Abstract
Uncontrolled inflammation is a unifying component of many chronic inflammatory diseases, such as arthritis. Resolvins (Rv) are a new family from the endogenous specialized pro-resolving lipid mediators (SPM) that actively stimulate resolution of inflammation. Herein, using lipid mediator (LM) metabololipidomics with murine joints we found a temporal regulation of endogenous SPM during self-resolving inflammatory arthritis. The SPMs present in self-resolving arthritic joints include the D-series resolvins, e.g. Resolvin (Rv) D1, RvD2, RvD3 and RvD4. Of note, RvD3 levels were reduced in inflamed joints from mice with delayed-resolving arthritis when compared to self-resolving inflammatory arthritis. RvD3 was also reduced in serum from rheumatoid arthritis (RA) patients compared to healthy controls. RvD3 administration reduced joint leukocytes as well as paw joint eicosanoids, clinical scores and edema. Together, these findings provide evidence for dysregulated endogenous RvD3 levels in inflamed paw joints and its potent actions in reducing murine arthritis.
Keywords: mediators, resolution of inflammation, eicosanoids, leukocytes
Introduction
Acute inflammation is a host-protective response against injury or infection, that when uncontrolled can lead to chronic inflammation, scarring and eventual loss of tissue function (1, 2). The ideal outcome of acute inflammation is complete resolution, an active process now known to be orchestrated by specialized pro-resolving mediators (SPM) that limit further neutrophil recruitment and counter-regulate proinflammatory mediators (e.g. leukotrienes (LT), prostaglandins (PG), and cytokines) (1), which mount the initiation and propagation of the initial acute inflammatory response (3, 4). This novel genus of bioactive lipid mediators (LM) biosynthesized from essential fatty acids (EFA) in resolving inflammatory exudates, includes lipoxins (LX), resolvins (Rv), protectins (PD) and maresins (MaR) (1, 5). They actively promote catabasis via potent anti-inflammatory and pro-resolving actions (e.g. stimulating leukocyte uptake of apoptotic cells (efferocytosis) and bacterial clearance (reviewed in (1)), providing a molecular basis for novel therapeutic approaches via promoting resolution (1).
Arthritis is a significant clinical problem characterized by exuberant inflammation leading to joint destruction and pain (6, 7). Emerging evidence indicates that failure to engage proresolving pathways may contribute to persistent chronic inflammation, such as in rheumatoid arthritis (RA) (1, 2). Current therapeutic approaches aim to disrupt the inflammatory response, slow disease progression and limit pain (2, 8). These include methotrexate, anti-TNF biologics and non-steroidal anti-inflammatory drugs (NSAIDs)(8). However, these treatments may have drawbacks in terms of infections and may prevent resolution and tissue repair (1, 8, 9). Hence, safe and effective treatments that can regulate arthritic inflammation are still in need. In that context, 17R-RvD1 and the D-series resolvin pathway marker 17R-HDHA, are log order more potent than opioids, cyclooxygenase (COX) inhibitors or glucocorticoids in modulating inflammatory pain in a preclinical model of arthritis in rats (10).
Resolvins of the D-series are enzymatically biosynthesized from the omega-3 EFA, docosahexaenoic acid (DHA), including RvD1, RvD2, RvD3 and RvD4 (1). Recently, we completed the stereochemistry of RvD3 (4S, 11R, 17S- trihydroxy-docosa-5Z, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid), established its potent pro-resolving actions and unique temporal profile late within the resolution phase (11), suggesting it may have a specific role in regulating resolution. Given these properties, we investigated the role and action of RvD3 in arthritis.
Materials and Methods
Animals
Male C57Bl/6 mice (8 wks old) were obtained from Charles River Laboratories (Newton, MA). All animal experiments were in accordance with the Harvard Medical Area Standing Committee on Animals (protocol no. 02570). For some experiments male C57Bl/6 (12 wks old) were obtained from Charles River, Kent or ALX/fpr2/3 knockout (KO) mice generated on a C57Bl/6 background (and back-backcrossed 10 times) in-house at the William Harvey Research Institute, Barts and the London School of Medicine, Queen Mary University of London, UK, as described (12). All animal experiments were performed in accordance with institutional guidelines
LC-MS-MS-based LM metabololipidomics
Deidentified serum from healthy volunteers and RA patients were obtained from DX Biosamples (San Diego, CA). Their demographics are shown in Supplemental Table 1. RA patients were diagnosed with stage III RA by board certified physician and were treated with standard therapies (Supplemental Table 1). For LM profiling, ice-cold methanol containing deuterated internal standards d8-5S-HETE, d4-LTB4, d5-LXA4, d4-PGE2 and d5-RvD2 representing each chromatographic region of identified LM (500 pg each) were added to samples prior to processing to facilitate quantification and assessment of sample recovery. LC-MS-MS based metabololipidomics were performed as described in (13). RvD3 was prepared and qualified by LC-MS-MS and spectroscopic analysis as in (11) and second synthetic RvD3 was obtained from Cayman Chemicals (Ann Arbor, MI). Also, the complete stereochemistry of RvD4 was recently determined (14). Multiple reaction monitoring (MRM) with signature ion pairs, Q1 (parent ion) - Q3 (characteristic daughter ion) for each molecule. Data acquisition was performed in negative ionization mode and identification was conducted in accordance with published criteria (13), with minimum of 6 diagnostic ions.
K/BxN Serum Transfer Inflammatory Arthritis Model
For self-resolving and. delayed-resolving arthritis: C57BL/6 male mice were administered arthritogenic K/BxN serum (100 µl, i.p.) at days 0 and 2 for self-resolving arthritis, and with additional challenge on day 8 for delayed-resolving arthritis. Clinical scores were assessed (score criteria per paw: 0, no signs of inflammation; 1, inflammation in one of the following aspects: individual phalange joints, localized wrist/ankle or swelling on surface of paw; 2, inflammation on two aspects of paw; 3, major swelling on all aspects of paw; maximum score = 12 per mouse) to monitor disease development. Joints were harvested at indicated time intervals, and LM metabololipidomics performed as described. In some experiments, mice were taken to moorFLPI-2 Laser Doppler imaging (Moor Instruments, Wilmington, DE) at day 15. For RvD3 treatments: Mice (, C57Bl/6 or ALX/fpr2/3 KO mice) were administered vehicle (0.1% ethanol) or RvD3 (100 ng) in 100 µl of saline i.p. daily, starting from day 0 until completion of experiments at day 6. Clinical scores were assessed as above and paw edema assessed by water displacement plethysmometry (Ugo Basile, Comerio, Italy). On termination of the experiments, arthritic paws and joints were collected for histology staining and LM metabololipidomics.
Partial least-squares discriminant analysis
Partial least squares-discriminant analysis (PLS-DA) was performed using SIMCA 13.0.3 software (Umetrics, San Jose, CA) following mean centering and unit variance scaling of LM amounts. The score plot shows the systematic clusters among the observations (closer plots presenting higher similarity in the data matrix). Loading plots describe the magnitude and the manner (positive or negative correlation) in which the measured LMs/SPMs contribute to the cluster separation in the score plot (15).
Histological Analysis
Joints were fixed and decalcified for 2 weeks prior to embedding in paraffin. Sections (8 µm) were stained with Haemotoxylin & Eosin and standard light microscopy used to determine the degree of neutrophil infiltration.
Ligand Selectivity Using Electric Cell-Substrate Impedance Sensing (ECIS)
hALX-transfected chinese hamster ovary (CHO) cells were plated at 1×105 cells/well in HAM-F 12 buffer containing 10% FCS and G418 (37°C) for 36 h. Then the medium was changed to serum free HAMs media and vehicle or RvD3 (100 nM) were added and impedance across the cell monolayer measured using an ECIS (Applied Biophysics, Troy, NY) to monitor ligand-receptor interactions as in (16).
Statistics
Data are presented as mean ± s.e.m. The criterion for statistical significance was P < 0.05 using Student's t test (two groups), one-way ANOVA (multiple groups) or two-way repeated measure ANOVA (two groups over time) followed by a post hoc Bonferroni test using GraphPad Prism 6.
Results
SPMs are temporally regulated in joints during self-limited inflammatory arthritis
To investigate whether SPMs are temporally regulated in joints during inflammation-resolution we used an established murine model relevant to human arthritis that is driven by the innate immune response (17, 18). Mice injected with K/BxN arthritogenic serum (100 µl/mouse, i.p.) at day 0 and day 2, developed a self-resolving polyarthritic inflammatory response that reached a maximum clinical score at day 11, and subsequently declined (Fig. 1a). The reduction in clinical scores corresponded with loss of leukocytes from the joints (Fig. 1b), a key histological feature of resolution (1).
Figure 1. SPM temporal regulation during self-limited inflammatory arthritis.
Mice were challenged with K/BxN serum (100 µl, i.p.) on days 0 and 2. Disease progression was monitored and paws were collected on the indicated intervals. (a) Clinical scores (b) H&E staining blue arrowheads denote leukocytic infiltration, black arrowheads denotes bone erosion. (c-d). LM levels were determined in paws at the indicated intervals using LC-MS-MS based LM profiling. (c) MS-MS spectrum employed for the identification of RvD3. (d) PCA of the lipid mediator profiles; left panel: 2D Score Plot, right panel: 2D Loading Plot. Results (a) are mean ± SEM. n=4 mice per interval. Results (b–d) are representative of n=16 mice.
Using liquid chromatography tandem mass spectrometry (LC-MS-MS)-based metabololipidomics we next investigated the temporal LM-SPM profiles in self-resolving joints. LM identified, include E and D-series resolvins, protectins, maresins from the omega-3 (i.e. EPA and DHA) bioactive metabolomes, and lipoxins as well as prostanoids and LT from the arachidonic acid bioactive metabolome (Fig. 1c, Supplemental Fig. 1). All LMs were identified in accordance with published criteria as illustrated for RvD3 (see Material and Methods; Fig. 1d; Supplemental Fig. 1).
Distinct LM profiles were identified in joints for each time interval prior to (day 0; naïve) and after induction of self-resolving arthritis using principal component analysis (PCA; Fig. 1d).) There were higher levels of RvD4, MaR1, RvE1 and 15R-LXA4 in naïve compared with arthritic paws. Following serum challenge there was an increase in pro-inflammatory eicosanoids including PGE2, PGD2 and TXB2, LTB4, with a contaminant decrease in most SPMs identified (Fig. 1d; Supplemental Fig. 1) demonstrated by a downward shift in the 4- and 8-day clusters (arthritis) on the score plot (Fig 1d, left). This was followed by an anti-clockwise shift in the 16 d cluster (resolution) back towards the 0 day cluster (Fig 1d). Of note, levels of specific SPM, including the D-series resolvins RvD1, RvD2 and RvD3 increased in arthritic joints during the resolution phase (day 16; Supplemental Fig 1). These findings demonstrate that endogenous SPM pathways are active in murine joints that are temporally and differentially regulated during self-resolving inflammatory arthritis.
Shorter resolution intervals and specific SPM in self-resolving arthritis
Since select SPMs were temporally regulated in self-resolving joint inflammation, we investigated their levels during delayed-resolving inflammatory arthritis to determine which of these tissue protective molecules may be directly involved in resolving joint inflammation. First, to provide quantitative analyses of resolution (i.e. remission) of joint inflammation we applied resolution indices, as are widely used to calculate the loss of neutrophils from site of inflammation (19), using clinical scores to quantify the resolution interval (Ri). Here, the Ri is the time interval between when clinical scores reach maximum (Tmax; day 8) and the time it reaches 50 % remission (T50; day 12), giving an Ri of 4 d (Fig. 2a). Additional challenge with K/BxN-serum at day 8 prolonged arthritis with delayed resolution, with a T50 of 17 d, or an Ri of 9 d (Fig. 1a). At day 15, mice were taken for laser Doppler imaging to further assess the degree of joint inflammation-resolution in comparison with unchallenged mice (without K/BxN serum, Fig. 2b). Increases in tissue perfusion monitored via laser Doppler were consistent with increases in joint inflammation. These results indicate that resolution of inflammatory arthritis is delayed following an additional K/BxN-serum challenge, which may be associated with an altered LM-SPM profile.
Figure 2. Delayed-resolving vs self-resolving arthritis: prolonged resolution interval and altered LM-SPM profile.
Mice were challenged with K/BxN serum (100 µl, i.p.) on days 0 and 2 (Self-resolving) or days 0, 2 and 8 (Delayed-resolving). (a) Clinical scores were determined and used to calculate the resolution interval (Ri; see Material and Methods). Results are mean ± SEM from n = 5 mice. (b) Doppler imaging from paws with arthritis (left), self-resolving arthritis (middle) and without K/BxN-serum (right). Results are expressed as Perfusion Units (which are arbitrary) and representative from n = 5 mice per group. LM from self-resolving (left) and delayed-resolving (right) paw joints was determined using LC-MS-MS-based LM metabololipidomics. (c) PLS-DA; Endogenous LM: 2D score (left) and 2D loading (right) plots of LM-SPM obtained from Self-resolving (blue) and Delayed-resolving (red) paw joints. LM with variable influence on the projection (VIP) scores >1.0 are color coded in accordance with associated cluster. Results are from four mice per group.
We next assessed the local endogenous LM-SPM profile in self-resolving joints and compared it to the delayed-resolving joints. Differences in local paw joint LM-SPM profiles were assessed using PLS-DA (Fig. 2c). To identify which variables were responsible for the separation between the clusters (Fig. 2c, left), the variable influence on the projection (VIP) score >1.0 was used to select the most significant variables contributing to the cluster separation. The self-resolving cluster was characterized by higher levels of RvD3, MaR1 and PD1 (VIP >1.0;Fig. 2c, right), whereas, the delayed-resolving cluster was associated with higher levels of pro-inflammatory LM, such as TxB2, and PGE2 (Fig. 2c right). Taken together, these findings indicate that delayed resolution dysregulates endogenous resolution programs in arthritic joints, increasing local pro-inflammatory mediators and diminishing SPM.
Dysregulated LM-SPM profiles in human rheumatoid arthritis patients
Toward human translation, we also compared the LM-SPM profile in serum from healthy individuals and patients with RA. All LM were identified in accordance with published criteria as illustrated for RvD1 and RvD3 (Fig. 3a). Quantification using MRM gave significantly lower levels of RvD3 (4.3 ± 1.4 vs. 17.7 ± 2.3 pg/mL, p=0.008) and PGD2 (46.8 ± 9.8 vs. 95.7 ± 13.9 pg/mL, p=0.046), with a similar trend for RvD4 (1.1 ± 0.3 vs. 3.3 ± 0.9 pg/mL, p=0.083), found in serum from RA patients compared with healthy individuals (Supplemental Table 1). It is important to note that a larger sample size is needed to confirm these. Following identification using the LC-MS-MS fractions with matched mass spectra diagnostic ions and retention time of authentic standards, the data was subjected to multivariate analysis. The PLS-DA score plot demonstrated a clear separation between the healthy and RA serum clusters (Fig. 3b, left). The loading plot, indicating the magnitude and the manner (positive or negative correlation) each of the LM-SPM contribute to the cluster separation, showed that serum from healthy individuals was characterized (VIP score ≥ 1.0) by higher RvD3, RvD4, RvE3, 15R-LXA4 and PGD2 levels, while the RA serum was associated with higher TxB2 levels (Fig. 3b, right). Hence, these results in human RA patients together with the in vivo findings in murine arthritis suggest that select SPMs, such as RvD3, are deregulated in arthritis.
Figure 3. Human arthritis patients: altered LM-SPM profiles.
LM obtained from deidentified serum from healthy individuals or RA patients were identified by LC-MS-MS-based LM metabololipidomics (see Material and Methods). (a) Representative MS/MS spectra employed for identification of, for example, RvD1 (left) and RvD3 (right; inset, diagnostic ions; M, molecular mass). (b) PLS-DA; Endogenous LM: 2D score (left) and 2D loading (right) plots of LM-SPM obtained from healthy (blue) and RA (red) serum. LM with variable influence on the projection (VIP) scores >1.0 are color coded in accordance with associated cluster. Results are from three individuals in each group.
RvD3 limits arthritis severity and joint inflammation in mice
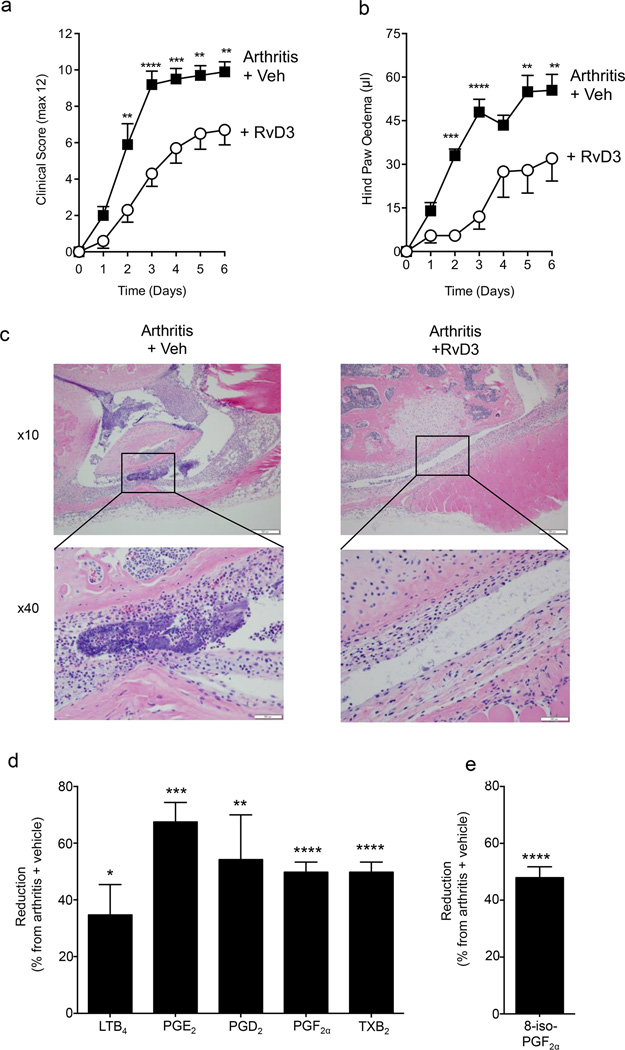
Having found that RvD3 was dysregulated both in delayed-resolving arthritic joints (Fig. 2c) and in human RA serum (Fig. 3), we next sought to determine its pharmacological actions in inflammatory arthritis. Mice were challenged with K/BxN-serum (100 µl, i.p.) on day 0 and 2. Daily administration of RvD3 (100 ng/mouse, i.p.) starting at day 0 significantly reduced clinical scores by 35–60% (Fig. 4a) and hind paw edema by 50–85% (Fig. 4b) during the course of the experiments, with a significant reduction observed as early as day 2 (Fig. 4a,b). Histological analysis showed fewer leukocytes at day 6 in joints from arthritic mice administered RvD3 (Fig. 4c). Hence, these results demonstrate that RvD3 alleviates arthritis progression, reducing edema and limiting leukocyte numbers in murine K/BxN-serum induced arthritis.
Figure 4. RvD3 limits joint inflammation in arthritic mice.
Mice were challenged with K/BxN serum (100 µl, i.p.) on days 0 and 2 for induction of arthritis and mice administered either vehicle (veh; saline plus 0.1% EtOH) or RvD3 (100 ng per mouse, i.p.) daily. (a) Clinical scores and (b) hind paw edema were assessed daily (see Material and Methods). (c) On day 6, joints were collected and taken for histological microscopic analysis with H&E: x10 magnification (top) and inset box shows leukocyte infiltrates with x40 magnification (bottom). Results are (a-b) mean ± SEM or (c) representative from n = 10 mice per group. LM were assessed in arthritic paws (day 6) using LC-MS-MS based LM metabololipidomics following solid phase extraction. (d) Reduction in joint eicosanoids with RvD3 treatment expressed as % reduction from arthritis plus vehicle mice. Results are mean ± SEM from n = 3 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. arthritis plus vehicle mice.
RvD3 reduces local eicosanoid levels in murine inflammatory arthritis
Since RvD3 reduces eicosanoid production in acute peritonitis (11), we next assessed its ability to regulate local joint eicosanoid levels in murine inflammatory arthritis. We preformed LC-MS-MS based LM metabololipidomics with murine arthritic joints (day 6) to identify potential mechanisms underlying the anti-arthritic actions of RvD3. Treatment with RvD3 resulted in significant (p< 0.01) reduction in local joint levels of LTB4 (34.7 ± 10.9%), PGE2 (67.7 ± 6.9%), PGD2 (54.3 ± 15.9%) PGF2α (61.0 ± 10.1 %) and TxB2 (50.0 ± 3.6 %; Fig. 4d). We also identified the non-enzymatic 8-iso-PGF2α, indicative of oxidative stress, which was also reduced in RvD3 treated mice (Fig. 4e). Taken together, these results indicate that RvD3’s potent actions limiting paw joint inflammation include regulating local eicosanoids, characteristic of SPMs (1).
The arthritis-protective actions of RvD3 are lost in ALX/FPR2 deficient mice
We next tested whether RvD3 activates the pro-resolving receptor ALX/FPR2, which mediates the actions of LXA4 and RvD1 (1). Expression of this receptor in transfected CHO cells demonstrated that RvD3 at 100 nM directly activated this receptor (Fig. 5a). Since mice lacking ALX/FPR2 exhibit a more pronounced and prolonged joint response compared to WT counterparts (20), we next investigated whether the arthritis-protective actions of RvD3 were altered in these mice. We found that, in ALX/FPR2 deficient mice, RvD3 did not reduce clinical scores, hind paw edema or LTB4 levels (Fig. 5b–d). Thus, taken together, these results suggest that RvD3 activates ALX/FPR2 in vivo in mice that, at least in part, mediates RvD3 protective actions in this murine model of experimental arthritis.
Figure 5. RvD3’s protective actions in arthritis are lost in receptor deficient mice.
(a) Ligand-receptor-dependent changes in impedance with CHO cells overexpressing human ALX continuously recorded with real-time monitoring across cell monolayers using an ECIS system. Results are representative from 4 independent traces obtained from incubations of RvD3 (100 nM) or vehicle with CHO-ALX/fpr-expressing cells. (b-d) ALX deficient (ALX/fpr2/3 KO) and wild type (WT) mice were challenged with K/BxN serum (100 µl, i.p.) on days 0 and 2 for induction of arthritis and mice administered either vehicle (veh; saline plus 0.1% EtOH) or RvD3 (100 ng per mouse) i.p. daily.. Reduction in (b) arthritis score, (c) joint edema, and (d) local LTB4 levels at day 6. Results are expressed as % reduction by RvD3 from vehicle treated mice in WT and ALX/fpr2/3 KO. Results are (b-d) mean ± SEM from n = 8 mice for treatment with vehicle (saline plus 0.1% EtOH), n = 7 mice for treatment with RvD3 in ALX/fpr2/3 KO mice and n = 10 for RvD3 or vehicle treatment in WT mice. *P < 0.05, **P < 0.01 vs. arthritis plus vehicle mice.
Discussion
In the present report, using LM metabololipidomics, we investigated the temporal regulation of LM-SPM in joints during self-limited inflammatory arthritis in mice. We identified RvD3 as an SPM that was deregulated in both delayed-resolving murine arthritis and serum obtained from RA patients. When administered to mice, RvD3 alleviated arthritis severity, reduced paw edema, joint leukocytes and eicosanoid levels, actions that were lost in ALX/FPR2 deficient mice. Together, these results implicate a protective role for RvD3 in arthritis.
Disruption of endogenous resolution programs may contribute to persistent chronic inflammation a pathological feature of many widely occurring disease, including RA (1, 2). The results presented herein are the first to investigate the temporal regulation of SPMs in self-resolving arthritis to potentially identify specific SPMs that may be upregulated in resolution of joint inflammation. In this context, evidence from experimental models of inflammatory arthritis suggests that disruption of SPM biosynthetic or signaling pathways leads to exacerbation of disease (20, 21). Arthritic mice deficient in 12/15-lipoxygenase, a key enzyme in SPM biosynthesis, have reduced LXA4 levels and exacerbated disease (21). Similarly, increased disease severity is observed in mice lacking the receptor ALX/FPR2 (20). In mice, RvD1 signals via ALX/FPR2 (16), an observation that also holds for RvD3, as demonstrated by loss of activity in ALX/FPR2 deficient mice (Fig. 5b–c). These findings are consistent with the notion that ALX/FPR2 is a key receptor in the resolution mechanisms in inflammation. These findings, taken together with the findings that SPMs are temporally regulated during resolution and its delay is associated with deregulation in specific SPMs in arthritic murine joints, implicate a role for these SPM and their pathways in modulating inflammatory arthritis.
RA is a chronic progressive disease with predominant inflammation driven by pro-inflammatory signals, such as PGs, LTB4 and TNFα (6). Most current drugs used to treat RA aim to reduce, antagonize or inhibit the production of these pro-inflammatory molecules (8, 22). Although these medications can help relieve symptoms they can also delay or prevent complete resolution and are thus considered resolution toxic, because they block the production of local PG that are important for resolution (1). One such example is the COX-2 inhibitor NS-398, which delays resolution of murine autoimmune arthritis via disrupting the PGE2--mediated LXA4 induction and production (9). Of note, when added back in animal disease models, SPMs, such as Rvs, PDs and LXs, actively counter-regulate inflammation and promote its termination (reviewed in (1)). In that context, 17R-RvD1 as well as a D-series resolution precursor, 17R-HDHA, potently alleviate joint stiffness, inflammation, and inflammatory pain in a preclinical model of arthritis in rats (10). Both LXA4 and 17R-RvD1 counter-regulate production of TNF-α and TNF-α actions, and are protective in experimental models of arthritis (10, 23, 24). Of interest, SPM, including RvD3, enhance host-directed microbial responses and thus do not provoke immune suppression (1, 19, 25), a potential major side effect associated with many current RA therapeutics (2, 8). Thus, taken together with our present results on RvD3’s potent anti-arthritic actions, SPMs may represent a new class of molecules that are well suited to treat inflammation and inflammatory pain associated with arthritis without compromising host-directed responses.
Omega-3 PUFA, such as EPA and DHA, are now appreciated to exert beneficial actions in several human diseases with underlying inflammatory condition, like RA (26, 27), but their mechanisms have remained a subject of interest. In this regard, recent randomized controlled human trials indicate that omega-3 intake elevated 17-HDHA and RvD1 plasma levels in patients with chronic kidney diseases (28) as well as in chronic knee effusions and plasma in arthritis patients (29). Thus, these and our present findings may have implications for human chronic inflammatory diseases. Moreover, SPMs are present at bioactive concentrations in synovial fluid from RA patient (30), including RvD1 and RvD3 (24). Therefore in clinical settings RvD3 may represent one of the mediators involved in the etiopathology of RA. When taken together with our present results demonstrating that RvD3 is dysregulated in both human and murine arthritis, enhancing the endogenous D-series Rv may provide new therapeutic approaches for controlling arthritis and inflammation-mediated diseases. Moreover, these results with D-series resolvins provide a new molecular basis that may be responsible for some of the beneficial actions of omega-3 EFA in human RA.
Supplementary Material
Acknowledgments
The authors thank M.H. Small for expert assistance in manuscript preparation and B. Schmidt, MD at the Dept. of Pathology, Children’s Hospital Boston, for expert histopathology and microscopic evaluation of the arthritic joints.
This work was supported in part by the U.S. National Institutes of Health (grants P01GM095467 and R01GM38765, both to C.N.S.), the Arthritis Foundation (postdoctoral fellowship to H.H.A.), and Arthritis Research UK (carrier development Fellowship 19909 to L.V.N). J. Dalli is funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant 107613/Z/15/Z).
Abbreviations used in this article
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- EFA
essential fatty acid
- EPA
eicosapentaenoic acid
- HDHA
hydroxydocosahexaenoic acid
- LC-MS-MS
liquid chromatography tandem mass spectrometry
- LM
lipid mediators
- LT
leukotriene
- LTB4
leukotriene B4, (5S, 12R-dihydroxy-eicosa-6Z, 8E, 10E, 14Z-tetraenoic acid)
- LX
lipoxin
- LXA4
lipoxin A4 (5S, 6R, 15S-trihydroxy-eicosa-7E, 9E, 11Z, 13E-tetraenoic acid)
- 15R-LXA4
15R-lipoxin A4 (5S, 6R, 15R-trihydroxy-eicosa-7E, 9E, 11Z, 13E-tetraenoic acid)
- MaR
maresin
- MaR1
maresin 1 (7R, 14S-dihydroxy-docosa-4Z, 8E, 10E, 12Z, 16Z, 19Z-hexaenoic acid)
- MRM
multiple reaction monitoring
- PLS-DA
Partial least squares discriminant analysis
- PD
protectin
- PD1
protectin D1 (10R, 17S-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid), also known as neuroprotectin D1 (NPD1)
- 17R-PD1
17R-protectin D1 (10R, 17R-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid), also known as aspirin triggered neuroprotectin D1 (NPD1)
- PG
prostaglandin
- PGD2
11-oxo-9α, 15S-dihydroxy-prosta-5Z, 13E-dien-1-oic acid
- PGE2
9-oxo-11α, 15S-dihydroxy-prosta-5Z, 13E-dien-1-oic acid
- PGF2α
9α, 11α, 15S-trihydroxy-prosta-5Z, 13E-dienoic acid
- Rv
resolvin
- RvD1
Resolvin D1 (7S, 8R, 17S-trihydroxy-docosa-4Z, 9E, 11E, 13Z, 15E, 19Z-hexaenoic acid)
- 17R-RvD1
17R-Resolvin D1 (7S, 8R, 17R-trihydroxy-docosa-4Z, 9E, 11E, 13Z, 15E, 19Z-hexaenoic acid)
- RvD2
Resolvin D2 (7S, 16R, 17S-trihydroxy-docosa-4Z, 8E, 10Z, 12E, 14E, 19Z-hexaenoic acid)
- RvD3
Resolvin D3 (4S, 11R, 17S- trihydroxy-docosa-5Z, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid)
- 17R-RvD3
17R-Resolvin D3 (4S, 11R, 17R- trihydroxy-docosa-5Z, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid)
- RvD4
Resolvin D4 (4S, 5R, 17S- trihydroxy-docosa-6E, 8E, 10Z, 13Z, 15E, 19Z-hexaenoic acid)
- RvD5
Resolvin D5 (7S, 17S-dihydroxy-docosa-4Z, 8E, 10Z, 13Z, 15E, 19Z-hexaenoic acid)
- RvE1
Resolvin E1 (5S, 12R, 18R-trihydroxy-eicosa-6Z, 8E, 10E, 14Z, 16E-pentaenoic acid)
- RvE2
Resolvin E2 (5S, 18R-dihydroxy-eicosa-6E, 8Z, 11Z, 14Z, 16E-pentaenoic acid)
- RvE3
Resolvin E3 (17R, 18R-dihydroxy-eicosa-5Z, 8Z, 11Z, 13E, 15E-pentaenoic acid)
- SPM
specialized pro-resolving mediator
- Tx
thromboxane
- TxB2
9α, 11, 15S-trihydroxy-thromba-5Z, 13E-dien-1-oic acid
References
- 1.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol. Chem. 2012;287:10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koopman J, Moreland L, editors. Arthritis and Allied Conditions: A Textbook of Rheumatology. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 7.Yacoubian S, Serhan CN. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat. Clin. Pract. Rheumatol. 2007;3:570–579. doi: 10.1038/ncprheum0616. [DOI] [PubMed] [Google Scholar]
- 8.Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol. Rev. 2015;67:280–309. doi: 10.1124/pr.114.009639. [DOI] [PubMed] [Google Scholar]
- 9.Chan MM, Moore AR. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J Immunol. 2010;184:6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufton N, Perretti M. Therapeutic anti-inflammatory potential of formyl-peptide receptor agonists. Pharmacol. Ther. 2010;127:175–188. doi: 10.1016/j.pharmthera.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler JW, Orr SK, Dalli J, Cheng CY, Sanger JM, Chiang N, Petasis NA, Serhan CN. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 2016;6:18972. doi: 10.1038/srep18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janes KA, Yaffe MB. Data-driven modelling of signal-transduction networks. Nat. Rev. Mol. Cell Biol. 2006;7:820–828. doi: 10.1038/nrm2041. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misharin AV, Cuda CM, Saber R, Turner JD, Gierut AK, Haines GK, 3rd, Berdnikovs S, Filer A, Clark AR, Buckley CD, Mutlu GM, Budinger GR, Perlman H. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell. Rep. 2014;9:591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monach P, Hattori K, Huang H, Hyatt E, Morse J, Nguyen L, Ortiz-Lopez A, Wu HJ, Mathis D, Benoist C. The K/BxN mouse model of inflammatory arthritis: theory and practice. Methods Mol. Med. 2007;136:269–282. doi: 10.1007/978-1-59745-402-5_20. [DOI] [PubMed] [Google Scholar]
- 19.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D'Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, Zarbock A, Koenders MI, Axmann R, Zwerina J, Baenckler HW, van den Berg W, Voll RE, Kuhn H, Joosten LA, Schett G. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J Immunol. 2009;183:3383–3389. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 22.Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin. Arthritis Rheum. 2005;34:19–22. doi: 10.1016/j.semarthrit.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Conte FP, Menezes-de-Lima O, Jr, Verri WA, Jr, Cunha FQ, Penido C, Henriques MG. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br. J. Pharmacol. 2010;161:911–924. doi: 10.1111/j.1476-5381.2010.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1:e85922. doi: 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norris PC, Arnardottir H, Sanger JM, Fichtner D, Keyes GS, Serhan CN. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot. Essent. Fatty Acids. 2016 doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed GW, Leung K, Rossetti RG, Vanbuskirk S, Sharp JT, Zurier RB. Treatment of rheumatoid arthritis with marine and botanical oils: an 18-month, randomized, and double-blind trial. Evid. Based Complement Alternat. Med. 2014;2014:857456. doi: 10.1155/2014/857456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012;107(Suppl 2):S171–S184. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- 28.Mas E, Barden A, Burke V, Beilin LJ, Watts GF, Huang RC, Puddey IB, Irish AB, Mori TA. A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin Nutr. 2016;35:331–336. doi: 10.1016/j.clnu.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot. Essent. Fatty Acids. 2016;107:24–29. doi: 10.1016/j.plefa.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, Serhan CN, Mayboroda OA. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim. Biophys. Acta. 2012;1821:1415–1424. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.