Abstract
Studies of nucleotide receptors (P2-receptors) in cells and tissues are complicated by cleavage of phosphate groups from nucleotide agonist ligands by ecto-nucleotidases. Some P2 receptor antagonists may also inhibit ecto-nucleotidases, making these studies even more complex. In order to systematically approach this problem, we investigated structure–activity relationships of pyridoxal-5′-phosphate-6-azophenyl-2,4-disulfonate (PPADS) and 14 derivatives, many potent as antagonists at P2 receptors, as inhibitors of ecto-nucleotidases. The compounds were tested for their ability to inhibit enzymatic nucleotide breakdown by CHO cells stably transfected with plasmids containing the cDNA for rat ecto-apyrase (NTPDase1) and rat ecto-ATPase (NTPDase2). All inhibitors were tested at a concentration of 100 µM and ATP hydrolysis was quantified by HPLC. Maximal inhibition obtained for ecto-apyrase and ecto-ATPase was 60% and 35%, respectively. Most PPADS analogs were better inhibitors of ecto-apyrase than of ecto-ATPase. Compound 8, a phosphate derivative, inhibited ecto-apyrase with no inhibition evident at ecto-ATPase. Comparison of pharmacological data of PPADS analogs at P2 receptors as previously determined showed that four PPADS analogs exhibited selectivity for P2X nucleotide receptors. None of these compounds inhibited ecto-ATPase, while two inhibited the ecto-apyrase. Compound 14, a bisphosphate derivative, inhibited ecto-ATPase without inhibition of ecto-apyrase. This compound only weakly antagonized P2X1 receptors and was inactive at P2X2 and P2Y1 receptors, thus bearing some selectivity for ecto-ATPase. Compound 7, a 5-methylphosphonate derivative, a potent antagonist of P2X1 receptors, was inactive at ecto-apyrase and only weakly inhibitory at ecto-ATPase. Thus, PPADS modifications that enhance selectivity among ecto-nucleotidases and P2 receptors have been identified.
Keywords: PPADS, enzyme-inhibition, ecto-ATPase, ecto-apyrase, CD39, P2 receptors
INTRODUCTION
Extracellular nucleotides (principally ATP1, UTP, and the corresponding diphosphates) can activate nucleotide receptors (P2 receptors) and thus alter cellular functions [Fredholm et al., 1994; North and Bernard, 1997; Ralevic and Burnstock, 1998; Abbracchio and Burnstock, 1998]. These signaling processes are terminated through the stepwise dephosphorylation of the nucleotides by endogenous ecto-nucleotidases. Many cell types, and presumably all tissues, express enzymes located at the cell surface to metabolize extracellular nucleotides and nucleosides [Zimmermann, 1996].
Members of the E-NTPDase family (ecto-nucleoside triphosphate diphosphohydrolase family) hydrolyze not only ATP and ADP but also other nucleoside 5′-triphosphates and nucleoside 5′-diphosphates. However, the preference for the individual type of nucleotide can vary considerably [Zimmermann, 1999; Zimmermann et al., 2000]. Individual members of the enzyme family may hydrolyze nucleoside-5′-triphosphates and nucleoside-5′-diphosphates about equally well (ecto-apyrase, NTPDase1) or have a high preference for nucleoside-5′-triphosphates (ecto-ATPase, NTPDase2). Studies of P2 receptors in cells and tissues are complicated by enzymatic degradation of nucleotide agonist ligands. Some P2 receptor antagonists may also inhibit ecto-nucleotidases, making these studies even more complex. This was found for the weak P2 antagonist Reactive Red 2 [Bültmann and Starke, 1995], Evans blue and related compounds [Wittenburg et al., 1996], small aromatic isothiocyanato-sulphonates [Bültmann et al., 1996a], compounds related to suramin [Bültmann et al., 1996b], and compounds related to Reactive Blue 2 [Tuluc et al., 1998]. Structure–activity relationships of pyridoxal-5′-phosphate-6-azophenyl-2,4-disulfonate (PPADS), a frequently used P2 receptor antagonist [Lambrecht et al., 1992], have been studied on P2 receptors [Jacobson et al., 1998; Kim et al., 1998, 2000]. Structural modifications of PPADS have been made through functional group substitution on the sulfophenyl ring and at the phosphate moiety through the inclusion of phosphonates, demonstrating that a phosphate linkage is not required for P2 receptor antagonism [Kim et al., 1998]. Phosphonates are not hydrolyzable and thus may be more stable than phosphate analogs in pharmacological studies. PPADS has been found to inhibit ecto-nucleotidases [Bültmann et al., 1999; Heine et al., 1999], but its structure–activity relationships for inhibition of ecto-nucleotidases have not previously been studied. In order to systematically approach this problem, we investigated structure–activity relationships of PPADS and 14 derivatives, many of which are potent as antagonists at P2 receptors, as inhibitors of recombinant rat ecto-apyrase and ecto-ATPase.
MATERIALS AND METHODS
Chemicals
ATP and other chemicals were obtained from Sigma (Dreisenhofen, Germany). PPADS and analogs were synthesized as previously described [Kim et al., 1998, 2000]. Reagent grade liquids for HPLC were purchased from Merck (Darmstadt, Germany).
Cloning of Rat ATPase cDNA and Rat Apyrase cDNA Into the Expression Vector
cDNA encoding ecto-ATPase (NTPDase2, CD39-L1) isolated from a rat brain Uni-Zap cDNA library (Stratagene, Heidelberg, Germany) as well as cDNA encoding ecto-apyrase (NTPDase1, CD39) obtained from rat brain mRNA using reverse transcription-PCR (RTPCR) were ligated into the mammalian expression vector pcDNA3 (Invitrogen, NV Leek, Netherlands) as previously described [Heine et al., 1999].
Stable Transfection of CHO Cells
CHO (Chinese hamster ovary) cells were cultured in HAM’s FC-12 medium containing 10% fetal calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco BRL, Eggenstein, Germany). They were transfected with plasmid-DNA containing ecto-ATPase or ecto-apyrase cDNA by electroporation, as previously described [Kegel et al., 1997]. Stable transfectants [Heine et al., 1999] were selected for neomycin resistance using 800 µg/ml of genectin G418 sulfate (Gibco BRL) and subcloned by limiting dilution. In control experiments, cells were transfected with the empty pcDNA3 vector and selected for neomycin resistance.
Measurement of Ecto-Nucleotidase Activity
Stable transfectants were seeded in multiwell plates (5 × 104 cells per well, 1.88 cm2). Surface-located enzyme activity of intact cells was determined 24 h later at 37°C. The culture medium was carefully aspirated and cells were washed twice with phosphate-free physiological saline solution (140 mM NaCl, 5 mM KCl, 10 mM glucose, 1 mM MgCl2, 2 mM CaCl2, 10 mM Hepes, pH 7,4). To start the reaction 1 mM ATP was added (final ATP-concentration 0.5 mM). After varying periods of time, saline solution (500 µl) was collected from the cells and transferred into Eppendorf tubes. Tubes were stored on ice until centrifugation at 300g for 10 min (4°C), followed by recentrifugation of the supernatant fraction at 14,000g for 45 min (4°C). The supernatant was immediately analyzed for ATP-breakdown by HPLC or stored at −20°C.
Inhibitor Studies
For determination of the inhibitory effect of PPADS and its analogs, cells were preincubated for 30 min with 250 µl phosphate-free saline solution supplemented with 100 µM of potential inhibitor. After 30 min, 250 µl of the same saline solution containing the potential inhibitor (100 µM) and ATP (final concentration 0.5 mM) was added. Saline solution (500 µl) was collected from the cells after certain periods of time and transferred into Eppendorf tubes. Concentration dependence of inhibition was investigated using inhibitor concentrations up to 300 µM. Measurement of ecto-nucleotidase activity in the absence of potential inhibitors served as a reference for each set of experiments.
Determination of ATP Breakdown by HPLC
For analysis of ATP-breakdown, ATP, ADP, and AMP were separated and quantified by HPLC. A 100 µl aliquot of supernatant diluted with 200 µl ultrapure water was injected into a Sepsil C18 reversed phase column (Jasco, Groβ-Umstadt, Germany) and eluted at 0.75 ml/min with the mobile phase consisting of 10 mM potassium-phosphate buffer (pH 7.4), 12% acetonitrile, and 2 mM tetrabutylammonium hydrogen sulfate. Absorbance at 260 nm was monitored continuously and nucleotide concentrations were determined from the area under each absorbance peak. Three time points, measured as duplicates, were taken for each determination and enzyme activity (nmol ATP per 106 cells*min−1) was calculated from the slope after linear regression. Enzyme activities are shown as percentage of ATP degradation in the absence of inhibitors (reference experiment).
RESULTS
Prior to measuring enzymatic inhibition, we established conditions that displayed a linear time course for the hydrolysis of ATP. ATP (0.5 mM) was added to intact CHO cells stably transfected with ecto-apyrase or ecto-ATPase. Samples were taken after 1–10 min and ATP degradation was determined by HPLC. The rate of ATP-hydrolysis was linear for both enzymes from 1–8 min of incubation time (Fig. 1). Vector-transfected CHO cells used as a control did not show any catalytic activity. Based on these results, the time points for collecting samples in the inhibition experiments were set to be 1, 4, and 8 min. Neither cells transfected with ecto-apyrase nor cells transfected with ecto-ATPase cleaved the phosphate moiety from PPADS (data not shown).
Fig. 1.
Determination of the time course of enzymatic ATP hydrolysis. CHO cells stably expressing ecto-apyrase or ecto-ATPase were incubated with 0.5 mM ATP. Samples were taken over a period of 10 min and analyzed for ATP-degradation by HPLC. Degradation of ATP was expressed as nmoles ATP per 106 cells (representative experiments with duplicate determinations ± range for each time point). Catalytic activity as determined for linear ATP-breakdown (up to 8 min) was 80.4 nmoles and 97.9 nmoles of ATP per 106 cells/min for ecto-ATPase and ectoapyrase, respectively.
Inhibition experiments were performed with an inhibitor concentration of 100 µM. For each individual set of experiments an additional curve for ATP-hydrolysis was obtained as a reference for the rate of ATP-hydrolysis in the absence of inhibitor (100% value). ATP degradation, as determined by HPLC, was plotted against time and analyzed by linear regression. The slope of the curve between 1 and 8 min was taken as the rate of ATP-hydrolysis and expressed as the percentage of the rate of ATP-hydrolysis in the absence of the inhibitor (Fig. 2). The structures of the pyridoxal derivatives tested as ecto-nucleotidase inhibitors are shown in Figure 3. Monophosphate (1–6, 8, 10, 12), phosphonate (7, 9, 11), bisphosphate (14), and cyclic phosphate analogs (13, 15) are included. The inhibition results for both enzymes are listed in Table 1. None of the tested PPADS analogs fully inhibited either ecto-apyrase or ecto-ATPase at 100 µM concentration. In the majority of cases the inhibition of ATP-hydrolysis by PPADS analogs was stronger for ecto-apyrase than for ecto-ATPase. Almost half of the tested compounds did not inhibit ecto-ATPase activity at 100 µM concentration. Only compound 14 showed inhibition of ecto-ATPase without inhibition of ecto-apyrase.
Fig. 2.
Effect of compounds 2, 8, and 10 on catalytic activity of ectoapyrase. The inhibitors were applied at a concentration of 100 µM at CHO cells stably expressing rat ecto-apyrase. After 30 min of preincubation ATP was added as the substrate to a final concentration of 0.5 mM. Samples were taken after 1, 4, and 8 min and degradation of ATP was analyzed by HPLC. The slope of the curves equal enzyme activities in µmoles/(106 cells × min) and were 0.0842 (= 100%) for the reference and 0.059 (= 70%), 0.043 (= 49%), and 0.036 (= 43%) for compounds 2, 8, and 10. Enzyme activities were expressed as percentage of enzyme activity in the absence of inhibitors. The values represent means ± standard deviation of two independent experiments with duplicate determinations. 100% values correspond to the hydrolysis of 84.2 nmoles of ATP per 106 cells/min.
Fig. 3.
Structures of pyridoxal-6-azoaryl-5-phosphate or phosphonate derivatives examined as inhibitors at ecto-apyrase and ecto-ATPase. a: Sites of modifications at varying positions of the diazophenyl group are indicated for compounds 1–11. b: Structure of compounds 12–15.
TABLE 1.
Enzyme Inhibition by PPADS and PPADS Analogs at 100 µM Concentration and Pharmacological Activities for P2 Receptors
| Compound | Ecto-apyrase % ATP degradation |
Ecto-ATPase % ATP degradation |
P2X1 recombinant IC50 (µM)a |
P2X2 recombinant IC50 (µM)a,b |
P2Y1 PLC assay IC50 (µM)a,b |
|---|---|---|---|---|---|
| Control | 100 | 100 | |||
| 1 | 81.6 ± 21.5 | 82.8 ± 1.3 | 0.042 ± 0.006 | 1.2 ± 0.2a | 22.5a |
| 2 | 71.6 ± 6.2 | 78.3 ± 2.1 | n.d. | 2.6 ± 0.0b | 25.1 ± 6.3b |
| 3 | 49.4 ± 1.2 | n.i.# | 0.009 ± 0.002 | 11.9 ± 1.4a | >100a |
| 4 | 53.9 ± 2.5 | n.i. | n.d. | 0.819 ± 0.279b | >100b |
| 5 (PPADS) | 83.7 ± 10.9 | 78.3 ± 8.1 | 0.099 ± 0.006 | 1.6 ± 0.1a | 16.6 ± 2.5a |
| 6 | 75.3 ± 10.4 | n.i. | 0.043 ± 0.018 | 0.398 ± 0.125a | 21.4 ± 9.0a |
| 7 | n.i. | 78.2 | 0.012 ± 0.003 | 1.1 ± 0.2a | 71% (100 µM)a |
| 8 | 50.7 ± 2.3 | n.i. | n.d. | n.d. | 12.7 ± 5.6b |
| 9 | 39.3 ± 5.2 | 65.4 ± 7.8 | n.d. | n.d. | 7.23 ± 2.10b |
| 10 | 40.7 ± 6.1 | 68.1 ± 10.9 | n.d. | n.d. | 13.8 ± 2.2b |
| 11 | 48.9 ± 5.7 | 81.1 | 0.011 ± 0.005 | 0.28 ± 0.03a | 14.0 |
| 12 | 82.0 | 76.4 ± 8.4 | n.d. | n.d. | n.d. |
| 13 | n.i. | n.i. | 10.2 ± 2.6 | inactivea | inactivea |
| 14 | n.i. | 65.6 ± 16.3 | 27 ± 7% (10 µM) | n.d. | >100a |
| 15 | 86.1 ± 0.2 | 68.4 | inactive | n.d. | inactivea |
All substances were applied at a final concentration of 100 µM.
N.i. no inhibition; ATP hydrolysis was not significantly different from control. Values represent means ± range or SEM of two to five different experiments each performed in duplicate or single experiments with duplicate determinations. n.d. not determined.
IC50 or percent displacement at the indicated concentration, values taken from Kim et al. [2000].
Values taken from Kim et al. [1998]. MRS numbers for selected compounds: compound 3, MRS 2159; compound 4, MRS 2160; compound 7, MRS 2191; compound 8, MRS 2157; compound 13, MRS 2220; compound 14, MRS 2235.
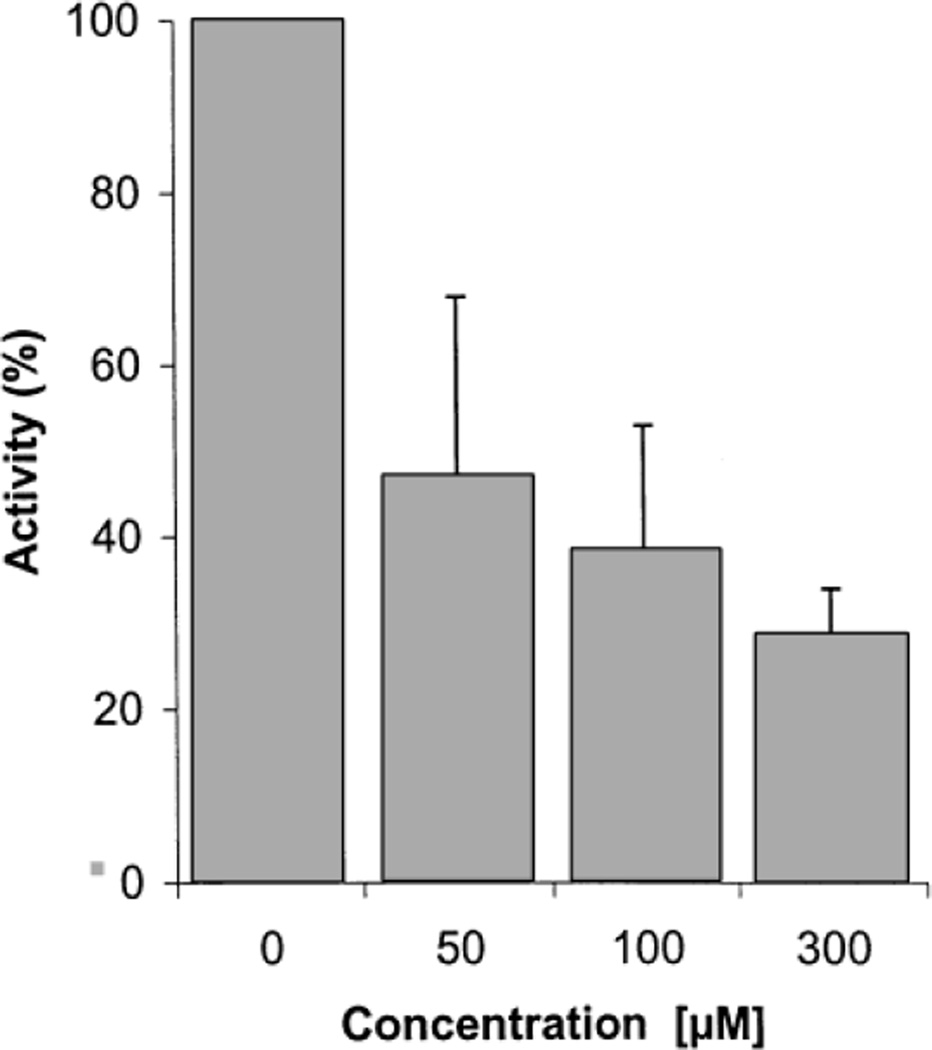
The concentration-dependent inhibition of ATP hydrolysis was determined for several inhibitory PPADS derivatives and yielded essentially identical results. Catalytic activity sharply declined at lower concentrations but increasing concentrations of inhibitor yielded only a small additional inhibitory effect. Identical results were obtained for cells transfected with ecto-ATPase and ecto-apyrase. Figure 4 shows as an example the concentration-dependent inhibition of ATP hydrolysis by ecto-apyrase for one of the most active inhibitors, compound 10. The concentration–response relationship is not linear and declines at higher inhibitor concentrations. The same profile was observed for the textile dye Evans blue, a strong inhibitor of ecto-ATPase and particularly of ecto-apyrase [Heine et al., 1999] (not shown). This precluded a reliable determination of IC50 values. For this reason all compounds were tested at the identical inhibitor concentration of 100 µM.
Fig. 4.
Dose–response relationship for the inhibition of ecto-apyrase by compound 10. ATP degradation by CHO cells stably expressing ecto-apyrase was determined in the presence of increasing concentrations of inhibitor. Enzyme activities are expressed as percentage of the catalytic activity in the absence of inhibitor. Values represent means ± standard deviation of two independent experiments with duplicate determinations. The 100% value corresponds to the hydrolysis of 87.8 nmoles ATP per 106 cells/min−1.
DISCUSSION
The analysis of nucleotide hydrolysis at the surface of viable transfected CHO cells has previously proven to be a reliable method for the determination of the catalytic properties of ecto-ATPase and ecto-apyrase [Kegel et al., 1997; Heine et al., 1999]. Since the concentration–response relationship was not linear, IC50 values could not be determined for individual compounds. The inhibitory effects of PPADS and its analogs at a fixed concentration of 100 µM permit, however, an estimation and comparison of the potency of the compounds. At present, the nonlinearity in the concentration–response relationship is not understood. It should be noted that the cell surface-located members of the E-NTPDase family are not monomeric enzymes but form homooligomeric complexes (dimers to tetramers) that are not linked by disulfide bonds. The state of oligomerization affects catalytic activity [Stout and Kirley, 1996; Wang et al., 1998; Grinthal and Guidotti, 2000]. It appears possible that the application of increasing inhibitor concentrations interferes with the state of enzyme oligomerization, resulting in an altered potency of the inhibitor.
In general, PPADS analogs containing the aldehyde group (compounds 1–11) were better inhibitors of ecto-apyrase than of ecto-ATPase. This holds true for all inhibitory compounds, except compounds 1, 5, and 7, which were equipotent at both enzymes or weakly inhibitory at ecto-ATPase. Alteration of the aldehyde group (compounds 12–15) led to decreased inhibition of ecto-apyrase and increased inhibition of ecto-ATPase (compare compounds 8 and 12, 6 and 13, 6 and 14, and 1 and 15) indicating that this functional group is beneficial for ecto-apyrase inhibition.
A comparison of pharmacological data of PPADS analogs at P2 receptors as previously determined [Kim et al., 1998, 2000; Jacobson et al., 1998] (see Table 1) showed that four compounds (3, 4, 7, and 13) exhibited selectivity for P2X nucleotide receptors. Only compound 7 weakly inhibited ecto-ATPase, while two compounds inhibited the ecto-apyrase (3, 4). Within the group of P2Xselective compounds, inhibition of ecto-apyrase only occurred for compounds that were substituted at the 4′-position in the azoaryl moiety (3, 4), while compounds with a double substitution at 2′ and 5′-positions did not inhibit ecto-apyrase (7, 13). However, para-substitution at the azoaryl moiety did not generally abolish the inhibition of ecto-apyrase. Compounds having two sulfonate groups in a para-orientation displayed weak (compound 6) or no inhibition of ecto-apyrase (compounds 7, 13, and 14), while para-substitution with a chlorine at the 2′-position (compounds 8, 9, and 10) resulted in effective ecto-apyrase inhibitors. Compound 8 inhibited ecto-apyrase without inhibiting ecto-ATPase, although this compound antagonized smooth muscle P2X receptors [Kim et al., 1998].
Compound 14, a bisphosphate derivative, was found to be the only compound in our study to significantly inhibit ecto-ATPase without inhibition of ecto-apyrase. This compound only weakly antagonized P2X1 receptors and was inactive at P2X2 and P2Y1 receptors, thus it bears some selectivity for ecto-ATPase. Compound 7, a 5-methylphosphonate derivative, is a useful probe of P2X1 receptors due to its potency at that receptor (IC50 12 nM) and inactivity at ecto-apyrase and only minor inhibition of ecto-ATPase. Compound 13, a cyclic phosphate derivative, is a weak, yet selective, P2X1 antagonist, which did not inhibit ecto-nucleotidases.
In conclusion, we have identified structural modifications of PPADS that enhance selectivity between the two ecto-nucleotidases and between ecto-nucleotidases and P2 receptors.
As reported [Kim et al., 2000], Brian F. King, Sean Brown and Geoffrey Burnstock (Royal Free Hospital, University College London) carried out measurements at recombinant P2X1 and P2X1 receptors, and José L. Boyer and Kendal Harden (University of North Carolina) at P2Y1 receptors.
Acknowledgments
Contract grant sponsor: Deutsche Forschungsgemeinschaft; Contract grant number: SFB 269, A4; Contract grant sponsor: the European Community; Contract grant number: BMH4-CT96-0676; Contract grant sponsor: the Fonds der Chemischen Industrie.
Abbreviations
- ADP
Adenosine 5′-diphosphate
- ATP
Adenosine 5′-triphosphate
- CHO
Chinese hamster ovary
- E-NTPDase
ecto-nucleoside triphosphate diphosphohydrolase
- PPADS
pyridoxal-5′-phosphate-6-azophenyl-2,4-disulfonate
- HPLC
high performance liquid chromatography
- RT-PCR
reverse transcriptase polymerase chain reaction
- UTP
Uridine 5′-triphosphate.
REFERENCES
- Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- Bültmann R, Starke K. Reactive Red 2: a P2Y-selective purinoceptor antagonist and an inhibitor of ecto-nucleotidase. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:477–482. doi: 10.1007/BF00169380. [DOI] [PubMed] [Google Scholar]
- Bültmann R, Pause B, Wittenburg H, Kurz G, Starke K. P2-purinoceptor antagonists. I. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by small aromatic isothiocyanato-sulphonates. Naunyn Schmiedebergs Arch Pharmacol. 1996a;354:481–490. doi: 10.1007/BF00168440. [DOI] [PubMed] [Google Scholar]
- Bültmann R, Wittenburg H, Pause B, Kurz G, Nickel P, Starke K. P2-purinoceptor antagonists. III. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by compounds related to suramin. Naunyn Schmiedebergs Arch Pharmacol. 1996b;354:498–504. doi: 10.1007/BF00168442. [DOI] [PubMed] [Google Scholar]
- Bültmann R, Trendelenburg M, Tuluc F, Wittenburg H, Starke K. Concomitant blockade of P2X-receptors and ecto-nucleotidases by P2-receptor antagonists: functional consequences in rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:339–344. doi: 10.1007/pl00005360. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors: a report from the IUPHAR subcommittee. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Grinthal A, Guidotti G. Substitution of His59 converts CD39 into an ADPase in a quartery structure dependent manner. Biochemistry. 2000;39:9–16. doi: 10.1021/bi991751k. [DOI] [PubMed] [Google Scholar]
- Heine P, Braun N, Heilbronn A, Zimmermann H. Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur J Biochem. 1999;262:102–107. doi: 10.1046/j.1432-1327.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Kim YC, Wildman SS, Mohanram A, Harden TK, Boyer JL, King BF, Burnstock G. A pyridoxine cyclic phosphate and its 6-azoaryl derivative selectively potentiate and antagonize activation of P2X1 receptors. J Med Chem. 1998;41:2201–2206. doi: 10.1021/jm980183o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel B, Braun N, Heine P, Maliszewski CR, Zimmermann H. An ecto-ATPase and an ecto-ATP diphosphohydrolase are expressed in rat brain. Neuropharmacology. 1997;36:1189–1200. doi: 10.1016/s0028-3908(97)00115-9. [DOI] [PubMed] [Google Scholar]
- Kim YC, Camaioni E, Ziganshin AU, Ji XD, King BF, Wildman SS, Rychkov A, Yoburn J, Kim H, Mohanram A, Harden T, Boyer JL, Burnstock G, Jacobson KA. Synthesis and structure-activity relationships of pyridoxal-6-arylazo-5′-phosphate and phosphonate derivatives as P2 receptor antagonists. Drug Dev Res. 1998;45:52–66. doi: 10.1002/(SICI)1098-2299(199810)45:2<52::AID-DDR2>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Brown SG, Harden TK, Boyer JL, Dubyak G, King BF, Burnstock G, Jacobson KA. Structure activity relationships of pyridoxal phosphate derivatives as potent and selective antagonists of P2X1 receptors. J Med Chem. 2000 doi: 10.1021/jm9904203. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht G, Friebe T, Grimm U, Windscheif U, Bungardt E, Hildebrandt C, Baumert HG, Spatzkumbel G, Mutschler E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur J Pharmacol. 1992;217:217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Stout JG, Kirley TL. Control of cell membrane ecto-ATPase by oligomerization state: intermolecular cross-linking modulates ATPase activity. Biochemistry. 1996;35:8289–8298. doi: 10.1021/bi960563g. [DOI] [PubMed] [Google Scholar]
- Tuluc F, Bültmann R, Glanzel M, Frahm AW, Starke K. P2-receptor antagonists. IV. Blockade of P2-receptor subtypes and ectonucleotidases by compounds related to Reactive Blue 2. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:111–120. doi: 10.1007/pl00005144. [DOI] [PubMed] [Google Scholar]
- Wang TF, Ou Y, Guidotti G. The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J Biol Chem. 1998;273:24814–24821. doi: 10.1074/jbc.273.38.24814. [DOI] [PubMed] [Google Scholar]
- Wittenburg H, Bültmann R, Pause B, Ganter C, Kurz G, Starke K. P2-purinoceptor antagonists. II. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by compounds related to Evans blue and trypan blue. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:491–497. doi: 10.1007/BF00168441. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular purine metabolism. Drug Dev Res. 1996;39:337–352. [Google Scholar]
- Zimmermann H. Two novel families of ecto-nucleotidases: molecular structures, catalytic properties, and a search for function. Trends Pharm Sci. 1999;20:231–236. doi: 10.1016/s0165-6147(99)01293-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Beaudoin AR, Bollen M, Goding JW, Guidotti G, Kirley TL, Robson SC, Sano K. Proposed nomenclature for two novel nucleotide hydrolyzing enzyme families expressed on the cell surface. In: Vanduffel L, Lemmens R, editors. Ecto-ATPases and related ectonucleotidases. Maastricht: Shaker Publishing; 2000. pp. 1–8. [Google Scholar]