Abstract
IL-22-IL-22R signaling plays a crucial role in regulating host defenses against extracellular pathogens particularly in the intestine, through the induction of antimicrobial peptides and chemotactic genes. However, the role of IL-22- IL-22R is understudied in Spn lung infection, a prevalent pathogen of pneumonia. This paper presents the findings of IL-22 signaling during a murine model of pneumococcal pneumonia and improvement of bacterial burden upon IL-22 administration. IL-22 was rapidly induced in the lung during pneumococcal infection in wild type mice and Il22−/− mice had higher pneumococcal burdens compared to controls. Additionally, mice with hepatic-specific deletion of Il22ra1 also had higher bacterial burdens in lungs compared to littermate controls after intrapulmonary pneumococcal infection, suggesting that IL-22 signaling in the liver is important to control pneumococcal pneumonia. Thus, we hypothesized that enhancement of IL-22 signaling would control pneumococcal burden in lung tissues in an experimental pneumonia model. Administration of recombinant IL-22 systemically to infected wild type mice decreased bacterial burden in lung and liver at 24 hours post infection. Our in vitro studies also showed that mice treated with IL-22 had increased C3 expression in the liver compared to the isotype control group. Furthermore, serum from mice treated with IL-22 had improved opsonic capacity by increasing C3 binding on Spn. Taken together, endogenous IL-22 and hepatic IL-22R signaling play critical roles in controlling pneumococcal lung burden and systemic IL-22 decreases bacterial burden in the lungs and peripheral organs by potentiating C3 opsonization on bacterial surfaces, through the increase of hepatic C3 expression.
Introduction
IL-22 is a secreted cytokine produced mainly by cells of the lymphoid lineage and innate lymphoid cells (1). Ligation of IL-22 to IL-22RA1 and IL-10R2 results in activation of JAK1/TYK2 kinases and downstream phosphorylation of STAT proteins, in particular STAT3 (2, 3). IL-22RA1 is characteristically expressed in epithelial cells, and its activation by IL-22 is involved in host defense against extracellular organisms including Klebsiella pneumoniae (4) Citrobacter rodentium (5, 6) and Candida albicans (7). Also, IL-22 signaling is related to maintaining barrier integrity and epithelial repair during influenza infection (8), mediating recruitment of leukocytes, and inducing antimicrobial peptides. IL-22RA1 is also abundantly expressed in hepatocytes and has been shown that IL-22 liver signaling is critical for liver repair in acute injury models such as acute alcoholic hepatitis (9) as well as fibrotic injury such as in exposure to carbon tetrachloride (10). Furthermore, IL-22 promotes resistance against pathobionts by regulating the complement system (11) in a mouse model of Clostridium difficile colitis.
However, the role of IL-22 is understudied during pneumococcal pneumonia. Streptococcus pneumoniae (Spn) accounts for 4 million illness episodes, 445,000 hospitalizations and 22,000 deaths annually in the US (12). Children younger than 2 years old (12-31 cases/100,000) and individuals older than 65 years (~37 cases/100,000) are the most vulnerable for invasive disease. Although much work has been done on investigating the local pulmonary response to control Spn infection, it has been shown that also the liver is key to pneumococcal host immunity by controlling bacterial burden and dissemination (13). When both, NF-κB RelA and STAT3 are selectively deleted in hepatocytes, mice showed higher pneumococcal burdens particularly in extrapulmonary tissues (13). Several cytokines have been shown to be critical for controlling this hepatic response in the infected lung including TNF-α, IL-1β and IL-6. The fact that the liver has an important role in controlling pneumococcal pneumonia is also supported by clinical evidence. In particular, patients with liver cirrhosis (alcoholic and non-alcoholic) have higher rates of pneumococcal pneumonia as well as a higher incidence of pneumococcal bacteremia and extrapulmonary dissemination (14, 15).
Having the evidence that IL-22 is important for the host response against certain extracellular pathogens as well as having evidence of IL-22RA1 expression in the liver, we first confirmed that IL-22 was induced in the lung after a pneumococcal lung infection model in wild type mice. We confirmed that IL-22KO mice were more susceptible to infection compared to control mice, which suggested that endogenous IL-22 played a role in controlling pneumococcal burden. Thus, we further hypothesized that enhancement of IL-22 signaling would control pneumococcal burden in lung tissues in an experimental pneumonia model. This paper describes the increased pneumococcal susceptibility in mice that have a liver specific deletion of IL-22RA1 as well as the improved bacterial burden not only in lung but also in extrapulmonary tissues after recombinant IL-22 administration. Complement component 3 (C3) was increased in liver tissues from mice treated with IL-22 compared to the isotype control group and serum from mice treated with IL-22 had improved opsonic capacity by increasing C3 binding on Spn. These findings suggest the importance of local IL-22 production upon pneumococcal infection, IL-22RA1 hepatic signaling in controlling bacterial burden in part by increasing hepatic C3 expression and increased C3 deposition on pneumococci, which translates into decreased bacterial burden in the host.
Material and Methods
Mice
Il22ra1fl/fl mice on a C57BL/6 background were designed in our laboratory and made at Ozgene. LoxP sites were engineered to flank exon 3. To determine functional recombination, Il22ra1fl/fl mice were injected in the tail vein with an E1-deleted adenovirus expressing Cre recombinase and liver DNA was extracted and subjected to PCR using primers spanning exon 3. To determine functional deletion of IL-22RA1 specifically in the liver of Il22ra1fl/fl mice, we crossed this line to B6.Cg-Tg(Alb-cre)21Mgn/J or Albumin-Cre mice (obtained from The Jackson Laboratory), which confirmed cre-mediated DNA recombination by allele specific PCR. Ccsp-Cre mice were obtained as a kind gift from Dr. Thomas Mariani and described before(16). Il22−/− mice were obtained from The Taconic Farms.
Animal Studies
8-10 week old mice were used for in vivo studies. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Experimental Spn mouse infection
One fresh colony of Spn serotype 3 (ATCC 6303) was grown in 100 ml of Todd-Hewitt broth for 10 hours at 37°C, 5%CO2. 1 ml aliquots were obtained and centrifuged at 17000 g for 1 minute and washed twice with PBS. Bacterial pellet was resuspended in PBS and concentration was determined by measuring the absorbance at 600 nm. An absorbance of 0.5 was about 2×108 CFU/ml. An inoculum of 106 CFU/mouse was given by intranasal aspiration (in 50 ul of PBS). The working stock was serially diluted and plated on trypticase soy agar with 5% sheep blood plates (TSAII) to determine the exact concentration of the inoculum.
Recombinant proteins
IL22:Fc 4 ug/mouse (Generon, Shanghai, China) or isotype control 4 ug/mouse (human IgG) were given by intraperitoneal injection 3-4 hours before Spn infection. Anti IL-23p19: 500ug/mouse by intraperitoneal injection right before infection with Spn. Anakinra: 100 mg/kg by intraperitoneal injection right before infection with Spn. Cobra venom factor (CVF) (EMD Millipore): 25 ug/mouse of CVF was administered intraperitoneally for 24 hours before infection with Spn.
Bacterial burden, mRNA, and protein analysis
To determine bacterial burden, lungs, livers and spleen were harvested in PBS, homogenized with glass homogenizer, and plated on TSA II plates in four serial dilutions. Colonies were counted after overnight incubation at 37°C, 5%CO2. To determine gene expression, lungs and livers were placed in TRIzol reagent (Invitrogen), homogenized and processed according to manufacturer’s protocol. One microgram of RNA was used to synthesize cDNA. Real-time PCR primers with SSOFast Probes Supermix probes were used. Threshold cycle (CT) values were normalized to Hprt. For protein analysis, tissues were placed in RIPA cell lysis buffer with protease inhibitor mixture tablets (Roche Applied Science) homogenized, and centrifuged at 10,000 3 g. IL-22 levels in supernatants were measured by ELISA (eBioscience) and normalized to total protein levels (Pierce BCA Protein Assay Kit; Thermo Fisher Scientific).
C3 binding assay
Serum was collected from mice 24h after i.p administration of IL-22:Fc or isotype control . 106 CFU of Spn were incubated for 20 min at 37°C (no CO2), with no rotation with 10 or 50 ul of serum (from IL-22:Fc treated mice or isotype control), followed by washing with PBS Tween 0.1% . Mix of bacteria and serum was resuspended FITC-conjugated anti-mouse C3 (1:300; MBL International) for 20 min at 4°C. The percentage of positively labeled bacteria was determined using flow cytometry. Background fluorescence was based on that exhibited by non- opsonized bacteria.
Lung cell sorting and Flow Cytometry
Lungs were digested with collagenase D (3 mg/ml) in PBS for 1 h at 37°C, crushed through 100-mm cell strainers, and treated with ammonium chloride to lyse RBCs . Single cell suspensions were stained with CD16/32 (eBioscience). Two pooled lungs of Spn infected mice were stained with fluorochrome-conjugated antibodies of the following surface antigens: CD4, CD8α, and TCR-γδ . Three pooled lungs of infected mice with Spn were stained with fluorochrome-conjugated antibodies of the following surface antigens: CD11c, CD11b, F4/80, CD19, Ter119, NK1.1, TCR-β, TCR-γδ, CD8b, CD4 and CD90.2. Live /dead fixable blue cell stain kit was used to exclude dead cells. For neutrophil staining, the following fluorochrome-conjugated antibodies were used: CD45, Ly6G, Cd11b and F4/80. Gating strategies are supplied in Supplement Figure S1 (A-C).
Statistical Analyses
Unpaired, one-tailed, Student’s t tests, p< 0.05, were used to assess whether the means of two normally distributed groups differed significantly. ANOVA or Kruskal-Wallis was used to compare multiple means. Significance is indicated as p <0.05.
Results
IL-22 is required for host control of Spn pulmonary infection
To assess whether Il22 is expressed upon pneumococcal pneumonia, wild type C57Bl/6 mice (B6) were infected with 106 CFU of Spn serotype 3 and groups were sacrificed at different time points: 5h, 12h, 24h and 48h. Bacterial burden had a direct correlation with time of infection, thus, the highest bacterial burden in the lungs was at 48 hours post infection, after which we observed more than 95% mortality (data not shown). Lung Il22 expression increased as early as 5 hours post infection (Figure 1A) and even though, liver bacterial burdens increased significantly at 48 hours, hepatic Il22 gene expression was undetectable across all time points (Figure 1B), which suggests that the liver does not produce IL-22 upon pneumococcal lung infection. Furthermore, IL-22 protein was detected in lung homogenates at 12 hours post infection and it was also present in serum (Figure 1C) at the same time point. To evaluate if endogenous IL-22 is required for control of Spn infection we infected IL22−/− mice or control B6 mice and sacrificed them at 24 hours post infection. IL22−/− mice had increased bacterial burdens at 24 hours post infection compared to B6 mice in both the lung and liver (Figure 1D). Thus, endogenous IL-22 is required for control of pneumococcal burden after intrapulmonary infection.
Figure 1. IL-22 is required for host control of Spn pulmonary infection.
A) Spn burden in lungs and livers at different time points. Mice were infected with Spn 106 CFU/mouse by oropharyngeal aspiration and groups were sacrificed at different time points. B) Il22 gene expression in lungs and livers. C) IL-22 protein expression detected by ELISA at different times points of infection in lung tissue homogenate and serum. * p <0.05, **p<0.01, *** p<0.001 (One-way ANOVA). D) Bacterial burden in lung and liver between B6 and IL-22 knockout (IL-22KO) mice. *** p<0.001 (unpaired t-test). Each circle represents one mouse. Experiments were repeated two times.
IL-22 is regulated by Spn induced IL-23 and IL-1β
IL-23 and IL-1β, which are produced by macrophages and dendritic cells, are induced during pneumococcal pneumonia (17, 18). These two cytokines are major inducers of IL-22 (19). To asses if IL-23 and IL-1R control IL-22 expression in the pneumococcal pneumonia model, mice received anti-IL23 subunit p19 antibody (anti IL-23p19), IL-1R antagonist (IL-1Ra) or isotype control by intraperitoneal administration, followed by Spn infection. Mice were sacrificed at 5 hours post infection, since at this time point lung bacterial burdens were similar among experimental groups. At 5 hours post infection the anti-IL-23p19 group had substantially decreased Il22, Lcn2 and Reg3g compared to the isotype control group (Figure 2). Mice that received IL-1Ra prior to pneumococcal infection also displayed decreased Il22 mRNA expression as well as, Il17 and Lcn2 compared to the control group.
Figure 2. IL-22 production is dependent on IL-23 and IL-1β upon Spn pneumonia.
Wild type mice received either anti IL-23 p19, IL-1Ra or isotype control. Mice were sacrificed at 5 hours post infection. Gene expression of Il22, Il17, Lcn2 and Reg3g were assessed by qPCR. Each circle represents one mouse. * p <0.05, **p<0.01, *** p<0.001 (One-way ANOVA).
Cellular source of Il22 during acute pneumococcal pneumonia
To evaluate the cellular sources of IL-22 during acute pneumococcal pneumonia, B6 mice were infected with Spn (106 CFU/mouse) and sacrificed 24 hours post infection. Single cell suspensions from lung tissue were stained and flow cytometry sorted for the following cell populations: CD4+, TCR-γδ+ and Lin− CD90.2+ The sorted groups of cells were evaluated for expression of Rorc, Il22, Il17, Gata3, Il13, Il5 and Ifng (Figure 3). In the lung, TCR-γδ+ cells showed the highest expression of Il22, followed by Lin− CD90.2+ cells, which likely represent innate lymphoid cells. Although our sorting strategy for ILCs did not differentiate between ILC2 and ILC3, genes expressed in ILC2 like Gata3, Il13 and Il5 were highly expressed in our sorted Lin− CD90.2+ lung cells, suggesting that this group may represent mostly ILC2 cells with a smaller population of ILC3 cells, which expressed Rorc and Il22. Thus, in this acute pneumococcal pneumonia model TCR-γδ + cells were the predominant IL-22 producing cells. The frequency (percentage) and absolute number of cells were similar in sorted TCR-γδ + and Lin−CD90.2+ (Supplement Table S1).
Figure 3. Cellular source of IL-22 during pneumococcal pneumonia.
Mice were infected with Spn for 24 hours. Lungs were collected and collagenase treated for single cell suspension. Two pooled lungs of Spn infected mice were stained with CD4, CD8, and TCR-γδ antibodies; CD4+ cells and TCR-γδ+ cells were sorted. Three pooled lungs of infected mice with Spn were stained with Lin (CD11c, CD11b, F4/80, CD19, Ter119, NK1.1, TCRβ, CD8, CD4) and CD90.2 antibodies; Lin− CD90.2+ cells were sorted. Cell populations underwent gene expression by qPCR. Data represent three separate experiments. * p <0.05 (One-way ANOVA).
IL-22RA1 signaling in the liver is required for control of pneumococcal pneumonia burden
We evaluated the requirement of IL-22RA1 in lung epithelium in our pneumococcal pneumonia model. For this purpose, conditional lung epithelium specific Il22ra1 deficient mice: Ccsp Cre+/−Il22ra1fl/fl (Il22ra1ΔCcsp) and Ccsp Cre−/− Il22ra1fl/fl (Il22ra1fl/fl) littermate control were used. Upon experimental lung infection, there was no difference in lung bacterial burden between Il22ra1fl/fl and Il22ra1ΔCcsp groups (Figure 4A), which suggested that IL-22 signaling in the CCSP+ cells is dispensable in controlling lung pneumococcal burden. As mentioned before, IL-22RA1 is highly expressed in the liver and can activate STAT3 (3, 20, 21). Thus, to address the contribution of hepatic IL-22RA1 signaling during pneumococcal lung infection, we generated a conditional liver specific Il22ra1 knockout: Alb-Cre+/−Il22ra1fl/fl (Il22ra1ΔHEP) and used Alb-Cre−/− Il22ra1fl/fl (Il22ra1fl/fl) as littermate controls (22). Upon experimental lung infection Il22ra1ΔHEP mice had increased bacterial burdens in the lung and liver at 24 hours post pneumococcal infection compared to Il22ra1fl/fl mice (Figure 4B).
Figure 4. IL-22RA1 signaling in the liver is required for control of pneumococcal pneumonia burden.
A) CcspCre−/− × Il22ra1fl/fl (Il22ra1fl/fl) and CcspCre−/+ × Il22ra1fl/fl (Il22ra1ΔCcsp) mice were infected with Spn 106 CFU/mouse and sacrificed at 24 hours post infection. B) AlbCre−/− × Il22ra1fl/fl (Il22ra1fl/fl) and AlbCre−/+ × Il22ra1fl/fl (Il22ra1ΔHEP) were infected with Spn 106 CFU/mouse and sacrificed at 24 hours post infection. *p<0.05 , ns: p>0.05 (unpaired t-test). Each circle represents one mouse. Experiments have been repeated twice.
IL-22 pre-treatment controls pneumococcal burden in lung tissue
Next, we determined if administration of exogenous IL-22 could lead to improved host bacterial burden. B6 mice were pretreated with IL-22:Fc or isotype control , followed by Spn infection (106 CFU/mouse) and sacrificed at 24 hours post infection. B6 mice that received pre-treatment with IL-22:Fc showed decreased lung, liver and spleen bacterial burdens at 24 hours post pneumococcal lung infection, compared to the isotype control group (Figure 5A). Gene expression analysis in lung tissues showed no difference in genes reported to be regulated by IL-22 including Lcn2, Reg3g, Saa2/1 or Saa3 (Figure 5B). On the other hand, in liver tissue, Saa2/1 was increased in the IL-22Fc group compared to isotype control group, while other genes such as Saa3 and Lcn2 were not different between groups (Figure 5C).
Figure 5. IL-22 pre-treatment controls pneumococcal burden in lung tissue.
A) Wild type mice were given 4ug of IL-22:Fc or Isotype by intraperitoneal route 4 hours before Spn infection (106 CFU/mouse) and sacrificed at 24 hours post infection. Each circle represents one mouse. *p< 0.05, **p< 0.01 (unpaired t-test). Gene expression in B) lungs and C) livers of naïve , Isotype or IL-22:Fc pre-treated mice followed by Spn infection. **p<0.01(One-way ANOVA).
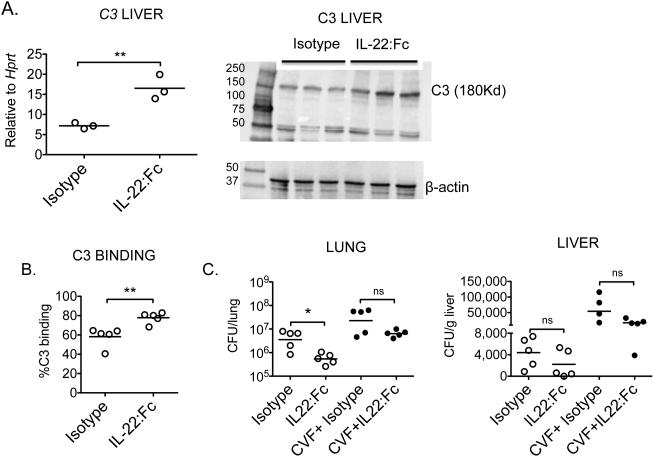
IL-22 increases hepatic C3 expression and serum opsonization capacity
Since pretreatment with IL-22:Fc decreased bacterial burden compared to isotype control and since IL-22RA1 in the liver is important for pneumococcal burden control, we first tested if IL-22:Fc would increase gene expression of Complement component 3 (C3) in liver tissue. For that B6 mice received either Isotype or IL-22Fc for 24 hours. Afterwards, liver tissues were retrieved and assessed for C3 gene and protein expression. IL-22:Fc treated mice had higher C3 expression in liver tissues compared to isotype group (Figure 6A). Furthermore, to asses if C3 would be contributing to increase bacterial opsonization, we performed an in vitro C3 binding assay, in which Spn was opsonized with serum from IL-22:Fc or Isotype treated mice. There was increase in C3 binding of Spn opsonized with IL-22:Fc serum compared to the Isotype control (Figure 6B). To determine the role of endogenous C3 upon infection, C3 was depleted in vivo by the administration of cobra venom factor (CVF) for 24 hours or PBS as control. These groups of mice were further randomized to IL-22:Fc or isotype pre-treatment for 3 hours, followed by Spn (106 CFU/mouse) and sacrificed at 24 hours post infection. Mice that received CVF and IL-22Fc had some decrease in lung bacterial burden compared to the group that received CVF and isotype (Figure 6C), but this difference did not reach statistical significance, which suggested that IL-22 had a partial rescue in bacterial burden.
Figure 6. IL-22 increases serum opsonization capacity.
A) C3 gene expression in liver tissue from mice treated with Isotype or IL-22:Fc for 24 hours , **p< 0.01 (unpaired t-test). Protein expression by western blot of same liver homogenates. B) C3 binding assay. C3 deposition was measured on serum-opsonized Spn by flow cytometry. Data represent mean of the percentage of C3 positive bacteria exposed to serum from different mice: Isotype, IL-22:Fc or Spn treated (n = 5/group). C) C3 was depleted using cobra venom factor (CVF) (25 ug/mouse i.p) for 24 hours , followed by IL-22:Fc or isotype control pre treatment for 3 hours and Spn infection for 24 hours. * p <0.05, *** p<0.001, ns: p>0.05 (One-way ANOVA). Experiments were repeated twice.
Discussion
In this study we investigated if IL-22 plays a role during pneumococcal pneumonia, hepatic IL-22RA1 signaling for control of bacterial burden and whether exogenous IL-22 administration offers protection to the infected host. Our findings show that IL-22 is required for control of pneumococcal burden, which is supported by Il22 gene expression in the lungs as early as 5 hours post infection. Il22 is expressed in the lungs along with other cytokines like Il6, Il1β and Tnfα during pneumococcal pneumonia. We showed that genetic deletion of IL-22 makes the host more susceptible, which supports the role of IL-22 in controlling pneumococcal burden. We also found that IL-22 produced during pneumococcal pneumonia is dependent on IL-23 and IL-1β signaling. This finding is supported by the well established role of IL-23 and IL-1β in controlling IL-22 expression using different models and routes of infection (4, 5).
In our experimental model, TCR- γδ+ cells were the predominant source of Il22 during the early stages of pneumococcal pneumonia. This was supported by higher gene expression of Il22, Rorc and Il17 in flow cytometry sorted TCR- γδ+ cells from the lungs of infected mice. The second population that expressed Il22 were ILCs (likely ILC3), although due to the small number of cells, we did not differentiate further between ILC2 and ILC3. Based on the higher levels of Gata3, Il13 and Il5 in this population, we assume it mostly represents ILC2 cells. ILC3 cells have been described to produce IL-22 during pneumococcal pneumonia (23), although in our study, the sorted population were not cultured ex-vivo for 3 days as reported by this other group. As the lungs have very low numbers of TCR- γδ+ cells and ILCs (24) in the lungs, we had to pool samples to overcome this issue and have adequate material for gene expression analysis.
In our pneumococcal infection model, the lack of IL-22RA1 signaling in the liver resulted in a higher bacterial burden after pulmonary inoculation. This suggests that engagement of IL-22 and hepatic IL-22RA1 is important for controlling pneumococcal lung burden and dissemination. Furthermore, pre-treatment with systemic exogenous IL-22 (IL-22:Fc) resulted in decreased bacterial burden in lung and peripheral tissues. The importance of the liver in controlling lung bacterial burden goes in line with previous studies that have found the importance of the liver response to control bacterial pneumonia (25, 26), as hepatic specific mutations of both STAT3 and NF-κB RelA abrogate the hepatic acute phase response and renders the host more susceptible to bacterial dissemination (13, 27). IL-22, also a potent acute phase reactant inducer (28, 29) is induced during pneumococcal pneumonia and in physiologic amounts likely acts in concert along with other cytokines such as IL-6, IL-1β, TNF-α. Nevertheless, contrary to other secreted cytokine’s receptors, IL-22RA1 is present at specific mucosal barrier sites, in non-immune cells, which makes its activity more targeted and potentially with less collateral damage when used as a therapeutic agent to help controlling pneumococcal pneumonia and dissemination. We also assessed if pretreatment with IL-IL-22:Fc in mice infected with a lower dose of Spn (LD50: 103CFU/mouse) would confer some advantage in survival at 10 days post infection (60% for IL-22:Fc treated versus 20% for isotype control) but this did not reach statistical significance. (Supplement Figure S2).
One of the mechanisms by which pretreatment with IL-22: Fc decreases bacterial burden at 24 hours in our study is by improving Complement factor 3 (C3) deposition on pneumococci. Recent evidence supports the role of IL-22 in regulating the complement system (11) and eliminating commensal bacteria during C. difficile infection, thus our finding goes in line with this report. Furthermore, our in vivo C3 depletion studies, using CVF showed that pretreatment with IL-22:Fc was associated with a slight reduction in bacterial burden in the lungs, although this did not reach statistical significance. This may suggest additional mechanisms in our model by which IL-22 is contributing to bacterial containment. To this end, one aspect to explore is the role of SAA 1 and 2 in our infection model, since they are markedly increased by IL-22 in our model (Figure 5C). The increase in C3 deposition may contribute to improved neutrophil phagocytosis. Thus we first tested if neutrophil recruitment was affected in the IL-22:Fc group compared to isotype control mice. Neutrophil recruitment into the BAL fluid was similar in both groups (Supplement Figure S3A-B). To determine the uptake of Spn we sorted Ly6G+,Cd11b+, F4/80− cells from total lungs (which represent lung neutrophils) and extracted total RNA from these cells. Sorted neutrophils from IL-22:Fc treated mice had increased detectable CpsA gene expression (a gene specifc for Spn, Supplement Figure S3C)(30), which suggests that neutrophils from the IL-22:Fc treated mice had improved bacterial phagocytosis consistent with the observed increase in C3 bacterial deposition. Furthermore, the Fc part of IL-22:Fc itself seems not to activate C3, as we retrieved sera from naïve mice and spiked them either with IL-22:Fc or Isotype control at 2ug/ml. Afterwards these spiked sera was used to opsonize Spn at 37oC for 20 minutes. C3 binding capacity was similar in both groups, suggesting that Fc portion of IL-22:Fc may not activate C3 in vitro (Supplement Figure S3D).
In conclusion, endogenous IL-22 plays a role in protecting the host during pneumococcal pneumonia and enhancement of IL-22-IL-22RA1 signaling is beneficial for the host to control pneumococcal pneumonia. IL-22:Fc which is currently in clinical trials for gut graft versus host disease (NCT02406651) and alcoholic hepatitis (NCT02655510) may be a useful adjunct therapy for severe pneumococcal infection.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Thomas Mariani for the CCSP-cre mice and the technical assistance of Ms. Kara Kracinovsky.
This work was supported by R37 HL079142 and R37HL079142-11S1.
References
- 1.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22:immunobiology and pathology. Annu Rev Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16:5894–5903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. The Journal of biological chemistry. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 4.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 6.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, Puccetti P, Romani L. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- 8.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 is essential for lung epithelial repair following influenza infection. The American journal of pathology. 2013;182:1286–1296. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–49. [PubMed] [Google Scholar]
- 11.Hasegawa M, Yada S, Liu MZ, Kamada N, Munoz-Planillo R, Do N, Nunez G, Inohara N. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity. 2014;41:620–632. doi: 10.1016/j.immuni.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, Finkelstein JA. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 13.Quinton LJ, Blahna MT, Jones MR, Allen E, Ferrari JD, Hilliard KL, Zhang X, Sabharwal V, Algul H, Akira S, Schmid RM, Pelton SI, Spira A, Mizgerd JP. Hepatocyte-specific mutation of both NF-kappaB RelA and STAT3 abrogates the acute phase response in mice. The Journal of clinical investigation. 2012;122:1758–1763. doi: 10.1172/JCI59408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viasus D, Garcia-Vidal C, Castellote J, Adamuz J, Verdaguer R, Dorca J, Manresa F, Gudiol F, Carratala J. Community-acquired pneumonia in patients with liver cirrhosis: clinical features, outcomes, and usefulness of severity scores. Medicine. 2011;90:110–118. doi: 10.1097/MD.0b013e318210504c. [DOI] [PubMed] [Google Scholar]
- 15.Preto-Zamperlini M, Farhat SC, Perondi MB, Pestana AP, Cunha PS, Pugliese RP, Schvartsman C. Elevated C-reactive protein and spontaneous bacterial peritonitis in children with chronic liver disease and ascites. Journal of pediatric gastroenterology and nutrition. 2014;58:96–98. doi: 10.1097/MPG.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 16.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ. Epithelial cell PPAR[gamma] contributes to normal lung maturation. FASEB J. 2006;20:1507–1509. doi: 10.1096/fj.05-5410fje. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Ma J, Charboneau R, Barke R, Roy S. Morphine inhibits murine dendritic cell IL-23 production by modulating Toll-like receptor 2 and Nod2 signaling. The Journal of biological chemistry. 2011;286:10225–10232. doi: 10.1074/jbc.M110.188680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma J, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. Journal of immunology. 2011;187:434–440. doi: 10.4049/jimmunol.1003143. [DOI] [PubMed] [Google Scholar]
- 19.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res. 2001;21:1047–1053. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- 22.Zheng M, Horne W, McAleer JP, Pociask D, Eddens T, Good M, Gao B, Kolls JK. Therapeutic Role of Interleukin 22 in Experimental Intra-abdominal Klebsiella pneumoniae Infection in Mice. Infect Immun. 2016;84:782–789. doi: 10.1128/IAI.01268-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Maele L, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, Chabalgoity JA, Renauld JC, Eberl G, Benecke AG, Trottein F, Faveeuw C, Sirard JC. Activation of Type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis. 2014;210:493–503. doi: 10.1093/infdis/jiu106. [DOI] [PubMed] [Google Scholar]
- 24.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr., Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilliard KL, Allen E, Traber KE, Yamamoto K, Stauffer NM, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. The Lung-Liver Axis: A Requirement for Maximal Innate Immunity and Hepatoprotection during Pneumonia. Am J Respir Cell Mol Biol. 2015;53:378–390. doi: 10.1165/rcmb.2014-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinton LJ, Mizgerd JP. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Physiol. 2015;77:407–430. doi: 10.1146/annurev-physiol-021014-071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinton LJ, Jones MR, Robson BE, Mizgerd JP. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect Immun. 2009;77:2417–2426. doi: 10.1128/IAI.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10144–10149. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, Fouser LA. IL-22 induces an acute-phase response. Journal of immunology. 2010;185:5531–5538. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- 30.Park HK, Lee SJ, Yoon JW, Shin JW, Shin HS, Kook JK, Myung SC, Kim W. Identification of the cpsA gene as a specific marker for the discrimination of Streptococcus pneumoniae from viridans group streptococci. J Med Microbiol. 2010;59:1146–1152. doi: 10.1099/jmm.0.017798-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.