Summary
WRN, the protein defective in Werner Syndrome (WS), is a multifunctional nuclease involved in DNA damage repair, replication and genome stability maintenance. It was assumed that the nuclease activities of WRN were critical for these functions. Here, we report a non-enzymatic role for WRN in preserving nascent DNA strands following replication stress. We found that lack of WRN led to shortening of nascent DNA strands after replication stress. Further, we discovered that the exonuclease activity of MRE11 was responsible for the shortening of newly replicated DNA in the absence of WRN. Mechanistically, the N-terminal FHA domain of NBS1 recruits WRN to replication-associated DNA double-stranded breaks to stabilize Rad51 and to limit the nuclease activity of its C-terminal binding partner MRE11. Thus, the previously unrecognized non-enzymatic function of WRN in the stabilization of nascent DNA strands sheds light on the molecular reason for the origin of genome instability in WS individuals.
Introduction
During DNA replication, moving replication forks may encounter obstacles like DNA lesions, DNA secondary structures, or protein-DNA complexes that can result in prolonged fork stalling and collapse to generate DNA double-strand breaks (DSBs). Alterations in the pathways involved in the recovery of stalled or collapsed replication forks cause genome instability and chromosomal rearrangements that are hallmarks of cancer cells (Bartkova et al., 2005; Petermann and Helleday, 2010). One of the multiple factors involved in DNA replication and repair is WRN, a protein defective in Werner Syndrome (WS). WS is a rare autosomal recessive disorder characterized by premature development of features that resemble aging. In addition, WS individuals have an increased cancer predisposition, leading primarily to rare cancers that are mesenchymal in origin (Friedrich et al., 2010; Goto, 1997). Primary cells derived from WS patients exhibit elevated levels of chromosomal translocations, inversions, and deletions of large segments of DNA and have a high spontaneous mutation rate (Fukuchi et al., 1989; Salk et al., 1981). Further, WS cells are hypersensitive to several types of DNA damaging agents including 4-nitroquinoline-1-oxide, cross-linking agents (such as mitomycin C and cisplatin), camptothecin, and hydroxyurea (Pichierri et al., 2001; Poot et al., 2002; Poot et al., 1999). Moreover, WS cells display a prolonged S-phase and impaired replication fork progression (Poot et al., 1992; Sidorova et al., 2008). Though these reports suggest that WRN plays a crucial role in one or more genome stability maintenance pathways, the exact contribution of WRN in preventing genome instability is unclear.
WRN belongs to the RecQ DNA helicase family. WRN is unique among known RecQ helicases in having an N-terminal 3′ to 5′ exonuclease activity (Huang et al., 1998). WRN exonuclease functions on a variety of structured DNA substrates, including bubbles, stem-loops, forks, and Holliday junctions, as well as on RNA-DNA duplexes, implying roles for WRN in DNA replication, recombination, and repair (von Kobbe et al., 2003). The 3′ to 5′ DNA helicase activity (Gray et al., 1997) of WRN shows substrate specificity similar to that for the exonuclease, suggesting that the two enzymatic activities may have coordinated functions. In addition to its nuclease activities, WRN also has nuclease-independent functions during DNA replication and repair (Chen et al., 2003; Kamath-Loeb et al., 2012), although these non-enzymatic activities are not well understood.
WRN forms several dynamic sub-complexes with different factors involved in multiple biological processes. WRN physically interacts with Nijmegen breakage syndrome protein (NBS1) via the forkhead-associated (FHA) domain of NBS1 in response to DSBs, and this interaction is important for the post-translational modification of WRN (Kobayashi et al., 2010). WRN interacts with MRE11 nuclease via NBS1 (Cheng et al., 2004); MRE11 promotes WRN helicase activity, but WRN does not modulate the nuclease activities of MRE11 (Cheng et al., 2004). WRN interacts directly with Rad51; however, this interaction does not affect the nuclease activities of WRN (Otterlei et al., 2006). Further, WRN directly and functionally associates with XPG, a DNA endonuclease, and this interaction stimulates the helicase activity of WRN (Trego et al., 2011). Furthermore, WRN not only interacts with NEIL1 but also stimulate its DNA glycosylase activities (Popuri et al., 2010). Importantly, mutations in majority of these genes lead to cancer prone disorders. However, the contributions of WRN and its interacting partners to the maintenance of genome stability are not well studied.
Though the nuclease and the non-nuclease activities of WRN have been implicated in a multitude of DNA metabolic pathways, how WRN acts at the molecular level to prevent genome instability has not been determined. In this study, we report a non-enzymatic role for WRN in the stabilization of nascent DNA strands at collapsed replication forks. We found that NBS1-mediated recruitment of WRN to the replication-associated DSBs stabilizes Rad51 and prevents the excessive degradation of nascent DNA strands mediated by MRE11. Significantly, stabilization of collapsed replication forks by the coordinated actions of WRN, NBS1, Rad51, and MRE11 prevents chromosome instability. In summary, our study reveals a previously uncharacterized non-catalytic role for WRN in the faithful duplication of the genome and provides new insights into the molecular reason for the development of genome instability in WS individuals.
Results
WRN maintains nascent DNA strands in response to replication stress
Faithful and complete replication of genome in human cells is essential for preventing the accumulation of cancer-promoting mutations. WS, a disorder of premature aging manifested in adolescence, is associated with an elevated risk of specific types of cancers. Cells derived from WS patients display elevated levels of chromosome instability. The WRN protein, which is defective in WS, has been implicated in replication fork progression and efficient restart of DNA replication (Ammazzalorso et al., 2010; Sidorova et al., 2013; Sidorova et al., 2008). To better understand the involvement of WRN in genome stability maintenance in response to replication stress, we used a single-molecule DNA fiber technique (Petermann et al., 2010; Schlacher et al., 2011) (Fig. S1A, Tables S1 and S2). In this assay, replicating DNA before and during replication stress induced by camptothecin (CPT) was sequentially labeled by incorporation of the thymidine analogs 5-iodo-2′deoxyuridine (IdU) and 5-chloro-2′-deoxyuridine (CldU), respectively (Fig. S1B). The assay was performed in hTERT-immortalized WS cells (WS) and in WS cells complemented with wild-type WRN (WS+WRN). In CPT-treated WS cells, 39±5.7% of all DNA fibers had both IdU and CldU tracts, whereas 73.2±7.8% fibers contained both IdU and CldU in CPT-treated WS+WRN cells (Fig. S1C). These results indicated that, as reported previously (Ammazzalorso et al., 2010), a greater proportion of replication forks fail to restart in WS cells in response to replication stress than in cells that express WRN. Furthermore, we observed significantly lower numbers of DNA fibers containing only CldU tracts, which represent new origins of replication, in WS cells compared to WS+WRN cells (28.7±0.09% and 62.6±5.8% in WS and WS+WRN cells, respectively, p<0.014, Fig. S1D). Importantly, we observed a significantly higher percentage of DNA fibers that contained only IdU tracts, which represent stalled forks, in CPT-treated WS cells relative to CPT-treated WS+WRN cells (61.02±2.8% and 26.81±3.9%, WS and WS+WRN, respectively, p<0.034, Fig. S1E). Our results suggest that a greater proportion of replication forks break in CPT-treated WS cells than in CPT-treated WS+WRN cells.
Interestingly, we noticed that DNA fibers that contained only IdU tracts were significantly shorter in CPT-treated WS cells than in CPT-treated WS+WRN cells (3.5±0.08 μm and 5.17±0.35 μm, respectively, P<0.022, Fig. S1F), implying that the collapsed replication forks are not maintained in WS cells. We further verified these results by labeling WS and WS+WRN cells with IdU for 30 min, after which replication stress was introduced and the IdU-labeled DNA fibers were detected using anti-BrdU (mouse monoclonal) antibodies (Fig. S1G). Under these conditions, the IdU tract lengths were the same in mock-treated WS and WS+WRN cells (4.92±0.01 μm and 5.08±0.1 μm, respectively, Fig. 1A). Similarly, the IdU tract lengths were comparable between cells treated with CPT and mock-treated WS+WRN cells (4.92±0.01 μm and 4.86±0.03 μm, respectively). In contrast, the IdU tract lengths were significantly shortened in CPT-treated WS cells relative to CPT-treated WS+WRN cells (2.71±0.05 μm and 4.86±0.03 μm, respectively, P<0.0003, Fig. 1A), reflecting a degradation of IdU tracts in WS cells. Thus, in addition to roles in replication fork progression and efficient restart, WRN also plays a role in the maintenance of nascent DNA strands in response to CPT-induced replication stress.
Figure 1. WRN stabilizes nascent DNA strands in response to replication stress.
(A) Collapsed replication forks are shortened in cells derived from WS patients. Replicating DNA in hTERT-immortalized WS and WS cells complemented with wild-type WRN (WS+WRN) were first labeled with IdU for 30 min and then treated with or without CPT (1 μm) for 5 hours. DNA fibers were immunostained with anti-BrdU antibodies. DNA fiber images were captured using a fluorescence microscopy and DNA fiber lengths were measured using Axiovision Software. The frequency distributions of lengths of more than 100 DNA fibers from three or four independent experiments in each group were calculated. Inset shows western blot for WRN expression in WS and WS+WRN cells. See also Figure S1, Tables S1 and S2.
(B) Nascent DNA tracts are shortened in the absence of WRN in HeLa cells exposed to CPT. HeLa cells were transfected with either WRN or control shRNA, labeled with IdU for 30 min, treated with CPT or mock-treated for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group were calculated. Inset shows western blot for WRN expression in HeLa cells transfected with WRN shRNA and control shRNA.
(C) Nascent DNA strands are shortened in WRN-deficient mouse embryonic fibroblasts (MEFs) treated with CPT. MEFs derived from WRN-defective and wild-type mice were labeled with IdU for 30 min, treated with 1 μm CPT or mock-treated for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group were calculated.
(D) Replication forks are stable in WS cells in response to low doses of CPT. WS and WS+WRN cells were labeled with IdU for 30 min, treated with 25 or 100 nM CPT for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(E) Replication forks are stable in WS cells in response to short HU treatment. WS and WS+WRN cells were labeled with IdU for 30 min, treated with 4 mM HU for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(F) Replication forks are shortened in BRCA2-deficient V-C8 cells in response to short HU treatment. V-C8 and V-C8+BRCA2 cells were labeled with IdU for 30 min, treated with 4 mM HU for 5 h, and the frequency distributions of lengths of DNA fibers were calculated.
Next, we verified whether the shortening of nascent DNA tracts is unique to cells derived from WS patients or whether shortening also happens in other cell types. We depleted WRN from HeLa cells using WRN-specific shRNA and examined the nascent DNA tract lengths. As in WS cells, nascent DNA tract lengths were significantly shortened in CPT-treated, WRN-depleted HeLa cells compared to CPT-treated HeLa cells transfected with a control shRNA (3.57±0.13 μm and 5.15±0.11 μm, respectively, P<0.0001, Fig. 1B). This result confirmed that WRN is required for the maintenance of nascent DNA strands in non-WS cells.
To further clarify whether the role of WRN in replication fork maintenance is limited to human cells, we performed experiments in mouse embryonic fibroblasts (MEF) derived from WRN-knockout mice (Lombard et al., 2000). As shown in Figure 1C, the nascent DNA tract lengths in CPT-treated WRN-/- MEFs were significantly shorter than in the CPT-treated wild-type MEFs (3.28±0.22 μm and 5.02±0.12 μm, respectively, P<0.0003). Thus, WRN is involved in the maintenance of nascent DNA strands in response to CPT-induced replication stress in different mammalian cell types.
Evidence indicates that CPT induces both replication stalling and DNA breaks in a concentration-dependent manner. Low doses (25-100 nM) of CPT induce replication fork slowing and reversal without inducing detectable levels of DSBs, and at higher concentrations (>100 nM) CPT induces DNA breaks (Berti et al., 2013; Ray Chaudhuri et al., 2012). To discriminate whether WRN is required for the stability of nascent DNA strands in response to replication fork stalling or replication-associated DSBs, we measured IdU tract lengths in cells treated with low-doses of CPT. As shown in Figure 1D, IdU tract lengths in WS cells treated with 25 and 100 nM CPT (5.04±0.01 μm and 5.00±0.05 μm, respectively) were similar to lengths in WS+WRN cells treated with 25 and 100 nM CPT (5.07±0.02 μm and 5.04±0.04 μm, respectively). These results suggest that WRN is not involved in the maintenance of nascent DNA tracts in response to replication fork stalling.
Treatment of cells for a short time with hydroxyurea (HU) causes replication fork stalling but it does not lead to DSBs formation (Petermann et al., 2010). To further rule out the possibility that WRN is not involved in the maintenance of nascent DNA strands in response to replication fork stalling, we exposed WS and WS+WRN cells to 4 mM HU for 5 hours and then quantified the nascent DNA tract lengths. Similar to low-dose CPT treatment, the nascent DNA tract lengths in HU-treated WS cells were comparable to those in HU-treated WS+WRN cells (4.58±0.03 μm and 4.89±0.05 μm, respectively, Fig. 1E). However, using similar HU treatment conditions, nascent DNA strands were significantly shortened in HU-treated BRCA2-defective V-C8 cells relative to V-C8 cells complemented with BRCA2 (3.26±0.06 μm and 5.16±0.01 μm, respectively, P<0.02, Fig. 1F), confirming a previous finding (Schlacher et al., 2011; Schlacher et al., 2012). Taken together, these results clearly reveal that, unlike BRCA2, WRN is not important for the stabilization of nascent DNA strands in response to replication fork stalling.
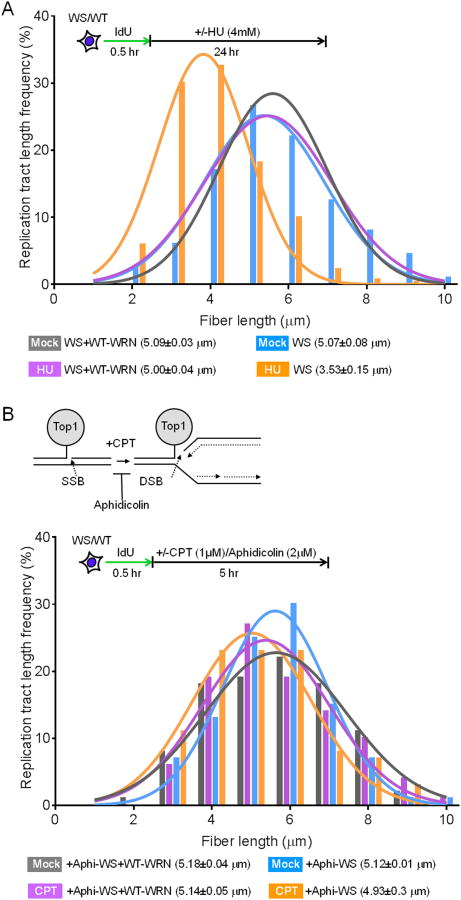
To further evaluate whether WRN is particularly important for the maintenance of nascent DNA strand in response to replication breaks or important only for replication breaks induced by CPT, we examined IdU tract lengths after treatment of cells for 24 hours with HU; under these conditions, the stalled replication forks is broken (Petermann et al., 2010). As shown in Figure 2A, IdU track lengths in HU treated WS+WRN cells were comparable to those in mock-treated WS+WRN cells (5.00±0.04 μm and 5.09±0.03 μm, respectively). In contrast, IdU track lengths were significantly shortened in HU-treated WS cells relative to HU-treated WS+WRN cells (3.53±0.15 μm and 5.0±0.04 μm, respectively, P<0.03, Fig. 2A). These results suggest that WRN is critical for the maintenance of nascent DNA strands in response to replication-associated DSBs and is not specific only to CPT-induced replication breaks.
Figure 2. WRN preserves nascent DNA strands in response to replication breaks.
(A) Replication forks are shortened in WS cells in response to long HU treatment. WS and WS+WRN cells were labeled with IdU for 30 min, treated with 4 mM HU for 24 h, and the frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(B) WRN stabilizes nascent DNA strands in response to replication-associated DSBs. WS and WS+WRN cells were labeled with IdU for 30 min and then exposed to 2 μm aphidicolin/1 μm CPT or CPT only for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
To further confirm the contribution of WRN to nascent DNA strand maintenance in response to replication breaks, we inhibited the formation of CPT-induced replication-mediated DSBs in cells using the DNA polymerase inhibitor aphidicolin (APH, Fig. 2B top) (Pommier et al., 2010; Takemura et al., 2006). Treatment of WS cells with 2 μg/ml of APH entirely prevented the CPT-dependent shortening of nascent DNA strands in WS cells (Fig. 2B bottom). The nascent DNA tract lengths in APH+CPT-treated WS cells were comparable to those in APH+CPT-treated WS+WRN cells (4.93±0.3 μm and 5.14±0.05 μm, respectively, Fig. 2B bottom). Collectively, these results reveal that WRN is required for the stabilization of nascent DNA strands in response to replication breaks.
WRN is recruited to the sites of replication-associated DSBs and it co-localizes with RPA2, Rad51, NBS1, and MRE11
Because WRN maintains newly synthesized DNA strands after replication stress, we next verified that WRN is recruited to the sites replication in response to CPT-induced replication stress. As shown in Figure S2A, WRN clearly juxtaposed with the EdU-labeled replication sites in CPT-treated early-, mid-, and late-S phase cells, indicating that WRN is indeed recruited to the sites of replication in all S-phase cells in response to replication stress. Further, as reported previously (Cheng et al., 2005; Kobayashi et al., 2010), we noticed that most WRN foci (82±8%) clearly overlapped with γH2AX foci in CPT-treated but not in mock-treated cells (Fig. S2B), confirming that WRN is recruited to the sites of replication-associated DSBs. As with previous reports (Futami et al., 2007; Patro et al., 2011; Sakamoto et al., 2001), we observed co-localization of 70±6% of WRN foci with RPA2 and Rad51 foci in CPT-treated cells (Figs. S2C and D). In addition, as reported previously (Cheng et al., 2004), we also observed co-localization of WRN and NBS1 foci (71±9%) in CPT-treated cells (Fig. S2E). Furthermore, 53±6% of WRN foci overlapped with MRE11 foci in CPT-treated cells (Fig. S2F), confirming a previous report (Pichierri and Franchitto, 2004). Taken together, these data demonstrate that WRN co-localizes with many of its known interacting partners at replication-associated DSBs.
Nuclease activities of WRN are not required for the stabilization of nascent DNA strands in response to replication-associated DSBs
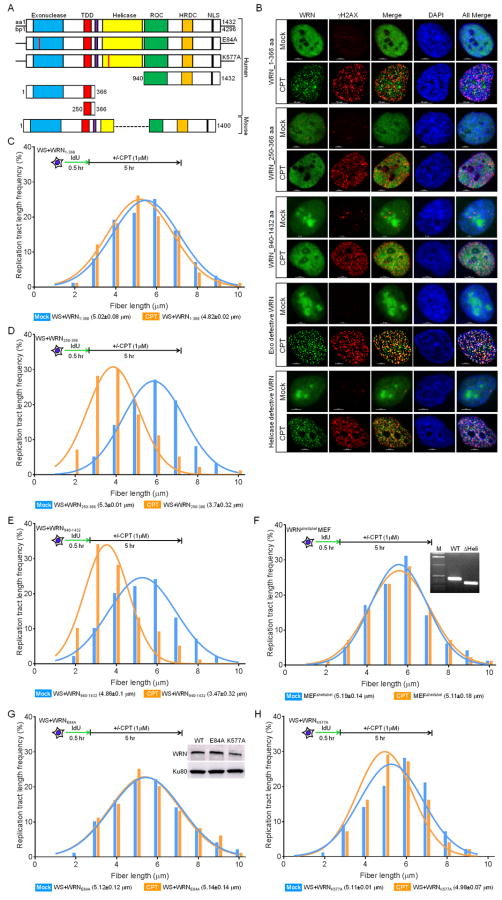
WRN contains multiple protein-protein interaction domains and has DNA binding motifs in the C-terminal domain (Lan et al., 2005; von Kobbe et al., 2003). It also has exonuclease (Huang et al., 1998) and helicase activities (Gray et al., 1997). We set out to identify the domain and the enzymatic activity that are involved in the maintenance of nascent DNA strands in response to replication breaks. We stably expressed Flag+EGFP tagged 1-366 (WRN1-366), 250-366 (WRN250-366), and 940-1432 (WRN940-1432) amino acid regions of WRN, and the exonuclease defective (WRNE84A) and the helicase defective (WRNK577A) full-length WRN constructs in WS cells (Figs. 3A and S3A-B) and used these cell lines to identify the WRN domain that is recruited to replication-associated DSBs. As shown in Figure 3B, WRN1-366, but not the WRN250-366 and WRN940-1432 domains, formed distinct foci in response to CPT-induced replication stress. Further, indirect immunostaining with anti-WRN antibodies revealed that both exonuclease (WRNE84A) and helicase (WRNk577A) defective WRNs formed distinct foci in response to CPT (Fig. 3B). In response to CPT treatment, 55±7.7% of WRN1-366, 71±5.4% of WRNE84A and 82±6.1% of WRNK577A foci overlapped with γH2AX foci. Thus, WRN1-366, exonuclease-defective and helicase-defective WRN, but not WRN250-366 and WRN940-1432, were recruited to the sites of replication-associated DSBs.
Figure 3. N-terminal region of WRN (1-366 amino acids) is sufficient to stabilize nascent DNA strands in response to replication-associated DSBs.
(A) Schematics show different functional regions of human and mouse WRN and the various WRN domains used in the study. TDD: WRN-WRN interaction domain; HRDC: helicase and RNase D C-terminal domain; RQC: RecQ conserved domain: NLS: nucleolar localization signal; E84A: exonuclease mutant; K577A: helicase mutant. See also Figure S3.
(B) N-terminal 1-366 aa domain, exonuclease-defective (E84A), and helicase-defective (K577A) WRN form foci in response to replication stress. WS cells stably expressing WRN1-366, WRN250-366, WRN940-1432, WRNE84A and WRNK577A were treated with 1 μM CPT for 1 h. After 5 h, WS cells expressing WRN1-366, WRN250-366 and WRN940-1432 were immunostained with anti-γH2AX, and WS cells expressing WRNE84A and WRNK577A were immunostained with anti-γH2AX and anti-WRN antibodies. Representative confocal microscope images are shown. Scale bars, 5 and 10 μm. DAPI, 4′6-diamidino-2-phenylindole.
(C) WRN1-366 is involved in the maintenance of nascent DNA tracts. SV40-immortalized WS cells stably expressing WRN1-366 were labeled with IdU for 30 min, treated with 1 μM CPT or mock-treated for 5 h and the frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group were calculated.
(D) WRN-WRN interaction domain (WRN250-366) is not sufficient to stabilize nascent DNA tracts. SV40-immortalized WS cells stably expressing WRN250-366 were labeled with IdU for 30 min, treated with 1 μm CPT or mock-treated for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(E) The C-terminal region containing HRDC and DNA binding motifs (940-1432 aa) of WRN is not involved in the maintenance of nascent DNA strands. SV40-immortalized WS cells stably expressing WRN940-1432 were labeled with IdU for 30 min, treated with 1 μM CPT or mock-treated for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group was calculated.
(F) The helicase domain of WRN is not required for the maintenance of nascent DNA strands. MEFs derived from helicase domain deficient WRN (WRNΔhel/Δhel) mice were labeled with IdU for 30 min, treated with 1 μM CPT or mock-treated for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group were calculated.
(G-H) The replication fork maintenance function of WRN is independent of its exonuclease and helicase activities. WS cells stably expressing exonuclease-defective (E84A, G) or helicase-defective (K577A, H) WRN were labeled with IdU for 30 min, treated with 1 μM CPT or mock-treated for 5 h, and the frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated. Inset shows western blot analyses for WT, E84A and K577A WRN expression in WS cells.
To verify that the recruitment of WRN1-366 to the sites of replication breaks functioned in the maintenance of nascent DNA strands, we evaluated lengths of IdU-labeled fragments in WS cells stably expressing WRN1-366. The IdU tract lengths in CPT-treated WS+WRN1-366 were comparable to those in mock-treated WS+WRN1-366 cells (4.82±0.02 μm and 5.02±0.08 μm, respectively, Fig. 3C). Therefore, the WRN1-366 fragment is required for the maintenance of nascent DNA strands in response to replication stress.
Since the WRN1-366 fragment contains both exonuclease (1-250 aa) and WRN-WRN interaction (250-366 aa) domains (Perry et al., 2010; Perry et al., 2006), we set out to further narrow down the region of WRN required for the maintenance of nascent DNA strands. The IdU tract lengths were significantly shortened in CPT-treated WS cells stably expressing WRN250-366 relative to mock-treated WS+WRN250-366 cells (3.7±0.32 μm and 5.3±0.01 μm, respectively, p<0.002, Fig. 3D). Thus the WRN-WRN interaction domain is not involved in the maintenance of nascent DNA strands.
We then examined the involvement of the C-terminal domain (940-1432 aa), which contains the DNA binding motifs (Lan et al., 2005; von Kobbe et al., 2003), and the HRDC domain in nascent DNA strand maintenance. We observed that the IdU tract lengths were significantly reduced in CPT-treated WS+WRN940-1432 cells as compared with the mock-treated WS+WRN940-1432 cells (3.47±0.32 μm and 4.86±0.1 μm, respectively, P<0.002, Fig. 3E,). Thus, the C-terminal domain (940-1432 aa) containing the DNA binding motifs and the HRDC domain of WRN are not required for the maintenance of nascent DNA strands.
To rule out the possibility that the lack of nascent DNA strand maintenance in CPT-treated WS+WRN250-366 and WS+WRN940-1432 cells could be due to improper folding, we examined the interaction of WRN250-366 with full-length WRN and WRN940-1432 with the Ku70/80 heterodimer. As previously demonstrated (Perry et al., 2010), the WRN250-366 domain interacted with full-length WRN in vivo (Fig. S3C). Similarly, the WRN940-1432 domain clearly interacted with Ku70/80 heterodimer in vivo (Fig. S3D), confirming a previous study (Cooper et al., 2000). These results clearly suggest that the lack of complementation of nascent DNA strand maintenance by WRN250-366 and WRN940-1432 domains is not be due to the improper folding of these constructs.
To determine the contribution of helicase domain, we used MEFs derived from WRN helicase domain-deficient mouse (WRNΔhel/Δhel) (Lebel and Leder, 1998). As shown in Figure 3F, the IdU tract lengths were similar in CPT-treated and mock-treated WRNΔhel/Δhel MEFs (5.11±0.18 μm and 5.19±0.14 μm, respectively), suggesting that the central helicase domain of WRN does not contribute to the stabilization of nascent DNA strands in response to replication stress. Next, we examined the involvement of exonuclease and helicase activities in the stabilization of replication forks. The nascent DNA strand lengths in CPT-treated WS cells expressing WRNE84A were similar to those in mock-treated WS+WRNE84A cells (5.12±0.12 μm and 5.14±0.14 μm, respectively, Fig. 3G). Similarly, the IdU tract lengths in CPT-treated WS cells expressing WRNK577A were comparable to those in mock-treated WS+WRNK577A cells (4.98±0.07 μm and 5.11±0.01 μm, respectively, Fig. 3H). Thus, the nuclease activities of WRN are not important for the stabilization of newly replicated genome.
Subsequently, we verified whether the physical presence of WRN at replication breaks is critical for the maintenance of nascent DNA strands. For this purpose, we disrupted the recruitment of WRN to replication breaks in HeLa cells by stably expressing the WRN-WRN interaction domain (Perry et al., 2010). As shown in Figure S4A, recruitment of endogenous WRN to the sites of replication breaks was attenuated in CPT-treated HeLa cells stably expressing WRN250-366. Further, we noticed that the nascent DNA strands were significantly shortened in CPT-treated HeLa+WRN250-366 cells relative to mock-treated HeLa+WRN250-366 cells (3.46±0.1 μm and 5.21±0.07 μm, respectively, p<0.0001, Fig. S4B). Therefore, it is not the nuclease activities of WRN but the physical presence of WRN at the sites of replication breaks is critical for the maintenance of nascent DNA strands.
NBS1 recruits WRN to stabilize collapsed replication forks
Though the WRN1-366 fragment lacks any known DNA binding motif, it is sufficient to maintain nascent DNA strands. Therefore, it is possible that this function of WRN is mediated indirectly via one of its binding partners. The FHA domain of NBS1 is known to recruit WRN to the sites of DSBs (Cheng et al., 2004; Kobayashi et al., 2010), and the WRN-NBS1 interaction is mediated through the N-terminal region of WRN (Cheng et al., 2004) (Fig. 4A). As shown in Figure 4B, WRN is recruited to replication-associated DSBs in CPT-treated NBS+WT NBS1 cells but not in NBS or NBS+ΔFHA-NBS1 cells. Further, DNA fiber length measurements revealed no substantial differences in the IdU tract lengths between CPT- and mock-treated NBS cells expressing full-length NBS1 (NBS+WT; 5.19±0.09 μm and 5.27±0.23 μm, respectively, Fig. 4C). In contrast, nascent DNA strand lengths were significantly shortened in CPT-treated NBS cells expressing ΔFHA-NBS1 relative to mock-treated NBS+ΔFHA-NBS1 cells (3.32±0.07 μm and 5.15±0.02 μm, respectively, P<0.00001) (Fig. 4D). Thus, the FHA domain of NBS1 recruits WRN to replication-associated DSBs, and this interaction is critical for the stabilization of newly replicated DNA.
Figure 4. Nascent DNA strand maintenance function of WRN is NBS1 dependent.
(A) Diagram shows different functional protein-protein interaction domains of NBS1 protein. FHA: Forkhead-associated domain; BRCT: BRCA1 C-terminus domain; WRN, ATM and MRE11: WRN, ATM and MRE11 interaction domains, respectively.
(B) FHA domain of NBS1 is essential for the recruitment of WRN to the sites of replication-associated DSBs. Representative confocal images show recruitment of WRN to the sites of replication-associated DSBs in NBS cells expressing full-length NBS1. NBS, NBS+NBS1, and NBS+ΔFHA-NBS1 cells were treated with 1 μM or were mock-treated for 5 h and then immunostained with anti-WRN and anti-γH2AX antibodies. Scale bars, 5 μm. DAPI, 4′6-diamidino-2-phenylindole.
(C) Nascent DNA strands are intact in NBS cells complemented with NBS1. NBS+NBS1 cells were labeled with IdU for 30 min, exposed to 1 μm CPT or were mock-treated for 5 h. Frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group were calculated.
(D) FHA domain of NBS1 is required for the maintenance of nascent DNA strands after replication breaks. NBS cells stably expressing ΔFHA NBS1 were labeled with IdU for 30 min, exposed to 1 μm CPT or were mock-treated for 5 h, and frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group were calculated. Inset shows western blot analysis for NBS1 and ΔFHA-NBS1 expression in NBS cells.
MRE11 degrades nascent DNA strands in the absence of WRN
When we examined the nascent DNA strand lengths in CPT-treated NBS cells, the IdU tract lengths were similar to those in mock-treated NBS cells (5.04±0.1 μm and 5.24±0.07 μm, respectively, Fig. 5A), implying that the newly replicated DNA is maintained in the absence of NBS1. Evidence suggests that the recruitment of MRE11 to the nucleus and damaged DNA sites is NBS1-dependent (Sakamoto et al., 2007). In the absence of full-length NBS1, MRE11 cannot be recruited to the replication-associated DSBs; hence, the nascent DNA strands are intact in NBS cells. In the NBS+ΔFHA-NBS1 cells, MRE11 is recruited to replication-associated DSBs, but WRN is not; therefore, the nascent DNA tracts are shortened in NBS+ΔFHA-NBS1 cells. Further, a number of studies have found that MRE11 degrades nascent DNA strands in the absence of BRCA2, Fanconi anemia (FA) factors, and Rad51 in response to replication stalling (Hashimoto et al., 2010; Schlacher et al., 2011; Schlacher et al., 2012). For these reasons, we hypothesize that MRE11 is the nuclease that degrades nascent DNA strands in the absence of WRN. To verify this hypothesis, we first determined the rate of nascent DNA strand degradation in WS cells. As shown in Figure 5B, exposure of WS cells to CPT for different times resulted in a gradual shortening of nascent DNA strands (5.1±0.16 μm, 4.53±0.4 μm, 3.7±0.12 μm and 2.71±0.05 μm, in mock-treated cells and in CPT-treated cells at 1, 2.5, and 5 hours, respectively). The calculated rate of nascent DNA strand degradation was 0.54±0.05 μm/hour. The slow kinetics of nascent DNA strand degradation and lack of replication fork degradation in NBS cells together reveal that the nuclease activities of MRE11 are responsible for the degradation of nascent DNA strands in the absence of WRN.
Figure 5. MRE11 degrades nascent DNA strands in response to collapsed replication forks in the absence of WRN.
(A) Nascent DNA tract lengths are maintained in NBS1-deficient cells. NBS cells were labeled with IdU for 30 min, treated with 1 μm CPT or were mock-treated for 5 h, and frequency distributions of lengths of more than 100 DNA fibers from three independent experiments in each group were calculated. Inset shows western blot analysis for NBS1 expression in NBS and NBS+NBS1 cells.
(B) Rate of nascent DNA strand degradation is slow in WS cells. WS cells were labeled with IdU for 30 min, treated with 1 μm CPT or were mock-treated for 1, 2.5, and 5 h, and frequency distributions of lengths of more than 100 DNA fibers from two-three independent experiments in each group were calculated.
(C) Nascent DNA strands are degraded from 3′-5′ direction in the absence of WRN. HeLa cells transfected with WRN shRNA were first labeled with CldU then with IdU for 20 min each. Cells were then treated with 1 μm CPT or were mock-treated for 5 h, and the IdU:CldU ratios of more than 100 DNA fibers from two independent experiments in each group were calculated.
(D) WRN blocks MRE11-dependent degradation of nascent DNA strands in response to replication-associated DSBs. WS cells were labeled with IdU for 30 min. Cells were then treated with or without 1 μM CPT and with or without 100 μM MRE11 exonuclease inhibitor mirin for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(E) WRN protects replication forks from MRE11-dependent degradation. WS cells were transfected with either control or MRE11 shRNA, labeled with IdU for 30 min, then treated with or without 1 μM CPT for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated. Inset shows western blot for MRE11 expression in WS cells transfected with control and MRE11 shRNAs.
(F) WRN prevents MRE11-mediated degradation of replication forks. MRE11-defective ATLD cells were transfected with either control or WRN shRNA. At 72 h after the transfection, cells were labeled with IdU for 30 min, then treated with or without 1 μM CPT for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated. Inset shows western blots for WRN and MRE11 expression in ATLD cells.
Subsequently, we examined the direction of nascent DNA strand degradation in WS cells by sequentially labeling the replicating DNA first with CldU and then with IdU. The IdU:CldU ratio was significantly smaller in CPT-treated WRN-depleted HeLa cells than in mock-treated WRN-depleted HeLa cells (0.62±0.09 and 1±0.01, respectively, P<0.0097, Fig. 5C). Importantly, we observed the shortening of very recently replicated DNA (i.e., IdU-labeled DNA) in CPT-treated WRN-depleted HeLa cells. MRE11 has 3′ to 5′ exonuclease and endonuclease activities (Shibata et al., 2013), and the nascent DNA strands were degraded from 3′-5′ direction, supporting our hypothesis that the degradation of nascent DNA strands in WRN-defective cells is mediated by the exonuclease activity of MRE11.
When the exonuclease activity of MRE11 was inhibited with mirin (Dupre et al., 2008), the IdU tract lengths were not shortened in CPT-treated cells, and the DNA fiber lengths in CPT+mirin-treated WS cells were comparable to that of mock+mirin-treated WS cells (4.87±0.01 μm and 5.37±0.42 μm, respectively, Fig.5D). To rule out the possibility of non-specific inhibitory activities of mirin, we depleted MRE11 in WS cells using MRE11 shRNA and then measured the nascent DNA tract lengths. As shown in Figure 5E, the DNA fiber lengths were similar in CPT-treated MRE11-depleted WS cells and in mock-treated WS+MRE11-shRNA cells (5.14±0.03 μm and 5.17±0.01 μm, respectively). To further validate these findings, we depleted WRN in MRE11-defective (ATLD) cells and found that the nascent DNA tract lengths were comparable in CPT-treated and in mock-treated WRN-depleted ATLD cells (5.11±0.01 μm and 5.14±0.01 μm, respectively; Fig. 5F). Collectively, these data indicate that MRE11 is the main nuclease that degrades nascent DNA strands after replication breaks in the absence of WRN.
WRN and Rad51 function additively to block MRE11-mediated degradation of nascent DNA strands
How does WRN limit the exonuclease activity of MRE11 on the nascent DNA strands at replication-associated DSBs? WRN may modulate either the nuclease activity of MRE11 or the stability of the interaction of Rad51 with replication breaks. Since, WRN neither stimulates nor inhibits the nuclease activities of MRE11 (Cheng et al., 2004), we excluded the possibility of WRN-mediated regulation of the nuclease activity of MRE11. As WRN directly interacts with Rad51 (Otterlei et al., 2006) and co-localizes with Rad51 at the replication-associated DSBs (Fig. S2D), it is possible that WRN and Rad51 function together in the stabilization of the broken replication forks. To determine whether WRN and Rad51 function co-operatively to protect nascent DNA strands, we first evaluated the involvement of Rad51 in the maintenance of nascent DNA strands using HT1080 cells that stably express tetracycline-inducible Rad51 shRNA. The DNA fiber lengths were significantly shortened in CPT-treated Rad51-depleted HT1080 cells relative to CPT-treated control HT1080 cells (3.36±0.11 μm and 5.09±0.01 μm, respectively, P<0.002, Fig. 6A), confirming a previous report (Hashimoto et al., 2010). Further, we validated these results using a small-molecule B02 that prevents the formation of Rad51 nucleofilaments (Huang et al., 2012). As shown in Figure S5A, pre-treatment of WS and WS+WRN cells with B02 prevented the formation of Rad51 foci in 77±11% of the cells. In cells pre-treated with B02, nascent DNA strands were shorter in CPT-treated than in mock-treated WS+WRN cells (3.4±0.26 μm and 5.1±0.02 μm, respectively, P<0.01, Fig. 6B). Moreover, similar to a previous finding (Hashimoto et al., 2010), treatment of WS+WRN cells with mirin, B02, and CPT completely prevented the degradation of IdU tracts. The nascent DNA tract lengths were comparable to those of mock+B02-treated WS+WRN cells (4.7±0.2 μm and 4.8±0.05 μm, respectively, Fig. 6B), implying that MRE11 degrades nascent DNA tracts in the absence of Rad51. Like WRN, Rad51 blocks the MRE11-meditated degradation of nascent DNA strands in response to CPT-induced replication stress.
Figure 6. WRN and Rad51 additively protect nascent DNA strands from MRE11-mediated degradation.
(A) Nascent DNA strands are shortened in the absence of Rad51. HT1080 cells stably expressing tetracycline-inducible Rad51 shRNA were labeled with IdU for 30 min, then treated with or without 1 μM CPT for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated. Inset shows analysis of Rad51 expression in HT1080 cells with and without doxycycline treatment for 72 hours.
(B) Rad51 protects replication forks from MRE11-mediated degradation in response to replication-associated DSBs. WS+WRN cells were pre-treated with 100 μM B02 for 8 h. Cells were labeled with IdU for 30 min and then treated with or without 1 μM CPT and with or without 100 μM mirin for 5 h. Subsequently, frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(C) WRN and Rad51 additively protect nascent DNA strands from MRE11-mediated degradation. HT1080 cells stably expressing tetracycline-inducible Rad51 shRNA were transfected with WRN shRNA. At 72 h after the transfection, cells were labeled with IdU for 30 min and then treated with or without 1 μM CPT and with or without 100 μM mirin for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two-three independent experiments in each group were calculated. Inset shows western blot for Rad51 and WRN expression in HT1080 cells.
(D) WRN and Rad51 additively block replication fork degradation mediated by MRE11. WS cells were pre-treated with B02 for 8 h. Cells were labeled with IdU for 30 min and then treated with or without 1 μM CPT and with or without 100 μM mirin for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two or three independent experiments in each group were calculated. See also Figure S5.
(E) Stabilization of DNA-Rad51 complex in WS cells protects nascent DNA strands. WS cells were transfected with Rad51 K133R mutant, labeled with IdU for 30 min, then treated with or without 1 μM CPT for 5 h. The frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated. Inset shows over-expression of wild-type and K133R Rad51 in WS cells. See also Figure S5.
(F) Over-expression of wild-type Rad51 in WS cells partially prevents nascent DNA strand degradation. WS cells were transfected with wild-type Rad51, labeled with IdU for 30 min, treated with or without 1 μM CPT for 5 h, and frequency distributions in lengths of more than 100 DNA fibers from two independent experiments in each group were calculated. Sell also Figure S5.
Recent reports indicate that BRCA2/FA factors and Rad51 act epistatically to protect the nascent DNA strands (Schlacher et al., 2011; Schlacher et al., 2012). Further, we noticed that the extent of nascent DNA strand degradation was comparable between WRN- and Rad51-deficient cells. To determine the relative contribution of WRN and Rad51 to the stability of replication forks, we down-regulated the expression of WRN and Rad51 in the same cell (Fig. 6C, inset). Surprisingly, we found that the nascent DNA tracts in CPT-treated WRN shRNA/Rad51 shRNA cells (2.6±0.3 μm and 5.03±0.1 μm, CPT and mock respectively, P<0.0002) were shorter than in CPT-treated cells that expressed only one of the shRNAs (Fig. 6C). Additionally, we observed similar results when WS cells were first treated with B02 and then exposed to CPT (2.73±0.21 μm and 4.78±0.06 μm, CPT and mock-treatment, respectively, P<0.0001, Fig. 6D). Further, addition of mirin prevented the degradation of nascent DNA strands in CPT-treated HT1080 cells that expressed both WRN and Rad51 shRNAs (5.03±0.1 μm and 4.9±0.15 μm, mock- and CPT-treatment, respectively, Fig. 6C) and in CPT-treated WS+B02 cells (4.78±0.06 μm and 4.58±0.19 μm, mock and mirin, respectively, Fig. 6D). Collectively, these results demonstrate that, unlike BRCA2/FA factors and Rad51, WRN and Rad51 function additively to protect nascent DNA strands after replication stress.
Additive effects of WRN and Rad51 in the protection of replication forks suggest that these proteins are functioning in independent pathways. If this is the case, then how does WRN protect nascent DNA strands in response to replication breaks? Evidence suggests that WRN directly interacts with Rad51 (Otterlei et al., 2006). Further, it has recently been shown that stabilization of Rad51 at stalled replication forks in BRCA2 and FA pathway-defective cells blocks MRE11-mediated degradation (Schlacher et al., 2011; Schlacher et al., 2012). Furthermore, the level of Rad51 expression in WS cells was similar to that of WS+WRN cells (Fig. S5B). Therefore, it is possible that WRN might stabilize Rad51 at collapsed replications forks to block the MRE11-mediated degradation of nascent DNA strands. To determine whether WRN is involved in the stabilization of Rad51 in response to replication stress, first we enumerated the number of Rad51 foci in WS and WS+WRN cells. We noticed that the number of Rad51 foci remain unchanged in WS+WRN cells exposed to CPT for 1, 2.5 and 5 hours (86.1±11.4, 79.8±7.1 and 83.8±12.4 foci per cell, respectively, Figs. S5C-D). On the contrary, similar to a previous report (Pichierri et al., 2001), the number of Rad51 foci was reduced significantly in WS cells (24.1±9.3 foci per cell) relative to WS+WRN cells exposed to CPT for 5 hr (Figs. S5C-D). Subsequently, we verified these results by a chromatin fractionation assay. As shown in Figure S5E, the level of chromatin-bound Rad51 was lower in CPT-treated WS cells relative to CPT-treated WS+WRN cells. Overall, these results suggest that WRN is somehow involved in the stabilization of Rad51 at replication-associated DSBs.
To test whether stabilization of Rad51 at replication breaks prevents MRE11-mediated degradation of nascent DNA strands, we expressed a Rad51 mutant (K133R) in WS cells (Fig. S5F). ATP hydrolysis by Rad51 is required for efficient dissociation of Rad51 from the DNA (van Mameren et al., 2009). The Rad51 K133R mutant lacks ATPase activity, and it forms stable DNA-Rad51 complexes and promotes strand exchange in vitro (Morrison et al., 1999). In WS cells over-expressing the Rad51 K133R mutant, the nascent DNA tract lengths were not shortened in CPT-treated cells compared to mock-treated cells (4.38±0.04 μm and 4.46±0.01 μm, respectively, Fig. 6E). Further, Rad51 levels are often elevated in tumor cells (Brown and Holt, 2009) and the formation of stable Rad51 filaments are observed in these cells (Raderschall et al., 2002). We tested whether over-expression of Rad51 in WS cells stabilizes Rad51 filaments and therefore protects replication forks (Fig. S5F). As shown in Figure 6F, over-expression of wild-type Rad51 partially protected the nascent DNA tracts in CPT-treated WS cells (3.99±0.12 μm and 4.52±0.02 μm, CPT and mock, respectively). Thus, the stabilization Rad51 at replication breaks in WRN-defective cells blocks MRE11-mediated degradation of nascent DNA strands.
WRN is critical for chromosome stability in response to CPT-treatment
To investigate the contribution of WRN to maintenance of genome stability in response to replication stress, we evaluated gross chromosomal aberrations in metaphase cells derived from WS and WS+WRN cells exposed to CPT for five hours. Classical chromosome analysis of metaphase spreads revealed that exposure of WS cells to CPT significantly elevated the number of chromosomal aberrations per mitotic cell relative to the number per mitotic WS+WRN cell (P=0.0006, Fig. S6A). The average number of aberrations per WS cell treated with CPT was 2.29±0.02, but it was only 0.56±0.03 per WS+WRN cell exposed to CPT. Further, the percent of metaphases with gaps, breaks, and radials was significantly higher in WS cells relative to WS+WRN cells (Fig. S6B). Numbers of gaps, breaks, tri-radial, chromosomal breaks, and end-end fusions were also significantly elevated in CPT-treated WS cells compared to CPT-treated WS+WRN cells (Fig. S6C). Thus, WRN plays an important role in the suppression of chromosome instability in response to replication stress.
WRN, NBS1, Rad51 and MRE11 assemble onto nascent DNA strands
To verify whether WRN, NBS1, Rad51 and MRE11 assemble onto nascent DNA strands, we carried out chromatin immunoprecipitation (ChIP) assay (Petermann et al., 2010). As shown in Figure Fig. 7A, WRN, NBS1, Rad51 and MRE11 clearly interacted with the nascent DNA strands in WS+WRN cells. Interestingly, we noticed that association of Rad51 with nascent DNA strands was reduced in CPT treated WS cells relative to WS+WRN cells (Fig. 7A), further supporting our observation on the reduced number of Rad51 foci and chromatin binding of Rad51 in WS cells. Evidences indicate that WRN directly interacts with NBS1 (Cheng et al., 2004) and Rad51 (Otterlei et al., 2006) and its interaction with MRE11 is mediated via NBS1 (Cheng et al., 2004). Therefore, it is possible that WRN, NBS1, Rad51 and MRE11 assemble onto nascent DNA strands not as a complex but via pair wise interactions.
Figure 7. WRN, NBS1, Rad51 and MRE11 assemble onto nascent DNA strands to maintain genome stability in response to replication stress.
(A) WRN, NBS1, Rad51 and MRE11 associate with nascent DNA strands. WS and WS+WRN cells were labeled with IdU for 60 min and then treated with 1 μM CPT for 5 hr. Cells were cross-linked with paraformaldehyde and the chromatin fraction (input) was subjected to co-immunoprecipitation using anti-BrdU mouse monoclonal antibodies. Western blots were probed with anti-WRN, anti-MRE11, anti-NBS1, anti-Rad51, and anti-Histone 3 antibodies.
(B) A model depicting the choreography of WRN, NBS1, Rad51 and MRE11 action in the maintenance of nascent DNA strands in response to replication-associated DSBs. See also Figure S6.
Discussion
Here, we report a previously uncharacterized non-enzymatic role for WRN in the stabilization of nascent DNA strands in response to replication stress. We found that the nuclease activities of WRN were not required for the maintenance of nascent DNA strands in response to replication breaks. The N-terminal FHA domain of NBS1 recruits WRN to the replication-associated DSBs to limit the nuclease activity of its C-terminal binding partner, MRE11, on the newly replicated genome. Notably, WRN functions together with Rad51 to block the MRE11-mediated degradation of nascent DNA strands. Overall, our findings reveal that WRN, NBS1 and Rad51 co-operatively protect collapsed replication forks to maintain genome stability (Fig. 7B). See also supplemental information.
A unique finding of our study is that the physical presence of WRN at replication-associated DSBs, rather than its enzymatic activities, is critical for the protection of nascent DNA strands. This is evident from our observation that the nascent DNA strands are not degraded in WS cells expressing exonuclease- or helicase-defective WRNs (Figs. 3G and H). The nascent DNA strands were degraded when recruitment of WRN to the replication-associated DSBs was blocked in wild-type cells by expression of the WRN-WRN interaction domain (Fig. S4B). In support of our findings, a previous report indicated that WRN plays a structural role during DSB repair by protecting DSB ends from enzymatic degradation (Chen et al., 2003) and another study found that WRN forms complexes with a range of DNA structures independently of its catalytic activities (Kamath-Loeb et al., 2012). Our identification of the non-enzymatic contribution of WRN in the stabilization of nascent DNA tracts clarifies why WS patients are symptomatic even though most of the WRN mutations identified result in premature termination of WRN protein leading to a loss of WRN nuclear localization rather than to mutations that eliminate exonuclease or helicase activity.
Our results revealed that nascent DNA strands are shortened more in CPT-treated cells deficient in WRN and Rad51 than in cells treated with CPT and either WRN or Rad51 shRNA alone (Figs. 6C and D), suggesting that WRN and Rad51 function additively to maintain newly replicated DNA. This is in contrast to replication fork protection functions of BRCA2 and FA factors. BRCA2 and FA factors functions are epistatic with Rad51 function (Schlacher et al., 2011; Schlacher et al., 2012). There are a number of potential explanations for this puzzling observation. Evidence indicates that CPT can induce both replication fork stalling and breaks. In the absence of WRN, Rad51 may collaborate with BRCA2 and FA factors to maintain the nascent DNA strands in response to CPT-induced stalling of replication forks. In support of this possibility, replication fork stalling caused by low-dose CPT or short-term HU treatments did not shorten the nascent DNA strands in WS cells (Figs. 1D and E). On the other hand, in the absence of Rad51, WRN can bind to replication breaks and may be sufficient to block the MRE11-mediated degradation of nascent DNA strands. Alternatively, WRN may interact with Rad52 via its C-terminal domain (Baynton et al., 2003) to protect newly synthesized DNA from MRE11. These possibilities can be tested by identifying and mutating the Rad51/Rad52 interaction domain in WRN and by examining the ability of WRN mutants to protect nascent DNA strands. Furthermore, in the absence of both WRN and Rad51, it is probable that both stalled and broken replication forks are degraded by the MRE11 and, therefore, more shortening of nascent DNA strands in CPT-treated WRN- and Rad51-deficient cells was observed than in cells deficient in either Rad51 or WRN. Despite unknowns, our study, together with other reports, clearly indicates that Rad51 together with BRCA2/FA factors or with WRN blocks MRE11-mediated degradation of both stalled and broken replication forks, respectively.
We found that WRN is somehow involved in the stabilization of Rad51 at replication-associated DSBs (Figs. S5C-E). This result was totally unexpected, since there is no evidence for the WRN-mediated stabilization of Rad51 at replication breaks. However, the mechanism by which WRN directly or indirectly stabilizes Rad51 at replication breaks is unclear. WRN is shown to directly interact with Rad51 (Otterlei et al., 2006) and this interaction may facilitate the stability of Rad51 at replication breaks. In the absence of WRN, Rad51 loads onto the replication breaks but might immediately be unloaded. This can happen either passively due to lack of WRN at the breaks or actively dislodged by other proteins. For example, BLM and/or RecQL5 might disrupt Rad51 filaments (Bugreev et al., 2007; Hu et al., 2007) in the absence of WRN; however, further experiments are required to support this notion. Nevertheless, our study together with other studies (Schlacher et al., 2011; Schlacher et al., 2012) suggest that Rad51 prevents MRE11-mediated degradation of nascent DNA strands through two parallel, yet cooperative, pathways: 1) Rad51 cooperates with BRCA2 and FA factors to stabilize nascent DNA strands in response to stalled replication forks (Schlacher et al., 2011; Schlacher et al., 2012) and 2) Rad51 collaborates with WRN to maintain nascent DNA strands in response to replication breaks.
Mutations in WRN, NBS1, and MRE11 genes lead to WS, Nijmegen breakage syndrome (NBS), and Ataxia telangiectasia-like disorder (ATLD), respectively. Individuals with these syndromes are all at risk for development of cancer. What is the link between unprotected replication forks and genome instability? The presence of a large number of chromosomal aberrations in CPT-treated WRN-defective cells suggests that maintenance of nascent DNA strands is critical for the prevention of chromosomal instability. Though exposure of WS cells to CPT compromises cell survival (Poot et al., 1999), some cells with chromosomal aberrations enter mitosis. Every subsequent round of replication is expected to increase the overall mutation level in surviving cells. Therefore, the biological significance of unprotected replication forks is high, since replication of a damaged genome can provide the opportunity for genomic rearrangements and can increase genomic instability leading to genetic changes required for progression from an initiated cell to a metastatic tumor cell.
Our data suggest a model for the interplay of WRN, NBS1, MRE11, and Rad51 in preserving genome integrity during replication (Fig. 7B). NBS1 recruits WRN through the N-terminal FHA domain and MRE11 via its C-terminal domain to the replication-associated DSBs. The physical presence of WRN at collapsed replication forks somehow stabilizes the interaction of Rad51 with replication breaks and that limits the excessive exonuclease activity of MRE11 on the newly replicated genome. Thus, the WRN, NBS1 and Rad51 co-operatively protect nascent DNA strands to maintain genome integrity during replication.
In summary, we have deciphered the molecular choreography of WRN, NBS1, MRE11, and Rad51 that occurs at nascent DNA strands to maintain chromosomal integrity during replication. The coordinated action of these factors prevents the accumulation of cancer-promoting mutations in humans. Our experiments revealed a non-enzymatic role for WRN in DNA replication and provide new insight into the molecular origin of genome instability in WS individuals.
Experimental Procedures
Cell lines
The simian virus 40 transformed control (AG07217A) and Werner Syndrome (AG11395) fibroblasts, HT1080 cells, and HeLa cells were obtained from the ATCC. hTERT-immortalized WS and WS cells complemented with WT, exonuclease defective (E84A), or helicase defective (K577A) WRN were described previously (Perry et al., 2006). NBS, NBS cells complemented with full-length or FHA-deleted NBS1, and ATLD cells were described previously (Kobayashi et al., 2010). Chinese hamster ovary cells defective in BRCA2 (V-C8) and V-C8 cells complemented with WT BRCA2 were described previously (Nagasawa et al., 2008).
Drugs
Aphidicolin, MRE11 inhibitor [5-(4-hydroxybenzylidene)-2-iminothiazolidin-4-one, (mirin)], IdU (I7125), CldU (C6891), hydroxyurea, and camptothecin were obtained from Sigma. Rad51 inhibitor [(E)-3-benzyl-2(2-(pyridine-3-yl)vinyl)quinazolin-4(3H)-one, (BO2)] was purchased from Calbiochem.
DNA fiber assay
DNA fiber technique was performed as previously described (Petermann et al., 2010; Schlacher et al., 2011). Briefly, 2.5×105 cells were labeled with IdU (150 μm) for 30 min, washed four times with warm PBS and exposed to 1 μm CPT for 1 to 5 h. After three washes with warm PBS, both labeled and unlabeled cells were trypsinized and mixed at 1:15 ratio (labeled:unlabeled), lysed on a clean glass slide in 20 μl of lysis buffer (0.5% SDS, 50 mM EDTA, and 200 mM Tris-HCl, pH 7.4) for 8 min, and slides were tilted slightly (∼15° angle) to help DNA spread slowly. Subsequently, slides were immunostained with anti-BrdU antibodies and the DNA fiber lengths were measured using Axiovision Software. See also supplemental information.
Statistical analysis
The Student's t-test was performed to calculate the level of significance and a value of p<0.05 was considered statistically significant. Graphpad Prism (version 6.0) was used to calculate DNA fiber lengths distribution and for making the graphs.
Supplementary Material
Figure S1, related to figure 1: A majority of replication forks collapse in WS cells in response to replication stress.
(A) Flow chart shows DNA fiber experimental setup, with incorporation of IdU and CldU shown in green and red, respectively.
(B) Representative immunofluorescence images of DNA fibers showing sequential labeling of IdU and CldU in mock-treated WS+WRN cells.
(C) Replication restart in CPT treated WS and WS+WRN cells was evaluated by the percentage of labeled tracts containing both IdU and CldU against the total number of labeled tracts. Each data point is the average of two independent experiments. Error bars represent STDEV.
(D) Firing of new origins in CPT treated WS and WS+WRN cells were evaluated by the percentage of labeled tracts containing only CldU. Each data point is the average of two independent experiments. Error bars represent STDEV.
(E) Collapsed replication forks in CPT treated WS and WS+WRN cells were evaluated by the percentage of labeled tracts containing only IdU. Each data point is the average of two independent experiments. Error bars represent STDEV.
(F) Collapsed replication forks are shorter in CPT treated WS cells relative to WS+WRN cells. Replicating DNA before and during CPT-induced replication stress in WS and WS+WRN was sequentially labeled by incorporation of the IdU and CldU, respectively. The length of DNA fibers that contained only IdU tracts was measured, and the frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(G) Representative DNA fiber images show intact (left) and shortened (right) DNA fibers in mock- and CPT-treated WS cells, respectively.
Figure S2, related to results section on the recruitment and association of WRN with RPA2, Rad51, NBS1, and MRE11 at replication-associated DSBs,
(A) WRN is recruited to the sites of replication in response to replication stress. WS cells stably expressing EGFP-tagged-WRN were pulse-labeled with 50 μm EdU for 90 min and then treated with 1 μm CPT for 1 h. After 5 h, cells were fixed with 4% paraformaldehyde and the Click-IT reaction was carried out to detect EdU signal. Representative three-dimensional deconvoluted confocal images are shown.
(B-F) A majority of WRN co-localizes with γH2AX, RPA2, RAD51, NBS1, and MRE11 at replication-associated DSBs. WS cells stably expressing EGFP-tagged WRN were treated with 1 μM CPT for 1 h. After 5 h, cells were fixed with 4% paraformaldehyde and subjected to indirect immunostaining with (B) anti-γH2AX, (C) anti-RPA2, (D) anti-RAD51, (E) anti-NBS1, or (F) anti-MRE11 antibodies. Representative three-dimensional deconvoluted confocal images are shown. All scale bars, 5 μm.
Figure S3, related to figure 3: WRN940-1432 and WRN250-366 domains interact with Ku70/80 heterodimer and WRN, respectively.
(A) Western blot shows expression of different domains of WRN in WS cells. Whole-cell extracts prepared from WS cells stably expressing Flag-EGFP-tagged WRN1-366, WRN250-366, WRN940-1432 and full-length WRN (1-1432aa) were separated onto a 10% SDS-PAGE and immunoblotted with anti-Flag monoclonal antibodies.
(B) Representative live cell images show localization of EGFP-tagged WRN1-366, WRN250-366, WRN940-1432 and full-length WRN in WS cells. Cells were seeded onto a glass-bottomed 35 mm dishes and the live cell images were captured using a fluorescent microscope. All scale bars, 5μm.
(C) Western blots shows in vivo interaction of WRN 250-366 domain with full length WRN. Total cell extract prepared from HeLa cells stably expressing flag EGFP-tagged-WRN-250-366 was subjected to immunoprecipitation using anti-Flag (M2) antibodies. Subsequently, the immune complex was separated onto a SDS-PAGE and immunoblotted with anti-WRN (endogenous WRN) and anti-Flag (WRN 250-366) antibodies.
(D) Western blots shows in vivo interaction of WRN 940-1432 domain with Ku70/80 heterodimer. Total cell extract prepared from WS cells stably expressing FLAG-EGFP-tagged-WRN 366-800 and WRN 940-1432 were subjected to immunoprecipitation using anti-Flag (M2) antibodies. Subsequently, the immune complex was separated onto a SDS-PAGE and immunoblotted with anti-Ku70, anti-Ku80 and anti-Flag antibodies.
Figure S4, related to figure 3: Expression of WRN-WRN interaction domain in HeLa cells results in the degradation of nascent DNA strands.
(A) Representative images show localization of endogenous WRN in HeLa cells stably expressing WRN-WRN interaction domain (WRN250-366). HeLa+WRN250-366 were treated with or without 1 μM CPT for 1 h and were fixed with 4% PFA. Subsequently, cells were immunostained with anti-γH2AX and anti-WRN antibodies and were imaged using a confocal microscopy. The result shows that the recruitment of WRN to the replication-associated DSBs is abrogated due to the expression of WRN250-366 domain. All scale bars, 5μm.
(B) Replication forks are shortened in HeLa cells expressing WRN250-366. HeLa+WRN250-366 cells were labeled with IdU for 30 min, treated with or without 1 μM CPT for 5 h. DNA fibers were immunostained with anti-BrdU antibodies. DNA fiber images were captured using a fluorescence microscope and DNA fiber lengths were measured using Axiovison Software. The frequency distribution of lengths of more than 100 DNA fibers from three independent experiments in each group was calculated.
Figure S5, related to figures 6 and 7: WRN plays a role in the stabilization of Rad51 at replication-associated DSBs.
(A) Recruitment of Rad51 to replication-associated DSBs is blocked by Rad51 inhibitor (B02). WS and WS+WRN cells were pre-treated with 50 μM B02 for 8 h and then treated with 1 μM CPT for 5 h. Cells were fixed with 4% paraformaldehyde and immunostained with anti-γH2AX and anti-Rad51 antibodies. Representative confocal microscope images are shown. All scale bars, 10 μm.
(B) Expression of Rad51 is not affected in WRN-defective cells. Nuclear extracts prepared from WS and WS+WRN cells were separated onto a 10% SDS-PAGE and immunoblotted with anti-WRN, anti-Rad51, and anti-Ku80 antibodies.
(C) Rad51 foci numbers are reduced in CPT treated WS cells but not in WS+WRN cells. WS and WS+WRN cells were exposed to 1 μM CPT for 5 hrs and were subjected to indirect immunostaining with anti-Rad51 and anti-γH2AX antibodies. All scale bars, 10 μm.
(D) Graph shows the number of Rad51 foci in CPT treated WS and WS+WRN cells. WS and WS+WRN cells were exposed to 1 μM CPT and were subjected to indirect immunostaining with anti-Rad51 antibodies at indicated times. Rad51 foci were counted in 50-100 cells from two independent experiments. Error bars represent STDEV.
(E) Biochemical fractionation of chromatin bound Rad51. WS and WS+WRN cells were treated with 1 μM CPT for 5 h and cells were subjected to chromatin fractionation. Chromatin fraction was separated onto a 4-15% gradient SDS-PAGE, transferred onto a nitrocellulose membrane and immunoblotted with anti-WRN, anti-Rad51, anti-Ku80 and anti-H3 antibodies. The signal intensity was quantified using Image J.
(F) Wild-type and K133R Rad51 are recruited to the sites of replication-associated DSBs in WS cells. WS cells transfected with FLAG-EYFP-WT and K133R Rad51 were treated with 1 μM CPT for 5 h and then were immunostained with anti-γH2AX and anti-Rad51 antibodies. All scale bars, 10 μm.
Figure S6, related to results section on the role for WRN in chromosome stability maintenance in response to CPT induced replication stress.
(A) Graph shows the number of gross-chromosomal aberrations in WS and WS+WRN cells treated with and without CPT.
(B) Graph shows the percentage of WS and WS+WRN cells with radial and non-radial type chromosomal aberrations in WS and WS+WRN cells treated with or without CPT.
(C) Graph shows different types of gross-chromosomal aberrations in WS and WS+WRN cells treated with and without CPT. Exponentially growing WS and WS+WRN cells stably expressing WRN were treated with 1 μM CPT for 5 h. After 20 h, chromosome preparations were made by accumulating mitotic cells in the presence of 0.1 mg/ml colcemid for 4 h. For each cell type, more than 50 metaphase spreads were counted. Each data point is the average of three independent experiments. Error bars represent STDEV. *-p=0.005; **-p=0.003; ***-p=0.0003; Qua-Radial-quadri-radial.
Table S1,related to figures 1, 2, 3, 4, 5, 6, S1 and S4: DNA fiber data analysis information
Table S2, related to figures 1, 2, 3, 4, 5, 6, S1 and S4: DNA fiber lengths distribution data analysis information
Acknowledgments
We would like to thank Dr. Brad Johnson and Dr. Philip Leder for the WRN knockout and WRNΔhel/Δhel mouse strains, respectively. We also thank Drs. Claudia Wiese and David Schild for Rad51 shRNA and Rad51 K133R mutant plasmids. This work was supported by a National Institutes of Health Grant CA134991 (to D.J.C) and the National Aeronautics and Space Association grants NNX13AD57G (to A.A.) and NNX11AC54G (to D.J.C.).
Footnotes
Supplemental Information includes extended experimental procedures and discussion, six figures, two tables and detailed figure legends, and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.10.025.
Author Contributions: F.S. and A.A. designed and performed the experiments, analyzed data and made the figures. S.M., Y.Y., E.M., S.B., S.M.Y., and J.K. performed experiments. D.J.C provided reagents/materials/analysis tools. A.A. conceived the study and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammazzalorso F, Pirzio LM, Bignami M, Franchitto A, Pichierri P. ATR and ATM differently regulate WRN to prevent DSBs at stalled replication forks and promote replication fork recovery. EMBO J. 2010;29:3156–3169. doi: 10.1038/emboj.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Baynton K, Otterlei M, Bjoras M, von Kobbe C, Bohr VA, Seeberg E. WRN interacts physically and functionally with the recombination mediator protein RAD52. J Biol Chem. 2003;278:36476–36486. doi: 10.1074/jbc.M303885200. [DOI] [PubMed] [Google Scholar]
- Berti M, Ray Chaudhuri A, Thangavel S, Gomathinayagam S, Kenig S, Vujanovic M, Odreman F, Glatter T, Graziano S, Mendoza-Maldonado R, et al. Human RECQ1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat Struct Mol Biol. 2013;20:347–354. doi: 10.1038/nsmb.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ET, Holt JT. Rad51 overexpression rescues radiation resistance in BRCA2-defective cancer cells. Molecular carcinogenesis. 2009;48:105–109. doi: 10.1002/mc.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugreev DV, Yu X, Egelman EH, Mazin AV. Novel pro- and anti-recombination activities of the Bloom's syndrome helicase. Genes & development. 2007;21:3085–3094. doi: 10.1101/gad.1609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang S, Lee L, Davalos A, Schiestl RH, Campisi J, Oshima J. WRN, the protein deficient in Werner syndrome, plays a critical structural role in optimizing DNA repair. Aging Cell. 2003;2:191–199. doi: 10.1046/j.1474-9728.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Sakamoto S, Fox JT, Komatsu K, Carney J, Bohr VA. Werner syndrome protein associates with gamma H2AX in a manner that depends upon Nbs1. FEBS Lett. 2005;579:1350–1356. doi: 10.1016/j.febslet.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Cheng WH, von Kobbe C, Opresko PL, Arthur LM, Komatsu K, Seidman MM, Carney JP, Bohr VA. Linkage between Werner syndrome protein and the Mre11 complex via Nbs1. J Biol Chem. 2004;279:21169–21176. doi: 10.1074/jbc.M312770200. [DOI] [PubMed] [Google Scholar]
- Cooper MP, Machwe A, Orren DK, Brosh RM, Ramsden D, Bohr VA. Ku complex interacts with and stimulates the Werner protein. Genes & development. 2000;14:907–912. [PMC free article] [PubMed] [Google Scholar]
- Dupre A, Boyer-Chatenet L, Sattler RM, Modi AP, Lee JH, Nicolette ML, Kopelovich L, Jasin M, Baer R, Paull TT, et al. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nature chemical biology. 2008;4:119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich K, Lee L, Leistritz DF, Nurnberg G, Saha B, Hisama FM, Eyman DK, Lessel D, Nurnberg P, Li C, et al. WRN mutations in Werner syndrome patients: genomic rearrangements, unusual intronic mutations and ethnic-specific alterations. Hum Genet. 2010;128:103–111. doi: 10.1007/s00439-010-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K, Martin GM, Monnat RJ., Jr Mutator phenotype of Werner syndrome is characterized by extensive deletions. Proc Natl Acad Sci U S A. 1989;86:5893–5897. doi: 10.1073/pnas.86.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami K, Takagi M, Shimamoto A, Sugimoto M, Furuichi Y. Increased chemotherapeutic activity of camptothecin in cancer cells by siRNA-induced silencing of WRN helicase. Biol Pharm Bull. 2007;30:1958–1961. doi: 10.1248/bpb.30.1958. [DOI] [PubMed] [Google Scholar]
- Goto M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mech Ageing Dev. 1997;98:239–254. doi: 10.1016/s0047-6374(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Gray MD, Shen JC, Kamath-Loeb AS, Blank A, Sopher BL, Martin GM, Oshima J, Loeb LA. The Werner syndrome protein is a DNA helicase. Nat Genet. 1997;17:100–103. doi: 10.1038/ng0997-100. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Ray Chaudhuri A, Lopes M, Costanzo V. Rad51 protects nascent DNA from Mre11-dependent degradation and promotes continuous DNA synthesis. Nature structural & molecular biology. 2010;17:1305–1311. doi: 10.1038/nsmb.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, Zheng L, Stark JM, Barnes EL, Chi P, Janscak P, et al. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes & development. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Mazina OM, Zentner IJ, Cocklin S, Mazin AV. Inhibition of homologous recombination in human cells by targeting RAD51 recombinase. Journal of medicinal chemistry. 2012;55:3011–3020. doi: 10.1021/jm201173g. [DOI] [PubMed] [Google Scholar]
- Huang S, Li B, Gray MD, Oshima J, Mian IS, Campisi J. The premature ageing syndrome protein, WRN, is a 3′→5′ exonuclease. Nat Genet. 1998;20:114–116. doi: 10.1038/2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath-Loeb A, Loeb LA, Fry M. The Werner syndrome protein is distinguished from the Bloom syndrome protein by its capacity to tightly bind diverse DNA structures. PloS one. 2012;7:e30189. doi: 10.1371/journal.pone.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J, Okui M, Asaithamby A, Burma S, Chen BP, Tanimoto K, Matsuura S, Komatsu K, Chen DJ. WRN participates in translesion synthesis pathway through interaction with NBS1. Mech Ageing Dev. 2010;131:436–444. doi: 10.1016/j.mad.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Nakajima S, Komatsu K, Nussenzweig A, Shimamoto A, Oshima J, Yasui A. Accumulation of Werner protein at DNA double-strand breaks in human cells. J Cell Sci. 2005;118:4153–4162. doi: 10.1242/jcs.02544. [DOI] [PubMed] [Google Scholar]
- Lebel M, Leder P. A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc Natl Acad Sci U S A. 1998;95:13097–13102. doi: 10.1073/pnas.95.22.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Beard C, Johnson B, Marciniak RA, Dausman J, Bronson R, Buhlmann JE, Lipman R, Curry R, Sharpe A, et al. Mutations in the WRN gene in mice accelerate mortality in a p53-null background. Mol Cell Biol. 2000;20:3286–3291. doi: 10.1128/mcb.20.9.3286-3291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C, Shinohara A, Sonoda E, Yamaguchi-Iwai Y, Takata M, Weichselbaum RR, Takeda S. The essential functions of human Rad51 are independent of ATP hydrolysis. Mol Cell Biol. 1999;19:6891–6897. doi: 10.1128/mcb.19.10.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Wilson PF, Chen DJ, Thompson LH, Bedford JS, Little JB. Low doses of alpha particles do not induce sister chromatid exchanges in bystander Chinese hamster cells defective in homologous recombination. DNA Repair (Amst) 2008;7:515–522. doi: 10.1016/j.dnarep.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Otterlei M, Bruheim P, Ahn B, Bussen W, Karmakar P, Baynton K, Bohr VA. Werner syndrome protein participates in a complex with RAD51, RAD54, RAD54B and ATR in response to ICL-induced replication arrest. J Cell Sci. 2006;119:5137–5146. doi: 10.1242/jcs.03291. [DOI] [PubMed] [Google Scholar]
- Patro BS, Frohlich R, Bohr VA, Stevnsner T. WRN helicase regulates the ATR-CHK1-induced S-phase checkpoint pathway in response to topoisomerase-I-DNA covalent complexes. J Cell Sci. 2011;124:3967–3979. doi: 10.1242/jcs.081372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JJ, Asaithamby A, Barnebey A, Kiamanesch F, Chen DJ, Han S, Tainer JA, Yannone SM. Identification of a coiled coil in werner syndrome protein that facilitates multimerization and promotes exonuclease processivity. J Biol Chem. 2010;285:25699–25707. doi: 10.1074/jbc.M110.124941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JJ, Yannone SM, Holden LG, Hitomi C, Asaithamby A, Han S, Cooper PK, Chen DJ, Tainer JA. WRN exonuclease structure and molecular mechanism imply an editing role in DNA end processing. Nature structural & molecular biology. 2006;13:414–422. doi: 10.1038/nsmb1088. [DOI] [PubMed] [Google Scholar]
- Petermann E, Helleday T. Pathways of mammalian replication fork restart. Nat Rev Mol Cell Biol. 2010;11:683–687. doi: 10.1038/nrm2974. [DOI] [PubMed] [Google Scholar]
- Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell. 2010;37:492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichierri P, Franchitto A. Werner syndrome protein, the MRE11 complex and ATR: menage-a-trois in guarding genome stability during DNA replication? Bioessays. 2004;26:306–313. doi: 10.1002/bies.10411. [DOI] [PubMed] [Google Scholar]
- Pichierri P, Franchitto A, Mosesso P, Palitti F. Werner's syndrome protein is required for correct recovery after replication arrest and DNA damage induced in S-phase of cell cycle. Mol Biol Cell. 2001;12:2412–2421. doi: 10.1091/mbc.12.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M, Gollahon KA, Emond MJ, Silber JR, Rabinovitch PS. Werner syndrome diploid fibroblasts are sensitive to 4-nitroquinoline-N-oxide and 8-methoxypsoralen: implications for the disease phenotype. Faseb J. 2002;16:757–758. doi: 10.1096/fj.01-0906fje. [DOI] [PubMed] [Google Scholar]
- Poot M, Gollahon KA, Rabinovitch PS. Werner syndrome lymphoblastoid cells are sensitive to camptothecin-induced apoptosis in S-phase. Hum Genet. 1999;104:10–14. doi: 10.1007/s004390050903. [DOI] [PubMed] [Google Scholar]
- Poot M, Hoehn H, Runger TM, Martin GM. Impaired S-phase transit of Werner syndrome cells expressed in lymphoblastoid cell lines. Exp Cell Res. 1992;202:267–273. doi: 10.1016/0014-4827(92)90074-i. [DOI] [PubMed] [Google Scholar]
- Popuri V, Croteau DL, Bohr VA. Substrate specific stimulation of NEIL1 by WRN but not the other human RecQ helicases. DNA Repair (Amst) 2010;9:636–642. doi: 10.1016/j.dnarep.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raderschall E, Stout K, Freier S, Suckow V, Schweiger S, Haaf T. Elevated levels of Rad51 recombination protein in tumor cells. Cancer Res. 2002;62:219–225. [PubMed] [Google Scholar]
- Ray Chaudhuri A, Hashimoto Y, Herrador R, Neelsen KJ, Fachinetti D, Bermejo R, Cocito A, Costanzo V, Lopes M. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol. 2012;19:417–423. doi: 10.1038/nsmb.2258. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Iijima K, Mochizuki D, Nakamura K, Teshigawara K, Kobayashi J, Matsuura S, Tauchi H, Komatsu K. Homologous recombination repair is regulated by domains at the N- and C-terminus of NBS1 and is dissociated with ATM functions. Oncogene. 2007;26:6002–6009. doi: 10.1038/sj.onc.1210428. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Nishikawa K, Heo SJ, Goto M, Furuichi Y, Shimamoto A. Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells. 2001;6:421–430. doi: 10.1046/j.1365-2443.2001.00433.x. [DOI] [PubMed] [Google Scholar]
- Salk D, Au K, Hoehn H, Martin GM. Cytogenetics of Werner's syndrome cultured skin fibroblasts: variegated translocation mosaicism. Cytogenet Cell Genet. 1981;30:92–107. doi: 10.1159/000131596. [DOI] [PubMed] [Google Scholar]
- Schlacher K, Christ N, Siaud N, Egashira A, Wu H, Jasin M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A, Moiani D, Arvai AS, Perry J, Harding SM, Genois MM, Maity R, van Rossum-Fikkert S, Kertokalio A, Romoli F, et al. DNA Double-Strand Break Repair Pathway Choice Is Directed by Distinct MRE11 Nuclease Activities. Mol Cell. 2013 doi: 10.1016/j.molcel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova JM, Kehrli K, Mao F, Monnat R., Jr Distinct functions of human RECQ helicases WRN and BLM in replication fork recovery and progression after hydroxyurea-induced stalling. DNA Repair (Amst) 2013;12:128–139. doi: 10.1016/j.dnarep.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova JM, Li N, Folch A, Monnat RJ., Jr The RecQ helicase WRN is required for normal replication fork progression after DNA damage or replication fork arrest. Cell Cycle. 2008;7:796–807. doi: 10.4161/cc.7.6.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H, Rao VA, Sordet O, Furuta T, Miao ZH, Meng L, Zhang H, Pommier Y. Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks. J Biol Chem. 2006;281:30814–30823. doi: 10.1074/jbc.M603747200. [DOI] [PubMed] [Google Scholar]
- Trego KS, Chernikova SB, Davalos AR, Perry JJ, Finger LD, Ng C, Tsai MS, Yannone SM, Tainer JA, Campisi J, et al. The DNA repair endonuclease XPG interacts directly and functionally with the WRN helicase defective in Werner syndrome. Cell Cycle. 2011;10:1998–2007. doi: 10.4161/cc.10.12.15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mameren J, Modesti M, Kanaar R, Wyman C, Peterman EJ, Wuite GJ. Counting RAD51 proteins disassembling from nucleoprotein filaments under tension. Nature. 2009;457:745–748. doi: 10.1038/nature07581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kobbe C, Thoma NH, Czyzewski BK, Pavletich NP, Bohr VA. Werner syndrome protein contains three structure-specific DNA binding domains. J Biol Chem. 2003;278:52997–53006. doi: 10.1074/jbc.M308338200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, related to figure 1: A majority of replication forks collapse in WS cells in response to replication stress.
(A) Flow chart shows DNA fiber experimental setup, with incorporation of IdU and CldU shown in green and red, respectively.
(B) Representative immunofluorescence images of DNA fibers showing sequential labeling of IdU and CldU in mock-treated WS+WRN cells.
(C) Replication restart in CPT treated WS and WS+WRN cells was evaluated by the percentage of labeled tracts containing both IdU and CldU against the total number of labeled tracts. Each data point is the average of two independent experiments. Error bars represent STDEV.
(D) Firing of new origins in CPT treated WS and WS+WRN cells were evaluated by the percentage of labeled tracts containing only CldU. Each data point is the average of two independent experiments. Error bars represent STDEV.
(E) Collapsed replication forks in CPT treated WS and WS+WRN cells were evaluated by the percentage of labeled tracts containing only IdU. Each data point is the average of two independent experiments. Error bars represent STDEV.
(F) Collapsed replication forks are shorter in CPT treated WS cells relative to WS+WRN cells. Replicating DNA before and during CPT-induced replication stress in WS and WS+WRN was sequentially labeled by incorporation of the IdU and CldU, respectively. The length of DNA fibers that contained only IdU tracts was measured, and the frequency distributions of lengths of more than 100 DNA fibers from two independent experiments in each group were calculated.
(G) Representative DNA fiber images show intact (left) and shortened (right) DNA fibers in mock- and CPT-treated WS cells, respectively.
Figure S2, related to results section on the recruitment and association of WRN with RPA2, Rad51, NBS1, and MRE11 at replication-associated DSBs,
(A) WRN is recruited to the sites of replication in response to replication stress. WS cells stably expressing EGFP-tagged-WRN were pulse-labeled with 50 μm EdU for 90 min and then treated with 1 μm CPT for 1 h. After 5 h, cells were fixed with 4% paraformaldehyde and the Click-IT reaction was carried out to detect EdU signal. Representative three-dimensional deconvoluted confocal images are shown.
(B-F) A majority of WRN co-localizes with γH2AX, RPA2, RAD51, NBS1, and MRE11 at replication-associated DSBs. WS cells stably expressing EGFP-tagged WRN were treated with 1 μM CPT for 1 h. After 5 h, cells were fixed with 4% paraformaldehyde and subjected to indirect immunostaining with (B) anti-γH2AX, (C) anti-RPA2, (D) anti-RAD51, (E) anti-NBS1, or (F) anti-MRE11 antibodies. Representative three-dimensional deconvoluted confocal images are shown. All scale bars, 5 μm.
Figure S3, related to figure 3: WRN940-1432 and WRN250-366 domains interact with Ku70/80 heterodimer and WRN, respectively.
(A) Western blot shows expression of different domains of WRN in WS cells. Whole-cell extracts prepared from WS cells stably expressing Flag-EGFP-tagged WRN1-366, WRN250-366, WRN940-1432 and full-length WRN (1-1432aa) were separated onto a 10% SDS-PAGE and immunoblotted with anti-Flag monoclonal antibodies.
(B) Representative live cell images show localization of EGFP-tagged WRN1-366, WRN250-366, WRN940-1432 and full-length WRN in WS cells. Cells were seeded onto a glass-bottomed 35 mm dishes and the live cell images were captured using a fluorescent microscope. All scale bars, 5μm.
(C) Western blots shows in vivo interaction of WRN 250-366 domain with full length WRN. Total cell extract prepared from HeLa cells stably expressing flag EGFP-tagged-WRN-250-366 was subjected to immunoprecipitation using anti-Flag (M2) antibodies. Subsequently, the immune complex was separated onto a SDS-PAGE and immunoblotted with anti-WRN (endogenous WRN) and anti-Flag (WRN 250-366) antibodies.
(D) Western blots shows in vivo interaction of WRN 940-1432 domain with Ku70/80 heterodimer. Total cell extract prepared from WS cells stably expressing FLAG-EGFP-tagged-WRN 366-800 and WRN 940-1432 were subjected to immunoprecipitation using anti-Flag (M2) antibodies. Subsequently, the immune complex was separated onto a SDS-PAGE and immunoblotted with anti-Ku70, anti-Ku80 and anti-Flag antibodies.
Figure S4, related to figure 3: Expression of WRN-WRN interaction domain in HeLa cells results in the degradation of nascent DNA strands.
(A) Representative images show localization of endogenous WRN in HeLa cells stably expressing WRN-WRN interaction domain (WRN250-366). HeLa+WRN250-366 were treated with or without 1 μM CPT for 1 h and were fixed with 4% PFA. Subsequently, cells were immunostained with anti-γH2AX and anti-WRN antibodies and were imaged using a confocal microscopy. The result shows that the recruitment of WRN to the replication-associated DSBs is abrogated due to the expression of WRN250-366 domain. All scale bars, 5μm.
(B) Replication forks are shortened in HeLa cells expressing WRN250-366. HeLa+WRN250-366 cells were labeled with IdU for 30 min, treated with or without 1 μM CPT for 5 h. DNA fibers were immunostained with anti-BrdU antibodies. DNA fiber images were captured using a fluorescence microscope and DNA fiber lengths were measured using Axiovison Software. The frequency distribution of lengths of more than 100 DNA fibers from three independent experiments in each group was calculated.
Figure S5, related to figures 6 and 7: WRN plays a role in the stabilization of Rad51 at replication-associated DSBs.
(A) Recruitment of Rad51 to replication-associated DSBs is blocked by Rad51 inhibitor (B02). WS and WS+WRN cells were pre-treated with 50 μM B02 for 8 h and then treated with 1 μM CPT for 5 h. Cells were fixed with 4% paraformaldehyde and immunostained with anti-γH2AX and anti-Rad51 antibodies. Representative confocal microscope images are shown. All scale bars, 10 μm.
(B) Expression of Rad51 is not affected in WRN-defective cells. Nuclear extracts prepared from WS and WS+WRN cells were separated onto a 10% SDS-PAGE and immunoblotted with anti-WRN, anti-Rad51, and anti-Ku80 antibodies.
(C) Rad51 foci numbers are reduced in CPT treated WS cells but not in WS+WRN cells. WS and WS+WRN cells were exposed to 1 μM CPT for 5 hrs and were subjected to indirect immunostaining with anti-Rad51 and anti-γH2AX antibodies. All scale bars, 10 μm.
(D) Graph shows the number of Rad51 foci in CPT treated WS and WS+WRN cells. WS and WS+WRN cells were exposed to 1 μM CPT and were subjected to indirect immunostaining with anti-Rad51 antibodies at indicated times. Rad51 foci were counted in 50-100 cells from two independent experiments. Error bars represent STDEV.
(E) Biochemical fractionation of chromatin bound Rad51. WS and WS+WRN cells were treated with 1 μM CPT for 5 h and cells were subjected to chromatin fractionation. Chromatin fraction was separated onto a 4-15% gradient SDS-PAGE, transferred onto a nitrocellulose membrane and immunoblotted with anti-WRN, anti-Rad51, anti-Ku80 and anti-H3 antibodies. The signal intensity was quantified using Image J.
(F) Wild-type and K133R Rad51 are recruited to the sites of replication-associated DSBs in WS cells. WS cells transfected with FLAG-EYFP-WT and K133R Rad51 were treated with 1 μM CPT for 5 h and then were immunostained with anti-γH2AX and anti-Rad51 antibodies. All scale bars, 10 μm.
Figure S6, related to results section on the role for WRN in chromosome stability maintenance in response to CPT induced replication stress.
(A) Graph shows the number of gross-chromosomal aberrations in WS and WS+WRN cells treated with and without CPT.
(B) Graph shows the percentage of WS and WS+WRN cells with radial and non-radial type chromosomal aberrations in WS and WS+WRN cells treated with or without CPT.
(C) Graph shows different types of gross-chromosomal aberrations in WS and WS+WRN cells treated with and without CPT. Exponentially growing WS and WS+WRN cells stably expressing WRN were treated with 1 μM CPT for 5 h. After 20 h, chromosome preparations were made by accumulating mitotic cells in the presence of 0.1 mg/ml colcemid for 4 h. For each cell type, more than 50 metaphase spreads were counted. Each data point is the average of three independent experiments. Error bars represent STDEV. *-p=0.005; **-p=0.003; ***-p=0.0003; Qua-Radial-quadri-radial.
Table S1,related to figures 1, 2, 3, 4, 5, 6, S1 and S4: DNA fiber data analysis information
Table S2, related to figures 1, 2, 3, 4, 5, 6, S1 and S4: DNA fiber lengths distribution data analysis information