Abstract
Acute lung injury is a life-threatening condition caused by disruption of the alveolar-capillary barrier leading to edema, influx of inflammatory leukocytes, and impaired gas exchange. Specialized proresolving mediators biosynthesized from essential fatty acids, such as docosahexaenoic acid, have tissue protective effects in acute inflammation. Herein, we found that the docosahexaenoic acid–derived mediator resolvin D3 (RvD3): 4S,11R,17S-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid was present in uninjured lungs, and increased significantly 24 to 72 hours after hydrochloric acid–initiated injury. Because of its delayed enzymatic degradation, we used aspirin-triggered (AT)-RvD3: 4S,11R,17R-trihydroxydocosa-5Z,7E,9E,13Z,15E,19Z-hexaenoic acid, a 17R-epimer of RvD3, for in vivo experiments. Histopathological correlates of acid injury (alveolar wall thickening, edema, and leukocyte infiltration) were reduced in mice receiving AT-RvD3 1 hour after injury. AT-RvD3–treated mice had significantly reduced edema, as demonstrated by lower wet/dry weight ratios, increased epithelial sodium channel γ expression, and more lymphatic vessel endothelial hyaluronan receptor 1-positive vascular endothelial growth factor receptor 3-positive lymphatic vessels. Evidence for counterregulation of NF-κB by RvD3 and AT-RvD3 was seen in vitro and by AT-RvD3 in vivo. Increases in lung epithelial cell proliferation and bronchoalveolar lavage fluid levels of keratinocyte growth factor were observed with AT-RvD3, which also promoted cutaneous re-epithelialization. Together, these data demonstrate protective actions of RvD3 and AT-RvD3 for injured mucosa that accelerated restoration of epithelial barrier and function.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are serious conditions characterized by loss of normal epithelial and endothelial barrier function, leading to pulmonary edema and infiltration of leukocytes culminating in significant impairments in gas exchange.1 ARDS and its milder form, ALI, are serious conditions characterized by loss of normal epithelial and endothelial barrier function, leading to pulmonary edema and infiltration of leukocytes culminating in significant impairments in gas exchange.1 ALI is a common condition, affecting approximately 200,000 patients a year in the United States alone.2 Although our understanding of the pathophysiological changes associated with ALI/ARDS has improved since its original description, effective management of this condition still proves difficult, and mortality remains approximately 40%.2 Aspiration pneumonia/pneumonitis is a common cause of ALI/ARDS characterized by direct damage to lung epithelia (because of the low pH of gastric contents) followed by extensive sloughing of the injured epithelial cells.3 Despite significant damage to the epithelial barrier, the condition is self-limited in many cases, suggesting endogenous mechanisms/factors play key roles in its timely repair.3
Specialized proresolving mediators (SPMs) are derived from essential fatty acids, and are actively biosynthesized in the resolution phase of acute inflammation, playing a key role in mucosal protection and, therefore, catabasis.4 Docosahexaenoic acid (DHA) is an ω-3 fatty acid present in airway mucosa.5 In addition, ω-3 fatty acids are detectable in exudates at sites of injury, consistent with entry from plasma in edema.6 The D-series resolvins are DHA-derived mediators originally discovered in lipidomic analysis of resolving exudates.7 Members of this series, including resolvin D1 (RvD1) and RvD2, have been shown to possess potent proresolving properties in models of acute inflammation, including the lung.8, 9, 10 Earlier work from our group demonstrated host-protective functions of aspirin-triggered (AT)-RvD1 in murine models of ARDS8 and allergic airway inflammation.11 Recently, the biosynthetic routes and complete stereochemistry for resolvin D3 (RvD3) and its aspirin-triggered epimer AT-RvD3 were elucidated.12 Interestingly, RvD3 is generated later than RvD1 and RvD2 in the course of peritonitis, suggesting unique proresolving roles for RvD3.12
Herein, in a self-limited murine model of ARDS, we present evidence demonstrating AT-RvD3′s ability to promote resolution of tissue injury through multiple mechanisms, including reduction of edema through actions on sodium channel expression and lymphatic vasculature, limiting infiltration of proinflammatory leukocytes into the lung and NF-κB activation, and accelerating recovery of epithelial barrier integrity and function by promoting proliferation of epithelial cells.
Materials and Methods
Acid-Induced Lung Injury in Mice
All animal protocols were reviewed and approved by the Harvard Medical School standing committee on animals (protocol 03618). Mice were housed in an American Association for Laboratory Animal Science–accredited facility. As described previously,13 male BALB/c mice (6 to 10 weeks; Charles River, Wilmington, MA) were anesthetized with ketamine/xylazine, and 50 μL of hydrochloric acid (pH 1.0, 0.1N, 0.22 μm filter-sterilized; Sigma-Aldrich, St. Louis, MO) was instilled selectively into the left mainstem bronchus. Some mice received AT-RvD3 (10 ng/mouse) or vehicle control [0.1% (v/v) ethanol] in saline via i.v. injection 1 hour after injury was induced. Mice were euthanized at 6, 24, 48, or 72 hours after injury. In a separate experiment, mice received AT-RvD3 (10 ng, i.v.) or vehicle [0.1% (v/v) ethanol] at 24 and 48 hours, and euthanized 72 hours after intratracheal HCl. To ensure molecular integrity of AT-RvD3 stock and determine concentration, the compound was assessed by UV-visible light (UV-vis) spectrophotometry before preparation of the working solution. A representative spectrogram of AT-RvD3 is shown in Supplemental Figure S1.
Identification of Endogenous RvD3 in Murine Lung
BALB/c mice were subjected to the acid-injury protocol, as described above, and allowed to recover for 24 to 72 hours. Briefly, lungs from acid-injured mice and uninjured controls were removed, gently homogenized in 1 mL ice-cold methanol, and lipid mediators were extracted, as described before,12, 14 using C18 columns for liquid chromatography tandem mass spectrometry analysis. RvD3 and the pathway markers 4- and 17-hydroxydocosahexaenoic acid (HDHA) were monitored and quantified by multiple reaction monitoring using diagnostic ion fragmentation patterns.
Histology and Immunohistochemistry
Lungs were perfusion fixed with zinc fixative (BD Pharmingen, San Diego, CA) at 20 cm H2O, as done previously.8 Tissues were embedded, divided into sections, and stained with hematoxylin and eosin by either the Dana Farber/Harvard Cancer Center Rodent Histopathology Core or the Brigham and Women's Pulmonary and Critical Care Medicine Histology Core. Tissue sections were deparaffinized and rehydrated in graded ethanols and water; a subset of slides was stained with hematoxylin and eosin following standard protocols. For immunohistochemistry using antibodies for pNF-κB and Ki-67, antigen retrieval was performed by boiling slides in 10 mmol/L citrate buffer, pH 6; antigen retrieval was not performed for lymphatic vessel endothelial hyaluronan receptor (LYVE) 1 and vascular endothelial growth factor receptor (VEGFR) 3 antibodies. Slides were blocked with 10% normal goat serum diluted in 1% bovine serum albumin dissolved in Tris-buffered saline with 0.025% Tween-20 for 1 hour, followed by avidin-biotin blocking (Invitrogen, Grand Island, NY). Primary antibodies were diluted in 1% bovine serum albumin dissolved in Tris-buffered saline with 0.025% Tween-20; samples were incubated at 4°C overnight: pNF-κB (S536) (Cell Signaling, Danvers, MA; 1:100), Ki-67 (Abcam, Cambridge, MA; 1:1000), LYVE1 (AngioBio, DelMar, CA; 1:10,000), VEGFR3 (R&D Systems, Minneapolis, MN; 1:2000). The secondary antibody used was goat anti-rabbit (Vector Laboratories, Burlingame, CA; 1:5000, 2 hours, room temperature). Slides were developed using a 3′,3′-diaminobenzidine kit (Invitrogen). Negative controls for immunohistochemistry consisted of incubating slides in the absence of primary antibody.
Enumeration of Ki-67+ Epithelial Cells and LYVE1+/VEGFR3+ Lymphatic Vessels
To quantitate proliferation in mouse lung epithelia, images of five distinct fields per injured lung were taken at ×400 magnification. Total and Ki-67+ cells were counted for each field; results are broken down for alveolar and airway epithelial compartments.
For identification and enumeration of LYVE1+VEGFR3+ lymphatic vascular structures, stained slides were scanned at the Dana Farber/Harvard Cancer Center Tissue Microarray and Imaging Core using an Aperio slide scanning device (Leica Biosystems, Inc., Buffalo Grove IL). Scanned slides were viewed using Aperio's ImageScope software version 12.1.0.5029 (Leica Biosystems, Inc.); lymphatic vessels were identified by LYVE1 and VEGFR3 positivity. Total numbers of LYVE1+ and VEGFR3+ vessels were counted in the left (injured) lung for each animal.
Lung Fluid Clearance
To determine differences in total lung water levels, we performed wet/dry weight ratio analyses. Lungs were removed at necropsy and weighed immediately. Lungs were dried for 48 hours at 55°C and reweighed. The wet/dry weight ratio was calculated by dividing the initial wet weight by the dry weight.
Western Blot Analysis
Whole lung lysates were prepared by homogenizing tissues in cold radioimmunoprecipitation assay buffer supplemented with Complete mini protease inhibitor cocktail and PhosSTOP phosphatase inhibitors (Sigma-Aldrich). Protein concentrations were measured using a BCA protein assay kit (Thermo Fisher, Cambridge, MA). Any kD precast gels were loaded with 40 μg/well protein and run at 100 V (Bio-Rad, Hercules, CA). Proteins were transferred to polyvinyl difluoride membranes overnight (4°C, 25 V). For detection of selected proteins, blots were blocked for 1 hour with 5% skim milk in Tris-buffered saline with 0.025% Tween-20. Primary antibodies were diluted in 5% milk–Tris-buffered saline with 0.025% Tween-20 buffer. We evaluated expression of surfactant proteins D and A (SP-D and SP-A) in bronchoalveolar lavage fluid (BALF); for these blots, 30 μL of BALF was loaded per well. Primary antibody sources and concentrations were as follows: phospho-NF-κB (S536) (Cell Signaling), 1:1000; NF-κB (BioLegend, San Diego, CA), 1:1000; epithelial sodium channel (ENaC) γ (Abcam), 1:1000; aquaporin 5 (Millipore, Billerica, MA), 1:1000; and α-tubulin (Abcam), 1:5000. Antibodies for SP-D and SP-A were a gift from Dr. David Douda (Brigham and Women's Hospital/Harvard Medical School), Dr. Nades Palaniyar, and Pascal Dijadeu (Hospital for Sick Children, Toronto, ON, Canada; 1:10,000). Blots for all antibodies except SP-D and SP-A were incubated overnight at 4°C; SP-D and SP-A blots were incubated with primary antibody for 2 hours at room temperature. A horseradish peroxidase–conjugated goat anti-rabbit antibody (1:10,000, 2 hours at 25°C) was used as a secondary antibody (Santa Cruz Biotechnology, Dallas, TX). Tissue lysates suggested in the antibody data sheets as positive or negative for specific proteins were used as controls. Chemiluminescence was developed using the Pierce Supersignal Pico Chemiluminescence substrate kit (Thermo Fisher, Cambridge, MA). Images were taken using the ChemiDoc XRS system (Bio-Rad). Densitometry was performed using ImageJ software version 1.49t (NIH, Bethesda, MD).
Growth Factor and Cytokine Analysis
IL-6 levels were measured using a LegendPlex Mouse Inflammation kit, following manufacturer's instructions (BioLegend). Keratinocyte growth factor (KGF; alias FGF7) was measured in BALF by enzyme-linked immunosorbent assay, per manufacturer's instructions (RayBiotech, Norcross, GA).
Bronchoalveolar Lavage Collection and Cytospin Preparation
Bilateral BAL was performed with two aliquots of phosphate-buffered saline + 0.6 mmol/L EDTA (24-hour time point). Total cell counts and leukocyte differentials were performed as described.15 Briefly, BALFs were spun down, and the supernatant collected for analyses of soluble mediators. The cell pellet was subjected to red cell lysis. Total cell counts were performed using a hemacytometer. A fraction of the BALF cells was used for cytospins; leukocyte differentials were performed after staining cytospin preparations with Wright-Giemsa (Sigma-Aldrich).
Cell Culture Experiments
NF-κB Reporter Assay
A549 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin (1000 U/mL penicillin; 100 μg/mL streptomycin) (Gibco, Grand Island, NY). Cells were stably transfected with a pNF-kB-DD-ZsGreen1 reporter vector (Clontech, Mountain View, CA). Cells were selected for stable expression of the reporter with antibiotics (G418, Sigma-Aldrich, 600 μg/mL). To ensure homogeneity of the population, cells surviving antibiotic selection were sorted by flow for ZsGreen fluorescence after stimulation with lipopolysaccharide (Invivogen, San Diego, CA) using a BD AriaFACS sorter (BD Biosciences, San Jose, CA). This reporter is tagged with a destabilization domain allowing rapid degradation of signal (ZsGreen1) in the absence of a stabilizing reagent (Shield1).16 For purposes of these experiments, 2 μmol/L Shield1 was added to culture media at the time of cell stimulation. A549s were seeded in 96-well plates and grown to approximately 90% to 95% confluence. Cells were serum starved overnight and stimulated with 1.56 to 100 ng/mL tumor necrosis factor-α for 24 hours. Fluorescence was measured on a Molecular Devices M5 microplate reader (λex 494, λem 525) (Molecular Devices, Sunnyvale, CA).
In Vitro Epithelial Wound Healing Assay
Calu3 cells were maintained in Eagle's minimal essential medium supplemented with 10% fetal bovine serum and penicillin/streptomycin (1000 U/mL penicillin; 100 μg/mL streptomycin) (Gibco, Grand Island, NY). The Electric Cell-Substrate Impedance Sensing system (Applied Biophysics, Troy, NY) was used to monitor wound healing of Calu3 epithelial cells in vitro.17 For this, 800,000 cells were seeded into each well of 8W1E PET electrode arrays (Applied Biophysics) and cultured for 2 days to reach confluence. The confluent monolayers were serum starved for 5 hours before wounding with a current of 1250 μA at 64,000 Hz for 30 seconds. The cells were washed with phosphate-buffered saline for removal of cellular debris and treated with 10 nmol/L AT-RvD3 or phosphate-buffered saline (vehicle control) in serum-free medium. Cell migration into the wounded region was monitored by measuring the change in impedance at 16,000 Hz for 18 hours. All results were normalized by the initial impedance values after wounding and before treatment.
Cutaneous Wound Healing Model and Treatment
After anesthetization, mice were shaved and subjected to a depilatory agent 1 day before dorsal wound generation. On the day of wound generation, shaved skin was cleaned three times with betadine antiseptic solution scrub and further cleaned with alcohol wipes. Two full-thickness circular wounds were made using a 5-mm punch biopsy tool.18 Wounds were splinted to prevent contracture using a prefabricated silicone splint that was attached using VetBond tissue adhesive (3M, St. Paul, MN). Wounds were treated daily, starting at day 0, topically with 10 μL of sterile saline with or without 100 ng of AT-RvD3 and covered using a semipermeable polyurethane dressing. Wounds and splints were then covered using self-adherent wrap. Re-epithelialization analysis was performed using ImageJ software and presented as a percentage re-epithelialization of original wound area. On day 5, wounds were collected, formalin fixed, paraffin embedded, and divided into sections. Embedding, dividing into sections, and hematoxylin and eosin staining were performed using standard protocols by Children's Hospital Boston Pathology Department. Images were obtained using a Nikon Eclipse E400 (Nikon Instruments, Inc., Melville, NY) equipped with SPOT acquisition software version 3.5 (SPOT Imaging, Sterling Heights, MI).
E. coli Pneumonia
Mice were prepared for intratracheal instillation of live bacteria to the left lung, as described above and previously.19 A total of 1 × 106 colony-forming units of Escherichia coli were introduced to the left mainstem bronchus (total volume, 25 μL). Mice were given AT-RvD3 (10 ng, i.v.) 1 hour after surgery. Mice were allowed to recover from anesthesia and returned to their cages. At 6 hours after surgery, mice were euthanized; lungs were harvested aseptically and homogenized in ice-cold sterile saline. To determine colony-forming units (CFUs), lungs were homogenized and serial dilutions were plated on Lysogeny broth agar plates. Colonies were counted after 24 hours' incubation at 37°C. Results are expressed as CFU per whole lung. Bacterial growth index was calculated as the ratio of lung CFU/CFU in the original inoculum.19
Statistical Analysis
Data analysis was performed using GraphPad Prism software version 5.01 (GraphPad Software, Inc., La Jolla, CA). Data are expressed as means ± SEM unless stated otherwise. Results were considered statistically significant if P ≤ 0.05. Comparisons were performed using t-test (for larger sample sizes with normal distribution) or Mann-Whitney U test (smaller sample sizes and/or nonnormally distributed data) unless otherwise noted. See Results and figure legends for details of specific tests performed.
Results
Endogenous RvD3 Is Generated in Response to Acid-Induced Lung Injury
To determine whether RvD3 was generated after acid-initiated injury of murine lungs, we performed lipidomics analysis on lung extracts (described in Materials and Methods). RvD3 and its biosynthetic pathway intermediates 4-HDHA and 17-HDHA were identified in accordance with published criteria,14 including retention time and MS-MS fragmentation spectra (Figure 1, A and B). Relative to uninjured lungs, 4-HDHA and 17-HDHA increased approximately twofold to threefold 24 hours after injury, returning to baseline levels at 72 hours (Figure 1C). RvD3 was present in uninjured lungs (26.3 ± 7.8 pg/lung) and significantly increased by approximately twofold at 24 hours (56.7 ± 5.8 pg/lung), and threefold by 72 hours (80.7 ± 15.7 pg/lung) in injured lungs (Figure 1D).
Figure 1.
RvD3 is generated endogenously in acid-injured murine lung. A: Multiple reaction chromatograms for endogenous D-series resolvins and docosahexaenoic acid (DHA) monohydroxy acids found in acid-injured mouse lung show the elution times of relevant compounds. B: Tandem mass spectrum used for the identification of RvD3 demonstrating diagnostic ion pattern. C: Amount of DHA-derived monohydroxy acids 4- and 17-hydroxydocosahexaenoic acid (HDHA) in injured left lung. D: Endogenous RvD3 identified and quantified in uninjured lungs (0 hour) and acid-injured lungs at 24 and 72 hours after injury. n = 3 per group (C and D). ∗P < 0.05, U-test.
AT-RvD3 Limits Acute Lung Injury
Because of its increased resistance to enzymatic degradation, we used AT-RvD3, a 17R-epimer of RvD3, for in vivo experiments.12, 20 Lung histology revealed interstitial and alveolar edema and inflammation in the left lung of acid-injured animals that was maximal at 24 hours and slowly decreased at 48 and 72 hours (Figure 2, A–D and F–H). Relative to vehicle alone (Figure 2, A–D), mice that received AT-RvD3 (10 ng/mouse, i.v., 1 hour after injury) had decreased histopathological correlates of ALI (n ≥ 3) (Figure 2, E–H). In mice treated with AT-RvD3, there were reductions in edema, alveolar wall thickening, inflammatory cell infiltrates, and hemorrhage (Figure 2, E–H). Higher-magnification images are shown for the 24-hour time point (Supplemental Figure S2).
Figure 2.
AT-RvD3 promotes restoration of normal lung tissue architecture in acid-injured mice and uninjured controls. Representative images (hematoxylin and eosin) for vehicle (top row) and AT-RvD3–treated (10 ng/mouse; i.v.) (bottom row) lungs at 0 hour (uninjured) (A and E), 24 hours (B and F), 48 hours (C and G), and 72 hours (D and H) after injury. n = 3 (A–H). Original magnification, ×100 (A–H).
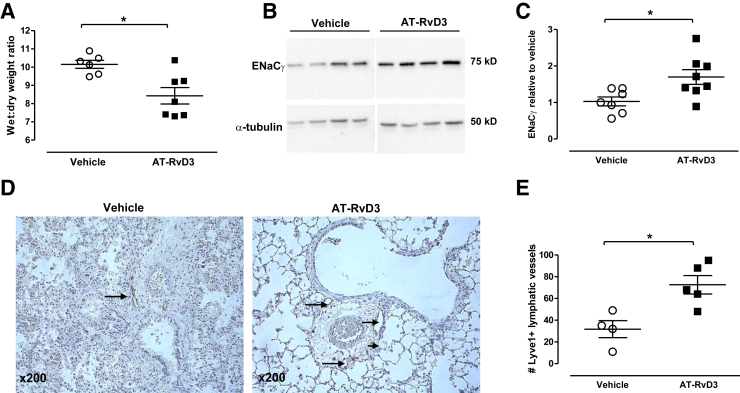
AT-RvD3 Enhances Resolution of Edema
In view of the histological changes in lung edema with AT-RvD3, lung wet/dry weight ratios were determined 24 hours after acid instillation. AT-RvD3 administration led to a significant reduction in lung water (Figure 3A). Because ENaC serves a major role in alveolar water clearance,21, 22 we next measured whole lung expression of the ENaCγ subunit (Figure 3B). Results were quantitated by densitometry and normalized to α-tubulin. Levels of ENaCγ were decreased from baseline (data not shown) in vehicle-treated mice; ENaCγ expression was significantly increased in the presence of AT-RvD3 (Figure 3C). We also measured expression of the water channel protein aquaporin 5 at 24 hours after injury by Western blot analysis; no significant difference was observed between the groups (Supplemental Figure S3).
Figure 3.
AT-RvD3–treated mice (10 ng/mouse, i.v., 1 hour after injury) show improved resolution of pulmonary edema. A: Wet/dry weight ratios in mice treated with AT-RvD3 (10 ng/mouse) or vehicle control. B: Western blot for epithelial sodium channel (ENaC) γ. Each lane represents lung lysate prepared from an individual mouse. C: Quantitation of ENaCγ was performed by comparison to the loading control α-tubulin; densitometry data are shown as relative to vehicle controls. D: Representative images of lymphatic vessel endothelial hyaluronan receptor (LYVE) 1 staining. Arrows denote lymphatic vessels staining positive for LYVE1. E: Quantitation of lymphatic vessels by LYVE1 staining. Data are presented as means ± SEM (A, C, and E). n ≥ 4 per group (E). ∗P < 0.05, U-test. AT, aspirin-triggered.
Because lymphatics serve an important function in the clearance of lung tissue edema,23 we determined if AT-RvD3 influenced the number of detectable lymphatic vessels. To visualize the lymphatics, lung tissue was stained for expression of LYVE1 (Figure 3, D and E) and VEGFR3 (Supplemental Figure S4). Seventy-two hours after injury, there were increased numbers of LYVE1+ lymphatic vessels detected in mice treated with AT-RvD3 relative to controls (Figure 3D). The lymphatics were enumerated and approximately 50% more LYVE1+ vessels were present with AT-RvD3 relative to vehicle (P < 0.02, n ≥ 4 per group, Mann-Whitney U test) (Figure 3E). VEGFR3+ vessels increased with AT-RvD3, but did not reach statistical significance with this sample size (n = 3) (Supplemental Figure S4).
AT-RvD3 Limits Leukocyte Infiltration to Acid-Injured Lung
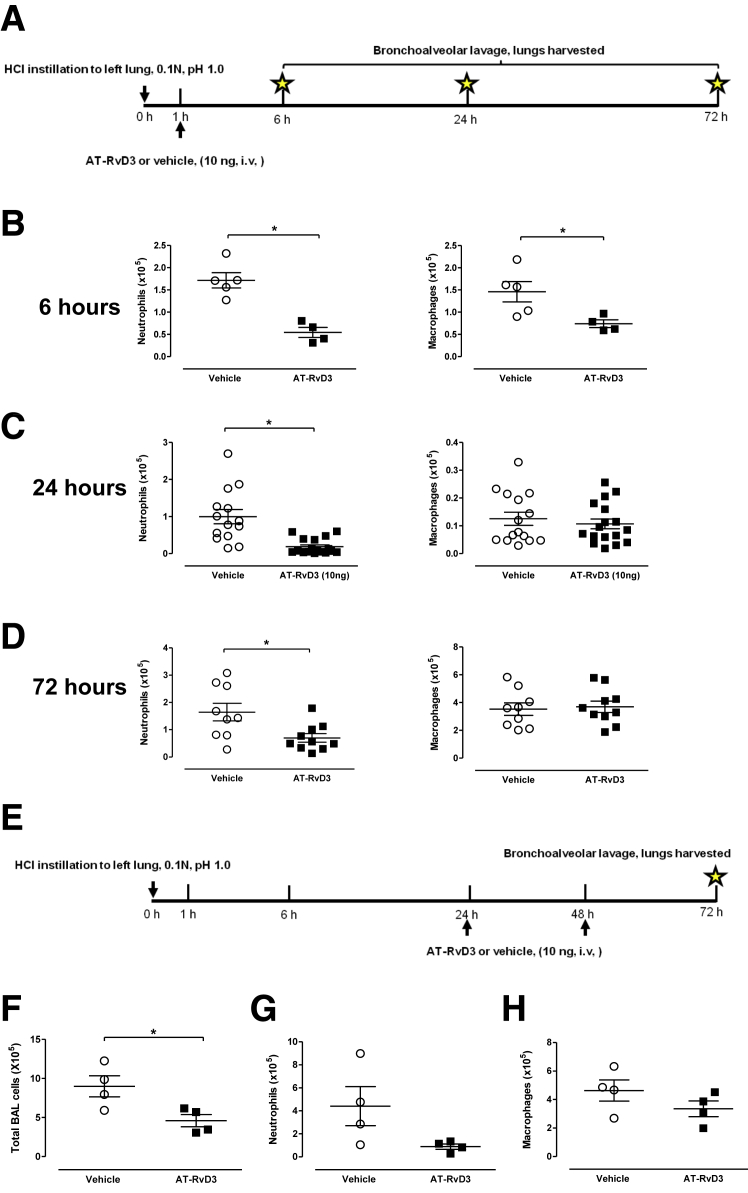
To examine the impact of AT-RvD3 on tissue inflammation, lung leukocyte subsets were enumerated in BALFs of mice 6, 24, and 72 hours after initiation of injury; mice were given AT-RvD3 or vehicle 1 hour after injury (described in Materials and Methods) (Figure 4A). At 6 hours after injury, AT-RvD3 led to an approximately threefold reduction in the number of neutrophils [171 ± 17.13 (vehicle); 54.53 ± 11.38 (AT-RvD3)] (Figure 4B). Macrophages were reduced almost twofold in AT-RvD3 treated mice at this time point [146 ± 23 (vehicle); 74 ± 8.63 (AT-RvD3)] (Figure 4B). At 24 hours after injury, AT-RvD3 led to a dramatic and statistically significant reduction in BALF neutrophils [0.99 × 105 ± 0.19 × 105 (vehicle) and 0.19 × 105 ± 0.06 × 105 (AT-RvD3)] (Figure 4C). At 24 hours, no significant difference in total numbers of BALF macrophages was evident with AT-RvD3 (Figure 4C). A statistically significant decrease in total lymphocytes was found with AT-RvD3 treatment [2.70 × 103 ± 0.37 × 103 (vehicle) versus 1.60 × 103 ± 0.32 × 103 (AT-RvD3); P < 0.04, n ≥ 14 mice per group] (data not shown). Seventy-two hours after injury, neutrophils are significantly reduced in the AT-RvD3–treated mice [0.70 × 105 ± 0.15 × 105 (AT-RvD3), 1.64 × 105 ± 0.32 × 105 (vehicle)] (Figure 4D); no significant difference was observed in the number of macrophages (Figure 4D).
Figure 4.
AT-RvD3 limits infiltration of leukocytes into the lung. A–D: AT-RvD3 or vehicle was administered to mice i.v. 1 hour after injury (10 ng/mouse). Total cells from bronchoalveolar lavage were enumerated by hemacytometer; major leukocyte subsets were identified on cytospins stained with Wright-Giemsa. Data are shown for neutrophils (left panels) and macrophages (right panels) for 6 hours (B), 24 hours (C), and 72 hours (D) after injury. Data reflect results of one to five experiments. E–H: Administration of AT-RvD3 at 24 and 48 hours reduces total bronchoalveolar lavage (BAL) leukocytes (F), neutrophils (G), and macrophages (H). Mice were subjected to the acid injury protocol as described, and two doses of AT-RvD3 (10 ng/mouse, i.v.) were given at 24 and 48 hours; mice were euthanized at 72 hours after injury (E). Data are presented as means ± SEM (B–D and F–H). n ≥ 4 mice per group (A–D); n = 4 per group (E–H). ∗P < 0.05, U-test. AT, aspirin-triggered. HCl, hydrochloric acid.
To determine the impact of administration of AT-RvD3 at later time points, mice were subjected to acid injury as described, and received doses of AT-RvD3 (10 ng/mouse, i.v.) at 24 and 48 hours after injury (Figure 4E). Total leukocytes were significantly increased in AT-RvD3–treated mice relative to vehicle (n = 4 per group; P = 0.028) (Figure 4F). Mice receiving AT-RvD3 had fewer neutrophils (0.89 × 105 ± 0.23 × 105) relative to controls (4.41 × 105 ± 0.89 × 105) (Figure 4G). Macrophage numbers were not significantly different between the groups (Figure 4H).
RvD3 and AT-RvD3 Limit Activation of the NF-κB Pathway
Lung injury elicits NF-κB activation as a key regulator of tissue inflammation,24 so we next explored the influence of AT-RvD3 on NF-κB expression and activation. First, in vitro, we used a lung epithelial cell line stably transfected with a fluorescent NF-κB reporter (described in Materials and Methods). Tumor necrosis factor-α gave a robust, dose-dependent increase in fluorescence (Figure 5, A and B). RvD3 and AT-RvD3 (10 nmol/L) significantly inhibited tumor necrosis factor-α–induced NF-κB activation (Figure 5, A and B). To detect NF-κB activation in vivo, we used a phospho-NF-κB (pNF-κB) antibody for serine 536 for Western blot analysis and immunohistochemistry (Figure 5, C–E). At 24 hours after injury, lung pNF-κB was detected by Western blot analysis (Figure 5C). Mice receiving AT-RvD3 had consistently decreased activation by approximately 1.5-fold (Figure 5D). To identify cellular sources of pNF-κB, we performed immunohistochemistry with the same antibody used for Western blot analysis. NF-κB activation was apparent in both epithelial cells and leukocytes, and was decreased with AT-RvD3 (Figure 5E). As a marker of functional regulation of NF-κB,25 BALF levels of IL-6 were determined. In response to AT-RvD3, levels of IL-6 were significantly reduced 24 hours after injury (Figure 5F).
Figure 5.
AT-RvD3 influences pNF-κB expression and signaling in injured epithelium. A and B: A549 epithelial cells stably transfected with an NF-κB reporter (pNF-κB-DD-ZsGreen1, described in Materials and Methods) were exposed to 10 nmol/L RvD3 (A), 10 nmol/L AT-RvD3 (B), or vehicle before stimulation with varying doses of TNF-α (1.56 to 100 ng/mL). Data are shown for vehicle versus 10 nmol/L RvD3 or AT-RvD3. C and D: Representative Western blots for pNF-κB (S536) and total NF-κB measured in whole lung lysates are shown. Quantitation of p-NF-κB (p65, S536) is shown in D. E: Representative images are shown for p-NF-κB (S536) immunohistochemistry in injured lungs of vehicle and AT-RvD3–treated (10 ng/mouse, i.v., 1 hour after injury) mice. Positive staining is denoted by arrows. F: Levels of IL-6 were measured in bronchoalveolar lavage fluids (BALFs) of acid-injured mice. Data are shown as means ± SEM (A–D). n ≥ 3 per group (A and B); n = 7 (C and D); n = 3 mice per group (E); n ≥ 4 per group (F). ∗P < 0.05, U-test. AT, aspirin-triggered.
AT-RvD3 Promotes Increased Proliferation of Epithelial Cells after Acid Injury
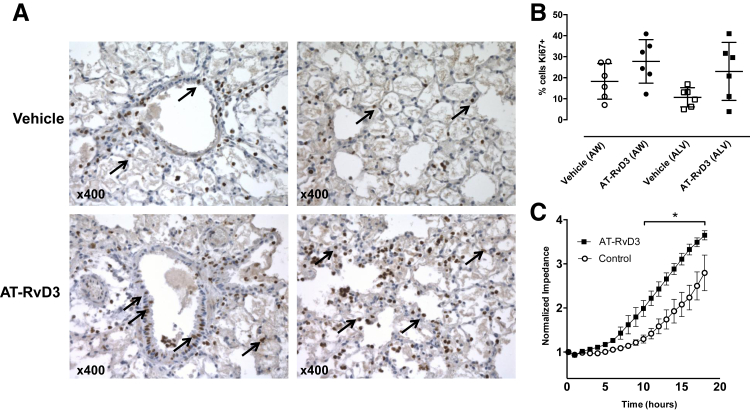
After acid injury, epithelial cell proliferation is required for restitution of the mucosal barrier. Airway epithelial cells stained positive for the nuclear protein Ki-67 24 hours after injury (Figure 6A). The percentage of Ki-67+ cells by immunohistochemistry was increased by AT-RvD3 in both airway and alveolar compartments (Figure 6, A and B). To quantitate the direct actions of AT-RvD3 on epithelial responses to injury, we used the Electrical Cell-Substrate Impedance Sensing in vitro assay with Calu3 cells that (unlike A549 cells) form a tight barrier (described in Materials and Methods). After induction of injury, Calu3 cells exposed to 10 nmol/L AT-RvD3 displayed an accelerated increase in restitution of an intact epithelial barrier; statistically significant differences between treatment groups were apparent as early as 10 hours after injury (Figure 6C).
Figure 6.
AT-RvD3 accelerates human lung epithelial cell proliferation in vivo and epithelial wound closure in vitro. A: Representative images of Ki-67 staining in vehicle and AT-RvD3–treated (10 ng/mouse, i.v., 1 hour after injury) acid-injured mouse lung. Images showing airway (left panels) and alveolar changes (right panels) are presented. Ki-67–positive cells are denoted by arrows. B: Quantitation of Ki-67–positive epithelial cells in acid-injured mouse lung. Data are shown as percentage Ki-67–positive cells at 24 hours after injury; airway (AW) and alveolar (ALV) compartments are shown (P = 0.066 and 0.08, for AW and ALV comparisons, respectively; Mann-Whitney U test). C: Repair of focal wounding of Calu3 lung epithelial cell monolayers was assessed with an elevated voltage using an Electric Cell-Substrate Impedance Sensing system. Cells were left untreated or treated with 10 nmol/L AT-RvD3 and wound closure was monitored in real-time over an 18-hour period; data are from three independent experiments with three to four replicates per experiment. Data are presented as normalized impedance ± SEM (C). n = 6 per group (B). ∗P < 0.05 by two-way analysis of variance. AT, aspirin-triggered.
AT-RvD3 Enhances Reparative Responses and Re-Epithelialization in Models of Lung Injury and Skin Wounding
KGF has been linked to epithelial restitution in both skin and lung,26, 27 so the influence of AT-RvD3 on BALF and whole lung lysate levels of this cytokine was next determined. AT-RvD3 led to significant increases in BALF KGF [116.4 ± 31.1 pg KGF/μg protein (AT-RvD3) versus 47.7 ± 7.0 pg KGF/μg protein (vehicle)] (Figure 7A). Similarly, AT-RvD3 treatment led to increased KGF in samples of whole lung lysates [234.4 ± 9.05 (vehicle) versus 295.4 ± 20.84 (AT-RvD3)] (Figure 7B). Messenger RNA for the KGF receptor Fgfr2 was also expressed in lung 24 hours after injury [mean ΔCt ± SEM: 4.29 ± 0.22 (AT-RvD3) and 4.46 ± 0.21 (vehicle)]. As KGF is also known to affect surfactant protein levels in response to lung injury, we measured levels of surfactant proteins A and D (SP-A and SP-D) in BALF from mice 24 hours after acid injury. At this time point, we did not observe any clear difference between vehicle and AT-RvD3 treated groups (Supplemental Figure S5).
Figure 7.
AT-RvD3 accelerates re-epithelialization in cutaneous excisional surgical injury and promotes generation of the mitogen keratinocyte growth factor in injured lung. A and B: Keratinocyte growth factor (KGF) was measured by enzyme-linked immunosorbent assay in bronchoalveolar lavage fluids (BALFs) of acid-injured mouse lung (A) and whole lung lysates (B). Data are presented as pg KGF per μg protein (U-test). C: Representative images of wounds treated with AT-RvD3 or saline at day 0 and 5 days after injury. Dotted lines indicate the epidermal border; the area within the dotted lines represents the area of the wound not yet covered by new epidermis. D: Quantitative analysis of re-epithelialization in splinted wounds of C57BL/6J mice treated with saline or AT-RvD3 (100 ng/day; topical) (two-way analysis of variance with Bonferroni multiple comparisons post-test). E: Histological analysis (hematoxylin and eosin stained) of wounds treated with AT-RvD3 or saline and harvested at day 5. Right panels show a higher magnification of the boxed areas in the left panels, with dotted lines indicating the epidermal border. n ≥ 3 per group (A and B); n = 5 mice per group (D). ∗P < 0.05. AT, aspirin-triggered.
Because KGF serves important roles in skin wound healing,27 we next determined whether epithelial responses to AT-RvD3 were exclusive to the lung or represented a more general action in promoting resolution. We used a model of full-thickness skin wounding that is characterized by initial re-epithelialization of the defect (described in Materials and Methods). AT-RvD3 (100 ng administered topically, once a day for 5 days) led to more rapid re-epithelialization than vehicle, as determined by visual inspection (Figure 7, C and D) and tissue histology (Figure 7E). The degree of re-epithelialization was significantly greater by day 3 after injury in mice given AT-RvD3 (Figure 7D). Representative histology revealed a more rapid restoration of normal, full-thickness epidermis in mice receiving AT-RvD3 (Figure 7E).
To determine the impact of AT-RvD3 on bacterial clearance, we subjected a group of mice to the E. coli pneumonia protocol (described in Materials and Methods). AT-RvD3 (10 ng, i.v.) did not lead to a worsening of infection, as assessed by measurement of CFU and bacterial growth index at 6 hours after instillation of bacteria (Supplemental Figure S6).
Discussion
Herein, we have provided evidence of epithelial cell-directed proresolving actions for RvD3 and AT-RvD3. We detected endogenous RvD3 production in vivo after acid-initiated lung injury, and determined that early administration of exogenous AT-RvD3 1 hour after injury led to reduced tissue injury and inflammation and promoted a more rapid recovery of barrier integrity. AT-RvD3 treatment in this model improved clearance of edema, which was associated with increased expression of ENaCγ, and increased numbers of LYVE1+ lymphatic vessels. AT-RvD3 decreased leukocyte infiltration and NF-κB activation. In addition, AT-RvD3 led to epithelial cell proliferation, KGF production, and cutaneous re-epithelialization. Taken together, these results support anti-inflammatory and proresolving roles for RvD3 and AT-RvD3 in the timely resolution of epithelial injury and mucosal protection.
The ω-3 polyunsaturated fatty acid DHA serves as a precursor for potent anti-inflammatory/proresolving mediators, and is known to be present in airway mucosa.5, 7 In addition, unesterified DHA is rapidly delivered to acutely inflamed sites via plasma exudation, providing multiple potential sources of DHA for enzymatic conversion to bioactive autacoids.6 Endogenous biosynthesis of RvD3 involves an initial lipoxygenase reaction converting DHA to 17(S)-hydroperoxy DHA, followed by a second lipoxygenase reaction yielding a hydroperoxide at the C4 position (4S-hydroperoxy-docosapentaenoic acid). 4S-HpDHA is subsequently converted to 4S,5S epoxide that is hydrolyzed to give RvD3.7, 12, 28, 29 The 17R epimer or AT form can be generated endogenously by aspirin-acetylated cyclooxygenase-2 performing the initial conversion of DHA; however, aspirin-independent biosynthesis of AT-RvD3 also occurs in mammalian tissues.7, 12, 28 Recent work established a method for total organic synthesis of RvD3 and AT-RvD3 that enabled elucidation of the stereochemistry of these mediators.12, 29 In addition to peritonitis,12 RvD3 biosynthesis also occurs after kidney ischemia and colitis.30, 31 Of interest, intervention with nanoparticles designed to deliver AT-RvD3 and AT-RvD1 is able to rescue age-related delays in the normal resolution process.32 For the work presented herein, we elected to focus on the 17R (AT) epimer of RvD3 for in vivo experiments on the basis of its longer half-life and favorable pharmacological properties relative to RvD3.12, 20 Lung edema is considered a defining characteristic of ALI/ARDS and understanding how to control its development and progression, as well as promote its resolution, remains an important clinical challenge.21 Damage to the epithelial barrier of the lung is an initiating event allowing leakage of fluid, protein, and leukocytes into the airspaces.1 A key mechanism for edema clearance from alveoli is the establishment of a Na+ gradient between the apical and basolateral cell membranes, which directs movement of water by osmotic forces out of the airspaces.21 ENaC and Na+-K+-ATPase are the two principal proteins involved in sodium transport, and their regulation has been shown to affect outcomes in ALI/ARDS.22, 33, 34, 35 In the present study, AT-RvD3 induced a significant increase in ENaCγ expression that coincided with significant reduction in lung edema. Additional SPMs, namely LXA4 and RvD1, also influence sodium transport proteins and fluid balance in the lung after lipopolysaccharide exposure,36, 37 so these actions on epithelial Na+ transport may represent a vital catabatic mechanism for SPMs.
The lymphatic vasculature of the lung is of considerable importance in maintaining tissue level fluid balance and serving as a conduit for leukocyte trafficking.23, 38, 39 Lung lymphatic vessels increase in size and number in various pathological states.23, 38, 39 The discovery of markers (LYVE1, VEGFR3) for lymphatic endothelial cells has facilitated identification of lymphatic vessels via immunostaining.40, 41 Herein, AT-RvD3 led to increased numbers of detectable lymphatic vessels at 72 hours after injury. The increased capacity of the lymphatics would be expected to enhance fluid clearance and leukocyte clearance from the injured lung during its catabasis.42, 43 Although perfusion fixation was routinely performed at 20 cm H2O, differences in lung compliance with AT-RvD3 may have affected lung volume–dependent measures; however, lung wet/dry weight differences support changes in edema clearance from the lung. In a model of corneal injury and repair, SPM limited hemangiogenesis, but not lymphangiogenesis.44 The results presented herein are the first to suggest that lymphatics are engaged by an SPM for tissue protection.
Exuberant lung inflammation is integral to the pathobiology of ALI/ARDS. Of interest, AT-RvD3 decreased BALF neutrophil and lymphocyte numbers 24 hours after injury and led to reductions in NF-κB activation and IL-6 levels. On tissue histology, AT-RvD3 also had a significant impact on lung inflammation. AT-RvD3 regulation of lung inflammation did not adversely affect host defense against the enteric pathogen E. coli. NF-κB is a transcription factor that can respond quickly to proinflammatory signals or injurious stimuli, and its activation through post-translational modifications (eg, phosphorylation of serine 536)45 leads to induction of several genes important in host defense.24, 46 Although NF-κB activation is initially host protective, promoting host resistance to pathogens, many of the genes it up-regulates have proinflammatory activity, and failure to counterregulate NF-κB signaling in a timely manner can lead to tissue damage.46 Herein, AT-RvD3 directly limited A549 epithelial cell activation of an NF-κB reporter in vitro. Results from this engineered A549 reporter assay were bolstered by reduced phosphorylation of NF-κB (S536) in vivo and the observation that AT-RvD3 decreased BALF IL-6, a proinflammatory target of pNF-κB. In some settings, NF-κB activation can be biphasic with late protective roles in repair. Herein, AT-RvD3 regulation of NF-κB serves as a protective mechanism to modulate the immune response to acid injury.8, 25
Restoration of a fully functioning epithelial barrier is critical for complete resolution of mucosal tissue injury.47 Impaired healing in general is a major clinical issue, and there is still a need to further our understanding of normal repair mechanisms to develop better approaches to promote successful tissue regeneration.48 In lung, as in other mucosal tissues, reestablishment of the epithelial barrier after injury or infection is a necessary step for accelerating a return to homeostasis.47, 49 In response to acid injury, epithelial cells slough and basal cells proliferate to reepithelialize the airway.49, 50 Herein, the proliferating airway and alveolar epithelium expressed Ki-67, with a trend for increase by AT-RvD3. Functionally, Calu3 cells injured by Electric Cell-Substrate Impedance Sensing had accelerated restitution in vitro with AT-RvD3. Calu3 cells were specifically selected for this assay, on the basis of their ability to form a tight barrier.51 Although several factors play a role in recovery of injured epithelium, KGF promotes epithelial protection in mouse and human studies of lung26, 52, 53 and skin27 injury. AT-RvD3 increased KGF levels in both BALF and whole lung lysates, suggesting a link for the SPM to this key protective signaling circuit for wounded epithelium. The protective epithelial actions for AT-RvD3 were not unique to the lung, as topical AT-RvD3 promoted more rapid re-epithelialization of skin wounds as well. Of interest, intervention with AT-RvD3 did not lead to epithelial hyperplasia.
In summary, we provide evidence that RvD3 is endogenously produced with a temporal delay and displayed epithelial-directed actions. AT-RvD3′s tissue protective actions included decreased lung edema and inflammation with enhanced restitution of epithelial barrier function. Molecular targets for AT-RvD3 included ENaCγ, NF-κB, and KGF. Together, these results highlight a multipronged mechanism for RvD3 and AT-RvD3 in lung epithelial cell responses and catabasis after injury, suggesting their potential roles in a proresolving therapeutic approach for ALI/ARDS or other conditions precipitated by epithelial disruption.
Acknowledgments
We thank Bonna Ith for assistance with lung histology, Children's Hospital Boston Pathology Department members for their help with skin histology, the Flow Cytometry Core at Brigham and Women's Hospital for help with cell sorting, and Dr. David N. Douda (Brigham and Women's Hospital) and Dr. Nades Palaniyar and Pascal Djiadeu (Toronto Hospital for Sick Children) for material and advice on immunoblotting with the surfactant protein D and A antibodies.
Footnotes
Supported by NIH grants PO1-GM095467 (C.N.S., B.D.L., and N.A.P.), 5T32 HL007633 (B.D.L. and J.K.C.), HL106173 (M.S.), HL116186 (J.H.), and HL119902 (S.E.-C.).
Disclosures: C.N.S. and B.D.L. are inventors on patents (resolvins) assigned to Brigham and Women's Hospital and licensed to Resolvyx Pharmaceuticals; their interests are managed by the Brigham and Women's Hospital and Partners HealthCare. C.N.S. was the scientific founder of Resolvyx Pharmaceuticals and owns founder stock in the company.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.03.011.
Supplemental Data
UV-visible light (UV-vis) absorption spectrogram of AT-RvD3. UV-vis spectrophotometry was used to measure the concentration of AT-RvD3 stock solution and confirm integrity of compound used in all experiments.
Impact of AT-RvD3 on lung histology after acid injury. Higher-power magnifications of acid-injured lung at 24 hours after injury. Representative images of vehicle (left panels) and AT-RvD3–treated mice (right panels) are shown. Asterisks denote edema present in airspaces; arrows, leukocytes.
Evaluation of the impact of AT-RvD3 on aquaporin 5 expression in acid-injured mice. A: Representative immunoblot for aquaporin 5. Mice were subjected to the acid injury protocol; AT-RvD3 (10 ng) or vehicle control were injected i.v. 1 hour after injury. Each column represents an individual animal. B: Densitometry of results shown in A. Results are presented as means ± SEM (B). n ≥ 5 (B). P = 0.36 (B).
Impact of AT-RvD3 on lymphatic vasculature, as demonstrated by vascular endothelial growth factor receptor (VEGFR) 3 immunohistochemistry. A and B: Representative images of VEGFR3 staining in vehicle (A) and AT-RvD3–treated (B) mouse lung 72 hours after injury. Mice were injured as described; AT-RvD3 or vehicle control were administered i.v., 1 hour after injury. Arrows denote VEGFR3+ lymphatic vessels. C: Quantification of VEGFR3+ vessels in injured mouse lung. Data are presented as means ± SEM (C). n = 3 per group (C). P = 0.1 by U-test (C).
Impact of AT-RvD3 on surfactant protein A and D (SP-A and SP-D, respectively) expression in the lung acid injury model. Representative immunoblots for SP-A (A) and SP-D (B) expression. Each lane represents a bronchoalveolar lavage fluid sample from an individual mouse.
Response to Escherichia coli pneumonia in mice given AT-RvD3 (10 ng i.v., 1 hour after injury). Mice received 1 × 106 bacteria intratracheally. Colony-forming units (CFUs) and bacterial growth index (BGI) were assessed 6 hours after instillation. A: CFUs of E. coli in mice given vehicle or AT-RvD3. B: BGI in mice given vehicle or AT-RvD3. Data are presented as means ± SEM (A and B). n ≥ 4 per group (A and B). P = 0.42 (A and B).
References
- 1.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Marik P.E. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 4.Serhan C.N., Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13:632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman S.D., Blanco P.G., Zaman M.M., Shea J.C., Ollero M., Hopper I.K., Weed D.A., Gelrud A., Regan M.M., Laposata M., Alvarez J.G., O'Sullivan B.P. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 6.Kasuga K., Yang R., Porter T.F., Agrawal N., Petasis N.A., Irimia D., Toner M., Serhan C.N. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eickmeier O., Seki H., Haworth O., Hilberath J.N., Gao F., Uddin M., Croze R.H., Carlo T., Pfeffer M.A., Levy B.D. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6:256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A., Flower R.J., Perretti M., Serhan C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang N., Fredman G., Backhed F., Oh S.F., Vickery T., Schmidt B.A., Serhan C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogerio A.P., Haworth O., Croze R., Oh S.F., Uddin M., Carlo T., Pfeffer M.A., Priluck R., Serhan C.N., Levy B.D. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalli J., Winkler J.W., Colas R.A., Arnardottir H., Cheng C.Y., Chiang N., Petasis N.A., Serhan C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukunaga K., Kohli P., Bonnans C., Fredenburgh L.E., Levy B.D. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 14.Dalli J., Serhan C.N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–e72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilberath J.N., Carlo T., Pfeffer M.A., Croze R.H., Hastrup F., Levy B.D. Resolution of Toll-like receptor 4-mediated acute lung injury is linked to eicosanoids and suppressor of cytokine signaling 3. FASEB J. 2011;25:1827–1835. doi: 10.1096/fj.10-169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banaszynski L.A., Chen L.C., Maynard-Smith L.A., Ooi A.G., Wandless T.J. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norling L.V., Spite M., Yang R., Flower R.J., Perretti M., Serhan C.N. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Y., Zhang M.J., Hellmann J., Kosuri M., Bhatnagar A., Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki H., Fukunaga K., Arita M., Arai H., Nakanishi H., Taguchi R., Miyasho T., Takamiya R., Asano K., Ishizaka A., Takeda J., Levy B.D. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 21.Matthay M.A. Resolution of pulmonary edema: thirty years of progress. Am J Respir Crit Care Med. 2014;189:1301–1308. doi: 10.1164/rccm.201403-0535OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson M.D., Widdicombe J.H., Allen L., Barbry P., Dobbs L.G. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci U S A. 2002;99:1966–1971. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Chemaly S., Levine S.J., Moss J. Lymphatics in lung disease. Ann N Y Acad Sci. 2008;1131:195–202. doi: 10.1196/annals.1413.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinton L.J., Mizgerd J.P. NF-kappaB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res. 2011;343:153–165. doi: 10.1007/s00441-010-1044-y. [DOI] [PubMed] [Google Scholar]
- 25.Libermann T.A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware L.B., Matthay M.A. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–L940. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 27.Yang J., Meyer M., Muller A.K., Bohm F., Grose R., Dauwalder T., Verrey F., Kopf M., Partanen J., Bloch W., Ornitz D.M., Werner S. Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J Cell Biol. 2010;188:935–952. doi: 10.1083/jcb.200910126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serhan C.N., Petasis N.A. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler J.W., Uddin J., Serhan C.N., Petasis N.A. Stereocontrolled total synthesis of the potent anti-inflammatory and pro-resolving lipid mediator resolvin D3 and its aspirin-triggered 17R-epimer. Org Lett. 2013;15:1424–1427. doi: 10.1021/ol400484u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffield J.S., Hong S., Vaidya V.S., Lu Y., Fredman G., Serhan C.N., Bonventre J.V. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 31.Hudert C.A., Weylandt K.H., Lu Y., Wang J., Hong S., Dignass A., Serhan C.N., Kang J.X. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnardottir H.H., Dalli J., Colas R.A., Shinohara M., Serhan C.N. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol. 2014;193:4235–4244. doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folkesson H.G., Kuzenko S.R., Lipson D.A., Matthay M.A., Simmons M.A. The adenosine 2A receptor agonist GW328267C improves lung function after acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2012;303:L259–L271. doi: 10.1152/ajplung.00395.2011. [DOI] [PubMed] [Google Scholar]
- 34.Dagenais A., Gosselin D., Guilbault C., Radzioch D., Berthiaume Y. Modulation of epithelial sodium channel (ENaC) expression in mouse lung infected with Pseudomonas aeruginosa. Respir Res. 2005;6:2. doi: 10.1186/1465-9921-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware L.B., Matthay M.A. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q., Lian Q.Q., Li R., Ying B.Y., He Q., Chen F., Zheng X., Yang Y., Wu D.R., Zheng S.X., Huang C.J., Smith F.G., Jin S.W. Lipoxin A(4) activates alveolar epithelial sodium channel, Na,K-ATPase, and increases alveolar fluid clearance. Am J Respir Crit Care Med. 2013;48:610–618. doi: 10.1165/rcmb.2012-0274OC. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q., Zheng X., Cheng Y., Zhang Y.L., Wen H.X., Tao Z., Li H., Hao Y., Gao Y., Yang L.M., Smith F.G., Huang C.J., Jin S.W. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J Immunol. 2014;192:3765–3777. doi: 10.4049/jimmunol.1302421. [DOI] [PubMed] [Google Scholar]
- 38.Baluk P., Tammela T., Ator E., Lyubynska N., Achen M.G., Hicklin D.J., Jeltsch M., Petrova T.V., Pytowski B., Stacker S.A., Yla-Herttuala S., Jackson D.G., Alitalo K., McDonald D.M. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori M., Andersson C.K., Graham G.J., Lofdahl C.G., Erjefalt J.S. Increased number and altered phenotype of lymphatic vessels in peripheral lung compartments of patients with COPD. Respir Res. 2013;14:65. doi: 10.1186/1465-9921-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podgrabinska S., Braun P., Velasco P., Kloos B., Pepper M.S., Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folpe A.L., Veikkola T., Valtola R., Weiss S.W. Vascular endothelial growth factor receptor-3 (VEGFR-3): a marker of vascular tumors with presumed lymphatic differentiation, including Kaposi's sarcoma, kaposiform and Dabska-type hemangioendotheliomas, and a subset of angiosarcomas. Mod Pathol. 2000;13:180–185. doi: 10.1038/modpathol.3880033. [DOI] [PubMed] [Google Scholar]
- 42.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N.A., Serhan C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwab J.M., Chiang N., Arita M., Serhan C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin Y., Arita M., Zhang Q., Saban D.R., Chauhan S.K., Chiang N., Serhan C.N., Dana R. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang X., Takahashi N., Matsui N., Tetsuka T., Okamoto T. The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J Biol Chem. 2003;278:919–926. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beers M.F., Morrisey E.E. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crosby L.M., Waters C.M. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonnans C., Fukunaga K., Levy M.A., Levy B.D. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen B.Q., Finkbeiner W.E., Wine J.J., Mrsny R.J., Widdicombe J.H. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am J Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- 52.Gomperts B.N., Belperio J.A., Fishbein M.C., Keane M.P., Burdick M.D., Strieter R.M. Keratinocyte growth factor improves repair in the injured tracheal epithelium. Am J Respir Cell Mol Biol. 2007;37:48–56. doi: 10.1165/rcmb.2006-0384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shyamsundar M., McAuley D.F., Ingram R.J., Gibson D.S., O'Kane D., McKeown S.T., Edwards A., Taggart C., Elborn J.S., Calfee C.S., Matthay M.A., O'Kane C.M. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med. 2014;189:1520–1529. doi: 10.1164/rccm.201310-1892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UV-visible light (UV-vis) absorption spectrogram of AT-RvD3. UV-vis spectrophotometry was used to measure the concentration of AT-RvD3 stock solution and confirm integrity of compound used in all experiments.
Impact of AT-RvD3 on lung histology after acid injury. Higher-power magnifications of acid-injured lung at 24 hours after injury. Representative images of vehicle (left panels) and AT-RvD3–treated mice (right panels) are shown. Asterisks denote edema present in airspaces; arrows, leukocytes.
Evaluation of the impact of AT-RvD3 on aquaporin 5 expression in acid-injured mice. A: Representative immunoblot for aquaporin 5. Mice were subjected to the acid injury protocol; AT-RvD3 (10 ng) or vehicle control were injected i.v. 1 hour after injury. Each column represents an individual animal. B: Densitometry of results shown in A. Results are presented as means ± SEM (B). n ≥ 5 (B). P = 0.36 (B).
Impact of AT-RvD3 on lymphatic vasculature, as demonstrated by vascular endothelial growth factor receptor (VEGFR) 3 immunohistochemistry. A and B: Representative images of VEGFR3 staining in vehicle (A) and AT-RvD3–treated (B) mouse lung 72 hours after injury. Mice were injured as described; AT-RvD3 or vehicle control were administered i.v., 1 hour after injury. Arrows denote VEGFR3+ lymphatic vessels. C: Quantification of VEGFR3+ vessels in injured mouse lung. Data are presented as means ± SEM (C). n = 3 per group (C). P = 0.1 by U-test (C).
Impact of AT-RvD3 on surfactant protein A and D (SP-A and SP-D, respectively) expression in the lung acid injury model. Representative immunoblots for SP-A (A) and SP-D (B) expression. Each lane represents a bronchoalveolar lavage fluid sample from an individual mouse.
Response to Escherichia coli pneumonia in mice given AT-RvD3 (10 ng i.v., 1 hour after injury). Mice received 1 × 106 bacteria intratracheally. Colony-forming units (CFUs) and bacterial growth index (BGI) were assessed 6 hours after instillation. A: CFUs of E. coli in mice given vehicle or AT-RvD3. B: BGI in mice given vehicle or AT-RvD3. Data are presented as means ± SEM (A and B). n ≥ 4 per group (A and B). P = 0.42 (A and B).