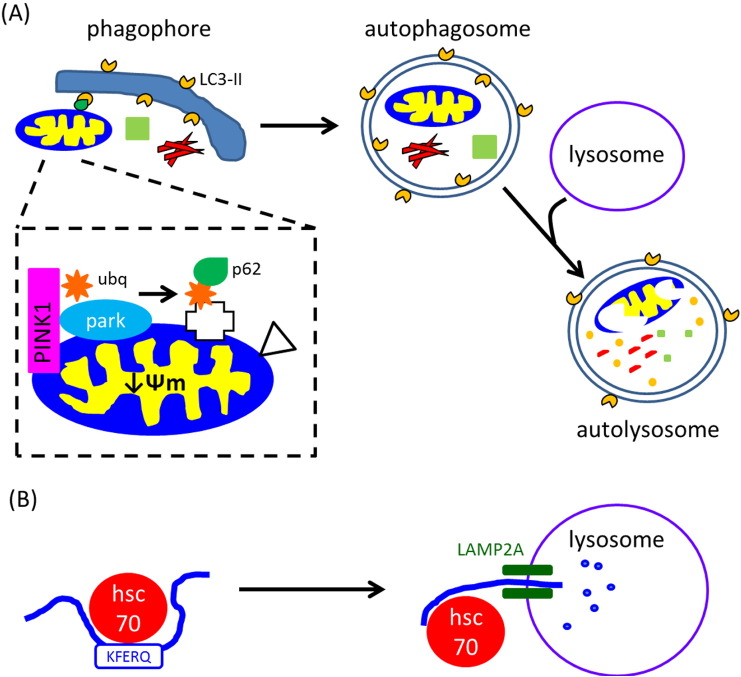
Fig. 1.
Macroautophagy and chaperone mediated autophagy pathways. (A) Macromolecules such as protein and lipids (green square), aggregated proteins (red fibrils) or damaged mitochondria (see inset) are recruited to phagophores by binding to LC3-II embedded in the membrane (orange segments). Phagophores mature into double membrane autophagosomes thus sequestering cargo for degradation. Following fusion with lysosomes to form an autolysosome, macromolecules and organelles are degraded by degradative enzymes from the lysosome. Inset: damaged mitochondria have decreased Ψm causing accumulation of full length PINK1 on the OMM. This recruits and phosphorylates the E3 ubiquitin ligase parkin (park) and ubiquitin (ubq; orange star), resulting in ubiquitination of proteins in the OMM (white cross). These proteins can then be bound by p62 (green teardrop) that enables binding to LC3-II on the phagophore. Other mitophagy receptors (white triangle) that can bind LC3-II such as FUNDC1, BNIP and cardiolipin are also up regulated following mitophagy induction. (B) Chaperone mediated autophagy degrades proteins with the pentapeptide motif KFREQ (approximately 30% of cellular proteins contain this motif). Unfolded protein is bound by hsc70, which then directly delivers protein to lysosomes for degradation via the integral protein LAMP2A.