Summary
A high percentage of transgenic mice developing from eggs microinjected with plasmids containing the SV40 early region genes and a metallothionein fusion gene develop tumors within the choroid plexus. A line of mice has been established in which nearly every affected animal succumbs to this brain tumor. Thymic hypertrophy and kidney pathology are also observed in some mice. SV40 T-antigen mRNA and protein are readily detected in affected tissues; however, SV40 T-antigen gene expression is barely detectable in unaffected tissues or in susceptible tissues prior to overt pathology, suggesting that tumorigenesis depends upon activation of the SV40 genes. Comparison of DNA from tumor tissue (or cell lines derived from tumors) with DNA from unaffected tissues reveals structural rearrangements as well as changes in DNA methylation of the foreign DNA. The SV40 genes are frequently amplified in tumor tissue, which further indicates that their expression is intimately involved in tumorigenesis in transgenic mice.
Introduction
Recent success in obtaining transgenic mice that express various genes (Brinster et al., 1981, 1983; Harbers et al., 1981; Wagner et al., 1981; Palmiter et al., 1982, 1983; Lacy et al., 1983; McKnight et al., 1983) prompted us to initiate studies with transforming or onc genes. A particularly well suited and interesting set of transforming genes is localized in the early region of the simian virus 40 (SV40) chromosome, which contains both the large T-antigen and small T-antigen coding sequences (Tooze, 1980). These gene products are required to transform cells in culture (Tegtmeyer, 1975; Sleigh et al., 1978). A variety of experiments have demonstrated that SV40 can also induce tumors in hamsters (Tooze, 1980). A subcutaneous injection of SV40 into newborn hamsters results primarily in fibrosarcomas at the site of injection (Eddy et al., 1962), while an intracerebral inoculation produces choroid plexus papillomas (see review by Janisch and Schrieber, 1977). An intravenous injection of high titers of SV40 into adult hamsters can produce a wide variety of tumors such as leukemia, lymphomas, osteosarcomas, and reticulum cell sarcomas (Diamondopoulos, 1972, 1978).
Until recently, SV40 was thought not to be oncogenic in mice (Tooze, 1980), but when specific inbred strains of mice were utilized, tumors could be detected after very long latency (Hargis and Malkiel, 1979; Abramczuk et al., 1984). Although the oncogenic potential of polyoma virus has been linked to the expression of the early region of the viral genome (Fried, 1965; Israel et al., 1979), it has proved experimentally difficult to determine the causal relationships between expression of the SV40 early region and the formation of tumors in animals. To date, this relationship is only suggested by the demonstrated role of the SV40 large and small T antigens in transformation of cells in culture (Tegtmeyer, 1975; Sleigh et al., 1978). Experimental systems designed to study the effects of the SV40 gene products in tumorigenesis have not produced tumors. For example, injection of SV40 virus or DNA into the blastocoel cavity of mouse embryos resulted in adult mice containing the SV40 genome, but no tumors were noted in these animals (Jaenisch and Mintz, 1974; Abramczuk, 1983). To develop an experimental system that would maximize for SV40 gene expression in transgenic mice, the SV40 early genes were placed in a plasmid containing a metallothionein fusion gene known to be expressed in transgenic animals (Brinster et al., 1981; Palmiter et al., 1983). When transgenic mice containing these constructions were obtained, we found that the SV40 T-antigen genes were expressed in a tissue-specific fashion and that characteristic tumors developed in many of the mice.
Results
Introduction of T-Antigen Genes into Mice
For these experiments, the SV40 early region (Kpn I–Bam HI), coding for the large and small T antigens and containing the two 72 bp enhancers, was introduced into a plasmid that already contained a metallothionein fusion gene (Figure 1). In one series of experiments, the fusion gene consisted of the metallothionein promoter/regulator fused to the thymidine kinase (TK) structural gene of herpes simplex virus, type 1. This fusion gene is called MK (Brinster et al., 1981). The SV40 early region and MK genes were inserted into the plasmid in opposite orientations in order to place an active transcription unit close to the promoter of the T-antigen genes. We know that MK is active in a high percentage of transgenic carriers (Palmiter et al., 1982), and we assumed that it would induce an open or expressible chromatin configuration. A similar construction employing a human growth hormone gene was also made because expression of this fusion gene is easily recognized by increased growth of the animals (Palmiter et al., 1983). Three sets of transgenic animals (a total of 25 mice) were generated for these experiments as summarized in Table 1. All of these mice died before 5 months of age; see legend to Table 1 for details.
Figure 1. Map of Plasmids pSV-MK and pSV-MGH.

Plasmid pSV-MK consists of the T-antigen gene from SV40 (Kpn I–Bam HI fragment) and a metallothionein–thymidine kinase fusion gene (Kpn I–Bam HI) cloned into the Bam HI site of pBXΔ, which is a derivative of pBR322 that deletes the Sal I to Pvu II region and has an Xho I linker inserted at about 3000 on the pBR322 map. The SV40 T-antigen gene was isolated from pSV3-ts58, which was obtained from R. Mulligan. The metallothionein–thymidine kinase fusion gene was created by substituting the Bgl II–Sst II fragment of HSV thymidine kinase gene for the comparable fragment of the mouse MT-I gene. The location of restriction sites used in this study is indicated; the solid circles indicate the position of Hpa II sites, and the open circles indicate the position of Hha I sites in the MT-I promoter and closest adjacent sites—there are about 30 additional Hpa II and Hha I sites in the TK and pBX regions and no sites in SV40. The polyadenylated primary transcripts from these genes are shown as solid arrows; the stippled regions represent MT-I gene sequences; pBR sequences are shown as a single line with the site of deletion indicated by Δ. This plasmid is 8.8 kb. pSV-MGH is similar to pSV-MK except that the Bam HI–Eco RI fragment of the human growth hormone gene is substituted for the Bgl II–Eco RI fragment of pSV-MK; the size is 8.4 kb.
Table 1.
Summary of Microinjection Experiments
| Experiment | Plasmida | Approx. No. of Plasmids Injected/Eggb | Mouse Strain | No. of Eggs Transferred to Foster Mothers | No. of Pups Born | No. of Transgenic Pupsc | No. of Premature Deathsc | Identifying Numbers of Mice Discussed in Texte |
|---|---|---|---|---|---|---|---|---|
| A | SV-MK | 240 | C57 | 160 | 23 | 9 | 4 (1) | 419, 422 |
| B | SV-MK | 240 | C57 × SJL | 214 | 41 | 6 | 4 (0) | 425, 427 |
| C | SV-MGH | 830 | C57 × SJL | 551 | 31 | 10 | 8 (3) | 340, 341, 344, 345, 410, 414 |
|
|
||||||||
| Totals: | 95 | 25 | 16 | |||||
See Figure 1.
SV-MK (linearized with Pvu I) or SV-MGH (Xho I–Bam HI fragment) were injected into the male pronucleus.
Transgenic pups were identified by dot hybridization to tail nucleic acids with nick-translated probes corresponding to TK, human GH, or SV40 as described by Palmiter et al. (1982). The number of gene copies ranged from 1 to 67. See also Table 2.
The number of transgenic pups that died prematurely (possibly due to SV40 early region expression) is shown; the number of confirmed tumors is indicated in parentheses. An experiment with 5-azacytidine accidently resulted in the death of 5 mice from experiment A and 2 mice from experiment B at 5 months of age. In addition, 2 young mice from experiment C were sacrificed while apparently healthy. Thus 9 mice did not have adequate chance to develop a tumor. All of the 16 remaining mice died prior to 5 months; 4 had identifiable brain tumors (see footnote e) but the remaining 12 could not be adequately examined, hence they could have died from SV40 early region expression.
In experiment A, mouse 422 died of a brain tumor (see Figure 2); mouse 419 was mated and eventually gave rise to a homozygous line that does not develop tumors; it was accidently killed with an overdose of 5-azacytidine but presumably would not have developed a tumor. In experiment B, mouse 425 was bred and her daughter developed a brain tumor but she was killed by 5-azacytidine before reaching the critical age. Mouse 427 was similarly killed by 5-azacytidine but she gave rise to the pedigree shown in Figure 3 before she died. In experiment C, mouse 340 developed a brain tumor and had an enlarged thymus (see Figure 2). Mouse 341 was bred and then sacrificed while still healthy; her offspring developed brain tumors. Mouse 344 was ill and was sacrificed at 1 month of age; no tumor was visible but a brain culture gave a transformed cell line that expressed SV40 early genes (see Table 2). Mouse 345 developed a brain tumor and had an enlarged thymus. Mouse 410 developed a brain tumor and showed signs of paralysis. Mouse 414 was weak at birth and was killed; a brain culture developed into transformed cells that expressed SV40 early genes (see Table 2).
Tumorigenesis in Transgenic Animals
Because the first animals produced contained the MK gene, we assayed for the presence of viral TK activity following cadmium induction (Palmiter et al., 1982). Although 15 animals were born from experiments A and B (Table 1), only 12 remained alive at 3 months of age when the assays were performed. Only one animal, 427, had significant viral TK activity and another mouse had marginally elevated levels of TK activity following induction. We normally observe that about 70% of the animals express viral TK (Palmiter et al., 1982). This unusual finding, in addition to the significant and unexplained mortality, indicated the possibility of an effect from the SV40 early region. By careful monitoring, we identified an animal, 422, with a bulged cranium. When this animal was sacrificed, the most apparent pathology was an abnormal mass in the left lateral ventricle of the brain.
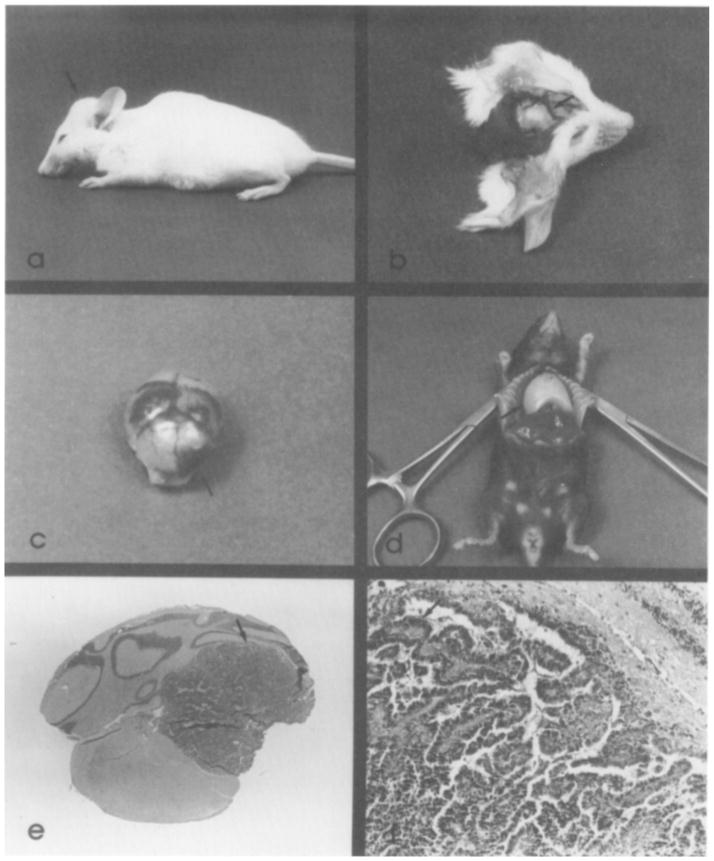
Subsequently, we identified several other mice with similar pathology (Figure 2a). The distention of the cranium was slight or quite distinct, and occasionally there were accompanying neurological signs (e.g. torticollis, loss of equilibrium, partial paralysis). Exposure of the cranium by reflection of the skin frequently revealed separated and hemorrhagic cranial suture lines (Figure 2b). When the cranium was removed large amounts of fluid often escaped and the brain collapsed. In some instances hemorrhage or columns of invading tumor cells were seen on the brain surface (Figure 2c). By dissecting the brain it was generally possible to locate one or more masses of pink tumor tissue in the ventricles of the brain. Histopathological examination identified these masses as choroid plexus papillomas or carcinomas (Figures 2e, 2f). Other body tissues were also sometimes abnormal. Frequently the thymus was enlarged, occasionally to enormous proportions (Figure 2d); however, microscopic examination revealed essentially normal tissue structure. Occasionally, kidney pathology also developed in these mice; for example, examination of mouse 410 revealed glomerular lesions.
Figure 2. Pathology Associated with Expression of SV40 Early Region in Mice.
(a) Photograph of mouse 427-4-2 (see Figure 3) showing the bulged cranium characteristic of brain tumor development. (b) The skin of 427-4-2 is reflected from the dorsal surface of the head to expose the separation of the cranial bones and hemorrhagic suture lines indicated by the arrow. (c) Brain of 427-4-2 showing extensive hemorrhages on the cerebral surface and a large tumor (arrow) in the right ventral cerebellum. (d) Thoracic cavity of an offspring of mouse 340 has been opened to reveal the enormous thymus. This mouse had difficulty breathing as well as a brain tumor. (e) A transverse section (stained with hematoxilin and eosin) through the cerebellar tumor area shown in (c). Note the distortion of normal cerebellar architecture and the apparent origin of the tumor from the choroid plexus of the IV ventricle. The arrow indicates the area from which the higher magnification shown in (f) was taken. (f) Photomicrograph showing tumor tissue in the center and lower left and compressed cerebellar tissue in the upper right. The arrow points to a typical papillary structure that is characteristic of these choroid plexus tumors. Magnification: 100X.
Rapidly growing cell lines were easily established from brain tumor tissue or hyperplastic thymus. Cell lines from either tissue looked similar and grew attached to the plastic dishes. The unattached thymic cells quickly died out, suggesting that the only cells capable of growing under our culture conditions were stromal cells. Both brain and thymus cells formed large colonies in soft agar; hence this technique was used to establish clonal lines. One transformed cell line (414) was established from the brain of a weak fetus taken at term.
Transmission of Tumorigenic Potential
We also established lines of animals in which the SV-MK gene was integrated. Some potentially interesting lines were lost because animals with tumors frequently died before progeny could be produced. However, three lines have been established. One line, 427, from experiment B, is a C57 × SJL hybrid line (Figure 3). The mice of this pedigree develop brain tumors, express viral TK, and produce SV40 T antigen in several tissues (see below). We have not been able to make this line homozygous because only two of 42 males have been fertile. In the second generation (427-2), mice 1, 2, 4, 7, and 9 all died from an identical type of brain tumor. Mouse 3 died at about the same age as the others, but rapid decomposition of the brain prevented definite identification of a tumor. Thus, in this generation, at least five of the six mice that inherited the SV40 genes developed a brain tumor. Mice in this pedigree died at an average age of 5 months (see legend to Figure 3). Offspring in another line, derived from mouse 341, also succumb to brain tumors. However, a third line (419) has been made homozygous, but no tumors have developed in any of the 35 animals positive for SV40 sequences. These mice do not express the MK gene, but some produce low levels of T-antigen mRNA in the liver (see Table 2).
Figure 3. Inheritance of Brain Tumors in Descendants of Transgenic Mouse 427.
This pedigree is designated 427; the second number indicates the generation and the last number represents the individual mouse; males are shown as boxes, females as circles; solid symbols signify the inheritance of SV-MK DNA (as determined by dot hybridization). For example, 427-2-1 is an SV-MK-positive male number 1 from the second generation of the 427 pedigree. s, signifies that the mouse was sacrificed while apparently healthy; †, signifies that the animal died with a brain tumor (bulged cranium or histological diagnosis); d, signifies that the mouse died without signs of pathology. The mean age (± SEM) at the time of death of those animals that developed tumors was 155 days (G0); 165 days (G1); 153 ± 11.3 days (G2); 152 ± 8.5 days (G3). Some of the mice shown on the bottom line of pedigree were just approaching the critical age for tumor development when this pedigree was drawn; mice 427-3-48 to 427-3-59 subsequently died of brain tumors. Most males in this pedigree are infertile.
Table 2.
Expression of Genes Coding for SV40 Early Region, MGH, and TK
| Mouse | Plasmida | Tissue | Gene Copy Numberb | T-Antigen mRNA Molecules/Cellc | MGH mRNA Molecules/Cellc | TK Activity Unitse |
|---|---|---|---|---|---|---|
| 340 | SV-MGH | Liver | 1 | <0.5 | 30 | |
| Tumor cells | 2–4 | 10–20 | 60 | |||
| 344 | SV-MGH | Liver | 26 | ~8 | 52 | |
| Tumor cells | 70 | 13 | 46 | |||
| 345 | SV-MGH | Liver | 3 | ~3 | 67 | |
| Thymus clone 2 | 4 | ND | 80 | |||
| Thymus clone 4 | 8 | ND | 320 | |||
| Thymus clone 5 | ND | 16 | 65 | |||
| Brain clone 1 | 26 | ND | 100 | |||
| Brain clone 3 | 25 | ND | 67 | |||
| Brain clone 5 | ND | 45 | 266 | |||
| 414 | SV-MGH | Brain + liver | 4 | ND | 38 | |
| Tumor cells | 11 | 58 | 73 | |||
| 419 | SV-MK | Liver | 7 | ~6 | 0 | |
| 422 | SV-MK | Liver | ND | 0d | 0 | |
| Muscle | 17 | 0d | ND | |||
| Tumor clones | 17 | 85–215 | ND | |||
| 427-2-1 | SV-MK | Liver | 2 | <2 | 22 | |
| Tumor | 20 | 19 | ND | |||
| Tumor cells | 44 | 57 | ND | |||
| 427-2-2 | Liver | 2 | 60 | 13 |
See Figure 1.
The gene copy number was determined by quantitative dot hybridization using the MT-I promoter fragment (Kpn I to Bgl II) or SV40 viral DNA as a probe.
SV40 T-antigen mRNA and MGH mRNA were measured by solution hybridization; see Experimental Procedures for details.
No SV40 mRNA was detected by dot hybridization, see Figure 4.
Mice were injected with CdSO4 (1 mg/kg) or ZnSO4 (3 mg/kg) to induce viral TK activity prior to partial hepatectomy. The activity is expressed as arbitrary units (cpm/μg/min) minus the endogenous activity. Endogenous activity was measured by adding an excess of antiserum specific for viral thymidine kinase; it averaged 3.0 units; see Palmiter et al. (1982) for details.
ND; not determined.
Analysis of SV40 T Antigen and mRNA
Dot hybridization was performed on RNA isolated from several tissues of mouse 422, and it was clear that the brain tumor contained significant amounts of RNA that hybridized with the SV40 probe; however, liver, kidney, and muscle appeared negative in this animal (Figure 4). Northern analysis of the RNA indicated that the tumor transcript was of the correct size for SV40 T-antigen mRNA when compared with RNA from SV40-transformed mouse cells (data not shown). Other mice, including progeny of some animals with tumors, have been analyzed for T-antigen mRNA in primary tissues and in derived cell populations or clonal lines (see Table 2). For routine quantitation of SV40 T antigen and MGH mRNA, a solution hybridization protocol was used. The abundance of MK mRNA is generally too low to measure easily; thus, we have relied on measuring viral TK activity. Table 2 shows that SV40 T-antigen mRNA was detected in all cell lines derived from brain tumors (from six different animals) as well as from thymus lines derived from mouse 345. All of the tumor cell lines also express either MGH mRNA or viral TK enzyme activity, with the exception of the 422 line. Thus, although the two genes are usually expressed together, expression of both the SV40 T-antigen gene and the closely linked metallothionein fusion gene is not essential for tumorigenesis.
Figure 4. Dot Hybridization of SV40 Early Region RNA in Tissues of Mouse 422.
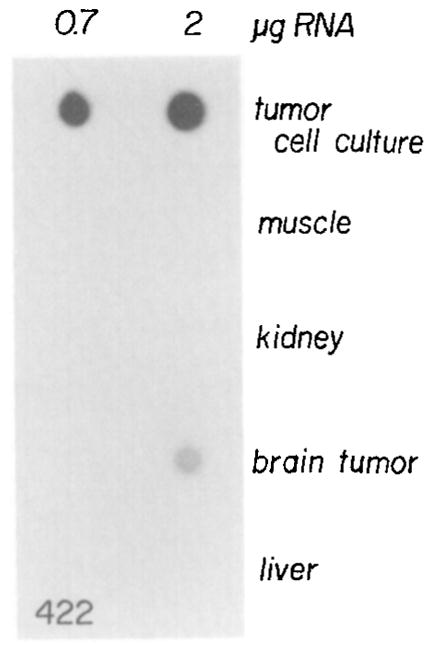
Total RNA was isolated from the brain tumor and various tissues of mouse 422 as well as from a tumor cell line that was established from the brain tumor of this mouse. RNA (0.7 and 2 μg) was applied to a nitrocellulose filter, hybridized with 32P-labeled SV40 viral DNA (2 × 108 cpm/μg), and washed, and then an autoradiograph of the filter was produced as described in Experimental Procedures.
The tissues of mouse 422 were examined for the presence of T-antigen protein by Western analysis employing a specific monoclonal antibody. The brain tumor had a high level of large T antigen that corresponds in molecular weight to the standard at approximately 94 kilodaltons (Figure 5A). With longer exposure of the autoradiogram (Figure 5B), bands corresponding to T antigen appeared in thymus, kidney, brain, and perhaps muscle. The T antigen in brain could represent contamination from infiltrating tumor cells. The protein in muscle was somewhat larger than expected and has not been seen in other animals. In several other mice, the brain tumors were always positive for T antigen, thymus and kidney were frequently positive, and very low levels of T antigen were sometimes detected in other tissues.
Figure 5. Detection of SV40 Large T Antigen in Tissues from Mouse 422.
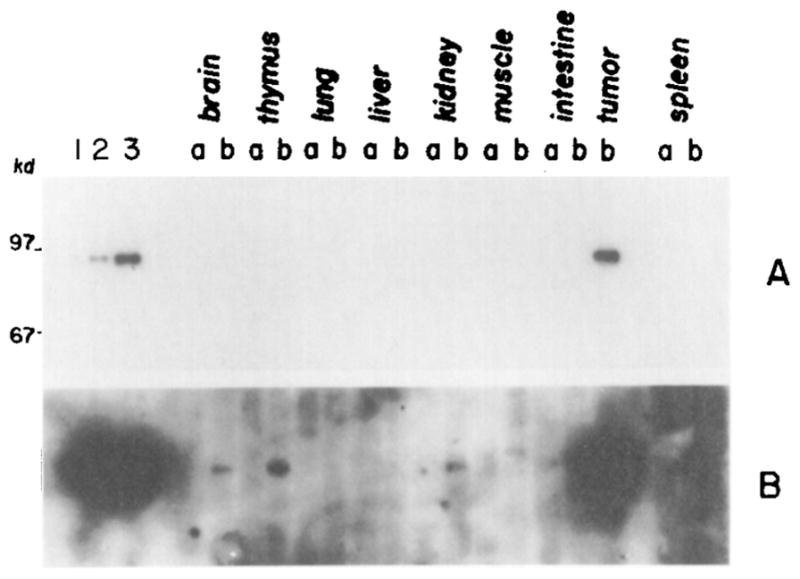
SV40 large T antigen was immunoprecipitated from soluble protein extracts from tissues of mouse 422 or a cell line isolated from the brain tumor. The immunoprecipitates were subjected to SDS-polyacrylamide gel electrophoresis; T antigen was detected by binding a specific antibody and 125I-protein A using a Western blot procedure as described in Experimental Procedures. Lanes 1, 2, and 3 contain SV40 large T antigen immunoselected from 0.5, 1.0, and 2.5 × 106 cells, respectively, of a clonal line of cells derived from the 422 brain tumor. In the other lanes, the samples labeled “a” are from tissues of a normal mouse and samples labeled “b” are from mouse 422. The autoradiogram shown in A is a 1 day exposure, while the autoradiogram shown in B is a 2 week exposure of the same filter.
Analysis of DNA
Table 2 summarizes the number of copies of SV-MK or SV-MGH genes per cell in the transgenic mice described here. These determinations were made by quantitative dot hybridization using probes specific for either the MT promoter or the SV40 T-antigen gene.
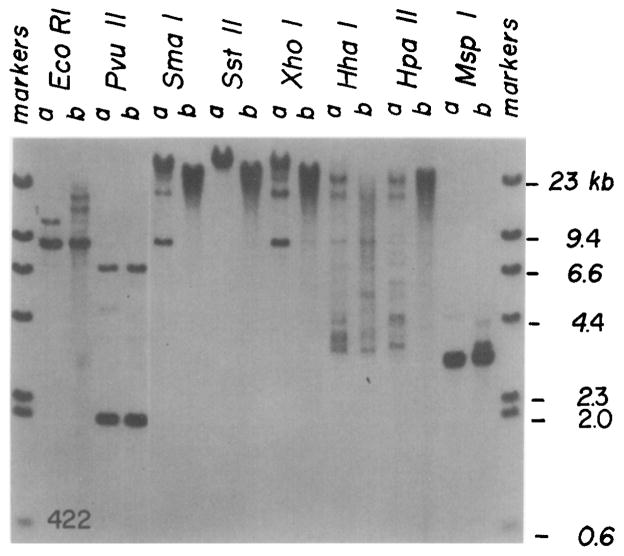
The DNA from mouse 422, the first transgenic animal with a brain tumor, was analyzed by Southern blotting following digestion with enzymes that are either sensitive or insensitive to DNA methylation. Figure 6 shows a comparison between DNA from brain tumor cells and muscle of this animal. Two of the enzymes that are sensitive to DNA methylation, and cut once within the injected DNA (Sma I, Xho I), result in the formation of an additional band in brain tumor that is not present in muscle. Apparently, most of the Sma I and Xho I sites are methylated in the muscle, but ~25% of these sites are unmethylated in tumor cells. Digestion at these unmethylated sites results in a band of 8.8 kb, the same length as the injected plasmid; a fainter dimer band is also visible. The presence of the unit-length band following digestion with enzymes that cut once within the injected fragment is expected for tandemly integrated copies of the injected DNA. The unique Sst II sites are predominantly methylated in both muscle and tumor DNA. Most of the Hpa II sites are methylated in the muscle DNA; however, many (but not all) of the more than 30 sites are unmethylated in the tumor cell DNA. Msp I digests show the size expected of completely unmethylated DNA. The DNA from the original tumor also indicated extensive hypomethylation of Hpa II sites (data not shown). There was no evidence for SV40 gene amplification in tumor DNA (Table 2 and Figure 6). Compare in particular the intensity of the bands in tumor cells and muscle following Eco RI, Pvu II, and Msp I digestion, where there are major bands of equal intensity. However, there are some subtle changes in restriction fragment sizes with enzymes that are insensitive to DNA methylation (e.g. Eco RI, Pvu II, and Msp I) that might reflect a small amount of DNA rearrangement.
Figure 6. Southern Blot Comparing DNA from Brain Tumor Cells and Skeletal Muscle of Mouse 422.
Brain tumor cells were passaged in cell culture for 5 weeks and then DNA was isolated for comparison with DNA isolated from skeletal muscle of the same animal. DNA (5 μg) from tumor cells (a) and muscle (b) was digested with enzymes sensitive to DNA methylation (Sma I, Sst II, Xho I, Hha I, Hpa II) or with enzymes insensitive to DNA methylation (Eco RI, Pvu II, Msp I), electrophoresed on a 1% agarose gel, transferred to nitrocellulose, and hybridized with nick-translated SV40. The sizes of λ markers are indicated.
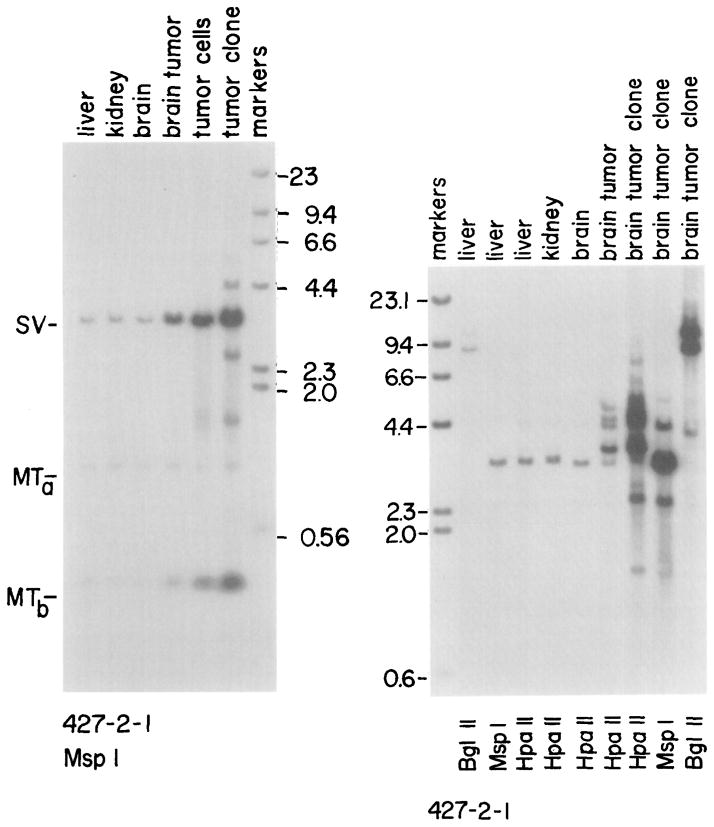
DNA from tumors and “normal” tissues of the 427 line of mice (Figure 3) have also been examined by the method of Southern. Following Msp I digestion, a major band appears at 4.0 kb when the DNA is probed for SV40 sequences. This band represents the entire SV40 early region (see Figure 1). The intensity of this band is similar for liver, kidney, and brain; however, its intensity is increased about 10-fold in the brain tumor and even more in the tumor clone (Figure 7A; Table 2). To show that equal amounts of DNA were present in each lane, the blot was reprobed with a metallothionein-I promoter sequence; Figure 7A shows bands of equal intensity at approximately 1 kb (labeled MTa) which correspond to the endogenous MT-I gene. The band labeled MTb confirms the results of the SV40 probe, since this probe detects the MT promoter sequence that is fused to the TK gene. Gene amplification has clearly taken place in the tumor and increased in the cultured tumor cells. The extra bands in Msp I digests of the tumor clone DNA presumably represent DNA rearrangements. In addition to amplification, the tumor DNA of this animal shows increased methylation (Figure 7B). The Hpa II digests of liver, kidney, and brain have a major band the same size as the Msp I band, indicating the DNA is not methylated at any Hpa II sites. However, the brain tumor DNA has several bands larger than the Msp I band. Most of the amplified copies in the tumor have become methylated at one or more Hpa II sites. The DNA amplification seen in Figure 7A is also apparent on this gel.
Figure 7. Southern Blots Showing Amplification and Methylation of SV-MK DNA in the Primary Brain Tumor and Brain Tumor Cell Lines of Mouse 427-2-1 Compared to “Normal” Tissues.
(A) DNA (5 μg) from liver, brain, primary brain tumor, a mixed brain tumor cell population, and a brain tumor cell clone selected in soft agar was digested with Msp I, electrophoresed on a 1.5% agarose gel, transferred to nitrocellulose, and hybridized with nick-translated SV40 and then with nick-translated Kpn I-Bgl II fragment corresponding to the MT-I promoter region. The band labeled SV corresponds to the entire T-antigen gene of SV-MK as detected with the SV40 probe; the band labeled MTb corresponds to the 260 bp fragment located between two black circles in the MT-I promoter (see Figure 1); the band labeled MTa corresponds to endogenous MT-I gene.
(B) DNA (5 μg) from “normal” tissues and tumor tissue or cells was digested with the indicated enzymes, electrophoresed on a 1% agarose gel, transferred to nitrocellulose, and hybridized with nick-translated SV40 viral DNA. The sizes (in kilobases) of λ Hind III markers are indicated.
DNA samples of several animals arising from integration of the SV-MGH construction were also examined. A Southern blot of DNA from liver and a brain tumor clone of mouse 344 is shown in Figure 8. A modest amplification (~ 2.5-fold) of the SV-MGH gene is apparent in the tumor clone. There is no evidence of rearrangement and the Hpa II sites of the SV-MGH DNA are highly methylated in both the liver and tumor clone as shown by a comparison of the Hpa II and Msp I lanes. In mouse 345, containing three copies of SV-MGH construction in the liver, significant amplification of the DNA occurred in one of the four thymus clones; clone 2 may be slightly higher than the liver, but clone 4 contains two to three times as many copies of the gene (Figure 9 and Table 2). However, the most dramatic increase in the number of genes has occurred in all of the brain tumor clones. Quantitation of the DNA indicates an 8 or 9 fold increase (Table 2). DNA from the brain tumor clone is highly methylated as shown by comparison of the Hpa II and Msp I digestions. Liver DNA from this animal was also high methylated (data not shown). Amplification of SV40 DNA was also observed in tumor cells from transgenic mice designated 340 and 414 (Table 2).
Figure 8. Southern Blot Showing Amplification and Methylation of SV-MGH DNA in a Brain Tumor Clone Derived from Mouse 344.

A brain tumor clone was selected in soft agar and then expanded in normal media. DNA (5 μg) from this clone and the liver of mouse 344 were digested with the indicated enzymes, electrophoresed on a 1% agarose gel, transferred to nitrocellulose, and hybridized with nick-translated SV40 viral DNA. The sizes of λ markers are indicated.
Figure 9. Southern Blot Showing Amplification of the SV-MGH Sequences in Cell Lines Derived from the Brain Tumor and Hypertrophied Thymus of Mouse 345.
DNA was isolated from liver, cultured thymus cells, cultured brain tumor cells, and several clones of thymus or brain tumor cells that were selected in soft agar. DNA (5 μg) was digested with Msp I, Hpa II, or Bgl II, electrophoresed on a 1% agarose gel, transferred to nitrocellulose, and hybridized with nick-translated SV40 probe. The sizes (in kilobases) of end-labeled λ Hind III markers are indicated.
Discussion
In the experiments described here, transgenic mice carrying SV40 T-antigen genes in all of their cells were obtained by injecting linearized plasmids into the male pronucleus of fertilized one-cell eggs. The frequency of obtaining viable pups in these experiments (Table 1) was not significantly different from previous experiments with different genes (Palmiter et al., 1982, 1983). Thus the SV40 early region genes do not appear to be toxic to mouse developmental processes. One reason for this could be that the SV40 early genes may be inactivated, perhaps as the result of developmental processes similar to those that occur when viruses infect preimplantation embryos (Jaenisch, 1976; Jahner et al., 1982) or teratocarcinoma cells (Swartzendruber and Lehman, 1975; Segal et al., 1979; Stewart et al., 1982; Niwa et al., 1983). This possibility is suggested by the observation that only one of 12 transgenic mice containing the SV-MK gene expressed high levels of TK activity, whereas 70% have expressed TK activity in other experiments where the MK gene was not linked to the SV40 early region (Palmiter et al., 1982). Although the data suggest that SV40 early region expression does not occur during early development, expression in the form of choroid plexus tumorigenesis is detected in many transgenic adults.
In previous experiments, SV40 DNA was injected into the blastocoel cavity of mouse embryos but the mice that developed never produced detectable tumors (Jaenisch and Mintz, 1974; Abramczuk, 1983). The major difference is that in the experiments described here the SV40 DNA was injected into fertilized eggs, which generally ensures the presence of the DNA in all the cells of a transgenic animal. However, injection of DNA into the blastocoel results in mosaic mice with SV40 DNA sequences in some tissues but not in others. In particular, only four of the ten transgenic mice analyzed by Jaenisch and Mintz (1974) contained SV40 sequences in brain tissue and two of their mice died unexpectedly. Thus the lack of tumorigenesis reported previously may reflect both the small sample size of transgenic mice with SV40 DNA in the appropriate tissue and the death of potentially affected mice prior to gaining knowledge of what symptoms to anticipate. We lost several transgenic mice carrying SV40 early region DNA because we were unprepared for what pathology to expect and because the brain tumors progress rapidly. However, it has now become easier to identity mice affected by SV40 T antigen.
Brain tumor development is the most common and striking finding associated with expression of SV40 early region in these transgenic mice. The bulged cranium is generally the first and most prominent physical sign. In fact, this change is sufficiently common to suggest that brain tumors may occur in all animals in which the gene is expressed. Unfortunately, many SV-MK mice were killed before brain tumors might have been expected (see legend to Table 1); however, in the 427 line, nearly all of the mice develop a bulged cranium due to a brain tumor (see Figure 3). In the SV-MGH mice, three of eight animals examined after death in our early experiment (Table 1) and three of four animals in a subsequent experiment had easily identifiable brain tumors. The primary cell type involved in the brain tumors has been histologically identified as a choroid plexus epithelial cell. The tumors appear to be either papillomas or carcinomas when examined microscopically. It is noteworthy that choroid plexus papillomas were also observed after intracerebral inoculation of SV40 into newborn hamsters (Janisch and Schrieber, 1977). Other organs, particularly thymus and kidney, may also undergo pathological alterations as a result of SV40 early gene expression. Initially cell lines were established from pathological specimens to study SV40 gene expression and DNA structural changes, but we subsequently found that other tissues, which appear normal in the mouse, also give rise to cell lines that grow well in culture and express SV40 T antigen (unpublished observations). Thus the establishment of cell lines from SV40 transgenic mice is not in itself a good criterion of oncogenesis.
A critical question raised by this study is why the oncogenic potential of the SV40 T antigens is expressed most often in the epithelial cells of the choroid plexus. Because the plasmids were injected into the fertilized egg, in most of the transgenic mice every cell carries identical, integrated copies of the SV-MK or SV-MGH genes. Yet, in many different transgenic mice, each with presumably random integration sites, the primary site of oncogenesis is the choroid plexus. These observations suggest that the site of integration is not important and that the MK or MGH fusion genes which were part of the injected plasmids are probably not involved. More recently, we have obtained identical choroid plexus tumors in six of seven mice developing from eggs injected with the SV40 early region alone, confirming the suspicion that the MT promoter is not involved via some unsuspected MT enhancer function. Three of the tumors developed in mice that received the wild-type SV40 early region, and three developed in mice that received the ts58 early region used in SV-MK and SV-MGH constructions. Furthermore, recent evidence suggests that the SV40 enhancer region is essential for development of these characteristic choroid plexus tumors (unpublished observations). It seems likely that oncogenicity of the integrated SV40 genes is due to a gene activation event. This is based on the observation that T-antigen protein and mRNA are barely detectable in normal tissues but are prominent in affected tissues (Figures 4 and 5, Table 2). Thus development of brain tumors is not a consequence of T antigen being present in all cells, with the choroid plexus being the most sensitive target tissue. Instead, it appears that the choroid plexus is more permissive for T-antigen gene activation. The implication of this line of reasoning is that some mechanism inactivates the injected SV40 genes during early development and then an infrequent event activates the gene in a few cells during later development.
Current evidence suggests that the SV40 early region gives rise to two mRNAs—one coding for large T and one coding for small t antigen. Large T appears to have both “immortalizing” and “transforming” activity; the role of small t is unknown (Tooze, 1980; Colby and Shenk, 1982). In the experiments described here, evidence for expression of large T antigen has been presented (Figure 5), but the expression of small t antigen has not been explored. However, we know that a mutant SV40 early region construct that encodes only an intact large T antigen, pSV11 (Colby and Shenk, 1982), is capable of initiating choroid plexus tumors in mice (unpublished observation), hence it is likely that small t antigen is dispensable in this model system. It is certainly possible that independent activation of other genes may also be required for development of a choroid plexus tumor. Alternatively, the choroid plexus may be the prime site of carcinoma development because other genes that can act in concert with SV40 T antigens are already expressed in these cells. The observation that most “normal” tissues from mice (without an overt tumor) of the 427 pedigree can easily give rise to transformed cell lines when subjected to tissue culture conditions is noteworthy. It seems to imply that activation of SV40 genes and/or appropriate selection processes can give rise to immortalized cell lines from any tissue. The thymic enlargement and kidney pathology that develop in some of these transgenic mice presumably represent secondary sites of T-antigen gene activation in which cellular conditions do not readily support development of a tumor. Alternatively, the lower levels of T antigen in kidney and thymic tissue (Figure 5) may not be sufficient for complete transformation in vivo.
Several different covalent modifications of c-onc genes have been associated with their activation, including promoter insertion by retroviruses (Hayward et al., 1981), enhancer insertion by retroviruses or by chromosomal translocation (Payne et al., 1982; Leder et al., 1983), gene amplification (Collins and Groudine, 1982; Dalla Favera et al., 1982; Schwab et al., 1983), and hypomethylation (Groudine et al., 1981; Jahner et al., 1982; Youssoufian et al., 1982). Because of these observations, changes in and around the SV40 gene integration sites have been examined. Evidence for DNA rearrangements, changes in methylation, and gene amplification has been observed. The most consistent observation is the amplification of the SV40 T-antigen genes. Five of six tumor cell lines examined showed from 2 to 20 fold gene amplification. In one case (427) amplification was also apparent in the primary tumor (Figure 7). Presumably, T-antigen gene amplification could provide a selective advantage if T-antigen is rate-limiting for transformation. It is not yet clear how much SV40 early region gene product is required for choroid plexus transformation, but the data in Table 2 suggest that about 10 to 20 early region mRNA molecules per cell are sufficient. The tumor cells from mouse 422, which did not show gene amplification, had the highest level of T-antigen mRNA that we have observed; this may account for the lack of gene amplification. Amplification generally involves all of the various fragments that we detect on a Southern blot; thus it is most likely that the unit of amplification includes the entire insert plus flanking DNA. SV40 does not replicate in mouse cells (Tooze, 1980); however, the possibility cannot be excluded that the presence of the SV40 origin of replication in these constructs contributes to the amplification process.
An interpretation of the other changes in the injected plasmid DNA is more problematical. The major difficulty is that there are multiple copies of the foreign DNA in most of these transgenic mice. Hence, we cannot determine whether the changes we observe are involved in gene activation or whether they are inconsequential changes in an inactive neighboring gene copy. Changes in methylation are frequently observed when comparing DNA from tumor cells with that from other unaffected cells, but the changes can be either in the direction of hypomethylation (e.g. mouse 422) or hypermethylation (e.g. mouse 427). A study of methylation patterns is also complicated by the fact that there are no readily assayable methylation sites within the T-antigen genes, promoter, or enhancer region. The one Hpa II site in SV40 DNA is not included in these constructs. Thus all of the observed changes in methylation occur in the flanking MK, MGH, or pBX sequences.
Thus, while the results from the DNA structural analyses have demonstrated that genes are modified in brain tumor tissue compared to normal tissues, a simple, systematic alteration has not been uncovered. Examples of hypomethylation, hypermethylation, rearrangement, and amplification of the inserted DNA have all been observed along with tumor formation. Possibly one or any of these phenomena may contribute to T-antigen gene activation or expression in a particular mouse. Which event is seen in the tumor could even depend upon which change happens to occur first. These changes in the plasmid DNA are not mutually exclusive, which could account for the observance of more than one change in a single tumor. Alternatively, the DNA structural changes described here may all be secondary to an as yet undetected gene modification. Analysis of the DNA from a large number of independent tumors developing within a line of mice containing a low number of integrated SV40 T-antigen genes (e.g. the 427 line) may provide valuable clues to the mechanism(s) of gene activation.
The approach for studying oncogenes described here is a logical extension from the transformation assays involving viral infection (Tooze, 1980; Cooper, 1982; Bishop, 1983) or DNA transfection of established cell lines (Shih et al., 1981; Krontiris and Cooper, 1981; Perucho et al., 1981; Pulciani et al., 1982) or primary cell lines (Land et al., 1983; Ruley, 1983). The technique ensures that the oncogene is exposed to every possible cellular environment; thus it should prove to be a valuable research tool for dissecting the mechanisms underlying oncogene activation and tissue specificity of tumor formation.
Experimental Procedures
Plasmid Construction and Microinjection
Plasmids SV-MK and SV-MGH were constructed as described in the legend to Figure 1. Although an SV40 ts58 early region (obtained from R. Mulligan) was used in these constructions, the T antigen produced after transfection of tissue culture cells with this plasmid is not temperature-sensitive. Comparison of restriction fragments generated from ts58 and wild-type SV40 has revealed an insertion of about 20 bp in ts58 between the Bam HI site (position 2469) and the Hinf I site (position 2760). These observations are not likely to be relevant to the phenomena described here because we have subsequently observed identical pathology with wild-type SV40 early region.
Plasmid SV-MK was linearized with Pvu I, whereas SV-MGH was digested with Bam HI and Xho I. The fragments were electrophoresed on a 1% agarose gel and then eluted by the NaClO4 method (Chen and Thomas, 1980); the concentration was determined fluorimetrically (Labarca and Paigen, 1980). A few hundred molecules of linearized plasmid were microinjected into the male pronucleus of either C57 or F2 hybrid eggs (obtained by mating C57 × SJL hybrid adults) as summarized in Table 1.
Histological Procedures
Tissues were fixed in 4% formaldehyde in 0.1 M phosphate buffer (pH 7.2) for at least 24 hr and then washed in phosphate buffer. Following fixation, the brain was cut transversely in 2 mm thick slices to facilitate localization of tumor tissue. Selected areas were then cut in 5 μm sections and stained for microscopic examination. Other tissues were sectioned and stained by standard techniques.
Cell Culture
Tumors and other tissues were minced finely and then placed in culture flasks with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum and antibiotics and incubated at 37°C in 5% CO2/air environment. Cells were replated at lower density when the flasks became confluent. For cloning, cells were suspended in 25 ml of 0.5% low melting point agarose (Bethesda Research Laboratories) and plated over 10 ml of 0.5% agar (Difco) that had already set; the medium was the same as above. Large, unattached colonies were picked several weeks later with a Pipetman tip, trypsinized, plated in 1 cm wells, then expanded in 10 cm petri dishes.
Western Blot Analysis of T-Antigen Protein
Tissues were obtained from mice, homogenized, and then sonicated for 10 sec in 2 ml of lysis buffer (150 mM NaCl; 50 mM Tris-HCl, pH 8.0; 5 mM EDTA; 0.5% NP40; 1 mM PMSF) using a Branson sonicator at a setting of 5.0. Soluble extracts were obtained by centrifugation for 5 min at 12,000 x g. The SV40 large T antigen was then immunoprecipitated from the extract using the monoclonal antibody PAb412, as described by Linzer et al. (1979). The resulting immunoprecipitates were solubilized and electrophoresed though a 12.5% discontinuous polyacrylamide gel in the presence of SDS (Laemmli, 1970). The proteins in the gel were transferred to nitrocellulose paper (Schleicher and Schuell) as described by Burnette (1981), then the blot was blocked, incubated with antibody, followed by incubation with 125I-protein A (New England Nuclear Corporation), and washed, and an autoradiogram was prepared as described by Reich et al. (1983). The monoclonal antibody PAb412 is directed against the SV40 large T antigen (Harlow et al., 1981).
Analysis of Nucleic Acids
Total nucleic acids were isolated from tissues or cell cultures by the SDS–proteinase K method (McKnight, 1978). For analysis of RNA, DNA was removed by centrifugation through 5.7 M CsCl cushion. Then 0.7 and 2 μg of RNA was denatured and spotted on nitrocellulose according to the method of Thomas (1980) or subjected to electrophoresis on 1.5% agarose and transferred to nitrocellulose as described by Thomas (1980). The nitrocellulose sheets were hybridized with nick-translated SV40 probes as described (Reich et al., 1983). Alternatively, SV40 T-antigen mRNA or MGH mRNA were quantitated in samples of total nucleic acid by solution hybridization as described by Durnam and Palmiter (1983). The MGH cDNA probe was the same as described by Palmiter et al. (1983). The SV40 cDNA probe corresponds to the region from Pvu II (position 3506) to Hind III (position 4002) of SV40. This same region was cloned into M13 as a hybridization standard.
Transgenic animals were detected by dot hybridization of tail nucleic acids as described by Palmiter et al. (1982); nick-translated SV40, TK, or hGH fragments were used as probes. For Southern analysis of DNA, the total nucleic acid samples were digested with RNAase A, followed by SDS–proteinase K, phenol–chloroform and ethanol precipitation. The DNA was redissolved in 10 mM Tris-HCl, 0.25 mM EDTA (pH 8), the concentration was measured (Labarca and Paigen, 1980), and 5 μg was digested for several hours with 10 to 15 units of restriction enzyme (New England Biolabs or Bethesda Research Laboratories) using assay conditions suggested by the supplier. The sample was diluted to 22 μl with loading buffer and loaded into the well of a 1% agarose gel, electrophoresed overnight, transferred to nitrocellulose, and hybridized with nick-translated probes prepared as described by Durnam and Palmiter (1983). The probes were either nick-translated SV40 viral DNA (to avoid contamination with pBR322 sequences) or a Kpn I–Bgl II fragment corresponding to the mouse MT-I promoter (see Figure 1).
Acknowledgments
We are grateful to M. Trumbauer and M. Yagle for valuable assistance in the experiments. We thank our colleagues for many constructive suggestions during the course of this study. This work was supported by grants from the National Institutes of Health and the National Science Foundation. A. M. was supported by a fellowship of the Smith, Kline and Beckman Education Fund.
References
- Abramczuk J. Attempts of permanent introduction of SV40 genome into mouse gametes and early embryos. In: Nagley P, Linnane AW, Peacock WJ, Pateman JA, editors. Manipulation and Expression of Genes in Eukaryotes. New York: Academic Press; 1983. pp. 355–356. [Google Scholar]
- Abramczuk J, Pan S, Maul G, Knowles BB. Tumor induction by simian virus 40 in the mouse is controlled by long term persistence of the viral genome and the immune response of the host. J Virol. 1984;49:540–548. doi: 10.1128/jvi.49.2.540-548.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JM. Cellular oncogenes and retroviruses. Ann Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Chen HY, Trumbauer M, Senear AW, Warren R, Palmiter RD. Somatic expression of herpes virus thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981;27:223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Ritchie KA, Hammer RE, O’Brien RL, Arp B, Storb U. Expression of a microinjected immunoglobulin gene in the spleen of transgenic mice. Nature. 1983;306:332–336. doi: 10.1038/306332a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette WN. “Western blotting” electrophoretic transfer of proteins from sodium dodecyl sulfate polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen CW, Thomas CA., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980;101:339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Colby WW, Shenk T. Fragments of the simian virus 40 transforming gene facilitate transformation of rat embryo cells. Proc Nat Acad Sci USA. 1982;79:5189–5193. doi: 10.1073/pnas.79.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982;298:679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Cooper GM. Cellular transforming genes. Science. 1982;218:801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- Dalla Favera R, Wong-Staal F, Gallo RC. One gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982;299:61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Diamondopoulos GT. Leukemia, lymphomas, and osteosarcoma induced in the Syrian golden hamster by simian virus 40. Science. 1972;176:173–175. doi: 10.1126/science.176.4031.173. [DOI] [PubMed] [Google Scholar]
- Diamondopoulos GT. Incidence, latency and morphological types of neoplasms induced by simian virus 40 inoculated intravenously into hamsters of three inbred strains and one outbred stock. J Nat Cancer Inst. 1978;60:445–448. doi: 10.1093/jnci/60.2.445. [DOI] [PubMed] [Google Scholar]
- Durnam DM, Palmiter RD. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983;131:385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Eddy BE, Borman GS, Grubbs GE, Young RD. Identification of the oncogenic substance in rhesus monkey kidney cell cultures as simian virus 40. Virology. 1962;17:65–75. doi: 10.1016/0042-6822(62)90082-x. [DOI] [PubMed] [Google Scholar]
- Fried M. Cell transforming ability of a temperature sensitive mutant of polyoma virus. Proc Nat Acad Sci USA. 1965;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M, Eisenman R, Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981;292:311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Harbers K, Jahner D, Jaenisch R. Microinjection of cloned retroviral genomes into mouse zygotes: integration and expression in the animal. Nature. 1981;293:540–542. doi: 10.1038/293540a0. [DOI] [PubMed] [Google Scholar]
- Harlow E, Crawford LV, Pim DC, Williamson N. Monoclonal antibodies specific for SV40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargis BJ, Malkiel S. Sarcomas induced by injection of simian virus 40 into neonatal CFW mice. J Nat Cancer Inst. 1979;63:965–968. [PubMed] [Google Scholar]
- Hayward WS, Neel BG, Astrin SM. Activation of a cellular one gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981;290:475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Israel MA, Chowdlury K, Ramseur J, Chandrasekaran K, Vanderrign DF, Martin MA. Tumorigenicity of polyoma virus in hamsters. Cold Spring Harbor Symp Quant Biol. 1979;44:591–596. doi: 10.1101/sqb.1980.044.01.061. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of Moloney leukemia virus. Proc Nat Acad Sci USA. 1976;73:1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Nat Acad Sci USA. 1974;71:1250–1254. doi: 10.1073/pnas.71.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahner D, Stuhlmann H, Stewart CL, Harbers K, Lohler J, Simon I, Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982;298:623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Janisch W, Schrieber D. In: In Experimental Tumors of the Central Nervous System. Bigner DD, Swenberg JA, editors. Kalamazoo, Michigan: Upjohn Company; 1977. p. 36. [Google Scholar]
- Krontiris TG, Cooper GM. Transforming activity of human tumor DNAs. Proc Nat Acad Sci USA. 78:1181–1185. doi: 10.1073/pnas.78.2.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lacy E, Roberts S, Evans EP, Burtenshaw MD, Costantini F. A foreign β-globin gene in transgenic mice: integration at abnormal chromosomal positions and expression in inappropriate tissues. Cell. 1983;34:343–358. doi: 10.1016/0092-8674(83)90369-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–601. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Leder P, Battey J, Lenoir G, Moulding C, Murphy W, Potter H, Stewart T, Taub R. Translocations among antibody genes in human cancer. Science. 1983;222:765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Linzer DH, Maltzman W, Levine AJ. The SV40 A-gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979;90:308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- McKnight GS. The induction of ovalbumin and conalbumin mRNA by estrogen and progesterone in chick oviduct explant cultures. Cell. 1978;14:403–413. doi: 10.1016/0092-8674(78)90125-3. [DOI] [PubMed] [Google Scholar]
- McKnight GS, Hammer RE, Kuenzal EA, Brinster RL. Expression of the chicken transferrin gene in transgenic mice. Cell. 1983;34:335–341. doi: 10.1016/0092-8674(83)90368-9. [DOI] [PubMed] [Google Scholar]
- Niwa O, Yokoda Y, Ishida H, Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983;32:1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Chen HY, Brinster RL. Differential regulation of metallothionein–thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982;29:701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Norstedt G, Gelinas RE, Hammer RE, Brinster RL. Metallothionein–human GH fusion genes stimulate growth of mice. Science. 1983;222:809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- Payne GS, Bishop JM, Varmus HE. Multiple arrangements of viral DNA in an activated host oncogene in bursal lymphomas. Nature. 1982;295:209–213. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Perucho M, Goldfarb M, Shimizu K, Lama C, Fogh J, Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981;27:467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Pulciani S, Santos E, Lauver AV, Long LK, Robbins KC, Barbacid M. Oncogenes in human tumor cell lines: molecular cloning of a tranforming gene from human bladder carcinoma cells. Proc Nat Acad Sci USA. 1982;79:2845–2849. doi: 10.1073/pnas.79.9.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich NC, Oren M, Levine AJ. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983;3:2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley HE. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983;304:602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schwab M, Alitalo K, Varmus HE, Bishop JM, George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983;309:497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Segal S, Levine AJ, Khoury G. Evidence for non-spliced SV40 RNA in undifferentiated murine teratocarcinoma stem cells. Nature. 1979;280:335–337. doi: 10.1038/280335a0. [DOI] [PubMed] [Google Scholar]
- Shih C, Padhy LC, Murray M, Weinberg RA. Transforming genes of carcinoma and neuroblastomas introduced into mouse fibroblasts. Nature. 1981;290:261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- Sleigh MJ, Topp WC, Hanich R, Sambrook JF. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978;14:79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Swartzendruber DE, Lehman JM. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975;85:179–188. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975;15:613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PS. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Nat Acad Sci USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J. The Molecular Biology of Tumor Viruses. 2. Part 2. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 1980. DNA Tumor Viruses. [Google Scholar]
- Wagner E, Stewart T, Mintz B. The human β-globin and a functional viral thymidine kinase gene in developing mice. Proc Nat Acad Sci USA. 1981;78:5016–5020. doi: 10.1073/pnas.78.8.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H, Hammer SM, Hirsch MS, Mulder C. Methylation of the viral genome in an in vitro model of herpes simplex virus latency. Proc Nat Acad Sci USA. 1982;79:2207–2210. doi: 10.1073/pnas.79.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]