Abstract
Inflammation in the brain is a prominent feature in Alzheimer’s disease (AD). Recent studies suggest that chronic inflammation can be a consequence of failure to resolve the inflammation. Resolution of inflammation is mediated by a family of lipid mediators (LMs), and the levels of these specialized pro-resolving mediators (SPMs) are reduced in the hippocampus of those with AD. In the present study we combined analysis of LMs in the entorhinal cortex (ENT) from AD patients with in vitro analysis of their direct effects on neurons and microglia. We probed ENT, an area affected early in AD pathogenesis, by liquid chromatography-tandem mass spectrometry (LC-MS-MS), and found that the levels of the SPMs maresin 1 (MaR1), protectin D1 (PD1) and resolvin (Rv) D5, were lower in ENT of AD patients as compared to age-matched controls, while levels of the pro-inflammatory prostaglandin D2 (PGD2) were higher in AD. In vitro studies showed that lipoxin A4 (LXA4), MaR1, RvD1 and protectin DX (PDX) exerted neuroprotective activity, and that MaR1 and RvD1 down-regulated Aβ42-induced inflammation in human microglia. MaR1 exerted a stimulatory effect on microglial uptake of Aβ42. Our findings give further evidence for a disturbance of the resolution pathway in AD, and indicate that stimulating this pathway is a promising treatment strategy for AD.
Keywords: Alzheimer, human, inflammation, lipoxin, maresin, microglia, neuroprotection, omega-3, resolvin, SPMs
Introduction
Lipids represent the primary structural component of the cell membrane and represent up to 50% of the brain dry weight. Lipid dysregulation has been found in Alzheimer’s disease (AD) [1–3], the most common type of dementia. In the AD brain, lower levels of the omega (ω)-3 fatty acid (FA) docosahexaenoic acid (DHA) were reported [4]. In elderly persons, a correlation between higher plasma levels of ω-3 FAs and less cognitive decline has been shown [5]. Prostaglandins (PGs) and leukotrienes (LTs) are classical pro-inflammatory lipid mediators (LMs) that mediate fever and pain. PGs and LTs are biosynthesized from arachidonic acid (AA) via cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) pathways, the activity of which have been shown to be elevated in AD [6,7]. Suppression of these pathways by inhibition or gene depletion of COX or 5-LOX successfully reduced AD-like pathologies in AD animal models [8].
The elevated levels of pro-inflammatory LMs are in line with abundant evidence of the inflammatory process in the AD brain [9–11], characterized by activated microglia, astrocyte proliferation and an increased production of other inflammatory factors such as cytokines. The major histopathological hallmarks of AD include amyloid plaques mainly composed of aggregated β-amyloid (Aβ) peptide [12], neurofibrillary tangles (NFTs) consisting of hyper-phosphorylated tau protein [13], and loss of neurons [12]. Aβ has been shown to induce neurotoxicity [14] and to activate microglia, which secrete pro-inflammatory mediators that can also cause neurotoxicity [15]. Notably, the production of Aβ is increased by inflammation [16], indicating the existence of a vicious circle of reciprocal stimulation in AD [15]. Moreover, an early epidemiological study suggested a lower prevalence of dementia in patients on long-term treatment with non-steroid anti-inflammatory drugs (NSAIDs) [17]. Recent studies have highlighted a role of LMs, not only in stimulating inflammation, but also in stimulating its resolution, i.e. termination of inflammation and restoration of the inflamed tissue. In resolution, a novel group of LMs termed specialized pro-resolving lipid mediators (SPMs) plays an important role. SPMs are biosynthesized from ω-3 and ω-6 FAs by the activities of LOXs and COXs [18]. Hence, there is a duality of these enzymes with regard to producing pro-inflammatory and pro-resolving LMs. Several series of SPMs have been identified: lipoxins (LXs) derived from AA [19], E-series resolvins (RvEs) derived from eicosapentaenoic acid (EPA) [20], and D-series resolvins (RvDs), protectin/neuroprotectin (PD/NPD) and maresins (MaRs) derived from DHA [21,22]. SPMs have potent pro-resolving actions, leading to cessation of immune cell infiltration, down-regulation of pro-inflammatory and up-regulation of anti-inflammatory mediators, and promotion of phagocytosis and of tissue regeneration [23–26]. Altogether, these activities promote the return to homeostasis. Resolution of inflammation is thus an actively controlled process, mediated by SPMs, rather than a passive dissipation of the inflammatory response due to cessation of activating stimuli [18].
The inflammatory response is a defense mechanism of the body to eliminate harmful stimuli, hence protective, and should be naturally ended or resolved upon succesful elimination. However, if left uncontrolled, inflammation may persist and result in chronic inflammation. Interventions based on inhibition of inflammation in AD patients have in most cases not been successful [27]. In addition, long-term use of NSAIDs may induce gastrointestinal and cardiovascular side effects [28]. Therefore, there is call for a novel strategy, not based on classical anti-inflammatory treatment, and therapeutic strategies based on resolving the inflammatory state in AD are therefore of interest. The inflammatory response would thereby be induced to progress to its natural end, promoting the beneficial and restorative aspects of inflammation, which may be lost in anti-inflammatory treatments. In a recent study, we characterized the resolution pathway in the hippocampus region of AD patients, and found altered levels of SPMs, their receptors, and of an enzyme involved in their synthesis [29]. In the present study, the aim was to investigate a wider spectrum of LMs, with a special focus on SPMs, using the liquid chromatography - tandem mass spectrometry (LC-MS-MS) based approach in post mortem tissue samples of entorhinal cortex (ENT), a brain region of importance for memory and affected early in AD pathogenesis [30]. To further understand the role of SPMs in the brain, and to evaluate the therapeutic potential, we also analyzed their actions in vitro on neuronal survival and microglial function.
Methods
Post mortem human brain samples
Post mortem tissue samples from the ENT were obtained from 7 subjects with neuropathologically confirmed AD (Braak stage 5–6/definite AD), and 7 non-demented control subjects, all from the Brain Bank at Karolinska Institutet. All AD patients were clinically diagnosed and confirmed by pathological examination. None of the control subjects had significant brain pathological changes consistent with AD neuropathology. The age of the subjects at the time of death, and the post mortem interval, did not differ significantly between the AD and control groups (see Table 1 for patient information). As part of the clinical routines the apolipoprotein E genotype was analyzed, and information on carriers of one or two E4 alleles is included in Table 1. The ENT tissue was dissected from the frontal pole of the temporal lobe on dry ice and kept at −80°C until further processing.
Table 1.
Patient information
| AD (n = 7) |
Control (n = 7) |
p value |
|
|---|---|---|---|
| Age (years) | 78.1 ± 5.3 | 82.9 ± 6.1 | NS |
| Gender (female/male) | 7/0 | 4/3 | - |
| PMI (hours ± SD)* | 18.6 ± 8.6 | 21.3 ± 7.5 | NS |
|
ApoE4 (hetero- + homozygotic) |
6 | 3 | - |
Data are expressed as mean ± SD.
PMI from one AD patient was missing. AD = Alzheimer’s disease, ApoE4 = apolipoprotein E 4 allele, NS = not significant, PMI = post mortem interval
Liquid mediator metabololipidomics
For LM extraction, human ENT tissues were thawed on ice, weighed, and then gently homogenized by a glass dounce. Internal deuterium-labeled standards d4-LTB4, d5-LXA4, d5-RvD2, and d4-PGE2 (500 pg each) in 2 ml of ice-cold methanol were added to each sample, in order to facilitate quantification and sample recovery. Next, the samples were kept at −20°C for 45 min for protein precipitation, and were then centrifuged at 1200 × g (4°C for 10 min). Supernatants were collected and brought to less than 1 ml of methanol content with nitrogen gas. An automated extraction system (Rapid Trace, Biotage, NC, USA) was used to extract the samples as in [31,32]. Briefly, acidified samples (pH 3.5 by HCl) were rapidly loaded onto solid-phase C18 cartridges, pre-conditioned with 3 ml methanol and 6 ml H2O, and then washed with 4 ml H2O and 5 ml hexane. The products were eluted with 9 ml methyl formate, and then brought to dryness with nitrogen gas. The dried products were immediately suspended in methanol-water (50:50 vol/vol) for LC- MS-MS automated injections.
The LC-MS-MS system included a LC-20AD HPLC and a SIL-20AC auto-injector (Shimadzu, Kyoto, Japan), paired with a QTrap 6500 (ABSciex, CA, USA). LMs were eluted through an Eclipse Plus C18 column (100 mm × 4.6 mm × 1.8 µm; Agilent, CA, USA). A mobile phase of methanol-water-acetic acid of 55:45:0.01 (vol/vol/vol) was ramped to 85:15:0.01 over 10 min, and then to 98:2:0.01 for the next 8 min. This was subsequently maintained at 98:2:0.01 for 2 min. The flow rate was 0.4 ml/min. The QTrap 6500 was operated in negative ionization mode. Scheduled multiple reaction monitoring (MRM) with a 90 s window was coupled with information-dependent acquisition (IDA) and an enhanced product ion scan (EPI) [31,32]. Each LM parameter was optimized individually.
Each LM was monitored by recently published criteria [31,32], including matching retention time to synthetic and authentic materials, and at least six diagnostic ions for each LM. Linear calibration curve with r2 value of 0.98 – 0.99 was obtained for each LM using authentic compound mixtures and deuterium-labeled LM at 0.78, 1.56, 3.12, 6.25, 12.5, 25, 50, 100, and 200 pg.
Cell cultures
Human SH-SY5Y neuroblastoma cells
SH-SY5Y neuroblastoma cells (ATCC, Sweden) were cultured in DMEM/F12 medium supplemented with Glutamax™ (Life Technologies, Stockholm, Sweden), supplemented with 10% heat-inactivated fetal calf serum (FCS) (Life Technologies, Stockholm, Sweden), at 37°C in humidified air containing 5% CO2. The cells were split at 80% confluence. Differentiated SH-SY5Y cells have been reported previously as a model of human neuronlike cells, which have morphological and biochemical similarity to primary neurons [33]. SH-SY5Y cells were differentiated with 10 µM retinoic acid (RA) (Sigma, Stockholm, Sweden) in complete medium for 5 days. Subsequently, the cells were harvested with enzyme-free cell dissociation buffer (Life Technologies, Stockholm, Sweden), and re-seeded at a density of 20,000 cells/well in 48-well plates coated with Matrigel reduced of growth factors (Becton Dickinson, USA). After allowing the cells to attach for one day, the culture medium was replaced with serum-free medium containing brain-derived neurotrophic factor (BDNF) (Life Technologies, Stockholm, Sweden) at a final concentration of 25 ng/ml. Experiments were performed after 5 days of differentiation in this medium. All treatments were prepared in fresh serum-free DMEM/F12 medium supplemented with Glutamax™
Human CHME3 microglial cells
Human microglial cells (CHME3) were kindly provided by Prof. M Tardieu, Neurologie pédiatrique, Hôpital Bicêtre, Hôpitaux de Paris, Paris, France. CHME3 cells were cultured in DMEM/high glucose medium (Life Technologies, Stockholm, Sweden) supplemented with GlutaMax™ (Life Technologies, Stockholm, Sweden) and 10% FCS. The culture medium was changed twice per week, and the cells were split at 80% confluence, and seeded into 6-well plates for experiment and propagation.
Immunocytochemistry
Differentiated SH-SY5Y cells and CHME3 microglia cells cultured on coverslips were fixed and processed for immunocytochemistry. The cells were fixed with 4% paraformaldehyde for 20 min at room temperature (RT), washed with phosphate-buffered saline (PBS) for 3 × 10 min, blocked with 5% normal goat serum for 45 min at RT, and incubated over night at 4 °C with primary antibodies. Neuroblastoma cells were stained with antibodies to G protein-coupled receptor 32 (GPR32), and microglia were stained with antibodies to GPR32 and LXA4 receptor/formyl peptide receptor 2 (ALX/FPR2). Both antibodies were diluted 1:100 in PBS containing 5% normal goat serum and 0.1% Triton-X100. After washing with PBS for 3 × 10 min, the coverslips were incubated for 2 h at RT with FITC-conjugated goat anti-rabbit IgG diluted 1:200 in PBS containing 5% normal goat serum. The coverslips were then washed with PBS for 3 × 10 min, mounted with fluorescence mounting medium (DAKO Sweden AB, Stockholm, Sweden), and analyzed in a Nikon E800 microscope.
Western blot
Analysis of ALX/FPR2 and GPR32 by western blotting was performed on differentiated SH-SY5Y cells and CHME3 microglia cells lysed with radio-immunoprecipitation assay (RIPA) buffer, supplemented with 1% protease inhibitor cocktail (Sigma-Aldrich, Stockholm, Sweden), and 1% phosphatase inhibitor cocktail (Thermo scientific, Stockholm, Sweden), and centrifuged at 11,000 rpm at 4°C for 20 min. The supernatant was collected and total protein concentration determined by a BCA assay kit (Thermo scientific, Stockholm, Sweden). Briefly, samples containing 40 µg protein each were mixed with equal volume of 2X Laemmli sample buffer (Thermo scientific, Stockholm, Sweden), and were boiled at 95°C for 5 min. The denatured samples were then loaded on a 10% SDS-PAGE gel, after which the proteins were transferred to a nitrocellulose membrane under 85 mA current over night at 4°C. The membranes were blocked with 5% non-fatty dry milk at room temperature for 45 min, and incubated with primary antibodies diluted (1:500 for anti-GPR32 and 1:1000 for anti-ALX/FPR2 antibodies) in Tris-buffered saline with 0.1% Tween20 (TBS-T) at 4°C over night. The binding of primary antibodies was probed with horseradish peroxidase (HRP)-conjugated secondary antibodies (GE healthcare, Stockholm, Sweden) diluted (1:1000) in TBS-T, followed by incubation with enzymatic chemiluminescence (ECL™ Prime, GE healthcare, Stockholm, Sweden) reagent for 5 min. A CCD camera (LAS-3000 luminescence image analyser, Fuji Film, Tokyo, Japan) was used to detect the signals.
Cell stimulation
SH-SY5Y cell stimulation
To analyze the effect of SPMs on neuronal survival, apoptosis was induced by incubating the differentiated SH-SY5Y cells with 100 nM staurosporine (STS) (Sigma, Stockholm, Sweden). The SPMs MaR1, LXA4, RvD1 and PDX (all from Cayman Chemical, MI, USA), at 0 – 0.5 µM, were added to the cultures immediately prior to addition of STS. The treatment with SPMs was repeated by addition to the cultures at 6 and 24 h, and viability and cell death were analyzed at 48 h. The effects of SPMs alone in the absence of STS were also analyzed.
CHME3 cell stimulation
To assess the effect of SPMs on phagocytosis of Aβ42, CHME3 cells were seeded into 6-well plates (100,000 cells/well), and incubated with 1 µg/ml HiLight488-conjugated Aβ42 (Anaspec, Fremont, USA) together with a range of concentrations (0 – 100 nM) of SPMs for 1 and 6 h. Phagocytosis of Aβ42 was analyzed by flow-cytometry.
To analyze the effects of SPMs on Aβ42-induced microglia activation, CHME3 cells were seeded into 6 well plates and stimulated with 10 µg/ml Aβ42 combined with 0 – 0.5 µM SPMs for 1 and 6 h, the cells were harvested at the end of the experiment for flow-cytometry analysis. To analyze the effects of MaR1 on microglial phenotype at basal unstimulated conditions, the CHME3 cells were seeded into 6-well plates and stimulated with 0 – 1 µM MaR1. After 6 h incubation, the cells were harvested for flow-cytometry analysis.
Assessment of cell death and viability
Cell death was assessed by lactate dehydrogenase (LDH) assay. A sample of 100 µl cell culture supernatant was transferred to a 96-well plate and incubated with LDH reagent (Roche, Stockholm, Sweden) for 30 min, after which the absorbance was measured at 340 nm. Cells lysed with 10% triton X-100 to induce 100% cell death served as positive control (PC). The absorbance of the medium from the other wells in the experiment was normalized to the PC. Cell viability was assessed by incubating the remaining cells for 2 h at 37°C with resazurin salt (Sigma, Stockholm, Sweden) at a final concentration of 0.01% in culture medium, after which the fluorescence intensity was analyzed at 590 nm. The fluorescence signal of the medium from the vehicle treatment served as control, and the signal measured in the other wells in the experiment was normalized to this control signal.
Flow-cytometry
For analysis of membrane-associated cellular markers, the cells were harvested as described previously [34] and fixed with 1% paraformaldehyde in PBS at room temperature for 45 min. The cells were washed once by adding 5 ml PBS, centrifuged at 400 × g for 10 min, and then resuspended with 1% bovine serum albumin (BSA, Sigma-Aldrich, Stockholm, Sweden) in PBS. Cell suspensions were incubated for 45 min on ice with fluorophore-conjugated antibodies against CD40, CD86, CD11b, CD33, CD163, CD206, and CD80, major histocompatibility complex class II (MHC-II)), at their respective working concentration (see Table 2). The cells were then washed once by adding 1 ml PBS and centrifuged at 500 × g for 5 min, resuspended in 200 µl 1% BSA, and analyzed in a FACS Calibur cytometer (Becton Dickinson, USA). The proportion of microglia positive for each cell surface marker was assessed by determining the percentage of cells showing a signal in the proper channel exceeding the signal of the isotype control. All flow-cytometric data were analyzed in Cell Quest Pro software.
Table 2.
Antibody information
| Antibodies | Company | Working dilution |
|---|---|---|
| ALX/FPR2 | Acris, Stockholm, Sweden | 1:100 or 1:1000 |
| CD11b | Biosite, Stockholm, Sweden | 1:50 |
| CD11b (activated) | Biosite, Stockholm, Sweden | 1:50 |
| CD33 | Biosite, Stockholm, Sweden | 1:25 |
| CD40 | Biosite, Stockholm, Sweden | 1:200 |
| CD80 | Becton Dickinson, Stockholm, Sweden | 1:10 |
| CD86 | Biosite, Stockholm, Sweden | 1:40 |
| CD163 | Biosite, Stockholm, Sweden | 1:25 |
| CD206 | Biosite, Stockholm, Sweden | 1:25 |
| GPR32 | Lifespan Bioscience, Stockholm, Sweden | 1:100 or 1:500 |
| MHCII | Becton Dickinson, Stockholm, Sweden | 1:25 |
Statistical analysis
Statistical analysis was performed in Statistica 12 (Dell Software, Aliso Viejo, USA), and SPSS software (version 21.0, IBM Corporation, NY, USA). Pairwise comparison between treatments/groups was analyzed by Mann-Whitney U test. All data from the in vitro experiments were normalized to the average of each individual experiment. Variance was analyzed by the Kruskal-Wallis test, and the post-hoc test built into the Kruskal-Wallis analysis in Statistica 12 was used for group comparisons. P < 0.05 was considered statistically significant.
Study approval
The use of human postmortem brain samples was approved by the Ethical committee at Karolinska Institutet (ethical permit number 2011/680-31/1).
Results
Identification and quantification of SPMs in the human entorhinal cortex (ENT)
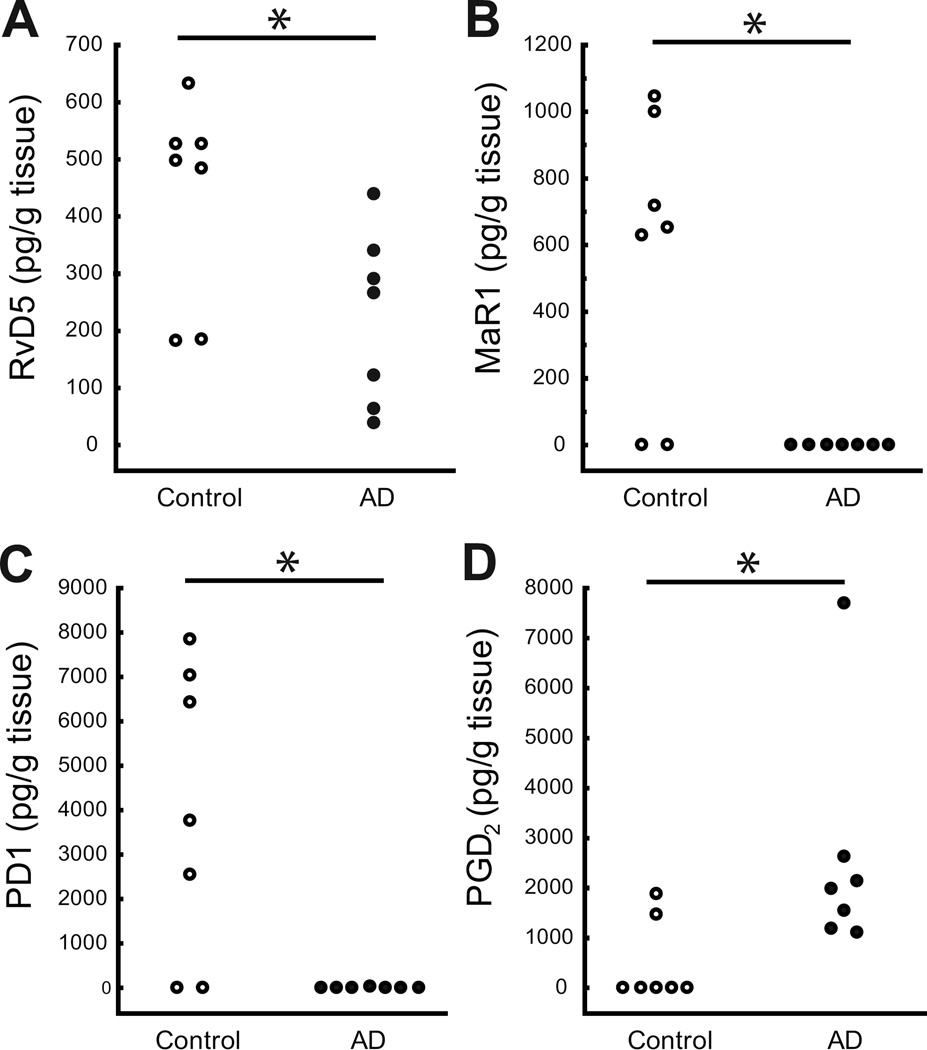
LC-MS-MS based profiling was used to analyze LMs in the human ENT from both AD patients and controls. Representative multiple reaction monitoring (MRM) profiles in control and AD ENT tissue are shown in Fig. 1a, along with MS-MS spectra employed for identification (Fig. 1b). The LMs were quantified by the targeted MRM method using specific ion pairs including the parent ion (Q1) and the diagnostic daughter ion (Q3). Previously, we have shown the occurrence of LXA4, RvD1 and MaR1 in the human hippocampus [29], and we present here evidence for the presence in the human brain of the SPMs RvE1, RvE2, RvD2 and RvD5, as well as other LMs (see Fig. 1 and Table 3). Comparisons between ENT from AD patients and controls did not reveal any difference with regard to LXs derived from AA (Table 3). However, levels of RvD5, MaR1 and PD1, all derived from DHA, were lower in the AD group than in the control group (Fig. 2a–c), whereas no apparent differences were observed with regard to RvD1 and RvD2 (Table 3).
Fig. 1.
a–b. Lipid mediator (LM) identification in post mortem entorhinal cortex (ENT) tissue from controls and Alzheimer’s disease (AD) patients. Tissues were extracted and subjected to LM-metabololipidomics. (a) Representative multiple reaction monitoring (MRM) chromatograms for the identified LMs in the ENT. (b) Related MS-MS spectra employed for identification of LMs, based on analysis of n = 14 ENT tissues. RvD5 = 7S,17S–dihydroxy-docosa-5Z,8E,10Z,13Z,15E,19Z–hexaenoic acid. PD1 = 10R,17S–dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z–hexaenoic acid. MaR1 = 7R,14S–dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z–hexaenoic acid. LXA4 = (5S,6R,7E,9E,11Z,13E,15S)-5,6,15-trihydroxyicosa-7,9,11,13-tetraenoic acid. LXB4 = (5S,6E,8Z,10E,12E,14R,15S)-5,14,15-trihydroxyicosa-6,8,10,12-tetraenoic acid. RvE1 = 5S,12R,18R–trihydroxy-6Z,8E,10E,14Z,16E–eicosapentaenoic acid.
Table 3.
Quantitative data from LC-MS-MS analysis
| pg/g tissue | Q1 | Q3 | AD | Control | p value |
|---|---|---|---|---|---|
| AA Bioactive Metabolome | |||||
| LXA4 | 351 | 115 | 934.1 ± 990.8 | 359.6 ± 188.5 | NS |
| LXB4 | 351 | 221 | 1369.0 ± 1024.7 | 3074.6 ± 2364.8 | NS |
| 5,15-diHETE | 335 | 115 | 3730.1± 4826.5 | 5437.6 ± 2171.1 | NS |
| LTB4 | 335 | 195 | 520.2 ± 453.0 | 782.0 ± 585.3 | NS |
| 20-OH-LTB4 | 351 | 195 | 155.1 ± 287.7 | 117.0 ± 220.4 | NS |
| PGD2 | 351 | 223 | 2609.7 ± 2304.8 | 476.5 ± 822.7 | 0.011 |
| PGE2 | 351 | 189 | 3718.7 ± 3308.3 | 1666.2 ± 2182.2 | NS |
| PGF2α | 353 | 193 | 1351.4 ± 1355.7 | 13738.3 ± 29679.1 | NS |
| TxB2 | 369 | 169 | 3581.5 ± 2597.2 | 2011.1 ± 2967.6 | NS |
| EPA Bioactive Metabolome | |||||
| RvE1 | 349 | 161 | 1174.3 ± 1469.9 | 6.1 ± 11.3 | NS |
| RvE2 | 333 | 115 | 64.0 ± 99.2 | 15.3 ± 26.2 | NS |
| DHA Bioactive Metabolome | |||||
| RvD1 | 375 | 215 | 80.7 ± 137.0 | 10.4 ± 27.5 | NS |
| RvD2 | 375 | 175 | 57.1 ± 95.1 | 11.8 ±20.3 | NS |
| RvD5 | 359 | 199 | 223 ± 150.6 | 433.6 ± 177.1 | 0.038 |
| MaR1 | 359 | 221 | * | 578.1 ± 426.9 | 0.026 |
| PD1 | 359 | 153 | 4.7 ± 12.5 | 3946.2 ± 3264.3 | 0.038 |
5,15-diHETE = 5, 15- dihydroxy- 6E, 8Z, 10Z, 13E- eicosatetraenoic acid; 20-OH-LTB4 = 20-Hydroxy-LTB4; LTB4 = leukotriene B4; LXA4 = lipoxin A4; LXB4 = lipoxin B4; PD1 = protectin D1; PGD2 = prostaglandin D2; PGE2 = prostaglandin E2; PGF2α = prostaglandin E2α; RvD1 = resolvin D1; RvD2 = resolvin D2; RvD5 = resolvin D5; RvE1 = resolvin E1; RvE2 = resolvin E2; MaR1 = maresin 1; TxB2 = thromboxane B2. Data are expressed as mean ± SD.
not detectable. NS = not significant.
Fig. 2.
a–b. Lipid mediator (LM) levels in the human entorhinal cortex (ENT). LMs were quantified by LC-MS-MS. The levels of resolvin D5 (RvD5) (a), maresin 1 (MaR1) (b) and protectin D1 (PD1) (c) were significantly lower in post mortem ENT tissue from Alzheimer’s disease (AD) patients (n = 7) than in tissue from controls (n = 7), while prostaglandin D2 (PGD2) (d) levels were higher in AD. * indicates p < 0.05. Results are expressed in pg/g tissue.
In the EPA bioactive metabolome, the ENT levels of RvE1 and RvE2 did not differ between AD and controls (Table 3). The levels of pro-inflammatory LMs, including LTB4, 20-OH-LTB4, dihydroxyeicosatetraenoic acid (diHETE), PGE2, PGF2α and thromboxane (Tx) B2, did not differ between the AD and control group, except for significantly higher levels of PGD2, in AD patients (Table 3 and Fig. 2d).
Among the subjects included in this study, 6 of the AD patients had 1 or 2 ApoE4 alleles and 3 of the controls (see Table 1). There was no correlation between ApoE4 genotype and the levels of SPMs (data not shown).
Actions of SPMs on STS-induced neuronal apoptosis
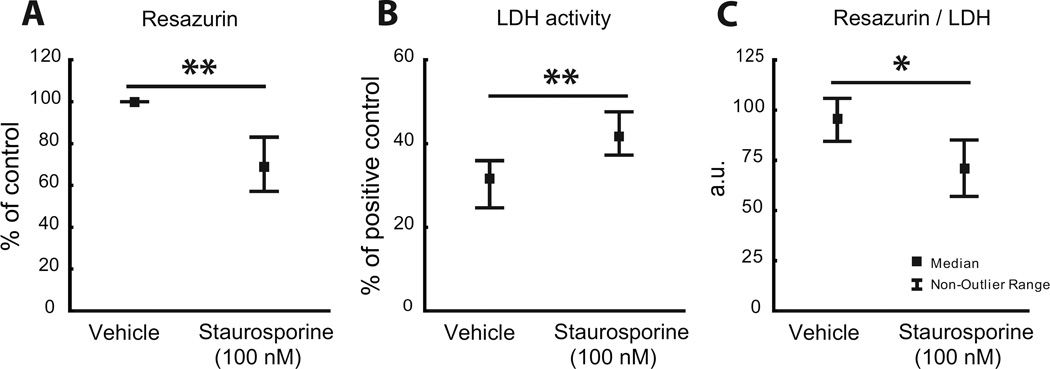
In view of the reduced levels of SPMs in the AD brain we further analyzed their direct effects on neurons with regard to potential protective or rescuing actions. A model of STS-induced neuronal apoptosis in differentiated neuroblastoma cells was used. Incubation of the cells with 100 nM STS resulted in significantly reduced cell viability and increased cell death as determined by resazurin (Fig. 3a) and LDH (Fig. 3b) assay, respectively. The cell survival, as defined by the ratio between data from the resazurin and LDH assays, was significantly decreased (Fig. 3c). These parameters were also used to assess the effects of LXA4, RvD1, MaR1 and PDX, in the STS-model. Treatment with LXA4 at 0.5µM, RvD1 at 0.005 µM and PDX at 0.05 µM, significantly reduced the decrease in cell viability caused by STS (Fig. 4a), whereas no effect was seen with MaR1. Neuronal cell death induced by STS was significantly reduced by RvD1 at 0.005 and 0.5 µM, by 0.05 and 0.5 µM MaR1, and by 0.005 and 0.05 µM PDX (Fig. 5b). No effect on cell death was observed after incubation with LXA4. The STS-induced decrease in cell survival (as defined by the ratio between data from resazurin and LDH assays) was significantly counteracted by 0.5 µM LXA4, by 0.005 and 0.5 µM RvD1, and by 0.05 µM MaR1, along with a significant protection also by PDX at 0.05 and 0.5 µM (Fig. 4c).
Fig. 3.
a–c. Staurosphorine (STS)-induced neurotoxicity in differentiated human SH-SY5Y neuroblastoma cells. A significant decrease in viability by 100 nM STS is seen as assessed by resazurin assay (a). Analysis with LDH assay shows increased cell death by STS (b). As an index of cell survival the ratio of data from the resazurin and LDH assays was calculated, showing a significant reduction by STS (c). * indicates p < 0.01 compared to vehicle, ** indicates p < 0.001 compared to vehicle. LDH = lactate dehydrogenase.
Fig. 4.
a–c. SPMs reduce STS-induced neurotoxicity in differentiated human SH-SY5Y neuroblastoma cells. The cells were incubated with 0 – 0.5 µM lipoxin A4 (LXA4), resolvin D1 (RvD1) and protectin DX (PDX), an isomer of protectin D1 (PD1), or maresin 1 (MaR1). STS at 100 nM was added immediately afterwards. Incubation with SPMs was repeated at 6 and 24 h, and cell viability and cytotoxicity were assessed at 48 h by resazurin (a) and LDH (b) assay, respectively. As an index of cell survival, the ratio between data from the resazurin and LDH assays was calculated, showing a significant effect by all SPMs tested (c). The data were normalized to the average of each individual experiment. The data are presented as median ± non-outlier range (n = 7). * indicates p < 0.05, ** indicates p < 0.01 and *** indicates p < 0.005, all compared to STS treatment alone. K-W = Kruskal-Wallis analysis of variance, LDH = lactate dehydrogenase, SPMs = specialized pro-resolving mediators, STS = staurosporine
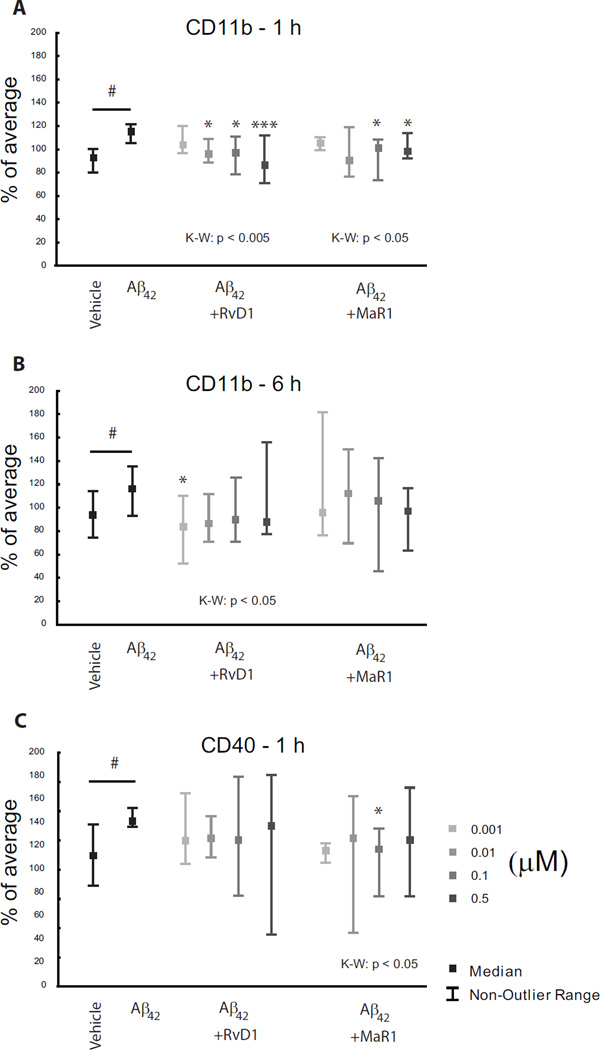
Fig. 5.
a–c. Actions of SPMs on Aβ42-induced microglial activation. Human CHME-3 microglia were incubated with 10 µg/ml Aβ42 in combination with 0 – 0.5 µM of the SPMs LXA4, RvD1, MaR1 and PDX. Flow-cytometry analysis showed an up-regulation of CD11b by Aβ42 at both 1 and 6 h (a, b). Both RvD1 and MaR1 counteracted this at 1 h (a), and RvD1 at 6 h (b). CD40 expression was increased at 6 h (c), and this was down-regulated by MaR1 at 0.1 µM. # indicates p < 0.01 compared with vehicle, * indicates p < 0.05 and *** indicates p < 0.005 compared to Aβ42 treatment. The data were normalized to the average of each individual experiment and are presented as median ± non-outlier range (n = 8). K-W = Kruskal-Wallis analysis of variance, LXA4 = lipoxin A4, MaR1 = maresin 1, PDX = protectin DX, RvD1 = resolvin D1, SPMs = specialized pro-resolving mediators.
The differentiated neuroblastoma cells were also incubated the LMs at basal conditions, and an increase in cell survival (as defined by the ratio between data from resazurin and LDH assays) was observed with 0.5 µM LXA4 and 0.005 µM RvD1 (see Supplementary Fig. S1a–c).
Actions of SPMs on microglial activation induced by Aβ42
Microglia represent the brain equivalent of monocytes/macrophages in the periphery, and SPMs have potent actions on these peripheral cells. In view of this, and the fact that microglial activation is observed in the AD brain, we used a model of human microglia incubated with Aβ42, known to activate microglia, to investigate potential actions of SPMs. Incubation of the human CHME-3 microglial cells with 10 µg/ml Aβ42 resulted in up-regulation of the levels of CD11b after 1 and 6 h incubation (Fig. 5a, b), as well as of the CD40 expression at 6 h (Fig. 5c). Aβ42 did not induce any significant change in CD163, CD206, CD200R and CD33 (data not shown). Incubation of the microglia with various concentrations of RvD1 and MaR1 for 1 h (Fig. 5a), and with RvD1 for 6 h (Fig. 5b), resulted in a decrease in the Aβ42-induced levels of CD11b. MaR1 significantly reduced the increase in CD40 caused by Aβ42 at 6 h (Fig. 5c).
Actions of SPMs on microglial phagocytosis of Aβ42
SPMs are known to stimulate non-phlogistic phagocytosis, and stimulating microglial phagocytosis of Aβ could conceivably be of benefit in AD. In order to investigate the effects of SPMs on phagocytosis of Aβ42, the human microglial cells (CHME3) were co-incubated with 0 – 100 nM SPMs and 1µg/ml Hilight-488-conjugated Aβ42 for 1 and 6 h. Quantification by flow-cytometry showed that MaR1 increased the phagocytosis of fluorophore-conjugated Aβ42 at 100 nM after 1 and 6 h incubation, and after 6 h there was an increase also by 1 nM MaR1 (Fig. 6). Kruskal-Wallis analysis showed a treatment effect at both 1 and 6 h (Fig. 6). There was no significant effect on Aβ42 phagocytosis with LXA4, RvD1 or PDX (data not shown).
Fig. 6.
MaR1 stimulates Aβ42 phagocytosis by human microglia. Human microglial cells (CHME3) were co-incubated with 0 – 100 nM MaR1 and 1 µg/ml Hilight-488-conjugated Aβ42 for 1 and 6 h. Phagocytosis was analyzed by flow-cytometry. Kruskal-Wallis analysis showed a dose-dependent increase in Aβ42 phagocytosis by MaR1. Pairwise comparison was analyzed by Mann-Whitney test. * indicates p < 0.05 and ** indicates p < 0.01 compared to vehicle. The data were normalized to the average of each individual experiment and presented as median ± non-outlier range (n = 5). MaR1 = maresin 1.
Actions of MaR1 on microglial phenotype
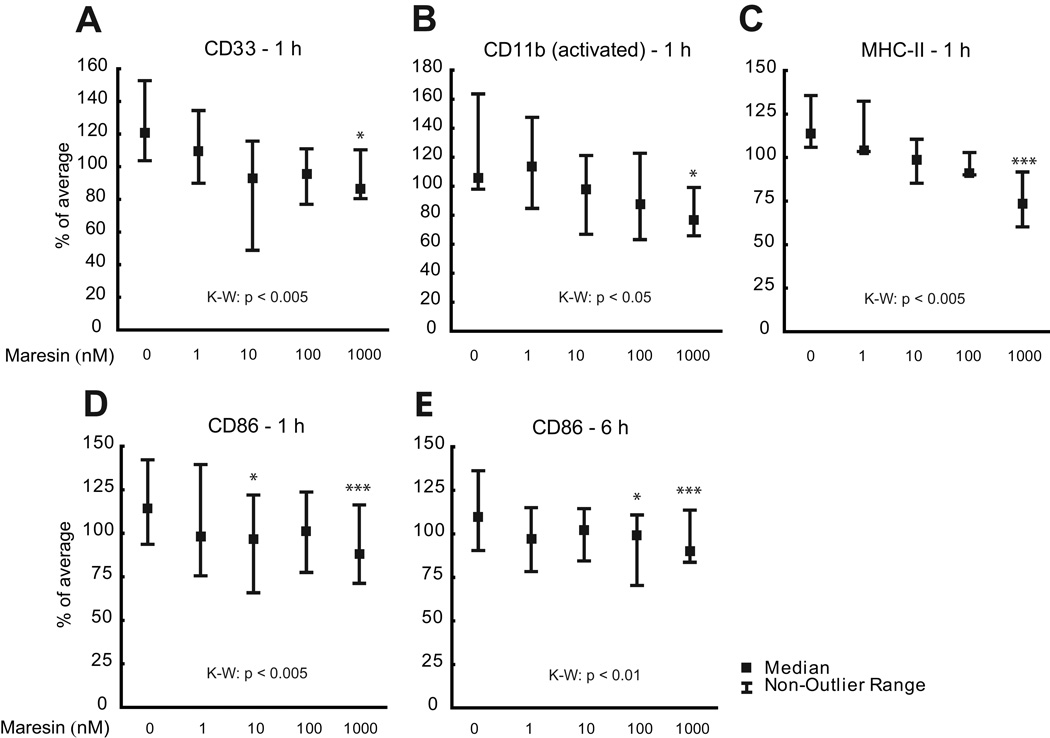
In view of the action of MaR1 on Aβ42 phagocytosis, as well as the deficiency of MaR1 in AD we show here, and in a previous study [29], the phenotype of the microglial cells upon MaR1 treatment was given special attention. A panel of interest, including the pro-(CD11bactivated, CD86, CD40, CD80, MHC-II) and anti-inflammatory (CD163, CD206) markers, was used in the assessment, as well as CD33, a member of the Siglec family of lectins involved in endocytosis. The CHME3 microglia were stimulated with 0 – 1000 nM MaR1 for 1 and 6 h, after which the effects on the markers were assessed by flow-cytometry. The treatment with MaR1 significantly decreased the levels of the pro-inflammatory markers CD11bactivated, MHC-II and CD86 (Fig. 7b–e), as well as the levels of CD33 (Fig. 7a). The levels of the anti-inflammatory markers CD163 and CD206 remained unchanged upon incubation with MaR1 (data not shown).
Fig. 7.
a–e. Actions of MaR1 on microglial phenotype. Human CHME3 microglia were treated with 0 – 1000 µM MaR1 for 1 and 6 h, and flow-cytometry analysis showed a down-regulation of CD33 (a), which is involved in inhibition of phagocytosis. MaR1 also down-regulated the pro-inflammatory markers activated form of CD11b (b), major histocompatibility complex class II (MHC-II), and (c) CD86 (d and e). * indicates p < 0.05 and *** indicates p < 0.005 compared to vehicle. The data were normalized to the average of each individual experiment and presented as median ± non-outlier. K-W = Kruskal-Wallis analysis of variance, MaR1 = maresin 1.
SPM receptors
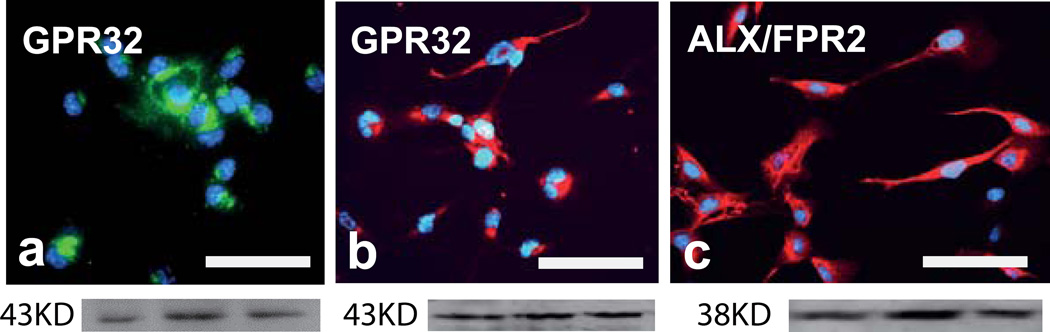
Only some of the receptors for SPMs have been determined so far, i.e. ALX/FPR2 and GPR32, both of which are receptors for LXA4 and RvD1, ChemR23, receptor for RvE1, and BLT1 or LTB4R, receptor for LTB4 and RvE1. However, to our knowledge the receptors for MaR1 and NPD1 have not been described. In the sections above, we present evidence for direct effects of SPMs, including LXA4 and RvD1, on differentiated neuroblastoma cells and on microglial cells, and immunohistochemical analysis demonstrates the occurrence of GPR32 in the neurons (Fig. 8a), and of both GPR32 and ALX/FPR2 in the microglia (Fig. 8b and c). The staining was validated by western blot analysis (Fig. 8a–c).
Fig. 8.
a–c. Expression of SPM receptors in differentiated neuroblastoma cells and in microglial cells. (a) Micrograph and underneath an immunoblot of differentiated human SH-SY5Y neuroblastoma cells with antibodies against G protein-coupled receptor 32 (GPR32). (b and c) Micrographs and underneath immunoblots of human CHME3 microglia cells with antibodies against LXA4 receptor/formyl peptide receptor 2 (ALX/FPR2) (b) and GPR32 (c), respectively. (a) Scale bar = 2.5 µm, (b and c) scale bar = 5 µm. LXA4 = lipoxin A4, SPM = specialized pro-resolving mediator.
Discussion
Chronic inflammation, a characteristic of the pathology in the AD brain [35,36], could be a consequence of failure in resolution of inflammation. The existence of a dysregulated resolution mechanism in the AD brain is indicated by previous reports on decreased levels of PD1 [4,29] and MaR1 [29] in AD hippocampus, and reduced levels of LXA4 in the hippocampus and CSF of AD patients [29]. This opens up for a possible treatment strategy based on replacement of pro-resolving LMs and/or stimulation of pro-resolving activities. A prerequisite for this is an understanding of the actions of SPMs on brain cells, as well as to investigate whether their reduction is common to other brain regions. In the present study, we combine these aspects by studies on the effects of SPMs on neurons and microglia (see below), and LC-MS-MS analysis of lipids in the ENT, a region that plays an important role in declarative memory, particularly spatial memory formation, and that is affected at an early stage of AD pathogenesis [30]. The LC-MS-MS analysis provided further evidence for a dysregulation of resolution. The analysis identified D- and E-series resolvins, maresins, protectins and AA-derived lipoxins in the human ENT. To our knowledge, neither RvD2 and RvD5 nor RvE1 and RvE2, have been shown so far in the human brain. Consistent with the earlier findings in the hippocampus [4,29], the levels of MaR1 and PD1 were reduced in post mortem ENT tissue from AD patients. Furthermore, we found evidence for reduced levels of RvD5 in AD. The PMI for the samples analyzed was 18–21 h, however, there was no significant difference between the AD and control group with regard to PMI, indicating that a potential confounding effect by degradation would affect both groups equally. ApoE4 is an important risk factor for AD, and also involved in cholesterol transport and lipid metabolism. However, correlation analysis did not reveal an association between the presence of one or two ApoE4 alleles with levels of the LMs measured here. A larger sample size would be required to determine with certainty if lower SPM levels in the AD brain are associated with a specific ApoE genotype.
The observed alterations in the hippocampus and ENT, as well as in the CSF [29], indicate that a LM profile, with an emphasis on reduction in SPMs, appears as a common feature in AD pathology, and may therefore be involved in development of AD-related neurodegeneration. However, the findings were not completely equivalent. In contrast to the findings in the hippocampus, the levels of LXA4 in the ENT did not differ between AD patients and controls, indicating a regional difference. Similarly to the hippocampus [29], the ENT had higher levels of the pro-inflammatory LM PGD2 in AD patients. PGD2 is produced by hematopoietic PGD synthase (HPGDS), an enzyme increased in glial cells within senile plaques in AD brains [7]. Clinical trials where human AD patients are given SPMs to correct a deficiency in the levels of SPMs have not yet been performed. However, results from a clinical trial on AD-patients treated with the SPM-precursors DHA and EPA showed a positive effect on cognition compared to placebo in a subgroup of AD patients having less severe cognitive decline (mini-mental state examination, MMSE > 27) [37].
So far, only few studies have analyzed the cellular origin of SPMs in the brain. In vitro studies demonstrated production of PD1 in mixed human neuron-glia cell cultures [4], and our studies on the human microglia cell line (CHME-3) showed LXA4 and RvD1 in the conditioned medium [38]. Immunohistochemical studies on human and murine brains have shown the occurrence of the biosynthetic enzymes 5-LOX and 15-LOX in neurons and glia [6,39–42]. Substrates for these enzymes are poly-unsaturated FAs (PUFAs) within the cellular membranes, DHA being the most abundant FA in the human brain. Altogether, this supports a local production of SPMs by neurons and glia in the brain. Earlier studies have shown higher levels of 5-LOX [8] and 12/15-LOX [42] in AD brains vs. controls, which may indicate an increased capacity to produce SPMs, and a contradiction to the reduced levels observed for SPMs. However, the LOX enzymes are also able to produce pro-inflammatory LMs, including LTs. Studies by Praticò showed that presence of 12/15-LOX was accompanied by higher levels of IL-12P40, a pro-inflammatory cytokine and lack of this enzyme correlated with protection against oxidative stress [42]. Under certain conditions, the production of pro-inflammatory LMs can be switched to the production of pro-resolving LMs, a process termed “class-switching” [43,44]. Hence, the production profile of the enzymatic pathway producing LMs appears to be “programmable” by the conditions in the tissue. Without knowledge on which production profile that is active in the brain, the consequences of altered levels are hard to foresee. The mechanisms behind class-switching are still not fully known. Cholinergic signaling through α7 nicotinic acetylcholine receptor (α7nAChR) has been suggested to stimulate a shift to pro-resolving LM production [45]. The deficiency in cholinergic signaling in the AD brain is well known. In a recent study on human microglia, we showed that inflammation induced by Aβ42 produced changes in resolution mechanisms, including the levels of α7nAChR, suggesting that inflammation caused by Aβ42 may be more difficult to resolve compared to the inflammation caused by a classical infectious stimulus such as lipopolysaccharide (LPS) [38]. Furthermore, the substrates for SPM biosynthesis, PUFAs, are reduced in the AD brain (see [46]), and also the enzymatic machinery in the liver for production of PUFAs from dietary sources of substrates such as a-linoleic acid, is compromised in AD patients [47]. A failure in class-switching mechanisms may thus prevent an increased production in SPMs in spite of higher levels of LOXs.
In order to better understand the consequences of a deficiency in the levels of SPMs with regard to neuronal cell death and overabundance of Aβ42, conditions intimately associated with AD, we performed in vitro experiments on monocultures of human neuronal and microglial cells. We found that all SPMs tested (LXA4, MaR1, PDX and RvD1) showed some degree of protection against neuronal cell death induced by STS. Furthermore, MaR1 had pronounced pro-resolving immunomodulatory effects on microglia, by stimulating phagocytosis of Aβ42 and downregulating pro-inflammarory markers. By connecting these pre-clinical data to the deficiencies in the levels of SPMs in the AD brain shown in the present study, as well as in previous studies on the hippocampus [4,29], we hypothesize that a deficiency in the levels of SPMs, particularly of MaR1, deprives the brain of protective and restorative signals which can contribute to the pathogenesis in AD. In addition to the present study, beneficial actions of SPMs have been shown in in vitro and in vivo models related to AD, including reduction of Aβ pathology and inflammation, and an improvement in cognitive function [4,48–50]. However, few studies have investigated the effects of SPMs on neuronal or glial cells in monocultures, in which observation of direct neuroprotective and/or immunomodulatory effects is possible. Direct effects require the presence of receptors for SPMs on the responding cells. RvD1 and LXA4 bind to the same receptor, ALX/FPR2, and RvD1 can also bind to GPR32 [51,52]. Receptors for MaR1, PD1 or PDX have not yet been described. We have shown the occurrence of ChemR23, a receptor for RvE1, and ALX/FPR2 in both neurons and glia in the human brain [29], as well as of ALX/FPR2 and GPR32 in the human microglial cell line analyzed in the present study [38], complemented here by immunocytochemical evidence. In the present study we also show the occurrence of GPR32 in the differentiated neuroblastoma cells used as a neuronal model, indicating that they can receive protective signals through this receptor. The signaling events downstream of receptors mediating pro-resolving effects of SPMs are not yet fully characterized. STS targets mitochondria and induces oxidative stress [53], which has been described to be associated with neuronal loss in AD (see e.g. [54]). The present results may therefore indicate that neuroprotection offered by SPMs is mediated at least in part via a mitochondrial pathway. This is in line with a study on murine and human macrophages in which pre-treatment with LXA4 diminished STS-induced apoptosis by promoting the PI3K/Akt and ERK/Nrf-2 pathway, and preserving mitochondrial function and integrity [55]. Reduction in oxidative stress has been shown to be involved in the protective effect of PD1 in in vitro models of neurodegeneration [56,57]. PDX, used in the present study, is an endogenous isomer of PD1, and differs from PD1 with regard to its biosynthetic route, chemical structure and potency [58]. PDX is less potent in inducing resolution of inflammation compared to PD1 [59], but has been shown to inhibit platelet aggregation via inhibition of COX-1 at nM concentrations, an action that is absent for PD1 [60]. PD1 was shown to protect retinal and neuronal cells through an increase in the anti-apoptotic Bcl-2 family, decreasing pro-apoptotic Bax and Bad expression, thus inhibiting caspase-3 activation [4,61,62] and stimulating the PI3K/Akt and mTOR/p70S6K pathways, which are supportive for cell survival [63]. It is currently not known to which degree the effects of PDX on intracellular signaling resemble those described for PD1, but the molecular similarity and the protective effects they share suggest that effects on anti- and pro-apoptotic proteins and PI3K/Akt mediate the neuroprotection by PDX. Peroxisome proliferator-associated receptor (PPAR)-γ is a receptor for several different FAs and LMs, including LXA4 [64] and RvD1 [65], as well as PD1, which was shown to exert neuroprotection via induction of neuronal PPAR-γ [50]. There are no published data available on the effects of MaR1 on PPAR-γ, although it is likely that MaR1, being similar in structure to PD1 and RvD1, can bind to PPAR-γ and explain its neuroprotective effect.
Aβ42 induces a predominantly pro-inflammatory phenotype in microglia [38,60,66]. It has previously been shown that Aβ42 stimulate the expression of cellular activation markers such as CD40 [67], and this effect was replicated in our model of human microglia. The increase in CD40 was significantly diminished when MaR1 or RvD1 were added to the cultures, while LXA4 and PDX were ineffective in this regard. The activity of the JAK1/2-STAT1 pathway is known to increase the expression of CD40, and inhibition of this pathway by lovastatin was shown to decrease CD40 in mouse microglia [68]. Although no studies have investigated whether SPMs have inhibitory effects on the JAK/STAT pathway, it is a potential mode of anti-inflammatory action for SPMs. The levels of CD40 is of particular importance in AD since it is increased in the AD brain [69], and when binding to its ligand CD40L promotes neuronal cell death [70], and stimulates production of Aβ [71]. CD11b interacts with CD18 and forms a heterodimer, macrophage antigen complex (Mac)-1, which is an integrin receptor present on eosinophils, neutrophils, monocytes, macrophages and microglia. Although it is mostly known for its role in adhesion of activated blood borne cells, Mac-1 has also been shown to be involved in microglial migration, phagocytosis and inflammation [72]. Upon activation, CD11b undergoes a change that causes hyperadhesion [73]. We show in the present study for the first time that activation of CD11b occurs in microglia exposed to Aβ42, and that MaR1 diminished this activation. Hyperadhesion is a consequence of activation in eosinophils [73], and neutrophils [74], and it is reasonable to assume that this is also true in microglia. Activation of CD11b also occurs in macrophages [75], where it is related to opsonic complement-dependent phagocytosis, and a similar modulatory effect on microglial phagocytosis is possible. The transcription of pro-inflammatory genes after activation of nuclear factor (NF)-κB and activator protein (AP)-1 is well-known and fundamental events in pro-inflammatory activation. PD1 and RvD1 have been shown to inhibit activation of NF-κB [65,76]. Inhibition by RvD1 was shown to be dependent on PPAR-γ [65]. LXA4 was shown to inhibit the activation of both NF-κB and AP-1 [76]. Activation of mitogen-activated protein kinases (MAPK) by phosphorylation is another well-known event in pro-inflammatory activation upstream of NF-κB activation, and we have previously shown an increase in the phosphorylation of c-jun n-terminal kinase (JNK) and p38 upon exposure of CHME-3 microglia to Aβ42 [38]. Therefore, it is likely that inhibition of MAPK phosphorylation contributes to the anti-inflammatory effects of SPMs. Support for this has been provided in a mouse model of traumatic brain injury (TBI) [77], where LXA4 decreased TBI-induced phosphorylation of JNK, and in a study on human macrophages, where MaR1 and RvD1 decreased zymosan-induced phosphorylation of JNK and p38, in parallel with inhibition of NF-κB activation [78]. Furthermore, activation of PPAR-γ has been shown to exert an anti-inflammatory effect on microglia in several studies [79–81], and in view of the ability of several SPMs to act as ligand for PPAR-γ, it is a likely mediator of their anti-inflammatory effects.
In a previous study, we showed an increase in Aβ42 phagocytosis upon stimulation of human CHME3 microglia with DHA and EPA, precursors for SPMs [34]. Conceivably, this effect may be indirect through conversion to SPMs, for which these cells express the relevant machinery [38].The data in the present study showed that the DHA-derivative MaR1, but not RvD1 or PDX, or the AA-derived LXA4, increased the phagocytosis of Aβ42, suggesting that MaR1 has a distinct role in microglia-mediated removal of Aβ. RvD1 has been reported previously to increase phagocytosis of the fibrillar form of Aβ42 by peripheral blood mononuclear cells (PBMCs) from AD-patients [49]. However, this effect was not observed in the present study on CHME-3 microglia, indicating different actions of SPMs in the periphery vs. the brain.
The prominence of MaR1 in stimulating phagocytosis as well as neuroprotection motivated further investigation. Consistent with the increased Aβ42 phagocytosis, we found that MaR1 significantly down-regulated the levels of CD33, a 67-kDa trans-membrane glycoprotein expressed by microglia, also shown to be elevated in AD, and associated with decreased cognitive function [82]. Studies by Griciuc et al showed that CD33 inhibits microglial uptake and clearance of Aβ42 [83]. In addition to cytoprotection, and the inhibitory effects on inflammatory markers, PPAR-γ has been shown in several studies to be a positive regulator of phagocytosis [84–86]. Similarly to the discussion on neuroprotection, it can be hypothesized that MaR1 exerts its stimulatory effects on phagocytosis by binding to PPAR-γ. We also tested the effects of MaR1 on an extended set of pro- and anti-inflammatory markers, and found that the levels of CD80, CD86, CD11b and MHC-II were decreased by incubation with MaR1. This finding is in line with an earlier study, in which an increase in the production of MaR1 was associated with decreased levels of the pro-inflammatory markers CD54 and CD80 in interferon (IFN)-γ- and LPS-stimulated macrophages, as well as with increased levels of the anti-inflammatory phenotype markers CD163 and CD206 [87].
Except for MaR1, for which data have not been presented so far, it appears that PPAR-γ is a mechanism in common for SPMs contributing to their cytoprotective and anti-inflammatory effects, as well as the stimulation of phagocytosis. It is reasonable to assume that the response of a cell is an outcome of the activation of PPAR-γ together with other additional signaling pathways. A tentative hypothesis is that binding of SPMs to membrane receptors such as GPR32 modulate the effects elicited by the activation of genes that follow the concurrent binding of SPMs to PPAR-γ into a protective and anti-inflammatory cellular (i.e. pro-resolving) response.
In conclusion, our results further strengthen the argument that there is a deficiency in the resolution of inflammation in the AD brain, and extend our previous findings to the ENT and in vitro mechanistic studies. In particular, the DHA-derivative MaR1 is consistently lower in AD. Our in vitro data showed an important role of MaR1 in maintaining homeostasis in the brain by promoting neuronal survival, microglial removal of Aβ42, and limiting microglial inflammation. Therefore, reduced levels of MaR1 and other SPMs may play an important role in the pathogenesis of AD, by resulting in decreased restorative and protective activities, as well as by perpetuating inflammation in the AD brain. Our results also indicate that restoring this deficiency by treatment with SPMs or analogues thereof is a promising treatment strategy for AD.
Supplementary Material
Acknowledgements
The authors are grateful for the support from the grants from The Swedish Research Council (22743, 22744); Swedish Brain Power; The Chinese Scholarship Council, P.R. China; The Knut and Alice Wallenberg Foundation; Karolinska Institutet research funds; Stiftelsen för Gamla Tjänarinnor; The Swedish Alzheimer Foundation; Gun och Bertil Stohnes Stiftelse; and Barmore Fund (MUSC). This work was also supported in part by National Institutes of Health (P01GM095467 and GM038765 to C.N.S.).
Footnotes
Conflict of interest
C.N.S. has the following disclosures: as inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals; scientific founder of Resolvyx Pharmaceuticals with equity ownership in the company; and has interests reviewed and managed by the Brigham and Women’s Hospital and Partners Health Care in accordance with their conflict of interest policies.
References
- 1.Farooqui AA, Wells K, Horrocks LA Breakdown of membrane phospholipids in Alzheimer disease. Involvement of excitatory amino acid receptors. Mol Chem Neuropathol. 1995;25(2–3):155–173. doi: 10.1007/BF02960910. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol Dis. 1996;3(1):51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 3.Skinner ER, Watt C, Besson JA, Best PV. Differences in the fatty acid composition of the grey and white matter of different regions of the brains of patients with Alzheimer’s disease and control subjects. Brain. 1993;116(Pt 3):717–725. doi: 10.1093/brain/116.3.717. [DOI] [PubMed] [Google Scholar]
- 4.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115(10):2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dullemeijer C, Durga J, Brouwer IA, van de Rest O, Kok FJ, Brummer RJ, van Boxtel MP, Verhoef P. n-3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr. 2007;86(5):1479–1485. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- 6.Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J Histochem Cytochem. 2008;56(12):1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohri I, Kadoyama K, Kanekiyo T, Sato Y, Kagitani-Shimono K, Saito Y, Suzuki K, Kudo T, Takeda M, Urade Y, Murayama S, Taniike M. Hematopoietic prostaglandin D synthase and DP1 receptor are selectively upregulated in microglia and astrocytes within senile plaques from human patients and in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2007;66(6):469–480. doi: 10.1097/01.jnen.0000240472.43038.27. [DOI] [PubMed] [Google Scholar]
- 8.Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Pratico D. 5-Lipoxygenase gene disruption reduces Aβ pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22(4):1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cacabelos R, Alvarez XA, Fernandez-Novoa L, Franco A, Mangues R, Pellicer A, Nishimura T. Brain interleukin-1β in Alzheimer’s disease and vascular dementia. Meth Find Exp Clin Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- 10.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79(1–2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 12.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 13.Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res. 2010;7(8):656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicklund L, Leao RN, Strömberg AM, Mousavi M, Hovatta O, Nordberg A, Marutle A. Aβ42 oligomers impair function of human embryonic stem cell-derived forebrain cholinergic neurons. PLoS One. 2010;5(12):e15600. doi: 10.1371/journal.pone.0015600. PMID: 21179413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Bo R, Angeretti N, Lucca E, De Simoni MG, Forloni G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and β-amyloid production in cultures. Neurosci Lett. 1995;188(1):70–74. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- 16.Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNFa plus IFNy induce the production of Alzheimer β-amyloid peptides and decrease the secretion of APPs. FASEB J. 1999;13:63–68. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- 17.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47(2):425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 18.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuelsson B, Dahlén SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 20.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S–resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278(17):14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 22.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40(3):315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu MX, Tan BC, Zhou W, Wei T, Lai WH, Tan JW, Dong JH. Resolvin D1, an endogenous lipid mediator for inactivation of inflammation-related signaling pathways in microglial cells, prevents lipopolysaccharide-induced inflammatory responses. CNS Neurosci Ther. 2013;19(4):235–243. doi: 10.1111/cns.12069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Xu ZZ, Liu XJ, Berta T, Park CK, Lu N, Serhan CN, Ji RR. NpD/PD1 protects against neuropathic pain in mice after nerve trauma. Annals of Neurology. 2013;74(3):490–495. doi: 10.1002/ana.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARγ-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29(12):3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cudaback E, Jorstad NL, Yang Y, Montine TJ, Keene CD. Therapeutic implications of the prostaglandin pathway in Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):565–572. doi: 10.1016/j.bcp.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham C, Skelly DT. Non-steroidal anti-inflammatory drugs and cognitive function: are prostaglandins at the heart of cognitive impairment in dementia and delirium? J Neuroimmune Pharmacol. 2012;7(1):60–73. doi: 10.1007/s11481-011-9312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zhu M, Hjorth E, Cortés-Toro V, Eyjolfsdottir H, Graff C, Nennesmo I, Palmblad J, Eriksdotter M, Sambamurti K, Fitzgerald JM, Serhan CN, Granholm A-C, Schultzberg M. Resolution of inflammation is altered in Alzheimer’s disease. Alzheimer’s & Dementia. 2015;11:40–50. doi: 10.1016/j.jalz.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17(2):304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014;307(1):C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–e72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75(3):991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 34.Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G, Wahlund LO, Basun H, Eriksdotter M, Schultzberg M. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheimers Dis. 2013;35(4):697–713. doi: 10.3233/JAD-130131. [DOI] [PubMed] [Google Scholar]
- 35.Aisen PS, Davis KL. Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry. 1994;151(8):1105–1113. doi: 10.1176/ajp.151.8.1105. [DOI] [PubMed] [Google Scholar]
- 36.Mann DM, Iwatsubo T, Fukumoto H, Ihara Y, Odaka A, Suzuki N. Microglial cells and amyloid β protein (Aβ) deposition; association with Aβ40-containing plaques. Acta Neuropathol (Berl) 1995;90(5):472–477. doi: 10.1007/BF00294808. [DOI] [PubMed] [Google Scholar]
- 37.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch of Neurol. 2006;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 38.Zhu M, Wang X, Hjorth E, Schultzberg M. Differential regulation of resolution in inflammation induced by Aβ42 and LPS in human microglia. J Alzheimers Dis. 2015;43:1237–1250. doi: 10.3233/JAD-141233. [DOI] [PubMed] [Google Scholar]
- 39.Haynes RL, van Leyen K. 12/15-lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia. Dev Neurosci. 2013;35(2–3):140–154. doi: 10.1159/000350230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lammers CH, Schweitzer P, Facchinetti P, Arrang JM, Madamba SG, Siggins GR, Piomelli D. Arachidonate 5-lipoxygenase and its activating protein: prominent hippocampal expression and role in somatostatin signaling. J Neurochem. 1996;66(1):147–152. doi: 10.1046/j.1471-4159.1996.66010147.x. [DOI] [PubMed] [Google Scholar]
- 41.Ohtsuki T, Matsumoto M, Hayashi Y, Yamamoto K, Kitagawa K, Ogawa S, Yamamoto S, Kamada T. Reperfusion induces 5-lipoxygenase translocation and leukotriene C4 production in ischemic brain. Am J Physiol. 1995;268(3 Pt 2):H1249–H1257. doi: 10.1152/ajpheart.1995.268.3.H1249. [DOI] [PubMed] [Google Scholar]
- 42.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164(5):1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo M, Jones SM, Phare SM, Coffey MJ, Peters-Golden M, Brock TG. Protein kinase A inhibits leukotriene synthesis by phosphorylation of 5-lipoxygenase on serine 523. J Biol Chem. 2004;279(40):41512–41520. doi: 10.1074/jbc.M312568200. [DOI] [PubMed] [Google Scholar]
- 44.Ye Y, Lin Y, Perez-Polo JR, Uretsky BF, Ye Z, Tieu BC, Birnbaum Y. Phosphorylation of 5-lipoxygenase at Ser523 by protein kinase A determines whether pioglitazone and atorvastatin induce proinflammatory leukotriene B4 or anti-inflammatory 15-epi-lipoxin A4 production. J of Immunol. 2008;181(5):3515–3523. doi: 10.4049/jimmunol.181.5.3515. [DOI] [PubMed] [Google Scholar]
- 45.De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of α7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2(1):4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15(12):771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 47.Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen DL, Head E, Cotman CW, Piomelli D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS One. 2010;5(9):e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, LaFerla FM. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am J Pathol. 2013;182(5):1780–1789. doi: 10.1016/j.ajpath.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizwicki MT, Liu G, Fiala M, Magpantay L, Sayre J, Siani A, Mahanian M, Weitzman R, Hayden E, Rosenthal MJ, Nemere I, Ringman J, Teplow DB. 1a,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-(3 phagocytosis and inflammation in Alzheimer’s disease patients. J Alzheimers Dis. 2013;34:155–170. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPAR-γ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE. 2011;6(1):e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272(11):6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 52.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107(4):1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deshmukh M, Johnson EM., Jr Staurosporine-induced neuronal death: multiple mechanisms and methodological implications. Cell Death Differ. 2000;7(3):250–261. doi: 10.1038/sj.cdd.4400641. [DOI] [PubMed] [Google Scholar]
- 54.Padurariu M, Ciobica A, Lefter R, Serban IL, Stefanescu C, Chirita R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr Danub. 2013;25(4):401–409. [PubMed] [Google Scholar]
- 55.Prieto P, Cuenca J, Traves PG, Fernandez-Velasco M, Martin-Sanz P, Bosca L. Lipoxin A4 impairment of apoptotic signaling in macrophages: implication of the PI3K/Akt and the ERK/Nrf-2 defense pathways. Cell Death Differ. 2010;17(7):1179–1188. doi: 10.1038/cdd.2009.220. [DOI] [PubMed] [Google Scholar]
- 56.Calandria JM, Asatryan A, Balaszczuk V, Knott EJ, Jun BK, Mukherjee PK, Belayev L, Bazan NG. NPD1-mediated stereoselective regulation of BIRC3 expression through cREL is decisive for neural cell survival. Cell Death Differ. 2015;22(8):1363–1377. doi: 10.1038/cdd.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calandria JM, Mukherjee PK, de Rivero Vaccari JC, Zhu M, Petasis NA, Bazan NG. Ataxin-1 poly(Q)-induced proteotoxic stress and apoptosis are attenuated in neural cells by docosahexaenoic acid-derived neuroprotectin D1. J Biol Chem. 2012;287(28):23726–23739. doi: 10.1074/jbc.M111.287078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balas L, Guichardant M, Durand T, Lagarde M. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie. 2014;99:1–7. doi: 10.1016/j.biochi.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176(3):1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 60.Liu M, Boussetta T, Makni-Maalej K, Fay M, Driss F, El-Benna J, Lagarde M, Guichardant M. Protectin DX, a double lipoxygenase product of DHA, inhibits both ROS production in human neutrophils and cyclooxygenase activities. Lipids. 2014;49(1):49–57. doi: 10.1007/s11745-013-3863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101(22):8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antony R, Lukiw WJ, Bazan NG. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A–dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J Biol Chem. 2010;285(24):18301–18308. doi: 10.1074/jbc.M109.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halapin NA, Bazan NG. NPD1 induction of retinal pigment epithelial cell survival involves PI3K/Akt phosphorylation signaling. Neurochem Res. 2010;35(12):1944–1947. doi: 10.1007/s11064-010-0351-8. [DOI] [PubMed] [Google Scholar]
- 64.Weinberger B, Quizon C, Vetrano AM, Archer F, Laskin JD, Laskin DL. Mechanisms mediating reduced responsiveness of neonatal neutrophils to lipoxin A4. Pediatr Res. 2008;64(4):393–398. doi: 10.1203/PDR.0b013e318180e4af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir Res. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovacs DM, Mancini R, Henderson J, Na SJ, Schmidt SD, Kim TW, Tanzi RE. Staurosporine-induced activation of caspase-3 is potentiated by presenilin 1 familial Alzheimer’s disease mutations in human neuroglioma cells. J Neurochem. 1999;73(6):2278–2285. doi: 10.1046/j.1471-4159.1999.0732278.x. [DOI] [PubMed] [Google Scholar]
- 67.Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, Mattson MP, Flavell RA, Mullan M. Microglial activation resulting from CD40-CD40L interaction after (3-amyloid stimulation. Science. 1999;286(5448):2352–2355. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- 68.Townsend KP, Shytle DR, Bai Y, San N, Zeng J, Freeman M, Mori T, Fernandez F, Morgan D, Sanberg P, Tan J. Lovastatin modulation of microglial activation via suppression of functional CD40 expression. J Neurosci Res. 2004;78(2):167–176. doi: 10.1002/jnr.20234. [DOI] [PubMed] [Google Scholar]
- 69.Togo T, Akiyama H, Kondo H, Ikeda K, Kato M, Iseki E, Kosaka K. Expression of CD40 in the brain of Alzheimer’s disease and other neurological diseases. Brain Res. 2000;885(1):117–121. doi: 10.1016/s0006-8993(00)02984-x. [DOI] [PubMed] [Google Scholar]
- 70.Ke ZJ, Calingasan NY, DeGiorgio LA, Volpe BT, Gibson GE. CD40-CD40L interactions promote neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. Neurochem Int. 2005;47(3):204–215. doi: 10.1016/j.neuint.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Volmar CH, Ait-Ghezala G, Frieling J, Weeks OI, Mullan MJ. CD40/CD40L interaction induces Aβ production and increases y-secretase activity independently of tumor necrosis factor receptor associated factor (TRAF) signaling. Exp Cell Res. 2009;315(13):2265–2274. doi: 10.1016/j.yexcr.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 72.Akiyama H, McGeer PL. Brain microglia constitutively express β2 integrins. J Neuroimmunol. 1990;30(1):81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- 73.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006;35(3):378–386. doi: 10.1165/rcmb.2006-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pillay J, Ramakers BP, Kamp VM, Loi AL, Lam SW, Hietbrink F, Leenen LP, Tool AT, Pickkers P, Koenderman L. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J Leukoc Biol. 2010;88(1):211–220. doi: 10.1189/jlb.1209793. [DOI] [PubMed] [Google Scholar]
- 75.Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, Nuccio SP, Crawford RW, Tukel C, Baumler AJ. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect Immun. 2011;79(2):830–837. doi: 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278(44):43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 77.Luo CL, Li QQ, Chen XP, Zhang XM, Li LL, Li BX, Zhao ZQ, Tao LY. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013;1502:1–10. doi: 10.1016/j.brainres.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Dalli J, Chiang N, Baron RM, Quintana C, Serhan CN. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by microRNA and proresolving mediators. Immunity. 2013;39(5):885–898. doi: 10.1016/j.immuni.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woster AP, Combs CK. Differential ability of a thiazolidinedione PPARγ agonist to attenuate cytokine secretion in primary microglia and macrophage-like cells. J Neurochem. 2007;103(1):67–76. doi: 10.1111/j.1471-4159.2007.04706.x. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Barger SW, Drew PD. The PPAR-γ agonist 15-deoxy-5-prostaglandin J(2) attenuates microglial production of IL-12 family cytokines: potential relevance to Alzheimer’s disease. PPAR Res. 2008:349185. doi: 10.1155/2008/349185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu J, Storer PD, Chavis JA, Racke MK, Drew PD. Agonists for the peroxisome proliferator-activated receptor-α and the retinoid X receptor inhibit inflammatory responses of microglia. J Neurosci Res. 2005;81(3):403–411. doi: 10.1002/jnr.20518. [DOI] [PubMed] [Google Scholar]
- 82.Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, Goate AM. Expression of novel Alzheimer’s disease risk genes in control and Alzheimer’s disease brains. PLoS One. 2012;7(11):e50976. doi: 10.1371/journal.pone.0050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid β. Neuron. 2013;78(4):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aronoff DM, Serezani CH, Carstens JK, Marshall T, Gangireddy SR, Peters-Golden M, Reddy RC. Stimulatory effects of peroxisome proliferator-activated receptor-γ on Fcy receptor-mediated phagocytosis by alveolar macrophages. PPAR Res. 2007;2007:52546. doi: 10.1155/2007/52546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Majai G, Sarang Z, Csomos K, Zahuczky G, Fesus L. PPAR-γ-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol. 2007;37(5):1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 86.Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32(48):17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. The novel 13S,14S–epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27(7):2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.