Abstract
Introduction
Reliable cerebrospinal fluid (CSF) biomarkers enabling identification of frontotemporal dementia (FTD) and its pathologic subtypes are lacking.
Methods
Unbiased high-resolution mass spectrometry–based proteomics was applied on CSF of FTD patients with TAR DNA-binding protein 43 (TDP-43, FTD-TDP, n = 12) or tau pathology (FTD-tau, n = 8), and individuals with subjective memory complaints (SMC, n = 10). Validation was performed by applying enzyme-linked immunosorbent assay (ELISA) or enzymatic assays, when available, in a larger cohort (FTLD-TDP, n = 21, FTLD-tau, n = 10, SMC, n = 23) and in Alzheimer's disease (n = 20), dementia with Lewy bodies (DLB, n = 20), and vascular dementia (VaD, n = 18).
Results
Of 1914 identified CSF proteins, 56 proteins were differentially regulated (fold change >1.2, P < .05) between the different patient groups: either between the two pathologic subtypes (10 proteins), or between at least one of these FTD subtypes and SMC (47 proteins). We confirmed the differential expression of YKL-40 by ELISA in a partly independent cohort. Furthermore, enzyme activity of catalase was decreased in FTD subtypes compared with SMC. Further validation in a larger cohort showed that the level of YKL-40 was twofold increased in both FTD pathologic subtypes compared with SMC and that the levels in FTLD-tau were higher compared to Alzheimer's dementia (AD), DLB, and VaD patients. Clinical validation furthermore showed that the catalase enzyme activity was decreased in the FTD subtypes compared to SMC, AD and DLB.
Discussion
We identified promising CSF biomarkers for both FTD differential diagnosis and pathologic subtyping. YKL-40 and catalase enzyme activity should be validated further in similar pathology defined patient cohorts for their use for FTD diagnosis or treatment development.
Keywords: Biomarkers, Cerebrospinal fluid, Proteomics, Frontotemporal dementia, Pathology, TDP-43, Tau, Differential diagnosis
1. Introduction
Frontotemporal dementia (FTD) is the second most prevalent dementia of patients aged <65 years that clinically presents with either behavior and personality changes or language disturbance. The disease is often misdiagnosed in the early stage, either as a psychiatric disorder or as a different type of dementia such as Alzheimer's dementia (AD). The pathology is characterized by two main distinct subtypes, i.e., tau pathology accounting for roughly one half of cases and TAR DNA-binding protein 43 (TDP-43)–pathology for the other half [1], [2]. The clinical spectrum of FTD does not correlate with the distinct pathologies, except when the underlying pathology of FTD is predicted by the presence of an autosomal dominant mutation, which is found in only 20%–30% of the patients [3]. Mutations in the C9orf72 and GRN genes correspond to TDP-43 pathology, and mutations in the microtubule-associated protein tau (MAPT) to tau pathology, but an autosomal dominant family history is found in only 20%–30% of the patients. In addition, FTD with amyotrophic lateral sclerosis or motor neuron disease is almost always associated with underlying TDP-43 pathology [4]. Correct diagnosis and subtyping is very relevant to determine patient management plans and boost therapy development, especially to develop treatments targeting either tau or TDP-43 pathologic mechanisms.
Thus far, no reliable biomarker or set of biomarker with both high sensitivity and high specificity is available for FTD, let alone its pathologic subtypes. The cerebrospinal fluid (CSF) biomarkers for AD, i.e., (p)Tau and amyloid β-42 (Aβ42), appear to be of limited value for the diagnosis of clinical FTD [5], [6], although a prognostic value of tau in diagnosed FTD patients has been reported [7]. A reduced CSF P-tau-181-to-tau ratio has recently been found to identify patients with TDP-43 pathology at a sensitivity and specificity of each 82% [8], which awaits independent validation.
A good technology to identify multiple novel biomarkers in body fluids is mass spectrometry–based proteomics [9]. So far, no comprehensive discovery at the protein level has been performed as previous proteomics studies used low resolution methods for profiling of a limited set of abundant CSF peptides or proteins in clinically defined FTD patient groups [10], [11], [12], [13], [14]. A recent immunoassay-based proteomics study focused on an analysis of 151 biomarkers for different pathologic subtypes of FTD. This has yielded several proteins that discriminated FTD-TDP-43 and FTD-tau, including interleukins (IL-23 and IL-7), which combined had an 86% sensitivity and 78% specificity [15].
In the present study, we aimed to identify novel pathology-specific biomarkers for FTD by in-depth protein profiling of antemortem collected CSF of FTD patients with known underlying pathology. We applied unbiased CSF proteomics methods [16] in patients with confirmed tau or TDP-43 pathology, either by genetic testing or postmortem analysis, and controls with subjective memory complaints (SMC). We validated the findings using alternative assays in a largely independent cohort and in patients with other dementias.
2. Methods
2.1. Patients
Patients with established FTD subtypes and SMC were included from the biobanks of the Amsterdam Dementia Cohort and from the Erasmus MC. The method to define the pathology to is outlined in Table 1, which shows that the majority was based on postmortem examination. FTD pathology was reviewed according to international criteria [17]. Pathologic examination was performed according to protocolized procedures by the Dutch brain bank, including specific immunostaining for TDP-43 pathology and tau pathology. Genetic testing was performed for mutations in the MAPT and progranulin genes and for the hexanucleotide repeat at C9orf72. The discovery cohort contained 30 patients, the validation cohort 53 patients, and 17 of the 53 patients in the validation cohort overlapped with the discovery cohort, as outlined in Table 1.
Table 1.
Characteristics of included patient populations
| Characteristics | Age (y) | Sex (female/male) | MMSE score | Disease duration (y) | n | Amsterdam/Rotterdam | Method pathology definition |
|---|---|---|---|---|---|---|---|
| FTD discovery cohort | |||||||
| SMC | 60.9 (15.7) | 5/5 | 10 | 5/5 | |||
| TDP-43-FTD | 60.9 (5.7) | 5/7 | 12 | 3/9 | PGRN mutation (n = 2)/pathology confirmed (n = 10) | ||
| Tau-FTD | 53.4 (6.7)∗ | 3/5 | 8 | 2/6 | MAPT mutation (n = 7)/Pathology confirmed (n = 1) | ||
| FTD validation cohort | |||||||
| SMC | 60.7 (10.1) | 12/9 | 27.3 (2.9) | 23 | 20/3 | ||
| TDP-43-FTD | 60.5 (5.8) | 10/12 | 26.2 (3.1) | 3.3 (2.5) (n = 18) | 20 | 12/8 | PGRN mutation (n = 2)/C9orf72 hexanucleotide repeat (n = 2)/FTD-MND (n = 2)/pathology confirmed (n = 14) |
| Tau-FTD | 55.2 (13.6)∗ | 6/3 | NA | 2.9 (1.0) (n = 8) | 10 | 4/6 | MAPT (n = 6)/pathology confirmed (n = 4) |
| Overlap discovery and validation cohort | |||||||
| SMC | 60.1 (19.2) | 5/2 | 7 | 5/2 | |||
| FTD-TDP-43 | 59.9 (4.7) | 2/5 | 7 | 4/3 | GRN (n = 2)/pathology confirmed (n = 5) | ||
| FTD-Tau | 51.5 (6.3) | 1/2 | 3 | 0/3 | MAPT (n = 3) | ||
| Dementia validation cohort | |||||||
| Alzheimer's disease (AD) | 63.7 (6.9) | 8/12 | 19.7 (6.9) | 20 | |||
| Dementia with Lewy bodies (DLB) | 62.9 (5.1) | 3/17 | 23.2 (4.8) | 20 | |||
| Vascular Dementia (VaD) | 63.6 (5.4) | 3/15 | 22.3 (4.8) | 18 | |||
Abbreviations: MMSE, mini-mental state examination; FTD, frontotemporal dementia; TDP-43, TAR DNA-binding protein 43; SMC, subjective memory complaints; MAPT, microtubule-associated protein tau; NA, not available; SD, standard deviation.
NOTE. Mean values (SD) are given.
Significantly different between Tau-FTD and the other two groups (P < .05).
All subjects underwent extensive dementia screening at baseline, including physical and neurologic examination, mini-mental state examination (MMSE), neuropsychological investigation, electroencephalogram, magnetic resonance imaging, and laboratory tests, including lumbar puncture. Dementia diagnoses were made by consensus in a multidisciplinary meeting according to standard criteria [18], [19]. Probable AD was diagnosed according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders association [20], and all patients met the core clinical National Institute on Aging-Alzheimer's Association criteria [21]. Definite FTD was diagnosed using the criteria of Rascovsky et al. [2]. Control groups of subjects with SMC consisted of individuals who presented with cognitive complaints, but cognitive and laboratory investigations, were normal and criteria for mild cognitive impairment, dementia, or any other neurologic or psychiatric disorders known to cause cognitive complaints were not met. CSF biomarkers abeta (1–42), tTau, and pTau were not used for the clinical diagnosis of any of the patients. Groups were matched for age and gender. Patient characteristics of the discovery and validation cohorts are presented in Table 1.
The study was performed according to the ethical principles of the Declaration of Helsinki and was approved by the local ethics committee. We obtained written informed consent from all subjects participating in the study.
2.2. CSF biobanking and proteomics analysis
CSF and blood were collected and stored at −80°C in polypropylene tubes (Sarstedt, Nümbrecht, Germany) after centrifugation within 1 hour after withdrawal according to international biobanking consensus guidelines optimized for CSF proteomics [22]. CSF Aβ42, total tau, and p-tau were measured with commercially available enzyme-linked immunosorbent assays (ELISAs; INNOTEST Fujirebio, Ghent, Belgium) on a routine basis as described previously [23].
Proteomics analysis was essentially performed as described previously [16] and details are presented in Supplementary File 1. Because this is a typical proteomics discovery study, where the candidates are to be validated, we did not apply a multiple testing correction and applied a P value of .05.
2.3. Independent assay validation
The following assays were used: human adipocyte fatty acid binding protein (FABP4) ELISA kit was obtained from BioVendor (Karasek, Czech Republic). The MicroVue YKL-40 enzyme immunoassay kit was from Quidel Corporation (San Diego, USA). Complement factor D (CFD) Quantikine ELISA and interleukin 1 receptor accessory protein (IL1RAP) DuoSet ELISA were from R&D Systems (Abingdon, UK). Human apolipoprotein L1 (APOL1) ELISA was from Proteintech (Manchester, UK). Catalase enzyme activity was measured using an EnzyChrom Assay from BioAssay Systems (Hayward, CA, USA). Total β-hexosaminidase (HexA) activity was measured using 3-mM 4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-glucopyranoside (4MUGlcNAc) as a substrate in 0.1-M citric acid/0.2-M disodium phosphate buffer. β-HexA was measured using 3-mM 4-Methylumbelliferyl 6-Sulfo-2-acetamido-2-deoxy-β-D-glucopyranoside in 0.1 M citric acid/0.2 M disodium phosphate buffer. α-Galactosidase A (GLA) was measured using 4.5-mM 4-methylumbelliferyl-α-D-galactoside in 0.2-M Na/acetate buffer.
Candidate biomarkers for validation were selected based on the following criteria: (1) fold change >1.2 and P value <.05; (2) mean spectral count in all patients in one of the patients groups >2; and (3) number of identified peptide sequences covering at least 20% of the protein. We searched for availability of ELISAs and selected those with a detailed validation report (i.e., recovery, linearity, and coefficients of variation (CV) presented of individual samples). We briefly tested the analytical performance of all assays for analysis of CSF as described in Supplementary File 1. We, furthermore, performed extensive validation of the FABP4 and YKL-40 assays according to the immunoassay validation SOP developed by the BIOMARKAPD project [24], the results of which are also presented in Supplementary File 1. Precision data of all assays are presented in Table 2.
Table 2.
Assay precision of all assays included in the validation
| Marker | Intra-assay CV (%) | Stdev | Inter-assay CV (%) | Stdev |
|---|---|---|---|---|
| APOL1 | 2.55 | 0.219 | 1.6–5.1 | 2.5 |
| Catalase | 10.33 | 0.17 | 34 | 0.1 |
| FABP4 | 1.9–3.0 | 0.5 | 5.7–8.8 | 1.8 |
| CFD | 3.2 | 2.1 | 4.9 | 2.8 |
| IL-RAcP | 3.82 | 3.99 | 4.14 | 0.017 |
| YKL-40 | 3.1–3.7 | 0.3 | 3.1–10.9 | 4.4 |
| β-Hexosaminidase-A | 3.24 | 0.73 | 3.15 | 0.70 |
| α-Galactosidase A | 5.68 | 2.43 | 5.73 | 1.41 |
Abbreviations: CV, coefficients of variation; FABP4, fatty acid binding protein; CFD, Complement factor D; Stdev, standard deviation.
2.4. Statistics
Statistics were performed in SPSS (version 20). None of the validation results had a normal distribution, as tested by the Shapiro-Wilk test. Differences in mean values between clinical groups obtained by ELISA were analyzed with one-way analysis of variance on the ranked values, correcting for age as indicated in the results. Spearman correlation analyses were performed to study correlations between markers or demographics. A P value <.05 was considered significant.
3. Results
3.1. Identification of discriminatory CSF biomarkers and functions
To facilitate in-depth coverage of the CSF proteome, CSF samples were subjected to high-abundant protein depletion followed by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) fractionation, in-gel tryptic digestion, and nanoLC-MS/MS analysis. In total, 1914 proteins were identified in CSF (Supplementary Table 1). The beta binomial test for comparison of the protein spectral counts in the three patient groups yielded 56 differentially regulated proteins (Table 3, Table 4, Table 5, Table 6, Table 7). Supplementary Fig. 1 shows a heat map of the relative concentrations of these proteins in each individual patient sample.
Table 3.
Proteins that were differentially regulated between TDP-43-FTD and Tau-FTD
| Gene name | tau versus SMC, fold change | P value | TDP-43 versus SMC fold change | P value | Tau versus TDP-43, fold change | P value |
|---|---|---|---|---|---|---|
| KLK7 | −2.064 | .029 | ||||
| F13A1 | −2.003 | .026 | ||||
| HSPA8 | −1.634 | .036 | ||||
| FSCN1 | −1.491 | .032 | ||||
| CLIC4 | −1.481 | .030 | ||||
| ABI3BP | −1.268 | .044 | ||||
| MOG | 1.295 | .042 | ||||
| HEXA | 1.309 | .009 | ||||
| CHID1 | 1.553 | .035 | ||||
| UBL3 | 2.168 | .045 |
Abbreviations: TDP-43, TAR DNA-binding protein 43; FTD, frontotemporal dementia; SMC, subjective memory complaints.
Table 4.
Proteins that were differentially regulated between FTD-tau and both FTD-TDP-43 and SMC
| Gene name | SMC versus tau, fold change | P value | SMC versus TDP-43, fold change | P value | TDP-43 versus tau, fold change | P value |
|---|---|---|---|---|---|---|
| MYOC | −1.817 | .011 | −2.054 | .016 | ||
| SHBG | −1.420 | .026 | −1.527 | .008 | ||
| FCGBP | −1.335 | .002 | −1.427 | .049 | ||
| IGFALS | 1.267 | .005 | 1.299 | .011 | ||
| NDRG4 | 3.459 | .000 | 2.729 | .000 | ||
| APOL1 | 5.127 | .001 | 2.595 | .009 |
Abbreviations: FTD, frontotemporal dementia; SMC, subjective memory complaints; TDP-43, TAR DNA-binding protein 43.
Table 5.
Proteins that were differentially regulated between Tau-FTD and SMC
| Gene name | SMC versus tau, fold change | P value | SMC versus TDP-43, fold change | P value | TDP-43 versus tau, fold change | P value |
|---|---|---|---|---|---|---|
| IL1RAP | −2.533 | .034 | ||||
| OLFML1 | −2.013 | .023 | ||||
| ISLR2 | −1.969 | .014 | ||||
| CNTNAP3 | −1.850 | .039 | ||||
| TENM2 | −1.655 | .012 | ||||
| GALNS | −1.462 | .041 | ||||
| F5 | −1.424 | .037 | ||||
| QDPR | −1.368 | .028 | ||||
| PCMT1 | −1.307 | .049 | ||||
| CTSH | −1.307 | .018 | ||||
| OLFML3 | −1.277 | .027 | ||||
| NOV | −1.266 | .011 | ||||
| LCAT | 1.347 | .032 | ||||
| FSTL5 | 1.406 | .045 | ||||
| CDH15 | 1.519 | .019 | ||||
| CASP14 | 1.660 | .043 | ||||
| ACAN | 1.671 | .026 | ||||
| FABP4 | 1.710 | .047 | ||||
| FAH | 1.731 | .018 | ||||
| VCL | 1.856 | .036 | ||||
| LRP8 | 1.901 | .013 | ||||
| MAT2A | 2.471 | .022 | ||||
| CA1 | 3.283 | .036 | ||||
| CAT | 4.473 | .031 | ||||
| S100A7 | 4.591 | .007 |
Abbreviations: FTD, frontotemporal dementia; SMC, subjective memory complaint; TDP-43, TAR DNA-binding protein 43; FABP4, fatty acid binding protein.
Table 6.
Proteins that were differentially regulated between TDP-43-FTD and SMC
| Gene name | SMC versus tau, fold change | P value | SMC versus TDP-43, fold change | P value | TDP-43 versus tau, fold change | P value |
|---|---|---|---|---|---|---|
| GLA | −5.512 | .026 | ||||
| LAMTOR2 | −2.547 | .035 | ||||
| MPZ | −1.813 | .043 | ||||
| TMEM132B | −1.792 | .017 | ||||
| IMPA1 | −1.480 | .024 | ||||
| DLD | −1.469 | .045 | ||||
| GDA | −1.205 | .034 | ||||
| CPVL | −1.351 | .013 | ||||
| CTSL1 | 1.215 | .014 | ||||
| ST6GAL2 | 1.611 | .042 |
Abbreviations: FTD, frontotemporal dementia; SMC, subjective memory complaints; TDP-43, TAR DNA-binding protein 43.
Table 7.
Proteins that were differentially regulated between FTD and SMC (no specific pathologic subtype)
| Gene name | SMC versus tau, fold change | P value | SMC versus TDP-43, fold change | P value | TDP-43 versus tau, fold change | P value |
|---|---|---|---|---|---|---|
| SPTBN5 | −2.482 | .040 | −2.947 | .045 | ||
| RAD23B | −1.901 | .016 | −1.539 | .044 | ||
| AP2B1 | −1.371 | .031 | −1.306 | .039 | ||
| CFD | −1.284 | .033 | −1.223 | .037 | ||
| YKL-40 | 1.243 | .020 | 1.276 | .009 |
Abbreviations: FTD, frontotemporal dementia; SMC, subjective memory complaints; TDP-43, TAR DNA-binding protein 43.
3.1.1. FTD-tau versus FTD-TDP-43
Ten proteins were differentially regulated between FTD-tau and FTD-TDP-43 patients (Table 3). The largest negative fold change was observed for KLK7 and F13A1 (<−2.0 fold), and the largest positive fold change was observed for UBL3 (>2.1-fold increase in FTD-TDP-43 compared with FTD-tau).
3.1.2. FTD-tau versus both SMC and TDP-43
In total, six proteins were differentially regulated between FTD-tau patients compared with both SMC and FTD-TDP-43 patients (Table 4). The proteins APOL1 and N-Myc downstream regulated gene (NDRG)4 had the highest positive fold change (>3.5 higher spectral counts in FTD-tau compared with both SMC and TDP-43).
3.1.3. FTD-tau versus SMC
In total, 25 proteins were differentially regulated between FTD-tau patients compared with SMC only (Table 5). The proteins IL1RAP, OLFML1 had the highest negative fold change (<−2.0 times decreased in FTD-tau compared with SMC). The proteins MAT2A, CA1, CAT, and S100A7 had the highest positive fold change (>2.4-fold increase in FTD-tau compared with SMC).
3.1.4. FTD-TDP-43 versus SMC
In total, 10 CSF proteins were differentially regulated (P < .05) between SMC and FTD-TDP-43 patients (Table 6). Of these, GLA and LAMTOR had the largest negative fold change (<−2.5-fold decrease in FTD-TDP-43 compared with SMC).
3.1.5. Both FTD-tau and FTD-TDP-43 versus SMC
In total, five proteins were differentially regulated between SMC and FTD-tau or FTD-TDP-43 patients (Table 6). Among these, SPTBN5 had the largest negative fold change (>2.5-fold decrease in both FTD subtypes compared with SMC).
3.2. Validation by independent assays
Validation was performed in a small subset of biomarker candidates for which independent assays were available commercially or via collaboration as specified in the method section.
Three of six tested ELISA assays met our validation criteria for reliable detection in CSF. These were FABP4, YKL-40, complement factor D, IL1RAP, and APOL1. No assays to analyze the concentration were available, but enzyme activity assays were available for catalase, total HexA, β-HexA, and GLA.
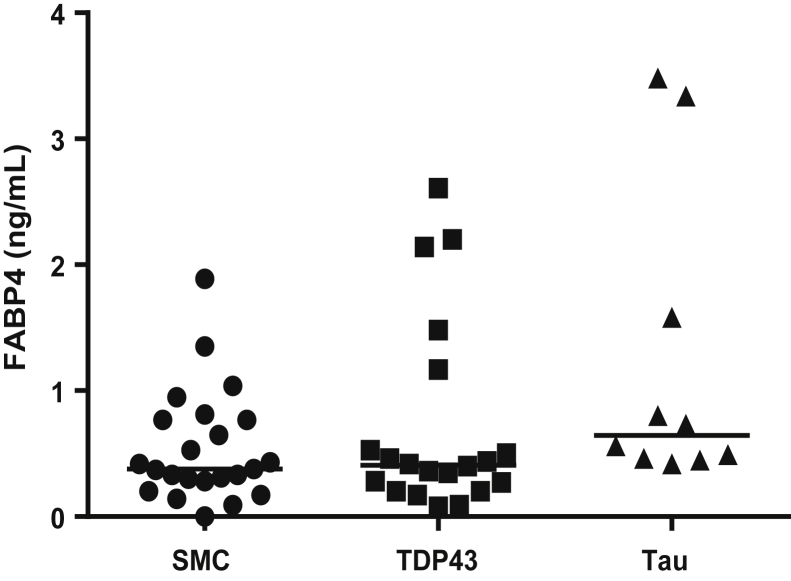
The results in Fig. 1 show that the levels of FABP4 were comparable between the FTD-patient groups and SMC.
Fig. 1.
FABP4 levels in CSF of different patient groups as measured by ELISA. Abbreviations: FABP4, fatty acid binding protein; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; SMC, subjective memory complaints; TDP-43, TAR DNA-binding protein 43.
The levels of the YKL-40 were twofold increased in both FTD pathologic subtypes compared with SMC (Fig. 2), which confirmed the increase in FTD-TDP-43 and FTD-tau compared with SMC of the discovery results.
Fig. 2.
YKL-40 levels in CSF of different patient groups as measured by ELISA. ***P < .001. Abbreviations: CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; SMC, subjective memory complaint; TDP-43, TAR DNA-binding protein 43; AD, Alzheimer's dementia; DLB, dementia with Lewy bodies; VaD, vascular dementia.
The complement factor D levels were comparable between the FTD patient groups and SMC (Fig. 3).
Fig. 3.
Complement factor D levels in CSF of different patient groups as measured by ELISA. Abbreviations: CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; SMC, subjective memory complaints; TDP-43, TAR DNA-binding protein 43.
The catalase enzyme activities were decreased in both the FTD-TDP-43 and FTD-tau groups compared with the controls (Fig. 4), which is a reverse relation compared to the increase observed in the protein concentration by proteomics.
Fig. 4.
Catalase enzyme activity in CSF of different patient groups. *P < .05, **P < .01, ***P < .001. Abbreviations: CSF, cerebrospinal fluid; SMC, subjective memory complaints; TDP-43, TAR DNA-binding protein 43; AD, Alzheimer's dementia; DLB, dementia with Lewy bodies; VaD, vascular dementia.
The total HexA and GLA activity was not significantly changed in any of the groups (data now shown).
3.3. Correlations with demographics and clinical characteristics and between validated biomarkers
CSF YKL-40 was positively correlated with age. We, therefore, included age as covariate in further analyses of this marker. CSF YKL-40 was positively correlated with t-tau and negatively to catalase enzyme activity levels (data not shown, Table 8). There were no correlations with MMSE or difference in levels between females and males.
Table 8.
Correlation between CSF biomarkers and demographics in all groups∗
| Demographics | CSF biomarker |
|
|---|---|---|
| YKL-40 | Catalase | |
| Age | 0.300** | 0.093 |
| Disease duration∗ | −0.133 | −0.222 |
| Abeta (1–42) | −0.100 | −0.058 |
| t-Tau | 0.397*** | 0.044 |
| p-Tau | 0.197* | 0.084 |
| YKL-40 | x | −0.240* |
Abbreviation: CSF, cerebrospinal fluid.
NOTE. Bivariate Spearman correlation coefficients were calculated (n = 88–101). *P < .05; **P < .01; ***P < .001.
n = 26 (YKL-40) and 19 (catalase).
3.4. Validation in other dementia types
We next continued validation of the proteins for which a differential expression was confirmed, i.e., YKL-40 and catalase, by analysis of the levels in other common dementia subtypes, AD, DLB, and vascular dementia (VaD).
The levels of YKL-40 (Fig. 2) were higher in FTD-tau compared with AD (P < .001), dementia with Lewy bodies (DLB, P < .001), and VaD (P = .001).
The levels of catalase (Fig. 4) were lower in FTD-tau or FTD-TDP-43 compared with AD (P < .001), DLB (P < .05), and in the FTD-tau group compared with VaD (P = .05).
3.5. Serum analysis
We analyzed YKL-40 concentrations in paired serum of the FTD patients available in the Amsterdam cohort (12 FTD-TDP-43, 4 FTD-Tau, and 18 SMC). There was no correlation between serum and CSF levels of YKL-40 and no differences in serum YKL-40 levels between the patient groups (data not shown).
4. Discussion
In this study, we identified 56 candidate biomarkers that were differentially regulated between the pathologic subtypes of FTD or between FTD and SMC, in CSF of homogeneous patient groups. The result could be confirmed for one of five biomarkers for which a robust ELISA was available, i.e., YKL-40. Moreover, enzyme activity of one of three tested proteins (catalase) was confirmed to be differentially regulated between the FTD subtypes and controls.
The strongest increase in the proteomics results (fold change >3) was observed for S100A7, GLA, APOL1, NDRG4, CAT, and CA1 (Table 3, Table 4, Table 5, Table 6, Table 7). Each of these specific proteins have not yet been related to FTD, and we here provide some more background information on these proteins.
S100A7, also called psoriasin, is increased in several cancers and has multiple functions, including inflammatory roles [25].
GLA and APOL1 are lysosomal proteins. They have not yet been related to FTD pathology but can be very promising as they confirm the previously revealed important role of the autophagy/lysosome system in the etiology of FTD. For instance, the GRN and CHMP2B genes that are directly related to FTD are associated with the autophagy/lysosomal pathway [26], [27]. Similarly, the discovered genetic risk factors for FTD, TMEM106b [28], [29], RAB38, and CTCS [30] are all associated with lysosomal pathways. These data support further research into the role of the identified CSF proteins in the FTD pathology.
Catalase and CA1 are relevant proteins for oxidative stress, but it remains to be determined if the increase is real and not an artifact, as a recent proteomics study suggested these two, besides hemoglobin and peroxiredoxin, as suitable biomarkers for blood contaminated CSF [31].
NDRG4 levels were increased specifically in the tau group. The NDRG4 protein expression is upregulated in aggressive meningioma [32]. In contrast, the levels were decreased in glioblastoma or colorectal cancer [33]. NDRG4 is specifically expressed in the brain and heart and plays a possible role in neuronal differentiation. Interestingly, the NDRG4 messenger RNA expression was decreased in Alzheimer brain tissue [34].
We validated a subset of biomarkers by immunoassays as independent methods. YKL-40 has already been proven to have a role in dementia, i.e., in Alzheimer's dementia [35], [36], and also in early stages of multiple sclerosis [37]. Our data expand findings in a recent report showing increased levels in clinically defined FTD patients and a positive correlation with CSF t-tau [38]. The 1.2-fold increase in CSF levels in AD is of similar magnitude as earlier reports, and lack of significant difference in our cohort is likely due to the smaller number of AD patients included in our study [39]. The elevation observed in other diseases and the correlation with t-tau, albeit moderate, indicate that YKL-40 cannot function as a single pathology-specific biomarker and is probably a sensitive biomarker for astrogliosis [37]. Interestingly, neuroinflammation is one of the explanatory mechanisms through which the progranulin haploinsufficiency can cause FTD [40]. Further studies should define the role of neuroinflammation and, in particular, astrogliosis, in FTD in more depth [40], [41], [42], [43].
Lumbar puncture may be perceived as inconvenient but gets common practice in the dementia field and risk complications as severe headache of 0.9%, typical postlumbar puncture headache of 9%, and back pain of 17% is generally accepted in this population [44]. Unfortunately, blood levels of YKL-40 were similar in FTD groups and SMC. This was, however, not surprising, as blood levels of these biomarkers have been related to inflammation in several other diseases, including cardiovascular disease and diabetes, which could mask brain-related alterations [45], [46], [47].
The discovery results on catalase were validated by an activity assay, due to the lack of assays to analyze the concentrations. It remains to be determined if the activity of this enzyme correlates to its concentrations, but the decreased activity observed in FTD is at least encouraging to perform future validation studies, including studies on the effects of blood contamination on the activity.
A major strength of the study was the study design, i.e., the start with pathologically homogeneous patient groups that were defined either by postmortem evaluation or genetic subtyping and the use of an unbiased, well-validated method for CSF proteomics [48]. Because clinical FTD is heterogeneous, which does not correlate strongly to its pathologic subtypes [49], cohorts with known pathologic subtypes are very relevant to increase knowledge on pathways, reflected in biomarkers changes, and therapy development. Another strength was the stringent screening of ELISA assays as independent methods and validation in independent cohort including other dementia types. The validation was successful for 20% of the biomarkers for which a CSF-validated assay was available, which may forecast a similar success rate for the remainder-identified proteins. The commercial availability and shown quality of these assays have important implications. First, these can be easily applied for independent replication of our study by other research groups, and second, implementation in clinical practice will be feasible.
A weakness of this study is that we did not further differentiate the pathologic subtypes, as the TDP-43 can be subdivided into at least four distinct pathologic subtypes, depending on the cellular location of the TDP-43 inclusions (e.g., cytoplasmic or axonal) or stage of the pathology [1], [50]. Further subtyping would have led to lower number of patients per subgroup, due to limited availability. Nevertheless, the here identified biomarkers can be further studied in relation to these different pathologies.
So far, there has been a lack of differential diagnostic biomarkers of the pathologic subtypes of FTD. An interesting recent report showed that a reduced ratio (<0.37) of p-tau to t-tau could be a biomarker to identify FTD-TDP-43 from FTD-tau, AD, and healthy seniors with 82% sensitivity and 82% specificity in cohorts of similar size as ours [8]. An imaging study showed that white matter volume could discriminate between these pathologic FTD subtypes [51]. Thus, studies focusing on pathologic subtypes of FTD are just emerging, and it will be interesting to evaluate the additional value combining different CSF biomarkers and different biomarker modalities for accurate diagnosis.
Taken together, YKL-40 should be studied further for its use in diagnosis of FTD, as it discriminated both FTD subtypes from SMC. Our study presented promising new candidate biomarkers for a correct and differential diagnosis of the FTD subtypes, which is important for inclusion of specific patients for either TDP-43 or tau-targeted treatments.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and citations within articles. Key words were frontotemporal dementia (FTD) and biomarkers and cerebrospinal fluid (CSF).
-
2.
Interpretation: Our findings led to the discovery and validation of several novel CSF biomarkers for the different pathologic subtypes of FTD. Validation of inflammatory biomarkers underscores the involvement of these pathways in FTD.
-
3.
Future directions: Future studies should aim at (1) independent multicenter validation of the biomarkers; (2) establish the pathways role of these identified key biomarkers for lysosomal and inflammatory pathways in FTD pathology; and (3) validation of biomarkers for which methods are to be developed.
Acknowledgments
The Association for Frontotemporal Degeneration and Alzheimer's Drug Discovery Foundation, and the ZonMw Memorabel program project “PRODIA,” as part of the Deltaplan Dementie, are acknowledged for their grant support of this study.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2015.12.004.
Supplementary data
References
- 1.Irwin D.J., Trojanowski J.Q., Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer's disease. Front Aging Neurosci. 2013;5:6. doi: 10.3389/fnagi.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seelaar H., Rohrer J.D., Pijnenburg Y.A., Fox N.C., van Swieten J.C. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: A review. J Neurol Neurosurg Psychiatry. 2011;82:476–486. doi: 10.1136/jnnp.2010.212225. [DOI] [PubMed] [Google Scholar]
- 4.Ng A.S., Rademakers R., Miller B.L. Frontotemporal dementia: A bridge between dementia and neuromuscular disease. Ann N Y Acad Sci. 2015;1338:71–93. doi: 10.1111/nyas.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pijnenburg Y.A., Janssen J.C., Schoonenboom N.S., Petzold A., Mulder C., Stigbrand T. CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer's disease and controls. Dement Geriatr Cogn Disord. 2007;23:225–230. doi: 10.1159/000099473. [DOI] [PubMed] [Google Scholar]
- 6.Schoonenboom N.S., Reesink F.E., Verwey N.A., Kester M.I., Teunissen C.E., van de Ven P.M. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 7.Borroni B., Benussi A., Cosseddu M., Archetti S., Padovani A. Cerebrospinal fluid tau levels predict prognosis in non-inherited frontotemporal dementia. Neurodegener Dis. 2014;13:224–229. doi: 10.1159/000353280. [DOI] [PubMed] [Google Scholar]
- 8.Hu W.T., Watts K., Grossman M., Glass J., Lah J.J., Hales C. Reduced CSF p-Tau181 to tau ratio is a biomarker for FTLD-TDP. Neurology. 2013;81:1945–1952. doi: 10.1212/01.wnl.0000436625.63650.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham T.V., Piersma S.R., Oudgenoeg G., Jimenez C.R. Label-free mass spectrometry-based proteomics for biomarker discovery and validation. Expert Rev Mol Diagn. 2012;12:343–359. doi: 10.1586/erm.12.31. [DOI] [PubMed] [Google Scholar]
- 10.Davidsson P., Sjogren M., Andreasen N., Lindbjer M., Nilsson C.L., Westman-Brinkmalm A. Studies of the pathophysiological mechanisms in frontotemporal dementia by proteome analysis of CSF proteins. Brain Res Mol Brain Res. 2002;109:128–133. doi: 10.1016/s0169-328x(02)00549-1. [DOI] [PubMed] [Google Scholar]
- 11.Mattsson N., Ruetschi U., Pijnenburg Y.A., Blankenstein M.A., Podust V.N., Li S. Novel cerebrospinal fluid biomarkers of axonal degeneration in frontotemporal dementia. Mol Med Rep. 2008;1:757–761. doi: 10.3892/mmr_00000025. [DOI] [PubMed] [Google Scholar]
- 12.Ruetschi U., Zetterberg H., Podust V.N., Gottfries J., Li S., Hviid S.A. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol. 2005;196:273–281. doi: 10.1016/j.expneurol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Schweitzer K., Decker E., Zhu L., Miller R.E., Mirra S.S., Spina S. Aberrantly regulated proteins in frontotemporal dementia. Biochem Biophys Res Commun. 2006;348:465–472. doi: 10.1016/j.bbrc.2006.07.113. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen A.H., McGuire J., Podust V.N., Hagnelius N.O., Nilsson T.K., Kapaki E. A novel panel of cerebrospinal fluid biomarkers for the differential diagnosis of Alzheimer's disease versus normal aging and frontotemporal dementia. Dement Geriatr Cogn Disord. 2007;24:434–440. doi: 10.1159/000110576. [DOI] [PubMed] [Google Scholar]
- 15.Hu W.T., Chen-Plotkin A., Grossman M., Arnold S.E., Clark C.M., Shaw L.M. Novel CSF biomarkers for frontotemporal lobar degenerations. Neurology. 2010;75:2079–2086. doi: 10.1212/WNL.0b013e318200d78d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fratantoni S.A., Piersma S.R., Jimenez C.R. Comparison of the performance of two affinity depletion spin filters for quantitative proteomics of CSF: Evaluation of sensitivity and reproducibility of CSF analysis using GeLC-MS/MS and spectral counting. Proteomics Clin Appl. 2010;4:613–617. doi: 10.1002/prca.200900179. [DOI] [PubMed] [Google Scholar]
- 17.Cairns N.J., Bigio E.H., Mackenzie I.R., Neumann M., Lee V.M., Hatanpaa K.J. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 19.McKeith I.G., Dickson D.W., Lowe J., Emre M., O'Brien J.T., Feldman H. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teunissen C.E., Petzold A., Bennett J.L., Berven F.S., Brundin L., Comabella M. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914–1922. doi: 10.1212/WNL.0b013e3181c47cc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulder C., Verwey N.A., van der Flier W.M., Bouwman F.H., Kok A., Van Elk E.J. Amyloid-beta(1-42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem. 2010;56:248–253. doi: 10.1373/clinchem.2009.130518. [DOI] [PubMed] [Google Scholar]
- 24.Andreasson U., Perret-Liaudet A., van Waalwijk van Doorn L.J., Blennow K., Chiasserini D., Engelborghs S. A practical guide to immunoassay method validation. Front Neurol. 2015;6:179. doi: 10.3389/fneur.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia J., Duan Q., Guo J., Zheng Y. Psoriasin, a multifunctional player in different diseases. Curr Protein Pept Sci. 2014;15:836–842. doi: 10.2174/138920371508141128152712. [DOI] [PubMed] [Google Scholar]
- 26.Smith K.R., Damiano J., Franceschetti S., Carpenter S., Canafoglia L., Morbin M. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urwin H., Authier A., Nielsen J.E., Metcalf D., Powell C., Froud K. Disruption of endocytic trafficking in frontotemporal dementia with CHMP2B mutations. Hum Mol Genet. 2010;19:2228–2238. doi: 10.1093/hmg/ddq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady O.A., Zheng Y., Murphy K., Huang M., Hu F. The frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function. Hum Mol Genet. 2013;22:685–695. doi: 10.1093/hmg/dds475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Deerlin V.M., Sleiman P.M., Martinez-Lage M., Chen-Plotkin A., Wang L.S., Graff-Radford N.R. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari R., Hernandez D.G., Nalls M.A., Rohrer J.D., Ramasamy A., Kwok J.B. Frontotemporal dementia and its subtypes: A genome-wide association study. Lancet Neurol. 2014;13:686–699. doi: 10.1016/S1474-4422(14)70065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You J.S., Gelfanova V., Knierman M.D., Witzmann F.A., Wang M., Hale J.E. The impact of blood contamination on the proteome of cerebrospinal fluid. Proteomics. 2005;5:290–296. doi: 10.1002/pmic.200400889. [DOI] [PubMed] [Google Scholar]
- 32.Kotipatruni R.P., Ren X., Thotala D., Jaboin J.J. NDRG4 is a novel oncogenic protein and p53 associated regulator of apoptosis in malignant meningioma cells. Oncotarget. 2015;6:17594–17604. doi: 10.18632/oncotarget.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S., Yang B., Li G., He S., Li Y. Downregulation of N-Myc downstream-regulated gene 4 influences patient survival in gliomas. Brain Tumor Pathol. 2013;30:8–14. doi: 10.1007/s10014-012-0092-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhou R.H., Kokame K., Tsukamoto Y., Yutani C., Kato H., Miyata T. Characterization of the human NDRG gene family: A newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001;73:86–97. doi: 10.1006/geno.2000.6496. [DOI] [PubMed] [Google Scholar]
- 35.Craig-Schapiro R., Perrin R.J., Roe C.M., Xiong C., Carter D., Cairns N.J. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsson B., Hertze J., Lautner R., Zetterberg H., Nagga K., Hoglund K. Microglial markers are elevated in the prodromal phase of Alzheimer's disease and vascular dementia. J Alzheimers Dis. 2013;33:45–53. doi: 10.3233/JAD-2012-120787. [DOI] [PubMed] [Google Scholar]
- 37.Comabella M., Fernandez M., Martin R., Rivera-Vallve S., Borras E., Chiva C. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain. 2010;133:1082–1093. doi: 10.1093/brain/awq035. [DOI] [PubMed] [Google Scholar]
- 38.Alcolea D., Carmona-Iragui M., Suarez-Calvet M., Sanchez-Saudinos M.B., Sala I., Anton-Aguirre S. Relationship between β-Secretase, inflammation and core cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis. 2014;42:157–167. doi: 10.3233/JAD-140240. [DOI] [PubMed] [Google Scholar]
- 39.Alcolea D., Vilaplana E., Pegueroles J., Montal V., Sanchez-Juan P., Gonzalez-Suarez A. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer's disease. Neurobiol Aging. 2015;36:2018–2023. doi: 10.1016/j.neurobiolaging.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Galimberti D., Scarpini E. Genetics and biology of Alzheimer's disease and frontotemporal lobar degeneration. Int J Clin Exp Med. 2010;3:129–143. [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold S.E., Han L.Y., Clark C.M., Grossman M., Trojanowski J.Q. Quantitative neurohistological features of frontotemporal degeneration. Neurobiol Aging. 2000;21:913–919. doi: 10.1016/s0197-4580(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 42.Rentzos M., Paraskevas G.P., Kapaki E., Nikolaou C., Zoga M., Rombos A. Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer's disease and frontotemporal dementia. J Neurol Sci. 2006;249:110–114. doi: 10.1016/j.jns.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 43.Sjogren M., Folkesson S., Blennow K., Tarkowski E. Increased intrathecal inflammatory activity in frontotemporal dementia: Pathophysiological implications. J Neurol Neurosurg Psychiatry. 2004;75:1107–1111. doi: 10.1136/jnnp.2003.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duits F.H., Martinez-Lage P., Paquet C., Engelborghs S., Lleo A., Hausner L. Performance and complications of lumbar puncture in memory clinics: Results of the multicenter lumbar puncture feasibility study. Alzheimers Dement. 2016;12:154–163. doi: 10.1016/j.jalz.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Rathcke C.N., Vestergaard H. YKL-40—An emerging biomarker in cardiovascular disease and diabetes. Cardiovasc Diabetol. 2009;8:61. doi: 10.1186/1475-2840-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thumser A.E., Moore J.B., Plant N.J. Fatty acid binding proteins: Tissue-specific functions in health and disease. Curr Opin Clin Nutr Metab Care. 2014;17:124–129. doi: 10.1097/MCO.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 47.Djousse L., Gaziano J.M. Plasma levels of FABP4, but not FABP3, are associated with increased risk of diabetes. Lipids. 2012;47:757–762. doi: 10.1007/s11745-012-3689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piersma S.R., Fiedler U., Span S., Lingnau A., Pham T.V., Hoffmann S. Workflow comparison for label-free, quantitative secretome proteomics for cancer biomarker discovery: Method evaluation, differential analysis, and verification in serum. J Proteome Res. 2010;9:1913–1922. doi: 10.1021/pr901072h. [DOI] [PubMed] [Google Scholar]
- 49.Josephs K.A., Hodges J.R., Snowden J.S., Mackenzie I.R., Neumann M., Mann D.M. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122:137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackenzie I.R., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMillan C.T., Irwin D.J., Avants B.B., Powers J., Cook P.A., Toledo J.B. White matter imaging helps dissociate tau from TDP-43 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2013;84:949–955. doi: 10.1136/jnnp-2012-304418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.