Summary
Background
Therapeutic treatments for schizophrenia do not alleviate symptoms for all patients and efficacy is limited by common, often severe, side-effects. Genetic studies of disease can identify novel drug targets, and drugs for which the mechanism has direct genetic support have increased likelihood of clinical success. Large-scale genetic studies of schizophrenia have increased the number of genes and gene sets associated with risk. We aimed to examine the overlap between schizophrenia risk loci and gene targets of a comprehensive set of medications to potentially inform and improve treatment of schizophrenia.
Methods
We defined schizophrenia risk loci as genomic regions reaching genome-wide significance in the latest Psychiatric Genomics Consortium schizophrenia genome-wide association study (GWAS) of 36 989 cases and 113 075 controls and loss of function variants observed only once among 5079 individuals in an exome-sequencing study of 2536 schizophrenia cases and 2543 controls (Swedish Schizophrenia Study). Using two large and orthogonally created databases, we collated drug targets into 167 gene sets targeted by pharmacologically similar drugs and examined enrichment of schizophrenia risk loci in these sets. We further linked the exome-sequenced data with a national drug registry (the Swedish Prescribed Drug Register) to assess the contribution of rare variants to treatment response, using clozapine prescription as a proxy for treatment resistance.
Findings
We combined results from testing rare and common variation and, after correction for multiple testing, two gene sets were associated with schizophrenia risk: agents against amoebiasis and other protozoal diseases (106 genes, p=0·00046, pcorrected =0·024) and antipsychotics (347 genes, p=0·00078, pcorrected=0·046). Further analysis pointed to antipsychotics as having independent enrichment after removing genes that overlapped these two target sets. We noted significant enrichment both in known targets of antipsychotics (70 genes, p=0·0078) and novel predicted targets (277 genes, p=0·019). Patients with treatment-resistant schizophrenia had an excess of rare disruptive variants in gene targets of antipsychotics (347 genes, p=0·0067) and in genes with evidence for a role in antipsychotic efficacy (91 genes, p=0·0029).
Interpretation
Our results support genetic overlap between schizophrenia pathogenesis and antipsychotic mechanism of action. This finding is consistent with treatment efficacy being polygenic and suggests that single-target therapeutics might be insufficient. We provide evidence of a role for rare functional variants in antipsychotic treatment response, pointing to a subset of patients where their genetic information could inform treatment. Finally, we present a novel framework for identifying treatments from genetic data and improving our understanding of therapeutic mechanism.
Introduction
Schizophrenia is a debilitating disease affecting 0·7% of the population worldwide.1 Although antipsychotics are effective treatments for schizophrenia, they do not alleviate all symptoms and often result in serious side-effects,2 reducing efficacy through poor adherence.3 In general, the reasons antipsychotic medications are ineffective for some patients remain unclear.
Genetic studies of schizophrenia have implicated genomic regions and genes with shared biological function. A genome-wide association study (GWAS) of 34 241 schizophrenia cases and 45 604 controls (36 989 cases and 113 075 controls with replication) identified 108 independently associated regions.4 An exome-sequencing study of 2536 schizophrenia cases and 2543 controls demonstrated a polygenic burden of rare variants (disruptive variants seen once in 10 158 chromosomes) in sets of genes related to synaptic transmission, calcium channels, and genes with de-novo mutations in schizophrenia probands.5
A challenge is how to use genomic data to understand drug efficacy, improve drug design, and identify opportunities for drug repurposing. Genetic studies have identified drug targets; for example two genes associated with LDL cholesterol, HMGCR6 and NPC1L1,7 are the main targets of the cholesterol-lowering drugs HMG-CoA reductase inhibitors (HMGCR) and ezetimibe (NPC1L1).8 DRD2, the main target of antipsychotics, is within one of the 108 associated regions reported in schizophrenia.4 Rare disease variation can also predict drug effects; a novel obesity drug that inhibits DGAT1 (a gene implicated in a form of severe congenital diarrhoea9) had treatment-limiting, dose-dependent gastrointestinal side-effects, in particular diarrhoea.10 Findings from a recent study11 showed that drugs with genetically supported mechanisms proceeded further along the development pipeline and were more likely to be clinically successful.11 Taken together, these findings suggest that direct assessment of the overlap between genetics and drug targets has the potential to inform our knowledge of both drug mechanisms and disease pathology.
Research in context.
Evidence before this study
Studies have shown a broad overlap between genes contributing to genetic risk of disease and the key targets of drugs that treat the disease. For example, the main gene targets of the cholesterol-lowering drugs statins and ezetimibe have been associated with cholesterol concentrations through genome-wide association studies. Further, drugs with direct genetic evidence supporting their targets are more likely to be clinically successful. There are also examples demonstrating consistent phenotypic outcomes from a drug targeting a gene and a specific variant within that gene. We sought articles related to schizophrenia that addressed whether plausible pharmacological interventions can be found in the overlap between disease risk-associated genes and known druggable targets, and whether deleterious mutations in genes targeted by a treatment can affect response to that treatment. We searched PubMed multiple times between June 1, 2013, and Aug 31, 2015, using combinations of search terms including “schizophrenia”, “antipsychotics”, “pharmacogenetics”, “genetics”, and “drug response”. Abstracts in English were reviewed and limited evidence for specific genes was found. A review article gave the strongest support to a few genes, including DRD3,which encodes the main target of all antipsychotics.
Added value of this study
Our systematic evaluation using all available data and information on genome-wide genetic risk factors and druggable targets identified antipsychotics as the class of drugs with most direct genetic support for treating schizophrenia. We show that efficacy of these drugs is mediated through a complex polygenic mechanism including many genes not previously known to be involved in antipsychotic drug response or believed to contribute only to off -target effects. Additionally, we show that rare deleterious variants in genes targeted by antipsychotics and those previously implicated in being relevant to the pharmacogenetics of antipsychotics are enriched in individuals with treatment-resistant schizophrenia.
Implications of all the available evidence
Our analysis identified both known and novel genes likely facilitating antipsychotic efficacy. This approach and these findings can be used to identify novel drugs with target profiles more directly supported by the genetics, or novel pharmacogenes for guiding individualised therapy in schizophrenia. The finding that treatment-resistant individuals are more likely to carry deleterious mutations in antipsychotic targets or key pharmacogenetic genes suggests a method for identifying patients whose clinical outcomes could be improved through earlier administration of clozapine.
A third of patients with schizophrenia do not respond to standard treatments,12 and three-quarters discontinue treatment within 18 months because of ineffectiveness or side-effects.13 Pharmacogenetic studies have yielded few reproducible findings, probably because of the limited availability of large samples with both treatment response and genetic data. Common variants in DRD2 and genes that metabolise antipsychotics such as CYP2D6 have been variably associated with efficacy of several antipsychotics.14 By contrast, pharmacogenetic studies in cancer have identified target-associated resistance for several therapies, often the result of rare functional mutations within gene targets of the chemotherapeutic agent.15 These results suggest that rare functional variants have a pharmacodynamic role in treatment response. Use of genetic data to identify the appropriate treatment for each patient could greatly improve efficacy and reduce time and morbidity during failed treatment periods.
Here, we aimed to intersect schizophrenia risk loci and gene targets of therapeutic agents to inform and potentially improve treatment of schizophrenia.
Methods
Study design and participants
In this analysis we tested schizophrenia risk loci from both common and rare variation for enrichment in gene targets of therapeutic agents to identify medications that were enriched for targeting these loci. Additionally, we linked the rare variation data with a national drug registry to assess the contribution of rare variants to treatment response. We identified rare variants from an exome-sequencing sample of schizophrenia cases and controls (Swedish Schizophrenia Study). Cases were selected from the Swedish Hospital Discharge Register, requiring at least two discharge diagnoses of schizophrenia.16 Controls were randomly selected from Swedish population registers, requiring no discharge diagnoses of schizophrenia or bipolar disorder. Diagnostic validity has been reported in previous publications4,16,17 and is supported by clinical, epidemiological, and genetic evidence. Samples were sequenced using either the SureSelect Human All Exon Kit (29 megabases [Mb] of sequence targeted) or the SureSelect Human All Exon version 2 Kit (33 Mb of sequence targeted; Agilent, Santa Clara, CA, USA). Sequencing was done by Genome Analyzer IIx or HiSeq 2000 (illumina, San Diego, CA, USA). Additional details of sample and sequencing have been previously reported.5
There are no formal diagnostic criteria for treatment-resistant schizophrenia. Research studies have defined it empirically as more than one antipsychotic trial at a particular dose and duration without improvement in symptoms; generally, two failed trials, with doses of 400–1000 chlorpromazine equivalent mg daily, for a duration of 4–6 weeks, with less than 20% improvement in the Positive and Negative Symptom Scale.18 In large population-based genetic studies of schizophrenia, ascertainment of such high-dimensional clinical data has proven difficult. Despite its proven efficacy,19 clozapine is usually reserved as a second-line or third-line therapy in schizophrenia because of side-effects. However, it is the only approved medication for treatment-resistant schizophrenia and as a result, a history of having been prescribed clozapine has been used as a proxy for treatment resistance.
All individuals provided written informed consent; institutional human subject committees at the Karolinska Institutet and the University of North Carolina at Chapel Hill approved the research.
Data sources
The Swedish Prescribed Drug Register20 contains data on all redeemed prescriptions from Jan 7, 2005, to Dec 31, 2012. For each individual, the following information was available: dates for first and last antipsychotic prescriptions, dates for first and last clozapine prescriptions, total number of antipsychotic pre scriptions, total number of clozapine prescriptions, and the number of unique antipsychotics prescribed. We classified any individual that had been prescribed clozapine at least once as treatment resistant.
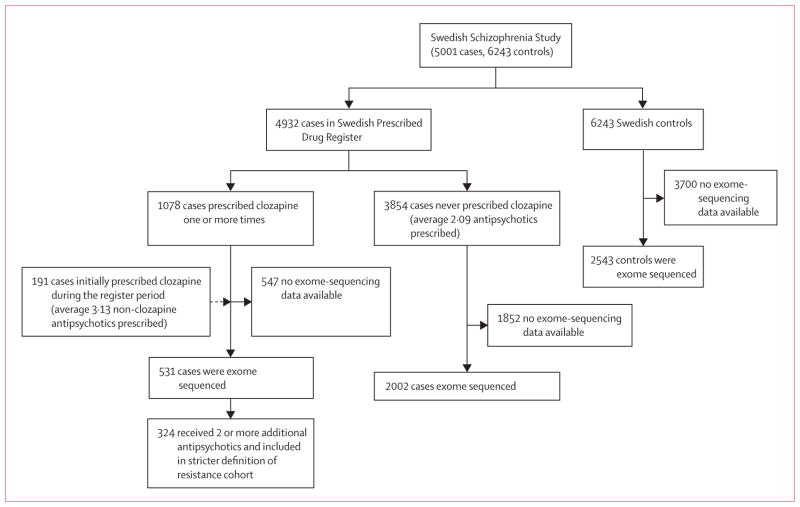
The Swedish Schizo phrenia Study contains data for 5001 schizophrenia cases and 6243 controls. In this analysis, we included 2543 controls with exome-sequencing data available. We included 4932 individuals with schizophrenia who were in the Swedish Prescribed Drug Register, of whom 2533 had exome-sequencing data available (figure 1).
Figure 1.
Description of sample and phenotypes for rare variant analyses
Of the 4932 individuals in the medication register, clozapine was prescribed and dispensed one or more times for 1078 individuals. 829 (77%) received clozapine as their first prescription in the register, implying that any initial antipsychotic treatments occurred before the beginning of register (Jan 7, 2005). Because our goal was to assess the prescription patterns leading to clozapine initiation, these individuals offered no information and were removed. 58 of the remaining 249 patients were on a non-clozapine antipsychotic at the end of the register. In these cases we cannot tell from the available data the temporal relationship of the other antipsychotics to clozapine initiation, so we removed these individuals from analysis of prescription patterns. The remaining 191 individuals could be used for an assessment of prescribing patterns before clozapine (figure 1). This subset of 191 cases received a mean of 3·13 (SD 1·73) unique antipsychotic prescriptions (not including clozapine) compared with 2·09 (1·50) for the 3854 patients with schizophrenia who were never prescribed clozapine, and the mean amount of time before the first clozapine prescription was 1126 days (SD 824 days). In our analysis of treatment resistance, we included 531 patients who had been prescribed clozapine at least once (not restricting to the subset of 191 patients where clozapine was initiated during the registry) and who had exome-sequencing data available. We also stratified cases into a more stringent class of treatment resistance, defined as having been prescribed clozapine and two or more additional antipsychotics (n=324 cases with exome sequencing).
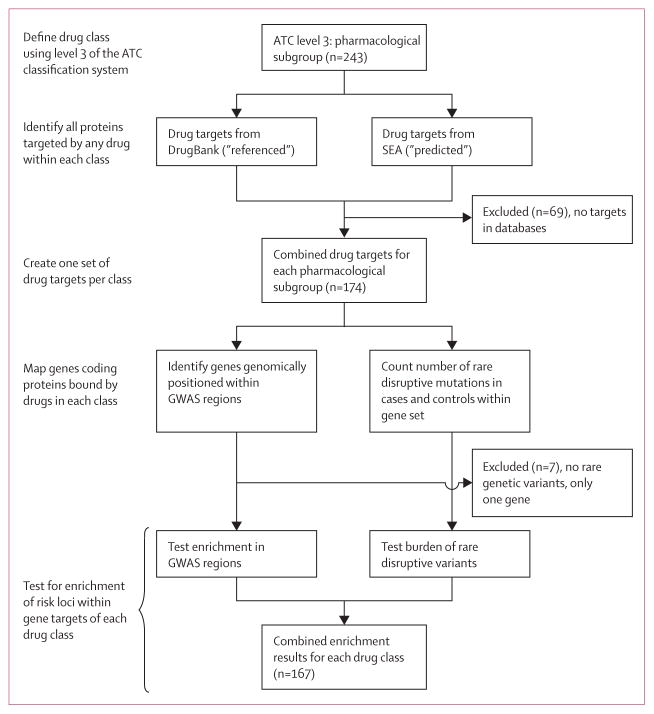
Drugs were classified according to the third level of the Anatomical Therapeutic Chemical classification system, a hierarchical classification system from anatomical group (level 1) to chemical substance (level 5). Level 3 represents the pharmacological subgroup (eg, antipsychotics) and contains 243 drug classes.
We used two databases to link drugs to gene targets (proteins). DrugBank version 4.121 is a widely used, publicly available catalogue of drug targets manually curated from the literature where each drug–protein target pair is referenced on average four times; Similarity Ensemble Approach (SEA)22 is an in-silico drug target prediction method using ligand similarity. Ligand-to-target training information for SEA was pulled from ChEMBL-14, a curated chemical database of more than 1·5 million compounds and 13·5 million activities. Although DrugBank represents the space of experimentally observed drug targets, SEA surveys the larger landscape of possible drug-to-target connections using chemical structure. By comparing the 603 drugs to all structural features in known reference ligands, and to random expectation,22,23 SEA assessed significance for all 1·6 million possible drug–target pairs retaining the 19 327 most significant drug–target predictions (1·2%), for which similarity was unlikely by random chance alone. Individually, DrugBank contains a unique set of 1048 drugs, 878 protein targets, and 4592 drug–target pairs; SEA consists of 603 drugs, 1440 protein targets, and 19 327 predicted drug–target pairs. We combined the databases, removing duplicates resulting in 1267 drugs, 1820 protein targets, and 23 383 drug–target pairs.
For each of the 243 classes of drugs, we created one set of all targets from both databases (requiring that the gene that codes for the target protein be one of 23 085 RefSeq genes). Of the 243 drug classes, 174 had at least one drug target.
PharmGKB24 is a database of pharmacogenetic information including genetic variants and their association with drug response. We extracted all references to any of the 64 antipsychotics from the Anatomical Therapeutic Chemical classification system. We identified 294 entries of genetic variants being associated with efficacy of an antipsychotic for 13 unique drugs and 91 unique genes.
Other genes will contribute to treatment response, for example cytochrome P450 (CYP) genes explain a large proportion of the known metabolic effects on efficacy of a broad range of drugs.25 Thus, we compiled the family of 65 cytochrome P450 genes (CYP) and a subset of 12 that account for nearly 75% of all phase I drug oxidation reactions (CYP-12).26 Additionally, we compiled a set of 32 core genes related to drug absorption, distribution, metabolism, and excretion (ADME core) and the full list of 298 genes including the extended ADME set. In total, we defined five pharmacogenetic gene sets to be tested.
Statistical analysis
We used two enrichment testing methods. To test for an excess of rare genetic case variants we used the statistic/matrix/permutation test,5 in which gene sets were evaluated on the empirical distribution of the sum of individual gene burden statistics. P values were corrected for exome-wide burden. Individuals were matched based on ancestry, sex, and experimental batch; permutation was done within clusters of matched individuals. To test for enrichment in genome-wide significant schizophrenia regions4, we used Inrich.27 Inrich performs gene set analysis by comparing the number of genes within associated regions to the number within randomly placed regions, accounting for gene size, marker density, and linkage disequilibrium between genes. Regions tested were required to be genome-wide significant and were defined by the furthest single nucleotide polymorphisms, in either genomic direction, having high linkage disequilibrium (r2>0·6) with the most significant single nucleotide polymorphism.
We applied an empirical multiple test correction procedure, randomly assigning genes from the list of 23 085 RefSeq genes to each drug class preserving the same number of genes. We performed 100 000 permutations testing the rare and common variation separately and then combined p-values using Fisher’s method. For each class, we counted the number of times the observed p-value was equal to or greater than the most significant permuted p-value across all classes. Empirical correction was performed over all 167 pharmacological gene sets having at least one rare variant and two drug targets.
For testing enrichment of rare variants in treatment-resistant cases, phenotypes were permuted 10 000 times and permutation-based gene burden statistics were combined. Corrected p values were calculated empirically across the five pharmacogenetic gene sets and targets of antipsychotics. The same approach was employed to test gene sets previously implicated in schizophrenia through rare variation for a role in treatment response.
Role of the funding source
The funders had no role in study design, execution, analysis, or manuscript preparation. The corresponding author had access to all the data in the study and had responsibility for the final decision to submit for publication.
Results
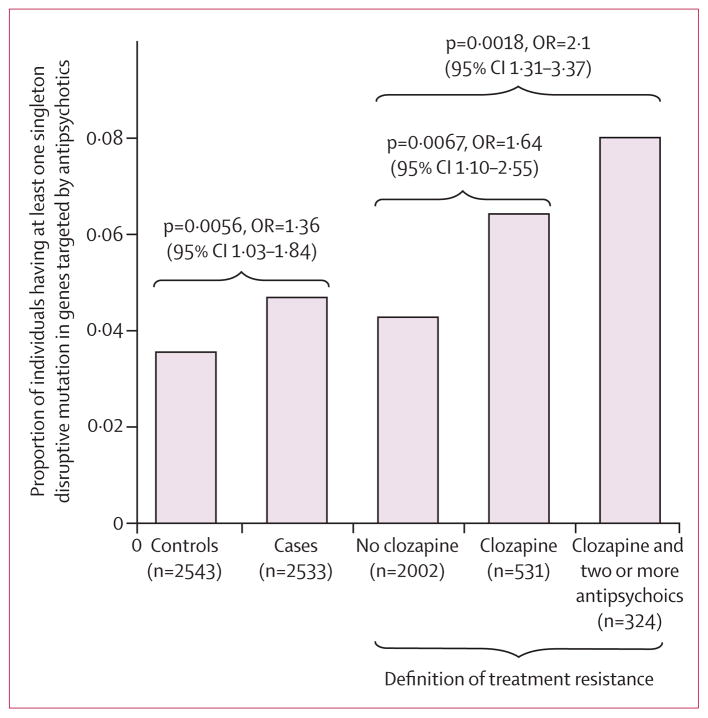
We tested for enrichment of schizophrenia risk loci within sets of genes coding for the proteins targeted by drugs within each of 167 pharmacological subgroups defined by the Anatomical Therapeutic Chemical classification system (figure 2). Enrichment of rare deleterious variants in the 167 gene sets was tested in an exome-sequenced sample of 2536 schizophrenia cases and 2543 controls.5 Previous analysis of these data demonstrated that rare variants found only once in the study and predicted to be disruptive of gene function (so-called singleton disruptives) contributed most to schizophrenia risk.5 Thus, we specifically focused on this rarest and most functionally deleterious class of variation. In total, 10 (6%) of 167 gene sets were enriched with rare variants in schizophrenia cases (p<0·05), with the strongest finding in the 347 genes targeted by antipsychotics (122 case mutations, 90 control mutations, p=0·0056, odds ratio 1·36 (1·03–1·84).
Figure 2. Construction of the analysis pipeline.
ATC=Anatomical Therapeutic Chemical. SEA=Similarity Ensemble Approach. GWAS=genome-wide association study.
Next, we tested common variants using the 108 genome-wide significant loci4 and found that 35 (21%) of the 167 gene sets were enriched for genes within schizophrenia associated loci (p<0·05). Several of the genes within the GWAS regions associated with schizophrenia are common drug targets for many drug classes and probably contribute to this enrichment, including DRD2 which is a target for at least one drug in 46 different non-antipsychotic pharmacological subgroups.
Two gene sets were associated with schizophrenia risk after an empirical multiple test correction on the combined results: agents against amoebiasis and other protozoal diseases (106 genes, p=0·00046, pcorrected=0·024), followed by antipsychotics (347 genes, p=0·00078, pcorrected=0·046). These were the only gene sets showing an association with schizophrenia risk for both rare and common variation (appendix). 56 genes were in both sets, representing 53% (56/106) of genes from the agents against amoebiasis and other protozoal diseases gene set and 16% (56/347) of genes from the antipsychotics gene set. The antipsychotics set still showed an association after removal of the 56 overlapping genes (antipsychotics, p=0·041; agents against amoebiasis and other protozoal diseases, p=0·63; appendix). Enrichment of rare variants persisted after removal of the 11 genes found in GWAS loci (p=0·024). Further, enrichment persisted within the 49% (170/347) of genes highly expressed in the brain (p=0·00006).5,28 Of the 11 genes in GWAS loci, five had singleton disruptive mutations in cases only (number of cases shown in parentheses): CACNA1C (2), CYP2D6 (2), GRIN2A (2), AKT3 (1), and HCN1 (1; appendix).
To test the contribution of targets of antipsychotics to enrichment for schizophrenia risk, we used drug target information from two sources: a database representing the wealth of all publicly known and literature referenced drug–target pairs (DrugBank) and a set of predicted targets (SEA) representing a broader pharmacological group of drug–target connections. 386 drugs, representing 37% of the referenced set and 64% of the predicted set were shared. On average, the referenced set had 4·4 gene targets per drug and the predicted set had 32. Of the 64 drugs listed as antipsychotics in level 3 of the Anatomical Therapeutic Chemical classification system (appendix), 40 had at least one target.
We identified enrichment for schizophrenia risk in both the set of predicted targets (313 genes, p=0·00071) and the set of referenced targets (70 genes, p=0·0078). After removing all referenced gene targets, enrichment remained in the genes that were novel predicted targets of antipsychotics (277 genes, p=0·0189; table).
Table.
Enrichment of genes targeted by antipsychotics
| Genes | Combined
|
Singleton disruptives
|
PGC2 GWAS
|
|||||
|---|---|---|---|---|---|---|---|---|
| p value | Corrected p value | Case/control | OR (95% CI) | p value | Number of GWAS loci | p value | ||
| Referenced | 70 | 0·00775 | 0·479 | 20/8 | 2·51 (1·06–6·62) | 0·0135 | 3 | 0·0724 |
|
| ||||||||
| Predicted | 313 | 0·00071 | 0·041 | 113/86 | 1·32 (0·99–1·80) | 0·0096 | 10 | 0·0070 |
|
| ||||||||
| Predicted without referenced | 277 | 0·01893 | 0·811 | 102/82 | 1·25 (0·93–1·71) | 0·0419 | 7 | 0·0655 |
|
| ||||||||
| Combined | 347 | 0·00078 | 0·046 | 122/90 | 1·36 (1·03–1·84) | 0·0056 | 10 | 0·0133 |
Table shows data for enrichment of genes in significant GWAS regions (p<5 × 10−8) and singleton disruptive mutations from exome-sequencing stratified by drug target database. GWAS=genome-wide association study.
We next examined the role of rare disruptive mutations in treatment resistance in schizophrenia. Of the 2536 schizophrenia cases in our sample that were exome sequenced, all but three had clozapine prescription information (figure 1): 531 had been prescribed clozapine (“clozapine group”) and 2002 had not (“no-clozapine group”). The 347 gene targets of antipsychotics were enriched for singleton disruptive mutations in the clozapine group compared with the no-clozapine group. This enrichment remained after empirical multiple test correction for the six total tests including the five pharmacogenetic sets tested (37 clozapine, 85 no-clozapine, p=0·0067, pcorrected=0·044). In total, 6·4% of the clozapine group carried a variant in these genes compared to 4·2% of the no-clozapine group (figure 3).
Figure 3. Proportion of individuals carrying singleton disruptive mutations within the 347 gene targets of antipsychotics stratified by case/control and treatment response.
OR=odds ratio.
Genes implicated in antipsychotic efficacy by PharmGKB and genes with known roles in ADME including a set of cytochrome P450 (CYP) genes were also tested. Enrichment was observed (figure 4, appendix) in the set of PharmGKB genes (91 genes, 14 clozapine, 21 no-clozapine, p=0·0029, pcorrected=0·018) but not in the ADME or CYP sets (figure 4, appendix) and no burden of rare singleton disruptive mutations existed exome-wide (p=0·28).
Figure 4. Enrichment results of singleton disruptive mutations for both schizophrenia and treatment resistance in antipsychotic targets and sets of previously identified genes enriched for rare variants in schizophrenia.
SCZ de novo (disruptive) relates to genes carrying disruptive mutations in schizophrenia probands. SCZ de novo (nonsyn) relates to genes carrying non-synonymous mutations in schizophrenia probands. SCZ de novo (CNV) relates to genes carrying de novo copy number variants in schizophrenia probands. Calcium channels relates to genes related to voltage-gated calcium ion channel functioning. ARC relates to signalling complex formed by the activity-regulated cytoskeleton-associated scaffold protein (ARC) of the postsynaptic density. NMDAR relates to N-methyl-D-aspartate receptor (NMDAR) postsynaptic signalling complex. PSD-95 relates to postsynaptic density set encoded by DLG3, FMRP targets (Darnell) relates to targets of the fragile X mental retardation protein from Darnell et al.29 Sets of pharmacogenetic genes described in the methods. p values presented are before multiple hypothesis correction.
Among the 324 individuals who had been prescribed clozapine and two or more other antipsychotics, 26 (8%) had at least one singleton disruptive mutation in an antipsychotic gene target (figure 3) compared with 85 in the no-clozapine group (4%, p=0·0018). The PharmGKB sets showed similar increased burden of singleton disruptive mutations in these cases (data not shown).
To identify genes with a role in both schizophrenia risk and treatment resistance, we tested for association to treatment response in gene sets that were previously enriched for schizophrenia singleton disruptive mutations.5 These sets included genes with either a disruptive, non-synonymous, or copy-number de-novo mutation, post-synaptic density genes (including activity-regulated cytoskeleton-associated scaffold protein [ARC], N-methyl-D-aspartate receptor [NMDAR], and postsynaptic density genes encoded by DLG4 [PSD-95]), and targets of the fragile X mental retardation protein (FMRP). None were enriched in the clozapine group compared with the no-clozapine group. Furthermore, none of the pharmacogenetic gene sets were enriched for schizophrenia risk alleles (figure 4, appendix). Targets of antipsychotics represented the only set showing enrichment for both disease risk and treatment resistance.
Discussion
In this analysis, we leveraged genetic findings in schizophrenia from large studies of both common and rare variation to ask which drugs are targeting proteins encoded by genes having the most genetic evidence for a role in schizophrenia. Among 167 drug sets, only two were enriched for both genes within GWAS regions associated with schizophrenia and case singleton disruptive mutations, and after removing overlapping targets only the class of antipsychotics retained an association. This result supports the hypothesis that the pathogenesis of schizophrenia and the mechanism of action of antipsychotics overlap. Crucially, this overlap is not solely through dopamine or serotonin receptors and no particular gene or even the set of all currently known antipsychotic targets fully accounts for the enrichment. This finding suggests that, similar to genetic risk, the underlying mechanisms that prove useful in treating schizophrenia are probably polygenic and modulated at least partly through unknown pathways. A corollary of this finding is that it could be hard for a single target to provide the perfect therapeutic.
Of the 347 gene targets of antipsychotics, only a subset is probably relevant to efficacy. Our results point to perhaps dozens of new targets contributing to treatment efficacy, including four predicted genes (CACNA1C, GRIN2A, AKT3, HCN1) that were identified as associated with schizophrenia through GWAS and carry only case singleton disruptive mutations representing putative novel moderators of the mode of action of these drugs. These results, together with research into identifying the genes relevant to efficacy or contributing to adverse effects, should inform future drug design and repurposing.
Clozapine is the most effective treatment for refractory schizophrenia. Individuals with treatment-resistant schizophrenia have more symptoms, are burdened with a higher prevalence of substance abuse, and have a lower quality of life.30 Early identification of treatment-resistant patients who would be candidates to receive clozapine sooner could have a meaningful impact. In this study, we have identified an enrichment of rare mutations in gene targets of antipsychotics and previously implicated pharmacogenetic genes in treatment-resistant patients, pointing to a method of identifying patients who might be candidates for earlier treatment with clozapine. Prospective trials are warranted to investigate this further. Of all the gene sets tested, including those previously implicated in schizophrenia risk and treatment efficacy, only the set of genes targeted by antipsychotics showed significant enrichment in both disease risk and treatment response.
The drug class of agents against amoebiasis and other protozoal diseases showed an association with schizophrenia that was no longer observed after removal of the overlap of genes with antipsychotics, which does not preclude relevance to schizophrenia. There have been links between the presence of protozoal antibodies and disease status.31 Alternatively, this overlap might account for the antiprotozoal properties of antipsychotics, indicating that antipsychotics might be candidate antiprotozoal drugs rather than that antiprotozoal drugs are potential antipsychotics.32,33
As genetic studies have become well powered, loci from these studies are being used to develop therapeutic targets (eg, imatinib for cancer). However, the advent of currently available antipsychotics long preceded genetic findings, meaning the drug–target data and genetic findings are orthogonal pieces of information and unlikely subject to the same biases. Limitations of our study include the fact that drug target and chemical structure data are incomplete and false-positive predictions remain, which could be inducing unknown biases and effects on power. The lack of additional clinical information limits our ability to further characterise treatment-resistant individuals with rare mutations. Although clozapine prescription is only a proxy for treatment resistance, we have used the prescription register to show that clozapine in this sample was reserved mainly for refractory illness; the average number of antipsychotics prescribed (3·13 non-clozapine antipsychotics) and amount of time before the first clozapine prescription (1126 days) suggests that clozapine prescription practices in our cohort met criteria for treatment-resistant schizophrenia. Although more stringent methods for defining treatment resistance exist, none allow for investigation of a large sample with available GWAS and exome-sequence data. Nonetheless, when we used a more standard definition (two or more antipsychotics and clozapine), we demonstrated an even larger burden of rare mutations, consistent with this sample representing a more severe group of treatment-resistant patients.
This work demonstrates the potential of using genomic findings of schizophrenia to inform and ultimately improve treatment through identifying more effective drug target profiles or classifying individuals as to whether they will respond to particular therapies.
Supplementary Material
Acknowledgments
Funding US National Institutes of Health.
We are grateful for the participation of all individuals contributing to this research, and to the collection team that worked to recruit them. We acknowledge funding support from National Institutes of Health (NIH)/National Institute of Mental Health (NIMH) ARRA Grand Opportunity grant NIMH RC2 MH089905 (SMP, PS), the Sylvan Herman Foundation, the Stanley Center for Psychiatric Research, the Stanley Medical Research Institute, NIDDK grant R01 DK098242 (JTD) and NCI U54 CA189201 (JTD), NIH/NIGMS grant R44 GM093456 (MJK), NIH/NIGMS grant R43 GM097912 (MJK), NIH/NIGMS grant K23 GM104401 (SAS), NIH/NIMH grant R01 MH099126 (SMP), NIH/NHGRI grant R01 HG005827 (SMP), NIH/NIMH grant R01 MH077139 (PFS), NIH/NIMH grant R01 MH095034 (PS), NIH/NIMH grant U01 MH096296 (PS), and the Swedish Research Council and Karolinska Institutet (CMH, AKK). Work at the Icahn School of Medicine at Mount Sinai was also supported by the Institute for Genomics and Multiscale Biology (including computational resources and staff expertise provided by the Department of Scientific Computing).
Footnotes
Contributors
DMR, AWC, JTD, and PS designed the study. DMR, AWC, BR, BAK, and SMP contributed to statistical analyses. AKK, JLM, CMH, and PFS contributed samples and phenotypes. MJK and SAS provided pharmacological data. DMR, AWC, PJK, SMP, JTD, and PS contributed to main interpretations. DMR, AWC, and PS performed the primary drafting of the report. All authors contributed to, read, and approved the final manuscript.
Declaration of interests
MJK is a co-founder and board member of SeaChange Pharmaceuticals. JTD reports personal fees for bioinformatics consulting from Janssen Pharmaceuticals. All other authors declare no competing interests.
References
- 1.McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 2.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 3.Higashi K, Medic G, Littlewood KJ, Diez T, Granstrom O, De Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3:200–18. doi: 10.1177/2045125312474019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–27. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Gen. 2008;40:189–97. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Calvo M, Lisnock J, Bull HG, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci USA. 2005;102:8132–37. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas JT, Winter HS, Lim E, et al. DGAT1 mutation is linked to a congenital diarrheal disorder. J Clin Invest. 2012;122:4680–84. doi: 10.1172/JCI64873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denison H, Nilsson C, Lofgren L, et al. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab. 2014;16:334–43. doi: 10.1111/dom.12221. [DOI] [PubMed] [Google Scholar]
- 11.Nelson MR, Tipney H, Painter JL, et al. The support of human genetic evidence for approved drug indications. Nature Genetics. 2015;47:856–60. doi: 10.1038/ng.3314. [DOI] [PubMed] [Google Scholar]
- 12.Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738–49. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JP, Malhotra AK. Pharmacogenetics and antipsychotics: therapeutic efficacy and side effects prediction. Expert Opin Drug Metab Toxicol. 2011;7:9–37. doi: 10.1517/17425255.2011.532787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 16.Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–282. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenstein P, Bjork C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med. 2006;36:1417–25. doi: 10.1017/S0033291706008385. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Remington G, Mulsant BH, et al. Treatment resistant schizophrenia and response to antipsychotics: a review. Schizophr Res. 2011;133:54–62. doi: 10.1016/j.schres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JP, Lencz T, Geisler S, DeRosse P, Bromet EJ, Malhotra AK. Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr Res. 2013;146:285–88. doi: 10.1016/j.schres.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–35. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 21.Law V, Knox C, Djoumbou Y, et al. DrugBank 4. 0: shedding new light on drug metabolism. Nucleic Acid Res. 2014;42:D1091–97. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keiser MJ, Setola V, Irwin JJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–81. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 24.Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92:414–17. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–62. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 26.Gordon AS, Tabor HK, Johnson AD, et al. Quantifying rare, deleterious variation in 12 human cytochrome P450 drug-metabolism genes in a large-scale exome dataset. Hum Mol Gen. 2014;23:1957–63. doi: 10.1093/hmg/ddt588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PH, O’Dushlaine C, Thomas B, Purcell SM. INRICH: interval-based enrichment analysis for genome-wide association studies. Bioinformatics. 2012;28:1797–99. doi: 10.1093/bioinformatics/bts191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang HJ, Kawasawa YI, Cheng F, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–89. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–99. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29:63–76. doi: 10.1097/YIC.0b013e32836508e6. [DOI] [PubMed] [Google Scholar]
- 31.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729–36. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson RD, Manian AA, Harcus JL, Hall D, Hewlett EL. Lethal effect of phenothiazine neuroleptics on the pathogenic protozoan Leishmania donovani. Science. 1982;217:369–71. doi: 10.1126/science.6124040. [DOI] [PubMed] [Google Scholar]
- 33.Fond G, Macgregor A, Tamouza R, et al. Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci. 2014;264:179–83. doi: 10.1007/s00406-013-0413-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.