1002/btpr.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver AC, Patrias LM, Finke JM, Loeffler DA. Specificity and sensitivity of the Abeta oligomer ELISA. J Neurosci Methods. 2011;195:249–254. doi: 10.1016/j.jneumeth.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Aβ oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Fujiwara H, Mann DMA, Giasson B, Baba M, Schmidt ML, Nee LE, O’Connell B, Pollen DA, St George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VMY, Iwatsubo T, Trojanowski JQ. Lewy Bodies Contain Altered α-Synuclein in Brains of Many Familial Alzheimer’s Disease Patients with Mutations in Presenilin and Amyloid Precursor Protein Genes. The American Journal of Pathology. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SM, Zhou C, Messer A. An scFv Intrabody against the Nonamyloid Component of α-Synuclein Reduces Intracellular Aggregation and Toxicity. J Mol Biol. 2008;377:136–147. doi: 10.1016/j.jmb.2007.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí M, Tolosa E, de la Cerda A. Dementia in Parkinson’s disease. J Neurol. 2007;254:41–48. [Google Scholar]
- Martin HL, Teismann P. Glutathione—a review on its role and significance in Parkinson’s disease. The FASEB Journal. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- Moreth J, Mavoungou C, Schindowski KZ. Is Abeta a sufficient Biomarker for monitoring anti-Abeta clinical studies? A critical review. Front Aging Neurosci. 2013:5. doi: 10.3389/fnagi.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Jang M, Chang S. Deleterious effects of soluble amyloid-β oligomers on multiple steps of synaptic vesicle trafficking. Neurobiol Dis. 2013;55:129–139. doi: 10.1016/j.nbd.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Noble W, Hanger DP. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology. 2014;76(Part A):1–8. doi: 10.1016/j.neuropharm.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Reitz C, Mayeux R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende R, Ferreiro E, Pereira C, Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1–42: Involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F, Soto C. Detection of Misfolded Aβ Oligomers for Sensitive Biochemical Diagnosis of Alzheimer’s Disease. Cell Reports. 2014;7:261–268. doi: 10.1016/j.celrep.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Sierks MR, Chatterjee G, McGraw C, Kasturirangan S, Schulz P, Prasad S. CSF levels of oligomeric alpha-synuclein and beta-amyloid as biomarkers for neurodegenerative disease. Integrative Biology. 2011;3:1188–1196. doi: 10.1039/c1ib00018g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinjoanu RC, Kleinschmidt S, Bitner RS, Brioni JD, Moeller A, Ferreira A. The novel calpain inhibitor A-705253 potently inhibits oligomeric beta-amyloid-induced dynamin 1 and tau cleavage in hippocampal neurons. Neurochem Int. 2008;53:79–88. doi: 10.1016/j.neuint.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proceedings of the National Academy of Sciences. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. [alpha]-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Swirski M, Miners JS, de Silva R, Lashley T, Ling H, Holton J, Revesz T, Love S. Evaluating the relationship between amyloid-β and α-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease. Alzheimers Res Ther. 2014;6:77. doi: 10.1186/s13195-014-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Davidowitz E, Lopez P, Emadi S, Moe J, Sierks M. Trimeric Tau Is Toxic to Human Neuronal Cells at Low Nanomolar Concentrations. Int J Cell Biol. 2013;2013:9. doi: 10.1155/2013/260787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX, Masliah E. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLoS One. 2008;3:e3135. doi: 10.1371/journal.pone.0003135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- van Helmond Z, Heesom K, Love S. Characterisation of two antibodies to oligomeric Aβ and their use in ELISAs on human brain tissue homogenates. J Neurosci Methods. 2009;176:206–212. doi: 10.1016/j.jneumeth.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Wang M, Boddapati S, Emadi S, Sierks M. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 2010;11:57. doi: 10.1186/1471-2202-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Schulz P, Sierks MR. A Sensitive phage-based capture ELISA for sub-femtomolar detection of protein variants directly from biological samples. Biotechnol Prog. 2014:n/a–n/a. doi: 10.1002/btpr.1987. [DOI] [PubMed] [Google Scholar]
- Williams SM, Venkataraman L, Tian H, Khan G, Harris BT, Sierks MR. Novel Atomic Force Microscopy Based Biopanning for Isolation of Morphology Specific Reagents against TDP-43 Variants in Amyotrophic Lateral Sclerosis. 2015:e52584. doi: 10.3791/52584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Bayer TA. α-Synuclein, Aβ and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:103–108. doi: 10.1016/S0278-5846(02)00339-1. [DOI] [PubMed] [Google Scholar]
- Wischik CM, Harrington CR, Storey JMD. Tau-aggregation inhibitor therapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:529–539. doi: 10.1016/j.bcp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:499–507. doi: 10.1016/j.bcp.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W, Emadi S, Williams S, Liu Q, Schulz P, He P, Alam N, Wu J, Sierks M. Toxic Oligomeric Alpha-Synuclein Variants Present in Human Parkinson’s Disease Brains Are Differentially Generated in Mammalian Cell Models. Biomolecules. 2015;5:1634. doi: 10.3390/biom5031634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Hong S, O’Malley T, Sperling RA, Walsh DM, Selkoe DJ. New ELISAs with high specificity for soluble oligomers of amyloid β-protein detect natural Aβ oligomers in human brain but not CSF. Alzheimer’s & Dementia. 2013;9:99–112. doi: 10.1016/j.jalz.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Schulz P, Sierks MR. Improved Affinity Selection of an scFv Antibody Fragment Against -amyloid Using Phage Display Technology and Off-rate Based Selection. Electronic Journal of Biotechnology. 2006;9:171–175. [Google Scholar]
- Zameer A, Kasturirangan S, Emadi S, Nimmagadda SV, Sierks MR. Anti-oligomeric Aβ Single-chain Variable Domain Antibody Blocks Aβ-induced Toxicity Against Human Neuroblastoma Cells. J Mol Biol. 2008;384:917–928. doi: 10.1016/j.jmb.2008.09.068. [DOI] [PubMed] [Google Scholar]
- Zhou C, Emadi S, Sierks MR, Messer A. A Human Single-Chain Fv Intrabody Blocks Aberrant Cellular Effects of Overexpressed [alpha]-Synuclein. Mol Ther. 2004;10:1023–1031. doi: 10.1016/j.ymthe.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chan KH, Chu LW, Kwan JSC, Song YQ, Chen LH, Ho PWL, Cheng OY, Ho JWM, Lam KSL. Plasma amyloid-β oligomers level is a biomarker for Alzheimer’s disease diagnosis. Biochem Biophys Res Commun. 2012;423:697–702. doi: 10.1016/j.bbrc.2012.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.