Abstract
During decision-making, neurons in multiple brain regions exhibit responses that are correlated with decisions1-6. However, it remains uncertain whether or not various forms of decision-related activity are causally related to decision-making7-9. Here we address this question by recording and reversibly inactivating the lateral intraparietal (LIP) and middle temporal (MT) areas of rhesus macaques performing a motion direction discrimination task. Neurons in area LIP exhibited firing rate patterns that directly resembled the evidence accumulation process posited to govern decision making2,10, with strong correlations between their response fluctuations and the animal's choices. Neurons in area MT, in contrast, exhibited weak correlations between their response fluctuations and animal choices, and had firing rate patterns consistent with their sensory role in motion encoding1. The behavioral impact of pharmacological inactivation of each area was inversely related to their degree of decision-related activity: while inactivation of neurons in MT profoundly impaired psychophysical performance, inactivation in LIP had no measurable impact on decision-making performance, despite having silenced the very clusters that exhibited strong decision-related activity. Although LIP inactivation did not impair psychophysical behavior, it did influence spatial selection and oculomotor metrics in a free-choice control task. The absence of an effect on perceptual decision-making was stable over trials and sessions, arguing against several forms of compensation, and was robust to changes in stimulus type and task geometry. Thus, decision-related signals in LIP do not appear to be necessary for computing perceptual decisions. Our findings highlight a dissociation between decision correlation and causation, showing that strong neuron-decision correlations may reflect secondary or epiphenomenal signals, and do not necessarily offer direct access to the neural computations underlying decisions.
We investigated the functional significance of decision-related activity by recording and inactivating neural activity in two well-studied cortical areas, MT and LIP, while rhesus monkeys performed a challenging motion discrimination task. On each trial, the monkey maintained stable visual fixation while discriminating the net direction of a visual motion stimulus, and then made a saccade to one of two choice targets to communicate their choice (Fig. 1a, b). For electrophysiological recordings in MT, we placed the motion stimulus in the receptive field of the neurons and aligned it with the preferred direction of one or more MT neurons on the multi-electrode array. For LIP, we placed one of the two targets in the response field of the neurons (as opposed to the visual motion stimulus), and the other target on the contralateral side of the visual field, consistent with previous studies of decision-related responses in LIP 11.
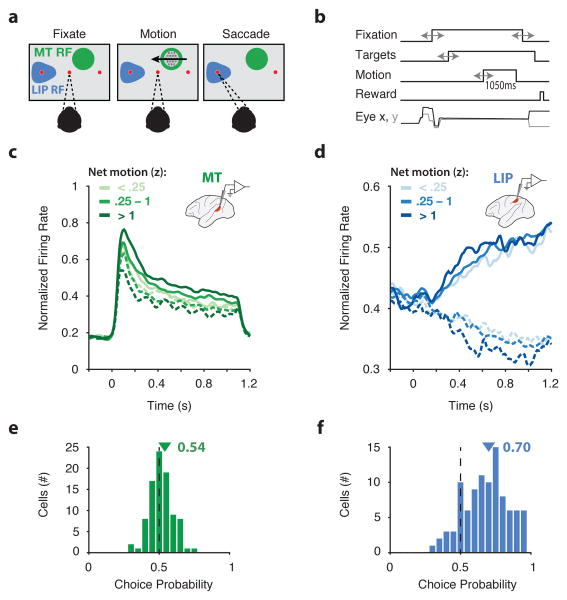
Figure 1. Task and neural responses during direction discrimination.
a, Monkeys were trained to discriminate the direction of visual motion and communicate their decision with a saccadic eye movement to one of two choice targets. For MT recordings, motion was placed in the MT receptive field (RF) (green patch). For LIP recordings, one of the saccade targets was placed in the LIP RF (blue patch). b, Sequence of task events. Gray arrows indicate temporal jitter. c, Average response of 94 MT neurons as a function of motion strength (grouped by z-scored net motion, see Methods) and direction (in versus opposite of cell's preferred direction, solid and dashed lines, respectively), aligned to motion onset. d, Average response of 113 LIP neurons as a function of motion strength and direction (in and out of cell's RF, solid and dashed lines, respectively), aligned to motion onset. e, Choice probability for 90 MT neurons computed during the motion epoch. Triangle indicates mean, 0.54. f, Choice probability for 96 LIP neurons computed during the motion epoch. Triangle indicates mean, 0.70. Only neurons with >20 repeats of identical stimuli were included in the choice probability analysis.
We recorded 157 MT neurons and 200 LIP neurons with either single electrodes or multi-electrode linear arrays. MT neurons that were well-targeted by the stimulus (n = 94) had average firing rates that depended on the motion strength and direction (Figure 1c). As expected in this area, responses increased sharply after motion onset and maintained a robust firing rate throughout motion viewing12. The average responses of well-targeted LIP neurons (n = 113) were also consistent with classical observations2,11, exhibiting ramp-like increases or decreases in firing rate whose slopes were proportional to motion strength, the primary physiological characteristic implicating LIP in reflecting the accumulation of evidence over time (Fig. 1d).
We further quantified the decision-related activity of MT and LIP using choice probability1 (CP), a measure of correlation between neural activity and choice behavior, independent of stimulus-driven responses. MT neurons were weakly but reliably correlated with the animal's choice on a trial-by-trial basis (mean CP = 0.54, p = 1e-5; Fig. 1e). LIP neurons were more strongly correlated with choices (mean CP = 0.70, p = 1e-21; Fig. 1f). Thus, the stimulus-dependent responses and choice probability in MT were consistent with its well-established role in representing the motion stimulus, and the response patterns in LIP resembled the timecourse of an evolving decision process. Together, these properties have given rise to a model where LIP neurons either integrate, or reflect the integration of, motion evidence from area MT in favor of a decision11,13.
Having confirmed the neurophysiological properties of areas MT and LIP and their differential degrees of correlations with decisions, we tested their respective causal contributions by performing reversible inactivations in each area and evaluating the impacts on psychophysical performance (hypothesized outcomes shown in Fig. 2a). We infused muscimol (a GABA-A agonist which hyperpolarizes cell bodies but not fibers of passage14) into either MT or LIP, 1mm away from a multi-electrode array (Fig. 2b). The injection cannula was targeted to locations that had yielded the largest number of canonical MT or LIP units during recording sessions (Extended Data Fig. 1). The multi-electrode array was used to confirm both pre-infusion physiological properties and post-infusion neural silencing, performed on every inactivation session. Silencing was typically observed across all recording channels of the array (Fig. 2b) and estimated to span a spherical volume of ∼2.5mm radius (see Methods).
Figure 2. Psychophysical performance before and after neural inactivations in areas MT and LIP.
a, Hypothesized consequences of inactivation on the psychometric function. Left, decreased psychophysical sensitivity would correspond to a decrease in slope. Right, changes in psychophysical bias would correspond to a shifted midpoint. Positive values in the x-axis (z-scored motion strength) refer to motion towards the target contralateral to the LIP under study. Correspondingly, the y-axis refers to the proportion of contralateral target choices. This convention is maintained throughout. b, Schematic of the inactivation protocol. Left, A multi-electrode array was lowered alongside the cannula to identify the targeted cortical location, to verify neural selectivity prior to infusion, and to confirm neural silencing after. Right, continuous voltage traces from an example inactivation session in which neural silencing is evident ∼10 minutes after infusion start. c, d, Psychophysical data for averaged pairs of baseline and muscimol treatment sessions in MT (c), and LIP (d). Insets illustrate the brain region inactivated (top) and the corresponding experimental geometry (bottom), along with the estimated inactivated field (gray cloud). Error bars on points show ±1 SEM across all sessions. e, The distribution of psychometric function parameters, slope (top) and shift (bottom), reflecting sensitivity and bias, respectively, for baseline (x-axis) and treatment (y-axis) session pairs for MT inactivations (green symbols), LIP inactivations (blue symbols), as well as LIP saline (open gray symbols) and sham/control experiments (filled gray symbols), for monkey N (diamonds) and monkey P (squares). Error bars show 95% confidence intervals for individual sessions. f, g, Psychophysical weighting, estimated via reverse correlation. Y-axis indicates how much the subject weighed each of the motion stimulus pulses over all baseline and inactivation session pairs in MT (f) and in LIP (g), for monkey N (top) and monkey P (bottom).
Inactivations in area MT exerted large effects on psychophysical performance. The motion stimulus was placed within a region of visual space retinotopically matched to the inactivated population of MT neurons (Fig. 2c). MT inactivations (n = 6; monkey N, 3; monkey P, 3) had a large and consistent impact on direction discrimination sensitivity (68.5% reduction from baseline, t(5) = -9.7, p = 0.002, paired t test). When the motion stimulus was moved outside the inactivated region within the same session (n = 3), psychophysical performance was restored, confirming that the effects were not due to general changes in arousal or vigilance (Extended Data Figure 2). These severe and specific impairments in direction discrimination performance were consistent with prior causal perturbations15,16.
In contrast, inactivations in area LIP (n = 21; monkey N, 12; monkey P, 9) did not exert compelling or substantial effects on psychophysical performance (Fig. 2d). In these experiments, we placed one choice target in the inactivated region of visual space, in line with previous electrophysiological investigations that placed a choice target (and not the visual motion stimulus) in the response fields of LIP neurons to elicit the area's canonical decision-related responses. Although we performed large inactivations in locations where LIP electrophysiology had mirrored the accumulation of evidence and demonstrated strong decision-related activity, we did not detect significant changes in either the animal's sensitivity or bias, as indicated by statistically-indistinguishable differences in the slope (3.7% reduction from baseline, t(20) = -1.4, p = 0.16, paired t test) or midpoint (-0.4% shift, t(20) = -0.08, p = 0.93, paired t test) of the psychometric functions. Saline and sham control experiments showed similar patterns to the main baseline vs. muscimol treatment comparison (Extended Data Table 1). Thus, while the impact of MT inactivation on sensitivity was substantial, an effect of LIP inactivation was not clearly identifiable using our techniques and task (Fig. 2e).
We also assessed whether inactivation affected the timing or strategy of evidence integration8,17,18. For example, if LIP supported the temporal integration of motion evidence, inactivation could alter the strategy to reflect “leakier” integration that might still support the same overall performance. Contrary to this possibility, inactivation in LIP did not lead to greater reliance on either early or late information (Fig. 2f and 2g), estimated via reverse correlation. Inactivations in area MT, in contrast, reduced the psychophysical weighting of motion roughly evenly over time.
Although inactivation in LIP had no measurable effect on direction discrimination, it did exert effects on a “free-choice” control task, which was performed on every inactivation session (Fig 3a and 3b). LIP inactivation biased choices away from the contralateral hemifield (8.88% reduction from baseline on average, t(33) = 3.4, p = 0.001, paired t test), (Fig. 3c and 3d), consistent with previous reports in monkeys19-21, rodents8, and parietal lesions in humans22. Thus, our electrophysiological confirmation of LIP inactivation was complemented by a behavioral consequence in this free-choice control task. In addition to causing a spatial bias, LIP inactivation led to an increase in endpoint error of saccades made to the hemifield contralateral to inactivation (0.36° on average, t(33) = 4.4, p < 0.0001, Fig. 3c and 3d). No systematic change was detected in other oculomotor metrics during the free-choice task (reaction time, peak velocity, or duration), and no effects on any oculomotor metrics were detected during the direction discrimination task. Despite observing a muscimol-induced effect in the free-choice task, effect magnitude in the free-choice task was not predictive of effect magnitude in the direction discrimination task (Extended Data Fig. 3a,b), nor was there a dose-response relationship between muscimol mass and behavioral performance (Extended Data Fig. 3c-e), suggesting that our large muscimol administrations were likely operating within a “ceiling” regime.
Figure 3. Performance in control tasks following LIP inactivation.
a, The “free-choice” task. Following a 200ms long presentation of two targets at random locations in space, monkeys were required to hold fixation for another 600-3,000ms, and then to move their eyes to the remembered location of either target. b, Task timing. Events in the task were presented in sequence and were jittered in time (gray arrows). c, The effect of LIP inactivation on choice bias and saccade accuracy in the free-choice task (example session): saccade landing points (black dots) have been aligned to target position (red dot), for contralateral (left) and ipsilateral target choices (right), during baseline (top) and inactivation (bottom). Both saccadic accuracy and percent contralateral choices (noted in text, top left) are reduced after LIP inactivation, to the contralateral hemifield. d, The effect of LIP inactivation on choice bias and saccade accuracy in the free-choice task, over all sessions. Histograms show baseline/inactivation differences in proportion contralateral choices (top) and saccade error (bottom), where positive numbers indicate an increase in metric following inactivation. Dark bars indicate sessions that took place on the same days as the main direction discrimination experiment (“Main experiment inactivations”, n=21; monkey N, 12; monkey P, 9); dark triangle indicates the median difference. Light bars include an additional 13 sessions that took place during other inactivation experiments under similar conditions (“All inactivations”, n=34; monkey N, 14; monkey P, 20); light triangle indicates median difference (visually occluded by dark triangle). e, Psychophysical data for pairs of baseline and muscimol treatment in LIP when both choice targets were placed within the inactivated field. Inset presents stimulus geometry and estimated inactivated field.
Because muscimol inactivations require comparisons across relatively long time scales, it remains logically possible that LIP normally plays a critical role in decision making, but that other areas are processing information in parallel and are able to quickly compensate when it is artificially inactivated. Although other techniques with faster time scales will allow for more direct tests of this possibility, we did not observe changes indicative of compensation either within a session or over sessions (Extended Data Fig. 4 and 5, respectively). We also tested for compensation involving the non-inactivated hemisphere23. We performed 6 inactivation experiments with both choice targets placed in a single hemifield (Fig 3e, inset), in order to maximize reliance on a single LIP and minimize involvement of the other hemisphere. Inactivation of the LIP corresponding to the two targets did not produce clear changes in behavioural performance (Fig 3e), indicating that inter-hemispheric compensation was unlikely in our main experiments. Previous LIP inactivation studies also find no evidence in support of compensation that manifests behaviourally (see Spatial and temporal extent of inactivation, Methods). We also found no disruption of decision making performance using the moving-dot stimulus used in previous studies of MT and LIP function during decision-making2,15 (Extended Data Fig. 6c).
Our results reveal a dissociation between decision-related activity in LIP and the causal role of such activity in decision-making. Instead, decision-related signals in LIP may be a result of feedback24, or an emergent phenomenon driven by extensive training25. Although one prior study observed effects of LIP microstimulation in a reaction time direction discrimination task26, such electrical perturbations can produce orthodromic (and antidromic) activation of connected areas, and their observed effects are reconcilable with multiple alternatives to evidence accumulation6. It remains possible that LIP contributes to decision-making in conjunction with associated brain regions, whose parallel and/or redundant processing simply renders LIP non-necessary in the particular tasks used to study its decision-related activity. Indeed, a growing body of work has observed decision-related activity in other brain areas3-6,9, consistent with the prospect of LIP playing a minor and/or nonessential role in decision-making. Our results mirror findings in rodent posterior parietal cortex, where inactivations did not affect decision-making despite electrophysiological correlates of evidence accumulation 8. Finally, a richer appreciation of LIP's contributions to decision-making might be gleaned from placing the motion stimulus itself (as opposed to the saccadic choice target) within the inactivated field, a configuration studied electrophysiologically in a categorization task27 but not yet causally investigated.
Taken together, decision-related activity is likely represented broadly across the brain, and may be “read out” by a flexible process to support behavior, in LIP or elsewhere7,18,28. Our results call for a broader consideration of both decision-making circuitry and the mechanisms for reading out decision-related activity— regardless of whether decisions are conveniently reflected, or actually computed, in the activity of a particular brain area23,29,30.
Methods
Monkey preparation
We performed electrophysiological recordings and reversible inactivations in the middle temporal (MT) and the lateral intraparietal (LIP) cortices of two rhesus macaques (subject N and subject P), female and male, aged 10 and 14 years, weighing 7.7 and 10 kg, respectively. Subject N had a custom titanium chamber that enabled access to both MT and LIP on the right hemisphere (L9, P2), guided by MRI. Subject P had a cilux chamber (Crist Instruments) over the right LIP (L12, P5) and another over the left V1 for a posterior approach to MT (L17, P17). Standard surgical procedures were applied31. All experimental protocols were approved by The University of Texas Institutional Animal Care and Use Committee and in accordance with National Institute of Health standards for care and use of laboratory animals.
The subject sat comfortably while head-posted in a primate chair (Crist Instruments), facing a linearized 55 inch LCD (LG) monitor (resolution = 1920 × 1080p, refresh rate = 60Hz, background luminance = 26.49 cd/m2) at a distance of 118cm, in a dark room. Eye position was recorded using an Eyelink 1000 eye tracker (SR Research), sampled at 1 kHz. A solenoid-operated reward system was used to deliver liquid reward to the monkey. Stimuli were generated by using the Psychophysics Toolbox32 in MATLAB (The MathWorks), and task events and neural responses were recorded (Plexon) using a Datapixx I/O box (Vpixx) for precise temporal registration. All of these systems were integrated using the PLDAPS system 33.
General procedure and experimental design
Recording sessions in either MT or LIP began by lowering an electrode to the known location of the area based on previous mapping and recording sessions. Anatomical identification (MR guided in monkey N; previously established in monkey P31) was followed by functional identification (mapping receptive/response fields (RF) of MT and LIP neurons, detailed below). Inactivations of either area began by lowering both a cannula and multichannel electrode array to the region of interest, collaterally, at least 1mm apart. The electrode array was used to (i) confirm that the cannula is within the target cortex, (ii) to record electrophysiological responses to relevant task events pre-infusion, and (iii) to confirm the electrophysiological silencing of neurons during and after the infusion. Thus, while it is not feasible to precisely measure the inactivated proportion of an area, we do confirm the silencing of a large swath (approximately 2.5mm in radius), on every session (detailed in Infusion Protocol, below).
MT inactivation was predicted to disrupt motion direction discrimination sensitivity within a specific region of contralateral space, consistent with MT retinotopic organization15,16. The behavioural consequence of MT inactivation was measured by comparing psychophysical performance in the direction-discrimination task, before and after muscimol infusion, within the same experimental session, with the motion stimulus placed inside the inactivated region of space. LIP inactivation was predicted to disrupt spatial selection to contralateral space more generally8,19-21,34,35, noting that LIP RF are large and that the topographic organization is less precise than in earlier visual areas36. The behavioural consequence of LIP inactivation was measured by comparing the proportion of contralateral choices in a double-target memory-guided “free-choice” task, before and after muscimol infusion, within the same session. To measure the impact of LIP inactivation in the direction-discrimination task, we compared psychophysical performance between pairs of sessions, baseline and treatment, in which the treatment session was a muscimol, saline, or sham infusion treatment. The paired sessions typically took place 1 day apart at the same time of day and after a similar number of tasks and trials, to minimize the impact of within-session fatigue or motivation on behaviour. Behavioural data were collected 15-30 minutes after muscimol/saline/sham infusion end, and always completed within 150 minutes. An additional 16 control pairs (without saline or sham manipulation) were collected to better estimate session-to-session variability. Statistical results do not depend on the inclusion/exclusion of these control session pairs.
Direction discrimination task
The principal task was a motion direction discrimination task. Subjects were required to discriminate the net direction of a motion stimulus and communicate their decision with an eye movement to one of two targets. The sequence of task events is presented in Fig. 1a. The timing of each event was randomly jittered from trial to trial (Fig. 1b). A trial began with the appearance of a fixation point. Once the monkey acquired fixation and held for 400-1200ms (uniform distribution), two targets appeared and remained visible until the end of the trial. 200-1000ms after target onset, the motion stimulus was presented at an eccentricity of 5-7° for 1050ms. The fixation point was extinguished 200-1000ms after motion offset, and the subject was required to shift its gaze towards one of the two targets within 600ms (saccade end points within 3° of the target location were accepted).
We used a reverse-correlation motion stimulus inspired by the classic moving dots stimulus15 in which motion was in either one direction or the opposite, with varying motion strength. The motion stimulus consisted of 19 non-overlapping Gabor elements arranged in a hexagonal grid (5-7° across, scaled by eccentricity). The individual elements were set to approximate the RF size of a V1 neuron and the entire motion stimulus approximated the RF size of an MT neuron. Motion was presented by varying the phase of the sine-wave carrier of the Gabors. Each Gabor underwent a sinusoidal contrast modulation with independent random phase to prevent perceptual “pop-out” of individual drifting elements. Gabor spatial frequency (0.9 cycles/°, sigma = 0.1 × eccentricity) and temporal frequency (7Hz for monkey N, 5Hz for money P, yielding velocities of 7.77 and 5.55 °/s, respectively) were selected to match the approximate sensitivity of MT neurons.
Each trial comprised seven consecutive motion pulses lasting 150ms each (9 video frames), producing a pulse sequence of 1050ms in duration. On any given pulse Xi, a number of Gabors would have their carrier sine waves drift in unison to produce motion (“signal” Gabors), and the remaining would counter-phase flicker (“noise” Gabors). Signal Gabors on pulse Xi were assigned at random within the grid and all signal Gabors drifted in the same direction.
Motion strength was defined as the proportion of signal Gabors out of the total, the value of which was drawn from a Gaussian distribution, Xi ∼ N(μk, σ) and rounded to the nearest integer, where μk was set to one of five values at random: -50%, -12%, 0%, 12%, and 50% (negative sign indicates motion in the opposite direction), and σ was set to 15%. Thus, while each pulse within a sequence could take on any value (or sign) from distribution N(μk, σ), the expectation of a sequence would be μk. Motion strength was then z scored over all sessions, for each monkey separately.
On the motion strength axis, we use positive values to indicate motion towards the hemifield contralateral to the LIP under study, and negative values to indicate motion towards the hemifield ipsilateral to the LIP understudy. We use the term “Proportion choices” to refer to the proportion of choices towards the contralateral target. For consistency, we maintain this convention throughout the paper, such that even on MT inactivations sessions, psychometric performance is evaluated in relation to the LIP under study.
The monkey was rewarded for selecting the target consistent with the sign of the motion pulse sequence sum (i.e., the net direction), independent of the distribution μk from which they were drawn. On trials that summed to exactly zero, the monkey was rewarded at random. 10% of trials consisted of a frozen random seed, generating identical pulse sequences. In addition to the direction discrimination task described here, we performed a subset of experiments (n=2) using the classical moving dots stimulus15 with motion coherence values of 0, 3.2, 6.4, 12.8, 25.6, 51.2% (Extended Data Fig. 6c).
Free choice task
A free choice task was used to measure spatial bias to one target over another and confirm a behavioural consequence of LIP inactivation8,21,35. The task was performed before and after every LIP inactivation experiment (n=21 during experiments using the standard direction discrimination task, n=13 during other experiments, see for example Extended Data Fig. 6c). The sequence of events within the free-choice task is illustrated in Fig. 3a and 3b. Trials began with the appearance of a central fixation point. At a random time after acquiring fixation (500-900ms), two targets were simultaneously flashed for a brief 200ms. Subjects were required to maintain fixation until the fixation point disappeared (600 to 3,000ms after target flash), and then saccade to either of the remembered locations of the two targets. On every trial, target position was determined independently from one another and at random, drawn from a 2D Gaussian with a mean of either [-12, 0] (left target) or [12, 0] (right), and a standard deviation of 2-4° for x and 3-5° for y position. Means and standard deviations were sometimes adjusted online to better position the distributions within the LIP RF (when recorded) or LIP inactivated field (when inactivated).
A trial was successfully completed when the monkey's saccade entered a circular window (unobservable to the monkey) around either target and held for 300-500ms (window radius scaled by 0.35° × eccentricity, minimum: 3°). Successfully completed free-choices were rewarded on 70% of trials irrespective of the target chosen for monkey N, and 100% of trials for monkey P. Monkey N also performed memory-guided saccades to single targets (30% of trials, randomly interleaved) that appeared randomly in space (uniform distribution), and were rewarded 100% of the time. The adjustments in subject N's task were performed to prevent a spatial bias and encourage exploration. Overall performance and inactivation effects were similar between monkeys despite subtle differences in task parameters.
Behavioural analysis
All analyses were performed in Matlab (The Mathworks). Responses in the direction discrimination task were analyzed with a maximum likelihood fit of a two parameter logistic function37 assuming a Bernoulli distribution of binary choices, in which the probability of a contralateral choice is P and ipsilateral choice is 1-P, where P is given by:
where x is the motion strength value (z-scored over all sessions for each monkey separately), α is the bias parameter (reflecting the midpoint of the function in units of motion strength), and β is the slope (i.e., sensitivity, in units of log-odds per motion strength). Error estimates on the parameters were obtained from the diagonal of the inverse Hessian (2nd derivative matrix) of the negative log-likelihood. A four-parameter model including sub-perfect response rates for the top and bottom asymptotes8 was also considered, but did not confer any advantage over the two-parameter model nor change analysis results, and so we focus on the simpler 2-parameter fit (Extended Data Table 1). The first 10-30 trials of every session were excluded from analysis because motion strength was maximal to “warm up” the animal. Median session length for all baseline and treatment sessions was 409 trials. Sessions were excluded from analysis if the animal either completed less than 250 trials or performed poorly (lapse rate >10%). For inactivation sessions, all sessions were included regardless of performance. A single inactivation session in monkey P was aborted due to a leak in the infusion system, and was not included in the analysis.
Animal strategy in the direction discrimination task (Fig. 2f and 2g) was measured by computing psychophysical weights via logistic regression, where the probability of the binary choice Y∈{0,1} on every trial is given by
where X is a matrix of the seven pulse values on each trial, augmented by a column of ones to capture the bias term, and w is a vector of the monkey's weights. We computed the maximum likelihood estimate of the weight vector w using Matlab's glmfit function.
In the free-choice task, spatial bias was computed as the proportion of choices to the target contralateral to the LIP under study. Saccade onset and offset were detected in every task by identifying the time at which eye velocity exceeded 30 °/sec (onset) and returned below 50 °/sec (offset). We only analyzed saccades on trials where the task was completed successfully (i.e. no broken fixations and no saccades outside of the target windows). Saccades were analyzed for reaction time, amplitude, duration, and error amplitude (i.e. distance of saccadic end point from saccadic target). Saccadic reaction times less than 100ms from the go signal were excluded to ensure that only task relevant saccades are analyzed.
Neuronal recordings
Recordings were performed in areas MT and LIP with either single-channel glass coated tungsten electrodes (Alpha Omega) or multi-electrode arrays (Plexon U or V Probe). Neuronal signals were amplified, bandpass filtered, digitized, and saved (Plexon MAP server). Neural waveforms passing a manually-set threshold were isolated for online mapping of their receptive fields (both MT and LIP) and directional tuning (MT).
MT RF locations were hand mapped using drifting dot stimuli in a circular aperture. Once the retinotopic location was identified, direction preference and selectivity were measured using drifting dot stimuli at 100% coherence in 12 directions. LIP RF locations were mapped with a memory-guided delayed saccade task38.
In monkey P, offline spike sorting was performed by hand refinement of a standard clustering algorithm (Plexon Offline Sorter v3). Single unit isolation quality was established using SNR39. In monkey N, spike sorting was performed by fitting a mixture of Gaussians model to clipped waveforms in a reduced dimensional space40. In both monkeys, sorting was refined by maximum a posteriori estimation of a model, where the multi-electrode voltage was the linear superposition of Gaussian white noise and the spike waveforms41,42.
Neuronal Analysis
Peri-stimulus time histograms (PSTHs) were computed by aligning spike times to events (motion onset or saccade time), binned at 10ms resolution, and smoothed with a Gaussian kernel with standard deviation of 25ms. Trial motion strengths were binned into three groups: between 0 and 0.25, between 0.25 and 1, and greater than 1. We averaged spike rates separately for the three motion strengths for each choice. Note that these motion strengths correspond to a narrower range than that used in previous studies11, selected to encourage longer integration times. This is evident in the PSTHs (narrow dynamic range) and psychometric functions (fewer data points in the asymptotic range of behavior).
Choice Probability
Choice probability (CP) is a metric used to measure the predictive relationship between neural responses and choice, independent of stimulus strength. It is defined as the area under the receiver operating characteristic curve (ROC) for a pair of spiking response distributions sorted by choice1,43. We quantified CP using trials that had zero expected motion and were repeated with identical random seeds (i.e. had no stimulus variation, “frozen noise”). Sometimes more than one random seed was repeated in a session, in which case we calculated the spiking response distributions for each seed separately, subtracted the mean, and then combined them, similar to an analysis known as Grand Choice Probability 1. Neurons with >20 “frozen” repeats were included (90/94 MT cells, 96/113 LIP cells), and significance testing against the null (i.e. CP=0.5) was performed using a Student's t test. In MT, we counted spikes during the motion epoch (1050ms), In LIP, we counted spikes over a 400ms window counting backwards from the 100ms before the saccade.
Infusion Protocol
Infusions were performed by lowering an infusion cannula into grid locations that had previously yielded the largest number of selective cells during the recording phase of the study (Extended Data Figure 1). The cannula (31-32 gauge) was lowered alongside a multi-electrode array, at least 1mm away (Fig. 2a). The two were lowered to target cortical areas where functional identification took place (mapping). Infusion was then performed, and electrophysiological silencing was confirmed on the recording electrodes, typically within 15 minutes of infusion start.
Infusions were performed with a syringe pump (Harvard Apparatus) through a single and direct line to the cannula (constant rate of 0.1-0.4μl/min, 15-30 minutes), in agreement with infusion parameters proposed by Noudoost and Moore44. We delivered 6.66-8μg/μl muscimol (in phosphate buffered saline) at volumes of 5-12 μl (mean 7.4μl), netting a total mass of 40-80μg (mean 56.4μg). This protocol was chosen to match the very high end of ranges used previously in order to maximize the probability of neural inactivation. Infusions were typically made at multiple depths within a single cannula track. On 5 of the 21 main LIP inactivation sessions, more than one cannula was lowered (Extended Data Table 2). Cannluae were left in situ for at least 15 minutes after infusion end. Saline infusions followed the same protocol and included both a cannula and multi-electrode array. Sham infusions included only a multi-electrode array but followed similar timings, including the operation of the syringe pump with no syringe attached.
Spatial and temporal extent of Inactivation
Previous analyses of the spatial extent of muscimol inactivation have estimated the functional silencing to cover a spherical radius of roughly 2-3mm34,45-47. The study most comparable to ours, Liu et al.34, co-infused muscimol and Manganese (Mn) into LIP of awake macaques and imaged the spread. They also estimated a cortical silencing of approximately 2-3mm in radius, in line with the linear dependence of volume distribution (mm3) on infusion volume (μl)48.
In our experiments, lowering both a multi-electrode array and infusion cannula collaterally (Fig. 2b) enables direct confirmation of neural silencing at known distances from the cannula tip. This places a lower bound on the spatial extent of functional inactivation. Although our standard protocol placed the multi-electrode array 1mm away from the cannula tip, we sometimes lowered a second array, 2 or 3mm away. On these sessions too, we observed silencing on most recording channels. Taken together, we conservatively estimate neural inactivation in LIP to span a radius of at least 2.5mm, silencing large swaths of LIP while primarily targeting its ventral portion34,49. For inactivations of this spatial magnitude there is no evidence that larger inactivations result in larger behavioural deficits20. Similarly, we did not observe a dose-response function in our own data (Extended Data Figure 3c-e).
On a few occasions, residual firing persisted despite near-complete silencing of electrophysiological activity (example shown in Fig. 2b, voltage traces, channels 5 and 6). We tested the selectivity of residual firing with the appropriate mapping task (motion for MT, memory guided saccades for LIP) and found that these spikes did not respond selectively, indicating that these residual spikes likely emanate from afferent fibers terminating within the inactivated area50.
Previous LIP inactivation studies found no evidence to support within-session compensation that manifests behaviourally19,20,34,47,51, but see Wilke et al.21 In fact, studies that report the temporal effect of LIP inactivation find an increase in the impact over time, not a decrease19,51. Regardless, we explored the time course of psychophysical performance within a session (Extended Data Fig. 4), and also measured for compensation on longer time scales, across sessions, to explore the possibility of increasing behavioural robustness to inactivation that might develop over time (Extended Data Fig. 5).
Extended Data
Extended Data figure 1.

Location of LIP recording and muscimol infusion sites The recording (blue circles) and infusion sites (red) for monkey N (panel a) and monkey P (b) along the medial-lateral (M/L) and posterior-anterior (P/A) axes within the chamber (demarcated by the ovals). Electrode and cannula tracks are represented by the gray lines (with a small jitter on the x-y plane for better visualization). The mean infusion depths were 7.12 ± 1.15 (monkey N) and 7.03 ± 1.39 (monkey P) (microdrive was zeroed below dura mater and just above the cortical surface). Given the estimated spread of muscimol described in the main text, the inactivations targeted a substantial territory of the ventral portion of LIP 49. Even though a functional distinction with depth has been proposed 34, we emphasize that the critical component of our protocol was targeting the precise locations at which we measured canonical decision-related activity in LIP.
Extended Data figure 2.
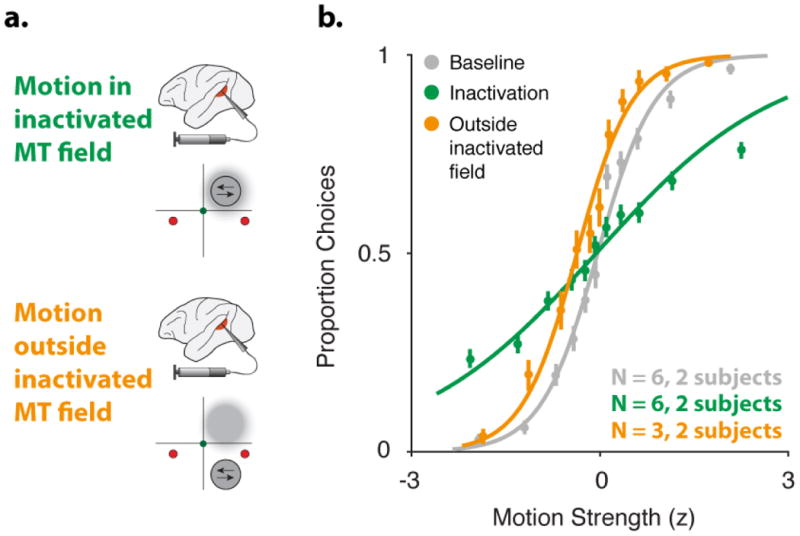
Direction discrimination sensitivity is restored when motion is placed outside of the inactivated MT field a. Illustration of MT inactivation along with the estimated inactivated field (gray cloud), for two experimental geometries: motion stimulus placed inside the inactivated MT field (top) and motion placed outside the inactivated MT field (bottom). b. Average psychophysical data for baseline and muscimol treatment pairs (gray and green, respectively, same data as Fig. 2c, n=6; monkey N, 3; monkey P, 3) and psychophysical data collected during muscimol treatment, with the motion stimulus outside of the inactivated MT field (orange, n=3). Direction discrimination sensitivity is restored to baseline levels in these sessions. Error bars on points show ±1 SEM across all sessions.
Extended Data figure 3.
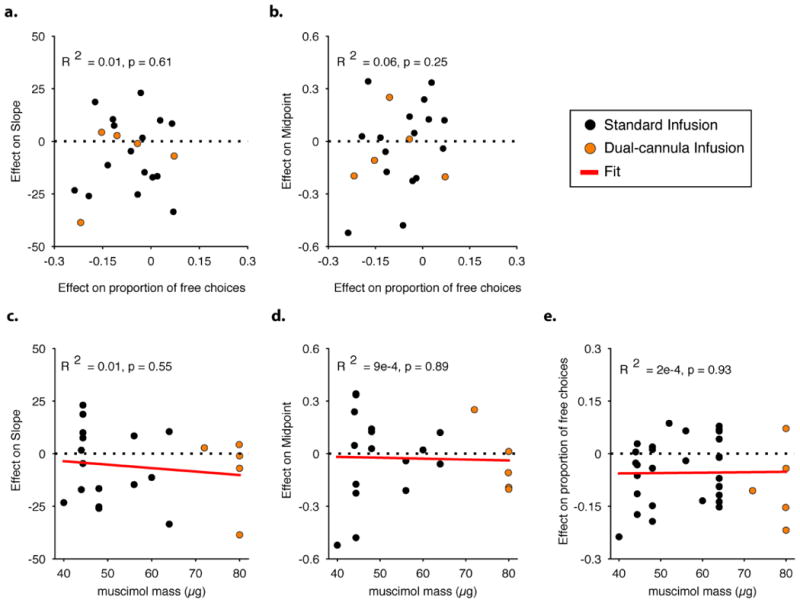
No relationship between effect magnitude in control task, effect magnitude in direction discrimination task, and muscimol mass a, b, The relationship between the effect of LIP inactivation in the free-choice task (i.e. shift in proportion of contralateral choices from baseline to muscimol treatment) and the effect of LIP inactivation in the direction discrimination task on sensitivity (i.e. %change in psychometric function slope, panel a) and bias (i.e. shift in normalized motion strength, panel b). R2 and associated p values of a Pearson correlation are indicated on individual plots (n=21; monkey N, 12; monkey P, 9). Orange data points indicate sessions in which muscimol was infused from two cannulae simultaneously into LIP. c, d, e, Dose-response functions between muscimol mass and the effect in the direction discrimination task on slope (c, same units as panel a), bias (d, same units as panel b), and the effect in the free-choice task (e, same units as a, b). For panel e we used free-choice sessions that took place on the same days as the direction discrimination task (n=21) along with an additional 13 session that took place during other inactivation experiments under similar conditions (n=34 in total; monkey N, 14; monkey P, 20; as in Fig. 3d). R2, associated p values and regression lines are indicated on the plots (linear regression).
Extended Data Figure 4. Time course of accuracy and bias within sessions.

Accuracy and bias in the direction discrimination task were computed over time by taking a running mean of correct and contralateral choices, respectively (sliding window of 40 trials). a, Inactivation in area MT (n=6, green curve; monkey N, 3; monkey P, 3) had a clear and consistent impact on behavioural accuracy compared to baseline (n=6, gray), but did not have systematic effects on bias (bottom), consistent with our results from the fitted psychometric functions (main text). Panels show data from trial 40 (sliding window size) to the median trial length of each group of experiments (variable session lengths contribute to increased variability at later trials). Error bars show ±1 SEM over experiments. b, Inactivations in area LIP (n=21, blue curve; monkey N, 12; monkey P, 9) yielded no systematic trends in either accuracy (top) or bias (bottom) compared to baseline (n=21, gray), indicating that within-session compensation is unlikely. Panel format same as in a. We also investigated whether compensation may have taken place before we began collecting the “inactivation” dataset, or perhaps during the first 10-30 low difficulty “warm-up” trials. On 13 of the 21 LIP inactivation sessions we collected a 3rd dataset (in addition to the standard paired baseline and inactivation datasets), in which psychophysical performance was monitored during the time muscimol was being infused (“during infusion”, orange curve). No systematic changes in accuracy or bias were observed in this exploratory dataset either, further arguing against compensation on the time scales of our manipulations and measurements.
Extended Data Figure 5. Psychophysical performance in the direction discrimination task across sessions.
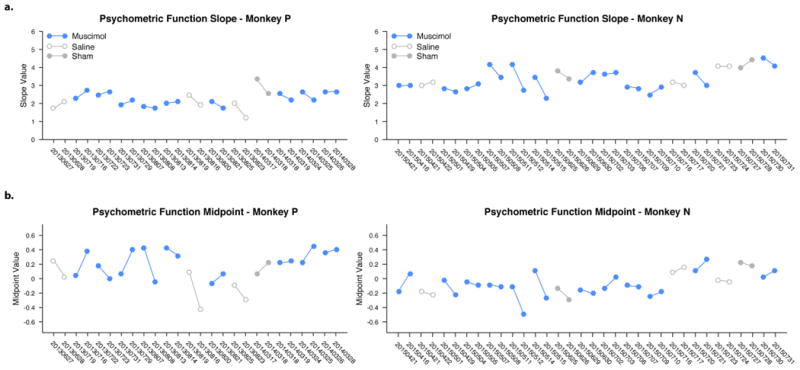
Panels show data from monkey P (left) and monkey N (right), for all baseline and treatment pairs: muscimol (blue, n=21), saline (unfilled gray, n=6) and sham (filled gray, n=3). Each pair consists of two sessions that took place in close succession (typically on consecutive days), at a similar time of day, after a similar number of preceding tasks and trials, and is represented by two markers connected by a line. (Additional control pairs with no saline/sham manipulation (n=16) are not presented, for visual clarity). a, Psychometric function slope over sessions. No significant change in slope was present over time, evaluated by linear regression, for either monkey P (p = 0.22) or N (p = 0.63). When considering the difference in slope between baseline and treatment pairs, monkey P exhibited a small decrease (regression line slope = -0.07, p = 0.023), indicating that inactivations may have affected monkey sensitivity gradually over time. However, a similar effect was seen in the interleaved controls (saline and sham, gray markers), indicating that this effect likely reflects nonspecific trends in performance across back-to-back pairs of experiments. Monkey N had no significant change (p = 0.92). b, Psychometric function midpoint over sessions. No significant change was observed in the session-to-session midpoint values, evaluated by linear regression, for either monkey P (p = 0.44) or monkey N (p = 0.24). When considering the difference in midpoint value for each dataset pair over time (i.e. muscimol treatment – baseline), no significant change was detected either (p = 0.98 and p = 0.4 for monkey P and N, respectively). X-axis dates are in yyyymmdd format.
Extended Data Figure 6. Psychophysical performance for all individual baseline and treatment session pairs.

All pairs of baseline and treatment sessions for all treatment types: muscimol, saline, and sham, (control pairs with no saline/sham manipulation are similar but not presented, for visual clarity) for all variants of the direction discrimination task: standard geometry (panel a), both targets in inactivated field (b), and Newsome dots (c), for both LIP and MT inactivation. In all panels, the abscissa represents motion strength towards the direction contralateral to the LIP under study, the ordinate represents the proportion of contralateral choices. The gray curve is baseline, and the coloured curve is treatment. The first panels in each section present mean psychophysical performance for each monkey over sessions. Subsequent panels present individual session pairs.
Extended Data Table 1. Parametric and nonparametric analysis of psychophysical data, for two- and four-parameter psychometric functions.
The table reports p-values for two types of statistical analyses: the parametric Student's t and the non-parametric Wilcoxon signed-rank sum test (WSRST). The tests were performed on model parameters fit to individual sessions. We present data for the standard two-parameter psychometric function (pmf2), and for an exploratory four-parameter psychometric function (pmf4). Muscimol infusions: Paired tests compared muscimol baseline sessions to muscimol treatment sessions. Control infusions: Paired tests compared saline/sham/control baseline sessions to saline/sham/control treatment sessions. Muscimol vs. Control infusion: Unpaired tests compared muscimol treatment sessions to saline/sham/control treatment sessions na: not enough data
| Muscimol Infusions | Control Infusions | Muscimol vs. Control Infusions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| Statistical test | model | monkey | midpoint | slope | minLapse | maxLapse | midpoint | slope | minLapse | maxLapse | midpoint | slope | minLapse | maxLapse |
| Student's t | pmf2 | N | 0.542 | 0.1734 | 0.986 | 0.2444 | 0.149 | 0.2367 | ||||||
| P | 0.6731 | 0.7982 | 0.2353 | 0.2166 | 0.0208 | 0.2461 | ||||||||
| Both | 0.9306 | 0.1659 | 0.3693 | 0.1092 | 0.2606 | 0.0704 | ||||||||
|
| ||||||||||||||
| pmf4 | N | 0.4028 | 0.0243 | 0.7585 | 0.2352 | 0.8213 | 0.2827 | 0.6065 | 0.6228 | 0.8213 | 0.2827 | 0.6065 | 0.6228 | |
| P | 0.6747 | 0.459 | 0.3554 | 0.8337 | 0.1377 | 0.3261 | 0.5675 | 0.3111 | 0.1377 | 0.3261 | 0.5675 | 0.3111 | ||
| Both | 0.8163 | 0.0552 | 0.3904 | 0.9091 | 0.2169 | 0.1595 | 0.7461 | 0.2734 | 0.2169 | 0.1595 | 0.7461 | 0.2734 | ||
|
| ||||||||||||||
| WSRST | pmf2 | N | 0.791 | 0.3394 | 0.9308 | 0.2305 | 0.184 | 0.1496 | ||||||
| P | 0.5703 | 0.9102 | na | na | 0.0503 | 0.3301 | ||||||||
| Both | 0.9032 | 0.3219 | 0.4432 | 0.1036 | 0.3898 | 0.0324 | ||||||||
|
| ||||||||||||||
| pmf4 | N | 0.6221 | 0.021 | 0.5186 | 0.2334 | 0.9032 | 0.2305 | 0.3754 | 0.4761 | 0.184 | 0.4887 | 0.184 | 0.5125 | |
| P | 0.4961 | 0.4258 | 0.8203 | 0.8203 | na | na | 0.875 | 0.625 | 0.0503 | 0.6042 | 0.8252 | 0.8252 | ||
| Both | 0.8213 | 0.0325 | 0.414 | 0.566 | 0.3533 | 0.1353 | 0.2758 | 0.3674 | 0.3543 | 0.0895 | 0.4942 | 0.2009 | ||
Extended Data Table 2. Infusion details for all treatment sessions.
The table presents all infusion sessions run over the course of the study for all infusion types (muscimol, saline, sham), in either MT or LIP. Infusions are sorted by date within each task, for each monkey separately. Positioning grid values (column 7) are relative to chamber centers (see Methods for stereotactic coordinates). Average depth (column 9) refers to the average depth across all infusion sites within a given cannula track. Total volume and total mass (columns 10 and 11, respectively) refer to the sum over all infusion sites and tracks.
| Task | Area | Monkey | Date | Treatment | Cannula Tracks (#) | Positioning Grid (x, y) | Infusion sites within Track (#) | Average depth (mm) | Total volume (μl) | Total mass (μg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Standard task geometry | MT | P | 20130801 | Muscimol | (2,-1) | 2 | 8 | 5 | 33.3 | |
| 20130826 | Muscimol | (2,0) | 2 | 10 | 9.7 | 64.3 | ||||
| 20130830 | Muscimol | (2,0) | 2 | 11.2 | 8.5 | 56.6 | ||||
| N | 20150414 | Muscimol | (5, -4) | 3 | 10.7 | 4 | 32 | |||
| 20150522 | Muscimol | (5, -4) | 2 | 10.5 | 5 | 40 | ||||
| 20150805 | Muscimol | (4, -4) | 2 | 6.9 | 5 | 40 | ||||
|
| ||||||||||
| LIP | P | 20130628 | Saline | (2,-1) | 1 | 7 | 6.7 | - | ||
| 20130716 | Muscimol | (3,0) | 1 | 6.5 | 6.7 | 44.4 | ||||
| 20130723 | Muscimol | (3,0) | 1 | 7 | 6.7 | 44.4 | ||||
| 20130729 | Muscimol | (2,-1) | 1 | 7 | 6.7 | 44.4 | ||||
| 20130808 | Muscimol | (3,0) | 1 | 6 | 6.7 | 44.4 | ||||
| 20130814 | Muscimol | (3,0) | 1 | 7 | 6.7 | 44.4 | ||||
| 20130816 | Saline | (3,0) | 1 | 6 | 6.7 | - | ||||
| 20130821 | Muscimol | 2 | (3,0); (0,3) | 2; 2 | 7; 7 | 12 | 79.9 | |||
| 20130823 | Saline | (3,0) | 1 | 7 | 6.7 | - | ||||
| 20140318 | Sham | - | - | - | - | - | ||||
| 20140319 | Muscimol | (3,0) | 1 | 7 | 5 | 40 | ||||
| 20140325 | Muscimol | (3,0) | 1 | 7 | 6 | 48 | ||||
| 20140328 | Muscimol | 2 | (3,0); (1,-3) | 2; 2 | 7.5; 7.5 | 10 | 80 | |||
| N | 20150416 | Muscimol | (2,4) | 2 | 6.3 | 6 | 48 | |||
| 20150422 | Saline | (2,4) | 1 | 7.6 | 5 | - | ||||
| 20150429 | Muscimol | (-2, 3) | 2 | 6.6 | 7.5 | 60 | ||||
| 20150505 | Muscimol | (-2, 3) | 2 | 6.5 | 5.5 | 44 | ||||
| 20150508 | Muscimol | (-1,3) | 2 | 8.6 | 5.5 | 44 | ||||
| 20150512 | Muscimol | 2 | (-2, 3); (3, 4) | 2; 2 | 7.9; 7.9 | 9 | 72 | |||
| 20150515 | Muscimol | (-2, 3) | 3 | 7.6 | 7 | 56 | ||||
| 20150626 | Sham | - | - | - | - | - | ||||
| 20150630 | Muscimol | (-2, 3) | 2 | 8 | 7 | 56 | ||||
| 20150703 | Muscimol | (-3, 2) | 3 | 7.1 | 8 | 64 | ||||
| 20150707 | Muscimol | 2 | (-3, 2); (2, 4) | 3; 3 | 7.9; 7.9 | 10 | 80 | |||
| 20150710 | Muscimol | 2 | (-3, 2); (2, 3) | 2; 2 | 6.2; 6 | 10 | 80 | |||
| 20150717 | Saline | (-3, 2) | 2 | 5.3 | 6 | - | ||||
| 20150721 | Muscimol | (-3, 2) | 2 | 6.1 | 8 | 64 | ||||
| 20150724 | Saline | (-3, 2) | 2 | 6 | 5 | - | ||||
| 20150728 | Sham | - | - | - | - | - | ||||
| 20150731 | Muscimol | (-3, 2) | 3 | 6 | 6 | 48 | ||||
|
| ||||||||||
| Both targets in inactivated field | LIP | P | 20140402 | Muscimol | (3,0) | 2 | 7.5 | 6 | 48 | |
| 20140407 | Sham | - | - | - | - | - | ||||
| 20140409 | Muscimol | (3,0) | 4 | 7 | 8 | 64 | ||||
| 20140509 | Muscimol | (3,0) | 3 | 9 | 8 | 64 | ||||
| 20140516 | Muscimol | (3,0) | 3 | 9 | 8 | 64 | ||||
| N | 20150821 | Sham | - | - | - | - | - | |||
| 20150826 | Sham | - | - | - | - | - | ||||
| 20150828 | Muscimol | (-3, 2) | 3 | 7 | 6 | 48 | ||||
| 20150831 | Sham | - | - | - | - | - | ||||
| 20150902 | Muscimol | (-3, 2) | 4 | 6.2 | 6.5 | 52 | ||||
|
| ||||||||||
| Newsome Dots | LIP | P | 20140425 | Muscimol | (3,0) | 3 | 7.2 | 8 | 64 | |
| 20140429 | Muscimol | (3,0) | 3 | 7.6 | 8 | 64 | ||||
Acknowledgments
We thank R. Krauzlis, C. Brody, E. Seidemann, L. Cormack, and R. Aldrich for comments on the manuscript. We thank the Brody lab (particularly C. Brody and J. Erlich) for motivating the experiments, the Mauk lab (particularly M. Mauk, F. Riusech, and H. Halverson) for assistance with muscimol preparation, and K. Mitchell for animal support. This research was supported by the Howard Hughes Medical Institute International Student Research Fellowship to L.N.K, the McKnight Foundation grant to J.W.P, the National Eye Institute (R01-EY017366) grant to both J.W.P and A.C.H, and the National Institutes of Health under Ruth L. Kirschstein National Research Service Awards T32DA018926 from the National Institute on Drug Abuse and T32EY021462 from the National Eye Institute.
Footnotes
Authoer Contributions: L.N.K, J.L.Y and A.C.H designed the experiment. L.N.K. and J.L.Y collected behavioral and electrophysiological data. L.N.K and J.L.Y performed pharmacological inactivations. L.N.K analyzed behavioral data. J.L.Y analyzed electrophysiological data. J.W.P and A.C.H guided data analysis. All authors discussed the results and wrote the manuscript.
References
- 1.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 2.Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. Journal of Neurophysiology. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 3.Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10:1038–1047. doi: 10.1038/nn1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding L, Gold JI. Perspective. Neuron. 2013;79:640–649. doi: 10.1016/j.neuron.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Gu Y, DeAngelis GC, Angelaki DE. Choice-related activity and correlated noise in subcortical vestibular neurons. Nat Neurosci. 2013;16:89–97. doi: 10.1038/nn.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanks TD, et al. Distinct relationships of parietal and prefrontal cortices to evidence accumulation. Nature. 2015;520:220–223. doi: 10.1038/nature14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitkow X, Liu S, Angelaki DE, DeAngelis GC, Pouget A. How Can Single Sensory Neurons Predict Behavior? Neuron. 2015;87:411–423. doi: 10.1016/j.neuron.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erlich JC, Brunton BW, Duan CA, Hanks TD, Brody CD. Distinct effects of prefrontal and parietal cortex inactivations on an accumulation of evidence task in the rat. Elife. 2015;4:8166. doi: 10.7554/eLife.05457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cumming BG, Nienborg H. Feedforward and feedback sources of choice probability in neural population responses. Current Opinion in Neurobiology. 2016;37:126–132. doi: 10.1016/j.conb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunton BW, Botvinick MM, Brody CD. Rats and Humans Can Optimally Accumulate Evidence for Decision-Making. Science. 2013;340:95–98. doi: 10.1126/science.1233912. [DOI] [PubMed] [Google Scholar]
- 11.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 12.Britten KH, Shadlen MN, Newsome WT, Movshon JA. Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci. 1993;10:1157–1169. doi: 10.1017/s0952523800010269. [DOI] [PubMed] [Google Scholar]
- 13.Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cerebral Cortex. 2003;13:1257–1269. doi: 10.1093/cercor/bhg097. [DOI] [PubMed] [Google Scholar]
- 14.Hess R, Murata K. Effects of glutamate and GABA on specific response properties of neurones in the visual cortex. Experimental Brain Research. 1974;21:285–297. doi: 10.1007/BF00235748. [DOI] [PubMed] [Google Scholar]
- 15.Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury SA, DeAngelis GC. Fine Discrimination Training Alters the Causal Contribution of Macaque Area MT to Depth Perception. Neuron. 2008;60:367–377. doi: 10.1016/j.neuron.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiani R, Hanks TD, Shadlen MN. Bounded Integration in Parietal Cortex Underlies Decisions Even When Viewing Duration Is Dictated by the Environment. Journal of Neuroscience. 2008;28:3017–3029. doi: 10.1523/JNEUROSCI.4761-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raposo D, Kaufman MT, Churchland AK. A category-free neural population supports evolving demands during decision-making. Nat Neurosci. 2014;17:1784–1792. doi: 10.1038/nn.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardak C, Olivier E, Duhamel JR. A Deficit in Covert Attention after Parietal Cortex Inactivation in the Monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- 20.Balan PF, Gottlieb J. Functional Significance of Nonspatial Information in Monkey Lateral Intraparietal Area. Journal of Neuroscience. 2009;29:8166–8176. doi: 10.1523/JNEUROSCI.0243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilke M, Kagan I, Andersen RA. Functional imaging reveals rapid reorganization of cortical activity after parietal inactivation in monkeys. Proceedings of the National Academy of Sciences. 2012;109:8274–8279. doi: 10.1073/pnas.1204789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerkhoff G. Spatial hemineglect in humans. Progress in Neurobiology. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Daie K, Svoboda K, Druckmann S. Robust neuronal dynamics in premotor cortex during motor planning. Nature. 2016;532:459–464. doi: 10.1038/nature17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe DA, et al. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci. 2013;16:1484–1491. doi: 10.1038/nn.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarma A, Masse NY, Wang XJ, Freedman DJ. Task-specific versus generalized mnemonic representations in parietal and prefrontal cortices. Nat Neurosci. 2015;19:143–149. doi: 10.1038/nn.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci. 2006;9:682–689. doi: 10.1038/nn1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature. 2006;443:85–88. doi: 10.1038/nature05078. [DOI] [PubMed] [Google Scholar]
- 28.Siegel M, Buschman TJ, Miller EK. Cortical information flow during flexible sensorimotor decisions. Science. 2015;348:1352–1355. doi: 10.1126/science.aab0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heitz RP, Schall JD. Neural chronometry and coherency across speed-accuracy demands reveal lack of homomorphism between computational and neural mechanisms of evidence accumulation. Philos Trans R Soc Lond, B, Biol Sci. 2013;368:20130071–20130071. doi: 10.1098/rstb.2013.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zandbelt B, Purcell BA, Palmeri TJ, Logan GD, Schall JD. Response times from ensembles of accumulators. Proceedings of the National Academy of Sciences. 2014;111:2848–2853. doi: 10.1073/pnas.1310577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meister MLR, Hennig JA, Huk AC. Signal Multiplexing and Single-Neuron Computations in Lateral Intraparietal Area During Decision-Making. Journal of Neuroscience. 2013 doi: 10.1523/JNEUROSCI.2984-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 33.Eastman KM, Huk AC. PLDAPS: A Hardware Architecture and Software Toolbox for Neurophysiology Requiring Complex Visual Stimuli and Online Behavioral Control. Front Neuroinform. 2012;6:1. doi: 10.3389/fninf.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Yttri EA, Snyder LH. Intention and attention: different functional roles for LIPd and LIPv. Nat Neurosci. 2010;13:495–500. doi: 10.1038/nn.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zirnsak M, Chen X, Lomber SG, Moore T. Effects of reversible inactivation of parietal cortex on the processing of visual salience in the frontal eye field. 2015 [Google Scholar]
- 36.Patel GH, et al. Topographic organization of macaque area LIP. Proceedings of the National Academy of Sciences. 2010;107:4728–4733. doi: 10.1073/pnas.0908092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit Perception & Psychophysics. 2001;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- 38.Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Experimental Brain Research. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- 39.Kelly RC, et al. Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. 2007;27:261–264. doi: 10.1523/JNEUROSCI.4906-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolias AS, et al. Recording Chronically From the Same Neurons in Awake, Behaving Primates. Journal of Neurophysiology. 2007;98:3780–3790. doi: 10.1152/jn.00260.2007. [DOI] [PubMed] [Google Scholar]
- 41.Pillow JW, et al. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature. 2008;454:995–999. doi: 10.1038/nature07140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillow JW, Shlens J, Chichilnisky EJ, Simoncelli EP. A Model-Based Spike Sorting Algorithm for Removing Correlation Artifacts in Multi-Neuron Recordings. PLoS ONE. 2013;8:e62123. doi: 10.1371/journal.pone.0062123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. Journal of Neuroscience. 1994;14:4109–4124. doi: 10.1523/JNEUROSCI.14-07-04109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noudoost B, Moore T. A reliable microinjectrode system for use in behaving monkeys. Journal of Neuroscience Methods. 2011;194:218–223. doi: 10.1016/j.jneumeth.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neuroscience Letters. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- 46.Arikan R, et al. A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. Journal of Neuroscience Methods. 2002;118:51–57. doi: 10.1016/s0165-0270(02)00143-7. [DOI] [PubMed] [Google Scholar]
- 47.Yttri EA, Wang C, Liu Y, Snyder LH. The parietal reach region is limb specific and not involved in eye-hand coordination. Journal of Neurophysiology. 2014;111:520–532. doi: 10.1152/jn.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heiss JD, Walbridge S, Asthagiri AR, Lonser RR. Image-guided convection-enhanced delivery of muscimol to the primate brain. J Neurosurg. 2010;112:790–795. doi: 10.3171/2009.7.JNS09652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis JW, Van Essen DC. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J Comp Neurol. 2000;428:79–111. doi: 10.1002/1096-9861(20001204)428:1<79::aid-cne7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 50.Chapman B, Zahs KR, Stryker MP. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. Journal of Neuroscience. 1991;11:1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubanek J, Li JM, Snyder LH. Motor role of parietal cortex in a monkey model of hemispatial neglect. Proceedings of the National Academy of Sciences. 2015;112:E2067–72. doi: 10.1073/pnas.1418324112. [DOI] [PMC free article] [PubMed] [Google Scholar]