Abstract
Cancer stem‐like cells (CSC)/cancer‐initiating cells (CIC) are defined as minor subpopulations of cancer cells that are endowed with properties of higher tumor‐initiating ability, self‐renewal ability and differentiation ability. Accumulating results of recent studies have revealed that CSC/CIC are resistant to standard cancer therapies, including chemotherapy, radiotherapy and molecular targeting therapy, and eradiation of CSC/CIC is, thus, critical to cure cancer. Cancer immunotherapy is expected to become the “fourth” cancer therapy. Cytotoxic T lymphocytes (CTL) play an essential role in immune responses to cancers, and CTL can recognize CSC/CIC in an antigen‐specific manner. CSC/CIC express several tumor‐associated antigens (TAA), and cancer testis (CT) antigens are reasonable sources for CSC/CIC‐targeting immunotherapy. In this review article, we discuss CSC/CIC recognition by CTL, regulation of immune systems by CSC/CIC, TAA expression in CSC/CIC, and the advantages of CSC/CIC‐targeting immunotherapy.
Keywords: Cancer stem cell, cancer testis antigen, cytotoxic T lymphocyte, immunotherapy, tumor antigen
Cancers are comprised of subpopulations of cells that are heterogeneous with regard to morphology, phenotype and functions. Several factors are associated with the heterogeneity of cancer, including genetic mutations, epigenetic changes, interaction with the microenvironment and metabolic changes. Two models have been proposed to explain the heterogeneity of cancer: (i) the clonal evolution model and (ii) the cancer stem cell model.1, 2 The acquisition of genetic mutations is the fundamental aspect of the clonal evolution model, in which cells with higher tumorigenic potential are dominant in the tumor. In the cancer stem cell model, heterogeneous cells make a hierarchical differentiation model and a small subset of cells are endowed with tumorigenicity. Highly tumorigenic cells are referred to as cancer stem‐like cells (CSC)/cancer‐initiating cells (CIC) due to their tumor‐initiating ability, self‐renewal ability and differentiation ability. Human CSC/CIC have been successfully isolated from several malignancies, including hematological malignancies, solid cancers and sarcomas. Accumulating results indicate that CSC/CIC are resistant to standard therapies, including chemotherapy, radiotherapy and molecular targeting therapy, because CSC/CIC are in a quiescent phase of the cell cycle and they have high expression levels of transporters and high expression levels of anti‐apoptosis proteins and are resistant to DNA damage.3 CSC/CIC are, thus, thought to be responsible for lethal events, including recurrence after treatment and distant metastasis. These distinct characters make CSC/CIC a reasonable and fascinating target for next‐generation cancer therapies.
Following the identification of human tumor‐associated antigens (TAA), cancer‐specific immunotherapy using TAA was initiated worldwide, and cancer immunotherapy is now expected to become the fourth cancer treatment following surgery, chemotherapy and radiotherapy.4, 5 We identified a novel antigenic peptide derived from survivin/BIRC5, which is a member of the family of inhibitors of apoptosis proteins (IAP), and started peptide vaccine therapy in 2004.6, 7, 8, 9, 10 We observed partial anti‐tumor effects in some cases of treatment‐refractory advanced cancer; however, there are still some aspects of current cancer therapy that need to be improved. We thus focused on CSC/CIC as a possible target of cancer immunotherapy to achieve more efficient cancer immunotherapy. Human CSC/CIC derived from colon, lung, prostate, ovary, kidney, head and neck cancers and sarcomas were isolated and analyzed.11, 12, 13, 14, 15, 16, 17, 18 We found that CSC/CIC express several TAA and that cytotoxic T lymphocytes (CTL) can recognize CSC/CIC both in vitro and in vivo.19, 20 Therefore, CSC/CIC are the next target for next‐generation cancer immunotherapy.
Immunological Phenotypes of Cancer Stem‐like Cells
Because CSC/CIC are resistant to standard therapies, susceptibility to effectors of immunity is a challenging issue. The immune system is composed of innate immunity and adaptive immunity. Phagocytes, natural killer (NK) cells and γδ T cells are effectors of innate immunity, and these effectors recognize target cells in an antigen non‐specific manner. CTL and antibodies are effectors of cellular and humoral immunity, respectively, and they recognize target cells in an antigen specific manner. NK cells, γδ T cells, CTL and antibodies have been reported to be able to recognize CSC/CIC, and only effectors of adaptive immunity can recognize CSC/CIC specifically.21
Antigen presentation of Cancer stem‐like cells/cancer‐initiating cells and recognition by cytotoxic T lymphocytes
Expression of antigenic peptides presented by major histocompatibility complex (MHC) (human leukocyte antigen [HLA] in human) class I is essential for recognition by CTL. MHC class I expression has long been thought to be related to disease progression and survival of cancer patients, and immune surveillance is believed to play a pivotal role in cancer progression.22, 23, 24, 25 Antigenic peptides are produced from fully folded mature proteins or unfolded immature amino acid fragments know as defective ribosomal products (DRiP). Oligo‐peptides are produced from proteins by proteasome in the cytosol and are transported into the endopoasmic reticulum (ER) through transporters associated with antigen processing‐1 and ‐2 (TAP1 and TAP2). Oligo‐peptides are cleaved by endoplasmic reticulum aminopeptidase associated with antigen processing (ERAAP) in the ER to an adequate length (antigenic peptide) and loaded onto MHC class I and β2‐microglobulin (B2M) complex. Several chaperone molecules, including calnexin, calreticulinm tapasin and ERp57, are necessary for antigenic peptide loading onto MHC class I, and the protein complex for peptide loading is referred to as peptide‐loading complex (PLC) (Fig. 1).26
Figure 1.
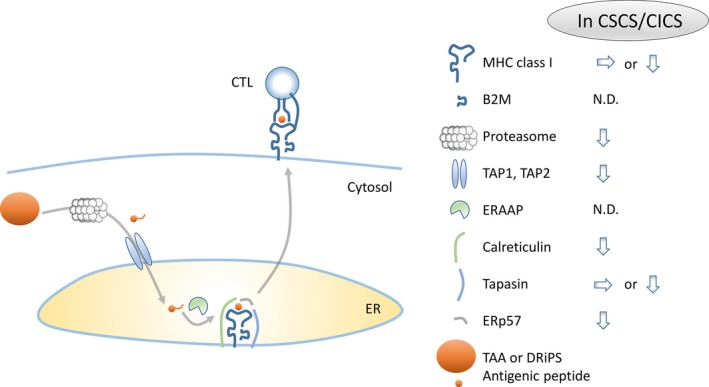
Antigen presentation of cancer stem‐like cells (CSC)/cancer‐initiating cells (CIC). Tumor‐associated antigens (TAA) are digested into oligopeptides by a protease complex, proteasome, in the cytosol. Oligopeptides enter the endopoasmic reticulum (ER) through a transporter complex, TAP. In the ER, oligopeptides are digested by an aminopeptidase, endoplasmic reticulum aminopeptidase associated with antigen processing (ERAAP), and then loaded onto major histocompatibility complex (MHC) class I with the help of chaperone proteins, calreticulin, tapasin and ERp57. The figure summarizes the expression of antigen presentation machinery proteins in CSC/CIC. N.D., not determined.
In our previous study, colon CSC/CIC derived from colon cancer cells as side population (SP) cells showed MHC class I expression, as did non‐CSC/CIC at similar levels.13 In contrast, another study showed that colon CSC/CIC isolated as sphere‐forming cells from colon cancer tissues had lower expression levels of MHC class I than did non‐CSC/CIC and were recognized by NK cells.27 Similar controversial results have been obtained in gliomas. Wei et al.28 report that glioblastoma multiform (GBM) stem cells isolated as sphere‐forming cells from GBM tissues showed significant expression of HLA class I. In contrast, Di Tomaso et al.29 report that GBM stem cells isolated as sphere‐forming cells from GBM tissues showed lower levels of HLA class I expression than the levels in GBM non‐stem cells. Wu et al.30 report that expression of HLA class I was not detected in CD133+ putative GBM stem cells. Melanoma stem cells isolated as ABCB5+ cells from melanoma tissues showed lower expression levels of HLA class I than those found in ABCB5− melanoma non‐stem cells.31 Sphere cultured cells derived from several cell lines showed lower levels of HLA class I expression than those found in serum cultured cells. Interestingly, sphere cultured cells did not respond to HLA class I upregulation by IFNγ treatment, suggesting impaired HLA class I induction machinery.32 The difference in results might be due to differences in CSC/CIC isolation methods, culture conditions and origins.
Several molecules are involved in antigenic peptide processing, transportion into the ER and loading onto MHC class I, and studies on antigen presentation machinery in CSC/CIC are limited. Busse et al.32 characterized sphere cultured cells derived from several cell lines and found that sphere‐forming cells show equal or higher expression levels of antigen‐presenting machinery molecules, including LMP2, LMP7, LMP10 (MECL1), TAP1 and TAP2. However, sphere cultured cells derived from primary GBM samples and colon cancer samples showed lower expression levels or no expression of antigen‐presenting machinery molecules, including LMP2, LMP7, LMP10, TAP1, TAP2, calnexin, calreticulin, ERp57 and tapasin.29, 33 Chikamatsu et al.34 report that CD44+ putative head and neck CSC/CIC showed expression levels of LMP2, LMP7, LMP10 (MECL1), TAP1 and tapasin that were equal to those in CD44− cells, whereas CD44+ cells showed a lower expression level of TAP2 than that in CD44− cells (Fig. 1).
In our previous study, SP cells derived from colon cancer cells showed susceptibility to CEP55, a colon cancer‐related antigen‐specific CTL clone #41, that was equal to that in non‐SP cells.13, 35 Furthermore, CTL clone #41 showed a significant anti‐tumor effect in immune‐deficient mice transplanted with SP cells. Similar results were obtained using a novel cancer testis (CT) antigen, DNAJB8. DNAJB8 is preferentially expressed in CSC/CIC and has an essential role in the maintenance of CSC/CIC derived from renal cell carcinoma cells and colon cancer cells.15, 36 These observations indicate that CSC/CIC have the ability to produce antigenic peptides and to be recognized by CTL both in vitro and in vivo. Ahmed et al.37 report that CD133+ GBM stem cells could be recognized by HER2‐specific CTL, as could CD133− GBM cells. Sphere‐forming GBM stem cells showed higher susceptibility to CTL than did NK cells.38 HER2‐specific peptide vaccination decreased aldehyde dehydrogenase (ALDH)‐positive breast cancer stem cells in MMTV‐PyMT transgenic breast cancer model mice.39 All of these observations in humans and mice suggest that treatment‐resistant CSC/CIC are sensitive to differentiated CTL both in vitro and in vivo.
Immune regulation by cancer stem‐like cells/cancer‐initiating cells
For induction of CTL from CD8+ naïve T cells, naïve T cells must encounter an antigenic peptide presented by antigen‐presenting cells, and then they are activated and clonally expand. Not only signaling through the T cell receptor (TCR) but also second signaling through co‐stimulatory molecules is necessary for full activation (Fig. 2).
Figure 2.

Immune regulation by cancer stem‐like cells (CSC)/cancer‐initiating cells (CIC). The first signal through T cell receptors and the second signal through co‐stimulatory molecules are essential for naïve CD8+ T cells to differentiate into cytotoxic T lymphocytes (CTL). Dendritic cells express both major histocompatibility complex (MHC) class I and co‐stimulatory molecules (B7.1, B7.2) and can induce CTL from naïve CD8+ T cells. On the other hand, CSC/CIC do not express or express at low levels co‐stimulatory molecules and cannot differentiate naïve T CD8+ T cells (#1). Furthermore, CSC/CIC express high levels of co‐stimulatory molecule ligand (PD‐L1) and inhibit CTL cytotoxicity. CSC/CIC secrete immune suppressive cytokines (TGF‐β, IL‐10) and inhibit naïve CD8+ T cell differentiation directly or by inducing Treg cells (#2). DC, dendritic cell; IL‐10, interleukin‐10; TGF‐β, transforming growth factor‐β.
Sphere‐forming GBM stem cells express low levels of ligands for second signals (B7.1 [CD80] and B7.2 [CD86]); however, they express high levels of the inhibitory co‐stimulatory molecule ligand PD‐L1 (CD274 or B7‐H1). Furthermore, GBM stem cells release immune suppressive cytokines, including IL‐10, IL‐13 and TGF‐β, and induce regulatory T cells (Treg cells) in vitro culture with peripheral blood CD4+ T cells.28 Similar observations were reported by another group.29 The inhibitory co‐stimulatory molecule PD‐L1 (B7‐H4) is expressed in non‐dividing glioma stem cells.40 Volonté et al.33 report that sphere‐forming colon CSC/CIC suppress the proliferation of peripheral blood T cells by membrane‐bound IL‐4 on the CSC/CIC. These immune suppressive profiles of co‐stimulatory molecules and cytokines suggest that CSC/CIC might contribute to making an immune‐suppressive microenvironment and escaping from CTL. Therefore, CSC/CIC might inhibit differentiation of CD8+ naïve T cells into CTL; however, CSC/CIC cannot inhibit the cytotoxicity of differentiated and activated CTL (Fig. 2).
Antigens Expressed in Cancer Stem‐like Cells
Antigen expression is essential for recognition by CTL, and antigen expression profiles in CSC/CIC have been reported in several malignancies. TAA can be classified into several groups, including (i) CT antigens; (ii) overexpressed antigens; (iii) mutated antigens (neoantigens); and (iv) differentiation antigens.41 Overexpressed antigens and differentiation antigens are expressed not only in cancer cells but also in normal somatic cells. CT antigens are expressed in cancer cells and germ cells. Germ cells do not express MHC class I molecules and CTL cannot access antigens expressed in germ cells. Thus, CT antigens and mutated antigens show strict expression in cancer cells and are thought to be more immunogenic (probably more effective in cancer immunotherapy) (Fig. 3).
Figure 3.

Matrix of expression of tumor‐associated antigens in cancer stem‐like cells (CSC)/cancer‐initiating cells (CIC) and normal cells. The antigens located in the right‐upper area are expressed in CSC/CIC and germ cells and are thought to be more effective in cancer immunotherapy. Tumor‐associated antigens (TAA) in red letters indicate that cytotoxic T lymphocytes (CTL) specific for TAA have been reported. TAA in blue letters indicate that CTL specific for TAA have not been reported.
Cancer testis antigens
Cancer testis antigens are as highly tumor‐specific antigens that are expressed in cancer cells and germ cells but not in normal cells.42 In transcriptome analysis of CSC/CIC, we found that some CT antigens are preferentially expressed in CSC/CIC. Therefore, we compared the expression of known CT antigens in SP cells and non‐SP cells derived from colon cancer cells, lung cancer cells and breast cancer cells and found that 18 CT antigens (MAGEA2, MAGEA3, MAGEA4, MAGEA6, MAGEA12, MAGEB2, GAGE1, GAGE8, SPANXA1, SPANXB1, SPANXC, XAGE2, SPA17, BORIS, PLU‐1, SGY‐1, TEX15 and CT45A1) are preferentially expressed in CSC/CIC.43 MAGEA2, MAGEA3, MAGEA4, MAGEA6, MAGEA12, GAGE1, GAGE8, XAGE2 and SPA17 have already been reported as targets of CTL. Thus, these CT antigens are potential candidates for CSC/CIC‐targeting immunotherapy. Novel CT antigens have also been identified by transcriptome analysis of CSC/CIC.15, 44 DNAJB8 (DnaJ [Hsp40] homolog, subfamily B, member 8) belongs to the heat shock protein (HSP) 40 family and is preferentially expressed in SP cells derived from kidney cancer cells.15 DNAJB8 has an essential role in the maintenance of kidney CSC/CIC, and a DNA vaccination model revealed the immunogenicity of DNAJB8. In the present study, we found that DNAJB8 is preferentially expressed in SP cells derived from colon cancer cells and that it has a role in the maintenance of colon CSC/CIC. HLA‐A24‐bound antigenic peptide was identified from the DNAJB8 amino acid sequence, and CTL specific for DNAJB8 peptide recognized SP cells of colon cancer cells more efficiently than did non‐SP cells of colon cancer cells.36 These observations indicate that DNAJB8‐derived antigenic peptides are promising candidates for colon and kidney CSC/CIC‐targeting immunotherapy. In contrast, Boiko et al.45 report that CD271+ melanoma stem cells show lower expression levels of MAGE‐C1/MAGE‐C2, CT antigens than the levels in CD271+ differentiated melanoma cells. However, they do not describe expression profiles of other CT antigens.
Overexpressed antigens
Cancer cells express TAA that are expressed in normal cells at low levels, and these TAA are named overexpressed antigens. CSC/CIC express several overexpressed antigens. Interestingly, CSC/CIC express antigens that are also expressed in normal stem cells, such as SOX2, OCT3/4 and ALDH1A1.12, 46, 47, 48 Expression of these antigens is shared by normal stem cells, and these antigens might, therefore, be sub‐categorized as stem cell‐related antigens. In generating CSC/CIC‐targeting immunotherapy, the severe side effect risks of targeting normal stem cells should be considered with stem cell‐related antigens.
Other types of overexpressed antigens have been reported to be expressed in CSC/CIC. Antigens of this category are expressed in ubiquitous normal organs at very low levels; they are expressed in CSC/CIC and often have pivotal roles in carcinogenesis. The apoptosis inhibitor proteins Survivin, Livin and Bcl‐2 are expressed in several CSC/CIC.15, 49, 50 hTERT is expressed in CD44+ breast cancer stem cells.51 HER2, a proto‐oncogene, is expressed in glioma stem cells.37 CEP55 and COA‐1 are expressed in colon CSC/CIC.13, 33 SP cells derived from breast cancer cells showed expression of MUC1.52 These overexpressed antigens are thought to be good candidates for CSC/CIC‐targeting immunotherapy.
Mutated antigens (neoantigens)
Mutated antigens are now known as neoantigens and are defined as antigens derived from tumor‐specific genomic DNA mutations.53 Antigens that belong to this category depend on the DNA mutations; therefore, the epitopes are case‐specific. The T cell pool specific for neoantigens may be less affected by T cell tolerance compared with nonmutated self‐antigens. Recent studies in mice revealed that the neoantigen‐specific CTL response has a role in tumor regression in checkpoint blockade cancer immunotherapy,54 and mass spectrometry and exome sequencing using next‐generation sequencer are expected to be powerful tools for identification of case‐specific neoantigens.55
Neoantigens may be promising targets of CSC/CIC‐targeting immunotherapy; however, there are several issues to be overcome. First, neoantigens are mostly derived from passenger gene mutations (not driver gene mutations); therefore, tumor cells carrying neoantigens of passenger gene mutations may lose the expression of neoantigens due to immune selection by CTL because passenger gene mutations do not play a role in tumor cell grow. Second, expression of neoantigens depends on the expression of mutated genes. Thus, limited numbers of neoantigens may be expressed in CSC/CIC, suggesting the difficulty of identification of CSC/CIC‐specific neoantigens.
Differentiation antigens
Tissue‐specific differentiation antigens from several organs are reported to be targets of CTL. CSC/CIC are thought to be in an immature state of cancer cell differentiation, and show lower expression levels of differentiation antigens. Melanoma differentiation antigens MART‐1 and tyrosinase are expressed at low levels in CD271+ melanoma stem cells and ABCB5+ melanoma stem cells.31, 45 A prostate differentiation antigen, PSA, is expressed at a low level in prostate CSC/CIC.56 Therefore, differentiation antigens are not suitable for CSC/CIC‐targeting immunotherapy.
Cancer stem‐like cell‐targeting immunotherapy: From bench to bedside
Susceptibility to CTL and expression of TAA in CSC/CIC potentially enable the design of CSC/CIC‐targeting immunotherapy, and several trials of both pre‐clinical and clinical settings have been reported. We previously reported that CTL can be isolated in in vitro culture with SP cells derived from malignant fibrous histiocytoma (MFH).57 Interestingly, a CTL clone preferentially recognized SP cells rather than non‐SP cells, indicating that this CTL clone recognizes MFH stem cell‐specific antigens. However, the antigen is not known. Xu et al.58 report that rats immunized with dendritic cells (DC) pulsed with sphere‐forming cells derived from 9L rat glioma cells demonstrated a significantly stronger anti‐tumor effect than rats immunized with DC pulsed with differentiated glioma cells. Similar results were obtained in a mouse immunization model using DC pulsed with ALDH+ cells derived from D5 mouse melanoma cells and SCC7 mouse squamous cell carcinoma cells.59 These observations indicate that a CSC/CIC‐specific CTL response can be induced and that a CSC/CIC‐specific CTL response might give rise to a better anti‐tumor effect than an all cancer cell‐reactive CTL response. To examine the efficacy of CSC/CIC‐specific antigens, we used a mouse DNA vaccination model. We immunized mice using DNAJB8‐coding DNA and Survivin‐coding DNA. DNAJB8 is a novel CT antigen, and the CTL response to DNAJB8 can target only CSC/CIC. In contrast, Survivin is an overexpressed antigen, and the CTL response to Survivin can target all cancer cells. Interestingly, vaccination using DNAJB8 DNA showed a significantly stronger anti‐tumor effect than that using Survivin DNA, indicating that a CSC/CIC‐specific CTL response corresponds to a better anti‐tumor effect.15
The results of a clinical trial based on these models of CSC/CIC‐targeting immunotherapy are reported. DC transfected with RNA isolated from sphere‐cultured GBM stem cells were used for immunotherapy in GBM patients. There were no severe adverse effects, and the patients had prolonged progression‐free survival compared with survival in the control group.60
Conclusion and Perspectives
Previous studies have shown that treatment‐resistant CSC/CIC are sensitive to cancer immunotherapy using differentiated CTL, and CT antigens are a reasonable and promising candidate for CSC/CIC‐targeting immunotherapy. However, several reports suggest that CSC/CIC might have immune suppressive potential. The expressions of immune suppressive cytokines and the inhibitory co‐stimulatory molecules in CSC/CIC are potentially major concerns. To overcome this problem, peptide vaccination at sites distant from cancer lesions using appropriate adjuvants, peptide vaccination using immune checkpoint inhibitors (CTLA‐4 antibody, PD‐1 antibody and PD‐L1 antibody), and adoptive transfer of differentiated CTL specific for CSC/CIC are possible options in the future.
Disclosure Statement
The authors have no financial conflict of interest to declare.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology and from the Ministry of Health, Labor and Welfare of Japan.
Cancer Sci 107 (2016) 12–17
Funding Information
Ministry of Education, Culture, Sports, Science and Technology Ministry of Health, Labor and Welfare of Japan
References
- 1. Nowell PC. The clonal evolution of tumor cell populations. Science 1976; 194: 23–8. [DOI] [PubMed] [Google Scholar]
- 2. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 2012; 10: 717–28. [DOI] [PubMed] [Google Scholar]
- 3. Park CY, Tseng D, Weissman IL. Cancer stem cell‐directed therapies: recent data from the laboratory and clinic. Mol Ther 2009; 17: 219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirohashi Y, Torigoe T, Inoda S et al The functioning antigens: beyond just as the immunological targets. Cancer Sci 2009; 100: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vacchelli E, Martins I, Eggermont A et al Trial watch: peptide vaccines in cancer therapy. Oncoimmunology 2012; 1: 1557–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirohashi Y, Torigoe T, Maeda A et al An HLA‐A24‐restricted cytotoxic T lymphocyte epitope of a tumor‐associated protein, survivin. Clin Cancer Res 2002; 8: 1731–9. [PubMed] [Google Scholar]
- 7. Tsuruma T, Hata F, Torigoe T et al Phase I clinical study of anti‐apoptosis protein, survivin‐derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med 2004; 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsuruma T, Iwayama Y, Ohmura T et al Clinical and immunological evaluation of anti‐apoptosis protein, survivin‐derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med 2008; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mizuguchi T, Torigoe T, Satomi F et al Trials of vaccines for pancreatic ductal adenocarcinoma: is there any hope of an improved prognosis? Surg Today 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Sato N, Hirohashi Y, Tsukahara T et al Molecular pathological approaches to human tumor immunology. Pathol Int 2009; 59: 205–17. [DOI] [PubMed] [Google Scholar]
- 11. Murase M, Kano M, Tsukahara T et al Side population cells have the characteristics of cancer stem‐like cells/cancer‐initiating cells in bone sarcomas. Br J Cancer 2009; 101: 1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakatsugawa M, Takahashi A, Hirohashi Y et al SOX2 is overexpressed in stem‐like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest 2011; 91: 1796–804. [DOI] [PubMed] [Google Scholar]
- 13. Inoda S, Hirohashi Y, Torigoe T et al Cytotoxic T lymphocytes efficiently recognize human colon cancer stem‐like cells. Am J Pathol 2011; 178: 1805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishida S, Hirohashi Y, Torigoe T et al Gene expression profiles of prostate cancer stem cells isolated by aldehyde dehydrogenase activity assay. J Urol 2012; 188: 294–9. [DOI] [PubMed] [Google Scholar]
- 15. Nishizawa S, Hirohashi Y, Torigoe T et al HSP DNAJB8 controls tumor‐initiating ability in renal cancer stem‐like cells. Cancer Res 2012; 72: 2844–54. [DOI] [PubMed] [Google Scholar]
- 16. Kuroda T, Hirohashi Y, Torigoe T et al ALDH1‐high ovarian cancer stem‐like cells can be isolated from serous and clear cell adenocarcinoma cells, and ALDH1 high expression is associated with poor prognosis. PLoS One 2013; 8: e65158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasuda K, Torigoe T, Morita R et al Ovarian cancer stem cells are enriched in side population and aldehyde dehydrogenase bright overlapping population. PLoS One 2013; 8: e68187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michifuri Y, Hirohashi Y, Torigoe T et al Small proline‐rich protein‐1B is overexpressed in human oral squamous cell cancer stem‐like cells and is related to their growth through activation of MAP kinase signal. Biochem Biophys Res Commun 2013; 439: 96–102. [DOI] [PubMed] [Google Scholar]
- 19. Hirohashi Y, Torigoe T, Inoda S, Morita R, Kochin V, Sato N. Cytotoxic T lymphocytes: sniping cancer stem cells. Oncoimmunology 2012; 1: 123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saijo H, Hirohashi Y, Torigoe T, Kochin V, Takahashi H, Sato N. Cytotoxic T lymphocytes: the future of cancer stem cell eradication? Immunotherapy 2013; 5: 549–51. [DOI] [PubMed] [Google Scholar]
- 21. Hirohashi Y, Torigoe T, Inoda S et al Immune response against tumor antigens expressed on human cancer stem‐like cells/tumor‐initiating cells. Immunotherapy 2010; 2: 201–11. [DOI] [PubMed] [Google Scholar]
- 22. Aptsiauri N, Cabrera T, Garcia‐Lora A, Lopez‐Nevot MA, Ruiz‐Cabello F, Garrido F. MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol 2007; 256: 139–89. [DOI] [PubMed] [Google Scholar]
- 23. Mariya T, Hirohashi Y, Torigoe T et al Prognostic impact of human leukocyte antigen class I expression and association of platinum resistance with immunologic profiles in epithelial ovarian cancer. Cancer Immunol Res 2014; 2: 1220–9. [DOI] [PubMed] [Google Scholar]
- 24. Kitamura H, Torigoe T, Honma I et al Effect of human leukocyte antigen class I expression of tumor cells on outcome of intravesical instillation of bacillus calmette‐guerin immunotherapy for bladder cancer. Clin Cancer Res 2006; 12: 4641–4. [DOI] [PubMed] [Google Scholar]
- 25. Tsukahara T, Kawaguchi S, Torigoe T et al Prognostic significance of HLA class I expression in osteosarcoma defined by anti‐pan HLA class I monoclonal antibody, EMR8‐5. Cancer Sci 2006; 97: 1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol 2013; 31: 443–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tallerico R, Todaro M, Di Franco S et al Human NK cells selective targeting of colon cancer‐initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol 2013; 190: 2381–90. [DOI] [PubMed] [Google Scholar]
- 28. Wei J, Barr J, Kong LY et al Glioma‐associated cancer‐initiating cells induce immunosuppression. Clin Cancer Res 2010; 16: 461–73. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Di Tomaso T, Mazzoleni S, Wang E et al Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res 2010; 16: 800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu A, Wiesner S, Xiao J et al Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol 2007; 83: 121–31. [DOI] [PubMed] [Google Scholar]
- 31. Schatton T, Schutte U, Frank NY et al Modulation of T‐cell activation by malignant melanoma initiating cells. Cancer Res 2010; 70: 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Busse A, Letsch A, Fusi A et al Characterization of small spheres derived from various solid tumor cell lines: are they suitable targets for T cells? Clin Exp Metastasis 2013; 30: 781–91. [DOI] [PubMed] [Google Scholar]
- 33. Volonté A, Di Tomaso T, Spinelli M et al Cancer‐initiating cells from colorectal cancer patients escape from T cell‐mediated immunosurveillance in vitro through membrane‐bound IL‐4. J Immunol 2014; 192: 523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chikamatsu K, Takahashi G, Sakakura K, Ferrone S, Masuyama K. Immunoregulatory properties of CD44+ cancer stem‐like cells in squamous cell carcinoma of the head and neck. Head Neck 2011; 33: 208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inoda S, Morita R, Hirohashi Y et al The feasibility of Cep55/c10orf3 derived peptide vaccine therapy for colorectal carcinoma. Exp Mol Pathol 2011; 90: 55–60. [DOI] [PubMed] [Google Scholar]
- 36. Morita R, Nishizawa S, Torigoe T et al Heat shock protein DNAJB8 is a novel target for immunotherapy of colon cancer‐initiating cells. Cancer Sci 2014; 105: 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed N, Salsman VS, Kew Y et al HER2‐specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res 2010; 16: 474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Avril T, Vauleon E, Hamlat A et al Human glioblastoma stem‐like cells are more sensitive to allogeneic NK and T cell‐mediated killing compared with serum‐cultured glioblastoma cells. Brain Pathol 2012; 22: 159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gil EY, Jo UH, Lee HJ et al Vaccination with ErbB‐2 peptides prevents cancer stem cell expansion and suppresses the development of spontaneous tumors in MMTV‐PyMT transgenic mice. Breast Cancer Res Treat 2014; 147: 69–80. [DOI] [PubMed] [Google Scholar]
- 40. Yao Y, Wang X, Jin K et al B7‐H4 is preferentially expressed in non‐dividing brain tumor cells and in a subset of brain tumor stem‐like cells. J Neurooncol 2008; 89: 121–9. [DOI] [PubMed] [Google Scholar]
- 41. Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014; 14: 135–46. [DOI] [PubMed] [Google Scholar]
- 42. van der Bruggen P, Traversari C, Chomez P et al A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254: 1643–7. [DOI] [PubMed] [Google Scholar]
- 43. Yamada R, Takahashi A, Torigoe T et al Preferential expression of cancer/testis genes in cancer stem‐like cells: proposal of a novel sub‐category, cancer/testis/stem gene. Tissue Antigens 2013; 81: 428–34. [DOI] [PubMed] [Google Scholar]
- 44. Takahashi A, Hirohashi Y, Torigoe T et al Ectopically expressed variant form of sperm mitochondria‐associated cysteine‐rich protein augments tumorigenicity of the stem cell population of lung adenocarcinoma cells. PLoS One 2013; 8: e69095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boiko AD, Razorenova OV, van de Rijn M et al Human melanoma‐initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010; 466: 133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dhodapkar KM, Feldman D, Matthews P et al Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci USA 2010; 107: 8718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV. SOX2‐specific adaptive immunity and response to immunotherapy in non‐small cell lung cancer. Oncoimmunology 2013; 2: e25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Visus C, Wang Y, Lozano‐Leon A et al Targeting ALDH(bright) human carcinoma‐initiating cells with ALDH1A1‐specific CD8(+) T cells. Clin Cancer Res 2011; 17: 6174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jin F, Zhao L, Zhao HY et al Comparison between cells and cancer stem‐like cells isolated from glioblastoma and astrocytoma on expression of anti‐apoptotic and multidrug resistance‐associated protein genes. Neuroscience 2008; 154: 541–50. [DOI] [PubMed] [Google Scholar]
- 50. Madjd Z, Mehrjerdi AZ, Sharifi AM, Molanaei S, Shahzadi SZ, Asadi‐Lari M. CD44+ cancer cells express higher levels of the anti‐apoptotic protein Bcl‐2 in breast tumours. Cancer Immun 2009; 9: 4. [PMC free article] [PubMed] [Google Scholar]
- 51. Chung SS, Aroh C, Vadgama JV. Constitutive activation of STAT3 signaling regulates hTERT and promotes stem cell‐like traits in human breast cancer cells. PLoS One 2013; 8: e83971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res 2008; 68: 2419–26. [DOI] [PubMed] [Google Scholar]
- 53. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 54. Gubin MM, Zhang X, Schuster H et al Checkpoint blockade cancer immunotherapy targets tumour‐specific mutant antigens. Nature 2014; 515: 577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yadav M, Jhunjhunwala S, Phung QT et al Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014; 515: 572–6. [DOI] [PubMed] [Google Scholar]
- 56. Qin J, Liu X, Laffin B et al The PSA(‐/lo) prostate cancer cell population harbors self‐renewing long‐term tumor‐propagating cells that resist castration. Cell Stem Cell 2012; 10: 556–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kano M, Tsukahara T, Emori M et al Autologous CTL response against cancer stem‐like cells/cancer‐initiating cells of bone malignant fibrous histiocytoma. Cancer Sci 2011; 102: 1443–7. [DOI] [PubMed] [Google Scholar]
- 58. Xu Q, Liu G, Yuan X et al Antigen‐specific T‐cell response from dendritic cell vaccination using cancer stem‐like cell‐associated antigens. Stem Cells 2009; 27: 1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ning N, Pan Q, Zheng F et al Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res 2012; 72: 1853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vik‐Mo EO, Nyakas M, Mikkelsen BV et al Therapeutic vaccination against autologous cancer stem cells with mRNA‐transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 2013; 62: 1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]


