Preface
Over the past ten years, preclinical studies implicating sustained androgen receptor (AR) signaling as the primary driver of castration resistant prostate cancer (CRPC) led to the development of novel agents targeting the AR pathway that are now in widespread clinical use. These drugs prolong survival of patients with late stage prostate cancer but are not curative. In this review, we highlight emerging mechanisms of acquired resistance to these contemporary therapies, which fall into the three broad categories of restored AR signaling, AR bypass signaling and complete AR independence. This diverse spectrum of resistance mechanisms presents new challenges for long term disease control, which may be addressable through early use of combination therapies guided by recent insights from genomic landscape studies of CRPC.
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed cancers among men in Western industrialized nations and a leading cause of cancer-related deaths 1 (http://seer.cancer.gov/statfacts/html/prost.html). In the era of routine screening of serum prostate specific antigen (PSA) levels, the majority of cancers are detected while organ-confined and hence potentially curable, while the incidence of lethal metastatic disease at the time of diagnosis has declined 2. Androgen synthesis is under the physiological regulation of the hypothalamic-pituitary-testicular axis (Figure 1A), with contributions from the adrenal glands resulting from de novo steroidogenesis (Figure 1B). Pioneering work by Charles Huggins in 1941 demonstrated the remarkable benefit of androgen deprivation therapy (ADT) via surgical castration for men with advanced metastatic PCa 3, establishing a clinical paradigm that continues to this day. Contemporary first-line ADT for PCa recurring after prostatectomy or radiotherapy typically involves chemical castration through the chronic use of gonadotropin-releasing hormone (GnRH) agonists or antagonists (Table 1) that lower testosterone levels by stable suppression of androgen secretion from the testes (Figure 1A). Combined androgen blockade incorporates the additional use of a competitive androgen receptor (AR) antagonist (an antiandrogen) (Table 1) to further impede AR signaling within the PCa cell (Figure 1C) and mitigate the effects of acute systemic testosterone surges resulting from the initial use of GnRH agonists 4. Although nearly all patients respond to hormonal therapy, response duration varies from months to years followed by uniform progression to a lethal stage of the disease, termed castration resistant prostate cancer (CRPC) (Figure 2A).
Figure 1. AR signaling is regulated by the hypothalamic-pituitary-testicular axis, adrenal gland steroidogenesis and prostate cell intrinsic factors.

A. The hormones gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) bind to their cognate receptors, resulting in testosterone secretion from Leydig cells of the testes. Chronic use of GnRH agonists leads to downregulation of the GnRH receptor (GnRH-R) while antagonists provide immediate GnRH-R blockade. Both agents suppress LH production causing a decline in serum testosterone to castrate levels. The adrenal glands secrete androgens dehydroepiandrosterone (-sulfate) (DHEA-S, predominantly), DHEA and androstenedione (AD) into the circulation. B. Adrenal androgen de novo steroidogenesis (enzymes in ovals). CYP17A has 17α-hydroxylation (red) and 17, 20-lyase (blue) activities; both inhibited by abiraterone. Dashed arrow indicates a weak effect. C. Prostate conversion of adrenal androgens to dihydrotestosterone (DHT). DHT binds to androgen receptor (AR) in the cytoplasm, triggering a conformational change leading to nuclear translocation 141, 142. DHT bound AR homodimerizes and with coactivators (CoA) and RNA polymerase II (RNA Pol II) or corepressors (not shown), binds DNA at cis androgen response elements to activate (shown) or repress AR target gene expression, respectively 143–146. Enzalutamide inhibits AR by competing with DHT for binding, blocking nuclear translocation, and blocking DNA and cofactor binding 7.
Table 1.
Pharmacological agents targeting Androgen Receptor (AR) signaling in advanced prostate cancer.
| Generic or Code Name | Brand Names or Other Code Names | FDA Approval Year (or clinical development stage) | Drug Class |
|---|---|---|---|
| FDA Approved | |||
| Leuprolide | Lupron Depot, Eligard | 1989 (Lupron Depot), 2002 (Eligard) | GnRH agonist |
| Goserelin | Zoladex | 1989 | GnRH agonist |
| Triptorelin | Trelstar | 2000 | GnRH agonist |
| Histrelin | Vantas | 2004 | GnRH agonist |
| Degarelix | Firmagon | 2008 | GnRH antagonist |
| Flutamide | 1989 | AR antagonist | |
| Bicalutamide | Casodex | 1995 | AR antagonist |
| Nilutamide | Nilandron | 1996 | AR antagonist |
| Enzalutamide | Xtandi | 2012 | AR antagonist |
| Abiraterone | Zytiga | 2011 | CYP17A1 inhibitor |
| Clinical Development | |||
| JNJ-56021927 | ARN-509 | Phase III | AR antagonist |
| BAY1841788 | ODM-201 | Phase III | AR antagonist |
| VT-464 | Phase II | CYP17A1 (lyase specific) inhibitor | |
| Galeterone | TOK-001 | Phase III | CYP17A1 (lyase specific) inhibitor, AR antagonist, AR degradation |
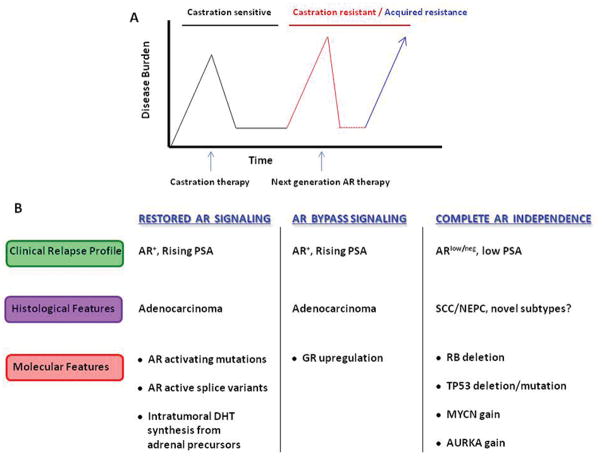
Figure 2. Overview of resistance mechanisms to next generation AR targeted therapies for CRPC.
A. Increasing disease burden following primary prostate cancer therapy is indicated by rising prostate specific antigen (PSA) and/or radiographic progression and is treated with medical castration. The castration resistant prostate cancer (CRPC) stage follows failure of castration therapy. Next generation androgen receptor (AR) inhibitors (abiraterone, enzalutamide) are initiated during CRPC, but acquired (or inherent) resistance mechanisms lead to disease recurrence and ultimately death. B. Heterogeneous patterns of resistance mechanisms to AR inhibitors include broad classes of restored AR signaling, AR bypass signaling, and complete AR independence. The majority of patients relapse with typical AR-positive adenocarcinoma with rising PSA levels. Although the incidence is not precisely defined, a subset of relapsing patients present with AR-low or negative tumors and low PSA. The histological classification of these cancers is an area of active investigation, but include classical small cell carcinoma (SCC), neuroendocrine prostate cancer (NEPC) in the absence of SCC-histological features, and potentially emerging, novel subtypes. Known or suspected molecular drivers of resistance are highlighted. Note that these molecular alterations are not mutually exclusive to each class, and some degree of overlap occurs in model systems and is likely in patients. AURKA, Aurora kinase A, DHT, dihydrotestosterone, GR, glucocorticoid receptor.
Just over a decade ago, it was generally believed that AR signaling was dispensable to the biology of CRPC. This led to the frequent usage of terms such as “androgen independent” or “hormone refractory” to describe this stage of the disease. An abundance of data acquired since then, however, has made it overwhelmingly clear that residual androgens remaining after castration and AR itself remain central both to the progression to CRPC and to its continued growth. An early indication of the possible contribution of AR to the progression towards CRPC came from the observation that 30% of CRPC patients harbored genomic amplification of the AR locus in late stage tumors but not in patient-matched tumor samples obtained prior to ADT 5. In in vitro and in vivo studies using the preclinical PCa models LNCaP and LAPC4 (Box 1), our laboratory established that AR overexpression was indeed a sufficient and principal driver of progression to CRPC, with these cells exhibiting acquired resistance to both ADT and to the primary antiandrogen in clinical usage at that time, bicalutamide 6. These findings provided the rationale for a drug discovery screen of novel antiandrogens that would maintain the ability to inhibit AR signaling in the setting of receptor overexpression, which led to the identification of enzalutamide (formerly MDV3100) 7. In parallel, others developed the CYP17A1 inhibitor, abiraterone acetate (hereafter, simply abiraterone), which targets this central enzyme in de novo steroidogenesis (Figure 1B) 8.
Box 1. Human Prostate Cancer Model Systems.
Research in the prostate cancer (PCa) field has historically been hampered by a limited number of human cell lines and xenograft models. Cell lines derived from non-metastatic, primary PCa are particularly poorly represented. Consequently, discoveries in the field generally result from the study of only a few major cell lines (detailed below). Nevertheless, clinically relevant resistance mechanisms have been identified through the use of these models. Finally, recent refinements in tissue culture methodologies, particularly serum free conditions for growth of tumor organoids, have enabled the development of several additional PCa lines that recapitulate common genomic alterations seen in PCa patients 126.
22Rv1 (also known as CWR22Rv1)127
Tissue of origin: CRPC derivative of CWR22 xenograft 128
AR: positive; mutation H875Y 21
AR splice variant: positive 37
CWR22Pc129
DU145130
Tissue of origin: prostate cancer brain metastasis (direct from patient)
AR: negative 131
LAPC-4132
Tissue of origin: prostate cancer lymph node metastasis (from mouse xenograft)
AR: positive; wild type
Tissue of origin: prostate cancer lymph node metastasis (direct from patient)
AR: positive; mutation T878A 13
Usage: The dominant model system in the field, LNCaP has been utilized to study the general biology of AR, to screen for novel AR antagonists, and to investigate mechanisms of progression to castration resistance in part through the generation of numerous derived sublines, such as C4-2 135.
MDA Pca 2a136
Tissue of origin: prostate cancer bone metastasis (direct from patient)
AR: positive; mutations L702H and T878A 137
PC-3138
Tissue of origin: prostate cancer bone metastasis (direct from patient)
AR: negative 131
VCaP139
Abiraterone and enzalutamide are both approved for treatment of CRPC in chemotherapy-naive and chemotherapy refractory patients, based on a series of phase III trials showing overall improved survival for either agent used alone versus placebo 9–12. Despite the success of these second-generation AR targeted therapies, inherent or acquired resistance remains a major clinical challenge. Here we will review the current understanding of resistance to contemporary AR inhibition strategies, which we group into the three general categories of restored AR signaling, bypass of AR and complete AR independence (Figure 2B). We also discuss implications for the development of the next generation of molecularly targeted therapies for PCa.
Restored Androgen Receptor Signaling
Androgen Receptor Ligand Binding Domain Mutations
Early investigations into the mechanisms of resistance to AR targeted therapies were facilitated by the identification of a point mutation (T878A) within the AR ligand binding domain (LBD) in the PCa cell line LNCaP 13, a finding that was soon validated in a CRPC patient 14. This discovery spurred inquiries by numerous groups to define the frequency of AR mutations in clinical PCa. Collectively, these efforts revealed that AR mutations were detectable in a minority of patients and were exclusively found in men with CRPC but not primary prostate cancer, a finding which was later validated in comprehensive genomic sequencing studies 15–19. Of note, these recent investigations, which used next generation sequencing protocols with deep coverage to discover somatic alterations, found very few mutations within the AR amino terminal transactivation domain (exon 1). This is in contrast to the plethora of exon 1 mutations reported in the older literature, primarily using PCR-based protocols, and catalogued in the Androgen Receptor Gene Mutations Database (http://androgendb.mcgill.ca/) 20. Instead, the LBD has emerged as a mutational hotspot (Figure 3A), with four principal point mutations recurring across multiple studies (L702H, W742C, H875Y, and T878A). Collectively, these recurrent AR mutants are present in ~15–20% of CRPC cases, a frequency that grows to greater than 60% when combined with AR gene amplification 16, 19.
Figure 3. Domain structure of AR, cancer associated missense mutations, and splice variants.

A. Androgen receptor (AR) is 920 amino acids long and consists of four functional domains encoded by eight exons: the ligand independent amino terminal transactivation domain (NTD, exon 1), DNA binding domain (DBD, exons 2–3), the hinge region (exon 4), and the ligand binding domain (LBD, exons 4–8). Note that exon 4 comprises both the hinge region and part of the LBD. The nuclear localization signal (NLS, pair of green bars) of AR is a bipartite motif contained within exons 3 and 4. Recurring missense mutations are noted beneath the AR schematic. These same mutations are also described in the literature with alternative numerical designations based on earlier genomic builds (that is, L701H, W741C, H874Y, T877A and originally published as T868A). B. The protein structures of representative androgen receptor splice variants (ARVs) are shown with the in-frame variant specific amino acids derived from the alternative splicing events.
In vitro characterization of T878A and H875Y revealed that both mutants are paradoxically activated, rather than inhibited, by the antiandrogens nilutamide and flutamide 13, 21. Hence, an AR antagonist behaves as an agonist in the context of these mutations, resulting in the transcriptional induction of AR target genes. In one report, T878A was detected only in those patients who had received combined androgen blockade with flutamide 22. It has been well documented that discontinuation of flutamide therapy can result in clinical improvement for a subset of patients who had previously responded to the antagonist, the so called “antiandrogen withdrawal syndrome” 23. In some patients this phenomenon is likely due to the presence of flutamide-activating AR mutations driving PCa growth while under treatment 24. The concept of AR mutations converting antiandrogens to AR agonists has extended to additional antiandrogens. W742C mutations were identified in LNCaP cells with acquired resistance to bicalutamide and lead to enhanced AR transcriptional activity driven by bicalutamide in a manner analogous to that caused by flutamide in conjunction with T878A or H875Y 25. Our group discovered the mutation F877L (reported as F876L in an earlier genomic build) in a random mutagenesis screen using LNCaP cells under selective pressure of enzalutamide 26. F877L causes enzalutamide and the antiandrogen ARN-509 27 to function as AR agonists and confers drug resistance across multiple models both in vitro and in vivo, presumably from a repositioning of the coactivator docking helix 12 26. Using circulating cell-free DNA, F877L was also identified in a small number of CRPC patients at the time of progression with ARN-509 28 or enzalutamide 29. Whether these AR mutations will emerge as a significant cause of clinical resistance remains to be determined but current evidence suggests they may be infrequent. For example, from four genomic studies of CRPC comprising a total of 262 cases, just three W742C mutations have been reported 16–19 despite the widespread clinical use of bicalutamide for nearly 20 years. It is too early to comment on the newer antiandrogen enzalutamide, but a recent genomic landscape study of 150 men with metastatic CRPC did not identify F877L despite prior exposure to enzalutamide in some of these patients 19.
Curiously, compared to W742C, the flutamide resistance mutations H875Y and T878A occur more frequently (21 of 262 cases) 16–19 despite the shift to bicalutamide over flutamide as the preferred antiandrogen in clinical practice more than a decade ago. One possible explanation is that these mutations, as well as L702H (which has not been linked to antiandrogen resistance), share the property of promiscuous activation by non-canonical steroid ligands such as adrenal androgens, estrogen, and progesterone 13, 21 or, in the case of L702H, by glucocorticoids 30, 31. Although there is currently no direct evidence that these alternative steroids can promote progression of AR mutant cancer cells to CRPC, the transcriptional activation data raises the possibility that these alternative steroids could play a substantial role in disease progression. For example, T878A mutations were found in 3/18 patients progressing on CYP17A1 inhibitors who had no prior flutamide treatment 32. The authors proposed increased systemic progesterone levels resulting from abiraterone treatment as a potential explanation (Figure 1B). A second group has also reported H875Y and T878A mutations in circulating cell-free DNA from 11% of CRPC patients, and all of these occurred in the context of progression on, or prior treatment with, abiraterone 29. In addition, L702H mutations (activated by glucocorticoids) have now been reported in patients receiving abiraterone, which is administered concurrently with the glucocorticoid prednisone to prevent mineralocorticoid excess syndrome caused by inhibition of the 17α-hydroxylase activity of CYP17A1 19, 33, 34. Therefore, the frequent identification of L702H, H875Y and T878A mutations in contemporary patient cohorts may be a consequence of promiscuity towards other steroid ligands rather than antiandrogen resistance.
Androgen Receptor Splice Variants
Alternative splicing of AR mRNA is another mechanism implicated in progression to CRPC as well as in resistance to abiraterone and enzalutamide 35, 36. Although expression of AR splice variants (ARVs) is clearly increased in resistant tumors, the evidence that ARVs play a causal role in resistance remains somewhat controversial, for reasons discussed below. Numerous ARVs have been identified in several PCa cell lines and xenograft tumors at the level of mRNA, some of which have been confirmed in clinical specimens 19, 37–42. All ARVs share the common structural feature of truncation or exon skipping of the complete carboxy-terminal LBD, typically with a small, variant-specific in-frame sequence added as a consequence of alternative splicing. Importantly, all ARVs retain the amino-terminal transactivation and DNA binding domains (Figure 3B). AR-V7 (also known as AR3) 39, 40 is the best characterized ARV, in part due to the availability of a variant-specific antibody which has enabled analysis of AR-V7 expression in patient samples using immunohistochemistry.
In principle, these structural properties could confer constitutive, androgen independent activity to all AR isoforms, but in practice, only some ARVs display this characteristic in AR transactivation reporter assays. This capability is correlated with constitutive, androgen independent nuclear localization 38, 42 and is explained in part by the presence of a bipartite AR nuclear localization signal located in exons 3 and 4 43. Only two ARVs, ARv567es 41 and murine mAR-V4 38, retain both exon 3 and 4 and thus have a complete nuclear localization signal. All other reported ARVs are truncated after exon 3 and are expected to be predominantly cytoplasmic, as exemplified by AR-V1 38. AR-V7 is a clear exception since it does have constitutive nuclear localization and transcriptional activity without a full nuclear localization signal by a mechanism yet to be precisely defined 38–40.
One point of confusion as to whether ARVs cause resistance is the fact that ARVs are expressed in normal prostate tissue 39, 44 and levels in PCa are physiologically increased in response to ADT. It has long been known that ADT leads to a rapid elevation of AR mRNA in prostate tissue which is reversed by androgen replacement 45, 46. Similar results, including proportional increases in ARV expression, are seen in human PCa xenograft tumors. Two days after castration of mice bearing VCaP tumors, protein levels of both AR-FL (the canonical full-length AR) and AR-V7 were elevated in a reversible manner, reaching a peak at two weeks. Importantly, at all time points analyzed, AR-V7 mRNA levels remained only a small fraction of AR-FL levels 38. Conversely, AR-FL and AR-V7 mRNA levels in castration-resistant and abiraterone-resistant VCaP tumors were decreased 24 hours after androgen treatment 47. One mechanism of AR autoregulation involves AR binding a classic androgen response element in an AR intron in conjunction with lysine-specific demethylase 1 (KDM1A), resulting in the repression of AR transcription 48. In VCaP and a derivative line of LNCaP, ADT can also promote a redistribution of splicing factors that enables synthesis of AR-V7 49. Therefore, physiologic feedback mechanisms triggered by ADT can be responsible for the increased levels of ARVs and AR-FL observed in PCa patients.
In contrast to these physiological explanations for ARV expression (which are unlikely to explain resistance because they would occur months to years before progression to CRPC), there are other contexts where ARV expression does confer resistance to antiandrogen therapy. The best evidence comes from the 22Rv1 cell line derived from the CWR22 xenograft, which express high levels of AR-V7 and are resistant to enzalutamide. Importantly, siRNA knockdown of AR-V7 restored sensitivity to enzalutamide 50. In this model, it seems that AR-V7 can completely replace the function of AR-FL. However, this is not the case in VCaP cells, which also express AR-V7 yet remain sensitive to androgen depletion or enzalutamide 7, 38, 47. One important difference is that AR-V7 levels are substantially higher in 22Rv1 cells, particularly when considered relative to AR-FL. The high levels in 22Rv1 cells may be explained by intragenic duplications and rearrangements within the AR locus, which has also been reported in some patients 51, 52. Confusingly, forced expression of AR-V7 at high levels in various ADT sensitive models is not sufficient to confer resistance to castration or to enzalutamide. Overexpression of AR-V7 in ARV-negative LNCaP cells did not confer resistance to enzalutamide in vitro and in vivo, even though AR-V7 promoted tumor growth in mice treated with castration alone 38. Furthermore, overexpression of AR-V7 53 or ARv567es 54 in the prostates of transgenic mice was not sufficient to block castration-induced apoptosis and glandular involution.
Recognizing the limitations of preclinical models, the confusion about the role of AR-V7 (and other ARVs) in resistance will only be resolved through carefully executed studies in patients. Two recent reports in small cohorts of men with heavily pre-treated CRPC (post-docetaxel, multiple lines of hormonal therapies) showed that AR-V7 expression correlated with primary resistance to abiraterone or enzalutamide 55, 56. It will be important to see if these results are confirmed in larger cohorts of men with less advanced disease, where the ratio of ARV expression relative to AR-FL is expected to be substantially lower 19, 38, 40, 44.
Adrenal Androgens and Intraprostatic Testosterone and DHT Synthesis
In normal men, testosterone is produced by the testes and is the principle circulating androgen. Within tissues, testosterone is converted into the more potent androgen, 5α-dihydrotestosterone (DHT) through one of two isoenzymes, steroid 5α-reductase 1 or steroid 5α-reductase 2 (SRD5A1 or SRD5A2, respectively), both of which are targets of the drug dutasteride 57. SRD5A2 predominates within the normal prostate, while the liver and skin primarily express SRD5A1 58. Medical or surgical castration reduces circulating levels of serum testosterone by >90% 59; however, physiologically significant amounts of intraprostatic androgens remain following ADT in localized PCa 59, 60, metastatic CRPC 61, and even in benign prostates from healthy men 62. A primary source of these residual prostatic androgens are the adrenal androgens dehydroepiandrosterone (DHEA) and androstenedione (AD) which are converted to testosterone and DHT in the prostate (Figure 1C). DHEA and AD are products of de novo steroidogenesis in the adrenal gland (as well as the testis) beginning with cholesterol. Cytochrome P450-C17 (CYP17A1), the target of abiraterone, regulates two successive reactions to convert pregnenolone to DHEA, while AD arises predominantly from DHEA (Figure 1B). DHEA also exists in a sulfated form (DHEA-S), which is the predominant adrenal androgen in circulation. Levels of both DHEA and AD are significantly reduced in CRPC patients treated with abiraterone, but a persistent pool of DHEA-S could serve as a precursor for conversion to testosterone and DHT in prostate tissue 63.
In prostate cells, AD is ultimately converted through the classic route to DHT by aldo-keto reductase family 1 member C3 (AKR1C3, also known as 17-β-hydroxysteroid dehydrogenase type 5) 64, 65, or by a testosterone-independent pathway (Figure 1C) 66. There is growing evidence that AKR1C3 could be a relevant drug target for CRPC, particularly in the context of enzalutamide resistant cell lines 67, 68, and several potential inhibitors have been reported 69. Another enzyme in androgen biosynthesis that has gained recent attention is HSD3B1, which converts DHEA to AD in prostate tissue as well as the adrenal gland. A gain of function allele of HSD3B1 is found in some CRPC patient samples and cell lines (LNCaP, VCaP); the resulting HSD3B1 protein has enhanced stability and therefore increases metabolic conversion of DHEA to intraprostatic DHT 70. This allele can exist as a heterozygous germline polymorphism, but there is strong evidence for somatic mutation based on the frequency of homozygous alleles in CRPC samples. Collectively, these examples underscore the potential value of further blockade of androgen biosynthesis downstream of CYP17A1.
Androgen Receptor Bypass Signaling
Recent work reveals a novel AR pathway resistance mechanism analogous to one originally described for kinase inhibitors, in which signaling downstream of the targeted kinase is restored by activation of a related kinase not targeted by the inhibitor 71. In the context of kinase inhibitors, the clinical impact of this escape mechanism is well established in epidermal growth factor receptor (EGFR)-mutant lung cancer and BRAF-mutant melanoma and is widely referred to as “bypass” signaling to emphasize the sustained importance of the initial oncogenic pathway now activated by a different driver. Two groups have now documented an analogous mechanism for hormone receptors 72, 73. [To avoid confusion, we note that the term “bypass” was used in earlier reviews of castration resistance to describe mechanisms completely independent of AR 74, 75. In light of the contemporary analogy with kinase inhibitors, we suggest that “bypass” in this context is better suited to refer to mechanisms in which downstream hormone receptor pathway signaling remains relevant but through activation by a different hormone receptor, as described below.] In the LNCaP xenograft model with exogenous AR overexpression (LNCaP-AR) 6, acquired resistance to enzalutamide or ARN-509 correlated with upregulation of the glucocorticoid receptor (GR) as revealed by transcriptome analysis 73. A LNCaP-AR subline termed LREX′, with acquired resistance to enzalutamide, was shown to be dependent on GR expression for enzalutamide-resistant growth. In VCaP cells, glucocorticoid mediated activation of the comparatively lower level of endogenous GR was sufficient to confer enzalutamide resistance. ChIP-seq and mRNA expression analysis for AR and GR revealed highly overlapping cistrome and transcriptome profiles for both receptors 73, 76. In the resistant LREX′ tumors, GR induction was associated with restored expression of a restricted subset of AR target genes that are presumed to mediate resistance. Analysis of bone marrow biopsies from patients treated with enzalutamide supported a role for GR induction in clinical resistance to enzalutamide 73. Recent data presented at the 2015 ASCO Annual Meeting suggested that GR bypass may occur in earlier stages of disease. Tumor cells in men with high-risk localized PCa with early resistance to neoadjuvant chemical castration plus abiraterone also expressed significant levels of GR 77. It is worth highlighting that active AR inhibition is necessary to maintain high levels of GR expression in preclinical models, due to active repression of GR expression by AR binding to the GR locus. For this reason, it may be important to obtain clinical specimens from patients undergoing active antiandrogen treatment to fully evaluate the importance of GR as a resistance mechanism 73.
At first glance, the hypothesis that GR can confer resistance may seem inconsistent with clinical evidence that glucocorticoid administration can be beneficial to some CRPC patients. This apparent paradox is explained by the fact that glucocorticoids inhibit adrenocorticotropic hormone (ACTH) production by the pituitary which results in reduced androgen levels (Figure 4A) 78. This androgen lowering activity explains declines in serum PSA level observed in men taking prednisone alone, which was documented in the comparator arm of the phase III clinical trial that led to abiraterone approval for chemotherapy naive CRPC 79. However, in men whose prostate cancers express high levels of GR, this androgen lowering benefit would be counteracted by GR activation in tumor cells (Figure 4B). In this setting, a more effective treatment strategy could be combined inhibition of AR and GR, as is currently being explored in an early phase clinical trial of enzalutamide with the GR antagonist mifepristone (NCT02012296). One potential confounder of this study is the fact that mifepristone also has a high binding affinity for AR and can function as an AR agonist 80. Therefore, mifepristone treatment could unintentionally result in AR activation through agonism by displacing the potent antagonism of enzalutamide. Also of concern is the fact that mifepristone treatment resulted in higher androgen levels in an earlier single agent phase II study 81, likely due to GR inhibition in the pituitary gland, with a subsequent increase in ACTH and adrenal androgens (the opposite effect of glucocorticoid administration). It will be important to document combined AR and GR inhibition in tumor cells from patients treated in the ongoing combination therapy trial; otherwise, a lack of clinical benefit could be due to AR re-activation by mifepristone.
Figure 4. Opposing roles of glucocorticoids in prostate cancer.

A. Glucocorticoids (GCs) negatively regulate adrenocorticotropic hormone (ACTH) production from the pituitary gland, which in turn diminishes adrenal androgen production. As a consequence, there is less conversion of adrenal androgens to dihydrotestosterone (DHT). This effect is observed clinically in some patients receiving exogenous GCs (such as prednisone, dexamethasone) by decline in androgen receptor (AR) activity as measured by prostate specific antigen (PSA) 78, 147. B. In other situations, GCs can directly stimulate tumor proliferation by activating AR target gene expression. One scenario is through outcompeting enzalutamide for binding to AR target genes in tumor clones carrying the ARL702H mutation, which is stimulated by GC. Another route is GC activation of tumors by direct activation of glucocorticoid receptor (GR) in tumors that acquire GR expression, thereby bypassing the blockage of AR target gene expression by enzalutamide.
In addition to GR, the progesterone receptor (PGR) and the mineralocorticoid receptor (MR) are also steroid hormone nuclear receptor family members structurally related to AR, sharing substantial homology within the DNA binding domain 82. As with GR, it is possible that PGR or MR could transcriptionally regulate a subset of AR target genes in PCa, and thereby bypass AR. PGR expression has been demonstrated in prostate tumor cells in some 83, 84 although not all 85, 86 studies. Interestingly, high PGR staining in primary PCa was associated with clinical recurrence in a recent, large retrospective analysis 83. There is less evidence implicating MR, but it is worth noting that MR was among the top upregulated genes in VCaP xenograft tumors with acquired resistance to abiraterone 47.
Complete Androgen Receptor Independence
It has long been known that metastatic CRPC displays a remarkable degree of inter- and intra-patient molecular heterogeneity 19, 87–89. Heterogeneity also extends to the distribution and intensity of AR expression, as revealed by immunohistochemical studies of CRPC bone metastases 88, 90–92. For example, in a study with 44 CRPC bone metastases, 58.1% of patients had moderate (30.4% of cases) to intense (69.6% of cases) AR staining in 76–100% of the tumor cells, whereas 8.8% of the patients in the study had AR staining in just 1–25% of tumor cells 92. Thus, metastatic CRPC can exist as a mixture of cells displaying a range of AR expression levels.
With the growing clinical use of abiraterone and enzalutamide, it is increasingly appreciated that some men can relapse with clinically aggressive variants of PCa with reduced or absent AR expression. While the precise histological classification of these subtypes continues to be refined, they are often found to express markers of neuroendocrine differentiation (NED) (chromogranin A, synaptophysin and neural cell adhesion molecule) and may also show histological features of small cell carcinoma (SCC), a rare variant of AR-negative primary PCa also displaying NED 93–95. Adding further complexity, tumor cells with NED can often be found mixed with usual adenocarcinoma cells 95, but the relevance of these subpopulations to the disease course is unclear. Molecular profiling of PCa with NED has revealed loss of RB1, PTEN, and TP53 mutations as well as amplification of MYCN and Aurora kinase A (AURKA) 96, 97. MYCN overexpression in LNCaP resulted in the induction of NED features concurrent with downregulation of AR and AR target genes 97. Conditional deletion studies in mice provide strong evidence of a causal role for loss of both RB1 and TP53 in the genesis of metastatic CRPC with NED, although tumors in these mice retained heterogeneous expression of AR and luminal epithelial cytokeratins 98. On the other hand, RB1, PTEN, and TP53 deletions and mutations are observed in CRPC with usual adenocarcinoma histology, so the association with NED is not absolute 19.
It is unclear whether AR-negative PCa arise directly from typical AR-positive adenocarcinomas by a process of transdifferentiation or instead from a population of AR-negative neuroendocrine cells present in the normal prostate. Evidence supporting transdifferentiation comes from multiple studies showing the presence of the AR-regulated TMPRSS2-ERG genomic translocation by fluorescence in situ hybridization in AR-negative SCC 99–101 at a frequency akin to that seen in AR-positive adenocarcinoma 102. These findings are consistent with earlier observations that LNCaP cells can acquire expression of some neuroendocrine markers upon prolonged ADT 103.
Since CRPC undergoes temporal clonal selection in response to treatment 33, there is concern that the widespread and long-term usage of next generation AR inhibitors could increase the prevalence of PCa with loss of AR and NED. At present, it is still too early to know whether this concern is justified. One recent genomic landscape study of 150 CRPC patients, including many with prior exposure to abiraterone or enzalutamide, suggests this may not be the case because more than 96% of cases had usual adenocarcinoma histology. Subtypes of adenocarcinoma with NED comprised just 2.9% of cases, while SCC was only 0.7% 19. However, another study presented at the 2015 ASCO Annual Meeting of 101 cases of CRPC resistant to abiraterone or enzalutamide reported that only 33% displayed typical adenocarcinoma histology, with 12% SCC and an intermediate histology distinct from either in 27% of cases 104. Further analyses of contemporary CRPC cohorts, with careful attention to potentially new emerging histological subtypes, will be critical in resolving this issue.
Future Therapeutic Options for CRPC
Despite the clinical success of abiraterone and enzalutamide as single agent therapy for metastatic CRPC, it is clear that additional therapeutic advances are needed. One possibility is that the efficacy of both drugs may increase substantially when they are used much earlier in the disease course, as has been seen routinely with kinase inhibitors for various cancers. Clinical trials addressing this question are underway in men with pre-metastatic CRPC (also called M0 disease) and as front line therapy in men with hormone sensitive disease (NCT02058706, NCT01664923, NCT02003924, NCT01927627, NCT01715285, NCT01957436, NCT02064582, NCT02023463, NCT02028988, NCT01751451, NCT01023061, NCT02203695). In addition, it is possible that combination therapy with abiraterone and enzalutamide may be more effective than either drug used alone since they attack AR signaling by distinct mechanisms. This hypothesis is also being tested in several clinical trials (NCT01949337, NCT01650194, NCT02268175, NCT02125357). Sequential studies using one of these agents, followed by second-line treatment with the other, have shown responses in some patients but the overall impact has been modest 105–110. Recent findings that a metabolite of abiraterone, Δ4-abiraterone, is also a potent AR antagonist on par with enzalutamide may provide an explanation for cross-resistance between enzalutamide and abiraterone 111.
Although sequential or combined abiraterone and enzalutamide therapy may be insufficient to control CRPC, recent genomic landscape studies employing either tumor biopsies or circulating cell-free DNA sampling underscore the sustained importance of AR in late stage disease, with AR amplification present in 45–52% of the cancers 19, 29. These results justify continued endeavors to discover novel AR focused treatment strategies. The most advanced efforts are with compounds that continue to be directed against the LBD of AR and CYP17A1, but it remains to be seen whether these agents can overcome the cross-resistance seen after prior abiraterone or enzalutamide treatment. Several novel AR targeting methods are under investigation that bypass the conventional approach of interfering with ligand-mediated AR activation. These include small molecule inhibitors directed against the amino terminal transactivation domain 112 or the DNA binding domain 113. These molecules offer the additional advantage of efficacy against all isoforms of AR, including ARVs. Another strategy is to pharmacologically trigger AR degradation, given the analogous success with the estrogen receptor degrader fulvestrant, used for the treatment of metastatic breast cancer progressing after failure of first-line antiestrogen therapy 114. AZD3514, a purported AR degrader, showed PSA declines in 13% of CRPC patients but clinical development was halted due to gastrointestinal toxicity 115.
In addition to the sustained role of AR, another important insight from CRPC genomic landscape studies is the number of molecular alterations in other actionable pathways, including PI3K-AKT-PTEN, RAF, WNT, DNA repair and the cell cycle 19 (cBioPortal; www.cbioportal.org). Numerous inhibitors of the PI3K-AKT-mTOR pathway have shown activity in preclinical models, often in combination with next generation AR therapy, and are under study in clinical trials in CRPC 116–118. Perhaps the most unexpected finding is the high frequency of germline and somatic mutations in genes encoding proteins in DNA repair pathways, such as BRCA1, BRCA2 and ATM. BRCA1 and BRCA2-deficient tumor cells are deprived of normal repair of DNA breaks through homologous recombination, and as such, become highly sensitized to inhibitors of the DNA repair enzyme, poly(ADP-ribose) polymerase (PARP1) 119, 120. One such inhibitor, olaparib, is now approved for treatment of advanced ovarian cancer in women with germline mutations in BRCA1 or BRCA2 and others are in late stage clinical development. The ovarian cancer experience has sparked analogous PARP inhibitor trials in CRPC 121–124. Although still early, overall response rates have been encouraging including several exceptional responses in men with BRCA mutations.
Looking forward, it seems likely that the standard of care for CRPC will evolve toward distinct molecular subclasses with individualized therapies, as has already occurred in other tumor types such as lung adenocarcinoma. We base this prediction on the unexpectedly high percentage of patients with potentially actionable mutations 19 together with the now demonstrable feasibility of testing for these mutations in biopsies of metastatic lesions as well as in circulating tumor cells and circulating cell-free DNA 29, 125. Analogous to lung cancer and EGFR inhibitors, this shift in clinical practice will be driven by a compelling new treatment option. Assuming the early clinical data are confirmed, this will likely be treatment with PARP inhibitors, and perhaps cisplatin chemotherapy, in patients with BRCA1 or BRCA2 mutations. We anticipate this approach will rapidly expand beyond traditional single gene tests (companion diagnostics) to multi-gene sequencing platforms capable of identifying an array of mutations that will steer patients to appropriate clinical trials. Based on the high frequency of mutations observed in PI3K pathway genes (PTEN, PIK3CA and PIK3CB) in CRPC, we predict increased focus on clinical trials of PI3K inhibitors, particularly those selective for the p110α and p110β isoforms, in this subset of patients. Patients with complete AR independence are more challenging as there is currently little insight into actionable mutations for this subset. With growing evidence that lineage plasticity or transdifferentiation (and associated changes in chromatin landscape) may precipitate the transition to complete AR independence, the focus may shift to preventing this transition with drugs targeting chromatin modifying enzymes. Whether AR-focused treatment will remain the backbone for all men with metastatic prostate cancer remains to be determined.
Summary
The survival advantage seen from treatment with either enzalutamide or abiraterone in patients with metastatic CRPC has further solidified the importance of AR even in late stage disease. However, inherent or acquired resistance to these agents remains a major clinical obstacle and a greater understanding of biomarkers of response as well as mechanisms of resistance is urgently needed. Multiple mechanisms of resistance to AR targeted therapies have been identified (AR overexpression with or without amplification, AR mutations, ARVs, intratumoral DHT synthesis, GR overexpression and loss of AR) and others undoubtedly remain to be discovered. Due to the multiclonal and heterogeneous nature of PCa, it is probable that multiple mechanisms of resistance may be operating concurrently in any given patient, and these may also change temporally in response to sequential treatments. Clinical trials with biopsies of metastases before the onset of a new treatment and again at emergence of resistance, coupled with integrative genomic analysis, should help to identify these evolving resistance mechanisms, which could then ideally be acted upon to improve patient outcomes. In addition, insights gained through the ongoing efforts to classify molecular subtypes of PCa according to their genomic profiles should continue to identify candidate driver mutations that will better inform clinical trial design. In the long term, success will most likely come from early and aggressive treatment of high risk patients with combinations tailored to prevent resistance before tumors evolve to genomically complex stages.
Supplementary Material
Key Points.
Prostate cancer (PCa) pathogenesis is dependent upon signaling through the steroid nuclear hormone Androgen Receptor (AR), which is activated after binding of the androgen ligands testosterone or dihydrotestosterone. Ligand bound AR translocates to the nucleus, where it serves to induce or repress gene expression through binding to chromatin at cis androgen response elements.
Medical castration to substantially deplete serum testosterone is the mainstay therapy for advanced prostate cancer that recurs following surgical removal of the prostate (prostatectomy) or radiotherapy. However, castration therapy is not curative, and patients will eventually progress to lethal castration resistant prostate cancer (CRPC).
Despite a castrate level of testosterone, CRPC almost uniformly remains dependent upon AR signaling. Next generation hormonal therapies for PCa, abiraterone and enzalutamide, are now in widespread clinical use and attack AR signaling through inhibition of extra-gonadal androgen biosynthesis and by directly interfering with androgen binding to AR, respectively.
Resistance mechanisms to these drugs have been identified that result in restoration of AR signaling through gain-of-function AR mutations, upregulation of constitutively active AR splice variants, or increased intratumoral androgen biosynthesis. Another resistance mechanism bypasses AR by switching to the related Glucocorticoid Receptor (GR) to maintain transcriptional regulation of a subset of the same genes.
At resistance, a subset of patients are now presenting with low or no AR in their tumors, suggesting that evolution to complex genomic states completely independent of AR could increasingly become a cause for concern.
Comprehensive analyses of late-stage CRPC are uncovering multiple genetic lesions in this patient cohort that indicate that it may be possible to eventually stratify patients based on their cancers’ genomic profiles. These efforts will aid in clinical trial design and facilitate the employment of rationally designed combination strategies to improve patient outcomes.
Acknowledgments
VKA is funded by a Young Investigator Award from the Prostate Cancer Foundation and a Physician Research Training Award from the Department of Defense (W81XWH-11-1-0274). CLS is funded by the Howard Hughes Medical Institute, by SU2C/AACR (DT0712), by grants from the NCI/NIH (R01 CA155169-04, R01 CA19387-01, T32 CA160001-05), NIH/MSKCC Spore in Prostate Cancer (P50 CA092629-14), and from the NCI/MSK Cancer Center Support Grant/Core Grant (P30 CA008748-49 and P30 CA008748-49 S2).
Glossary Terms
- Androgen
The male sex steroid hormones, of which testosterone and dihydrotestosterone (DHT) are the principal members that bind to and activate the Androgen Receptor.
- ChIP-seq
Chromatin immunoprecipitation sequencing. A technique to ascertain the cistrome of a transcription factor of interest through the combination of immunoprecipitation followed by massive parallel sequencing.
- Cistrome
The collection of DNA elements within a genome that are bound by a transcription factor.
- CYP17A1
The gene encoding for cytochrome P450, family 17, subfamily A, polypeptide 1. CYP17A1 possess both 17α-hydroxylase and 17, 20-lyase activities and is a key enzyme in the synthesis of the steroid hormones.
- Docetaxel
Antineoplastic taxane that disrupts microtubule disassembly, resulting in inhibition of mitosis. Docetaxel is FDA approved for use in men with metastatic CRPC.
- Glucocorticoid
A class of steroid hormones produced by the adrenal gland that are involved in the regulation of metabolism and possess anti-inflammatory activity. The physiological effects of glucocorticoids are mediated through the Glucocorticoid Receptor.
- Gonadotropin-releasing hormone (GnRH)
Additionally known as luteinizing hormone-releasing hormone (LHRH), GnRH is a small peptide hormone produced in the hypothalamus that stimulates the secretion of luteinizing hormone and follicle-stimulating hormone by the pituitary gland.
- Luteinizing hormone (LH)
Secreted by the pituitary gland in response to stimulation by GnRH, LH in turn stimulates receptors on Leydig cells of the testes which leads to synthesis and secretion of testosterone.
- Neuroendocrine
A rare subtype of prostate cell found in both the normal and cancerous prostate, which is noted for the secretion of numerous neuropeptides.
- Prostate specific antigen (PSA)
An androgen regulated serine protease encoded by the gene KLK3, PSA is produced by epithelial cells of the normal and cancerous prostate. Serum levels of PSA are widely used in the clinic as a screening tool for prostate cancer, as well as to monitor cancer recurrence in the post-treatment setting.
Biographies
Philip A. Watson, PhD is a Senior Research Scientist in the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center. His research interests are in identifying novel drivers of castration resistant prostate cancer.
Vivek K. Arora, MD, PhD is an Assistant Professor in the Division of Medical Oncology at the Washington University School of Medicine in St. Louis. His research interests are in defining molecular drivers of genitourinary malignancies.
Charles L. Sawyers, MD has served as the Chair of the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center since 2006, where he is also an Investigator of the Howard Hughes Medical Institute conducting cancer research. Sawyers studies mechanisms of cancer drug resistance with an eye toward developing novel therapies. He co-discovered the antiandrogen drug enzalutamide that was approved by the FDA in 2012 for treatment of advanced prostate cancer and shared the 2009 Lasker~DeBakey Clinical Medical Research Award for his work on ABL kinase inhibitors for patients with chronic myeloid leukemia.
Footnotes
Competing interests statement
PAW: Stock ownership in Tokai Pharmaceuticals.
CLS: Inventor of patents covering enzalutamide and ARN509 and is entitled to royalties. Serves on the Board of Directors of Novartis.
VKA: Declares no competing interests.
References
- 1.Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15:3866–3871. [PMC free article] [PubMed] [Google Scholar]
- 2.Buzzoni C, et al. Metastatic prostate cancer incidence and prostate-specific antigen testing: new insights from the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 4.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001–1006. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 5.Visakorpi T, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. This paper was the first to establish that the androgen receptor undergoes frequent genomic amplification in prostate cancer during progression to castration resistance. [DOI] [PubMed] [Google Scholar]
- 6.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. Modest overexpression of the androgen receptor in prostate cancer cells was sufficient to confer resistance to androgen receptor inhibition, in part by facilitating the conversion of an antiandrogen into a transcriptional agonist. [DOI] [PubMed] [Google Scholar]
- 7.Tran C, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–1246. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. In a phase III trial, enzalutamide prolonged the survival of patients with metastatic castration resistant prostate cancer refractory to docetaxel by nearly five months. [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. In a phase III trial, abiraterone conferred a nearly four month survival advantage to patients with metastatic castration resistant prostate cancer refractory to docetaxel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan CJ, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 12.Beer TM, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldscholte J, et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, et al. Androgen receptor gene mutations in human prostate cancer. J Steroid Biochem Mol Biol. 1993;46:759–765. doi: 10.1016/0960-0760(93)90316-o. [DOI] [PubMed] [Google Scholar]
- 15.Barbieri CE, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltran H, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013;63:920–926. doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grasso CS, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. A multi-institutional effort that generated whole-exome and transcriptome sequencing from 150 patients with metastatic castration resistant prostate cancer. This study identified clinically actionable genomic alterations in 89% of the patients, highlighting possible avenues for personalized medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33:887–894. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- 21.Tan J, et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11:450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 22.Taplin ME, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–2515. [PubMed] [Google Scholar]
- 23.Scher HI, Kelly WK. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:1566–1572. doi: 10.1200/JCO.1993.11.8.1566. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, et al. Codon 877 mutation in the androgen receptor gene in advanced prostate cancer: relation to antiandrogen withdrawal syndrome. Prostate. 1996;29:153–158. doi: 10.1002/1097-0045(199609)29:3<153::aid-pros2990290303>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Hara T, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–153. [PubMed] [Google Scholar]
- 26.Balbas MD, et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. Elife. 2013;2:e00499. doi: 10.7554/eLife.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clegg NJ, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph JD, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 29.Azad AA, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–2324. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 30.Zhao XY, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–706. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 31.van de Wijngaart DJ, et al. Systematic structure-function analysis of androgen receptor Leu701 mutants explains the properties of the prostate cancer mutant L701H. J Biol Chem. 2010;285:5097–5105. doi: 10.1074/jbc.M109.039958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen EJ, et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res. 2015;21:1273–1280. doi: 10.1158/1078-0432.CCR-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carreira S, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. 2014;6:254ra125. doi: 10.1126/scitranslmed.3009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Attard G, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–516. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 35.Ware KE, Garcia-Blanco MA, Armstrong AJ, Dehm SM. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocr Relat Cancer. 2014;21:T87–T103. doi: 10.1530/ERC-13-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakazawa M, Antonarakis ES, Luo J. Androgen receptor splice variants in the era of enzalutamide and abiraterone. Horm Cancer. 2014;5:265–273. doi: 10.1007/s12672-014-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson PA, et al. Inaugural Article: Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. Overexpression of ligand binding domain deleted androgen receptor splice variants was not sufficient to confer growth resistance to enzalutamide, suggesting that in certain cellular contexts the androgen receptor variants are not fully capable of recapitulating the function of the full-length receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Z, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu R, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun S, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71:1656–1667. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenster G, Trapman J, Brinkmann AO. Nuclear import of the human androgen receptor. Biochem J. 1993;293(Pt 3):761–768. doi: 10.1042/bj2930761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hornberg E, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quarmby VE, Yarbrough WG, Lubahn DB, French FS, Wilson EM. Autologous down-regulation of androgen receptor messenger ribonucleic acid. Mol Endocrinol. 1990;4:22–28. doi: 10.1210/mend-4-1-22. [DOI] [PubMed] [Google Scholar]
- 46.Shan LX, Rodriguez MC, Janne OA. Regulation of androgen receptor protein and mRNA concentrations by androgens in rat ventral prostate and seminal vesicles and in human hepatoma cells. Mol Endocrinol. 1990;4:1636–1646. doi: 10.1210/mend-4-11-1636. [DOI] [PubMed] [Google Scholar]
- 47.Yu Z, et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20:1590–1600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai C, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457–471. doi: 10.1016/j.ccr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu LL, et al. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33:3140–3150. doi: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. In prostate cancer cells with naturally high expression of ligand binding domain truncated androgen receptor splice variants and inherent antiandrogen resistance, variant specific knockdown conferred sensitivity to enzalutamide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, et al. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 2011;71:2108–2117. doi: 10.1158/0008-5472.CAN-10-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, et al. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31:4759–4767. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun F, et al. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J Biol Chem. 2014;289:1529–1539. doi: 10.1074/jbc.M113.492140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu G, et al. AR variant ARv567es induces carcinogenesis in a novel transgenic mouse model of prostate cancer. Neoplasia. 2013;15:1009–1017. doi: 10.1593/neo.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Efstathiou E, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antonarakis ES, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bramson HN, et al. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997;282:1496–1502. [PubMed] [Google Scholar]
- 58.Steers WD. 5alpha-reductase activity in the prostate. Urology. 2001;58:17–24. doi: 10.1016/s0090-4295(01)01299-7. discussion 24. [DOI] [PubMed] [Google Scholar]
- 59.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 60.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 61.Montgomery RB, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page ST, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 63.Tamae D, et al. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chem Biol Interact. 2015;234:332–338. doi: 10.1016/j.cbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:568–574. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- 65.Koh E, Noda T, Kanaya J, Namiki M. Differential expression of 17beta-hydroxysteroid dehydrogenase isozyme genes in prostate cancer and noncancer tissues. Prostate. 2002;53:154–159. doi: 10.1002/pros.10139. [DOI] [PubMed] [Google Scholar]
- 66.Chang KH, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108:13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C, et al. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015;75:1413–1422. doi: 10.1158/0008-5472.CAN-14-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai C, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adeniji AO, Chen M, Penning TM. AKR1C3 as a target in castrate resistant prostate cancer. J Steroid Biochem Mol Biol. 2013;137:136–149. doi: 10.1016/j.jsbmb.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang KH, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–1084. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawyers CL. In: DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. DeVita VTD Jr, Lawrence TS, Rosenberg SA, editors. Wolters Kluwer Health; Philadelphia, USA: 2015. pp. 237–247. [Google Scholar]
- 72.Isikbay M, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5:72–89. doi: 10.1007/s12672-014-0173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arora VK, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. By acquiring expression of the glucocorticoid receptor, prostate cancer cells were shown to evade the antiproliferative effects of enzalutamide or ARN-509 by utilizing this related steroid hormone receptor to cross regulate a subset of androgen receptor regulated target genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 75.Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 76.Sahu B, et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73:1570–1580. doi: 10.1158/0008-5472.CAN-12-2350. [DOI] [PubMed] [Google Scholar]
- 77.Efstathiou E, et al. Biological heterogeneity in localized high-risk prostate cancer (LHRPC) from a study of neoadjuvant abiraterone acetate plus leuprolide acetate (LHRHa) versus LHRHa. ASCO Annual Meeting. 2015:abstract 5005. [Google Scholar]
- 78.Tannock I, et al. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989;7:590–597. doi: 10.1200/JCO.1989.7.5.590. [DOI] [PubMed] [Google Scholar]
- 79.Ryan CJ, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song LN, Coghlan M, Gelmann EP. Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor. Mol Endocrinol. 2004;18:70–85. doi: 10.1210/me.2003-0189. [DOI] [PubMed] [Google Scholar]
- 81.Taplin ME, et al. A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones. BJU Int. 2008;101:1084–1089. doi: 10.1111/j.1464-410X.2008.07509.x. [DOI] [PubMed] [Google Scholar]
- 82.Lu NZ, et al. International Union of Pharmacology. LXV The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- 83.Grindstad T, et al. High progesterone receptor expression in prostate cancer is associated with clinical failure. PLoS One. 2015;10:e0116691. doi: 10.1371/journal.pone.0116691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Progesterone receptor expression in human prostate cancer: correlation with tumor progression. Prostate. 2001;48:285–291. doi: 10.1002/pros.1108. [DOI] [PubMed] [Google Scholar]
- 85.Mobbs BG, Liu Y. Immunohistochemical localization of progesterone receptor in benign and malignant human prostate. Prostate. 1990;16:245–251. doi: 10.1002/pros.2990160308. [DOI] [PubMed] [Google Scholar]
- 86.Yu Y, et al. Expression and function of the progesterone receptor in human prostate stroma provide novel insights to cell proliferation control. J Clin Endocrinol Metab. 2013;98:2887–2896. doi: 10.1210/jc.2012-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roudier MP, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 88.Shah RB, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 89.Gundem G, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hobisch A, et al. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 1995;55:3068–3072. [PubMed] [Google Scholar]
- 91.Efstathiou E, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crnalic S, et al. Nuclear androgen receptor staining in bone metastases is related to a poor outcome in prostate cancer patients. Endocr Relat Cancer. 2010;17:885–895. doi: 10.1677/ERC-10-0059. [DOI] [PubMed] [Google Scholar]
- 93.Palmgren JS, Karavadia SS, Wakefield MR. Unusual and underappreciated: small cell carcinoma of the prostate. Semin Oncol. 2007;34:22–29. doi: 10.1053/j.seminoncol.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 94.Deorah S, Rao MB, Raman R, Gaitonde K, Donovan JF. Survival of patients with small cell carcinoma of the prostate during 1973–2003: a population-based study. BJU Int. 2012;109:824–830. doi: 10.1111/j.1464-410X.2011.10523.x. [DOI] [PubMed] [Google Scholar]
- 95.Epstein JI, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–767. doi: 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan HL, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. 2014;20:890–903. doi: 10.1158/1078-0432.CCR-13-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beltran H, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Z, et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
- 99.Lotan TL, et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol. 2011;24:820–828. doi: 10.1038/modpathol.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo CC, et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum Pathol. 2011;42:11–17. doi: 10.1016/j.humpath.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williamson SR, et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod Pathol. 2011;24:1120–1127. doi: 10.1038/modpathol.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 103.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 104.Small EJ, et al. Characterization of neuroendocrine prostate cancer (NEPC) in patients with metastatic castration resistant prostate cancer (mCRPC) resistant to abiraterone (Abi) or enzalutamide (Enz): Preliminary results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT) ASCO Annual Meeting. 2015:abstract 5003. [Google Scholar]
- 105.Schrader AJ, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65:30–36. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 106.Noonan KL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–1807. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 107.Badrising S, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120:968–975. doi: 10.1002/cncr.28518. [DOI] [PubMed] [Google Scholar]
- 108.Zhang T, et al. Exploring the clinical benefit of docetaxel or enzalutamide after disease progression during abiraterone acetate and prednisone treatment in men with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2015;13:392–399. doi: 10.1016/j.clgc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Bianchini D, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50:78–84. doi: 10.1016/j.ejca.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 110.Brasso K, et al. Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. Eur Urol. 2015;68:317–324. doi: 10.1016/j.eururo.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 111.Li Z, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523:347–351. doi: 10.1038/nature14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andersen RJ, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 113.Dalal K, et al. Selectively targeting the DNA-binding domain of the androgen receptor as a prospective therapy for prostate cancer. J Biol Chem. 2014;289:26417–26429. doi: 10.1074/jbc.M114.553818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90(Suppl 1):S2–6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Omlin A, et al. AZD3514, an oral selective androgen receptor down-regulator in patients with castration-resistant prostate cancer - results of two parallel first-in-human phase I studies. Invest New Drugs. 2015;33:679–690. doi: 10.1007/s10637-015-0235-5. [DOI] [PubMed] [Google Scholar]
- 116.Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr Relat Cancer. 2013;20:R83–99. doi: 10.1530/ERC-12-0394. [DOI] [PubMed] [Google Scholar]
- 117.Carver BS, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schwartz S, et al. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell. 2015;27:109–122. doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 120.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 121.Kaufman B, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fong PC, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 123.Sandhu SK, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 124.Mateo J, et al. DNA repair defects and antitumor activity with PARP inhibition: TOPARP, a phase II trial of olaparib in metastatic castration resistant prostate cancer. American Association for Cancer Research. 2015;CT322 [Google Scholar]
- 125.Lohr JG, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479–484. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gao D, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sramkoski RM, et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim. 1999;35:403–409. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- 128.Pretlow TG, et al. Xenografts of primary human prostatic carcinoma. J Natl Cancer Inst. 1993;85:394–398. doi: 10.1093/jnci/85.5.394. [DOI] [PubMed] [Google Scholar]
- 129.Dagvadorj A, et al. Androgen-regulated and highly tumorigenic human prostate cancer cell line established from a transplantable primary CWR22 tumor. Clin Cancer Res. 2008;14:6062–6072. doi: 10.1158/1078-0432.CCR-08-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 131.Mitchell S, Abel P, Ware M, Stamp G, Lalani E. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 2000;85:932–944. doi: 10.1046/j.1464-410x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 132.Klein KA, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 133.Horoszewicz JS, et al. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 134.Horoszewicz JS, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 135.Wu HC, et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 136.Navone NM, et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3:2493–2500. [PubMed] [Google Scholar]
- 137.Zhao XY, et al. Two mutations identified in the androgen receptor of the new human prostate cancer cell line MDA PCa 2a. J Urol. 1999;162:2192–2199. doi: 10.1016/S0022-5347(05)68158-X. [DOI] [PubMed] [Google Scholar]
- 138.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 139.Korenchuk S, et al. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15:163–168. [PubMed] [Google Scholar]
- 140.Liu W, et al. Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia. 2008;10:897–907. doi: 10.1593/neo.08428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Georget V, et al. Trafficking of the androgen receptor in living cells with fused green fluorescent protein-androgen receptor. Mol Cell Endocrinol. 1997;129:17–26. doi: 10.1016/s0303-7207(97)04034-3. [DOI] [PubMed] [Google Scholar]
- 142.Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41:11824–11831. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- 143.Wong CI, Zhou ZX, Sar M, Wilson EM. Steroid requirement for androgen receptor dimerization and DNA binding. Modulation by intramolecular interactions between the NH2-terminal and steroid-binding domains. J Biol Chem. 1993;268:19004–19012. [PubMed] [Google Scholar]
- 144.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 145.Wang Q, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sahu B, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011;30:3962–3976. doi: 10.1038/emboj.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Attard G, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.